Abstract
Background
While the relationship between pollen and respiratory allergies is well‐documented, the role of short‐term pollen exposure in food allergy and eczema flares has not previously been explored. We aimed to investigate these associations in a population‐based sample of children.
Methods
We investigated 1‐ (n = 1108) and 6‐year‐old (n = 675) children in the grass pollen season from the HealthNuts cohort. Grass pollen concentrations were considered on the day of testing (lag 0), up to three days before (lag 1‐lag 3) and cumulatively (lag 0–3). Associations between grass pollen and food skin‐prick test reactivity (SPT ≥ 2 mm at age 1 year and ≥ 3 mm at age 6 years), eczema flares, challenge‐confirmed food allergy, reaction threshold to oral food challenges (OFC), and serum food‐specific IgE levels were analyzed using either logistic or quantile regression models. Atopy and family history of allergic disease were considered as potent effect modifiers.
Results
Grass pollen at lag 0–3 (every 20 grains/m3 increase) was associated with an up to 1.2‐fold increased odds of food SPT reactivity and eczema flares in 6‐year‐olds. In 1‐year‐olds, the associations were only observed for peanut in those with a family history of food allergy. Increasing grass pollen concentrations were associated with a lower reaction threshold to OFC and higher serum IgE levels in peanut‐allergic 1‐year‐olds only.
Conclusion
Increasing grass pollen concentration was associated with increased risk of food SPT reactivity and eczema flares in children. The associations in peanut‐allergic infants may be related to immune activation and/or peanut and grass pollen cross‐reactivity leading to a lower reaction threshold.
Keywords: eczema, food allergy, food sensitization, grass, pediatrics, pollen
Short abstract

Abbreviations
- IgE
Immunoglobulin E
- MAPCAH
Melbourne Air Pollen Children and Adolescent Health Study
- LRT
likelihood ratio test
- OFC
oral food challenge
- RAST
radioallergosorbent test
- SCORAD
SCORing Atopic Dermatitis
- SPTs
skin‐prick tests
Key Message.
This population‐based study is the first to investigate the relationship between short‐term grass pollen exposure, food skin‐prick test (SPT) reactivity, food allergy outcomes, and eczema flares. We found that persistent grass pollen exposure may trigger a general state of heightened immune responsiveness leading to increased odds of food SPT reactivity and eczema flares in children, but the impact may be greater if peanut allergy is already present. Research into SPTs and oral food challenges should consider pollen concentrations to limit residual confounding and/or effect modification, and additional caution for peanut avoidance may need to be taken for peanut‐allergic children during the grass pollen season.
1. INTRODUCTION
Globally, childhood food allergy and eczema continue to be growing public health problems. Australia is particularly affected, with approximately 10% and 25% of children under five showing food allergy and eczema, respectively. 1 , 2 Little is known about the impacts of outdoor pollen on these conditions. Our systematic review showed that short‐term outdoor pollen exposure can enhance type‐2 immune responses such as immunoglobulin E (IgE), mast cell, and eosinophil production in the airways, especially among those with asthma and/or seasonal allergic rhinitis. 3 Although this is the current evidence, investigating further the adverse effects of outdoor pollen within the community is warranted because thunderstorm asthma events showed that people with no known asthma were also susceptible. 4 , 5 The priming hypothesis indicates that increased sensitivity towards pollen is possible with persistent exposure. 6 Therefore, we hypothesized that high pollen exposure may result in an inflammatory response that may lower the threshold for manifestation of other allergic diseases such as food allergy and eczema, and this may include those without pre‐existing respiratory conditions or known grass pollen sensitization.
There is mounting evidence that pollen exposure may be associated with eczema and food allergy. Direct application of grass pollen onto the skin has been shown to induce eczematous lesions. 7 , 8 Krämer et al. 9 also demonstrated that children with eczema experienced more symptoms on days with high grass pollen counts. Previously, studies including our own have found that exposure to grass pollen in utero and the first few months of life was associated with food sensitization and allergy. 10 , 11 , 12 We have also recently demonstrated that increased residential greenness exposure was associated with an increased risk of challenge‐confirmed peanut and egg allergy in infants, and this could be related to the higher pollen concentrations. 13 Antibody cross‐reactivity between a specific pollen (e.g. grass) and plant‐based foods that contain similar proteins (e.g. Ara h 9 of peanut) can also occur resulting in the pollen‐food allergy syndrome. 14 , 15 , 16 Hence, outdoor pollen exposure may be linked to broad priming across all food allergens or limited to known cross‐reactive food allergens only.
Here, we aimed to determine whether outdoor grass pollen concentration was associated with food skin‐prick test (SPT) reactivity, food allergy, and eczema flares in children aged 1 and 6 years, and their potential modifiers. Additionally, we also investigated whether grass pollen concentration was associated with oral food challenge (OFC) outcomes and/or serum food‐specific IgE levels in food allergic children. We have focused on grass pollen a priori because our study population consisted of children who were based in Melbourne, Australia, and grass pollen was the most abundant aeroallergen in the city. 17
2. METHODS
2.1. Study sample
The study sample consisted of participants from the HealthNuts cohort. 18 The study methodology is detailed elsewhere, 19 but briefly, a total of 5276 1‐year‐old infants were recruited at local council‐run immunization clinics across Melbourne, Australia, from 2007 to 2011, and underwent skin‐prick testing (SPT) to food allergens. A follow‐up was conducted at age 6 years (2013–2016) where 3233 children underwent clinical examinations. This clinic visit included SPT to food and pollen extracts, as well as eczema assessment. At both follow‐ups, participants with a food SPT ≥ 1 mm or recent history of food allergy were offered OFC. Those with food allergy were also offered blood draw for serum food‐specific IgE.
The present work was restricted to participants who underwent testing during the Melbourne grass pollen season (October to January). To investigate any potential selection/participation bias, we have compared the characteristics of participants tested during the grass pollen season vs. out of the grass pollen season (Table 1). By excluding participants outside of the pollen season, we reduced possible bias of the estimates toward the null as the time of observation and of potential grass pollen exposure would not have coincided. 20 Nonetheless, a separate analysis was conducted involving all participants, where grass pollen season of testing was investigated as an exposure.
TABLE 1.
Characteristics of the HealthNuts participants
| Variables | 1‐year cohort | 6‐year cohort | |||||
|---|---|---|---|---|---|---|---|
| Present study sample | p‐value | Present study sample | p‐value | ||||
| Tested outside of grass pollen season a | Tested during the grass pollen season (Oct‐Jan) a | Tested outside of grass pollen season | Tested during the grass pollen season (Oct‐Dec) a | ||||
| N (Total) | 2013 | 1108 | – | 2551 | 675 | – | |
| Male sex | 990 (49.2%) | 573 (51.7%) | .22 | 1325 (51.9%) | 356 (52.7%) | .69 | |
| Recent history of eczema b | 762 (37.9%) | 411 (37.1%) | .67 | 554 (21.7%) | 160 (23.7%) | .38 | |
| Eczema in the past 12 months c | 602 (29.9%) | 326 (29.4%) | .82 | 400 (15.7%) | 121 (17.9%) | .21 | |
| Ever wheeze | 315 (15.6%) | 179 (16.2%) | .57 | – | – | – | |
| Current asthma d | – | – | – | 333 (13.1%) | 97 (14.4%) | .43 | |
| Current hay fever e | – | – | – | 348 (13.6%) | 116 (17.2%) | .02 | |
| Family history of food allergy | 204 (10.1%) | 137 (12.4%) | .05 | 173 (6.8%) | 55 (8.1%) | .12 | |
| Family history of eczema | 600 (29.8%) | 348 (31.4%) | .35 | 493 (19.3%) | 145 (21.5%) | .09 | |
| Grass pollen sensitized g | – | – | – | 235 (9.2%) | 104 (15.4%) | <.001 | |
| SEIFA quintiles f | Least disadvantaged | 419 (20.8%) | 211 (19.0%) | .62 | 499 (19.6%) | 107 (15.9%) | .25 |
| 2 | 383 (19.0%) | 211 (19.0%) | 505 (19.8%) | 139 (20.6%) | |||
| 3 | 426 (21.2%) | 258 (23.3%) | 546 (21.4%) | 159 (23.6%) | |||
| 4 | 408 (20.3%) | 220 (19.9%) | 498 (19.5%) | 137 (20.3%) | |||
| Most disadvantaged | 371 (18.4%) | 204 (18.4%) | 501 (19.6%) | 131 (19.4%) | |||
| SPT reactivity g | Peanut | 146 (7.3%) | 83 (7.5%) | .81 | 152 (6.0%) | 52 (7.7%) | .12 |
| Cashew | – | – | – | 106 (4.2%) | 40 (5.9%) | .06 | |
| Hazelnut | – | – | – | 68 (2.7%) | 24 (3.6%) | .24 | |
| Almond | – | – | – | 57 (2.2%) | 17 (2.5%) | .70 | |
| Sesame | 31 (1.5%) | 25 (2.3%) | .15 | 17 (0.7%) | 17 (2.5%) | <.001 | |
| Egg | 260 (12.9%) | 149 (13.4%) | .67 | 58 (2.3%) | 17 (2.5%) | .76 | |
| Milk | 34 (1.7%) | 23 (2.1%) | .21 | 15 (0.6%) | 2 (0.3%) | .34 | |
Note: The 1‐year cohort in and outside of grass pollen season do not add up to n = 5,276 (at recruitment) because some participants were recruited before pollen data were available (i.e. prior to the MAPCAH study which commenced in September 2009). The 6‐year cohort in and outside of grass pollen season do not add up to n = 3,233 because some children did not have their date of assessment recorded. Participants tested in and out of grass pollen season were compared using chi‐squared tests. – = Information not collected.
Sample with pollen data available.
Recent history of eczema= Nurse‐observed eczema or history of parent‐reported doctor‐diagnosed eczema/itchy rash that had been treated with topical steroids (age 1 year); Non‐zero SCORAD score or parent‐reported itchy rash that had affected typical locati.
Eczema in the past 12 months = History of parent‐reported doctor‐diagnosed eczema/itchy rash that had been treated with topical steroids (age 1 year); Parent‐reported itchy rash that had affected typical locations related to eczema such as knees and folds of elbows in the past 12 months (age 6 years).
Current asthma= Parent‐reported doctor‐diagnosed asthma plus either wheeze/asthma medication use in the past 12 months (age 6 years).
Current hay fever= Sneezing/runny/blocked nose plus itchy eyes without cold/flu in the past 12 months (age 6 years).
SEIFA= Socioeconomic status was defined using the Socio‐Economic Indexes for Areas (SEIFA) measures, which was based on the participants’ home postcodes.
SPT reactivity/sensitised = SPT ≥ 2 mm (age 1 year); SPT ≥ 3 mm (age 6 years).
2.2. Ethics
Ethics approval was obtained from the Victorian Department of Human Services (reference no. 10/07), the Office for Children (reference no. CDF/07/492), and the Royal Children's Hospital Human Research Ethics Committee (reference no. 27047 & no. 32294). The parents provided written informed consent on behalf of their children.
2.3. Outcome collection and definitions
The study samples varied depending on the outcome and are defined in Table S1.
2.3.1. Food SPT reactivity
SPTs were conducted by placing a single drop of the allergen extract (ALK‐Abelló, Spain at age 1 year; ALK‐Abelló, Spain, Hollister‐Stier, USA, and Stallergènes, France, at age 6 years) on the infant's or child's back and then pricked with a lancet (Stallergènes, France). Positive and negative controls were histamine (10 mg/ml) and saline, respectively.
Age 1 – SPT reactivity was defined as wheal ≥ 2 mm, which is the common cut‐off used in young infants. 21 , 22 A sensitivity analysis was conducted using the 3 mm cut‐off, and no significant differences were found (data not shown). The food allergens considered were peanut, egg, sesame, and cow's milk.
Age 6 – SPT reactivity was defined as wheal ≥ 3 mm. Food allergens considered were peanut, egg, sesame, cashew, hazelnut, and almond.
2.3.2. Serum food‐specific IgE
Only those with food allergy were offered blood draw for serum food‐specific IgE. We collected blood samples and separated the child's plasma for serum IgE assays on the same day. The serum food‐specific IgE antibodies were then analyzed using ImmunoCAP System FEIA (Phadia AB, Uppsala, Sweden). 23 We only had sufficient sample size to assess this outcome in 1‐year‐old peanut or egg allergic infants. An infant was considered allergic if he or she had (i) a positive OFC (at least three non‐contact urticaria lasting >5 min, angioedema, vomiting, or anaphylaxis within two hours of last dose 2 ), or a history of a reaction (consistent with OFC stopping criteria) in the past month for egg and 2 months for peanut plus; (ii) a positive SPT (≥ 2 mm) and/or a positive in vitro test defined as ≥ 0.35kUA/L. 24 , 25
2.3.3. Food allergy outcomes
The method of OFC administration was described elsewhere. 18 , 19 We investigated (i) the presence of challenge‐confirmed food allergy, defined by a positive OFC, and (ii) the reaction threshold to OFC, defined as the cumulative total dose (ml) given to a child before a reaction occurred.
Age 1 – The food allergens considered were peanut and egg.
Age 6 – We only had sufficient power to investigate the presence of challenge‐confirmed allergy to peanut.
2.3.4. Eczema flares on the day of assessment
Age 1 – This was determined by nurses who have undergone online SCORing Atopic Dermatitis (SCORAD) training photo tutorials on different appearances of eczema and skin pigmentation. During a participant's assessment, the nurses were asked to identify cardinal signs of eczema, such as excoriation, erythema, edema/papulation, vesiculation, and lichenification and used clinical judgment (e.g. erythema on the cheeks from crying). 26
Age 6 –This was defined as having a non‐zero SCORAD 27 total score (based on area affected, intensity, and subjective symptoms 28 ).
2.4. Grass pollen data collection
A Burkard volumetric trap was used to collect daily grass pollen. This single trap is a good proxy for exposure for those living up to 50 km from the site. 29 All participants lived within this radius, and the clinical testing site was within a short distance of the trap. The methods of data collection were described elsewhere. 10 , 30 This present work examined grass pollen concentrations on the day of health examination (lag 0) and up to three days before (lag 1, lag 2, lag 3) for each participant. This was because of previous evidence of a lagged association (up to 3 days) between pollen and asthma. 30 , 31 We also considered each participant's cumulative grass pollen exposure over the 4 days (lag 0–3).
Age 1 – Grass pollen data were obtained from the Melbourne Air Pollen Children and Adolescent Health (MAPCAH) study. 32 Only data collected during the grass pollen season (October to January) were utilized in this present study.
Age 6 – Grass pollen data were obtained directly from the School of Biosciences. However, data were only available in October to December.
2.5. Potential confounders: weather
Daily temperature and humidity data were obtained from the Bureau of Meteorology. We considered temperature and humidity as potential confounders because they could influence the concentrations of allergenic pollens, the timing of pollen seasons, and other allergic diseases such as asthma and eczema exacerbations. 33 , 34 , 35 , 36
2.6. Potential modifiers: allergic disease and genetic susceptibility
Given allergic disease 37 , 38 , 39 and genetic susceptibility 40 , 41 could modify the relationships between risk factors and food SPT reactivity and/or eczema, we investigated potential interactions between grass pollen at all lags and recent history of eczema, wheeze, food sensitization, asthma, hay fever, grass pollen sensitization, and family history of either food allergy or eczema, depending on the outcome. These potential modifiers are defined in Table S2.
2.7. Statistical analysis
The associations between daily grass pollen concentrations (continuous), food SPT reactivity, challenge‐confirmed food allergy, and eczema flares were assessed using logistic regression models. Likelihood ratio tests (LRTs) comparing general additive 42 linear models to non‐linear models indicated that the linear models were appropriate to assess the above associations. Pooled analyses of data from both follow‐ups were not conducted, because there was evidence that the associations differed across the two follow‐ups (p‐value for interaction <.1). Interactions were explored separately using LRTs.
Meanwhile, the associations between grass pollen concentrations, the reaction threshold to OFC, and serum food‐specific IgE levels were assessed using quantile regression models for the median. We chose to model median rather than mean outcome due to the highly skewed distributions of these outcomes and the model residuals not meeting ordinary linear regression assumptions. LRTs indicated that the associations with reaction threshold to OFC and serum food‐specific IgE levels were best fit with non‐linear and linear, respectively.
Where the associations were linear, we interpreted the results as the estimated effect size per IQR (i.e. 29 grains/m3 at the 1‐year follow‐up; 20 grains/m3 at the 6‐year follow‐up) increase in grass pollen. This interpretation using the IQR is commonly used in studies investigating continuous environmental variables and adverse outcomes. 43 , 44 Food SPT reactivity and allergy models were not adjusted for temperature and humidity because there was limited understanding of the relationship and sensitivity analyses indicated little to no change in the effect size or statistical significance between the univariate (Table 2) and multivariate models (Table S3). For the eczema model, temperature and humidity were adjusted for as a priori confounders. 36
TABLE 2.
Associations between grass pollen concentrations at all lags and food SPT reactivity and eczema flares on the day of assessment in 6‐year‐old children
| Outcomes | Lag 0 | Lag 1 | Lag 2 | Lag 3 | Lag 0–3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p‐value | OR (95% CI) | p‐value | OR (95% CI) | p‐value | OR (95% CI) | p‐value | OR (95% CI) | p‐value | |
| SPT reactivity | ||||||||||
| Peanut | 1.0 (0.8, 1.5) | .64 | 0.9 (0.7, 1.2) | .65 | 1.0 (0.8, 1.8) | .63 | 0.9 (0.7, 1.2) | .66 | 1.17 (1.0, 1.3) | .002 |
| Cashew | 1.0 (0.7, 1.5) | .76 | 0.9 (0.7, 1.5) | .76 | 0.9 (0.5, 1.5) | .80 | 1.0 (0.7, 1.5) | .86 | 1.13 (1.0, 1.24) | .036 |
| Hazelnut | 0.9 (0.5, 1.5) | .81 | 1.2 (0.7, 1.8) | .55 | 1.2 (0.7, 2.2) | .65 | 0.8 (0.5, 1.5) | .50 | 1.15 (1.0, 1.35) | .048 |
| Almond | 0.9 (0.5, 1.5) | .80 | 0.8 (0.4, 1.5) | .66 | 1.5 (0.7, 2.7) | .33 | 0.96 (0.5, 1.5) | .87 | 1.17 (1.0, 1.37) | .047 |
| Egg | 1.0 (0.7, 1.8) | .77 | 0.98 (0.5, 1.8) | .94 | 1.2 (0.7, 2.2) | .52 | 0.8 (0.4, 1.5) | .48 | 1.22 (1.0, 1.43) | .01 |
| Sesame | 1.2 (0.7, 1.8) | .67 | 1.0 (0.5, 2.2) | .77 | 1.2 (0.5, 2.2) | .74 | 0.8 (0.4, 1.5) | .36 | 1.17 (1.0, 1.4) | .048 |
| Eczema flares | 1.2 (0.8, 1.8) | .22 | 0.7 (0.5, 1.0) | .10 | 0.7 (0.4, 1.2) | .14 | 1.2 (0.96, 1.8) | .08 | 1.13 (1.0, 1.22) | .02 |
Note: No confounders were adjusted for in the food SPT reactivity models. Temperature and humidity were adjusted for in the eczema models. Results were interpreted as the effect size per IQR (20 grains/m3) increase in grass pollen.
A separate second set of analyses was performed involving the whole cohort, where the associations between grass pollen season of testing (categorical) and the outcomes were investigated at age 1 year (n = 5276) and age 6 years (n = 3226), separately. Here, the results were interpreted as the estimated effect size in those tested in the grass pollen season compared to those tested outside of the grass pollen season.
All statistical analyses were carried out in Stata IC 16.1 (College Station, TX: StataCorp LLC) for the logistic regressions and R for the general additive 42 and quantile regressions. 45
3. RESULTS
3.1. Sample and environmental characteristics
In total, 1108 1‐year‐old infants and 675 6‐year‐old children participated during the grass pollen seasons and were included in the primary analyses. Most socio‐demographic characteristics were similar to participants outside of the grass pollen seasons (Table 1). The summary statistics for grass pollen, temperature, and humidity are presented in Table S4. At each follow‐up, there were 40 high pollen days (≥ 50 grains/m3). 46
Summary of the findings can be found in Table S1.
3.2. Food SPT reactivity
Age 1 – There was no evidence of an overall association, but interactions were observed with family history of food allergy for peanut (p‐int = 0.03). In infants with a family history of food allergy, grass pollen concentrations were associated with a 1.3‐fold increased odds of peanut SPT reactivity at lag 0 (95% CI = 1.0, 2.4) (Table S5).
Age 6 – Grass pollen at lag 0–3 was associated with an up to 1.2‐fold increased odds of SPT reactivity to peanut (p = .002), cashew (p = .036), hazelnut (p = .048), almond (p = .047), egg (p = .01), and sesame (p = .048) (Table 2). There was no consistent evidence of interactions with any of the potential effect modifiers. Stratified analyses showed that the associations were not restricted to or amplified in grass pollen‐sensitized children, but slightly higher risks were observed for non‐grass pollen‐sensitized children (Table S6).
3.3. Serum food‐specific IgE
Age 1 – Grass pollen concentrations were associated with a higher median serum food‐specific IgE levels in peanut‐allergic infants at lag 1 (Coef = 5.8 [95% CI = 0.3, 12] kUA/L; p = .04) and lag 0–3 (Coef = 1.2 [95% CI = 0.6, 2.0] kUA/L; p = .001) (Table S7), but no evidence was found in egg‐allergic infants (data not shown).
3.4. Food allergy outcomes
Age 1 – There was no evidence of an association between grass pollen concentrations and challenge‐confirmed peanut or egg allergy (data not shown).
In peanut‐allergic infants (n = 27), grass pollen concentrations at lag 0 (p < 0.001) were non‐linearly associated with a lower median reaction threshold to OFC (Figure 1). There was some evidence at lag 3, but the direction of association was inconsistent. No evidence of an association was found in egg‐allergic infants.
FIGURE 1.
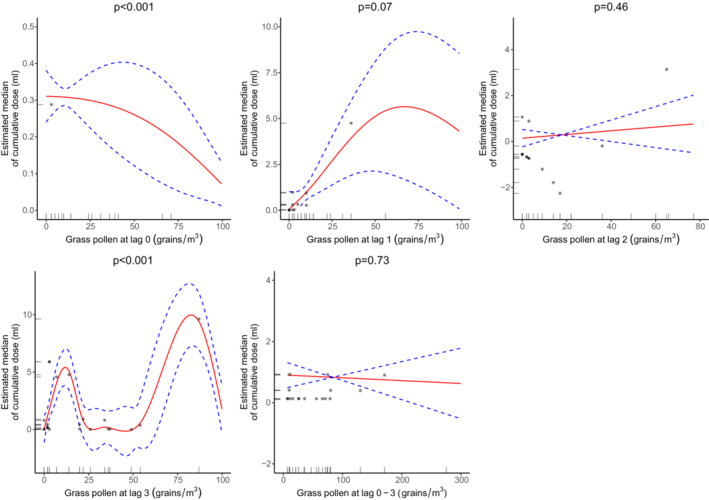
The associations between grass pollen concentrations at all lags and the estimated median of cumulative dose (ml) ingested by 1‐year‐old infants before they had a reaction to OFC with peanut (n = 27). The dotted blue lines represent the 95% confidence intervals, while the black dots represent the observations
Age 6 – There was no evidence of an association between grass pollen concentrations and challenge‐confirmed peanut allergy (data not shown).
3.5. Eczema on the day of assessment
Age 1 – There was no evidence of an association between grass pollen concentrations and eczema flares nor was there consistent evidence of interactions with any of the potential effect modifiers. Stratified analyses indicated that the association was not restricted to or amplified in children with reported eczema in the past 12 months (Table S8).
Age 6 – Grass pollen at lag 0–3 was associated with a 1.13‐fold increased odds of eczema flares (p = .02) (Table 2). There was no consistent evidence of interactions with any of the potential effect modifiers, and stratified analyses indicated that the association was not restricted to or amplified in children with reported eczema in the past 12 months or grass pollen sensitization (Table S9).
3.6. Pollen season of testing as an exposure for all outcomes
The results are presented in Tables S10–S12.
Age 1 – There was no evidence of an association between pollen season of testing and all outcomes.
Age 6 – Participants in the grass pollen season had a relatively higher odds of food SPT reactivity compared to participants outside of grass pollen season. However, the association was only significant for sesame (p < .001). No evidence of an association was found with the other outcomes.
4. DISCUSSION
Overall, we found cumulative exposure to grass pollen over 4 days to be associated with an up to 1.2‐fold increased odds of SPT reactivity to all food allergens and eczema flares in 6‐year‐olds. In 1‐year‐old infants, an association was only observed with peanut in those with a family history of food allergy. We also found grass pollen concentrations to be associated with a higher serum food‐specific IgE levels and lower reaction threshold to OFC in peanut‐allergic 1‐year‐olds. For clinical relevance, we have used the common cut‐offs of SPT ≥ 2 mm at age 1 year and SPT ≥ 3 mm at age 6 years to define food SPT reactivity, but this does not imply that short‐term grass pollen exposure could lead to the development of new food sensitization in children.
It is well‐known that exposure to high ambient grass pollen enhances mast cell activity, 47 so we speculate that this could explain why we observed increased odds of food SPT reactivity or eczema flares in 6‐year‐old children. Conversely, the associations between grass pollen concentration and food SPT reactivity were not restricted to or amplified in children with grass pollen sensitization. Similarly, the associations with eczema flares were not limited to children with reported eczema in the past 12 months or grass pollen sensitization. There could be several reasons for these unexpected findings. Firstly, there may have been small sample bias or residual confounding for which we were unable to control for. Plausibly, increased awareness in parents of children with allergic disease may have led to adoption of preventive measures during the pollen season, such as limiting time spent outdoors, utilizing asthma, hay fever, or eczema medications (which may not have been entirely captured at testing), using HEPA filters and/or closing windows to reduce the burden of exposure. Our recently published study of the same cohort also showed no evidence of an association between daily grass pollen concentrations and lung function changes in children sensitized to ryegrass and/or Bermuda grass. 48 Limited data on other grass pollen sensitization may have led to misclassification and subsequently influenced the associations observed in this cohort. Also, lack of data on serum allergen‐specific IgE for grass pollen did not allow investigation into the nuances that may be provided by the quantitative nature of this measure.
Furthermore, we observed the risk of food SPT reactivity at cumulative lag 0 to 3 to be higher in non‐grass pollen‐sensitized children at age 6 years. Evidence shows that people are more likely to become sensitized to inhalant allergens over time, 49 , 50 so we may have studied children who were in the process of becoming pollen sensitized. In terms of the potential mechanisms that could trigger pollen‐induced immune responses in non‐sensitized participants, we hypothesize that persistent grass pollen exposure may lead to a general state of heightened immune responsiveness. Experimental studies have shown that pollen can trigger both the innate and adaptive immune responses in allergic and non‐allergic people. 51 For example, pollen grains have non‐allergenic properties; they can release pro‐inflammatory eicosanoid‐like substances such as bioactive lipid mediators, upon contact with respiratory or mucous membranes. 51 These lipid mediators can activate polymorphonuclear leukocytes independent of sensitization status. 52 Studies involving other allergens such as house dust mite, cat and dog, have reported that exposure to high levels of these domestic allergens can significantly increase non‐specific airway reactivity and trigger production of antibody responses in people who were not sensitized to them. 53 , 54 , 55 We believe that the impact of pollen on health is broader than just the induction of allergy. To support this, pollen has also been shown to be associated with non‐allergic diseases, such as chronic obstructive pulmonary disease, cardiovascular disease, and pneumonia. 56 This is akin to air pollution effects, suggesting that particles of biological origin can have similar health impacts. Nevertheless, more studies are required to replicate our findings.
It is not clear why we found fewer associations in 1‐year‐olds. We postulate that young infants have lower SPT reactivity, spend less time outdoors with less opportunity for grass pollen exposure, and/or had their eczema well‐controlled at the time of observation. Nonetheless, we did observe the main findings on eczema flares and food SPT reactivity to be in line at both follow‐ups. Furthermore, we found grass pollen concentrations to be associated with an increased odds of peanut SPT reactivity in infants with a family history of food allergy. This indicates that genetic susceptibility and/or shared household/lifestyle factors may accentuate the effects of grass pollen on the immune system in infants. However, it is important to note that these results were based on a small subgroup, and without pollen sensitization data available in this age group, it was difficult to untangle the relationship.
Moreover, grass pollen concentration was not associated with the presence of challenge‐confirmed food allergy, indicating that while persistent grass pollen exposure may trigger an immune response leading to increased odds of food SPT reactivity, it may not necessarily lead to increased odds of food allergy. Alternatively, there could be lack of power to detect associations as some participants with probable food allergy chose not to undergo OFCs. Nonetheless, infants with definite peanut allergy had a lower reaction threshold to OFC and higher serum food‐specific IgE levels with increased grass pollen concentrations. This suggests that grass pollen exposure may trigger type‐2 immune responses through immune activation and/or peanut and grass pollen cross‐reactivity, but only if peanut allergy was already present. However, more research is needed because the associations with the reaction threshold to OFC outcome were inconsistent, and the confidence intervals were wide. This could be due to the small number of observations and/or that the outcome only had a few distinct values, which would negatively affect any model fit. In addition, we only had data for IgE and not T cell subsets or cytokines. No evidence of an association was found between grass pollen concentrations and OFC outcomes in egg‐allergic infants, indicating that the reaction to non‐cross‐reactive allergens or non‐plant‐derived foods may be milder, but more research is warranted to support this hypothesis.
4.1. Strengths and limitations
This population‐based study is the first to investigate the relationship between short‐term grass pollen exposure, food SPT reactivity, food allergy outcomes, and eczema flares. Validated questionnaires and gold standard measurements were utilized, limiting measurement bias. Furthermore, investigation into clinically relevant outcomes and potential effect modifiers was possible due to the range of data collected in this study.
However, some study limitations need to be acknowledged. Our cross‐sectional study design does not allow us to infer causal relationships. Although repeated measures before and after high pollen exposure could strengthen the conclusions, it would not completely address the causal question because there are likely to be other environmental differences. Nonetheless, our findings generated novel hypotheses that could be tested in future studies. Additionally, grass pollen was only measured at a single site, and this may not reflect individual‐level exposure. Furthermore, data on T‐cell subsets, cytokines, and sensitization to peanut allergen components were also not collected, and this limited outcome assessment. We also could not comprehensively investigate the role of grass pollen sensitization or allergy in the observed associations because SPTs to grass pollen were not performed at age 1 year and data on serum allergen‐specific IgE levels for grass pollen were not collected at ages 1 and 6 years. Furthermore, SPTs were only performed for ryegrass and Bermuda grass, not other grass pollen species. Moreover, we had limited power to assess important outcomes such as the severity of OFC reactions (e.g. anaphylaxis), reaction thresholds to OFC and food‐specific IgE levels in 6‐year‐olds, severity of SCORAD‐based eczema, and mutually exclusive food sensitization groups, for example SPT reactivity to egg but not peanut. Lastly, there were some inconsistencies in the results between pollen season of testing and daily grass pollen concentrations as the exposures, but daily grass pollen concentrations correspond to a more accurate representation of exposure as the magnitude of pollen exposures may vary between the years and therefore, may not be directly comparable to pollen season of testing. 3
In conclusion, persistent grass pollen exposure may trigger dysregulation of immune responses leading to increased odds of food SPT reactivity and eczema flares in children, but the impact on food may be greater if peanut allergy is already present. These findings suggest the need for future research to better understand the physiological mechanisms that take place in the days following high grass pollen exposure. If these findings are confirmed in other studies, additional caution for peanut avoidance may be necessary for peanut‐allergic children during the grass pollen season to reduce the burden of this exposure. Investigations involving SPT, OFC, food‐specific IgE levels, and eczema flares in children may also need to consider pollen concentrations to minimize residual confounding and/or effect modification on observed associations.
AUTHOR CONTRIBUTIONS
Nur Sabrina Idrose: Formal analysis (lead); investigation (lead); writing – original draft (lead); writing – review and editing (lead). Caroline J Lodge: Conceptualization (equal); supervision (equal); writing – review and editing (equal). Rachel Louise Peters: Data curation (equal); funding acquisition (equal); resources (equal); writing – review and editing (equal). Jo Douglass: Supervision (equal); writing – review and editing (equal). Jennifer Julia Koplin: Data curation (equal); funding acquisition (equal); resources (equal); supervision (equal); writing – review and editing (equal). Adrian J Lowe: Data curation (equal); funding acquisition (equal); resources (equal); writing – review and editing (equal). Kirsten Perrett: Data curation (equal); funding acquisition (equal); resources (equal); writing – review and editing (equal). Mimi Tang: Data curation (equal); funding acquisition (equal); resources (equal); writing – review and editing (equal). Ed Newbigin: Data curation (equal); funding acquisition (equal); resources (equal); writing – review and editing (equal). Michael Abramson: Data curation (equal); funding acquisition (equal); resources (equal); writing – review and editing (equal). Bircan Erbas: Data curation (equal); funding acquisition (equal); resources (equal); writing – review and editing (equal). Don Vicendese: Conceptualization (equal); formal analysis (equal); investigation (equal); supervision (equal); writing – review and editing (equal). Shyamali C Dharmage: Conceptualization (lead); data curation (equal); funding acquisition (equal); resources (equal); supervision (lead); writing – review and editing (equal).
FUNDING INFORMATION
NSI is supported by the Centre for Food and Allergy Research (NHMRC Centre of Research Excellence) PhD scholarship and the Melbourne Children's LifeCourse top‐up PhD scholarship (Royal Children's Hospital Foundation grant #2018–984). SCD, RLP, and AJL are supported by the Australian National Health and Medical Research Council (NHMRC). CJL is supported by a University of Melbourne Developing Research Momentum grant. The HealthNuts study was funded by the National Health & Medical Research Council (NHMRC GNT 491233 and 1,006,215) of Australia, the Ilhan Food Allergy Foundation, AnaphylaxiStop, the Charles and Sylvia Viertel Medical Research Foundation and the Victorian Government's Operational Infrastructure Support Program.
CONFLICTS OF INTEREST
MLKT has received speaker fees from Nestle Health Science, is a consultant to Bayer Pharmaceuticals, and has received research funding from NHMRC, Bayer Pharmaceuticals, Abbot Nutrition, and Prota Therapeutics. JAD has received honoraria for educational presentations from Astra‐Zeneca, GSK, Novartis, Alphapharm, Shire, and CSL. She has served on advisory boards: Sanofi‐Aventis, Novartis, GSK, Astra‐Zeneca, Shire, Immunosis, and CSL. She has also undertaken contracted or investigator‐initiated research on behalf of GSK, Novartis, Immunosis, Astra‐Zeneca, Sanofi‐Aventis, Grifols, CSL, BioCryst & Equilium. She has a personal superannuation shareholding in CSL. MJA holds investigator‐initiated grants from Pfizer and Boehringer‐Ingelheim for unrelated research. He has undertaken an unrelated consultancy for and received assistance with conference attendance from Sanofi. He has also received a speaker's fee from GSK. SCD, AJL & CJL have received an investigator‐initiated grant from GSK for unrelated work. Other authors have no conflicts to disclose.
5.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pai.13862.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
We thank the children and parents who participated in the HealthNuts Study as well as the staff of Melbourne's Local Government Areas for access to community Immunization Clinics. We thank ALK Abello, S.A. Madrid, Spain, Hollister‐Stier, USA, and Stallergènes, France, for supplying the SPT reagents and the HealthNuts safety committee: Associate Professor Noel Cranswick (Australian Pediatric Pharmacology Research Unit, Murdoch Children's Research Institute), Dr Jo Smart (Department of Allergy and Immunology, Royal Children's Hospital, Melbourne, Australia), and Professor Jo Douglass (Director, Department of Allergy and Immunology, Royal Melbourne Hospital, Melbourne, Australia). Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
Idrose NS, Lodge CJ, Peters RL, et al. The role of short‐term grass pollen exposure in food skin‐prick test reactivity, food allergy, and eczema flares in children. Pediatr Allergy Immunol. 2022;33:e13862. doi: 10.1111/pai.13862
Don Vicendese and Shyamali C. Dharmage equal senior authors.
Editor: Motohiro Ebisawa
This article is commented on by Motohiro Ebisawa and Philippe Eigenmann. To view this editorial comment visit https://onlinelibrary.wiley.com/doi/10.1111/pai.13866.
REFERENCES
- 1. Tang ML, Mullins RJ. Food allergy: is prevalence increasing? Intern Med J. 2017;47(3):256‐261. [DOI] [PubMed] [Google Scholar]
- 2. Peters RL, Koplin JJ, Gurrin LC, et al. The prevalence of food allergy and other allergic diseases in early childhood in a population‐based study: HealthNuts age 4‐year follow‐up. J Allergy Clin Immunol. 2017;140(1):145‐53.e8. [DOI] [PubMed] [Google Scholar]
- 3. Idrose NS, Walters EH, Zhang J, et al. Outdoor pollen‐related changes in lung function and markers of airway inflammation: a systematic review and meta‐analysis. Clin Exp Allergy. 2021;51:636‐653. [DOI] [PubMed] [Google Scholar]
- 4. Idrose NS, Dharmage SC, Lowe AJ, et al. A systematic review of the role of grass pollen and fungi in thunderstorm asthma. Environ Res. 2020;181:108911. [DOI] [PubMed] [Google Scholar]
- 5. Thien F, Beggs PJ, Csutoros D, et al. The Melbourne epidemic thunderstorm asthma event 2016: an investigation of environmental triggers, effect on health services, and patient risk factors. Lancet Planet Health. 2018;2(6):e255‐e263. [DOI] [PubMed] [Google Scholar]
- 6. Andersson M, Svensson C, Andersson P, Pipkorn U. Objective monitoring of the allergic inflammatory response of the nasal mucosa in patients with hay fever during natural allergen exposure. Am Rev Respir Dis. 1989;139(4):911‐914. [DOI] [PubMed] [Google Scholar]
- 7. Darsow U, Vieluf D, Ring J. Evaluating the relevance of aeroallergen sensitization in atopic eczema with the atopy patch test: a randomized, double‐blind multicenter study. Atopy patch test study group. J Am Acad Dermatol. 1999;40(2 Pt 1):187‐193. [DOI] [PubMed] [Google Scholar]
- 8. Ring J, Darsow U, Behrendt H. Role of aeroallergens in atopic eczema: proof of concept with the atopy patch test. J Am Acad Dermatol. 2001;45(1 Suppl):S49‐S52. [DOI] [PubMed] [Google Scholar]
- 9. Krämer U, Weidinger S, Darsow U, Möhrenschlager M, Ring J, Behrendt H. Seasonality in symptom severity influenced by temperature or grass pollen: results of a panel study in children with eczema. J Invest Dermatol. 2005;124(3):514‐523. [DOI] [PubMed] [Google Scholar]
- 10. Susanto NH, Lowe AJ, Salim A, et al. Associations between grass pollen exposures in utero and in early life with food allergy in 12‐month‐old infants. Int J Environ Health Res. 2020;32(4):1‐11. [DOI] [PubMed] [Google Scholar]
- 11. Tanaka K, Matsui T, Sato A, et al. The relationship between the season of birth and early‐onset food allergies in children. Pediatr Allergy Immunol. 2015;26(7):607‐613. [DOI] [PubMed] [Google Scholar]
- 12. Pyrhönen K, Läärä E, Hiltunen L, Kaila M, Hugg T, Näyhä S. Season of the first trimester of pregnancy predicts sensitisation to food allergens in childhood: a population‐based cohort study from Finland. J Epidemiol Community Health. 2012;66(1):49‐56. [DOI] [PubMed] [Google Scholar]
- 13. Peters RL, Sutherland D, Dharmage SC, et al. The association between environmental greenness and the risk of food allergy: a population‐based study in Melbourne, Australia. Pediatr Allergy Immunol. 2022;33(2):e13749. [DOI] [PubMed] [Google Scholar]
- 14. Arkwright PD, Summers CW, Riley BJ, Alsediq N, Pumphrey RSH. IgE sensitization to the nonspecific lipid‐transfer protein Ara h 9 and Peanut‐associated bronchospasm. Biomed Res Int. 2013;2013:746507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vereda A, van Hage M, Ahlstedt S, et al. Peanut allergy: clinical and immunologic differences among patients from 3 different geographic regions. J Allergy Clin Immunol. 2011;127(3):603‐607. [DOI] [PubMed] [Google Scholar]
- 16. Brown CE, Katelaris CH. The prevalence of the oral allergy syndrome and pollen‐food syndrome in an atopic paediatric population in south‐West Sydney. J Paediatr Child Health. 2014;50(10):795‐800. [DOI] [PubMed] [Google Scholar]
- 17. Erbas B, Chang JH, Dharmage S, et al. Do levels of airborne grass pollen influence asthma hospital admissions? Clin Exp Allergy. 2007;37(11):1641‐1647. [DOI] [PubMed] [Google Scholar]
- 18. Koplin JJ, Wake M, Dharmage SC, et al. Cohort profile: the HealthNuts study: population prevalence and environmental/genetic predictors of food allergy. Int J Epidemiol. 2015;44(4):1161‐1171. [DOI] [PubMed] [Google Scholar]
- 19. Osborne NJ, Koplin JJ, Martin PE, et al. Prevalence of challenge‐proven IgE‐mediated food allergy using population‐based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127(3):668‐676.e2. [DOI] [PubMed] [Google Scholar]
- 20. Hernán MA, McAdams M, McGrath N, Lanoy E, Costagliola D. Observation plans in longitudinal studies with time‐varying treatments. Stat Methods Med Res. 2009;18(1):27‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peters RL, Allen KJ, Dharmage SC, et al. Differential factors associated with challenge‐proven food allergy phenotypes in a population cohort of infants: a latent class analysis. Clin Exp Allergy. 2015;45(5):953‐963. [DOI] [PubMed] [Google Scholar]
- 22. Ménardo JL, Bousquet J, Rodière M, Astruc J, Michel F‐B. Skin test reactivity in infancy. J Allergy Clin Immunol. 1985;75(6):646‐651. [DOI] [PubMed] [Google Scholar]
- 23. Peters RL, Allen KJ, Dharmage SC, et al. Skin prick test responses and allergen‐specific IgE levels as predictors of peanut, egg, and sesame allergy in infants. J Allergy Clin Immunol. 2013;132(4):874‐880. [DOI] [PubMed] [Google Scholar]
- 24. Martin PE, Eckert JK, Koplin JJ, et al. Which infants with eczema are at risk of food allergy? Results from a population‐based cohort. Clin Exp Allergy. 2015;45(1):255‐264. [DOI] [PubMed] [Google Scholar]
- 25. Chan JCK, Peters RL, Koplin JJ, et al. Food challenge and community‐reported reaction profiles in food‐allergic children aged 1 and 4 years: a population‐based study. The journal of allergy and clinical immunology. In Pract. 2017;5(2):398‐409.e3. [DOI] [PubMed] [Google Scholar]
- 26. Martin PE, Koplin JJ, Eckert JK, et al. The prevalence and socio‐demographic risk factors of clinical eczema in infancy: a population‐based observational study. Clin Exp Allergy. 2013;43(6):642‐651. [DOI] [PubMed] [Google Scholar]
- 27. Kunz B, Oranje A, Labreze L, Stalder J‐F, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European task force on atopic dermatitis. Dermatology. 1997;195(1):10‐19. [DOI] [PubMed] [Google Scholar]
- 28. Oranje A, Glazenburg E, Wolkerstorfer A, Waard‐van D, der Spek F. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three‐item severity score. Br J Dermatol. 2007;157(4):645‐648. [DOI] [PubMed] [Google Scholar]
- 29. Silver JD, Spriggs K, Haberle S, Katelaris CH, Newbigin EJ, Lampugnani ER. Crowd‐sourced allergic rhinitis symptom data: the influence of environmental and demographic factors. Sci Total Environ. 2020;705:135147. [DOI] [PubMed] [Google Scholar]
- 30. Idrose NS, Tham RCA, Lodge CJ, et al. Is short‐term exposure to grass pollen adversely associated with lung function and airway inflammation in the community? Allergy. 2020;76(4):1136‐1146. [DOI] [PubMed] [Google Scholar]
- 31. Erbas B, Jazayeri M, Lambert KA, et al. Outdoor pollen is a trigger of child and adolescent asthma emergency department presentations: a systematic review and meta‐analysis. Allergy. 2018;73(8):1632‐1641. [DOI] [PubMed] [Google Scholar]
- 32. Erbas B, Dhamarge S, O'Sullivan M, Akram M, Newbigin E, Taylor P. A case‐crossover design to examine the role of aeroallergens and respiratory viruses on childhood asthma exacerbations requiring hospitalization: the MAPCAH study. J Biomet Biostat S7‐018 Doi. 2012;10:2155‐6180. [Google Scholar]
- 33. Poole JA, Barnes CS, Demain JG, et al. Impact of weather and climate change with indoor and outdoor air quality in asthma: a work group report of the AAAAI environmental exposure and respiratory health committee. J Allergy Clin Immunol. 2019;143(5):1702‐1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gross L, Weber R, Wolf M, Crooks JL. The impact of weather and climate on pollen concentrations in Denver, Colorado, 2010‐2018. Ann Allergy Asthma Immunol. 2019;123(5):494‐502.e4. [DOI] [PubMed] [Google Scholar]
- 35. Mireku N, Wang Y, Ager J, Reddy RC, Baptist AP. Changes in weather and the effects on pediatric asthma exacerbations. Ann Allergy Asthma Immunol. 2009;103(3):220‐224. [DOI] [PubMed] [Google Scholar]
- 36. Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133(7):1752‐1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dharmage SC, Martin PE, Osborne NJ, et al. The epidemiology of food sensitization‐associated eczema in infancy in HealthNuts, a population‐based study. J Allergy Clin Immunol. 2011;127(2):AB35. [Google Scholar]
- 38. Roberts G, Lack G. Food allergy and asthma—what is the link? Paediatr Respir Rev. 2003;4(3):205‐212. [DOI] [PubMed] [Google Scholar]
- 39. Gray CL. Allergies in eczema: review article. Curr Allergy Clin Immunol. 2011;24(4):185‐191. [Google Scholar]
- 40. Koplin JJ, Allen KJ, Gurrin LC, et al. The impact of family history of allergy on risk of food allergy: a population‐based study of infants. Int J Environ Res Public Health. 2013;10(11):5364‐5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Böhme M, Wickman M, Lennart Nordvall S, Svartengren M, Wahlgren CF. Family history and risk of atopic dermatitis in children up to 4 years. Clin Exp Allergy. 2003;33(9):1226‐1231. [DOI] [PubMed] [Google Scholar]
- 42. Hastie T, Tibshirani R. Generalized additive models for medical research. Stat Methods Med Res. 1995;4(3):187‐196. [DOI] [PubMed] [Google Scholar]
- 43. Lee SW, Yon DK, James CC, et al. Short‐term effects of multiple outdoor environmental factors on risk of asthma exacerbations: age‐stratified time‐series analysis. J Allergy Clin Immunol. 2019;144(6):1542‐50.e1. [DOI] [PubMed] [Google Scholar]
- 44. Lambert KA, Katelaris C, Burton P, et al. Tree pollen exposure is associated with reduced lung function in children. Clin Exp Allergy. 2020;50(10):1176‐1183. [DOI] [PubMed] [Google Scholar]
- 45. Fasiolo M, Wood SN, Zaffran M, Nedellec R, Goude Y. qgam: Bayesian non‐parametric quantile regression modelling in R. arXiv preprint arXiv:200703303. 2020.
- 46. Erbas B, Akram M, Dharmage SC, et al. The role of seasonal grass pollen on childhood asthma emergency department presentations. Clin Exp Allergy. 2012;42(5):799‐805. [DOI] [PubMed] [Google Scholar]
- 47. He S‐H, Zhang H‐Y, Zeng X‐N, Chen D, Yang P‐C. Mast cells and basophils are essential for allergies: mechanisms of allergic inflammation and a proposed procedure for diagnosis. Acta Pharmacol Sin. 2013;34(10):1270‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Idrose NS, Vicendese D, Peters RL, et al. Children with food allergy are at risk of lower lung function on high‐pollen days. J Allergy Clin Immunol Pract. 2022;10(8):2144‐2153. [DOI] [PubMed] [Google Scholar]
- 49. Wickman M, Asarnoj A, Tillander H, et al. Childhood‐to‐adolescence evolution of IgE antibodies to pollens and plant foods in the BAMSE cohort. J Allergy Clin Immunol. 2014;133(2):580‐582. [DOI] [PubMed] [Google Scholar]
- 50. Nissen SP, Kjaer HF, Høst A, Nielsen J, Halken S. The natural course of sensitization and allergic diseases from childhood to adulthood. Pediatr Allergy Immunol. 2013;24(6):549‐555. [DOI] [PubMed] [Google Scholar]
- 51. Traidl‐Hoffmann C, Kasche A, Menzel A, et al. Impact of pollen on human health: more than allergen carriers? Int Arch Allergy Immunol. 2003;131(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 52. Traidl‐Hoffmann C, Kasche A, Jakob T, et al. Lipid mediators from pollen act as chemoattractants and activators of polymorphonuclear granulocytes. J Allergy Clin Immunol. 2002;109(5):831‐838. [DOI] [PubMed] [Google Scholar]
- 53. Langley SJ, Goldthorpe S, Craven M, Woodcock A, Custovic A. Relationship between exposure to domestic allergens and bronchial hyperresponsiveness in non‐sensitised, atopic asthmatic subjects. Thorax. 2005;60(1):17‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Platts‐Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population‐based cross‐sectional study. Lancet. 2001;357(9258):752‐756. [DOI] [PubMed] [Google Scholar]
- 55. Sporik R, Squillace SP, Ingram JM, Rakes G, Honsinger RW, Platts‐Mills TAE. Mite, cat, and cockroach exposure, allergen sensitisation, and asthma in children: a case‐control study of three schools. Thorax. 1999;54(8):675‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brunekreef B, Hoek G, Fischer P, Spieksma FT. Relation between airborne pollen concentrations and daily cardiovascular and respiratory‐disease mortality. Lancet. 2000;355(9214):1517‐1518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


