Abstract
CD40 ligand (CD40L) is a potent inducer of interleukin-12 (IL-12) production from macrophages and dendritic cells. We show that combining CD40L with antigen derived from Leishmania is an effective way to preferentially induce type 1 immune responses to the antigen and to vaccinate mice against subsequent challenge with virulent organisms. Mice vaccinated in this way had smaller lesions, with more than 1,000-fold fewer parasites within them. To improve the efficiency of CD40L-induced immunopotentiation, we attempted to specifically direct CD40L to macrophages. We developed transfected cells expressing CD40L and a single Leishmania antigen, gp63. These cells bound efficiently to macrophages and induced robust IL-12 production. Vaccination with these cotransfected cells provided a significant degree of protection against challenge with virulent organisms. CD40L was also adsorbed to the surface of virulent Leishmania. These organisms induced only modest lesions in genetically susceptible mice, and the lesions had an average of 105-fold fewer organisms within them relative to control mice. These studies suggest that CD40L could be exploited to improve vaccines against intracellular pathogens, especially those organisms that reside within cells expressing CD40 on their surface.
Clinical cutaneous leishmaniasis can range from a relatively mild self-healing form of the disease to a severe form that fails to resolve and disseminates throughout the dermis (4). To date, there are no effective vaccines against Leishmania, and drugs used to treat the disease in humans are generally expensive, toxic, and difficult to administer. There is a well-established murine model of cutaneous Leishmania which mimics many aspects of the human disease. This powerful model has been used to demonstrate that the preferential activation and/or expansion of subsets of either Th1 or Th2 cells can predict the resolution or progression of murine cutaneous leishmaniasis. Expansion of the Th1 subset results in the production of gamma interferon (IFN-γ) and results in disease resolution, whereas expansion of the Th2 subset produces interleukin-4 (IL-4) and leads to progressive disease (32). The major surface protein on Leishmania is designated gp63. gp63 is a glycoprotein metalloprotease that is expressed by all species of Leishmania. The abundance of gp63 expression on Leishmania has been correlated with parasite virulence (6). This protein has been shown to facilitate the binding of parasites to macrophages (10).
A cytokine that plays a central role in the generation of the type 1 immune response is IL-12. IL-12 is a heterodimeric cytokine produced primarily by antigen-presenting cells. IL-12 has been shown to stimulate the secretion of IFN-γ by T and NK cells (22, 40, 45). In the murine model of leishmaniasis, the administration of exogenous IL-12 can promote the development of a type 1 immune response to Leishmania antigen and can afford protection against experimental infection (1, 36, 38).
CD40 ligand (CD40L) is a member of the tumor necrosis factor family. It is a 39-kDa type 2 glycoprotein that is expressed primarily on activated T lymphocytes. This molecule was initially implicated in B-cell development. The cognate interaction of CD40L with CD40 on B lymphocytes is essential for germinal center formation, isotype switching, and memory B-cell generation (3). A mutation in the human CD40L gene results in the failure to produce immunoglobulin G (IgG) and IgA in response to antigen (2, 7). CD40-CD40L interactions have also been shown to play an important role in driving cell-mediated immune responses. CD40L is a potent inducer of IL-12 from macrophages and dendritic cells (20, 21). Mice lacking CD40L exhibited impaired T-cell activation and deficient IFN-γ production (15, 16). Importantly, CD40L−/− mice were more susceptible to Leishmania infection (8, 41). Furthermore, the administration of an agonistic antibody to CD40 was shown to induce IL-12 and to protect mice from Leishmania infection (14).
The induction of IL-12 by antigen-presenting cells stimulated with CD40L (20, 21) suggested that CD40L could be used as a vaccine adjuvant to preferentially drive type 1 immune responses. To test this, we immunized mice with CD40L in combination with crude soluble Leishmania antigen and confirmed that CD40L preferentially induces a type 1 immune response and protects vaccinated mice from subsequent challenge with virulent parasites. CD40L was directed specifically to macrophages by coexpressing it on the surface of cells along with a recombinant Leishmania antigen or by adsorbing it to intact parasites. These approaches were effective in inducing IL-12 and preventing disease progression. Thus, directing CD40L to macrophages may be a particularly effective way to provide protection against intracellular pathogens that reside within them.
MATERIALS AND METHODS
Parasites.
Promastigotes of Leishmania major Friedlin strain, clone V1 (MHOM/IL/80/Friedlin), and Leishmania amazonensis (RAT/BA/72/LV78) were maintained in Schneider's complete medium consisting of Schneider's Drosophila Medium (GIBCO/BRL, Rockville, Md.) supplemented with 20% heat-inactivated fetal bovine serum (HI-FCS), 100 U of penicillin G per ml, 100 μg of streptomycin per ml, and 2 mM glutamine (GIBCO/BRL). Amastigotes of Leishmania were isolated from footpads of BALB/c mice which were infected 6 to 8 weeks earlier, as described previously (26). Briefly, the excised foot was forced through nylon mesh by using the plunger from a 10-ml syringe while being washed with 10 ml of Schneider's complete medium. The mixture was then subjected to repeated passages through 21-, 23-, and 25-gauge needles to release amastigotes from infected mononuclear cells. Cellular debris was removed from the mixture by centrifugation at 50 × g for 5 min. Amastigotes were separated by centrifugation at 600 × g for 10 min and washed prior to use.
Macrophages.
Bone marrow-derived macrophages (BMMϕ) were established as previously described (42). Briefly, femurs were flushed with cation-free Dulbecco's phosphate-buffered saline (PBS) (GIBCO/BRL) using a 23-gauge needle. Cells were grown in Dulbecco's modified Eagle's medium (Mediatech, Herndon, Va.) containing 10% HI-FCS, 2 mM l-glutamine, 100 U of penicillin G per ml, and 100 μg of streptomycin (D-10) per ml, supplemented with 20% L929 cell-conditioned medium. Cells were incubated at 37°C in 5% CO2 for 5 to 7 days on petri plates until uniform monolayers of macrophages were established. Cells were removed from the original dishes by EDTA treatment 12 h before use. For reverse transcription (RT)-PCR, a total of 106 cells were plated per well of tissue culture-treated six-well plates (Nunc, Naperville, III.). For enzyme-linked immunosorbent assay (ELISA), a total of 105 cells were plated per well of 24-well plates (Nunc).
Dendritic cells.
Bone marrow-derived dendritic cells were isolated as previously described (19). Briefly, femurs were flushed with cation-free Dulbecco's PBS (GIBCO/BRL) using a 23-gauge needle. Cells were cultured in 24-well plates at 1.0 × 106 per ml in RPMI 1640 medium (Mediatech) containing 10% HI-FCS, 2 mM l-glutamine, 100 U of penicillin G per ml, and 100 μg of streptomycin (R-10) per ml, with the addition of 50 μM 2-mercaptoethanol and 750 U of murine recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems, Minneapolis, Minn.) per ml. On day 2 and day 4, 75% of the culture supernatants were taken off and replaced with fresh medium containing 750 U of GM-CSF per ml. On day 6, total supernatants containing nonadherent cells were harvested and gently overlaid onto a column of 50% FCS–50% RPMI medium in 15-ml conical tubes. After 20 min, the supernatants and the top 1 ml of the column's contents were removed and discarded. The remaining cells were diluted with RPMI medium and centrifuged at 300 × g for 5 min. The pellet was resuspended and plated onto individual wells of 24-well plates at a concentration of 3.0 × 105 per well. Nonadherent cells were harvested the following day for use.
Transfected cell lines expressing human CD40L and Leishmania gp63.
Control L929 cells and CD40L-transfected L929 cells were a kind gift from Brian Kelsall (National Institutes of Health). These cells were cultured in RPMI 1640 (Mediatech) containing 10% HI-FCS, 15 mM HEPES buffer (Mediatech), 5 × 10−2 mM 2-mercaptoethanol (GIBCO/BRL), 2 mM l-glutamine, 100 U of penicillin G per ml, and 100 μg of streptomycin per ml. Transfected cells were grown in 1 mg of Geneticin (G418) (GIBCO/BRL) per ml to maintain CD40L expression. The construct encoding the L. major gp63 gene with modifications for mammalian cell expression (27) was developed and generously provided to us by R. McMaster (University of British Columbia, Vancouver, Canada). The gp63 gene was subcloned into the pCEP4 vector (Invitrogen, San Diego, Calif.) and transfected into L cells with Lipofectin (GIBCO) according to the manufacturer's protocol. gp63-transfected L929 cells were selected in medium containing 250 μg of hygromycin per ml.
To measure the binding of transfected cells to macrophages, BMMϕ were incubated in vitro with a 5:1 ratio of transfected L cells, which were prelabeled with 50 μM 5-chloromethyl-fluorescein diacetate (cmfDA; Molecular Probes, Eugene, Oreg.) for 30 min at 37°C, washed, and incubated for an additional 30 min at 37°C. Transfected L cells expressing CD40L alone or CD40L and gp63 were added to macrophage monolayers on a gently rotating platform for 5 to 15 min at 37°C. After being gently washed, monolayers were fixed and the number of adherent cells was determined microscopically. IL-12 production by macrophages following in vitro stimulation with transfected cells was quantitated by ELISA and visualized by intracellular immunofluorescence staining using a phycoerythrin (PE) conjugated C15.6 monoclonal antibody (MAb) to IL-12 (BD Pharmingen, San Diego, Calif.) as previously described (31).
Macrophage stimulation.
To induce the secretion of IL-12, lipopolysaccharide (LPS) (Escherichia coli O127:B8; Sigma, St. Louis, Mo.) was added to monolayers at a final concentration of 100 ng/ml in D-10. L cells expressing CD40L were added to monolayers at a ratio of 1:1. To infect macrophages with parasites, Leishmania promastigotes or amastigotes were washed three times in D-10 prior to their addition to monolayers at a ratio of 10 parasites per cell. In some assays (data not shown) parasites were added to macrophages at a ratio of 25:1. Stimulated cells were cultivated for an additional 6 or 24 h to detect cytokine mRNA or protein, respectively.
Flow cytometry.
Flow cytometry was used to detect CD40L and gp63 on transfected L cells. Cells were brought to a concentration of 5 × 106 cells per ml in Hanks balanced salt solution (HBSS) containing 1% bovine serum albumin and 0.05% sodium azide. To detect CD40L, cells were incubated with a 1:100 dilution of purified MR1 MAb to CD40L (Pharmingen). To detect gp63, cells were incubated in MAb #96 to L. major gp63 (5), kindly provided by R. McMaster. After 45 min at 4°C in the primary antibody, the cells were washed with cold buffer and incubated with either fluorescein isothiocyanate (FITC)-conjugated goat anti-hamster IgG (Jackson ImmunoResearch, West Grove, Pa.) for CD40L or FITC-conjugated goat anti-mouse IgG (Jackson ImmunoResearch) for gp63 for 45 min at 4°C. Cells were fixed in 1% paraformaldehyde in PBS and analyzed on an Epics Elite flow cytometer (Coulter Diagnostics, Hialeah, Fla.).
RT-PCR.
Total RNA was extracted using RNAzol B (Biotecx, Houston, Tex.) according to the manufacturer's instructions. Four micrograms of RNA was reverse transcribed using Superscript II RT (GIBCO) and random hexamer primers (Promega, Madison, Wis.) according to the GIBCO cDNA synthesis protocol. PCR was performed using a multiple cytokine-containing competitor named PQRS (33), generously provided by Steven Reiner (University of Pennsylvania). Sample cDNAs were normalized with the constitutively expressed gene hypoxanthine phosphoribosyltransferase (HPRT). HPRT-normalized cDNAs were then used to quantitate cytokine levels using cytokine-specific primers and a fixed concentration of competitor in each reaction. Some cytokine PCRs were performed in the absence of competitor (data not shown). The primers used for amplification were as follows (33): HPRT, 5′-GTTGGATACAGGCCAGACTTTGTTG, 3′-GAGGGTAGGCTGGCCTATAGGCT; IL-12 (p40), 5′-ATGGCCATGTGGGA-GCTGGAGAAAG, 3′-GTGGAGCAGCAGATGTGAGTGGCT. cDNA was amplified for 35 cycles (94°C for 40 s, 60°C for 20 s, 72°C for 40 s) using Taq polymerase (Boehringer Mannheim, Indianapolis, Ind.). Amplification products were resolved on 1.8% ethidium-stained agarose gels.
ELISA.
Cytokine production in cell culture supernatants was measured by ELISA as previously described (43). Murine IL-12 (p40) levels were measured with MAbs C15.6 and biotinylated C17.8 (Pharmingen) as ELISA capture and detection antibodies, respectively. Murine IL-12 (p70) levels were measured with MAbs C18.2 and biotinylated C17.15 (Pharmingen). Murine IL-4 levels were measured with MAbs 11B11 and biotinylated BVD6-24G2, and IFN-γ levels were measured with MAbs R4-6A2 and biotinylated XMG1.2 (Pharmingen). Recombinant murine IL-12 (Genzyme Corp., Cambridge, Mass.), IL-4, and IFN-γ (Pharmingen) were used as standards for the ELISAs.
Affinity chromatography to isolate CD40L.
Human CD40L was isolated by affinity chromatography according to the previously described method (29). Hybridoma cells secreting MR1 MAb to CD40L (23) were generously provided by R. J. Noelle (Dartmouth Medical School). MR1 was coupled to CNBr-activated Sepharose 4B (Pharmacia) according to the manufacturer's protocol. Briefly, MR1 was dialyzed overnight against coupling buffer (0.1 M NaHCO3 [pH 8.3] and 0.5 M NaCl) and mixed with 1.5 g of washed, preswelled, CNBr-activated Sepharose 4B overnight at 4°C. After excess ligand was washed away, the gel was packed, washed with three cycles of alternating pH, and equilibrated with loading buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 2 mM MgCl2, 0.1% Triton X-100, and 0.05% octyl-β-glucoside) prior to the addition of cell lysates. To generate cell lysates, CD40L cells were pelleted and resuspended in lysis buffer containing 100 mM Tris-HCl (pH 8.0), 150 mM NaCl, 2 mM MgCl2, 1% Triton X-100 (Sigma), and 0.05% octyl-β-glucoside (Pierce, Rockford, Ill.) with 5 μM α-tosyl-l-lysine chloro-methyl ketone, 0.2 U of aprotinin per ml, 1 mM phenylmethylsulfonyl fluoride, 5 mM iodoactamide, and 1.5 μM leupeptin (Roche Molecular Biochemicals, Indianapolis, Ind.). Samples were solubilized on a rotating shaker for 2 h at 4°C and centrifuged at 10,000 rpm for 30 min in a Beckman ultracentrifuge. Samples were then loaded onto an equilibrated affinity column, and unbound material was washed from the column with buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 2 mM MgCl2, and 0.5% octyl-β-glucoside. Bound material was eluted with elution buffer containing 50 mM triethylamine, 300 mM NaCl (pH 10.4), 2 mM MgCl2, and 0.5% octyl-β-glucoside. Fractions containing CD40L were assayed for their ability to induce BMMϕ IL-12 production in vitro. Positive fractions were pooled and dialyzed in HBSS containing 0.05% octyl-β-glucoside.
Infection of BALB/c mice with CD40L and Leishmania.
Purified CD40L was mixed with 5 × 105 L. major promastigotes for 1 h at room temperature. An aliquot of parasites was washed and examined by immunofluorescence microscopy (Zeiss) using MR1 to visualize CD40L adsorbed to the surface of parasites. Parasites with adsorbed CD40L were injected directly into the footpads of BALB/c mice. For controls, the same amount of L. major promastigotes were mixed in saline and injected into parallel mice. Footpad swelling measurements were recorded twice weekly using calipers and expressed as the net swelling, determined by subtracting the diameter of the contralateral uninfected foot. Parasite numbers were determined by serial dilution (44). Footpad homogenates were serially diluted in 96-well flat-bottomed microtiter plates containing Schneider's complete medium. All dilutions were performed in triplicate. The number of viable parasites was determined by the highest dilution at which promastigotes were growing following a 7-day incubation at 26°C.
Immunization.
Female BALB/c mice were immunized with CD40L and soluble Leishmania antigen (SLA). SLA was obtained from a suspension of approximately 2 × 1010 late-stationary-phase promastigotes of L. major. The suspension was frozen for 2 min in a dry ice-ethyl alcohol bath and then thawed in 37°C water bath. This procedure was repeated six times until promastigotes were lysed. After a brief centrifugation at 10,000 × g, supernatants were filtered with a 0.2-μm-pore-size filter and frozen at −20°C before use. Mice were injected in their hind footpad and intradermally in the back with 2 × 106 cells expressing CD40L in combination with 50 μg of SLA. Mice were boosted twice with the same injection 2 and 3 weeks later. Vaccinated mice were challenged with 5 × 105 L. major promastigotes in the footpad 1 week later.
C57BL/6 mice were vaccinated with 2 × 106 irradiated transfected L929 cells expressing either gp63 alone or coexpressing gp63 and CD40L. Vaccinated mice were boosted twice with the same injection 2 and 3 weeks later and were challenged with 5 × 105 L. major promastigotes in the footpad 1 week later.
Analysis of data.
Statistical analysis of all data was performed using the Student's t test, with statistical significance defined as a P value of ≤0.05.
RESULTS
Leishmania fails to induce IL-12 production from macrophages.
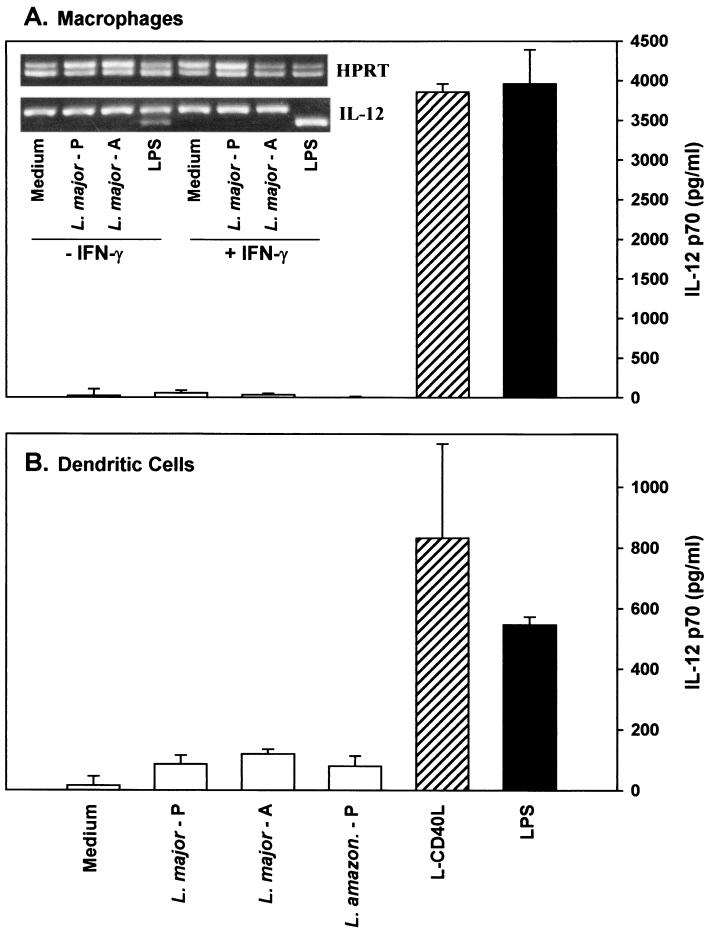
We examined IL-12 production by resting bone marrow-derived macrophages and dendritic cells following their interaction with Leishmania. Cytokine secretion was measured by ELISA for IL-12 p70 (Fig. 1A and B), and mRNA accumulation was measured by competitive RT-PCR (Fig. 1A, inset). By both criteria, infection of macrophages with either of the two species, L. major or L. amazonensis, or the developmental forms, promastigotes or amastigotes, failed to induce the production of significant quantities of IL-12. Even at high multiplicities of infection (25:1; data not shown) with IFN-γ-primed macrophages and even when the RT-PCR analysis was done in the absence of competitor (data not shown), virtually no IL-12 mRNA was detected following infection with Leishmania. In contrast to Leishmania organisms, transfected cells expressing CD40L on their surface were potent inducers of IL-12 from macrophages (Fig. 1A) and dendritic cells (Fig. 1B). The accumulation of IL-12 following CD40 ligation was comparable to that induced by bacterial LPS, the positive control for these experiments (Fig. 1). Both LPS and CD40L resulted in a rapid accumulation of mRNA (note the lower band in the Fig. 1A inset), and both stimuli induced the secretion of relatively large amounts of IL-12 into the supernatant, as previously reported by others (20, 28).
FIG. 1.
IL-12 production by bone marrow-derived macrophages and dendritic cells. Bone marrow-derived macrophages (A) or bone marrow-derived dendritic cells (B) were infected in vitro with promastigotes (L. major - P) or amastigotes (L. major - A) of L. major or promastigotes of L. amazonensis (L. amazon - P) or were stimulated with CD40L-transfected L cells (L-CD40L, striped bars) or LPS (solid bars). Stimulated macrophages were cultivated for an additional 6 or 24 h to measure cytokine mRNA or protein, respectively. Protein levels were determined by ELISA specific for IL-12 p70 on cells which were primed with 100 U of IFN-γ per ml. Determinations were performed in triplicate, and values are expressed as means ± standard deviations. (A, inset) IL-12 mRNA levels were determined by semiquantitative competitive RT-PCR. These experiments are representative of three or more independent determinations.
Vaccination of susceptible BALB/c mice with CD40L.
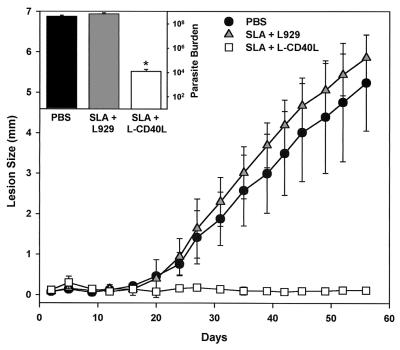
Because of the power of CD40L to induce IL-12, BALB/c mice were vaccinated three times with irradiated cells expressing CD40L on their surface along with SLA. Control mice immunized in parallel with SLA and untransfected L929 cells developed progressive legions that eventually ulcerated. This result was expected, because previous studies have shown that in this susceptible strain of mice, vaccination with SLA alone failed to induce protective type 1 immune responses (39). Mice that were immunized with SLA and CD40L-transfected cells, in contrast, had only minimal increases in lesion size (Fig. 2), and the parasite burdens in these mice were an average of approximately 104-fold lower than those in unvaccinated mice or mice vaccinated with SLA and control L cells (Fig. 2, inset). Thus, the coadministration of CD40L to mice along with SLA resulted in a significant degree of protection from subsequent challenge with virulent organisms.
FIG. 2.
Vaccination of BALB/c mice with SLA in the presence of CD40L. Mice were vaccinated and boosted twice with cells expressing CD40L in combination with 50 μg of SLA (open squares). Infections in mice vaccinated with 50 μg of SLA with untransfected control cells (grey triangles) or mock vaccination with saline (solid circles) were also examined. Mice were challenged with 5 × 105 L. major promastigotes in the footpad, and the course of the disease was monitored by measuring footpad swelling twice weekly with a metric caliper. Results are presented as differences in footpad thickness between the infected and noninfected footpads. (Inset) At week 8, mice were sacrificed and parasite numbers (burdens) in the footpads were measured. The values shown are means ± standard deviations of five mice per group. Statistical significance at the 95% confidence level (P ≤ 0.05), determined by the Student's t test, is denoted by an asterisk.
CD40L vaccination enhances Th1 cytokine production in BALB/c mice.
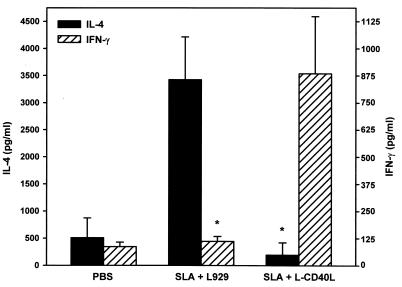
A critical step in the immune response to L. major is the induction of parasite-specific T cells producing type 1 cytokines. To examine T-cell responses in vaccinated mice, IFN-γ and IL-4 production from popliteal lymph node cells (LNC) stimulated in vitro with SLA was examined (Fig. 3). LNC were taken from infected BALB/c mice following immunization with SLA alone or with SLA and CD40L (as described above). Vaccination with SLA alone produced relatively high levels of IL-4 and lower levels of IFN-γ, consistent with this being a susceptible strain of mice (37). Conversely, LNC from mice immunized with CD40L and SLA produced higher amounts of IFN-γ and lower levels of IL-4 (Fig. 3). Control LNC from noninfected mice (saline) did not develop specific T-cell responses against Leishmania antigen and produced low levels of both IFN-γ and IL-4, as expected (Fig. 3).
FIG. 3.
Cytokine production by T cells taken from mice vaccinated with SLA and CD40L. Groups of five mice were sacrificed at day 3 after challenge with Leishmania, and cells from lymph nodes were stimulated with SLA (final concentration, 50 μg/ml) for 72 h. Supernatants were collected, and IL-4 and IFN-γ were measured by ELISA. Data represent means ± standard deviations from five mice per group. Asterisks denote statistical significance at the 95% confidence level (P ≤ 0.05) compared to that of the control (determined by the Student's t test).
Vaccination with L929 cells expressing Leishmania gp63 and murine CD40L.
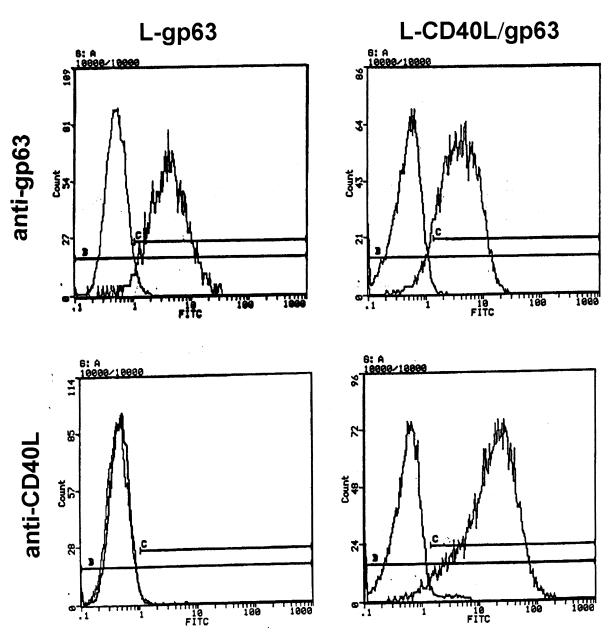
To improve the efficiency of vaccination, we specifically directed CD40L to macrophages. L929 cells were developed that coexpress human CD40L and a single Leishmania surface antigen, gp63 (L-CD40L–gp63). By flow cytometry, these L-CD40L–gp63 cells expressed relatively high levels of both antigens on their surface (Fig. 4), and the cotransfected cells expressed levels of gp63 that were comparable to those of cells expressing gp63 alone (L-gp63).
FIG. 4.
The expression of antigens on the surface of transfected L929 cells. Transfected L cells were incubated with MR1 MAb to CD40L or MAb #96 to Leishmania gp63 and were stained with FITC-conjugated anti-IgG, as described in Materials and Methods. After being extensively washed, cells were fixed in 1% paraformaldehyde in PBS and analyzed on an Epics Elite flow cytometer. Profiles of cells stained with each antibody were overlaid and compared to those of cells stained with secondary antibody alone.
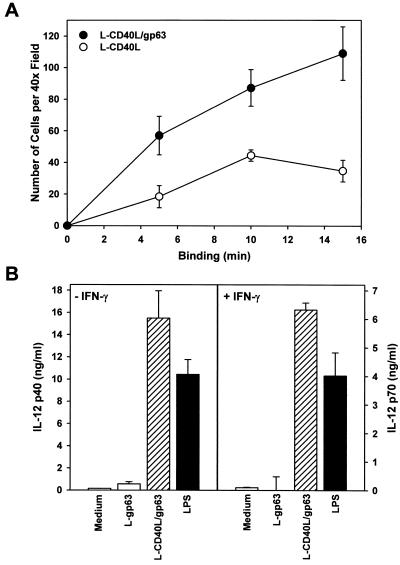
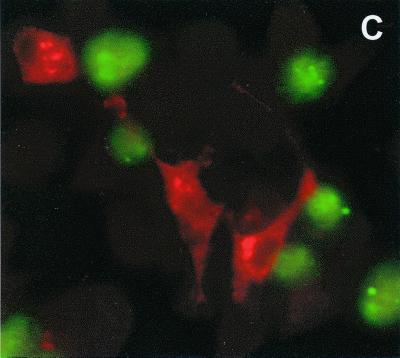
To show that gp63-expressing cells will target directly to macrophages, transfected cells were added to a monolayer of macrophages for increasing amounts of time. Transfected cells expressing both gp63 and CD40L bound efficiently to macrophages even when the assays were performed in the absence of exogenous serum (Fig. 5A). Cells expressing both gp63 and CD40L bound more efficiently to macrophages than did cells expressing gp63 alone (Fig. 5A). Control untransfected cells failed to bind to macrophages, as expected (data not shown). The binding of transfected cells to macrophages resulted in the efficient production of IL-12 from BMMϕ. By ELISA, nanogram amounts of IL-12 p40 and p70 were produced by unprimed and IFN-γ-primed macrophages, respectively (Fig. 5B). The amounts of IL-12 produced in response to transfected cells were in excess of that observed following LPS administration. By intracellular staining, the macrophages producing the highest levels of IL-12 were those to which the transfected cells were bound (Fig. 5C). Thus, targeting CD40L to macrophages by coupling its expression to gp63, a macrophage-specific ligand, efficiently induces macrophage IL-12 production.
FIG. 5.
The binding of transfected L929 cells to macrophage monolayers. (A) Transfected cells expressing either CD40L alone (open symbols) or both gp63 and CD40L (solid symbols) were prelabeled with CM-FDA (see Methods and Materials) and added to monolayers of BMMϕ. At the designated times (x axis), monolayers were gently washed and fixed with paraformaldehyde. The average number of transfected cells bound per 40× field was determined by fluorescence microscopy. (B) Transfected L cells expressing both CD40L and gp63 (striped bars) or gp63 alone (open bars) were added to monolayers of resting (left) or IFN-γ-primed (right) BMMϕ. Stimulated macrophages were cultivated for an additional 24 h before IL-12 p40 and p70 levels were determined by ELISA. IL-12 p40 levels (left) were determined in unprimed cells, and p70 levels (right) were determined in cells that were primed with 100 U of IFN-γ per ml. Protein determinations were performed in triplicate, and values are expressed as the means ± standard deviations. (C) Macrophages were incubated with L-CD40L–gp63 cells and stained for intracellular IL-12 accumulation. A total of 5 × 105 L-CD40L–gp63 cells (green) were labeled with CM-FDA and added to 105 BMMϕ adhered to glass coverslips. The cells were coincubated at 37°C for 4 h and treated with 2 μM monensin for the last 2 h. Monolayers were washed, fixed with paraformaldehyde, stained with a PE-conjugated anti-IL-12 antibody (red), and analyzed by immunofluorescence microscopy.
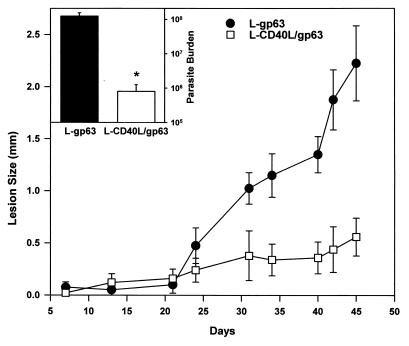
C57BL/6 mice were vaccinated with L-CD40L–gp63 cells and then infected with L. amazonensis promastigotes. L. amazonensis was used for these studies, because unlike L. major, this species causes relatively large lesions in C57BL/6 mice. Mice immunized with L cells expressing gp63 alone (L-gp63) developed progressive lesions (Fig. 6) with relatively high numbers of parasites within them (Fig. 6, inset). In contrast, mice that were immunized with L-CD40L–gp63 had only modest increases in the size of their lesions (Fig. 6), and their parasite burdens at the end of the observation period were more than 100-fold lower than those in control mice (Fig. 6, inset). Thus, CD40L protects two different strains of mice, BALB/c and C57BL/6, against two different species of Leishmania, L. major and L. amazonensis. The later studies were done with C57BL/6 mice because the gp63 molecule that was used as the antigen for these studies is poorly immunogenic in strains expressing the H2D molecule, such as BALB/c mice (25). In fact, vaccination of BALB/c mice with L-CD40L–gp63 was not protective (data not shown), verifying that this was an antigen-specific effect.
FIG. 6.
Vaccination of mice with L cells expressing CD40L and gp63. Mice were injected and boosted twice with irradiated cells expressing both CD40L and gp63 (open squares) or with cells expressing gp63 alone (solid circles). Vaccinated mice were challenged with 5 × 105 L. amazonensis promastigotes in the footpad 1 week after the final boost. Footpad swelling was measured twice weekly using a metric caliper. Results are presented as differences in footpad thickness between the infected and noninfected footpads. (Inset) At week 8, mice were sacrificed and footpad parasite burdens were measured. The values shown are means ± standard deviations of five mice per group. Statistical significance at the 95% confidence level (P ≤ 0.05), determined by the Student's t test, is denoted by an asterisk.
CD40L reduces L. major infection.
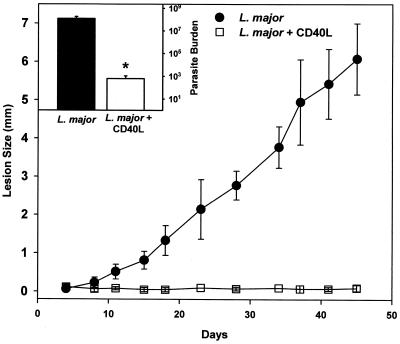
To determine whether the addition of CD40L directly to virulent Leishmania could confer protection against infection in normally susceptible BALB/c mice, Leishmania organisms were coincubated with purified CD40L for an hour, and then the mixture was injected into mice. This procedure resulted in parasites which stained positively for CD40L (data not shown), indicating that some of the CD40L had become adsorbed to the parasite surface. Lesion size was measured over time, and at the end of the analysis parasite burden was quantitated. The addition of CD40L to Leishmania parasites dramatically diminished lesion development (Fig. 7) and significantly reduced parasite numbers in lesions. At the end of the analysis (day 45 postinfection), there were fewer than 103 parasites in the lesions of mice receiving parasites with CD40L (Fig. 7, inset). Parallel mice infected with mock-adsorbed parasites developed progressive lesions which contained more than 108 parasites within them (Fig. 7, inset). Thus, the addition of CD40L to Leishmania organisms prevented them from forming progressive lesions in susceptible mice.
FIG. 7.
Footpad lesions caused by L. major parasites to which CD40L had been adsorbed. Purified CD40L (open square) or control saline (solid circle) was mixed with 5 × 10 5 L. major promastigotes for 1 h at room temperature. The mixture was then injected into the left hind footpad of BALB/c mice. Footpad swelling was measured twice weekly using a metric caliper. At week 7 mice were sacrificed and parasite burdens (inset) were measured by serial dilution. The number of parasites from control (solid bars) and CD40L-coinjected mice (open bars) were determined. The results show the means ± standard deviations from five mice per group. Statistical significance at the 95% confidence level (P ≤ 0.05), determined by the Student's t test, is denoted by an asterisk.
DISCUSSION
CD40L is a 39-kDa glycoprotein that is expressed primarily on activated T cells. The ligation of CD40 on macrophages and dendritic cells can induce the production of high levels of IL-12 from these cells (20, 21), suggesting that CD40L may play a role in directing cell-mediated immunity. Recent studies have shown that mice receiving plasmid DNA encoding CD40L and nominal antigen preferentially develop type 1 immune responses to antigen, characterized by enhanced cytotoxic T-lymphocyte activity and IgG2a production (17). Indeed, treatment of mice with an agonistic antibody to CD40 or cDNA encoding CD40L renders them relatively resistant to Leishmania infection (8, 14, 17). In the present study, we directed CD40L to macrophages to induce IL-12 production from these cells and to limit Leishmania infection.
To examine the feasibility of using CD40L as a vaccine adjuvant, we first vaccinated mice with a soluble Leishmania antigen in the presence of cells expressing CD40L. Mice vaccinated and boosted with antigen and CD40L were partially protected from disease. They had smaller lesions with fewer parasites within them relative to control mice receiving antigen alone. The protection was associated with the emergence of a Th1-type T-cell population in the CD40L-vaccinated mice. This is consistent with the general finding that IL-12-dependent production of IFN-γ is the key mechanism in controlling Leishmania infection (13). The importance of IL-12 in this disease has been well established. Exogenous IL-12 protects susceptible BALB/c mice from L. major infection (38), and conversely antibodies to IL-12 exacerbate infection in resistant mice (18, 36). However, some controversy exists as to whether Leishmania can directly induce IL-12 production from infected macrophages. Previous studies have indicated that Leishmania promastigotes (35) and amastigotes (34) induce IL-12 production from macrophages, whereas other studies demonstrate that they do not (9). In the present studies, Leishmania-infected macrophages produced virtually no IL-12 at either the mRNA or the protein level. This lack of IL-12 production was especially evident compared to parallel populations of cells stimulation with CD40 ligand. Even when macrophages were primed with IFN-γ before infection with L. major, little or no IL-12 was detected. This suggests that Leishmania parasites infect macrophages by a quiescent mechanism that does not elicit substantial cytokine production.
To further examine the potential of CD40L as an adjuvant, we attempted to specifically direct CD40L to macrophages by coupling its expression to that of a parasite molecule that binds to macrophages. We chose a single Leishmania antigen, gp63, for these studies. gp63 is one of the most abundant molecules on the surface of the parasite (11, 24). Previous studies by us and others have shown that this molecule can influence complement fixation and parasite adhesion to macrophages (6, 10). Leishmania gp63 was coexpressed along with human CD40L on the surface of a mammalian cell. These cotransfected cells bound efficiently to macrophages and stimulated macrophage IL-12 production, as demonstrated by both ELISA and intracellular cytokine staining. Mice were vaccinated with irradiated cells expressing these antigens and then were infected with virulent parasites. Lesion progression in vaccinated mice was compared to mice vaccinated with cells expressing the gp63 antigen alone. Previous studies have shown that mice vaccinated with purified Leishmania gp63 protein (30) failed to restrict lesion development. In fact, in some cases mice vaccinated in this way had even larger numbers of parasites within their lesions (1). We confirm these previous studies and show that vaccination with gp63 alone was ineffective in protecting susceptible mice against Leishmania infection. However, mice vaccinated with cells coexpressing both gp63 and CD40L were partially protected against infection with virulent organisms. Their lesions were smaller and they contained 100-fold fewer organisms within them. These studies were done with L. amazonensis and the C57BL/6 strain of mice. Our reason for moving these studies to this second strain of mice instead of BALB/c was the previous observation of others (25) that BALB/c mice, a H2D haplotype strain, react poorly to the gp63 antigen. Mice expressing other major histocompatibility complex haplotypes, however, recognized this antigen and efficiently presented it to T cells. The present result is similar to that of a previous study in which Mycobacterium bovis BCG expressing recombinant gp63 partially protected CBA/J mice against L. amazonensis but failed to protect BALB/c mice from L. major infection (12). The failure to induce immunity in BALB/c mice with gp63 is consistent with previous observations of others that vaccination of mice with recombinant IL-12 and gp63 failed to confer protection (30), whereas vaccination with SLA and recombinant IL-12 was fully protective (1, 30). Thus, the combinatorial use of CD40L and an appropriate Leishmania antigen can be an effective vaccine.
In addition to functioning as an effective vaccine, the adsorption of CD40L to virulent organisms rendered them avirulent in BALB/c mice. These organisms formed minimal lesions in a genetically susceptible strain of mice that would normally contract a progressively fatal form of the disease. This dramatic effect suggests that the induction of IL-12 by CD40 ligation at the time of parasite infection of macrophages may be responsible for resolution. We hypothesize that the tethering of CD40L to the parasite itself may accentuate this effect; however, we acknowledge that the preparation was not washed prior to administration, so the free CD40L in this preparation may have also contributed to this dramatic effect. Nevertheless, this observation suggests that the ligation of CD40 on macrophages at the time of Leishmania phagocytosis will prevent the parasites from establishing a successful infection.
In summary, CD40L induces macrophages and dendritic cells to produce relatively high levels of IL-12. This in vitro observation was applied to an infectious model using the intracellular pathogen Leishmania, and two general conclusions were made. First, the combination of CD40L with Leishmania antigens preferentially induced type 1 immune responses to those antigens. Second, the presence of CD40L prevented virulent Leishmania from establishing an infection in a susceptible mouse strain. These observations suggest that viable Leishmania parasites expressing human CD40L on their surface would not only be strongly immunogenic but that they would also be avirulent—two properties of an excellent vaccine.
REFERENCES
- 1.Afonso L C, Scharton T M, Vieira L Q, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 2.Allen R C, Armitage R J, Conley M E, Rosenblatt H, Jenkins N A, Copeland N G, Bedell M A, Edelhoff S, Disteche C M, Simoneaux D K, et al. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993;259:990–993. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi J P, van Kooten C, Liu Y J, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 4.Berman J D. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 5.Brittingham A, Chen G, McGwire B S, Chang K P, Mosser D M. Interaction of Leishmania gp63 with cellular receptors for fibronectin. Infect Immun. 1999;67:4477–4484. doi: 10.1128/iai.67.9.4477-4484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brittingham A, Morrison C J, McMaster W R, McGwire B S, Chang K P, Mosser D M. Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion, and resistance to complement-mediated lysis. J Immunol. 1995;155:3102–3111. [PubMed] [Google Scholar]
- 7.Callard R E, Armitage R J, Fanslow W C, Spriggs M K. CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol Today. 1993;14:559–564. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- 8.Campbell K A, Ovendale P J, Kennedy M K, Fanslow W C, Reed S G, Maliszewski C R. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996;4:283–289. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 9.Carrera L, Gazzinelli R T, Badolato R, Hieny S, Muller W, Kuhn R, Sacks D L. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J Exp Med. 1996;183:515–526. doi: 10.1084/jem.183.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang K P, Chaudhuri G, Fong D. Molecular determinants of Leishmania virulence. Annu Rev Microbiol. 1990;44:499–529. doi: 10.1146/annurev.mi.44.100190.002435. [DOI] [PubMed] [Google Scholar]
- 11.Colomer-Gould V, Glvao Quintao L, Keithly J, Nogueira N. A common major surface antigen on amastigotes and promastigotes of Leishmania species. J Exp Med. 1985;162:902–916. doi: 10.1084/jem.162.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connell N D, Medina-Acosta E, McMaster W R, Bloom B R, Russell D G. Effective immunization against cutaneous leishmaniasis with recombinant bacille Calmette-Guerin expressing the Leishmania surface proteinase gp63. Proc Natl Acad Sci USA. 1993;90:11473–11477. doi: 10.1073/pnas.90.24.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etges R, Muller I. Progressive disease or protective immunity to Leishmania major infection: the result of a network of stimulatory and inhibitory interactions. J Mol Med. 1998;76:372–390. doi: 10.1007/s001090050230. [DOI] [PubMed] [Google Scholar]
- 14.Ferlin W G, von der Weid T, Cottrez F, Ferrick D A, Coffman R L, Howard M C. The induction of a protective response in Leishmania major-infected BALB/c mice with anti-CD40 mAb. Eur J Immunol. 1998;28:525–531. doi: 10.1002/(SICI)1521-4141(199802)28:02<525::AID-IMMU525>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 15.Grewal I S, Foellmer H G, Grewal K D, Xu J, Hardardottir F, Baron J L, Janeway C A, Jr, Flavell R A. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science. 1996;273:1864–1867. doi: 10.1126/science.273.5283.1864. [DOI] [PubMed] [Google Scholar]
- 16.Grewal I S, Xu J, Flavell R A. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 17.Gurunathan S, Irvine K R, Wu C Y, Cohen J I, Thomas E, Prussin C, Restifo N P, Seder R A. CD40 ligand/timer DNA enhances both humoral and cellular immune responses and induces protective immunity to infectious tumor challenge. J Immunol. 1998;161:4563–4571. [PMC free article] [PubMed] [Google Scholar]
- 18.Heinzel F P, Rerko R M, Ahmed F, Pearlman E. Endogenous IL-12 is required for control of Th2 cytokine responses capable of exacerbating leishmaniasis in normally resistant mice. J Immunol. 1995;155:730–739. [PubMed] [Google Scholar]
- 19.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman R M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato T, Hakamada R, Yamane H, Nariuchi H. Induction of IL-12 p40 messenger RNA expression and IL-12 production of macrophages via CD40-CD40 ligand interaction. J Immunol. 1996;156:3932–3938. [PubMed] [Google Scholar]
- 21.Kelsall B L, Stuber E, Neurath M, Strober W. Interleukin-12 production by dendritic cells. The role of CD40-CD40L interactions in Th1 T-cell responses. Ann N Y Acad Sci. 1996;795:116–126. doi: 10.1111/j.1749-6632.1996.tb52660.x. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi M, Fitz L, Ryan M, Hewick R M, Clark S C, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laman J D, Claassen E, Noelle R J. Functions of CD40 and its ligand, gp39 (CD40L) Crit Rev Immunol. 1996;16:59–108. doi: 10.1615/critrevimmunol.v16.i1.40. [DOI] [PubMed] [Google Scholar]
- 24.Lepay D A, Nogueira N, Cohn Z. Surface antigens of Leishmania donovani promastigotes. J Exp Med. 1983;157:1562–1572. doi: 10.1084/jem.157.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez J A, Reins H A, Etges R J, Button L L, McMaster W R, Overath P, Klein J. Genetic control of the immune response in mice to Leishmania mexicana surface protease. J Immunol. 1991;146:1328–1334. [PubMed] [Google Scholar]
- 26.Love D C, Mentink Kane M, Mosser D M. Leishmania amazonensis: the phagocytosis of amastigotes by macrophages. Exp Parasitol. 1998;88:161–171. doi: 10.1006/expr.1998.4232. [DOI] [PubMed] [Google Scholar]
- 27.Macdonald M H, Morrison C J, McMaster W R. Analysis of the active site and activation mechanism of the Leishmania surface metalloproteinase gp63. Biochim Biophys Acta. 1995;1253:199–207. doi: 10.1016/0167-4838(95)00155-5. [DOI] [PubMed] [Google Scholar]
- 28.McDyer J F, Wu C Y, Seder R A. The regulation of IL-12: its role in infectious, autoimmune, and allergic diseases. J Allergy Clin Immunol. 1998;102:11–15. doi: 10.1016/s0091-6749(98)70047-8. [DOI] [PubMed] [Google Scholar]
- 29.Mosser D M, Springer T A, Diamond M S. Leishmania promastigotes require opsonic complement to bind to the human leukocyte integrin Mac-1 (CD11b/CD18) J Cell Biol. 1992;116:511–520. doi: 10.1083/jcb.116.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mougneau E, Altare F, Wakil A E, Zheng S, Coppola T, Wang Z-E, Waldmann R, Locksley R M, Glaichenhaus N. Expression cloning of a protective Leishmania antigen. Science. 1995;268:563–566. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 31.Prussin C, Metcalf D D. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anticytokine antibodies. J Immunol Methods. 1995;188:117–128. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 32.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 33.Reiner S L, Zheng S, Corry D B, Locksley R M. Constructing polycompetitor cDNAs for quantitative PCR. J Immunol Methods. 1993;165:37–46. doi: 10.1016/0022-1759(93)90104-f. . (Errata, 173:133, 1994, and 175:275, 1994.) [DOI] [PubMed] [Google Scholar]
- 34.Reiner S L, Zheng S, Wang Z, Stowring L, Locksley R M. Leishmania promastigotes evade IL-12 induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med. 1994;179:447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sartori A, Oliveira M A, Scott P, Trinchieri G. Metacyclogenesis modulates the ability of Leishmania promastigotes to induce IL-12 production in human mononuclear cells. J Immunol. 1997;159:2849–2857. [PubMed] [Google Scholar]
- 36.Scharton-Kersten T, Afonso L C, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J Immunol. 1995;154:5320–5330. [PubMed] [Google Scholar]
- 37.Scharton-Kersten T, Scott P. The role of the innate immune response in Th1 cell development following Leishmania major infection. J Leukoc Biol. 1995;57:515–522. doi: 10.1002/jlb.57.4.515. [DOI] [PubMed] [Google Scholar]
- 38.Scott P. IL-12: initiation cytokine for cell-mediated immunity. Science. 1993;260:496–497. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- 39.Scott P, Trinchieri G. IL-12 as an adjuvant for cell-mediated immunity. Semin Immunol. 1997;9:285–291. doi: 10.1006/smim.1997.0084. [DOI] [PubMed] [Google Scholar]
- 40.Seder R A, Gazzinelli R, Sher A, Paul W E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soong L, Xu J C, Grewal I S, Kima P, Sun J, Longley B J, Jr, Ruddle N Y, McMahon-Pratt D, Flavell R A. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 42.Sutterwala F S, Noel G J, Clynes R, Mosser D M. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 1997;185:1977–1985. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutterwala F S, Noel G J, Salgame P, Mosser D M. Reversal of proinflammatory responses by ligating the macrophage Fcγ receptor type I. J Exp Med. 1998;188:217–222. doi: 10.1084/jem.188.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. III. Validity tests for the single-hit Poisson model. J Immunol Methods. 1984;72:29–40. doi: 10.1016/0022-1759(84)90430-7. [DOI] [PubMed] [Google Scholar]
- 45.Tripp C S, Wolf S F, Unanue E R. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]