ABSTRACT
Objective
We aimed to explore fetal cortical brain development by neurosonography in fetuses conceived by assisted reproductive technology (ART), including frozen and fresh embryo transfer (ET), compared with those conceived spontaneously (SC), and to investigate its association with infant neurobehavior at 12 months of age.
Methods
This was a prospective cohort study of 210 singleton pregnancies, including 70 SC pregnancies, 70 conceived by in‐vitro fertilization (IVF) following frozen ET and 70 conceived by IVF after fresh ET. Fetal neurosonography was performed at 32 ± 2 gestational weeks to assess cortical development. Sulci depths were measured offline and normalized by biparietal diameter (BPD). Ages and Stages Questionnaires (ASQ) were completed postnatally, at 12 ± 1 months of corrected age. Neurosonographic findings were adjusted by regression analysis for maternal age, ethnicity, parity, fetal sex and fetal‐weight centile and gestational age at scan, and ASQ scores were adjusted for maternal age, ethnicity, parity, educational level and employment status, gestational age at birth, breastfeeding, infant sex and infant age at the ASQ evaluation.
Results
Overall, in comparison to the SC fetuses, fetuses conceived by ART showed statistically significant differences in cortical development, with reduced parieto‐occipital sulci depth adjusted for BPD (mean ± SD: fresh ET, 12.5 ± 2.5 vs frozen ET, 13.4 ± 2.6 vs SC, 13.4 ± 2.6, P < 0.001), cingulate sulci depth adjusted for BPD (median (interquartile range (IQR)): fresh ET, 5.8 (4.2–7.4) vs frozen ET, 5.8 (4.1–7.5) vs SC, 6.5 (4.8–7.8), P = 0.001) and calcarine sulci depth adjusted for BPD (median (IQR): fresh ET, 13.5 (10.1–16.1) vs frozen ET, 14.5 (12.1–15.8) vs SC, 16.4 (14.3–17.9), P < 0.001), together with lower Sylvian fissure grading score. Changes in cortical development were more pronounced in the fresh ET than in the frozen ET group. ART infants showed lower ASQ scores as compared to SC infants, particularly in the fresh ET group (mean ± SD global ASQ Z‐score: fresh ET, –0.3 ± 0.4 vs frozen ET, –0.2 ± 0.4 vs SC, 0 ± 0.4, P < 0.001).
Conclusions
Fetuses conceived by ART show a distinctive pattern of cortical development and suboptimal infant neurodevelopment, with more pronounced changes in those conceived following fresh ET. These findings support the existence of in‐utero brain reorganization associated with ART and warrant follow‐up studies to assess its long‐term persistence. © 2022 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: ART, cortical folding, fetal brain, in‐vitro fertilization, IVF, mode of conception, neurodevelopment, neurosonography, prenatal imaging
Short abstract
This article's abstract has been translated into Spanish and Chinese. Follow the links from the abstract to view the translations.
RESUMEN
Neurosonografía fetal y neurocomportamiento infantil tras la concepción por tecnología de reproducción asistida con transferencia de embriones frescos o congelados
Objetivo El objetivo del estudio fue explorar el desarrollo cortical del cerebro fetal mediante neurosonografía en fetos concebidos mediante tecnología de reproducción asistida (TRA), incluyendo la transferencia de embriones (TE) congelados y frescos, en comparación con los concebidos espontáneamente (CE), e investigar su asociación con el neurocomportamiento infantil a los 12 meses de edad
Métodos La investigación fue un estudio de cohorte prospectivo de 210 embarazos con feto único, entre ellos 70 embarazos CE, 70 concebidos in vitro (FIV) tras una TE congelados y 70 concebidos por FIV tras una TE frescos. La neurosonografía fetal se realizó a las 32±2 semanas de gestación para evaluar el desarrollo cortical. La profundidad de las cisuras se midió fuera de línea y se normalizó mediante el diámetro biparietal (DBP). Postnatalmente se completaron Cuestionarios de Edades y Etapas (CEE) a los 12±1meses de edad corregida. Los hallazgos neurosonográficos se ajustaron mediante un análisis de regresión en función de la edad materna, la etnia, la paridad, el sexo del feto y el percentil de peso fetal y la edad gestacional en el momento de la exploración, y las puntuaciones del CEE se ajustaron en función de la edad materna, la etnia, la paridad, el nivel educativo y la situación laboral, la edad gestacional en el momento del nacimiento, la lactancia materna, el sexo del bebé y la edad del bebé en el momento de la evaluación del CEE.
Resultados En general, en comparación con los fetos de CE, los fetos concebidos por TRA mostraron diferencias estadísticamente significativas en el desarrollo cortical, con una menor profundidad de las cisuras parieto‐occipitales ajustada al DBP (media±SD: TE frescos, 12,5±2,5 frente a TE congelados, 13,4±2,6 frente a CE, 13,4±2,6, P<0,001), la profundidad de las cisuras cinguladas ajustada al DBP (mediana [rango intercuartil (RIQ)]: TE frescos, 5,8 (4,2–7,4) frente a TE congelados, 5,8 (4,1–7,5) frente a CE, 6,5 (4,8–7,8), P=0,001) y la profundidad de los cisuras calcarinas ajustada al DBP (mediana (RQI): TE frescos, 13,5 (10,1–16,1) frente a TE congelados, 14,5 (12,1–15,8) frente a EC, 16,4 (14,3–17,9), P<0,001), junto con una menor puntuación de la cisura de Silvio. Los cambios en el desarrollo cortical fueron más pronunciados en el grupo de TE frescos que en el de congelados. Los lactantes concebidos mediante TRA mostraron puntuaciones en los CEE más bajas que los lactantes CE, en particular en el grupo de TE frescos (media±DT de la puntuación estándar global del CEE: TE frescos, ‐0,3±0,4 frente a TE congelados, ‐0,2±0,4 frente a CE, 0±0,4, P<0,001).
Conclusiones Los fetos concebidos mediante TRA muestran un patrón distintivo de desarrollo cortical y un neurodesarrollo infantil subóptimo, con cambios más pronunciados en los concebidos tras una TE frescos. Estos resultados apoyan la existencia de una reorganización cerebral en útero asociada con la TRA y justifican la realización de estudios de seguimiento para evaluar su persistencia a largo plazo.
摘要
通过辅助生殖技术采用新鲜或冷冻胚胎移植受孕后的胎儿神经声像图和婴儿神经行为
目的 本研究旨在通过神经声像图探讨通过辅助生殖技术(ART),包括冷冻和新鲜胚胎移植(ET)受孕的胎儿与自然受孕(SC)的胎儿相比大脑皮质的发育情况,并研究其与12月龄婴儿神经行为的关系。
方法 本研究为针对210例单胎妊娠的前瞻性队列研究,包括70例自然受孕、70例冷冻胚胎移植后体外受精(IVF)受孕和70例新鲜胚胎移植后体外受精受孕。在32±2孕周时进行了胎儿神经声像图检查,以评估大脑皮质的发育情况。脑沟采用离线测量,并根据双顶径(BPD)进行标准化。年龄和阶段调查表(ASQ)在产后12±1个月的矫正月龄完成。通过回归分析,根据母亲年龄、种族、胎次、胎儿性别和胎儿体重百分位数以及扫描时的胎龄对神经声像结果进行了调整,ASQ评分则根据母亲年龄、种族、胎次、教育水平和就业状况、出生时胎龄、母乳喂养、婴儿性别和ASQ评估时的婴儿年龄进行了调整。
结果 总体而言,与自然受孕胎儿相比,辅助生殖技术受孕的胎儿在皮质发育方面显示出显著的统计学差异,根据双顶径调整后的顶枕沟深度减少(平均值±SD:新鲜ET,12.5±2.5 vs 冷冻ET,13.4±2.6 vs SC,13.4±2.6,P<0.001),根据双顶径调整后的扣带沟深度减少(中位数(四分位数范围(IQR)):新鲜ET,5.8 (4.2‐7.4) vs 冷冻ET, 5.8 (4.1‐7.5) vs SC, 6.5 (4.8‐7.8), P=0.001)和根据双顶径调整后的距状沟深度减少(中位数(IQR):新鲜ET, 13.5 (10.1‐16.1) vs 冷冻ET, 14.5 (12.1‐15.8) vs SC, 16.4 (14.3‐17.9) , P<0.001),以及较低的外侧裂分级评分。新鲜ET组的皮质发育变化比冷冻ET组更明显。与SC婴儿相比,ART婴儿的ASQ评分较低,特别是在新鲜ET组(平均±SD全局ASQ Z评分:新鲜ET,‐0.3±0.4 vs 冷冻ET,‐0.2±0.4 vs SC,0±0.4,P<0.001)。
结论 通过ART受孕的胎儿表现出独特的皮质发育模式和不够理想的婴儿神经发育,在新鲜ET后受孕的胎儿中变化更为明显。这些研究结果支持存在与ART相关的子宫内脑功能重组,并需要进行后续研究以评估其长期的持久性。
CONTRIBUTION —
What are the novel findings of this work?
Comparison of brain cortical folding in fetuses conceived by assisted reproductive technology (ART) and fetuses conceived spontaneously showed distinct differences, with reduced sulci depth and lower Sylvian fissure grading score in ART fetuses, and more pronounced changes in the fetuses conceived by ART using fresh embryo transfer (ET) than those conceived using frozen ET. ART infants also showed lower Ages and Stages Questionnaire scores, especially in the fresh ET group.
What are the clinical implications of this work?
Neurosonography is an appropriate tool to identify subtle brain differences among fetuses conceived by ART. These findings suggest the existence of in‐utero brain reorganization associated with ART, and support neurodevelopmental follow‐up in offspring conceived by ART.
INTRODUCTION
The number of pregnancies conceived by assisted reproductive technologies (ART) is increasing worldwide 1 . There is a growing interest in the neurodevelopment of people conceived by ART, and this has been studied mostly in children and adolescents. Some follow‐up studies have suggested suboptimal neurodevelopment in ART offspring compared with the general population, although there are inconsistencies in the literature 2 , 3 , 4 . Fresh and frozen embryo transfer (ET) in in‐vitro fertilization (IVF) cycles show different perinatal risk profiles 5 , 6 , 7 , and some registry‐based studies have revealed poorer neurologic results for those conceived using frozen compared with fresh ET in singleton pregnancies 8 . This inconsistency between previous studies reporting postnatal neurodevelopment in ART offspring might be explained partially by the influence of socioeconomic and educational levels during childhood and adolescence that limit direct comparisons with spontaneously conceived (SC) offspring.
Prenatal neurosonography enables accurate evaluation of fetal brain cortical folding, a surrogate marker of brain maturation, prior to any influence of postnatal factors. Interestingly, only two previous studies have explored the central nervous system in fetuses conceived by ART vs SC, with contradictory results, reporting differences between these groups in first‐trimester brain volumes 9 and no differences during the second trimester 10 . However, no study has evaluated fetal brain cortical development in the third trimester of pregnancy, which would be the optimal time period in which to study prenatal brain maturation.
We aimed to explore fetal cortical brain development by neurosonography in fetuses conceived by ART, including frozen and fresh ET, compared with SC fetuses, and to investigate its association with infant neurobehavior at 12 months of age.
SUBJECTS AND METHODS
Study populations and protocol
We conducted a prospective cohort study of 210 singleton pregnancies between 2017 and 2020, including 70 SC pregnancies and 140 conceived by IVF after frozen ET (n = 70) or fresh ET (n = 70). All ART pregnancies were recruited from a single center during the first trimester (Assisted Reproduction Unit, Hospital Clínic de Barcelona), ensuring homogeneity among the study participants with respect to ovarian stimulation and endometrial preparation protocols, laboratory procedures and embryo culture conditions. In addition, a group of SC pregnancies was recruited during the third trimester from fertile couples (with a time‐to‐pregnancy of no longer than 12 months) who were attending the BCNatal Barcelona Center for Maternal Fetal and Neonatal Medicine (Hospital Clínic and Hospital Sant Joan de Déu). Enrolment of the SC population started 11 months after the beginning of recruitment of the ART participants, and each SC pregnancy was matched to both a fresh and a frozen ET pregnancy by maternal age (± 1 year) and gestational age at neurosonography (± 1 week). Pregnancies conceived after oocyte‐donation cycles were not eligible for inclusion. Intrauterine infection, fetal malformation or chromosomal anomaly were considered exclusion criteria. Figure 1 summarizes the study population.
Figure 1.
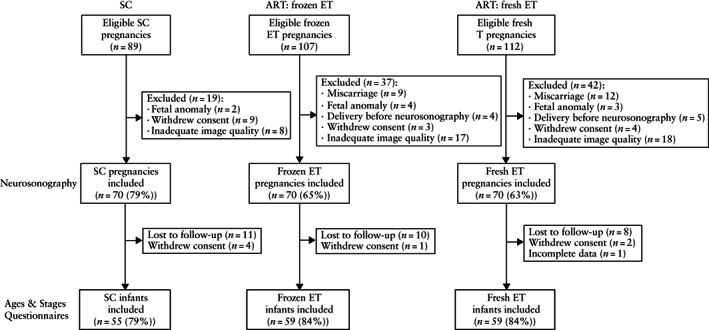
Flow diagram summarizing the study populations of pregnancies conceived spontaneously (SC) or using assisted reproductive technology (ART) with fresh or frozen embryo transfer (ET).
Maternal demographics, ART‐related variables, obstetric variables and, following delivery, perinatal outcomes, were collected directly by patient interview and by review of medical records. Gestational age was calculated according to crown–rump length (CRL) measurement at first‐trimester ultrasound examination 11 . Small‐for‐gestational age was defined as birth weight < 10th centile and large‐for‐gestational age as birth weight > 90th centile 12 , according to local standards 13 . Preterm birth was defined as delivery prior to the 37th week of gestation. Pre‐eclampsia was defined by new‐onset hypertension (≥ 140 mmHg systolic blood pressure and/or ≥ 90 mmHg diastolic blood pressure, on two occasions at least 4 h apart, after 20 weeks' gestation) together with proteinuria (≥ 300 mg protein or protein/creatinine ratio ≥ 0.3 in 24‐hour urine sample) or, in the absence of proteinuria, new onset of maternal thrombocytopenia, renal insufficiency, liver dysfunction, pulmonary edema or neurological features 14 . Gestational diabetes was defined as glucose intolerance with either onset or first recognition during pregnancy, and was diagnosed by means of a pathologic oral glucose tolerance test (usually indicated after determination of an altered fasting glucose or an altered glucose challenge test from the second trimester onwards, according to the National Diabetes Data Group (NDDG) guidelines 15 . Major neonatal morbidity was defined by the presence of at least one of the following: bronchopulmonary dysplasia, necrotizing enterocolitis, intraventricular hemorrhage, periventricular leukomalacia, retinopathy, persistent ductus arteriosus and sepsis. Minor neonatal morbidity was defined by the presence of at least one of the following: respiratory distress, hyperbilirubinemia and anemia. Perinatal mortality was defined by either intrauterine fetal death after 22 weeks of pregnancy or neonatal death within the first 28 days of age.
The study protocol included performance of fetal neurosonography in the third trimester and completion of Ages and Stages Questionnaires 16 (ASQ) at 12 months of corrected age. This study was conducted according to the Declaration of Helsinki for Medical Research involving Human Subjects 17 ; the study protocol was evaluated and approved by the local ethics committee (HCB/2017/0714) and all participants provided their written informed consent.
ART protocols
Ovarian stimulation
The ovarian stimulation protocol for IVF and the gonadotropin doses were chosen according to the woman's age and ovarian reserve markers. Long agonist or antagonist protocols were used. Ovarian stimulation was achieved with daily doses of 150–300 IU recombinant follicle stimulating hormone (FSHr) (Gonal‐F®; Merck‐Serono S.A., Madrid, Spain), alone or with the addition of 75 IU recombinant luteinizing hormone (LHr) (Luveris®; Merck‐Serono S.A.) or human menopausal gonadotropin (HMG) (Menopur®; Ferring SA, Madrid, Spain). Administration of human chorionic gonadotropin (hCG) (Ovitrelle®; 250 mg s.c., Merck‐Serono S.A.) was indicated in the presence of two or more follicles ≥ 18 mm in diameter, with four or more follicles measuring ≥ 14 mm in association with a consistent rise in serum estradiol concentration. Ultrasound‐guided transvaginal oocyte retrieval was performed 36 h after hCG administration.
ART laboratory procedures
After fertilization by IVF or intracytoplasmic sperm injection (ICSI), embryo culture was carried out in microdrops of Global Media (LifeGlobal, CooperSurgical, Målov, Denmark) under mineral oil at 37°C in an atmosphere of 6.5% carbon dioxide (CO2) and 7% oxygen (O2). Embryo quality was assessed according to the Asociación Española para el estudio de la Biología Reproductiva (ASEBIR) criteria 18 . The quality of blastocysts was assessed according to the criteria of Gardner et al. 19 .
Vitrification and warming protocols of both cleavage embryos and blastocysts were performed using commercially available kits (Kitazato, Tokyo, Japan) according to the method described by Kuwayama 20 . After warming, embryos were cultured in Global Media containing 10% protein substitute supplement (LifeGlobal) until ET. Cleavage embryos with at least 50% of their cells intact immediately after warming and further development after a 24‐h culture period were considered as surviving embryos and transferred. Survival of blastocysts was defined by their re‐expanding or starting to re‐expand within 2 h after warming.
In cases undergoing preimplantation genetic testing for monogenic defects, embryos underwent biopsy on day 3 and unaffected embryos were transferred or cryopreserved 2 days later at blastocyst stage. In those undergoing preimplantation genetic testing for aneuploidies, trophectoderm biopsy at blastocyst stage and subsequent vitrification was performed.
ET protocols
In pregnancies undergoing IVF with fresh ET, vaginal natural progesterone was started the morning after oocyte retrieval (200 mg per 8 h). Cleavage embryos were transferred on day 3 and blastocysts on day 5.
Frozen ET was performed either during the woman's natural cycle or using an endometrial preparation protocol. The choice of natural cycle depended on the regularity of the patient's menstrual cycle and their preference. For frozen ET with natural cycle, ultrasound surveillance was started on day 8–9 of the cycle (depending on the cycle duration) and, once the dominant follicle reached a mean diameter of 17–18 mm, daily follow‐up was carried out until its disappearance, that day then being defined as day 0. Frozen ET with endometrial preparation was achieved using transdermal estrogen (Evopad 50 µg, three patches replaced every 72 h (Janssen, Toledo, Spain)) or oral estradiol valerate (Progynova, 2 mg every 8 h (Bayer, Barcelona, Spain)). Estrogen was started on the first day of the cycle and ultrasound monitoring was performed after 12–15 days of treatment. Vaginal natural progesterone (200 mg every 8 h (Progeffik®, Effik, Alcobendas, Spain or Utrogestan®, SEID, Barcelona, Spain)) was added when endometrial thickness was ≥ 7 mm on ultrasound. The first day of progesterone treatment was considered day 0. Cleavage embryos were thawed on day 3 and transferred on day 4, and blastocyst embryos were thawed and transferred on day 5. Supplementation with estrogens and progesterone was performed until the 12th week of pregnancy.
Pregnancy was diagnosed by a positive serum β‐hCG test 12 days after ET and transvaginal ultrasound examination was performed in all pregnancies at 5–6 weeks of gestation.
Fetal neurosonography
Neurosonographic acquisition
A detailed two‐dimensional neurosonographic examination was performed in all fetuses during the third trimester (32 ± 2 weeks), using a Voluson 730 Expert (GE Healthcare, Zipf, Austria) ultrasound machine. Structural brain normality was confirmed using a standardized protocol, following the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) guidelines 21 . This included axial planes (transventricular and transthalamic), obtained by a transabdominal approach, and coronal planes (transthalamic, transcaudate and transcerebellar), obtained by a transvaginal approach in fetuses with cephalic presentation and transabdominally in fetuses with breech presentation. We excluded patients with image quality that was insufficient for delineation of measurements (mainly due to fetal presentation or patient intolerance of the transvaginal approach, but also because of the presence of severe endometriosis, previous abdominopelvic surgery, high maternal body mass index or placenta previa).
Image processing, linear measurements and assessment of cortical folding
Measurements were performed offline, using OsiriX MD 12.0 imaging software (Pixmeo SARL, Geneva, Switzerland), by a single experienced examiner, who was blinded to the study group. To provide rigorous measurements that were perpendicular to the midline, a straight line lying along the interhemispheric fissure was traced in every plane, from frontal to occipital bone in axial views and from cranial to caudal bone in coronal views. Brain structures were measured according to previous studies 22 , 23 , 24 , 25 . Briefly, fissures and sulci depths were measured in millimeters (Figure 2) and values were corrected by dividing by the biparietal diameter and multiplying by 100 to normalize them according to head size 22 , 23 . Parieto‐occipital sulcus depth was evaluated in a plane slightly cranial to the transventricular plane, where the full depth or triangle shape of the sulcus could be visualized, drawing a perpendicular line from the midline to the apex of the sulcus 23 . Cingulate sulcus depth was measured in the coronal transthalamic plane, drawing a perpendicular line from the midline to the apex of the sulcus. Calcarine sulcus depth was measured in the coronal view, using the transcerebellar plane, drawing a perpendicular line from the midline to the apex of the sulcus. All sulci measurements were performed excluding the cortex and only the side distal to the transducer was measured.
Figure 2.
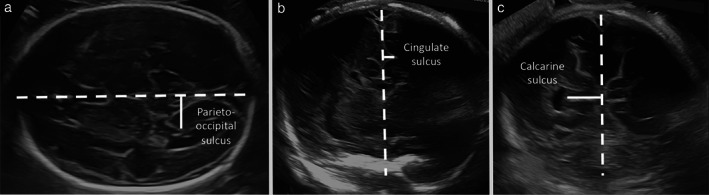
Ultrasound images demonstrating fetal neurosonographic measurements (solid lines): (a) axial transthalamic plane showing measurement of parieto‐occipital sulcus depth; (b) coronal transthalamic plane showing measurement of cingulate sulcus; and (c) coronal transcerebellar plane showing measurement of calcarine sulcus depth. Dashed line corresponds to the interhemispheric fissure.
The degree of cortical development of the Sylvian fissure and parieto‐occipital, cingulate and calcarine sulci was evaluated according to methodology described previously 26 , assigning grading scores on a scale from 0 (no maturation) to 5 (maximum maturation).
For the sulci depth measurements, an intraobserver intraclass correlation coefficient (ICC) of 0.685–0.971 and an interobserver ICC of 0.773–0.917 were reported in our previous publication 24 . In the same report, the Cohen's kappa coefficient calculated for the sulci grading scores lay between 0.894 and 0.955 for intraobserver variability and between 0.765 and 0.906 for interobserver variability.
Postnatal ASQ assessment
Postnatal neurobehavior was assessed at a mean age of 12 ± 1 months of corrected age by means of a Spanish version of the ASQ (2nd edition), a first‐level comprehensive screening program used widely to determine children's performance compared with standards taken from typically developing children of the same age, and validated for its application by both parents and primary caregivers 16 , 27 . The ASQ screening system comprises several questionnaires designed for use from 4 to 60 months, providing information on five different domains at each age: communication, personal‐social, problem‐solving, gross‐motor and fine‐motor skills. In most cases, these questionnaires identify accurately infants or young children who need further evaluation to determine whether they require early intervention services. For the current study, parents completed the questionnaires and emailed them to the researchers. In order to compare the ASQ score results among groups, we calculated Z‐scores using the SC population as a reference group.
Statistical analysis
Data management and statistical analyses were performed using STATA 16 software (Statacorp, College Station, TX, USA). The prespecified study outcomes were fetal neurosonographic measurements and postnatal neurobehavioral scores per domain. The independent or exposure variable of interest was the mode of conception (spontaneous, ART with frozen ET, ART with fresh ET). Normal distribution of continuous variables was checked using the Shapiro–Wilk test and histograms. Descriptive statistics and results were expressed as mean (± SD), median (interquartile range) or n (%), as appropriate. After checking the fulfilment of each test's assumptions, comparisons among the study groups were made by ANOVA or Kruskal–Wallis tests with Bonferroni correction for continuous variables and Pearson's chi‐square test for categorical variables. Neurosonographic findings between groups were adjusted for confounding factors (maternal age, ethnicity, parity, fetal sex and fetal weight centile and gestational age at scan) by multiple regression analyses. Normalized neurobehavioral Z‐scores from each domain (communication, gross‐motor, fine‐motor, problem‐solving and personal‐social skills) and from the global performance (sum of the five domains) were calculated using the correspondent SC group mean and SD by means of the following formula: Z‐score = (score − mean score for SC)/SD for SC. Neurobehavioral comparisons among groups were adjusted by linear regression for maternal age, ethnicity, parity, educational level and employment status, gestational age at birth, breastfeeding, infant sex and infant age at postnatal evaluation. Correlations between normalized neurosonographic and ASQ findings were investigated using the Spearman correlation coefficient, ρ. All reported P‐values are two‐sided. The significance level was set at 0.05 for all statistical tests.
Sample size calculation
Since there are no data on cortical brain assessment by ultrasound in ART compared with SC fetuses, the sample size was calculated based on a previous publication that acknowledged ultrasonographic changes in cortical folding between third‐trimester fetuses with ventriculomegaly vs controls 24 . In a proportion of 1 : 1, for a two‐sided 95% CI and 80% power, samples of 36 and 63 patients per experimental group were calculated for detecting a 15% difference in normalized calcarine and parieto‐occipital sulci depths, respectively. Therefore, the study design aimed for an initial recruitment of at least 70 patients per study group.
RESULTS
Baseline and perinatal characteristics
Baseline characteristics (Table 1) and perinatal outcomes (Table 2) were similar among the study groups, with the exception of higher rates of nulliparity and induction of labor among the ART groups compared with the SC group and a higher proportion of Caucasian ethnicity in the fresh ET population. Regarding causes of infertility, there was a higher proportion of unexplained infertility in the fresh ET than in the frozen ET group, with no difference in the rates of endometriosis, tubal obstruction, male factor and preimplantation genetic diagnosis, nor in the rate of ICSI or the number of embryos transferred. There was a higher proportion of blastocysts transferred in the frozen ET than in the fresh ET group. Frozen ET was performed in programmed cycles in 78.6% (n = 55) of cases and in natural cycles in 21.4% (n = 15). There were no significant differences between groups regarding the gestational age at CRL assessment for pregnancy dating (mean ± SD: SC group, 12.5 ± 0.6 weeks vs ART frozen ET, 12.5 ± 0.6 weeks vs ART fresh ET, 12.5 ± 0.5 weeks, P = 0.505).
Table 1.
Baseline and fertility characteristics in pregnancies conceived spontaneously (SC) or using assisted reproductive technology (ART) with fresh or frozen embryo transfer (ET)
| Characteristic | ART | ||
|---|---|---|---|
| SC (n = 70) | Frozen ET (n = 70) | Fresh ET (n = 70) | |
| Maternal characteristics | |||
| Age (years) | 34.4 (30.1–37.3) | 35.8 (33.7–37.6) | 35.9 (33.9–37.7) |
| Body mass index (kg/m2) | 23.2 (20.6–25.7) | 23.4 (20.3–25.9) | 23.1 (20.9–27.2) |
| Caucasian | 48 (68.6) | 58 (82.9) | 63 (90.0)* |
| Nulliparous | 40 (57.1) | 58 (82.9)* | 65 (92.9)* |
| Chronic hypertension | 1 (1.4) | 2 (2.9) | 0 |
| Cardiovascular disease | 1 (1.4) | 2 (2.9) | 3 (4.3) |
| Diabetes | 0 | 1 (1.4) | 1 (1.4) |
| Autoimmune disease | 3 (4.3) | 3 (4.3) | 2 (2.9) |
| Thyroid disease | 2 (2.9) | 2 (2.9) | 5 (7.1) |
| Kidney disease | 1 (1.4) | 1 (1.4) | 0 |
| Epilepsy | 1 (1.4) | 0 | 1 (1.4) |
| Psychopharmaceutical exposure during pregnancy | 3 (4.3) | 1 (1.4) | 3 (4.3) |
| Self‐reported smoking habit during pregnancy | 13 (18.6) | 9 (12.9) | 11 (15.7) |
| Self‐reported alcohol intake during pregnancy | 9 (12.9) | 8 (11.4) | 5 (7.1) |
| Self‐reported drug use during pregnancy | 0 | 0 | 0 |
| University‐level education | 37 (52.9) | 41 (58.6) | 40 (57.1) |
| Employment rate | 46 (65.7) | 52 (74.3) | 56 (80.0) |
| Fertility and ART characteristics | |||
| Cause of infertility/indication for ART | |||
| Unexplained | — | 20 (28.6) | 35 (50.0)† |
| Endometriosis | — | 7 (10.0) | 11 (15.7) |
| Tubal obstruction | — | 7 (10.0) | 7 (10.0) |
| Male factor | — | 35 (50.0) | 27 (38.6) |
| Preimplantation genetic diagnosis | — | 4 (5.7) | 5 (7.1) |
| Number of embryos transferred | — | 2.0 (1.0–2.0) | 2.0 (2.0–2.0) |
| Transfer of ICSI embryo | — | 68 (97.1) | 68 (97.1) |
| Transfer in blastocyst stage | — | 41 (58.6) | 14 (20.0)† |
| Vanishing twin | — | 29 (41.4) | 14 (20.0) |
Data are given as median (interquartile range) or n (%).
P < 0.05 vs SC.
P < 0.05 vs ART with frozen ET. ICSI, intracytoplasmic sperm injection.
Table 2.
Perinatal characteristics in pregnancies conceived spontaneously (SC) or using assisted reproductive technology (ART) with fresh or frozen embryo transfer (ET)
| Characteristic | ART | ||
|---|---|---|---|
| SC (n = 70) | Frozen ET (n = 70) | Fresh ET (n = 70) | |
| Pregnancy complications | |||
| Preterm delivery | 2 (2.9) | 5 (7.1) | 2 (2.9) |
| Pre‐eclampsia | 2 (2.9) | 7 (10.0) | 2 (2.9) |
| Gestational diabetes | 4 (5.7) | 5 (7.1) | 6 (8.6) |
| Placenta previa | 0 | 1 (1.4) | 0 |
| Placental abruption | 0 | 1 (1.4) | 0 |
| Aspirin exposure from first trimester onwards | 9 (12.9) | 10 (14.3) | 4 (5.7) |
| Prenatal corticoid exposure | 1 (1.4) | 4 (5.7) | 2 (2.9) |
| Delivery data | |||
| Gestational age at birth (weeks) | 40.0 (39.0–40.4) | 39.5 (38.4–41.0) | 40.2 (39.1–41.0) |
| Birth weight (g) | 3280 (3030–3540) | 3280 (3050–3550) | 3235 (2876–3630) |
| Birth‐weight centile | 41 (19–67) | 40 (18–60) | 36 (11–64) |
| Birth weight < 10th centile | 11 (15.7) | 6 (8.6) | 12 (17.1) |
| Birth weight > 90th centile | 4 (5.7) | 5 (7.1) | 5 (7.1) |
| Induction of labor | 24 (34.3) | 40 (57.1)* | 35 (50.0)* |
| Cesarean section | 21 (30.0) | 29 (41.4) | 19 (27.1) |
| Female gender | 37 (52.9) | 28 (40.0) | 39 (55.7) |
| Neonatal outcome | |||
| Admission to neonatal intensive care unit | 9 (12.9) | 6 (8.6) | 2 (2.9) |
| Minor neonatal morbidity | 11 (15.7) | 11 (15.7) | 4 (5.7) |
| Major neonatal morbidity | 0 | 0 | 0 |
| Neonatal mortality | 0 | 0 | 0 |
Data are given as n (%) or median (interquartile range).
P < 0.05 vs SC.
Fetal neurosonography
Gestational age and estimated fetal weight were similar at neurosonographic assessment in all three groups (Table 3). Cephalic dimensions were similar between groups. Both ART populations showed less profound cingulate and calcarine sulci depths compared with the SC group, and the fresh ET group presented less profound parieto‐occipital sulci depth compared with both the SC and the frozen ET groups. Lower cortical grading scores were also observed in both ART groups at the level of the Sylvian fissure and the parieto‐occipital and calcarine sulci as compared with the SC population (Figure 3). Overall, differences in cortical development, as reflected in sulci depth, were more pronounced in the fresh ET compared with the frozen ET group (Table 3). These reported neurosonographic differences were statistically significant after adjustment for maternal age, ethnicity, parity, fetal sex and gestational age and estimated fetal weight centile at ultrasound scan.
Table 3.
Fetal neurosonographic results in pregnancies conceived spontaneously (SC) or using assisted reproductive technology (ART) with fresh or frozen embryo transfer (ET)
| Characteristic | ART | |||
|---|---|---|---|---|
| SC (n = 70) | Frozen ET (n = 70) | Fresh ET (n = 70) | P | |
| Gestational age at scan (weeks) | 31.6 (29.3–32.5) | 31.2 (29.3–32.2) | 30.5 (29.4–32.0) | 0.192 |
| EFW at scan (g) | 1775 ± 435 | 1716 ± 340 | 1678 ± 346 | 0.325 |
| EFW at scan (centile) | 43 (20–79) | 57 (33–82) | 46 (21–72) | 0.303 |
| EFW < 10th centile | 8 (11.4) | 8 (11.4) | 9 (12.9) | 0.967 |
| EFW > 90th centile | 16 (22.9) | 11 (15.7) | 8 (11.4) | 0.188 |
| Cephalic presentation | 60 (85.7) | 64 (91.4) | 58 (82.9) | 0.315 |
| Left laterality | 42 (60.0) | 45 (64.3) | 39 (55.7) | 0.585 |
| Biparietal diameter (mm)* | 78.3 ± 6.4 | 78.1 ± 4.5 | 78.4 ± 4.5 | 0.910 |
| Occipitofrontal diameter (mm)* | 102.9 ± 6.9 | 101.8 ± 5.3 | 101.1 ± 5.9 | 0.171 |
| Head circumference (mm)* | 289.8 ± 19.6 | 288.0 ± 14.4 | 286.8 ± 15.1 | 0.401 |
| Normalized parieto‐occipital sulcus depth*, † | 13.4 ± 2.6 | 13.4 ± 2.6 | 12.5 ± 2.5‡, § | < 0.001 |
| Normalized cingulate sulcus depth*, † | 6.5 (4.8–7.8) | 5.8 (4.1–7.5)‡ | 5.8 (4.2–7.4)‡ | 0.001 |
| Normalized calcarine sulcus depth*, † | 16.4 (14.3–17.9) | 14.5 (12.1–15.8)‡ | 13.5 (10.1–16.1)‡, § | < 0.001 |
Data are given as median (interquartile range), mean ± SD or n (%).
P‐values adjusted by maternal age, ethnicity, parity, fetal sex and gestational age and fetal weight centile at scan.
Data normalized by dividing by biparietal diameter and multiplying by 100.
P < 0.05 vs SC.
P < 0.05 vs ART with frozen ET. EFW, estimated fetal weight.
Figure 3.
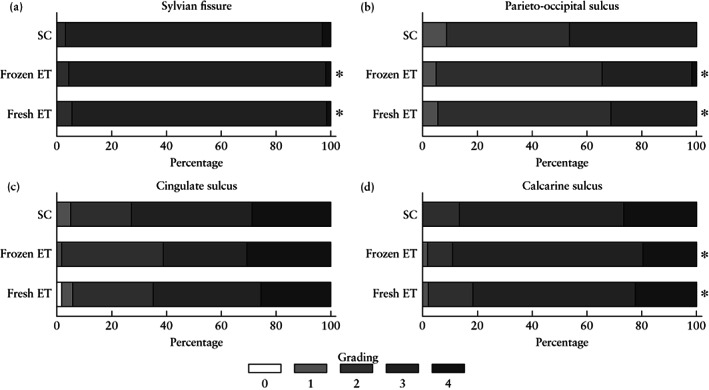
Distribution of main sulci and Sylvian fissure grading scores in fetuses of pregnancies conceived spontaneously (SC) or using assisted reproductive technology with fresh or frozen embryo transfer (ET): (a) Sylvian fissure; (b) parieto‐occipital sulcus; (c) cingulate sulcus; and (d) calcarine sulcus. P‐values adjusted for maternal age, ethnicity, parity, fetal sex, and gestational age and fetal weight centile at scan. *P < 0.05 vs SC.
Infant ASQ scores
Infant ASQ results were available in 173 cases (Figure 4). The fresh ET group presented lower global ASQ scores as compared with the SC and the ART with frozen ET populations (mean ± SD global ASQ Z‐score: fresh ET, –0.3 ± 0.4 vs frozen ET, –0.2 ± 0.4 vs SC, 0 ± 0.4, P < 0.001). Moreover, the fresh ET group showed significantly lower scores for communication, motor and problem‐solving skills compared with both the SC group and the frozen ET group. Both groups of infants conceived by ART showed significantly lower scores compared with the SC group in the personal‐social domain. Results were statistically significant after adjustment for maternal age, ethnicity, parity, educational level, employment status, breastfeeding, infant sex, gestational age at birth and infant age at postnatal evaluation. The distribution of cases with Z‐scores below −2 SD from the spontaneously conceived study population mean is given according to domain in Table S1.
Figure 4.

Adjusted Z‐scores of infant Ages & Stages Questionnaire domains at 12 months of age among study groups according to mode of conception, either spontaneously (SC) (n = 55) or using assisted reproductive technology (ART) with fresh or frozen embryo transfer (ET) (n = 59 each): (a) global score; (b) communication skills; (c) personal‐social skills; (d) gross‐motor skills; (e) fine‐motor skills; and (f) problem‐solving skills. Z‐score values and P‐values adjusted for maternal age, ethnicity, parity, educational level and employment status, gestational age at delivery, breastfeeding, infant sex and infant age at evaluation. Individual ( ) and median (
) and median ( ) values are shown. *P < 0.05 vs SC. †P < 0.05 vs ART with frozen ET.
) values are shown. *P < 0.05 vs SC. †P < 0.05 vs ART with frozen ET.
Fetal neurosonographic cortical features and infant global ASQ scores showed a weak but statistically significant positive correlation in the overall population with (parieto‐occipital sulcus depth: ρ = 0.23, P = 0.003; cingulate sulcus depth: ρ = 0.21, P = 0.008; calcarine sulcus depth: ρ = 0.32, P < 0.001) and within the ART population (parieto‐occipital sulcus depth: ρ = 0.22, P = 0.017; cingulate sulcus depth: ρ = 0.20, P = 0.032; calcarine sulcus depth: ρ = 0.29, P = 0.001). In addition, there were statistically significant positive correlations between these parameters and each of the ASQ domains, except for the cingulate sulcus in motor and problem‐solving areas (Table S2).
DISCUSSION
We report third‐trimester neurosonographic results in fetuses conceived by ART which suggest association with a distinctive pattern of cortical development, along with suboptimal infant neurobehavior at 12 months of age. To our knowledge, this is the first study assessing prenatal brain cortical development in ART offspring. We report less profound parieto‐occipital, cingulate and calcarine sulci depths, together with lower cortical grading scores, in ART fetuses.
Cortical folding is a complex process of prenatal brain organization, in which the smooth brain surface evolves into a system of sulci and gyri associated with rapid cortical expansion in functional areas in ventricular and subventricular zones 28 . Parieto‐occipital, cingulate and calcarine sulci increase in depth with gestational age 23 , 26 and the Sylvian fissure undergoes a process of operculization 29 . Changes in cortical development have been described in fetuses with growth restriction (FGR), congenital heart defects (CHD) and non‐severe ventriculomegaly. Whereas FGR, with or without pre‐eclampsia, has been associated with only reduced Sylvian fissure depth 25 , 30 , 31 , fetuses with CHD 32 or isolated ventriculomegaly 24 , 33 have been reported to experience a widespread suboptimal cortical folding process, with shallower sulci depths and delayed operculization, similar to that observed in our ART population.
Our reported differences in fetal cortical development in ART pregnancies correlated weakly with suboptimal neurobehavior in infancy, in communication, personal‐social, problem‐solving and motor domains. Complex functions at the cortical level are distributed across several neural networks 34 . The calcarine sulcus is part of the primary visual cortex. The parieto‐occipital sulcus participates in visuospatial working memory 35 . The cingulate sulcus is involved in cognitive 36 , motor 37 , emotional and social‐behavioral 38 , 39 processing; its deficiency has been described in psychiatric disorders such as schizophrenia, attention deficit hyperactivity disorder and autism spectrum disorder 40 , 41 . Therefore, the changes observed in our population in these structures may have contributed partially to the neurobehavioral features found at 12 months. Previous follow‐up studies in infants and children conceived by ART showed inconsistent cognitive, psychomotor and behavioral results 42 , 43 , 44 , 45 , 46 , 47 . These inconsistencies may be explained by differences in the ART populations, the influence of cofactors such as infertility, multiple gestation, prematurity or other perinatal complications associated with ART 8 , 48 , 49 , 50 , 51 , 52 , and the effect of different postnatal socioeconomic and educational levels 50 . Couples undergoing fertility treatment may differ intrinsically from those conceiving spontaneously in demographic characteristics such as age, educational level and socioeconomic position 50 , and also in the way in which they encourage learning 53 , 54 and acknowledge health issues in their offspring 55 . As many factors influence neurodevelopment after birth, the uniqueness of the current study is our description of fetal brain changes in utero, occurring before exposure to postnatal influences.
It has been proposed that neurodevelopment in fetuses conceived by ART might be influenced by the underlying parental subfertility, ovarian stimulation and/or IVF procedures. The observed differences between our three study groups could have been triggered by their different intrauterine vasoactive and hormonal milieu 56 , 57 , 58 , 59 , 60 , 61 , but also by changes in cardiac function: brain and heart development take place simultaneously in utero and often share morphogenetic programs 62 . Fetuses conceived by ART show differences in growth and cardiac shape and function compared with SC fetuses, fresh ET being associated with fetal smallness 6 , 63 and cardiac remodeling and dysfunction 64 , 65 , and frozen ET with macrosomia 63 , hypertensive disorders of pregnancy 5 , 66 and milder cardiac changes 65 , 67 .
Interestingly, our findings were more pronounced in the fresh ET than in the frozen ET group, contrary to a registry‐based study reporting a statistically higher risk of neurodevelopmental delay associated with ART (particularly ICSI using ejaculated sperm with fresh and frozen ET), with higher risk for ICSI with frozen ET, when restricting the analysis to singletons 8 . Nevertheless, no differences were reported between fresh and frozen ET when comparing academic performance in adolescents, in an uncontrolled follow‐up study, in which, however, mild conditions may have been underrepresented 68 . A birth cohort study with a 2‐year follow‐up reported reassuring results for ART, but it was underpowered to detect differences between the fresh and frozen ET modalities 69 .
Strengths and limitations
Among the strengths of our study is that it is the first to examine cortical development in fetuses conceived by ART. We present a well‐phenotyped cohort from a single center, with all study groups having been included prospectively. All patients underwent a detailed neurosonographic examination to exclude any additional abnormality of the central nervous system, preventing the inclusion of conditions that could potentially bias our results. Another strength of this study is the use of both sulci depth measurements and grading of fissures and sulci, providing quantitative objective data along with cortical maturation status. To ensure high image quality and accurate assessment of cortical folding parameters, ultrasonographic measurements were performed offline, following a strict and reliable protocol, by a single trained neurosonographer who was blinded to the mode of conception. Finally, we adjusted for potential confounders in our models for both prenatal and postnatal outcomes.
Among the limitations of our study is that the potential contribution of infertility factors to outcome cannot be separated from the contribution of the ART procedure itself. Moreover, regarding embryonic stage at ET, there was a different proportion of blastocysts transferred in the two ART groups. We therefore performed a sub‐analysis, excluding the cases with transfer at the blastocyst stage (Tables S3 and S4 and Figure S1) and, even though the power decreased, significant differences between the study groups remained. Furthermore, the frozen ET group itself was heterogeneous, with 15% of ET performed with the woman's natural cycle. The conditions associated with decreased fertility were likely to have been underdiagnosed in the SC population. The reported neurosonographic differences were subtle, with most outcomes lying within normal ranges; their postnatal persistence needs to be investigated. Due to technical reasons, particularly shadowing from the fetal skull, we measured only the side of the brain distal to the transducer, which could have biased our results in case of asymmetry. The ASQ used to assess postnatal performance at 12 months is mainly a screening tool, subject to reporting bias, and it was applied only once, rather than longitudinally. Finally, although the mass‐significance effect was attenuated by applying Bonferroni correction to each comparison, we cannot exclude that some of the apparent differences could have been due to chance.
Conclusions
Fetuses conceived by ART showed a distinctive pattern of cortical development and suboptimal infant neurodevelopment at 12 months, with more pronounced changes in those conceived following fresh ET. Our results provide new evidence for the existence of in‐utero brain reorganization associated with ART and for the importance of neurodevelopmental follow‐up and assessment of the long‐term consequences in ART offspring.
Supporting information
Figure S1 Distribution of cortical grading scores of main sulci in fetuses of pregnancies conceived spontaneously or using assisted reproductive technology with fresh or frozen embryo transfer, excluding cases of transfer in the blastocyst stage.
Table S1 Distribution of crude Ages and Stages Questionnaire Z‐scores below −2 SD from the spontaneously conceived study population mean
Table S2 Pearson's correlations between each Ages & Stages Questionnaire domain score and sulci depth in entire study population of pregnancies conceived spontaneously or using assisted reproductive technology (ART) with fresh or frozen embryo transfer, and in only those conceived by ART
Tables S3 and S4 Baseline, fertility and perinatal characteristics (Table S3) and fetal neurosonographic assessment (Table S4) of pregnancies conceived spontaneously or using assisted reproductive technologies with fresh or frozen embryo transfer, excluding cases of transfer in the blastocyst stage
Acknowledgments
We thank the study participants for their personal time and commitment to this project. This project was partially funded with support from the Erasmus+ Programme of the European Union (Framework Agreement number: 2013‐0040). This publication reflects the views only of the author, and the Commission cannot be held responsible for any use, which may be made of the information contained therein. Additionally, the research leading to these results received funding from ‘la Caixa’ Foundation under grant agreement LCF/PR/GN18/10310003, the Instituto de Salud Carlos III (PI15/00130, PI16/00861, PI17/00675, PI18/00073, INT21/00027), cofunded by the European Union, Cerebra Foundation for the Brain Injured Child (Carmarthen, Wales, UK) and AGAUR 2017 SGR grant no 1531. E.E. received funding from the Departament de Salut under grant SLT008/18/00156.
This article's abstract has been translated into Spanish and Chinese. Follow the links from the abstract to view the translations.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Wyns C, De Geyter C, Calhaz‐Jorge C, Kupka MS, Motrenko T, Smeenk J, Bergh C, Tandler‐Schneider A, Rugescu IA, Vidakovic S, Goossens V. ART in Europe, 2017: results generated from European registries by ESHRE. Hum Reprod Open 2021; 3: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berntsen S, Söderström‐Anttila V, Wennerholm U‐B, Laivuori H, Loft A, Oldereid NB, Bente Romundstad L, Bergh C, Pinborg A. The health of children conceived by ART: “the chicken or the egg?” Hum Reprod Update 2019; 25: 137–158. [DOI] [PubMed] [Google Scholar]
- 3. Pinborg A. Short‐ and long‐term outcomes in children born after assisted reproductive technology. BJOG 2019; 126: 145–148. [DOI] [PubMed] [Google Scholar]
- 4. Bergh C, Wennerholm UB. Long‐term health of children conceived after assisted reproductive technology. Ups J Med Sci 2020; 125: 152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ernstad EG, Wennerholm U‐B, Khatibi A, Petzold M, Bergh C. Neonatal and maternal outcome after frozen embryo transfer: Increased risks in programmed cycles. Am J Obstet Gynecol 2019; 221: 126.e1–18. [DOI] [PubMed] [Google Scholar]
- 6. Maheshwari A, Pandey S, Raja EA, Shetty A, Hamilton M, Bhattacharya S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta‐analysis provide a definitive answer? Hum Reprod Update 2018; 24: 35–58. [DOI] [PubMed] [Google Scholar]
- 7. Pinborg A, Loft A, Aaris Henningsen AK, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: The Danish National Cohort Study 1995–2006. Fertil Steril 2010; 94: 1320–1327. [DOI] [PubMed] [Google Scholar]
- 8. Sandin S, Nygren KG, Iliadou A, Hultman CM, Reichenberg A. Autism and mental retardation among offspring born after in vitro fertilization. JAMA 2013; 310: 75–84. [DOI] [PubMed] [Google Scholar]
- 9. Husen SC, Koning IV, Go ATJI, Groenenberg IAL, Willemsen SP, Rousian M, Steegers‐Theunissen RPM. IVF with or without ICSI and the impact on human embryonic brain development: the Rotterdam Periconceptional Cohort. Hum Reprod 2021; 36: 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yin L, Xu Y, Li H, Ling C, Wai Choy K, Xia F, Deng X. Effect of assisted reproductive technology on fetal brain development assessed by prenatal ultrasonography. J Perinat Med 2015; 43: 103–109. [DOI] [PubMed] [Google Scholar]
- 11. Robinson HP. Sonar Measurement of Fetal Crown‐Rump Length as Means of Assessing Maturity in First Trimester of Pregnancy. Br Med J 1973; 4: 28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salomon LJ, Alfirevic Z, Da Silva Costa F, Deter RL, Figueras F, Ghi T, Glanc P, Khalil A, Lee W, Napolitano R, Papageorghiou A, Sotiradis A, Stirnemann J, Toi A, Yeo G. ISUOG Practice Guidelines: ultrasound assessment of fetal biometry and growth. Ultrasound Obstet Gynecol 2019; 53: 715–723. [DOI] [PubMed] [Google Scholar]
- 13. Figueras F, Meler E, Iraola A, Eixarch E, Coll O, Figueras J, Francis A, Gratacos E, Gardosi J. Customized birthweight standards for a Spanish population. Eur J Obstet Gynecol Reprod Biol 2008; 136: 20–24. [DOI] [PubMed] [Google Scholar]
- 14. Gestational Hypertension and Preeclampsia : ACOG Practice Bulletin, Number 222. Obstet Gynecol 2020; 135: e237–260. [DOI] [PubMed] [Google Scholar]
- 15. National Diabetes Data Group . Guide to diagnosis and classification of diabetes mellitus and other categories of glucose intolerance. Diabetes Care 1997; 20: 1039–1057. [Google Scholar]
- 16. Bricker D, Squires J, Mounts L, Potter L, Nickel R, Twombly E, Farrell J. Ages & Stages Questionnaires: A Parent‐Completed Child‐Monitoring System ‐ Spanish Version, 2nd edn. Paul H Brookes Publishing Co.: Baltimore, MD, USA, 1999. [Google Scholar]
- 17. World Medical Association . Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 18. Cuevas Saiz I, Carme Pons Gatell M, Vargas MC, Delgado Mendive A, Rives Enedáguila N, Moragas Solanes M, Carrasco Canal B, Teruel López J, Busquets Bonet A, Hurtado de Mendoza Acosta MV. The Embryology Interest Group: updating ASEBIR's morphological scoring system for early embryos, morulae and blastocysts. Med Reprod y Embriol Clínica 2018; 5: 42–54. [Google Scholar]
- 19. Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: Towards a single blastocyst transfer. Fertil Steril 2000; 73: 1155–1158. [DOI] [PubMed] [Google Scholar]
- 20. Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: The Cryotop method. Theriogenology 2007; 67: 73–80. [DOI] [PubMed] [Google Scholar]
- 21. Paladini D, Malinger G, Birnbaum R, Monteagudo A, Pilu G, Salomon LJ, Timor‐Tritsch IE. ISUOG Practice Guidelines (updated): sonographic examination of the fetal central nervous system. Part 2: performance of targeted neurosonography. Ultrasound Obstet Gynecol 2021; 57: 661–671. [DOI] [PubMed] [Google Scholar]
- 22. Egaña‐Ugrinovic G, Sanz‐Cortes M, Figueras F, Bargalló N, Gratacós E. Differences in cortical development assessed by fetal MRI in late‐onset intrauterine growth restriction. Am J Obstet Gynecol 2013; 209: 126.e1–8. [DOI] [PubMed] [Google Scholar]
- 23. Alonso I, Borenstein M, Grant G, Narbona I, Azumendi G. Depth of brain fissures in normal fetuses by prenatal ultrasound between 19 and 30 weeks of gestation. Ultrasound Obstet Gynecol 2010; 36: 693–699. [DOI] [PubMed] [Google Scholar]
- 24. Hahner N, Puerto B, Perez‐Cruz M, Policiano C, Monterde E, Crispi F, Gratacos E, Eixarch E. Altered cortical development in fetuses with isolated nonsevere ventriculomegaly assessed by neurosonography. Prenat Diagn 2018; 38: 365–375. [DOI] [PubMed] [Google Scholar]
- 25. Paules C, Miranda J, Policiano C, Crovetto F, Youssef L, Hahner N, Nakaki A, Crispi F, Gratacós E, Eixarch E. Fetal neurosonography detects differences in cortical development and corpus callosum in late‐onset small fetuses. Ultrasound Obstet Gynecol 2021; 58: 42–47. [DOI] [PubMed] [Google Scholar]
- 26. Pistorius LR, Stoutenbeek P, Groenendaal F, De Vries L, Manten G, Mulder E, Visser G. Grade and symmetry of normal fetal cortical development: A longitudinal two‐ and three‐dimensional ultrasound study. Ultrasound Obstet Gynecol 2010; 36: 700–708. [DOI] [PubMed] [Google Scholar]
- 27. Sarmiento Camposa JA, Squires J, Ponte J. Universal developmental screening: Preliminary studies in Galicia, Spain. Early Child Dev Care 2011; 181: 475–485. [Google Scholar]
- 28. Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell 2011; 146: 18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quarello E, Stirnemann J, Ville Y, Guibaud L. Assessment of fetal Sylvian fissure operculization between 22 and 32 weeks: A subjective approach. Ultrasound Obstet Gynecol 2008; 32: 44–49. [DOI] [PubMed] [Google Scholar]
- 30. Husen SC, Koning IV, Go ATJI, Van Graafeiland AW, Willemsen SP, Groenenberg IAL, Steegers‐Theunissen RPM. Three‐dimensional ultrasound imaging of fetal brain fissures in the growth restricted fetus. PLoS One 2019; 14: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Basso A, Youssef L, Nakaki A, Paules C, Miranda J, Casu G, Salazar L, Gratacos E, Eixarch E, Crispi F, Crovetto F. Fetal neurosonography at 31–35 weeks reveals altered cortical development in pre‐eclampsia with and without small‐for‐gestational‐age fetus. Ultrasound Obstet Gynecol 2022; 59: 737–746. [DOI] [PubMed] [Google Scholar]
- 32. Lee FT, Seed M, Sun L, Marini D. Fetal brain issues in congenital heart disease. Transl Pediatr 2021; 10: 2182–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hahner N, Benkarim OM, Aertsen M, Perez‐Cruz M, Piella G, Sanroma G, Bargallo N, Deprest J, Gonzalez Ballester MA, Gratacos E, Eixarch E. Global and regional changes in cortical development assessed by MRI in fetuses with isolated nonsevere ventriculomegaly correlate with neonatal neurobehavior. Am J Neuroradiol 2019; 40: 1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nieuwenhuys R. The insular cortex. A review. Prog Brain Res 2012; 195: 123–163. [DOI] [PubMed] [Google Scholar]
- 35. Pollmann S. Working memory dependence of spatial contextual cueing for visual search. Br J Psychol 2019; 110: 372–380. [DOI] [PubMed] [Google Scholar]
- 36. Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain 2014; 137: 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Michelet T, Badets A. The anterior midcingulate cortex might be a neuronal substrate for the ideomotor mechanism. Exp Brain Res 2021; 239: 2345–2355. [DOI] [PubMed] [Google Scholar]
- 38. Hadland KA, Rushworth MFS, Gaffan D, Passingham RE. The effect of cingulate lesions on social behaviour and emotion. Neuropsychologia 2003; 41: 919–931. [DOI] [PubMed] [Google Scholar]
- 39. Lockwood PL. The anatomy of empathy: Vicarious experience and disorders of social cognition. Behav Brain Res 2016; 311: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chiu PH, Kayali MA, Kishida KT, Tomlin D, Klinger LG, Klinger MR, Montague PR. Self Responses along Cingulate Cortex Reveal Quantitative Neural Phenotype for High‐Functioning Autism. Neuron 2008; 57: 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rubia K, Alegría AA, Brinson H. Brain abnormalities in attention‐deficit hyperactivity disorder: a review. Rev Neurol 2014; 58 (Suppl 1): S3–16. [PubMed] [Google Scholar]
- 42. Middelburg KJ, Heineman MJ, Bos AF, Hadders‐Algra M. Neuromotor, cognitive, language and behavioural outcome in children born following IVF or ICSI ‐ A systematic review. Hum Reprod Update 2008; 14: 219–231. [DOI] [PubMed] [Google Scholar]
- 43. Bay B, Mortensen EL, Kesmodel US. Assisted reproduction and child neurodevelopmental outcomes: A systematic review. Fertil Steril 2013; 100: 844–853. [DOI] [PubMed] [Google Scholar]
- 44. Hart R, Norman RJ. The longer‐term health outcomes for children born as a result of IVF treatment. Part II‐mental health and development outcomes. Hum Reprod Update 2013; 19: 244–250. [DOI] [PubMed] [Google Scholar]
- 45. Liu L, Gao J, He X, Cai Y, Wang L, Fan X. Association between assisted reproductive technology and the risk of autism spectrum disorders in the offspring: A meta‐analysis. Sci Rep 2017; 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rumbold AR, Moore VM, Whitrow MJ, Oswald TK, Moran LJ, Fernandez RC, Barnhart KT, Davies MJ. The impact of specific fertility treatments on cognitive development in childhood and adolescence: A systematic review. Hum Reprod 2017; 32: 1489–1507. [DOI] [PubMed] [Google Scholar]
- 47. Rönö K, Rissanen E, Bergh C, Wennerholm UB, Opdahl S, Romundstad LB, Henningsen AKA, Spangmose AL, Pinborg A, Gissler M, Tiitinen A. The neurodevelopmental morbidity of children born after assisted reproductive technology: a Nordic register study from the Committee of Nordic Assisted Reproductive Technology and Safety group. Fertil Steril 2022; 117: 1026–1037. [DOI] [PubMed] [Google Scholar]
- 48. Kissin DM, Zhang Y, Boulet SL, Fountain C, Bearman P, Schieve L, Yeargin‐Allsopp M, Jamieson DJ. Association of assisted reproductive technology (ART) treatment and parental infertility diagnosis with autism in ART‐conceived children. Hum Reprod 2015; 30: 454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Drenth Olivares M, Kuiper DB, Haadsma ML, Heineman KR, Heineman MJ, Hadders‐Algra M. IVF procedures are not, but subfertility is associated with neurological condition of 9‐year‐old offspring. Early Hum Dev 2019; 129: 38–44. [DOI] [PubMed] [Google Scholar]
- 50. Carson C, Kelly Y, Kurinczuk JJ, Sacker A, Redshaw M, Quigley MA. Effect of pregnancy planning and fertility treatment on cognitive outcomes in children at ages 3 and 5: Longitudinal cohort study. BMJ 2011; 343: 7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hvidtjørn D, Grove J, Schendel D, Sværke C, Schieve LA, Uldall P, Ernst E, Jacobsson B, Thorsen P. Multiplicity and early gestational age contribute to an increased risk of cerebral palsy from assisted conception: A population‐based cohort study. Hum Reprod 2010; 25: 2115–2123. [DOI] [PubMed] [Google Scholar]
- 52. Källén AJB, Finnström OO, Lindam AP, Nilsson EME, Nygren KG, Otterblad Olausson PM. Is there an increased risk for drug treated attention deficit/hyperactivity disorder in children born after in vitro fertilization? Eur J Paediatr Neurol 2011; 15: 247–253. [DOI] [PubMed] [Google Scholar]
- 53. Agarwal P, Loh SKE, Lim SB, Sriram B, Daniel ML, Yeo SH, Heng D. Two‐year neurodevelopmental outcome in children conceived by intracytoplasmic sperm injection: Prospective cohort study. BJOG 2005; 112: 1376–1383. [DOI] [PubMed] [Google Scholar]
- 54. Spangmose AL, Malchau SS, Schmidt L, Vassard D, Rasmussen S, Loft A, Forman J, Pinborg A. Academic performance in adolescents born after ART ‐ A nationwide registry‐based cohort study. Hum Reprod 2017; 32: 447–456. [DOI] [PubMed] [Google Scholar]
- 55. Källén B, Finnström O, Nygren KG, Olausson PO. In vitro fertilization in Sweden: Child morbidity including cancer risk. Fertil Steril 2005; 84: 605–610. [DOI] [PubMed] [Google Scholar]
- 56. Zhou CL, Xu GF, Yang Q, Wang HH, Guo MX, Xiong YM, Guo XY, Hou M, Jin LY, Sheng JZ, He L, Jin L, Huang HF. Diminished verbal ability among children conceived through ART with exposure to high serum estradiol in utero. J Assist Reprod Genet 2020; 37: 1931–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen X, Kong L, Piltonen TT, Gissler M, Lavebratt C. Association of polycystic ovary syndrome or anovulatory infertility with offspring psychiatric and mild neurodevelopmental disorders: a Finnish population‐based cohort study. Hum Reprod 2020; 35: 2336–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mainigi M, Rosenzweig JM, Lei J, Mensah V, Thomaier L, Talbot CC, Olalere D, Ord T, Rozzah R, Johnston MV, Burd I. Peri‐Implantation Hormonal Milieu: Elucidating Mechanisms of Adverse Neurodevelopmental Outcomes. Reprod Sci 2016; 23: 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gore AC, Martien KM, Gagnidze K, Pfaff D. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr Rev 2014; 35: 961–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lester BM, Marsit CJ. Epigenetic mechanisms in the placenta related to infant neurodevelopment. Epigenomics 2018; 10: 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li H, Liu L, Dang M, Zhang W, Liu J. Increased susceptibility of mice obtained from in vitro fertilization to global cerebral ischemia‐reperfusion injury: possible role of hydrogen sulphide and its biosynthetic enzymes. Int J Neurosci 2020; 130: 533–540. [DOI] [PubMed] [Google Scholar]
- 62. McQuillen PS, Miller SP. Congenital heart disease and brain development. Ann N Y Acad Sci 2010; 1184: 68–86. [DOI] [PubMed] [Google Scholar]
- 63. Terho AM, Pelkonen S, Opdahl S, Romundstad LB, Bergh C, Wennerholm UB, Henningsen AA, Pinborg A, Gissler M, Tiitinen A. High birth weight and large‐for‐gestational‐age in singletons born after frozen compared to fresh embryo transfer, by gestational week: a Nordic register study from the CoNARTaS group. Hum Reprod 2021; 36: 1083–1092. [DOI] [PubMed] [Google Scholar]
- 64. Valenzuela‐Alcaraz B, Serafini A, Sepulveda‐Martínez A, Casals G, Rodríguez‐López M, Garcia‐Otero L, Cruz‐Lemini M, Bijnens B, Sitges M, Balasch J, Gratacós E, Crispi F. Postnatal persistence of fetal cardiovascular remodelling associated with assisted reproductive technologies: a cohort study. BJOG 2019; 126: 291–298. [DOI] [PubMed] [Google Scholar]
- 65. Boutet ML, Casals G, Valenzuela‐Alcaraz B, García‐Otero L, Crovetto F, Cívico MS, Borrás A, Manau D, Gratacós E, Crispi F. Cardiac remodeling in fetuses conceived by ARTs: fresh versus frozen embryo transfer. Hum Reprod 2021; 36: 2697–2708. [DOI] [PubMed] [Google Scholar]
- 66. Sites CK, Wilson D, Barsky M, Bernson D, Bernstein IM, Boulet S, Zhang Y. Embryo cryopreservation and preeclampsia risk. Fertil Steril 2017; 108: 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rizzo G, Pietrolucci ME, Mappa I, Bitsadze V, Khizroeva J, Makatsariya A, D'Antonio F. Fetal Cardiac Remodeling Is Affected by the Type of Embryo Transfer in Pregnancies Conceived by in vitro Fertilization: A Prospective Cohort Study. Fetal Diagn Ther 2020; 47: 772–778. [DOI] [PubMed] [Google Scholar]
- 68. Spangmose AL, Malchau SS, Henningsen AA, Forman JL, Rasmussen S, Loft A, Schmidt L, Pinborg A. Academic performance in adolescents aged 15–16 years born after frozen embryo transfer compared with fresh embryo transfer: a nationwide registry‐based cohort study. BJOG 2019; 126: 261–269. [DOI] [PubMed] [Google Scholar]
- 69. Balayla J, Sheehy O, Fraser WD, Séguin JR, Trasler J, Monnier P, MacLeod AA, Simard MN, Muckle G, Bérard A. Neurodevelopmental outcomes after assisted reproductive technologies. Obstet Gynecol 2017; 129: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Distribution of cortical grading scores of main sulci in fetuses of pregnancies conceived spontaneously or using assisted reproductive technology with fresh or frozen embryo transfer, excluding cases of transfer in the blastocyst stage.
Table S1 Distribution of crude Ages and Stages Questionnaire Z‐scores below −2 SD from the spontaneously conceived study population mean
Table S2 Pearson's correlations between each Ages & Stages Questionnaire domain score and sulci depth in entire study population of pregnancies conceived spontaneously or using assisted reproductive technology (ART) with fresh or frozen embryo transfer, and in only those conceived by ART
Tables S3 and S4 Baseline, fertility and perinatal characteristics (Table S3) and fetal neurosonographic assessment (Table S4) of pregnancies conceived spontaneously or using assisted reproductive technologies with fresh or frozen embryo transfer, excluding cases of transfer in the blastocyst stage
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


