Abstract
Ctenostome bryozoans are unmineralized and mostly marine. Their lack of calcified skeletal features requires other characters to be considered for systematic and phylogenetic considerations. As a continuation of an ongoing series of studies, we herein investigate the morphology of Amphibiobeania epiphylla, a unique bryozoan inhabiting mangrove leaves that are highly exposed to tidal cycles and regular dry events according to the tidal cycle. Besides this interesting mode of life, the species was originally interpreted to be a weakly mineralized cheilostome bryozoan, whereas molecular data place it among ctenostome bryozoans. To elucidate the systematic and phylogenetic position of the genus and also find morphological adaptations to an extreme habitat, we investigated the morphology of A. epiphylla in detail. Zooids show a lophophore with eight tentacles and a simple gut with a prominent caecum, lophophoral anus and most notably a distinct gizzard in the cardiac region. Gizzard teeth are multiple, simple homogeneous cuticular structures. The cuticle of the zooid is rather uniform and shows no respective thickenings into opercular flaps or folds. Likewise, apertural muscles are represented by a single pair of muscles. There are no specific closing muscles in the apertural area like the operculum occlusors of cheilostomes. Most prominent within zooids is a spongiose tissue filling most of the body cavity. Although not properly understood, this tissue may aid in keeping animals moist and hydrated during prolonged dry times. In summary, all morphological characters support a ctenostome rather than a cheilostome affinity, possibly with Vesicularioidea or Victorelloidea. In addition, we provide new molecular data that clearly supports such a closer relationship.
Keywords: ctenostome evolution, gizzard, Gymnolaemata, intertidal, mangroves
Schematic drawing of a zooid of Amphibiobeania epiphylla showing its main morphological features.

Research highlight
This study analyses the morphology of a unique bryozoan, Amphibiobeania epiphylla, which was originally described as cheilostome bryozoan. Molecular results indicate, however, that this species is in fact a ctenostome. Our detailed morphological analyses support this conclusion.
1. INTRODUCTION
Bryozoa is a moderately large phylum of lophotrochozoans comprising more than 6000 recent and 15,000 fossil species. In general, all species are colonial and consist of iterated clonal modules, termed zooids. The latter are traditionally divided into a ‘cystid’, which is the body wall and a ‘polypide’ that comprises most organs involved in suspension‐feeding. Typical for each individual polypide is its ability to retract into the cystid via prominent retractor muscles. Ctenostomes are a paraphyletic assemblage of gymnolaemate bryozoans (Todd, 2000; Waeschenbach et al., 2012) that occur mostly in marine or brackish habitats, but some live in freshwater (Schwaha, 2020a).
Ctenostomes lack mineralized skeletons and usually have chitinized cuticles. The lack of such skeletons, in contrast to the cyclostomes and cheilostomes, renders them rather featureless with only few external characters. In addition, many characters observed at colony and zooid levels are subject to high plasticity (see Jebram, 1986a, 1987; Jebram & Everitt, 1982). A series of recent studies have started to explore ctenostome soft‐body morphology on a broader scale for drawing systematic and phylogenetic inferences (Schwaha, 2021; Schwaha & De Blauwe, 2020; Schwaha et al., 2020a, 2021, 2022).
Amphibiobeania epiphylla is a bryozoan specialized in colonizing mangrove leaves. It is unique in being able to survive longer periods of desiccation than most other bryozoans because the mangroves are highly exposed to tidal cycles. During the Northern Territory dry season, when the tidal amplitude is diminished, colonies can be maximally emergent for as long as 2‒3 days (Metcalfe et al., 2007), making this bryozoan virtually amphibious. Such a specialized habitat and lifestyle invite detailed morphological analyses to better understand specific adaptations. Moreover, originally described as cheilostomatous, newer results indicated a ctenostome affinity of this species and genus (Cook et al., 2018). Consequently, this study aims to understand not only the necessary adaptations in morphology required for this unique lifestyle but also their use in clarifying its systematic position.
2. MATERIALS AND METHODS
2.1. Specimen collection
Amphibiobeania epiphylla Metcalfe et al. 2007 was collected from the Lim Chu Kang mangrove habitat on Johor Strait, Singapore, on May 8, 2019. Colony samples were fixed in 2% glutaraldehyde in 0.1 mol l−1 phosphate buffer for morphological analyses. A separate specimen (tissue code AW766; GenBank accession number OP538578) was allocated for molecular analysis. Additional ctenostome species were collected for molecular analysis as follows: Sundanella sibogae (tissue code AW764; GenBank accession number OP538576); Amathia sp. (tissue code AW765; GenBank accession number OP538580), both collected by DPG from Lim Chu Kang, Johor Strait, Singapore on 1 May 2019; Vesicularia spinosa (tissue code AW761; GenBank accession number OP538579), collected by HDB from Zeebrugge, Belgium on 17 November 2012; Anguinella palmata (tissue code AW096; GenBank accession number OP538577), collected by AW from Harwich, UK, on 17 October 2001.
2.2. Morphological analysis
Samples were first rinsed in several washes with phosphate buffer. Then they were analysed and documented with a Nikon SMZ25 stereomicroscope equipped with a Nikon Ri2 microscope camera (Nikon, Tokyo, Japan). Small cut pieces of leaves with colonies were subsequently cut into smaller pieces and postfixed in 1% osmiumtetroxide. Afterwards they were dehydrated in acidified dimethoxypropane and subsequently embedded into Agar Low Viscosity Resin (Agar). Cured resin blocks were serially sectioned with a Diatome HistoJumbo diamond knife on a Leica UC6 ultramicrotome (Leica Microsystems, Wetzlar, Germ; see also Ruthensteiner, 2008). Serial sections of 1 µm thickness were stained with toluidine blue and photographed with a Nikon NiU compound microscope, afterward processed in FIJI (Schindelin et al., 2012) and imported into the three‐dimensional reconstruction software Amira (ThermoFisher). Features of interest such as the digestive tract or the lophophore were semiautomatically segmented and afterward visualized as optimized surface models. Surrounding structures were depicted as volume renderings.
2.3. Illumina sequencing, assembly and annotation
Total genomic DNA (gDNA) was extracted from ethanol‐preserved specimens using the DNeasy Blood & Tissue Kit (Qiagen) following the manufacturer's instructions. Double‐stranded (ds) DNA concentration was quantified using a Qubit™ fluorometer using either the Qubit™ dsDNA BR (Broad Range) or dsDNA HS (High Sensitivity) assay kits. Dual‐indexed libraries were prepared using the TruSeq DNA Nano Library Prep Kit (Illumina, Inc.). Sequencing was performed by the Natural History Museum Sequencing Facility on the Illumina NextSeq. 500 platform (Illumina, Inc.), mid‐output, using 2 × 150 bp paired‐end sequencing. Paired‐end reads were trimmed using Trimmomatic v.0.39 (Bolger et al., 2014) and assembled de novo using SPAdes v.3.13.0 (Bankevich et al., 2012) with k‐mer sizes of 33, 55, 77, 99 and 127. 18S rDNA contigs were identified by conducting blast searches (Altschul et al., 1990) against a local custom database of reference bryozoan sequences in Geneious v.11.1.4 (https://www.geneious.com). 18S rDNA gene boundaries were identified using RNAmmer v.1.2 (Lagesen et al., 2007).
2.4. Phylogenetic analysis
The newly generated sequences were analysed in the context of published 18S rDNA data (for GenBank accession numbers, see Figure 1). Outgroups were the phylactolaemate Pectinatella magnifica and the cyclostome Exidmonea atlantica. Nucleotide alignments were produced using MAFFT v.7.453 (Katoh & Standley, 2013) using the—maxiterate 1000 option and the—genafpair algorithm. Unambiguously aligned positions were identified using the stand‐alone version of Gblocks v.91b (Castresana, 2000; Talavera & Castresana, 2007) using the following settings: minimum number of sequences for a conserved position = lowest; minimum number of sequences or a flank position = lowest; maximum number of contiguous nonconserved positions = 10; minimum length of a block = 5; allowed gap positions = with half. Following the exclusion of ambiguously aligned positions, the best‐fitting nucleotide substitution model was determined using ModelTest‐NG v.0.1.6 (Darriba et al., 2020). Phylogenetic maximum likelihood (ML) analysis was carried out under the GTRCAT model with fast bootstrap replicates (1000) using RAxML v.8.2.12 (Stamatakis, 2014).
Figure 1.

Maximum likelihood analysis of 18S rDNA carried out under the GTRCAT model in RAxML v.8.2.12. Nodal values indicate fast bootstrap support from 1000 replicates. Branch length scale bar indicates number of substitutions per site.
3. RESULTS
3.1. Colony structure, general zooidal form and body cavity
A. epiphylla forms encrusting sheets of colonies on mangrove leaves (Figure 2). Zooids are serially arranged with the distal zooid extending from the basal side of its mother zooid (Figures 2, 3 and 4b). In addition, each zooid possesses two tubular interzooidal connections on one lateral side (Figures 2, 4a,d and 5a,b,e–g). It seems that this pattern of two interconnected zooids alternates between consecutive zooids in proximo‐distal direction. Most conspicuously is the silt‐like covering of the entire colony (Figure 2), which is also prominent in section images (e.g., Figure 5).
Figure 2.

Amphibiobeania epiphylla, general overview of (a) colony on a mangrove leaf (b) detail showing multiple zooidal apertures with the zooids mostly covered in a silty organic layer. ap, aperture.
Figure 3.

Schematic drawing of Amphibiobeania epiphylla. a, anus; am, apertural muscles; ap, aperture; cae, caecum; cd, cauda; es, esophagus; fm, funicular muscle; int, intestine; l, lophophore; nz, neighboring zooid; ph, pharynx; pop, pore plate; rm, retractor muscle; vw, vestibular wall.
Figure 4.

Amphibiobeania epiphylla, three‐dimensional reconstruction. (a) Frontal view of a zooid showing the thin proximal cauda, two unilateral interzooidal connections and the flattened area of the aperture in the distal area. (b) Lateral view of a zooid showing internal features of the polypide (lophophore: blue, gut: green, muscles: red) and surrounding tissues transparently. (c) Obliquely distal view showing the vestibular area transparently and the four bundles of apertural muscles. (d) Frontal view with superficial layers of the rendering cut off. Note the obliquely oriented vestibular wall in shape of a butterfly. (e) Basal view of the polypide showing the different regions of the gut and funicular muscles. a, anus; am, apertural muscles; ap, aperture; ca, cardia; cae, caecum; cd, cauda; cg, cerebral ganglion; es, esophagus; fm, funicular muscle; int, intestine; l, lophophore; nz, neighboring zooid; ph, pharynx; py, pylorus; rm, retractor muscle; v, vestibulum; vw, vestibular wall.
Figure 5.

Amphibiobeania epiphylla, pore plates. (a) Cross‐section of a pore showing the special cells passing through the interzooidal pore plate. (b) Section showing more intensely stained special cells in the right zooid directly adjacent to the spongiose tissue of the body cavity. (c, d) Sections of the same pore at different levels showing the rim of surrounding limiting cells (c) and a cross‐section of the narrow portions of the eight special cells passing through the pore. (e) Longitudinal section of a pore complex. (f) Longitudinal section of a pore complex and associated elongated cells (arrows), possibly funicular tissue. (g) Section of two neighboring zooids showing dense cell accumulations near the pore complex. Asterisks marks an example of assembled round cells. am, apertural muscles; cae, caecum; ect, ectocyst; ed, epidermis; li, limiting cell; ph, pharynx; pop, pore plate; sil, silt‐layer on body wall; spe, special cell; spo, spongiose tissue; vw, vestibular wall; vwm, vestibular wall muscles.
Most prominent is the presence of an internal spongiose tissue (or cells) that fills most of the body cavity between the zooidal wall and the retracted polypide (Figures 5b, 6b–d, 7a–c and 8a). It consists of a polygonal mesh of cells that appear void but usually contain some inclusions and most frequently a greyish‐blackish inclusion, possibly lipids.
Figure 6.
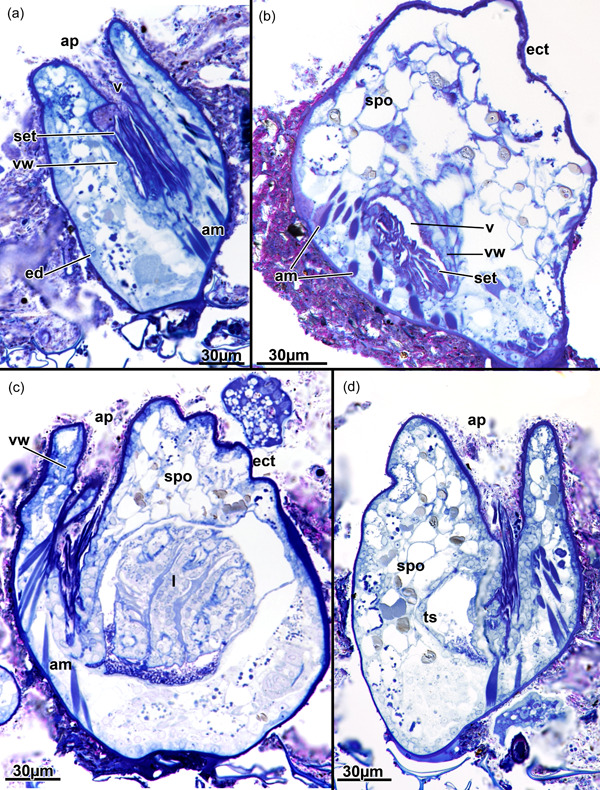
Amphibiobeania epiphylla, apertural area. (a) Longitudinal section of the aperture showing a prominent setigerous collar. (b) Cross‐section of the vestibular wall showing the collar. (c) Longitudinal section of the aperture showing the associated apertural muscles and their insertion at the lower third of the vestibular wall. Note also the regular thin cuticle around the aperture lacking any distinct thickening. (d) Longitudinal section showing densely arranged spongiose tissue surrounding the aperture. ap, aperture; am, apertural muscles; ect, ectocyst; ed, epidermis; l, lophophore; set, setigerous collar; spo, spongiose tissue; ts, tentacle sheath; v, vestibulum; vw, vestibular wall.
Figure 7.

Amphibiobeania epiphylla, lophophore and digestive tract. (a) Cross‐section of the retracted lophophore showing a few thick tentacles located within the tentacle sheath. Note also the large amount of spongiose tissue in the body cavity. (b) Cross‐section of retracted lophophore next to the large caecum. (c) Section of various gut areas including the denticulate cardia or gizzard showing multiple densely stained teeth. (d) Section of the foregut and the gizzard transitioning into the caecum. cae, caecum; ect, ectocyst; ed, epidermis; fg, foregut; gz, gizzard; l, lophophore; lb, lophophoral base; ph, pharynx; pl, peritoneal lining; rm, retractor muscle; spo, spongiose tissue; ts, tentacle sheath; v, vestibulum; vw, vestibular wall.
Figure 8.

Amphibiobeania epiphylla, histological details of the spermatogenic tissue. (a) Ripe sperm tissue associated with spermatogenic tissue at the body wall. (b) More undifferentiated earlier spermiogenic tissue. (c, d) Sections showing multiple (c) or few (d) spermatogenic cisterns associated with the developing spermatogenic tissue. bb, brown body; cae, caecum; ect, ectocyst; hg, hindgut; l, lophophore; spm, sperm; spo, spongiose tissue; spt, spermatogenic tissue; spy, spermatogenic cistern; ts, tentacle sheath.
Besides the spongiose tissue, the remaining body cavity is commonly lined by thin inconspicuous epidermal cells (Figure 7). In other areas, the lining can be more prominent (Figure 5a), sometimes even with large aggregation of round cells (Figure 5g). In addition, spermatogenic tissue was found in some zooids associated with the body wall (Figure 8). A gradual distinction from undifferentiated spermatogenic clusters closer to the body wall (Figure 8b) to more ripe sperm toward the body cavity of the zooid (Figure 8a) appears to be present. More unusual are tubular epithelial cords or cisterns associated with the spermatogenic tissue (Figure 8c,d). The cisterns appear filled with a homogeneous content with few granular inclusions.
3.2. Cuticle and apertural area
The cuticle in A. epiphylla is consistently thin over the entire zooid, but overall heavily covered by irregular silt‐like particles, slightly less on the basal side where it is attached to the substrate (Figures 5, 6, 7, 8). It lacks any lamellar structure and is present as a compact lining.
The apertural area is almost circular from the external view with some slight irregular folds (Figure 4a). Below the orifice, the vestibular wall is obliquely bilateral with four distinct folds, which gives the vestibular wall the shape of a butterfly (Figures 3 and 4d). The vestibular wall extends toward the basal side of the zooid (Figure 4b) where it transitions via the diaphragm into the more thin‐walled tentacle sheath, which surrounds the retracted lophophore. At the diaphragm, a prominent setigerous collar is situated and fills approximately half of the vestibulum, the cavity lined by the vestibular wall (Figure 6a,b). The latter itself carries a loose mesh of circular and longitudinal muscle fibres (Figure 5a). In addition to these muscles, a single set of apertural muscles consisting of four bundles reflecting the vestibulum shape extend from the basal side of each zooid to the lower half of the vestibular wall (Figures 3, 4b,c and 6c). Duplicature bands are entirely lacking.
At least four interzooidal pore plates seem to be present for each zooid. One distal and proximal and two laterally on the same side. The pore complex consists of multiple (up to eight have been encountered) special cells passing through the interzooidal pore plate (Figure 5a–e,g). The special cells commonly show a translucent cytoplasm in histological sections (Figure 5a,d,e), but also can show more intense staining (Figure 5b,c,g). Surrounding the pore‐plugging special cells is a layer of limiting cells (Figure 5a,c,e,g). Associated with the pore complex are often elongated, cord‐like cells, which perhaps are funicular cells (Figure 5f,g). Also, the pore complex is often associated with prominent cell clusters (Figure 5a,c,f,g).
3.3. Lophophore and digestive tract
The lophophore of A. epiphylla is short and only carries eight tentacles, which in retracted zooids are distally folded within the tentacle sheath (Figures 3, 4b–d and 6c). From the inconspicuous lophophoral base, the digestive tract starts with a foregut consisting of a pharynx and short esophagus (Figures 3 and 4e). The adjoining midgut consist of cardia, caecum and pylorus (Figure 4e). The cardia is present as a gizzard with multiple, short teeth lining the inner gut lumen. The teeth appear solid and stain, on semithin sections, as a single entity without any differentiation (Figure 7c,d). The caecum is comparatively large and its proximal end stretches far proximally to the level of the esophagus. A short funicular muscle extends from the mid‐caecal region to the body wall (Figure 4e). From the ciliated pylorus, the midgut enters the hindgut or intestine which exits via a lophophoral anus into the tentacle sheath close to the lophophoral base (Figures 3 and 4e; see also Schwaha, 2020b).
3.4. Molecular phylogeny
The complete 18S rDNA alignment was composed of 1933 positions, of which 138 were excluded due to their ambiguous alignment. ML phylogenetic analysis showed that Amphibiobeania formed a moderately supported clade (71% bootstrap support) with vesicularid ctenostome taxa Amathia sp. and V. spinosa (Figure 1). As in previous analyses (e.g., Waeschenbach et al., 2012) the ctenostomes were paraphyletic to the inclusion of the cheilostomes.
4. DISCUSSION
4.1. General colony and zooidal morphology
The general colony structure observed in the current study corresponds to the original description (Metcalfe et al., 2007). Only the number of lateral connections differs in being two in the current study and a single one from the first description. Also, the lateral connections appear longer in the original study than in the current observations. Originally, A. epiphylla was described from the Northern Territory of Australia, close to Darwin, whereas the material of the current study was collected in Singapore. Potentially, there is either a certain degree of plasticity in colony structure or even separate species are present. Currently, we have more or less no information on the reproductive modes of A. epiphylla and its potential for dispersal. Given the extreme habitat and probable high‐substrate specificity, we think that short‐lived fast‐settling larvae appear more plausible in this species.
In its original description, A. epiphylla was described as having boat‐shaped zooids with distinct area of frontal wall (Metcalfe et al., 2007). This finding is not supported by our current observations and we suggest that the original interpretation might have been based on collapse of part of the body wall during histological preparation—a common feature in ctenostomes (see also Hayward, 1985; Schwaha, 2020a). For example, in retracted zooids, on which all histological studies on A. epiphylla have been conducted, the frontal membrane should be more flexed outwards rather than inwards.
The most unusual histological structure observed in A. epiphylla is the ‘spongiose’ tissue filling most of the body cavity. Such a highly vacuolated filling of the body cavity has so far never been reported for any other bryozoan. The frequent inclusion of dark‐greyish droplets indicates that lipids are often included in this tissue.
Spermatogenic tissue is usually associated with the body wall (Ostrovsky, 2020). In this respect, A. epiphylla is not any different. However, the current observation of the tubular epithelial structures associated with the spermatogenic tissue seems rather unusual or even unique. We had only a few zooids showing male gametes and no female ones, but reproduction is an important aspect in this species that merits additional studies, especially with material collected at different times of the year.
4.2. Cuticle and aperture
Cuticles among gymnolaemates usually vary from mostly thin in many ctenostome bryozoans (e.g., Braem, 1951) to rather thick in others (e.g., S. H. Decker et al., 2021; Schwaha, 2021; Schwaha et al., 2021). Cheilostome bryozoans were never reported to possess a thick cuticle (e.g., Banta, 1968). Sclerotized rims are, however, typical for the opercular flap (Martha et al., 2020) and such sclerotization is entirely lacking in A. epiphylla. Among ctenostomes, thin cuticles are typical for victorelloideans (Braem, 1951) and vesicularioideans (Bobin, 1964). Calcification, or at the least the presence of calcium in the body wall has been found in A. epiphylla (Metcalfe et al., 2007); however, histologically we were not able to observe any distinctions in the cuticle of A. epiphylla. Some early publications had reported the presence of calcium carbonate in ctenostome bryozoans (Kraepelin, 1887), but these rare observations require verification and newer analyses.
Pore plate complexes are a typical feature of all gymnolaemates (Cheetham & Cook, 1983). In cheilostomes, pores are usually plugged by a small number of cells, ranging from 1 to 2 per pore (Banta, 1969), whereas a variable number is found in ctenostomes bryozoans (Schwaha et al., 2022). A. epiphylla has eight cells plugging each pore—a feature resembling vesicularioidean ctenostomes (Schwaha et al., 2022).
The aperture and vestibular wall of A. epiphylla is circular from the outside and lacks and distinct cuticular reinforcements or opercular flap. Consequently, the lack of an operculum, contrary to the original description (Metcalfe et al., 2007), rather supports a ctenostome affinity rather than a cheilostome one. The general impression of a concave indentation of the cuticle and obscure aperture easily conveys the impression of an operculum in A. epiphylla.
Apertural muscles can show a high range of variability among gymnolaemates (see Schwaha, 2020c; Schwaha et al., 2011), especially among ctenostomes (e.g., S. Decker et al., 2020; Pröts et al., 2019; Schwaha, 2021; Schwaha & De Blauwe, 2020; Schwaha et al., 2020a, 2021). In the general Bauplan, there are duplicature bands as peritoneal bands coming from the tentacle sheath and attaching at the lateral distal body wall and separate thick muscles generally termed vestibular muscles. The latter are often present as two separate muscles, one inserting at the diaphragm (parieto‐diaphragmatic muscles as antagonist to the diaphragmatic sphincter) and a more distal set inserting at the vestibular wall (parieto‐vestibular muscles), that facilitate opening the aperture for polypide protrusion (see Schwaha et al., 2011). In cheilostome bryozoans both of these muscles are present, with the parieto‐vestibular muscles being modified as the prominent operculum‐occlusors. Also, duplicature bands are present in all analysed cheilostomes. The situation in A. epiphylla shows a single set of muscles as apertural muscles—in pairs of four according to the shape of the vestibular wall—and a complete lack of duplicature bands. Lack of duplicature bands is typical for three groups of ctenostome bryozoans: hislopioideans, vesicularioideans and Victorellidae (Schwaha, 2020c). In summary, the situation of apertural muscles favor a victorelloidean‐vesicularidioidean ctenostome affinity and clearly lack any similarities to cheilostomes.
In addition, a prominent, setigerous collar, reported here for the first time in A. epiphylla, is reminiscent of other ctenostome genera (McKinney & Dewel, 2002; Schwaha, 2020a). Collars are ubiquitous among gymnolaemates but appear redundant among cheilostomes that possess an operculum, in which the collar is often reduced or vestigial (Schwaha et al., 2020b). Setigerous collars as observed in A. epiphylla are typical, again, for victorelloidean or vesicularioidean ctenostomes (McKinney & Dewel, 2002).
4.3. Lophophore and digestive tract
The lophophore of A. epiphylla carries only eight tentacles, a feature which is prominent in many cyclostomes and also numerous ctenostome bryozoans (Jebram, 1986b). Among ctenostomes, it is common for vesicularioidean or victorelloidean ctenostomes (Schwaha, 2020a).
The digestive tract of A. epiphylla consists of the same common regions that are found in other bryozoans including ctenostomes: foregut, midgut and hindgut (Schwaha, 2020c; Silen, 1944). Only the first section of the midgut, the cardia, shows a mentionable specialization in the form of gizzard teeth. In general, many ctenostomes show distinct muscular condensations in this area. In its most simple form, condensed circular muscles are present as a cardiac constrictor as found in victorellids (Braem, 1951), nolellids, walkeriids (Schwaha & Wanninger, 2018) or the genus Arachnidium (Schwaha & De Blauwe, 2020). More complex cardiac regions show a thick cuticular internal lining usually together with a bulbous shape to form a proventriculus as seen in hislopiids (Schwaha, 2020a). In several ctenostomes and also a few cyclostomes and cheilostomes, the internal cuticle forms distinct teeth to form a gizzard (Gordon, 1975a; Schäfer, 1986). In vesicularioidean ctenostomes, these are usually large and are structurally identical to annelid setae (Gordon, 1975b), which can also be seen with light‐microscopical techniques (Schwaha, 2020c). The gizzard teeth of A. epiphylla appear to lack such a structure, although higher‐resolving methods such as electron microscopy could reveal more details.
The anus of A. epiphylla is lophophoral, being closer to the lophophoral base rather than the vestibular wall (see Schwaha, 2020b). Such a condition is the ancestral one and only in alcyonidioidean and walkerioidean ctenostomes does a vestibular anus seem to be persistent (Schwaha, 2020b).
Funicular muscles, besides the typical funicular cords of cheilostomes (see Schwaha et al., 2020b), are present in numerous ctenostomes (Schwaha et al., 2020b) and also cheilostomes (Lutaud, 1962). Short funicular muscles extending laterally to the body wall were not recently detected in any ctenostome. Position and location resemble the caecal ligament/muscles observed in some cheilostomes (Schwaha et al., 2020b). In other ctenostomes, there are sometimes longer funicular muscle bundles projecting proximally or distally (Schwaha, 2021; Schwaha et al., 2021) or they have short fibres similar to A. epiphylla projecting to the basal side as in Arachnidium fibrosum (Schwaha & De Blauwe, 2020).
5. CONCLUSIONS
The overall assessment of morphological features agrees with a previous indication that A. epiphylla is a ctenostome rather than a cheilostome bryozoan (see Cook et al., 2018, p. 116), which is also supported by our molecular phylogeny. There is no indication of any operculum as previously mentioned (Metcalfe et al., 2007) and neither is there a similarity of the apertural area including its musculature to cheilostomes. The denticulate gizzard, the low tentacle number of eight, pore plate structure and lack of duplicature bands is indicative of a vesicularioidean or victorellid affinity, but details on its exact placement within ctenostomes remains ambiguous. As possible adaptation to extended dry periods, the spongiose tissue of the body cavity appears as a distinct feature that remains unknown for any other bryozoan.
ACKNOWLEDGEMENTS
The recollection of material from Singapore in 2019 was funded by a grant to Dr. Lee Hsiang Liow (University of Oslo) by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement No 724324 to L.H. Liow). Laboratory processing and preservation at the National University of Singapore was supported by Dr. Danwei Huang and Sudhanshi Jain. We thank the Natural History Museum (NHM) Sequencing Facility staff for preparing Illumina libraries and for operating the Illumina NextSeq. 500. We also thank Zichen Zhou for identifying 18S rDNA contigs amongst assemblies and Mary Spencer Jones for providing NHM collections accession numbers for voucher specimens. Funding for sequencing was provided by the NHM Special Funds.
Schwaha, T. , Waeschenbach, A. , De Blauwe, H. , & Gordon, D. P. (2022). Morphology of ctenostome bryozoans: 6. Amphibiobeania epiphylla . Journal of Morphology, 283, 1505–1516. 10.1002/jmor.21519
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Altschul, S. F. , Gish, W. , Miller, W. , Myers, E. W. , & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Bankevich, A. , Nurk, S. , Antipov, D. , Gurevich, A. A. , Dvorkin, M. , Kulikov, A. S. , Lesin, V. M. , Nikolenko, S. I. , Pham, S. , Prjibelski, A. D. , Pyshkin, A. V. , Sirotkin, A. V. , Vyahhi, N. , Tesler, G. , Alekseyev, M. A. , & Pevzner, P. A. (2012). SPAdes: A new genome assembly algorithm and its applications to single‐cell sequencing. Journal of Computational Biology, 19, 455–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta, W. C. (1968). The body wall of cheilostome Bryozoa. I. The ectocyst of Watersipora nigra (Canu and Bassler). Journal of Morphology, 125, 497–507. [DOI] [PubMed] [Google Scholar]
- Banta, W. C. (1969). The body wall of cheilostome Bryozoa. II. Interzoidal communication organs. Journal of Morphology, 129, 149–170. [Google Scholar]
- Bobin, G. (1964). Cytologie des rosettes de Bowerbankia imbricata (Adams) (Bryozaire Cténostome, Vesicularine) Hypothese sur leur fonctionnement. Archives de Zoologie Experimentale et Generale, 104, 1–43. [Google Scholar]
- Bolger, A. M. , Lohse, M. , & Usadel, B. (2014). Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics, 30, 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braem, F. (1951). Über Victorella und einige ihrer nächsten Verwandten, sowie über die Bryozoenfauna des Ryck bei Greifswald. Zoologica, 102, 1–59. [Google Scholar]
- Castresana, J. (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution, 17, 540–552. [DOI] [PubMed] [Google Scholar]
- Cheetham, A. H. , & Cook, P. L. (1983). General features of the class Gymnolaemata. In Robinson R. A. (Ed.), Treatise on invertebrate paleontology. Part G: Bryozoa (pp. 138–207). Geological Society of America and University of Kansas. [Google Scholar]
- Cook, P. L. , Bock, P. E. , Hayward, P. J. , & Gordon, D. P. (2018). Class Gymnolaemata, Order Cheilostomata. In Cook P. L., Bock P. E., Gordon D. P., & Weaver H. J. (Eds.), Australian Bryozoa (Vol. 2, pp. 61–280). CSIRO Publishing. [Google Scholar]
- Darriba, D. , Posada, D. , Kozlov, A. M. , Stamatakis, A. , Morel, B. , & Flouri, T. (2020). ModelTest‐NG: A new and scalable tool for the selection of DNA and protein evolutionary models. Molecular Biology and Evolution, 37, 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker, S. , Wanninger, A. , & Schwaha, T. (2020). Morphology and life cycle of an epiphytic pherusellid ctenostome bryozoan from the Mediterranean Sea. Organisms Diversity & Evolution, 20, 417–437. 10.1007/s13127-020-00443-2 [DOI] [Google Scholar]
- Decker, S. H. , Gordon, D. P. , Spencer Jones, M. E. , & Schwaha, T. (2021). A revision of the ctenostome bryozoan family Pherusellidae, with description of two new species. Journal of Zoological Systematics and Evolutionary Research, 59, 963–980. [Google Scholar]
- Gordon, D. P. (1975a). The occurence of a gizzard in a bryozoan of the order Cheilostomata. Acta Zoologica, 56, 279–282. [Google Scholar]
- Gordon, D. P. (1975b). The resemblance of bryozoan gizzard teeth to “annelid‐like” setae. Acta Zoologica, 56, 283–289. [Google Scholar]
- Hayward, P. J. (1985). Ctenostome bryozoans. E. J. Brill/Dr. W. Backhuys for The Linnean Society of London & The Estuarine and Brackish‐Water Sciences Association. [Google Scholar]
- Jebram, D. (1986a). The ontogenetical and supposed phylogenetical fate of the parietal muscles in the Ctenostomata (Bryozoa). Journal of Zoological Systematics and Evolutionary Research, 24, 58–82. [Google Scholar]
- Jebram, D. (1986b). Arguments concerning the basal evolution of the Bryozoa. Journal of Zoological Systematics and Evolutionary Research, 24, 266–290. [Google Scholar]
- Jebram, D. (1987). Thigmic growth reactions in Victorella. Zoologischer Jahrbücher. Abtheilung für Anatomie und Ontogenie der Tiere, 115, 255–262. [PubMed] [Google Scholar]
- Jebram, D. , & Everitt, B. (1982). New Victorellids (Bryozoa, Ctenostomata) from North America: The use of parallel cultures in bryozoan taxonomy. The Biological Bulletin, 163, 172–187. [Google Scholar]
- Katoh, K. , & Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution, 30, 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin, K. (1887). Die deutschen Süßwasser‐bryozoen. 1. Anatomisch‐systematischer Teil. Abhandlungen aus dem Gebiete der . Naturwissenschaften, hrsg. vom Naturwissenschaftlicher Verein in Hamburg, 10, 168. [Google Scholar]
- Lagesen, K. , Hallin, P. , Rødland, E. A. , Stærfeldt, H.‐H. , Rognes, T. , & Ussery, D. W. (2007). RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Research, 35, 3100–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutaud, G. (1962). Sur la presence d'un muscle du caecum chez les bryozoaires chilostomes. Bulletin de le Soci zoologique de France, 87, 410–418. [Google Scholar]
- Martha, S. O. , Vieira, L. M. , Souto‐Derungs, J. , Grischenko, A. V. , Gordon, D. P. , & Ostrovsky, A. N. (2020). Gymnolaemata, Cheilostomata. In Schwaha T. (Ed.), Handbook of zoology, Phylum Bryozoa (pp. 317–424). de Gruyter. [Google Scholar]
- McKinney, F. K. , & Dewel, R. A. (2002). The ctenostome collar—An enigmatic structure. In Wyse Jackson P. N., Buttler C. J., & Spencer‐Jones M. E. (Eds.), Bryozoan studies 2001 (pp. 191–197). A. A. Balkema Publishers. [Google Scholar]
- Metcalfe, K. , Gordon, D. P. , & Hayward, E. (2007). An amphibious Bryozoan from living mangrove leaves—Amphibiobeania new genus (Beaniidae). Zoological Science, 24, 563–570. [DOI] [PubMed] [Google Scholar]
- Ostrovsky, A. N. (2020). Sexual reproduction in Bryozoa. In Schwaha T. (Ed.), Handbook of zoology. Bryozoa (pp. 101–122). de Gruyter. [Google Scholar]
- Pröts, P. , Wanninger, A. , & Schwaha, T. (2019). Life in a tube: Morphology of the ctenostome bryozoan Hypophorella expansa . Zoological Letters, 5(1), 28. 10.1186/s40851-019-0142-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthensteiner, B. (2008). Soft part 3D visualization by serial sectioning and computer reconstruction. Zoosymposia, 1, 63–100. [Google Scholar]
- Schäfer, P. (1986). On the gizzard of the bryozoan genus Diaperoecia Canu (order Tubuliporata). Senckenbergiana Marit, 17, 253–277. [Google Scholar]
- Schindelin, J. , Arganda‐Carreras, I. , Frise, E. , Kaynig, V. , Longair, M. , Pietzsch, T. , Preibisch, S. , Rueden, C. , Saalfeld, S. , Schmid, B. , Tinevez, J. Y. , White, D. J. , Hartenstein, V. , Eliceiri, K. , Tomancak, P. , & Cardona, A. (2012). Fiji: An open‐source platform for biological‐image analysis. Nature Methods, 9, 676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaha, T. (2020b). O anus, where art thou? An investigation of ctenostome bryozoans. Journal of Morphology, 281, 914–922. 10.1002/jmor.21146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaha, T. (2021). Morphology of ctenostome bryozoans. 3. Elzerina, Flustrellidra, Bockiella . Journal of Morphology, 282, 633–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaha, T. , & De Blauwe, H. (2020). Morphology of ctenostome bryozoans: 1. Journal of Morphology, 281(12), 1598–1606. 10.1002/jmor.21275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaha, T. , Grischenko, A. V. , & Melnik, V. P. (2020a). Morphology of ctenostome bryozoans: 2. Haywardozoon pacificum, with implications of the phylogenetic position of the genus. Journal of Morphology, 281(12), 1607–1616. 10.1002/jmor.21272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaha, T. , Grischenko, A. V. , & Melnik, V. P. (2021). Morphology of ctenostome bryozoans: 4. Journal of Morphology, 282(5), 746–753. 10.1002/jmor.21344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaha, T. , Ostrovsky, A. N. , & Wanninger, A. (2020b). Key novelties in the evolution of aquatic colonial phylum Bryozoa: Evidence from soft body morphology. Biological Reviews, 95, 696–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaha, T. (2020a). Ctenostomata. In Schwaha T. (Ed.), Handbook of zoology. Bryozoa (pp. 269–316). de Gruyter. [Google Scholar]
- Schwaha, T. (2020c). Morphology of bryozoans. In Schwaha T. (Ed.), Handbook of zoology: Bryozoa (pp. 57–100). DeGruyter. [Google Scholar]
- Schwaha, T. , Winston, J. E. , & Gordon, D. P. (2022). Morphology of ctenostome bryozoans: 5. Sundanella, with description of a new species from the Western Atlantic and the multiporata concept. Journal of Morphology, 283, 1139–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaha, T. , Wood, T. S. , & Wanninger, A. (2011). Myoanatomy and serotonergic nervous system of the ctenostome Hislopia malayensis: Evolutionary trends in bodyplan patterning of Ectoprocta. Frontiers in Zoology, 8, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaha, T. F. , & Wanninger, A. (2018). Unity in diversity: A survey of muscular systems of ctenostome Gymnolaemata (Lophotrochozoa, Bryozoa). Frontiers in Zoology, 15, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silen, L. (1944). On the division and movements of the alimentary canal of the Bryozoa. Arkiv foer Zoologi, 35A(12), 1–41. [Google Scholar]
- Stamatakis, A. (2014). RAxML version 8: A tool for phylogenetic analysis and post‐analysis of large phylogenies. Bioinformatics, 30, 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera, G. , & Castresana, J. (2007). Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology, 56, 564–577. [DOI] [PubMed] [Google Scholar]
- Todd, J. A. (2000). The central role of ctenostomes in bryozoan phylogeny. In Herrera Cubilla A., & Jackson J. B. C. (Eds.), Proceedings of the 11th International Bryozoology Association Conference (pp. 104–135). Smithsonian Tropical Research Institute. [Google Scholar]
- Waeschenbach, A. , Taylor, P. D. , & Littlewood, D. T. J. (2012). A molecular phylogeny of bryozoans. Molecular Phylogenetics and Evolution, 62(2), 718–735. 10.1016/j.ympev.2011.11.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


