Abstract
Global warming is increasing mean temperatures and altering temperature variability at multiple temporal scales. To better understand the consequences of changes in thermal variability for ectotherms it is necessary to consider thermal variation at different time scales (i.e., acute, diel, and annual) and the responses of organisms within and across generations. Thermodynamics constrain acute responses to temperature, but within these constraints and over longer time periods, organisms have the scope to adaptively acclimate or evolve. Yet, hypotheses and predictions about responses to future warming tend not to explicitly consider the temporal scale at which temperature varies. Here, focusing on multicellular ectothermic animals, we argue that consideration of multiple processes and constraints associated with various timescales is necessary to better understand how altered thermal variability because of climate change will affect ectotherms.
Keywords: acute temperature change, annual cycles, climate change, diel cycles, ectotherms, temporal scale, thermal variability
Global warming is increasing mean temperatures and altering temperature variability at multiple temporal scales, such as acute, diel, and annual. Organisms have various (potential) constraints and responses to thermal stress, and the importance of these constraints and responses is temporally dependent. Consequently, we argue that organisms' responses and vulnerability to changes in thermal variability resulting from climate change, should be assessed using approaches that explicitly consider different temporal scales.

1. INTRODUCTION
Anthropogenic climate change is causing increases in mean temperatures and altering patterns of temperature variability globally (Coumou & Rahmstorf, 2012; Diffenbaugh et al., 2017; Duan et al., 2017; Geerts, 2003; Qian & Zhang, 2015; Thorne et al., 2016; Wallace & Osborn, 2002; Wang & Dillon, 2014; see Table S1 for a summary of changes in temporal variability). Ectotherms vary widely in their tolerance to temperature (Angilletta & Angilletta, 2009), and there has been extensive research on the response of these organisms to changes in mean temperature (Marshall & Sinclair, 2012; Thompson et al., 2013). Increasingly, research has also considered how changes in temperature variability shape tolerance and vulnerability (Colinet et al., 2015; Deutsch et al., 2008; Dowd et al., 2015; Estay et al., 2014; Huey et al., 2012; Sinclair et al., 2016; Sunday et al., 2014; Thompson et al., 2013; Vasseur et al., 2014). Ectotherms respond to temperature variability on multiple time scales (Box 1). In the shortest time, acute changes in body temperature are assumed to determine biological rates and organismal performance via thermodynamic effects on biochemical and cellular processes (Dillon et al., 2010; Gillooly et al., 2006; Payne & Smith, 2017). At intermediate time scales, thermal variation within the lifespan of organisms occurs on diel, successive diel cycles, and within and between seasons. These are assumed to shape organismal performance through a combination of thermodynamics (Colinet et al., 2015; Dowd et al., 2015; Estay et al., 2014; Stoks et al., 2017; Vasseur et al., 2014), buffering (e.g., behavioral thermoregulation) and plastic responses of hardening and, as the time scale increases, acclimation (Bowler, 2005; Havird et al., 2020; Teets & Denlinger, 2013). Finally, at the longest time scale considered in this paper, across generations, seasonal changes in temperature over the annual cycle are thought to shape adaptive evolutionary changes in thermal breadth and traits associated with plastic change in thermal tolerance, like acclimation ability (Janzen, 1967; Shah, Funk, et al., 2017). Yet many studies do not explicitly distinguish between the effects of acute, diel, and seasonal temperature variability in determining organisms' responses to temperature variability, and thus vulnerability to climate change (Deutsch et al., 2008; Huey et al., 2012; Sinclair et al., 2016; Sunday et al., 2014; Thompson et al., 2013). Indeed, to date, relatively little research has considered the consequences for climate change impacts of temperature varying on multiple temporal scales (Box 1).
BOX 1. Scales of temporal thermal variability and extreme thermal events.
Temporal variability in temperature occurs at various scales. (1) Acute thermal variability is typically considered to be (near) instantaneous changes in temperature. Outside of experiments, such variability occurs from such things as clouds passing across the sun and the arrival of a cold (or warm) front that abruptly changes temperature. Importantly, acute temperature changes are assumed to occur too quickly for compensatory plastic responses (e.g., hardening, acclimation) or adaptive evolution that would alter an organism's thermal sensitivity (Cossins & Bowler, 1987; Sinclair et al., 2016), although environments with predictable acute temperature change may lead to adaptive evolution of thermal tolerance and other associated traits (see main text). (2) Diel thermal variability refers to temperature change occurring over a 24‐h cycle as a result of a single rotation of the Earth. There is generally predictable diel temperature variability in terrestrial and shallow aquatic habitats. Most multicellular organisms in terrestrial and shallow aquatic habitats experience successive cycles of diel variability and have consequently evolved various traits such as buffering, including behavioral thermoregulation, within‐generation plasticity, periods of inactivity, and the ability to accumulate and recover from physiological damage (Ørsted et al., 2022). Due to the movements of weather systems (Marshall & Sinclair, 2012), there is variability between these successive diel temperature cycles. (3) Seasonal change in temperature is a result of an annual cycle in temperature and most multicellular organisms live long enough to experience at least some degree of seasonal change. This seasonal variability is greater in temperate and polar regions than in tropical latitudes due to the tilt of the Earth's axis (Janzen, 1967; Wang & Dillon, 2014). Seasonal change in temperature due to the annual cycle is the longest scale that we will consider in this paper. There are, however, longer cyclic patterns of variability that organisms with multi‐year cycle experience, for example, El Niño‐Southern Oscillation (McPhaden et al., 2006) and the Interdecadal Pacific Oscillation (Henley et al., 2015).
An important point is that variability within a given period contributes to thermal variability across longer periods. For example, the annual temperature range (i.e., difference between minimum and maximum temperature) in the tropics can be the result of successive diel cycles and not seasonal change (Shah, Gill, et al., 2017). Although measures such as annual temperature range include variability in temperature at all sub‐annual scales, different biological responses are likely to occur with different scales of temperature variability (see main text).
Another temporal scale of thermal variation occurs in reference to unusually high or low temperatures (Dole et al., 2014; Hoerling et al., 2013), which are often referred to as extreme thermal events. There is no consistent definition of extreme thermal events (Broska et al., 2020). They have been defined in terms of probability of occurrence (Milly et al., 2002) and based on biological impact, for example, causing substantial mortality (Gutschick & BassiriRad, 2003). The magnitude, duration, and frequency of extreme thermal events are predicted to increase because of climate change (Coumou & Rahmstorf, 2012). Extreme thermal events have disproportionate impacts on biological systems compared with their frequency. Here, we consider extreme events only in the context of how they are mediated by organisms' previous exposures to different scales of thermal variability.
Why should we care about organismal responses to different temporal scales of temperature variation? Briefly, not only are global temperatures increasing, but temperature variability is also changing. Various studies have shown that since the year 1700 the magnitude of temperature variability has changed to varying amounts depending on the data series examined, the temporal scale of variation (annual vs. diel) and study location (see Table S1). For example, Wang and Dillon (2014) estimate that between 1975 and 2013, there was a 1.4°C increase in the amplitude of diel variability in polar regions, a 1.0°C increase in temperate regions, and a 0.3°C increase in tropical regions. By contrast, their analysis of the same data that show amplitude of annual temperature variability has decreased by 0.6°C in polar regions, increased by 0.4°C in temperate regions, and has not changed in tropical regions. Thus, organisms across the globe are experiencing the differential effects of climate change on thermal variability across both diel and annual scales.
An appreciation of how organisms respond to different temporal scales of thermal variation is, thus, critical to understanding and projecting the biological responses to changing temperature. For example, differences in annual thermal variability are predicted to affect the breadth of thermal tolerance and species' latitudinal and elevational range, which in turn shape the geographic scale at which populations experience climatic conditions (Ghalambor et al., 2006; Gill et al., 2016; Janzen, 1967; Sheldon et al., 2018). Yet, annual thermal variability alone is not a good predictor of responses to temperature that occur on the scale of hours or minutes. Similarly, differences in the magnitude of diel variation can either expand or constrain critical functions to favorable periods of the diel cycle (Gilchrist, 1995) but may or may not inform variation in the thermal sensitivity of biological rates. Finally, acute thermal variation above some thermal optimum is thought to be particularly detrimental to organism performance, such as growth because of the asymmetrical shape of thermal performance curves (TPCs; Colinet et al., 2015; Dowd et al., 2015; Vasseur et al., 2014) but do not explain the evolutionary divergence in thermal sensitivity across species. Yet, despite the general recognition that such biological responses vary at different temporal scales, most attempts to predict how climate change will impact ectotherms tend to consider only a single time scale or fail to explicitly recognize the consequences of responses to different time scales. Thus, whether differences in scale of temporal temperature variability generate different organismal responses remains an unresolved problem in global change biology.
Here, we briefly review current conceptual frameworks used to understand ectotherm responses to temperature variability at acute, diel, and annual temporal scales. We primarily focus on literature pertaining to multicellular ectothermic animals but also briefly consider relevant literature from microbes (see Boyd et al., 2016; Cabrerizo & Marañón, 2021 for recent reviews of the effects of regarding fluctuating temperature on phytoplankton) and plants. We review these perspectives because responses to temperature variability at these scales will, in part, determine how organisms respond to changes in thermal variability from climate changes and thus their vulnerability. We discuss the effects of acute and annual temperature variability first, as these have been relatively well described in the published literature. We then focus on the diel scale, which has received less attention. We finally attempt to synthesise existing hypotheses for considering the potential effects of changes in temperature variability across temporal scales.
2. ACUTE TEMPERATURE CHANGE
Acute temperature changes refer to the near‐instantaneous variation that occurs in short time scales and are not usually cyclical in nature. These changes are generally assumed to occur too quickly for organisms to actively shift their thermal tolerances (Cossins & Bowler, 1987; Sinclair et al., 2016) certainly via whole organism acclimation (Havird et al., 2020). A key concept in describing organismal response to temperature is TPCs and their derived metrics.
2.1. Thermal performance curves
Thermal performance curves (Figure 1) describe the relationship between temperature and performance at the sub‐organism or whole‐organism scale (Colinet et al., 2015; Dowd et al., 2015; Huey & Kingsolver, 1989; Schulte, 2015; Schulte et al., 2011) but have also been extended to population level metrics (Estay et al., 2014). The TPC is heuristic in nature, intended to illustrate the expected relationship between temperature and performance, based in part on the thermal dependence of biological rates. This relationship depicts performance increasing exponentially from zero at the critical thermal minimum (CTmin), to a point of theoretical maximum performance, the thermal optimum (T opt). Performance then rapidly decreases toward zero at a critical thermal maximum (CTmax). The difference between an organism's CTmax and CTmin is referred to as thermal breadth (T br). Jørgensen et al. (2021) have recently developed a framework to allow comparisons of CTmin and CTmax produced using different protocols.
FIGURE 1.
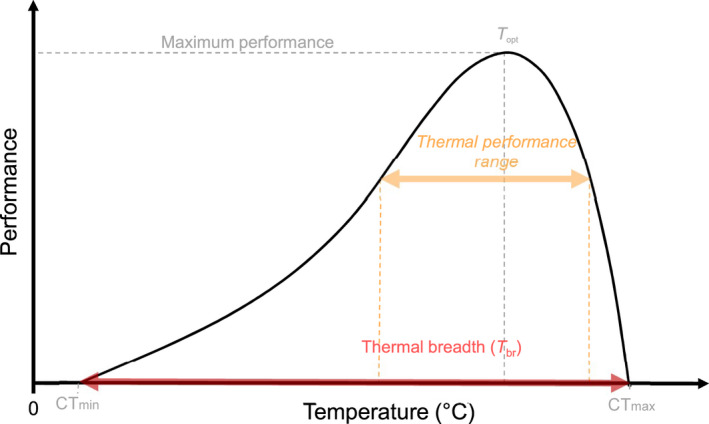
Generalised thermal performance curve showing change in individual performance (e.g., locomotion, development, growth) with varying temperature.
2.2. Consequences of acute thermal changes
Acute changes in body temperature occur too quickly for acclimation and other related active plastic responses. Thus, thermal tolerances and the shape of TPCs are assumed to be fixed for a given organism's life stage, nutritional status, etc. when exposed to acute temperature changes (Cossins & Bowler, 1987; Sinclair et al., 2016). The most obvious negative effect of acute changes in body temperature away from T opt is the cost of reduced performance (Figure 1). However, this effect will be dependent on the magnitude of the temperature change relative to T opt (Figure 1).
A characteristic of TPCs is that they have both a concave (ascending limb of curve) and convex (descending limb) component (Figure 1). Due to this asymmetry, Jensen's inequality, which predicts thermal variability below an organism's T opt can either increase or decrease the performance relative to the performance at the mean temperature experienced (Colinet et al., 2015; Dowd et al., 2015; Vázquez et al., 2017). However, thermal variability above T opt will always result in a decrease in performance because of the steeper decline in performance. For more detailed reviews of the consequences of varying temperatures in the context of Jensen's inequality see (Colinet et al., 2015; Dowd et al., 2015; Estay et al., 2014; Vázquez et al., 2017).
Where environments have had predictable levels of acute thermal change over multiple generations, then the level of acute thermal change has the potential to be an agent of selection, leading to adaptive evolution in thermal tolerance. Various artificial selection experiments have shown that selecting for individuals that are tolerant of high temperatures results in the evolution of increased tolerance or optimal temperatures in subsequent generations of bacteria (Bennett & Lenski, 1993), phytoplankton (Baker et al., 2018; Listmann et al., 2016; Schaum et al., 2022), and fruit fly (Gilchrist & Huey, 1999; Hoffmann et al., 1997; McColl et al., 1996). However, other selection experiments have failed to induce an evolutionary response. For example, in fish, when Heterandria formosa were selected for acute heat or cold tolerance there was no cross‐generational change in CTmin nor CTmax (Baer & Travis, 2000), whereas in Danio rerio moderate increases in CTmax were observed (Morgan et al., 2020). It would appear, that in some species there is sufficient genetic variation for adaptive evolution for tolerance to acute thermal change, whereas in others there is not. Rapid evolution in thermal tolerance and associated traits, over a relatively few generations, may be much more constrained than that occurring over millions of years (Bennett et al., 2021). Thus, how populations evolve in response to selection in nature appears to depend on various factors, including the predictability of acute thermal change, whether there are trade‐offs associated with adaptation to acute thermal tolerance, (McColl et al., 1996) and constraints on the upper limits to thermal tolerance (Hoffmann et al., 2013; Morgan et al., 2020). Yet, acute thermal change resulting in strong selection will also likely result in circumstances where acute temperatures are so high that they exceed organisms' tolerances, leading to mortality (Bennett & Lenski, 1993).
2.3. Shortcomings of TPCs
Using TPCs to predict ectotherm responses to changes in temperature involves a range of assumptions (Schulte et al., 2011; Sinclair et al., 2016). A key assumption is that performance affects evolutionary fitness, defined as the individual's survival and reproductive success or the average contribution to the gene pool of the next generation (Orr, 2009). Because fitness is difficult to measure directly, fitness is most often assessed by traits that are assumed to be positively correlated with fitness. The choice of these traits is important. Life‐history traits, such as survival, growth rate, fecundity, and generation time, are likely to be more correlated with fitness. However, TPCs for commonly used traits such as locomotion speed, feeding rates, and physiological or biochemical metrics may not have simple relationships with fitness (Ørsted et al., 2022; Sinclair et al., 2016). Different traits for the same organisms frequently have different TPCs (Barton et al., 2020; Bozinovic et al., 2020; Clarke, 2004; Dell et al., 2011; Iverson et al., 2020; Schulte, 2015; Sinclair et al., 2016) and the shape of TPCs can be altered by acclimation (Schulte et al., 2011). The utility of TPCs to understand the responses of organisms to temperature change can be aptly characterised by the aphorism “all models are wrong, but some are useful” (Box, 1979). TPCs are simplifications, yet they are a useful heuristic for conceptualising organisms' responses to temperature changes. Thus, the challenge is to recognize the utility and limitations of TPCs. For example, there is strong evidence that below T opt most biological rates increase exponentially with increasing temperature (Dillon et al., 2010; Gillooly et al., 2006; Payne & Smith, 2017)—the concept that “hotter is better” (Angilletta et al., 2010); however, the decrease in rates beyond T opt is less clear. Nevertheless, factors other than temperature impact performance. Animals that exhibit increasing performance as a function of temperature must also be able to supply exponential increases in resources, that is, food, water, and oxygen, to drive the increased demand of biological rates. The limitation of oxygen (Pörtner, 2010) or food/nutrients (Huey & Kingsolver, 2019; Thomas et al., 2017) can, therefore, interact with increasing temperatures to negatively impact many organismal functions, including locomotion, behavior, feeding, and predator avoidance. If such oxygen or food limitation is pronounced, death will occur not necessarily because of a lack of direct thermal tolerance, but through these cumulative responses. Such complicating factors represent only some of the complications when considering a fixed TPC as the sole predictor of vulnerability to climate change.
3. ANNUAL TEMPERATURE CHANGE
Annual cycles in temperature, as a result of seasonal changes, are an important component of thermal regimes, particularly in temperate and polar regions that have high annual temperature variability relative to the tropics (Geerts, 2002; Wang & Dillon, 2014). Annual temperature cycles have been relatively constant over recent evolutionary time (i.e., the last 10,000–12,000 years of the Holocene, Viau et al., 2006). Here, we consider the consequences that these predictable changes in annual temperature variability have for adaptive evolution of organisms and evolutionary strategies in response to high annual temperature variability. We then consider how universal thermodynamic constraints may interact with these evolutionary responses to limit an organisms' ability to cope with both high temperature and high‐temperature variability. These different perspectives are the basis for competing hypotheses around what are the primary constraints on organisms' responses to annual thermal variability.
3.1. Adaptation of organisms to annual temperature variability
The predictability of annual temperature variability is generally thought to be one of the main selective pressures driving evolutionary divergence in patterns of thermal sensitivity and thermal breadth. Janzen's (1967) The climate variability hypothesis (CVH) is a well‐established and influential explanation for how annual temperature variation shapes the evolution of thermal breadth, acclimation ability, and other traits, allowing organisms to tolerate a changing seasonal thermal regime and in turn impact patterns of dispersal, and species turnover across elevational gradients (Ghalambor et al., 2006; Perez et al., 2016; Sheldon et al., 2018). This hypothesis is grounded in the assumption that organisms evolve a thermal breadth (T br = CTmax − CTmin, Figures 1 and 2) and acclimation ability reflecting the annual temperature range experienced over their evolutionary history. Janzen based the CVH on the observation that tropical latitudes display bands of largely non‐overlapping temperatures along elevation gradients, which he argued should favor narrower thermal breadths (Janzen, 1967). Consequently, tropical organisms restricted to the lowlands experience a narrow range of warm temperatures year‐round, while their high‐elevation counterparts experience a narrow range of cool temperatures year‐round (Figure 2a). In contrast, at temperate and polar latitudes large annual shifts in temperature result in greater annual overlap in temperatures across elevations, exposing all organisms to a broader range of temperatures (Figure 2a). If T br has evolved to be as narrow as possible to minimize maintenance costs of varying temperatures (Pörtner & Farrell, 2008), then selection is predicted to cause narrower T br in tropical versus temperate regions (Figure 2b,c). Differences in T br lead to four predictions: (1) that elevation creates a thermal dispersal barrier for tropical organisms, which in turn (2) results in narrower elevational ranges (Figure 2b) and (3) a higher degree of genetic structuring between populations, which (4) should contribute to higher speciation rates in the tropics. There is support for many of these predictions at macrogeographic scales (e.g., Ghalambor et al., 2006; Gill et al., 2016; Markle & Kozak, 2018; Polato et al., 2018; Shah, Gill, et al., 2017; Sunday et al., 2011; but see Chen, 2015). Nevertheless, a shortcoming of simply looking at the relationship between annual variability and thermal breadth is the inability to incorporate the responses to temperature variation at other temporal scales.
FIGURE 2.
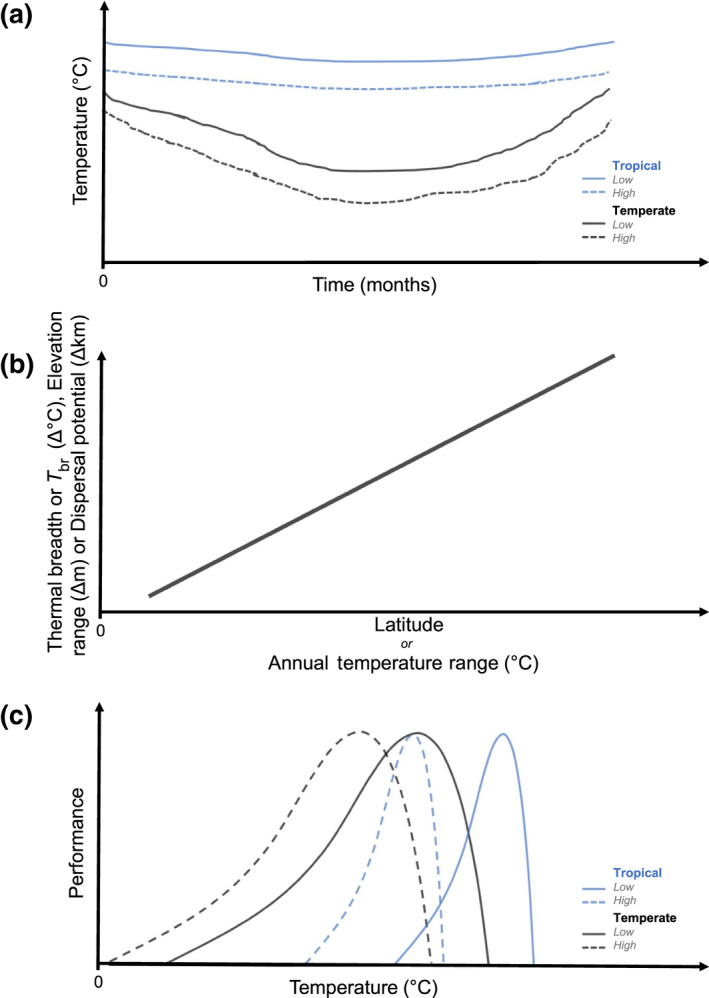
Conceptual model showing the predictions of Janzen's (1967) climate variability hypothesis (CVH). (a) Generalised pattern in annual air temperature variability at tropical and temperate locations at low‐elevation (solid lines) and high‐elevation (dotted lines) sites proposed by the CVH. (b) Pattern in organisms' responses intraspecific genetic structuring (dashed darker line and y‐axis on left), thermal breath (T br), elevational range and dispersal potential (grey solid line and y‐axis on right) predicted by the CVH as a result of latitude, or annual temperature range. (c) A prediction by the CVH for organisms active year‐round without significant seasonal acclimation or shift in thermal tolerance of organisms' thermal performance curves from low and high elevations at temperate and tropic locations.
3.2. Evolutionary strategies for dealing with thermal variation
Tests of the CVH have largely focused on quantifying T br because it is relatively straightforward to measure and compare, but how annual thermal variability influences the shape of the TPC (e.g., slopes or optimum temperatures) remains under‐explored. Multiple alternative evolved strategies have been proposed to cope with temperature variability (Figure 3). For example, thermal generalists (eurytherms) should persist across a relatively wide range of temperatures and thus require a wide but flat TPC. These organisms are hypothesized to trade off maximum performance to be able to live across a wider range of temperatures. In contrast, thermal specialists (stenotherms) should occupy a relatively narrow range of temperatures and would thus be expected to have narrower TPCs. Such differences between eurytherms and stenotherms in tolerance are suspected to be predictive of their vulnerability to climate warming (Somero, 2010). However, such generalizations can be overly simplistic as some stenotherms can persist in environments with a wide range of temperatures by restricting critical functions (e.g., growth, feeding, reproduction) to a narrow temperature range (Huey et al., 2012). Organisms can also exhibit “seasonal escape” whereby they avoid periods of unfavorable temperatures by inactivity (e.g., diapause, hibernation) or migration (Huey & Kingsolver, 1993; Tüzün & Stoks, 2018). A third strategy can evolve whereby organisms have relatively narrow TPCs but track shifts in seasonal temperature change—referred to here as the “seasonal shift” strategy—either via life‐stage specific thermal sensitivity (Uno & Stillman, 2020) or acclimation (Fangue & Bennett, 2003). This strategy has the advantage of a narrow TPC at any given time while not requiring seasonal escape. However, this strategy assumes low costs of shifting thermal tolerance (Shah, Funk, et al., 2017), and the rate of seasonal temperature change needs to be slower than the organism's ability to shift its thermal tolerance (Havird et al., 2020). It would be expected that species exhibiting a seasonal shift strategy would be particularly vulnerable to acute or sub‐seasonal temperature extremes (Stoks et al., 2017; Thompson et al., 2013). The variety of strategies that organisms might adopt to cope with thermal variability (Figure 3) could be limited by fundamental thermodynamic constraints. So the range of values and interrelationships between their thermal traits such as CTmin, CTmax, T br, and T opt (Angilletta et al., 2010; Huey & Kingsolver, 1993) and acclimation ability (Somero, 2010; Stillman, 2003) are likely somewhat restricted.
FIGURE 3.
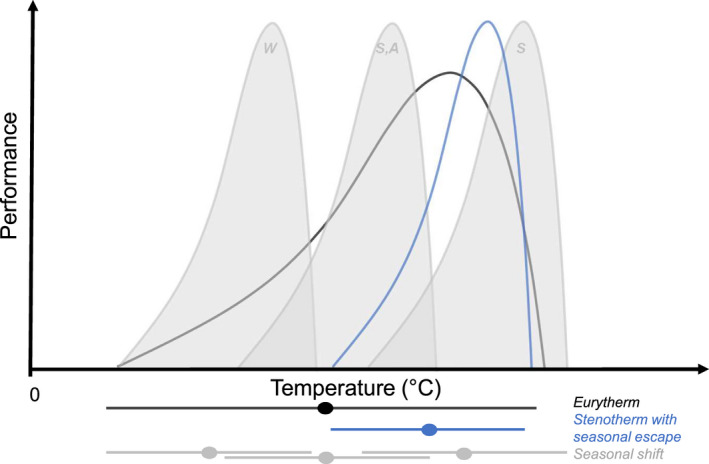
Three generalised strategies to cope with annual temperature variability expressed as thermal performance curves (TPCs): (i) black line—eurytherm, (ii) blue line—stenotherm with seasonal escape, and (iii) grey lines—seasonal shift in TPC shown for winter (W), spring and autumn (S‐A), and summer (S). Horizontal lines below the graph show the temperatures at which an organism is active under the three strategies, as well as the seasonal shift in TPC strategy for the different seasons.
3.3. High temperatures may constrain organisms' ability to tolerate thermal variability
Recently, Payne and Smith (2017) challenged the CVH to explain the narrower thermal (and thus elevational) ranges of tropical species. Following Angilletta et al. (2010), we refer to this alternative as the thermal constraint hypothesis (TCH). Thermodynamics results in most physiological rates (e.g., resting respiration) increasing exponentially with increasing temperature up to some limit. Consequently, Payne and Smith (2017) argue that species occupying warmer environments, such as the lowland tropics, will have narrower thermal tolerances because of the greater sensitivity of physiological rates at high temperatures. Thus, species living in warm environments may be physiologically incapable of tolerating both high temperatures and a wide thermal range due to their closer proximity to T opt and the descending part of the TPC (see also Dillon et al., 2010). In contrast, the biological rates of species occupying cooler environments are further from T opt meaning they are less sensitive to acute effects of increasing temperatures, resulting in a greater thermal tolerance range (Figure 1).
Debate over the validity of thermodynamic constraints versus adaptive evolution in thermal tolerance and acclimation ability, as proposed by the CVH, is part of a wider debate over the factors that shape responses to temperature (Angilletta et al., 2010; Bozinovic et al., 2020; Clarke, 2004; Clarke & Fraser, 2004; Gillooly et al., 2006; Havird et al., 2020; Seebacher et al., 2015). Unlike the CVH, the TCH assumes physiological rates and the shape of the TPC are largely fixed and not altered through acclimation (Havird et al., 2020; Seebacher et al., 2015) and/or adaptative evolution (Bennett et al., 2021; Gardiner et al., 2010; Nielsen et al., 1999). Some authors argue that adaptation and acclimation ability to different thermal regimes determine thermal sensitivity within limits (Clarke, 2004; Clarke & Fraser, 2004; Gilchrist, 1995; Seebacher et al., 2015), whereas others assume thermal sensitivity is constrained solely by universal thermodynamic laws (Dillon et al., 2010; Gillooly et al., 2006; Payne & Smith, 2017; see also Perez et al., 2016). Support for this latter view comes from some studies that find consistent TPCs (e.g., Bronikowski et al., 2001; Tüzün & Stoks, 2018) or T br (Chen, 2015) among populations or species living in different thermal environments. In contrast, there is ample evidence for differences in thermal tolerance or acclimation ability of populations or species across thermal regimes (e.g., Bennett et al., 2021; Ghalambor et al., 2006; Havird et al., 2020; Shah, Funk, et al., 2017; Shah et al., 2021; Sunday et al., 2011; Thomas et al., 2012). Such apparent contradictions may reflect the responses to temperature at different biological levels (e.g., biochemical, cellular, physiological, or whole organism responses; Bozinovic et al., 2020). Furthermore, the TPCs for different traits of organisms will be affected differently by evolutionary and acclimation history (Ørsted et al., 2022; Schulte, 2015; Sinclair et al., 2016), thereby contributing to this apparent contradiction.
Regardless, the CVH and the TCH offer fundamentally different views on what determines vulnerability to climate warming. Under the TCH, vulnerability increases simply as a function of environmental temperatures approaching T opt; thus, ectotherms in warmer environments are more vulnerable than those occupying colder environments. In contrast, if species have the capacity to evolve different T br or shapes of the TPC, then predictions about vulnerability cannot simply be made based on temperature. Instead, the CVH predicts vulnerability is a function of T br, such that even species occupying cold environments can be vulnerable to warming if they are adapted to a narrow range of temperatures, such as high‐elevation tropical species (Shah, Gill, et al., 2017).
The TCH additionally suggests that increased mean temperature from climate change will decrease an organism's tolerance to annual thermal variability. Consequently, the TCH predicts that if climate change increases both mean temperature and annual thermal variability, organisms will be more thermally stressed than if only mean temperature were increased. By contrast, the CVH predicts that organisms' responses to changes in mean temperature and temperature variability are dependent on the temperature regimes where they evolved.
Payne and Smith (2017) couch their TCH in terms of annual scale variability; however, this hypothesis is arguably more applicable at shorter time scales, such as acute and diel change. Acute responses are considered to occur too fast for plastic responses like acclimation to occur (Cossins & Bowler, 1987; Sinclair et al., 2016), meaning thermal tolerances from acute variability are likely to be relatively fixed. We propose that Payne and Smith's (2017) TCH, and its predictions of synergistic effects, will be most applicable at the acute scale and will reduce in importance as time scales increase. Whether this hypothesis retains some importance at the annual scale is uncertain.
3.4. Disentangling the CVH and TCH
As the CVH and the TCH predict profoundly different consequences for climate change vulnerability, it is important to determine the contributions each make to best describe the effects of annual scale thermal variability. Separating between these hypotheses is challenging; however, in principle, the relative importance of the CVH and TCH can be tested by measuring the T br (Figure 1) of organisms from locations with contrasting mean temperature and annual temperature variability. The CVH predicts that T br of organisms will vary with annual temperature variation but not with mean temperature. In contrast, the TCH predicts that organisms' T br will change with mean temperature but not with annual temperature variation. Attempts to test these hypotheses to date have confounded annual mean temperature and annual temperature variation, thereby precluding discrimination between the CVH and TCH (Payne & Smith, 2017). However, by studying thermal tolerance ranges along elevation gradients at different latitudes (Shah, Gill, et al., 2017), coastal versus continental sites, and/or in different hemispheres it is possible to contrast sites with similar mean annual temperatures that differ in annual variation. Consequently, it is possible to collect data that can determine if the CVH or the TCH better describes species' thermal tolerances.
Various scenarios can complicate the testing of CVH and TCH. Behavioral thermoregulation will lead to organism body temperatures not directly tracking the thermal environment (Domínguez‐Guerrero et al., 2019; Muñoz & Bodensteiner, 2019; Muñoz et al., 2014). Snapshot measurements of T br would also be unable to capture an organisms' realized T br where they have adopted a seasonal shift strategy (Figure 3). Testing the CVH and TCH is, therefore, best achieved by studying organisms that do not have seasonal escape and have limited ability to thermoregulate behaviorally, such as small aquatic ectotherms that are active year‐round and T br (and other metrics of thermal tolerance) are measured in multiple seasons.
4. EFFECTS OF DIEL TEMPERATURE VARIABILITY
Predictable changes in temperature also characterize the daily transition between day and night. Like the geographic variation in annual temperatures, the magnitude of diel variation also varies considerably (Geerts, 2003). High diel temperature variability (e.g., >20°C) is a feature of many ecosystems, including many temperate locations (Marshall & Sinclair, 2012). Diel temperature variability is especially pronounced in low‐humidity environments (e.g., deserts) and locations remote from large water bodies (Geerts, 2003). In extreme cases, temperature range can exceed 50°C within 24 h (e.g., https://www.nps.gov/glac/learn/nature/weather.htm), and shallow aquatic habitats can exhibit larger diel shifts than their surrounding air temperature due to radiative warming of benthic sediments (e.g., Marchant et al., 2011; Podrabsky & Somero, 2004). While it is generally assumed that diel temperature cycling is less than the annual temperature variability, this is not universal (Wang & Dillon, 2014). For example, some tropical mountains can have nightly frosts and snowfalls, morning thaws and can reach midday “summer” temperatures (Mani, 1968). Below, we discuss how diel thermal variability can influence ectotherm thermal physiology through (1) within generation plasticity, (2) changes in the TPC, (3) buffering of body temperatures against unfavorable temperatures, and (4) coping with consequences of stressful diel fluctuations. These aspects of thermal physiology are likely the product of adaptive evolution to variable diel thermal regimes, although less attention has been given to them in the context of climate change.
4.1. Within‐generation plastic responses to diel variability
Unlike acute temperature changes (Cossins & Bowler, 1987; Sinclair et al., 2016), organisms may be able to adapt to diel thermal variability using within‐generation plastic responses, but not all, plastic responses are relevant for diel variability. Different plastic responses occur at different time scales. Many organisms can change ventilation rates almost instantaneously (Schulte et al., 2011), so they can supply enough oxygen to meet metabolic demands at their new temperature. Organisms can also differentially express genes regulating the production of heat shock proteins within minutes of exposure to stressful temperatures (Schulte et al., 2011). Cold or heat hardening, in response to near fatal temperature exposure, can occur within minutes to hours. However, acclimation of whole organisms takes much longer (Bowler, 2005; Teets & Denlinger, 2013). For example, acclimation of metabolic rates following temperature changes generally takes multiple days to months (Havird et al., 2020), and it is, thus, unlikely to be a useful response to diel variability. While it may be tempting to see such plastic changes to an organism's thermal biology as occurring along a continuum (i.e., ventilation rates to whole organism acclimation), different physiological mechanisms appear to be involved (Bowler, 2005; Teets & Denlinger, 2013). Moreover, cold/heat hardening requires temperatures approaching lethal levels, whereas acclimation occurs at more moderate temperatures (Bowler, 2005), including those that permit growth and reproduction (Teets & Denlinger, 2013). Thus, some plastic responses (e.g., hardening and heat shock, Bowler, 2005) may be useful for organisms to cope with diel variability approaching lethal limits (Moyen et al., 2020, 2022), whereas acclimation will generally not help organisms cope with diel variability.
Although not well studied, there is no evidence that exposure of individuals to increased diel variability evokes a plastic response that helps organisms tolerate a wider range of temperatures. While exposure to increased diel temperature variability increased performance at higher temperatures in a terrestrial isopod, Porcellio laevis, performance decreased at cool temperatures (Bozinovic et al., 2016). The eurythermal fish, Austrofundulus limnaeus, differently expressed various genes in individuals with long‐term acclimation to constant or diel fluctuating temperature (Podrabsky & Somero, 2004), although whether these expressions improved whole organism traits (such as survival probability and fecundity) under fluctuating temperature is unknown. Thus, it is unlikely that most organisms will use acclimation as a strategy in response to the daily cycle of warming and cooling and they are more likely to respond through evolutionary changes in T br.
4.2. Adaptive evolution of organisms to diel temperature variability
If the magnitude of annual temperature variability is an evolutionary driver of T br (Janzen, 1967), then predictable differences in diel thermal variation (Wang & Dillon, 2014) should also act as a selection pressure. Yet, few studies have attempted to quantify how the thermal biology of species occupying habitats that predictably differ in seasonal and diel temperature variation responds (but see Chan et al., 2016; Gutiérrez‐Pesquera et al., 2016; Shah, Gill, et al., 2017).
The predicted relationship between diel thermal variation and T br can result in different patterns. On the one hand, a simple expectation is that T br should evolve to match the range of diel variation (Shah, Gill, et al., 2017). However, evolutionary models of performance predict a strategy of conducting critical functions (e.g., feeding, reproduction, dispersal) over a narrow or wide range of temperatures depending on whether temperature cycles are less than or greater than one generation (Gilchrist, 1995). Specifically, under conditions of high thermal variability within a generation, typical of diel variability, a specialized narrow thermal performance range is predicted to evolve (Gilchrist, 1995). Put simply, over multiple generations individuals cannot postpone critical functions to cope with persistent thermal change, but there can be selection to avoid unfavorable short‐term temperatures and restrict vital functions to periods of favorable temperatures. We refer to this as the “diel narrowing hypothesis” (DNH; Figure 4a), which is premised on organisms confining critical functions to a narrower, favorable temperature range, provided that such favorable temperatures occur sufficiently frequently for an overall positive energy balance. So, a nocturnal (or diurnal) habit allows organisms to exploit favorable conditions and avoid conducting critical functions across the full diel thermal range. Consequently, the DNH predicts that organisms adapted to high diel thermal variability should have a narrow thermal performance range (Gilchrist, 1995). There is some evidence that organisms do adopt diel narrowing as predicted by the DNH. For example, Chan et al. (2016) invoked the DNH to explain the negative correlation between elevational range size of species and diel temperature variation. They argue that, with increasing diel variation, species become increasingly physiologically specialized to a narrower elevational range rather than evolving to be generalists with a broader elevational range (but see a critique by Qian et al., 2017). As a specific example, the tree, Eucalyptus parramattensis, reduced photosynthesis by 95% during the hottest part of the day during an experimental heatwave, despite the high‐temperature tolerances of their leaves (photosystem II integrity) not being exceeded (Drake et al., 2018). This tree appears to be confining a critical function, photosynthesis, to a narrow range of temperatures while its body temperature varied more widely (Gilchrist, 1995). Organisms adopting diel narrowing need to either have a sufficiently wide T br to survive the periods of inactivity or use buffering (e.g., behavioral thermoregulation) to keep within critical limits (Stillman, 2003).
FIGURE 4.
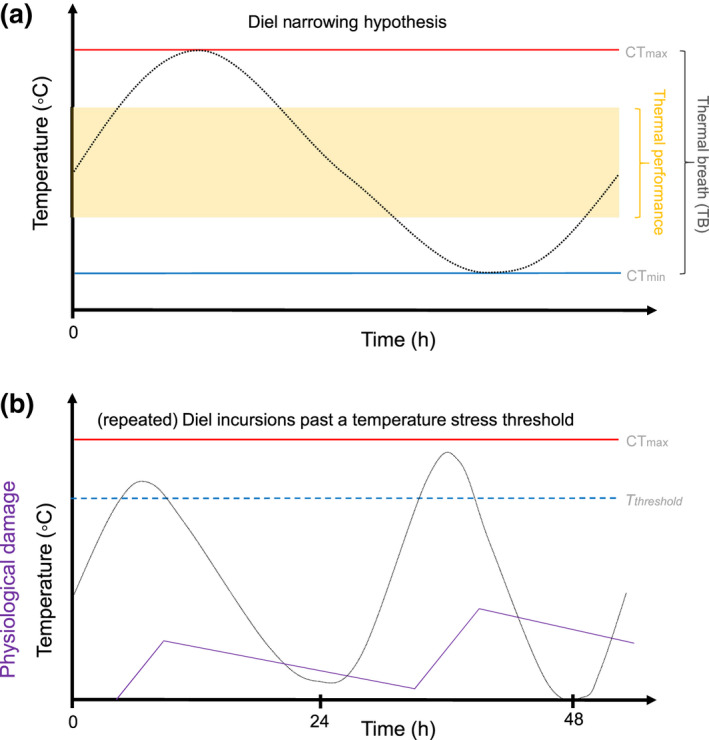
Some mechanisms for how diel thermal variability affects organisms. (a) The diel narrowing hypothesis based on Gilchrist (1995). (b) Repeated diel cycles into stressful temperatures, conceptually showing the accumulation of physiological damage when temperature is beyond T threshold and recovery from this physiological damage when below this threshold.
4.3. Buffering of diel temperature variability
Many organisms use a range of strategies to buffer against unfavorable meteorological diel temperature variation. These include the use of thermal microhabitats, for example, under vegetation, soil (Klinges & Scheffers, 2021; Woods et al., 2015) or snow (Marshall & Sinclair, 2012) and building burrows, nests, galls, etc. (Woods et al., 2021) to avoid the full range of diel temperature variability. Many mobile ectothermic animals move between thermal micro‐habitats to behaviorally thermoregulate and reduce their experienced diel temperature variability (Huey et al., 2012). The degree to which this is possible depends on the availability of thermal refugia and the thermoregulatory benefits relative to the costs (e.g., reduced foraging opportunities and predation risk, Besson & Cree, 2010; Muñoz & Bodensteiner, 2019). In aquatic habitats, particularly those with well‐mixed waters, there is typically less spatial thermal heterogeneity compared with terrestrial environments, and this reduces the opportunity for behavioral thermoregulation. Nevertheless, some freshwater fish use cool groundwater seeps as thermal refugia (Boulton & Hancock, 2006; Sauter et al., 2001) and mobile intertidal animals hide under rocks during low tide (Stillman, 2003). In the absence of buffering, ectotherms must cope with diel fluctuations in body temperature.
4.4. Coping with diel variability in body temperature
Despite experiencing variation in acute temperatures (see Section 2 above), most ectotherms experience body temperatures that vary around T opt (Dowd et al., 2015). As with acute temperature changes, non‐linear TPCs and Jensen's inequality cause increased diel thermal variability below T opt to be either negative or beneficial, but above T opt diel thermal variability will always be negative (Vasseur et al., 2014). Lowland tropical species typically experience temperatures close to or above their T opt (Dillon et al., 2010; Dowd et al., 2015; Payne & Smith, 2017) meaning that increased diel variability will always have some negative cost by exceeding T opt and increasing vulnerability (Estay et al., 2014; Martin & Huey, 2008; Vasseur et al., 2014). In contrast, species occupying environments cooler than T opt are likely to benefit from thermal variability and be less vulnerable to warming that does not exceed T opt (Deutsch et al., 2008).
In addition to the implications of Jensen's inequality for diel temperature variability, an organism's body temperatures may have repeated incursions into stressful temperature ranges over successive diel cycles (Figure 4b). For example, a fish in a well‐mixed stream might experience 6 h at stressfully hot temperatures during one especially hot day, then only 30 min on the next day and then 2 h the following day, with physiological damage accumulating over successive incursions into stressful temperatures (Jørgensen et al., 2019, 2021; Ørsted et al., 2022; Pörtner, 2010; Rezende et al., 2014, 2020). The effect of such repeated incursions should be dependent on many attributes of repeated diel temperature variation, including the frequency, magnitude, and duration of thermally stressful conditions, intervals between stressful temperature exposures (Marshall & Sinclair, 2015), and capacity for recovery between incursions (Figure 4b). With many attributes being involved, it is a challenge to understand how climate change altered patterns in repeated diel cycles will affect organisms' vulnerability. Nevertheless, we will next consider a hypothesis of how individual organisms might plausibly respond to conditions where they dip in and out of stressful temperatures on a diel basis. This hypothesis could be expanded to consider survival probability functions (Jørgensen et al., 2019, 2021).
Prolonged exposure to sub‐lethal temperatures results in a reduction in the lethal temperature for an organism (Santos et al., 2011). Pörtner (2010) proposed that organisms have thresholds for both cold and warm temperature exposure. He suggests that if their body temperature is above the warm temperature threshold or below the cold‐temperature threshold, they begin to accumulate physiological damage. The absolute difference between the relevant threshold temperature and the exposure temperature (°C) multiplied by the exposure period (e.g., minutes or hours) determines the amount of physiological damage incurred. Damage continues to accumulate, until death occurs. For example, if an organism starts to accumulate damage at 35°C, and it can survive for 10 h at 37°C (i.e., at 2°C above this threshold), it would be able to survive 5 h at 39°C (i.e., 4°C above the threshold). These threshold temperatures are not necessarily fixed and may vary due to acclimation, life stage and other factors (Pörtner, 2010). Cold temperature stress is generally less well understood than heat stress and ectotherms potentially have multiple values for their cold tolerance depending on the physiological state (e.g., active vs. diapause; Rezende et al., 2014). Crossing freeze threshold temperatures can also change the temperatures at which organisms freeze (Marshall & Sinclair, 2015).
Rezende et al. (2014) suggest that the temperature–time relationship should place time on a log10 scale via the following relationships for stressfully warm and cold temperatures, respectively:
| (1) |
| (2) |
where T ko is the temperature (°C), which results in death or knockdown, CTmax and CTmin are the 1 min exposure critical maximum and minimum temperatures (°C, Figure 1), respectively (Santos et al., 2011), and z and z′are constants of thermal sensitivity to exposure period (Rezende et al., 2014, 2020). Values of z and z’ can be obtained by measuring CTmax and CTmin, respectively, and T kn following exposure to thermally stressful temperatures for multiple periods (Jørgensen et al., 2019, 2021). High values of z and z′ imply that there are large differences in the T ko with brief exposures compared with lengthy exposures, while for low values of z and z′ the effect of exposure period on T ko is relatively smaller. Rezende et al. (2014) suggests that z is positively correlated with CTmax across species comparisons and influenced by body size (Peralta‐Maraver & Rezende, 2021), but Jørgensen et al. (2019) suggest that this may be an analytical artifact.
When organisms are exposed to successive cycles of diel variability, which dip into and out of thermally stressful conditions, the simplest model would be to assume that accumulated physiological damage (Biederman et al., 2019; Chou et al., 2017; Kim et al., 2017; Pörtner, 2002, 2010; Pörtner & Farrell, 2008; Pörtner et al., 2007) is irreversible, such that mortality ensues when sufficient damage accumulates. For example, using Pörtner's (2010) model, if an organism perishes after 20 h at 2°C past the threshold, and it experiences 2 h at this temperature each day, it will perish after 10 diel cycles. However, there is evidence that insects have greater survival rates during stressful cold periods if they experience regular “breaks” from damaging temperatures (Colinet et al., 2006; Lalouette et al., 2011; Leopold et al., 1998; Marshall & Sinclair, 2010, 2012; Nedvéd et al., 1998; Renault et al., 2004; Tollarová‐Borovanská et al., 2009). During these intervals, the antioxidant system (Lalouette et al., 2011), ion homeostasis (Koštál et al., 2007), and potentially other mechanisms (Tollarová‐Borovanská et al., 2009) are activated to allow for tissue repair and enzymatic recovery. Marshall and Sinclair (2010) found that Drosophila melanogaster had greater survival when exposed repeatedly to a stressfully cold diel temperature (−0.5°C) compared with a single exposure to this temperature for the same total period. However, the D. melanogaster individuals experiencing these repeated exposures had reduced fecundity, energy reserves, and their intrinsic rate of population increased, relative to those exposed to a single period of the same total length. Suggesting that, although D. melanogaster was able to repair physiological damage during periods of non‐stressful temperature, this repair resulted in a fitness cost (Marshall & Sinclair, 2010). Consequently, consideration of the build‐up of cold damage, recovery from this damage, and the costs of this recovery is needed to understand how organisms might respond to shifting levels of successive cycles of diel thermal variability under climate change.
It is not known whether a parallel mechanism exists whereby cool “breaks” extend tolerance to stressful high temperatures. This is feasible, as enzymes exposed to heating initially undergo reversible inactivation, before experiencing denaturation (Schulte, 2015). Organisms are also capable of synthesising new enzymes when heat stress is removed. Consequently, we suggest that organisms will generally have mechanisms to repair physiological damage caused by cold or heat stress (see also Ørsted et al., 2022).
We recognise that organisms accumulate physiological damage, either as described by Pörtner's (2010) or Rezende et al.'s (2014; Equations 1 or 2) models (purple line, when rising, in Figure 4b). However, we hypothesize that as they “dip” out of stressful body temperatures they have some ability to recover from physiological stress (purple line, when falling, in Figure 4b). During successive diel cycles when organisms are dipping in and out of physiological stressful temperatures, their accumulating damage is described by the sum of the damage received during each dial cycle minus the sum of recovery achieved during each diel cycle. Following Pörtner's (2010) model, the damage will be the integral of the temperature function above the warm temperature threshold during the ith cycle for stressful hot temperatures (Figure 4b), or below the cold threshold for stressful non‐freezing cold temperatures. Alternatively, when following the Rezende et al. (2020) model (Equations 1 and 2), damage needs exposure time on a log10 scale. Cumulative damage can continue to increase over repeated cycles up to the point that it exceeds some limit after which mortality occurs. However, there may be sub‐lethal consequences of physiological recovery from repeated temperature extremes (Marshall & Sinclair, 2010).
Little is known about the rate at which recovery might occur (Ørsted et al., 2022). For cold injuries, 2 h per day at warm temperatures (19–25°C) were sufficient to reduce the effects of stressful cold temperatures in various insects (Colinet et al., 2006; Leopold et al., 1998; Nedvéd et al., 1998; Renault et al., 2004; Tollarová‐Borovanská et al., 2009), with greater benefit from more frequent (Colinet et al., 2006) or longer (Bale et al., 2001; Lalouette et al., 2011) intervals. A complicating factor is that recovery rate itself could be temperature dependent (Ørsted et al., 2022), presumably being greatest at or near T opt (Figure 1).
The importance of such hypothesised models is that they demonstrate multiple mechanisms (i.e., damage, recovery, and sub‐lethal costs of recovery, Marshall & Sinclair, 2010) that are dependent on the frequency, magnitude, and duration of temperatures that exceed critical thresholds. Although such models have limitations (Box, 1979), they highlight that the capacity for damage and subsequent recovery may be important traits related to vulnerability, yet these traits are poorly quantified (Ørsted et al., 2022; Peralta‐Maraver & Rezende, 2021; Rezende et al., 2014).
5. IMPLICATIONS FOR CLIMATE CHANGE OF CONSIDERING TEMPERATURE VARIATION AND ORGANISMAL RESPONSES AT MULTIPLE TEMPORAL SCALES
5.1. Organisms' responses to thermal variability is time scale dependent
We have reviewed how ectotherms respond to temporal variability in temperature on different times scales via a range of mechanisms (Figure 5). Reduced performance from not being at T opt, the nonlinear effects of TPCs from Jensen's inequality (Colinet et al., 2015; Dowd et al., 2015; Vasseur et al., 2014), and the consequences of exponentially increasing biological rates up to T opt (Dillon et al., 2010; Gillooly et al., 2006) are most relevant at the acute scale and become progressively less important as the scale of thermal variability increases because of a range of other strategies, such as acclimation, and adaptative evolution in thermal tolerance in response to predictable thermal variability. Such responses may nevertheless still have some, admittedly reduced, importance at longer time scales (Payne & Smith, 2017). In contrast, we suggest that adaptive evolution of thermal tolerance is generally most important with thermal variability at longer time scales (e.g., annual) and becomes progressively less important at shorter time scales of thermal variability, although likely playing some role in environments with predicable patterns in diel variability (Gilchrist, 1995). All traits, including the ability to seasonally escape, plastically change thermal tolerance, adopt diel narrowing, buffer organisms body temperature against larger environmental thermal change and accumulate and recover from physiological damage, have the potential to evolve in response to natural selection if there is sufficient genetic variation. Adaptive evolution of thermal tolerance may even have some importance for acute thermal changes if environments have had long‐term and predictable history of acute thermal changes (Gilchrist & Huey, 1999; Hoffmann et al., 1997; McColl et al., 1996). Various other responses are most important at intermediate time scales of temporal variability (Figure 5).
FIGURE 5.
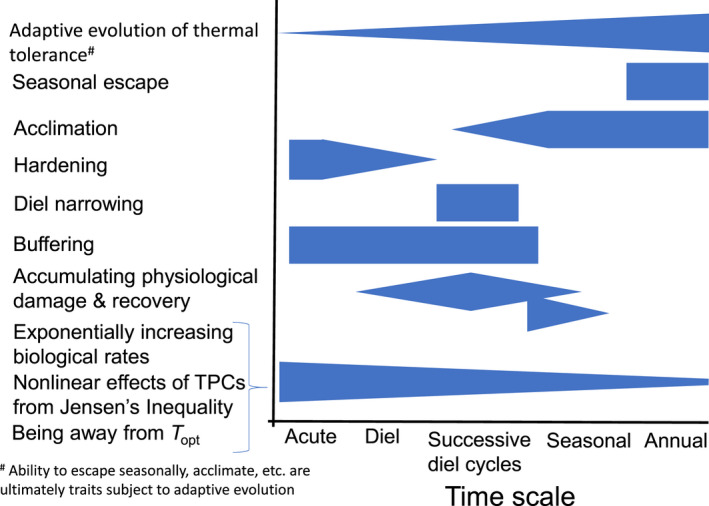
Hypothesised importance of responses to thermal variability along a continuous time scale from acute (near instantaneous) to annual (1 year). Vertical width of blue triangles or bars indicate indicates the approximate relative general importance of the indicated response across the time scale. We acknowledge that there will be some differences among taxa (Shah et al., 2021), evolutionary history (Bennett et al., 2021), habitats, etc. In some habitats, for example, well mixed shallow aquatic systems, there will be little capacity for buffering. Long‐term and predictable temperature change at any time scale that is an agent of selection will result in adaptive evolution; however, there is more evidence that adaptive evolution is more relevant as a result of annual scale variability.
While these different degrees of temporal variation and organismal responses are known to thermal physiologists, the relative importance of acute versus acclimated versus evolved responses in the face of increasing mean and changing variability in environmental temperatures remains a major unresolved problem for global change biologists. Consequently, we argue that more attention must be given to both the temporal scale of temperature variability and the organismal responses, if we seek to accurately determine species vulnerability. For example, in response to increased diel temperature variability (Wang & Dillon, 2014), whole organism acclimation is unlikely to be important, as acclimation takes too long (Havas & Adovokaat, 1995), while buffering, such as behavioral thermoregulation, is more likely to be an important response (Figure 5). Thus, quantifying variation among species in the opportunity or capacity for behavioral thermoregulation is likely to be far more informative for predicting vulnerability to increased diel temperature variability than measuring acclimation.
Organisms' responses to changes in diel and annual scale under climate change may be fundamentally different. Chan et al. (2016) proposed that high annual thermal variability would increase organisms T br based on the CVH (Janzen, 1967), while high diel variability would decrease organisms' thermal performance ranges (Figure 1) based on the DNH (Gilchrist, 1995). With somewhat different responses from organisms to thermal variability at different time scales (Figure 5), there is the potential for interactive effects (Duncan & Kefford, 2021) between variation at different scales. The cumulative effect of both hardening and acclimation on organisms' thermal tolerances appears to be additive (Bowler, 2005; Teets & Denlinger, 2013), but synergistic or antagonistic effects are also possible. Indeed, Payne and Smith's (2017) TCH implies a synergistic effect for the cumulative effect of both increasing mean temperatures and increasing temperature variability. If this synergistic effect exists, then increases in temperature variability from climate change will have greater effects in warm than cooler environments. Also, climate change will have much greater effects where it increases both mean and temperature variability than where it only affects one of these metrics.
5.2. Accumulating and recovering from damage of successive diel cycles
The extent of diel variability may be critical for determining organisms' responses to increased maximum temperatures under climate change. In climates with high diel variability, for example, inland locations with low humidity (Geerts, 2003), organisms may have (some) ability for recovery from high temperature induced physiological damage during the hottest part of the day during relatively cooler parts of the diel cycle. In contrast, in environments with low diel temperature variability, there may be relatively less respite where physiological repairs can take place. While in environments with no diel variability, for example, many deep aquatic systems, organisms would continue to accumulate physiological damage from climate change induced high temperature throughout the diel cycle. Models of future climate warming that incorporate diel variability and the potential for recovering from temperature induced physiological damage will need to be developed to further explore these ideas.
The hypothesis we suggest for organisms accumulating and recovering physiological damage over successive diel cycles is also likely useful for considering the effects of extreme thermal events. In many extreme thermal events in terrestrial and shallow aquatic systems, temperatures will not exceed thermal thresholds for physiological damage over the entire event. Rather, temperature will dip in and out of physiological damaging levels over successive diel cycles with damage accumulating over repeated incursions into stressful temperatures and the potential for some recovery during periods when temperature is not physiologically stressful.
5.3. Extreme thermal events
The relative extent of diel and annual variability differs rather predictably globally with latitude and elevation (Geerts, 2002, 2003; Wang & Dillon, 2014), as well as between aquatic and terrestrial habitats, between microhabitats (Klinges & Scheffers, 2021; Sheldon et al., 2018) and depth below ground (Spötl et al., 2005). We would expect in such circumstances that organisms would be generally adapted to the acute, diel, and annual variability they have experienced over evolutionary time. How organisms might differentially respond to changes in thermal variability at these locations and habitats is not clear and deserves research. We suggest how previous exposure to thermal temporal variability influences organisms' responses to extreme events will be highly dependent on matching the temporal scale(s) of this variability with the scale of the extreme events.
Evolution of organisms to live in environments with large seasonal variation in temperature (Janzen, 1967) is unlikely to equip them well to cope with heatwaves or cold snaps over days to weeks if they occur during the hottest/coldest period of the year. Where temperatures do not exceed acute tolerances, organisms adapted to deal with high levels of diel thermal variability are more likely to have an evolutionary advantage in dealing with extreme thermal events than are organisms that have evolved in environments with limited diel thermal variability. Therefore, we would expect organisms living in tropical (nonseasonal) locales with high diel variation will generally fare better during extreme thermal events lasting days to weeks than those organisms in low diel variation sites, as the former organisms are more likely to have evolved strategies to deal with a wider range of temperatures across succussive days.
6. CONCLUDING REMARKS AND FUTURE DIRECTIONS
We have highlighted that different thermodynamic and biological processes are important for organisms to deal with thermal variation occurring at different temporal scales, and we have examined a range of different responses associated with thermal variation at these various temporal scales. Future progress will depend on our ability to develop robust predictive models that explicitly incorporate the temporal scales at which temperature and organismal responses vary.
Although numerous challenges remain in predicting the outcomes of various changes in thermal variability (e.g., acute, diel, and annual scales), there are some general lessons that emerge. The first is that there are substantial geographic differences in the temporal scale at which organisms are experiencing thermal variation. This variation occurs across well‐known gradients of latitude and elevation, but also less appreciated gradients such as, in terrestrial environments, distance to major water bodies, and trends in humidity that alter diel temperature variation. In aquatic environments, there are also variations across depth, size of the aquatic environment, and distance to large terrestrial environments. Thus, no single time scale or biological response can be expected to apply universally. The second lesson is that depending on the time scale of thermal variation, there is a need to look beyond T br as a singular metric of vulnerability and incorporate the diversity of responses that organisms use. For example, the use of behavioral thermoregulation, the potential for recovery following exposure to thermal stress, and the ability to restrict activity to favorable times of the diel cycle. Many of these responses are (somewhat) relevant across several time scales (Figure 5) and will likely vary between taxa (Shah et al., 2021), evolutionary history (Bennett et al., 2021), habitat spatial temperature variability, and other factors.
We suggest three broad areas that future research that should advance our understanding of how organisms will respond to changing thermal variability at multiple temporal scales. First comparative studies from locations with contrasting mean temperature and acute, diel, and annual variability can aid in understanding organisms' responses to temperature regimes. Such studies can take advantage of thermal variability with latitude and elevation but need to be carefully designed to avoid confounding effects of annual mean temperatures and annual variability (Payne & Smith, 2017). This can be achieved by comparing coastal versus continental terrestrial sites, coastal versus open‐ocean marine habitats, small versus large lakes and center versus fringe habitats. In both terrestrial and aquatic environments, having sites in both hemispheres will also be helpful. Information from such comparative studies will indicate organism responses to temperature variability at different temporal scales and how climate change induced changes in temperature variability might affect organism vulnerability.
Second, studies should incorporate measurements of microclimatic conditions to understand the role of refugia and thermal heterogeneity in promoting persistence (Klinges & Scheffers, 2021; Woods et al., 2015, 2021). Organisms in relatively spatially homogeneous thermal environments may respond to temporal temperature regimes differently from those in high spatially heterogeneous thermal environments where there may be greater opportunity for behavioral regulation and diel narrowing. Comparative studies of behavioral thermoregulation and acclimation for species sharing a similar macroclimate, but different microclimate refugia could be informative in determining how organisms from different habitats will respond to changing thermal variability at different time scales. Finally, the capacity to accumulate physiological damage during periods of stressful temperature (Pörtner, 2010; Rezende et al., 2014, 2020) and then recover during periods of less stressful temperature (Marshall & Sinclair, 2015) are two potentially critical traits for predicting vulnerability to successive diel cycles of temperature variation and extreme thermal events and measurements of these traits are urgently needed to understand organisms' vulnerability to changing patterns in diel variability and extreme thermal events.
AUTHOR CONTRIBUTIONS
Development of the conceptual framework: Ben J. Kefford, Cameron K. Ghalambor, N. LeRoy Poff, Ross Thompson, and Jane Hughes. Writing of a first draft of the MS: Ben J. Kefford. Writing other parts: Cameron K. Ghalambor, Ross Thompson. Making of figures: Ben J. Kefford, Beatrice Dewenter, and Ross Thompson. Literature searches: Ben J. Kefford, Jollene Reich, Beatrice Dewenter, and Cameron K. Ghalambor. Commenting and editing the MS and approval of final version: all.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
Table S1.
ACKNOWLEDGMENTS
Our co‐author Beatrice Dewenter passed away unexpectedly for medical reasons during the revisions of this manuscript. We acknowledge her contributions to the broader body of work represented here and dedicate this paper to her. We are grateful for funding from an Australian Research Council Discovery Project (DP180102016) held by BKJ, RT, NLP, and JH. JR and BD received scholarships from these funds and the University of Canberra. CKG was supported by a grant from the National Science Foundation (IOS 1457383). We thank George Wang, Michael Dillon, and Peter Thorne for answering questions about their papers and Enrico Rezende and anonymous reviewers for their thoughtful comments. We thank the organisers of the University of Canberra writer's retreat where a first draft of this paper was prepared. Open access publishing facilitated by University of Canberra, as part of the Wiley ‐ University of Canberra agreement via the Council of Australian University Librarians.
Kefford, B. J. , Ghalambor, C. K. , Dewenter, B. , Poff, N. L. , Hughes, J. , Reich, J. , & Thompson, R. (2022). Acute, diel, and annual temperature variability and the thermal biology of ectotherms. Global Change Biology, 28, 6872–6888. 10.1111/gcb.16453
[Correction added on 29‐November‐2022, after first online publication: funding statement has been added.]
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Angilletta, M. J., Jr. , & Angilletta, M. J. (2009). Thermal adaptation: A theoretical and empirical synthesis. Oxford University Press. [Google Scholar]
- Angilletta, M. J., Jr. , Huey, R. B. , & Frazier, M. R. (2010). Thermodynamic effects on organismal performance: Is hotter better? Physiological and Biochemical Zoology, 83, 197–206. [DOI] [PubMed] [Google Scholar]
- Baer, C. F. , & Travis, J. (2000). Direct and correlated responses to artificial selection on acute thermal stress tolerance in a livebearing fish. Evolution, 54, 238–244. [DOI] [PubMed] [Google Scholar]
- Baker, K. G. , Radford, D. T. , Evenhuis, C. , Kuzhiumparam, U. , Ralph, P. J. , & Doblin, M. A. (2018). Thermal niche evolution of functional traits in a tropical marine phototroph. Journal of Phycology, 54, 799–810. [DOI] [PubMed] [Google Scholar]
- Bale, J. , Worland, M. , & Block, W. (2001). Effects of summer frost exposures on the cold tolerance strategy of a sub‐Antarctic beetle. Journal of Insect Physiology, 47, 1161–1167. [DOI] [PubMed] [Google Scholar]
- Barton, S. , Jenkins, J. , Buckling, A. , Schaum, C.‐E. , Smirnoff, N. , Raven, J. A. , & Yvon‐Durocher, G. (2020). Evolutionary temperature compensation of carbon fixation in marine phytoplankton. Ecology Letters, 23, 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, A. F. , & Lenski, R. E. (1993). Evolutionary adaptation to temperature II. Thermal niches of experimental lines of Escherichia coli . Evolution, 47, 1–12. [DOI] [PubMed] [Google Scholar]
- Bennett, J. M. , Sunday, J. , Calosi, P. , Villalobos, F. , Martínez, B. , Molina‐Venegas, R. , Araújo, M. B. , Algar, A. C. , Clusella‐Trullas, S. , & Hawkins, B. A. (2021). The evolution of critical thermal limits of life on Earth. Nature Communications, 12, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson, A. A. , & Cree, A. (2010). A cold‐adapted reptile becomes a more effective thermoregulator in a thermally challenging environment. Oecologia, 163, 571–581. [DOI] [PubMed] [Google Scholar]
- Biederman, A. M. , Kuhn, D. E. , O'Brien, K. M. , & Crockett, E. L. (2019). Physical, chemical, and functional properties of neuronal membranes vary between species of Antarctic notothenioids differing in thermal tolerance. Journal of Comparative Physiology B, 189, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton, A. , & Hancock, P. (2006). Rivers as groundwater‐dependent ecosystems: A review of degrees of dependency, riverine processes and management implications. Australian Journal of Botany, 54, 133–144. [Google Scholar]
- Bowler, K. (2005). Acclimation, heat shock and hardening. Journal of Thermal Biology, 30, 125–130. [Google Scholar]
- Box, G. E. (1979). All models are wrong, but some are useful. Robustness in Statistics, 202, 549. [Google Scholar]
- Boyd, P. W. , Cornwall, C. E. , Davison, A. , Doney, S. C. , Fourquez, M. , Hurd, C. L. , Lima, I. D. , & McMinn, A. (2016). Biological responses to environmental heterogeneity under future ocean conditions. Global Change Biology, 22, 2633–2650. [DOI] [PubMed] [Google Scholar]
- Bozinovic, F. , Cavieres, G. , Martel, S. I. , Alruiz, J. M. , Molina, A. N. , Roschzttardtz, H. , & Rezende, E. L. (2020). Thermal effects vary predictably across levels of organization: Empirical results and theoretical basis. Proceedings of the Royal Society B, 287, 20202508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozinovic, F. , Sabat Opazo, P. , Rezende, E. L. , & Canals Lambarri, M. (2016). Temperature variability and thermal performance in ectotherms: Acclimation, behaviour, and experimental considerations. Evolutionary Ecology Research, 17, 111–124. [Google Scholar]
- Bronikowski, A. M. , Bennett, A. F. , & Lenski, R. E. (2001). Evolutionary adaptation to temperature. VIII. Effects of temperature on growth rate in natural isolates of Escherichia coli and Salmonella enterica from different thermal environments. Evolution, 55, 33–40. [DOI] [PubMed] [Google Scholar]
- Broska, L. H. , Poganietz, W.‐R. , & Vögele, S. (2020). Extreme events defined—A conceptual discussion applying a complex systems approach. Futures, 115, 102490. [Google Scholar]
- Cabrerizo, M. J. , & Marañón, E. (2021). Temperature fluctuations in a warmer environment: Impacts on microbial plankton. Faculty Reviews, 10, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, W.‐P. , Chen, I.‐C. , Colwell, R. K. , Liu, W.‐C. , Huang, C.‐Y. , & Shen, S.‐F. (2016). Seasonal and daily climate variation have opposite effects on species elevational range size. Science, 351, 1437–1439. [DOI] [PubMed] [Google Scholar]
- Chen, B. (2015). Patterns of thermal limits of phytoplankton. Journal of Plankton Research, 37, 285–292. [Google Scholar]
- Chou, H. , Pathmasiri, W. , Deese‐Spruill, J. , Sumner, S. , & Buchwalter, D. B. (2017). Metabolomics reveal physiological changes in mayfly larvae (Neocloeon triangulifer) at ecological upper thermal limits. Journal of Insect Physiology, 101, 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, A. (2004). Is there a universal temperature dependence of metabolism? Functional Ecology, 18, 252–256. [Google Scholar]
- Clarke, A. , & Fraser, K. (2004). Why does metabolism scale with temperature? Functional Ecology, 18, 243–251. [Google Scholar]
- Colinet, H. , Renault, D. , Hance, T. , & Vernon, P. (2006). The impact of fluctuating thermal regimes on the survival of a cold‐exposed parasitic wasp, Aphidius colemani . Physiological Entomology, 31, 234–240. [Google Scholar]
- Colinet, H. , Sinclair, B. J. , Vernon, P. , & Renault, D. (2015). Insects in fluctuating thermal environments. Annual Review of Entomology, 60, 123–140. [DOI] [PubMed] [Google Scholar]
- Cossins, A. , & Bowler, K. (1987). Temperature biology of animals. Springer. [Google Scholar]
- Coumou, D. , & Rahmstorf, S. (2012). A decade of weather extremes. Nature Climate Change, 2, 491–496. [Google Scholar]
- Dell, A. I. , Pawar, S. , & Savage, V. M. (2011). Systematic variation in the temperature dependence of physiological and ecological traits. Proceedings of the National Academy of Sciences of the United States of America, 108, 10591–10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch, C. A. , Tewksbury, J. J. , Huey, R. B. , Sheldon, K. S. , Ghalambor, C. K. , Haak, D. C. , & Martin, P. R. (2008). Impacts of climate warming on terrestrial ectotherms across latitude. Proceedings of the National Academy of Sciences of the United States of America, 105, 6668–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffenbaugh, N. S. , Singh, D. , Mankin, J. S. , Horton, D. E. , Swain, D. L. , Touma, D. , Charland, A. , Liu, Y. , Haugen, M. , & Tsiang, M. (2017). Quantifying the influence of global warming on unprecedented extreme climate events. Proceedings of the National Academy of Sciences of the United States of America, 114, 4881–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon, M. E. , Wang, G. , & Huey, R. B. (2010). Global metabolic impacts of recent climate warming. Nature, 467, 704–706. [DOI] [PubMed] [Google Scholar]
- Dole, R. , Hoerling, M. , Kumar, A. , Eischeid, J. , Perlwitz, J. , Quan, X.‐W. , Kiladis, G. , Webb, R. , Murray, D. , & Chen, M. (2014). The making of an extreme event: Putting the pieces together. Bulletin of the American Meteorological Society, 95, 427–440. [Google Scholar]
- Domínguez‐Guerrero, S. F. , Muñoz, M. M. , de Jesús Pasten‐Téllez, D. , Arenas‐Moreno, D. M. , Rodríguez‐Miranda, L. A. , Manríquez‐Morán, N. L. , & Méndez‐de la Cruz, F. R. (2019). Interactions between thermoregulatory behavior and physiological acclimatization in a wild lizard population. Journal of Thermal Biology, 79, 135–143. [DOI] [PubMed] [Google Scholar]
- Dowd, W. W. , King, F. A. , & Denny, M. W. (2015). Thermal variation, thermal extremes and the physiological performance of individuals. The Journal of Experimental Biology, 218, 1956–1967. [DOI] [PubMed] [Google Scholar]
- Drake, J. E. , Tjoelker, M. G. , Vårhammar, A. , Medlyn, B. E. , Reich, P. B. , Leigh, A. , Pfautsch, S. , Blackman, C. J. , López, R. , & Aspinwall, M. J. (2018). Trees tolerate an extreme heatwave via sustained transpirational cooling and increased leaf thermal tolerance. Global Change Biology, 24, 2390–2402. [DOI] [PubMed] [Google Scholar]
- Duan, J. , Esper, J. , Büntgen, U. , Li, L. , Xoplaki, E. , Zhang, H. , Wang, L. , Fang, Y. , & Luterbacher, J. (2017). Weakening of annual temperature cycle over the Tibetan Plateau since the 1870s. Nature Communications, 8, 14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, R. P. , & Kefford, B. J. (2021). Interactions in statistical models: Three things to know. Methods in Ecology and Evolution, 12, 2287–2229. [Google Scholar]
- Estay, S. A. , Lima, M. , & Bozinovic, F. (2014). The role of temperature variability on insect performance and population dynamics in a warming world. Oikos, 123, 131–140. [Google Scholar]
- Fangue, N. A. , & Bennett, W. A. (2003). Thermal tolerance responses of laboratory‐acclimated and seasonally acclimatized Atlantic stingray, Dasyatis sabina . Copeia, 2003, 315–325. [Google Scholar]
- Gardiner, N. M. , Munday, P. L. , & Nilsson, G. E. (2010). Counter‐gradient variation in respiratory performance of coral reef fishes at elevated temperatures. PLoS One, 5, e13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts, B. (2002). Empirical estimation of the annual range of monthly‐mean temperatures. Theoretical and Applied Climatology, 73, 107–132. [Google Scholar]
- Geerts, B. (2003). Empirical estimation of the monthly‐mean daily temperature range. Theoretical and Applied Climatology, 74, 145–165. [Google Scholar]
- Ghalambor, C. K. , Huey, R. B. , Martin, P. R. , Tewksbury, J. J. , & Wang, G. (2006). Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integrative and Comparative Biology, 46, 5–17. [DOI] [PubMed] [Google Scholar]
- Gilchrist, G. W. (1995). Specialists and generalists in changing environments. I. Fitness landscapes of thermal sensitivity. The American Naturalist, 146, 252–270. [Google Scholar]
- Gilchrist, G. W. , & Huey, R. B. (1999). The direct response of Drosophila melanogaster to selection on knockdown temperature. Heredity, 83, 15–29. [DOI] [PubMed] [Google Scholar]
- Gill, B. A. , Kondratieff, B. C. , Casner, K. L. , Encalada, A. C. , Flecker, A. S. , Gannon, D. G. , Ghalambor, C. K. , Guayasamin, J. M. , Poff, N. L. , Simmons, M. P. , Thomas, S. A. , Zamudio, K. R. , & Funk, W. C. (2016). Cryptic species diversity reveals biogeographic support for the ‘mountain passes are higher in the tropics’ hypothesis. Proceedings of the Royal Society B, 283, 20160553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly, J. , Allen, A. , Savage, V. , Charnov, E. , West, G. , & Brown, J. (2006). Response to Clarke and Fraser: Effects of temperature on metabolic rate. Functional Ecology, 20, 400–404. [Google Scholar]
- Gutiérrez‐Pesquera, L. M. , Tejedo, M. , Olalla‐Tárraga, M. , Duarte, H. , Nicieza, A. , & Solé, M. (2016). Testing the climate variability hypothesis in thermal tolerance limits of tropical and temperate tadpoles. Journal of Biogeography, 43, 1166–1178. [Google Scholar]
- Gutschick, V. P. , & BassiriRad, H. (2003). Extreme events as shaping physiology, ecology, and evolution of plants: Toward a unified definition and evaluation of their consequences. New Phytologist, 160, 21–42. [DOI] [PubMed] [Google Scholar]
- Havas, M. , & Adovokaat, E. (1995). Can sodium regulation be used to predict the relative acid‐sensitivity of various life‐stages and differenct species of aquatic fauna? Water, Air and Soil Pollution, 85, 865–870. [Google Scholar]
- Havird, J. C. , Neuwald, J. L. , Shah, A. A. , Mauro, A. , Marshall, C. A. , & Ghalambor, C. K. (2020). Distinguishing between active plasticity due to thermal acclimation and passive plasticity due to Q10 effects: Why methodology matters. Functional Ecology, 34, 1015–1028. [Google Scholar]
- Henley, B. J. , Gergis, J. , Karoly, D. J. , Power, S. , Kennedy, J. , & Folland, C. K. (2015). A tripole index for the interdecadal Pacific oscillation. Climate Dynamics, 45, 3077–3090. [Google Scholar]
- Hoerling, M. , Kumar, A. , Dole, R. , Nielsen‐Gammon, J. W. , Eischeid, J. , Perlwitz, J. , Quan, X.‐W. , Zhang, T. , Pegion, P. , & Chen, M. (2013). Anatomy of an extreme event. Journal of Climate, 26, 2811–2832. [Google Scholar]
- Hoffmann, A. A. , Chown, S. L. , & Clusella‐Trullas, S. (2013). Upper thermal limits in terrestrial ectotherms: How constrained are they? Functional Ecology, 27, 934–949. [Google Scholar]
- Hoffmann, A. A. , Dagher, H. , Hercus, M. , & Berrigan, D. (1997). Comparing different measures of heat resistance in selected lines of Drosophila melanogaster . Journal of Insect Physiology, 43, 393–405. [DOI] [PubMed] [Google Scholar]
- Huey, R. B. , Kearney, M. R. , Krockenberger, A. , Holtum, J. A. , Jess, M. , & Williams, S. E. (2012). Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology and adaptation. Philosophical Transactions of the Royal Society B: Biological Sciences, 367, 1665–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey, R. B. , & Kingsolver, J. G. (1989). Evolution of thermal sensitivity of ectotherm performance. Trends in Ecology & Evolution, 4, 131–135. [DOI] [PubMed] [Google Scholar]
- Huey, R. B. , & Kingsolver, J. G. (1993). Evolution of resistance to high temperature in ectotherms. The American Naturalist, 142, S21–S46. [Google Scholar]
- Huey, R. B. , & Kingsolver, J. G. (2019). Climate warming, resource availability, and the metabolic meltdown of ectotherms. The American Naturalist, 194, E140–E150. [DOI] [PubMed] [Google Scholar]
- Iverson, E. N. , Nix, R. , Abebe, A. , & Havird, J. C. (2020). Thermal responses differ across levels of biological organization. Integrative and Comparative Biology, 60, 361–374. [DOI] [PubMed] [Google Scholar]
- Janzen, D. H. (1967). Why mountain passes are higher in the tropics. The American Naturalist, 101, 233–249. [Google Scholar]
- Jørgensen, L. B. , Malte, H. , Ørsted, M. , Klahn, N. A. , & Overgaard, J. (2021). A unifying model to estimate thermal tolerance limits in ectotherms across static, dynamic and fluctuating exposures to thermal stress. Scientific Reports, 11, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen, L. B. , Malte, H. , & Overgaard, J. (2019). How to assess Drosophila heat tolerance: Unifying static and dynamic tolerance assays to predict heat distribution limits. Functional Ecology, 33, 629–642. [Google Scholar]
- Kim, K. S. , Chou, H. , Funk, D. H. , Jackson, J. K. , Sweeney, B. W. , & Buchwalter, D. B. (2017). Physiological responses to short‐term thermal stress in mayfly (Neocloeon triangulifer) larvae in relation to upper thermal limits. Journal of Experimental Biology, 220, 2598–2605. [DOI] [PubMed] [Google Scholar]
- Klinges, D. H. , & Scheffers, B. R. (2021). Microgeography, not just latitude, drives climate overlap on mountains from tropical to polar ecosystems. The American Naturalist, 197, 75–92. [DOI] [PubMed] [Google Scholar]
- Koštál, V. , Renault, D. , Mehrabianova, A. , & Bastl, J. (2007). Insect cold tolerance and repair of chill‐injury at fluctuating thermal regimes: Role of ion homeostasis. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 147, 231–238. [DOI] [PubMed] [Google Scholar]
- Lalouette, L. , Williams, C. , Hervant, F. , Sinclair, B. J. , & Renault, D. (2011). Metabolic rate and oxidative stress in insects exposed to low temperature thermal fluctuations. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 158, 229–234. [DOI] [PubMed] [Google Scholar]
- Leopold, R. A. , Rojas, R. R. , & Atkinson, P. W. (1998). Post pupariation cold storage of three species of flies: Increasing chilling tolerance by acclimation and recurrent recovery periods. Cryobiology, 36, 213–224. [DOI] [PubMed] [Google Scholar]
- Listmann, L. , LeRoch, M. , Schlüter, L. , Thomas, M. K. , & Reusch, T. B. (2016). Swift thermal reaction norm evolution in a key marine phytoplankton species. Evolutionary Applications, 9, 1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani, M. S. (1968). Ecology and biogeography of high altitude insects. Springer Science & Business Media. [Google Scholar]
- Marchant, R. , Kefford, B. J. , Wasley, J. , King, C. K. , Doube, J. , & Nugegoda, D. (2011). Response of stream invertebrate communities to vegetation damage from overgrazing by exotic rabbits on subantarctic Macquarie Island. Marine and Freshwater Research, 62, 404–413. [Google Scholar]
- Markle, T. M. , & Kozak, K. H. (2018). Low acclimation capacity of narrow‐ranging thermal specialists exposes susceptibility to global climate change. Ecology and Evolution, 8, 4644–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, K. E. , & Sinclair, B. J. (2010). Repeated stress exposure results in a survival–reproduction trade‐off in Drosophila melanogaster . Proceedings of the Royal Society B: Biological Sciences, 277, 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, K. E. , & Sinclair, B. J. (2012). The impacts of repeated cold exposure on insects. Journal of Experimental Biology, 215, 1607–1613. [DOI] [PubMed] [Google Scholar]
- Marshall, K. E. , & Sinclair, B. J. (2015). The relative importance of number, duration and intensity of cold stress events in determining survival and energetics of an overwintering insect. Functional Ecology, 29, 357–366. [Google Scholar]
- Martin, T. L. , & Huey, R. B. (2008). Why “suboptimal” is optimal: Jensen's inequality and ectotherm thermal preferences. The American Naturalist, 171, E102–E118. [DOI] [PubMed] [Google Scholar]
- McColl, G. , Hoffmann, A. A. , & McKechnie, S. W. (1996). Response of two heat shock genes to selection for knockdown heat resistance in Drosophila melanogaster . Genetics, 143, 1615–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhaden, M. J. , Zebiak, S. E. , & Glantz, M. H. (2006). ENSO as an integrating concept in earth science. Science, 314, 1740–1745. [DOI] [PubMed] [Google Scholar]
- Milly, P. C. D. , Wetherald, R. T. , Dunne, K. , & Delworth, T. L. (2002). Increasing risk of great floods in a changing climate. Nature, 415, 514–517. [DOI] [PubMed] [Google Scholar]
- Morgan, R. , Finnøen, M. H. , Jensen, H. , Pélabon, C. , & Jutfelt, F. (2020). Low potential for evolutionary rescue from climate change in a tropical fish. Proceedings of the National Academy of Sciences of the United States of America, 117, 33365–33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyen, N. E. , Crane, R. L. , Somero, G. N. , & Denny, M. W. (2020). A single heat‐stress bout induces rapid and prolonged heat acclimation in the California mussel, Mytilus californianus . Proceedings of the Royal Society B, 287, 20202561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyen, N. E. , Somero, G. N. , & Denny, M. W. (2022). Effects of heat acclimation on cardiac function in the intertidal mussel Mytilus californianus: Can laboratory‐based indices predict survival in the field? Journal of Experimental Biology, 225, jeb243050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz, M. , & Bodensteiner, B. (2019). Janzen's hypothesis meets the Bogert effect: Connecting climate variation, thermoregulatory behavior, and rates of physiological evolution. Integrative Organismal Biology, 1, oby002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz, M. M. , Stimola, M. A. , Algar, A. C. , Conover, A. , Rodriguez, A. J. , Landestoy, M. A. , Bakken, G. S. , & Losos, J. B. (2014). Evolutionary stasis and lability in thermal physiology in a group of tropical lizards. Proceedings of the Royal Society B: Biological Sciences, 281, 20132433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedvéd, O. , Lavy, D. , & Verhoef, H. (1998). Modelling the time–temperature relationship in cold injury and effect of high‐temperature interruptions on survival in a chill‐sensitive collembolan. Functional Ecology, 12, 816–824. [Google Scholar]
- Nielsen, M. , Elmes, G. , & Kipyatkov, V. (1999). Respiratory Q10 varies between populations of two species of Myrmica ants according to the latitude of their sites. Journal of Insect Physiology, 45, 559–564. [DOI] [PubMed] [Google Scholar]
- Orr, H. A. (2009). Fitness and its role in evolutionary genetics. Nature Reviews Genetics, 10, 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørsted, M. , Jørgensen, L. B. , & Overgaard, J. (2022). Finding the right thermal limit: A framework to reconcile ecological, physiological and methodological aspects of CTmax in ectotherms. Journal of Experimental Biology, 225(19), jeb244514. [DOI] [PubMed] [Google Scholar]
- Payne, N. L. , & Smith, J. A. (2017). An alternative explanation for global trends in thermal tolerance. Ecology Letters, 20, 70–77. [DOI] [PubMed] [Google Scholar]
- Peralta‐Maraver, I. , & Rezende, E. L. (2021). Heat tolerance in ectotherms scales predictably with body size. Nature Climate Change, 11, 58–63. [Google Scholar]
- Perez, T. M. , Stroud, J. T. , & Feeley, K. J. (2016). Thermal trouble in the tropics. Science, 351, 1392–1393. [DOI] [PubMed] [Google Scholar]
- Podrabsky, J. E. , & Somero, G. N. (2004). Changes in gene expression associated with acclimation to constant temperatures and fluctuating daily temperatures in an annual killifish Austrofundulus limnaeus . Journal of Experimental Biology, 207, 2237–2254. [DOI] [PubMed] [Google Scholar]
- Polato, N. R. , Gill, B. A. , Shah, A. A. , Gray, M. M. , Casner, K. L. , Barthelet, A. , Messer, P. W. , Simmons, M. P. , Guayasamin, J. M. , Encalada, A. C. , Kondratieff, B. C. , Flecker, A. S. , Thomas, S. A. , Ghalambor, C. K. , Poff, N. L. , Funk, W. C. , & Zamudio, K. R. (2018). Narrow thermal tolerance and low dispersal drive higher speciation in tropical mountains. Proceedings of the National Academy of Sciences of the United States of America, 115, 12471–12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pörtner, H.‐O. (2002). Climate variations and the physiological basis of temperature dependent biogeography: Systemic to molecular hierarchy of thermal tolerance in animals. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 132, 739–761. [DOI] [PubMed] [Google Scholar]
- Pörtner, H.‐O. (2010). Oxygen‐and capacity‐limitation of thermal tolerance: A matrix for integrating climate‐related stressor effects in marine ecosystems. Journal of Experimental Biology, 213, 881–893. [DOI] [PubMed] [Google Scholar]
- Pörtner, H. O. , & Farrell, A. P. (2008). Physiology and climate change. Science, 322, 690–692. [DOI] [PubMed] [Google Scholar]
- Pörtner, H. O. , Peck, L. , & Somero, G. (2007). Thermal limits and adaptation in marine Antarctic ectotherms: An integrative view. Philosophical Transactions of the Royal Society of London B, 362, 2233–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, C. , & Zhang, X. (2015). Human influences on changes in the temperature seasonality in mid‐ to high‐latitude land areas. Journal of Climate, 28, 5908–5921. [Google Scholar]
- Qian, H. , Field, R. , Zhang, J. , & Zhang, Y. (2017). Does daily climate variation have an effect on species' elevational range size? Journal of Biogeography, 44, 2432–2436. [Google Scholar]
- Renault, D. , Nedved, O. , Hervant, F. , & Vernon, P. (2004). The importance of fluctuating thermal regimes for repairing chill injuries in the tropical beetle Alphitobius diaperinus (Coleoptera: Tenebrionidae) during exposure to low temperature. Physiological Entomology, 29, 139–145. [Google Scholar]
- Rezende, E. L. , Bozinovic, F. , Szilágyi, A. , & Santos, M. (2020). Predicting temperature mortality and selection in natural Drosophila populations. Science, 369, 1242–1245. [DOI] [PubMed] [Google Scholar]
- Rezende, E. L. , Castañeda, L. E. , & Santos, M. (2014). Tolerance landscapes in thermal ecology. Functional Ecology, 28, 799–809. [Google Scholar]
- Santos, M. , Castaneda, L. E. , & Rezende, E. L. (2011). Making sense of heat tolerance estimates in ectotherms: Lessons from Drosophila . Functional Ecology, 25, 1169–1180. [Google Scholar]
- Sauter, S. T. , McMillan, J. , & Dunham, J. B. (2001). Salmonid behavior and water temperature . Final report to the policy workgroup of the EPA region 10 water temperature criteria guidance project, EPA 910‐D‐01‐001. United States Environmental Protection Agency, Region 10 Office of Water, Seattle, WA, 36 pp.
- Schaum, C.‐E. , Buckling, A. , Smirnoff, N. , & Yvon‐Durocher, G. (2022). Evolution of thermal tolerance and phenotypic plasticity under rapid and slow temperature fluctuations. Proceedings of the Royal Society B, 289, 20220834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte, P. M. (2015). The effects of temperature on aerobic metabolism: Towards a mechanistic understanding of the responses of ectotherms to a changing environment. Journal of Experimental Biology, 218, 1856–1866. [DOI] [PubMed] [Google Scholar]
- Schulte, P. M. , Healy, T. M. , & Fangue, N. A. (2011). Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integrative and Comparative Biology, 51, 691–702. [DOI] [PubMed] [Google Scholar]
- Seebacher, F. , White, C. R. , & Franklin, C. E. (2015). Physiological plasticity increases resilience of ectothermic animals to climate change. Nature Climate Change, 5, 61–66. [Google Scholar]
- Shah, A. A. , Funk, W. C. , & Ghalambor, C. K. (2017). Thermal acclimation ability varies in temperate and tropical aquatic insects from different elevations. Integrative and Comparative Biology, 57, 977–987. [DOI] [PubMed] [Google Scholar]
- Shah, A. A. , Gill, B. A. , Encalad, A. C. , Flecker, A. S. , Funk, W. C. , Guayasamin, J. M. , Kondratieff, B. C. , Poff, N. L. , Thomas, S. A. , Zamudio, K. R. , & Ghalambor, C. K. (2017). Climate variability predicts thermal limits of aquatic insects across elevation and latitude. Functional Ecology, 31, 2118–2127. [Google Scholar]
- Shah, A. A. , Woods, H. A. , Havird, J. C. , Encalada, A. C. , Flecker, A. S. , Funk, W. C. , Guayasamin, J. M. , Kondratieff, B. C. , Poff, N. L. , & Thomas, S. A. (2021). Temperature dependence of metabolic rate in tropical and temperate aquatic insects: Support for the climate variability hypothesis in mayflies but not stoneflies. Global Change Biology, 27, 297–311. [DOI] [PubMed] [Google Scholar]
- Sheldon, K. S. , Huey, R. B. , Kaspari, M. , & Sanders, N. J. (2018). Fifty years of mountain passes: A perspective on Dan Janzen's classic article. The American Naturalist, 191, 553–565. [DOI] [PubMed] [Google Scholar]
- Sinclair, B. J. , Marshall, K. E. , Sewell, M. A. , Levesque, D. L. , Willett, C. S. , Slotsbo, S. , Dong, Y. , Harley, C. D. , Marshall, D. J. , & Helmuth, B. S. (2016). Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecology Letters, 19, 1372–1385. [DOI] [PubMed] [Google Scholar]
- Somero, G. (2010). The physiology of climate change: How potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. Journal of Experimental Biology, 213, 912–920. [DOI] [PubMed] [Google Scholar]
- Spötl, C. , Fairchild, I. J. , & Tooth, A. F. (2005). Cave air control on dripwater geochemistry, Obir Caves (Austria): Implications for speleothem deposition in dynamically ventilated caves. Geochimica et Cosmochimica Acta, 69, 2451–2468. [Google Scholar]
- Stillman, J. H. (2003). Acclimation capacity underlies susceptibility to climate change. Science, 301, 65. [DOI] [PubMed] [Google Scholar]
- Stoks, R. , Verheyen, J. , Van Dievel, M. , & Tüzün, N. (2017). Daily temperature variation and extreme high temperatures drive performance and biotic interactions in a warming world. Current Opinion in Insect Science, 23, 35–42. [DOI] [PubMed] [Google Scholar]
- Sunday, J. M. , Bates, A. E. , & Dulvy, N. K. (2011). Global analysis of thermal tolerance and latitude in ectotherms. Proceedings of the Royal Society B: Biological Sciences, 278, 1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday, J. M. , Bates, A. E. , Kearney, M. R. , Colwell, R. K. , Dulvy, N. K. , Longino, J. T. , & Huey, R. B. (2014). Thermal‐safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proceedings of the National Academy of Sciences of the United States of America, 111, 5610–5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teets, N. M. , & Denlinger, D. L. (2013). Physiological mechanisms of seasonal and rapid cold‐hardening in insects. Physiological Entomology, 38, 105–116. [Google Scholar]
- Thomas, M. K. , Aranguren‐Gassis, M. , Kremer, C. T. , Gould, M. R. , Anderson, K. , Klausmeier, C. A. , & Litchman, E. (2017). Temperature–nutrient interactions exacerbate sensitivity to warming in phytoplankton. Global Change Biology, 23, 3269–3280. [DOI] [PubMed] [Google Scholar]
- Thomas, M. K. , Kremer, C. T. , Klausmeier, C. A. , & Litchman, E. (2012). A global pattern of thermal adaptation in marine phytoplankton. Science, 338, 1085–1088. [DOI] [PubMed] [Google Scholar]
- Thompson, R. M. , Beardall, J. , Beringer, J. , Grace, M. , & Sardina, P. (2013). Means and extremes: Building variability into community‐level climate change experiments. Ecology Letters, 16, 799–806. [DOI] [PubMed] [Google Scholar]
- Thorne, P. W. , Donat, M. G. , Dunn, R. J. H. , Williams, C. N. , Alexander, L. V. , Caesar, J. , Durre, I. , Harris, I. , Hausfather, Z. , Jones, P. D. , Menne, M. J. , Rohde, R. , Vose, R. S. , & Davy, R. (2016). Reassessing changes in diurnal temperature range: Intercomparison and evaluation of existing global data set estimates. Journal of Geophysical Research: Atmospheres, 121, 5138–5158. [Google Scholar]
- Tollarová‐Borovanská, M. , Lalouette, L. , & Koštál, V. (2009). Insect cold tolerance and repair of chill‐injury at fluctuating thermal regimes: Role of 70 kDa heat shock protein expression. CryoLetters, 30, 312–319. [PubMed] [Google Scholar]
- Tüzün, N. , & Stoks, R. (2018). Evolution of geographic variation in thermal performance curves in the face of climate change and implications for biotic interactions. Current Opinion in Insect Science, 29, 78–84. [DOI] [PubMed] [Google Scholar]
- Uno, H. , & Stillman, J. H. (2020). Lifetime eurythermy by seasonally matched thermal performance of developmental stages in an annual aquatic insect. Oecologia, 192, 647–656. [DOI] [PubMed] [Google Scholar]
- Vasseur, D. A. , DeLong, J. P. , Gilbert, B. , Greig, H. S. , Harley, C. D. G. , McCann, K. S. , Savage, V. , Tunney, T. D. , & O'Connor, M. I. (2014). Increased temperature variation poses a greater risk to species than climate warming. Proceedings of the Royal Society B, 281, 20132612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez, D. P. , Gianoli, E. , Morris, W. F. , & Bozinovic, F. (2017). Ecological and evolutionary impacts of changing climatic variability. Biological Reviews, 92, 22–42. [DOI] [PubMed] [Google Scholar]
- Viau, A. , Gajewski, K. , Sawada, M. , & Fines, P. (2006). Millennial‐scale temperature variations in North America during the Holocene. Journal of Geophysical Research: Atmospheres, 111, D9. [Google Scholar]
- Wallace, C. J. , & Osborn, T. J. (2002). Recent and future modulation of the annual cycle. Climate Research, 22, 1–11. [Google Scholar]
- Wang, G. , & Dillon, M. E. (2014). Recent geographic convergence in diurnal and annual temperature cycling flattens global thermal profiles. Nature Climate Change, 4, 988–992. [Google Scholar]
- Woods, H. A. , Dillon, M. E. , & Pincebourde, S. (2015). The roles of microclimatic diversity and of behavior in mediating the responses of ectotherms to climate change. Journal of Thermal Biology, 54, 86–97. [DOI] [PubMed] [Google Scholar]
- Woods, H. A. , Pincebourde, S. , Dillon, M. E. , & Terblanche, J. S. (2021). Extended phenotypes: Buffers or amplifiers of climate change? Trends in Ecology & Evolution, 36, 889–898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


