Abstract
Background
Limited evidence supports the omission of routine bone marrow (BM) examination (biopsy and aspiration) in patients with nasal‐type extranodal NK/T‐cell lymphoma (ENKTCL). This study was aimed at assessing whether BM examination provides valuable information for positron emission tomography/computed tomography (PET/CT)–based staging in this patient population.
Patients and Methods
Patients newly diagnosed with ENKTCL who underwent initial staging with both PET/CT and BM examination between 2013 and 2020 were retrospectively identified in two Chinese institutions. Overall, 742 patients were included; the BM examination was positive in 67 patients.
Results
Compared with BM biopsy alone, the combination of BM biopsy and aspiration assessment did not afford any additional diagnostic value. No patient with a positive BM biopsy was found to have early‐stage disease by PET/CT. BM biopsy or PET/CT led to upstaging from stage III to IV as a result of BM involvement in 21 patients. In 135 patients with distant organ involvement, BM involvement was associated with worse overall survival (OS) and progression‐free survival (PFS) compared with the corresponding durations in patients without BM involvement (2‐year OS: 35.9% vs. 60.4%, p < .001; PFS: 26% vs. 40.7%, p = .003). No difference in survival was noted between groups judged positive based on PET/CT and BM biopsy.
Conclusion
Compared with aspiration, BM biopsy led to the detection of more BM lesions. Baseline PET/CT can be safely used to exclude BM involvement in early‐stage disease. Overall, routine BM examination affords diagnostic or prognostic value over PET/CT in patients with advanced‐stage nasal‐type ENKTCL.
Keywords: bone marrow examination, NK/T‐cell lymphoma, PET/CT, prognosis, survival
Short abstract
Baseline positron emission tomography/computed tomography (PET/CT) can be safely used to exclude bone marrow (BM) involvement in patients with early‐stage nasal‐type extranodal NK/T‐cell lymphoma (ENKTCL). Compared with PET/CT alone, routine BM examination can afford better diagnostic and prognostic value in advanced‐stage nasal‐type ENKTCL.
INTRODUCTION
Extranodal NK/T‐cell lymphoma (ENKTCL) nasal type is a rare neoplasm that is primarily prevalent in East Asia and less frequently in Western countries. 1 , 2 , 3 At initial diagnosis, 70% to 90% of patients have early‐stage disease, and most can be cured by nonanthracycline (non‐ANT)‐based chemotherapy and upfront radiotherapy. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 On the other hand, patients with advanced‐stage disease who were treated with primary chemotherapy showed extremely poor survival. 13 , 14 , 15 , 16 , 17 , 18 Thus, accurate staging and risk stratification of disease are critical in the context of risk‐adapted treatment strategies. 5 , 7 , 19 , 20 , 21
Although some new prognostic indexes or staging systems have been used for more accurate risk stratification, 22 , 23 , 24 , 25 , 26 the Ann Arbor classification remains an essential tool for the assessment of disease burden in the era of non–ANT‐based regimens. Bone marrow (BM) involvement is considered a sign of generalized disease and is associated with a poor prognosis for ENKTCL and other lymphomas, because it signifies stage IV according to the Ann Arbor staging system. 27 , 28 For many decades, the BM examination (biopsy and aspiration) has been the benchmark test for the evaluation of BM status and a cornerstone of the routine staging workup for lymphomas. Whole‐body positron emission tomography/computed tomography (PET/CT) is now commonly used as a standard staging modality for fluorodeoxyglucose (FDG)‐avid aggressive lymphomas, including ENKTCLs. 5 , 29 , 30 , 31 However, routine BM examination is no longer considered necessary in the normal staging of Hodgkin lymphoma (HL), because the occasionally missed BM involvement by PET/CT rarely changes the risk assessment and management. 30 , 32 , 33 , 34
In ENKTCLs, because of a lack of high‐level evidence, the staging of BM involvement is largely based on experience with other non‐HLs (NHLs). The wide range of reported detection rates (6%‐20%) for BM involvement raises questions about applied methodologies and possible sample collection bias. 18 , 22 , 24 , 26 , 35 , 36 , 37 In addition, unlike for other NHLs, there are insufficient data available on the sensitivity and specificity of PET/CT for detecting BM involvement in ENKTCL. 38 , 39 , 40 For these reasons, recent guidelines recommending routine BM examination for all patients with ENKTCL who have undergone staging using PET/CT have been controversial. 41 , 42 Therefore, we designed a large‐scale retrospective study to quantify the diagnostic and prognostic value afforded by routine BM examination in patients with newly diagnosed nasal‐type ENKTCL who have undergone PET/CT staging.
PATIENTS AND METHODS
Study population
Patients newly diagnosed with nasal‐type ENKTCL at Fujian Medical University Union Hospital from January 2013 to December 2020 were retrospectively reviewed to establish the primary cohort. Initial staging evaluations included history taking and a physical examination; endoscopic examinations of the upper aerodigestive tract; CT scans of the head and neck, chest, abdomen, and pelvis; magnetic resonance imaging of the head and neck; and unilateral posterior iliac crest BM examination. PET/CT was recommended for all patients. Staging was performed using the Ann Arbor staging system. Patients with the following characteristics were excluded from this study: (1) those who had not undergone prior treatment or had disease relapse; (2) those with other lymphomas or malignant diseases; and (3) those who had not undergone BM examination.
The validation cohort was retrospectively recruited at the Sun Yat‐Sen University Cancer Center between January 2013 and December 2020. After the BM examination for ENKTCL, the results obtained in the primary cohort were validated in an independent cohort of patients who were not included in the primary cohort. The prognostic value of BM status was validated in this validation cohort. All aspects of this study were reviewed and approved by the institution's review boards, which waived the requirement for signed informed consent because of the retrospective nature of the study.
BM examination and PET/CT scanning
All patients were diagnosed with ENKTCL according to criteria of the World Health Organization classification of lymphomas. 43 Routine blind unilateral posterior iliac crest trephine biopsy and/or aspiration was performed before treatment. The diagnosis of lymphoma was based on standard morphology and immunohistochemistry locally by dedicated hematopathologists. CD56, CD3ε, and Epstein‐Barr virus‐encoded RNA were examined in specific cases of BM involvement. The baseline PET scans were visually evaluated. A positive BM examination result was defined as focal FDG uptake higher than that in normal liver that could not be attributed to any other cause (e.g., bone fractures, elevated uptake caused by BM biopsy) at least one site. Diffusely increased FDG uptake without focal lesions was considered indicative of a negative BM examination result; otherwise, it was considered positive.
Statistical methods
Continuous variables are summarized as median values and ranges, and categorical variables are summarized as frequencies and percentages. Statistical comparisons of categorical variables were usually performed using the Pearson χ2 test. The Mann‐Whitney U test and Pearson χ2 test were used to compare continuous and categorical variables, respectively. The diagnostic performances of PET and BM aspiration were assessed considering only BM biopsy as the gold standard. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy of PET and BM aspiration for detecting BM involvement were assessed considering BM biopsy results as reference standards. Clopper‐Pearson exact confidence intervals were calculated for sensitivity, specificity, PPV, NPV, and accuracy.
Overall survival (OS) was defined as the time from the diagnosis of ENKTCL until death from any cause or the date of the last follow‐up when the patient was alive. Progression‐free survival (PFS) was defined as the time from the diagnosis of ENKTCL until the first instance of disease progression, relapse, or death from any cause. OS and PFS were estimated using the Kaplan–Meier method, and differences between survival curves were compared using the log‐rank test. A two‐sided p value < .05 was considered significant. All statistical analyses were performed using SPSS, version 26.0 (IBM Inc), and R, version 4.1.2 (http://www.r‐project.org/).
RESULTS
Baseline characteristics
The final analyses included 742 patients from two institutions (Figure 1): the primary cohort (Fujian Medical University Union Hospital, n = 186) and the validation cohort (Sun Yat‐Sen University Cancer Center, n = 556). The baseline clinical characteristics and treatments are listed in Table 1. In the primary cohort, the median age at diagnosis was 47 years (interquartile range, 35‐58 years); and 33 (18%) of the 186 patients were older than 60 years. More than 50% of patients had early‐stage disease (stage I‐II), and most patients (74.7%) had undergone initial PET/CT scans. The validation cohort included a higher proportion of patients with favorable parameters, such as stage I or II disease (422 [75.9%]), good performance status (847 [98.6%]), more lactate dehydrogenase normal (369 [66.4%]), and less BM involvement (22 [4%]) compared with those in the primary cohort (Table 1).
FIGURE 1.
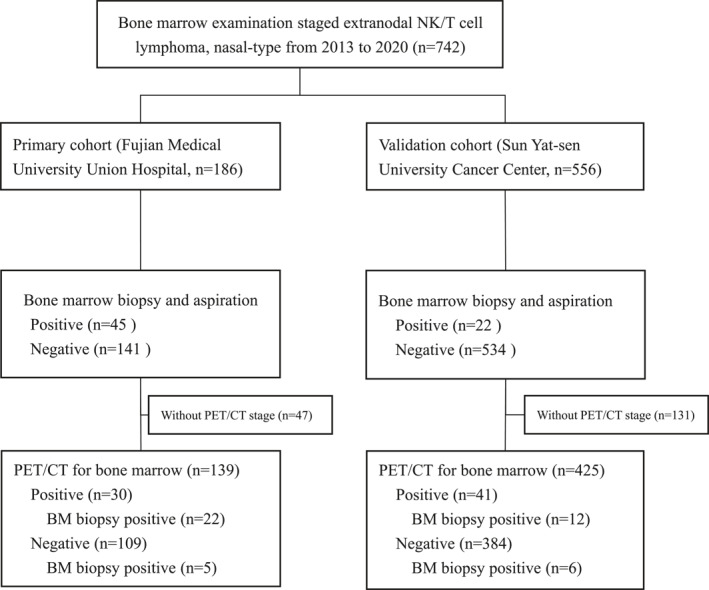
CONSORT diagram
TABLE 1.
Clinical characteristics of enrolled patients with extranodal NK/T‐cell lymphoma, nasal‐type (n = 742)
| Primary cohort | Validation cohort | |
|---|---|---|
| Characteristics | No. (%) | No. (%) |
| All | 186 | 556 |
| Sex | ||
| Male | 140 (75.3) | 391 (70.3) |
| Female | 46 (24.7) | 165 (29.7) |
| Age, y | ||
| ≤60 | 153 (82.3) | 472 (84.9) |
| >60 | 33 (17.7) | 84 (15.1) |
| Primary site | ||
| UADT | 143 (76.9) | 489 (87.9) |
| Extra‐UADT | 43 (23.1) | 67 (12.1) |
| Regional lymph nodes | ||
| Yes | 73 (39.2) | 271 (48.7) |
| No | 113 (60.8) | 285 (51.3) |
| Distant lymph nodes | ||
| Yes | 51 (27.4) | 52 (9.4) |
| No | 135 (72.6) | 504 (90.6) |
| B symptoms | ||
| Yes | 120 (64.5) | 186 (33.5) |
| No | 66 (35.5) | 370 (66.5) |
| Elevated LDH | ||
| Yes | 101 (54.3) | 187 (33.6) |
| No | 85 (45.7) | 369 (66.4) |
| ECOG score | ||
| 0‐1 | 140 (75.3) | 548 (98.5) |
| ≥2 | 46 (24.7) | 8 (1.5) |
| Ann Arbor stage | ||
| I‐II | 100 (53.8) | 423 (76.1) |
| III‐IV | 86 (46.2) | 133 (23.9) |
| Bone marrow aspiration | ||
| Negative | 156 (83.9) | 547 (98.4) |
| Positive | 30 (16.1) | 9 (1.6) |
| Bone marrow biopsy | ||
| Negative | 141 (75.8) | 534 (96.0) |
| Positive | 45 (24.2) | 22 (4.0) |
| PET/CT | ||
| Yes | 139 (74.7) | 425 (76.4) |
| No | 47 (25.3) | 131 (23.6) |
Abbreviations: CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; PET, positron emission tomography; UADT, upper aerodigestive tract.
BM biopsy and aspiration findings
All patients in the primary cohort whose BM biopsy and aspiration data were available were included in the evaluation of the sensitivity, specificity, PPV, NPV, and accuracy of BM aspiration. In all, 45 (24%) patients had BM involvement as established by BM biopsy and aspiration (n = 30) or BM biopsy alone (n = 15). When regarding BM biopsy results as the reference standards, aspiration showed a sensitivity, specificity, PPV, and NPV of 66.7% (95% CI, 51.1‐80.0), 100% (95% CI, 97.4‐100), 100%, and 90.4% (95% CI, 86.1‐93.4), respectively (Table 2). In the validation cohort, 556 patients whose BM biopsy and aspiration data were available were included in the evaluation of the sensitivity, specificity, PPV, NPV, and accuracy of BM aspiration. A total of 22 (4.0%) patients had BM involvement detected by biopsy and aspiration (n = 9) or biopsy alone (n = 13). Aspiration showed a sensitivity, specificity, PPV, and NPV of 40.9% (95% CI, 20.7‐63.6), 100% (95% CI, 99.3‐100), 100%, and 97.6% (95% CI, 96.7‐98.3), respectively (Table 2). The overall diagnostic accuracy (the proportion of true‐positive and true‐negative results over the total number) was 91.9% (95% CI, 87‐95.4) and 97.7% (95% CI, 96.0‐98.7) for BM aspiration in the primary and validation cohorts, respectively.
TABLE 2.
Sensitivity, specificity, PPV, NPV, and accuracy of BM aspiration and PET/CT for detection of BM involvement
| BM involvement by aspiration a | BM involvement by PET/CT a | |||
|---|---|---|---|---|
| Absolute No. | % (95% CI) | Absolute No. | % (95% CI) | |
| Primary cohort | ||||
| Sensitivity | 30/45 | 66.7 (51.1‐80.0) | 22/27 | 81.5 (61.9‐93.7) |
| Specificity | 141/(141 + 0) | 100 (97.4‐100) | 104/(104 + 8) | 92.9 (86.4‐96.6) |
| PPV | 30/(30 + 0) | 100 | 22/(22 + 8) | 73.3 (57.9‐84.6) |
| NPV | 141/(141 + 15) | 90.4 (86.1‐93.4) | 104/(104 + 5) | 95.4 (90.4‐97.9) |
| Accuracy | (30 + 141)/186 | 91.9 (87.0‐95.4) | (22 + 104)/139 | 90.6 (84.5‐94.9) |
| Validation cohort | ||||
| Sensitivity | 9/22 | 40.9 (20.7‐63.6) | 12/18 | 66.7 (41.0‐86.7) |
| Specificity | 534/(534 + 0) | 100 (99.3‐100) | 378/(378 + 29) | 92.9 (90.0‐95.2) |
| PPV | 9/(9 + 0) | 100 | 12/(12 + 29) | 29.3 (20.4‐40.1) |
| NPV | 534/(534 + 13) | 97.6 (96.7‐98.3) | 378/(378 + 6) | 98.4 (97.0‐99.2) |
| Accuracy | (9 + 534)/556 | 97.7 (96.0‐98.7) | (12 + 378)/425 | 91.8 (88.7‐94.2) |
Abbreviations: BM, bone marrow; NPV, negative predictive value; PET/CT, positron emission tomography/computed tomography; PPV, positive predictive value.
BM biopsy is considered the gold standard in this analysis.
Diagnostic performance of PET/CT for BM status
Figure 1 shows that 22 of the 30 patients (73.3%) showing positive PET/CT results had positive BM biopsy findings, whereas five of the 109 patients (4.5%) with negative PET/CT results had positive BM biopsy findings. When regarding BM biopsy as the reference standard, PET/CT showed a sensitivity, specificity, PPV, and NPV of 81.5% (95% CI, 61.9‐93.7), 92.9% (95% CI, 86.4‐96.6), 73.3% (95% CI, 57.9‐84.6), and 95.4% (95% CI, 90.4‐97.9), respectively. In the validation cohort, only 12 of the 30 patients (40.0%) with positive PET/CT results had positive BM biopsy findings, whereas six of the 384 patients (1.6%) with negative PET/CT results had positive BM biopsy findings. PET/CT showed a sensitivity, specificity, PPV, and NPV of 66.7% (95% CI, 41.0‐86.7), 92.9% (95% CI, 90.0‐95.2), 29.3% (95% CI, 20.4‐40.1), and 98.4% (95% CI, 97.0‐99.2), respectively (Table 2). The overall diagnostic accuracy for PET/CT was 90.6% (95% CI, 84.5‐94.9) and 91.8% (95% CI, 88.7‐94.2) in the primary and validation cohorts, respectively. None of the early‐stage patients staged with PET/CT were upstaged to stage IV because of positive BM biopsy findings.
Prognostic performance of BM biopsy and PET/CT in advanced‐stage patients
To further evaluate the prognostic value of BM biopsy in advanced‐stage (stage III‐IV) patients staged with PET/CT, we combined 61 patients from the primary cohort with 113 patients from the validation cohort. Among the 48 patients with positive BM biopsy findings, 43 (89.6%) already showed evidence of advanced disease by PET/CT alone. Overall, 21 patients were upstaged from stage III to stage IV because of positive BM biopsy findings (12.2%, n = 5/38) or BM involvement by PET/CT (42.1%, 16/38) without any other distant organ involvement.
Furthermore, in the 135 patients with other distant organ involvement by PET/CT, 61 (45.2%) already showed evidence of BM involvement by PET/CT (n = 21) or BM biopsy findings (n = 40). The 2‐year OS was 35.9% (95% CI, 24.1‐53.6) for those with BM‐positive results (n = 61) and 60.4% (95% CI, 48.7‐74.8) for those with BM‐negative results (n = 74, p < .001). The 2‐year PFS was 26% (95% CI, 15.9‐42.4) and 40.7% (95% CI, 30.0‐55.2), respectively (p = .003) (Figure 2A,B). The 2‐year OS was 25.7% (95% CI, 12.2‐54) for BM biopsy‐positive results (n = 40) and 50% (95% CI, 31.1‐80.4) for those with BM PET–positive results (n = 21, p = .250). The 2‐year PFS was 15.5% (95% CI, 6.4‐37.4) and 45.2% (95% CI, 26.9‐75.8), respectively (p = .110) (Figure 2C,D).
FIGURE 2.
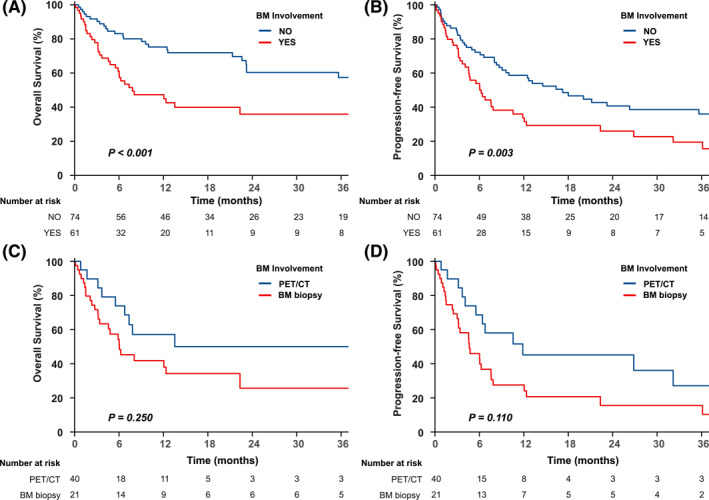
OS and PFS stratified by BM status and detection methods. (A) OS and (B) PFS for BM involvement vs. no BM involvement in patients with other distant organ involvement. (C) OS and (D) PFS of BM PET/CT‐positive vs. biopsy‐positive status in patients with other distant organ involvement. BM indicates bone marrow; CT, computed tomography; OS, overall survival; PET, positron emission tomography; PFS, progression‐free survival
DISCUSSION
To our knowledge, this is the largest study to examine the value of the BM examination in PET/CT‐staged patients with an initial diagnosis of ENKTCL. Our results show that BM biopsy had greater accuracy than BM aspiration for identifying BM involvement. In addition, no patient showed early‐stage disease on PET/CT but positive BM biopsy findings. In patients already considered to have stage III‐IV disease by PET/CT, BM biopsy appears to be necessary because 12.2% of the patients were upstaged to stage IV disease and BM involvement by PET or BM biopsy was identified as a prognostic factor in this study.
For many decades, BM aspiration and biopsy have been the gold standard tests to detect bone marrow involvement for lymphomas, which may upstage some patients from early stage to advanced stage. 27 , 28 However, BM aspiration may examine only a small volume of the liquid BM and may easily miss a patchy lymphoma infiltration. In our study, all cases showing BM involvement by aspiration can be confirmed by biopsy. On the other hand, aspiration missed 33% to 60% of the cases showing BM involvement in the present study. However, BM biopsy is recognized to have several limitations, including the likelihood of missing a patchy pattern of BM involvement, which is supported by the fact that the bilateral BM biopsy found more BM involvement. 28 In addition, BM biopsy was associated with the risk of complications such as pain, anxiety, infection, and bleeding. 44 , 45
PET/CT for detection of BM disease has recently been evaluated in several studies in both HL and NHL. 32 , 33 , 34 , 46 , 47 , 48 The 2014 Lugano classification proposes omitting routine BM biopsy in patients with HL based on data from the Danish Lymphoma Registry study. 30 However, insufficient data are available for the sensitivity and specificity of PET/CT for detection of BM involvement in ENKTCL; therefore, routine BM biopsy remains endorsed by European Society for Medical Oncology and National Comprehensive Cancer Network guidelines. 41 , 42 Three previous studies investigating the utility of PET/CT for BM status in ENKTCL reported sensitivities of 57% to 100%. 38 , 39 , 40 This variation in sensitivities between these studies may be explained by the proportion of advanced‐stage disease and the number of patients with positive BM biopsy findings. These ENKTCL studies included less than 10 patients with positive BM biopsy findings, 39 , 40 whereas the present study included 67 patients with positive BM disease. Consistent with previous studies on HL and ENKTCL, we demonstrated that no ENKTCL patients with positive BM biopsy findings were assessed as having early‐stage disease by PET/CT (NPV, 100%). 32 , 34 , 39 , 40 The high NPV of 100% makes PET/CT a reliable tool for identifying early‐stage patients in whom a BM biopsy is not likely to add any diagnostic value and does not influence assignment to therapy.
In theory, BM biopsy upstages some patients from stage III to stage IV, which can add prognostic value and change the potential management. In our study, BM biopsy upstaged 5 (12.2%) patients with stage III who had negative PET/CT findings for BM involvement. Similar results were reported in other studies for stage III HL and NHL. 34 , 46 , 48 Nevertheless, more than 80% of patients with positive BM biopsy findings already had evidence of advanced disease for ENKTCL, DLBCL, and HL, 34 , 46 and any attempts to confirm the prognostic value of BM status will be extremely challenging. In our cohort, the prognostic value of BM status was separately evaluated in different cases of stage IV disease. For patients with other distant organ involvement, BM involvement detected by PET/CT or biopsy resulted in worse OS and PFS. However, there was no difference in the OS and PFS rates between PET/CT and biopsy groups. Considering the lower sensitivity and survival differences between BM‐positive and BM‐negative cases, it would appear reasonable to routinely use BM biopsy for patients who had already been assigned stage IV by PET/CT.
The limitations of the present analysis include the following. First, the present analysis is retrospective in nature and PET/CT imaging was not mandatory and without a central review. Even though we aimed to include all patients with ENKTCL during the study period, a small proportion of patients may not have undergone both PET/CT and BM examinations at diagnosis. However, this study used data generated in routine clinical settings, which reflect the type of daily clinical practice. Second, directed biopsies of FDG‐avid focal BM lesions were not obtained. Although routine BM biopsy can erroneously miss BM involvement in patients with FDG‐avid patchy lesions outside the biopsy area, PET‐guided biopsies are not feasible from each site of focal FDG uptake. The evaluation of directed biopsies of FDG‐avid focal BM lesions versus routine BM biopsy may be explored in a future study. Third, although 742 patients with ENKTCL were pooled from two independent institutions, more work is required to externally validate the diagnostic and prognostic value of BM examination using independent data from nonendemic areas.
In conclusion, BM biopsy appears to identify BM lesions with a higher sensitivity than BM aspiration. Diagnostically, BM examination did not lead to important changes in the distribution of stage for early‐stage patients undergoing upfront PET/CT staging. It is reasonable to reserve BM biopsy as the routine test for advanced‐stage patients because of changes in the prognosis for these patients.
AUTHOR CONTRIBUTIONS
Yong Yang: Conception and design. financial support, administrative support, study material or patient provision, data collection and assembly, and data analysis and interpretation. Ji‐Jin Wang: Conception and design, study material or patient provision, data collection and assembly, and data analysis and interpretation. Rui‐Zhi Zhao: Study material or patient provision and data collection and assembly. Cheng Huang: Study material or patient provision and data collection and assembly. Gui‐Qing Shi: Study material or patient provision. Hao Zheng: Data collection and assembly. Tian‐Lan Tang: Study material or patient provision. Si‐Qin Liao: Study material or patient provision. Jin‐Hua Chen: Study material or patient provision, data collection and assembly, data analysis and interpretation. Jian‐Zhen Shen: Study material or patient provision. Ting‐Bo Liu: Conception and design, administrative support, study material or patient provision, and data analysis and interpretation. Ben‐Hua Xu: Conception and design, financial support, administrative support, provision of study material or patients, and data analysis and interpretation. Yu‐Jing Zhang: Conception and design, financial support, administrative support, provision of study material or patients, and data analysis and interpretation. All authors: manuscript writing, final manuscript approval, and all aspects of the work.
CONFLICT OF INTEREST
All authors declare no competing interests.
ACKNOWLEDGMENT
This work was supported by grants from the Fujian provincial health technology project (No. 2021CXA011). The funding sources had no influence on the design, performance, or reporting this study.
Yang Y, Wang J‐J, Zhao R‐Z, et al. The value of routine bone marrow examination in patients with extranodal NK/T‐cell lymphoma staged with PET/CT. Cancer. 2022;128(22):3943‐3950. 10.1002/cncr.34473
Yong Yang, Ji‐Jin Wang, Rui‐Zhi Zhao, and Cheng Huang contributed equally as first authors.
Contributor Information
Ting‐Bo Liu, Email: 13706998835@139.com.
Ben‐Hua Xu, Email: benhuaxu@163.com.
Yu‐Jing Zhang, Email: zhangyj@sysucc.org.cn.
REFERENCES
- 1. Liu W, Ji X, Song Y, et al. Improving survival of 3760 patients with lymphoma: experience of an academic center over two decades. Cancer Med. 2020;9(11):3765‐3774. 10.1002/cam4.3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams SV, Newcomb PA, Shustov AR. Racial patterns of peripheral T‐cell lymphoma incidence and survival in the United States. J Clin Oncol. 2016;34(9):963‐971. 10.1200/jco.2015.63.5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun J, Yang Q, Lu Z, et al. Distribution of lymphoid neoplasms in China: analysis of 4, 638 cases according to the World Health Organization classification. Am J Clin Pathol. 2012;138(3):429‐434. 10.1309/ajcp7yltqpusdq5c [DOI] [PubMed] [Google Scholar]
- 4. Li YX, Yao B, Jin J, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T‐cell lymphoma. J Clin Oncol. 2006;24(1):181‐189. 10.1200/jco.2005.03.2573 [DOI] [PubMed] [Google Scholar]
- 5. Qi SN, Li YX, Specht L, et al. Modern radiation therapy for extranodal nasal‐type NK/T‐cell lymphoma: risk‐adapted therapy, target volume, and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2021;110(4):1064‐1081. 10.1016/j.ijrobp.2021.02.011 [DOI] [PubMed] [Google Scholar]
- 6. Qi SN, Yang Y, Song YQ, et al. First‐line non‐anthracycline‐based chemotherapy for extranodal nasal‐type NK/T‐cell lymphoma: a retrospective analysis from the CLCG. Blood Adv. 2020;4(13):3141‐3153. 10.1182/bloodadvances.2020001852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qi SN, Yang Y, Zhang YJ, et al. Risk‐based, response‐adapted therapy for early‐stage extranodal nasal‐type NK/T‐cell lymphoma in the modern chemotherapy era: a China Lymphoma Collaborative Group study. Am J Hematol. 2020;95(9):1047‐1056. 10.1002/ajh.25878 [DOI] [PubMed] [Google Scholar]
- 8. Zhu Y, Tian S, Xu L, et al. GELAD chemotherapy with sandwiched radiotherapy for patients with newly diagnosed stage IE/IIE natural killer/T‐cell lymphoma: a prospective multicentre study. Br J Haematol. 2022;196(4):939‐946. 10.1111/bjh.17960 [DOI] [PubMed] [Google Scholar]
- 9. Yamaguchi M, Suzuki R, Oguchi M, et al. Treatments and outcomes of patients with extranodal natural killer/T‐cell lymphoma diagnosed between 2000 and 2013: a Cooperative Study in Japan. J Clin Oncol. 2017;35(1):32‐39. 10.1200/jco.2016.68.1619 [DOI] [PubMed] [Google Scholar]
- 10. Zhang Y, Ma S, Cai J, et al. Sequential P‐GEMOX and radiotherapy for early‐stage extranodal natural killer/T‐cell lymphoma: a multicenter study. Am J Hematol. 2021;96(11):1481‐1490. 10.1002/ajh.26335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwong YL, Kim SJ, Tse E, et al. Sequential chemotherapy/radiotherapy was comparable with concurrent chemoradiotherapy for stage I/II NK/T‐cell lymphoma. Ann Oncol. 2018;29(1):256‐263. 10.1093/annonc/mdx684 [DOI] [PubMed] [Google Scholar]
- 12. Yang Y, Cao J‐Z, Lan S‐M, et al. Association of improved locoregional control with prolonged survival in early‐stage extranodal nasal‐type natural killer/T‐cell lymphoma. JAMA Oncol. 2017;3(1):83‐91. 10.1001/jamaoncol.2016.5094 [DOI] [PubMed] [Google Scholar]
- 13. Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T‐cell lymphoma, nasal type. Blood. 2018;131(23):2528‐2540. 10.1182/blood-2017-12-791418 [DOI] [PubMed] [Google Scholar]
- 14. Liu W, Yang Y, Qi S, et al. Treatment, survival, and prognosis of advanced‐stage natural killer/T‐cell lymphoma: an analysis from the China Lymphoma Collaborative Group. Front Oncol. 2020;10:583050. 10.3389/fonc.2020.583050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li X, Cui Y, Sun Z, et al. DDGP versus SMILE in newly diagnosed advanced natural killer/T‐cell lymphoma: a randomized controlled, multicenter, open‐label study in China. Clin Cancer Res. 2016;22(21):5223‐5228. 10.1158/1078-0432.ccr-16-0153 [DOI] [PubMed] [Google Scholar]
- 16. Lim SH, Hong JY, Lim ST, et al. Beyond first‐line non‐anthracycline‐based chemotherapy for extranodal NK/T‐cell lymphoma: clinical outcome and current perspectives on salvage therapy for patients after first relapse and progression of disease. Ann Oncol. 2017;28(9):2199‐2205. 10.1093/annonc/mdx316 [DOI] [PubMed] [Google Scholar]
- 17. Allen PB, Lechowicz MJ. Management of NK/T‐cell lymphoma, nasal type. J Oncol Pract. 2019;15(10):513‐520. 10.1200/jop.18.00719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fox CP, Civallero M, Ko YH, et al. Survival outcomes of patients with extranodal natural‐killer T‐cell lymphoma: a prospective cohort study from the international T‐cell Project. Lancet Haematol 2020;7(4):e284‐e294. 10.1016/s2352-3026(19)30283-2 [DOI] [PubMed] [Google Scholar]
- 19. Liu X, Wu T, Zhu SY, et al. Risk‐dependent conditional survival and failure hazard after radiotherapy for early‐stage extranodal natural killer/T‐cell lymphoma. JAMA Netw Open. 2019;2(3):e190194. 10.1001/jamanetworkopen.2019.0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu T, Yang Y, Zhu SY, et al. Risk‐adapted survival benefit of IMRT in early‐stage NKTCL: a multicenter study from the China Lymphoma Collaborative Group. Blood Adv. 2018;2(18):2369‐2377. 10.1182/bloodadvances.2018021311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang Y, Zhu Y, Cao JZ, et al. Risk‐adapted therapy for early‐stage extranodal nasal‐type NK/T‐cell lymphoma: analysis from a multicenter study. Blood. 2015;126(12):1424‐1432. 10.1182/blood-2015-04-639336 [DOI] [PubMed] [Google Scholar]
- 22. Lee J, Suh C, Park YH, et al. Extranodal natural killer T‐cell lymphoma, nasal‐type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006;24(4):612‐618. 10.1200/jco.2005.04.1384 [DOI] [PubMed] [Google Scholar]
- 23. Yang Y, Zhang YJ, Zhu Y, et al. Prognostic nomogram for overall survival in previously untreated patients with extranodal NK/T‐cell lymphoma, nasal‐type: a multicenter study. Leukemia. 2015;29(7):1571‐1577. 10.1038/leu.2015.44 [DOI] [PubMed] [Google Scholar]
- 24. Kim SJ, Yoon DH, Jaccard A, et al. A prognostic index for natural killer cell lymphoma after non‐anthracycline‐based treatment: a multicentre, retrospective analysis. Lancet Oncol 2016;17(3):389‐400. 10.1016/s1470-2045(15)00533-1 [DOI] [PubMed] [Google Scholar]
- 25. Chen SY, Yang Y, Qi SN, et al. Validation of nomogram‐revised risk index and comparison with other models for extranodal nasal‐type NK/T‐cell lymphoma in the modern chemotherapy era: indication for prognostication and clinical decision‐making. Leukemia. 2021;35(1):130‐142. 10.1038/s41375-020-0791-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hong H, Li Y, Lim ST, et al. A proposal for a new staging system for extranodal natural killer T‐cell lymphoma: a multicenter study from China and Asia Lymphoma Study Group. Leukemia. 2020;34(8):2243‐2248. 10.1038/s41375-020-0740-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han T, Stutzman L, Rogue AL. Bone marrow biopsy in Hodgkin's disease and other neoplastic diseases. JAMA. 1971;217(9):1239‐1241. 10.1001/jama.1971.03190090061014 [DOI] [PubMed] [Google Scholar]
- 28. Brunning RD, Bloomfield CD, McKenna RW, Peterson LA. Bilateral trephine bone marrow biopsies in lymphoma and other neoplastic diseases. Ann Intern Med. 1975;82(3):365‐366. 10.7326/0003-4819-82-3-365 [DOI] [PubMed] [Google Scholar]
- 29. Elstrom R, Guan L, Baker G, et al. Utility of FDG‐PET scanning in lymphoma by WHO classification. Blood. 2003;101(10):3875‐3876. 10.1182/blood-2002-09-2778 [DOI] [PubMed] [Google Scholar]
- 30. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059‐3068. 10.1200/jco.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barrington SF, Trotman J. The role of PET in the first‐line treatment of the most common subtypes of non‐Hodgkin lymphoma. Lancet Haematol 2021;8(1):e80‐e93. 10.1016/s2352-3026(20)30365-3 [DOI] [PubMed] [Google Scholar]
- 32. Voltin CA, Goergen H, Baues C, et al. Value of bone marrow biopsy in Hodgkin lymphoma patients staged by FDG PET: results from the German Hodgkin Study Group trials HD16, HD17, and HD18. Ann Oncol. 2018;29(9):1926‐1931. 10.1093/annonc/mdy250 [DOI] [PubMed] [Google Scholar]
- 33. Adams HJ, Kwee TC, de Keizer B, et al. Systematic review and meta‐analysis on the diagnostic performance of FDG‐PET/CT in detecting bone marrow involvement in newly diagnosed Hodgkin lymphoma: is bone marrow biopsy still necessary? Ann Oncol. 2014;25(5):921‐927. 10.1093/annonc/mdt533 [DOI] [PubMed] [Google Scholar]
- 34. El‐Galaly TC, d'Amore F, Mylam KJ, et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography‐staged treatment‐naive patients with Hodgkin lymphoma. J Clin Oncol. 2012;30(36):4508‐4514. 10.1200/jco.2012.42.4036 [DOI] [PubMed] [Google Scholar]
- 35. Kim TM, Lee SY, Jeon YK, et al. Clinical heterogeneity of extranodal NK/T‐cell lymphoma, nasal type: a national survey of the Korean Cancer Study Group. Ann Oncol. 2008;19(8):1477‐1484. 10.1093/annonc/mdn147 [DOI] [PubMed] [Google Scholar]
- 36. Vose J, Armitage J, Weisenburger D. International peripheral T‐cell and natural killer/T‐cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124‐4130. 10.1200/jco.2008.16.4558 [DOI] [PubMed] [Google Scholar]
- 37. Chen Y, Luo L, Zheng X, et al. Clinical characteristics and survival of extranodal natural killer/T‐cell lymphoma: a single‐center 12‐year retrospective analysis. Leuk Lymphoma. 2020;61(14):3306‐3318. 10.1080/10428194.2020.1808207 [DOI] [PubMed] [Google Scholar]
- 38. Fujiwara H, Maeda Y, Nawa Y, et al. The utility of positron emission tomography/computed tomography in the staging of extranodal natural killer/T‐cell lymphoma. Eur J Haematol. 2011;87(2):123‐129. 10.1111/j.1600-0609.2011.01645.x [DOI] [PubMed] [Google Scholar]
- 39. Zhou Z, Chen C, Li X, et al. Evaluation of bone marrow involvement in extranodal NK/T cell lymphoma by FDG‐PET/CT. Ann Hematol. 2015;94(6):963‐967. 10.1007/s00277-014-2289-4 [DOI] [PubMed] [Google Scholar]
- 40. Wang Y, Xie L, Tian R, et al. PET/CT‐based bone‐marrow assessment shows potential in replacing routine bone‐marrow biopsy in part of patients newly diagnosed with extranodal natural killer/T‐cell lymphoma. J Cancer Res Clin Oncol. 2019;145(10):2529‐2539. 10.1007/s00432-019-02957-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. NCCN clinical practice guidelines in oncology (NCCN guidelines): Non‐Hodgkin's lymphomas, Version 2.0, 2022. http://www.nccn.org [DOI] [PubMed]
- 42. d'Amore F, Gaulard P, Trumper L, et al. Peripheral T‐cell lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2015;26(suppl 5):v108‐v115. 10.1093/annonc/mdv201 [DOI] [PubMed] [Google Scholar]
- 43. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375‐2390. 10.1182/blood-2016-01-643569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Salem P, Wolverson MK, Reimers HJ, Kudva GC. Complications of bone marrow biopsy. Br J Haematol. 2003;121(6):821. 10.1046/j.1365-2141.2003.04328.x [DOI] [PubMed] [Google Scholar]
- 45. Malempati S, Joshi S, Lai S, Braner DA, Tegtmeyer K. Videos in clinical medicine. Bone marrow aspiration and biopsy. N Engl J Med. 2009;361(15):e28. 10.1056/nejmvcm0804634 [DOI] [PubMed] [Google Scholar]
- 46. Alzahrani M, El‐Galaly TC, Hutchings M, et al. The value of routine bone marrow biopsy in patients with diffuse large B‐cell lymphoma staged with PET/CT: a Danish‐Canadian study. Ann Oncol. 2016;27(6):1095‐1099. 10.1093/annonc/mdw137 [DOI] [PubMed] [Google Scholar]
- 47. Alderuccio JP, Isrow D, Reis IM, et al. Diagnostic bone marrow biopsy in patients with stage I EMZL treated with radiation therapy: needed or not? Blood. 2020;135(15):1299‐1302. 10.1182/blood.2019003236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nakajima R, Moskowitz AJ, Michaud L, et al. Baseline FDG‐PET/CT detects bone marrow involvement in follicular lymphoma and provides relevant prognostic information. Blood Adv. 2020;4(8):1812‐1823. 10.1182/bloodadvances.2020001579 [DOI] [PMC free article] [PubMed] [Google Scholar]


