Abstract
The frequency of aggressive subtypes of B‐cell non‐Hodgkin lymphoma (B‐NHL), such as high‐grade B‐cell lymphomas (HGBL) with MYC and BCL2 and/or BCL6 rearrangement (HGBL‐DH/TH) or Burkitt‐like lymphoma (BL) with 11q aberration, is not well known in the HIV setting. We aimed to characterise HIV‐associated aggressive B‐NHL according to the 2017 WHO criteria, and to identify genotypic and phenotypic features with prognostic impact. Seventy‐five HIV‐associated aggressive B‐NHL were studied by immunohistochemistry (CD10, BCL2, BCL6, MUM1, MYC, and CD30), EBV‐encoded RNAs (EBERs), and fluorescence in situ hybridisation (FISH) to evaluate the status of the MYC, BCL2, and BCL6 genes and chromosome 11q. The 2017 WHO classification criteria and the Hans algorithm, for the cell‐of‐origin classification of diffuse large B‐cell lymphomas (DLBCL), were applied. In DLBCL cases, the frequencies of MYC and BCL6 rearrangements (14.9 and 27.7%, respectively) were similar to those described in HIV‐negative patients, but BCL2 rearrangements were infrequent (4.3%). MYC expression was identified in 23.4% of DLBCL cases, and coexpression of MYC and BCL2 in 13.0%, which was associated with a worse prognosis. As for BL cases, the expression of MUM1 (30.4%) conferred a worse prognosis. Finally, the prevalence of HGBL‐DH/TH and BL‐like with 11q aberration are reported in the HIV setting. The phenotypic and genotypic characteristics of HIV‐associated aggressive B‐NHL are similar to those of the general population, except for the low frequency of BCL2 rearrangements in DLBCL. MYC and BCL2 coexpression in DLBCL, and MUM‐1 expression in BL, have a negative prognostic impact on HIV‐infected individuals.
Keywords: genetics, phenotype, HIV, lymphoma

Introduction
Aggressive B‐cell non‐Hodgkin lymphomas (B‐NHL), such as diffuse large B‐cell lymphoma (DLBCL) and Burkitt's lymphoma (BL), have an increased incidence in the HIV‐infected population. 1 HIV‐infected patients are currently treated with the same regimens as those given to the noninfected, achieving similar response rates. 1 , 2 However, HIV‐infected patients have impaired immunosurveillance. This is due to the direct effect of HIV on CD4+ T cells and the further imbalance of cellular populations and levels of cytokines. 3 HIV‐encoded proteins, like p17, can activate PI3K/Akt signalling pathways, leading to the expansion of B cells. 4 HIV is responsible for the chronic activation of B lymphocytes, thus increasing the probability of genetic alterations promoted by activation‐induced cytidine deaminase (AID), such as chromosomal translocations involving MYC and BCL6 mutations. Moreover, HIV‐infected patients are prone to coviral infections by Epstein–Barr virus (EBV) and human herpes virus‐8 (HHV8), which are known to enhance tumour cell growth and survival, and induce lymphomagenesis. 5 Therefore, it would be expected to find phenotypic and genotypic differences between HIV‐associated lymphomas and their corresponding immunocompetent counterparts.
DLBCL can be divided into two subtypes according to the cell of origin (COO): germinal centre B‐cell like (GCB) and activated B‐cell like (ABC), the latter being associated with poorer outcomes. 6 It is currently recommended to study the COO subtype in all DLBCL, and immunohistochemistry (IHC)‐based algorithms are widely used when gene expression technologies are not available. 7
Both the 2008 and 2017 WHO classifications of tumours of haematopoietic and lymphoid tissues include a category for lymphomas associated with HIV infection. This category comprises subtypes occurring more specifically in HIV‐positive patients, in other immunodeficient states, and lymphomas also occurring in immunocompetent individuals. 7 , 8 The most frequent lymphoma subtypes among HIV‐infected individuals are DLBCL and BL. The 2017 WHO classification introduced new categories in the general population, such as Burkitt‐like lymphoma with 11q aberration, high‐grade B‐cell lymphomas (HGBL) with MYC and BCL2 and/or BCL6 rearrangement (HGBL‐DH/TH), and HGBL NOS, the former with a dismal prognosis. Moreover, it has been found that coexpression of MYC and BCL2 in the absence of a double‐hit also impacts prognosis. However, most of the studies on HIV‐associated lymphomas are not based on the new classifications and data regarding gene translocations, and the expression of MYC and BCL2 in these lymphomas is scarce. We aimed to ascertain how HIV‐associated lymphomas are distributed according to the WHO 2017 classification. Therefore, we reviewed the diagnosis of a comprehensive group of HIV‐associated aggressive B‐cell lymphomas applying IHC and fluorescent in situ hybridisation (FISH) studies currently recommended for the diagnosis of these lymphomas. This study provides a useful snapshot of the genotypic and phenotypic features of HIV‐associated aggressive B‐NHL and identifies markers with prognostic implications.
Materials and methods
Study population
Seventy‐five patients previously diagnosed with HIV‐associated aggressive B‐NHL including DLBCL, BL, and B‐cell lymphoma unclassifiable with features intermediate between DLBCL and classical BL (BCLU) were selected based on the availability of formalin‐fixed and paraffin‐embedded (FFPE) material. Cases with inconclusive FISH analyses were excluded. Other aggressive lymphoma subtypes, occurring more specifically in HIV‐infected patients, were not included. Patients were diagnosed in several hospitals in Spain and the United Kingdom. All cases were retrospectively reviewed, and the main clinical‐biological variables were retrieved. The study was approved by the Institutional Research Board of the Hospital Germans Trias I Pujol HGTIP (Reference: EO‐12‐072) in accordance with the Declaration of Helsinki and biomedical research legislation (Law 14/2007).
Immunohistochemical analyses
Haematoxylin–eosin‐stained slides were reviewed and representative tumour cell‐rich cores were taken in triplicate from formalin‐fixed and paraffin‐embedded (FFPE) blocks to build tissue microarrays (TMA). Immunohistochemical stains were performed with Dako EnVision system (Dako, Glostrup, Denmark) following the manufacturer's protocol. The following antibodies (clone, concentration; brand) were used: CD10 (56C6, 1/20; Novocastra, Newcastle, UK), BCL6 (PG‐B6P, 1/30; Dako), MUM1 (clone MUM1p, 1/40; Dako), BCL2 (124, 1/200; Dako) MYC (Y69, 1/100, Epitomics, Burlingame, CA, USA) and CD30 (clone 1G12, 1/20, Novocastra). BCL2 and MYC were scored in 5% increments, and other markers as positive or negative. The cutoff of 30% was applied for CD10, BCL6 and MUM1 according to Hans criteria. 9 For CD30, the cutoff was 20% and focal positivity was recorded. 10
In situ hybridisation analyses
The presence of EBV was assessed in all cases by means of EBV‐encoded RNA (EBER) detection by in situ hybridisation with the INFORM EBER PROBE assay (Ventana Medical Systems, Tucson, AZ, USA) and the ISH IVIEWBLUE detection system (Ventana Medical Systems) in a BenchMark ULTRA automated instrument (Ventana Medical Systems).
Fluorescent in situ hybridisation (FISH) analyses were performed in all cases for the presence of MYC, BCL2, and BCL6 rearrangements, according to published methods. 11 MYC rearrangements were evaluated with Vysis MYC Break Apart and Vysis IGH/MYC/CEP8 Tri‐Colour Dual Fusion probes (Abbott Molecular., Abbot Park, IL, USA). Cases with MYC rearrangements detected with the break‐apart probe but not with the IGH/MYC/CEP8 were hybridised with a mixture of Vysis LSI MYC SpectrumGold probe (Abbott Molecular) and IGL or IGK FISH DNA Split Signal probe (Dako). Cases with IGL or IGK FISH‐split pattern colocalised with a MYC FISH‐split signal were considered as IGL‐MYC or IGK‐MYC rearranged, respectively. Vysis BCL2 and BCL6 dual‐colour break‐apart probes (Abbott Molecular) were used for detection of BCL2 and BCL6 translocations. The 11q alteration was studied with ZytoLight SPEC 11q gain/loss Triple Colour Probe (ZytoVision, Bremerhaven, Germany).
Statistical analyses
A descriptive study of the variables was performed. Chi‐square, Fisher's exact test, or median test were used for comparisons. Overall survival (OS) and progression‐free survival (PFS) were defined according to standard criteria. 12 Survival probabilities were estimated according to the Kaplan–Meier method and comparisons were performed with the log‐rank test. Univariate cox regression analyses for OS and PFS were performed to calculate hazard ratios with confidence intervals. P ≤ 0.05 was considered statistically significant. Analyses were performed using SPSS v24.0 (IBM, Somer, NY, USA).
Results
Diagnostic revision and clinical features
This study included 75 patients with HIV‐associated aggressive B‐NHL diagnosed between 1998 and 2013.
The clinical features of the 75 patients with complete FISH are in Table 1. Information regarding previous combined antiretroviral therapy (cART) was available in 72 out of the 75 cases. Analysing the whole series, patients who were on cART (n = 45), when lymphoma was diagnosed, had worse OS than those who were not (n = 27); OS probability (95% confidence interval [CI]) 59% (44%, 74%) versus 78% (62%, 94%) (P: 0.038). No relationship was found between previous cART and any of the clinical or biological variables, including degree of immunosuppression, HIV‐load, previous AIDS, and year of lymphoma diagnosis.
Table 1.
Clinical features of the HIV‐associated lymphoma series
| Clinical features | DLBCL N = 47 | BL N = 24 | HGBL DH/TH N = 3 | P‐value |
|---|---|---|---|---|
| Males, N (%) | 37 (78.7) | 21 (87.5) | 2 | 0.287 |
| Age in years, median (min, max) | 44 (30, 68) | 46 (33, 76) | 47 (41, 54) | 0.394 |
| ECOG ≥2, N (%) | 21/46 (45.7) | 10 (41.7) | 2 | 0.750 |
| Ann Arbor stage III‐IV, N (%) | 32 (68.1) | 18 (75.0) | 2 | 0.546 |
| Extranodal sites ≥2, N (%) | 12 (25.5) | 11 (45.8) | 1 | 0.084 |
| B‐symptoms, N (%) | 20 (42.6) | 18 (75.0) | 1 | 0.010 |
| Bulky disease, N (%) | 9 (8.5) | 10 (41.7) | 1 | 0.043 |
| Elevated ß2M, N (%) | 28/32 (87.5) | 10/15 (66.7) | 2 | 0.100 |
| Elevated serum LDH, N (%) | 29 (61.7) | 24 (100) | 2 | <0.001 |
| IPI ≥3, N (%) | 19/46 (41.3) | 15 (60) | 2 | 0.092 |
| Previous OI, N (%) | 16/33 (48.5) | 3/20 (15) | 3 | 0.014 |
| Previous AIDS, N (%) | 21/43 (48.8) | 7 (29.2) | 2 | 0.118 |
| Previous cART, N (%) | 27/45 (60.0) | 14/23 (60.9) | 3 | 0.945 |
| Detectable HIV‐load, N (%) | 32/41 (78.0) | 13/20 (65) | 1 | 0.277 |
| CD4+ T‐cell counts/uL, median (min, max) | 139 (6, 1198) | 199 (12, 697) | 499 (353, 600) | 0.679 |
| Treatment with R, N (%) | 43 (91.5) | 20/23 (87) | 3 (100) | 0.418 |
ß2M, beta2‐microglobulin; BL, Burkitt lymphoma; cART, combination antiretroviral therapy; DLBCL, diffuse large B‐cell lymphoma; ECOG, Eastern Cooperative Oncology Group; HGBL DH/TH, high grade B‐cell lymphoma with MYC and BCL2 and/or BCL6 rearrangement; IPI, International Prognostic index; LDH, lactate dehydrogenase; OI, opportunistic infection; R, rituximab.
P‐values were calculated comparing DLBCL and BL groups and statistically significant P‐values are highlighted. HGBL DH/TH were not included in the comparison due to the low number of cases. The case of Burkitt‐like lymphoma with 11q aberration is not included in the table.
In the following sections we describe the phenotypic and genotypic characteristics of each category of aggressive B‐NHL according to the 2017 WHO classification and their prognostic impact. Of note, no cases of HGBL NOS were identified.
HIV‐Associated high‐grade B‐cell lymphoma with MYC , BCL2, and/or BCL6 rearrangement
The three HIV‐infected patients diagnosed with HGBL‐DH/TH presented all the possible patterns of rearrangements of this category.
One case was a triple hit, harbouring MYC‐IGH translocation, besides BCL2 and BCL6 rearrangements. The tumour had a DLBCL‐like morphology, and lymphoma cells expressed BCL6, MUM1, BCL2, and MYC (40%), whereas they were negative for CD10, CD30, and EBER. Therefore, it was classified as nongerminal centre (non‐GC). The patient presented with bulky disease, without extranodal involvement, and an International Prognostic index (IPI) of 3. After six cycles of RCHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) with concomitant cART, the patient achieved a complete remission that persists, so far, more than 10 years after diagnosis.
The second case showed an MYC‐IGH translocation and a BCL2 rearrangement (Figure 1). The tumour had a DLBCL‐like morphology, and tumour cells expressed MUM1, BCL2, and MYC (95%) and were negative for CD10, BCL6, CD30, and EBER; hence, it was classified as non‐GC. The patient presented with multiple adenopathies, bone marrow and skin involvement, and an IPI of 4. The patient died due to lymphoma progression after the 5th cycle of RCHOP.
Figure 1.
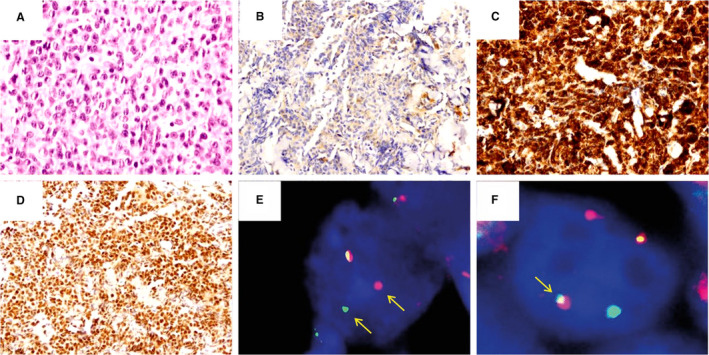
High‐grade B‐cell lymphoma with MYC and BCL2 rearrangement. Diffuse proliferation of large lymphoid cells with centroblastic morphology on H&E (A, ×400). The neoplastic cells were negative for CD10 (B, ×200) and positive for BCL2 (C, ×200). Positivity for MYC in >90% of the neoplastic cells (D, ×200). FISH studies revealed a BCL2 rearrangement (E) and an MYC‐IGH fusion (F).
The third case was a double‐hit lymphoma with MYC‐non‐Ig and BCL6 rearrangements (Figure 2). The tumour had a BL‐like morphology, and cells were positive for BCL6, MYC (80%), and EBER, and negative for CD10, MUM1, BCL2, and CD30; hence, it had a GC phenotype. The patient presented with Ann Arbor stage II and IPI of 0. The lymphoma was treated with an immunochemotherapy regimen for BL with concomitant cART and the patient is in complete remission for more than 5 years. 13
Figure 2.
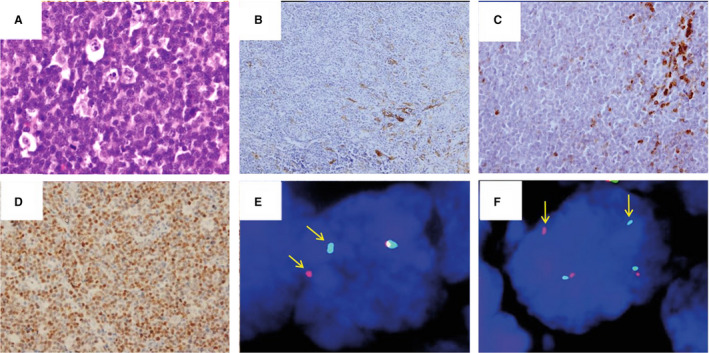
High‐grade B‐cell lymphoma with MYC and BCL6 rearrangements. Diffuse proliferation of medium‐sized lymphocytes with blastoid morphology and a starry‐sky pattern on H&E (A, ×400). The cells were negative for CD10 (B, ×200) and BCL2 (C, ×200). MYC (D, ×200) was positive in >95% of the neoplastic cells. FISH studies with break apart probes highlighted both BCL6 (E) and MYC rearrangements (F).
HIV‐associated Burkitt lymphoma
Patients with BL were treated with intention to cure as follows: intensive treatment for BL (Burkimab) 13 (cyclophosphamide, prednisone, rituximab, vincristine, methotrexate, iphosphamide, dexamethasone, teniposide, cytarabine, doxorubicine, vindesine, etoposide) (n = 13), RCHOP (n = 6), RHyperCVAD (rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, and cytarabine) (n = 3), and CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) (n = 2). All patients received combined antiretroviral therapy (cART) concomitantly with immune‐chemotherapy or chemotherapy.
An MYC‐IGH translocation was detected in 21 out of 24 HIV‐associated BL cases (one of them detected with fusion probes) and an MYC‐IGL in 2 out of 24. The remaining case was an aggressive lymphoma with morphology and phenotype consistent with BL (CD10+, BCL6+, BCL2−, MUM1−, EBER−), without BCL2 or BCL6 translocation. As MYC rearrangement was not detected, FISH analysis for 11q alterations was performed and ruled out. Despite a negative MYC rearrangement, the remaining clinicopathological features were consistent with BL.
Phenotypic features and gene translocations were analysed in the 24 BL cases. The GC markers CD10 and BCL6 were expressed in 100% and 95.7% of the cases, respectively. Notably, 54.2% of the cases were positive for EBER, and all cases were negative for CD30. Four HIV‐associated BL (16.7%) strongly expressed BCL2 (90% of the cells in the mean). These four cases had otherwise typical clinicopathological and phenotypic features of BL, including MYC‐IGH translocation, without concomitant BCL2 or BCL6 rearrangement. All four had monomorphic medium‐sized lymphocytes with fine chromatin and several basophilic nucleoli in a starry‐sky pattern. Patients had extranodal involvement and advanced stage at presentation in all cases, and bulky mass in three of them.
MUM1 was expressed in 30.4% of the cases (7 out of 23). Importantly, HIV‐associated BL expressing MUM1 had worse OS and PFS (Figure 4A,B). The rest of the main clinical and biological variables did not show any prognostic impact on OS in the univariate analyses (Table 2). On the other hand, PFS was influenced by the level of immunosuppression, having patients with CD4 + lymphocytes ≤200/μl a significantly lower 5‐year PFS probability (Table 2). However, multivariate analyses were not performed due to the low number of BL cases (n = 24), especially MUM‐1 positive (n = 7), and hence, a lack of statistical power.
Figure 4.
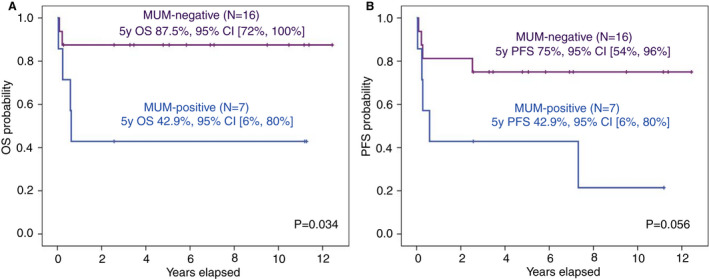
Outcomes of patients with HIV‐associated Burkitt lymphoma according to MUM1 expression. (A) Kaplan–Meier curves for overall survival (OS) of HIV‐associated Burkitt lymphoma by MUM1 expression. (B) Kaplan–Meier curves of progression‐free survival (PFS) of HIV‐associated Burkitt lymphoma by MUM1 expression. Of note, the Burkitt‐like lymphoma with 11q case was removed from the survival analyses.
Table 2.
Univariate analyses for overall survival and progression‐free survival of patients with Burkitt lymphoma
| Variable | 5 year OS (95% CI) | P | 5 year PFS (95% CI) | P | |
|---|---|---|---|---|---|
| ECOG | ≤1 | 70% (45%, 95%) | 0.857 | 57% (31%, 83%) | 0.427 |
| >1 | 70% (42%, 98%) | 70% (42%, 98%) | |||
| Ann Arbor stage | I‐II | 63% (21%, 85%) | 0.708 | 50% (10%, 90%) | _ |
| III‐IV | 72% (51%, 93%) | 67% (45%, 89%) | |||
| Serum LDH | Normal | _ (n = 0) | _ | _ (n = 0) | _ |
| High | 70% (51%, 89%) | 63% (44%, 82%) | |||
| Number of extranodal sites | ≤1 | 84% (64%, 100%) | 0.126 | 69% (44%, 94%) | 0.251 |
| >1 | 55% (26%, 84%) | 55% (26%, 84%) | |||
| Bone marrow involvement | No | 77% (57%, 97%) | 0.271 | 67% (54%, 80%) | 0.590 |
| Yes | 50% (10%, 90%) | 50% (10%, 90%) | |||
| IPI score | 0–2 | 76% (47%, 100%) | 0.627 | 67% (36%, 98%) | 0.680 |
| 3–5 | 67% (43%, 91%) | 60% (35%, 85%) | |||
| CD4 | ≤200 | 62% (33%, 91%) | 0.175 | 46% (17%, 75%) | 0.045 |
| >200 | 90% (71%, 100%) | 90% (71%, 100%) | |||
| CD4 | ≤100 | 57% (20%, 94%) | 0.177 | 57% (20%, 94%) | 0.489 |
| >100 | 86% (68%, 100%) | 71% (47%, 95%) | |||
| HIV‐load | Non‐detectable | 71% (37%, 100%) | 0.454 | 57% (20%, 94%) | 0.158 |
| Detectable | 85% (65%, 100%) | 77% (54%, 100%) | |||
| Previous cART | No | 89% (68%, 100%) | 0.154 | 78% (51%, 100%) | 0.199 |
| Yes | 63% (37%, 100%) | 57% (31%, 83%) | |||
| EBERs | Negative | 71% (43%, 99%) | 0.791 | 64% (36%, 92%) | 0.779 |
| Positive | 69% (44%, 94%) | 62% (36%, 88%) | |||
| MUM1 expression | No | 87.5% (72%, 100%) | 0.034 | 75% (54%, 96%) | 0.056 |
| Yes | 43% (6%, 80%) | 43% (6%, 80%) | |||
BL, Burkitt lymphoma; cART, combination antiretroviral therapy; DLBCL, diffuse large B‐cell lymphoma; EBER, Epstein–Barr virus–encoded small RNAs; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase.
Burkitt‐like lymphoma with 11q aberration
One patient with a previous diagnosis of BL without demonstrable MYC rearrangement showed losses of 11q24.1‐ter by FISH, being reclassified as Bukitt‐like lymphoma with 11q aberration (Figure 3). He was a 57‐year‐old male who presented with extranodal disease and adenopathies with an IPI of 0. The lymphoma cells were positive for CD10 and BCL6, and negative for BCL2, MUM1, CD30, and EBER. MYC expression was inconclusive. The patient received six cycles of RCHOP with concomitant cART, achieving a complete remission and died 5 years later of lung non‐small‐cell carcinoma.
Figure 3.
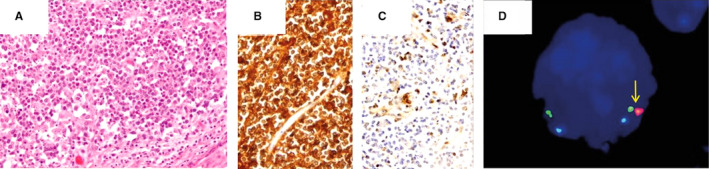
Burkitt‐like lymphoma with 11q alterations. (A, H&E, ×200) diffuse proliferation of medium‐sized lymphocytes with a starry‐sky pattern, with expression of CD10 (B, ×200) negativity for BCL2 (C, ×200). FISH studies highlighted distal loss on chromosome 11q (D, arrow).
HIV‐associated diffuse large B‐cell lymphoma
Patients with DLBCL were treated with intention to cure as follows: RCHOP (n = 40), CHOP (n = 4), intensive treatment for BL (Burkimab) (n = 2), and R‐EPOCH (etoposide, prednisone, cyclophosphamide, vincristine, and doxorubicine) (n = 1). All patients received cART concomitantly with immune‐chemotherapy or chemotherapy.
Rearrangements involving MYC were present in 7 out of 47 (14.9%) of DLBCL cases. Five cases had an MYC‐IGH rearrangement, one case had an MYC‐IGK, and one case was negative for MYC‐IGH rearrangement and inconclusive for MYC‐IGL and MYC‐IGK (Table 4). Rearrangements of the BCL6 gene were found in 13 cases (27.7%), whereas BCL2 rearrangements were detected only in two (4.3%).
Table 4.
Genotype and phenotype of HIV‐associated diffuse large B‐cell lymphoma by cell‐of‐origin group assessed by Hans algorithm
| Total N = 47 | GC N = 17 (36.2%) | Non‐GC N = 30 (63.8%) | P‐value | |
|---|---|---|---|---|
| TMYC, N (%) | 7 (14.9) | 5 (29.4) | 2 (6.7) | 0.049 |
| TBCL2, N (%) | 2 (4.3) | 0 | 2 (6.7) | 0.402 |
| TBCL6, N (%) | 13 (27.7) | 1 (5.9) | 12 (40) | 0.011 |
| CD10, N (%) | 14 (29.8) | 14 (82.4) | 0 | <0.001 |
| BCL6, N (%) | 32 (68.1) | 17 (100) | 15 (50) | <0.001 |
| MUM1, N (%) | 28 (59.6) | 3 (17.6) | 25 (83.3) | <0.001 |
| CD30, N (%) | 10 (21.3) | 0 | 10 (33.3) | 0.006 |
| EBER, N (%) | 10 (21.3) | 2 (11.8) | 8 (26.7) | 0.206 |
| BCL2, N (%) | 19/46 (41.3) | 1 (5.9) | 18/29 (62.1) | <0.001 |
| MYC, N (%) | 11 (23.4) | 6 (35.3) | 5 (16.7) | 0.138 |
| DE, N (%) | 6/46 (13.0) | 1 (5.9) | 5/29 (17.2) | 0.266 |
DE, double expressers considering positive cutoffs of BCL2 ≥ 50% and MYC ≥40%; GC, germinal center; GCB, germinal center B‐cell; non‐GC, nongerminal center; TBCL2, BCL2 rearrangement; TBCL6, BCL6 rearrangement; TMYC, MYC rearrangement.
P‐values from Chi‐square or Fisher's exact test are disclosed except for the comparison of Hans algorithm results, whose P‐value is from McNemar's test. Statistically significant P‐values are highlighted.
The immunophenotype of HIV‐associated DLBCL is described in Table 4. Almost two‐thirds were non‐GC phenotype according to the Hans algorithm. No differences were found between the two groups regarding the proportion of patients who were on cART at lymphoma diagnosis. Of note, CD30 was positive in 10 out of 47 cases (21,3%), four of them only focally, and 41.3% were BCL2‐positive. Although there is no consensual cutoff for BCL2 positivity, the 50% cutoff was applied, as previously reported. 14
EBER was positive in 21.3% cases, and it was not associated with CD30 expression. As expected, BCL6 expression was associated with BCL6 rearrangements (P = 0.025). Eleven out of 47 cases (23.4%) expressed MYC protein, and six cases (12.8%) were double expressors (DE) of MYC and BCL2.
Then we compared genotype and phenotype of GC and non‐GC cases (Table 4). MYC rearrangements were more frequent in HIV‐associated DLBCL with a GC phenotype, whereas BCL6 rearrangements, CD30 and BCL2 expression were almost exclusive of the non‐GC subtype.
Survival analyses were performed including only HIV‐associated DLBCL treated with RCHOP (N = 40). Concomitant expression of MYC and BCL2 had a strong negative impact on survival, particularly PFS (Figure 5). HIV‐associated DLBCL cases with a non‐GC phenotype tended to have a worse OS and PFS than the cases with the GC phenotype (Table 3). Moreover, cases that had BCL6 rearrangements tended to have worse OS and PFS (Table 3). All cases with a BCL6 rearrangement, except one, had a non‐GC phenotype. Hence, the impact of BCL6 rearrangement on outcomes was studied in this phenotype‐subset, but there was none (Supplementary Figure S1).
Figure 5.
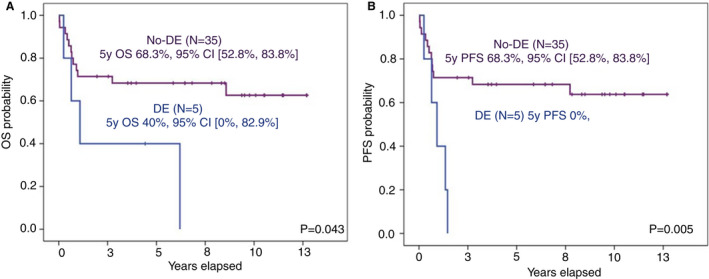
Phenotypic features with impact on outcomes of patients with HIV‐associated diffuse large B‐cell lymphoma (DLBCL). (A) Kaplan–Meier curves for overall survival (OS) of HIV‐associated DLBCL for double expressers (DE) and no‐DE. (B) Kaplan–Meier curves for progression‐free survival (PFS) of HIV‐associated DLBCL for double expressers (DE) and no‐DE.
Table 3.
Univariate analyses for overall survival and progression‐free survival of the 40 patients with diffuse large B‐cell lymphoma treated with RCHOP
| 5 year OS (95% CI) | P | 5 year PFS (95% CI) | P | ||
|---|---|---|---|---|---|
| ECOG | ≤1 | 67% (47%, 87%) | 0.658 | 67% (47%, 87%) | 0.488 |
| >1 | 63% (41%, 85%) | 52% (29%, 75%) | |||
| Ann‐Arbor stage | I‐II | 91% (74%, 100%) | 0.091 | 91% (74%, 100%) | 0.064 |
| III‐IV | 55% (37%, 73%) | 48% (30%, 66%) | |||
| Serum LDH | Normal | 77% (57%, 97%) | 0.331 | 66% (44%, 88%) | 0.452 |
| High | 55% (34%, 76%) | 55% (34%, 76%) | |||
| N extranodal sites | ≤1 | 66% (49%, 83%) | 0.582 | 63% (46%, 80%) | 0.570 |
| >1 | 60% (30%, 90%) | 50% (19%, 81%) | |||
| Bone marrow involvement | No | 63% (47%, 79%) | 0.999 | 63% (47%, 79%) | 0.505 |
| Yes | 80% (45%, 100%) | 40% (0, 83%) | |||
| IPI score | 0–2 | 69% (50%, 88%) | 0.420 | 65% (45%, 85%) | 0.518 |
| 3–5 | 59% (36%, 82%) | 53% (29%, 77%) | |||
| CD4 | ≤200 | 69% (50%, 88%) | 0.284 | 69% (50%, 88%) | 0.157 |
| >200 | 63% (39%, 87%) | 50% (25%, 75%) | |||
| CD4 | ≤100 | 58% (34%, 82%) | 0.636 | 58% (34%, 82%) | 0.839 |
| >100 | 73% (54%, 92%) | 64% (44%, 84%) | |||
| HIV‐load | Non‐detectable | 86% (60%, 100%) | 0.261 | 62% (27%, 97%) | 0.478 |
| Detectable | 61% (43%, 79%) | 61% (43%, 79%) | |||
| cART before lymphoma | No | 71% (49%, 93%) | 0.276 | 71% (49%, 93%) | 0.201 |
| Yes | 59% (38%, 80%) | 50% (29%, 71%) | |||
| EBERs | Negative | 67% (50%, 84%) | 0.596 | 61% (44%, 78%) | 0.746 |
| Positive | 56% (23%, 89%) | 56% (23%, 89%) | |||
| CD30 expression | Negative | 71% (43%, 99%) | 0.791 | 58% (41%, 75%) | 0.965 |
| Positive | 69% (44%, 94%) | 67% (36%, 98%) | |||
| MYC expression | No | 70% (53%, 86%) | 0.153 | 70% (53%, 86%) | 0.055 |
| Yes | 50% (19%, 81%) | 30% (2%, 58%) | |||
| Double expresser status | No | 68% (53%, 83%) | 0.043 | 68% (53%, 83%) | 0.005 |
| Yes* | 40% (0, 83%) | 0 | |||
| COO subtype | GC | 79% (57%, 100%) | 0.082 | 79 (57%, 100%) | 0.061 |
| Non‐GC | 57% (38%, 76%) | 50 (30%, 69%) | |||
| MYC rearrangement | No | 65% (49%, 81%) | 0.656 | 62% (46%, 78%) | 0.716 |
| Yes | 67% (29%, 100%) | 50% (10%, 90%) | |||
| BCL6 rearrangement | No | 74% (58%, 91%) | 0.060 | 67% (49%, 84%) | 0.092 |
| Yes | 45% (17%, 72%) | 45% (17%, 72%) | |||
BL, Burkitt lymphoma; cART, combination antiretroviral therapy; COO, cell of origin; DLBCL, diffuse large B‐cell lymphoma; EBER, Epstein–Barr virus–encoded small RNAs; ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic index; LDH, lactate dehydrogenase.
The rest of the important clinical and biological variables did not show any prognostic impact on OS and PFS in the univariate analyses (Table 3). Multivariate analyses were not performed due to the low number of double‐expresser cases (n = 5) and the lack of statistical power.
MYC expression in HIV‐associated aggressive B‐cell lymphomas
MYC expression studies were successful in 71 out of the 75 HIV‐associated HGBL; MYC studies were inconclusive in three HIV‐associated BL cases and in the Burkitt‐like lymphoma with 11q aberration case. MYC expression was considered positive when at least 40% of the cells were positive for MYC staining. MYC was positive in all three HIV‐associated HGBL‐DH/TH cases (100%), 23 out of 24 (95.8%) BL, and 11 out of 47 (23.4%) DLBCL cases (Figure 6). As expected, MYC rearrangements were associated with MYC expression (P < 0.001). However, as reported previously in non‐HIV‐associated lymphomas, the correlation was not perfect 15 ; two cases with MYC‐Ig rearrangement (one DLBCL and one BL) did not express MYC protein, and six cases without MYC rearrangements (five DLBCL and one BL) showed high MYC protein expression.
Figure 6.
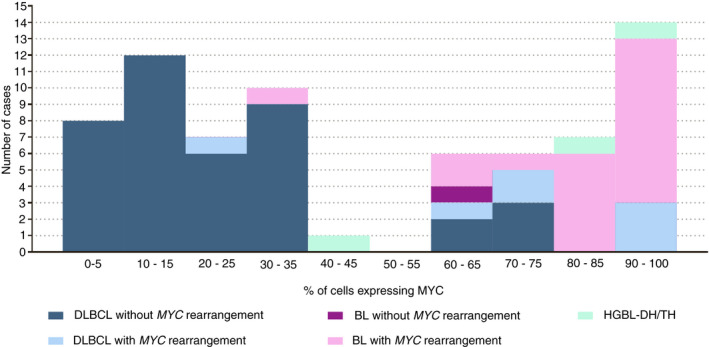
Relationship between the percentage of tumour cells expressing MYC and the presence or not of rearrangements involving MYC. BL, Burkitt lymphoma; DLBCL, diffuse large B‐cell lymphoma; HGBL‐DH/TH, high grade B‐cell lymphoma with MYC and BCL2 and/or BCL6 rearrangement.
Discussion
The present study included 75 HIV‐associated aggressive B‐NHL cases with previous diagnoses of DLBCL, BL, or BCLU (2008 WHO classification) that were reviewed and reclassified according to the 2017 WHO classification. MYC, BCL2, and BCL6 rearrangements were studied in all cases, and 11q alterations were evaluated in cases with BL features without MYC rearrangements. Although these genetic abnormalities have been already reported in HIV‐infected patients, to the best of our knowledge this is the first systematic study by FISH in a large series of HIV‐associated lymphoma, leading to understand the real prevalence of HGBL‐DH/TH and BL‐like with 11q aberration in the HIV‐setting. 16 , 17 , 18 , 19 Importantly, we studied the prognostic impact of COO subtypes and of some phenotypic markers, including MYC, BCL2 and DE, in the subset of patients with DLBCL treated with RCHOP.
After a comprehensive study by FISH and immunohistochemistry we succeeded in reviewing the diagnosis of 75 cases. In our series, 4% of the cases were HGBL‐DH/TH, a percentage similar to that reported in immunocompetent patients. 20 , 21 The reduced number of HGBL‐DH/TH prevented survival analyses. All three cases were DE and two had a non‐GC phenotype. Although the 2017 WHO classification states that “a double‐hit status should be investigated in all DLBCLs, NOS, using cytogenetic or molecular cytogenetic studies,” some centres prefer to look for rearrangements only in cases with a GC phenotype and DE, or in cases with a BL‐like morphology. 22 Our results would support the need for universal screening for gene rearrangements.
Around 50% of HIV‐associated BL were EBER‐positive. However, we observed BL cases with peculiarities so far only reported in the immunocompetent population. First, 16.7% of the HIV‐associated BL cases expressed BCL2, even with an intense and diffuse pattern. BCL2 is usually negative or weak in BL. Whereas the high expression of BCL2 in an aggressive lymphoma with BL morphology suggests a BCL2 rearrangement consistent with a diagnosis of HGBL‐DH/TH, around 20% of BL cases show BCL2 expression in the immunocompetent population and a diagnosis of BL can be made after excluding an HGBL‐DH/TH. 23 Second, we observed MUM1 expression in a subset of HIV‐associated BL (30.4%). It is generally assumed that BL do not express late/post GC markers, thus MUM1 has been scarcely studied in these lymphomas. However, MUM1 expression has been detected in 10% to 41% of BL, including the HIV‐associated BL series. 24 , 25 , 26 , 27 , 28 Importantly, we observed that MUM1 expression negatively impacted outcomes of HIV‐associated BL, in line with a recent study on BL in immunocompetent individuals. 29
One case previously classified as BL, without detectable MYC rearrangements by FISH, was tested for 11q alterations and finally was reclassified as a Burkitt‐like lymphoma with 11q aberration. This lymphoma category is a recently described type of aggressive lymphoma that shares clinical and phenotypic characteristics with BL, but lacks MYC rearrangements and carries gains in 11q23.2–23.3 and/or losses in 11q24.1‐ter. 30 This lymphoma lacks the genetic profile of BL and is similar to the GCB subtype of DLBCL or high‐grade B‐cell lymphoma. 31 , 32 This lymphoma subtype has been rarely reported in the HIV setting, and our results show that the full spectrum of aggressive B‐cell lymphoma can be seen in HIV‐infected patients.
Gene rearrangements involving MYC were detected in 15% of HIV‐associated DLBCL cases, a frequency that is similar to that reported in the immunocompetent population. 33 , 34 , 35 Moreover, MYC rearrangements were more frequently found in cases with a GC phenotype. The impact of MYC rearrangements on DLBCL prognosis is controversial, but it seems that cases with MYC rearrangements treated with RCHOP have a poorer prognosis. 33 , 34 , 35 In our series of HIV‐associated DLBCL cases, the presence of MYC rearrangements was not significantly associated with worse outcomes. The frequency of BCL6 rearrangements found in DLBCL of HIV‐infected patients (28%) was also similar to that described in non‐HIV‐infected patients (around 30%) and were mainly detected in non‐GC cases, as reported in the general population. 20 , 34 , 35 , 36 , 37 , 38 , 39 The prognostic impact of BCL6 rearrangements is controversial in immunocompetent DLBCL. 7 , 20 , 36 , 37 In this series, cases with BCL6 rearrangement showed a trend for shorter PFS and OS. Due to the BCL6 rearrangements mainly present in non‐GC cases, we studied the prognostic impact of this genetic aberration in this subgroup of patients and we did not find any. Interestingly, BCL2 rearrangements were scarcely detected in our series (4.3%), an incidence substantially lower than that reported in immunocompetent patients (20% to 30%). 34 , 35 , 38 , 39
The distribution of HIV‐associated DLBCL cases in the GC and non‐GC categories is quite inconsistent among studies, but the criteria employed for COO assignment also. 3 , 40 , 41 , 42 , 43 , 44 , 45 Some authors argue that HIV‐associated DLBCL show an immunophenotype intermediate between the GC and activated B‐cell phenotypes of DLBCL of immunocompetent patients, which could also explain those discrepancies. 3 , 44 , 45 In line with this, we have previously showed a disagreement, in an HIV‐associated case series, between the results of Hans algorithm and a commonly used molecular‐based method for COO assignment developed by Nanostring. 38 , 46 Herein, in line with a previous report by Chao et al., we identified a higher frequency of non‐GC types in HIV‐associated DLBCL than that reported in immunocompetent patients. 41 Regarding the prognostic impact of COO, two previous studies on HIV‐associated DLBCL showed that the Hans algorithm could identify cases with worse outcomes. 43 , 47 In the present study, a larger series of HIV‐associated DLBCL treated with RCHOP we observed that non‐GC cases tended to have a worse OS and PFS than GC cases, although without statistical significance.
Among DLBCL cases, 21% of cases were positive for EBER, a percentage slightly lower than that previously reported (around 30%). 40 , 43 , 48 EBER was expressed slightly more frequently in non‐GC cases, and none of the EBV‐positive cases had BCL6 rearrangements. 40 , 43 , 48 Although the context of HIV‐associated immunosuppression excludes the diagnosis of EBV‐positive DLBCL NOS, the features above described are similar to those found in EBV‐positive DLBCL NOS, which rarely show BCL6 rearrangements and are generally non‐GC. 49 EBER was not associated with CD30 expression in the present series and, as in other studies including patients treated with immunochemotherapy, EBV showed no impact on outcomes of HIV‐associated DLBCL. 40 , 43 , 48
In DLBCL of the immunocompetent population, 12% to 19% of the cases are positive for CD30 (≥20% cutoff) and it is expressed both in GC and non‐GC cases. 10 , 50 , 51 , 52 Interestingly, our results showed that around 20% of HIV‐associated DLBCL express CD30, and this result could drive therapeutic decisions, as CD30 is effectively targetable. 53 In our series, all CD30‐positive cases were of the non‐GC phenotype, and in line with the results in non‐GC DLBCL of the general population, it had no prognostic impact.
In line with previous studies on HIV‐associated DLBCL, around 40% of the studied cases in our series expressed BCL2. 40 , 41 , 54 BCL2 was mainly expressed in non‐GC cases, as described in DLBCL of immunocompetent patients. 14 Our survival analyses showed that BCL2 expression had no impact on outcomes.
An MYC expression was detected in 23.4% of HIV‐associated DLBCL, and 13% were DE, most of them of the non‐GC phenotype. This incidence is slightly lower than that reported in DLBCL series of immunocompetent patients (20–30%). 14 , 55 , 56 , 57 Of note, MYC positivity tended to be associated with worse PFS, and DE was significantly associated with shorter probability of OS and PFS. These results support the consideration, made in general DLBCL, that MYC and BCL2 coexpression, but not MYC expression alone, is associated with a worse prognosis. 56 The negative impact of DE on prognosis of DLBCL affecting immunocompetent individuals is well established. 10 , 58 However, to our knowledge, only one study has previously assessed MYC expression and DE in HIV‐associated DLBCL, but HGBL‐DH/TH cases were not excluded and patients were not treated with immunochemotherapy. 41 These authors reported that 64% of HIV‐associated DLBCL expressed MYC and 25% were DE. However, the cutoff employed for MYC positivity was 10% instead of 40% (recommended by the WHO classification and used herein). On the other hand, a recent study showed that patients with MYC‐positive HIV‐associated DLBCL, treated with REPOCH, had a significantly lower EFS. In this study, no differences were found for both OS and PFS between single MYC+ patients and double expressers (MYC+/BCL2+). 59
In summary, this study provides a useful characterisation of HIV‐associated aggressive B‐cell lymphomas according to the 2017 WHO criteria. An important message to retrieve from this study is that new entities such as Burkitt‐like lymphoma with 11q aberration and HGBL‐DH/TH can be found in the HIV‐setting, with similar frequencies to those observed in the immunocompetent population. Some similarities and differences were observed between HIV‐associated DLBCL and general DLBCL. MUM1 in BL and MYC/BCL2 coexpression in DLBCL were found to be markers with prognostic significance in HIV‐infected patients. Therefore, the diagnostic criteria and some of the prognostic markers used for the study of aggressive B‐NHL in immunocompetent patients should also be taken into account when these lymphomas occur in the setting of HIV infection.
Authors contributions
M‐J.B, G.T. J‐L.M., and J‐T.N. designed the study; M‐J.B., G.T., and J‐T.N. wrote the article; A‐M.M‐M. and J.M. performed laboratory methods: M‐J.B., J.M., and O.G. performed statistical analyses; S.M., J. G., M.C., A.M., L.V., A.M‐T., T. A., J.M., M.J., A.F., M.A., J.B., E.G‐B., F.C., A.M., J‐M.M., M.P., P.A., E. A., L., C.G‐B, M.G‐C, J‐M.S., and J‐M. R. provided patient data and samples.
Conflict of Interest
J.G. has received honoraria from Abbvie, Acerta/Astra Zeneca, Gilead, and Janssen; and grant funding from Celgene, Janssen, and AstraZeneca. M.J.T. discloses consultancy in Janssen, Roche, Astra Zeneca, Abbvie, and research funding from Janssen and Gilead. J.B. discloses honoraria from Roche, Takeda, Celgene, Novartis, and Gilead; research funding from Celgene and Roche; consultation or advisory role in Takeda, Jansen, Celgene, and Gilead/Kyte; and travel and accommodations expenses from Roche, Takeda, Celgene, Jansen, and Gilead. A.M. has received a speaker honorarium and travel grants from Roche, Abbvie, and Janssen and has participated in advisory boards for Roche, Abbvie, and Janssen. M.P. discloses advisory board BMS, Astra Zeneca, Takeda, MSD, and Roche. P.A. has received honoraria from Janssen, Roche, Celgene, and Abbvie. J.M.S. discloses honoraria from Roche, Janssen, Celgene, Novartis, Gilead, Takeda, Servier, and Mundipharma; consultation or advisory role in Roche, Janssen, Celgene, Novartis, Gilead, Celltrion, and Sandoz; speakers' bureau in Roche and Celgene; and travel and accommodations expenses from Roche. J.M.R. discloses honoraria and consultation or advisory role in Celgene, Novartis, Takeda, Servier, Incyte, Amgen, and Pfizer; speakers' bureau in Amgen and Pfizer; and travel and accommodations expenses from Amgen, Pfizer, and Incyte. J.T.N. discloses honoraria from Novartis; consultation or advisory role in EUSA Pharma; research funding from Gilead and EUSA Pharma; and travel and accommodation expenses from Roche. The remaining authors report no conflicts of interest.
Supporting information
Figure S1. Impact of BCL6 rearrangements on outcomes of patients with HIV‐associate nongerminal center diffuse large B‐cell lymphoma (DLBCL). (a) Kaplan–Meier curves for overall survival of HIV‐associate nongerminal center (non‐GC) DLBCL according to the presence of BCL6 rearrangements. (b) Kaplan–Meier curves for progression free survival of HIV‐associate nongerminal center (non‐GC) DLBCL according to the presence of BCL6 rearrangements.
Acknowledgements
The authors thank the staff from the Department of Pathology of Hospital Germans Trias i Pujol and from the Biobanking of tumour samples from Institut Germans Trias i Pujol for their collaboration. This work was supported in part by Instituto de Salud Carlos III, Ministerio de Economia y Competividad, Spain (PI19/01588); Gilead Sciences S.L., Spain (GLD19/00121); 2017 SGR288 (GRE) from AGAUR Programme/Generalitat de Catalunya, and by funds from Josep Carreras International Foundation and “la Caixa” Foundation.”
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Barta SK, Samuel MS, Xue X et al. Changes in the influence of lymphoma‐ and HIV‐specific factors on outcomes in AIDS‐related non‐Hodgkin lymphoma. Ann. Oncol. 2015; 26; 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baptista MJ, Garcia O, Morgades M et al. HIV‐infection impact on clinical‐biological features and outcome of diffuse large B‐cell lymphoma treated with R‐CHOP in the combination antiretroviral therapy era. AIDS 2015; 29; 811–818. [DOI] [PubMed] [Google Scholar]
- 3. Dolcetti R, Gloghini A, Caruso A, Carbone A. A lymphomagenic role for HIV beyond immune suppression? Blood 2016; 127; 1403–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giagulli C, Marsico S, Magiera AK et al. Opposite effects of HIV‐1 p17 variants on PTEN activation and cell growth in B cells. PLoS ONE 2011; 6; e17831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Linke‐Serinsöz E, Fend F, Quintanilla‐Martinez L. Human immunodeficiency virus (HIV) and Epstein‐Barr virus (EBV) related lymphomas, pathology view point. Semin. Diagn. Pathol. 2017; 34; 352–363. [DOI] [PubMed] [Google Scholar]
- 6. Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A, Staudt LM. A gene expression‐based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc. Natl. Acad. Sci. U. S. A. 2003; 100; 9991–9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swerdlow S, Campo E, Harris N et al. eds. WHO classification of tumours of haematopoietic and lymphoid tissues. revised. 4th ed. Lyon, France: IARC Press, 2017. [Google Scholar]
- 8. Swerdlow SH, Campo E, Harris NL et al. eds. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press, 2008. [Google Scholar]
- 9. Hans CP, Weisenburger DD, Greiner TC et al. Confirmation of the molecular classification of diffuse large B‐cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004; 103; 275–282. [DOI] [PubMed] [Google Scholar]
- 10. Hu S, Xu‐Monette ZY, Balasubramanyam A et al. CD30 expression defines a novel subgroup of diffuse large B‐cell lymphoma with favorable prognosis and distinct gene expression signature: a report from the international DLBCL rituximab‐CHOP consortium program study. Blood 2013; 121; 2715–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muñoz‐Mármol AM, Sanz C, Tapia G, Marginet R, Ariza A, Mate JL. MYC status determination in aggressive B‐cell lymphoma: the impact of FISH probe selection. Histopathology 2013; 63; 418–424. [DOI] [PubMed] [Google Scholar]
- 12. Cheson BD, Pfistner B, Juweid ME et al. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007; 25; 579–586. [DOI] [PubMed] [Google Scholar]
- 13. Ribera J‐M, García O, Grande C et al. Dose‐intensive chemotherapy including rituximab in Burkitt's leukemia or lymphoma regardless of human immunodeficiency virus infection status: final results of a phase 2 study (Burkimab). Cancer 2013; 119; 1660–1668. [DOI] [PubMed] [Google Scholar]
- 14. Johnson NA, Slack GW, Savage KJ et al. Concurrent expression of MYC and BCL2 in diffuse large B‐cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Clin. Oncol. 2012; 30; 3452–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tapia G, Lopez R, Muñoz‐Mármol AM et al. Immunohistochemical detection of MYC protein correlates with MYC gene status in aggressive B cell lymphomas. Histopathology 2011; 59; 672–678. [DOI] [PubMed] [Google Scholar]
- 16. Hinkle C, Makar GS, Brody JD, Ahmad N, Zhu GG. HIV‐associated ‘double‐hit’ lymphoma of the tonsil: a first reported case. Head Neck Pathol. 2020; 14; 1129–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chapman JR, Bouska AC, Zhang W et al. EBV‐positive HIV‐associated diffuse large B cell lymphomas are characterized by JAK/STAT (STAT3) pathway mutations and unique clinicopathologic features. Br. J. Haematol. 2021; 194; 870–878. [DOI] [PubMed] [Google Scholar]
- 18. Ferreri AJM, Cattaneo C, Lleshi A et al. A dose‐dense short‐term therapy for human immunodeficiency virus/acquired immunodeficiency syndrome patients with high‐risk Burkitt lymphoma or high‐grade B‐cell lymphoma: safety and efficacy results of the ‘CARMEN’ phase II trial. Br. J. Haematol. 2021; 192; 119–128. [DOI] [PubMed] [Google Scholar]
- 19. Gebauer N, Witte HM, Merz H et al. Aggressive B‐cell lymphoma cases with 11q aberration patterns indicate a spectrum beyond Burkitt‐like lymphoma. Blood Adv. 2021; 5; 5220–5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akyurek N, Uner A, Benekli M, Barista I. Prognostic significance of MYC, BCL2, and BCL6 rearrangements in patients with diffuse large B‐cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Cancer 2012; 118; 4173–4183. [DOI] [PubMed] [Google Scholar]
- 21. Chen B J, Fend F, Campo E, Quintanilla‐Martinez L. Aggressive B‐cell lymphomas—From morphology to molecular pathogenesis. Annals of Lymphoma; Vol 3 (January 2019): Annals of Lymphoma 2019; 3. http://aol.amegroups.com/article/view/4949 (accessed 1 Jan2019), 3.
- 22. Sesques P, Johnson NA. Approach to the diagnosis and treatment of high‐grade B‐cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood 2017; 129; 280–288. [DOI] [PubMed] [Google Scholar]
- 23. Masqué‐Soler N, Szczepanowski M, Kohler CW et al. Clinical and pathological features of Burkitt lymphoma showing expression of BCL2‐‐an analysis including gene expression in formalin‐fixed paraffin‐embedded tissue. Br. J. Haematol. 2015; 171; 501–508. [DOI] [PubMed] [Google Scholar]
- 24. Chuang S‐S, Ye H, Du M‐Q et al. Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B‐cell lymphoma with very high proliferation index and with or without a starry‐sky pattern: a comparative study with EBER and FISH. Am. J. Clin. Pathol. 2007; 128; 558–564. [DOI] [PubMed] [Google Scholar]
- 25. Nasr MR, Rosenthal N, Syrbu S. Expression profiling of transcription factors in B‐ or T‐acute lymphoblastic leukemia/lymphoma and burkitt lymphoma: usefulness of PAX5 immunostaining as pan‐pre‐B‐cell marker. Am. J. Clin. Pathol. 2010; 133; 41–48. [DOI] [PubMed] [Google Scholar]
- 26. Gualco G, Queiroga EM, Weiss LM, Klumb CEN, Harrington WJ, Bacchi CE. Frequent expression of multiple myeloma 1/interferon regulatory factor 4 in Burkitt lymphoma. Hum. Pathol. 2009; 40; 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ye Z, Xiao Y, Shi H et al. Sporadic Burkitt lymphoma in southern China: 12 years' experience in a single institution in Guangzhou. J. Clin. Pathol. 2011; 64; 1132–1135. [DOI] [PubMed] [Google Scholar]
- 28. Carbone A, Gloghini A, Larocca LM et al. Expression profile of MUM1/IRF4, BCL‐6, and CD138/syndecan‐1 defines novel histogenetic subsets of human immunodeficiency virus‐related lymphomas. Blood 2001; 97; 744–751. [DOI] [PubMed] [Google Scholar]
- 29. Satou A, Asano N, Kato S et al. Prognostic impact of MUM1/IRF4 expression in Burkitt lymphoma (BL): a reappraisal of 88 BL patients in Japan. Am. J. Surg. Pathol. 2017; 41; 389–395. [DOI] [PubMed] [Google Scholar]
- 30. Horn H, Kalmbach S, Wagener R et al. A diagnostic approach to the identification of Burkitt‐like lymphoma with 11q aberration in aggressive B‐cell lymphomas. Am. J. Surg. Pathol. 2021; 45; 356–364. [DOI] [PubMed] [Google Scholar]
- 31. Gonzalez‐Farre B, Ramis‐Zaldivar JE, Salmeron‐Villalobos J et al. Burkitt‐like lymphoma with 11q aberration: a germinal center‐derived lymphoma genetically unrelated to Burkitt lymphoma. Haematologica 2019; 104; 1822–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wagener R, Seufert J, Raimondi F et al. The mutational landscape of Burkitt‐like lymphoma with 11q aberration is distinct from that of Burkitt lymphoma. Blood 2019; 133; 962–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosenwald A, Bens S, Advani R et al. Prognostic significance of MYC rearrangement and translocation partner in diffuse large B‐cell lymphoma: a study by the Lunenburg lymphoma biomarker consortium. J. Clin. Oncol. 2019; 37; 3359–3368. [DOI] [PubMed] [Google Scholar]
- 34. Tzankov A, Xu‐Monette ZY, Gerhard M et al. Rearrangements of MYC gene facilitate risk stratification in diffuse large B‐cell lymphoma patients treated with rituximab‐CHOP. Mod. Pathol. 2014; 27; 958–971. [DOI] [PubMed] [Google Scholar]
- 35. Copie‐Bergman C, Cuillière‐Dartigues P, Baia M et al. MYC‐IG rearrangements are negative predictors of survival in DLBCL patients treated with immunochemotherapy: a GELA/LYSA study. Blood 2015; 126; 2466–2474. [DOI] [PubMed] [Google Scholar]
- 36. Barrans SL, O'Connor SJM, Evans PAS et al. Rearrangement of the BCL6 locus at 3q27 is an independent poor prognostic factor in nodal diffuse large B‐cell lymphoma. Br. J. Haematol. 2002; 117; 322–332. [DOI] [PubMed] [Google Scholar]
- 37. Iqbal J, Greiner TC, Patel K et al. Distinctive patterns of BCL6 molecular alterations and their functional consequences in different subgroups of diffuse large B‐cell lymphoma. Leukemia 2007; 21; 2332–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scott DW, Mottok A, Ennishi D et al. Prognostic significance of diffuse large B‐cell lymphoma cell of origin determined by digital gene expression in formalin‐fixed paraffin‐embedded tissue biopsies. J. Clin. Oncol. 2015; 33; 2848–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Staiger AM, Ziepert M, Horn H et al. Clinical impact of the cell‐of‐origin classification and the MYC/ BCL2 dual expresser status in diffuse large B‐cell lymphoma treated within prospective clinical trials of the German high‐grade non‐Hodgkin's lymphoma study group. J. Clin. Oncol. 2017; 35; 2515–2526. [DOI] [PubMed] [Google Scholar]
- 40. Chadburn A, Chiu A, Lee JY et al. Immunophenotypic analysis of AIDS‐related diffuse large B‐cell lymphoma and clinical implications in patients from AIDS malignancies consortium clinical trials 010 and 034. J. Clin. Oncol. 2009; 27; 5039–5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chao C, Silverberg MJ, Xu L et al. A comparative study of molecular characteristics of diffuse large B‐cell lymphoma from patients with and without human immunodeficiency virus infection. Clin. Cancer Res. 2015; 21; 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xicoy B, Ribera J‐M, Mate J‐L et al. Immunohistochemical expression profile and prognosis in patients with diffuse large B‐cell lymphoma with or without human immunodeficiency virus infection. Leuk. Lymphoma 2010; 51; 2063–2069. [DOI] [PubMed] [Google Scholar]
- 43. Dunleavy K, Little RF, Pittaluga S et al. The role of tumor histogenesis, FDG‐PET, and short‐course EPOCH with dose‐dense rituximab (SC‐EPOCH‐RR) in HIV‐associated diffuse large B‐cell lymphoma. Blood 2010; 115; 3017–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Madan R, Gormley R, Dulau A et al. AIDS and non‐AIDS diffuse large B‐cell lymphomas express different antigen profiles. Mod. Pathol. 2006; 19; 438–446. [DOI] [PubMed] [Google Scholar]
- 45. Hoffmann C, Tiemann M, Schrader C et al. AIDS‐related B‐cell lymphoma (ARL): correlation of prognosis with differentiation profiles assessed by immunophenotyping. Blood 2005; 106; 1762–1769. [DOI] [PubMed] [Google Scholar]
- 46. Baptista MJ, Tapia G, Morgades M et al. Using the Lymph2Cx assay for assessing cell‐of‐origin subtypes of HIV‐related diffuse large B‐cell lymphoma. Leuk. Lymphoma 2019; 60; 1087–1091. [DOI] [PubMed] [Google Scholar]
- 47. Philippe L, Lancar R, Laurent C et al. In situ BCL2 expression is an independent prognostic factor in HIV‐associated DLBCL, a LYMPHOVIR cohort study. Br. J. Haematol. 2020; 188; 413–423. [DOI] [PubMed] [Google Scholar]
- 48. Chao C, Silverberg MJ, Martínez‐Maza O et al. Epstein‐Barr virus infection and expression of B‐cell oncogenic markers in HIV‐related diffuse large B‐cell lymphoma. Clin. Cancer Res. 2012; 18; 4702–4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marques‐Piubelli ML, Salas YI, Pachas C, Becker‐Hecker R, Vega F, Miranda RN. Epstein‐Barr virus‐associated B‐cell lymphoproliferative disorders and lymphomas: a review. Pathology 2020; 52; 40–52. [DOI] [PubMed] [Google Scholar]
- 50. Xu J, Oki Y, Saksena A et al. CD30 expression and prognostic significance in R‐EPOCH‐treated patients with diffuse large B‐cell lymphoma. Hum. Pathol. 2017; 60; 160–166. [DOI] [PubMed] [Google Scholar]
- 51. Slack GW, Steidl C, Sehn LH, Gascoyne RD. CD30 expression in de novo diffuse large B‐cell lymphoma: a population‐based study from British Columbia. Br. J. Haematol. 2014; 167; 608–617. [DOI] [PubMed] [Google Scholar]
- 52. Salas MQ, Climent F, Tapia G et al. Clinicopathologic features and prognostic significance of CD30 expression in de novo diffuse large B‐cell lymphoma (DLBCL): results in a homogeneous series from a single institution. Biomarkers 2020; 25; 69–75. [DOI] [PubMed] [Google Scholar]
- 53. van der Weyden CA, Pileri SA, Feldman AL, Whisstock J, Prince HM. Understanding CD30 biology and therapeutic targeting: a historical perspective providing insight into future directions. Blood Cancer J. 2017; 7; e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morton LM, Kim CJ, Weiss LM et al. Molecular characteristics of diffuse large B‐cell lymphoma in human immunodeficiency virus‐infected and ‐uninfected patients in the pre‐highly active antiretroviral therapy and pre‐rituximab era. Leuk. Lymphoma 2014; 55; 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Horn H, Ziepert M, Becher C et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B‐cell lymphoma. Blood 2013; 121; 2253–2263. [DOI] [PubMed] [Google Scholar]
- 56. Ott G, Rosenwald A, Campo E. Understanding MYC‐driven aggressive B‐cell lymphomas: pathogenesis and classification. Blood 2013; 122; 3884–3891. [DOI] [PubMed] [Google Scholar]
- 57. Hwang J, Suh C, Kim K et al. The incidence and treatment response of double expression of MYC and BCL2 in patients with diffuse large B‐cell lymphoma: a systematic review and meta‐analysis. Cancers (Basel) 2021; 13; 3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Green TM, Young KH, Visco C et al. Immunohistochemical double‐hit score is a strong predictor of outcome in patients with diffuse large B‐cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Clin. Oncol. 2012; 30; 3460–3467. [DOI] [PubMed] [Google Scholar]
- 59. Ramos JC, Sparano JA, Chadburn A et al. Impact of Myc in HIV‐associated non‐Hodgkin lymphomas treated with EPOCH and outcomes with vorinostat (AMC‐075 trial). Blood 2020; 136; 1284–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Impact of BCL6 rearrangements on outcomes of patients with HIV‐associate nongerminal center diffuse large B‐cell lymphoma (DLBCL). (a) Kaplan–Meier curves for overall survival of HIV‐associate nongerminal center (non‐GC) DLBCL according to the presence of BCL6 rearrangements. (b) Kaplan–Meier curves for progression free survival of HIV‐associate nongerminal center (non‐GC) DLBCL according to the presence of BCL6 rearrangements.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


