Abstract
Dispersal is a central biological process tightly integrated into life‐histories, morphology, physiology and behaviour. Such associations, or syndromes, are anticipated to impact the eco‐evolutionary dynamics of spatially structured populations, and cascade into ecosystem processes. As for dispersal on its own, these syndromes are likely neither fixed nor random, but conditional on the experienced environment. We experimentally studied how dispersal propensity varies with individuals' phenotype and local environmental harshness using 15 species ranging from protists to vertebrates. We reveal a general phenotypic dispersal syndrome across studied species, with dispersers being larger, more active and having a marked locomotion‐oriented morphology and a strengthening of the link between dispersal and some phenotypic traits with environmental harshness. Our proof‐of‐concept metacommunity model further reveals cascading effects of context‐dependent syndromes on the local and regional organisation of functional diversity. Our study opens new avenues to advance our understanding of the functioning of spatially structured populations, communities and ecosystems.
Keywords: context‐dependent dispersal, dispersal strategy, distributed experiment, predation risk, resource limitation
Dispersal is a central biological process tightly integrated into life histories, morphology, physiology and behaviour. Such associations, or syndromes, are anticipated to impact the eco‐evolutionary dynamics of spatially structured populations, and cascade into ecosystem processes. With a coordinated distributed experiment involving five laboratories across Europe and 15 focal study organisms covering the tree of life, we demonstrate that dispersal is generally positively related to three ubiquitous and functionally important traits and that the harshness of environment strengthens the link between dispersal and morphological traits.

INTRODUCTION
Species and populations consist of phenotypically variable individuals with potentially different ecological functions. This heterogeneity can result from neutral processes and environmental constraints, or from adaptive processes in response to environmental variation during life or evolutionary history (Ridley, 2004). Phenotypic heterogeneity can extend beyond single traits and manifest as suites of correlated traits in phenotypic syndromes (Raffard et al., 2017; Sih et al., 2004). This intraspecific variation shapes individual strategies and fitness, and is at the nexus of evolutionary and ecological dynamics (Hendry, 2017). Indeed, evidence is accumulating on the importance of phenotypic variation for the functioning and stability of populations, communities and ecosystems, and their responses to changing environments (Bolnick et al., 2011; Moran et al., 2016; Raffard et al., 2019). More phenotypically variable populations may for example less vulnerable to environmental changes, better colonise novel environments and are less prone to extinction. This influence of intraspecific variation should be further magnified in challenging and stressful environments (Forsman & Wennersten, 2016; Hämäläinen et al., 2021). With ongoing global changes, it is thus of prime importance to better understand the expressed variation in functional traits within and across populations, taxonomic groups and environments.
A particularly relevant intraspecific variation pertains to dispersal strategies (e.g. dispersing vs. philopatric, short‐ vs. long‐distance dispersal), because dispersal determines population dynamics and gene flow throughout landscapes (Clobert et al., 2012), and thereby eco‐evolutionary dynamics (Bonte & Dahirel, 2017; Legrand et al., 2017), species range dynamics (Fronhofer et al., 2017) and local adaptation patterns. Variation in dispersal strategies often results from covariation between dispersal and other morphological, physiological and behavioural traits, referred to as dispersal syndromes (Clobert et al., 2009). These syndromes can be observed at population and species levels (Comte & Olden, 2018; Cote, Bestion, et al., 2017; Le Galliard et al., 2012; Stevens et al., 2014) and can strongly influence metapopulation dynamics (Jacob et al., 2019) and community and ecosystem functioning (Cote, Brodin, et al., 2017; Little et al., 2019). They are determined by the genetic background, the environmentally induced plasticity or their combination (Goossens et al., 2020). Contrary to many theoretical simplifications, dispersal syndromes are thus not necessarily fixed, but conditional on the environment (Bonte & Dahirel, 2017; Cote, Bestion, et al., 2017). A more in‐depth understanding of dispersal syndromes and their dependence on contrasting environments is hence fundamental to predict species persistence under global change (Cote, Bestion, et al., 2017; Raffard et al., 2021; Travis et al., 2013).
The costs and benefits of dispersal are often determined by the harshness of encountered environments, and therefore dispersal syndromes may vary in magnitude between challenging and benign environments as supported by a few empirical studies (Bestion et al., 2014; Byers, 2000; Gilliam & Fraser, 2001; Kim, 2000). Benign conditions may buffer individual variation in the costs of philopatry and dispersal while challenging conditions may amplify that variation. Even though different types and intensity of stress may result in different filtering of phenotypes during dispersal, challenging conditions should generally accentuate the strength of covariation between dispersal and phenotypic traits compared with benign situations (Figure 1). Context‐dependent dispersal syndromes could strongly influence population, community and ecosystem functioning through non‐random flows and distributions of phenotypes and genotypes throughout landscapes. It could, for example, lead to adaptive dispersal, where individuals select habitats according to their expected fitness. This habitat choice allows for a match between the phenotype and the local environment and increases individual fitness as well as the mean fitness in the landscape. Such adaptive dispersal may further reduce spatial variation in fitness and stabilise metapopulation dynamics (Figure 1). So far, conditional dispersal syndromes have only been investigated in a species‐by‐species approach with a narrow taxonomic range and focused on a few traits and to a single dimension of the environment (reviewed in Cote, Bestion, et al., 2017). Accordingly, the few comparative studies on dispersal syndromes have demonstrated links between species‐averaged phenotypic traits and dispersal propensity across taxa rather than within‐species syndromes and their environmental dependencies (Comte & Olden, 2018; Le Galliard et al., 2012; Stevens et al., 2012, 2014). It is yet to be determined if within‐species dispersal syndromes can be generalised across taxa and how these syndromes vary with environmental conditions (e.g. competition and predation risk). General rules across taxonomic groups would guide efforts of predictive modelling and biodiversity management.
FIGURE 1.
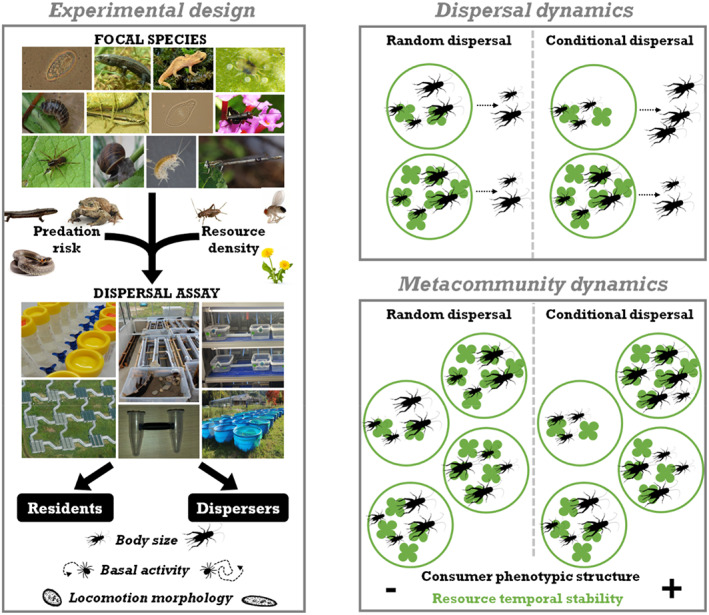
The experimental design testing for cross‐species conditional syndromes and an example of potential consequences on dispersal and metacommunity dynamics. The experimental design (left panel) manipulates the predation risk and resource density for 15 species in multi‐patch systems to obtain dispersers and residents which were then characterised for their body size, basal activity and locomotion morphology. The right panel shows the dispersal dynamics for scenarios where dispersal was random (left column) or dependent on individual phenotype (here the body size of crickets, right column) and local conditions (here the amount of clover plants in a patch) on top and theoretical consequences of such dispersal modes for metacommunity dynamics on the bottom. In the random scenario, the dispersal rate and body size of dispersers do not vary with patch resources; in the conditional dispersal scenario, dispersers are more numerous and bigger when patch resources are low. Context‐ and phenotype‐dependent dispersal results in the spatial structuring of consumers' population size and phenotype (body size) where patches with more resources host more and bigger consumers and equalise per capita consumption rate whereas random dispersal does not. We expect context‐ and phenotype‐dependent dispersal to equalise the resource densities between patches and stabilise metacommunities.
We performed a meta‐experiment investigating the generality of dispersal syndromes involving three phenotypic traits in benign and challenging environments across 15 species ranging from protists to vertebrates (Table S1). This experiment extends our previous research on the generality of context‐dependent dispersal strategies (Fronhofer et al., 2018) by analysing phenotypic differences between dispersers and residents, addresses how they vary with local conditions, and extends their consequences for metacommunity dynamics (Figure 1). Using experimental systems connecting habitat patches through a hostile matrix, we tested whether body size, locomotion morphology and basal activity level, three ubiquitous traits impacting local dynamics, vary with individuals' dispersal behaviour. We compared groups of dispersers and residents of each species for their average phenotype and their correlations between the three phenotypic traits. Furthermore, we compared these differences between benign and challenging environments by manipulating two stressors: predation risk and local competition for resources. We find cross‐species relationships between intraspecific variation of dispersal and phenotypic traits, and this trait covariation depends on local environmental contexts. To illustrate how a context‐dependent dispersal syndrome could influence ecological dynamics, we developed a simple proof‐of‐concept metacommunity model. We studied the consumption rate and density of a sessile resource interacting with a consumer dispersing across a patchy landscape according to its body size and local resource availability, following our main empirical results, and discussed the role of dispersal syndromes on the stability of this consumer‐resource system (Figure 1).
MATERIALS AND METHODS
Study organisms
This meta‐experiment involves five laboratories across Europe, initially focusing on dispersal rate in 21 aquatic, terrestrial and aerially dispersing species of protists, arthropods, molluscs and vertebrates (Fronhofer et al., 2018). Here, we expanded the focus to study phenotypic differences between dispersers and residents for a subset of 15 species for which phenotypic traits could be measured (Table S1). Resources and predators of these focal species were chosen for their natural co‐occurrences (see SI).
Experimental design
We used the same experimental procedure for all species with multi‐patch micro‐ or semi‐natural mesocosms systems (three patches for minnows and two patches for other species), densities and living conditions adapted to the studied species to mimic natural conditions (see SI). Multi‐patch systems were characterised by the presence of a ‘hostile matrix’ connecting patches suitable for the studied species. It guaranteed that inter‐patch relocation represents dispersal decisions rather than routine foraging movement.
We applied a full factorial design crossing two levels of resource availability and predation risk. We provided either ad libitum resources (‘standard’ condition) or limited resources. Predation risk was represented by the presence or absence of chemical cues (coupled with auditory and visual cues for damselflies) of a natural predator of the focal species. The treatments were applied to the patch of origin while reference conditions (standard resources, no risk) were applied to the initially empty ‘target’ patch.
After populating the ‘origin’ patch, we allowed an acclimation phase without dispersal. It lasted approximately a quarter of the subsequent dispersal phase duration and treatments were applied throughout acclimation. The absolute durations of acclimation and dispersal phases were adapted to species (see SI). Due to experimental feasibility, the numbers of replicates per treatment varied among species, and were 2 (1 species), 4 (1 species), 5 (5 species), 6 (7 species) and 8 (1 species).
Data collection
After the dispersal phase, the number, the body size, the locomotion morphology and the basal activity level of residents (individuals that never left their ‘origin’ patch) and dispersers (individuals that left their ‘origin’ patch and reached the target patch) for each population (i.e. replicate) were measured using video analysis or direct measurements. The exact methods to measure traits were adjusted to the specific species (see SI). Locomotion morphology was taken as either the length or weight of the locomotory apparatus relative to body size (e.g. legs in lizards and newts, wings in damselflies or foot in molluscs), and body elongation (e.g. in protists, fish or woodlice). Basal activity was measured without stressors in independent assays from dispersal as the swimming velocity (protists), time spent moving during a given time (e.g. in fishes or damselflies), or distance moved during a given time (e.g. in mites or slugs, Noonan et al., 2019). Activity level was measured alone or in a group (i.e. dispersers and residents of each population separately) depending on the species due to methodological constraints. We checked that methodological differences for locomotion morphology and basal activity did not influence the results (Tables S22–S24).
Statistical analysis
All analyses were done on mean body size, locomotion morphology and basal activity per population and dispersal status (i.e. dispersers or residents within each replicate). Traits were log‐transformed when needed to achieve normality and standardised to have comparable metrics across species and analyses insensitive to differences in variance between species and to species‐specific units (e.g. pixels or centimetres for body size). For a consistent interpretation of locomotion morphology, we needed positive values of the trait to be associated with higher predicted moving ability for each species. When this was not the case, we multiplied it by −1.
Statistical analyses were performed using R version 3.6.1 (R Core Team, 2013). We first analysed effects of treatments and dispersal status on all species using linear mixed effects models (lme4; Bates et al., 2019) on body size, locomotion morphology and activity level separately (see Tables S2–S4). Fixed effects included resource availability, predation risk, dispersal status and the two‐way interaction between dispersal status and each environmental condition. We did not include three‐way interactions because of the limited sample size for each species in each combination. As random intercepts, we included population identity (replicate) nested within experimental blocks of replicates within species within taxon (‘protists’, ‘arthropods’, ‘molluscs’ and ‘vertebrates’) to account for potential phylogenetic non‐independence (see Table S1). However, we also ran models with only species and population as random effects to check that over‐parameterised random structure would not lead to spurious results and that a simple random structure led to the same results (Tables S12–S15). To prevent unbalanced effects among species, we ran analyses at the population (i.e. replicate) level rather than at the individual level, and the difference in sample size (i.e. number of populations) among species was accounted for by adding the inverse of the square root of the sample size (i.e. number of populations × dispersal status) as a weighting parameter. Results were similar with and without weight (Tables S16–S19). Model selection using AICc was performed on a set of models including dispersal status, resource availability, predation risk, the interaction between resource availability and dispersal status and the interaction between predation risk and dispersal status to the intercept model (Tables S2–S4). The top best models (ΔAICc < 2) were used in a model averaging method. We provide conditional average parameter estimates and their statistical tests (z‐test for averaged best models and t‐test for single best models) and relative variable importance in averaged models for each retained variable.
We confirmed the results observed in Table 1 using a meta‐analysis framework. We first calculated, for each species, effects sizes and standard errors of the dispersal syndrome. We used general linear mixed models with each phenotypic trait as a dependent variable, population identity as a random intercept, and dispersal status, interactions between dispersal status and environmental conditions as fixed effects. When a conditional dispersal syndrome was identified in best models (Table 1), we also obtained effect sizes of dispersal syndrome in each environmental condition (Table 2). We analysed the variation and the mean of effect sizes using random effects meta‐analyses with a restricted maximum likelihood and inverse‐variance weights (Metafor package; Viechtbauer, 2010). We ran this analysis on the effect sizes for dispersal syndromes over all conditions and on each condition when a conditional dispersal syndrome was identified. We report average estimates, confidence intervals, Wald‐type tests and residual heterogeneity I 2 of effect sizes (Table 2). I 2 can be interpreted as the proportion of the total variation of the effect size among species and is using the Q statistics to estimate within‐study sampling variance (Higgins & Thompson, 2002).
TABLE 1.
Dispersal syndromes for body size, movement morphology and basal activity level depending on environmental contexts
| Dependent variable | Parameter | Estimate | SE | p‐value | Relative importance |
|---|---|---|---|---|---|
| Body size | Intercept | 0.43 | 0.10 | <0.001 | — |
| RA | −0.28 | 0.12 | 0.027 | 1.00 | |
| PRED | −0.12 | 0.08 | 0.131 | 1.00 | |
| DISP | −0.64 | 0.11 | <0.001 | 1.00 | |
| RA × DISP | 0.43 | 0.16 | 0.007 | 1.00 | |
| Movement morphology | Intercept | 0.21 | 0.10 | 0.029 | — |
| RA | −0.08 | 0.08 | 0.321 | 1.00 | |
| PRED | 0.18 | 0.13 | 0.167 | 1.00 | |
| DISP | −0.37 | 0.12 | 0.002 | 1.00 | |
| PRED × DISP | −0.27 | 0.15 | 0.084 | 0.61 | |
| Basal activity level | Intercept | 0.24 | 0.09 | 0.008 | — |
| RA | 0.08 | 0.10 | 0.429 | 1.00 | |
| PRED | −0.07 | 0.09 | 0.408 | 1.00 | |
| DISP | −0.45 | 0.10 | <0.001 | 1.00 | |
| RA × DISP | 0.08 | 0.17 | 0.629 | 0.28 |
Notes: Parameter estimates, standard error and relative importance from the best model or from averaged models (when multiple models with ∆AIC < 2) of dispersal syndromes. Estimates for resource availability (RA), predation risk (PRED) and dispersal status (DISP, residents vs. dispersers) are given for standard resources, presence of predation risk and residents respectively. p‐values are estimated with z‐test for averaged best model (i.e. morphology and activity) and with t‐test for single best models (i.e. body size). The best models explained 25%, 23% and 25% of the marginal variance and 41%, 50% and 25% of the conditional variance, respectively, for body size, movement morphology and basal activity level (calculated on the best model or on the model with the same structure as averaged best models). Random intercepts are population identity within experimental block within species within taxon, population identity within treatments. See full model selection in Tables S2–S4.
TABLE 2.
Effect sizes from random effects meta‐analyses of dispersal syndromes, that is bias of phenotypic values for dispersers compared to residents
| Phenotypic trait | Parameter | Estimate [CI] | I 2 [CI] |
|---|---|---|---|
| Body size | ALL | 0.23 ± 0.08 [0.08–0.39], p = 0.003 | 78.4 [58.5–90.4] |
| ABU | 0.17 ± 0.11 [−0.04–0.39], p = 0.117 | 82.3 [65.7–92.2] | |
| LOW | 0.34 ± 0.09 [0.17–0.51], p = 0.001 | 70.3 [41.9–86.3] | |
| Locomotion morphology | ALL | 0.24 ± 0.07 [0.11–0.38], p = 0.004 | 75.4 [53.1–89.4] |
| NOPRED | 0.25 ± 0.09 [0.07–0.42], p = 0.006 | 80.9 [59.7–91.3] | |
| PRED | 0.31 ± 0.10 [0.12–0.50], p = 0.001 | 84.2 [69.5–93.2] | |
| Activity | ALL | 0.25 ± 0.10 [0.06–0.43], p = 0.009 | 88.9 [77.0–95.2] |
Notes: Only effect sizes with substantial effects in best averaged models (see statistical and results sections and Table 1) are reported. Estimates and confidence intervals (CI) and heterogeneity index I 2 of effect sizes among species (%) are given for each phenotypic trait overall environmental conditions (i.e. ALL) or in each environmental condition (ABU, abundant resources; LOW, low resources; NOPRED, without predation risk; PRED, with predation risk) depending on averaged models (Table 1).
Finally, we investigated how correlations between phenotypic traits varied between dispersers and residents. We used three linear mixed models in which one of the three phenotypic traits was the dependent variable and fixed effects were one of the remaining two phenotypic traits, dispersal status, resource availability, predation risk and up to three‐way interactions (Tables S5–S11). Random intercepts were population identity nested within experimental block within species within taxon. All models were tested and the top best models (ΔAICc < 2) were used in a model averaging method. We provide conditional average parameter estimates and their statistical tests (z‐test or t‐test) and relative variable importances in averaged models for each retained variable. We checked that the choice of dependent variable when studying the covariation between two phenotypic traits did not matter by re‐running the analysis with dependent and explanatory variables switched (Tables S20–S22).
For all linear mixed models, we checked for the assumptions of general linear model and the robustness of results to the exclusion of one potential outlier identified on QQ‐plots for activity level (Tables S23–S24). We also calculated marginal and conditional R 2 (R 2 m and R 2 c, Nakagawa & Schielzeth, 2013) on the best model or the model with the same structure as averaged models.
Resource‐consumer metacommunity model
We modelled a simple resource‐consumer metacommunity subject to spatio‐temporal environmental variability. The metacommunity is composed of 10 identical patches populated by consumers that can disperse between patches. Individuals can be either a disperser or a resident from a random draw with a probability p D and independently of the parental dispersal status. Individuals consume resource, so that the amount of resource available in a patch decreases with the number of individuals present and with the per capita resource consumption. We compared four dispersal scenarios exploring our main results on body size and resource‐dependent dispersal (Figure 2a). Given the association between body size and resource consumption (Peters, 1986), we factorially explored the consequences of dependencies of emigration rate to body size, resource or both on the rate and variability of resource consumption (Figure 1): (1) Dispersers are of similar size, and therefore consume as much as residents, and leave their patch regardless of the resource availability in the patch (random dispersal); (2) Dispersers are of similar size but emigrate more from their patch when resources are low (resource‐dependent dispersal); (3) Dispersers are larger, and therefore consume more than residents, and leave their patch regardless of the resource availability in the patch (size‐dependent dispersal) and (4) Dispersers are larger and emigrate more from their patch when resources are low (size‐ and resource‐dependent dispersal). Dispersers choose their new patch uniformly at random. We compared the different scenarios for a range of dispersal probability p D. When comparing scenarios and when varying p D, we fixed the mean resource consumption per individual in the metacommunity. We also kept the variation in consumption rates similar in scenarios with and without a syndrome.
FIGURE 2.
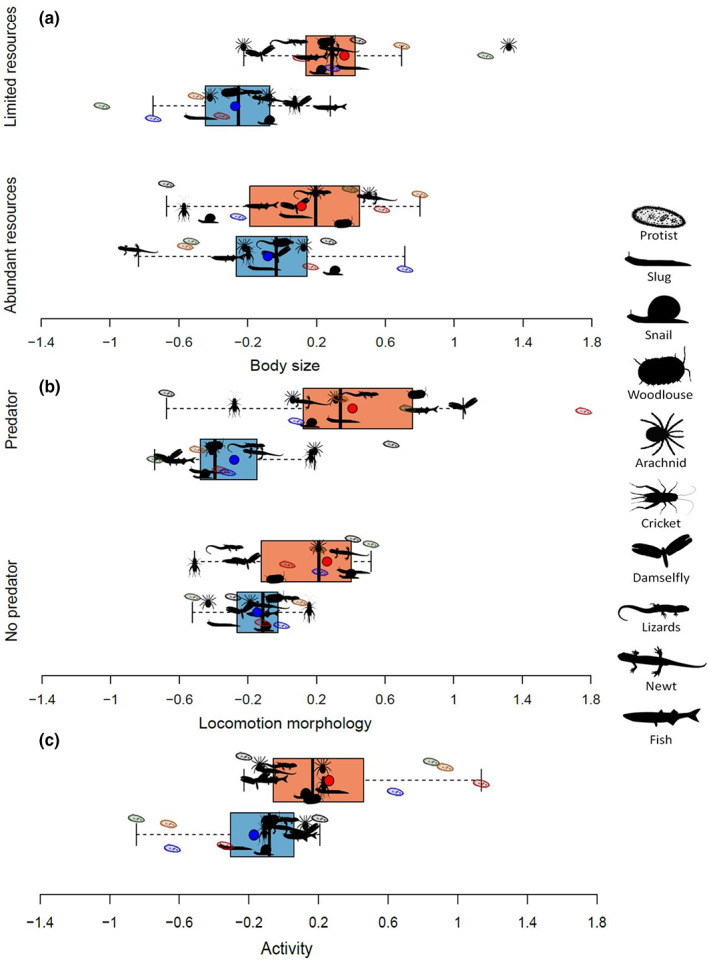
Dispersal syndrome conditional to environmental context. (a) Size dispersal syndrome according to resource availability. (b) Locomotion morphology dispersal syndrome according to predation risk. (c) Activity dispersal syndrome. Horizontal boxplots for dispersers (light red bars) and residents (light blue bars) are built from the mean traits for each species and the mean trait value (dark red and dark blue dots) are shown. To isolate the phenotypic differences between dispersers and residents, we used residuals of a model controlling for resource and predation treatments as fixed effects and population identity nested within experimental block within species within taxon as a random intercept, but not for dispersal status and its interaction with resources and predation treatments. The colours of protist pictograms represent different species (black: Colpidium sp., red: Dexiostoma sp., orange: Tetrahymena thermophila, blue: Tetrahymena elliotti, green: Tetrahymena pyriformis).
We subjected the metacommunity to a random series of local extinctions at a similar rate in all scenarios. After extinction, the patch is recolonised by dispersers. This sequence of extinction and recolonisation events determines the metacommunity dynamics. We compared the response of the metacommunity to these perturbations for the four scenarios. Simulations were performed until reaching a stationary state. We computed the consumer and resource density per patch and the mean individual and patch consumption rate and their spatial and temporal coefficient of variation of the patches. Here, we show the results of patches consumption rate and resource density (see SI for a full description, Figure S1). Results for spatial and temporal variation were similar. We ran 100 simulations per dispersal probability value and used 100 time points per simulation to compute temporal statistics (see SI for details of the model).
RESULTS
The three traits substantially differed between dispersers and residents (Tables 1 and 2, Tables S2–S4, Figure 2): dispersers were overall larger, with a marked locomotion morphology (e.g. larger wings, longer legs, more elongated cells/bodies) and had higher basal activity levels than residents. However, we observed marked variation in these resident‐disperser differences among phenotypic traits (Table 1), environmental contexts (Table 1) and species (I 2, indices of heterogeneity among species, Table 2, Figures S2–S4). The size difference between dispersers and residents was larger when resources were limited in the patch of departure than when resources were abundant (Tables 1 and 2, Figure 2a). The difference in locomotion morphology was larger with higher predation risk in the patch of departure, but this environmental dependency was of much lower magnitude than the dependency of size difference on resource limitation (Table 2, Figure 2b). While a dependency on resources for basal activity appeared in the averaged best models, this effect was marginal according to the relative importance and effect sizes (Table 1, Table S4, Figure S5). Aside from these general patterns, the strength, direction and environmental dependency of dispersal syndromes varied among species (I 2 in Table 2, Figures S2–S4).
We then compared correlations among the three traits between dispersers and residents. The correlation between body size and locomotion morphology depended on dispersal status and predation risk (Tables S5 and S6, Figure 3). In a predation risk context, body size and locomotion morphology were positively correlated in dispersers (estimate: 0.26 ± 0.09, Chi2 = 8.92, p‐value = 0.003, Table S7) and negatively correlated in residents (estimate: −0.18 ± 0.07, Chi2 = 5.99, p‐value = 0.015, Table S7), while not correlated in the absence of predation risk (Table S7, Figure 3). The correlation between basal activity and body size also depended on dispersal status, but not on environmental conditions (Tables S8 and S9, Figure S6). The two traits were positively correlated in dispersers (estimate: 0.22 ± 0.06, Chi2 = 11.87, p‐value < 0.001) but not in residents (estimate: 0.04 ± 0.06, Chi2 = 0.53, p‐value = 0.468). Finally, the correlation between basal activity and locomotion morphology was independent from dispersal status but depended on predation risk (Tables S10 and S11). The two traits were correlated in presence of predation risk (estimate: 0.18 ± 0.06, Chi2 = 7.47, p‐value = 0.006), but not in absence (estimate: 0.001 ± 0.06, Chi2 = 0.00, p‐value = 0.985).
FIGURE 3.
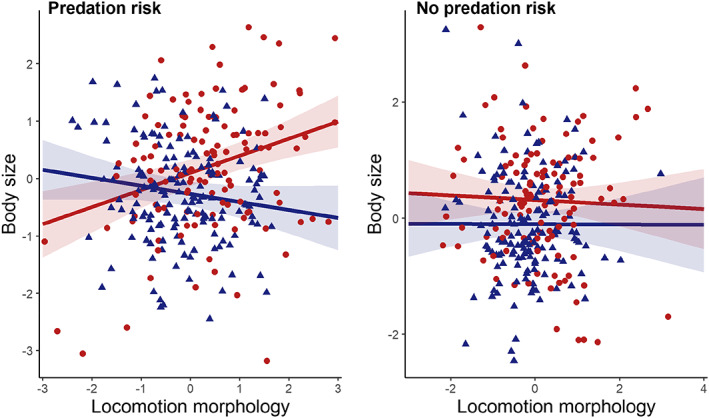
Correlations between body size and locomotion morphology between dispersers and depending on predation risk. All raw data points and predicted lines (± 95% CI) for dispersers (light red ribbons) and residents (light blue ribbons) in the predation risk treatments are shown.
Our metacommunity model illustrates the impact on the spatiotemporal variability of a consumer‐resource metacommunity of one of our empirical results: the dependencies of dispersal on body size and patch resource availability (Figure 2a), assuming that individual consumption rate was positively related to body size (Peters, 1986). We compared temporal mean and coefficient of variation of resource consumption and population density per patch for four scenarios in which consumers' dispersal was dependent on body size and/or local resource availability or not (Figure 4). Differences among dispersal scenarios emerge for intermediate probabilities of dispersal. Compared to scenarios where dispersal was independent of phenotypes and resources, the dependencies of dispersal on individual body size, resource availability or both had positive additive effects on the temporal mean of consumption rate, and consequently negative effects on resource density in patches (Figure 4a,c). Importantly for metacommunity stability, the temporal variation in consumption rate and resource density was strongly decreased by the tested context‐dependent dispersal syndrome (Figure 4b,d).
FIGURE 4.
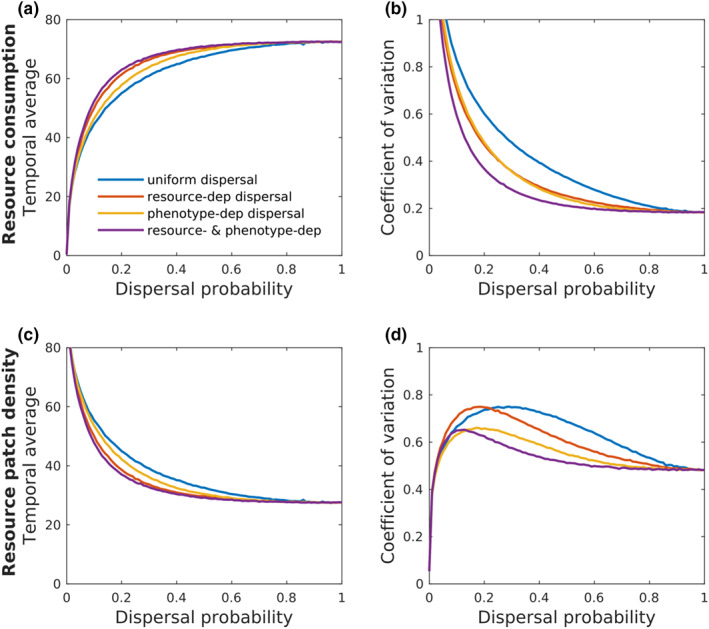
Theoretical illustration of resource consumption and density in relation to consumers' dispersal dependencies. The dispersal of consumers was either independent of individual consumption rate and patch resource density (blue line, ‘uniform dispersal’) or positively covaried with patch resource density (orange line, ‘resource‐dep dispersal’), individual consumption rate (yellow line, ‘phenotype‐dep dispersal’), or both (purple line, ‘resource & phenotype‐dep dispersal’). Response variables are shown as the temporal average and coefficient of temporal variation of patches resource consumption (a & b respectively) and resource patch density (c & d respectively).
DISCUSSION
Dispersal influences ecological and evolutionary processes on broad temporal, spatial and biological scales from demography to macroevolution, local dynamics to species range expansion, and genetic diversity to ecosystem functioning (Clobert et al., 2012). Across major taxonomic groups, we demonstrated similar covariations between dispersal strategies (here categorised as dispersal and philopatry) and three universal phenotypic traits. Some of these covariations were strengthened under local resource limitation or predation risk. As a plastic response to adverse local conditions, dispersal shapes the dynamics and stability of trophic interactions and more generally of metacommunities and metaecosystems (Haegeman & Loreau, 2014; Massol et al., 2017). These consequences will likely be modulated by the observed coupling of dispersal with functionally important traits such as body size or basal activity.
We found a general within‐species dispersal syndrome across a wide range of species, from protists to vertebrates. Dispersers were larger with a morphology better adapted to movements and more active than residents. These differences are suitable phenotypic specialisations allowing dispersers to move farther and/or faster (Bonte et al., 2012), corroborating results obtained from species‐by‐species and single trait approaches (reviewed in Cote, Bestion, et al., 2017). By reducing the costs of dispersing and opening up a broad range of potential habitats to settle in, these phenotypic specialisations should enhance dispersers' fitness (Bonte et al., 2014). The resulting balanced fitness between dispersers and residents is expected to maintain the large variation in dispersal strategies (and associated traits) that is often reported in wild populations (Clobert et al., 2012). The dispersal specialisations with respect to body size, and to a lower extent to locomotion morphology, were accentuated when local conditions were adverse, while the activity syndrome was not conditional on local conditions.
Dispersers and residents differed in body size only when resources were low (‘harsh conditions’). Potential explanations lie in the size‐dependence of competitive ability, energy availability and energetic needs. It is generally presumed that only large individuals or individuals in good condition may have enough energy reserves to overcome the costs and risks of dispersal (Bonte et al., 2012) and disperse over long distances to settle in better habitats. On the other hand, smaller individuals are often less competitive and might disperse earlier or more (Bowler & Benton, 2005). The resulting dispersal decision will thus depend on how individuals' phenotype, particularly body size and condition, tip the balance between dispersal costs and the strength of local competition. These dispersal decisions can result in positive, negative, or quadratic relationships between dispersal and body size or condition (e.g. Le Galliard et al., 2012). In habitats with limited resources and so with high levels of competition, the weaker competitors, the smaller individuals, may disperse more. Alternatively only bigger individuals with more energy available and larger energetic needs may be able to search for habitats with more resources without increasing their mortality risk. Our results suggest that increasing competition for resources tips the balance towards a positive dispersal‐size syndrome.
Dispersers also had a more locomotion‐oriented morphology than residents (e.g. longer legs, elongated shape), which should give them better ability to move farther and faster. This syndrome was slightly stronger under predation risk. Such a pattern is consistent with the few previous studies showing that predation risk modifies the relationship between phenotype and dispersal, either reinforcing it (Bestion et al., 2014; Gilliam & Fraser, 2001) or diminishing it (Cote et al., 2013). It could be explained by individuals with a more locomotion‐oriented morphology being better able to escape predators while moving through a landscape of fear, or more at risk in habitats with higher predation risk. In contrast, the harshness of environment did not accentuate the basal activity difference between residents and dispersers. We expected a positive association between activity and dispersal in benign environments because more active individuals should disperse faster or at lower cost, and a reinforcement of this syndrome in challenging environments because more active individuals might have lower expected fitness when resources are limited (i.e. high activity level requires more energy) and when predation risk is high (i.e. high activity level increases encounter rates with predators). We hypothesize that the independence of syndrome to local environments results from a variation in species' strategies to mitigate adversity. High activity level can reduce the impact of limited resources and predation risk in some species while a low level of activity might do so in other species. For example, in presence of predators, a positive association between dispersal and activity might allow ciliates to escape predation during dispersal, while snails might rather opt for slow and understated movements. Alternatively, the context‐independency of activity syndrome could be explained by differences in metrics. We indeed chose activity and morphology metrics to match species biology and literature rather than to force homogeneity of metrics among species. Locomotion morphology was measured with organ size (six species) or body elongation (nine species) and basal activity was measured with distance travelled per time unit, proportion of time spent moving or speed (five species each). However, we scaled phenotypic traits at the species level, and controlling for metrics used did not change results (Tables S25–S27). We therefore believe such a methodological artefact is unlikely.
We further show that the correlations between phenotypic traits vary with dispersal status and/or environmental conditions. While dispersal syndromes theoretically involve a suite of phenotypic specialisations, studies generally focus on the correlation between one trait and dispersal, but surprisingly rarely investigate the correlations among different traits (e.g. Bonte & Dahirel, 2017; Jacob et al., 2020) and never investigate changes in trait correlations between dispersers and residents. This is even more surprising considering the general fitness consequences of negative and positive correlations among traits (Laughlin & Messier, 2015). Here, the correlation between basal activity and body size was stronger in dispersers than in residents and the correlation between body size and locomotion morphology was positive in dispersers and negative in residents in presence of predation risk. This suggests that, aside from being on average larger, morphologically adapted to movement and more active individuals, there is still some variation in these traits that covary positively among dispersers. This means that dispersers are distributed, at least in presence of predation risk, along phenotypic axes from more active, larger and with a more locomotion‐oriented morphology to less active, smaller and with a less locomotion‐oriented morphology. This repertoire of dispersal strategies in a species may result in immigration to varying patches (e.g. more or less distant from the departure patch, or with more or less competitors) and may contribute to global patch occupancy in a landscape and to metapopulation persistence. Overall, dispersers cannot be pictured as a unique kind of individual (Junker et al., 2021), suggesting the existence of a range of dispersal strategies to move across adverse environmental conditions.
Consistent with hypotheses on pace‐of‐life syndromes (Hämäläinen et al., 2021), the relationships between dispersal status and two phenotypic traits are accentuated in some harsh and challenging conditions (Figure 1). Our results on within‐species syndromes generally align with previous comparative studies investigating these relationships at the species level for body size and, to a smaller extent, for locomotion apparatus and basal activity (Comte & Olden, 2018; Le Galliard et al., 2012; Stevens et al., 2012, 2014). This supports our prediction of broad and deterministic processes optimising the association between dispersal and other traits. These comparative studies, however, reported a large variation of syndromes among species with positive, null and quadratic relationships at the species level between body size and dispersal propensity among taxonomic groups (Stevens et al., 2014), and when comparing dispersers and residents in a few vole species (Le Galliard et al., 2012). We suggest that this heterogeneity may be explained by an overall dependency of dispersal syndromes on local conditions and strong variation among species. Indeed, we found substantial variation among species in the dispersal syndrome and their dependencies on environmental conditions (heterogeneity of effects sizes I 2 in Table 2 and Figures S1–S3). This is particularly true for predation risk, which matches our previous results showing a larger heterogeneity of dispersal rates between species according to predation risk than to resource scarcity (Fronhofer et al., 2018).
Our proof‐of‐concept model shows potential effects on metacommunity stability caused by a context‐dependent dispersal syndrome as evidenced for body size by our meta‐experiment. Local resource limitation increased consumer departure rate, particularly of large consumers. Through the relationships between body size, energetic needs and consumption rates (Peters, 1986), this dependence of dispersal on resource availability and body size may influence consumer–resource dynamics. Our metacommunity model indeed shows that the observed dispersal syndromes and their dependence on local resources increase the spatiotemporal variability of consumers' individual consumption rate (Figure S1) and largely reduce local fluctuations of patches resource consumption (Figure 4). This conditional dispersal syndromes thus stabilize resource density over time. Together with previous models featuring context‐dependent dispersal rates (Fronhofer et al., 2018; Quévreux et al., 2021), our results point the importance of context‐dependent dispersal rates and syndromes in equalising resource consumption among patches of heterogeneous landscapes and therefore in stabilising resource metapopulation. The metacommunity model in Fronhofer et al. (2018) shows the stabilising effect of context‐dependent dispersal rates by dampening local temporal fluctuations in resources and consumers population sizes and by desynchronising fluctuations among patches. We show here additive effects of the context‐dependency of syndromes (Figure 4, yellow and purple lines) and of dispersal rate (Figure 4, red lines) on the variation of patches consumption rate and resource density with potential consequences on metacommunity stability that should be further studied with more complex models integrating the full palette of context‐dependent dispersal syndromes.
Our study shows that the intraspecific variation in dispersal strategies, resulting from the joint action of internal and external forces, is widespread and departs from the neutral assumption. Although broadening the taxonomic coverage should be considered by adding for example endotherm or bacteria species, the first principles shaping within‐species dispersal syndromes and its dependence on the environment should apply to a large proportion of biodiversity. The observed phenotypic differences between dispersers and residents may either result from plastic changes induced by local conditions and/or movement itself, from selective phenotypic bias during dispersal, or from covariation between pre‐dispersal phenotypic variation and the propensity to disperse from different local environments (Cote, Bestion, et al., 2017). While these explanations have different evolutionary consequences, which should be further investigated, they can all cause changes in phenotypic distribution and functional biodiversity across landscapes. This may have considerable consequences on demography, food webs and ecosystem functioning especially in a changing and more heterogeneous world (Bolnick et al., 2011; Dahirel et al., 2017; Moran et al., 2016). We advocate for the inclusion of this variation—the context‐dependency of dispersal syndromes—in theoretical and empirical studies forecasting biodiversity and the influence of dispersal on ecological and evolutionary dynamics.
AUTHOR CONTRIBUTIONS
F.A., D.B., A.C., J.C., M.D., F.D.L., E.A.F., D.L., S.J., E.L., S.M., Fr.P. and N.S. designed the research. This research was designed during a meeting of the dispNet group (https://dispnet.github.io/) organised at UC Louvain by N.S. and D.B. F.A., A.A., S.B., D.B., J.C., M.D., F.D.L., J.D.R., L.D.G., E.A.F., D.L., S.J., O.K., E.L., C.J.L., L.M., F.M., S.M., Fe.P., Fr.P., N.S., L.T., A.V. and L.W. performed the experiments (see Supplementary Information for details). J.C. analysed the experimental data and M.D. reviewed the analysis code. B.H., J.C. and D.L. designed the model and B.H. analysed the model. J.C. and D.L. drafted the manuscript and F.A., S.B., D.B., A.C., M.D., F.D.L., E.A.F., S.J., O.K., E.L., C.J.L., F.M., S.M., Fe.P., Fr.P., N.S., A.V. and L.W. commented on the drafts. Detailed authors contributions can be found at the beginning of each species' Supplementary methods section.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ele.14124.
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
FA thanks the Swiss National Science Foundation (grants no. PP00P3 150698 310030_197410 and PP00P3_179089 and the University of Zurich Research Priority Programme URPP on Global Change and Biodiversity). FrP thanks the Swiss National Science Foundation for support (Grant 310030_197811). DB and SM thank the FWO (Fonds Wetenschappelijk Onderzoek | grant no. G018017N). SJ, EL and NS thank UCLouvain and F.R.S.‐FNRS; SJ acknowledges a ‘Move‐In Louvain’ postdoc position at UCLouvain; NS is Senior Research Associate of F.R.S.‐FNRS. DL, MD, JC and LW thank Fyssen Foundation for funding. JDR thanks the FWO (Research Foundation Flanders—grant n. FWO14/ASP/075). FDL was supported by the ARC DIVERCE, a concerted research action from the special research fund (Convention 18/23‐095). FrP was financially supported by Swiss National Science Foundation Grant 31003A 159498. JC was supported by the ERA‐Net BiodivERsA, with the national funder ONEMA, part of the 2012‐2013 BiodivERsA call for research proposals, and a funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement no. 817779). DL, AC, SJ, SB and JC are supported by the French Laboratory of Excellence project “TULIP” (ANR‐10‐LABX‐41). This work was supported by an Investissements d'Avenir programme from the Agence Nationale de la Recherche (no. ANR‐11‐INBS‐0001AnaEE‐Services). DL and JC thank Audrey Trochet and Olivier Calvez for their valuable input in experiments involving newts, toads and snakes. MD, AA and LM are especially grateful to Christelle Van Gheluwe for running the experiments, and to Maryvonne Charrier for providing Deroceras reticulatum slugs. This is publication ISEM‐2022‐227 of the Institut des Sciences de l'Evolution—Montpellier, and BRC395 of the Biodiversity Research Centre—UCLouvain. We thank Elvire Bestion for an external review of the analysis code. We thank Eugene W. Schupp, Remo Ryser and the editor for their constructive comments on the manuscript.
Cote, J. , Dahirel, M. , Schtickzelle, N. , Altermatt, F. , Ansart, A. & Blanchet, S. et al. (2022) Dispersal syndromes in challenging environments: A cross‐species experiment. Ecology Letters, 25, 2675–2687. Available from: 10.1111/ele.14124
Editor: Eelke Jongejans
DATA AVAILABILITY STATEMENT
The data reported in this paper and the codes used to analyse the data are available at zenodo (DOI 10.5281/zenodo.7107088) and were made available at submission as a supplementary material file.
REFERENCES
- Bates, D. , Maechler, M. , Bolker, B. , Walker, S. , Christensen, R.H.B. , Singmann, H. et al. (2019) lme4: Linear mixed‐effects models using “Eigen” and S4 . Available from: https://CRAN.R‐project.org/package=lme4 [Accessed 5th March 2019].
- Bestion, E. , Teyssier, A. , Aubret, F. , Clobert, J. & Cote, J. (2014) Maternal exposure to predator scents: offspring phenotypic adjustment and dispersal. Proceedings of the Royal Society B: Biological Sciences, 281, 20140701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick, D.I. , Amarasekare, P. , Araújo, M.S. , Bürger, R. , Levine, J.M. , Novak, M. et al. (2011) Why intraspecific trait variation matters in community ecology. Trends in Ecology & Evolution, 26, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonte, D. & Dahirel, M. (2017) Dispersal: a central and independent trait in life history. Oikos, 126, 472–479. [Google Scholar]
- Bonte, D. , De Roissart, A. , Wybouw, N. & Van Leeuwen, T. (2014) Fitness maximization by dispersal: evidence from an invasion experiment. Ecology, 95, 3104–3111. [Google Scholar]
- Bonte, D. , Van Dyck, H. , Bullock, J.M. , Coulon, A. , Delgado, M. , Gibbs, M. et al. (2012) Costs of dispersal. Biological Reviews, 87, 290–312. [DOI] [PubMed] [Google Scholar]
- Bowler, D.E. & Benton, T.G. (2005) Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biological Reviews, 80, 205–225. [DOI] [PubMed] [Google Scholar]
- Byers, J.E. (2000) Effects of body size and resource availability on dispersal in a native and a non‐native estuarine snail. Journal of Experimental Marine Biology and Ecology, 248, 133–150. [DOI] [PubMed] [Google Scholar]
- Clobert, J. , Baguette, M. , Benton, T.G. & Bullock, J.M. (2012) Dispersal. Ecology and evolution. New York, NY: Oxford University Press. [Google Scholar]
- Clobert, J. , Le Galliard, J.F. , Cote, J. , Meylan, S. & Massot, M. (2009) Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecology Letters, 12, 197–209. [DOI] [PubMed] [Google Scholar]
- Comte, L. & Olden, J.D. (2018) Evidence for dispersal syndromes in freshwater fishes. Proceedings of the Royal Society B, 285, 20172214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote, J. , Bestion, E. , Jacob, S. , Travis, J. , Legrand, D. & Baguette, M. (2017) Evolution of dispersal strategies and dispersal syndromes in fragmented landscapes. Ecography, 40, 56–73. [Google Scholar]
- Cote, J. , Brodin, T. , Fogarty, S. & Sih, A. (2017) Non‐random dispersal mediates invader impacts on the invertebrate community. The Journal of Animal Ecology, 86, 1298–1307. [DOI] [PubMed] [Google Scholar]
- Cote, J. , Fogarty, S. , Tymen, B. , Sih, A. & Brodin, T. (2013) Personality‐dependent dispersal cancelled under predation risk. Proceedings of the Royal Society B: Biological Sciences, 280, 20132349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahirel, M. , Dierick, J. , De Cock, M. & Bonte, D. (2017) Intraspecific variation shapes community‐level behavioral responses to urbanization in spiders. Ecology, 98, 2379–2390. [DOI] [PubMed] [Google Scholar]
- Forsman, A. & Wennersten, L. (2016) Inter‐individual variation promotes ecological success of populations and species: evidence from experimental and comparative studies. Ecography, 39, 630–648. [Google Scholar]
- Fronhofer, E.A. , Gut, S. & Altermatt, F. (2017) Evolution of density‐dependent movement during experimental range expansions. Journal of Evolutionary Biology, 30, 2165–2176. [DOI] [PubMed] [Google Scholar]
- Fronhofer, E.A. , Legrand, D. , Altermatt, F. , Ansart, A. , Blanchet, S. , Bonte, D. et al. (2018) Bottom‐up and top‐down control of dispersal across major organismal groups. Nature Ecology & Evolution, 1, 1859–1863. [DOI] [PubMed] [Google Scholar]
- Gilliam, J.F. & Fraser, D.F. (2001) Movement in corridors: enhancement by predation threat, disturbance, and habitat structure. Ecology, 82, 258–273. [Google Scholar]
- Goossens, S. , Wybouw, N. , Van Leeuwen, T. & Bonte, D. (2020) The physiology of movement. Movement Ecology, 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman, B. & Loreau, M. (2014) General relationships between consumer dispersal, resource dispersal and metacommunity diversity. Ecology Letters, 17, 175–184. [DOI] [PubMed] [Google Scholar]
- Hämäläinen, A.M. , Guenther, A. , Patrick, S.C. & Schuett, W. (2021) Environmental effects on the covariation among pace‐of‐life traits. Ethology, 127, 32–44. [Google Scholar]
- Hendry, A.P. (2017) Eco‐evolutionary dynamics. Princeton, NJ: Princeton University Press. [Google Scholar]
- Higgins, J.P.T. & Thompson, S.G. (2002) Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine, 21, 1539–1558. [DOI] [PubMed] [Google Scholar]
- Jacob, S. , Chaine, A.S. , Huet, M. , Clobert, J. & Legrand, D. (2019) Variability in dispersal syndromes is a key driver of metapopulation dynamics in experimental microcosms. The American Naturalist, 194, 613–626. [DOI] [PubMed] [Google Scholar]
- Jacob, S. , Laurent, E. , Morel‐Journel, T . & Schtickzelle, N . (2020). Fragmentation and the context‐dependence of dispersal syndromes: matrix harshness modifies resident‐disperser phenotypic differences in microcosms. Oikos, 129, 158–169. [Google Scholar]
- Junker, A.D. , Jacob, S. , Philippe, H. , Legrand, D. & Pearson, C.G. (2021) Plastic cell morphology changes during dispersal. iScience, 24, 102915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K.W. (2000) Dispersal behaviour in a subsocial spider: group conflict and the effect of food availability. Behavioral Ecology and Sociobiology, 48, 182–187. [Google Scholar]
- Laughlin, D.C. & Messier, J. (2015) Fitness of multidimensional phenotypes in dynamic adaptive landscapes. Trends in Ecology & Evolution, 30, 487–496. [DOI] [PubMed] [Google Scholar]
- Le Galliard, J.F. , Remy, A. , Ims, R.A. & Lambin, X. (2012) Patterns and processes of dispersal behaviour in arvicoline rodents. Molecular Ecology, 21, 505–523. [DOI] [PubMed] [Google Scholar]
- Legrand, D. , Cote, J. , Fronhofer, E.A. , Holt, R.D. , Ronce, O. , Schtickzelle, N. et al. (2017) Eco‐evolutionary dynamics in fragmented landscapes. Ecography, 40, 9–25. [Google Scholar]
- Little, C.J. , Fronhofer, E.A. & Altermatt, F. (2019) Dispersal syndromes can impact ecosystem functioning in spatially structured freshwater populations. Biology Letters, 15, 20180865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massol, F. , Altermatt, F. , Gounand, I. , Gravel, D. , Leibold, M.A. & Mouquet, N. (2017) How life‐history traits affect ecosystem properties: effects of dispersal in meta‐ecosystems. Oikos, 126, 532–546. [Google Scholar]
- Moran, E.V. , Hartig, F. & Bell, D.M. (2016) Intraspecific trait variation across scales: implications for understanding global change responses. Global Change Biology, 22, 137–150. [DOI] [PubMed] [Google Scholar]
- Nakagawa, S. & Schielzeth, H. (2013) A general and simple method for obtaining R 2 from generalized linear mixed‐effects models. Methods in Ecology and Evolution, 4, 133–142. [Google Scholar]
- Noonan, M.J. , Fleming, C.H. , Akre, T.S. , Drescher‐Lehman, J. , Gurarie, E. , Harrison, A.‐L. et al. (2019) Scale‐insensitive estimation of speed and distance traveled from animal tracking data. Movement Ecology, 7, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, R.H. (1986) The ecological implications of body size. Cambridge: Cambridge University Press. [Google Scholar]
- Quévreux, P. , Pigeault, R. & Loreau, M. (2021) Predator avoidance and foraging for food shape synchrony and response to perturbations in trophic metacommunities. Journal of Theoretical Biology, 528, 110836. [DOI] [PubMed] [Google Scholar]
- R Core Team . (2013) R: A language and environment for statistical computing . Available from: http://www.R‐project.org/ [Accessed 5th July 2019].
- Raffard, A. , Bestion, E. , Cote, J. , Haegeman, B. , Schtickzelle, N. & Jacob, S. (2021) Dispersal syndromes can link intraspecific trait variability and meta‐ecosystem functioning. Trends in Ecology & Evolution, 37, 322–331. [DOI] [PubMed] [Google Scholar]
- Raffard, A. , Lecerf, A. , Cote, J. , Buoro, M. , Lassus, R. & Cucherousset, J. (2017) The functional syndrome: linking individual trait variability to ecosystem functioning. Proceedings of the Royal Society B, 284, 20171893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffard, A. , Santoul, F. , Cucherousset, J. & Blanchet, S. (2019) The community and ecosystem consequences of intraspecific diversity: a meta‐analysis. Biological Reviews, 94, 648–661. [DOI] [PubMed] [Google Scholar]
- Ridley, M. (2004) Evolution, 3rd edition. Malden, MA: Blackwell Publishing. [Google Scholar]
- Sih, A. , Bell, A.M. , Johnson, J.C. & Ziemba, R.E. (2004) Behavioral syndromes: an integrative overview. Quarterly Review of Biology, 79, 241–277. [DOI] [PubMed] [Google Scholar]
- Stevens, V.M. , Trochet, A. , Van Dyck, H. , Clobert, J. & Baguette, M. (2012) How is dispersal integrated in life histories: a quantitative analysis using butterflies. Ecology Letters, 15, 74–86. [DOI] [PubMed] [Google Scholar]
- Stevens, V.M. , Whitmee, S. , Le Galliard, J.‐F. , Clobert, J. , Böhning‐Gaese, K. , Bonte, D. et al. (2014) A comparative analysis of dispersal syndromes in terrestrial and semi‐terrestrial animals. Ecology Letters, 17, 1039–1052. [DOI] [PubMed] [Google Scholar]
- Travis, J.M.J. , Delgado, M. , Bocedi, G. , Baguette, M. , Bartoń, K. , Bonte, D. et al. (2013) Dispersal and species' responses to climate change. Oikos, 122, 1532–1540. [Google Scholar]
- Viechtbauer, W. (2010) Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software, 36, 1–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data reported in this paper and the codes used to analyse the data are available at zenodo (DOI 10.5281/zenodo.7107088) and were made available at submission as a supplementary material file.


