Abstract
In this study different inbred strains of mice appeared to control and contain a low dose aerosol infection with Mycobacterium tuberculosis in a similar manner, giving rise to a chronic state of disease. Thereafter, however, certain strains gradually began to show evidence of regrowth of the infection, whereas others consistently did not. Using C57BL/6 mice as an example of a resistant strain and CBA/J mice as an example of a strain susceptible to bacterial growth, we found that these animals revealed distinct differences in the cellular makeup of lung granulomas. The CBA/J mice exhibited a generally poor lymphocyte response within the lungs and vastly increased degenerative pathology at a time associated with regrowth of the infection. As a possible explanation for these events, it was then observed that the CBA/J mouse strain was also less able to upregulate adhesion molecules, including CD11a and CD54, on circulating lymphocytes. These results therefore suggest that a failure to control a chronic infection with M. tuberculosis may reflect an inability to localize antigen-specific lymphocytes within the lung.
Disease caused by Mycobacterium tuberculosis is often not due to primary infection but instead is caused by reactivation of a latent or dormant infection that the patient may have carried for many years (20). It is unclear however, how the host initially expresses resistance in the lung and why this resistance is eventually lost, thus allowing bacterial regrowth. In this regard, the mouse is a useful model of specific resistance to M. tuberculosis infection. After low or moderate doses of bacilli are delivered by either intravenous or aerogenic routes, an apparently stable chronic disease state is established after a few weeks in the infected organs of the animal (3, 24). This chronic infection continues for a prolonged period in the C57BL/6 mouse strain until influenced by immunosenescence (23). It is currently speculated that during the chronic phase of infection, the bacteria remain in some form of latent state.
That this assumption may be wrong, however, is suggested by recent evidence indicating that chronic M. tuberculosis infection in the lung is in fact a dynamic event (27), at least in terms of granuloma pathology, which changes dramatically over the life span of the animal. In addition, studies directed toward understanding the role of the Bcg gene (Nramp1) have shown that certain inbred strains of mice clearly differ in their ability to survive a chronic M. tuberculosis infection (16, 18), in confirmation of much earlier reports of this phenomenon (15, 26).
The results of the current study confirm and extend these findings by showing that certain strains of mice, although able to initially control a low-dose aerosol infection with M. tuberculosis, eventually succumb during the chronic phase of disease. The current study shows that this early mortality was associated with an increased bacterial burden within the lung and occurred prior to events that could be attributed to immunosenesence (23). Comparison of the pathology in reactivation-prone mice and those which were able to maintain the chronic disease state highlighted profound differences as early as the first few weeks into infection. Of these, the most obvious difference was the predominance of macrophages, combined with a minimal lymphocyte influx, within the lesions of the susceptible mouse strains. This absence of lymphocytes was associated with a failure to upregulate the T-cell adhesion molecules CD11a and CD54 on circulating lymphocytes. These data thus imply that strains of mice prone to this regrowth or reactivation phenomenon are less able to recruit lymphocytes to the site of infection and that this inability then predisposes the animal to an increased likelihood of bacterial regrowth at a later time.
MATERIALS AND METHODS
Mice.
These studies were performed using specific-pathogen-free C57BL/6, DBA/2, or CBA/J female mice (Jackson Laboratories, Bar Harbor, Maine) at 6 to 8 weeks of age. Mice were kept in ABL-3 biohazard conditions throughout the study and maintained on sterile chow and water ad libitum. The specific-pathogen-free nature of the mouse colonies was demonstrated by testing sentinel animals. These were shown to be negative for 12 known mouse pathogens.
Bacteria.
M. tuberculosis strains Erdman, H37Rv, and CSU22 were grown from low-passage seed lots in Proskauer-Beck liquid media containing 0.02% Tween 80 to mid-log phase and then separated into aliquots and frozen at −70°C until use.
Bacterial infections.
Mice were infected via the aerosol route with a low dose (102) or a high dose (103) of bacteria. Briefly, the nebulizer compartment of a Middlebrook airborne infection apparatus (Glas-col, Terre Haute, Ind.) was filled with 5 ml of distilled water containing a suspension of bacteria known to deliver approximately 100 or 1,000 bacteria per lung.
Enumeration of bacteria.
The numbers of viable bacteria in the lungs were monitored over time by plating serial dilutions of individual whole-organ homogenates onto nutrient Middlebrook 7H11 agar and counting the bacterial colony formations after 21 days incubation at 37°C. The data was expressed as the log10 value of the mean number of bacteria recovered (n = 4 animals).
Estimation of mouse morbidity.
Infected mice were monitored regularly, and those exhibiting signs of recrudescent disease were euthanized. Markers for poor health were loss of weight, poor coat condition, and lethargy.
Histology.
The lower right lung lobe from each mouse was inflated with 10% formal saline. Tissues were prepared routinely and sectioned for light microscopy with lobe orientation designed to allow for the maximum surface area of each lobe to be seen. Consecutive sections were stained with hematoxylin and eosin or with Ziehl-Neelsen stain for the detection of acid-fast bacilli. Sections were examined by a veterinary pathologist without prior knowledge of the experimental groups and evaluated at least twice to verify the reproducibility of the observations.
Flow cytometry.
A single cell suspension was prepared from spleens as described previously (9). Cells from each individual mouse were incubated with specific antibody (fluorescein isothiocyanate [FITC], phycoerythrin [PE], PerCP, or allophycocyanin [APC] labeled at 25 μg/ml) for 30 min at 4°C and in the dark. After two washes in D-RPMI lacking biotin and phenol red (Irvine Scientific), cells were washed three times prior to analysis on a Becton Dickinson FACSCalibur. Lymphocytes were gated by forward and side scatter, and CD4+ and CD8+ T cells were characterized by the presence of specific fluorescence labeled antibody. Cell surface markers analyzed were FITC-labeled CD3ɛ (clone 145-2C11), CD11a (clone 2D7), or CD54 (clone 3E2); PE-labeled CD3ɛ (clone 145-2C11); PerCP-labeled CD8 (clone 53-6.7); and APC-labeled CD4 (clone RM4-5). Appropriate isotype control antibodies were included in each analysis. All antibodies were purchased from Pharmingen (San Diego, Calif.). Data were analyzed using CellQuest (Becton Dickinson, San Diego, Calif.).
Bone marrow-derived macrophage cultures.
Bone marrow-derived macrophages were prepared 1 week prior to use. Mice were sacrificed, and the femurs and tibias were removed by dislocation from the joint and extraction using forceps and dissection scissors. The bones were placed into Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum, 10% L-929 fibroblast conditioned supernatant, 1% HEPES buffer (1 M; Sigma), 1% l-glutamine (200 nM; Sigma), and 2% Minimal essential medium-nonessential amino acids (100×; Sigma). The femurs were sheared at the proximal end and flushed with 5 ml of the supplemented media (S-DMEM). The tibias were snipped above the ankle joint, sheared in a similar manner to the femur at the proximal end, and flushed with 5 ml of S-DMEM. The suspension was centrifuged, and the pellets were resuspended in S-DMEM. Cells were seeded into petri dishes (Corning, N.Y.) for 6 days before transfer to flat-bottom 96-well tissue culture plates at a concentration of 2 × 105/well. Wells were replenished with S-DMEM every 48 h.
CD4 overlay assays.
One day before the CD4 T-cell purification, the S-DMEM medium was replaced with medium lacking L-929, and the macrophages were pulsed with culture filtrate proteins (CFP) (10 μg/ml) (supplied by J. Belisle [under NIH contract AI-75320], Department of Microbiology, Colorado State University) or ovalbumin (10 μg/ml). The following day the spleens were harvested from infected mice and gently dispersed through a mesh sieve. Red blood cells were lysed using Geys lysis buffer and resuspended in phosphate-buffered saline plus 2.5% bovine serum albumin. Cells were incubated with anti-CD4 microbeads for 15 min (Miltenyi Biotec, Auburn, Calif.). CD4 T cells were purified using a magnetic column (Miltenyi Biotec) and resuspended at 106 cells/ml in DMEM (Sigma, St. Louis, Mo.) plus supplements. Pure CD4 T cells (>95% pure, assessed by flow cytometry) were overlaid onto macrophages prepulsed with antigen. Cultures were incubated for 72 h at 37°C, 5% CO2 prior to cytokine analysis.
IFN-γ ELISA.
Supernatants were harvested from CD4 T cell overlay cultures and assayed for the presence of gamma interferon (IFN-γ) by enzyme-linked immunosorbent assay (ELISA). Antibodies were purchased from Pharmingen. Briefly, the primary antibody (Pharmingen clone R4-6A2) was incubated overnight in 96-well round-bottom Immulon 2 plates in carbonated coating buffer. Excess antibody was washed away using PBS-Tween 20 (PBS-T). The wells were blocked with 3% bovine serum albumin in PBS-T. The samples were dispensed, in duplicate, into the wells. A standard curve for IFN-γ (Genzyme) was prepared for each individual plate. Cytokine production was detected by the addition of a secondary biotinylated antibody (Pharmingen clone XMG1.2), followed by avidin-peroxidase (Zymed Labs, Inc., San Francisco, Calif.) and TMB substrate (Dako, Carpinteria, Calif.).
Statistical analysis.
Statistical significance was carried out using the Student t test and found to be significant (P < 0.05) or highly significant (P < 0.005).
RESULTS
Increased susceptibility to tuberculosis is characterized by a regrowth of bacteria in the lung.
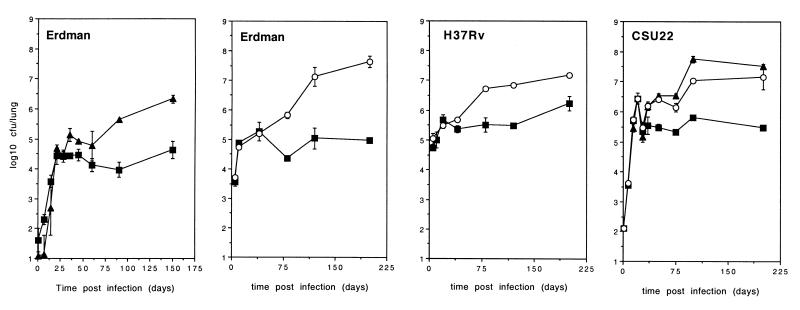
As previously described (16, 18), mouse strains differ in their susceptibility to M. tuberculosis, even in the aerosol model (17). By comparing the progression of aerosol infection between C57BL/6, DBA/2, and CBA/J mice, we were able to confirm these observations by detecting increased bacterial numbers in the lungs of the recrudescence-susceptible CBA/J and DBA/2 strains (Fig. 1). This increase in numbers correlated with decreased survival (mean survival time from high-dose [103] aerosol infection with M. tuberculosis Erdman; C57BL/6, 345 ± 23 days; CBA/J, 232 ± 26 days; and DBA/2, 186 ± 23 days). Increased bacterial numbers within the lungs of CBA/J and DBA/2 mice was also highly reproducible for other strains of M. tuberculosis (Fig. 1). Interestingly, the CBA/J and DBA/2 mouse strains were clearly able to stabilize the infection within the lung for at least 60 days, although often at a higher bacterial load. These mice were unable to maintain the chronic infection, however, and eventually succumbed. Hereafter, C57BL/6 and CBA/J mice are documented as representative examples.
FIG. 1.
Growth of M. tuberculosis in C57BL/6, DBA/2, and CBA/J mouse strains. C57BL/6 (■), CBA/J (▴), and DBA/2 (○) mice were aerogenically infected with M. tuberculosis Erdman, H37Rv, or CSU22. The bacterial load was calculated by plating partial organ homogenate onto 7H11 agar and counting colonies after 21 days at 37°C. The infective dose was calculated by plating lung homogenate 1 day after infection. The data are expressed as the mean ± the standard error of the mean from four mice per group. The graphs are representative of five (Erdman), two (CSU22), or two (H37Rv) independent experiments.
Progression of lung pathology in different mouse strains.
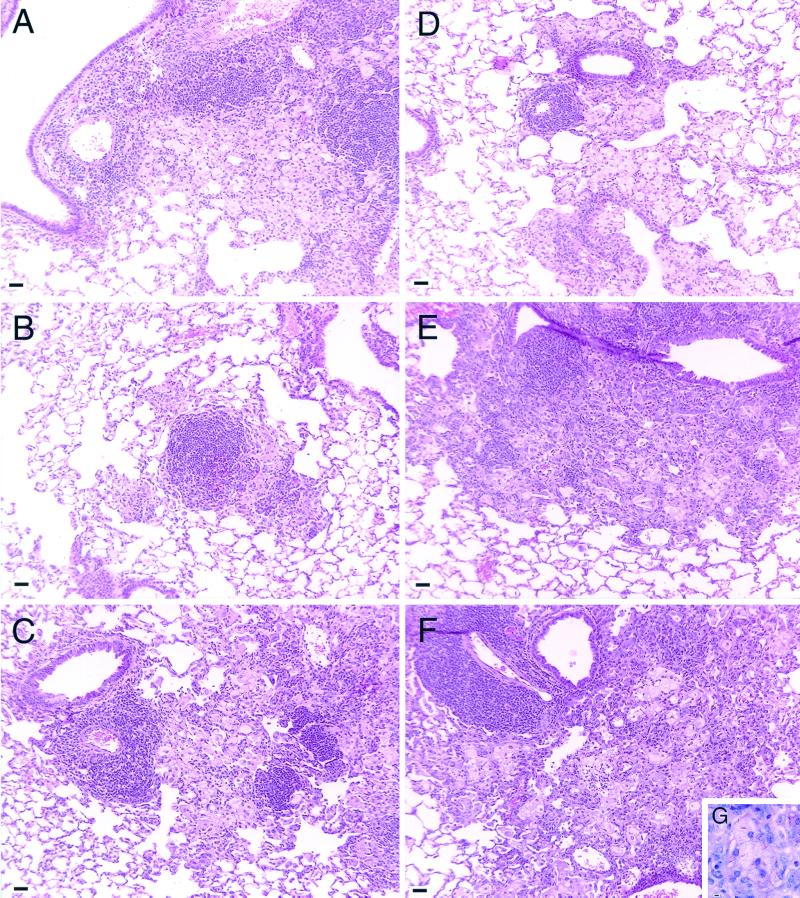
Examination of the lungs of the different mouse strains given a low-dose aerosol infection with M. tuberculosis Erdman revealed dramatic differences in the development and degeneration of the granulomatous response. In C57BL/6 mice, the lung lesions developed as we have previously described in detail (27). Briefly, moderately sized lesions were observed over the first 3 to 4 weeks after infection. The lesions consisted predominantly of macrophages. Some lymphocytes could be seen associated with these lesions, although most were perivascular. By day 60 (Fig. 2A), these perivascular cuffs were prominent, and large aggregates of lymphocytes were seen within the epithelioid macrophage fields. The development of organized multifocal granulomas containing lymphocytes and macrophages progressed through days 150 (Fig. 2B) and 295 (Fig. 2C).
FIG. 2.
Histological representation of lung lesions from infected mice. Formalin-fixed lung tissue was prepared and sectioned for light microcopy. The tissue was stained with hematoxylin and eosin, or serial sections were stained with Ziehl-Neelsen to detect acid-fast bacilli. (A) Lung lesions from C57BL/6 mice consisted of lymphocyte aggregates within epithelioid macrophage fields on day 60. Well-organized lesions consisting of both macrophages and lymphocytes developed through 150 (B) and 295 (C) days postinfection. (D) Lung lesions from CBA/J mice were smaller and contained many foamy macrophages by day 60. As the infection progressed through 150 days (E), the lesions became large and macrophage dominated, with extensive cholesterol deposition and tissue necrosis by 295 days after infection (F). (G) Degenerating tissue was associated with numerous acid-fast bacteria. Magnification, ×34.4; bar = 10 μm. The images are representative of three independent experiments.
The pathology seen in the CBA/J strain was markedly different. Early in infection, similar macrophage lesions were developing, but in general they appeared to be appreciably smaller than those seen in the C57BL/6 mice (Fig. 2D, 60 days postinfection). These lesions dramatically increased in size as the infection progressed, becoming much larger than those seen in the C57BL/6 mice. Most lesions contained many “foamy” macrophages, identified by their large size and abundant clear, small vacuoles filling the cytoplasm. Although some lymphocytes could be seen within the lesions, the majority of the lymphocytes were perivascular, and the lesions were largely devoid of lymphocyte aggregates. Degenerating macrophages, aggregates of neutrophils, and obvious cellular debris were evident, most likely a consequence of the increased bacterial numbers within the lungs.
Between day 150 (Fig. 2E) and 295 (Fig. 2F), this pathology gradually worsened in the CBA/J strain. Lesions became very large, consolidating much of the lung tissue. Scattered, small aggregates of lymphocytes were seen, but the majority of the lesions were dominated by degenerating macrophages with extensive cholesterol deposits indicative of macrophage necrosis. Areas of foamy macrophages, many associated with acid-fast bacilli, were infiltrated by clumps of neutrophils (Fig. 2G).
Circulating CD4 T lymphocytes from CBA/J mice can respond to CFP from M. tuberculosis.
The absence of lymphocyte foci within the lung lesions of CBA/J mice could have been a consequence of an inability of these mice to mount a strong antigen-specific T-cell response. To address this point, we purified CD4+ T cells from the splenic cell population and cultured them with CFP from M. tuberculosis. The purified CD4+ T cells from both the C57BL/6 and CBA/J mouse strains were capable of generating an IFN-γ response against macrophages pulsed with CFP throughout the course of the experiment (Table 1). Responses to ovalbumin throughout the experiment were equivalent to uninfected control data (not shown).
TABLE 1.
CFP-specific IFN-γ secretion by CD4+ T cells
| Time postinfection (days) | Mean IFN-γ secretion (pg/ml) ± SEM in:
|
|
|---|---|---|
| C57BL/6 mice | CBA/J mice | |
| 0 | 364 ± 196 | 1,021 ± 548 |
| 28 | 691 ± 151 | 1,160 ± 366 |
| 35 | 701 ± 154 | 778 ± 220 |
| 150 | 1,921 ± 697 | 3,825 ± 1,198 |
Splenocytes were harvested from infected C57BL/6 or CBA/J mice and cultured with bone marrow-derived macrophages which had been prepulsed with CFP from M. tuberculosis. Data are expressed as the mean values from four mice per group. The data are representative of two individual experiments.
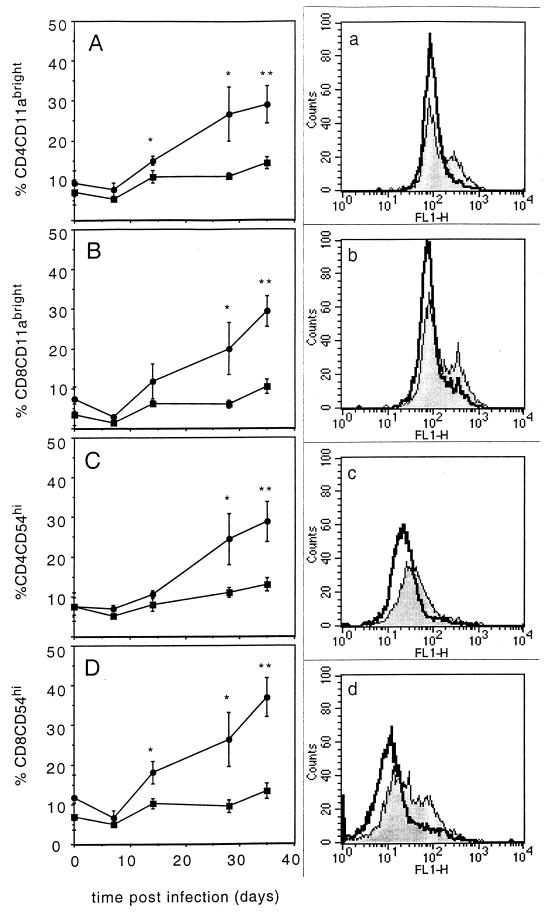
The expression of T-cell homing molecules is reduced on circulating lymphocytes from CBA/J mice.
To assess whether the circulating lymphocytes were capable of expressing the adhesion molecules associated with cellular migration to inflammatory sites, we analyzed splenic T cells from the different mouse strains for the upregulation of several adhesion molecules (Fig. 3). The most striking difference throughout the first 35 days of infection was the observation that circulating lymphocytes from the C57BL/6 mice expressed increasing levels of the adhesion molecule CD11a on their circulating CD4+ (Fig. 3A) and CD8+ (Fig. 3B) T cells. This shift to CD11abright expression was absent on the circulating T cells from CBA/J mice. In addition, lymphocytes from C57BL/6 mice demonstrated brighter expression of ICAM-1 (CD54) on both the CD4+ T cells (3C) and the CD8+ T cells (3D) than did the lymphocytes from CBA/J mice.
FIG. 3.
Upregulation of adhesion molecules on T cells. Splenocytes were harvested from infected C57BL/6 (circles and shaded histogram) and CBA/J (squares and open histogram) mice and stained for the expression of either CD4 in combination with CD11a (A) or CD54 (C) or else CD8 in combination with CD11a (B) or CD54 (D). Graphed data are expressed as the mean ± the standard error of the mean from four individual mice, and the histograms are representative of the experimental group after 35 days of M. tuberculosis infection. Statistical significance between molecule expression on T cells from C57BL/6 compared to CBA/J mice was calculated using the Student t test: ∗, P < 0.05; ∗∗, P < 0.005.
DISCUSSION
This study confirms and extends the findings of Medina and North (16, 18), who demonstrated that several mouse strains were more susceptible to M. tuberculosis infection than other mouse strains. As shown here, CBA/J and DBA/2 mice were able to control an aerosol infection, initially leading to a chronic disease state indistinguishable from that seen in the C57BL/6 mice. However, by about 60 days postinfection the bacterial load began to increase, a result which was associated with higher mortality rates in these mouse strains.
The development of disease in susceptible mouse strains was characterized by lung lesions consisting predominantly of macrophages and a few lymphocyte aggregates. As the infection progressed and the bacterial load increased, the lesions became degenerative, with evidence of macrophage breakdown and an influx of neutrophils. The data obtained support the hypothesis that the failure to adequately recruit lymphocytes into the infected lung was not due to a lack of antigen-specific immunity but instead was potentially associated with a failure to upregulate adhesion molecules that are associated with cellular localization of T cells to inflammatory sites (28, 29). Irregardless of whether lymphocytes were capable of producing IFN-γ in response to antigen, the failure to focus lymphocytes within infected tissue would compromise the ability to directly deliver this cytokine to its target.
Human genetic studies (10, 30) and the strikingly disparate results obtained from BCG vaccination trials (5, 6, 31) suggest that genetic linkage with susceptibility to M. tuberculosis infection may be an important issue. A recent study in Africa has identified markers on chromosomes 15q and Xq (2), which supports the hypothesis that susceptibility to M. tuberculosis is multifactoral in nature. What is clear from these studies is that it is extremely difficult to highlight one specific genetic trait which confers susceptibility to tuberculosis. Therefore, the importance of identifying biological correlates of protection is paramount in the identification of infected individuals who will later go on to develop reactivation disease.
In this study we demonstrate that the CBA/J mouse strain, despite evidence that it possessed the capacity to generate antigen-specific T lymphocytes, failed to upregulate the expression of two adhesion molecules, CD11a (LFA-1 component) and CD54 (ICAM-1), on the surface of circulating T lymphocytes. The inability to alter the expression of these two molecules was apparent on both the CD4+ and the CD8+ T-cell populations, which would clearly effect the ability of T lymphocytes to enter the lung. Indeed, the isolation of small numbers of cells from the infected lung shows that even CD4+ T cells from the lungs of CBA/J mice had significantly lower CD11a expression than similar cells isolated from the C57BL/6 mouse strain (unpublished observations).
The importance of interactions between adhesion molecules during M. tuberculosis infection has been highlighted recently in a study which demonstrated that blocking of the α4 integrin led to a reduction in lymphocyte-dominated lesions within the lung and the development of granulocyte-dominated lesions (4). The α4β7 integrin has been reported to be required for the subsequent engagement of LFA-1 (CD11a) and rolling of lymphocytes across HEV (1), and therefore the lack of α4 integrin interactions may also influence upstream events in the recruitment of lymphocytes into inflammatory sites. ICAM-1 is also upregulated on the epithelium and endothelium of cells infected with other bacteria (7, 12), thus suggesting a role for this molecule in cellular localization around infected tissue.
That differences in CD11a and CD54 expression could be a downstream event in CBA/J and DBA/2 mice needs to be addressed. The upregulation of these molecules is dependent on the responsiveness to inflammatory chemokines such as tumor necrosis factor or MCP-1 (8, 25). Chemokine mRNA within the lungs of infected C57BL/6 and CBA/J mice was equivalent (unpublished observations); however, we cannot as yet rule out the possibility that CBA/J and DBA/2 mice have deficiencies in the expression of chemokine receptors. A deficiency in C5a, another inflammatory mediator, has recently been reported in the A/J mouse strain (11), and C5 deficiency has been linked to susceptibility to infection with M. tuberculosis (11). Further studies will need to be carried out to address whether C5a deficiency is responsible for the susceptibility to M. tuberculosis seen in the CBA/J mouse or, indeed, in humans.
Different mouse strains have been used extensively in studies of M. tuberculosis immunity, the majority of which have demonstrated that several strains are indeed more susceptible to M. tuberculosis than others (15, 18, 21, 26). Our results are in keeping with those of Medina and North, who demonstrated that, after a low-dose infection with M. tuberculosis, all susceptible mouse strains survived for only 80 days after intravenous infection and for 120 days after aerosol infection (18). Examination of lung lesions showed that the DBA/2 mouse strain had bigger lesions consisting of more foamy epithelioid macrophages, as we also demonstrate in more detail here with the CBA/J mouse (16). In contrast, recent studies by Nikonenko et al. have shown that the I/St and A/Sn mouse strains express different susceptibility phenotypes much earlier (21). Although these two genetically similar strains are useful for identifying particular genetic determinants, the M. tuberculosis inoculum used was very high and the survival time was extremely reduced even in the resistant strain. High doses of M. tuberculosis administered into the lung will induce severe inflammation, and therefore this is not a useful model for the control of chronic M. tuberculosis infection.
The genetic determinants of resistance to M. tuberculosis have been long sought after, and these susceptible mouse models have been useful tools in aiding this search. Despite the obvious coassociation of Nramp1 expression in the susceptible mouse strains (18), it has been clearly demonstrated that Nramp1 alone is not able to confer an M. tuberculosis-resistant phenotype to a naturally susceptible strain (17, 19, 22). The resistant phenotype is evidently dominant (17, 18) and has been suggested to have H-2 linkages in the mouse, although non-major-histocompatibility-complex genes seem to also play a major role (18). The susceptible phenotype thus appears to be multifactoral in nature.
In support of this hypothesis, recent evidence has identified several gene linkages associated with susceptibility in the mouse, including genes associated with weight loss and short survival time on chromosomes 9 and 3 of the I/St mouse strain (14). In studies using the C57BL/6 and C3HeB/FeJ mouse strains, susceptibility has also been associated with a 9-centimorgan interval on mouse chromosome 1 named sst1 (13), which is located close to but not including the Nramp1 site and which confers some degree of resistance to pulmonary tuberculosis. Whether this gene plays a role in other M. tuberculosis- susceptible mouse strains has yet to be addressed. What is certain is that these single genes alone are not able to confer susceptibility.
In conclusion, we have demonstrated that the CBA/J mouse strain, previously characterized as susceptible to M. tuberculosis infection (18), develops lung lesions consisting predominantly of macrophages. The absence of lymphocytic infiltration was associated with a failure to upregulate CD11a and CD54 molecules on the surface of the T lymphocytes, therefore compromising the ability of these cells to migrate to inflammatory sites and focus into distinct lesions normally observed in the resistant C57BL/6 mouse strain. Therefore, the upregulation of CD11a and CD54 in response to M. tuberculosis challenge may be a useful correlate of protection and resistance to reactivation diseases which could be tested in the field.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI-44072, AI-40488, and AG-06946.
REFERENCES
- 1.Bargatze R F, Jutila M A, Butcher E C. Distinct roles of L-selectin and integrins alpha 4 beta 7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 2.Bellamy R, Beyers N, McAdam K P W J, Ruwende C, Gie R, Samaai P, Bester D, Meyer M, Corrah T, Collin M, Camidge D R, Wilkinson D, Helden E H-V, Whittle H C, Amos W, Helden P V, Hill A V S. Genetic susceptibility to tuberculosis in Africans: a genome-wide scan. Proc Natl Acad Sci USA. 2000;97:8005–8009. doi: 10.1073/pnas.140201897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardona P J, Cooper A, Luquin M, Ariza A, Filipo F, Orme I M, Ausina V. The intravenous model of murine tuberculosis is less pathogenic than the aerogenic model owing to a more rapid induction of systemic immunity. Scand J Immunol. 1999;49:362–366. doi: 10.1046/j.1365-3083.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- 4.Feng C G, Britton W J, Palendira U, Groat N L, Briscoe H, Bean A G D. Up-regulation of VCAM-1 and differential expansion of β integrin-expressing T lymphocytes are associated with immunity to pulmonary Mycobacterium tuberculosis infection. J Immunol. 2000;164:4853–4860. doi: 10.4049/jimmunol.164.9.4853. [DOI] [PubMed] [Google Scholar]
- 5.Fine P E M. Immunities in and to tuberculosis: implications for pathogenesis and vaccination. In: Porter J D H, McAdam K P W J, editors. Tuberculosis: back to the future. London, England: John Wiley & Sons, Ltd.; 1994. pp. 53–71. [Google Scholar]
- 6.Fine P E M, Clayton D. Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet. 1996;348:17–24. [PubMed] [Google Scholar]
- 7.Frick A G, Joseph T D, Pang L, Rabe A M, St. Geme R J W, Look D C. Haemophilus influenzae stimulates ICAM-1 expression on respiratory epithelial cells. J Immunol. 2000;164:4185–4196. doi: 10.4049/jimmunol.164.8.4185. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Amaro R, Sanchez-Madrid F. Cell adhesion molecules: selectins and integrins. Crit Rev Immunol. 1999;19:389–429. [PubMed] [Google Scholar]
- 9.Griffin J P, Orme I M. Evolution of CD4 T-cell subsets following infection of naive and memory immune mice with Mycobacterium tuberculosis. Infect Immun. 1994;62:1683–1690. doi: 10.1128/iai.62.5.1683-1690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill A V. The immunogenetics of human infectious diseases. Annu Rev Immunol. 1998;16:593–617. doi: 10.1146/annurev.immunol.16.1.593. [DOI] [PubMed] [Google Scholar]
- 11.Jagannath C, Hoffman H, Sepulveda E, Actor J K, Wetsel R A, Hunter R L. Hypersusceptibility of A/J mice to tuberculosis is in part due to a deficiency of the fifth complement component. Scand J Immunol. 2000;52:369–379. doi: 10.1046/j.1365-3083.2000.00770.x. [DOI] [PubMed] [Google Scholar]
- 12.Jarvis G A, Li J, Swanson K V. Invasion of human mucosal epithelial cells by Neisseria gonorrhoeae upregulates expression of intercellular adhesion molecule 1 (ICAM-1) Infect Immun. 1999;67:1149–1156. doi: 10.1128/iai.67.3.1149-1156.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramnik I, Dietrich W F, Demant P, Bloom B R. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2000;97:8560–8565. doi: 10.1073/pnas.150227197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavebratt C, Apt A S, Nikonenko B V, Schalling M, Schurr E. Severity of tuberculosis in mice is linked to distal chromosome 3 and proximal chromosome 9. J Infect Dis. 1999;180:150–155. doi: 10.1086/314843. [DOI] [PubMed] [Google Scholar]
- 15.Lynch C J, Pierce-Chase C H, Dubos R. A genetic study of susceptibility to experimental tuberculosis in mice infected with mammalian tubercle bacilli. J Exp Med. 1965;121:1051–1069. doi: 10.1084/jem.121.6.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medina E, North J. Genetically susceptible mice remain proportionally more susceptible to tuberculosis after vaccination. Immunology. 1999;96:16–21. doi: 10.1046/j.1365-2567.1999.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina E, North R J. Evidence inconsistent with a role for the Bcg gene (Nramp1) in resistance of mice to infection with virulent Mycobacterium tuberculosis. J Exp Med. 1996;183:1045–1051. doi: 10.1084/jem.183.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medina E, North R J. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology. 1998;93:270–274. doi: 10.1046/j.1365-2567.1998.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medina E, Rogerson B J, North R J. The Nramp1 antimicrobial resistance gene segregates independently of resistance to virulent Mycobacterium tuberculosis. Immunology. 1996;88:479–481. doi: 10.1046/j.1365-2567.1996.d01-700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray C J L, Styblo K, Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Int Union Tuberc Lung Dis. 1990;65:6–24. [PubMed] [Google Scholar]
- 21.Nikonenko B V, Averbakh J M M, Lavebratt C, Schurr E, Apt A S. Comparative analysis of mycobacterial infections in susceptible I/St and resistant A/Sn inbred mice. Tuberc Lung Dis. 2000;80:15–25. doi: 10.1054/tuld.1999.0225. [DOI] [PubMed] [Google Scholar]
- 22.North R J, LaCourse R, Ryan L, Gros P. Consequence of Nramp1 deletion to Mycobacterium tuberculosis infection in mice. Infect Immun. 1999;67:5811–5814. doi: 10.1128/iai.67.11.5811-5814.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orme I M. Aging and immunity to tuberculosis: increased susceptibility of old mice reflects a decreased capacity to generate mediator T lymphocytes. J Immunol. 1987;138:4414–4418. [PubMed] [Google Scholar]
- 24.Orme I M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138:293–298. [PubMed] [Google Scholar]
- 25.Petruzzelli L, Takami M, Humes H D. Structure and function of cell adhesion molecules. Am J Med. 1999;106:467–476. doi: 10.1016/s0002-9343(99)00058-3. [DOI] [PubMed] [Google Scholar]
- 26.Pierce C, Dubos R J, Middlebrook G. Infection of mice with mammalian tubercle bacilli grown in Tween-albumin liquid medium. J Exp Med. 1947;86:159–175. doi: 10.1084/jem.86.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhoades E R, Frank A A, Orme I M. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuberc Lung Dis. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 28.Springer T A. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 29.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 30.Stead W W, Senner J W, Reddick W T, Lofgren J P. Racial differences in susceptibilities to infection by Mycobacterium tuberculosis. N Engl J Med. 1990;322:422–427. doi: 10.1056/NEJM199002153220702. [DOI] [PubMed] [Google Scholar]
- 31.Tripathy S P. Fifteen year follow-up of the Indian BCG prevention trial. Bull Int Union Tuberc Lung Dis. 1987;62:69–72. [Google Scholar]