Abstract
The awn of grasses is a long, conspicuous outgrowth of the floral bracts in a grass spikelet. It is known to impact agricultural yield, but we know little about its broader ecological function, nor the selective forces that lead to its evolution. Grass awns are phenotypically diverse across the extant ~12,000 species of Poaceae. Awns have been lost and gained repeatedly over evolutionary time, between and within lineages, suggesting that they could be under selection and might provide adaptive benefit in some environments. Despite the phylogenetic context, we know of no studies that have tested whether the origin of awns correlates with putative selective forces on their form and function. Presence or absence of awns is not plastic; rather, heritability is high. The awns of grasses often are suggested as adaptations for dispersal, and most experimental work has been aimed at testing this hypothesis. Proposed dispersal functions include soil burial, epizoochory, and aerial orientation. Awns may also protect the seed from drought, herbivores, or fire by helping it become buried in soil. We do not fully understand the fitness or nutrient costs of awn production, but in some species awns function in photosynthesis, providing carbon to the seed. Here we show that awns likely provide an adaptive advantage, but argue that studies on awn function have lacked critical phylogenetic information to demonstrate adaptive convergent evolution, are taxonomically biased, and often lack clear alternative hypotheses.
Keywords: adaptation, agriculture, evolutionary ecology, exaptation, florivory, geniculate, grass ecology, herbivore defence, herbivory prevention, Poaceae, spikelet
GRASSES, GRASS FLOWERS, AND AWNS
Poaceae (the grass family) are an agriculturally important, ecologically diverse, and species‐rich plant family. The ~12,000 species of Poaceae (Soreng et al., 2017) collectively cover approximately 31–43% of global land surface in almost all habitat types, including open, dry savannah, dry woodlands, and wet tropical rainforests (Gibson, 2009; Lehmann et al., 2019).
The flowers of grasses are tiny, with each one subtended by a (relatively) large bract, the lemma; together, the flower itself plus the lemma are known as a floret (Schrager‐Lavelle et al., 2017). An individual floret comprises a uniovular gynoecium (female reproductive parts) and three to six stamens (pollen‐producing organs). The structures in the position of petals are known as lodicules, which typically become turgid at anthesis to force the floret open and then shrivel afterward. Outside the lodicules are two grass‐specific structures, the palea and the lemma (Figure 1). The palea has historically been interpreted as a bract or prophyll, although increasing evidence suggests that it represents highly modified adaxial outer tepals and, as such, is part of the flower (Kellogg, 2015; Schrager‐Lavelle et al., 2017). The lemma generally encloses the other organs. One or more florets are then aggregated into a larger structure, the spikelet, which is subtended by bracts (usually two) known as glumes (Figure 1).
Figure 1.

Simplified diagram of a grass spikelet to illustrate the position of nonfloral structures. The four example spikelets show positions where awns commonly develop and the possible variations in number and form. Awns can develop from lemma or glumes or more rarely paleas. The type of awn can be mixed within a single spikelet, e.g., bent and straight.
In many grasses, the lemma, one or both glumes, or occasionally the palea, may end in a sharp point, or bear a short, pointed extension (a mucro), or bear a longer extension known as an awn (Figure 1) (Gould and Shaw, 1983; Kellogg, 2000). The distinction between a mucro and awn is arbitrary; for this review, we define any structure over 1 mm long as an awn. The awn is most commonly an extension of the mid‐vein and is usually apical, but in some species forms on the abaxial side of the lemma. It is generally vascularized and usually tapers to a sharp tip. In some species, additional awns form from lateral veins, with their position, number, and morphology being species‐specific (Clayton et al., 2006–2021; Cavanagh et al., 2019). The awn may be straight, curved, once‐ or twice‐geniculate (bent like a knee because of a reverse twist), coiled, or even (in a few taxa) branched.
Despite the ubiquity of awns, their function is not fully known. Here, we optimize awn presence and structure over a previously published phylogeny, showing that awns have been repeatedly and independently gained and lost in grass evolution. We then review literature showing that awns are (1) generally fixed as present or absent within a species, (2) are costly to produce, and (3) may have roles in carbon capture, seed dispersal, seed defence, and seed burial. Taken together, these observations suggest that awns could confer a selective advantage. We also note that existing data are sparse and taxonomically biased. Finally, we conclude with suggestions for future research.
AWNS ARE REPEATEDLY GAINED FROM AN AWNLESS ANCESTRAL STATE
To provide a phylogenetic context for the remainder of this review, we mapped presence/absence of awns on the grass plastome phylogeny of Saarela et al. (2018), which is well supported and includes all grass subfamilies. For each species in the phylogeny, we retrieved awn data from descriptions in GrassBase (Clayton et al., 2006–2021) and augmented this information where necessary with our own observations of herbarium specimens at GBIF.org (2021) (Appendix S1). We optimized awn presence/absence using maximum likelihood in the ace function as part of the R package phytools (Revell, 2012; R Core Team, 2022). We compared Equal Rates (ER) and All Rates Different (ARD) models. For the questions addressed here, the models produced similar results so only the ER results are shown. With this sample of species, we found that awns appear to have been gained at least 12 times in the history of Poaceae, but once gained may often be lost (Figure 2; Appendix S2).
Figure 2.

Presence or absence of awns in Poaceae regardless of developmental origin (e.g., glume or lemma) optimized on a phylogeny from Saarela et al. (2018). Outer names indicate subfamily classification; inner names indicate tribe level classifications. Dark green: awned species or genera; blue: no awn. An awn is defined as present if it is ≥1 mm long. Full species names are in Appendix S2.
We noted whether awns were borne on the lemma, palea, glumes, or some combination (Appendix S1). Glume awns usually evolve in groups that have already developed lemma awns (Figure 3), but appear independent of ancestral lemma awns in at least three instances (marked with arrows on the figure). Thus, the lemma awn is not an evolutionary or genetic prerequisite for the evolution of glume awns, and glume awns may be a response to independent and distinct selection in those lineages. For this sample of species, palea awns have only a single origin, in Oryzeae (rice tribe), although a broader species sample might well uncover more instances. Lemma awns are common within the Oryzeae, and our analysis suggests they were likely present in the ancestors of the group.
Figure 3.
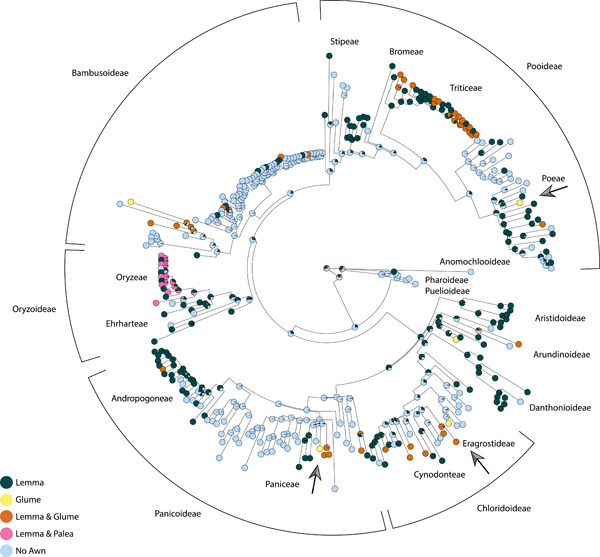
Morphological origin of awns on the spikelet, optimized on a phylogeny from Saarela et al. (2018). Outer names indicate subfamily classification; inner names indicate tribe level classifications. Light blue: species or genera without awns; dark green: lemma awn; yellow: glume awn, pink: palea awn, orange: both lemma and glume awns. Arrows specify taxa in which glume awns appear to have evolved independently of any other awn.
We mapped awn morphology on the phylogeny (Figure 4), using the awn shape character states straight, curved (falcate) and geniculate, following Cavanagh et al. (2019), except that we combined once‐ and twice‐geniculate into the single category of geniculate. Geniculate and straight awns are most common, with only a few awn types not fitting into either category (branched and coiled). Rarely, the two types co‐occur on a single spikelet; in which case, the geniculate awn is generally the central awn on the lemma, and the straight awns are either lateral on the lemma or form on paleas or glumes (Figure 1). Geniculate awns are rarely evolutionarily derived from straight awns. Of the six groups bearing geniculate awns, only two (noted with arrows on the figure and both in the large subfamily Pooideae) convincingly suggest ancestral awns were straight (Figure 4). The reverse appears even rarer, with geniculate awns not leading to straight awns anywhere in the phylogeny. Two unique awn morphologies appear in Aristida and Streptochaeta, coiled and branched, respectively. We do not know whether the coiled awns of Streptochaeta represent early awn evolution or a unique derived state in that tribe (Figure 4). However, Aristida appears to represent an independent evolution of branched awns, unique to that group and any other known extant awns (Figure 4).
Figure 4.
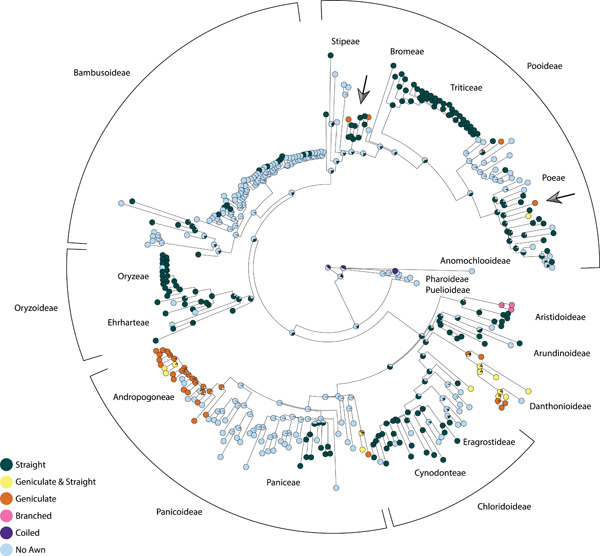
Type of awns optimized on a phylogeny from Saarela et al. (2018). Outer names indicate subfamily classification; inner names indicate tribe level classifications. Light blue: species or genera without awns, orange: geniculate (twisted and bent) awns, dark green; straight awns, yellow: mixed geniculate and straight awns, pink: others (branched or coiled). Arrows specify places where geniculate awns have evolved in lineages of straight awns.
Repeated gains and losses (i.e., convergent evolution) often suggest that a trait has been subject to natural selection (Losos, 2011; Stayton, 2015). The limited phylogenetic analyses here provide evidence that the selective forces acting on awn position and awn type may vary and provide context for the functional data described below. A phylogenetic analysis with a larger sample of taxa and a broader range of models will ultimately be needed, but the evidence of multiple origins and convergent morphology appears robust.
Awn presence is not a plastic response
Presence or absence of awns appears to be insensitive to environmental conditions and is largely fixed for a given genotype. Greenhouse‐grown plants do not lose or gain awns when moved indoors or outdoors, and awn characters are stable when plants are grown in different environments (K. B. Petersen and E. A. Kellogg, personal observations). Species descriptions also indicate that awn presence or absence is fixed within the species, although a few polymorphic species have been recorded (Clayton et al., 2006–2021). In wild Hyparrhenia diplandra and domesticated Sorghum bicolor, Oryza sativa, Triticum aestivum, and Hordeum vulgare, awn traits were highly heritable (Garnier and Dajoz, 2001; Zhang et al., 2015; Magwa et al., 2016; Sanad et al., 2019; Wang et al., 2019 [Preprint]), with broad sense heritability of awn presence over 40% and awn length 63–98%, depending on the species, environment, and degree of stress. Awn length of H. diplandra varied slightly with and between populations, but presence of awns is fixed. Furthermore, awn length appears to be under polygenic control, with multiple genes controlling length but not abolishing awns completely (Liu et al., 2007; Toriba et al., 2010; Yuo et al., 2012; Hua et al., 2015; Bessho‐Uehara et al., 2016; Jin et al., 2016).
POSSIBLE FUNCTIONS OF AWNS
Awns capture carbon but are costly to produce
The growth and presence of an awn on a spikelet has a cost, although it is unclear if costs are carbon‐driven or the result of other nutrient limitations. A negative relationship between awn presence and seed number has clearly been shown in some cultivated species (Oryza sativa, O. sativa subsp. japonica × O. sativa subsp. indica, Triticum aestivum, Hordeum vulgare) (Gu et al., 2015; Rebetzke et al., 2016; Liller et al., 2017; De Leon et al., 2020), and in wild species (Oryza rufipogon and Elymus sibiricus) (Gu et al., 2015; Ntakirutimana et al., 2019). In wild Siberian wildrye (Elymus sibiricus), awn length is negatively correlated with florets per inflorescence, percentage seed set, and total seeds per inflorescence (Ntakirutimana et al., 2019). However, even though fewer seeds and flowers are produced with the production of larger awns, the seeds were significantly larger. We hypothesize that the development of awns represents a shift in reproductive life strategy, from production of many small seeds to fewer large well‐provisioned seeds.
Awns are not necessarily a nutrient sink. Awns of some grasses may photosynthesize, offsetting carbon costs and provisioning seeds (Atkins and Finney, 1957; Grundbacher, 1963; Faris, 1974; Olugbemi, 1978; Tambussi et al., 2007). Photosynthetic awns are common among species in the tribe Triticeae and in subfamily Oryzoideae (Grundbacher, 1963; Tambussi et al., 2007). In both groups, experiments that block photosynthesis (e.g., by covering the awn or removing it) reduce yield; conversely, tracing labelled carbon shows movement of photosynthate from the awn to the seed (reviewed by Grundbacher [1963] and Tambussi et al. [2007]).
Not all awns photosynthesize, however (Moskalenko, 1930; Grundbacher, 1963; AuBuchon‐Elder et al., 2020). In particular, awns on seed‐bearing spikelets in Andropogoneae (Panicoideae) show no evidence of carbon assimilation (AuBuchon‐Elder et al., 2020). Seed‐bearing spikelets in that tribe are paired with awnless sterile spikelets, which assimilate carbon that is then transferred to the seed. In this tribe then, awn function in photosynthesis appears to be replaced by the sterile spikelet.
The carbon provided to developing seeds from awns (and neighboring underdeveloped spikelets, where present) suggests that awns are an adaptation to provide carbon to the seed. If a cost to produce awns exists, it may be offset by selective advantage of a larger seed.
Awns as aids in dispersal
The awns of grasses may function in dispersal in three ways: hygroscopically active awns move in response to changes in moisture, moving the diaspore (the seed and all other dispersed spikelet structures) into or across the soil, awns position the diaspore in the air and to enhance germination on landing, and awns attach to animals (epizoochory). The three are not mutually exclusive, and none is universal. Evidence for epizoochory remains the weakest, with static awns positioning in the air column and hygroscopic awn movement having better experimental support.
The distinctive hygroscopic movement of awns was noticed as early as in the 19th century (Quekett, 1844, p. 25):
“There is another act performed by the aid of moisture and dryness which approaches somewhat near to locomotion: this occurs in … the parts surrounding the fruit of Avena fatua, commonly known as the “animated oat,” where, by the twisting by dryness and untwisting by moisture of the awn, change of place is the result.”
Hygroscopic awns take up moisture from the air and then dry out. Each time the awn gets wet, it straightens, but as it dries, it twists and moves the diaspore. The underlying cellular structures that drive this motion were investigated in the 19th century by Zimmermann (1879), Hildebrand (1873), and briefly summarized by Murbach (1900) for species of Avena and Stipa. Curiously, this aspect of grass awns has not been studied since, although recent studies in Geraniaceae (Evangelista et al., 2011; Jung et al., 2014) are likely relevant. Many such twisted awns also exhibit a reverse twist, such that the lower (proximal) part of the awn twists one direction and the upper (distal) part twists the other. This bidirectional twist creates a flat section where the twist reverses, producing a pronounced bend or knee (hence the term geniculate). The physical forces involved in such reverse helices and their reversion point have been studied extensively in coiled tendrils (e.g., McMillen and Goriely [2002] Gerbode et al. [2012]) but not in grass awns.
Geniculate twisting is not the only known form of hygroscopic movement in grass awns. Elbaum et al. (2007) also showed that the long lemma awns from two adjacent florets of wheat actively propelled the seed into the soil. Cellulose in the awns is arranged such that different layers of cells expanded differently depending on ambient humidity conditions. The result of this expansion is that the awns move outward when drying and inward with increased humidity. The action of the awns causes the diaspore to push down into the soil, burying it deeper (Elbaum et al., 2007). The hygroscopic movement in wheat (Triticeae) may be an example of convergence in function for burial in awns. Whether the hygroscopic mechanism of wheat awns occurs in other species is unknown.
Hygroscopic awn movement, the role of passive inactive awns, burial ability, development, and their direct ecological importance have been investigated experimentally for ca. 75 species of Poaceae (Table 1) or 1% of the family (Peart, 1979, 1984; Peart and Clifford, 1987; Adams and Tainton, 1990; Ghermandi, 1995; Garnier and Dajoz, 2001; Schöning et al., 2004; Elbaum et al., 2007; Molano‐Flores, 2012; Drizin, 2013; Cavanagh et al., 2021). The data are heavily biased toward Australian species, representing only a few tribes (notably Andropogoneae). Hygroscopic awns in particular may function in germination and establishment within communities of mixed grass species (Peart, 1984; Peart and Clifford, 1987; Cavanagh et al., 2021). However, function has generally been extrapolated from limited and taxonomically biased species sampling without considering phylogenetic relationships (Stebbins, 1971; van der Pijl, 1982).
Table 1.
Species mentioned in this paper, reference, tribe, and subfamily. Species are sorted by tribe. The first species name listed is as written in cited literature (in at least one paper when an older name is listed). Synonyms are the currently accepted species names, following POWO (2021). Authority is for the accepted name, not synonyms.
| Species and Authority | References | Tribe | Subfamily |
|---|---|---|---|
| Andropogon gerardi Vitman | Rosas et al. (2008) | Andropogoneae | Panicoideae |
| Andropogon schirensis Hochst. ex A. Rich. | AuBuchon‐Elder et al. (2020) | Andropogoneae | Panicoideae |
| Bothriochloa bladhii (Retz.) S.T. Blake | Cavanagh et al. (2021) | Andropogoneae | Panicoideae |
| Bothriochloa macra (Steud.) S.T. Blake | Cavanagh et al. (2021) | Andropogoneae | Panicoideae |
| Chrysopogon fallax S.T. Blake | Cavanagh et al. (2021) | Andropogoneae | Panicoideae |
| Cymbopogon refractus (R.Br.) A. Camus | Peart (1984) | Andropogoneae | Panicoideae |
| Dichanthium sericeum (R.Br.) A. Camus | Peart (1979); Peart and Clifford (1987); Cavanagh et al. (2021) | Andropogoneae | Panicoideae |
| Heteropogon contortus (L.) P. Beauv. ex Roem. & Schult. | Peart (1979); Peart and Clifford (1987) | Andropogoneae | Panicoideae |
| Hyparrhenia diplandra (Hack.) Stapf | Garnier and Dajoz (2001) | Andropogoneae | Panicoideae |
| Schizachyrium fragile (R.Br.) A. Camus | Peart (1979) | Andropogoneae | Panicoideae |
| Sorghum bicolor (L.) Moench | Zhang et al. (2015); AuBuchon‐Elder et al. (2020) | Andropogoneae | Panicoideae |
| Themeda triandra Forssk. | Agnew and Flux (1970); Peart (1979); Adams and Tainton (1990); AuBuchon‐Elder et al. (2020); Cavanagh et al. (2021) | Andropogoneae | Panicoideae |
| Aristida jerichoensis (Domin) Henrard | Cavanagh et al. (2021) | Aristideae | Aristidoideae |
| Aristida latifolia Domin | Cavanagh et al. (2021) | Aristideae | Aristidoideae |
| Aristida ramosa R.Br. | Cavanagh et al. (2021) | Aristideae | Aristidoideae |
| Aristida vagans Cav. | Peart (1981, 1984) | Aristideae | Aristidoideae |
| Bromus tectorum L. | Kelrick et al. (1986); Ceradini and Chalfoun (2017); Lucero and Callaway (2018) | Bromeae | Pooideae |
| Bouteloua curtipendula (Michx.) Torr. | Laughlin (2003); Titulaer et al. (2018) | Cynodonteae | Chloridoideae |
| Bouteloua gracilis | Titulaer et al. (2018) | Cynodonteae | Chloridoideae |
| Chloris truncata R.Br. | Cavanagh et al. (2021) | Cynodonteae | Chloridoideae |
| Enteropogon acicularis (Lindl.) Lazarides | Cavanagh et al. (2021) | Cynodonteae | Chloridoideae |
| Leptochloa dubia syn. Disakisperma dubium (Kunth) P.M. Peterson & N. Snow | Titulaer et al. (2018) | Cynodonteae | Chloridoideae |
| Leptothrium senegalense (Kunth) Clayton | Agnew and Flux (1970) | Cynodonteae | Chloridoideae |
| Tragus berteronianus Schult. | Agnew and Flux (1970) | Cynodonteae | Chloridoideae |
| Triodia danthonioides (F. Muell.) Lazarides | Cavanagh et al. (2021) | Cynodonteae | Chloridoideae |
| Triodia schinzii (Henrard) Lazarides | Cavanagh et al. (2021) | Cynodonteae | Chloridoideae |
| Danthonia penicillata syn. Rytidosperma penicillatum (Labill.) Connor & Edgar | Simpson (1952) | Danthonieae | Danthonioideae |
| Danthonia tenuior syn. Rytidosperma tenuius (Steud.) O.E. Erikss., A. Hansen & Sunding | Peart (1979) | Danthonieae | Danthonioideae |
| Rytidosperma caespitosum (Gaudich.) Connor & Edgar | Humphreys et al. (2011); Cavanagh et al. (2021) | Danthonieae | Danthonioideae |
| Rytidosperma oreophilum H.P. Linder & N.G. Walsh | Cavanagh et al. (2021) | Danthonieae | Danthonioideae |
| Rytidosperma pallidum (R.Br.) A.M. Humphreys & H.P. Linder | Cavanagh et al. (2021) | Danthonieae | Danthonioideae |
| Microlaena stipoides (Labill.) R.Br. | Peart (1981, 1984) | Ehrharteae | Oryzoideae |
| Enneapogon nigricans (R.Br.) P. Beauv. | Cavanagh et al. (2021) | Eragrostideae | Chloridoideae |
| Eragrostis brownii (Kunth) Nees | Peart (1984) | Eragrostideae | Chloridoideae |
| Eragrostis lehmanniana Nees | Titulaer et al. (2018) | Eragrostideae | Chloridoideae |
| Harpachne schimperi Hochst. ex A. Rich. | Agnew and Flux (1970); Hovstad et al. (2009) | Eragrostideae | Chloridoideae |
| Oryza sativa L. | Liu et al. (2007); Bessho‐Uehara et al. (2016); Magwa et al. (2016) | Oryzeae | Oryzoideae |
| Oryza rufipogon Griff. | Gu et al. (2015) | Oryzeae | Oryzoideae |
| Melinis repens (Willd.) Zizka | Titulaer et al. (2018) | Paniceae | Panicoideae |
| Panicum bisulcatum Thunb. | Liang et al. (2019) | Paniceae | Panicoideae |
| Pennisetum ciliare syn. Cenchrus ciliaris L. | Titulaer et al. (2018) | Paniceae | Panicoideae |
| Agrostis capillaris L. | Hovstad et al. (2009) | Poeae | Pooideae |
| Avena fatua L. | Quekett (1844) | Poeae | Pooideae |
| Cynosurus echinatus L. | Cavanagh et al. (2021) | Poeae | Pooideae |
| Deschampsia cespitosa (L.) P.Beauv. | Hovstad et al. (2009) | Poeae | Pooideae |
| Deyeuxia fauriei (Hack.) Ohwi | Clayton et al. (2006−2021) | Poeae | Pooideae |
| Dichelachne micrantha (Cav.) Domin | Peart (1979) | Poeae | Pooideae |
| Echinopogon ovatus (G. Forst.) P. Beauv | Cavanagh et al. (2021) | Poeae | Pooideae |
| Festuca idahoensis Elmer | Lucero and Callaway (2018) | Poeae | Pooideae |
| Festuca ovina L. | Hovstad et al. (2009) | Poeae | Pooideae |
| Sesleria korabensis (Kümmerle & Jáv.) Deyl | Clayton et al. (2006–2021) | Poeae | Pooideae |
| Austrostipa aristiglumis (F. Muell.) S.W.L. Jacobs & J. Everett | Cavanagh et al. (2021) | Stipeae | Pooideae |
| Austrostipa bigeniculata (Hughes) S.W.L. Jacobs & J. Everett | Cavanagh et al. (2021) | Stipeae | Pooideae |
| Austrostipa blackii (C.E. Hubb.) S.W.L. Jacobs & J. Everett | Cavanagh et al. (2021) | Stipeae | Pooideae |
| Austrostipa mollis (R.Br.) S.W.L. Jacobs & J. Everett | Cavanagh et al. (2021) | Stipeae | Pooideae |
| Austrostipa nitida (Summerh. & C.E. Hubb.) S.W.L. Jacobs & J. Evertett | Cavanagh et al. (2021) | Stipeae | Pooideae |
| Austrostipa nivicola (J.H. Willis) S.W.L. Jacobs & J. Everett | Cavanagh et al. (2021) | Stipeae | Pooideae |
| Austrostipa nodosa (S.T. Blake) S.W.L. Jacobs & J. Everett | Cavanagh et al. (2021) | Stipeae | Pooideae |
| Austrostipa rudis (Spreng.) S.W.L. Jacobs & J. Everett | Cavanagh et al. (2021) | Stipeae | Pooideae |
| Austrostipa scabra (Lindl.) S.W.L. Jacobs & J. Everett | Cavanagh et al. (2021) | Stipeae | Pooideae |
| Hesperostipa spartea (Trin.) Barkworth | Molano‐Flores (2012) | Stipeae | Pooideae |
| Nassella neesiana (Trin. & Rupr.) Barkworth | Cavanagh et al. (2021) | Stipeae | Pooideae |
| Oryzopsis hymenoides, syn. Achnatherum hymenoides, syn. Eriocoma hymenoides (Roem. & Schult.) Rydb. | Kelrick et al. (1986); Lucero and Callaway (2018) | Stipeae | Pooideae |
| Stipa parviflora, syn. Achnatherum parviflorum (Desf.) M. Nobis | Schöning et al. (2004) | Stipeae | Pooideae |
| Stipa tenacissima, syn. Macrochloa tenacissima (L.) Kunth | Schöning et al. (2004) | Stipeae | Pooideae |
| Stipa verticillata, syn. Austrostipa verticillata (Nees ex Spreng.) S.W.L. Jacobs & J. Everett | Peart (1979); Cavanagh et al. (2021) | Stipeae | Pooideae |
| Stipa viridula, syn. Eriocoma lettermanii (Vasey) Romasch. | Kelrick et al. (1986) | Stipeae | Pooideae |
| Aegilops geniculata Roth | Clayton et al. (2006–2021) | Triticeae | Pooideae |
| Anthosachne scabra (R. Br.) Nevski | Cavanagh et al. (2021) | Triticeae | Pooideae |
| Elymus elymoides (Raf.) Swezey | Lucero and Callaway (2018) | Triticeae | Pooideae |
| Hordeum vulgare L. | Faris (1974); Liller et al. (2017) | Triticeae | Pooideae |
| Pascopyrum smithii, syn. Elymus smithii (Rydb.) Gould | Kelrick et al. (1986) | Triticeae | Pooideae |
| Pseudoroegneria spicata (Pursh) Á. Löve | Lucero and Callaway (2018) | Triticeae | Pooideae |
| Triticum aestivum L. | Sanad et al. (2019); AuBuchon‐Elder et al. (2020) | Triticeae | Pooideae |
| Elymus sibiricus L. | Ntakirutimana et al. (2019) | Triticeae | Pooideae |
Hygroscopic awns in some species aid burial and soil surface movement. Awn removal reduced successful germination 12‐fold in Danthonia penicillata (=Rytidosperma penicillatum) (Simpson, 1952) or showed significantly less movement (Cavanagh et al., 2021). (Here and throughout this paper, we cite the species name used in the original paper followed by the currently accepted name in parentheses if it is different.) Using the methods of Simpson (1952), multiple investigators have tested burial ability by placing spikelets (with or without modified awns) of various species on soil and observed the subsequent soil–spikelet interaction (Peart, 1979, 1981; Adams and Tainton, 1990; Garnier and Dajoz, 2001; Elbaum et al., 2007; Cavanagh et al., 2021). In these studies, removal or modification of awns changes the proportion of spikelets buried, the germination rate, and overall movement distance. In diaspores bearing both a hygroscopic and passive straight awn, removal of one awn did not significantly affect the ability of spikelets to move to a microsite location, but a significantly lower proportion of spikelets became permanently lodged or buried (Peart, 1979). However, removal of awns produced inconsistent results in Dichelachne micrantha and Themeda triandra, suggesting the awn alone does not determine burial success (Peart, 1979; Adams and Tainton, 1990).
The depth of burial and distance moved by diaspores correlates with awn length (r = 0.61, P < 0.001), as shown by a large Australian study on 30 species representing five of the 12 grass subfamilies (Cavanagh et al., 2021). The deepest burial was achieved by diaspores with hygroscopically active awns, with T. triandra, Bothriochloa macra, and Anthosachne scabra being buried 12.4, 6.4, and 5.9 mm, respectively (Cavanagh et al., 2021). The species with the lowest frequency of burial was Cynosurus echinatus, which has a single straight inactive awn. In an unrelated study, diaspores of the African grass Hyparrhenia diplandra were also buried deeper if they had longer awns (Garnier and Dajoz, 2001). Notably, the starting conditions in burial experiments were often artificial, with diaspores pushed slightly into the soil at the start, removing the need for the diaspore to initiate its own burial.
Soil type and heterogeneity of the surface may be important for the function of large hygroscopic awns (Humphreys et al., 2011). For example, T. triandra diaspores move and are effectively buried in heterogeneous soils (Peart, 1979; Cavanagh et al., 2021), but bury themselves poorly in fine homogeneous soils (Adams and Tainton, 1990). In fine soils over 80% of de‐awned diaspores were buried, but none of the awned diaspores achieved burial (Adams and Tainton, 1990). Slightly increasing the heterogeneity of the soil increased burial of awned T. triandra spikelets to 30% from zero. Introducing the large awned T. triandra diaspores to a soil surface with high heterogeneity led to an increase in burial depth, and adding solid objects to the soil surface was required for many species to achieve burial at all (Cavanagh et al., 2021). In northern Australia, heavily clay‐based soils with many cracks have significantly more species with hygroscopically active, awned diaspores than unawned diaspores (Peart and Clifford, 1987). Conversely, sandier sites with fewer deep cracks had a significantly higher species abundance of unawned diaspores (Peart and Clifford, 1987). In another study, seed length, awn length and width, and seed biomass of the grass Hesperostipa spartea were significantly different between diaspores originating from loam and sandy soils (Molano‐Flores, 2012), although all diaspores were significantly better at burying themselves in loam soils, regardless of diaspore origin. Diaspores originating in sandy soils were smaller and had significantly shorter awns, but buried themselves more deeply than the diaspores of loam origin. These results suggest an unclear and complex interaction between soil type, soil surface structure, and awn design (Humphreys et al., 2011).
Inactive passive awns do not appear to interact with the environment in the same way as hygroscopic awns, and frequency of diaspores with passive awns does not correlate with any particular soil type (Peart and Clifford, 1987). Straight awns are commonly passive‐inactive, but there are exceptions such as wheat (Elbaum et al., 2007). Most phylogenetic origins of straight awns are independent of geniculate hygroscopic ones (Figure 4). The passive awns of grasses may function in positioning spikelets on the soil or in the air column. The spikelets of Aristida vagans and Microlaena stipoides, which have rigid passive awns, fall consistently with the callus (base of the spikelet where the radicle emerges) downward when dropped from height (Peart, 1981). Removal of awns significantly decreases callus‐down landing and significantly decreases germination (Peart, 1981). Other studies on terminal velocity and falling orientation of diaspores found similar results, with hairs and rigid awns guiding the diaspores to fall almost straight down (Rabinowitz and Rapp, 1981). Furthermore, observations suggest that diaspores of passive‐awned species are often found in a standing position (Peart, 1984). However, there is no evidence to suggest that aerial orientation is a trait unique to straight, rigid awns.
Awns may also aid epizoochory, the transport of seed on the skin or fur of animals (Sorensen, 1986; Chambers and MacMahon, 1994; Tackenberg et al., 2006; Will et al., 2007; Costa et al., 2014; Quick et al., 2017). Epizoochory has been documented primarily on mammals and is rare in birds (Costa et al., 2014), but even the numbers of diaspores recorded on mammals are small, making the data anecdotal rather than statistical. Epizoochory undoubtedly occurs in grasses, but whether awn structures are selected specifically for this purpose is not clear. Some domesticated animals such as sheep (Mouissie et al., 2005) will carry any diaspores in the environment, and the unawned diaspores of Agrostis capillaris represent the most common grass on Galloway's cattle in Belgium (Couvreur et al., 2004). However, because sheep and cattle are rarely raised in environments where they are native, they cannot account for selection over evolutionary time.
In the few studies of wild mammals, diaspores of high‐abundance grass species attach more often than those of low‐abundance ones (Fischer et al., 1996; Couvreur et al., 2004; Hovstad et al., 2009). In a North American prairie, 622 diaspores of Andropogon gerardi (big bluestem), a dominant tallgrass prairie species, were found on 67 individual bison, with Bromus species the second most common grasses on the animals (331 diaspores on 65 individuals) (Rosas et al., 2008). Diaspores of both species are awned; awns of A. gerardi are hygroscopically active, whereas those of Bromus are straight and presumably passive. Bison also dispersed Bouteloua curtipendula (Laughlin, 2003), whose diaspores bear several straight awns; bison fur retained more diaspores than did fur of elk, deer, coyote, rabbit, and fox (down to 20% retention). However, pushing a fox dummy (using a real fox coat) through a meadow led to notably different results in Norway (Hovstad et al., 2009). High retention rates were observed for Deschampsia caespitosa (hairy and awned), and Festuca ovina (short awned and few hairs) on the fox coat. Overall in this study's field site, 19 of the 29 local species diaspores attached to the fox coat to some extent (Hovstad et al., 2009). However, even with an impressive variety of grasses attached to the coats, natural behavior of the animals was not taken into account, nor did the study provide a clear indication of selection. The long‐awned T. triandra where common in Kenya showed limited attachment to hares, even though grasses were the most common diaspore type on their coats (Agnew and Flux, 1970). The diaspores of T. triandra seemed to drill into the skin of the animal, and the natural outcome of this action is unclear, but appears to be an accidental consequence of hygroscopic function. The abundant hook‐covered diaspore of Tragus berteronianus was commonly found attached to hare fur, but the very similar diaspore of Latipes senegalensis (=Leptothrium senegalense) was not found at all. The lack of attachment of morphologically similar diaspores suggests that animal behavior may play a big part in which diaspores attach, in addition to (or rather than) diaspore morphology.
Overall, the existing literature documenting epizoochory in Poaceae species lacks a clear testable hypothesis involving awns and does not present convincing evidence of awns' involvement in animal attachment.
Awns as a defense mechanism
Awns may provide protection from fire by enhancing burial or deterring herbivores. Fire is common in grass‐dominated habitats (Mouillot and Field, 2005; Linder et al., 2018), but the ability of different grass lineages to survive fire varies markedly (Lehmann et al., 2019). Fire‐driven awn adaptation has only received attention in the literature once, with evidence for fire interaction with awns coming primarily from Andropogoneae (Garnier and Dajoz, 2001). Andropogoneae make up 37% of all grassy vegetation and tolerate chronic fire regimes better than any other grass tribe with a return interval of only 2 years (Lehmann et al., 2019; Simpson et al., 2021). Soil is an effective insulator with depths of 50 mm or more significantly reducing dangerous temperatures for seeds (Bradstock and Auld, 1995). However, a considerable burial depth requirement might suggest that only longer awns are suitable for achieving sufficient insulation. Longer awns were correlated with burial depth in Hyparrhenia diplandra in a savanna on the Ivory Coast that burned every year, but because fire intensity is unpredictable, fire could sustain the variable awn sizes within the population (35–70 mm) (Garnier and Dajoz, 2001). However, presence of awns does not seem to guarantee a germination advantage after a fire. In years preceding fire in Australia, awned species (awns 6–16 mm) were substantially more abundant than unawned species (Peart, 1984), but seedling abundance of awned species post fire dropped substantially, being replaced by unawned species (Peart, 1984). Alternatively, burial via hygroscopic awns may be too limited to provide an effective defence against fire, or possibly, a minimum size of awns is required to offer any protection from fire.
Herbivory from insects causes significant damage to reproductive structures of grasses in both natural (Bertness et al., 1987; Bertness and Shumway, 1992; Kelly and Sullivan, 1997) and agricultural environments (Naresh and Smith, 1984; Singh, 1987; Tindall et al., 2005; Awuni et al., 2015), with loss of reproductive output both before seed dispersal (Bertness et al., 1987; Singh, 1987; Bertness and Shumway, 1992; Kelly and Sullivan, 1997) and afterward (Reed et al., 2004; Schöning et al., 2004). While pre‐dispersal herbivory on grains is substantial and well documented (Naresh and Smith, 1984; Singh, 1987; Tindall et al., 2005; Parsons and Munkvold, 2010; Awuni et al., 2015), there is no information whether awns help prevent such herbivory. Pre‐dispersal herbivory is rarely studied in natural populations despite being a potentially important selective force (Bertness et al., 1987; Bertness and Shumway, 1992; Kelly and Sullivan, 1997). In an herbivore‐exclusion study, Bertness et al. (1987) found up to 80% of ovules were lost before dispersal in natural populations of Spartina patens (=Sporobolus pumilus), Spartina alterniflora (=Sporobolus alterniflorus), and Distichlis spicata, three unawned North American grass species. Loss was likely because of predation by the grasshopper Conocephalus spartinae. To our knowledge, no one has tested whether such herbivory might be less extensive on spikelets with awns.
The role of awns in preventing post‐dispersal herbivory is better documented. Like pre‐dispersal loss, the loss of dispersed seeds to herbivores can be high (Capon and O'Connor, 1990; Reed et al., 2004; Schöning et al., 2004). Awns may deter herbivores by increasing the time they spend handling the seeds (Schöning et al., 2004; Titulaer et al., 2018). Seed preference of sparrows around Chihuahua, Mexico varied considerably among six species of grass: Bouteloua gracilis, B. curtipendula, Leptochloa dubia (=Disakisperma dubium), Melinis repens, Eragrostis lehmanniana, and Pennisetum ciliare (=Cenchrus ciliaris), comprising three native and three exotic grasses, respectively (Titulaer et al., 2018). Cenchrus ciliaris and E. lehmanniana were largely avoided by sparrows. The former has multiple bent, long bristles (awn‐like and usually twice as long as any other structure) and was only exploited by one species of sparrow, while the latter is awnless; the low preference is attributed to its small size (difficulty of handling and low nutrient value). Bouteloua gracilis has six to nine awns (three per floret in a spikelet) but was consumed preferentially by the sparrows. The awns of B. gracilis, unlike C. ciliaris, are short, rarely reaching past the end of the spikelet, and fewer in number. Proportionate length and numbers of awns could be important to predatory deterrence, although importantly, this study was observational rather than experimental, so the relationship between predation and awn structure remains speculative.
Herbivory by ants (Messor spp.) is reduced by large hygroscopic awns in Stipa tenacissima (=Macrochloa tenacissima) and S. parviflora (=Achnatherum parviflorum) (Schöning et al., 2004). Handling time of the M. tenacissima diaspore was significantly longer when the awn was intact than when it was removed (P < 0.001). Ants were significantly more likely to take diaspores of A. parviflorum with awns removed than with awns intact when provided a choice (P < 0.001). Hygroscopic awns may also allow diaspores to move or actively bury themselves before ants have the chance to consume them (Schöning et al., 2004). However, in another study in sagebrush desert in Wyoming, United States, ants showed little preferential removal of seeds, and only Purshia tridentata (awnless Rosaceae) was significantly preferred over Bromus tectorum (large awned Poaceae) and Artemisia tridentata (awnless Asteraceae) (Kelrick et al., 1986). Ants in other studies were observed abandoning seeds and returning to their nest (Willard and Crowell, 1965; Rissing, 1981; Kelrick et al., 1986). Thus, diaspores with awns and developed seeds may not have been always consumed by ants.
Awns of Bromus tectorum may increase handling time for small rodents and thus are more likely to be avoided (Kelrick et al., 1986; Ceradini and Chalfoun, 2017). In a comparison of four Poaceae in subfamily Pooideae (Stipa viridula [=Eriocoma lettermanii], Pascopyrum smithii [=Elymus smithii], Oryzopsis hymenoides [=Eriocoma hymenoides], and Bromus tectorum) and two other local plant species (Purshia tridentata and Artemisia tridentata) from Wyoming, the large‐awned B. tectorum and unawned E. smithii were least preferred by small mammals along with the dicot A. tridentata (Kelrick et al., 1986). However, the fine awns of E. lettermanii did not appear to discourage herbivores.
Nutrient value of seeds has been considered a potential factor for herbivores, but the high nutritional value of B. tectorum does not appear to matter (Lucero and Callaway, 2018). Lucero and Callaway (2018) removed awns from B. tectorum and all other plants in their study (Festuca idahoensis, Pseudoroegneria spicata, Elymus elymoides, and Achnatherum hymenoides [=Eriocoma hymenoides]). Regardless of awn state, B. tectorum was always significantly less consumed by rodents than other seed, suggesting nutritional value alone cannot necessarily explain herbivore choice. In summary, the link between awns and herbivory prevention requires further investigation. In particular, a more experimental approach is needed along with experimental designs that consider the possibility that biochemical and nutritional differences among species are more important than presence or absence of awns.
MAJOR QUESTIONS AND NEEDS FOR FUTURE AWN RESEARCH
Expansion of phylogenetic sampling in analysis of awn trait evolution
A crucial step for understanding convergence is determining if observed traits are the result of convergence or simply phylogenetic relatedness. Our phylogenetic analyses indicate that major awn traits have arisen multiple times independently, suggesting that they are the result of convergent evolution and could be adaptive (Figures 2, 3, 4). Nonetheless, these analyses are a shallow examination of the diversity of Poaceae and complex questions of adaptation or exaptation cannot be confidently concluded from this or previous published studies. Incorporating an explicit phylogenetic structure for experimental design would be helpful for future studies. For example, although AuBuchon‐Elder et al. (2020) focused largely on Sorghum bicolor, they compared it to several other distantly related species of Andropogoneae and showed that none had awns that supplied carbon to the grain. Because of the position of the species in the phylogeny, they could reasonably infer that their conclusions applied to most if not all awned Andropogoneae. Likewise, Tambussi et al. (2007) reported on awns in several species in Triticeae, all of which are an important source of carbon for the grain; based on their choice of species and the similarities among them, we can infer that awns in all members of the tribe are likely to function similarly. Thus, awn function differs in Triticeae and Andropogoneae, but whether that difference characterizes their respective subfamilies or can be extended to larger clades is unknown. Because most studies on awn function have been based on the assemblage of species in a local community, rather than on species chosen phylogenetically, such extrapolation awaits future studies.
Fitness and energetic costs of awns
Awns are clearly not beneficial in all habitats, and losses of awns are not uncommon across Poaceae (Clayton et al., 2006–2021; Humphreys et al., 2011; Teisher et al., 2017; McAllister et al., 2019). Repeated loss of awns in a lineage suggests a fitness and or energetic cost associated with awn production and maintenance. However, the repeated evolution and persistence of awns suggest that they can offer significant fitness advantages in certain environments. Soil type may be an important factor for awn benefit (Peart and Clifford, 1987; Humphreys et al., 2011; Liang et al., 2019). The major questions that arise are: If awn production is limited by nutrient availability, which nutrient(s) are most important? If awns are costly to produce, what benefit did the original awns provide to the plant? Does awn production consistently cause a shift from producing many small seeds to fewer larger seeds, and if so, what are the conditions under which this shift is advantageous? The results from Ntakirutimana et al. (2019) in Elymus sibiricus suggest a trade‐off may occur, although more than a single study must exist for any generalisation across Poaceae to be possible. Nonetheless, from this available data, we hypothesize that the development of awns represents a shift in reproductive life strategy, from production of many small seeds to fewer large, well‐provisioned seeds.
Genetic basis of awns and plasticity
Awn length and presence are highly heritable in species that have been investigated, suggesting much of the observed variation is genetic. Extensive population‐ and individual‐level analyses are required to fully understand the controls of awn length within individuals and species. A few species produce awned, mixed, and unawned plants, but do not appear plastic in response (e.g., Sorghum halepense [Johnsongrass]; K. B. Petersen and E. A. Kellogg, personal observations). What advantage does a mixed awn system confer? These species present an opportunity to test whether yield and seed size are correlated with differences observed in other awned grasses. All studies of genetic control for awns that we know of to date have included only a handful of species and investigated only lemma awns (see recent review by Schrager‐Lavelle et al. [2017]). A few studies have found that a single gene functions as a genetic switch, with one allele allowing awns to develop and the other allele preventing awn formation. Such genes include the rice gene Awn‐1 (An‐1), which encodes a bHLH transcription factor that controls cell division at the tip of the lemma, thereby regulating the presence or absence of awns (Luo et al., 2013). Similarly, OsETTIN2, an auxin response gene, eliminates awns completely if silenced, depending on the genetic background (Toriba and Hirano, 2014). If the presence or absence of awns is regulated by a single gene, it is appropriate to treat it phylogenetically as a single qualitative binary trait as in Figure 2. It remains unclear whether genes that control awn production in paleas and glumes are the same as those in lemmas.
CONCLUSIONS
The diversity of extant Poaceae makes understanding their various ecological interactions an enormous task, although mapping the presence and absence of awns on a more comprehensive phylogeny should be relatively straightforward. The diversity in structure and the loss and reappearance of awns in different lineages has not inspired as much research as one would expect given the ubiquity and accessibility of the plants. Only a few species (e.g., Themeda triandra) have been included in more than one study to test dispersal mechanisms. However, these studies and others ignore the complex evolutionary history and diversity of awns in other grass species. Assumptions of adaptive convergence are made between species that are both closely related and completely unrelated. Consideration of ancestral traits is critical in understanding whether traits are a result of adaptive convergence or simply exaptation of existing traits. Furthermore, the adaptive origin of awns has been relatively ignored, and focus has been placed on complex functions such as burial in hygroscopic geniculate awns. We are unconvinced that awns were originally selected for burial; rather they may still retain another less complex adaptation such as carbon fixation or herbivore deterrence. Despite the range of potential functions for awns, research has disproportionately focused on burial and spikelet movement, particularly in a limited sample of geniculate‐awned plants. Evidence for epizoochory, herbivory, and pre‐dispersal defense remains weak. Fire has been suggested to be a selection force acting on particular aspects of awns, but the evidence is mostly circumstantial and may only apply to some species of Andropogoneae. The fitness or nutrient cost of producing awns is poorly understood, although carbon assimilation is well documented in awns for two clades of Poaceae. Furthermore, there is no evidence to suggest awn characteristics are plastic in any grasses, supporting independent and distinct selection occurring in grass lineages to evolve awns. Functional convergence in species‐rich families of angiosperms such as Poaceae (grasses) provides significant opportunity to explore adaptation and convergent evolution of floral structures.
AUTHOR CONTRIBUTIONS
K.B.P. produced initial structure, drafted the manuscript, did the data analyses and prepared the figures. E.A.K. developed the trait data set for the phylogenies. Both authors made extensive edits and revisions and prepared the document for final submission.
Supporting information
Appendix S1. Awn traits assembled from GrassBase.
Appendix S2. Alternative version of Figure 2 with species visible on tree tips.
ACKNOWLEDGMENTS
We thank the National Science Foundation (NSF) Grant #1929514 for funding to K.B.P. and E.A.K. We also thank review editor Kasey Barton for her guidance in developing this review, Aelys Humphreys and an anonymous reviewer for their detailed and constructive suggestions,and Sona Pandey, Ken Olsen, and the Kellogg lab for their insight and helpful comments; all substantially improved the final product.
Petersen, K. B. , and Kellogg E. A.. 2022. Diverse ecological functions and the convergent evolution of grass awns. American Journal of Botany 109(9): 1331–1345. 10.1002/ajb2.16060
This article is part of the AJB Synthesis series to showcase the work of early‐career scientists. One Synthesis article will be chosen annually to win the Synthesis prize. For more information, see https://botany.org/home/awards/awards-forearly-career-scientists/ajb-synthesis-papers-andprize.html.
REFERENCES
- Adams, K. M. , and Tainton N. M.. 1990. The function of the hygroscopic awn of Themeda triandra . Journal of the Grassland Society of Southern Africa 7: 271–273. [Google Scholar]
- Agnew, A. D. Q. , and Flux J. E.. 1970. Plant dispersal by hares (Lepus capensis L.) in Kenya. Ecology 51: 735–737. [Google Scholar]
- Atkins, I. M. , and Finney K. F.. 1957. Quality characteristics of two pairs of isogenic lines of wheat. Agronomy Journal 49: 351–353. [Google Scholar]
- AuBuchon‐Elder, T. , Coneva V., Goad D. M., Jenkins L. M., Yu Y., Allen D. K., and Kellogg E. A.. 2020. Sterile spikelets contribute to yield in Sorghum and related grasses. Plant Cell 32: 3500–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awuni, G. A. , Gore J., Cook D., Musser F., Catchot A., and Dobbins C.. 2015. Impact of Oebalus pugnax (Hemiptera: Pentatomidae) infestation timing on rice yields and quality. Journal of Economic Entomology 108: 1739–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertness, M. D. , and Shumway S. W.. 1992. Consumer driven pollen limitation of seed production in marsh grasses. American Journal of Botany 71: 288‐293. [Google Scholar]
- Bertness, M. D. , Wise C., and Ellison A. M.. 1987. Consumer pressure and seed set in a salt marsh perennial plant community. Oecologia 71: 190–200. [DOI] [PubMed] [Google Scholar]
- Bessho‐Uehara, K. , Wang D. R., Furuta T., Minami A., Nagai K., Gamuyao R., Asano K., et al. 2016. Loss of function at RAE2, a previously unidentified EPFL, is required for awnlessness in cultivated Asian rice. Proceedings of the National Academy of Sciences, USA 113: 8969–8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradstock, R. A. , and Auld T. D.. 1995. Soil temperatures during experimental bushfires in relationship to fire intensity: consequences for legume germination and fire management in south‐eastern Australia. Journal of Applied Ecology 32: 76–84. [Google Scholar]
- Capon, M. H. , and O'Connor T. G.. 1990. The predation of perennial grass seeds in Transvaal savanna grasslands. South African Journal of Botany 56: 11–15. [Google Scholar]
- Cavanagh, A. M. , Godfree R. C., and Morgan J. W.. 2019. An awn typology for Australian native grasses (Poaceae). Australian Journal of Botany 67: 309–334. [Google Scholar]
- Cavanagh, A. M. , Morgan J. W., and Godfree R. C.. 2021. Awn morphology influences dispersal, microsite selection and burial of Australian native grass diaspores. Frontiers in Ecology and Evolution 8: 581967. [Google Scholar]
- Ceradini, J. P. , and Chalfoun A. D.. 2017. Species’ traits help predict small mammal responses to habitat homogenization by an invasive grass. Ecological Applications 27: 1451–1465. [DOI] [PubMed] [Google Scholar]
- Chambers, J. C. , and MacMahon J. A.. 1994. A day in the life of a seed: movements and fates of seeds and their implications for natural and managed systems. Annual Review of Ecology and Systematics 25: 263–292. [Google Scholar]
- Clayton, W. D. , Vorontsova M. S., Harman K. T., and Williamson H.. 2006. ‐2021. GrassBase ‐ the online world grass flora. Website: https://www.kew.org/data/grasses-db.html [accessed 16 March 2021].
- Costa, J. M. , Ramos J. A., da Silva L. P., Timoteo S., Araújo P. M., Felgueiras M. S., Rosa A., et al. 2014. Endozoochory largely outweighs epizoochory in migrating passerines. Journal of Avian Biology 45: 59–64. [Google Scholar]
- Couvreur, M. , Christiaen B., Verheyen K., and Hermy M.. 2004. Large herbivores as mobile links between isolated nature reserves through adhesive seed dispersal. Applied Vegetation Science 7: 229–236. [Google Scholar]
- De Leon, T. B. , Pruthi R., Jampala B., Borjas A. H., and Subudhi P. K.. 2020. Genetic determinants for agronomic and yield‐related traits localized on a GBS‐SNP linkage map from a japonica × indica cross in rice. Plant Gene 24: 100249. [Google Scholar]
- Drizin, J. M. 2013. Hygroscopic awns of two prairie grasses, Andropogon gerardii and Sorghastrum nutans . Ph.D. dissertation, Northwestern University, Evanston, IL, USA.
- Elbaum, R. , Zaltzman L., Burgert I., and Fratzl P.. 2007. The role of wheat awns in the seed dispersal unit. Science 316: 884–886. [DOI] [PubMed] [Google Scholar]
- Evangelista, D. , Hotton S., and Dumais J.. 2011. The mechanics of explosive dispersal and self‐burial in the seeds of the filaree, Erodium cicutarium (Geraniaceae). Journal of Experimental Biology 214: 521–529. [DOI] [PubMed] [Google Scholar]
- Faris, D. G. 1974. Yield component development in four isogenic barley lines differing in awn length. Canadian Journal of Plant Science 54: 315–322. [Google Scholar]
- Fischer, S. F. , Poschlod P., and Beinlich B.. 1996. Experimental studies on the dispersal of plants and animals on sheep in calcareous grasslands. Journal of Applied Ecology 33: 1206–1222. [Google Scholar]
- Garnier, L. K. , and Dajoz I.. 2001. Evolutionary significance of awn length variation in a clonal grass of fire‐prone savannas. Ecology 82: 1720–1733. [Google Scholar]
- GBIF . 2021. Global Biodiversity Information Facility (GBIF) home page. Website: https://GBIF.org [accessed 2 September 2021]. GBIF, Copenhagen, Denmark.
- Gerbode, S. J. , Puzey J. R., McCormick A. G., and Mahadevan L.. 2012. How the cucumber tendril coils and overwinds. Science 337: 1087–1091. [DOI] [PubMed] [Google Scholar]
- Ghermandi, L. 1995. The effect of the awn on the burial and germination of Stipa speciosa (Poaceae). Acta Oecologica (Montrouge) 16: 719–728. [Google Scholar]
- Gibson, D. J. 2009. Grasses and grassland ecology. Oxford University Press, NY, NY, USA. [Google Scholar]
- Gould, F. W. , and Shaw R. B.. 1983. Grass systematics. Texas A & M University Press, College Station, TX, USA. [Google Scholar]
- Grundbacher, F. J. 1963. The physiological function of the cereal awn. Botanical Review 29: 366–381. [Google Scholar]
- Gu, B. , Zhou T., Luo J., Liu H., Wang Y., Shangguan Y., Zhu J., et al. 2015. An‐2 encodes a cytokinin synthesis enzyme that regulates awn length and grain production in rice. Molecular Plant 8: 1635–1650. [DOI] [PubMed] [Google Scholar]
- Hovstad, K. A. , Borvik S., and Ohlson M.. 2009. Epizoochorous seed dispersal in relation to seed availability – an experiment with a red fox dummy. Journal of Vegetation Science 20: 455–464. [Google Scholar]
- Hua, L. , Wang D. R., Tan L., Fu Y., Liu F., Xiao L., Zhu Z., et al. 2015. LABA1, a domestication gene associated with long, barbed awns in wild rice. Plant Cell 27: 1875–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys, A. M. , Antonelli A., Pirie M. D., and Linder H. P.. 2011. Ecology and evolution of the diaspore “burial syndrome.” Evolution 65: 1163–1180. [DOI] [PubMed] [Google Scholar]
- Jin, J. , Hua L., Zhu Z., Tan L., Zhao X., Zhang W., Liu F., et al. 2016. GAD1 encodes a secreted peptide that regulates grain number, grain length, and awn development in rice domestication. Plant Cell 28: 2453–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, W. , Kim W., and Kim H.‐Y.. 2014. Self‐burial mechanics of hygroscopically responsive awns. Integrative and Comparative Biology 54: 1034–1042. [DOI] [PubMed] [Google Scholar]
- Kellogg, E. A. 2000. Molecular and morphological evolution in the Andropogoneae. In Jacobs S. W. L. and Everett J. [eds.], Grasses: systematics and evolution, 149–158. CSIRO Publishing, Collingwood, Australia. [Google Scholar]
- Kellogg, E. A. 2015. Flowering plants. Monocots: Poaceae. In Kubitzki K. [ed.], The families and genera of vascular plants, 1–416. Springer, London, UK. [Google Scholar]
- Kelly, D. , and Sullivan J. J.. 1997. Quantifying the benefits of mast seeding on predator satiation and wind pollination in Chionochloa pallens (Poaceae). Oikos 78: 143–150. [Google Scholar]
- Kelrick, M. I. , MacMahon J. A., Parmenter R. R., and Sisson D. V.. 1986. Native seed preferences of shrub‐steppe rodents, birds and ants: the relationships of seed attributes and seed use. Oecologia 68: 327–337. [DOI] [PubMed] [Google Scholar]
- Laughlin, D. C. 2003. Geographic distribution and dispersal mechanismsof Bouteloua curtipendula in the Appalachian mountains. American Midland Naturalist 149: 268–281. [Google Scholar]
- Lehmann, C. E. R. , Griffith D. M., Simpson K. J., Anderson T. M., Archibald S., Beerling D. J., Bond W. J., et al. 2019. Functional diversification enabled grassy biomes to fill global climate space. BioRxiv. 10.1101/583625 [Preprint]. [DOI]
- Liang, W. , Liu Z., Liu M., Qin X., Xin Z., Lv Y., Li X., et al. 2019. How do diaspore traits, wind speed and sand surface configuration interact to determine seed burial during wind dispersal? Plant and Soil 440: 357–368. [Google Scholar]
- Liller, C. B. , Walla A., Boer M. P., Hedley P., Macaulay M., Effgen S., von Korff M., et al. 2017. Fine mapping of a major QTL for awn length in barley using a multiparent mapping population. Theoretical and Applied Genetics 130: 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder, H. P. , Lehmann C. E., Archibald S., Osborne C. P., and Richardson D. M.. 2018. Global grass (Poaceae) success underpinned by traits facilitating colonization, persistence and habitat transformation. Biological Reviews 93: 1125–1144. [DOI] [PubMed] [Google Scholar]
- Liu, B. , Chen Z., Song X., Liu C., Cui X., Zhao X., Fang J., et al. 2007. Oryza sativa Dicer‐like4 reveals a key role for small interfering RNA silencing in plant development. Plant Cell 19: 2705–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losos, J. B. 2011. Convergence, adaptation, and constraint. Evolution 65: 1827–1840. [DOI] [PubMed] [Google Scholar]
- Lucero, J. E. , and Callaway R. M.. 2018. Native granivores reduce the establishment of native grasses but not invasive Bromus tectorum . Biological Invasions 20: 3491–3497. [Google Scholar]
- Luo, J. , Liu H., Zhou T., Gu B., Huang X., Shangguan Y., Zhu J., et al. 2013. An‐1 encodes a basic helix‐loop‐helix protein that regulates awn development, grain size, and grain number in rice. Plant Cell 25: 3360–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magwa, R. A. , Zhao H., Yao W., Xie W., Yang L., Xing Y., and Bai X.. 2016. Genomewide association analysis for awn length linked to the seed shattering gene qSH1 in rice. Journal of Genetics 95: 639–646. [DOI] [PubMed] [Google Scholar]
- McAllister, C. A. , McKain M. R., Li M., Bookout B., and Kellogg E. A.. 2019. Specimen‐based analysis of morphology and the environment in ecologically dominant grasses: the power of the herbarium. Philosophical Transactions of the Royal Society, B, Biological Sciences 374: 20170403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen, T. , and Goriely A.. 2002. Tendril perversion in intrinsically curved rods. Journal of Nonlinear Science 12: 241–281. [Google Scholar]
- Molano‐Flores, B. 2012. Diaspore morphometrics and self‐burial in Hesperostipa spartea from loam and sandy soils. Journal of the Torrey Botanical Society 139: 56–62. [Google Scholar]
- Moskalenko, G. L. 1930. Investigation of the dependence between awnedness and the elements of productivity, on hybrids of winter wheat. Proceedings of the U.S.S.R. Congress of Genetics, Plant and Animal Breeding 4: 227–241. [Google Scholar]
- Mouillot, F. , and Field C. B.. 2005. Fire history and the global carbon budget: a 1° × 1° fire history reconstruction for the 20th century. Global Change Biology 11: 398–420. [Google Scholar]
- Mouissie, A. M. , Lengkeek W., and Van Diggelen R.. 2005. Estimating adhesive seed‐dispersal distances: field experiments and correlated random walks. Functional Ecology 19: 478–486. [Google Scholar]
- Murbach, L. 1900. Note on the mechanics of the seed‐burying awns of Stipa avenacea . Botanical Gazette 30: 113–117. [Google Scholar]
- Naresh, J. S. , and Smith C. M.. 1984. Feeding preference of the rice stink bug on annual grasses and sedges. Entomologia Experimentalis et Applicata 35: 89–92. [Google Scholar]
- Ntakirutimana, F. , Xiao B., Xie W., Zhang J., Zhang Z., Wang N., and Yan J.. 2019. Potential effects of awn length variation on seed yield and components, seed dispersal and germination performance in Siberian wildrye (Elymus sibiricus L.). Plants 8: 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olugbemi, L. B. 1978. Distribution of carbon‐14 assimilated by wheat awns. Annals of Applied Biology 90: 111–114. [Google Scholar]
- Parsons, M. W. , and Munkvold G. P.. 2010. Relationships of immature and adult thrips with silk‐cut, fusarium ear rot and fumonisin B1 contamination of maize in California and Hawaii. Plant Pathology 59: 1099–1106. [Google Scholar]
- Peart, M. H. 1979. Experiments on the biological significance of the morphology of seed‐dispersal units in grasses. Journal of Ecology 67: 843–863. [Google Scholar]
- Peart, M. H. 1981. Further experiments on the biological significance of the morphology of seed‐dispersal units in grasses. Journal of Ecology 69: 425–436. [Google Scholar]
- Peart, M. H. 1984. The effects of morphology, orientation and position of grass diaspores on seedling survival. Journal of Ecology 72: 437–453. [Google Scholar]
- Peart, M. H. , and Clifford H. T.. 1987. The influence of diaspore morphology and soil‐surface properties on the distribution of grasses. Journal of Ecology 75: 569–576. [Google Scholar]
- POWO . 2021. Plants of the world online. Facilitated by the Royal Botanic Gardens, Kew, UK. Website: https://powo.science.kew.org [accessed 13 September 2021].
- Quekett, E. J. 1844. On the structure of some tissues possessing hygrometric properties. Transactions of the Microscopical Society of London 1: 22–31. [Google Scholar]
- Quick, Z. I. , Houseman G. R., and Büyüktahtakin İ. E.. 2017. Assessing wind and mammals as seed dispersal vectors in an invasive legume. Weed Research 57: 35–43. [Google Scholar]
- Rabinowitz, D. , and Rapp J. K.. 1981. Dispersal abilities of seven sparse and common grasses from a Missouri prairie. American Journal of Botany 68: 616–624. [Google Scholar]
- Rebetzke, G. , Bonnett D., and Reynolds M.. 2016. Awns reduce grain number to increase grain size and harvestable yield in irrigated and rainfed spring wheat. Journal of Experimental Botany 67: 2573–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, A. W. , Kaufman G. A., and Kaufman D. W.. 2004. Influence of fire, topography, and consumer abundance on seed predation in tallgrass prairie. Canadian Journal of Zoology 82: 1459–1467. [Google Scholar]
- Revell, L. J. . 2012. phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Rissing, S. W. 1981. Foraging specializations of individual seed‐harvester ants. Behavioral Ecology and Sociobiology 9: 149–152. [Google Scholar]
- Rosas, C. A. , Engle D. M., Shaw J. H., and Palmer M. W.. 2008. Seed dispersal by Bison bison in a tallgrass prairie. Journal of Vegetation Science 19: 769–778. [Google Scholar]
- Saarela, J. M. , Burke S. V., Wysocki W. P., Barrett M. D., Clark L. G., Craine J. M., Peterson P. M., et al. 2018. A 250 plastome phylogeny of the grass family (Poaceae): topological support under different data partitions. PeerJ 6: e4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanad, M. N. , Smertenko A., and Garland‐Campbell K. A.. 2019. Differential dynamic changes of reduced trait model for analyzing the plastic response to drought phases: a case study in spring wheat. Frontiers in Plant Science 10: 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöning, C. , Espadaler X., Hensen I., and Roces F.. 2004. Seed predation of the tussock‐grass Stipa tenacissima L. by ants (Messor spp.) in south‐eastern Spain: the adaptive value of trypanocarpy. Journal of Arid Environments 56: 43‐61. [Google Scholar]
- Schrager‐Lavelle, A. , Klein H., Fisher A., and Bartlett M.. 2017. Grass flowers: an untapped resource for floral evo‐devo. Journal of Systematics and Evolution 55: 525‐541. [Google Scholar]
- Simpson, K. J. , Jardine E. C., Archibald S., Forrestel E. J., Lehmann C. E., Thomas G. H., and Osborne C. P.. 2021. Resprouting grasses are associated with less frequent fire than seeders. New Phytologist 230: 832‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, M. 1952. Value of the awn in establishing seed of Danthonia penicillata (Labill.) Palisot. New Zealand Journal of Science and Technology 34: 360‐364. [Google Scholar]
- Singh, B. U. 1987. Varietal resistance in sorghum to midge, Contarinia sorghicola Coquillett (Diptera: Cecidomyiidae). International Journal of Tropical Insect Science 8: 129‐144. [Google Scholar]
- Soreng, R. J. , Peterson P. M., Romaschenko K., Davidse G., Teisher J. K., Clark L. G., Barberá P., et al. 2017. A worldwide phylogenetic classification of the Poaceae (Gramineae) II: an update and a comparison of two 2015 classifications. Journal of Systematics and Evolution 55: 259‐290. [Google Scholar]
- Sorensen, A. E. 1986. Seed dispersal by adhesion. Annual Review of Ecology and Systematics 17: 443‐463. [Google Scholar]
- Stayton, C. T. 2015. What does convergent evolution mean? The interpretation of convergence and its implications in the search for limits to evolution. Interface Focus 5: 20150039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins, G. L. 1971. Adaptive radiation of reproductive characteristics in angiosperms, II: seeds and seedlings. Annual Review of Ecology and Systematics 2: 237‐260. [Google Scholar]
- Tackenberg, O. , Römermann C., Thompson K., and Poschlod P.. 2006. What does diaspore morphology tell us about external animal dispersal? Evidence from standardized experiments measuring seed retention on animal‐coats. Basic and Applied Ecology 7: 45‐58. [Google Scholar]
- Tambussi, E. A. , Bort J., Guiamet J. J., Nogués S., and Araus J. L.. 2007. The photosynthetic role of ears in C3 cereals: metabolism, water use efficiency and contribution to grain yield. Critical Reviews in Plant Sciences 26: 1‐16. [Google Scholar]
- R Core Team . 2022. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Website: https://www.R-project.org/ [Google Scholar]
- Teisher, J. , McKain M., Schaal B., and Kellogg E.. 2017. Polyphyly of Arundinoideae (Poaceae) and evolution of the twisted geniculate lemma awn. Annals of Botany 120: 725‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindall, K. V. , Williams B. J., Stout M. J., Geaghan J. P., Leonard B. R., and Webster E. P.. 2005. Yield components and quality of rice in response to graminaceous weed density and rice stink bug populations. Crop Protection 24: 991‐998. [Google Scholar]
- Titulaer, M. , Melgoza‐Castillo A., Macías‐Duarte A., and Panjabi A. O.. 2018. Seed size, bill morphology, and handling time influence preferences for native vs. nonnative grass seeds in three declining sparrows. Wilson Journal of Ornithology 130: 445‐456. [Google Scholar]
- Toriba, T. , and Hirano H.. 2014. The DROOPING LEAF and OsETTIN2 genes promote awn development in rice. Plant Journal 77: 616‐626. [DOI] [PubMed] [Google Scholar]
- Toriba, T. , Suzaki T., Yamaguchi T., Ohmori Y., Tsukaya H., and Hirano H.. 2010. Distinct regulation of adaxial–abaxial polarity in anther patterning in rice. Plant Cell 22: 1452–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pijl, L. 1982. Principles of dispersal, 3rd ed. Springer‐Verlag, Berlin, Germany. [Google Scholar]
- Wang, D. , Yu K., Jin D., Sun L., Chu J., Wu W., Xin P., et al. 2019. ALI‐1, candidate gene of B1 locus, is associated with awn length and grain weight in common wheat. bioRxiv . 10.1101/688085 [Preprint]. [DOI]
- Will, H. , Maussner S., and Tackenberg O.. 2007. Experimental studies of diaspore attachment to animal coats: predicting epizoochorous dispersal potential. Oecologia 153: 331–339. [DOI] [PubMed] [Google Scholar]
- Willard, J. R. , and Crowell H. H.. 1965. Biological activities of the harvester ant, Pogonomyrmex owyheei, in central Oregon. Journal of Economic Entomology 58: 484–489. [Google Scholar]
- Yuo, T. , Yamashita Y., Kanamori H., Matsumoto T., Lundqvist U., Sato K., Ichii M., et al. 2012. A SHORT INTERNODES (SHI) family transcription factor gene regulates awn elongation and pistil morphology in barley. Journal of Experimental Botany 63: 5223–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. , Kong W., Robertson J., Goff V. H., Epps E., Kerr A., Mills G., et al. 2015. Genetic analysis of inflorescence and plant height components in sorghum (Panicoidae) and comparative genetics with rice (Oryzoidae). BMC Plant Biology 15: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Awn traits assembled from GrassBase.
Appendix S2. Alternative version of Figure 2 with species visible on tree tips.


