Abstract
The coronavirus (COVID-19) global pandemic resulted in the breakdown of traditional supply chains responsible for providing essential equipment to hospitals and personal protective equipment (PPE) for health and social care workers. The three-dimensional (3D) printing community has responded to emerging need by recognizing shortages across health care systems and providing innovative solutions in real time, circumventing short-term global supply issues. A systematic review was undertaken to investigate the role of 3D printing in the COVID-19 pandemic in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using the MEDLINE, EMBASE, and World Health Organization (WHO) COVID-19 databases. Newspaper and internet article sources were identified using the NEXIS media database. All studies and articles on the application of 3D printed solutions during the peak of the COVID-19 pandemic were included. The literature search identified 26 related articles, and 13 studies met inclusion criteria and were suitable for full-text review. One thousand two hundred and one unique digital media articles were identified; after removal of duplicates and screening of headlines for the inclusion and exclusion criteria, 460 articles were suitable for full-text review. The cross-collaboration between the 3D printing community and health care systems has aided in the provision of innovative solutions to combat the COVID-19 crisis. The applications for 3D printing ranged from oxygenation equipment to noninvasive and invasive ventilatory parts and innovative solutions for infection control and quarantine hubs. This review has identified that 3D printing technology has made the biggest contribution to the production of PPE in particular face shields, mirroring the areas of greatest shortage and need. Additive manufacturing has played a pivotal role in aligning disciplines in engineering, science, and medicine for the greater good. We have witnessed the rapid reconfiguration of traditional supply chains to circumvent global shortages, while making advancements in effort to limit the impact of this and future pandemics.
Keywords: 3D printing, additive manufacturing, COVID-19, PPE, ventilators, infection control
Introduction
Mass production keeps the world divided between consumer and producer. Demassification of production may hasten the speed towards an era of prosumers.
—Michael Petch, 3D Printing: Rise of the Third Industrial Revolution
The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2), is the biggest global health challenge of our generation, with the scale of humanitarian efforts not seen since the H1N1 influenza pandemic in 1918. The early exponential spread of the disease has significantly impacted the supply of in-demand hospital equipment and resources, such as respiratory adjuncts for ventilators and personal protective equipment (PPE) for health care professionals. The pandemic has placed considerable strain on health care systems globally and now, more than ever, highlights the importance of innovation and cross-collaboration between medicine and technology.
The global scale of the COVID-19 pandemic, travel restrictions,1 and the halt in exports from China, as the main producer and supplier, led to an early depletion of PPE reserves with global demand outweighing production capacity.2 The consequence of this imbalance was that health care workers were left exposed and vulnerable, particularly in the face of emerging evidence that exposure to high viral loads could be linked to infection severity.3 The primary means of SARS-CoV-2 transmission via droplets and aerosol resulted in an unprecedented need for PPE, including respirator masks and eye protection.4,5 Without the correct protection, health and social care professionals become vehicles for further transmission, propagating the transmission cycle. This is reflected by the rapid depletion of PPE stocks during the peak of the pandemic in many countries, with over 20% of health care workers becoming infected in some cases.6–8
The Imperial College COVID-19 response team stipulated that there was a 30% risk of intensive care admission for those requiring hospitalization and a 50% mortality thereafter.9 Most hospital admissions require some form of ventilatory support from ward-based oxygen delivery, to both noninvasive and invasive forms of ventilation for acute respiratory distress syndrome, a potential sequela of the virus. This placed extraordinary strain on health care systems, resulting in widespread ventilator shortages and rationing of care.10
Recognizing the global shortages of PPE and hospital oxygenation and ventilation equipment, the World Health Organization (WHO) called on manufacturers to address the surge in demand by increasing production by 40%, coupled with a plea to governments to relax restrictions. Alongside this response, multidisciplinary cross-collaborative efforts between scientists, engineers, clinicians, and industry diverted their efforts toward finding solutions to aid health care systems and workers worldwide. Three-dimensional (3D) printing, with its ability to manufacture customized components on demand through a spectrum of methods and materials (Table 1), has been at the forefront of innovative solutions to this global crisis. This is largely due to the ability of local additive manufacturing, which overcomes the disruption to traditional supply chains. Contributing factors include global demand and limitations to movement and production of vital equipment due to enforced quarantine measures. The 3D printing community has united in generating multiple open source stereolithography (STL) files and sharing ideas and solutions through open access platforms.11–13 This review explores the areas of health care need arising from the evolving COVID-19 pandemic that were being met by 3D printing solutions.
Table 1.
Summary of Three-Dimensional Printing Methods and Materials
| 3D printing method | Mechanism | Key features | Exemplar materials |
|---|---|---|---|
| (1) Stereolithography | UV laser causes polymerization of liquid resin in layers. | Industrial use; prototyping; detailed parts; high-quality surface finish | Liquid resin |
| (2) SLS | Laser sintering (forming a solid by applying heat-not reaching melting point) of powdered material into a solid. | Strong; durable; rougher finish | Nylon; polyamide |
| (3) SLM | Similar to SLS but material reaches melting point, metals only. | Medical devices, aerospace, automotive industry; detailed parts, expensive, post-processing required | Metals |
| (4) Digital light processing | Curing by a digital projector of a photopolymer resin. | Detailed work; nonfunctional prototypes | Photopolymer resin |
| (5) Laminated object manufacturing | Sheets of material bound together and cut to shape. | Prototyping; rapid product, not utilized for production | Adhesive paper, plastic, and metal |
| (6) Fused deposition modeling | Filament is heated into molten material and deposited in layers based on construct co-ordinates. | Widely used technology in home 3D printers; cost-effective | ABS; PLA |
| (7) Electron beam melting | Electron beam utilized to melt powered material. | Fast; post-processing required; typically, less accuracy when compared with SLM | Titanium; chromium cobalt |
3D, three-dimensional; ABS, acrylonitrile butadiene styrene; PLA, polylactic acid; SLM, selective laser melting; SLS, selective laser sintering; UV, ultraviolet.
Methods
A systematic review was undertaken to investigate the role of 3D printing in tackling the COVID-19 pandemic in accordance with the PRISMA guidelines. MEDLINE (via PubMed), Embase, and WHO COVID-19 databases were searched. Newspaper and internet article sources were identified using the media database, Nexis (https://www.lexisnexis.com/en-us/products/nexis.page). The search terms utilized were broadly (“3D Printing”[mesh] OR “additive manufacturing”[mesh] (AND “COVID-19”[mesh]); see Appendix Table A1 and A2 for the full search methodology. There were no restrictions on publication type. This search was complemented by an exhaustive review of the references of key articles. Results were restricted to English language articles.
Table 4.
Total Numbers of Media Articles (Nexis Search)
| Categories | Total Numbers | % |
|---|---|---|
| Adjuncts for ventilation | 28 | 6 |
| Ventilation | 58 | 12.6 |
| Patient care/infection control | 19 | 4.1 |
| PPE | 355 | 77.2 |
Inclusion and exclusion criteria
All studies on the application of 3D printing during the COVID-19 pandemic were included. This included articles that reported on 3D printing for manufacture of PPE, adjuncts to ventilation, ventilators (invasive and noninvasive), testing kits, and infection control innovations. Non-English articles and transcripts were excluded.
Data extraction and assessment of study quality
Two authors independently assessed all articles for the inclusion and exclusion criteria and extracted data with a third author resolving conflicts. Data were extracted and subdivided into different areas of pandemic need. The data extraction was independently checked by the senior author. The following baseline data were extracted from each study: first author, year of publication, geographic location, and type of article. Data were extracted on the area of 3D printing application (PPE/ventilators/ventilator adjuncts/testing kits/infection control).
Narrative synthesis
Marked heterogeneity in study design and types was noted during the emerging COVID-19 pandemic; a narrative synthesis was performed according to the Guidance on the Conduct of Narrative Synthesis in Systematic Reviews.14 Three authors summarized each article, focusing specifically on the area of need being addressed by 3D printing during the COVID-19 pandemic. The senior author identified and grouped key themes, which were divided into subthemes and a tabulated summary of each article produced.
Results
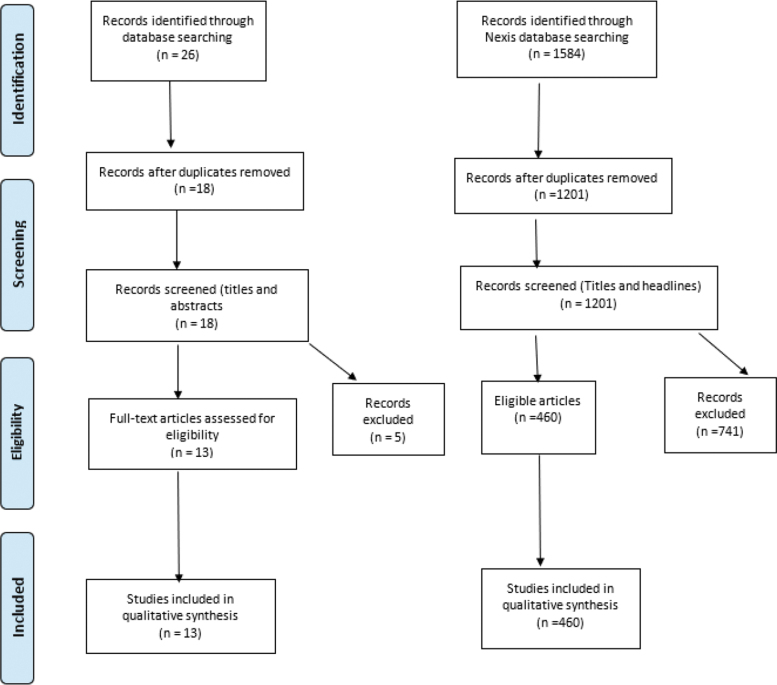
A total of 26 articles were identified from the literature search, leaving 18 for screening of titles and abstracts following removal of duplicates (n = 8). Thirteen studies met the inclusion criteria and were included for full-text review in the narrative synthesis (Fig. 1). A total of 1201 unique digital media articles were identified after removal of duplicates (n = 383). After screening the headlines for the inclusion and exclusion criteria, 460 articles were suitable for full-text review. Table 2 highlights the reviewed articles, and Tables 3 and 4 summarize the finding of the media search.
FIG. 1.
PRISMA flow diagram for database literature and media articles on 3D printing for COVID-19 crisis. 3D, three-dimensional; COVID-19, coronavirus; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 2.
Summary of Articles
| Title | First author | Year of publication | Geographic location | Type of article | 3D printed product |
|---|---|---|---|---|---|
| (1) 3-D Printed Protective Equipment during COVID-19 Pandemic43 | Wesemann | April 24, 2020 | Germany | Original article | Face shields |
| (2) 3D Printing beyond Dentistry during COVID 19 Epidemic: A Technical Note for Producing Connectors to Breathing Devices44 | Cavallo | April 7, 2020 | Italy | Technical note | Charlotte and Dave valves-Easybreath noninvasive ventilation |
| (3) 3D-Printed Face Protective Shield in Interventional Radiology: Evaluation of an Immediate Solution in the Era of COVID-19 Pandemic45 | Sapoval | April 2020 (online before print) | France | Technical note | Face shields |
| (4) Additive Manufacturing Can Assist in the Fight Against COVID-19 and Other Pandemics and Impact on the Global Supply Chain46 | Larrañeta | April 23, 2020 | Ireland | Editorial | PPE; ventilatory adjunct; NIV; patient care, infection control; ventilation/mechanical bagger |
| (5) Applications of 3D Printing Technology to Address COVID-19 Related Supply Shortages47 | Ishack | April 21, 2020 | America | Editorial | Face mask; face shields Venturi Medications Specimen collection kits |
| (6) COVID-19 and the Role of 3D Printing in Medicine48 | Tino | April 27, 2020 | Australia | Editorial | Face mask; face shields Venturi; splitters door handles; nasopharyngeal swabs |
| (7) Helmet Modification to PPE with 3D Printing During the COVID 19 Pandemic at Duke University Medical Center: A Novel Technique49 | Erickson | April 14, 2020 | America | Technical note | Modification of the Stryker Flyte orthopedic helmet |
| (8) How 3D Printing Can Prevent Spread of Covid-19 Between Health Care Professionals During Times of Critical Shortage of Protective Personal Equipment50 | Maracaja | April 13, 2020 | America | Letter | Oxy frame-positive airway PPE frame combined with shield |
| (9) The Role of Additive Manufacturing and Antimicrobial Polymers in the COVID-19 Pandemic51 | Zuniga | April 30, 2020 | America | Editorial | Antimicrobial face masks (copper impregnated); splitters |
| (10) 3D-Printing to Address COVID-19 Testing Supply Shortages52 | Cox | May 9, 2020 | America | Report | Nasopharyngeal swabs |
| (11) Challenges and Solutions in Meeting up the Urgent Requirement of Ventilators for COVID-19 Patients53 | Iyengar | May 5, 2020 | United Kingdom | Narrative review | Ventilation/mechanical bagger |
| (12) Custom-Made 3D-Printed Face Masks in Case of Pandemic Crisis Situations with a Lack of Commercially Available FFP2/3 Masks54 | Swennen | April 2, 2020 | Belgium | Proof of concept | Reusable custom-made face mask |
| (13) Open Science Takes on the Coronavirus Pandemic55 | Zastrow | April 24, 2020 | South Korea | Feature | Face shield; nasopharyngeal swabs |
COVID-19, coronavirus; PPE, personal protective equipment.
Table 3.
Summary of Media Articles (Nexis Search)
| Categories | Subcategories | § | % of Total |
|---|---|---|---|
| Adjuncts for ventilation | |||
| Not specific | 1 | 0.2 | |
| Valves for ventilators | 1 | 0.2 | |
| Venturi valves | 19 | 4.1 | |
| Breathing circuits | 1 | 0.2 | |
| Breathing simulators | 3 | 0.7 | |
| Oxygen hoods | 1 | 0.2 | |
| Splitters | 2 | 0.4 | |
| Ventilation | |||
| Ventilators/mechanical baggers | 16 | 3.5 | |
| Noninvasive ventilation—snorkel mask CPAP | 30 | 6.5 | |
| Ventilator parts | 10 | 2.2 | |
| Unspecified | 2 | 0.4 | |
| Patient care/infection control | |||
| Copper handles | 2 | 0.4 | |
| Door handles | 7 | 1.5 | |
| Key | 1 | 0.2 | |
| Swabs | 6 | 1.3 | |
| 4D printing for drug targeting | 1 | 0.2 | |
| Quarantine hospital wards | 1 | 0.2 | |
| Copper-printed surfaces | 1 | 0.2 | |
| PPE | |||
| Face mask | 33 | 7.2 | |
| Mask ear saver | 5 | 1.1 | |
| Face shields | 273 | 59.3 | |
| Not specific | 11 | 5 | |
| Mask-snorkel adapted with filter | 3 | 0.7 | |
| Goggles | 4 | 0.9 | |
| Other | |||
| Not specific | 26 | 5.7 | |
| Total numbers of news articles | 460 | ||
§, number of news articles; 4D, four-dimensional; CPAP, continuous positive airway pressure.
Within the media search performed, the focus pertained to the 3D manufacturing of PPE, with 77% (355/460) of articles. Specifically, within PPE, face shields comprised the largest subcategory of additive manufacturing with 59% of the total number of articles (273/460), followed by face masks 7.2% (33/460). The ventilation category comprised 12.6% (58/460) of the total number, specifically manufacturing of connectors for scuba mask adaptation for noninvasive ventilation 6.5% (30/460), and 3.5% (16/460) for ventilators. Adjuncts for ventilation comprised 6% with Venturi valves 4.1% being the mainstay of focus within the media. The systematic review gives a broader overview of the published literature on role of additive manufacturing during the pandemic, summarized in Table 2.
Discussion
3D printed solutions for the COVID-19 response
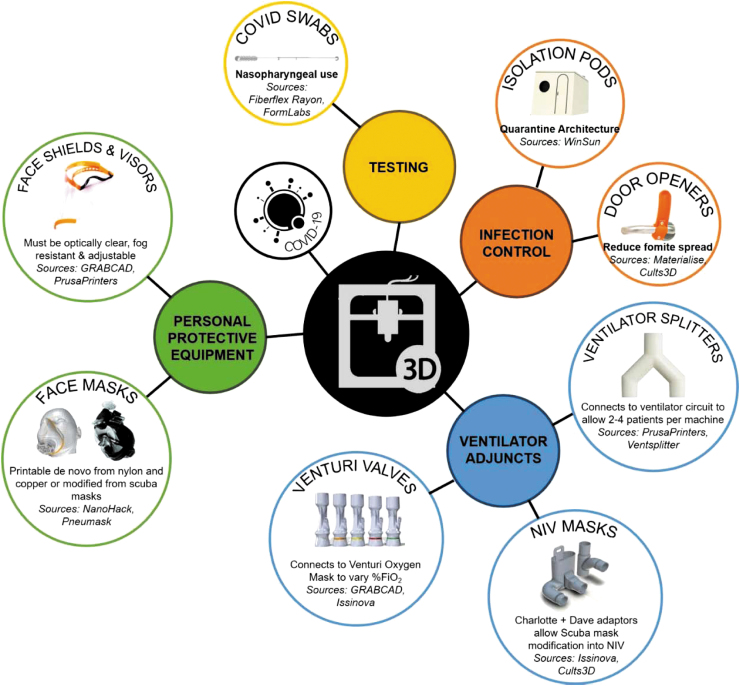
Through its on-demand fabrication capability, 3D printing is able to respond to local emergent needs and therefore ideally placed to bridge supply chain gaps in essential equipment.15 Here, we highlight the findings from our systematic review and explore the impact of 3D printing in augmenting (1) PPE (77.2%), (2) adjuncts for ventilation, (6%) (3) ventilation assistance (portions of emergency respiratory devices) (12.6%), and (4) patient care/infection control (4.1%) during the COVID-19 pandemic (Fig. 2).
FIG. 2.
Diagrammatic summary of search results relating to 3D printing applications during the COVID-19 pandemic. COVID-19, coronavirus.
Personal protective equipment
Face shields/visors
An area that has been highly publicized through media campaigns and crowdfunding is the production of face shields, which during the height of the pandemic was a necessity for most patient interactions, as outlined by Public Health England and WHO.16 Production has ranged from homemade shields utilizing open sources files, engineering departments in large universities, to established 3D printing manufacturers. These products can be mass produced to need, with the potential for subtle modifications to address specific indications.
Currently, the vast majority of manufactured devices are not CE marked, which is the European safety standard, and for this reason, they cannot be sold. In light of the legislation, it remains the responsibility of the user to decide whether they use 3D printed face shields. Such items may have bypassed the rigorous quality assurance tests that occur in ordinary circumstances. In such extraordinary circumstances, this pandemic has portrayed the need to circumvent the “red tape” restrictions to maximize patient and practitioner safety, meanwhile accepting that protective devices of the highest quality should nonetheless be pursued and produced where possible.
Respirator masks
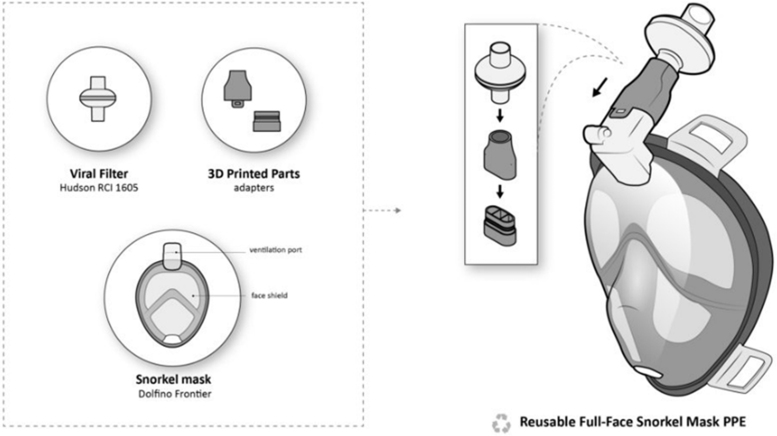
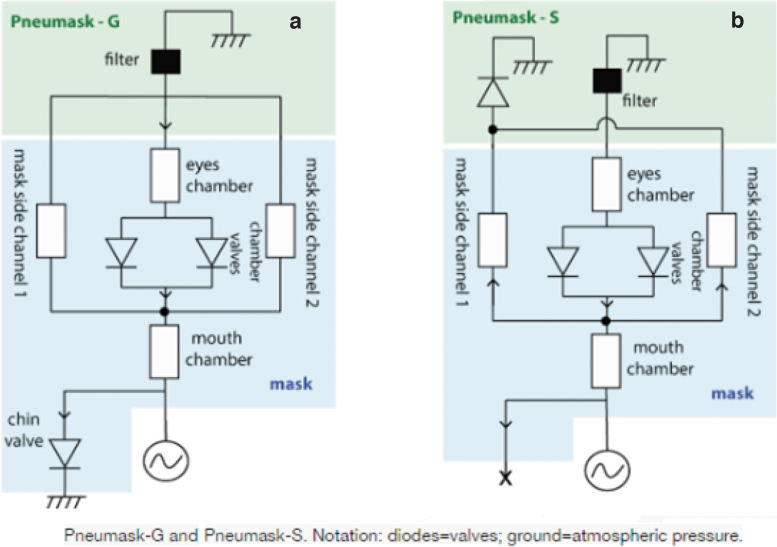
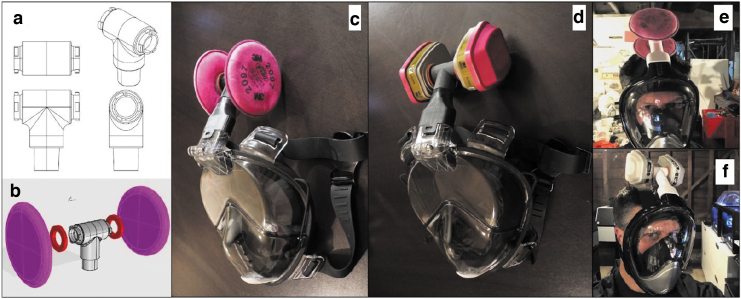
As the number of cases has become more prevalent, the demand for N95/FFP2/FFP3 masks has increased sharply, leaving health care workers on the frontline having to compromise safety by using suboptimal masks or even reusing surgical masks: a practice that has been shown to be potentially counter-effective if the masks remain moist.5 A good example of a successful “repurposing approach” relates to freely available snorkel masks,17 which have been adapted via free online tutorials to incorporate either a medical-grade or an industrial-grade filter via a 3D printed coupler. The Pneumask, designed by the Prakash Lab at Stanford University,18 illustrated in Figures 3–5, shows variations by which a medical-grade viral filter (i.e., Hudson RCI 1605 inline filter) can be used. A variety of industrial filters may also be fitted, should supplies of medical filter become deplete, that is, the P100 3M particulate filters.19 The mask has passed initial qualitative and quantitative fit mask testing and is sterilizable. Although this product is not FDA approved, Emergency Use Authorization (EUA) guidelines have been released, which relaxes testing if the product is correctly labeled with set criteria.20
FIG. 3.
Pneumask, a reusable full-face snorkel mask PPE, displayed 3D printed coupler and medical-grade filter (https://www.pneumask.org). PPE, personal protective equipment.
FIG. 5.
(a, b) Schematic of flow within the Pneumask (G) for general use and (S) for sterile use by occluding chin valve (www.pneumask.org).
FIG. 4.
(a–d) Illustrating varying industrial-grade filters jointed onto the same 3D printed coupler (https://www.pneumask.org). (e, f) Demonstrate Pneumask being worn.
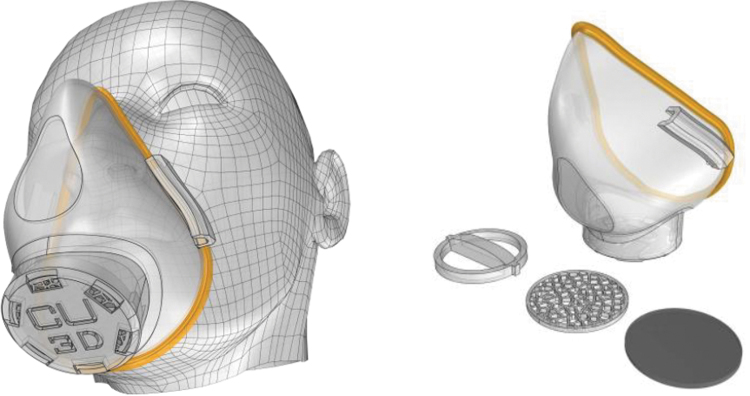
The “Stopgap” nylon face mask produced by the Veterans Health Administration has been published on the National Institute of Health 3D print exchange site,21 an online repository for bioscientific research and medicine, permitting open access to the STL files. The mask consists of two printed components: the main body of the mask and the filter cover, both are reusable and can be disinfected. The elastic strap and filter are single use and changed between applications. The product specification suggests the use of a high fluid resistant surgical mask to make four filters (Fig. 6) and was designed out of necessity to be used in the absence of fully approved products.
FIG. 6.
Stopgap face mask (https://3dprint.nih.gov/discover/3dpx-013429).
The most commonly used polymer for 3D printing is polylactic acid, derived directly from renewable resources.22 However, in light of the requirements of PPE to be sterilized, worn, and reused, other printable materials have been used including Nylon PA 2200: a robust durable and strong plastic, based on polyamide 12, with suitable chemical resistance for cleaning and sterilization.23 The addition of copper nanoparticles to conventional polymers used in 3D printing may have promising applications in producing antiviral consumables for clinical use.24,25 Although not inherently antimicrobial, polymers can be combined with copper nanoparticles to facilitate the adsorption of microorganisms. The copper ions and associated hydroxyl radicals produce DNA and RNA damage, thereby deactivating the virus.24 Nanohack 2.0 designed by Copper3D is an example of 3D printed open source mask, which attempts to incorporate the antimicrobial and antiviral effects of copper. The filtration system consists of three layers of nonwoven polypropylene embedded with nanocopper. The mask also houses a third-party filtration material (Fig. 7). The open source website clearly states that this should not be considered as PPE except for last resort situations and is not deemed equivalent to an N95 mask.
FIG. 7.
Nanohack 2.0 mask, nanocopper-embedded polypropylene and third part filtration (https://copper3d.com/hackthepandemic)
Adjuncts for ventilation
Venturi valves
The Venturi valve allows a set percentage of oxygen delivery to a patient through the Bernoulli principle. Isinova, a start-up company, was able to reverse engineer the valve quickly, in a time of life-threatening shortage, which had immediate success in a Northern Italian Hospital during their crisis. The Italian government agreed the files could be shared with health care systems across the globe in instances of proven urgency and unmet need.26 This measure was implemented with the intention of preserving the manufacturer's USP and intellectual property. Despite this, an open source replica was made available on GrabCAD27: an open source 3D printing depository. Despite allegations in media coverage,28 no legal action has been taken against the Italian start-up company.
Noninvasive ventilation masks
Following their early success with Venturi valves, Isinova went on to repurpose a commercial full-face snorkel mask as a continuous positive airway pressure mask, which were in short supply and crucial for the early management of those with COVID-19 infection. Termed “Easy COVID-19,” a Decathlon “Easybreath” mask was adapted utilizing the “Charlotte” and “Dave” valve. With the assistance of Decathlon, the CAD files were supplied, and the mask was dismantled, studied, and adapted.
Neyman and Irvin in 200629 anticipated the need to upscale the use of ventilators in disaster events and proposed the use of two flow splitters to enable a single ventilator to simultaneously ventilate up to four patients. This was a simulated in a feasibility study and was not tested on patients. Despite its limitations, this device was utilized in humans during the Las Vegas shooting in 2017 by Dr. Kevin Menes, an Emergency Physician. Multiple single ventilators were used temporarily to ventilate two patients at a time. Patients were roughly matched for size, and tidal volumes were doubled. Splitters were obtained from emergency respiration kits; however, purpose-built splitters have now been constructed by the 3D community. One example of this is the construct published by Leitat who designed the “Multivent” made from polyamide 12. This consists of one 22 mm female inlet, which gives two male outlets. To the best of our knowledge, this has not been used thus far in the COVID-19 crisis.
Ventilators/Mechanical Baggers
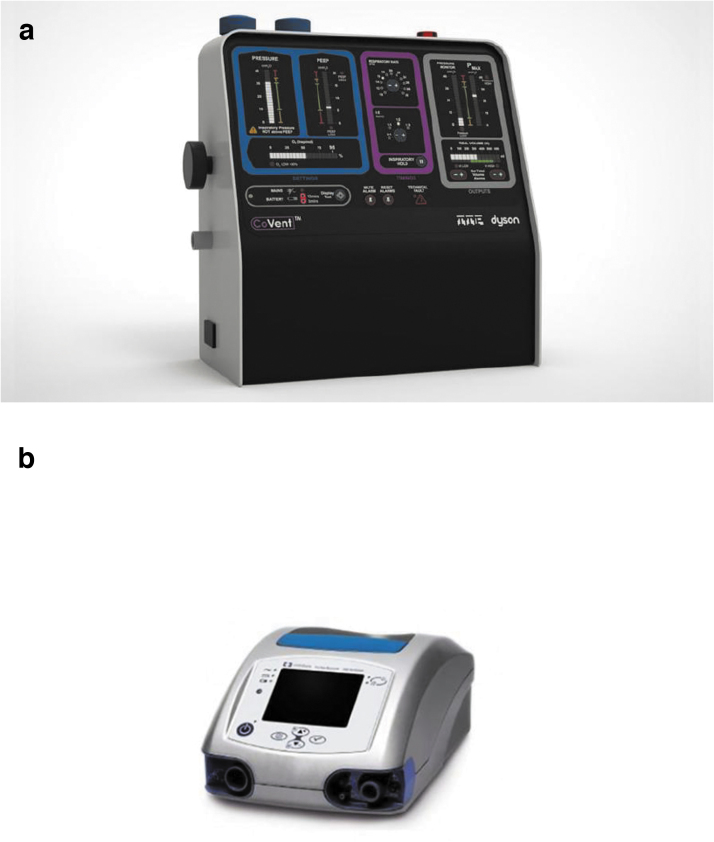
In the initial stages of the pandemic, ventilator shortages were highlighted globally. These are typically complex pieces of equipment, which can make fine adjustments to oxygen delivery based on a patient's physiology. Globally, shortages have been addressed in a multitude of methods ranging from the use of elective ventilators in theatres, short-term use of travel ventilators, to repurposing of large animal ventilators. The UK Government requested the manufacturing industry to turn their hand to construction of ventilators after the Department of Health and Social Care released a detailed specification. Such companies that have taken up this mantle include Dyson, with the construction of “Co-vent” (Fig. 8). However, such ventilators take time to manufacture. In emergency situations, a significantly less complex “ventilator/mechanical bagger” has again been developed by several institutions. An open source unofficial file has been constructed with all known emergency ventilator projects available, ranking them on their readiness for deployment.30 There are a vast number of ongoing projects, some of which are highlighted below.
FIG. 8.
(a) Dyson co-vent and (b) Medtronic Puritan Bennett 560 ventilator.
Medtronic, one of the largest medical device companies, released the design specifications for their Puritan Bennett 560 ventilator31 to enable manufacturers to rapidly reproduce the item (Fig. 8). All 3D CAD files were detailed, some of which can be 3D printed to expedite the production line. Unlike the Puritan Bennett 560, most of the other designs are based on the traditional bagging method of ventilation. Simply, an ambulatory bag provides oxygenation through a mechanical squeezing action.
Leitat announced that in a period of less than 1 week they were able to construct a medically validated and industrially reproducible 3D printed emergency bagger (Fig. 9), which has been tested on a COVID-19 patient in Parc Tauli Hospital. Their website claims that up to 100 units can be constructed in a day. The emergency bagger can provide volume-controlled ventilation with parameters, such as respiratory rate, inspiratory/expiratory ratio, tidal volumes, expiratory pressures, in-built pressure sensors, and positive end-expiratory pressure. Parameters can also be set to alarm if the patient deviates. This is not available as an open source file, and hence, the exact details regarding which portions are 3D printed are unclear currently.
FIG. 9.
Leitat 1 mechanical bagger.
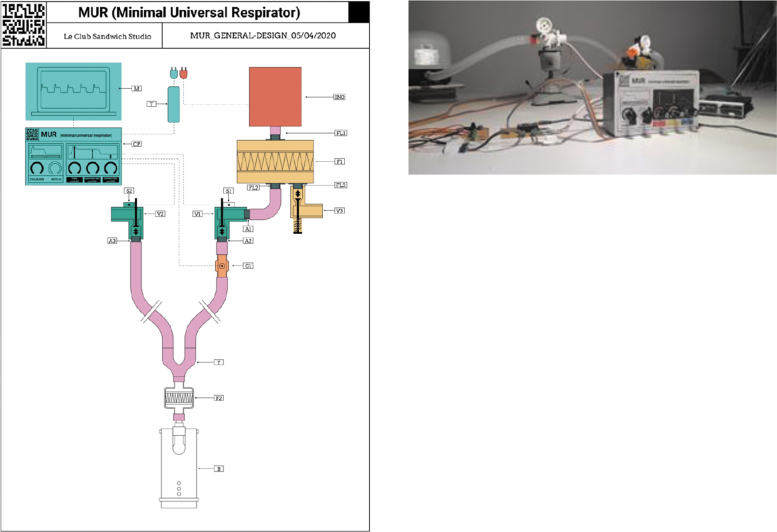
An example of a more traditional ventilator is the minimal universal respirator (M.U.R) produced by the club sandwich studio (Fig. 10). Their aim was to make a ventilator, which had a minimal number of expensive components, easily reproducible, and rapidly deployable.32 This device is still in the prototype stages; however, the website alludes to an approaching completion and testing. Two of the listed 10 components are 3D printed, both of which are valves.
FIG. 10.
Schematic of the M.U.R schematic and prototype. M.U.R, minimal universal respirator.
Patient Care/Infection Control
Hands-free door handles
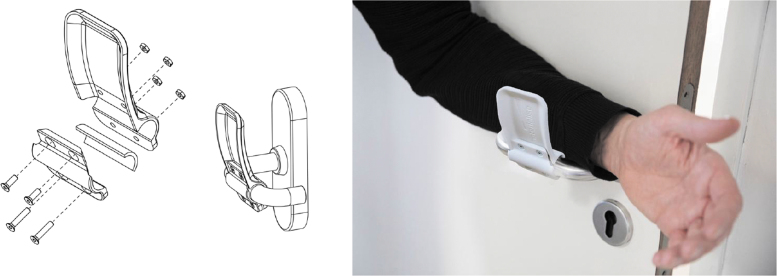
Door handles are subjected to repeated physical contact over the course of a day, especially in public spaces such as hospitals, making them a transmission hot spot via fomites and subsequent touching of the face. In response, the 3D-printable add-on to door handles has been developed by Materialise (Fig. 11). This allows users to carry out the lever action required to open most modern doors using their elbows and arms. This large 3D printing firm has manufactured a host of different handles to suit most doors; these resources are freely available to download in a host of formats.33
FIG. 11.
Left, schematic of Materialise door handles to reduce hand contact. Right, lever action to open doors hands-free.
Viral testing swabs
USF Health and Northwell have designed a 3D-printable nasal swab (Fig. 12). The protocol has been designed for emergency use only in an attempt to follow the approach that has been adopted by many of those nations with lower death tolls, such as South Korea, to test and contact trace: “Find, isolate, test and treat every case to break the chain of COVID transmission” Dr. Tedros Adhanom Ghebreyesus.
FIG. 12.
3D printed nasopharyngeal swab.
Quarantine pods
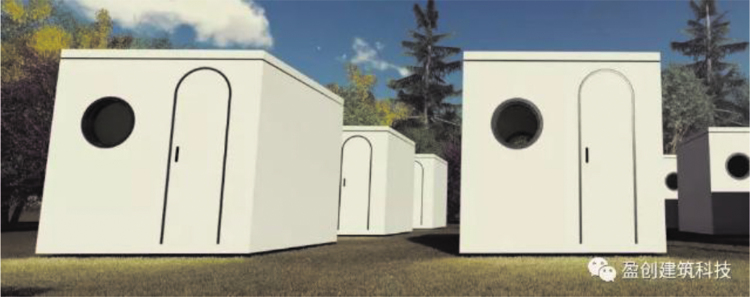
Winsun, a company based in China, represents one of the global leaders in 3D printed architecture. During the height of the pandemic, Winsun printed and constructed 15 quarantine pods to supply Xianning Central Hospital in the Hubei Province to relieve pressure on the hospital for both patients and staff. Large robotic arms mounted onto a railway tracks deposit successive layers of concrete, which sets quickly, to build quarantine pods in layers from the base up (Fig. 13).
FIG. 13.
Additive manufacturing of quarantine pods utilizing concrete.
Our Experience
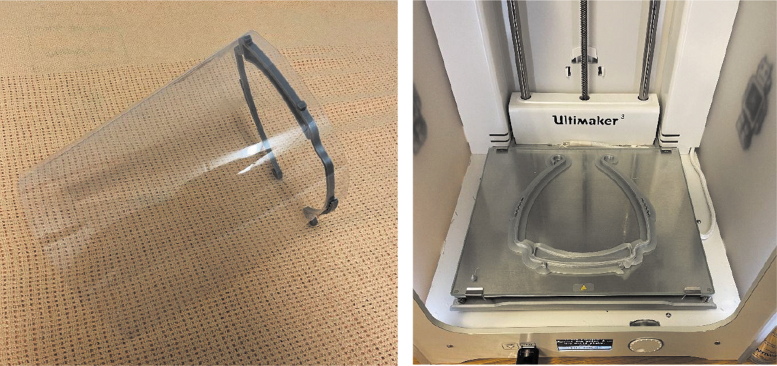
The Reconstructive Surgery and Regenerative Medicine Research Group of clinical academic researchers, based across Swansea University and Morriston Hospital, a tertiary care facility serving the population of South and West Wales, house on-site 3D printing facilities. Our experiences of having these facilities integrated within a health care environment, together with 3D printing know-how related to our bioprinting research and collaborative academic contacts through the South Wales Additive and Rapid Manufacturing (SWARM) Consortium,34 meant as products become in short supply, we were well placed to be able to implement a rapid solution. This ranged from 3D printed visors (Fig. 14) to Venturi valves (Fig. 15), made possible by the availability of open source 3D printing files and produced within hours of request. The coronavirus pandemic has highlighted the advantages to embedding technology and academia within health care settings, the symbiosis of which will hopefully outlive the span of the COVID-19 pandemic. Similar examples of 3D printing collaborations and consortia have been witnessed internationally, bringing together industry and academia to respond to emerging health care needs by producing medical equipment.35,36
FIG. 14.
In-house-made visors on the Ultimaker 3.
FIG. 15.
In-house Venturi valve.
Regulatory Hurdles
Devices made using 3D printing technology are subjected to regulatory requirements, both premarket and postmarket, in the same way as devices using other manufacturing methods.37 Recognizing the current urgency, governments have relaxed legislation around the production of both PPE and medical devices. The COVID-19 EUA38 or waivers35 for Medical Devices created single point-of-entry websites for innovators and product developers to submit descriptions of their technologies39 to shorten the time to apply for product licensure. It must be recognized, however, that many of these devices will not have encountered the rigorous testing that underpin routine practice in normal circumstances. Hence, medical disclaimers are a common feature. Medicolegally, this is a gray area, and the Medicine and Healthcare products Regulatory Agency (MHRA) released instructions regarding manufacturing during the pandemic; it also published the following: “In this exceptional situation, MHRA may authorise you to supply a non-CE marked device in the interest of the protection of health.” Every effort should be taken to mitigate risk such as on the National Institute of Health 3D print exchange.
Conclusions
The advent of 3D printing can be traced back as early as the 1980s with the use of additive manufacturing with photopolymers to build a solid model in layers. However, never has the impact of this emerging technology been more evident than during this time of crisis of the COVID-19 pandemic. When we look back on the current health crisis, there is no doubt that we will recognize this as a time of innovation, through the culmination of different industries for a common good. This skill set has ranged from enthusiasts to universities, tech firms, and industrial companies rising to the challenge of designing, replicating, enhancing, and innovating some of the most in-demand products in a time of need. This review has identified that 3D printing technology has made the biggest contribution to the production of PPE and garnered significant media interest as demonstrated by 460 media articles in the study period. This is likely a product of the overwhelming shortages of PPE faced internationally at the advent of the outbreak and the relative ease and accessibility of producing 3D printed alternatives. This was especially evident with the production of face shields, followed by ventilators, ventilatory adjuncts, and nasopharyngeal swabs: mirroring the areas of greatest need. As with the wartime efforts, a world in crisis inspires revolution, and 3D printing has played a pivotal role in aligning the disciplines of engineering, science, and medicine. We have seen a reconfiguration of traditional supply chains through necessity, advancements in additive manufacturing techniques, and the advent of antimicrobial polymers to allow the production of customizable medical devices with inherent antiviral properties,40 pushing the boundaries of innovation. A myriad of open source files are now available online for any individual with a 3D printer to replicate much needed items in a relatively short period of time, coined the “citizen supply chain,” personally funded or through crowdfunding.41,42 Ultimately, this is unlikely to be our final pandemic: lessons must be learned, adaptations must be implemented, and opportunities for multidisciplinary cross-collaboration must be harnessed. 3D printing has proven its versatility in responding to this global pandemic and warrants further exploration into how its role may be applied to routine medical practice in addition to disaster management.
Acknowledgments
3D printing and Coronavirus. Rhys Whelan. (May 1, 2020). Abertawe/Swansea, United Kingdom: Bwrdd Iechyd Prifysgol Bae Abertawe Library Services.
Appendix
Appendix Table A1.
Medline Search Criteria
| Results | ||
|---|---|---|
| (0) | Medline | 0 |
| (1) | exp *betacoronavirus/or exp *Coronavirus infection/ | 12,748 |
| (2) | ((corona* or corono*) adj1 (virus* or viral* or virinae*)).ti,ab. | 527 |
| (3) | ((novel or new or nouveau or “2019”) adj2 (coronavirus* or “corona virus*” or coronovirus* or coronavirinae*)).ti,ab. | 3180 |
| (4) | (Wuhan* or Hubei* or Huanan or “2019-nCoV” or 2019 nCoV or nCoV2019 or “nCoV-2019” or “COVID-19” or COVID19 or “CORVID-19” or CORVID19 or “WN-CoV” or WNCoV or “HCoV-19” or HCoV19 or CoV or “2019 novel*” or Ncov or “n-cov” or “SARS-CoV-2” or “SARSCoV-2” or “SARSCoV2” or “SARS-CoV2” or SARSCov19 or “SARS-Cov19” or “SARSCov-19” or “SARS-Cov-19” or Ncovor or Ncorona* or Ncorono* or NcovWuhan* or NcovHubei* or NcovChina* or NcovChinese*).ti,ab. | 16,134 |
| (5) | ((“seafood market*” or “food market*”) adj10 (Wuhan* or Hubei* or China* or Chinese* or Huanan*)).ti,ab. | 58 |
| (6) | 1 or 2 or 3 or 4 or 5 | 25,003 |
| (7) | exp Printing, Three-Dimensional/ | 5007 |
| (8) | “Three-Dimensional Printing”.ti,ab. | 1215 |
| (9) | “Three Dimensional Printing”.ti,ab. | 1215 |
| (10) | “3 Dimensional Printing”.ti,ab. | 200 |
| (11) | “3D Printing”.ti,ab;. | 4839 |
| (12) | “3-D Printing”.ti,ab. | 217 |
| (13) | “digital manufacturing”.ti,ab. | 34 |
| (14) | “Additive Manufacturing”.ti,ab. | 1886 |
| (15) | “3D Prototyping”.ti,ab. | 20 |
| (16) | “3D Fabrication”.ti,ab. | 84 |
| (17) | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 | 9687 |
| (18) | 6 and 17 | 8 |
3D, three-dimensional; SARS-Cov-2, severe acute respiratory syndrome coronavirus 2.
Appendix Table A2.
Embase Search Criteria
| (0) | Embase | 0 |
| (1) | exp *betacoronavirus/or exp *Coronavirus infection/ | 10,493 |
| (2) | ((corona* or corono*) adj1 (virus* or viral* or virinae*)).ti,ab. | 631 |
| (3) | ((novel or new or nouveau or “2019”) adj2 (coronavirus* or “corona virus*” or coronovirus* or coronavirinae*)).ti,ab. | 2902 |
| (4) | (Wuhan* or Hubei* or Huanan or “2019-nCoV” or 2019 nCoV or nCoV2019 or “nCoV-2019” or “COVID-19” or COVID19 or “CORVID-19” or CORVID19 or “WN-CoV” or WNCoV or “HCoV-19” or HCoV19 or CoV or “2019 novel*” or Ncov or “n-cov” or “SARS-CoV-2” or “SARSCoV-2” or “SARSCoV2” or “SARS-CoV2” or SARSCov19 or “SARS-Cov19” or “SARSCov-19” or “SARS-Cov-19” or Ncovor or Ncorona* or Ncorono* or NcovWuhan* or NcovHubei* or NcovChina* or NcovChinese*).ti,ab. | 17,700 |
| (5) | ((“seafood market*” or “food market*”) adj10 (Wuhan* or Hubei* or China* or Chinese* or Huanan*)).ti,ab. | 54 |
| (6) | 1 or 2 or 3 or 4 or 5 | 23,766 |
| (7) | exp Printing, Three-Dimensional/ | 10,407 |
| (8) | “Three-Dimensional Printing”.ti,ab. | 1364 |
| (9) | “Three Dimensional Printing”.ti,ab. | 1364 |
| (10) | “3 Dimensional Printing”.ti,ab. | 230 |
| (11) | “3D Printing”.ti,ab. | 6031 |
| (12) | “3-D Printing”.ti,ab. | 280 |
| (13) | “digital manufacturing”.ti,ab. | 37 |
| (14) | “Additive Manufacturing”.ti,ab. | 1742 |
| (15) | “3D Prototyping”.ti,ab. | 27 |
| (16) | “3D Fabrication”.ti,ab. | 73 |
| (17) | 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 | 12,549 |
| (18) | 6 and 17 | 7 |
Author Disclosure Statement
No competing financial interests exist.
Funding Information
I.S.W. would like to acknowledge funding from the Scar Free Foundation, Health & Care Research Wales, and the European Association of Plastic Surgeon/The American Association of Plastic Surgeons Academic Scholarship. T.J. would like to acknowledge funding from Action Medical Research and the Vocational Training Charitable Trust Foundation (GN2782) and the British Association of Plastic, Reconstructive and Aesthetic Surgeons Paton Masser Fund. Z.M.J. would like to acknowledge funding from Medical Research Council MR/N002431/1.
References
- 1. Devi S. Travel restrictions hampering COVID-19 response. Lancet 2020;395:1331–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Shortage of personal protective equipment endangering health workers worldwide. https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide (accessed May 16, 2020).
- 3. Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jessop ZM, Dobbs TD, Ali SR, et al. . Personal protective equipment (PPE) for surgeons during COVID-19 pandemic: A systematic review of availability, usage, and rationing. Br J Surg 2020;107:1262–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelkar US, Gogate B, Kurpad S, et al. . How effective are face masks in operation theatre? A time frame analysis and recommendations. Int J Infect Control 2013;9:1–6. [Google Scholar]
- 6. The Lancet. COVID-19: Protecting health-care workers. Lancet 2020;395:922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campbell D. One in three UK surgeons lacks enough protective kit, survey finds. The Guardian.
- 8. Smyth C, Swinford S, Lay K. Coronavirus facemasks for public ‘risk NHS shortage.’ April 21, 2020. https://www.thetimes.co.uk/article/facemasks-for-public-risk-nhs-shortage-q5bkzvzql (accessed April 22, 2020).
- 9. Ferguson NM, Laydon D, Nedjati-Gilani G, et al. . Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand. London: Imperial College London, 2020; pp. 3–20. [Google Scholar]
- 10. Ranney ML, Griffeth V, Jha AK. Critical supply shortages—The need for ventilators and personal protective equipment during the COVID-19 pandemic. N Engl J Med 2020;382:e41. [DOI] [PubMed] [Google Scholar]
- 11. 3D Printing Media Network. Open source projects. https://www.3dprintingmedia.network/forums/forum/am-in-the-time-of-covid-19-forum/open-source-projects (accessed May 16, 2020).
- 12. U.S. Department of Health and Human Services—National Institutes of Health. US National Institutes of Health 3D Print Exchange. https://3dprint.nih.gov/collections/covid-19-response (accessed May 16, 2020).
- 13. COVID-19 Call to Action. 3D Systems. https://uk.3dsystems.com/covid-19-response (accessed May 16, 2020).
- 14. Popay J, Roberts H, Sowden A, et al. Developing guidance on the conduct of narrative synthesis in systematic reviews. A Product from the ESRC Methods Program. Lancaster: Institute for Health Research, Lancaster University, 2005. [Google Scholar]
- 15. US Food and Drug Administration (FDA). Coronavirus (COVID-19) supply chain update. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-supply-chain-update (accessed April 12, 2020).
- 16. UK Government. COVID-19: Infection prevention and control (IPC). https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control (accessed April 12, 2020).
- 17. Decathalon. Subea surface snorkelling mask easybreath 500. https://www.decathlon.co.uk/mask-easybreath-500-turquoise-id_8491269.html (accessed April 12, 2020).
- 18. Prakash Lab. The pneumask project. https://www.pneumask.org/ (accessed April 12, 2020).
- 19. 3M. 3M™ particulate filter 2091/07000(AAD), P100 100 https://www.3m.com/3M/en_LB/p/d/v000093499/ (accessed April 12, 2020).
- 20. US Food and Drug Administration (FDA). Emergency use authorization. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization (accessed April 12, 2020).
- 21. VHA. Stopgap surgical face mask (SFM). https://3dprint.nih.gov/discover/3dpx-013429 (accessed April 12, 2020).
- 22. Roundtable. NRC (US) CS. Commodity polymers from renewable resources: Polylactic acid. https://www.ncbi.nlm.nih.gov/books/NBK44131 (accessed April 23, 2020).
- 23. EOS. Polyamide 12 in 3D printing. https://www.eos.info/de/additive-fertigung/3d-druck-kunststoffe/polymer-material-werkstoffe/polyamid-pa-12-alumide (accessed April 12, 2020).
- 24. Palza H. Antimicrobial polymers with metal nanoparticles. Int J Mol Sci 2015;16:2099–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palza H, Nuñez M, Bastías R, et al. . In situ antimicrobial behavior of materials with copper-based additives in a hospital environment. Int J Antimicrob Agents 2018;51:912–917. [DOI] [PubMed] [Google Scholar]
- 26. Feldman A. Meet the Italian engineers 3D-printing respirator parts for free to help keep coronavirus patients aliveitle. March 19, 2020. https://www.forbes.com/sites/amyfeldman/2020/03/19/talking-with-the-italian-engineers-who-3d-printed-respirator-parts-for-hospitals-with-coronavirus-patients-for-free/#588db89278f1 (accessed April 29, 2020).
- 27. Grabcad Community. Venturi valve. https://grabcad.com/library/respirator-free-reanimation-venturi-s-valve-rev-4-1 (accessed April 12, 2020).
- 28. Kent C. Covid-19: Start-up that saved lives with 3D-printed valve may face legal action. March 18, 2020. https://www.medicaldevice-network.com/news/3d-printed-valves-covid-19-italy (accessed March 29, 2020).
- 29. Neyman G, Irvin CB. A single ventilator for multiple simulated patients to meet disaster surge. Acad Emerg Med 2006;13:1246–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Evaluation of known projects. https://github.com/PubInv/covid19-vent-list (accessed May 16, 2020).
- 31. Medtronic. Medtronic releases all CAD files and PCB schematics for PB560 ventilator manufacturing. https://www.3dprintingmedia.network/medtronic-releases-pb560-ventilator-files (accessed April 12, 2020).
- 32. Club Sandwich Studio. M.U.R minimal universal respirator. https://mur-project.org/en-home (accessed April 12, 2020).
- 33. Materialise. Hands-free 3D-printed door openers to help against the spread of coronavirus. https://www.materialise.com/en/hands-free-door-opener (accessed April 23, 2020).
- 34. Swansea University. South Wales industry consortium established to support the NHS COVID 19 response. https://www.swansea.ac.uk/press-office/news-events/news/2020/03/south-wales-industry-consortium-established-to-support-the-nhs-covid-19-response.php (accessed June 7, 2020).
- 35. CECIMO. Additive manufacturing. https://www.cecimo.eu/machine-tools/additive (accessed June 7, 2020).
- 36. Copper3D. #HackThePandemic. https://copper3d.com/hackthepandemic (accessed June 8, 2020).
- 37. Clark L. Leading the way. https://www.harvardbusiness.org/innovation-in-a-time-of-crisis (accessed June 7, 2020).
- 38. US Food and Drug Administration (FDA). Medical devices and the COVID-19 pandemic. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/medical-devices-and-covid-19-pandemic (accessed June 7, 2020).
- 39. Medical Countermeasures.gov. 2019 Novel coronavirus. https://www.medicalcountermeasures.gov/Request-BARDA-TechWatch-Meeting (accessed June 7, 2020).
- 40. González-Henríquez CM, Sarabia-Vallejos MA, Hernandez JR. Antimicrobial polymers for additive manufacturing. Int J Mol Sci 2019;20:1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. JustGiving. 3D printed face shields. https://www.justgiving.com/crowdfunding/3d-printed-face-visor-covid19 (accessed May 16, 2020).
- 42. Crowdfunder. 3D PPE prints for Covid-19—Highlands. Available from: https://www.crowdfunder.co.uk/covid-19-highlands (accessed May 16, 2020).
- 43. Wesemann C, Pieralli S, Fretwurst T, et al. . 3-D printed protective equipment during COVID-19 pandemic. Materials (Basel) 2020;13:1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cavallo L, Marcianò A, Cicciù M, et al. . 3D printing beyond dentistry during COVID 19 epidemic: A technical note for producing connectors to breathing devices. Prosthesis 2020;2:46–52. [Google Scholar]
- 45. Sapoval M, Gaultier AL, Del Giudice C, et al. . 3D-printed face protective shield in interventional radiology: Evaluation of an immediate solution in the era of COVID-19 pandemic. Diagn Interv Imaging 2020;101:413–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Larrañeta E, Dominguez-Robles J, Lamprou DA. Additive manufacturing can assist in the fight against COVID-19 and Other Pandemics and Impact on the Global Supply Chain. 3D Print Addit Manuf 2020;7:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ishack S, Lipner SR. Applications of 3D printing technology to address COVID-19 related supply shortages. Am J Med 2020;133:771–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tino R, Moore R, Antoline S, et al. . COVID-19 and the role of 3D printing in medicine. 3D Print Med 2020;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Erickson MM, Richardson ES, Hernandez NM, et al. . Helmet modification to PPE with 3D printing during the COVID-19 pandemic at Duke University Medical Center: A novel technique. J Arthroplasty 2020;35:S23–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maracaja L, Blitz D, Maracaja DLV, et al. . How 3D printing can prevent spread of Covid-19 between health care professionals during times of critical shortage of Protective Personal Equipment. J Cardiothorac Vasc Anesth 2020;34:2847–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zuniga JM, Cortes A. The role of additive manufacturing and antimicrobial polymers in the COVID-19 pandemic. Expert Rev Med Devices 2020;17:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cox JL, Koepsell SA. 3D-printing to address COVID-19 testing supply shortages. Lab Med 2020;51:e45–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iyengar K, Bahl S, Vaishya R, et al. . Challenges and solutions in meeting up the urgent requirement of ventilators for COVID-19 patients. Diabetes Metab Syndr Clin Res Rev 2020;14:499–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Swennen GRJ, Pottel L, Haers PE. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg 2020;49:673–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zastrow M. Open science takes on the coronavirus pandemic. Nature 2020;581:109–110. [DOI] [PubMed] [Google Scholar]