Abstract
Background
Thrombosis is common among patients with cancer. Primary thromboprophylaxis guided by the Khorana score is endorsed by guidelines but recommendations rely mainly on data from patients treated with chemotherapy.
Objectives
To explore if the Khorana score could risk stratify patients with cancer treated with immune checkpoint inhibitors according to risk of venous and arterial thrombosis.
Patients/Methods
The study population and Khorana score were defined using administrative Danish health registries. The primary outcome was 6‐month risk of venous thromboembolism after initiation of checkpoint inhibitor treatment. Secondary outcomes were arterial thrombosis and any thromboembolic event. Death was considered a competing risk event.
Results
Among 3946 patients with cancer initiating checkpoint inhibitor treatment without other indications for anticoagulation, the overall 6‐month incidence of venous thromboembolism was 2.6% (95% confidence interval [CI]: 2.1–3.1). Risks were 2.1% (95% CI: 1.5–3.0), 2.6% (95% CI: 2.0–3.4), and 3.7% (95% CI: 2.1–5.9) in low (score 0), intermediate (score 1–2), and high risk (score ≥3) Khorana categories, respectively. Among patients eligible for primary thromboprophylaxis according to guidelines (Khorana score ≥2), risk of venous thromboembolism was 4.1% (95% CI: 3.1–5.4). Higher Khorana risk category was also associated with higher 6‐month risk of both arterial thrombosis and any thromboembolic events.
Conclusions
The Khorana score was able to risk stratify patients with cancer treated with immune checkpoint inhibitors according to 6‐month risk of thromboembolic events. Risks of venous thromboembolism were lower than in randomized thromboprophylaxis trials, thus questioning the absolute benefit of routine primary thromboprophylaxis in an unselected population of patients treated with immune checkpoint inhibitors.
Keywords: anticoagulants, immune checkpoint inhibitors, neoplasms, thrombosis, venous thromboembolism
Essentials.
Whether the Khorana score predicts thrombosis in cancer patients on checkpoint inhibitors is uncertain.
This was explored in a Danish cohort identified and followed in administrative health registries.
The Khorana score was able to risk stratify such patients according to venous and arterial thrombosis.
The Khorana score may guide thromboprophylaxis during checkpoint inhibitor treatment.
1. INTRODUCTION
Venous thromboembolism remains a frequent and potentially serious complication among patients with cancer. 1 , 2 , 3 , 4 In recent years, guidelines have recommended primary thromboprophylaxis with anticoagulation in selected cancer patients initiating systemic treatment following risk stratification with the Khorana score, a simple, point‐based risk score originally developed to assess risk of venous thromboembolism in patients initiating chemotherapy. 5 , 6 The benefit of primary anticoagulant thromboprophylaxis guided by the Khorana score has even been explored in randomized trials, but not specifically for patients treated with immune checkpoint inhibitors, and the Khorana score was developed in an era prior to clinical use of these agents. 7 , 8 , 9 Immune checkpoint inhibitors, which enhance the cytotoxic effects of T‐cells, have since become a widely used treatment modality with an increasing number of indications. 10 Some reports have suggested that checkpoint inhibitors, in addition to their unique toxicity profile, are also associated with a particularly high risk of both venous and arterial thrombosis. 11 , 12 , 13 , 14 , 15
Few studies have investigated the ability of the Khorana score to predict venous thromboembolism in patients treated with immune checkpoint inhibitors, and with conflicting results. 12 , 16 , 17 , 18 , 19 Nonetheless, some guidelines (see Table 4 for details) endorse use of primary anticoagulant thromboprophylaxis for ambulatory cancer patients irrespective of type of systemic medical cancer treatment, but concerns have been raised regarding the applicability of the Khorana score as a universally applicable risk score. 20 Also, considering that randomized trial data indicate that anticoagulant thromboprophylaxis prevents not only venous thromboembolic events, but also arterial events, the role of the Khorana score in predicting the cumulative thromboembolic burden in patients with cancer treated with checkpoint inhibitors is underexplored. 9
TABLE 4.
Guideline recommendations on primary thromboprophylaxis in ambulatory patients with cancer treated with immune checkpoint inhibitors
| Guideline | Recommends primary thromboprophylaxis during checkpoint inhibitor treatment? | Which Khorana score threshold? | Recommended treatment duration |
|---|---|---|---|
| NCCN 2022 39 | Yes, any systemic treatment | Khorana score ≥2 | Six months (“longer if risk persists”) |
| ASH 2021 40 | Yes, any systemic treatment | Both intermediate and high‐risk patients according to Khorana score (i.e., score ≥1) | Not specified |
| ASCO 2020 41 | No, only chemotherapy | Khorana score ≥2 | “Discuss with patient” |
| ITAC 2022 42 | Yes, any systemic treatment | Khorana score ≥2 | Not specified |
| ISTH 2019 43 | No, only chemotherapy | Khorana score ≥2 | Six months |
Abbreviations: ASCO, American Society of Clinical Oncology; ASH, American Society of Hematology; ISTH, International Society on Thrombosis and Haemostasis; ITAC, International Initiative on Thrombosis and Cancer; NCCN, National Comprehensive Cancer Network.
We aimed to describe the incidence of venous and arterial thrombosis in cancer patients treated with immune checkpoint inhibitors who were not using anticoagulation, while also exploring whether the Khorana score would be a valid tool for identifying patients at high risk of such thrombotic events.
2. METHODS
2.1. Design and setting
A cohort of cancer patients initiating treatment with immune checkpoint inhibitors (PD‐1, PD‐L1, or CTLA4 inhibitors) in Danish hospitals was identified using administrative Danish health registries. 21 Specifically, all Danish residents have a unique national identification number, which was used to link individual‐level health data from several registries: The Danish Civil Registration System, which holds information on vital and migration status; the Danish National Patient Register, containing information on hospital diagnosis and treatments; the National Prescription Register storing information on all claimed prescription drugs in Denmark; and the Register of Laboratory Results for Research database, which collects information on laboratory tests performed both in primary and secondary care. 22 , 23 , 24 The study was reported in accordance with TRIPOD (transparent reporting of a multivariable prediction model for individual prognosis or diagnosis) recommendations. 25
2.2. Study population
The study population consisted of cancer patients initiating treatment with immune checkpoint inhibitors before October 2020. Checkpoint inhibitors targeting both PD‐1/PD‐L1 (pembrolizumab, nivolumab, atezolizumab, avelumab, and durvalumab) and CTLA4 (ipilimumab) were included. Codes used to extract information about checkpoint inhibitor treatment are available in Table S1 in supporting information. The positive predictive value of treatment codes for identifying medical cancer treatment is high in the National Patient Register (95%). 26 Patients with primary brain cancer or multiple myeloma, patients already treated with anticoagulation or who had a potential existing indication for anticoagulation, and patients with missing laboratory values within 30 days prior to treatment initiation were excluded (see Figure S1 in supporting information for details) The latter criterion was because the registration of laboratory values in the Register of Laboratory Results for Research did not commence simultaneously for all Danish regions, but it has been reported that overall venous thromboembolism risk was identical for patients with and without available laboratory values. 20
2.3. Khorana score
The components used to calculate the Khorana score were identified as done previously. 20 Points are attributed to cancer type (stomach or pancreas [2 points]; lung, lymphoma, gynecologic, bladder, or testicular cancer [1 point]), platelet count ≥350x109/L (1 point), hemoglobin level < 10 g/dL or use of erythrocyte growth factors (1 point), leukocyte count >11x109/L (1 point), and body mass index ≥35 kg/m2 (1 point). 5 Laboratory values were pretreatment values obtained prior to treatment with checkpoint inhibitors and were obtained from the Register of Laboratory Results for Research. Information on cancer type was obtained using the National Patient Register. Body mass index ≥35 kg/m2 was defined using International Classification of Diseases (ICD) 10 codes. Codes used to define the components can be found in Table S1. The Khorana score was evaluated in two versions: 1) by the original categorization into low risk (score 0), intermediate risk (score 1–2), and high risk (≥3); and 2) dichotomous according to the most widely recommended score threshold for initiation of thromboprophylaxis (score 0–1 versus ≥2). 5 , 6
2.4. Outcomes
The primary outcome was a composite of a primary or secondary diagnosis of deep vein thrombosis or pulmonary embolism given in combination with a relevant imaging procedure. The positive predictive value of inpatient diagnoses given in combination with an imaging procedure is high (>90%). 27 Both inpatient and ambulatory diagnoses were included. In a sensitivity analysis, a broad definition of venous thromboembolism was applied, defined as any venous thromboembolic diagnosis given irrespective of imaging procedure; see Table S1 for details.
Arterial thrombosis was defined as the occurrence of either myocardial infarction, ischemic stroke, or systemic embolism. Ischemic stroke was defined as an inpatient diagnosis of either ischemic or unspecified stroke as most unspecified strokes have been shown to be of ischemic origin. The positive predictive value of an inpatient diagnosis of ischemic stroke is high (>85%). 28 , 29 Transient ischemic attack was not included due to low positive predictive value. 30 The positive predictive value of a diagnosis of myocardial infarction in the Danish National Patient Register is high. 27 , 31
2.5. Statistics
Baseline characteristics were presented using medians with interquartile range for continuous variables and as proportions for categorical variables. The cumulative incidence of venous thromboembolism was calculated using competing risk analysis considering death a competing event. 32 Patients were followed until the occurrence of the relevant thromboembolic event, death, emigration, or end of follow‐up (April 2021). In the main analysis, end of follow‐up was 6 months following treatment initiation because most guidelines recommend basing the decision on initiation of primary thromboprophylaxis on 6‐month risk estimates. 6 Because patients treated with checkpoint inhibitors are often treated for longer time periods, risk estimates were also reported for up to 1 year of follow‐up.
The associations between Khorana categories and risk of outcomes were compared using the Fine and Gray subdistribution hazard model considering death a competing event. 33 Analyses were performed using Stata version 16.
3. RESULTS
We identified 3946 patients initiating checkpoint inhibitor therapy who did not have an existing indication for anticoagulation. Baseline characteristics are presented in Table 1. Median age was 67 years, and 49% were females. The majority were treated with a PD‐1 inhibitor (82.4%) while 8.8% received double checkpoint inhibitor treatment. Concomitant chemotherapy was registered for 6.7% of the population, and most patients (79.1%) had received other medical anticancer treatment prior to initiation of checkpoint inhibitor therapy. Approximately 10% had a Khorana score of 3 or above, while 29.4% had a score of 2 or higher. During 6 months of follow‐up, 17.5% of the patients died, with mortality rising to 30.9% after 1 year.
TABLE 1.
Baseline characteristics of patients with cancer initiating treatment with an immune checkpoint inhibitor
| Patients, n | 3946 |
| Female sex | 49.0 (1934) |
| Age, years, median (quartile 1–3) | 67 (59–74) |
| Months since cancer diagnosis, median (quartile 1–3) | 16 (5–49) |
| Medical anticancer treatment | |
| PD‐1 inhibitor | 82.4 (3251) |
| PD‐L1 inhibitor | 8.3 (326) |
| CTLA4 inhibitor | 18.1 (715) |
| Double checkpoint inhibitor treatment | 8.8 (346) |
| Concomitant chemotherapy a | 6.7 (266) |
| Any previous medical anticancer treatment b | 79.1 (3120) |
| Khorana score level | |
| 0 | 36.0 (1421) |
| 1 | 34.6 (1366) |
| 2 | 19.6 (772) |
| 3 | 8.0 (315) |
| 4+ | 1.8 (72) |
| Khorana risk category | |
| Low risk (0) | 36.0 (1421) |
| Intermediate risk (1–2) | 54.2 (2138) |
| High risk (3+) | 9.8 (387) |
| Khorana guideline threshold | |
| 0–1 | 70.6 (2787) |
| 2+ | 29.4 (1159) |
| Khorana components | |
| Cancer type | |
| Stomach | 0.3 (12) |
| Pancreatic | 1.9 (74) |
| Lung | 39.1 (1543) |
| Lymphoma | 0.7 (27) |
| Gynecologic | 1.2 (48) |
| Bladder | 3.5 (137) |
| Testicular | n/a (<5) |
| Pre‐treatment laboratory values | |
| Hemoglobin level <10 g/dl or use of erythropoiesis stimulating agent | 8.7 (343) |
| Platelet count ≥ 350 x 109/L | 30.1 (1189) |
| Leukocyte count > 11 x 109/L | 16.4 (646) |
| Body mass index ≥ 35 kg/m2 | 1.1 (43) |
Patients registered with a treatment code for chemotherapy ±7 days in relation to date of checkpoint inhibitor treatment.
Defined as any anticancer treatment given >30 days prior to checkpoint inhibitor treatment.
3.1. Thrombotic events at 6‐month follow‐up
The cumulative incidences of both venous and arterial thrombotic events over a 6‐month period stratified by Khorana categories are depicted in Figure 1. During 6 months of follow‐up, 99 venous thromboembolic events occurred corresponding to an overall 6‐month incidence of 2.6% when considering death as a competing event; see Table 2. Six‐month incidence of venous thromboembolism was higher with higher Khorana risk category, with an incidence among high‐risk patients of 3.7%. Risk among those eligible for thromboprophylaxis according to current guidelines (Khorana score ≥2) was 4.1%. Conclusions were similar when applying a broad definition of venous thromboembolism, with 6‐month risk of 5.5% among those with Khorana score ≥3 and 5.2% among those with score ≥2; see Table S2 in supporting information.
FIGURE 1.
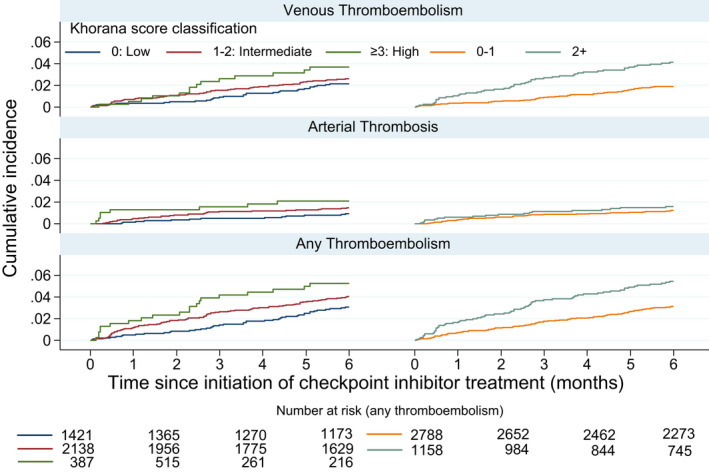
Six‐month cumulative incidence of venous, arterial, and any thromboembolic events in patients with cancer initiating treatment with immune checkpoint inhibitors
TABLE 2.
Events, cumulative incidence, and subdistribution hazard ratios (sHR) with 95% confidence interval (CI) for incident thrombotic events in patients with cancer initiating checkpoint inhibitor treatment: 6‐month follow‐up
| Venous thromboembolism | Arterial thrombosis | Any tromboembolism | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Events, n | Cumulative incidence a | sHR (95% CI) | Events, n | Cumulative incidence a | sHR (95% CI) | Events, n | Cumulative incidence a | sHR (95% CI) | |
| Overall | 99 | 2.6% | 52 | 1.3% | 148 | 3.8% | |||
| Single versus double checkpoint inhibitor treatment | |||||||||
| Single therapy | 89 | 2.5% | Ref. | 46 | 1.3% | Ref. | 132 | 3.7% | Ref. |
| Double therapy | 10 | 3.0% | 1.19 (0.62–2.30) | 6 | 1.8% | 1.38 (0.59–3.24) | 16 | 4.8% | 1.29 (0.77–2.17) |
| Khorana risk category | |||||||||
| 0: Low risk | 30 | 2.1% | Ref. | 13 | 0.9% | Ref. | 43 | 3.1% | Ref. |
| 1–2: Intermediate risk | 55 | 2.6% | 1.23 (0.79–1.92) | 31 | 1.5% | 1.60 (0.84–3.05) | 85 | 4.0% | 1.33 (0.92–1.92) |
| ≥3: High risk | 14 | 3.7% | 1.75 (0.93–3.30) | 8 | 2.1% | 2.31 (0.96–5.58) | 20 | 5.3% | 1.76 (1.03–2.99) |
| Guideline recommended threshold | |||||||||
| Score 0–1 | 52 | 1.9% | Ref. | 34 | 1.2% | Ref. | 86 | 3.1% | Ref. |
| Score ≥2 | 47 | 4.1% | 2.22 (1.50–3.30) | 18 | 1.6% | 1.28 (0.73–2.27) | 62 | 5.4% | 1.77 (1.28–2.46) |
Based on the cumulative incidence function considering death as competing risk.
A total of 52 arterial events occurred during the initial 6 months yielding an overall cumulative incidence of 1.3%, and the Khorana score suggested a gradation of risk according to such events irrespective of the choice of categorization, although the confidence intervals also included the possibility of no difference between the groups; see Table 2. Accordingly, the Khorana categories also showed a clear correlation with the composite thromboembolic outcome including both venous and arterial thrombosis, with an incidence of 5.4% at 6‐month follow‐up among patients with Khorana score ≥2.
Incidence of any thromboembolism was highest among those receiving dual checkpoint inhibitor treatment versus single drug treatment (4.8% vs. 3.7%), but broadly similar regardless of whether concomitant chemotherapy was given, subdistribution hazard ratio 0.89 (95% confidence interval [CI] 0.46–1.75) for patients with versus without concomitant chemotherapy.
3.2. One‐year follow
Results after 1 year of follow‐up are available in Table 3. Generally, the ability of the Khorana score to risk stratify patients according to risk of venous thromboembolism was preserved at 1‐year follow‐up, whereas there was no clear correlation between Khorana score categories and risk of arterial events.
TABLE 3.
Events, cumulative incidence, and subdistribution hazard ratios (sHR) with 95% confidence interval (CI) for incident thrombotic events in patients with cancer initiating checkpoint inhibitor treatment: 1‐year follow‐up
| Venous thromboembolism | Arterial thrombosis | Any thromboembolism | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Events, n | Cumulative incidence a | sHR (95% CI) | Events, n | Cumulative incidence a | sHR (95% CI) | Events, n | Cumulative incidence a | sHR (95% CI) | |
| Overall | 142 | 3.8% | 72 | 1.9% | 211 | 5.6% | |||
| Single versus double checkpoint inhibitor treatment | |||||||||
| Single therapy | 129 | 3.7% | Ref. | 62 | 1.8% | Ref. | 188 | 5.4% | Ref. |
| Double therapy | 13 | 4.1% | 1.10 (0.62–1.94) | 10 | 2.8% | 1.75 (0.90–3.41) | 23 | 7.2% | 1.34 (0.87–2.07) |
| Khorana risk category | |||||||||
| 0: Low risk | 42 | 3.0% | Ref. | 20 | 1.5% | Ref. | 62 | 4.6% | Ref. |
| 1–2: Intermediate risk | 82 | 4.0% | 1.31 (0.91–1.90) | 44 | 2.2% | 1.48 (0.87–2.50) | 125 | 6.1% | 1.36 (1.00–1.84) |
| ≥3: High risk | 18 | 4.5% | 1.61 (0.93–2.80) | 8 | 2.1% | 1.51 (0.66–3.43) | 24 | 6.1% | 1.47 (0.91–2.36) |
| Guideline recommended threshold | |||||||||
| Score 0–1 | 77 | 2.9% | Ref. | 51 | 1.9% | Ref. | 128 | 4.8% | Ref. |
| Score ≥2 | 65 | 5.8% | 2.09 (1.50–2.90) | 21 | 1.9% | 1.00 (0.60–1.66) | 83 | 7.5% | 1.60 (1.21–2.11) |
Based on the cumulative incidence function considering death as competing risk.
4. DISCUSSION
In an unselected population of patients with cancer initiating treatment with an immune checkpoint inhibitor, the Khorana score was able to risk stratify patients according to 6‐month risk of both venous and arterial thromboembolism. Among patients eligible for primary thromboprophylaxis according to guidelines, the 6‐month risk of any thromboembolic event was 5.4%. At 1‐year follow‐up, the ability of the Khorana score to risk stratify was preserved only for venous thromboembolism.
4.1. Comparison to existing literature
A study of 176 patients with lung cancer treated with checkpoint inhibitors found a lower risk of venous thromboembolism in those with Khorana score ≥2 versus score 1, but outcome events were few and the estimates thus highly sensitive to random variation. 16 In line with these findings, another study found that higher Khorana score was not associated with higher risk of venous thromboembolism during treatment with immune checkpoint inhibitors. 12 In this study, the subdistribution hazard ratio per Khorana score point increase was 0.93, 95% CI 0.60–1.46 and 0.69, 95% CI 0.32–1.51 comparing those with score ≥2 versus score 0–1. 12 In contrast, we found that higher Khorana score category suggested higher risk of venous thromboembolism. The overall 6‐month incidence in our study was 2.6% compared to 5.0% in the previous study. Generally, risk estimates from various studies vary substantially, underlining the need for further exploration of this population. 17 , 34 Reasons for this variation may include limited sample sizes yielding imprecise estimates, varying length of follow‐up (cumulative incidence estimates inherently increase over time), choice of methodology (ignoring competing risk from death overestimates thrombosis risk 20 ), and variation across cancer types, varying in‐ and exclusion criteria including use of anticoagulation. For example, among 481 patients with lung cancer treated with a checkpoint inhibitor who were followed for a median of 9.8 months, a Khorana score of 0–1 versus ≥2 was not found to be associated with venous thromboembolism. 18 This is in line with previous findings of limited discriminatory capacity of the Khorana score in ambulatory patients with lung cancer. 20 , 35
One study did not find the Khorana score useful for venous thromboembolism risk stratification in stage 4 cancer patients, but did also include patients using anticoagulation. 19 Another study found a higher incidence of venous thromboembolism with dual immunotherapy versus single treatment, 36 which was also indicated in the present study. Whether this has the potential to provide clinically meaningful refinement if added to the Khorana score warrants further investigation.
The overall 6‐month incidence of venous thromboembolism in the present study (2.6%) was comparable to the incidence observed in a cancer population initiating chemotherapy in another Danish cohort study using similar methodology (2.5%). 20 The present study therefore does not support the emerging concept of a particularly high incidence of venous thromboembolism among patients treated with immune checkpoint inhibitors, as also concluded elsewhere. 37
4.2. Clinical implications
Two randomized trials have been conducted assessing the benefit and harms of primary prophylaxis in ambulatory cancer patients with Khorana scores ≥2, both demonstrating reduced risk of venous thromboembolism with a higher risk of bleeding during 180 days of treatment. 7 , 8 , 9 The AVERT trial only included patients initiating chemotherapy 7 while the CASSINI trial applied a broader inclusion criterion of systemic cancer treatment. 38 Nonetheless, some guidelines do allow for thromboprophylaxis irrespective of type of systemic treatment; see Table 4 for guideline overview.
Although the present unselected clinical population data confirm a substantial risk of thromboembolic events in patients treated with checkpoint inhibitors, they do not specifically support the concept that primary thromboprophylaxis will yield a positive net clinical benefit. A post hoc study of the AVERT primary thromboprophylaxis trial suggested that the protective effect from anticoagulation among patients with a 6‐month risk of venous thromboembolism below 8% was outweighed by bleeding risk.44 In this study, patients eligible for anticoagulation according to guidelines had a 6‐month risk of venous thromboembolism of only 4.1%. In the placebo arm of the CASSINI trial the total 6‐month cumulative incidence of thromboembolic events was 11.6%, which also contrasts with the corresponding risk estimate from the present study of 5.4%. 9 Nonetheless, the 6‐month incidence of arterial events was similar in the CASSINI trial and the present study (1.7% and 1.6%, respectively). This suggests that arterial events are not particularly common during checkpoint inhibitor treatment compared to the incidence in a population treated with various anti‐cancer agents.
Importantly, there is no evidence from randomized trials to support primary thromboprophylaxis beyond 6 months, although treatment duration with checkpoint inhibitors may be longer. Nonetheless, some guidelines do allow for extended treatment defined rather arbitrarily as “if risk persists,” 39 while other guidelines do not specify treatment duration at all (see Table 4 for guideline overview). 40 In the present study, venous thromboembolism also occurred after the initial 6‐month period reaching a cumulative incidence of 3.8% after 1 year, but whether this entails net clinical benefit from extended treatment is unknown due to the inherent bleeding risk. In general, well‐validated and clinically useful tools to assess bleeding risk during anticoagulation therapy among cancer patients are lacking. 45
It should be noted that this study focused on risk stratification and therefore does not elucidate whether immune checkpoint inhibitors are the underlying cause of thrombosis, although mechanisms for a causal relationship have been proposed. 17
4.3. Strengths and limitations
The use of large, administrative health registries allowed us to study a representative population from routine clinical practice with minimal impact from random variation. Body mass index is insufficiently coded in the registries, and previous studies have shown that the prevalence of body mass index ≥35 kg/m2 in a cancer cohort is underestimated to a degree that 4% of the present population are likely to have their Khorana score level underestimated by 1 point. 20 , 46 The distribution of patients into Khorana score levels was nonetheless comparable to previous studies, suggesting that the registries allow for sufficiently accurate estimation of Khorana score levels. 5 Although the positive predictive values of venous thromboembolism, myocardial infarction, and stroke are generally high in the Danish National Patient Register, the sensitivity of using ICD codes to identify such events is not well described; that is, to what extent do treating physicians forget to register a relevant ICD code. 27 , 30 , 31 However, we consider it unlikely that the positive predictive value or sensitivity of ICD codes vary according to Khorana score levels. The observed differences in risk across Khorana score levels are unlikely to be due to information bias. The aim was to describe thrombotic events in a cohort not using anticoagulation, but low molecular weight heparin is not registered on individual patient level in the Danish registries. We therefore excluded all patients with a recent prescription claim of oral anticoagulants as well as with any potential existing indication for anticoagulation. Primary thromboprophylaxis in an ambulatory setting is not routinely used in Denmark, and we have previously shown that venous thromboembolic risk in ambulatory cancer patients was identical before and after the first mention in Danish guidelines allowing for use of primary thromboprophylaxis in 2017 (which also only applies to patients treated with chemotherapy). 20 , 47 We therefore consider it unlikely that a substantial proportion of patients would have been using anticoagulation during follow‐up.
Patients were followed in nationwide administrative registries, ensuring virtually complete follow‐up, but some patients are likely to have died from undiagnosed pulmonary embolism, which would underestimate risk estimates. 48
Indications for use of checkpoint inhibitors continue to expand and they are now used also in neoadjuvant and adjuvant settings. 49 The current cohort describes risk patterns mainly in patients treated with checkpoint inhibitors in palliative situations, as reflecting contemporary treatment patterns during the study period. Risk patterns in patients treated with checkpoint inhibitors in a curative setting may differ, although risk of venous thromboembolism has been reported to be high also in neoadjuvant settings. 50
4.4. Remaining knowledge gaps
In opposition to the present study, some previous validation studies concluded that the Khorana score is not useful as a clinical risk stratification tool in this population. These discrepancies should be explored further before primary thromboprophylaxis guided by the Khorana score should be recommended routinely in the population, or whether alternative prediction models are needed.
Clinically significant bleeding during checkpoint inhibitor treatment can occur even in the absence of anticoagulation. 51 However, whether the bleeding risk profile during concomitant anticoagulation and checkpoint inhibitor treatment is comparable with the corresponding risk during chemotherapy is not well described. Hence, estimates of net clinical benefit from treatment derived from populations treated with chemotherapy may not apply to patients treated with checkpoint inhibitors. Some guidelines specify treatment duration for primary thromboprophylaxis to be 6 months. 39 , 43 Immune checkpoint inhibitors are often used for an extended time period of up to 2 years, but whether thromboprophylaxis would remain beneficial beyond 6 months is unknown.
5. CONCLUSIONS
The thromboembolic burden in patients with cancer was substantial, but similar to previous reports of patients treated with chemotherapy. The Khorana score was able to risk stratify patients with cancer according to 6‐month risk of venous as well as arterial thrombosis, but the cumulative incidence of venous thromboembolism was markedly lower than reported in placebo arms of randomized primary thromboprophylaxis trials. Thus, the net clinical benefit of primary thromboprophylaxis observed in randomized trials may be smaller if applied in an unselected population of patients treated with immune checkpoint inhibitors.
AUTHOR CONTRIBUTIONS
Concept and design: Thure F. Overvad, Anne G. Ording, Flemming Skjøth, Peter B. Nielsen. Statistical analysis: Thure F. Overvad, Flemming Skjøth. Manuscript Drafting: Thure F. Overvad. Data interpretation, critical revision, and final approval: Thure F. Overvad, Anne G. Ording, Flemming Skjøth, Peter B. Nielsen, Torben B. Larsen, G. Piazza, Simon Noble.
CONFLICTS OF INTEREST
P.B. Nielsen reports speaking fees from Daiichi‐Sankyo, consulting fees from Bayer and Daiichi‐Sankyo, and grant support from BMS/Pfizer and Daiichi‐Sankyo. G. Piazza has received research support from BMS/Pfizer, Bayer, Janssen, Alexion, Amgen, and Boston Scientific Corporation, and consulting fees from Pfizer, Boston Scientific Corporation, Janssen, and Amgen. T.B. Larsen has been a speaker for Bayer, BMS/Pfizer, Janssen Pharmaceuticals, and Roche Diagnostics, and on an advisory board for Bayer and Roche Diagnostics. S. Noble has been on the speaker bureau for Leo Pharma, BMS/ Pfizer, and Bayer, and received grant support from Leo Pharma. F. Skjøth received consultancy fees from Bayer. Other authors: no conflicts declared.
Supporting information
Table S1‐S2‐Figure S1
Overvad TF, Skjøth F, Piazza G, et al. The Khorana score and venous and arterial thrombosis in patients with cancer treated with immune checkpoint inhibitors: A Danish cohort study. J Thromb Haemost. 2022;20:2921‐2929. doi: 10.1111/jth.15883
Manuscript handled by: Jeffrey Zwicker
Final decision: Jeffrey Zwicker, 15 September 2022
REFERENCES
- 1. Ording AG, Skjøth F, Søgaard M, et al. Increasing incidence and declining mortality after cancer‐associated venous thromboembolism: a nationwide cohort study. Am J Med. 2021;134:868‐876.e5. [DOI] [PubMed] [Google Scholar]
- 2. Mulder FI, Horváth‐Puhó E, van Es N, et al. Venous thromboembolism in cancer patients: a population‐based cohort study. Blood. 2021;137:1959‐1969. [DOI] [PubMed] [Google Scholar]
- 3. Kevane B, O'Neill AM. Venous thromboembolism: the patient perspective. Thromb Update. 2021;5:100082. [Google Scholar]
- 4. Albertsen IE, Nielsen PB, Søgaard M, et al. Risk of recurrent venous thromboembolism: a Danish nationwide cohort study. Am J Med. 2018;131:1067‐1074.e4. [DOI] [PubMed] [Google Scholar]
- 5. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy‐associated thrombosis. Blood. 2008;111:4902‐4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Streiff MB, Abutalib SA, Farge D, Murphy M, Connors JM, Piazza G. Update on guidelines for the management of cancer‐associated thrombosis. Oncologist. 2021;26:e24‐e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carrier M, Abou‐Nassar K, Mallick R, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711‐719. [DOI] [PubMed] [Google Scholar]
- 8. Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for thromboprophylaxis in high‐risk ambulatory patients with cancer. N Engl J Med. 2019;380:720‐728. [DOI] [PubMed] [Google Scholar]
- 9. Khorana AA, McNamara MG, Kakkar AK, et al. Assessing full benefit of rivaroxaban prophylaxis in high‐risk ambulatory patients with cancer: thromboembolic events in the randomized CASSINI trial. TH Open. 2020;04:e107‐e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stein‐Merlob AF, Rothberg MV, Ribas A, Yang EH. Cardiotoxicities of novel cancer immunotherapies. Heart. 2021;107:1694‐1703. [DOI] [PubMed] [Google Scholar]
- 11. Roopkumar J, Swaidani S, Kim AS, et al. Increased incidence of venous thromboembolism with cancer immunotherapy. Med. 2021;2:423‐434.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moik F, Chan WE, Wiedemann S, et al. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood. 2021;137:1669‐1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drobni ZD, Alvi RM, Taron J, et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142:2299‐2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li H, Sun X, Sun D, et al. Thromboembolic events associated with immune checkpoint inhibitors: A real‐world study of data from the food and drug administration adverse event reporting system (FAERS) database. Int Immunopharmacol. 2021;98:107818. [DOI] [PubMed] [Google Scholar]
- 15. Dolladille C, Akroun J, Morice PM, et al. Cardiovascular immunotoxicities associated with immune checkpoint inhibitors: a safety meta‐analysis. Eur Heart J. 2021;42:4964‐4977. [DOI] [PubMed] [Google Scholar]
- 16. Icht O, Darzi N, Shimony S, et al. Venous thromboembolism incidence and risk assessment in lung cancer patients treated with immune checkpoint inhibitors. J Thromb Haemost. 2021;19:1250‐1258. [DOI] [PubMed] [Google Scholar]
- 17. Goel A, Khorana A, Kartika T, et al. Assessing the risk of thromboembolism in cancer patients receiving immunotherapy. Eur J Haematol. 2022;108:271‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alma S, Eloi D, Léa V, et al. Incidence of venous thromboembolism and discriminating capacity of Khorana score in lung cancer patients treated with immune checkpoint inhibitors. J Thromb Thrombolysis. 2022;54:287‐294. [DOI] [PubMed] [Google Scholar]
- 19. Kewan T, Ko T, Flores M, Sallam Y, Haddad A, Daw H. Prognostic impact and risk factors of cancer‐associated thrombosis events in stage‐IV cancer patients treated with immune checkpoint inhibitors. Eur J Haematol. 2021;106:682‐688. [DOI] [PubMed] [Google Scholar]
- 20. Overvad TF, Ording AG, Nielsen PB, et al. Validation of the Khorana score for predicting venous thromboembolism in 40 218 patients with cancer initiating chemotherapy. Blood Adv. 2022;6:2967‐2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541‐549. [DOI] [PubMed] [Google Scholar]
- 22. Schmidt M, Schmidt S, Sandegaard J, Eherenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pottegård A, Schmidt SAJ, Wallach‐Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: the Danish national prescription registry. Int J Epidemiol. 2017;46:798‐798f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arendt JFH, Hansen AT, Ladefoged SA, Sørensen HT, Pedersen L, Adelborg K. Existing data sources in clinical epidemiology: laboratory information system databases in Denmark. Clin Epidemiol. 2020;12:469‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moons KG, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1‐W73. [DOI] [PubMed] [Google Scholar]
- 26. Broe MO, Jensen PB, Mattsson TO , Pottegård A. Validity of antineoplastic procedure codes in the Danish national patient registry: the case of colorectal cancer. Epidemiology. 2020;31:599‐603. [DOI] [PubMed] [Google Scholar]
- 27. Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish national patient registry: a validation study. BMJ Open. 2016;6:e012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wildenschild C, Mehnert FF, Thomsen RW, et al. Registration of acute stroke: validity in the Danish Stroke Registry and the Danish National Registry of Patients. Clin Epidemiol. 2013;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lühdorf P, Overvad K, Schmidt EB, Johnsen SP, Bach FW. Predictive value of stroke discharge diagnoses in the Danish National Patient Register. Scand J Public Health. 2017;45:630‐636. [DOI] [PubMed] [Google Scholar]
- 30. Krarup LH, Boysen G, Janjua H, Prescott E, Truelsen T. Validity of stroke diagnoses in a national register of patients. Neuroepidemiology. 2007;28:150‐154. [DOI] [PubMed] [Google Scholar]
- 31. Joensen AM, Jensen MK, Overvad K, et al. Predictive values of acute coronary syndrome discharge diagnoses differed in the Danish National Patient Registry. J Clin Epidemiol. 2009;62:188‐194. [DOI] [PubMed] [Google Scholar]
- 32. Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004;4:103‐112. [Google Scholar]
- 33. Austin PC, Fine JP. Practical recommendations for reporting Fine‐Gray model analyses for competing risk data. Stat Med. 2017;36:4391‐4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gervaso L, Dave H, Khorana AA. Venous and arterial thromboembolism in patients with cancer. JACC CardioOncology. 2021;3:173‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Noble S, Alikhan R, Robbins A, Macbeth F, Hood K. Predictors of active cancer thromboembolic outcomes: validation of the Khorana score among patients with lung cancer: comment. J Thromb Haemost. 2017;15:590‐591. [DOI] [PubMed] [Google Scholar]
- 36. Kartolo A, Yeung C, Moffat GT, Hanna L, Hopman W, Baetz T. Venous thromboembolism events in patients with advanced cancer on immune checkpoint inhibitors. Immunotherapy. 2022;14:23‐30. [DOI] [PubMed] [Google Scholar]
- 37. Deschênes‐Simard X, Richard C, Galland L, et al. Venous thrombotic events in patients treated with immune checkpoint inhibitors for non‐small cell lung cancer: a retrospective multicentric cohort study. Thromb Res. 2021;205:29‐39. [DOI] [PubMed] [Google Scholar]
- 38. Khorana AA, Vadhan‐Raj S, Kuderer NM, et al. Rivaroxaban for preventing venous thromboembolism in high‐risk ambulatory patients with cancer: Rationale and design of the CASSINI trial. Thromb Haemost. 2017;117:2135‐2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Angelini D, Ashrani A, Elshoury A, Fanikos J, Fertrin K, Fogerty AE, Gangaraju R, Gao S, Goldhaber SZ, Gundabolu K, Ibrahim I, Kraut E, Leavitt AD, Lee A, Lee JT, Lim M, Mann J, Martin K, McMahon B, Moriarty J, et al. NCCN guidelines version 1. 2022 cancer‐associated venous thromboembolic disease; 2022.
- 40. Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5:927‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38:496‐520. [DOI] [PubMed] [Google Scholar]
- 42. Farge D, Frere C, Connors JM, et al. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID‐19. Lancet Oncol. 2022;23:e334‐e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang TF, Zwicker JI, Ay C, et al. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: Guidance from the SSC of the ISTH. J Thromb Haemost. 2019;17:1772‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kumar V, Shaw JR, Key NS, et al. D‐dimer enhances risk‐targeted thromboprophylaxis in ambulatory patients with cancer. Oncologist. 2020;25:1075‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Poénou G, Tolédano E, Helfer H, et al. In search of the appropriate anticoagulant‐associated bleeding risk assessment model for cancer‐associated thrombosis patients. Cancers (Basel). 2022;14:1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Søgaard M, Heide‐Jørgensen U, Nørgaard M, Johnsen SP, Thomsen RW. Evidence for the low recording of weight status and lifestyle risk factors in the Danish National Registry of Patients, 1999–2012. BMC Public Health. 2015;15:1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rasmussen MS, Dorff MH, Holt MI, Grove EL, Hvas AM. Cancer and Venous Thromboembolism. Guideline by Danish Society of Thrombosis and Haemostasis and Danish Society of Clinical Oncology. 2017.
- 48. Gimbel IA, Mulder FI, Bosch FTM, et al. Pulmonary embolism at autopsy in cancer patients. J Thromb Haemost. 2021;19:1228‐1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of FDA‐approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel). 2020;12:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Garas SN, McAlpine K, Ross J, et al. Venous thromboembolism risk in patients receiving neoadjuvant chemotherapy for bladder cancer. Urol Oncol Semin Orig Invest. 2022;40:381.e1‐381.e7. [DOI] [PubMed] [Google Scholar]
- 51. Kewan T, Covut F, Ahmed R, Haddad A, Daw H. Clinically significant bleeding with immune checkpoint inhibitors: a retrospective cohort study. Eur J Cancer. 2020;137:285‐287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2‐Figure S1


