Abstract
The International Myeloma Working Group (IMWG) guidelines recommend using electrophoresis and immunofixation to define response and progressive disease (PD) in immunoglobulin (Ig) secretory multiple myeloma (Ig‐MM), whereas the role of serum‐free light chain (sFLC) is controversial. We retrospectively analyzed the value of adding sFLC assays in the definition of response and PD according to IMWG criteria in 339 Ig‐MM patients treated with a first‐line novel agent‐based therapy (median follow‐up 54 months). sFLC PD was defined according to conventional criteria plus increased sFLC levels, or sFLC escape (sFLCe); progression/sFLCe‐free survival (ePFS) was the time from the start of treatment to the date of first PD or sFLCe, or death; overall survival after PD/sFLCe (OS after Pe) was the time from first PD or sFLCe to the date of death. 148 (44%) patients achieved a complete response and 198 (60%) a normal sFLC ratio (sFLCR). sFLCR normalization was an independent prognostic factor for extended PFS (HR = 0.46, p = 0.001) and OS (HR = 0.47, p = 0.006) by multivariable analysis. 175 (52%) patients experienced PD according to the IMWG criteria, whereas 180 (53%) experienced PD or sFLCe. Overall, a sFLCe was observed in 31 (9%) patients. Median PFS and ePFS were both equal to 36 (95% CI = 32–42, and 32–40, respectively) months. sFLC PD adversely affected the OS after Pe compared to PD with increasing monoclonal Ig only (HR = 0.52, p = 0.012). Our results support the inclusion of the sFLC assay for defining response and PD in Ig‐MM.
1. INTRODUCTION
The serum‐free light chain (sFLC) assay was introduced in 2001 in the diagnostic work‐up of multiple myeloma (MM) to measure concentrations of circulating kappa and lambda light chains unbound to heavy chains. 1 As a marker of clonality and expression of tumor burden, the sFLC assay has proved invaluable for screening monoclonal gammopathies, 2 establishing the diagnosis of MM, 3 assessing and monitoring the response to therapy, 4 and for prognostic stratification. 5
An imbalance in the sFLC ratio (sFLCR) can be detected at diagnosis in approximately 70% of patients otherwise defined as having non‐secretory MM, in nearly all patients with light chain only MM and in 90% of immunoglobulin (Ig) secreting MM (Ig‐MM). 6 , 7 , 8 Notably, the coexistence of an abnormal sFLCR with monoclonal Ig (M‐Ig) can reflect their secretion by a single plasma cell clone, or intra‐clonal heterogeneity. 9 , 10 However, the use of sFLC assays for monitoring Ig‐MM remains an area of debate. Indeed, the International Myeloma Working Group (IMWG) guidelines 11 recommend the use of serum and urine protein electrophoresis (EP) and immunofixation (IF) to assess the quality of response and progression of the disease (PD) in secretory MM while the measurement of sFLC is required to meet the definition of stringent complete response (sCR), and for assessing the response or PD in oligo/non‐secretory MM. Nevertheless, it is well recognized that the sFLC assay might also provide additional information to evaluate the depth of response in Ig‐MM. 12 , 13 , 14 Moreover, increasing levels of sFLC might precede or occur concurrently with a raise in M‐Ig, and is associated with worse prognosis. 10 , 15 Increasing evidence also suggests that sFLC assay may have a greater sensitivity than urine EP and 24‐hour urine collection for the evaluation of response in light chain only MM. 16 , 17
Based on evidence supporting the value of sFLC measurement for monitoring MM, empirical modified response criteria including sFLC assay, previously established only for oligo/non‐secretory MM, have recently been proposed for the assessment of response and PD in secretory MM patients. 17 According to these proposed modified criteria, a normal sFLCR would be included in the definition of complete response (CR) along with the existing conventional criteria, while an increase of at least 25% over the lowest difference between involved and uninvolved sFLC levels ‐ the absolute increase being at least of 100 mg/L ‐ would be considered as a marker of PD. 11 , 17 However, despite the evidence in favor of incorporating the sFLC assay into disease monitoring, data regarding the clinical implications of these modified criteria are still limited, and their validation by additional studies is needed. To this aim, we analyzed the added value of incorporating sFLC assay into IMWG criteria 11 to redefine the assessment of response and PD in Ig‐MM patients treated with novel agents. We focused on the implications of these modified criteria on defining outcomes and the prognostic impacts of sFLC levels.
2. METHODS
We retrospectively identified all Ig‐MM patients (baseline serum M‐Ig ≥10 g/L) 11 who received first‐line novel agent‐based therapies at a single center between October 2006 and December 2018. The analysis was then restricted to 339 patients for whom coupled data of serum and urine EP plus IF, and sFLC assay were collected at diagnosis, and were thereafter available at each subsequent time point (i.e., every 3–4 months) until the last contact date. Demographic and disease baseline characteristics were gathered, including sex, age, MM isotype, International Staging System (ISS), 18 serum beta‐2‐microglobulin, albumin, creatinine, lactate dehydrogenase, C‐reactive protein, hemoglobin, bone marrow plasma cells and fluorescence in situ hybridization (FISH) for t(4;14), t(14;16) and del(17p). sFLC were measured by nephelometry using the Freelite test (The Binding Site, Birmingham, United Kingdom). 1 For the definition of sFLCR normalization, the established reference range for the kappa/lambda ratio of 0.26–1.65 was used. 19
Responses, progression‐free survival (PFS), and overall survival (OS) were conventionally defined. 11 To evaluate the implications of modified criteria including sFLC assay, 17 the following definitions and endpoints were also included. IMWG uniform criteria of PD in oligo/non‐secretory MM 11 were used to identify an increase in sFLC levels. 17 sFLC escape (sFLCe) was defined as an increase in sFLC, without any additional IMWG criteria of PD. 10 , 17 A new subcategory of sFLC PD was defined by an increase in sFLC, either combined with conventional IMWG criteria of PD or as sFLCe, and was distinguished from the subcategories of PD, with an increase in M‐Ig without increase in sFLC, or PD, with organ damage and no change in M‐Ig and sFLC. Time from the date of starting upfront treatment to the date of first PD or sFLCe, or death, identified the progression/sFLCe‐free survival (ePFS). The second time to PD/sFLCe (2nd TTPe) was the time from the date of first PD or sFLCe to that of the second PD or sFLCe. OS after PD/sFLCe (OS after Pe) was calculated from the date of first PD, or sFLCe, to the date of death.
During this retrospective analysis, data were collected by means of a Ms. Spreadsheet especially devised for this research. A thorough examination of the data collected was conducted upfront to ensure completeness and data entry accuracy.
All variables with a continuous distribution were described by means of the median and its interquartile range (IQR) or by mean and standard deviation, as appropriate. On the other hand, variables with a discrete distribution were reported noting their absolute and relative frequency.
X2 or Fisher tests were adopted to compare categorical variables; otherwise, the t‐test was adopted to compare continuously normal‐distributed variables, and the Mann–Whitney test in case of not‐normal distributions.
Time‐to‐event analysis was performed following the Kaplan–Meier method with respect to endpoint estimations and survival curves. Our univariate analysis adopted the semi‐parametric Cox regression analysis methodology in order firstly to identify the factor significantly affecting outcomes and secondly to list any significant variables to be tested for Cox multivariable regression analysis.
All analyses were conducted using R language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria). An alfa error of 0.05 was taken as the cut‐off for two‐sided p‐value statistical significance and all confidence intervals (CI) were reported as 95% CI.
The study was approved by the independent ethics committee and was conducted in accordance with the International Conference on Harmonization guidelines on Good Clinical Practice and the principles of the Declaration of Helsinki. All patients provided informed written consent.
3. RESULTS
A total of 339 patients with newly diagnosed Ig‐MM were included in this study. The main demographic and disease characteristics at baseline are detailed in Table 1. The median age was 67 (range: 59–74) and 60% were male. FISH results were available in 222 (65%) patients, of whom 32% (70 patients) had a high‐risk cytogenetic profile defined as the presence of del(17p) and/or t(4;14) and/or t(14;16). 20 Overall, 311 (92%) patients had an abnormal sFLCR and 231 (68%) had sFLC‐measurable disease defined by an abnormal sFLCR and involved sFLC ≥100 mg/L. 11 The presence of an sFLC‐measurable disease at baseline, as compared with the absence of this feature, was associated with higher rates of creatinine level ≥1.2 mg/dL (81 out of 231 [35%] patients vs. 20 out of 108 [19%], p = 0.002), hemoglobin level ≤ 10.5 g/dL (119 out of 231 [52%] patients vs. 40 out of 108 [37%], p = 0.016), bone marrow plasma cells >60% (81 out of 196 [41%] evaluable patients vs. 22 out of 93 [24%], p = 0.005), and ISS stage II or III (141 out of 192 [73%] evaluable patients vs. 49 out of 90 [54%], p = 0.002). First‐line treatments included proteasome‐inhibitors (PIs) in 141 (42%) patients, immunomodulatory drugs (IMiDs) in 45 (13%), or both these agents in the remaining 153 (45%). First‐line treatment strategies, including or not PIs or IMiDs, were equally distributed among patients with sFLC‐measurable or unmeasurable disease. Autologous stem cell transplantation was performed in 164 (48%) patients.
TABLE 1.
Main demographic and disease characteristics of patients at baseline
| Characteristics | n°= 339 |
|---|---|
| Median age, years (IQR) | 67 (59–74) |
| Male, n (%) | 203 (60) |
| Myeloma subtype, n (%) | |
|
•IgG •IgA •IgD •IgM |
253 (75) 81 (24) 4 (1) 1 (<1) |
| Involved sFLC, n (%) | |
|
•kappa •lambda |
228 (67) 111 (33) |
| sFLCR, n (%) | |
|
•abnormal •normal (0.26–1.65) |
311 (92) 28 (8) |
| Involved sFLC >100 mg/L with abnormal sFLCR, n (%) | 231 (68) |
| Serum creatinine ≥1.2 mg/dL, n (%) | 101 (30) |
| ISS stage, n (%) a | |
|
•I •II •III |
92 (33) 99 (35) 91 (32) |
| Centralized FISH analysis for CA, n (%) b | |
|
•del(17p) and/or t(4;14) and/or t(14;16) positivity •del(17p) and t(4;14) and t(14;16) negativity |
70 (32) 152 (69) |
| First‐line treatment, n (%) | |
|
•PIs •IMiDs •PIs + IMiDs •ASCT |
141 (42) 45 (13) 153 (45) 164 (48) |
Abbreviations: ASCT, autologous stem cell transplantation; CA, cytogenetic abnormalities; FISH, fluorescence in situ hybridization; IMiDs, immunomodulatory drugs; ISS, international staging system; PIs, proteasome inhibitors; sFLC, serum‐free light chain; sFLCR, serum‐free light chain ratio.
282 patients were available for assessment.
222 patients were available for assessment.
Among the study population, 148 (44%) patients achieved CR as their best response to first‐line therapy, 105 (31%) had a very good partial response and 62 (18%) partial response, while in the remaining 24 (7%) stable disease (22 patients) or PD (2 patients) were documented. In total, 238 (70%) patients achieved (or maintained) a normal sFLCR as the best sFLC response to up‐front therapy. Of these, 144 (61%) were in CR, whereas 94 (39%) had an M‐Ig still detectable by EP or IF (p < 0.001). Patients with a baseline sFLC‐measurable disease had a similar rate of best CR (97 out of 231 [42%]) to that of patients without sFLC‐measurable disease (51 out of 108 [47%], p not significant). Conversely, the probability of normalizing the sFLCR with first‐line therapy was significantly lower in the sFLC‐measurable subgroup (138 out of 231 [60%] patients) compared to the other subgroup (100 out of 108 [93%] patients, p < 0.001). Patients who achieved conventionally defined CR and normal sFLCR were not classified as having sCR due to the lack of bone marrow clonality data.
At a median follow‐up of 54 (IQR 25–84) months, 175 (52%) patients experienced a conventional PD, 11 as either increased M‐Ig (137 patients, 78%) or development of organ damage without changes in M‐Ig (38 patients, 22%) (Figure 1). Among the whole study population, 31 (9%) patients experienced a sFLCe; of these, 26 (84%) had a subsequent conventional PD after a median time of 42 (IQR 31–133) days, while 3 (10%) started very shortly a second‐line therapy due to rapid rise in sFLC 21 and before criteria for conventional PD were met. Finally, the last 2 (6%) patients did not experience a conventional PD nor required salvage therapy after a median follow‐up of 33 (IQR: 26–39) months from sFLCe. After inclusion of the sFLC measurement as an additional criterion of PD, 17 180 (53%) patients were categorized as progressed and three subgroups could be identified based on: (1) the increase of sFLC, either with additional criteria of PD, or as sFLCe (i.e., sFLC PD) (77 patients, or 43%); (2) the increase of M‐Ig without increased sFLC (72 patients, or 40%); (3) organ damage without any change in M‐Ig and sFLC (31, or 17%) (Figure 1). A flowchart detailing the different relapse patterns, based on both the IMWG criteria and the proposed modified criteria including sFLC measurements, is shown in Figure 2. Median values of PFS and ePFS were similar, both being 36 (95% CI = 32–42, and 32–40, respectively) months. The median value of OS was 98 (95% CI = 79‐not estimable) months.
FIGURE 1.
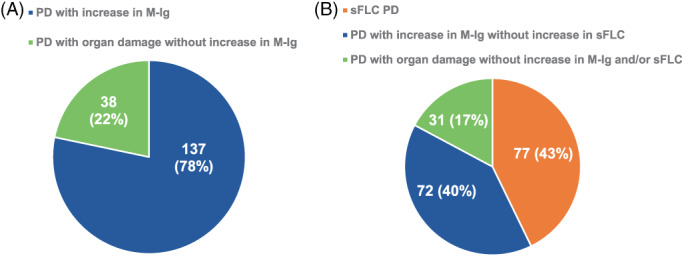
Different patterns of progressive disease (PD): (A) Conventionally defined PD, according to IMWG criteria, (B) Modified PD, according to IMWG criteria integrated with sFLC data and including a new subcategory of sFLC PD defined by an increase in sFLC either combined with conventional IMWG criteria of PD or as sFLC escape. M‐Ig, monoclonal immunoglobulin; PD, progressive disease; sFLC, serum‐free light chain. [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
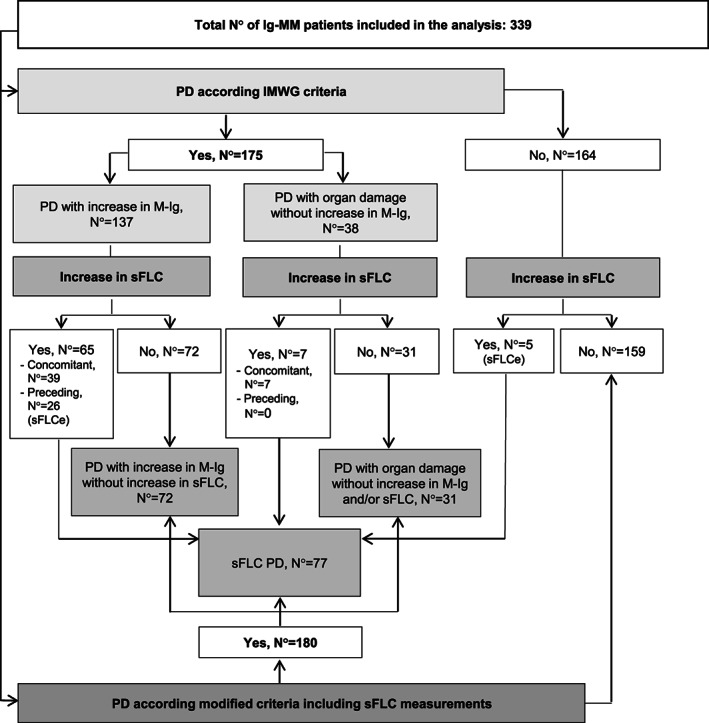
Flowchart detailing the different patterns of progressive disease. Ig‐MM, immunoglobulin secretory multiple myeloma; M‐Ig, monoclonal immunoglobulin; N°, number; PD, progressive disease; sFLC, serum‐free light chain; sFLCe, serum‐free light chain escape.
The presence of sFLC‐measurable disease at baseline predicted the worse outcomes in comparison with unmeasurable disease. Median PFS and ePFS for these subgroups were 32 (95% = CI 28–37) versus 46 (95% CI = 38–60) months (HR = 1.58, 95% CI = 1.16–2.17, p = 0.004), and 31 (95% CI = 28–36) versus 46 (95% CI = 38–60) months (HR = 1.66, CI = 1.21–2.26, p = 0.002), respectively. The OS estimates at 54 months for these subgroups were 65% (95% CI = 57–73) versus 83% (95% CI = 76–92) (HR = 1.98, 95% CI = 1.25–3.12, p = 0.003). Conversely, the achievement of sFLCR normalization as best response after first‐line therapy, predicted for extended survival outcomes, with median PFS of 45 (95% CI = 39–57) versus 17 (95% CI = 15–21) months (HR = 0.21, 95% CI = 0.15–0.28, p < 0.001), median ePFS of 44 (95% CI = 39–55) versus 16 (95% CI = 14–20) months (HR = 0.19, 95% CI = 0.14–0.26, p < 0.001), and 54‐month OS rate of 80% (95% CI = 75–87) versus 44% (95% CI = 32–60) (HR = 0.27, 95% CI = 0.18–0.41, p < 0.001), as compared with failure to achieve this target. Notably, achieving a normal sFLCR maintained an independent prognostic value by multivariable analysis for both PFS (HR = 0.46, 95% CI = 0.28–0.73, p = 0.001), and OS (HR = 0.44, 95% CI = 0.26–0.76, p = 0.003) (Table 2).
TABLE 2.
Multivariable analysis of factors associated with extended progression free survival (PFS), and overall survival (OS)
| HR | [95% CI] | p | |
|---|---|---|---|
| PFS | |||
| Absence of extramedullary disease | 0.10 | 0.03–0.32 | <0.001 |
| Treatment program including autotransplant | 0.46 | 0.30–0.73 | 0.001 |
| Achievement of sFLCR normalization | 0.46 | 0.28–0.73 | 0.001 |
| Achievement of CR | 0.48 | 0.30–0.78 | 0.003 |
| ISS stage I/II | 0.54 | 0.36–0.80 | 0.003 |
| Absence of t(4;14) and t(14;16) and del(17p) | 0.57 | 0.38–0.84 | 0.005 |
| OS | |||
| Absence of extramedullary disease | 0.04 | 0.01–0.14 | <0.001 |
| Achievement of sFLCR normalization | 0.44 | 0.26–0.76 | 0.003 |
| ISS stage I/II | 0.48 | 0.30–0.78 | 0.003 |
| Treatment program including autotransplant | 0.44 | 0.25–0.77 | 0.004 |
| Creatinine <1.2 mg/dL | 0.61 | 0.37–0.99 | 0.046 |
Abbreviations: CR, complete response; ISS, international staging system; sFLCR, serum‐free light chain ratio.
To evaluate the prognostic role of increasing levels of sFLC, patients with sFLC PD were compared to patients who relapsed with increased M‐Ig or organ damage but lacking a raise in sFLC. An sFLC PD was associated with more advanced age (median 71 [IQR 63–76] vs. 66 [IQR 60–73] years, p = 0.009) and higher rates of creatinine levels ≥1.2 mg/dL (31 out of 77 [40%] patients vs. 26 out of 103 [25%], p = 0.048), del(17p) positivity (16 out of 55 [29%] evaluable patients vs. 9 out of 88 [10%], p = 0.008), and sFLC measurable disease (71 out of 77 [92%] patients vs. 52 out of 103 [51%], p < 0.001) at baseline, in comparison with other patterns of PD. Moreover, patients with sFLC PD showed a higher rate of creatinine ≥1.2 mg/dL (28 out of 77 [39%] patients vs. 23 out of 103 [23%], p = 0.041) at relapse, with respect to patients with different patterns of PD. No difference was observed between patients with sFLC PD compared with other patterns of PD in terms of first‐line treatment strategies including or not including PIs or IMiDs. Organ damage at relapse was observed in 40 (55%) out of the 77 patients with an sFLC PD and 44 (61%) out of 72 patients relapsing with increased M‐Ig without raised sFLC (p not significant). No difference was observed between the two groups in terms of the type of organ damage, including bone lesions (observed in 25 [32%] vs. 35 [49%] patients, respectively), anemia (12 [16%] vs. 13 [18%] patients), renal impairment (8 [10%] vs. 3 [4%] patients), hypercalcemia (3 [4%] vs. 0 patients), and development of extramedullary disease (5 [6%] vs. 1 [<1%] patients).
We then analyzed the clinical outcomes after PD or sFLCe. Overall, 160 patients out of the 180 (89%) who experienced PD or sFLCe started a second‐line therapy, which included IMiDs in 60 (38%) patients, PIs in 23 (14%), both PI and IMiDs in 42 (26%), monoclonal antibodies with either PI or IMiDs in 24 (15%), and chemotherapy in 11 (7%) patients. Second‐line treatments were equally distributed among patients with sFLC PD and other patterns of relapse. Median 2nd TTPe was 14 (95% CI = 10‐not estimable) months for patients with PD with organ damage without any M‐Ig and/or sFLC changes, 20 (95% CI = 17‐not estimable) months for those with sFLC PD, and 26 (95% CI = 20–38) months for those with increased M‐Ig without sFLC changes (p not significant) (Figure 3). Conversely, curves of OS after Pe were significantly different between these subgroups, with median values of 29 (95% CI = 15‐not estimable), 30 (95% CI = 26–41) and 48 (95% CI = 42‐not estimable) months (p = 0.019), respectively (Figure 4). Notably, sFLC PD was confirmed as adversely affecting OS after Pe as compared with M‐Ig PD without increased sFLC (HR = 0.52, 95% CI = 0.32–0.87, p = 0.012).
FIGURE 3.
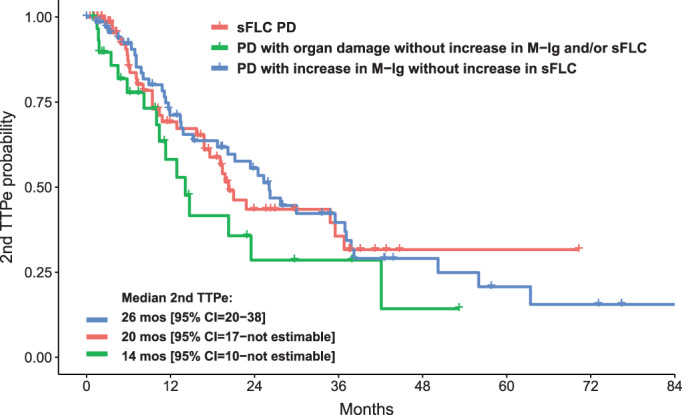
Second time to PD/sFLCe (2nd TTPe) according to the pattern of relapse. CI, confidence interval; M‐Ig, monoclonal immunoglobulin; mos, months; PD, progressive disease; sFLC, serum‐free light chain; sFLCe, serum‐free light chain escape; TTPe, time to PD/sFLCe. [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
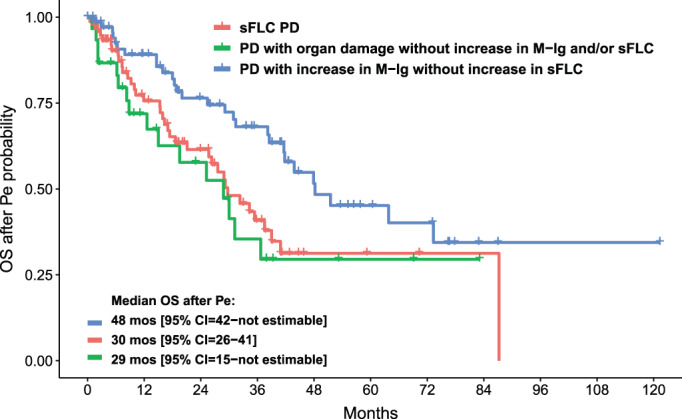
Overall survival after PD/sFLCe (OS after Pe) according to the pattern of relapse. CI, confidence interval; M‐Ig, monoclonal immunoglobulin; mos, months; PD, progressive disease; sFLC, serum‐free light chain; sFLCe, serum‐free light chain escape. [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
The current availability of highly effective treatment options for MM has led to a clinical need for sensitive monitoring of the disease. The most recent update of the IMWG consensus criteria has introduced new response categories of minimal residual disease negativity, defined on the basis of next‐generation flow or next‐generation sequencing and integrated with the imaging‐based study of extramedullary disease. 22 However, no major changes have been made to monoclonal protein monitoring from the recommendations established in 2006, 11 though a number of publications have addressed the role of sFLC immunoassays as an additional tool to evaluate the response and PD, along with serum and urine EP plus IF, also in secretory MM. 9 , 12 , 13 , 14 , 15 , 16 , 17 Modified response criteria modeled on those currently recommended by the IMWG, and including sFLC assay, have therefore been proposed. 17 However, none of the clinical trials supporting approval of novel agents has included the sFLC assay in the definition of response and PD for Ig‐MM patients, and the impact of these modified criteria on clinical outcomes evaluation, as well as their prognostic implications, should be assessed. We thereby designed this retrospective study to specifically analyze the value of including sFLC assays in the assessment of response and PD for Ig‐MM treated with a first‐line novel agent therapy.
In accordance with previous data, 6 , 7 , 8 an imbalance in the sFLCR was detected in approximately 90% of the patients at diagnosis, and two‐thirds of the patients showed an sFLC‐measurable disease as defined by the IMWG criteria, 11 along with M‐Ig. The presence of an sFLC‐measurable disease at baseline was associated with features of increased tumor burden and adverse prognostic factors, including higher frequency of ISS stage II or III, higher bone marrow plasma cell infiltration, lower hemoglobin concentration and increased creatinine levels. Consensually, patients with an sFLC‐measurable disease showed a reduced probability of normalizing the sFLCR with first‐line therapy and significantly shorter survival outcomes. These data are concordant with several previous reports showing that abnormal sFLC, or sFLCR, using different cut‐off levels, correlate with a worse prognosis in the context of conventional chemotherapy or novel agents, 12 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 and highlights the role of sFLC as an additional biomarker of the disease.
As a marker of clonality, several studies have evaluated the prognostic value of normalizing the sFLCR during and after treatment. The simultaneous presence of normal sFLCR and CR, together with the absence of clonal plasma cells on bone marrow biopsy using immunohistochemistry or immunofluorescence, is defined as sCR. 11 Conflicting results have been reported concerning the relevance of sFLCR normalization in defining the depth of response within sCR, based on the increasing sensitivity of flow cytometry‐based approaches for detecting residual myeloma cells. 4 , 31 , 32 , 33 , 34 On the other hand, the prognostic value of sFLCR normalization, regardless of the depth of response, has previously been reported. A study conducted on patients with MM who had not reached a CR after first‐line therapy showed favorable outcomes associated with the achievement of normal sFLCR than with failure to meet that target, including the context of partial response. 13 Similarly, we have previously shown that the achievement of sFLCR normalization after first‐line treatment with a bortezomib‐based regimen correlates with significant benefits in terms of PFS and OS, regardless of the positivity or negativity of serum and urine EP plus IF tests. 12 Additionally, a time‐dependent sFLCR normalization at any time point prior to the start of maintenance therapy significantly prolonged PFS and OS among patients treated within the GMMG‐MM5 trial. 14 In the present analysis, the achievement of sFLCR normalization as best sFLC response with a novel agent‐based first‐line therapy resulted in an independent prognostic variable associated with extended survival outcomes, regardless of the achievement of a CR, the ISS stage, the cytogenetics, the creatinine level, the presence of extramedullary disease, and the treatment program including or not autotransplant. Taken in aggregate, these data suggest that even though the role of the sCR category is probably becoming less relevant, given the availability of sensitive flow cytometry‐ and molecular‐based approaches for detecting minimal residual disease, 22 , 31 , 34 sFLC monitoring should be considered complementary with standard EP and IF as a tool to evaluate conventional response, since both negative IF and normal sFLCR are required to confirm the absence of laboratory clonal biomarkers. 17 As a marker of clonality, it was also recently highlighted that the definition of an abnormal sFLCR should be restricted to those patients with elevation of the light chain involved. 33 Additionally, mass spectrometry methodologies are emerging as a new sensitive way of monitoring monoclonal proteins in the peripheral blood. 35 , 36 , 37
One of the most debated issues is the role of the sFLC assay in defining PD. sFLCe is a widely described and known phenomenon and previous studies 10 , 15 have reported that a pattern of relapse characterized by increasing levels of sFLC, with or without concomitant rise in M‐Ig, is associated with worse survival outcomes than a relapse with increase in M‐Ig alone. However, although sFLC monitoring is already included in clinical practice, an isolated increase in sFLC levels without other laboratory or clinical signs of relapse is not currently considered as a marker of PD for Ig‐MM patients. 11 This aspect is of great interest considering that modified criteria for PD 17 could affect the evaluation of survival outcomes used as the main endpoints in clinical trials, which may also be geared to novel agent approval. Unlike previous reports, we included sFLCe as an event in the definition of PD 17 , making some important differences: first, the timing of PD detection could prove different; moreover, increasing sFLC levels not followed by conventional criteria for PD were also included as events. In our analysis, a sFLCe was detected in 9% of patients, in line with previous reports. 10 The introduction of sFLCe in the definition of PD did not significantly modify the evaluation of survival outcomes, as ePFS and PFS were almost the same. This absence of difference could be explained by the short median time (i.e., 42 days) between the sFLCe and the appearance of other signs of conventional PD, as well as by the small number of patients (i.e., 2 in our analysis, <1% among the whole study populations) who experienced a sFLCe without subsequent signs of conventional PD or need to start salvage therapy. At the same time, both these aspects support the role of increasing sFLC as a marker of relapse and highlight the importance of monitoring sFLC assay in order not to miss any early signs of progression.
Notably, an sFLC PD, defined as an increase in sFLC levels at the time of conventional relapse, or as sFLCe, showed also prognostic implications. Indeed, an sFLC PD was observed in about one‐quarter of the patients ‐ more frequently, but not exclusively, in patients with a baseline sFLC‐measurable disease ‐ and correlated with more advanced age, higher creatinine levels, and higher frequency of del(17p) positivity, at baseline. No significant difference was observed in terms of 2nd TTPe between patients with sFLC PD and other patterns of relapse by using these modified criteria for PD. This conflicts with a previous study where we observed that a relapse characterized by an increase in sFLC correlated with worse 2nd TTP. 15 However, it should be noted that, compared with conventional criteria, the use of modified criteria including sFLCe anticipates the detection of relapse ‐ and therefore the starting point for outcomes after relapse ‐ for patients with an sFLC pattern of PD. Moreover, a reduced OS after relapse was previously reported for patients progressing with increased sFLC levels, as compared with those progressing with only M‐Ig, 10 , 15 and this phenomenon was explained in terms of intraclonal heterogeneity and evolutive capacity of myeloma clones. Using modified criteria including sFLCe in the definition of PD, our analysis confirmed that an sFLC PD was associated with significantly shorter OS after Pe than when M‐Ig PD occurred without increased sFLC, supporting the evidence that a PD characterized by elevated sFLC levels correlates with a more aggressive clinical course. Notably, this difference in post‐relapse survival highlights the importance of including sFLC measurement to detect PD. Indeed, a proper identification of PD and an adequate characterization of the aggressiveness of the disease can lead to consider early rescue interventions.
Our study has some limitations, the major weakness being associated with the retrospective nature of the analysis. Indeed, although we only considered patients treated with a first‐line novel agent‐based therapy, anti‐MM treatments were not uniform. Moreover, the unavailability of bone marrow clonality data hampers conclusions about the prognostic value of sCR. Despite these limitations, the main contribution comes from the availability of sFLC evaluations at multiple time points in a large series of newly diagnosed Ig‐MM patients.
In conclusion, our results highlight the relevance of routine sFLC evaluations in monitoring Ig‐MM patients. Particularly, our data extend the existing body of evidence 12 , 13 , 14 , 15 , 16 , 17 suggesting the role of the sFLC assay as a laboratory marker of clonality complementary with standard EP and IF for assessing response and monitoring the disease. Indeed, our results support the inclusion of a normal sFLCR in the definition of CR along with the existing conventional criteria, while an increase of at least 25% over the lowest difference between involved and uninvolved sFLC levels ‐ the absolute increase being at least of 100 mg/L ‐ would be considered as a marker of PD. Moreover, to the best of our knowledge, we analyzed for the first time how the inclusion of the sFLCe in the definition of PD can impact the assessment of survival outcomes. We have demonstrated that PFS and ePFS are almost similar. On the other hand, we have shown that a pattern of sFLC PD negatively impacts the OS after Pe, thereby suggesting more aggressive clinical features. Overall, these data strongly support the inclusion of the sFLC assay in the definition of conventional response and PD in patients with an M‐Ig of 10 g/L or higher. Additional studies are necessary to determine the prognostic impact of the sFLC assay for patients with lower intact M‐Ig as well as those with light chain disease. On‐going clinical trials will provide further insights into the role of more sensitive reliable tools to assess minimal residual disease and drive treatment strategies.
FUNDING INFORMATION
The work reported in this publication was funded by the Italian Ministry of Health, RC‐2022‐2773323.
CONFLICT OF INTEREST
Paola Tacchetti has received Honoraria from Amgen, Bristol‐Myers Squibb/Celgene, Janssen, Takeda, AbbVie, Sanofi, GlaxoSmithKline, and Oncopeptides; Serena Rocchi receives Honoraria from Amgen, GlaxoSmithKline, and Janssen; Elena Zamagni has received honoraria from Janssen, Bristol‐Myers Squibb, Amgen, Takeda; Ilaria Rizzello has received Honoraria from Amgen, GlaxoSmithKline, and Sanofi; Lucia Pantani has received Honoraria from Janssen and Amgen; Katia Mancuso has received Honoraria from Celgene, Takeda, Amgen, Sanofi and Janssen; Michele Cavo has received Honoraria from Janssen, Celgene, Amgen, Bristol‐Myers Squibb, Takeda, AbbVie, Sanofi, Adaptive Biotechnologies, and is a member of Janssen's and Celgene's Speaker's Bureau; Simona Barbato, Gabriella De Cicco, Alessio Fusco, Luca Dozza, Margherita Ursi, Emanuele Favero, Carolina Terragna and Nicoletta Testoni declare no potential conflicts of interest.
ACKNOWLEDGMENT
Open access funding provided by BIBLIOSAN.
Tacchetti P, Rocchi S, Zamagni E, et al. Role of serum‐free light chain assay for defining response and progression in immunoglobulin secretory multiple myeloma. Am J Hematol. 2022;97(12):1607‐1615. doi: 10.1002/ajh.26747
Funding information Italian Ministry of Health, Grant/Award Number: RC‐2022‐2773323
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author Michele Cavo.
REFERENCES
- 1. Bradwell AR, Carr‐Smith HD, Mead GP, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47(4):673‐680. [PubMed] [Google Scholar]
- 2. Katzmann JA, Kyle RA, Benson J, et al. Screening panels for detection of monoclonal gammopathies. Clin Chem. 2009;55(8):1517‐1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538‐e548. [DOI] [PubMed] [Google Scholar]
- 4. Kapoor P, Kumar SK, Dispenzieri A, et al. Importance of achieving stringent complete response after autologous stem‐cell transplantation in multiple myeloma. J Clin Oncol. 2013;31(36):4529‐4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mateos MV, Kumar S, Dimopoulos MA, et al. International myeloma working group risk stratification model for smoldering multiple myeloma (SMM). J Blood Cancer J. 2020;10(10):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bradwell AR, Carr‐Smith HD, Mead GP, Harvey TC, Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet. 2003;361(9356):489‐491. [DOI] [PubMed] [Google Scholar]
- 7. Drayson M, Tang LX, Drew R, Mead GP, Carr‐Smith H, Bradwell AR. Serum free light‐chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood. 2001;97(9):2900‐2902. [DOI] [PubMed] [Google Scholar]
- 8. Mead GP, Carr‐Smith HD, Drayson MT, Morgan GJ, Child JA, Bradwell AR. Serum free light chains for monitoring multiple myeloma. Br J Haematol. 2004;126(3):348‐354. [DOI] [PubMed] [Google Scholar]
- 9. Ayliffe MJ, Davies FE, de Castro D, Morgan GJ. Demonstration of changes in plasma cell subsets in multiple myeloma. Haematologica. 2007;92(8):1135‐1138. [DOI] [PubMed] [Google Scholar]
- 10. Brioli A, Giles H, Pawlyn C, et al. Serum free light chain evaluation as a marker for the impact of intra‐clonal heterogeneity on the progression and treatment resistance in multiple myeloma. Blood. 2014;123(22):3414‐3419. [DOI] [PubMed] [Google Scholar]
- 11. Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467‐1473. [DOI] [PubMed] [Google Scholar]
- 12. Tacchetti P, Cavo M, Rocchi S, et al. Prognostic impact of serial measurements of serum‐free light chain assay throughout the course of newly diagnosed multiple myeloma treated with bortezomib‐based regimens. Leuk Lymphoma. 2016;57(9):2058‐2064. [DOI] [PubMed] [Google Scholar]
- 13. Moustafa MA, Rajkumar SV, Dispenzieri A, et al. Utility of serum free light chain measurements in multiple myeloma patients not achieving complete response to therapy. Leukemia. 2015;15:S238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klein EM, Tichy D, Salwender HJ, et al. Prognostic impact of serum free light chain ratio normalization in patients with multiple myeloma treated within the GMMG‐MM5 trial. Cancers (Basel). 2021;13(19):4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tacchetti P, Pezzi A, Zamagni E, et al. Role of serum free light chain assay in the detection of early relapse and prediction of prognosis after relapse in multiple myeloma patients treated upfront with novel agents. Haematologica. 2017;102(3):e104‐e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dejoie T, Attal M, Moreau P, Harousseau JL, Avet‐Loiseau H. Comparison of serum free light chain and urine electrophoresis for the detection of the light chain component of monoclonal immunoglobulins in light chain and intact immunoglobulin multiple myeloma. Haematologica. 2016;101(3):356‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dejoie T, Corre J, Caillon H, Moreau P, Attal M, Avet LH. Responses in multiple myeloma should be assigned according to serum, not urine, free light chain measurements. Leukemia. 2019;33:313‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412‐3420. [DOI] [PubMed] [Google Scholar]
- 19. Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48:1437‐1444. [PubMed] [Google Scholar]
- 20. Sonneveld P, Avet‐Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high‐risk cytogenetics: a consensus of the international myeloma working group. Blood. 2016;127(24):2955‐2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the international myeloma workshop consensus panel 1. Blood. 2011;117(18):4691‐4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar S, Paiva B, Anderson KC, et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016. Aug;17(8):e328‐e346. [DOI] [PubMed] [Google Scholar]
- 23. van Rhee F, Bolejack V, Hollmig K, et al. High serum‐free light chain levels and their rapid reduction in response to therapy define an aggressive multiple myeloma subtype with poor prognosis. Blood. 2007;110(3):827‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kyrtsonis MC, Vassilakopoulos TP, Kafasi N, et al. Prognostic value of serum free light chain ratio at diagnosis in multiple myeloma. Br J Haematol. 2007;137(3):240‐243. [DOI] [PubMed] [Google Scholar]
- 25. Dispenzieri A, Zhang L, Katzmann JA, et al. Appraisal of immunoglobulin free light chain as a marker of response. Blood. 2008;111(10):4908‐4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu Y, Sui W, Deng S, et al. Further stratification of patients with multiple myeloma by international staging system in combination with ratio of serum free kappa to lambda light chains. Leuk Lymphoma. 2013;54(1):123‐132. [DOI] [PubMed] [Google Scholar]
- 27. Khoriaty R, Hussein MA, Faiman B, Kelly M, Kalaycio M, Baz R. Prediction of response and progression in multiple myeloma with serum free light chains assay: corroboration of the serum free light chain response definitions. Clin Lymphoma Myeloma Leuk. 2010;10(1):E10‐E13. [DOI] [PubMed] [Google Scholar]
- 28. Snozek CL, Katzmann JA, Kyle RA, et al. Prognostic value of the serum free light chain ratio in newly diagnosed myeloma: proposed incorporation into the international staging system. Leukemia. 2008;22(10):1933‐1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maltezas D, Dimopoulos MA, Katodritou I, et al. Re‐evaluation of prognostic markers including staging, serum free light chains or their ratio and serum lactate dehydrogenase in multiple myeloma patients receiving novel agents. Hematol Oncol. 2013;31(2):96‐102. [DOI] [PubMed] [Google Scholar]
- 30. Du J, Lu J, Gao W, et al. Serum‐free light chains combined with the revised international staging system could further distinguish the superior and inferior clinical outcome of multiple myeloma patients. Ann Hematol. 2020;99:1779‐1791. [DOI] [PubMed] [Google Scholar]
- 31. Martínez‐López J, Paiva B, López‐Anglada L, et al. Spanish multiple myeloma group/program for the study of malignant Blood diseases therapeutics (GEM/PETHEMA) cooperative study group. Critical analysis of the stringent complete response in multiple myeloma: contribution of sFLC and bone marrow clonality. Blood. 2015;126(7):858‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lopez‐Anglada L, Cueto‐Felgueroso C, Rosiñol L, et al. GEM (Grupo Español de MM)/PETHEMA (Programa Para el Estudio de la Terapéutica en Hemopatias Malignas) cooperative study group. Prognostic utility of serum free light chain ratios and heavy‐light chain ratios in multiple myeloma in three PETHEMA/GEM phase III clinical trials. PLoS One. 2018;13(9):e0203392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abdallah N, Kapoor P, Murray DL, et al. Utility of serum free light chain ratio in response definition in patients with multiple myeloma. Blood Adv. 2020;4(2):322‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cedena MT, Martin‐Clavero E, Wong S, et al. The clinical significance of stringent complete response in multiple myeloma is surpassed by minimal residual disease measurements. PLoS One. 2020;15(8):e0237155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giles HV, Drayson MT, Wright N, et al. Residual monoclonal free light chain positivity by mass spectrometry identifies patients at increased risk of early relapse following first‐line anti‐myeloma treatment. Blood. 2021;138(Supplement 1):820. [Google Scholar]
- 36. Puig N, Contreras MT, Agulló C, et al. Mass spectrometry vs immunofixation for treatment monitoring in multiple myeloma. Blood Adv. 2022;6(11):3234‐3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murray DL, Puig N, Kristinsson S, et al. Mass spectrometry for the evaluation of monoclonal proteins in multiple myeloma and related disorders: an International Myeloma Working Group Mass Spectrometry Committee Report. Blood Cancer J. 2021;11(2):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author Michele Cavo.


