Summary
Background
Food insecurity affects diet quality (DQ) and is associated with non‐alcoholic fatty liver disease (NAFLD). While racial and ethnic disparities in NAFLD exist, the relationship between food insecurity and DQ by race and ethnicity is unknown.
Aim
To examine the relationship between food insecurity and DQ in adults with NAFLD and significant fibrosis by race and ethnicity in a nationally representative cohort
Methods
We performed a cross‐sectional analysis of U.S. adults (≥20 years) in the National Health and Nutrition Examination Survey 2017–2018 with vibration‐controlled transient elastography (VCTE), DQ, and food security (FS) measurements. NAFLD and significant fibrosis were defined using validated VCTE cut‐offs. We assessed total and component DQ by the healthy eating index (HEI)‐2015, with poor scores defined as <25th percentile. We used multivariable linear and logistic regression to examine associations of FS and race/ethnicity with DQ.
Results
Of 1351 adults with NAFLD (17% food insecure; 248 with fibrosis), mean (standard error [SE]) DQ score was 49 (1) and 47(1.2) for food secure and insecure groups, respectively. Mean (SE) DQ was lowest for White (47[1.1]), followed by Black (49[0.9]), Hispanic (50[1.2]) and Asian persons (56[2.1]). In multivariable models, there was an inverse relationship between FS and DQ, although this did not reach statistical significance (estimated difference [coef]:‐1.8 mean HEI score, 95% CI: −4.3–0.7; p = 0.14). Adjusted mean DQ scores were higher for Black (coef:+3.0, 95% CI:0.5–5.5; p = 0.02), Asian (coef:+7.4, 95% CI:3.4–11.5; p = 0.001) and Hispanic (coef: +4.3, 95% CI: 0.6–7.9; p = 0.03) compared to White persons. Greatest differences in DQ components by food security status were seen in White persons.
Conclusion
Among adults with NAFLD, White persons had poorer DQ than other races/ethnicities. The influence of food insecurity on DQ may be potentiated in this group. Exploration of the sociocultural factors influencing DQ is needed to mitigate NAFLD disparities.

1. INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a hepatic manifestation of the metabolic syndrome and is increasing in prevalence in the United States. 1 , 2 It is estimated that over 35% of U.S. adults have NAFLD, 3 mirroring rising rates of metabolic comorbidities, including obesity and diabetes. NAFLD can progress to cirrhosis and hepatocellular carcinoma and has become a leading indication for liver transplantation. 4 Adherence to a healthy diet is the cornerstone of management. Recent studies have demonstrated an association between higher diet quality (DQ) and lower risk of NAFLD. 5 Additionally, lifestyle interventions, including dietary modifications, may be effective in lowering steatosis and inflammation in adults with NAFLD. 6
The ability to eat a healthy diet is largely determined by access to affordable, healthy food options, a consequence of the social conditions in which one lives. 7 People who are socioeconomically vulnerable may face food insecurity, or the limited or uncertain availability of nutritionally adequate foods. 7 Food insecurity affects 40 million Americans and over 25% of U.S. adults with NAFLD. 8 In recent years, food insecurity has been shown to be an independent risk factor for NAFLD and significant fibrosis and has negative long‐term consequences in this population. 8 , 9 Indeed, food‐insecure adults with NAFLD have a greater risk of mortality compared to food‐secure adults, even after accounting for other socioeconomic factors such as poverty and education level. 8 While it has been hypothesised that the negative impact of food insecurity on health outcomes is a result of poorer diet quality, this hypothesis has not been previously explored.
Additionally, racial and ethnic disparities in NAFLD incidence and outcomes have been recently described. A prior meta‐analysis showed that NAFLD prevalence was highest in Hispanic and lowest in Black persons. 10 Whether differences in social and lifestyle factors, including diet quality or food insecurity, contribute to these disparities is unknown. Identifying contributing factors is critical to designing targeted interventions that mitigate health inequities. Leveraging a large, nationally representative sample of U.S. adults, we aimed to examine the relationship between food insecurity and diet quality in adults with NAFLD and significant fibrosis by race and ethnicity.
2. METHODS
2.1. Study population
The study population included adult participants (age 20 and older) from the National Health and Nutrition Examination Survey (NHANES), 2017–2018. The NHANES is a large, nationally representative health survey of the non‐institutionalised U.S. population conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control. It utilises a stratified, multi‐stage clustered probability sampling design to develop a population sample that is representative of the United States based on age, sex and race and ethnicity. Starting in the 2017–2018 survey year, liver ultrasound elastography was performed on all participants aged 12 years and older. Participants were excluded if they were ineligible (including those pregnant) or did not have valid transient elastography data (N = 755) or if they had viral hepatitis B (positive hepatitis B surface antigen, N = 27) or hepatitis C (positive hepatitis C antibody, N = 43). Of the eligible NAFLD population, participants were further excluded if they reported extreme caloric intakes (<500 or >5000 kilocalories per day, N = 30) as this may have been due to potential misreporting of dietary intake, or if they were missing the following pertinent data: food insecurity status (N = 83), poverty status (N = 140) or diet quality data (N = 103). We also excluded the ‘other’ race category because of the heterogeneity of races represented within this group (N = 73). The NHANES protocols were approved by the National Center for Health Statistics ethics review board and all participants provided written informed consent.
2.2. Assessment of NAFLD and significant fibrosis
Ultrasound elastography was performed using vibration controlled transient elastography (FibroScan® Model 502 V2 Touch equipped with a medium (M) or extra‐large (XL) probe). Health technicians performing examinations were trained and certified by NHANES staff and the equipment manufacturer (Echosens™ North America). Fibroscan® was performed according to a detailed procedural manual under manufacturer guidelines 11 and quality and control were periodically monitored by NHANES staff. Liver stiffness measurements (LSM) were assessed in kilopascals (kPA) and controlled attenuation parameter (CAP) was measured in decibels per metre (dB/m). Both LSM and CAP were obtained using the same liver volume and CAP was only calculated if the LSM was valid. Participants were excluded if they were pregnant, unable to lie on an examination table, had an implanted device or refused examination. Examinations were considered invalid if participants fasted for less than 3 h. An examination was considered complete when 10 valid measurements were taken with an interquartile range of <30%.
LSM and CAP were considered both continuous and categorical variables. NAFLD was defined as a CAP score ≥280 dB/m in the absence of viral hepatitis or heavy alcohol intake (>4 drinks per day in men and >3 drinks per day in women; N = 25). Significant fibrosis was defined as a median LSM of ≥8 kPA among those with NAFLD. These cut‐offs have been previously validated as optimal sensitivity and specificity of CAP and LSM values for histologic grade of steatosis and fibrosis, respectively, in biopsy‐confirmed NAFLD patients.12, 13, 14, 15
2.3. Assessment of diet quality (outcome variable)
Dietary intake was assessed using 24‐h dietary recall surveys conducted by trained interviewers. Only the first day's data were used to calculate diet quality (DQ) in this analysis. The most recent iteration of the HEI, the Healthy Eating Index (HEI)‐2015, was used to assess DQ scores as it was created to reflect key recommendations from the 2015–2020 U.S. Dietary Guidelines for Americans. The HEI‐2015 consists of 13 different components that are based on nutrient content and food serving equivalents. Of these, nine components assess dietary adequacy: total fruits, whole fruits, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins and fatty acids; and four dietary components address moderation: refined grains, sodium, added sugars, saturated fats. For the adequacy components, higher levels of intake result in higher scores (i.e., the higher the dietary components, the healthier the diet). For the moderation components, lower levels of intake result in higher scores (i.e., the lower the dietary components, the healthier the diets). Each component is scored on a density basis out of 1000 calories (except fatty acids, which is the ratio of unsaturated to saturated fatty acids) and scored on a scale of 0–10 (for seven of the components) or 0–5 (for six of the components). The total HEI‐2015 score is obtained by summing the scores for all 13 components and ranges from 0 to 100, with higher scores reflecting better diet quality. For our analysis, total HEI‐2015 scores were classified as continuous. Poor diet quality was defined as <25th percentile of possible scores for the 13 individual components.
2.4. Assessment of food security status
Food insecurity was assessed using the 18‐item U.S. Household Food Security Survey Module, a well‐validated questionnaire developed by the U.S. Department of Agriculture (USDA) to measure household food security over the prior 12 months. The survey includes three questions about food security conditions of the whole household, seven questions about food security conditions of adults in the household and eight questions about food security conditions of children in the household. These questions address anxiety over insufficient household food budget or food supply, the experience of running out of food without money to obtain more, perceptions that food is inadequate in quality or quantity and changes to food quality (i.e., substitutions of cheaper, higher calorie, nutrient‐poor foods) or reduced food intake. 16 Using the USDA validated cutpoints, adults were categorised as high, marginal, low and very low food security based on the number of affirmative responses. For our analysis, we grouped full and marginal food security into a combined ‘food secure’ category and categorised ‘low’ and ‘very low’ food security as ‘food insecure’, as has been done in prior analyses. 17
2.5. Covariate assessment
Race and ethnicity were categorised as White, Hispanic, Black or Asian. Participants were considered smokers if they reported at least 100 cigarettes used over the lifetime. Educational attainment was grouped as less than high school, high school or equivalent, or college or higher. Insurance status was categorised as private, public or none. Family poverty‐to‐income ratios were calculated by dividing family income by the poverty guidelines. Poverty‐to‐income ratio <1 was considered below the poverty line. Alcohol consumption was obtained from the NHANES alcohol questionnaire and was categorised as ever alcohol use during the survey year. These variables were all collected by self‐report. Coffee intake was obtained from the 24‐hour dietary recall interview and categorised as ≤3 cups versus >3 cups consumed, as greater than three cups of coffee daily has been associated with lower liver stiffness measurements. 18 Sugar‐sweetened beverages (SSBs) were also captured given their known association with increased risk of steatosis and fibrosis and categorised as 0, 1–2 and >2 drinks per day. 19 SSBs included soft drinks, fruit drinks with added sugar, sweetened coffee and tea, sport drinks, flavoured milk and sweetened water. Coffee, tea, unsweetened milk and 100% fruit juice were not categorised as SSBs. Fast food consumption was included given its known association with poor diet quality. Consistent with previous analyses, items reported as restaurant fast food/pizza were considered as fast food and described as the percentage of total calories from fast food. 20 , 21 Hypertension was defined as systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥ 80 mm Hg on physical exam and/or self‐reported previous use of antihypertensive medication. Hyperlipidaemia was defined as total cholesterol>240 mg/dl, low‐density lipoprotein (LDL) ≥ 160 mg/dl or high‐density lipoprotein (HDL) < 40 mg/dl by laboratory data, or self‐reported history of oral cholesterol medication use. Obesity was defined by a body mass index (BMI) ≥ 30 kg/m2. Diabetes mellitus included those with a history of diabetes diagnosis and/or treatment with a hypoglycaemic agent. Laboratory data included total bilirubin, platelet count, albumin, creatinine, AST, ALT and haemoglobin A1c at the time of exam.
2.6. Statistical analysis
Given the complex survey design, all statistical analyses were performed in accordance with NHANES guidelines. 22 Specifically, sample weights accounted for differential sampling probabilities and nonresponse to produce unbiased national estimates and variance calculated by Taylor series linearisation accounted for survey design parameters to produce accurate standard errors (SE) and confidence intervals (CI). Sociodemographic characteristics were estimated as weighted means and percentages with SE by food security status in each group (NAFLD and significant fibrosis). HEI‐2015 scores were calculated as means with SE among NAFLD adults stratified by race and ethnicity and food security status. To better visualise the multidimensional qualities of the HEI‐2015, the 13 component scores were graphed using radar charts. The mean of each component score was plotted as the percentage of the maximum score; the outer edge of the circle represented a score of 100% of the maximum score for that component and the centre of the circle represented a score of 0% for any given component.
Univariable linear regression was used to estimate (1) the mean differences in the HEI‐2015 scores and (2) the mean difference in the proportion with low diet quality for the 13 components by household food security. Multivariable linear regression models estimated the mean differences in HEI‐2015 scores adjusted for covariates selected a priori for their known association with diet quality or potential confounding effect in relation to food security, regardless of statistical significance. The following covariates were included: age, gender, race/ethnicity, poverty, coffee consumption, fast food intake, sugar‐sweetened beverage consumption and education level. Analyses were further stratified by race and ethnicity. We then performed multivariable logistic regression to examine the associations of poor diet quality based on the 13 individual components of HEI with race and ethnicity and among NAFLD participants.
Statistical tests were two‐sided and p < 0.05 was considered statistically significant. All analyses were performed using survey procedures in STATA/MP 16.1.
3. RESULTS
3.1. Cohort characteristics
Of 1351 adults with NAFLD, 248 had significant fibrosis (Table 1). Among NAFLD participants (17% food insecure), food insecure adults were more likely to be Black (11% vs. 8%), Hispanic (35% vs. 17%), foreign born (27% vs. 18%), live in poverty (37% vs. 6%) or have public or no insurance (65% vs. 29%). Among participants with significant fibrosis, those who were food insecure were more likely to be Hispanic (30% vs. 17%), foreign born (23% vs. 13%), have less than a high school education (33% vs. 11%) or have public or no insurance (64% vs. 29%). Emergency food assistance (i.e., from a church, food pantry, food bank or soup kitchen) was utilised by 33% of subjects with NAFLD and 28% of those with significant fibrosis who were food insecure.
TABLE 1.
Sociodemographic characteristics of NHANES participants from 2017–2018 with NAFLD according to food security status.
| NAFLD (n = 1351) | NAFLD with significant fibrosis (n = 248) | |||
|---|---|---|---|---|
| Characteristic of participants (% or mean ± SE) | Food secure (n = 1046) | Food insecure (n = 305) | Food secure (n = 193) | Food insecure (n = 55) |
| Age (years) | 52 (0.8) | 46 (1.3) | 53 (2.2) | 49 (2.5) |
| Male gender, % | 56 (0.02) | 42 (0.05) | 57 (0.1) | 56 (0.1) |
| Race/ethnicity, % | ||||
| White | 69 (0.03) | 51 (0.05) | 69 (0.1) | 58 (0.1) |
| Black | 8 (0.01) | 11 (0.03) | 8 (0.02) | 10 (0.05) |
| Asian | 6 (0.01) | 3 (0.01) | 5 (0.02) | 2 (0.02) |
| Hispanic | 17 (0.02) | 35 (0.06) | 17 (0.03) | 30 (0.08) |
| Foreign born, % | 18 (0.02) | 27 (0.04) | 13 (0.03) | 23 (0.07) |
| Smoking history, % | 41 (0.03) | 51 (0.04) | 39 (0.05) | 63 (0.07) |
| Poverty income ratio < 1, % | 6 (0.01) | 37 (0.03) | 5 (0.02) | 46 (0.09) |
| HEI‐2015 composite score | 49 (0.9) | 46 (1.4) | 46 (1.1) | 46 (2.7) |
| Utilised emergency food assistance, % | 3 (0.01) | 33 (0.04) | 3 (0.01) | 28 (0.08) |
| Married, % | 72 (0.02) | 57 (0.05) | 67 (0.06) | 53 (0.1) |
| Employed, % | 60 (0.02) | 55 (0.04) | 63 (0.06) | 48 (0.1) |
| Education level, % | ||||
| <High school | 7 (0.01) | 23 (0.03) | 11 (0.03) | 33 (0.1) |
| High school/GED | 31 (0.02) | 38 (0.05) | 37 (0.1) | 36 (0.1) |
| >High school | 62 (0.02) | 39 (0.03) | 53 (0.06) | 31 (0.1) |
| Insurance category, % | ||||
| Private | 71 (0.02) | 35 (0.02) | 70 (0.1) | 36 (0.1) |
| Public | 21 (0.02) | 46 (0.03) | 21 (0.04) | 46 (0.1) |
| None | 8 (0.02) | 19 (0.03) | 9 (0.03) | 18 (0.07) |
| History of alcohol use in survey year, % | 77 (0.02) | 70 (0.03) | 72 (0.1) | 62 (0.1) |
| Sugar‐sweetened beverage consumption (>2 beverages per day), % | 38 (0.03) | 49 (0.03) | 45 (0.1) | 56 (0.1) |
| Coffee consumption (>3 cups per day), % | 26 (0.02) | 16 (0.04) | 15 (0.05) | 15 (0.1) |
| Fast food consumption % of calories from fast food) | 14 (1.4) | 14 (2.1) | 17 (2.3) | 20 (6.1) |
| BMI (kg/m2) | 33 (0.4) | 35 (0.6) | 39 (1.0) | 39 (1.0) |
| Obese, % | 68 (0.03) | 74 (0.03) | 88 (0.03) | 90 (0.04) |
| Waist circumference (cm) | 111 (0.9) | 112 (1.2) | 124 (2.1) | 124 (2.3) |
| HbA1c, % | 6.0 (0.1) | 6.0 (0.1) | 6.3 (0.1) | 6.6 (0.3) |
| Diabetic, % | 22 (0.02) | 21 (0.03) | 42 (0.04) | 43 (0.1) |
| Hyperlipidaemia, % | 51 (0.03) | 47 (0.05) | 54 (0.1) | 58 (0.1) |
| Hypertension, % | 65 (0.02) | 61 (0.04) | 74 (0.03) | 71 (0.1) |
| Total bilirubin (mg/dl) | 0.5 (0.02) | 0.4 (0.02) | 0.5 (0.03) | 0.5 (0.06) |
| Platelet count (109/L) | 248 (4.0) | 256 (6.9) | 245 (5.2) | 244 (10.0) |
| Albumin (g/dl) | 4.1 (0.02) | 4.0 (0.04) | 4.0 (0.05) | 4.0 (0.1) |
| Creatinine (mg/dl) | 0.9 (0.02) | 0.8 (0.02) | 0.9 (0.03) | 0.9 (0.04) |
| ALT (U/L) | 28 (0.8) | 29 (2.9) | 36 (2.6) | 39 (8.4) |
| AST (U/L) | 24 (0.7) | 23 (1.3) | 30 (2.5) | 32 (4.3) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GED, general education development; HEI‐2015, healthy eating index‐2015; NAFLD, nonalcoholic fatty liver disease.
3.2. Total diet quality component scores by race and ethnicity in NAFLD
Mean total HEI‐2015 scores for participants with NAFLD by food security status are shown in Table 2. Mean total HEI (SE) was lowest for White (47, 1.1), followed by Black (49, 0.9), Hispanic (50, 1.2) and Asian (56, 2.1) persons, who had the highest diet quality scores. Among the NAFLD cohort, food secure participants had higher mean (SE) HEI compared to food insecure participants (food secure: 49 (0.9); food insecure: 46 (1.4); p = 0.10), but this did not reach statistical significance. In White persons, mean (SE) HEI was significantly greater in food secure (mean: 48, SE: 1.1) as compared to food insecure subjects (mean: 42 [1.6]; p = 0.003). There were no significant differences in mean HEI scores by food security status among Black, Asian or Hispanic persons.
TABLE 2.
Mean total HEI‐2015 scores by food security status in participants with NAFLD stratified by race and ethnicity (n = 1351).
| All adults | White | Black | Hispanic | Asian | |
|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | |
| Food secure | 49 (0.9) | 48 (1.1) | 49 (1.0) | 49 (1.4) | 56 (2.2) |
| Food insecure | 46 (1.4) | 42 (1.6) | 47 (1.6) | 51 (1.8) | 55 (3.1) |
| Difference between groups | −2.5 (1.4) | −5.7 (1.6) | −1.5 (1.6) | 1.5 (2.0) | −0.4 (3.2) |
| p‐value | 0.10 | 0.003 | 0.37 | 0.45 | 0.90 |
Abbreviations: HEI‐2015: healthy eating index‐2015; NAFLD: nonalcoholic fatty liver disease.
3.3. Associations between food insecurity with diet quality in participants with NAFLD
After adjustment for demographic, socioeconomic and metabolic factors, there was an inverse relationship between food security and diet quality among those with NAFLD, although this did not reach statistical significance (estimated difference [coef] in HEI score for food insecure compared to food secure subjects: −1.8, 95% CI −4.3 to 0.7, p = 0.14; Table 3). Adjusted mean HEI scores were significantly higher for Black (coef: +3.0 mean HEI score, 95% CI 0.5–5.5, p = 0.02), Asian (coef +7.4, 95% CI 3.4–11.5, p = 0.001) and Hispanic (coef +4.3, 95% CI 0.6–7.9, p = 0.03) compared to White persons, reflecting better diet quality scores. Older age was also associated with a higher diet quality score (coef +0.2, 95% CI 0.1–0.2, p < 0.001). Greater fast food caloric intake (coef −0.1 per percent increase in calories from fast food, 95% CI −0.1 to −0.03, p = 0.003) and SSB consumption (coef −5.7 for >2 vs. 0 beverages, 95% CI −8.0 to −3.6, p < 0.001) were associated with poorer diet quality.
TABLE 3.
Multivariable linear regression of factors associated with total HEI‐2015 diet quality score (outcome) in adults with NAFLD.
| Coefficient a | 95% CI | p‐value | |
|---|---|---|---|
| Age (per year increase) | 0.2 | 0.1 to 0.2 | <0.001 |
| Female gender | −0.3 | −2.9 to 2.3 | 0.82 |
| Food insecure (ref: food secure) | −1.8 | −4.3 to 0.7 | 0.14 |
| Race/ethnicity (ref: White) | |||
| Black | 3.0 | 0.5 to 5.5 | 0.02 |
| Asian | 7.4 | 3.4 to 11.5 | 0.001 |
| Hispanic | 4.3 | 0.6 to 7.9 | 0.03 |
| Poverty | 1.2 | −1.0 to 3.3 | 0.26 |
| Coffee (>3 cups per day) | −1.4 | −3.1 to 0.4 | 0.12 |
| Fast food intake (per percent increase in calories from fast food) | −0.1 | −0.1 to −0.03 | 0.003 |
| Sugar‐sweetened beverages (ref: 0) | |||
| 1–2 SSBs | −3.7 | −6.0 to −1.4 | 0.004 |
| >2 SSBs | −5.7 | −8.0 to −3.6 | <0.001 |
| Education (ref: <high school) | |||
| High school grad/GED | −2.2 | −5.0 to 0.6 | 0.11 |
| Some college or college grad | 1.8 | −1.7 to 5.3 | 0.29 |
Abbreviations: GED, general education development; HEI‐2015, healthy eating index‐2015; NAFLD, nonalcoholic fatty liver disease; SSBs, sugar‐sweetened beverages.
The coefficients reflect the mean differences in total HEI‐2015 score per unit change for continuous variables and compared to the reference group for categorical variables.
Among subjects with significant fibrosis (Table 4), higher caloric intake from fast food consumption was associated with a lower diet quality score (coef −0.1, 95% CI −0.1 to −0.03, p = 0.01). Black (coef: +5.2, 95% CI 0.8–9.5, p = 0.02) and Asian persons (coef: +10.0, 95% CI 4.0–16.1, p = 0.003) had higher diet quality scores compared to White persons. Food insecurity was not associated with diet quality score (coef 0.5, 95% CI −4.4 to 5.4, p = 0.14).
TABLE 4.
Multivariable linear regression of factors associated with HEI‐2015 diet quality score (outcome) in adults with significant fibrosis.
| Coefficienta | 95% CI | p‐value | |
|---|---|---|---|
| Age (per year increase) | 0.1 | −0.01 to 0.3 | 0.06 |
| Female gender | −1.8 | −6.9 to 3.3 | 0.46 |
| Food insecure (ref: food secure) | 0.5 | −4.4 to 5.4 | 0.14 |
| Race/ethnicity (ref: White) | |||
| Black | 5.2 | 0.8 to 9.5 | 0.02 |
| Asian | 10.0 | 4.0 to 16.1 | 0.003 |
| Hispanic | −1.0 | −5.2 to 3.2 | 0.62 |
| Poverty | 0.8 | −2.4 to 4.0 | 0.60 |
| Coffee (>3 cups per day) | −3.4 | −12.3 to 5.4 | 0.42 |
| Fast food calorie intake (per % increase in calories from fast food) | −0.1 | −0.1 to −0.03 | 0.01 |
| Sugar‐sweetened beverages (ref: 0) | |||
| 1–2 SSBs | −4.5 | −9.7 to 0.8 | 0.09 |
| >2 SSBs | −5.4 | −9.4 to −1.4 | 0.01 |
| Education (ref: <high school) | |||
| High school grad/GED | −7.4 | −13.2 to −1.6 | 0.02 |
| Some college or college grad | 0.7 | −5.0 to 3.5 | 0.73 |
Abbreviations: GED, general education development; HEI‐2015, healthy eating index‐2015; NAFLD, nonalcoholic fatty liver disease; SSBs, sugar‐sweetened beverages.
The coefficients reflect the mean differences in total HEI‐2015 score per unit change for continuous variables and compared to the reference group for categorical variables.
To assess whether there was a differential impact of food insecurity on diet quality among different racial/ethnic groups, we tested for interactions between food insecurity and race and ethnicity (Table S1) and found a significant interaction between food insecurity and Black race. We subsequently evaluated the associations of food insecurity with diet quality stratified by race and ethnicity. Among White participants with NAFLD (Table 5), food insecurity was associated with poorer diet quality (coef −3.2, 95% CI −6.3 to −0.1, p = 0.047). Among Black participants with NAFLD (Table 5), food insecurity was also associated with poorer diet quality but did not reach statistical significance (coef −3.3, 95% CI −7.2 to 0.6, p = 0.09), likely due to small sample size. In both White and Black participants, fast food calorie intake was associated with poorer diet quality. In Asian (Table 5) and Hispanic (Table 5) NAFLD cohorts, neither food insecurity nor fast food intake was associated with diet quality.
TABLE 5.
Association of food insecurity with diet quality among those with NAFLD stratified by race and ethnicity.
| White | Black | Asian | Hispanic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p‐value | Coefficient | 95% CI | p‐value | Coefficient | 95% CI | p‐value | Coefficient | 95% CI | p‐value | |
| Age (per year increase) | 0.2 | 0.1 to 0.3 | <0.001 | 0.04 | −0.1 to 0.1 | 0.36 | 0.2 | 0.1 to 0.3 | 0.01 | 0.1 | −0.04 to 0.2 | 0.17 |
| Female gender | −1.2 | −4.9 to 2.5 | 0.49 | 2.8 | −3.2 to 8.9 | 0.33 | −1.62 | −7.3 to 4.0 | 0.55 | 1.6 | −1.2 to 4.4 | 0.25 |
| Food insecure (ref: food secure) | −3.2 | −6.3 to −0.1 | 0.047 | −3.3 | −7.2 to 0.6 | 0.09 | 1.9 | −0.9 to 4.7 | 0.17 | 0.8 | −3.2 to 4.8 | 0.68 |
| Poverty | 2.6 | −1.9 to 7.0 | 0.24 | 1.5 | −3.0 to 6.1 | 0.49 | 9.2 | −0.5 to 19.0 | 0.06 | −2.1 | −6.9 to 2.6 | 0.36 |
| Alcohol use | −3.2 | −8.4 to 2.0 | 0.21 | 3.1 | −5.4 to 11.6 | 0.45 | −2.2 | −8.1 to 3.6 | 0.43 | −2.4 | −9.2 to 4.5 | 0.47 |
| Coffee (>3 cups per day) | −1.1 | −3.4 to 1.2 | 0.32 | 0.9 | −5.5 to 7.3 | 0.77 | −5.7 | −12.5 to 1.0 | 0.09 | −2.7 | −7.6 to 2.2 | 0.26 |
| Fast food intake (per % increase in calories from fast food) | −0.1 | −0.1 to −0.01 | 0.03 | −0.1 | −0.2 to −0.04 | 0.01 | 0.0003 | −0.1 to 0.1 | 0.997 | −0.1 | −0.2 to 0.01 | 0.10 |
| Sugar‐sweetened beverages (ref: 0) | ||||||||||||
| 1–2 SSBs | −4.1 | −7.2 to −1.0 | 0.012 | −0.4 | −6.8 to 6.1 | 0.91 | −4.6 | −11.3 to 2.1 | 0.16 | −4.6 | −11.0 to 1.8 | 0.14 |
| >2 SSBs | −5.8 | −8.7 to −3.0 | 0.001 | −0.7 | −5.3 to 3.9 | 0.75 | −3.3 | −10.3 to 3.6 | 0.32 | −8.2 | −11.1 to −5.4 | <0.001 |
| Education | ||||||||||||
| High school grad/GED | −3.6 | −9.8 to 2.7 | 0.24 | −2.5 | −7.6 to 2.7 | 0.32 | −4.7 | −12.8 to 3.4 | 0.23 | −0.3 | −5.3 to 4.7 | 0.89 |
| Some college or college grad | 1.8 | −5.5 to 9.0 | 0.62 | −0.4 | −5.5 to 4.7 | 0.88 | 7.2 | −2.3 to 16.6 | 0.13 | −1.4 | −6.2 to 3.3 | 0.52 |
Abbreviations: GED, general education development; NAFLD, nonalcoholic fatty liver disease; SSBs, sugar‐sweetened beverages.
The coefficients reflect the mean differences in total HEI‐2015 score per unit change for continuous variables and compared to the reference group for categorical variables.
Total diet quality component scores by race and ethnicity in NAFLD.
The 13 HEI component scores by race and ethnicity in subjects with NAFLD are shown in Figure 1 as a ratio of the mean to maximum HEI score. Among those with food insecurity, White participants had the lowest mean scores (indicating poorer compliance with dietary recommendations) for greens and beans, whole and total fruits, saturated fats, fatty acids and added sugars compared to the other racial/ethnic groups. There were fewer differences between groups in the food secure cohort. The proportion of NAFLD participants with low diet quality scores by race and ethnicity stratified by food security status is shown in Table S2. Greatest differences in individual DQ components by food security status were seen among White persons.
FIGURE 1.
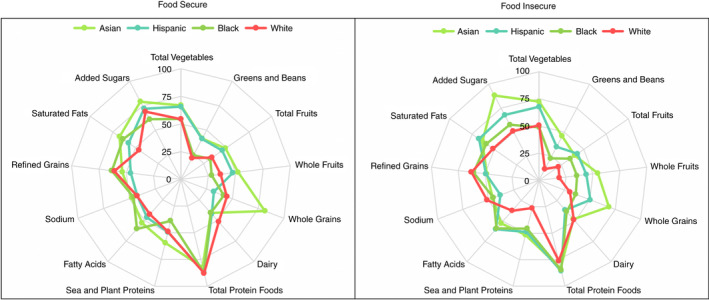
Ratios of HEI‐2015 component mean scores to maximum scores for adults with NAFLD by race and ethnicity. HEI, Healthy Eating Index.
In multivariable analysis controlling for sociodemographic factors, poverty, alcohol use and consumption of coffee or SSBs (Table 6), Hispanic, compared to White, participants had a lower odds of poorer diet quality for the following components: total vegetables (OR = 0.59, 95% CI: 0.37–0.94), whole fruit (OR = 0.41, 95% CI: 0.23–0.73), total fruit (OR = 0.39, 95% CI: 0.23–0.67), saturated fats (OR = 0.38, 95% CI: 0.24–0.62) and greens and beans (OR = 0.33, 95% CI: 0.23–0.49), but higher odds of poor diet quality for refined grains (OR = 1.81, 95% CI: 1.08–3.04). Black and Asian participants had lower odds of poor diet quality in the saturated fats and fatty acids categories compared to White participants.
TABLE 6.
Multivariable logistic regression a evaluating the association of race and ethnicity with poorer diet quality components among participants with NAFLD.
| Race/ethnicity (ref: White) | Total vegetables | Whole fruit | Total fruit | Whole grain | Total protein | Saturated fats | Added sugars |
|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Black | 1.08 (0.79–1.49) | 0.98 (0.52–1.87) | 0.99 (0.57–1.73) | 1.06 (0.57–1.97) | 1.06 (0.55–2.02) | 0.35 (0.22–0.54) | 1.28 (0.77–2.13) |
| Asian | 0.78 (0.38–1.62) | 0.52 (0.25–1.08) | 0.55 (0.27–1.09) | 0.57 (0.29–1.11) | 2.84 (1.16–6.92) | 0.35 (0.15–0.80) | 1.10 (0.35–3.42) |
| Hispanic | 0.59 (0.37–0.94) | 0.41 (0.23–0.73) | 0.39 (0.23–0.67) | 1.06 (0.65–1.73) | 0.73 (0.23–2.28) | 0.38 (0.24–0.62) | 0.89 (0.49–1.62) |
| Race/ethnicity (ref: White) | Greens and beans | Total dairy | Sea/plant protein | Fatty acids | Sodium | Refined grain |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Black | 0.71 (0.48–1.04) | 2.13 (1.57–2.90) | 1.19 (0.73–1.95) | 0.53 (0.34–0.82) | 0.87 (0.55–1.39) | 0.68 (0.43–1.09) |
| Asian | 0.37 (0.22–0.62) | 2.65 (1.47–4.78) | 0.67 (0.39–1.13) | 0.51 (0.27–0.97) | 0.77 (0.44–1.37) | 1.80 (1.00–3.24) |
| Hispanic | 0.33 (0.23–0.49) | 1.72 (1.08–2.74) | 0.79 (0.51–1.22) | 0.74 (0.51–1.07) | 1.22 (0.61–2.43) | 1.81 (1.08–3.04) |
Model also controlled for food security status, poverty, age, gender, education level, alcohol use, coffee consumption, sugar‐sweetened beverage intake, fast food intake.
3.4. Diet quality and food insecurity associations by NAFLD status
To better understand the influence of NAFLD on both diet quality and food insecurity, we also examined the relationship between food insecurity and diet quality among all persons with and without NAFLD. We found that food insecurity remained associated with poorer diet quality in the overall model (coefficient: −2.55, SE:1.1, p = 0.04). This analysis also demonstrated that diet quality decreases significantly for NAFLD participants compared to non‐NAFLD and showed a dose response with fibrosis (among NAFLD participants without fibrosis: coefficient: −2.03, SE: 0.8, p = 0.02) and NAFLD participants with fibrosis (among NAFLD participants with fibrosis: coefficient: −3.21, SE: 1.5, p = 0.053). We next examined the interaction between NAFLD status and food insecurity and found no significant interaction between these two variables, suggesting that the effect of food insecurity on diet quality does not differ between non‐NAFLD versus NAFLD groups. Given that food insecurity is associated with NAFLD in the NHANES, 9 our findings suggest that there may be other factors besides diet quality that mediate this association and warrant further exploration.
4. DISCUSSION
In this large prospective cohort study of a nationally representative sample of U.S. adults, food insecurity is associated with lower diet quality among adults with NAFLD and this association was potentiated for White persons, even after controlling for other sociodemographic factors including poverty and education level. We also found that Black, Hispanic and Asian persons with NAFLD all had better diet quality than their White counterparts regardless of food security status. These findings are among the first that show differences in diet quality, as well as the impact of food insecurity on diet quality, by race and ethnicity in adults with NAFLD.
We observed an interaction between food insecurity and race, which we had hypothesised; however, our results among different racial/ethnic groups were surprising. Given the greater prevalence of food insecurity among Black and Hispanic persons, we expected to see more pronounced effects of food insecurity on diet quality among Black and Hispanic persons with NAFLD. Instead, we found that White persons had lower diet quality scores with greater food insecurity. This was true for total diet quality as well as for individual components, including added sugars, total and whole fruits, total vegetables and seafood/plant proteins.
These findings are inconsistent with prior work in non‐NAFLD cohorts that have reported a more deleterious effect of low socioeconomic status on diet quality among minority groups compared to White persons. For example, according to the behavioural risk factor surveillance survey, African Americans had the lowest intake of fruits and vegetables of any U.S. racial or ethnic group. 23 Brown et al. reported that for U.S. adults aged >25 years, the number of Black persons with poor diet was greater than the number of White persons by a magnitude of 7%–12% points from 1988 to 2010. 24 Interestingly, in an analysis of non‐NAFLD participants from the Healthy Aging in Neighborhoods of Diversity across the Life Span Study, HEI scores were similar across races in the absence of food insecurity but were substantially lower in White compared to Black persons in those with food insecurity. 25 Similar to our study, the study researchers concluded that the influence of food insecurity on diet quality may be potentiated for White, but not Black, participants.
Among NAFLD cohorts, while data on diet quality are more limited, no significant differences between racial and ethnic groups have been previously identified. A retrospective analysis by Park et al. in the multiethnic cohort (MEC) showed no racial/ethnic differences in diet quality among people with NAFLD. 26 One limitation of the MEC, however, is the lack of generalisability of the cohort, which is predominantly comprised of Japanese and White adults from California and Hawaii and therefore may not reflect the heterogeneity of the U.S. population.
There are a few potential explanations for the more pronounced detrimental impact of food insecurity on diet quality in White persons with NAFLD. One possible reason is the lower rates of Federal Supplemental Nutrition Assistance Program (SNAP) participation among White persons. This program, formerly referred to as ‘food stamps’, provides food assistance distributed by state agencies to participating low‐ and no‐income households. 7 In the present analysis, only 17% of food insecure White persons with NAFLD utilised SNAP or emergency food assistance compared to 34% of Black and 25% of Hispanic persons with NAFLD. Prior studies indicate that receiving SNAP benefits can reduce food insecurity by as much as 20–35%; 27 thus, receipt of SNAP may have attenuated the impacts of food insecurity on diet quality.
Additionally, while this analysis only examined food insecurity over a 1‐year period, racial disparities in food insecurity prevalence have been present for a long time, and likely for generations due to centuries of structural racism and economic disadvantage for minority groups, including Black Americans. It is possible that this historical context has borne greater resilience and more effective coping skills in minority groups (i.e., cultural resilience) compared to White persons that gets passed down through the generations. 28 These coping skills may yield greater success in overcoming socioeconomic barriers to accessing higher quality foods (i.e., purchasing better quality foods on a tighter budget or in a geographic region with a low density of grocery stores or supermarkets). Furthermore, strong social support networks have been described in minority communities, including Black, Hispanic and Asian communities; 28 , 29 it is possible those who are struggling with limited food access may turn to their social support systems to relieve their temporary need for food. Ultimately, qualitative studies investigating sociocultural determinants of NAFLD disparities are needed to identify targets for food interventions that reduce racial/ethnic disparities and improve health outcomes.
Our findings in Hispanic participants were also surprising. Prior studies have shown a significantly higher prevalence of NAFLD in Hispanic persons as compared to White persons. 10 This may be due in part to genetic factors; in particular, single‐nucleotide polymorphisms in PNPLA3 are strongly associated with hepatic fat content and occurs most frequently in Hispanic persons. 30 Sociocultural factors have also been implicated but have not been previously explored. We found that Hispanic persons with NAFLD had better diet quality than their White counterparts, and that there were no differences between these two groups in diet quality among the subgroup with significant liver fibrosis. These findings suggest that diet quality plays less of a role in explaining the racial and ethnic disparities in NAFLD prevalence. Our findings should prompt further exploration into other potential sociocultural factors that may be contributing to the NAFLD epidemic among Hispanic persons.
We acknowledge several limitations. First, the cross‐sectional nature of the data does not allow for an examination of the duration of food insecurity and its impact on diet quality, or changes in food insecurity and diet quality, over time. Additionally, the HFSS addresses food security over the preceding 12 months, while dietary intake was assessed at the time of the survey. This may have led to misclassification of food security, as food insecurity is a transient condition and can vary over time. The NHANES also excludes people experiencing homelessness, a group at high risk for food insecurity. Future studies examining food insecurity in liver disease should include this high‐risk population. In addition, HEI‐2015 scores were calculated using the simple scoring method using day one of the 24‐hour dietary recalls and may not reflect usual dietary intakes of the individual. However, this method is recommended by the NCI for analyses relating independent predictors to HEI scores and components. Lastly, the NHANES does not capture data on nationality or immigration generation; therefore, we could not fully capture differences within racial/ethnic groups nor the impact of geographic origin and acculturation on diet quality.
There were many strengths of our study. We used a nationally representative sample of U.S. adults that is generalisable to the general population. Steatosis and fibrosis were measured using VCTE, which has been well validated in the NHANES and other chronic liver disease populations. 31 Food security status was evaluated using the HFSS Module, which has been validated and utilised extensively in many national data sets including in the NHANES. 16
In conclusion, food insecurity is associated with poorer diet quality among adults with NAFLD, and this relationship is most pronounced among White persons compared to other racial and ethnic groups. A better understanding of the sociocultural factors that influence these relationships is needed to design targeted food interventions that mitigate NAFLD disparities and reduce disease burden.
ACKNOWLEDGEMENT
Declaration of interests: AK and JLD report no conflicts of interest. NAT has received institutional grant support from Gilead Sciences, GlaxoSmithKline, Helio Health, Roche‐Genentech, and DURECT Corp and served as a consultant for Moderna.
AUTHORSHIP
Guarantor of the article: Ani Kardashian.
AUTHOR CONTRIBUTIONS
Ani Kardashian: Conceptualization (lead); formal analysis (supporting); funding acquisition (equal); investigation (equal); methodology (equal); writing – original draft (lead); writing – review and editing (lead). Jennifer L. Dodge: Data curation (lead); formal analysis (lead); methodology (equal); writing – review and editing (equal). Norah Terrault: Conceptualization (supporting); formal analysis (equal); methodology (equal); supervision (lead); writing – review and editing (equal).
FUNDING INFORMATION
This study was funded by the USC CTSI KL2 Award (Kardashian; KL2TR001854) and supported in part by the USC Research Center for Liver Diseases (Dodge; P30DK048522). These funding agencies played no role in the analysis of the data or the preparation of this manuscript.
CONFLICT OF INTEREST
None.
Supporting information
Table S1
Table S2
Kardashian A, Dodge JL, Terrault NA. Racial and ethnic differences in diet quality and food insecurity among adults with fatty liver and significant fibrosis: a U.S. population‐based study. Aliment Pharmacol Ther. 2022;56:1383–1393. 10.1111/apt.17219
The Handling Editor for this article was Professor Grace Wong, and it was accepted for publication after full peer‐review.
REFERENCES
- 1. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57. [DOI] [PubMed] [Google Scholar]
- 2. Kumar R, Priyadarshi RN, Anand U. Non‐alcoholic fatty liver disease: growing burden, adverse outcomes and associations. J Clin Transl Hepatol. 2020;8:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harrison SA, Gawrieh S, Roberts K, Lisanti CJ, Schwope RB, Cebe KM, et al. Prospective evaluation of the prevalence of non‐alcoholic fatty liver disease and steatohepatitis in a large middle‐aged US cohort. J Hepatol. 2021;75:284–91. [DOI] [PubMed] [Google Scholar]
- 4. Kwong AJ, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, et al. OPTN/SRTR 2019 Annual data report: liver. Am J Transplant. 2021;21(Suppl 2):208–315. [DOI] [PubMed] [Google Scholar]
- 5. Vilar‐Gomez E, Nephew LD, Vuppalanchi R, Gawrieh S, Mladenovic A, Pike F, et al. High‐quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology. 2021;75:1491–506. 10.1002/hep.32207 [DOI] [PubMed] [Google Scholar]
- 6. Vilar‐Gomez E, Martinez‐Perez Y, Calzadilla‐Bertot L, Torres‐Gonzalez A, Gra‐Oramas B, Gonzalez‐Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378.e5. [DOI] [PubMed] [Google Scholar]
- 7. Coleman‐Jensen A, Rabbitt MP, Gregory CA, & Singh A. Household food security in the United States in 2017, ERR‐256. Washington DC: U.S. Department of Agriculture Economic Research Service; 2018. [Google Scholar]
- 8. Kardashian A, Dodge JL, Terrault NA. Food Insecurity is associated with mortality among U.S. adults with nonalcoholic fatty liver disease and advanced fibrosis. Clin Gastroenterol Hepatol. 2021;S1542‐3565(21):01267–2. [DOI] [PubMed] [Google Scholar]
- 9. Golovaty I, Tien PC, Price JC, Sheira L, Seligman H, Weiser SD. Food insecurity may be an independent risk factor associated with nonalcoholic fatty liver disease among low‐income adults in the United States. J Nutr. 2020;150:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2018;16:198–210.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Health and Nutrition Examination Survey (NHANES) . Liver Ultrasound Transient Elastography Procedures Manual. 2018. Accessed January 10, 2022.
- 12. Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, et al. Individual patient data meta‐analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–30. [DOI] [PubMed] [Google Scholar]
- 13. Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717–30. [DOI] [PubMed] [Google Scholar]
- 14. Mózes FE, Lee JA, Selvaraj EA, Jayaswal ANA, Trauner M, Boursier J, et al. Diagnostic accuracy of non‐invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta‐analysis. Gut. 2022;71:1006–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai‐Sun Wong V, Wright E, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021;161:1657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to measuring household food security, revised 2000. Food and Nutrition Service: U.S. Department of Agriculture; 2000. [Google Scholar]
- 17. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low‐income NHANES participants. J Nutr. 2010;140:304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Niezen S, Mehta M, Jiang ZG, Tapper EB. Coffee consumption is associated with lower liver stiffness: a nationally representative study. Clin Gastroenterol Hepatol. 2021;S1542‐3565(21):2032–2040.e6. 10.1016/j.cgh.2021.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leung CW, Tapper EB. Sugar‐sweetened beverages are associated with increased liver stiffness and steatosis among apparently healthy adults in the United States. Clin Gastroenterol Hepatol. 2021;S1542‐3565(21):00591–7. 10.1016/j.cgh.2021.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fryar CD, Hughes JP, Herrick KA, Ahluwalia N. Fast food consumption among adults in the United States, 2013–2016. NCHS Data Brief. 2018;322:1–8. [PubMed] [Google Scholar]
- 21. Fryar CD, Carroll MD, Ahluwalia N, Ogden CL. Fast food intake among children and adolescents in the United States, 2015–2018. NCHS Data Brief. 2020;375:1–8. [PubMed] [Google Scholar]
- 22. Chen T‐C, Clark J, Riddles MK, Mohadjer LK, Fakhouri THI. National health and nutrition examination survey, 2015‐2018: sample design and estimation procedures. Vital Health Stat. 2020;2:1–35. [PubMed] [Google Scholar]
- 23. Moore LV, Dodd KW, Thompson FE, Grimm KA, Kim SA, Scanlon KS. Using behavioral risk factor surveillance system data to estimate the percentage of the population meeting US Department of Agriculture Food Patterns Fruit and Vegetable Intake Recommendations. Am J Epidemiol. 2015;181:979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brown AF, Liang L‐J, Vassar SD, Escarce JJ, Merkin SS, Cheng E, et al. Trends in racial/ethnic and nativity disparities in cardiovascular health among adults without prevalent cardiovascular disease in the United States, 1988 to 2014. Ann Intern Med. 2018;168:541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allen AJ, Kuczmarski MF, Evans MK, Zonderman AB, Waldstein SR. Race differences in diet quality of Urban Food‐Insecure Blacks and Whites Reveals Resiliency in Blacks. J Racial Ethn Health Disparities. 2016;3:706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park S‐Y, Noureddin M, Boushey C, Wilkens LR, Setiawan VW. Diet quality association with nonalcoholic fatty liver disease by cirrhosis status: the multiethnic cohort. Curr Dev Nutr. 2020;4:nzaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng XH, Jo Y, Kim J. Heterogeneous impact of supplemental nutrition assistance program benefit changes on food security by local prices. Am J Prev Med. 2020;58:e97–e103. [DOI] [PubMed] [Google Scholar]
- 28. Daly A, Jennings J, Beckett JO, Leashore BR. Effective coping strategies of African Americans. Soc Work. 1995;40:240–8. [Google Scholar]
- 29. Gallo LC, Penedo FJ, de los Monteros KE, Arguelles W. Resiliency in the face of disadvantage: do Hispanic cultural characteristics protect health outcomes? J Pers. 2009;77:1707–46. [DOI] [PubMed] [Google Scholar]
- 30. Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Siddiqui MS, Vuppalanchi R, van Natta M, Hallinan E, Kowdley KV, Abdelmalek M, et al. Vibration‐controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:156–163.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


