Abstract
Aim
Leaves support a large diversity of fungi, which are known to cause plant diseases, induce plant defences or influence leaf senescence and decomposition. To advance our understanding of how foliar fungal communities are structured and assembled, we assessed to what extent leaf flush and latitude can explain the within‐ and among‐tree variation in foliar fungal communities.
Location
A latitudinal gradient spanning c. 20 degrees in latitude in Europe.
Taxa
The foliar fungal community associated with a foundation tree species, the pedunculate oak Quercus robur.
Methods
We examined the main and interactive effects of leaf flush and latitude on the foliar fungal community by sampling 20 populations of the pedunculate oak Quercus robur across the tree's range. We used the ITS region as a target for characterization of fungal communities using DNA metabarcoding.
Results
Species composition, but not species richness, differed between leaf flushes. Across the latitudinal gradient, species richness was highest in the central part of the oak's distributional range, and foliar fungal community composition shifted along the latitudinal gradient. Among fungal guilds, the relative abundance of plant pathogens and mycoparasites was lower on the first leaf flush, and the relative abundance of plant pathogens and saprotrophs decreased with latitude.
Conclusions
Changes in community composition between leaf flushes and along the latitudinal gradient were mostly a result of species turnover. Overall, our findings demonstrate that leaf flush and latitude explain 5%–22% of the small‐ and large‐scale spatial variation in the foliar fungal community on a foundation tree within the temperate region. Using space‐for‐time substitution, we expect that foliar fungal community structure will change with climate warming, with an increase in the abundance of plant pathogens and mycoparasites at higher latitudes, with major consequences for plant health, species interactions and ecosystem dynamics.
Keywords: community composition, foliar fungi, growing season, latitude, leaf flush, Quercus robur
1. INTRODUCTION
Leaves support a large diversity of fungi, both on their surface and within and between their cells (Jumpponen & Jones, 2009; Laforest‐Lapointe & Whitaker, 2019; Redford et al., 2010; Turner et al., 2013; U'Ren et al., 2019; Vacher et al., 2016). These fungi are known to cause plant diseases, induce plant defences in response to biotic and abiotic stresses (Arnold et al., 2003; Jaber & Enkerli, 2017) and influence leaf senescence and decomposition (Vacher et al., 2016). While these fungi are crucial for plant health and ecosystem functioning, we lack insights into the patterns and drivers of within‐ and among‐tree variation in the foliar fungal community (Agan et al., 2021; Allen et al., 2020; Millberg et al., 2015; Moler & Aho, 2018; Piepenbring et al., 2015; Sokolski et al., 2017; Terhonen et al., 2011; Unterseher et al., 2018). One factor that might explain within‐tree variation in the foliar fungal community is the co‐existence of leaves from different leaf flushes within individual trees. Many plant species produce multiple leaf flushes during the growing season, and newly produced leaves might differ from older leaves in chemistry and resistance against plant enemies (Agan et al., 2021; Piepenbring et al., 2015; Unterseher et al., 2018). At larger spatial scales, the foliar fungal community might vary along the distributional range of the host plant. For example, foliar fungal communities might be more diverse at the southern range (e.g. latitudinal diversity gradient hypothesis; Mittelbach et al., 2007) or at the centre of the range (centre‐periphery hypothesis; Pironon et al., 2017). Since the frequency of multiple leaf flushes is expected to change with global warming, and latitudinal gradients are commonly a proxy for climate, it is important to examine how the production of multiple leaf flushes and latitude jointly shape the structure of foliar fungal communities.
Many plant species produce leaves continuously, or in distinct flushes, during the growing season (Auerbach & Simberloff, 1984; Fuenzalida et al., 2019; Moles & Westoby, 2000; Prado et al., 2014). The production of multiple leaf flushes is linked to many factors, such as climatic conditions, plant age and herbivory (Bobinac et al., 2012; Hilton et al., 1987; Moles & Westoby, 2000). Physical and chemical differences between leaves from different flushes might explain within‐tree variation in the foliar fungal community. If the establishment of foliar fungi is limited by dispersal, we might expect that leaves from earlier flushes have a higher diversity of fungi due to the accumulation of species over time. Alternatively, as young and developing leaves are often more susceptible to colonization, and the development of second and subsequent flushes frequently coincides with peaks of spore production, they might have a higher species diversity than leaves from the first leaf flush. As an illustrative example, the severity of oak powdery mildew on Quercus robur has been found to be lower on leaves from the first flush than on leaves from the second flush (Call & St. Clair, 2017). The only two studies that we are aware of that assessed the impact of the production of multiple leaf flushes on the foliar fungal community have found the highest species richness in leaves from the first leaf flush as well as differences in community composition between leaf flushes (Table S1; Agan et al., 2021; Unterseher et al., 2018). Knowing which guilds are more likely to grow in each leaf flush would further help us to understand how foliar fungal communities are distributed and structured within trees. Whereas a previous study focused on differences in the abundance of a single fungal guild (plant pathogens) between leaf flushes (Piepenbring et al., 2015), no study so far has examined differences in the relative abundance of other fungal guilds between leaf flushes.
The foliar fungal community associated with a given plant species might vary across its range (Millberg et al., 2015; Moler & Aho, 2018). Based on the latitudinal diversity gradient hypothesis, we might expect that the diversity of fungi associated with a host plant is highest at the southern part of the distributional range (Mittelbach et al., 2007). As an alternative, the environmental conditions might be suboptimal at the margins of a plant species' distributional range, in which case we would expect that the largest proportion of the fungal community is present in the core of the distributional range of the host species (Pironon et al., 2017). As another alternative, there might be marginal asymmetry, where the fungal diversity is lower at the northernmost (expanding) margin, corresponding to newly colonized areas, whereas it is the same at the southernmost margin, where the species has been for much longer time. While few studies have examined latitudinal clines in the diversity of foliar fungal communities associated with a given host species, the existing reports are contradictory (Table S2), with studies showing increases (Sokolski et al., 2017), decreases (Terhonen et al., 2011) or no changes in the diversity of the foliar fungal community with latitude (Allen et al., 2020; Millberg et al., 2015). Likewise, reports on latitudinal changes in community composition are variable (Table S2). Millberg et al. (2015) and Moler and Aho (2018) showed that foliar fungal communities in needles from Pinus sylvestris and Pinus albicaulis changed with latitude, while Allen et al. (2020) reported that the foliar fungal community of reed (Phragamites australis) did not change with latitude. Importantly, previous studies only focused on restricted parts of the distributional range of the plant species (in particular, the northern or middle part of the range; see Table S2) and did not examine non‐linear relationships between latitude and the structure of the foliar fungal community. Likewise, we lack information on how the relative abundance of fungal guilds changes with latitude. The relationship between the foliar fungal community and latitude might also differ between the leaf flushes. For example, we might expect more fungal species linked to secondary leaf flushes in southern Europe, where one can find the highest abundance of plants with multiple leaf flushes. As latitudinal clines are often characterized by large changes in climate, they might inform us about future changes in the climate (i.e. space‐for‐time substitution; De Frenne et al., 2013). It is thus important to understand the patterns and mechanisms behind latitudinal clines in the diversity and composition of the foliar fungal community across the plant's distribution.
Differences in community composition between leaves belonging to different flushes and along latitudinal gradients can be due to species turnover or nestedness (Baselga, 2010). These two components are related to different ecological processes influencing fungal community assembly (Baselga, 2012; Hewitt, 1999; Svenning & Skov, 2007). For example, changes in the foliar fungal community composition due to species turnover can reflect species sorting by the environment or dispersal limitation, while species nestedness can reflect priority effects or differences in the level of specialization (Debray et al., 2021; Thébault & Fontaine, 2010; Toju et al., 2015; Weidlich et al., 2021). By knowing the relative contribution of species turnover and nestedness in shaping changes in community composition, we can thus further our understanding of how foliar fungal communities are structured and assembled.
We investigated the main and interactive effects of leaf flush and latitude on the foliar fungal community for a foundation tree species (the pedunculate oak, Quercus robur) along a latitudinal gradient of c. 20 degrees in Europe during a full growing season. For this, we sampled leaves from 20 oak populations from northern Spain to southwestern Finland during the early, mid and late season. More specifically we addressed the following questions:
Do species richness, evenness and community composition of the foliar fungal community differ between leaf flushes during the early, mid and late season?
How do species richness, evenness and community composition of foliar fungal communities change with latitude across the species' range? Is this pattern similar for all leaf flushes?
Does the relative abundance of functional guilds (plant pathogens, mycoparasites, saprotrophs and symbiotrophs) differ between leaf flushes or along the latitudinal gradient?
Are differences in the foliar fungal community composition between leaf flushes and along the latitudinal gradient a product of species turnover or nestedness?
2. MATERIALS AND METHODS
2.1. Natural history
The pedunculate oak Quercus robur is a deciduous tree belonging to the Fagaceae that grows in a wide range of climatic conditions from northern Spain to southern Finland (Petit et al., 2002). The leaves of the pedunculate oak harbour a highly diverse fungal community on their surfaces (epiphytes) and within and between their cells (endophytes; Cordier et al., 2012; Jakuschkin et al., 2016). These foliar fungi differ strongly in their functional roles (Faticov et al., 2021; Jumpponen & Jones, 2009). Foliar fungi can be classified into several functional groups (sensu Nguyen et al., 2016), such as plant pathogens, mycoparasites (i.e. species that feed on cells of leaf‐associated fungi), saprotrophs (i.e. species that feed on dead or decaying matter) and symbiotrophs (i.e. species that derive their nourishment from a symbiotic relationship with the plant). Notably, there is large variation in our knowledge of fungal species: There are species that are well described and whose ecology is studied extensively, such as pathogen species from the Erysiphe genus (Desprez‐Loustau et al., 2011; Faticov et al., 2022), whereas other species are not described yet or their ecology is still unknown.
The pedunculate oak flushes its first leaves from April in the southern part of its distribution area and from the end of May in the northern part of its distribution area. Leaf senescence usually starts in September in the northern part of its distribution area and in October in the southern part of the distribution area (Crawley & Akhteruzzaman, 1988; Ekholm et al., 2019; Wenden et al., 2020). Pedunculate oaks are well known for having multiple leaf flushes during a single growing season (Hilton et al., 1987). The frequency of second and subsequent leaf flushes is strongly dependent on the climate. During the growing season, pedunculate oaks generally have three or four leaf flushes in southern Europe, two or three leaf flushes in central and western Europe, and one or two leaf flushes in northern Europe (Beikircher & Mayr, 2013; Hilton et al., 1987). The production of multiple leaf flushes can also be due to other factors, such as plant age, plant genetics, environmental conditions or as a compensation to herbivory in the first leaf flush (Beikircher & Mayr, 2013; Bobinac et al., 2012).
2.2. Field sampling and sample preparation
To assess the influence of the production of multiple leaf flushes on the foliar fungal community across a latitudinal gradient in Europe during a full growing season, we selected 20 oak populations (avoiding urbanized areas) from seven countries across a latitudinal gradient in Europe with a minimum distance of 20 km among populations (Figure S1). In each population, we selected three adult (reproductive) oaks. From each oak, we randomly collected five fully expanded leaves from the first and (if present) second leaf flush, in both the early (8–12 June), mid (27–31 July) and late season (7–18 September). The five leaves were then placed together in a Ziploc bag with 70 g of silica gel for drying. From each leaf, we took eight 5 mm diameter leaf discs, four from each side of the midrib. Leaf discs were punched in a laminar flow hood with a metal corer, which was sterilized with 95% ethanol and flamed over a Bunsen burner after each sample. Leaf discs for each combination of tree and leaf flush (i.e. n = 40 leaf discs) were pooled into a single sample.
2.3. Molecular work
We milled the leaf discs using two metal beads in a TissueLyser II (QIAGEN) and extracted DNA from 20 mg per sample using the Macherey‐Nagel NucleoSpin Plant II kit, following the standard protocol. To characterize the foliar fungal communities, we used the forward primer fITS7 (Ihrmark et al., 2012) and the reverse primer ITS4a (White et al., 1990), which target a 250–450 bp fragment of the ITS2 region (Schoch et al., 2012). For full details on the molecular methods and bioinformatics, see Supplementary Text S1. Our final dataset contained 2,291,469 sequences assigned to 7030 amplicon sequence variants (ASVs). Foliar fungal communities were represented by 261 samples with an average of 8813 reads per sample. Each ASV was identified to species level by comparison with species hypotheses (SHs) in the UNITE database V8 released on 12/08/2021 (Abarenkov et al., 2010). To calculate species richness (number of ASVs per sample) and Pielou's evenness (Pielou, 1966), we rarefied each sample to 1000 reads, omitting those samples with less reads and retaining 223 samples. From these 223 samples, 146 samples were from the first flush and 77 from the second leaf flush. We calculated species richness and evenness using the function estimate_richness from the R‐package Phyloseq, which performs standard alpha diversity estimates by operating on the cumulative population of each sample (McMurdie & Holmes, 2013). We used MetagenomeSeq's cumulative sum scaling (CSS) in the analyses of community composition as a normalization method to account for uneven sequencing depth (Paulson et al., 2013). Sequencing data have been deposited at SRA NCBI with the accession number (to be added upon manuscript acceptance).
2.4. Statistical analysis
As a general approach, we used linear mixed models and permutational multivariate analysis of variance (PERMANOVAs). For the univariate response variables, we fitted linear mixed models using the function lmer in the R‐package lme4 (Bates et al., 2015; R Core Team, 2019), specifying a Gaussian distribution with an identity link, and testing for significance using the function Anova in the R‐package car (Weisberg, 2019). We inspected the distribution of the residuals after running each model to check for normality and heteroscedasticity, and no model required transformation of the response variable. We calculated the marginal R 2 for the models using the function r.squaredGLMM in the R‐package MuMIn (Barton, 2009). For the multivariate response variable community composition, we used the function adonis2 in the R‐package vegan (Oksanen et al., 2015), with Bray–Curtis dissimilarity for the count data and Sørensen dissimilarity for presence‐absence data. Since random factors cannot be specified in adonis2, the described random factors (below) were added as fixed factors in the community composition models. We used the function emmeans from the R‐package emmeans (Lenth, 2020) for paired contrasts between leaf flushes or seasons.
To test if species richness, evenness and community composition of the foliar fungal community, as well as the relative abundance of the different guilds (proportion of reads belonging to each guild out of the total number of reads in each sample) differed between leaf flushes and changed with latitude during the growing season, we modelled each response variable as a function of leaf flush identity, latitude and season. As differences between leaf flushes might differ between the early, mid and late season, we included the interaction between leaf flush identity and season. To account for non‐linear relationships along the latitudinal gradient, we included the quadratic term of latitude. As differences between leaf flushes might change with latitude, we included the interaction term between leaf flush and latitude, and between leaf flush and the quadratic term of latitude. To account for the hierarchical sampling design, we included the random factor population, and to account for repeated measurements, we included tree as a random factor. When we found a significant interaction between leaf flush and season, we used season‐specific models to further investigate differences between leaf flushes in the early, mid and late season, where we included the fixed factor leaf flush identity and the random factors population and tree. To examine which species differed in abundance between leaf flushes, we conducted differential abundance analyses with the extension Deseq2 (Love et al., 2014) in the R‐package Phyloseq (McMurdie & Holmes, 2013), adjusting P‐values using the Benjamini–Hochberg's procedure to account for multiple comparisons.
To test to what extent differences in community composition between leaf flushes and along the latitudinal gradient are explained by species turnover or nestedness, we used the R‐package betapart (Baselga & Orme, 2012). We analysed differences in community composition between leaf flushes by computing dissimilarity values between the first and second leaf flush, both for the full growing season and separately for the individual seasons. We analysed changes in community composition along the latitudinal gradient by computing dissimilarity values between countries, in a series of pairwise comparisons of each country with its neighbouring countries. In both analyses, we partitioned the total β‐diversity (Sørensen dissimilarity) into two indices, where β‐STU is the turnover component and β‐SNE is the species nestedness component (Culp et al., 2019).
3. RESULTS
3.1. Differences in the foliar fungal community between leaf flushes throughout the growing season
We obtained reads from 7030 ASVs, from which 3676 ASVs were identified up to species level with high confidence and 2576 ASVs could be assigned to functional group (sensu Nguyen et al., 2016). Species richness of the foliar fungal community increased with the progression of the growing season but did not differ between the leaf flushes (Figure 1a, Table 1). Evenness did not differ between either seasons or leaf flushes (Table 1). Differences in community composition between the early, mid and late season explained 2% of the variation in the foliar fungal community, and differences between leaf flushes explained 5%–7% of the variation (Figure 2, Table 1).
FIGURE 1.
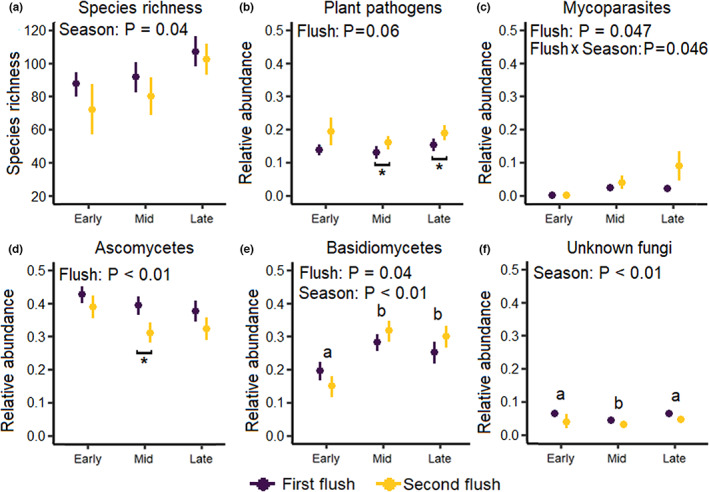
The impact of leaf flush on (a) species richness and relative abundance of (b) plant pathogens, (c) mycoparasites, (d) ascomycetes with unknown functions, (e) basidiomycetes with unknown functions and (f) unknown fungi of the foliar fungal community of the pedunculate oak Quercus robur during the early, mid and late season. Statistically significant p‐values (p < 0.05) and trends are shown in the top‐left of each panel. Circles represent means and bars represent standard errors. Asterisks below bars represent differences between flushes, and letters above bars represent differences between seasons. Only panels with statistically significant results are presented; for the full set of panels, see Figure S4.
TABLE 1.
The impact of leaf flush, season, latitude (linear and squared) and its interactions on species richness, evenness and composition of the foliar fungal community of the pedunculate oak (Quercus robur). Shown are χ 2 values (models on richness and evenness), F‐values (models on community composition), degrees of freedom, p‐values and marginal R 2 values. For community composition, results are given for both absolute counts and presence‐absence of species. Statistically significant results (p < 0.05) are shown in bold
| χ 2/F | df | p | R 2 | |
|---|---|---|---|---|
| Richness | 0.09 | |||
| Leaf flush | 1.19 | 1 | 0.28 | |
| Season | 6.40 | 2 | 0.041 | |
| Leaf flush × season | 0.07 | 2 | 0.97 | |
| Latitude | 0.06 | 1 | 0.81 | |
| Latitude2 | 11.16 | 1 | <0.01 | |
| Leaf flush × Latitude | 0.07 | 1 | 0.79 | |
| Leaf flush × Latitude2 | 0.18 | 1 | 0.67 | |
| Evenness | 0.20 | |||
| Leaf flush | 0.46 | 1 | 0.50 | |
| Season | 2.94 | 2 | 0.23 | |
| Leaf flush × season | 1.06 | 2 | 0.59 | |
| Latitude | 19.15 | 1 | <0.01 | |
| Latitude2 | 8.85 | 1 | <0.01 | |
| Leaf flush × Latitude | 1.07 | 1 | 0.30 | |
| Leaf flush × Latitude2 | 1.22 | 1 | 0.27 | |
| Community composition (absolute) | ||||
| Leaf flush | 2.35 | 1, 182 | <0.01 | 0.07 |
| Season | 2.86 | 2, 182 | <0.01 | 0.02 |
| Leaf flush × season | 0.96 | 2, 182 | 0.23 | <0.01 |
| Latitude | 3.08 | 19, 182 | <0.01 | 0.22 |
| Latitude2 | 3.08 | 19, 182 | <0.01 | 0.22 |
| Leaf flush × Latitude | 1.11 | 16, 182 | 0.76 | 0.06 |
| Leaf flush × Latitude2 | 1.11 | 16, 182 | 0.76 | 0.06 |
| Community composition (presence‐absence) | ||||
| Leaf flush | 1.69 | 1, 182 | <0.01 | 0.05 |
| Season | 2.10 | 2, 182 | <0.01 | 0.02 |
| Leaf flush × season | 1.06 | 2, 182 | 0.22 | <0.01 |
| Latitude | 2.21 | 19, 182 | <0.01 | 0.17 |
| Latitude2 | 2.21 | 19, 182 | <0.01 | 0.17 |
| Leaf flush × Latitude | 0.98 | 37, 182 | 0.70 | 0.06 |
| Leaf flush × Latitude2 | 0.98 | 37, 182 | 0.70 | 0.06 |
FIGURE 2.
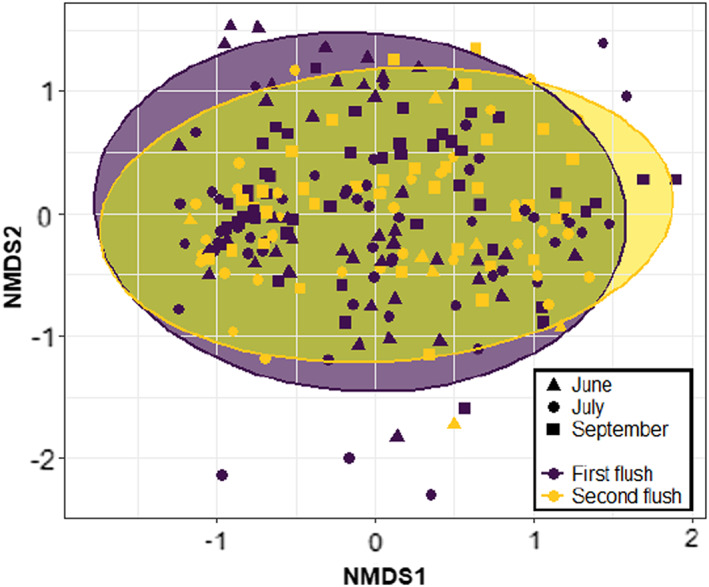
Differences in the community composition of foliar fungi between leaf flushes of the pedunculate oak Quercus robur. Visualization is based on non‐metric multidimensional scaling (NMDS) using Bray–Curtis metrics with a stress value = 0.7. Triangles, circles and squares indicate samples from the early, mid and late season respectively.
The relative abundance of plant pathogens tended to be lower in leaves from the first flush than in leaves from the second flush (Figure 1b, Tables S3 and S4). The relative abundance of mycoparasites was lower in leaves from the first flush than in leaves from the second flush, but only during the late season (Figure 1c, Tables S3 and S4). The relative abundance of saprotrophs and symbiotrophs did not differ between leaf flushes or throughout the growing season (Table S3). The relative abundance of ascomycetes with unknown function was generally higher in leaves from the first than in those from the second leaf flush (Figure 1d, Tables S3 and S4). The relative abundance of basidiomycetes with unknown functions increased from the early to the mid‐season and differed between leaf flushes (Figure 1e, Tables S3–S5). The relative abundance of unknown fungi was higher in the early and late season than during the mid‐season, and tended to be higher in the first leaf flush (Figure 1f, Tables S3 and S5).
The pathogens Didymella maydis, Microstroma bacarum and Oidiodendron griseum were more abundant in leaves from the second flush than in those from the first flush, even though these differences became non‐significant when adjusting p‐values for multiple comparisons (Figure S2, Table S6). While mycoparasites were generally more common in leaves from the second flush, the only species that significantly differed between the two leaf flushes was Sporobolomyces phaffii, which was more abundant in leaves from the first flush (Figure S2, Table S6). From the ascomycetes with unknown functions, Aureobasidium subglaciale and Gnomoniopsis paraclavulata were more abundant in leaves from the first flush than in those from the second flush (Figure S2, Table S6).
3.2. Changes in the foliar fungal community across the latitudinal gradient
Species richness was highest in the central part of the latitudinal gradient, a pattern similar for leaves from the first and the second flush (Figure 3a, Table 1). Evenness increased from southern Europe to the central part of the latitudinal gradient and then levelled off at high latitudes (Figure 3b, Table 1). The foliar fungal community composition changed non‐linearly with latitude, with a particularly steep change in community composition at higher latitudes, a pattern that was similar for leaves from the first and second leaf flush (Figure 3c, Table 1).
FIGURE 3.
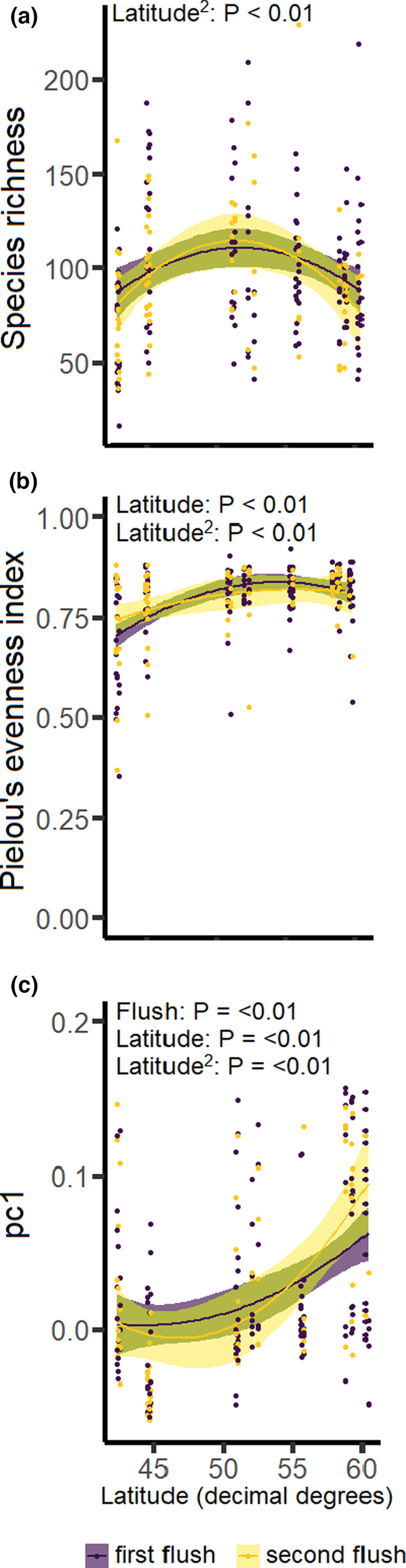
The relationship between latitude and the foliar fungal community on the pedunculate oak Quercus robur, separately for each leaf flush, across a latitudinal gradient in Europe. Panels show the relationship between latitude in decimal degrees and (a) species richness, (b) evenness, and (c) PCA axis 1 of the foliar fungal community composition using Bray‐Curtis metrics. Statistically significant p‐values are shown in the top‐left of each panel. Shown are non‐linear (quadratic) regression lines with their associated standard error (shaded area), separately for each leaf flush. Dots represent a leaf flush on a single tree.
The relative abundance of plant pathogens decreased with latitude, while the relative abundance of basidiomycetes with unknown function and unknown fungi followed the opposite pattern (Figure 4a,c,d, Table S3). The relative abundance of saprotrophs changed non‐linearly with latitude, but differently for the two leaf flushes: the relative abundance of saprotrophs in leaves from the first flush was lowest in the central part of the latitudinal gradient, whereas the relative abundance of saprotrophs in leaves from the second flush exhibited the opposite pattern (Figure 4b, Table S3). Mycoparasites, symbiotrophs and ascomycetes with unknown functions did not change in relative abundance along the latitudinal gradient (Table S3).
FIGURE 4.
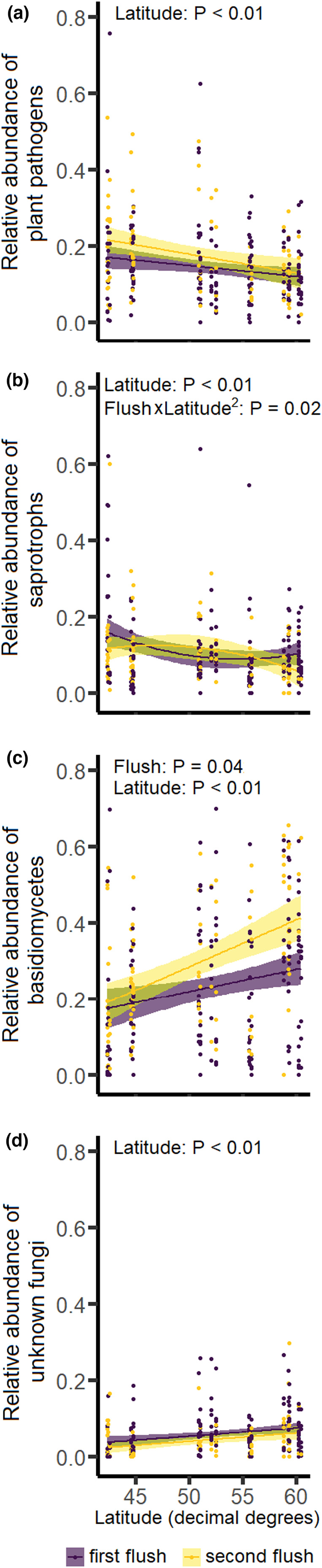
The relationship between latitude and the foliar fungal community on the pedunculate oak Quercus robur, separately for each leaf flush, across a latitudinal gradient in Europe. Panels show the relationship between latitude in decimal degrees and the relative abundance of (a) plant pathogens, (b) saprotrophs, (c) basidiomycetes with unknown functions, and (d) unknown fungi. Statistically significant p‐values are shown in the top‐left of each panel. Shown are regression lines with their associated standard error (shaded area), separately for each leaf flush. Dots represent a leaf flush on a single tree. Only guilds with statistically significant results are presented; for the full set of panels, see Figure S5.
3.3. Species turnover or nestedness?
Overall, differences in community composition between leaves from the first and second flush were mainly a result of turnover and only to a minor extent a result of nestedness (Figure 5a). The exception was the early season, when both components contributed equally (Figure S3). Shifts in community composition along the latitudinal gradient were mostly explained by species turnover and only to a lesser extent by species nestedness, a pattern that was particularly pronounced at lower latitudes (Figure 5b).
FIGURE 5.
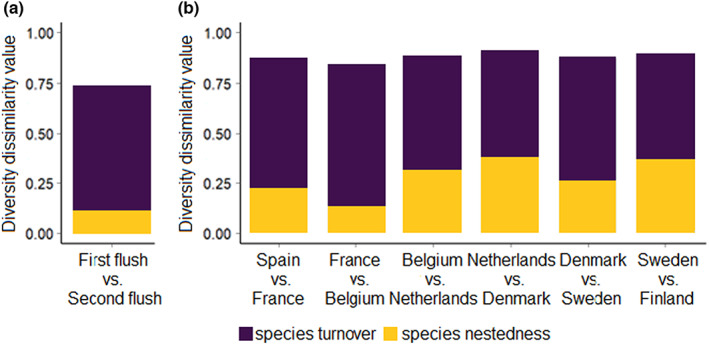
Partitioning of the total β diversity in the foliar fungal communities on the pedunculate oak Quercus robur between (a) leaf flushes and (b) neighbouring countries into the components of species turnover (purple) and nestedness (yellow) in pairwise comparisons.
4. DISCUSSION
To examine the effects of leaf flush and latitude on the foliar fungal community, we sampled leaves from the first and second flush in 20 oak populations across a latitudinal gradient in Europe during the early, mid and late season. Species composition, but not species richness and evenness, differed between the first and second leaf flush. Species richness was highest in the central part of the latitudinal gradient and the foliar fungal community composition changed with latitude, and these latitudinal patterns were similar for both leaf flushes. For the fungal guilds, the relative abundance of plant pathogens and mycoparasites was lower in leaves from the first flush than in those from the second flush, and the relative abundance of plant pathogens and saprotrophs decreased with latitude. Shifts in community composition between leaf flushes and across the latitudinal gradient were mostly a result of turnover and only to a minor extent a result of species nestedness. Our findings demonstrate that the co‐existence of multiple leaf flushes and environmental variables associated with latitude play a key role in shaping small‐ and large‐scale spatial variation in the foliar fungal community of a foundation tree in the temperate forests, with major consequences for plant health, species interactions and ecosystem dynamics.
We found that community composition, but not species richness and evenness, differed between oak leaf flushes, a pattern that was consistent between the early, mid and late season. The lack of a difference in species richness between leaf flushes in our study contrasts with the two previous studies on this topic, which all found a higher species richness on leaves from the first flush than on leaves from the second flush. Unterseher et al. (2018) found a higher species richness of foliar fungi in leaves from the first flush than in those from the second flush in tropical plants in Thailand, and Agan et al. (2021) found a higher species richness in Pinus sylvestris‐needles from the current year than in needles produced in previous years in Estonia and Norway. While very speculative, this might suggest that there are differences in the effects of the production of multiple leaf flushes on the species richness of the foliar fungal community between tropical and temperate regions, or between coniferous trees (where leaves from different years co‐exist) and deciduous trees. For evenness, no comparable studies exist. Our finding of a strong difference in the composition of the foliar fungal community between leaf flushes matches the two previous studies (Agan et al., 2021; Piepenbring et al., 2015; Unterseher et al., 2018). When investigating differences in functional guilds between leaf flushes, we found that plant pathogens and mycoparasites were more abundant in leaves from the second flush, while ascomycetes with unknown functions were more abundant in leaves from the first flush. In contrast to our finding of a higher relative abundance of plant pathogens in leaves from the second flush, Piepenbring et al. (2015) reported that plant pathogens in southwestern Panama were more likely to occur in older leaves than in young leaves. The difference in the relative abundance of plant pathogens between leaf flushes might be due to differences in the effect of leaf flush between tropical and temperate regions, but might also be explained by the identity of the host tree species. For example, in previous studies on oak trees, leaves from the second flush have been shown to be more susceptible to oak powdery mildew (Erysiphe spp.) infection (Gaytán et al., 2022; Marçais et al., 2009; Marçais & Desprez‐Loustau, 2014), and this relationship between leaf flush and susceptibility might differ among plant species (Jain et al., 2019).
Our finding of a higher species richness of the foliar fungal community in the central part of the latitudinal range of the pedunculate oak supports the centre‐periphery hypothesis, which states that the performance of species decreases from the centre to the margins of their distributional ranges (Pironon et al., 2017). This pattern might emerge either because the range of the oak‐associated microorganisms matches that of the oak trees (e.g. for specialists), or because the lower performance of oaks at their distributional margins indirectly and negatively affects the performance of the associated microorganisms. While previous studies have shown increases (Sokolski et al., 2017), decreases (Terhonen et al., 2011) and no changes in the diversity of the foliar fungal community with latitude (Allen et al., 2020; Millberg et al., 2015), these studies did not target the full distributional range of the focal plant species, and did not examine non‐linear latitudinal relationships. Evenness increased from the southern to the central part of the latitudinal range and then levelled off at high latitudes. Since there are no comparable studies for evenness of foliar fungal communities across latitudinal gradients, we cannot assess the generality of these results. Foliar fungal community composition changed with latitude throughout the distributional range, and this change was particularly pronounced at higher latitudes. Similarly, Millberg et al. (2015) and Moler and Aho (2018) reported changes in the foliar fungal communities (on Pinus albicaulis and P. sylvestris) across a latitudinal gradient, but both only focused on the mid and northern part of the host range, thereby corroborating our results on the mid and northern distributional range of oaks. Among functional guilds, the relative abundance of plant pathogens decreased towards higher latitudes, while basidiomycetes with unknown functions and unknown fungi exhibited the opposite pattern. The higher relative abundance of plant pathogens at lower latitudes matches the prediction that climate warming might increase the richness and severity of many plant pathogens at higher latitudes (Liu et al., 2019; Velásquez et al., 2018). We only found an interactive effect of leaf flush and latitude on the foliar fungal community for the guild of saprotrophic fungi: leaves from the first flush had the lowest relative abundance of saprotrophs in the central part of the latitudinal gradient, while saprotrophs in leaves from the second flush exhibited the opposite pattern. The foraging ascomycete hypothesis states that saprotrophs go through an endophytic stage in leaves to be able to colonize other substrates or survive times of resource scarcity (Nelson et al., 2020). Our results then suggest that leaves from the first flush are more likely to support the endophytic stage of saprotrophs in the edges of the distributional range of oaks, while leaves from the second flush are more likely to support the endophytic stage of saprotrophs in the central part of the distributional range of oaks.
Changes in community composition between leaf flushes and along the latitudinal gradient were mostly a product of species turnover, and only to a lesser degree the result of species nestedness. The high contribution of species turnover in explaining differences in the foliar fungal community between leaf flushes might indicate that foliar fungi have a relatively narrow (realized) niche, and that the physical and chemical differences between leaves from different leaf flushes are important in the spatial separation of foliar fungi, and particularly so for plant pathogens and mycoparasites. The high contribution of species turnover in explaining the latitudinal change in community composition supports a previous meta‐analysis on fungal communities, which showed that fungi are locally diverse, but have a high spatial turnover at the biogeographic scale (Meiser et al., 2014). Taken together, the high species turnover suggests that both leaf flush and environmental variation related to latitude contribute to the high regional diversity of fungi. Importantly, different mechanisms might cause the same patterns: for example, turnover can be due to the strong dependency of spore dispersal and leaf colonization on environmental and climatic conditions (Ahanger et al., 2013; Linnakoski et al., 2017; Tibpromma et al., 2021), but also be due to strong interactions among fungal species, both of which are well‐known for structuring fungal communities (Thébault & Fontaine, 2010; Toju et al., 2015). Overall, we need experimental studies to assess if changes in community composition of foliar fungi are mostly a product of the fundamental or realized niche, and whether this pattern differs among species living in different biogeographic regions with strong differences in mean and variance of climates, such as the tropics, temperate region and the arctic.
Overall, we identified leaf flush and latitude as two important mechanisms in shaping the distribution of foliar fungi at small and large spatial scales, respectively, in a foundation tree species in the temperate region. Using space‐for‐time substitution (sensu De Frenne et al., 2013), we can expect that the structure of the foliar fungal community on oaks will change with climate warming, with a notable increase in the relative abundance of plant pathogens and mycoparasites at higher latitudes. The increase in the relative abundance of plant pathogens might be even more pronounced due to the increase in the frequency of second and subsequent leaf flushes with climate warming, as at least the second leaf flush is predicted to have higher levels of plant pathogens. While leaf flush and latitude are rarely taken into account in studies on foliar fungal communities, our findings illustrate that these factors contribute to the maintenance of a high regional diversity in foliar fungi, and are important for our understanding of spatial variation in the foliar fungal community across multiple spatial scales.
5. CONFLICT OF INTEREST
All authors declare no conflict of interest.
BIOSKETCH
Álvaro Gaytán – PhD at Stockholm University, where I study the community of organisms linked to oaks. One of my main interest is to understand how spatial and temporal variation in climate affects the life cycle of a diverse community of herbivores and fungi on oak, and the consequences for species interactions within this food web.
Author contributions: AG, KG and AJMT conceived and designed the experiment. AG, XM, BC, IVH, PDF, CM, BGHT, JPJGTH, PUR, NB, RJ, PP and SS carried out the field work. AG, MF and KP carried out the molecular work. MF and AA conducted the bioinformatic analyses. AG analysed the data with support from MF, AA and AJMT. AG wrote the first draft and all authors contributed to the final manuscript.
Supporting information
Data S1
ACKNOWLEDGEMENTS
We acknowledge the guidance of Fede Berckx, Emilia Regazzoni and Nadia Binte Obaid during lab work. This research was supported by grants from the Bolin Centre for Climate Research (RA8) and the Swedish Research Council (2021‐03784) to AJMT. We are grateful for support from the National Genomics Infrastructure in Stockholm funded by Science for Life Laboratory, the Knut and Alice Wallenberg Foundation and the Swedish Research Council, and SNIC/Uppsala Multidisciplinary Center for Advanced Computational Science for assistance with massively parallel sequencing and access to the UPPMAX computational infrastructure. PDF and CM received funding from the European Research Council (ERC) under the European Union's Horizon 2020 Research and Innovation Programme (ERC Starting Grant FORMICA 757833). No permits were needed to conduct this research.
Gaytán, Á. , Abdelfattah, A. , Faticov, M. , Moreira, X. , Castagneyrol, B. , Van Halder, I. , De Frenne, P. , Meeussen, C. , Timmermans, B. G. H. , Ten Hoopen, J. P. J. , Rasmussen, P. U. , Bos, N. , Jaatinen, R. , Pulkkinen, P. , Söderlund, S. , Gotthard, K. , Pawlowski, K. , & Tack, A. J. M. (2022). Changes in the foliar fungal community between oak leaf flushes along a latitudinal gradient in Europe. Journal of Biogeography, 49, 2269–2280. 10.1111/jbi.14508
Handling Editor: Jacob Heilmann‐Clausen
DATA AVAILABILITY STATEMENT
Data are available from the Dryad Digital Repository (Gaytan et al., 2022). https://doi.org/10.5061/dryad.931zcrjpd.
REFERENCES
- Abarenkov, K. , Henrik Nilsson, R. , Larsson, K. , Alexander, I. J. , Eberhardt, U. , Erland, S. , Høiland, K. , Kjøller, R. , Larsson, E. , Pennanen, T. , Sen, R. , Taylor, A. F. S. , Tedersoo, L. , Ursing, B. M. , Vrålstad, T. , Liimatainen, K. , Peintner, U. , & Kõljalg, U. (2010). The UNITE database for molecular identification of fungi – recent updates and future perspectives. The New Phytologist, 186, 281–285. 10.1111/j.1469-8137.2009.03160.x [DOI] [PubMed] [Google Scholar]
- Agan, A. , Solheim, H. , Adamson, K. , Hietala, A. M. , Tedersoo, L. , & Drenkhan, R. (2021). Seasonal Dynamics of Fungi Associated with Healthy and Diseased Pinus sylvestris Needles in Northern Europe. Microorganisms, 9, 1757. 10.3390/microorganisms9081757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahanger, R. A. , Bhat, H. A. , Bhat, T. A. , Ganie, S. A. , Lone, A. A. , Ganai, S. A. , Haq, S. , Khan, O. A. , Junaid, J. M. , & Bhat, T. A. (2013). Impact of climate change on plant diseases. Animal Science, 1, 105–115. [Google Scholar]
- Allen, W. J. , DeVries, A. E. , Bologna, N. J. , Bickford, W. A. , Kowalski, K. P. , Meyerson, L. A. , & Cronin, J. T. (2020). Intraspecific and biogeographical variation in foliar fungal communities and pathogen damage of native and invasive Phragmites australis . Global Ecology and Biogeography, 29, 1199–1211. 10.1111/geb.13097 [DOI] [Google Scholar]
- Arnold, A. E. , Mejia, L. C. , Kyllo, D. , Rojas, E. I. , Maynard, Z. , Robbins, N. , & Herre, E. A. (2003). Fungal endophytes limit pathogen damage in a tropical tree. Proceedings of the National Academy of Sciences of the United States of America, 100, 15649–15654. 10.1073/pnas.2533483100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach, M. , & Simberloff, D. (1984). Responses of leaf miners to atypical leaf production patterns. Ecological Entomology, 9, 361–367. 10.1111/j.1365-2311.1984.tb00831.x [DOI] [Google Scholar]
- Barton, K. (2009). Mu‐MIn: Multi‐model inference. R package version 0.12.2/r18. http://R‐Forge.R‐project.org/projects/mumin/
- Baselga, A. (2010). Partitioning the turnover and nestedness components of beta diversity. Global Ecology and Biogeography, 19, 134–143. 10.1111/j.1466-8238.2009.00490.x [DOI] [Google Scholar]
- Baselga, A. (2012). The relationship between species replacement, dissimilarity derived from nestedness, and nestedness: Species replacement and nestedness. Global Ecology and Biogeography, 21, 1223–1232. 10.1111/j.1466-8238.2011.00756.x [DOI] [Google Scholar]
- Baselga, A. , & Orme, C. D. L. (2012). betapart: An R package for the study of beta diversity: Betapart package . Methods in Ecology and Evolution, 3, 808–812. 10.1111/j.2041-210X.2012.00224.x [DOI] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Beikircher, B. , & Mayr, S. (2013). Winter peridermal conductance of apple trees: Lammas shoots and spring shoots compared. Trees, 27, 707–715. 10.1007/s00468-012-0826-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobinac, M. , Batos, B. , Miljkovic, D. , & Radulovic, S. (2012). Polycyclism and phenological variability in the common oak (Quercus robur L.). Archives of Biological Sciences, 64, 97–105. [Google Scholar]
- Call, A. C. , & St. Clair, S. B. (2017). Outbreak of Drepanopeziza fungus in aspen forests and variation in stand susceptibility: Leaf functional traits, compensatory growth and phenology. Tree Physiology, 37, 1198–1207. 10.1093/treephys/tpx088 [DOI] [PubMed] [Google Scholar]
- Cordier, T. , Robin, C. , Capdevielle, X. , Fabreguettes, O. , Desprez‐Loustau, M.‐L. , & Vacher, C. (2012). The composition of phyllosphere fungal assemblages of European beech (Fagus sylvatica) varies significantly along an elevation gradient. The New Phytologist, 196, 510–519. 10.1111/j.1469-8137.2012.04284.x [DOI] [PubMed] [Google Scholar]
- Crawley, M. J. , & Akhteruzzaman, M. (1988). Individual variation in the phenology of oak trees and its consequences for herbivorous insects. Functional Ecology, 2, 409. 10.2307/2389414 [DOI] [Google Scholar]
- Culp, J. M. , Lento, J. , Curry, R. A. , Luiker, E. , & Halliwell, D. (2019). Arctic biodiversity of stream macroinvertebrates declines in response to latitudinal change in the abiotic template. Freshwater Science, 38, 465–479. 10.1086/704887 [DOI] [Google Scholar]
- De Frenne, P. , Graae, B. J. , Rodríguez‐Sánchez, F. , Kolb, A. , Chabrerie, O. , Decocq, G. , Kort, H. , Schrijver, A. , Diekmann, M. , Eriksson, O. , Gruwez, R. , Hermy, M. , Lenoir, J. , Plue, J. , Coomes, D. A. , & Verheyen, K. (2013). Latitudinal gradients as natural laboratories to infer species' responses to temperature. Journal of Ecology, 101, 784–795. 10.1111/1365-2745.12074 [DOI] [Google Scholar]
- Debray, R. , Herbert, R. A. , Jaffe, A. L. , Crits‐Christoph, A. , Power, M. E. , & Koskella, B. (2021). Priority effects in microbiome assembly. Nature Reviews. Microbiology, 20, 109–121. 10.1038/s41579-021-00604-w [DOI] [PubMed] [Google Scholar]
- Desprez‐Loustau, M.‐L. , Feau, N. , Mougou‐Hamdane, A. , & Dutech, C. (2011). Interspecific and intraspecific diversity in oak powdery mildews in Europe: Coevolution history and adaptation to their hosts. Mycoscience, 52, 165–173. 10.1007/S10267-010-0100-5 [DOI] [Google Scholar]
- Ekholm, A. , Tack, A. J. M. , Bolmgren, K. , & Roslin, T. (2019). The forgotten season: The impact of autumn phenology on a specialist insect herbivore community on oak. Ecological Entomology, 44, 425–435. 10.1111/een.12719 [DOI] [Google Scholar]
- Faticov, M. , Abdelfattah, A. , Roslin, T. , Vacher, C. , Hambäck, P. , Blanchet, F. G. , Lindahl, B. D. , & Tack, A. J. M. (2021). Climate warming dominates over plant genotype in shaping the seasonal trajectory of foliar fungal communities on oak. The New Phytologist, 231, 1770–1783. 10.1111/nph.17434 [DOI] [PubMed] [Google Scholar]
- Faticov, M. , Desprez‐Loustau, M. , Kiss, L. , Massot, M. , d'Arcier, J. F. , Mutz, J. , Németh, M. Z. , Roslin, T. , & Tack, A. J. M. (2022). Niche differentiation within a cryptic pathogen complex: Climatic drivers and hyperparasitism at multiple spatial scales. Ecography, 2022, ecog.06062. 10.1111/ecog.06062 [DOI] [Google Scholar]
- Fuenzalida, T. I. , Hernández‐Moreno, Á. , & Piper, F. I. (2019). Secondary leaves of an outbreak‐adapted tree species are both more resource acquisitive and more herbivore resistant than primary leaves. Tree Physiology, 39, 1499–1511. 10.1093/treephys/tpz083 [DOI] [PubMed] [Google Scholar]
- Gaytan, A. , Abdelfattah, A. , Faticov, M. , Moreira, X. , Castagneyrol, B. , Van Halder, I. , De Frenne, P. , Meeussen, C. , Timmermans, B. G. H. , Ten Hoopen, J. P. J. G. , Rasmussen, P. U. , Bos, N. , Jaatinen, R. , Pulkkinen, P. , Söderlund, S. , Gotthard, K. , Pawlowski, K. , & Tack, A. J. M. (2022). Data from: Changes in the foliar fungal community between oak leaf flushes along a latitudinal gradient in Europe. Dryad Digital Repository. 10.5061/dryad.931zcrjpd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytán, Á. , Moreira, X. , Castagneyrol, B. , Van Halder, I. , De Frenne, P. , Meeussen, C. , Timmermans, B. G. H. , Ten Hoopen, J. P. J. G. , Rasmussen, P. U. , Bos, N. , Jaatinen, R. , Pulkkinen, P. , Söderlund, S. , Covelo, F. , Gotthard, K. , & Tack, A. J. M. (2022). The co‐existence of multiple oak leaf flushes contributes to the large within‐tree variation in chemistry, insect attack and pathogen infection. The New Phytologist, 235, 1615–1628. 10.1111/nph.18209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt, G. M. (1999). Post‐glacial re‐colonization of European biota. Biological Journal of the Linnean Society, 68, 87–112. 10.1111/j.1095-8312.1999.tb01160.x [DOI] [Google Scholar]
- Hilton, G. M. , Packham, J. R. , & Willis, A. J. (1987). Effects of experimental defoliation on a population of pedunculate oak (Quercus robur L.). The New Phytologist, 107, 603–612. 10.1111/j.1469-8137.1987.tb02930.x [DOI] [Google Scholar]
- Ihrmark, K. , Bödeker, I. T. M. , Cruz‐Martinez, K. , Friberg, H. , Kubartova, A. , Schenck, J. , Strid, Y. , Stenlid, J. , Brandström‐Durling, M. , Clemmensen, K. E. , & Lindahl, B. D. (2012). New primers to amplify the fungal ITS2 region – evaluation by 454‐sequencing of artificial and natural communities. FEMS Microbiology Ecology, 82, 666–677. [DOI] [PubMed] [Google Scholar]
- Jaber, L. R. , & Enkerli, J. (2017). Fungal entomopathogens as endophytes: Can they promote plant growth? Biocontrol Science and Technology, 27, 28–41. 10.1080/09583157.2016.1243227 [DOI] [Google Scholar]
- Jain, A. , Sarsaiya, S. , Wu, Q. , Lu, Y. , & Jingshan, S. (2019). A review of plant leaf fungal diseases and its environment speciation. Bioengineered, 1, 409–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakuschkin, B. , Fievet, V. , Schwaller, L. , Fort, T. , Robin, C. , & Vacher, C. (2016). Deciphering the pathobiome: Intra‐ and interkingdom interactions involving the pathogen Erysiphe alphitoides . Microbial Ecology, 72, 870–880. 10.1007/s00248-016-0777-x [DOI] [PubMed] [Google Scholar]
- Jumpponen, A. , & Jones, K. L. (2009). Massively parallel 454 sequencing indicates hyperdiverse fungal communities in temperate Quercus macrocarpa phyllosphere. The New Phytologist, 184, 438–448. 10.1111/j.1469-8137.2009.02990.x [DOI] [PubMed] [Google Scholar]
- Laforest‐Lapointe, I. , & Whitaker, B. K. (2019). Decrypting the phyllosphere microbiota: Progress and challenges. American Journal of Botany, 106, 171–173. 10.1002/ajb2.1229 [DOI] [PubMed] [Google Scholar]
- Lenth, R. (2020). emmeans: Estimated marginal means, aka least‐squares means.
- Linnakoski, R. , Forbes, K. M. , Wingfield, M. J. , Pulkkinen, P. , & Asiegbu, F. O. (2017). Testing projected climate change conditions on the Endoconidiophora polonica/Norway spruce pathosystem shows fungal strain specific effects. Frontiers in Plant Science, 8, 883. 10.3389/fpls.2017.00883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Ma, Z. , Cadotte, M. W. , Chen, F. , He, J. , & Zhou, S. (2019). Warming affects foliar fungal diseases more than precipitation in a Tibetan alpine meadow. The New Phytologist, 221, 1574–1584. 10.1111/nph.15460 [DOI] [PubMed] [Google Scholar]
- Love, M. , Huber, W. , & Anders, S. (2014). Moderated estimation of fold change and dispersion of RNA‐seq data in DESeq2. Genome Biology, 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marçais, B. , & Desprez‐Loustau, M.‐L. (2014). European oak powdery mildew: Impact on trees, effects of environmental factors, and potential effects of climate change. Annals of Forest Science, 71, 633–642. 10.1007/s13595-012-0252-x [DOI] [Google Scholar]
- Marçais, B. , Kavkova, M. , & Desprez‐Loustau, M. L. (2009). Phenotypic variation in the phenology of ascospore production between European populations of oak powdery mildew. Annals of Science, 66, 814. [Google Scholar]
- McMurdie, P. , & Holmes, S. (2013). Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome consensus data. PLoS ONE, 8, e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser, A. , Balint, M. , & Schmitt, I. (2014). Meta‐analysis of deep‐sequenced fungal communities indicates limited taxon sharing between studies and the presence of biogeographic patterns. The New Phytologist, 201, 623–635. 10.1111/nph.12532 [DOI] [PubMed] [Google Scholar]
- Millberg, H. , Boberg, J. , & Stenlid, J. (2015). Changes in fungal community of Scots pine (Pinus sylvestris) needles along a latitudinal gradient in Sweden. Fungal Ecology, 17, 126–139. [Google Scholar]
- Mittelbach, G. G. , Schemske, D. W. , Cornell, H. V. , Allen, A. P. , Brown, J. M. , Bush, M. B. , Harrison, S. P. , Hurlbert, A. H. , Knowlton, N. , Lessios, H. A. , McCain, C. M. , McCune, A. R. , McDade, L. A. , McPeek, M. A. , Near, T. J. , Price, T. D. , Ricklefs, R. E. , Roy, K. , Sax, D. F. , … Turelli, M. (2007). Evolution and the latitudinal diversity gradient: Speciation, extinction and biogeography. Ecology Letters, 10, 315–331. 10.1111/j.1461-0248.2007.01020.x [DOI] [PubMed] [Google Scholar]
- Moler, E. R. V. , & Aho, K. (2018). Whitebark pine foliar fungal endophyte communities in the southern Cascade Range, USA: Host mycobiomes and white pine blister rust. Fungal Ecology, 33, 104–114. 10.1016/j.funeco.2018.02.003 [DOI] [Google Scholar]
- Moles, A. T. , & Westoby, M. (2000). Do small leaves expand faster than large leaves, and do shorter expansion times reduce herbivore damage? Oikos, 90, 517–524. 10.1034/j.1600-0706.2000.900310.x [DOI] [Google Scholar]
- Nelson, A. , Vandegrift, R. , Carroll, G. C. , & Roy, B. A. (2020). Double lives: Transfer of fungal endophytes from leaves to woody substrates. PeerJ, 8, e9341. 10.7717/peerj.9341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, N. H. , Song, Z. , Bates, S. T. , Branco, S. , Tedersoo, L. , Menke, J. , Schilling, J. S. , & Kennedy, P. G. (2016). FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecology, 20, 241–248. 10.1016/j.funeco.2015.06.006 [DOI] [Google Scholar]
- Oksanen, J. , Blanchet, F. G. , Kindt, R. , Legendre, P. , Minchin, P. R. , O'Hara, R. B. , Simpson, G. L. , Solymos, P. , Stevens, M. H. H. , & Wagner, H. (2015). vegan: Community ecology package.
- Paulson, J. N. , Stine, O. C. , Bravo, H. C. , & Pop, M. (2013). Differential abundance analysis for microbial marker‐gene surveys. Nature Methods, 10, 1200–1202. 10.1038/nmeth.2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit, R. J. , Csaikl, U. M. , Bordacs, S. , Burg, K. , Coart, E. , Cottrell, J. , van Dam, B. , Deans, J. D. , Dumolin‐Lapegue, S. , Fineschi, S. , Finkeldey, R. , Gillies, A. , Glaz, I. , Goicoechea, P. G. , Jensen, J. S. , Konig, A. O. , Lowe, A. J. , Madsen, S. F. , Matyas, G. , … Taurchini, D. (2002). Chloroplast DNA variation in European white oaks Phylogeography and patterns of diversity based on data from over 2600 populations. Forest Ecology and Management, 156, 2–26. [Google Scholar]
- Pielou, E. C. (1966). The measurement of diversity in different types of biological collections 14.
- Piepenbring, M. , Hofmann, T. , Miranda, E. , Cáceres, O. , & Unterseher, M. (2015). Leaf shedding and weather in tropical dry‐seasonal forest shape the phenology of fungi ‐ Lessons from two years of monthly surveys in southwestern Panama. Fungal Ecology, 18, 83–92. [Google Scholar]
- Pironon, S. , Papuga, G. , Villellas, J. , Angert, A. L. , García, M. B. , & Thompson, J. D. (2017). Geographic variation in genetic and demographic performance: New insights from an old biogeographical paradigm: The centre‐periphery hypothesis. Biological Reviews, 92, 1877–1909. 10.1111/brv.12313 [DOI] [PubMed] [Google Scholar]
- Prado, A. , Sierra, A. , Windsor, D. , & Bede, J. C. (2014). Leaf traits and herbivory levels in a tropical gymnosperm, Zamia stevensonii (Zamiaceae). American Journal of Botany, 101, 437–447. 10.3732/ajb.1300337 [DOI] [PubMed] [Google Scholar]
- R Core Team . (2019). R: A language and environment for statistical computing. [WWW Document]. https://www.R‐project.org/
- Redford, A. J. , Bowers, R. M. , Knight, R. , Linhart, Y. , & Fierer, N. (2010). The ecology of the phyllosphere: Geographic and phylogenetic variability in the distribution of bacteria on tree leaves: Biogeography of phyllosphere bacterial communities. Environmental Microbiology, 12, 2885–2893. 10.1111/j.1462-2920.2010.02258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch, C. L. , Seifert, K. A. , Huhndorf, S. , Robert, V. , Spouge, J. L. , Levesque, C. A. , Chen, W. , & Consortium, F. B. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proceedings of the National Academy of Sciences, 109, 6241–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolski, S. , Berner‐Cardou, M. , Piche, Y. , & Berube, J. (2017). Black spruce (Picea mariana) foliage hosts numerous and potentially endemic fungal endophytes. Canadian Journal of Forest Research, 37, 1737–1747. 10.1139/X07-037 [DOI] [Google Scholar]
- Svenning, J.‐C. , & Skov, F. (2007). Could the tree diversity pattern in Europe be generated by postglacial dispersal limitation? Ecology Letters, 10, 453–460. 10.1111/j.1461-0248.2007.01038.x [DOI] [PubMed] [Google Scholar]
- Terhonen, E. , Marco, T. , Sun, H. , Jalkanen, R. , Kasanen, R. , Vuorinen, M. , & Asiegbu, F. (2011). The effect of latitude, season and needle‐age on the mycota of Scots pine (Pinus sylvestris) in Finland. Silva Fennica, 45, 301–317. 10.14214/sf.104 [DOI] [Google Scholar]
- Thébault, E. , & Fontaine, C. (2010). Stability of ecological communities and the architecture of mutualistic and trophic networks. Science, 329, 853–856. 10.1126/science.1188321 [DOI] [PubMed] [Google Scholar]
- Tibpromma, S. , Dong, Y. , Ranjitkar, S. , Schaefer, D. A. , Karunarathna, S. C. , Hyde, K. D. , Jayawardena, R. S. , Manawasinghe, I. S. , Bebber, D. P. , Promputtha, I. , Xu, J. , Mortimer, P. E. , & Sheng, J. (2021). Climate‐fungal pathogen modeling predicts loss of up to one‐third of tea growing areas. Frontiers in Cellular and Infection Microbiology, 11, 610567. 10.3389/fcimb.2021.610567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toju, H. , Guimarães, P. R. , Olesen, J. M. , & Thompson, J. N. (2015). Below‐ground plant–fungus network topology is not congruent with above‐ground plant–animal network topology. Science Advances, 1, e1500291. 10.1126/sciadv.1500291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, T. , James, E. , & Poole, P. (2013). The plant microbiome. Genome Biology, 14, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterseher, M. , Karunarathna, S. C. , Cruz, G. R. , Dagamac, N. H. , Dahl, M. B. , Dool, S. E. , Galla, M. , Herbst, L. , Nilsson, R. H. , Puechmaille, S. J. , Schöner, C. , Schöner, M. , Siddique, A. B. , Teltewskoi, A. , Wicke, K. , Würth, D. G. , Wurzbacher, C. , & Hyde, K. D. (2018). Mycobiomes of sympatric Amorphophallus albispathus (Araceae) and Camellia sinensis (Theaceae) – a case study reveals clear tissue preferences and differences in diversity and composition. Mycological Progress, 17, 489–500. 10.1007/s11557-018-1375-8 [DOI] [Google Scholar]
- U'Ren, J. M. , Lutzoni, F. , Miadlikowska, J. , Zimmerman, N. B. , Carbone, I. , May, G. , & Arnold, A. E. (2019). Host availability drives distributions of fungal endophytes in the imperilled boreal realm. Nature Ecology and Evolution, 3, 1430–1437. 10.1038/s41559-019-0975-2 [DOI] [PubMed] [Google Scholar]
- Vacher, C. , Hampe, A. , Porté, A. J. , Sauer, U. , Compant, S. , & Morris, C. E. (2016). The phyllosphere: Microbial jungle at the plant–climate interface. Annual Review of Ecology, Evolution, and Systematics, 47, 1–24. 10.1146/annurev-ecolsys-121415-032238 [DOI] [Google Scholar]
- Velásquez, A. C. , Castroverde, C. D. M. , & He, S. Y. (2018). Plant–pathogen warfare under changing climate conditions. Current Biology, 28, R619–R634. 10.1016/j.cub.2018.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidlich, E. W. A. , Nelson, C. R. , Maron, J. L. , Callaway, R. M. , Delory, B. M. , & Temperton, V. M. (2021). Priority effects and ecological restoration. Restoration Ecology, 29, e13317. 10.1111/rec.13317 [DOI] [Google Scholar]
- Weisberg, F. (2019). An R companion to applied regression (3rd ed.). Sage. [Google Scholar]
- Wenden, B. , Mariadassou, M. , Chmielewski, F. , & Vitasse, Y. (2020). Shifts in the temperature‐sensitive periods for spring phenology in European beech and pedunculate oak clones across latitudes and over recent decades. Global Change Biology, 26, 1808–1819. 10.1111/gcb.14918 [DOI] [PubMed] [Google Scholar]
- White, T. J. , Bruns, T. , Lee, S. , & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In White T. J., Innis M. A., Gelfand D. H., & Sninsky J. J. (Eds.), PCR protocols (pp. 315–322). Elsevier. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
Data are available from the Dryad Digital Repository (Gaytan et al., 2022). https://doi.org/10.5061/dryad.931zcrjpd.


