Abstract
Background and Aims
Moisture damage increases the risk for respiratory disorders in childhood. Our aim was to determine whether early age residential exposure to inspector‐observed moisture damage or mold is associated with different wheezing phenotypes later in childhood.
Methods
Building inspections were performed by civil engineers, in a standardized manner, in the children's homes—mostly single family and row houses (N = 344)—in the first year of life. The children were followed up with repeated questionnaires until the age of 6 years and wheezing phenotypes—never/infrequent, transient, intermediate, late onset, and persistent—were defined using latent class analyses. The multinomial logistic regression model was used for statistical analysis.
Results
A total of 63% (n = 218) had infrequent or no wheeze, 23% (n = 80) had transient and 9.6% (n = 21) had a persistent wheeze. Due to the low prevalence, results for intermediate (3.8%, n = 13) and late‐onset wheeze (3.5%, n = 12) were not further evaluated. Most consistent associations were observed with the persistent wheeze phenotype with an adjusted odds ratio (95% confidence intervals) 2.04 (0.67–6.18) for minor moisture damage with or without mold spots (present in 23.8% of homes) and 3.68 (1.04–13.05) for major damage or any moisture damage with visible mold in a child's main living areas (present in 13.4% of homes). Early‐age moisture damage or mold in the kitchen was associated with transient wheezing.
Conclusion
At an early age, residential exposure to moisture damage or mold, can be dose‐dependently associated especially with persistent wheezing phenotype later in childhood.
Keywords: childhood, indoor, latent class analysis, moisture damage, mold, wheezing phenotype
Abbreviations
- ALSPAC
Avon Longitudinal Study of Parents and Children
- aOR
adjusted odds ratio
- BAMSE
Barn/Child Allergy Milieu Stockholm Epidemiology
- CCAAPS
Cincinnati Childhood Allergy and Air Pollution Study
- CI95%
95% confidence interval
- LCA
Latent Class Analyses
- PASTURE
Protection against Allergy Study in Rural Environments
- PIAMA
The Prevention and Incidence of Asthma and Mite Allergy (PIAMA) birth cohort study
Highlights.
Exposure to moisture damage is considered a risk factor for respiratory disorders.
Exposure to moisture damage in infancy was associated especially with persistent wheezing phenotype.
Persistent wheeze is strongly associated with a later doctor diagnosis of asthma.
Key Message.
This is the first study to assess the association of moisture damage and mold observations recorded during standardized building inspections with different asthma phenotypes obtained by latent class analysis in a general population. Early age exposure to moisture damage was associated with persistent wheezing.
1. INTRODUCTION
Moisture damage and mold growth in buildings increase the risk of asthma exacerbation. 1 Previous reviews 2 , 3 , 4 have also concluded that there is sufficient evidence for the association of moisture damage or mold with asthma development, a finding reported also in the present birth cohort. 5 , 6 However, asthma itself represents a complex spectrum of phenotypes from early childhood into adulthood. In this context, children might be diagnosed as asthmatics at an early age due to experiencing wheezing symptoms, for instance during respiratory infection episodes, but might lose these symptoms later on, so‐called transient wheezing. 7 This may lead to a false positive classification of asthma status in epidemiological studies.
Two analytical approaches have been widely used to identify distinct classifications of asthma and wheezing in birth cohorts: the epidemiological and clinical phenotype definitions. 8 The clinical phenotype definition approach is based on the onset or overall timing of wheezing symptoms and the number of lower respiratory tract illnesses over a certain time period, 9 whereas the epidemiological approach uses hypothesis‐free latent class analysis (LCA), utilizing parent‐reported respiratory symptoms in different time points. 8 , 10 Previous studies have differentiated wheezing and asthma phenotypes into six classes by various analytical and data‐driven approaches. 9 , 11 , 12 , 13 , 14 , 15
Previous research has suggested that fungal components including cell fragments or spores released during fungal growth in buildings affected by moisture or mold damage may provoke inflammatory responses, 16 , 17 and in turn, induce respiratory disorders including wheezing and asthma. 8 , 18 Due to the heterogeneity of asthma, it is further important to pinpoint those wheezing phenotypes, which may be associated with exposure to moisture damage and mold at an early age.
The aim of the present study was to evaluate prospectively, whether wheezing phenotypes as defined by LCA until the age of 6 years were associated with exposure to building inspection confirmed moisture damage and mold in the home during infancy in a Finnish birth cohort study from the general population.
2. MATERIAL AND METHODS
2.1. Study population and study area
The study population consisted of the Finnish LUKAS birth cohort that has been prospectively followed up from the third trimester of pregnancy. 5 Briefly, the study population includes 442 children that were born between September 2002 and May 2005 in Middle and Eastern Finland. The first half of the study population (N = 214) has been recruited from rural areas and is part of a European birth cohort (the Finnish arm of PASTURE), 19 the second half of the cohort comes mainly from suburban areas (N = 228). 5 For the current analysis, children whose homes have been investigated for moisture damage and mold at an early age (mostly between 2 and 5 months of age), 5 and who had information on wheezing phenotypes determined by LCA 8 were included (N = 344). The ethical permission for the study was granted by the Research Ethics Committee of the Hospital District of Northern Savo, Kuopio, Finland (ORG number: IORG00005196). The number for LUKAS is 299/2017 (33/2002) and for LUKAS2 is 300/2017 (48/2004). Written consent were acquired from the parents of the participating children.
2.2. Follow‐up
Questionnaires at 12, 18, and 24 months of age and thereafter annually, 6 enquired about any wheezing and other symptoms or diseases for the time period after the preceding questionnaire. Confounding factors were asked in the parents' questionnaires and in the 2‐ and 12‐months follow‐up questionnaires. Questionnaire‐based information about housing characteristics were collected during the home inspection.
2.3. Immunoglobulin E (IgE) against inhalant allergens
Venous blood samples have been taken at the age of 6 years and analyzed for specific immunoglobulin E (sIgE) to 19 common allergens by administering the Allergy Screen Test Panel for Atopy (Mediwiss Analytic). 20 , 21 The cut‐off level to define specific sensitization to 13 inhalant allergens was 0.70 kU/L. 5
2.4. Home inspection
The method of home inspection has been described earlier. 6 , 22 , 23 Briefly, trained civil engineer(s) inspected the homes for moisture damage, including detailed documentation of signs of excess moisture and/or mold on the surfaces and building structures using a pre‐designed checklist. In this context, “Children's main living area” encompasses the child's bedroom, the living room, and/or the kitchen. Children were 5 months old on average (mean 5.42 months, SD 6.00) during the home inspection and the results of the home inspection were reported to the parents.
2.5. Classification of moisture damage
During the building inspection, each sign of excess moisture was graded using a 6‐point “need for repair” estimation scale in addition to the area of the damage. 6 , 23 A description of the exposure assessment has been reported earlier in detail. 5 , 6 “No damage” was defined as no need for repair or need for repair was only cosmetics (need for repair classes 0 or 1, respectively). “Major damage” was defined in three instances: (A) a repair of surface materials was needed (class 2) with the area of moisture damage ≥1 m2; (B) a repair of structural components was needed (class 3) with the area of damage ≥0.1 m2; and (C) a need for repair was more extensive than structural components (classes 4 or 5). Other damage than the above was classified as “minor damage.” The presence of mold odor or visible mold was recorded in connection with each damage observation. The variable “Moisture damage or mould in the child's main living areas” was created using combined information on signs of moisture damage and visible mold in the child's bedroom, the home's living room, and kitchen. As in previous publications, 6 , 24 observation of visible mold only on silicone sealants in the kitchen or in the bathroom was classified as “no mold.”
2.6. Wheezing phenotypes
Wheezing phenotypes in PASTURE (N = 953, including the first half of the LUKAS study population) were originally defined using LCA, as defined in more detail in Depner et al. 8 This was done based on repeated parental reports on wheezing symptoms at 12, 18, and 24 months of age and thereafter annually up to the age of 6 years, using the following question: “How often has your child wheezed during the last 12 months?” or during the respective time period. The answer categories were “never,” “less than once a month,” “once a month,” and “at least twice a month.” Children with any wheeze at the respective time period were defined as wheezers. In consequence of varying time periods between questionnaires, each follow‐up period was recoded to cover a 12 months period. Only those who had no or at most one missing time‐point from the wheezing reports were included in the analyses. The Bayesian Information Criterion was used for determining the optimal number of five classes and the children were assigned to their respective classes based on the highest posterior probability of belonging. For the current analysis, the same LCA was repeated, now additionally including the second half of the LUKAS study population (N = 185), which resulted in five wheezing patterns as observed in the earlier analysis. 8 LCA was conducted in MPLUS Version 5 (Muthén and Muthén).
2.7. Statistical analysis
Multinomial logistic regression 25 was used to determine associations between moisture damage with or without mold and the five wheezing phenotypes. All models were adjusted for the following, a priori selected covariates: study cohort, maternal history of allergic diseases (asthma, atopic dermatitis, or allergic rhinitis), gender, number of older siblings (≥2, 1 vs. no siblings), smoking during pregnancy, and living on a farm. A more detailed phenotype description according to disease status and socio‐demographics was done in supplementary analyses. Due to the low number of observations in the groups of intermediate and late‐onset wheezing, we only report analytical findings for moisture damage and mold with no or infrequent wheezing, transient, and persistent wheezing phenotype in the manuscript (N = 319). The data were analyzed using SAS 9.3 for Windows (SAS Institute).
3. RESULTS
3.1. Descriptive findings
The main population characteristics are shown in Table 1. The low number of children in different subgroups prevented further analyses on the associations between wheezing phenotypes and the association with current disease status (e.g., asthma or atopy at 6 years) and socio‐demographic information (Table 1). Minor moisture damage in the child's main living areas was reported for 80 (24%) of the houses and major moisture damage or any moisture damage with visible mold was recorded in 46 homes (13%) (Table 2). During the 6‐year follow‐up, 63.4% (n = 218) of the children were categorized according to no or infrequent wheezing, 23.3% (n = 80) transient, 3.8% (n = 13) intermediate, 3.5% (n = 12) late onset, and 6.0% (n = 21) persistent wheezing (Figure 1).
TABLE 1.
Study population characteristics by wheezing phenotype
| N | Never/infrequent wheeze | Transient wheeze | Intermediate wheeze | Late onset wheeze | Persistent wheeze | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | ||
| Farmer | |||||||||||
| No | 245 | 145 | 67 | 63 | 79 | 10 | 77 | 9 | 75 | 18 | 86 |
| Yes | 99 | 73 | 34 | 17 | 21 | 3 | 23 | 3 | 25 | 3 | 14 |
| Cohort | |||||||||||
| Finnish arm of PASTURE | 166 | 110 | 51 | 35 | 44 | 9 | 42 | 5 | 41.7 | 7 | 33 |
| Extended cohort | 178 | 108 | 49 | 45 | 56 | 4 | 58 | 7 | 58.3 | 14 | 67 |
| Gender | |||||||||||
| Girl | 173 | 119 | 55 | 33 | 41 | 4 | 31 | 8 | 66.7 | 9 | 43 |
| Boy | 171 | 99 | 45 | 47 | 59 | 9 | 69 | 4 | 33.3 | 12 | 57 |
| Maternal smoking | |||||||||||
| Never | 187 | 125 | 57 | 40 | 50 | 7 | 54 | 5 | 41.7 | 10 | 48 |
| Not during pregnancy | 109 | 68 | 31 | 24 | 30 | 5 | 39 | 5 | 41.7 | 7 | 33 |
| During pregnancy | 48 | 25 | 12 | 16 | 20 | 1 | 8 | 2 | 16.7 | 4 | 19 |
| Maternal history of allergic disease | |||||||||||
| No | 152 | 107 | 49 | 31 | 39 | 5 | 39 | 3 | 25 | 6 | 29 |
| Yes | 192 | 111 | 51 | 49 | 61 | 8 | 62 | 9 | 75 | 15 | 71 |
| Paternal history of allergic disease | |||||||||||
| No | 178 | 121 | 56 | 40 | 51 | 3 | 25 | 6 | 50 | 8 | 38 |
| Yes | 161 | 95 | 44 | 38 | 49 | 9 | 75 | 6 | 50 | 13 | 62 |
| Number of siblings | |||||||||||
| None | 120 | 82 | 38 | 26 | 33 | 2 | 15 | 6 | 50 | 4 | 19 |
| One | 115 | 75 | 34 | 27 | 34 | 1 | 8 | 5 | 41.7 | 7 | 33 |
| Two or more | 109 | 61 | 28 | 27 | 34 | 10 | 77 | 1 | 8.3 | 10 | 48 |
| Maternal education level | |||||||||||
| Low | 105 | 62 | 28 | 26 | 33 | 7 | 54 | 2 | 16.7 | 8 | 38 |
| Middle | 160 | 101 | 46 | 38 | 48 | 4 | 31 | 8 | 66.6 | 9 | 43 |
| High | 79 | 55 | 25 | 16 | 20 | 2 | 15 | 2 | 16.7 | 4 | 19 |
| Asthma ever 6 years | |||||||||||
| No | 281 | 202 | 93 | 65 | 81 | 2 | 15 | 6 | 50 | 6 | 29 |
| Yes | 62 | 15 | 7 | 15 | 19 | 11 | 85 | 6 | 50 | 15 | 71 |
| Inhalant atopy age 6 year | |||||||||||
| No (<0.70 kU/L) | 178 | 120 | 66 | 34 | 55 | 7 | 54 | 7 | 58 | 10 | 59 |
| Yes (≥0.70 kU/L) | 109 | 63 | 34 | 28 | 45 | 6 | 46 | 5 | 42 | 7 | 41 |
TABLE 2.
Adjusted associations between different types of moisture damage or mold in the main locations of the home and the risk of transient and persistent wheezing phenotypes. Never or infrequent wheeze is always used as the comparison group.
| N | Never/infrequent wheeze | Transient wheeze | Persistent wheeze | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | aOR (95% CI) | n | % | aOR (95% CI) | ||
| Child's main living area (child's bedroom, living room, and kitchen) | |||||||||
| No moisture damage and no mold | 200 | 144 | 72 | 46 | 23 | 1 | 10 | 5 | 1 |
| Minor damage with/without mold spots | 76 | 48 | 63 | 22 | 29 | 1.59 (0.85–2.99) | 6 | 8 | 2.04 (0.67–6.18) |
| Major/any moisture damage with visible mold | 43 | 26 | 61 | 12 | 28 | 1.75 (0.78–3.94) | 5 | 11 | 3.68 (1.04–13.05), p = .0435 |
| Kitchen | |||||||||
| No moisture damage (reference) | 237 | 170 | 72 | 53 | 22 | 1 | 14 | 6 | 1 |
| Minor | 67 | 40 | 60 | 22 | 33 | 1.93 (1.02–3.68), p = .0447 | 5 | 8 | 1.65 (0.53–5.16) |
| Major | 15 | 8 | 53 | 5 | 33 | 2.43 (0.73–8.14) | 2 | 13 | 3.53 (0.61–20.5) |
| No moisture damage with mold (reference) | 307 | 213 | 69 | 74 | 24 | 1 | 20 | 7 | 1 |
| Spots | 4 | 1 | 25 | 2 | 50 | 4.17 (0.36–48.7) | 1 | 25 | 5.38 (0.28–104) |
| Visible mold | 8 | 4 | 50 | 4 | 50 | 2.62 (0.59–11.7) | 0 | 0 | – a |
| Living room | |||||||||
| No moisture damage (reference) | 260 | 177 | 68 | 70 | 27 | 1 | 13 | 5 | 1 |
| Minor | 40 | 28 | 70 | 7 | 18 | 0.67 (0.27–1.68) | 5 | 13 | 2.75 (0.81–9.29) |
| Major | 19 | 13 | 68 | 3 | 16 | 0.71 (0.19–2.64) | 3 | 16 | 6.24 (1.34–29.0), p = .0197 |
| No moisture damage with mold (reference) | 309 | 212 | 69 | 79 | 26 | 1 | 18 | 6 | 1 |
| Spots | 5 | 4 | 80 | 0 | 0 | – a | 1 | 20 | 2.38 (0.22–26.2) |
| Visible mold | 5 | 2 | 4 | 1 | 20 | 1.94 (0.16–23.5) | 2 | 40 | 32.3 (2.81–370), p = .0053 |
| Child's bedroom | |||||||||
| No moisture damage (reference) | 271 | 183 | 68 | 71 | 26 | 1 | 17 | 6 | 1 |
| Minor | 40 | 30 | 75 | 8 | 20 | 0.76 (0.33–1.78) | 2 | 5 | 0.78 (0.16–3.69) |
| Major | 8 | 5 | 63 | 1 | 13 | 0.69 (0.08–6.41) | 2 | 25 | 6.91 (0.89–53.6), p = .0647 |
| No moisture damage with mold (reference) | 305 | 209 | 69 | 79 | 26 | 1 | 17 | 6 | 1 |
| Spots | 6 | 4 | 67 | 0 | 0 | – a | 2 | 33 | 6.10 (0.85–43.6), p = .0715 |
| Visible mold | 8 | 5 | 63 | 1 | 13 | 0.71 (0.08–6.62) | 2 | 25 | 5.98 (0.83–42.8), p = .0752 |
Note: Models are adjusted for study cohort, living on a farm, gender, maternal history of allergic diseases (hay fever, atopic dermatitis, and/or asthma), smoking during pregnancy, and the number of siblings. The reference group in phenotypes is Never/Infrequent wheeze and in the exposure group as stated in the table.
aCannot be estimated.
A general estimate of the degree of damage was defined as “need for repair” class of the damage in the whole house.
Bold value indicates significant p ‐ value.
FIGURE 1.
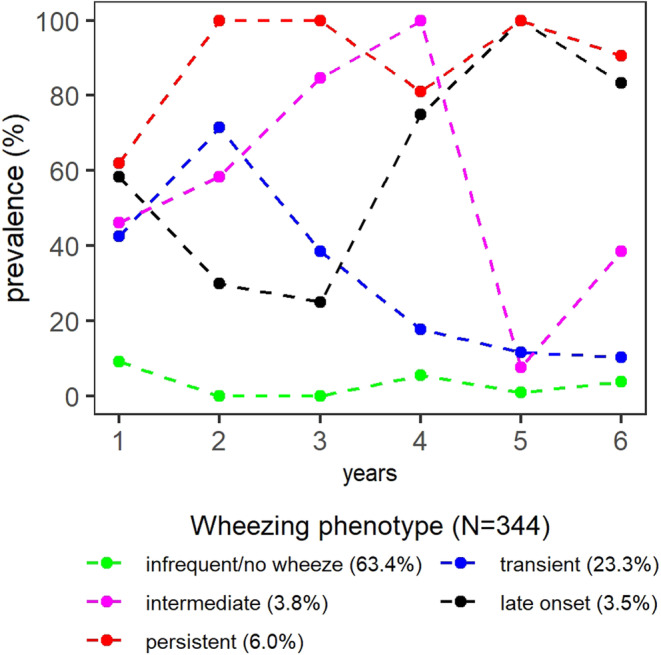
Prevalence of wheezing in different time points during the first 6 years of life in five wheezing phenotypes analyzed by latent class analyses (LCA) reproduction.
3.2. Analytical findings
3.2.1. Persistent wheezing
Moisture damage or mold in the child's main living area was dose‐dependently associated with an increased risk of persistent wheeze in adjusted analyses (Figure 2; Table 2 ). The persistent wheezing phenotype tended to be also associated with moisture damage and moisture damage with mold in the living room, in the child's bedroom (Table 2) and in the other main living areas (other than kitchen, living room, and child's bedroom) (Table 3), but due to small numbers confidence intervals are wide. No associations were found between moisture damage or moisture damage with mold in the bathrooms, other interior spaces, or in the whole house and persistent wheezing (Table 3). If children diagnosed with asthma (n = 45) were excluded from the current analyses (resulting in 6 persistent wheezers, 65 transient, and 203 no/infrequent wheezers in the model, N = 274), the association between moisture damage in the child's main living area and persistent wheeze phenotype showed the same trend. However, due to the small number of persistent wheezers in the model (n = 6), a meaningful interpretation of the results is questionable.
FIGURE 2.
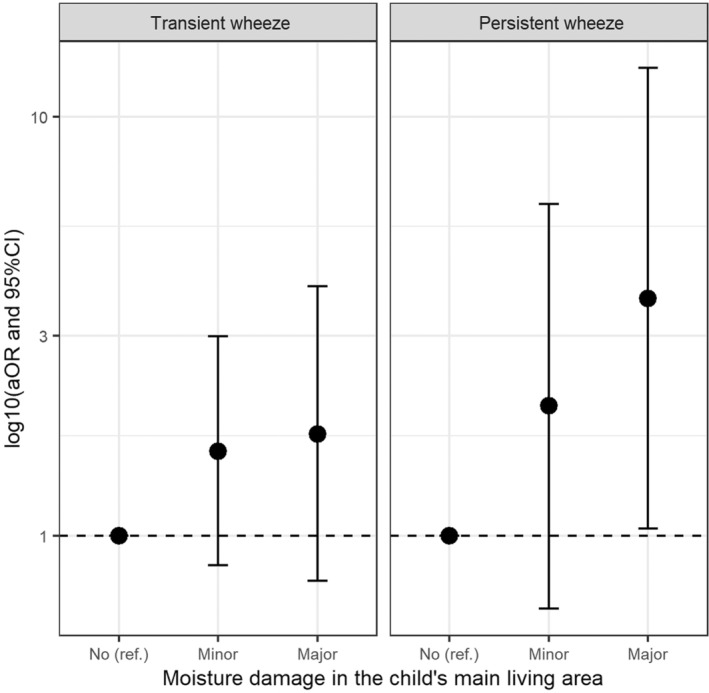
Adjusted associations between moisture damage in the child's main living area and transient or persistent wheezing phenotypes compared to no or infrequent wheezing phenotype. No moisture damage (reference category) = no damage or mold in the child's main living areas (minor = minor moisture damage with or without mold spots; major = major moisture damage or any moisture damage with visible mold. Multinomial logistic regression models are adjusted for study cohort, living on a farm, gender, maternal history of allergic diseases (hay fever, atopic dermatitis, and/or asthma), smoking during pregnancy, and the number of siblings. *p < .05.
TABLE 3.
Adjusted associations between characteristics of moisture damage or mold in further locations of the home and transient as well as persistent wheezing phenotypes compared with never or infrequent wheeze.
| N | Never/infrequent wheeze | Transient wheeze | Persistent wheeze | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | aOR (95% CI) | n | % | aOR (95% CI) | ||
| Other main living areas (other than the kitchen, living room, and child's bedroom) | |||||||||
| No moisture damage (reference) | 243 | 166 | 68 | 66 | 27 | 1 | 11 | 5 | 1 |
| Minor | 45 | 32 | 71 | 7 | 16 | 0.58 (0.24–1.42) | 6 | 13 | 3.59 (1.08–10.9), p = .0368 |
| Major | 31 | 20 | 65 | 7 | 23 | 0.87 (0.33–2.29) | 4 | 13 | 3.59 (0.90–14.3), p = .0690 |
| No moisture damage with mold (reference) | 293 | 200 | 68 | 76 | 26 | 1 | 17 | 6 | 1 |
| Spots | 9 | 6 | 68 | 1 | 11 | 0.41 (0.05–3.60) | 2 | 22 | 3.72 (0.58–23.9) |
| Visible mold | 17 | 12 | 71 | 3 | 18 | 0.74 (0.19–2.84) | 2 | 12 | 1.91 (0.34–10.8) |
| Bathrooms | |||||||||
| No moisture damage (reference) | 127 | 85 | 67 | 35 | 28 | 1 | 7 | 6 | 1 |
| Minor | 97 | 68 | 70 | 22 | 23 | 0.84 (0.44–1.62) | 7 | 7 | 1.56 (0.49–4.98) |
| Major | 95 | 65 | 68 | 23 | 24 | 0.99 (0.50–1.95) | 7 | 7 | 1.84 (0.55–6.12) |
| No moisture damage with mold (reference) | 285 | 192 | 67 | 74 | 26 | 1 | 19 | 7 | 1 |
| Spots | 11 | 8 | 73 | 2 | 18 | 0.46 (0.09–2.30) | 1 | 9 | 0.92 (0.10–8.42) |
| Visible mold | 23 | 18 | 78 | 4 | 17 | 0.45 (0.14–1.45) | 1 | 4 | 0.35 (0.04–3.08) |
| Other interior spaces | |||||||||
| No moisture damage (reference) | 240 | 160 | 67 | 63 | 26 | 1 | 17 | 7 | 1 |
| Minor | 15 | 12 | 80 | 3 | 20 | 0.75 (0.20–2.87) | 0 | 0 | – a |
| Major | 64 | 46 | 72 | 14 | 22 | 0.95 (0.47–1.93) | 4 | 6 | 1.08 (0.32–3.68) |
| No moisture damage with mold (reference) | 289 | 194 | 67 | 75 | 26 | 1 | 20 | 7 | 1 |
| Spots | 3 | 3 | 100 | 0 | 0 | – a | 0 | 0 | – a |
| Visible mold | 27 | 21 | 78 | 5 | 19 | 0.64 (0.22–1.85) | 1 | 4 | 0.49 (0.06–4.18) |
| Whole house | |||||||||
| Need for repair scale: Class 0 or 1 (reference) | 93 | 64 | 67 | 25 | 27 | 1 | 4 | 4 | 1 |
| Class 2 | 119 | 81 | 68 | 29 | 24 | 0.98 (0.51–1.89) | 9 | 8 | 2.21 (0.61–7.92) |
| Class ≥3 | 107 | 73 | 68 | 26 | 24 | 1.00 (0.50–2.01) | 8 | 8 | 2.31 (0.60–8.81) |
| No moisture damage with mold (reference) | 202 | 138 | 68 | 52 | 26 | 1 | 12 | 6 | 1 |
| Only spots | 46 | 32 | 70 | 9 | 20 | 0.63 (0.27–1.48) | 5 | 11 | 1.37 (0.41–4.58) |
| Visible mold | 71 | 48 | 68 | 19 | 27 | 1.17 (0.58–2.37) | 4 | 6 | 1.03 (0.28–3.82) |
Note: Models are adjusted for study cohort, living on a farm, gender, maternal history of allergic diseases (hay fever, atopic dermatitis, and/or asthma), smoking during pregnancy, and the number of siblings. The reference group in phenotypes is Never/Infrequent wheeze and in the exposure group as stated in the table.
aCannot be estimated.
The general estimate of the degree of damage was defined as “need for repair” class of the damage in the whole house, p‐value ^<.1, *<.05.
Bold value indicates significant p ‐ value.
3.2.2. Transient wheezing
Moisture damage or mold in the child's main living area was non‐significantly associated with transient wheeze (aOR (95% CI) 1.59 (0.85–2.99)) for minor damage with or without mold spots and 1.75 (0.78–3.94) for major moisture damage or any moisture damage with visible mold (Figure 2; Table 2). This association resulted from exposure to moisture damage or mold in the kitchen because no association was found for moisture damage or mold in the living room or in the child's bedroom (Table 2). No other associations were observed between moisture and mold categories and transient wheezing (Table 3).
4. DISCUSSION
4.1. Summary of main findings
In this study, we applied LCA to questions about current wheeze from birth until age 6 years, resulting in five wheezing phenotypes; infrequent/no wheeze, transient wheeze, intermediate wheeze, late‐onset wheeze, and persistent wheeze. We found that moisture damage and mold observations in the child's main living areas, as determined by building inspections performed during the child's first year of life, were significantly associated especially with the persistent wheezing phenotype until the age of 6 years. Exposure to moisture damage or mold in the kitchen area was associated with transient wheeze only. No associations were observed for moisture observation in the bathrooms, in the other interior spaces or when considering the entire house.
4.2. Validation of wheezing phenotype identification
It is crucial to determine the underlying mechanisms to prevent the continuation of symptoms and a subsequent asthma diagnosis later in childhood by targeted intervention measures beforehand. 26 To distinguish between different wheezing phenotypes in early childhood, epidemiological studies including ours, have mostly used a hypothesis‐free LCA approach to evaluating long‐term trajectories of wheezing outcomes or wheezing classes throughout childhood. 15 , 27 Martinez and colleagues 9 identified four patterns of early‐life wheezing (never wheeze, transient early wheeze, late‐onset wheeze, and persistent wheeze) on the basis of clinical observation among children from the Tucson Children's Respiratory Study in 1995. These wheezing patterns have generally been confirmed and further refined in subsequent investigations using clinical and epidemiological approaches. 10 , 11 , 13 , 27 The identified wheezing phenotypes in our study are consistent and similar in magnitude to those obtained in the Tuscon, 9 the ALSPAC (Avon Longitudinal Study of Parents and Children) birth cohort study, 10 and the Melbourne Atopy Cohort Study. 28 In addition, they were identical to those ascertained from the Dutch PIAMA birth cohort. 11 Consensus over the identification of wheezing phenotypes is important as previous findings indicate that in particular persistent and recurrent wheezing during early childhood years is associated with an asthma diagnosis and lung function decline throughout later childhood. 29 , 30 In our study, early age exposure to moisture damage and visible mold in the child's main living areas (living room, kitchen, and child's bedroom) was most notably associated with the persistent wheezing phenotype. This underlines the importance of measures to repair moisture damages at home and helps to target those efforts.
4.3. Results in comparison with previous findings
In line with the present findings, we reported previously from the same study population that early age exposure to residential moisture damage and mold in the child's main living area was associated with an increased risk of asthma ever and asthma at the age of 6 years. 6 The latter is likely to remain symptomatic until adulthood. 31 Accordingly, excluding asthmatics from the analyses still showed the same trend, however, a meaningful interpretation was hampered due to the small numbers.
In the current study, moisture damage or mold in the child's main living areas further tended to be associated with the transient wheezing phenotype, mainly driven by associations with moisture observations in the kitchen. We have reported earlier that moisture damage or mold in the kitchen was associated with asthma or asthmatic bronchitis in the first 18 months of life, 5 but was not associated with persistent asthma at the age of 6 years. 6 We can only speculate about the possible reasons for this specific finding, in addition to small numbers. Depending on the age of the child, their occupancy patterns with respect to time spent in different rooms of the apartment are likely to change. Transient wheezing was associated with wheezing that occurs at an early age when children typically spend more time with their parents, including in the kitchen when the parents prepare food. At the later age of the child, this aspect might become less important and the kitchen, for example, might no longer be as relevant with respect to exposure duration. In contrast, if the moisture damage or mold is located in the main living areas, such as the child's bedroom or the living room, the exposure time can be assumed to remain somewhat similar throughout childhood. In addition, it has been suggested that atopic children might be more affected by environmental exposures and thus prone to persistent wheezing. Compared to that, transient wheezing is often associated with early life respiratory infections, thus less affected by the environment. 11 , 28
To the best of our knowledge, this is the first study looking at objectively assessed early age moisture and mold exposure in relation to different wheezing phenotypes obtained by hypothesis‐free LCA in a general population. A very similar study protocol was followed in an atopic cohort (the Cincinnati Childhood Allergy and Air Pollution Study, CCAAPS), which used home investigation for moisture damage and mold at an early age, but used clinical asthma phenotypes defined by parental‐reported records instead of hypothesis‐free LCA. 32 Similarly, they observed that children had a significantly increased risk of persistent wheezing until the age of 7 years upon exposure to objectively observed severe moisture and mold damage in the home at an early age. 32 However, the weakness of the study was that the results were not as generalizable due to the nature of an atopic cohort as compared to the results from our cohort. In another birth cohort study (Barn/Child Allergy Milieu Stockholm Epidemiology, BAMSE) with the follow‐up up to the age of 16 years, parents reported mold odor, visible mold, or dampness in the whole home at an early age, and clinical asthma phenotypes defined by parental‐reported records was used. 33 Among children exposed to any parent‐reported mold or dampness at an early age, a significantly increased risk was observed for persistent, but not for the transient or late‐onset asthma phenotype, as was found in our cohort with shorter follow‐up time. Among 18 months old children from the PARIS (Pollution and Asthma Risk: An Infant Study) birth cohort, three wheezing phenotypes were identified by LCA. 34 Parent‐reported visible mold and/or moldy smell in the home was a risk factor for the atopic severe phenotype, which was associated with recurrent wheezing, other respiratory as well as allergic symptoms and an increased usage of medical and hospital‐based care. Due to the short follow‐up period, it is hard to separate children from early transient wheezing phenotype to persistent phenotype.
Based on the evidence from our study and others, early age exposure to moisture damage and mold is associated specifically with a more severe, partly atopic wheezing phenotype, characterized by persistency and frequent episodes. In addition, there are a few studies looking at the association between wheezing phenotypes and exposure to moisture and/or mold without using an a priori data‐driven approach. According to Civelek et al., 32 current but not early age exposure to mold and dampness was associated with frequent wheezing in a multi‐center cross‐sectional analysis among over 6000 elementary school children in Turkey. Finally, Lezmi and colleagues showed in a French multicenter observational cohort that exposure to residential mold was significantly associated with severe recurrent wheezing in preschoolers that were at high risk of asthma. 35 Taken together, the findings of our and previous investigations consistently suggest that exposure to moisture damage and mold in early and throughout infancy are associated in particular with persistent and recurrent wheezing, which in turn is a risk factor for later asthma outcomes.
4.4. Strengths and limitations
Our study used information on moisture damage and mold observations from a home inspection carried out by trained civil engineers following standardized protocols, which is a more objective assessment compared to for instance parent reports. Due to detailed records of the observations, we were able to estimate the dose of the exposure using mostly three‐level indicators and information on location. We also collected the information on wheezing prospectively, and thus, neither recall bias nor the well‐known difficulties with asthma diagnoses—that is, asthma diagnoses at an early age may lead to a false positive classification of asthma—could have affected our results. As mentioned, this is the first study investigating inspector observed early age exposure to moisture damage and mold in relation to wheezing phenotypes ascertained by latent class analyses in population‐based birth cohorts. Although there are limitations due to a limited sample size, our work confirmed earlier findings from similar studies in different geographical regions, underlining that moisture and mold damage in early infancy might contribute to later asthma outcomes. However, due to the low numbers of observations in the individual phenotypes in our study, some estimates may be unstable and the results need to be interpreted with caution.
5. CONCLUSION
Our results add to earlier findings that exposure during infancy to moisture damage and mold in the child's main living areas in the home, increases the risk of especially persistent wheezing, thereby potentially contributing to asthma outcomes later in life. These findings emphasize the importance of providing a healthy home environment free of major moisture damages during early childhood, in an effort to reduce the risk of later asthma.
AUTHOR CONTRIBUTIONS
Martin Taubel: Writing – review and editing (equal). Pirkka Kirjavainen: Writing – review and editing (equal). Martin Depner: Writing – review and editing (equal). Anne Hyvärinen: Writing – review and editing (equal). Eija Piippo‐Savolainen: Writing – review and editing (equal). Juha Pekkanen: Conceptualization (lead); funding acquisition (lead); writing – review and editing (equal). Anne Karvonen: Conceptualization (lead); formal analysis (lead); methodology (lead); writing – review and editing (supporting).
FUNDING INFORMATION
This study was supported by research grants from the European Union QLK4‐CT‐2001‐00250; EVO‐ and VTR‐funding; the Academy of Finland (grant 139021, 287675, 338679, 339666); the Juho Vainio Foundation; the Yrjö Jahnsson foundation; Päivikki and Sakari Sohlberg foundation; the Finnish Cultural Foundation; and by the Finnish Institute for Health and Welfare, Finland.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ACKNOWLEDGMENTS
We gratefully acknowledge the contribution of our fieldworkers Raija Juntunen, Riikka Juola, and Seija Antikainen, civil engineers Juho Halla‐aho and Jari Koivisto, as well as Timo Kauppila and Asko Vepsäläinen, for help with data management. We further like to thank Dr. Sabina Illi for her help and support in the statistical analysis. Ultimately, we would like to thank the families for their participation in the study.
Tischer C, Täubel M, Kirjavainen PV, et al. Early‐life residential exposure to moisture damage is associated with persistent wheezing in a Finnish birth cohort. Pediatr Allergy Immunol. 2022;33:e13864. doi: 10.1111/pai.13864
Juha Pekkanen and Anne M. Karvonen shared last authorship.
Editor: Jon Genuneit
REFERENCES
- 1. Kanchongkittiphon W, Mendell MJ, Gaffin JM, Wang G, Phipatanakul W. Indoor environmental exposures and exacerbation of asthma: an update to the 2000 review by the Institute of Medicine. Environ Health Perspect. 2015;123(1):6‐20. doi: 10.1289/ehp.1307922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J. Respiratory and allergic health effects of dampness, mold, and dampness‐related agents: a review of the epidemiologic evidence. Environ Health Perspect. 2011;119(6):748‐756. doi: 10.1289/ehp.1002410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tischer C, Chen C‐M, Heinrich J. Association between domestic mould and mould components, and asthma and allergy in children: a systematic review. Eur Respir J. 2011;38(4):812‐824. doi: 10.1183/09031936.00184010 [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . WHO guidelines for indoor air quality: dampness and mould. Accessed March 5, 2021. https://www.who.int/airpollution/guidelines/dampness‐mould/en/ [PubMed]
- 5. Karvonen AM, Hyvärinen A, Roponen M, et al. Confirmed moisture damage at home, respiratory symptoms and atopy in early life: a birth‐cohort study. Pediatrics. 2009;124(2):e329‐e338. doi: 10.1542/peds.2008-1590 [DOI] [PubMed] [Google Scholar]
- 6. Karvonen AM, Hyvärinen A, Korppi M, et al. Moisture damage and asthma: a birth cohort study. Pediatrics. 2015;135(3):e598‐e606. doi: 10.1542/peds.2014-1239 [DOI] [PubMed] [Google Scholar]
- 7. Fuchs O, Bahmer T, Rabe KF, von Mutius E. Asthma transition from childhood into adulthood. Lancet Respir Med. 2017;5(3):224‐234. doi: 10.1016/S2213-2600(16)30187-4 [DOI] [PubMed] [Google Scholar]
- 8. Depner M, Fuchs O, Genuneit J, et al. Clinical and epidemiologic phenotypes of childhood asthma. Am J Respir Crit Care Med. 2014;189(2):129‐138. doi: 10.1164/rccm.201307-1198OC [DOI] [PubMed] [Google Scholar]
- 9. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332(3):133‐138. doi: 10.1056/NEJM199501193320301 [DOI] [PubMed] [Google Scholar]
- 10. Henderson J, Granell R, Heron J, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid‐childhood. Thorax. 2008;63(11):974‐980. doi: 10.1136/thx.2007.093187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Savenije OE, Granell R, Caudri D, et al. Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. J Allergy Clin Immunol. 2011;127(6):1505‐12.e14. doi: 10.1016/j.jaci.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 12. Sbihi H, Koehoorn M, Tamburic L, Brauer M. Asthma trajectories in a population‐based birth cohort. Impacts of air pollution and greenness. Am J Respir Crit Care Med. 2017;195(5):607‐613. doi: 10.1164/rccm.201601-0164OC [DOI] [PubMed] [Google Scholar]
- 13. Spycher BD, Silverman M, Brooke AM, Minder CE, Kuehni CE. Distinguishing phenotypes of childhood wheeze and cough using latent class analysis. Eur Respir J. 2008;31(5):974‐981. doi: 10.1183/09031936.00153507 [DOI] [PubMed] [Google Scholar]
- 14. Panico L, Stuart B, Bartley M, Kelly Y. Asthma trajectories in early childhood: identifying modifiable factors. PLoS One. 2014;9(11):e111922. doi: 10.1371/journal.pone.0111922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reddy MB, Covar RA. Asthma phenotypes in childhood. Curr Opin Allergy Clin Immunol. 2016;16(2):127‐134. doi: 10.1097/ACI.0000000000000252 [DOI] [PubMed] [Google Scholar]
- 16. Hirvonen MR, Ruotsalainen M, Roponen M, et al. Nitric oxide and proinflammatory cytokines in nasal lavage fluid associated with symptoms and exposure to moldy building microbes. Am J Respir Crit Care Med. 1999;160(6):1943‐1946. doi: 10.1164/ajrccm.160.6.9903023 [DOI] [PubMed] [Google Scholar]
- 17. Roponen M, Meklin T, Rintala H, Hyvärinen A, Hirvonen MR. Effect of moisture‐damage intervention on the immunotoxic potential and microbial content of airborne particles and on occupants' upper airway inflammatory responses. Indoor Air. 2013;23(4):295‐302. doi: 10.1111/ina.12032 [DOI] [PubMed] [Google Scholar]
- 18. Strunk RC, Szefler SJ, Phillips BR, et al. Childhood Asthma Research and Education Network of the National Heart, Lung, and Blood Institute. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J Allergy Clin Immunol. 2003;112(5):883‐892. doi: 10.1016/j.jaci.2003.08.014 [DOI] [PubMed] [Google Scholar]
- 19. Mutius E v, Schmid S. The PASTURE project: EU support for the improvement of knowledge about risk factors and preventive factors for atopy in Europe. Allergy. 2006;61(4):407‐413. doi: 10.1111/j.1398-9995.2006.01009.x [DOI] [PubMed] [Google Scholar]
- 20. Herzum I, Blümer N, Kersten W, Renz H. Diagnostic and analytical performance of a screening panel for allergy. Clin Chem Lab Med. 2005;43(9):963‐966. doi: 10.1515/CCLM.2005.165 [DOI] [PubMed] [Google Scholar]
- 21. Tischer C, Karvonen AM, Kirjavainen PV, et al. Early age exposure to moisture and mould is related to FeNO at the age of 6 years. Pediatr Allergy Immunol. 2021;32(6):1226‐1237. doi: 10.1111/pai.13526 [DOI] [PubMed] [Google Scholar]
- 22. Pekkanen J, Hyvärinen A, Haverinen‐Shaughnessy U, Korppi M, Putus T, Nevalainen A. Moisture damage and childhood asthma: a population‐based incident case‐control study. Eur Respir J. 2007;29(3):509‐515. doi: 10.1183/09031936.00040806 [DOI] [PubMed] [Google Scholar]
- 23. Nevalainen A, Partanen P, Jääskeläinen E, et al. Prevalence of moisture problems in Finnish houses. Indoor Air. 1998;8(S4):45‐49. doi: 10.1111/j.1600-0668.1998.tb00007.x [DOI] [Google Scholar]
- 24. Karvonen AM, Tischer C, Kirjavainen PV, et al. Early age exposure to moisture damage and systemic inflammation at the age of 6 years. Indoor Air. 2018;28(3):450‐458. doi: 10.1111/ina.12454 [DOI] [PubMed] [Google Scholar]
- 25. Lee K, Ahn H, Moon H, Kodell RL, Chen JJ. Multinomial logistic regression ensembles. J Biopharm Stat. 2013;23(3):681‐694. doi: 10.1080/10543406.2012.756500 [DOI] [PubMed] [Google Scholar]
- 26. Oksel C, Granell R, Mahmoud O, Custovic A, Henderson AJ. Causes of variability in latent phenotypes of childhood wheeze. J Allergy Clin Immunol. 2019;143(5):1783‐1790.e11. doi: 10.1016/j.jaci.2018.10.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oksel C, Granell R, Haider S, et al. Distinguishing wheezing phenotypes from infancy to adolescence. A pooled analysis of five birth cohorts. Ann Am Thorac Soc. 2019;16(7):868‐876. doi: 10.1513/AnnalsATS.201811-837OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lodge CJ, Zaloumis S, Lowe AJ, et al. Early‐life risk factors for childhood wheeze phenotypes in a high‐risk birth cohort. J Pediatr. 2014;164(2):289‐294.e1‐2. doi: 10.1016/j.jpeds.2013.09.056 [DOI] [PubMed] [Google Scholar]
- 29. Belgrave DCM, Buchan I, Bishop C, Lowe L, Simpson A, Custovic A. Trajectories of lung function during childhood. Am J Respir Crit Care Med. 2014;189(9):1101‐1109. doi: 10.1164/rccm.201309-1700OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duijts L, Granell R, Sterne JAC, Henderson AJ. Childhood wheezing phenotypes influence asthma, lung function and exhaled nitric oxide fraction in adolescence. Eur Respir J. 2016;47(2):510‐519. doi: 10.1183/13993003.00718-2015 [DOI] [PubMed] [Google Scholar]
- 31. Grad R, Morgan WJ. Long‐term outcomes of early‐onset wheeze and asthma. J Allergy Clin Immunol. 2012;130(2):299‐307. doi: 10.1016/j.jaci.2012.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Civelek E, Cakir B, Orhan F, et al. Risk factors for current wheezing and its phenotypes among elementary school children. Pediatr Pulmonol. 2011;46(2):166‐174. doi: 10.1002/ppul.21346 [DOI] [PubMed] [Google Scholar]
- 33. Thacher JD, Gruzieva O, Pershagen G, et al. Mold and dampness exposure and allergic outcomes from birth to adolescence: data from the BAMSE cohort. Allergy. 2017;72(6):967‐974. doi: 10.1111/all.13102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Herr M, Just J, Nikasinovic L, et al. Risk factors and characteristics of respiratory and allergic phenotypes in early childhood. J Allergy Clin Immunol. 2012;130(2):389‐96.e4. doi: 10.1016/j.jaci.2012.05.054 [DOI] [PubMed] [Google Scholar]
- 35. Lezmi G, Lejeune S, Pin I, et al. Factors associated with asthma severity in children: data from the French COBRAPed cohort. J Allergy Clin Immunol Pract. 2021;9(5):1969‐1979. doi: 10.1016/j.jaip.2020.12.027 [DOI] [PubMed] [Google Scholar]


