Abstract
Until recently, three-dimensional (3D) printing/additive manufacturing has not been used extensively to create medical devices intended for actual clinical use, primarily on patient safety and regulatory grounds. However, in recent years there have been advances in materials, printers, and experience, leading to increased clinical use. The aim of this study was to perform a structured systematic review of 3D-printed medical devices used directly in patient treatment. A search of 13 databases was performed to identify studies of 3D-printed medical devices, detailing fabrication technology and materials employed, clinical application, and clinical outcome. One hundred and ten papers describing one hundred and forty medical devices were identified and analyzed. A considerable increase was identified in the use of 3D printing to produce medical devices directly for clinical use in the past 3 years. This is dominated by printing of patient-specific implants and surgical guides for use in orthopedics and orthopedic oncology, but there is a trend of increased use across other clinical specialties. The prevailing material/3D-printing technology used were titanium alloy/electron beam melting for implants, and polyamide/selective laser sintering or polylactic acid/fused deposition modeling for surgical guides and instruments. A detailed analysis across medical applications by technology and materials is provided, as well as a commentary regarding regulatory aspects. In general, there is growing familiarity with, and acceptance of, 3D printing in clinical use.
Keywords: 3D printing, medical device, medicine, patient, clinical, treatment
Introduction
Three-dimensional (3D) printing, also referred to as additive manufacturing (AM), has been the focus of considerable interest in the field of medical applications over the past decade.1 Such technology has been used to produce medical instruments for diagnostics, surgical instruments and guides, anatomical models for surgical planning, education and training, implants, prostheses and orthoses, tissue engineering scaffolds, tissue models, and pharmaceuticals.2–4 Initially, custom 3D printing was used primarily for education, but a remarkable growth in 3D printing of medical devices used in the direct treatment of patients is evident from published reports.1,5 SmarTech Publishing estimates that the production of 3D-printed implantable devices will experience a 29% compound annual growth through 2026.4
In the production of medical devices, 3D printing offers several advantages over traditional subtractive manufacturing techniques. Most notable is the possibility of individual patient-specific/personalized treatment, which is especially advantageous in the context of rare diseases or uncommon anatomy.3 3D printing also allows for high geometrical and structural complexity of designs with little influence on part-production time6; cost-effective small-scale, on-demand fabrication of highly specific, customized products due to favorable unit production costs and short product lead time6–9; and the possibility of in-house or localized production of medical devices and fabrication in remote areas subject to supply chain limitations.10
However, there are evident risks associated with 3D printing, including, but not limited to, potential adverse events related to bioavailability and bioactivity of component materials and microbiology safety concerns with respect to 3D-printed devices for placement inside the body. Therefore, regulatory issues regarding on-demand manufacturing of patient-specific 3D-printed medical devices are complex, challenging, and are still evolving.1,11 In 2017, the Food and Drug Administration (FDA) published draft guidelines for 3D printing of medical instruments with specifications of design, manufacturing, and device testing.12 Zhou and Bhaduri4 published a list of medical-device-related products that had recently been granted FDA approval.
A 2019 review of 71 articles by Culmone et al.3 focused on 3D-printed medical instruments for examining or treating patients. However, that study excluded, among others, prostheses, orthoses and surgical guides, and medical devices were meant to stay in the body. The review also included devices in the early prototyping phase that were not tested on live patients, and were thus not necessarily subjected to requirements regarding postprocessing for removal of debris and sterilization.3
The aim of the present study was to perform a systematic review of medical devices, as defined by the European Medical Device Regulation (EU) 2017/745 (MDR),13 that were produced by means of 3D printing, and employed for the direct treatment of medical conditions in human patients. Specifically of interest were the device types and fields of application, 3D-printing technology and materials employed, and the clinical outcomes of device use. The review also captures references made to particular regulatory aspects of 3D printing in the selected studies.
Materials and Methods
Literature search and study selection
A systematic literature search was performed in August 2020 of the following databases: Cochrane, EBSCOhost (including Academic Search Complete, Business Source Complete, CINAHL Complete, EconLit with Full Text, MEDLINE, OmniFile Full Text Mega, and Regional Business News), EMBASE, PubMed, Science Direct, Scopus, and Web of Science.
Of interest were all article that included the following keywords: “3D print*” or “3D-print*” or “dimensional print*” or “additive manufacturer*” in the title, and “medical device*” or “instrument*”, and “patient*” or “subject*” or “case report” or “case study” in the title, abstract or keywords. If necessary, the search string was adapted to meet the search options of the specific databases. The study selection was limited to full scientific articles in the English language that described 3D printing of medical devices, and use of these devices on living human patients.
Studies performed on healthy volunteers, animals and in vitro, as well as those where 3D printing was only employed for the production of tools/molds for medical device fabrication (i.e., indirect AM) were excluded. Further excluded were reviews and overviews, papers regarding directives, regulations, and economic aspects of the use of 3D printing, studies involving anatomical models for surgical planning or training/education, 3D printing of pharmaceuticals, and descriptions of new 3D-printing materials, material properties, and physical properties of 3D-printed parts. Due to the unique technological, clinical, ethical, and regulatory considerations, studies of implants produced by means of bioprinting and organ/tissue engineering were also excluded.
The review protocol was designed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines14 (Fig. 1). T.K. performed the searches, and A.S. confirmed search outcomes. L.W.O.S. resolved any disagreement between T.K. and A.S.
FIG. 1.
PRISMA flow diagram of literature search and study selection. PRISMA, preferred reporting items for systematic reviews and meta-analyses. Template adopted from Moher et al.14
Data extraction and synthesis
The following data were extracted from the selected studies: (1) medical-device description and field of application, (2) 3D-printing technology and materials used for device fabrication, (3) number, sex, age, and medical condition(s) of patients treated, and (4) clinical procedure and outcome of device use. The details of regulatory approval of the devices were extracted if included in the studies. In cases where information on the 3D-printing technology used was incomplete, but sufficient detail was provided (e.g., 3D-printer name, name of the company that produced the device), the missing information was obtained online, or from the corresponding authors of the papers.
Results
Overview of research activity regarding the use of 3D printing for medical device fabrication
One hundred and ten relevant papers were identified regarding direct 3D printing of medical devices and their use in treatment of patients. In 4 papers,15–18 several individual studies were described, and in 18 papers, more than one type of medical device was fabricated; one study19 was included in three different papers, and one20 in two different papers. Thus, a total of 119 studies detailing 140 medical devices were reviewed.
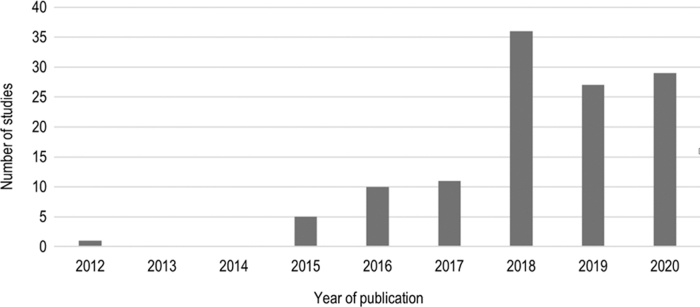
The majority of studies were published in 2018 (36), followed by 2020 (29 to date) and 2019 (27). There were considerably fewer studies before 2018 that met the inclusion criteria (Fig. 2).
FIG. 2.
Studies of three-dimensional printed medical device use on patients by publication year.
Fields of application and medical conditions addressed by direct 3D printing of medical devices
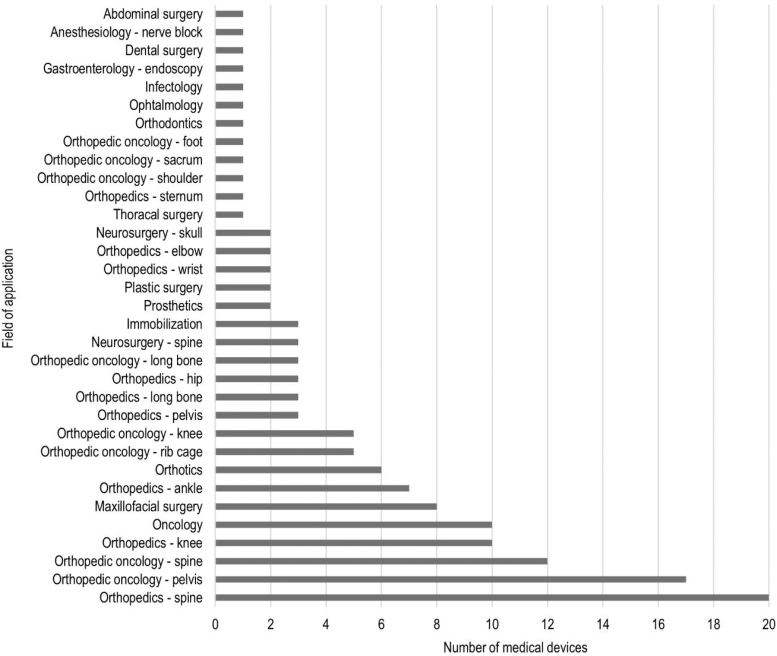
The use of 3D printing for medical device fabrication was most prevalent in surgery, especially in orthopedics (36%) and orthopedic oncology (32%), followed by maxillofacial surgery (6%), neurosurgery (4%), and plastic surgery (1%). Among nonsurgical applications, 3D printing was used in oncology (7%), followed by orthotics (4%), immobilization (2%), and prosthetics (1%). Single cases of 3D printing were reported in abdominal, thoracic, and dental surgery, anesthesiology, gastroenterology, infectology, ophthalmology, and orthodontics (Fig. 3).
FIG. 3.
Number of studies by medical application.
The majority of participants treated with 3D-printed medical devices were orthopedic patients, predominantly undergoing spinal, knee, or pelvic/hip surgery. Patients with spinal complaints presented with congenital or degenerative conditions causing spinal instability, neurological symptoms, pain, fractures, or deformities. At the knee, patients suffered from end-stage osteoarthritis, rheumatoid arthritis, post-traumatic and postoperative osteoarthrosis; and at the pelvis/hip from pain or instability due to fracture, endoprosthesis loosening, or bone defects. Other orthopedic diagnoses were limb deformity, traumatic fracture, fragment nonunion, severe arthritis or instrument failure after fracture reduction, epiphysiodesis, avascular necrosis, femoral head osteonecrosis, and osteomyelitis.
The second most frequent use of 3D printing was in relation to pelvic/sacral and spinal tumors. Other diagnoses in orthopedic oncology were primary or secondary sternum/rib, upper and lower limb tumors. In oncology, the patients presented with small peripheral lung nodules; carcinoma or mycosis fungoides of the head; and pancreatic, liver, or breast cancer. Airway collapse due to tracheobronchomalacia was prevented in infant patients, as were complications after hypotonic perioral musculature and macroglossia. Abdominal surgery was performed to plug an enteroatmospheric fistula. In gastroenterology, endoscopic treatment was facilitated for gastric and esophageal conditions.
In maxillofacial surgery, the conditions treated were tumor-related bone defects and osteoarthritis of temporomandibular joints. In dental surgery, agenesis of mandibular premolars was treated in teenage patients. In neurosurgery, patients were treated for hydrocephalus, spinal compression, and neurogenic constipation. Heminasal deformities and unilateral microtia were treated in plastic surgery.
Immobilization was performed on patients with traumatic wrist and lower-limb fractures. Orthoses were used to alleviate plantar fasciitis, bilateral flatfoot, and impairment of hand/wrist function due to stroke or spinal cord injury. Prostheses were made for patients with traumatic index finger amputation, and for a patient after rhinectomy. In anesthesiology, a supraclavicular block was facilitated on a patient with morbid obesity. Different conditions of the cornea were treated with keratoplasty in ophthalmology, and a nasal swab was used to diagnose SARS-CoV-2 infection. For detailed information on participants' medical conditions, see Tables 1–3.
Table 1.
Reviewed Studies Detailing Three-Dimensional-Printed Implantable Devices
| Field of application | Device description[Ref.] | 3D-printing technology Printer (producer) Material | Patients Medical condition | Device use and clinical outcome |
|---|---|---|---|---|
| Maxillofacial surgery | Patient-specific mandibular prosthesis for reconstruction after mandibular en bloc resection.133 | EBM Arcam Q10 (Arcam AB) Ti6Al4V Manufactured by Prototal AB |
♀, 84 yrs Postinitial ablative surgery due to squamous cell carcinoma of the left mandible. |
Successful mandible reconstruction, with short operating time, good esthetic outcome, implant stability, and high level of patient satisfaction. Reduced recovery period, and no complications at the 9-month follow-up. |
| Patient-specific temporomandibular joint prosthesis (condylar component).25 | SLM Eden 260V (Stratasys) Titanium-64 |
♀, 48 yrs Grade-5 osteoarthritis of temporomandibular joint. |
Successful prosthesis implantation, reduced patient pain, and increased intercisal opening distance. | |
| Patient-specific osteosynthesis plates for jaw reconstruction after tumor resection.23 | SLM N/A Pure titanium grade 2 |
9♀, 1♂, 22–75 yrs (mean 53) Bone defect due to osteoma, osteosarcoma, squamous cell carcinoma, ameloblastoma resection, and secondary mandibular defect due to clear cell carcinoma treatment. |
Successful, simplified, and highly accurate reconstruction, with precise adaptation of plates to bone surface without the need for intraoperative bending of plates, and no major adverse events at the 6.5-month follow-up. | |
| Patient-specific single-unit maxillary reconstruction plate for midface reconstruction with free fibula flap after tumor resection—TruMatch CMF Solutions (DePuy Synthes, West Chester, PA).24 | N/A N/A Titanium |
♀, 62 Mucoepidermoid carcinoma of maxilla. |
Successful, complication-free maxilla and midface reconstruction, with no unplanned surgical manipulation and shorter operating time. | |
| Orthopedic oncology | ||||
| Rib cage | Patient-specific sternum and rib implant.15 | EBM Arcam A1 (Arcam) Titanium Manufactured by Anatomics (Melbourne, Australia) |
♂, 54 yrs Chondrosarcoma of the sternum and ribs. |
Successful tumor resection, and replacement of sternum and ribs. |
| Patient-specific sternum and rib implant.15 | EBM Arcam A1 (Arcam) Titanium Manufactured by Anatomics |
N/A Mediastinal germ-cell tumor infiltrating the sternum. |
Successful tumor resection, and replacement of sternum and ribs. | |
| Patient-specific sternal endoprosthesis for reconstruction after major sternum resection.21 | EBM Arcam A1 (Arcam AB) Titanium/PoreStar Manufactured by Anatomics |
♀, 39 yrs Sternal metastases of breast adenocarcinoma. |
Successful en bloc resection of tumor and rib-cage reconstruction, with return to normal physical and respiratory function, exceptional cosmetic result, no sign of implant infection, seroma formation, or loosening at the 3-month follow-up. | |
| Shoulder | Patient-specific shoulder prosthesis for revision total shoulder arthroplasty with severe bone defects. Shoulder anatomical model.26 | EBM N/A Ti6Al4V |
♂, 47 yrs Loosening of prosthesis with severe bone defects 6 yrs after shoulder chondrosarcoma surgery. |
Successful reconstruction of shoulder, no change of prosthetic position at the 12-month follow-up, and satisfactory shoulder function. |
| Spine | Patient-specific self-stabilizing artificial vertebral body for spine reconstruction after C2 spondylectomy.34 | EBM N/A Titanium alloy |
♂, 12 yrs Ewing sarcoma of cervical spine. |
Successful tumor resection and replacement of C2 vertebral body. Implant osseointegration without implant subsidence or displacement, no residual neurologic deficits at the 12-month follow-up. |
| Patient-specific self-stabilizing artificial vertebral body for reconstruction of spine after radical resection of C2–C4 metastatic lesion.33 | EBM N/A (Arcam AB) Ti6Al4V |
♀, 53 yrs Metastatic C2–C4 spinal lesion from papillary thyroid carcinoma. |
Successful tumor resection and spine reconstruction. Removal of skull-neck-thorax orthosis without implant displacement or subsidence at the 12-month follow-up. | |
| Patient-specific spine fusion implant.15 | EBM Arcam A1 (Arcam) Titanium Manufactured by Anatomics |
♂, 63 yrs C1–C2 destructive chordoma. |
Successful spine fusion. | |
| Patient-specific vertebral-body implant for reconstruction of segmental defects after en bloc spinal tumor resection.15 | EBM N/A (Arcam AB) Ti6Al4V Manufactured by Anatomics |
7♀, 6♂, 18–73 yrs (mean 47) Primary spinal tumor, solitary bone metastasis in the thoracolumbar spine. |
Successful resection of vertebral bodies and reconstruction with implants. Subsidence into the adjacent vertebral bodies at bone–implant interfaces in all patients, clinically relevant only in 1 case. |
|
| Patient-specific L1–L3 prosthesis for reconstruction after total en bloc spondylectomy.42 | N/A N/A Titanium alloy Manufactured by AK Medical |
♂, 51 yrs Recurrent giant cell tumor 3 yrs after Th12-L4 fusion due to primary tumor. |
Successful lumbo-sacral spine reconstruction with significant functional recovery from neurologic deficits and improved mobility at the 3-month follow-up, no instrumentation failure at the 8-month follow-up. | |
| Patient-specific spinal implant filled with allogenic bone graft for reconstruction. Anatomical model for preoperative planning.16 | N/A N/A Ti6Al4V Manufactured by 4WEB Medical |
♂, 18 yrs Progressive L5 giant cell tumor |
Better preoperative planning, simplified operative procedure, and improved reconstruction. | |
| Patient-specific spinal implant with locking mechanism to the adjacent vertebrae.16 | N/A N/A Ti6Al4V Manufactured by 4WEB Medical |
♀, 13 yrs Ewing sarcoma of C3 vertebra. |
Better preoperative planning, simplified operative procedure, and improved reconstruction. | |
| Patient-specific spinal implant for reconstruction and deformity correction.16 | N/A N/A Ti6Al4V Manufactured by 4WEB Medical |
♂, 17 yrs Tumor recurrence and local kyphotic deformity 2 yrs after posterior decompression of T3 aggressive hemangioma. |
Better preoperative planning, simplified operative procedure, and improved reconstruction. | |
| Patient-specific implant for occipitocervical screw fixation.41 | SLM AM250 (Renishaw) Ti64 Manufactured by Anatomics |
♀, 79 yrs Pathologic fracture of C1 with subluxation due to metastatic breast cancer. |
Satisfactory screw placement, simplified procedure, no intra-/postoperative complications. Satisfactory occipitocervical alignment and functional outcome, no evidence of implant loosening or dysfunction at the 6-month follow-up. |
|
| Patient-specific prosthesis for upper cervical reconstruction after C2 spondylectomy.32 | EBM N/A (Arcam AB) Ti6Al4V Manufactured by AK Medical |
7♀, 2♂, 12–59 yrs (mean 31) Upper cervical-spine Ewing sarcoma, giant cell tumor, paraganglioma, hemangioendothelioma, and chordoma. |
Successful spine reconstruction with reliable primary immediate postoperative stability, and possible ambulation on first postoperative day with a Philadelphia collar. Larger prosthesis-bone contact area and superior match of interfacing surfaces. Osseointegration from adjacent vertebra and normal activity without pain associated with spinal instability at the 12-month follow-up. | |
| Patient-specific cervical-spine interbody implant packed with allograft.35 | EBM Arcam A1 (Arcam AB) Titanium alloy (Grade 5) Manufactured by Anatomics and CSIRO |
1♀, 2♂, 56–72 yrs Lytic lesion of C2 vertebra due to thyroid medullary carcinoma metastasis, multiple myeloma spread, and rheumatoid arthritis. |
Successful resection and reconstruction of anterior cervical column with preserved atlanto-occipital mobility, and without pain, neurological or radiological abnormalities at 4-/6-/14-month follow-up. | |
| Sacrum | Patient-specific sacral endoprosthesis for spinopelvic-continuity reconstruction.43 | DMLS N/A Ti6AI4V Manufactured by 3D Systems using K2M's Lamellar 3D Titanium Technology |
♀, 67 yrs Pseudarthrosis and instrumentation failure of the lumbosacral junction after TES and reconstruction due to sacral chordoma. |
Successful spinopelvic-continuity reconstruction with excellent bony incorporation and assisted short-distance walking at the 18-month follow-up. |
| Pelvis | Patient-specific pelvic implant for prosthetic reconstruction of the bone defect after partial acetabular resection.53 | SLM N/A Ti6Al4V (medical grade) |
♂, 65 yrs Osteolytic destruction due to pelvic chondrosarcoma. |
Successful pelvic resection and reconstruction without neurovascular complications or wound infection. Walking with full weight bearing 4 weeks after surgery. Satisfactory implant alignment, no evidence of implant loosening, and independent walking without pain at 10 months follow-up. |
| Patient-specific implant for reconstruction after pelvic tumor resection.50 | EBM Arcam A1 (Arcam) Titanium alloy |
62 yrs Primary bone sarcoma of the pelvis. |
Successful pelvic resection and reconstruction with the implant. | |
| Patient-specific sacral endoprosthesis for spinopelvic continuity reconstruction after TES.45 | EBM N/A Titanium alloy |
♂, 62 yrs Local recurrence of sacral chordoma 2 yrs postsurgery |
Two fractured screws at 8 months follow-up, asymptomatic; no pain or spinopelvic instability at the 1-year follow-up. | |
| Patient-specific hemisacral implant for pelvic reconstruction after hemisacrectomy due to osteosarcoma.44 | EBM Arcam A1 (ArcamAB) Ti-6Al-4V (extra-low interstitial, medical grade) |
♀, 16 yrs Chondroblastic osteosarcoma of the sacrum, after neoadjuvant chemotherapy. |
Successful sacrum reconstruction with the implant, slight postoperative pain, and walking without assistance at 2 weeks postoperatively. Excellent bony union on the densely structured strut surface and loosely structured porous mesh at the 1-year follow-up. | |
| Patient-specific hemipelvic prosthesis for pelvis reconstruction after en bloc resection of peri-acetabular tumor.54 | N/A N/A Titanium alloy |
6♀, 5♂, 21–63 yrs (mean 47) Peri-acetabular malignant bone tumor. |
Successful en bloc resection and pelvis reconstruction. Acceptable functional results without severe complications, alleviation of pain 2 weeks after surgery, and 2 cases of hip dislocation. No evidence of aseptic loosening, bone resorption, or periprosthetic fractures at 6–24 months' follow-up. | |
| Sacral endoprosthesis for spinopelvic continuity reconstruction after TES.46 | EBM N/A Titanium alloy |
Endoprosthesis group: 3♀, 7♂; 15–68 yrs (mean 40) Control group: 22 patients Malignant tumor of sacrum |
Spinopelvic stability better than with conventional reconstructive methods. No statistically significant differences in intraoperative hemorrhage and perioperative complication rates. Three cases of implant failure due to breakage of screws 7, 9, and 16 months postoperation; 1 re-operation required; and long-term spinopelvic stability secured by bone-prosthesis osseointegration at the porous bone-implant interface. | |
| Modified anatomic template for reconstruction after zone II and III borderline pelvic tumor resection.51 | EBM N/A Titanium alloy |
16♀, 22♂, 38–92 yrs 19 guide, 19 control Zone II and III borderline malignant pelvic tumor. |
Greater accuracy of tumor resection, simplified operation, shorter operating time, smaller intraoperative blood loss, lower tumor recurrence rate, and significantly higher rate of implant loosening compared with the conventional approach. | |
| Patient-specific sacral implant for reconstruction after total piecemeal tumor resection.47 | SLM EOS M290 (EOS) Ti-6Al-4V (medical-grade) |
2♀, 3♂, 31–53 yrs (mean 42) Giant cell tumor of the sacrum. |
Successful total piecemeal resection and reconstruction of sacrum without serious complications, neurogenic bladder dysfunction, fecal incontinence, or gait disturbance. Significant relief of pain, walking as early as 2 weeks postoperatively. No instrumentation failure at ∼17-month follow-up. Satisfactory bone fusion in CT. | |
| Patient-specific pelvic implant for reconstruction of bone defects after wide tumor resection.18 | N/A N/A Titanium Manufactured by Instrumentaria |
2♂, 13–15 yrs; ♀, 67 yrs Ewing sarcoma, Chondrosarcoma of pelvis. |
No implant-related complications in 2 cases, infectious hip dislocation in a 67-year old patient. All lethal outcomes due to disease 10–36 months after surgery. | |
| Hip | Patient-specific hemipelvic replacement implant.15 | EBM Arcam A1 (Arcam) Titanium Manufactured by Anatomics |
♀, 17 yrs Failure of viable bone postradiation of pelvic iliac crest sarcoma, and fixation breaking in situ. |
Successful implantation, precise compatibility with off-the-shelf hip device. |
| Knee | Patient-specific proximal tibia block for bone defect closure in combination with standard knee prosthesis.56 | EBM N/A Ti6Al4V Manufactured by AK Medical |
3♀, 1♂, 35–68 yrs Giant cell tumor of proximal tibia. |
Successful en bloc resection of the proximal tibia and closure of bone defect. No signs of prosthesis fracture, loosening, or other relevant complications at the 7-month follow-up. |
| Patient-specific uncemented prosthesis for reconstruction of tibial metaphyseal defects with epiphysis preservation.57 | EBM ARCAM Q10 (Arcam AB) Titanium alloy |
♂, 18 yrs Massive tibial metaphyseal defect 33 months after resection of tibial metaphyseal osteosarcoma and 12 months after surgery due to chronic allograft rejection. |
Successful reconstruction of tibial metaphyseal defect with good integration of prosthesis, and no prosthesis-related complication. Notable functional improvement with satisfactory limb function, no loosening or breakage of prosthesis at the 26-month follow-up. | |
| Patient-specific intercalary prosthesis for reconstruction after joint-preserving intercalary resection of knee metaphyseal bone tumor.58 | EBM Arcam A1 (Arcam AB) Titanium alloy Manufactured by Thytec Shanghai |
N/A of 5♀, 7♂, 7–59 yrs (mean 37.3) Knee metaphyseal malignant bone tumor. |
Accurate en bloc resection and matching between residual bone and prosthesis, without prosthetic-related complications (aseptic loosening, peri-prosthetic fracture) at follow-up. Reliable reconstruction with possible early partial weightbearing. Overall, 10 satisfactory functional outcomes and 2 cases of unsatisfactory knee RoM at 7–32 months' follow-up. |
|
| Patient-specific intercalary prosthesis for reconstruction after joint-preserving intercalary resection of knee metaphyseal bone tumor.58 | SLM EOS M290 (EOS) Titanium alloy Manufactured by Thytec Shanghai |
N/A of 5♀, 7♂, 7–59 yrs (mean 37.3) Knee metaphyseal malignant bone tumor. |
Accurate en bloc resection and matching between residual bone and prosthesis, without prosthetic-related complications (aseptic loosening, peri-prosthetic fracture) at follow-up. Reliable reconstruction with possible early partial weightbearing. Overall, 10 satisfactory functional outcomes and 2 cases of unsatisfactory knee RoM at 7–32 months' follow-up. | |
| Ankle | Patient-specific calcaneus implant.15 | EBM Arcam A1 (Arcam) Titanium Manufactured by Anatomics |
♂, 71 yrs Chronic calcaneal pain due to chondrosarcoma. |
Successful replacement of calcaneal bone. |
| Long bone | Patient-specific humeral implant for reconstruction of bone defects after wide tumor resection.18 | N/A N/A Titanium Manufactured by Instrumentaria |
3♀, 3♂, 5–16 yrs (mean 13) Osteosarcoma, Ewing sarcoma of humerus. |
No implant-related complications in 4 cases. Limited shoulder and elbow range of motion after total humeral endoprosthesis in a 5-year-old patient, dislocation of partial humerus endoprosthesis in a 13-year-old patient. One lethal outcome due to disease 12 months after surgery. |
| Patient-specific radial implant for reconstruction of bone defects after wide tumor resection.18 | N/A N/A N/A Manufactured by Instrumentaria |
2♀, 5–12 yrs Osteosarcoma, Ewing sarcoma of radius. |
Successful tumor resection and reconstruction. One distal radius implant dislocation during growth in the 12-year-old patient. | |
| Orthopedics | ||||
| Elbow | Patient-specific prosthesis for reconstruction of elbow joint with severe bone defect.27 | EBM Arcam Q10 plus (Arcam AB) Ti6Al4V |
♂, 60 yrs Fracture displacement and fixator breakage in distal humerus 10 years' post-traumatic fracture reduction. |
Successful total elbow arthroplasty without severe complications during the 36-month follow-up. |
| Hip | Ossis® patient-specific tri-flanged acetabular implant for reconstruction of severe acetabular defects in total hip arthroplasty. Plastic implant trial.52 | EBM N/A Ti6Al4V Manufactured by Ossis |
16♀, 20♂, 43–89 yrs (mean 68) (follow-up patients) Severe osteolysis after total hip arthroplasty; acetabular loosening due to infection, metallosis, or trauma; acetabular defects due to tumor or multiple surgeries for hip dysplasia. |
Successful acetabular reconstruction, with improvement in functional and radiographic outcomes comparable to similar designs. No aseptic loosening at 24–108 months' follow-up. |
| Hip femur | Patient-specific aMace® Acetabular Revision System (Mobelife NV) for reconstruction of extensive acetabular defects in revision total hip arthroplasty.49 | SLM N/A Titanium |
8♀, 1♂, 40–79 yrs (mean 67) Extensive acetabular defects with aseptic loosening of the acetabular component after total hip arthroplasty and 1–8 prior revisions. |
9 revision total hip arthroplasties followed by 5 revision surgeries due to nonimplant-associated complications, and 1 due to implant failure. |
| Trabecular bone reconstruction system for femoral head osteonecrosis reconstruction.55 | EBM N/A (Arcam AB) Ti6Al4V |
11♀, 19♂, 22–54 yrs (mean 42) Early femoral head osteonecrosis. |
Successful trabecular bone reconstruction without signs of stress shielding, trabecular bone enhancement, infection, or rejection. Significant improvement in joint function and mobility, and decreased pain at the 12-month follow-up; slight decline in joint function and mobility; and increase in pain at the 24-month follow-up. Postoperative improvement rates were 100% for ARCO stage IIA, 70% for IIB, and 0% in cases with a large necrotic area (IIC). | |
| Knee | Cementless highly porous titanium-coated baseplate Triathlon Tritanium Tibial Baseplate for total knee arthroplasty59 | N/A N/A N/A (Titanium coating) Manufactured by Stryker Orthopaedics |
363♀, 133♂, 33–88 yrs (mean 66) Knee osteoarthritis, rheumatoid arthritis, and osteonecrosis. |
Overall, 568 successful cementless total knee arthroplasty procedures with 99% implant survivorship rate at 3 yrs' follow-up. 4 implant failures due to aseptic loosening, and no signs of baseplate-related complications in other patients at 24–48 months' follow-up. |
| Rib cage | Patient-specific sternum and rib implant.20 | EBM Arcam A1 (Arcam) Titanium/PoreStar |
♂, 54 yrs 5 yrs postpartial removal of sternum and temporary reconstruction with an autologous muscle flap due to osteomyelitis. |
Successful reconstruction of the sternum with uncomplicated recovery and improved chest wall/respiratory function. |
| Spine | Patient-specific spinal interbody fusion cage, packed with bone graft.29 | N/A N/A Titanium Manufactured by Anatomics |
♀, 52 yrs Congenital L5 hemivertebra, segmental kyphosis with loss of lordosis, and degenerative changes. |
Successful spinal reconstruction with significant improvements in back and leg visual analog scale scores, disability index, and with solid mature fusion, no failure of fixation or subsidence at the 12-month follow-up. |
| Spine sacroiliac | Patient-specific spinal fusion cage and adaptor for implant insertion tool augmentation.15 | SLM SLM-250 (SLM Solutions) Titanium Manufactured by Anatomics |
♀, 39 yrs Congenital deformity of L5 with associated loss of spinal curvature and subsequent degeneration. |
Successful spinal fusion with no implant failure, movement, or subsidence at the 1-year follow-up. |
| Stereotactic patient-specific implant for atlantoaxial spine stabilization.36 | EBM Arcam A1 (Arcam) Ti6AlV4 |
3♀, 65–76 yrs Unilateral atlantoaxial osteoarthritis, unsuccessfully treated by conservative therapy. |
Successful placement of patient-specific implants with C1–C2 transarticular and C1 posterior arch screws. | |
| Patient-specific contoured iliolumbar (L2-pelvis) implant for revision lumbosacral surgery.37 | EBM Arcam A1 (Arcam AB) Ti64 Manufactured by Anatomics |
♀, 72 yrs Sciatica, complex L5–S1 pseudoarthrosis, 2 months after L2–S1 fixation surgery for symptomatic degenerative scoliosis. |
Successful revision lumbosacral surgery, resolution of symptoms without implant dysfunction at the 6-month follow-up. | |
| Patient-specific implantable interbody cages filled with allografts for interbody fusion in osteoporotic lumbar-spine fractures.39 | EBM Arcam A1 (Arcam AB) Ti6Al4V Manufactured by Anatomics |
♀, 74 yrs Vertebral collapse, lateral recess, and foraminal stenosis due to previous osteoporotic fractures at L2 and L3. |
Successful reconstruction of lumbar spine with minimal invasion, accurate fit between the implant and recipient surface, restoration of lost disk space and segmental lordosis, and improved coronal deformity. Uneventful recovery, mobilization from the first postoperative day. Significant clinical improvement and evidence of interbody fusion at 6 months' follow-up. | |
| Patient-specific prosthesis for vertebral-body replacement due to single-/multilevel cervical spondylotic myelopathy.28 | SLS EOSINT P800 (EOS) PEKK (polyether ketone ketone) Manufactured by Medicrea |
1♀, 5♂, 54–81 yrs (mean 67) Single-/double-level cervical spondylotic myelopathy. |
Successful spine resection and reconstruction, with favorable clinical and radiological outcomes, no intraoperative/postoperative complications, or hyperlordotic/kyphotic deformation. Overall, 2 cases of subsidence (1- and 3-mm) in anterior corporeal height at the 12-month follow-up, possibly due to mispositioning of the prosthesis at implantation. | |
| Personalized implantable prosthesis for anterior spine stabilization.30 | DMLS DMP320 (3D Systems) Ti6Al4V ELI grade 23 |
1♂, 16 yrs, 1♀, 68 yrs Incomplete paralysis due to severe thoracic-spine kyphotic deformity from neurofibromatosis type 1. Paralysis due to severe cervicothoracic dissociation from Gorham's vanishing bone disease. |
Successful implantation of prosthesis 6 months/6 weeks after emergency posterior stabilization surgery, with incorporation of prosthesis and no signs of loosening at 6-/25-month follow-up. | |
| Medussa-PL (Medyssey) spacer for posterior lumbar interbody fusion.40 | EBM N/A Ti6Al-4V ELI |
Overall, 40 patients, 51–73 yrs (mean 64) Lumbar degenerative spondylolisthesis, isthmic spondylolisthesis, degenerative spinal stenosis, disk disease, and failed diskectomy syndrome. |
Successful 1-/2-level posterior lumbar interbody fusion of 53 segments with satisfactory radiographic and clinical results, and without significant complications. No significant changes in interbody height, segmental instability, incomplete bone bridge formation, or pseudarthrosis at the 12-month follow-up. | |
| Fenestrated triangular titanium implant for invasive sacroiliac joint fusion—iFuse-3D™ (SI-BONE)38 | EBM N/A (Arcam AB) Ti6Al4V ELI |
39♀, 12♂, 21–70 yrs (mean 53 yrs) Patients with sacroiliac-joint pain, ≥6 months irresponsive to conservative treatment, Oswestry Disability Index score ≥30%, average pain score ≥50 (0–100 mm VAS). |
Overall, 46 unilateral, 5 bilateral SIJFs. No technical complications, device malfunctions, or adverse events during procedure. Significant rapid and sustained improvement in pain, disability and quality of life scores, with functional improvements, substantial opioid use reduction, and radiographic evidence of accelerated bony fusion. | |
| Ankle | Patient-specific truss cage with tibiotalocalcaneal arthrodesis for salvage of persistent distal tibia nonunion.62 | N/A N/A Titanium Manufactured by 4WEB Medical |
♂, 63 yrs Distal tibia nonunion 1 year post-traumatic fracture, external fixator, and cast treatment. |
Successful ankle reconstruction with minimal pain, no wound complications, and ability to ambulate and work independently without an assistive device at the 1-year follow-up. Subtle anterior translation of the foot on the tibia, which may disrupt the long-term load distribution of the foot and ankle. |
| Patient-specific truss cage with talar component, filled with bone allograft for revisional total ankle replacement.61 | N/A N/A Titanium Manufactured by 4WEB Medical |
♂, 54 yrs Osteolysis and severe subsidence of talar component 7 yrs after total ankle replacement due to post-traumatic ankle and subtalar-joint arthritis. |
Successful total ankle replacement, with excellent alignment and placement of the implant, no subsidence or osteolysis, and good bony ingrowth into the trabecular portion of the talar truss at the 4-month follow-up. Full activity with no restrictions at the 11-month follow-up. | |
| Patient-specific, ligament-compatible ankle prosthesis (based on BOX® ankle prosthesis [MatOrtho, United Kingdom] design).60 | N/A N/A Cr-Co-Mo |
♂, 57 yrs Severe post-traumatic ankle–joint arthritis. |
Successful bone resection and prosthesis implantation. Good implant positioning and alignment. Excellent clinical scores and functional abilities at 4 months' follow-up, satisfactory joint moment, and normal muscle-activation timing. | |
| Patient-specific implant cage for treatment of foot and ankle pathologies.63 | N/A N/A Ti6Al4V Manufactured by 4WEB Medical |
9♀, 6♂, 22–74 yrs (mean 53) Complex large bony defects after talus/tibia trauma, tibial/tibiotalocalcaneal nonunion, talus avascular necrosis, failed total ankle arthroplasty, and hindfoot valgus deformity. |
Overall, 15 reconstructions of tibia, ankle, or hindfoot, with significant improvement in pain and functional outcome, radiographic fusion in 13 patients at 2.6–8.2-months. 2 failures: 1 early deep infection 2 weeks postoperation, 1 nonunion at the 24-month follow-up. No cases of hardware failure. | |
| Patient-specific bioresorbable external airway splint that adjusts to tissue growth for prevention of airway collapse during respiration.22 | SLS Formiga P100 (EOS) 96% CAPA 6501 PCL, 4% hydroxyapatite |
9♀, 6♂, 3–25 months (median 8 months) Severe tracheobronchomalacia with high risk of death/permanent disability, 14 tracheostomy- and ventilator-dependent patients. |
Overall, 10 tracheal, 19 main-stem bronchus splints implanted in 15 children. Follow-up at 0.3–77.1 months: 12 long-term survivors, significant clinical benefits in all surviving patients (resolution of pulmonary and extra-pulmonary complications), continued growth of primary airways. Overall, 1 death possibly due to splint displacement. Higher success rate compared with alternative approaches using internal stents and prostheses for airway stabilization. | |
3D, three-dimensional; ABS, acrylonitrile butadiene styrene; Cr-Co-Mo, cobalt chromium molybdenum; DLP, digital light processing; DMLS, direct metal laser sintering; EBM, electron beam melting; EVA, ethylene vinyl acetate; FDM, fused deposition modeling; MJ, material jetting; N/A, information not provided; PA, polyamide (nylon); PCL, polycaprolactone; PEKK, polyetherketoneketone; PETG, polyethylene terephthalate glycol; PLA, polylactic acid; PMMA, polymethyl methacrylate; PPSF, polyphenylsulfone; SLA, stereolithography; SLM, selective laser melting; SLS, selective laser sintering; TES, total en bloc sacrectomy; TPU, thermoplastic polyurethane; yrs, years.
Table 3.
Reviewed Studies Detailing Other Three-Dimensional-Printed Medical Devices
| Field of application | Device description[Ref.] | 3D-printing technology Printer (producer) Material | Patients Medical condition | Device use and clinical outcome |
|---|---|---|---|---|
| Abdominal surgery | Patient-specific hollow, curving pipe stent to plug enteroatmospheric fistula.106 | FDM N/A TPU |
♂, 33 yrs Enteroatmospheric fistula with intermittent high fever and cachexia. |
Successful implantation of stent into the bowel with no obstruction around the fistulous-tract orifice, decrease in enteric fistula effluent amount, and increased stool frequency and capacity. Enteral nutrition restored by nasal feeding 4 days after surgery, without abnormal or subjective discomfort throughout the process. No sign of pyrexia or obvious infection, improved general condition at the 7-day follow-up. |
| Dental surgery | Patient-specific donor tooth replica to guide the preparation of artificial tooth sockets before donor tooth extraction for autotransplantation.103 | SLM LayerWise (Layer-Wise NV, 3D Systems) Titanium alloy grade 23 |
2♀, 1♂, 11–13 yrs Agenesis of mandibular premolars, patients indicated for orthodontic extraction therapy of maxillary premolars. |
Successful autotransplantation of 5 premolars, with immediate good fit (single fitting attempts), and decreased extraalveolar (15–45 s) and procedural times (20–30 min). |
| Gastro-enterology | Tailored endoscope caps for mucosal resection, submucosal dissection, Trucut biopsy, and peroral endoscopic myotomy.105 | MJ Objet260 Connex (Stratasys) Silicone |
14♀, 21♂, 33–78 yrs (mean 56) Gastric epithelial neoplasia, esophageal subepithelial tumor, and esophageal achalasia. |
Increased ease and shorter duration of endoscopic procedure, with successful outcomes and without complications. |
| Immobilization | ||||
| Wrist | Personalized hand–wrist–arm cast.110 | FDM F370 (Stratasys) Stratasys ABS M30 |
Five pediatric patients Wrist fracture. |
Patient-specific cast for wrist immobilization produced and applied by clinicians in day hospital, using a system for 3D scanning and semi-automatic 3D-modeling; manual generation of ventilation holes by an expert CAD modeler. |
| Leg | Customized external fixator in treating long bone fractures (Q-Fixator).112 | SLA SPS600B (Shaanxi Hengtong Intelligent Machine) Photosensitive resin |
3♂, 25–36 yrs Traumatic tibial shaft fracture. |
Minimally invasive, accurate, experience-independent reduction and appropriate fixation without exposure to X-rays. Successful fracture healing without fixation pin loosening, pin site infection, or other complications at the 1-year follow-up. Fixator removal after 20–25 weeks. |
| Patient-specific fracture external fixator.111 | SLA SPS600B (Shaanxi Hengtong Intelligent Machine) Photosensitive resin |
3 patients Tibial fracture |
Successful repositioning of fragments, based on prior computer simulation of fracture reduction, without intraoperative exposure to X-rays. Fracture healing success monitoring during 20–25 weeks of external fixator use. | |
| Infectology | Nasal swab.123 | SLS N/A PA2200 |
Overall, 50 hospital staff, 2 patients with COVID-19 | Sample collection with 3D-printed and conventional swab (Copan ESwab) in each patient. No significant differences in discomfort (median 5 points on a 10-point scale) were found between swabs. Overall, 67% participants preferred the 3D-printed swab, and 19% the conventional swab. According to health care providers, the swabs are easy to use, moderately easy to snap at the breakpoint, and provide a good balance between flexibility and rigidity. |
| Neurosurgery | ||||
| Skull | Single-use, nonmetallic self-retaining skin and soft tissue retractor for insertion of ventricular catheter for treatment of hydrocephalus. Adaptable shunt retainer.104 | N/A N/A UV-curable liquid resin |
♂, 85 yrs Normotensive hydrocephalus |
Successful ventricular shunt placement after unsuccessful attempts with standard stainless-steel retractor that caused intraoperative loss of signal of electromagnetic neuronavigation system due to interference with local magnetic field. |
| Orthopedics | ||||
| Spine | Patient-specific self-docking tubular retractors for the minimally invasive transforaminal approach in revision lumbosacral surgery.37 | SLS Eosint (EOS) PA-12 Manufactured by Anatomics |
♀, 72 yrs Sciatica, complex L5–S1 pseudoarthrosis, 2 months after L2–S1 fixation surgery for symptomatic degenerative scoliosis. |
Successful revision lumbosacral surgery, resolution of symptoms without implant dysfunction at the 6-month follow-up. |
| Oncology | ||||
| Radiotherapy | Patient-specific bolus for desired dose distribution in photon/modulated electron radiotherapy.17 | FDM MakerBot Z18 (MakerBot) PLA |
♂, 74 yrs Recurrent squamous cell carcinoma of nasal septum, basal cell carcinoma of posterior pinna, and upper face mycosis fungoides. |
Highly conformal bolus for radiotherapy, relative sparing of all the organs at risk distal to the target volume, while maintaining similar target volume coverage. |
| Patient-specific bolus for desired dose distribution in photon/modulated electron radiotherapy.17 | FDM LulzBot TAZ 5 (Aleph Objects) NinjaFlex TPU |
1♂, 1♀, 67–68 yrs Recurrent squamous cell carcinoma of nasal septum, basal cell carcinoma of posterior pinna, and upper face mycosis fungoides. |
Highly conformal bolus for radiotherapy, relative sparing of all the organs at risk distal to the target volume, while maintaining similar target volume coverage. | |
| Patient-specific bolus cap for delivering a uniform dose in total scalp irradiation.107 | MJ PolyJet J750 (Stratasys) Agilus-60 |
♂, 78 yrs Squamous cell carcinoma of the scalp. |
High conformality of bolus to patient scalp, 5.3% difference between measured and planned doses. The one-piece bolus was faster and easier to setup, with higher reproducibility of daily treatment compared with existing methods. | |
| Patient-specific bolus for chest wall radiotherapy.108 | FDM LulzBot TAZ 5 (Aleph Objects) PLA |
16♀, 38–83 yrs (median 61) Breast cancer, postmastectomy. |
Minimum 4 treatments with bolus. Better fit to the chest wall compared with standard sheet bolus. No difference in agreement with the treatment planning system. Reduced setup time, considerable time for fabrication, and quality assurance. | |
| Patient-specific radiotherapy bolus.109 | FDM Maker-Gear M2 (MakerGear) PLA/PHA |
4♀, 6♂, 55–84 yrs (mean 68) Basal cell carcinoma, plasmacytoma of nose, nasal cavity, lacrimal gland, ear, scalp, knee, and tibia. |
Successful use of boluses without issues with fit or comfort. In 9 of 12 cases, bolus bulk density within 3% of reference value, density uniformity as good as or better compared with traditional sheet bolus material. | |
| Brachytherapy | Patient-specific applicator for desired dose distribution in surface high-dose rate brachytherapy.17 | FDM LulzBot TAZ 5 (Aleph Objects) NinjaFlex TPU |
♂, 75 yrs Rapidly growing squamous cell carcinoma of nose and face. |
Highly conformal applicator for surface brachytherapy with adequate coverage. Relatively high dose to the left eye, owing to its proximity to the tumor. |
| Ophtalmology | Smart storage glide for preservation, transport, and easy insertion of lenticules into the recipient eye in Descemet stripping automated endothelial keratoplasty.122 | MJ Projet 3510 HD plus (3DZ) N/A |
Overall, 14 patients Fuchs' dystrophy, pseudophakic bullous keratopathy, posterior polymorphous dystrophy, and previous keratoplasty failure. |
Successful corneal transplantation, without difference in visual outcomes, postoperative endothelial cell loss, and complication rates compared with conventional procedures. Reduced surgical time and required surgical tools, eliminated complications related to tissue preparation and loading onto delivery tools. |
| Orthodontics | Patient-specific orthodontic palatal stimulation plate for hypotonic musculature stimulation, and improved tongue position/tonus in infants.121 | DLP Solflex 170 (VOCO) Methacrylate-based photosensitive resin |
One patient, 13 months Hypotonic perioral musculature and macroglossia due to Trisomy 21. |
Better fit, stayed in a place longer without adhesive cream compared with a conventionally produced plate. |
| Orthotics | ||||
| Hand | Patient-specific fingerboard for poststroke limb rehabilitation and prevention/treatment of finger spasm.115 | FDM N/A PLA |
5♀, 8♂, 68.3 ± 4.9 yrs ∼2 months poststroke |
Wearing of fingerboard for 2 h after rehabilitation exercises 3 times/day. Three patients discontinued use at the 3-week follow-up, and 2 patients at the 3-month follow-up. No skin allergy or hand swelling, improved grip strength, hand function and range of motion by varying degrees, decreased muscular tension by varying degrees, and no fingerboard failure at the 3-month follow-up. |
| EMG-controlled hand orthosis to enhance tenodesis grip.117 | FDM Moment2 (Moment) PLA |
1♀, 9♂, 31–65 yrs Chronic spinal cord injury (C4–C7) with stable impairment of hand function. |
Evaluation of strength and stability of grasp, palmar grasp torque, lateral pinch force, and eccentric load that the grasp could sustain, and functional independence in daily living. Significant improvement in the eating category of functional independence, no significant improvement grooming, bathing, clothing, or using small or relatively flat objects. Rating of orthosis dimensions 3.2/5.0, weight 3.8/5.0, adjustments 3.4/5.0, safety 4.1/5.0, durability 3.8/5.0, simplicity of use 3.9/5.0, comfort 3.8/5.0, effectiveness 4.5/5.0. 1 participant with spinal cord injury for 28 yrs, and flexor contracture of fingers and wrist performed worse using the orthosis. | |
| Dynamic hand device for improving dexterity and hand force in patients after stroke.118 | N/A UP Box (Go Hot Technologies) N/A |
Orthosis group: 5♂, 60 ± 8 yrs Control group: 1♀, 4♂, 57 ± 8 yrs >6 months poststroke with upper limb hemiparalysis. |
30-min onsite training (daily life tasks) 2 times/week, ≥30-min/day home training for the rest of the week for 4 weeks. Significant improvement in Box and Blocks Test, grasp force, lateral pinch force, and palmar pinch force after training; no significant differences compared with the control group. Larger motivation for training: improvement consistent at the 2-week follow-up; deterioration in control group. | |
| Wrist | Wrist orthosis for spasticity in chronic hemiparetic stroke.116 | N/A N/A Photosensitive resin |
3D-printing group: 5♀, 15♂, 55.2 ± 14.5 yrs Control group: 4F, 16M, 60.3 ± 9.8 yrs Chronic poststroke hemiparesis with wrist flexor spasticity. |
Overall, 6 weeks of wearing orthoses 4–8 h per day, for at least 30 min. Greater changes in reducing spasticity and swelling of the wrist, improving motor function and passive range of wrist extension compared with low-temperature thermoplastic plate orthosis. No differences in pain. No feeling of increased spasticity or skin allergy reactions throughout the wearing process. |
| Foot | Patient-specific therapeutic foot insole.114 | N/A Bodyarch X1 EVA |
3D-printing group: 15♀, 15♂; 31–58 yrs (mean 40) Control group: 15F, 15M; 33–60 yrs (mean 43) Bilateral plantar fasciitis. |
Overall, 9 weeks of insole use. Higher peak pressure in hallux and first metatarsal area, lower in mid-heel and lateral heel area at week 0 compared with conventional prefabricated insole. Lower comfort scores at week 8 compared with control group. |
| Patient-specific insole for midfoot load distribution.113 | N/A Bodyarch X1 Printer EVA Manufactured by Bodyarch |
Experimental group: 20♀, 20♂, 26–55 yrs (mean 39) Control group: 20♀, 20♂, 29–60 yrs (mean 42) Symptomatic bilateral flatfoot. |
Midfoot peak pressure significantly higher, contact areas of third and fourth metatarsal areas significantly smaller, no significant difference in heel and toe pressure, force, and contact area; significant improvement in comfort at week 8, significantly higher comfort score compared with prefabricated insoles. | |
| Plastic surgery | Patient-specific framework and contour guide for paramedian forehead flap heminasal reconstruction.101 | MJ Object30 Prime (Stratasys) MED 610 |
Guide group: 7♀, 3♂, 29–78 yrs (mean 43.6) Control group: 1♀, 9♂, 33–78 yrs (mean 49.3) Basal cell carcinoma, squamous cell carcinoma, congenital melanocytic nevus, benign alar tumor, alar trauma, and congenital deformity. |
Successful heminasal reconstruction with reduced operative time, no significant differences in alar height or base width between the native and reconstructed sides, and significant improvement in alar width and area symmetry compared with the conventional approach. |
| Cartilaginous-framework template for auricle reconstruction with autogenous costal cartilage and tissue-expanding technique.102 | FDM MakerBot Replicator 2 (MakerBot Industries) PLA |
10♀, 30♂, 6–29 yrs Unilateral microtia. |
Auricular reconstruction with superior accuracy and decreased surgical time compared with the conventional approach. No surgery-related complications. | |
| Prosthetics | Patient-specific antibacterial finger prosthesis.119 | FDM Ultimaker 2 extended (Ultimaker B.V.) PLACTIVE™ (PLA +1% Cu nanoparticles) |
2♂, 65 and 40 yrs Traumatic nondominant index finger amputation at the proximal phalanx. |
1-min Box and Block Test to assess unilateral gross dexterity; 2 weeks of prosthesis use for 12–15 h/week. Increase in manual gross dexterity, high patient satisfaction scores (dimensions 4.2–5.0, weight 4.7, adjustments 4.3, safety 5.0, durability 4.5, ease of use 4.5–5.0, comfort 5.0, effectiveness 4.5–4.6, device satisfaction 4.6–4.8). Effectiveness against bacteria: 98.95% MRSA, 99.99% S. aureus, and 95.03–99.99% E. coli. |
| Nasal prosthesis.120 | MJ (multi-material jetting) Objet Connex 500 (Objet Geometries) TangoPlus |
One patient 2 yrs after rhinectomy due to cancer. |
Only the indirectly-produced prosthesis was judged clinically viable and worth rating in terms of esthetic quality. Likert 5-point scale for evaluation of positional accuracy, shape, color, and quality of edge; 19 evaluators unaware of the manufacturing procedure. Significantly better esthetics compared with conventional prosthesis, especially edge quality. |
Technology employed in direct 3D printing of medical devices
In the reviewed literature, the most frequently used 3D-printing processes for medical device fabrication were electron beam melting (EBM; 29) and fused deposition modeling (FDM; 29), followed by selective laser sintering (SLS; 13), stereolithography (SLA; 9), selective laser melting (SLM; 10), and material jetting (MJ; 9); direct metal laser sintering and digital light processing (DLP) were only used twice. In 35 cases, the 3D-printing process was not specified.
The most common materials for medical devices were titanium/titanium alloy (including titanium/PoreStar composite and titanium coating; 55), polylactic acid (PLA; including PLA/polyhydroxyalkanoate [PHA] and PLACTIVE™; 19), polyamide (PA; 17), and photosensitive resin (including Dental SG and Somos® XC11122; 13). Other less used materials include acrylonitrile butadiene styrene (ABS), Acrylate resin, Agilus-60, cobalt chromium molybdenum (Cr-Co-Mo) alloy, ethylene vinyl acetate, high impact polystyrene (HIPS), MED610, polycaprolactone (PCL), polyetherketoneketone (PEKK), polyethylene terephthalate glycol (PETG), polymethyl methacrylate (PMMA), polyphenylsulfone, silicone, TangoPlus, tantalum, thermoplastic polyurethane (TPU; including NinjaFlex), and ULTEM™ 1010. In 11 cases, the material was not specified.
Overview of 3D-printed medical devices tested on patients
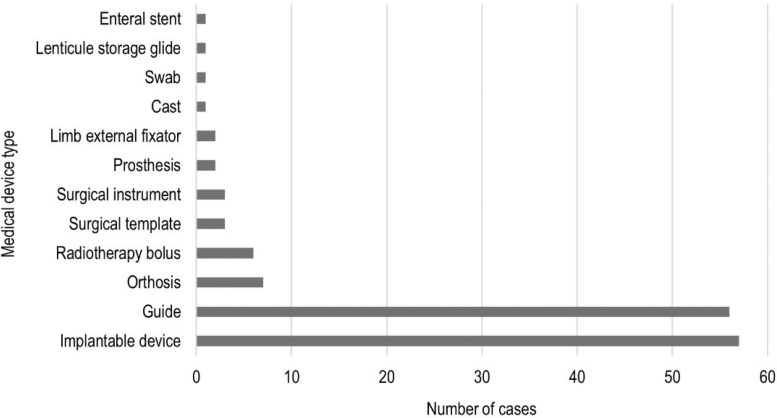
In the reviewed studies, 3D printing was employed to produce 57 implantable devices, 56 surgical guides, 6 radiotherapy boluses, 7 orthoses, 3 surgical templates, 3 surgical instruments, 3 immobilization devices, and 2 prostheses. A nasal swab, lenticule storage glide, and an enteral stent were also manufactured (Fig. 4). Several studies reported the use of 3D-printed anatomical models for presurgical planning, but these are beyond the scope of the present review.
FIG. 4.
Types of medical devices described in the reviewed studies.
The data extracted from the reviewed studies are provided in Table 1 for implantable devices, see Table 2 for guides, and Table 3 for other medical devices.
Table 2.
Reviewed Studies Detailing Three-Dimensional-Printed Cutting, Drill, and Navigation Guides
| Field of application | Device description[Ref.] | 3D-printing technology Printer (producer) Material | Patients Medical condition | Device use and clinical outcome |
|---|---|---|---|---|
| Anesthe-siology | Single-use articulating needle guide for in-plane ultrasound-guided nerve blocks.89 | SLA N/A (Formlabs) ABS |
1 patient, 54 yrs Morbid obesity (BMI = 56) |
Successful supraclavicular block with echogenic needle on first insertion. |
| Maxillofacial surgery | Patient-specific cutting and drill guides for jaw reconstruction after tumor resection.23 | MJ N/A MED610 Resin |
N/A of 9♀, 1♂, 22–75 yrs (mean 53) Bone defect due to osteoma, osteosarcoma, squamous cell carcinoma, ameloblastoma resection, and secondary mandibular defect due to clear cell carcinoma treatment. |
Successful, simplified, highly accurate reconstruction, with precise adaptation of plates to bone surface without the need for intraoperative bending of plates, and no major adverse events at the 6.5-month follow-up. |
| Patient-specific cutting and drill guides for jaw reconstruction after tumor resection.23 | FDM N/A ULTEM™ 1010 |
N/A of 9♀, 1♂, 22–75 yrs (mean 53) Bone defect due to osteoma, osteosarcoma, squamous cell carcinoma, ameloblastoma resection, and secondary mandibular defect due to clear cell carcinoma treatment. |
Successful, simplified, and highly accurate reconstruction, with precise adaptation of plates to bone surface without the need for intraoperative bending of plates, and no major adverse events at the 6.5-month follow-up. | |
| Patient-specific cutting guide for maxillary tumor resection—TruMatch CMF Solutions (DePuy Synthes).24 | N/A N/A PA |
♀, 62 Mucoepidermoid carcinoma of maxilla. |
Successful, complication-free tumor resection, with no unplanned surgical manipulation and shorter operating time. | |
| Patient-specific drill guide for midface reconstruction with patient-specific maxillary reconstruction plate—TruMatch CMF Solutions (DePuy Synthes).24 | N/A N/A Titanium |
♀, 62 Mucoepidermoid carcinoma of maxilla. |
Successful, complication-free maxilla and midface reconstruction, with no unplanned surgical manipulation and shorter operating time. | |
| Patient-specific osteotomy guides for minimally invasive mandible resection and reconstruction with a vascularized fibula flap.76 | FDM N/A (Ultimaker) PLA |
4♀, 3♂, 28–59 yrs (mean 49.3) Oral ameloblastoma, squamous cell carcinoma, myxoma. |
Successful mandible reconstruction with excellent fit and satisfactory footprint of the guides, and high correlation between the virtual and actual osteotomies. Overall, 2 guides were partially broken due to excessive manipulation, but they were still usable. No complications up to the 16-month follow-up. | |
| Neurosurgery | ||||
| Skull | Navigation guide for brainstem hematoma puncture drainage.95 | FDM N/A (Shandong Ruihua Electronic Technology) PLA (in text also ABS) |
4♀, 3♂, 40–56 yrs Brainstem hemorrhage |
Individualized, precise hematoma puncture under local anesthesia, with early hematoma compression relief. Slightly lower accuracy compared with stereotactic technology. Minimal operation trauma compared with craniotomy hematoma evacuation, significantly shorter time, and cost of severe brainstem hemorrhage treatment. |
| Spine | Test-needle guide for trans-foraminal (S2–S4) implantation of electrodes for sacral neuromodulation.92 | DLP Lite300 (UnionTech) Liquid photopolymer (Somos® XC11122) |
2 patients Intractable constipation, irresponsive to conservative treatment. |
Successful insertion of test needle into target sacral foramen at the first attempt of puncture without further adjustment. Under 20 min for implanting tined lead. |
| Spine localizer for use with portable lateral radiograph to determine the optimal location of skin incision in lumbar microsurgery.94 | FDM Desktop 3D printer PLA |
20♀, 23♂, 22–85 yrs (mean 60) Patients undergoing 1-level lumbar microsurgical decompression procedures (laminotomy with or without discectomy or foraminotomy) |
Higher accuracy of target spine segment location using the localizer (100%) compared with the surgeon's estimate based on palpation (81%). Inaccuracy of the surgeon's estimate was associated with higher BMI and transitional lumbosacral anatomy. | |
| Patient-specific navigation template for sacral-neuromodulation electrode placement.100 | N/A N/A N/A Manufactured by Beijing ThousandMed Innovation Technology |
Experimental group: 5♀, 5♂, 56.1 ± 15.8 yrs Control group: 8♀, 6♂, 40.5 ± 18.1 yrs Refractory lower urinary tract dysfunction. |
Successful electrode placement with greater accuracy, significantly fewer puncture repetitions, shorter procedure time, and lower X-ray exposure compared with conventional procedure. | |
| Oncology | Navigational guide for localizing small percutaneous lung nodules in lung cancer screening.91 | SLS FS251P (Farsoon) PA3200 |
Template group: 74♀, 26♂, 54 ± 15 yrs (13 excluded) CT group: 73♀, 27♂, 52 ± 11 yrs (5 excluded) Small peripheral lung nodules in early-stage lung cancer. |
Efficacy and safety of small peripheral lung nodule localization comparable to the CT-guided approach, significantly more simple, faster, and with less patient radiation exposure. |
| Needle guide for Iodine-125 seed implantation in treatment of liver tumor.93 | N/A N/A Photosensitive resin (1122-type) |
Guide group: 7♀, 8♂, 42–78 yrs (median 61) Control group: 11♀, 14♂, 37–82 yrs (median 57) Primary liver cancer, bile-duct carcinoma, and metastatic malignant liver tumor. |
Successful iodine-125 seed implantation, with shorter procedure time, less complication, dose closer to the planned one, and more precisely distributed. | |
| Coplanar needle guide for Iodine-125 seed implantation in treatment of pancreatic cancer.99 | N/A N/A PMMA |
Guide group: 6♀, 6♂, 48–81 yrs (median 66) Control group: 7♀, 6♂, 47–84 yrs (median 64) Unresectable advanced pancreatic carcinoma. |
Successful iodine-125 seed implantation in a safe and effective manner, and with improved accuracy and similarity between treatment planning values and postoperative dosimetric parameters. | |
| Patient-specific reference frame for intraoperative tracking of the patient's and instruments' position during palatal tumor resection.96 | FDM Ultimaker 3 Extended (Ultimaker B.V.) PLA |
1 patient Adenoid cystic carcinoma. |
Successful tumor resection with accurate results (∼1 mm errors in resection margins recorded compared with postoperative CT imaging). | |
| Orthopedic oncology | ||||
| Rib cage | Patient-specific sternum and rib resection template.15 | FDM N/A N/A Manufactured by Anatomics |
♂, 54 yrs Chondrosarcoma of sternum and ribs. |
Successful tumor resection, and replacement of sternum and ribs. |
| Patient-specific sternum and rib resection template.15 | FDM N/A N/A Manufactured by Anatomics |
N/A Mediastinal germ-cell tumor infiltrating the sternum. |
Successful tumor resection, and replacement of sternum and ribs. | |
| Spine | Patient-specific drill guide for occipitocervical screw fixation.41 | SLS Eosint (EOS) PA 12 Manufactured by Anatomics |
♀, 79 yrs Pathologic fracture of C1 with subluxation due to metastatic breast cancer. |
Satisfactory screw placement, simplified procedure, and no intra-/postoperative complications. Satisfactory occipitocervical alignment and functional outcome, no evidence of implant loosening or dysfunction at the 6-month follow-up. |
| Pelvis | Patient-specific cutting guide for partial acetabular resection.53 | SLS N/A PA (medical grade) |
♂, 65 yrs Osteolytic destruction due to pelvic chondrosarcoma. |
Successful pelvic resection and reconstruction without neurovascular complications or wound infection. Walking with full weight bearing 4 weeks after surgery. Satisfactory implant alignment, no evidence of implant loosening, and independent walking without pain at 10 months' follow-up. |
| Patient-specific osteotomy guide plate for en bloc resection of peri-acetabular tumor.54 | N/A N/A N/A |
6♀, 5♂, 21–63 yrs (mean 47) Peri-acetabular malignant bone tumor. |
Successful en bloc resection and pelvis reconstruction. Acceptable functional results without severe complications, alleviation of pain 2 weeks after surgery, and 2 cases of hip dislocation. No evidence of aseptic loosening, bone resorption, or periprosthetic fractures at 6–24 months' follow-up. | |
| Patient-specific guide for zone II and III borderline pelvic tumor resection.51 | N/A N/A PLA |
16♀, 22♂, 38–92 yrs 19 guide, 19 control Zone II and III borderline malignant pelvic tumor. |
Greater accuracy of tumor resection, simplified operation, shorter operating time, smaller intraoperative blood loss, lower tumor recurrence rate, and significantly higher rate of implant loosening compared with the conventional approach. | |
| Patient-specific cutting and acetabular-cup placement guides for hemipelvectomy and endoprosthetic reconstruction.72 | FDM N/A High-strength PETG |
♀, 75 yrs High-grade pelvic epithelioid hemangioendothelioma. |
Successful internal hemipelvectomy and reconstruction, with pain-free, unassisted walking at the 18-month follow-up. Acute immediate postoperative infection. | |
| Patient-specific osteotomy guide plates for sacral tumor resection.47 | FDM UP BOX (Tiertime) PLA |
2♀, 3♂, 31–53 yrs (mean 42) Giant cell tumor of the sacrum. |
Successful total piecemeal resection and reconstruction of sacrum without serious complications, neurogenic bladder dysfunction, fecal incontinence, or gait disturbance. Significant relief of pain, walking as early as 2 weeks postoperatively. No instrumentation failure at ∼17-month follow-up. Satisfactory bone fusion in CT. | |
| Patient-specific implant for pelvic reconstruction after tumor resection.48 | N/A N/A Tantalum (Xi'an Sailong Metal Materials) |
♀, 30 yrs Recurrent iliac low-grade chondrosarcoma. |
Successful tumor resection and pelvic reconstruction. Walking with crutches at the 1-month follow-up, without supportive brace at the 2-month follow-up without pain at the 6-month follow-up. Walking without assistance, and no tumor recurrence, instrumentation failure, or implant loosening at the 12-month follow-up. | |
| Knee | Patient-specific cutting guide for joint-preserving intercalary resection of knee metaphyseal bone tumor.58 | N/A N/A PA Manufactured by Thytec Shanghai |
5♀, 7♂, 7–59 yrs (mean 37.3) Knee metaphyseal malignant bone tumor. |
Accurate en bloc resection and matching between residual bone and prosthesis, no prosthetic-related complications (aseptic loosening, peri-prosthetic fracture) at the follow-up. Reliable reconstruction with possible early partial weightbearing. Overall, 10 satisfactory functional outcomes, 2 unsatisfactory knee RoM at 7–32 months' follow-up. |
| Limb | Patient-specific bone tumor resection guide.66 | MJ Objet30 Prime (Stratasys) MED610 |
5♀, 7♂, 23–70 yrs (median 49) Primary bone sarcoma, intermediate bone tumor, and bone metastases of the pelvis, sacrum, femur, tibia, calcaneus, and radius/ulna. |
Successful tumor resection with histologically negative margins and 0–3 mm cutting error. One case of local recurrence at the soft tissue. |
| Orthopedics | ||||
| Spine | Patient-specific drill guide for pedicle-screw placement in thoracic-spine surgery.87 | SLM EOSINT M270 (EOS) Titanium |
30♀, 6♂, 11–19 yrs (mean 15); Scoliosis. 4♀, 43–77 yrs (mean 55); Ossification of posterior longitudinal ligament. |
Successful placement of 466 pedicle screws with 98.6% and 100% success rates for patients with scoliosis and ligament ossification, respectively. |
| Patient-specific drill guide for cervical fusion surgery with pedicle, laminar, or lateral mass screws. Anatomical spine model.83 | SLA RS6000 (Union Technology) Photosensitive resin |
5♀, 5♂, 12–72 yrs (mean 51) Os odontoideum and atlanto-axial dislocation, cervical tumor, cervical spine fracture, cervical spondylotic myelopathy, and cervical instability. |
Accurate, radiation-free insertion of 46 of 48 screws. One significant deviation from planned screw trajectory due to template splitting by rough handling. No vascular or neurologic complications, injuries, infection, fracture of bone structure, screw loosening, or failure at 1–23 months' follow-up. | |
| Stereotactic patient-specific drill guide for atlantoaxial spine stabilization.36 | SLS N/A (Eosint) Nylon-12 |
3♀, 65–76 yrs Unilateral atlantoaxial osteoarthritis, unsuccessfully treated by conservative therapy. |
Successful placement of patient-specific implants with C1–C2 transarticular and C1 posterior arch screws. | |
| Patient-specific stereotactic drill guide for revision lumbosacral surgery.37 | SLS Eosint (EOS) PA-12 Manufactured by Anatomics |
♀, 72 yrs Sciatica, complex L5–S1 pseudoarthrosis, 2 months after L2–S1 fixation surgery for symptomatic degenerative scoliosis. |
Successful revision lumbosacral surgery, resolution of symptoms without implant dysfunction at the 6-month follow-up. | |
| Patient-specific surgical drill template (pedicle guider) for pedicle-screw placement in treatment of severe congenital scoliosis.86 | FDM Replicator 2 (MakerBot) PLA |
Pedicle guider group: 9♀, 6♂, 12 ± 3 yrs Control (freehand) group: 12♀, 5♂, 14 ± 4 yrs Severe congenital scoliosis |
Successful insertion of 244 of 254 pedicle screws under the guidance of 127 guides (96% success rate). Significantly higher proportion of accurately placed screws compared with freehand (93% vs. 78%). Significant decrease in operative time, single- and total-screw placement time. | |
| Patient-specific ad hoc surgical template for thoracic (Th5-Th6-Th7) pedicle screw insertion in spinal arthrodesis.80 | SLA Form 2 (Formlabs Inc.) Dental SG (Class 1 biocompatible resin) |
Three patients Thoracic scoliosis with hernial protrusion and/or vertebral canal stenosis |
Successful placement of pedicle screws with shorter screw application time, fewer X-ray shots per vertebra, and good screw placement accuracy (<2 mm deviation of 1 screw axis from planned trajectory). | |
| Patient-specific MySpine MC drill pilot guide for circumferential posterior interbody fusion with cortical bone trajectory screws.85 | N/A N/A PA 12 Manufactured by Medacta International |
Eleven patients, 42–57 yrs (mean 49) Spinal degenerative disease. |
Successful placement of 44 screws with accuracy comparable to traditional approach. Two pedicle perforations, no perforations >2 mm. Significant clinical improvement without new neurological deficits or radiological pathological findings at 6 months' follow-up. | |
| Patient-specific drill guides for corrective surgery of complex spinal deformities with pedicle screws.78 | FDM Mojo (Stratasys) ABS P430 |
Guide group: 6♀, 4♂, 16.6 ± 4.9 yrs Freehand group: 7♀, 3♂, 15.5 ± 3.8 yrs Congenital scoliosis, adolescent idiopathic scoliosis, and post-tubercular kyphosis. |
Successful placement of 137 vertebral screws with higher accuracy, enhanced safety, shorter operative time, less intraoperative radiation exposure, and blood loss compared with freehand technique. | |
| Patient-specific guides for pedicle screw implantation in spinal deformity correction.88 | N/A N/A MySpine guide (Medacta International) |
Guide group: 12♀, 2♂, 34 ± 15 yrs Freehand group: 14♀, 1♂, 26 ± 17 yrs Adolescent idiopathic scoliosis, adult degenerative scoliosis, and congenital spinal deformities. |
Higher accuracy of screw placement with lower intraoperative radiation dose, and shorter implantation time compared with freehand technique. | |
| Patient-specific drilling template for vertebral arthrodesis.81 | SLA Form 2 (Formlabs) Dental SG |
8♀, 12♂, 50–70 yrs Degenerative disease of the lumbar spine. |
Successful arthrodesis of 30 vertebrae with reduced operative time (63%) and X-ray exposure (92%), and the same or higher precision compared with freehand procedure. | |
| Patient-specific multi-level drill guide for posterior pedicle screw insertion in spinal deformity correction.84 | SLA SLA600 (N/A) Photosensitive resin |
6♀, 4♂, 13–23 yrs (mean 18) Severe, rigid idiopathic/congenital thoracic scoliosis. |
Successful placement of 152 screws, without neurologic damage, vascular injury, implant failure, infection, or other complications. Accurate placement of 45 of 48 screws (93.8%) using the drill guide, and 82 of 104 screws (78.8%) by free hand. | |
| Pelvis | Patient-specific drill guide for pelvic reconstruction with patient-specific acetabular endoprosthesis—aMace Acetabular Revision System (Mobelife NV/Materialise, Leuven, Belgium).49 | SLS N/A N/A |
8♀, 1♂, 40–79 yrs (mean 67) Extensive acetabular defects with aseptic loosening of the acetabular component after total hip arthroplasty and 1–8 prior revisions. |
Nine revision total hip arthroplasties followed by 5 revision surgeries due to nonimplant-associated complications. |
| Surgical template for external fixation of pelvic fracture with iliosacral screws.82 | SLA SLA-Lite 450 HD (UnionTech) Photosensitive resin |
Template group: 11♀, 11♂, 51.7 ± 15.2 yrs Control group: 8♀, 10♂, 50.1 ± 13.7 yrs Traumatic posterior pelvic fracture type B, C |
Successful pelvic fixation using 37 screws in template group and 28 screws in control group. No significant difference in quality of reduction between groups, significantly lower rate of screw perforation, significantly shorter operative time per screw, and significantly lower radiation exposure dose compared with freehand technique. | |
| Hip | Patient-specific acetabular jig to guide cup placement in total hip arthroplasty.98 | N/A N/A PLA |
Acetabular jig group: 18 patients Control group: 18 patients Patients indicated for total hip replacement. |
Higher accuracy of acetabular cup placement without significant increase in operating time or blood loss. |
| Knee | Patient-specific cutting guide and wedge spacers for distal femur varus osteotomy, K-wire positioning guide. Anatomical distal femur model.75 | FDM Witbox (BQ) PLA |
Guide group: 9♀, 3♂, 34–60 yrs (mean 44) Control group: 15♀, 5♂, 33–57 yrs (mean 41) Lateral compartment osteoarthritis of the knee with valgus malalignment. |
Higher accuracy of deformity correction, with increased ease of procedure, shorter operative time, less radiation exposure, and lower costs compared with classical technique. |
| Patient-specific guide for high tibial osteotomy in medial degeneration of the knee due to osteoarthritis.64 | SLA N/A (Formlabs) Dental SG |
6♀, 4♂, 56–79 yrs (mean 67) Medial osteoarthritis of the knee with varus deformity. |
Precise osteotomy with good short-term results. | |
| Patient-specific cutting guides for femoral and tibial resections in total knee arthroplasty.69 | SLS N/A PA (high-resolution) Manufactured by Wright Medical Group |
118♀, 70♂, 46–90 yrs (mean 67.7) End-stage knee arthrosis. |
Successful 201 total-knee arthroplasties with satisfactory accuracy, clinical and radiological outcome, no intraoperative complications, and no infections at 23.8 months' follow-up. An error in one resection jig (jammed sliding mechanism) caused a larger resection than planned. | |
| Patient-specific guide for distal femoral and tibial osteotomy to improve implant positioning in total knee replacement surgery.67 | SLS EOS P760 (EOS) EOS Pa2200 |
Guide group: 15♀, 5♂, 68.6 ± 8.6 yrs Control group: 16♀, 4♂, 70.5 ± 7.1 yrs End-stage gonarthrosis. |
Successful total knee replacement without significant differences in average postoperative mechanical femorotibial, femoral, coronal, and tibial coronal angles; significantly fewer patients with marked femorotibial malalignment. | |
| Patient-specific intramedullary guide to control femoral component rotation in total knee arthroplasty.73 | FDM UP BOX (Tiertime) PLA |
Guide group: 32♀, 8♂, 57–80 yrs, (mean 69) Control group: 33♀, 7♂, 55–82 yrs (mean 68) Terminal stage of knee osteoarthritis. |
Less postoperative drainage volume, better postoperative patella transverse axis-femoral transepicondylar axis angle and posterior condylar angle than conventional group. No significant difference in drainage duration, postoperative range of motion after surgery, but longer operation time than conventional group. | |
| Patient-specific surgical guide for total knee arthroplasty.74 | FDM UP BOX (Tiertime) PLA |
Guide group: 21♀, 9♂, 55–76 yrs (mean 69) Control group: 42♀, 18♂, 54–80 yrs (mean 68) End-stage knee osteoarthritis. |
Gait analysis at follow-up. Successful total knee arthroplasty with larger knee maximum flexion angle in the swing phase of gait, and smaller mean patella transverse axis-femoral transepicondylar axis angle at ∼12-month follow-up. | |
| Patient-specific distal femoral osteotomy guide plate model for total knee arthroplasty.70 | N/A N/A PA |
Guide group: 12♀, 7♂, 70.2 ± 8.4 yrs Control group: 23♀, 10♂, 68.6 ± 7.1 yrs Degenerative knee arthritis, no obvious/poor therapeutic effects after a stage of treatments. |
Procedure highly consistent of the preoperative software simulation plan. Significantly lower operation time, more accurate coronal force line recovery. | |
| Patient-specific osteotomy guide plate for total knee arthroplasty.77 | FDM Creator Pro (FlashForge) N/A |
Guide-plate group: 8♀, 2♂, 59–71 yrs (mean 60) Control group: 9♀, 1♂, 60–71 yrs (mean 61) Valgus knee deformity due to osteoarthritis or rheumatoid arthritis. |
Successful total knee arthroplasty with significantly lower operation time, intraoperative blood loss, and postoperative mean femorotibial angle, and significantly higher clinical and functional scores of the knee compared with the control group. | |
| Patient-specific guide for positioning of cutting blocks in total knee arthroplasty.65 | FDM Replicator 2 (MakerBot), Ultimaker 2 extended plus (Ultimaker) HIPS |
5♀, 1♂, 48 ± 9 yrs Post-traumatic gonarthrosis stage III–IV with severe varus deformity and severe knee-extensor contracture. |
Successful resection and reconstruction of the knee, with significant improvement in knee functioning and lower limb axis at the 6-month follow-up. One case of >3° deviation from the lower-limb frontal plane mechanical axis. |
|
| Ankle | Patient-specific cutting guides for ankle resection.60 | N/A N/A PA12 (biocompatible polyamide) |
♂, 57 yrs Severe post-traumatic ankle–joint arthritis. |
Successful bone resection and prosthesis implantation. Good implant positioning and alignment. Excellent clinical scores and functional abilities at 4 months' follow-up, satisfactory joint moment, and normal muscle-activation timing. |
| Patient-specific cutting guide for treatment of foot and ankle pathologies.63 | N/A N/A N/A Manufactured by 4WEB Medical (Frisco, TX) |
9♀, 6♂, 22–74 yrs (mean 53) Complex large bony defects after talus/tibia trauma, tibial/tibiotalocalcaneal nonunion, talus avascular necrosis, failed total ankle arthroplasty, and hindfoot valgus deformity. |
Overall, 15 reconstructions of tibia, ankle, or hindfoot, with significant improvement in pain and functional outcome, radiographic fusion in 13 patients at 2.6–8.2-months. Two failures: 1 early deep infection 2 weeks postoperation, 1 nonunion at the 24-month follow-up. No cases of hardware failure. | |
| Patient-specific guide for subtalar joint arthrodesis.97 | FDM UP BOX (Tiertime) PLA |
16♀, 13♂ Experimental group: 14 patients, 52 ± 19 yrs Control group: 16 patients, 50 ± 18 yrs Traumatic arthritis, severe osteoarthritis of the subtalar joint. |
Successful subtalar arthrodesis with significantly shorter operating and intraoperative fluoroscopy time, and fewer drilling repetitions compared with the conventional approach. No neurovascular injury or other complications occurred in either group. | |
| Long bone | Patient-specific cutting and drill guides for corrective osteotomies of long bones.68 | SLS N/A PA |
Ten patients Malunion of long bones (2 femur, 2 tibia, 4 humerus, 1 radius, and 1 radius/ulna). |
Undercorrection of femur and tibia, adequate osteotomies, and screw entry points. Adequate humerus coronal, but not axial and sagittal correction angles, adequate osteotomies, and screw entry points. Undercorrection of forearm in multiple planes, adequate screw entry points. |
| Patient-specific drill guide for internal fixation surgery of tibial plateau fracture.79 | FDM Replicator 2X (MakerBot) Acrylate resin |
2♀, 4♂, 33–52 yrs Schatzker classification V or VI tibial plateau fracture. |
Successful internal fixation surgeries with 33 screws and 6 locking plates. Screw lengths, entry points, and direction consistent with preoperative plan. | |
| Patient-specific osteotomy guide for reconstructive lengthening of the radius with autograft and Alians Radius™ osteosynthesis plate.71 | N/A N/A PA2200 uspcl6 Manufactured by Newclip Technics |
♂, 16 yrs Post-traumatic epiphysiodesis of the radius with severe radial loss of length, ulnocarpal impingement, and pain. |
Successful longitudinal lengthening of radius, with consolidation, improved radioulnar index, and without clinical deformation, ulnocarpal impingement, pain, or neurologic symptoms at the 6-month follow-up. More secure procedure, reduced radiation exposure, surgery duration, scar size, and postoperative pain at the iliac crest harvesting site. | |
| Patient-specific osteotomy guide for cubitus varus correctional surgery124 | N/A N/A N/A Manufactured by Metaklinik |
♂, 18 yrs 8 yrs' postsupracondylar fracture of humerus resulting in 40◦cubitus varus deformity with 20◦ flexion and extension deficits. |
Successful varus correction, perfectly matching preoperative plans. Elbow mobilization 1 week after surgery, full range of motion, no pain, and osteotomy site union at the 3-month follow-up. | |
| Wrist | Patient-specific guide plate for mini-invasive percutaneous internal screw fixation of fractured scaphoid.90 | MJ N/A MED610 |
4♂, 30–53 yrs Traumatic scaphoid fracture. |
Successful fixation with shorter operative time and fewer radiological exposures. |
BMI, body mass index; HIPS, high impact polystyrene.
Patient-specific surgical guides and implants were the most often produced devices, with the largest number of implants in orthopedic oncology (33), and surgical guides in orthopedics (31). With the exception of a vertebral-body endoprosthesis (PEKK printed by SLS), an airway splint (PCL printed by SLS), an ankle prosthesis (Cr-Co-Mo), and an implant for pelvic reconstruction (tantalum), all implantable devices were printed in titanium or titanium alloy by using either EBM (29) or SLM (8). In two cases,20,21 the titanium endoprosthesis was coated with porous High-density Polyethylene (PoreStar) for lightweight and structural enhancement. In 16 cases, the 3D-printing process was not reported.
Virtually all titanium endoprostheses were designed to provide a roughened or porous surface at the interface with the bone to improve the chance of bone ingrowth and enhanced implant stability. In seven cases, the implants had a cage construction filled with allogenic bone graft to further facilitate osseointegration.
Surgical guides were most often manufactured using either PA (15; 9 with SLS), PLA (12; 10 with FDM), photosensitive resin (including Dental SG, Somos XC11122, Med610, and ULTEM 1010; 6 with SLA, 4 with MJ, 1 with DLP, 1 technology not specified), PETG with FDM (2), and titanium (2; 1 with SLM). In single cases, ABS P430, acrylate resin, and HIPS were printed with FDM, and PMMA with a 3D-printing technology not specified.
Of the other devices in contact with internal tissues, surgical instruments were manufactured by using PA with SLS, silicone with MJ, or photosensitive resin with unspecified 3D-printing technology; templates for auricular reconstruction were printed by using PLA with FDM, for nose contouring using MED610 with MJ, and a tooth replica using titanium alloy with SLM; an enteral stent was printed by using TPU with FDM, and a lenticule storage glide by using an unspecified material with MJ.
Most devices in contact with the face were made of flexible, elastomeric materials to ensure patients' comfort. Radiotherapy boluses were printed in TPU (NinjaFlex) with FDM, or Agilus-60 with MJ, and a nasal prosthesis in TangoPlus with MJ. Two other boluses were fabricated by using PLA with FDM, and one using PLA/PHA with FDM. A nasal swab was printed in PA with SLS. Immobilization devices, limb orthoses, and prostheses were manufactured from polymer-based materials by using DLP, FDM, or SLA. Materials and 3D-printing technology used for the production of the reviewed medical devices are presented in Table 4.
Table 4.
Three-Dimensional-Printing Technology and Materials by Medical Device Type
| Medical device | Material | 3D-printing technology | Field of application | Study |
|---|---|---|---|---|
| Implant | ||||
| Airway splint | PCL | SLS | Thoracic surgery | 22 |
| Jaw | Titanium | SLM | Maxillofacial surgery | 23 |
| N/A | Maxillofacial surgery | 24 | ||
| Titanium alloy | SLM | Maxillofacial surgery | 25a | |
| Shoulder | Titanium alloy | EBM | Orthopedic oncology | 26 |
| Humerus | Titanium | N/A | Orthopedic oncology | 18 |
| Elbow | Titanium alloy | EBM | Orthopedics | 27 |
| Radius | N/A | N/A | Orthopedic oncology | 18 |
| Rib cage | Titanium | EBM | Orthopedic oncology | 15 |
| Titanium/PoreStar | EBM | Orthopedic oncology | 21 | |
| Orthopedics | 20 | |||
| Spine | PEKK | SLS | Orthopedics | 28 |
| Titanium | EBM | Orthopedic oncology | 15 | |
| SLM | Orthopedics | 15 | ||
| N/A | Orthopedics | 29 | ||
| Titanium alloy | DMLS | Orthopedics | 30 | |
| EBM | Orthopedic oncology | 31–35 | ||
| Orthopedics | 36–40 | |||
| SLM | Orthopedic oncology | 41 | ||
| N/A | Orthopedic oncology | 16,42 | ||
| Sacrum | Titanium alloy | DMLS | Orthopedic oncology | 43 |
| EBM | Orthopedic oncology | 44–46 | ||
| SLM | Orthopedic oncology | 47 | ||
| Sacroiliac | Titanium alloy | EBM | Orthopedics | 38 |
| Pelvis/acetabulum | Tantalum | N/A | Orthopedic oncology | 48 |
| Titanium | EBM | Orthopedic oncology | 15 | |
| SLM | Orthopedics | 49 | ||
| N/A | Orthopedic oncology | 18 | ||
| Titanium alloy | EBM | Orthopedic oncology | 50,51 | |
| Orthopedics | 52 | |||
| SLM | Orthopedic oncology | 53 | ||
| N/A | Orthopedic oncology | 54 | ||
| Femoral head | Titanium alloy | EBM | Orthopedics | 55 |
| Knee | Titanium alloy | EBM | Orthopedic oncology | 56–58 |
| SLM | Orthopedic oncology | 58 | ||
| Titanium coating | N/A | Orthopedics | 59 | |
| Foot | Titanium | EBM | Orthopedic oncology | 15 |
| Ankle | Cr-Co-Mo | N/A | Orthopedics | 60 |
| Titanium | N/A | Orthopedics | 61,62 | |
| Titanium alloy | N/A | Orthopedics | 63 | |
| Guide | ||||
| Cutting | Dental SG | SLA | Orthopedics | 64 |
| HIPS | FDM | Orthopedics | 65 | |
| MED610 | MJ | Orthopedic oncology | 66 | |
| Maxillofacial surgery | 23 | |||
| PA | SLS | Orthopedic oncology | 53 | |
| Orthopedics | 67–69 | |||
| N/A | Maxillofacial surgery | 24 | ||
| Orthopedic oncology | 58 | |||
| Orthopedics | 60,70,71 | |||
| PETG | FDM | Orthopedic oncology | 72 | |
| PLA | FDM | Orthopedics | 73–75 | |
| Orthopedic oncology | 47 | |||
| Maxillofacial surgery | 76 | |||
| N/A | Orthopedic oncology | 51 | ||
| ULTEM 1010 | MJ | Maxillofacial surgery | 23 | |
| N/A | FDM | Orthopedic oncology | 15 | |
| Orthopedics | 77 | |||
| N/A | Orthopedic oncology | 54 | ||
| Orthopedics | 63 | |||
| Drill | ABS P430 | FDM | Orthopedics | 78 |
| Acrylate resin | FDM | Orthopedics | 79 | |
| Dental SG | SLA | Orthopedics | 80,81 | |
| Photosensitive resin | SLA | Orthopedics | 82–84 | |
| PA | SLS | Orthopedic oncology | 41 | |
| Orthopedics | 36,37,68 | |||
| N/A | Orthopedics | 85 | ||
| PETG | FDM | Orthopedic oncology | 72 | |
| PLA | FDM | Orthopedics | 86 | |
| Titanium | SLM | Orthopedics | 87 | |
| N/A | Maxillofacial surgery | 24 | ||
| N/A | N/A | Orthopedics | 88 | |
| Navigation | ABS | SLA | Anesthesiology | 89a |
| MED610 | MJ | Orthopedics | 90 | |
| PA | SLS | Oncology | 91 | |
| Somos XC11122 | DLP | Neurosurgery | 92 | |
| Photosensitive resin | N/A | Oncology | 93 | |
| PLA | FDM | Neurosurgery | 94,95 | |
| Oncology | 96 | |||
| Orthopedics | 97 | |||
| N/A | Orthopedics | 98 | ||
| PMMA | N/A | Oncology | 99 | |
| N/A | N/A | Neurosurgery | 100 | |
| Template | ||||
| Nose contour | MED610 | MJ | Plastic surgery | 101 |
| Auricle frame | PLA | FDM | Plastic surgery | 102 |
| Tooth replica | Titanium alloy | SLM | Dental surgery | 103 |
| Instrument | ||||
| Retractor | PA | SLS | Orthopedics | 37 |
| Photosensitive resin | N/A | Neurosurgery | 104 | |
| Endoscopic cap | Silicone | MJ | Gastroenterology | 105a |
| Enteral stent | TPU | FDM | Abdominal surgery | 106 |
| Radiotherapy bolus | ||||
| Face | NinjaFlex TPU | FDM | Oncology | 17 |
| PLA | FDM | Oncology | 17 | |
| Scalp | Agilus-60 | MJ | Oncology | 107 |
| Chest | PLA | FDM | Oncology | 108 |
| Various body parts | PLA/PHA | FDM | Oncology | 109 |
| Immobilization device | ||||
| Cast—wrist | ABS M30 | FDM | Immobilization | 110 |
| External fixator | Photosensitive resin | SLA | Immobilization | 111,112 |
| Orthosis | ||||
| Insole | EVA | N/A | Orthotics | 113,114 |
| Fingerboard | PLA | FDM | Orthosis | 115 |
| Wrist | Photosensitive resin | N/A | Orthosis | 116 |
| Hand, dynamic | PLA | FDM | Orthotics | 117 |
| N/A | N/A | Orthotics | 118 | |
| Prosthesis | ||||
| Finger | PLACTIVE | FDM | Prosthetics | 119 |
| Nasal | TangoPlus | MJ | Prosthetics | 120 |
| Palatal plate | Photosensitive resin | DLP | Orthodontics | 121 |
| Lenticule storage glide | N/A | MJ | Ophtalmology | 122 |
| Swab | ||||
| Nasal | PA | SLS | Infectology | 123 |
The current authors are unfamiliar with these combinations of materials and technologies.
In the field of facial prosthetics, 3D printing was primarily used for fabrication of molds, as opposed to direct manufacturing of prostheses, due to the poor mechanical properties of the available 3D-printing materials (e.g., TangoPlus) compared with the benchmark silicone.120
Implantable airway splints were manufactured by using PCL, a biocompatible polyester that is bioresorbable after 2–3 years.19,22 The choice of material was of key importance in this procedure, as the conformational change due to material degradation over time in combination with an open cylindrical design allowed for accommodation of airway growth in infant patients who received the splint.
Clinical procedures and outcomes involving 3D-printed medical devices
All medical devices included in this review were used to directly treat patients. The number of participants in the studies ranged from 1 to 496 (1–16 for female only, 1–5 for male only, and 2–496 for both sexes). Forty-two studies were performed on single patients.
In orthopedics, orthopedic oncology, and maxillofacial surgery, the patients were treated with tumor/bone resection by using personalized surgical guides, and reconstruction of bone deficits and/or deformities with custom osteosynthetic material or endoprostheses. In all cases of orthopedic implants, satisfactory osseointegration and precise compatibility with the target bone surface, or off-the-shelf implantable devices was observed. It was noted that maximizing the contact area between the implant and bone improves stability and lessens postoperative pain.44
Additively-manufactured devices were also used for invasive and noninvasive immobilization of extremities in the treatment of bone fractures. In neurosurgery, 3D-printed instruments were used to facilitate the insertion of therapeutic devices to target location (i.e., ventricular catheter for hydrocephalus treatment, puncture for brainstem hematoma drainage, electrodes for sacral neuromodulation), and to localize the point of skin incision in lumbar microsurgery. Tailored endoscopic caps increased the ease and decreased the duration of endoscopic procedures. In abdominal surgery, an enteral stent was used to plug an enteroatmospheric fistula. In thoracic surgery, airway splints relieved the (extra)pulmonary complications due to tracheobronchomalacia. In oncology, patient-specific navigational guides were used to quickly and safely localize small percutaneous lung nodules in cancer screening.
Esthetically superior outcomes and reduced operative times were achieved with template use in plastic surgery for heminasal reconstruction and auricle reconstruction. Artificial tooth sockets were successfully prepared with the use of donor tooth replicas before autotransplantation in dental surgery, resulting in decreased extraalveolar and procedural times. Reduced surgical time and eliminated complications related to tissue preparation were also noted with the use of a smart storage glide for preservation, transport, and insertion of lenticules in keratoplasty. Additively manufactured nasal swabs were equally successful in diagnosing COVID-19 infections as conventional swabs, while being preferred by the majority of participants.
All invasive devices and the nasal swab were sterilized before use, either chemically (ethylene oxide, hydrogen peroxide), with low-temperature plasma sterilization, moist heat (autoclave), or cobalt-60 irradiation.
In general, invasive medical procedures using 3D-printed devices were successfully performed with favorable short- and long-term outcomes. For implantable devices, very few cases of failure (e.g., fracture, loosening, movement, subsidence) were reported up to 108 months postsurgery.
However, specific complications were reported in 15 studies. These included 1 case (of 51) of sacroiliac-joint-fusion implant malposition38; 2 cases (of 6) of subsidence of patient-specific PEKK prostheses for vertebral-body replacement, possibly due to mispositioning of the prosthesis at implantation28; 1 (of 13) clinically relevant vertebral-body implant subsidence into the adjacent vertebral body.31
Also reported were 2 cases (of 11) of hip dislocation with patient-specific hemipelvic prostheses54; 2 cases (of 12) of unsatisfactory knee range of movement with the use of intercalary prostheses for joint-preserving intercalary tumor resection58; 3 cases (of 10) of sacral endoprosthesis failure due to screw breakage46; 1 (of 9) aMace® Acetabular Revision System failure49; 4 (of 568) titanium-coated knee baseplate failures due to aseptic loosening59; significantly higher rates of loosening of a modified anatomic template for pelvic reconstruction compared with the conventional approach51; 1 (of 15) early deep infection and one nonunion with patient-specific ankle implant cages63; limited shoulder and elbow range of motion with total humeral endoprosthesis, a dislocation of partial humerus endoprosthesis, and a distal radius implant dislocation during growth in a teenage patient;18 and undercorrection in corrective osteotomies of long bones with patient-specific cutting and drill guides.68
In 15 infants receiving airway splints, one death was possibly associated with splint displacement.22 One (of 188) resection jig error caused a larger resection than planned,69 and 1 (of 10) significant deviation from the planned screw trajectory was noted due to a drill guide being split by rough handling.83
Regarding less invasive medical devices, patient-specific boluses were produced with superior density uniformity, and successfully used for controlled dose distribution in radiotherapy. Moreover, a patient-specific palatal plate ensured better fit and longer time to displacement without the use of adhesive cream.121 Limb orthoses and prostheses improved patients' conditions to varying degrees; nonetheless, high patient satisfaction scores119 and larger motivation for rehabilitation training118 were reported.
Twenty-six studies were performed with control groups of patients undergoing similar procedures performed with conventional techniques. Of these, 13 were in orthopedics,51,67,70,73,74,77,78,86,88,91,97–99 4 in orthopedic oncology,46,67,86,114 and 3 in orthotics,75,113,118 and single studies were in anesthesiology,82 gastroenterology,73 maxillofacial surgery,116 neurosurgery,100 oncology,98 and plastic surgery.101 In comparison with conventional approaches, the use of 3D-printed patient-specific implants and guides leads to increased accuracy of the procedure, reduced operating time, lower radiation exposure, comparable or smaller intra-operative blood loss, smaller number of surgical errors, comparable or improved quality of outcomes, less postoperative drainage volume, and fewer postoperative complications.
Lador et al.16 reported that a titanium lattice structure created significantly less radiologic interference when compared with other implants, allowing for better adjuvant radiation therapy and local disease recurrence monitoring. On the other hand, Chatain and Finn43 pointed out that a titanium sacral implant for pelvic reconstruction in orthopedic oncology may be suboptimal when follow-up imaging is needed due to extensive radiological metal artifact. Surgical guides facilitated preoperative planning and simplified the operative procedure, which was highly accurate and consistent with the plan. Positive feedback from patients was noted in several studies. One study reported high satisfaction rates among patients up to 60 months postsurgery, and up to 83% of these patients expressed the willingness to have the procedure again if needed.38
Regulatory aspects of 3D-printed medical device use
In 29 studies, medical devices were manufactured by the following certified companies: Anatomics (Melbourne, Australia),15,21,29,35,37,39,41 4WEB Medical (Frisco, TX),16,61–63 AK Medical (Beijing, China),32,42,56 Medacta International (Castel San Pietro, Switzerland),85,88 3D Systems,43 Beijing ThousandMed Innovation Technology (Beijing, China),100 Bodyarch (China),113 DePuy Synthes (West Chester, PA),24 Instrumentaria (Sesvete, Croatia),18 Mobelife NV (Materialise, Leuven, Belgium),49 Medicrea (Rillieux la Pape, France),28 Newclip Technics (France),71 Ossis (Christchurch, New Zealand),52 Stryker Orthopaedics (Mahwah, NJ),59 Thytec Shanghai (Shanghai, China),58 Wright Medical Group (Arlington, TN),69 and Metaklinik (Metaklinik.com).124 One study used titanium implants iFuse-3D™ (SI-BONE), cleared by the US FDA in 2017 for sacroiliac joint fusion,38 and another used off-the-shelf implants Medussa-PL (Medyssey) for lumbar interbody fusion.40 In these cases, additional approval for the use of the devices was not required.
Seven studies addressed regulatory aspects of 3D-printed medical device use directly. One study on spine-stabilization endoprostheses detailed the process of ensuring compliance with the EU Medical Device Regulation, including the required procedural blueprint and a technical file with a thorough description of all steps and procedures.30 A patient-specific titanium truss cage received compassionate use approval by an institutional review board for a single-time use to avoid below-knee amputation.62 Similarly, a patient-specific endoprosthesis in a case of sacral chordoma received FDA approval via the emergency and compassionate use of unapproved devices.43 The authors in that study noted the long duration from surgeon request to implantation (4 months) due to the complexity of implant concept modeling and the FDA approval process.43
A study of implantable airway splints for pediatric patients reported that to receive the splint, the compliance of each patient with the criteria for the FDA Emergency Use Exemption was verified by an impartial third-party physician.19 By virtue of the FDA guidelines, investigational devices were restricted to a limited number of uses before the formal regulatory approval process had to be pursued, thus limiting the initial number of patients recruited to 3. One tantalum patient-specific implant was designed under the parameters of “Personalized Additive Manufactured Medical Device Technical Censoring Guidelines” by the Center for Medical Device Evaluation of China.48 According to the authors of the study, a needle guide for in-plane ultrasound-guided nerve blocks falls under the FDA designation of a medical device Class I, and its use in their hospital did not require registration with the FDA, investigational device exemption, or Institutional Review Board approval, because it was not for sale, or used in a study or experiment.89 Finally, one study reported using a non-FDA approved endoprosthesis.31
Discussion
Dominant and emerging fields of application of 3D printing for medical device production
The largely dominant fields of 3D-printing application in medicine are orthopedics and orthopedic oncology, with increasing numbers of studies on patient-specific surgical guides and/or implants published since 2015. Often, the simultaneous use of both is reported, and medical-device companies that produce patient-specific implants typically offer corresponding cutting/drill guides to avoid mismatch between the size of the implants and defects after resection.
The emerging use of 3D printing is for patient- and/or procedure-specific medical devices to facilitate precision procedures in neurosurgery and oncology,91,92,94,95,100 and ensure planned dose distribution in radiotherapy.17,99,107–109
Technology use trends in medical-device production
Certain trends regarding the use of materials and 3D-printing processes were identified. Based on the reviewed studies, the most common material for 3D-printed implants is titanium alloy (Ti-6Al-4V), known for its excellent biocompatibility, biological inertness, favorable strength-to-density ratio, and superior corrosion resistance.125 It is of note that the final strength of titanium parts varies depending on the design and printing technology used, and it is not a priori guaranteed.44
The vast majority of implants were printed using EBM. A few studies preferred SLM due to the higher accuracy and superior mechanical properties of the printed parts.23 Nevertheless, additional heat treatment is needed after SLM to eliminate high residual stresses,125 and the surface roughness resulting from EBM represents an advantage for medical applications where tissue incorporation is desired.126 Technical difficulties were noted during the insertion of rough-surface implants without causing neural or cartilage tissue injury,40 and a possible risk of infection with the development of biofilm on titanium was also reported.63
Single-use surgical guides and instruments were mainly printed in biocompatible polymer-based materials that are less costly and more accessible. Medical-grade PA was the material of choice due to its high production strength and accuracy, temperature-, corrosion-, and deformation-resistance, and resistance to breakage.70 Among the noteworthy 3D-printing-related adverse events during surgery were splitting of an SLA-printed resin drill guide83 and an FDM-printed PLA cutting guide76 by rough handling, and an unplanned over-resection due to a jammed sliding mechanism in an SLS-printed PA resection jig.69 For retractors, PC was found to be too brittle, whereas resin models were much sturdier and capable of withstanding pressurized steam sterilization without deformation.104
For noninvasive devices that did not require sterilization, the choice of material depended on their specific use. Radiotherapy boluses in prolonged contact with facial skin were fabricated in soft, flexible materials (e.g., Agilus-60, NinjaFlex) to ensure comfort during therapy, and conformality to complex anatomy.107 It was also important that the boluses were printed at 100% infill to create a solid, homogeneous object with radiological properties resembling those of water.17 In orthotics, resistance to breakage was one of the key requirements, thus PLA (FDM) was found to be most appropriate.
Advantages and drawbacks of the use of 3D printing for medical device fabrication
The use of 3D printing for medical-device production was consistently viewed as advantageous across the reviewed studies. Generally reported were increased procedure accuracy, reduced duration, and improved quality of outcomes compared with traditional approaches. An important advantage of medical-device personalization is also the possibility of solving rare, unconventional medical problems.33,127 In several studies, patient-specific guides were described as easy to use, even by less-experienced operators; and an additional benefit was in orthopedic oncology, where they provided the possibility of close, but tumor-free margin resection while preserving the native joints.58
Although the benefits of 3D-printing for medical-device fabrication have been addressed in previous reviews, certain economic and clinical concerns were also expressed in the reviewed studies that need mentioning. Among the most common were the cost and duration of patient-specific device production, especially when outsourced to specialized commercial entities.23,28,29,39,49,62,63,72,76 The price of 3D-printed implants is estimated to be two- to five-times higher compared with conventional implants,28,128,129 and additional costs include preoperative virtual planning, device design, and the production of trials or implant variations.
Outsourcing is virtually inevitable for titanium implants due to their complex structure and the high cost of EBM machines. However, studies involving relatively simple devices printed in polymer-based materials also reported notably lower cost compared with conventional devices,76,104 and successful in-house design and fabrication by medical staff.23,76,110
Longer waiting periods for custom implantable devices may predispose patients to malignant changes, disease progression, or anatomical alterations, which can complicate implantation.21,31,43 Manufacturing times would also need to improve to include acute cases41 or contingency use. Cases of overnight production of medical devices for urgent interventions have already been reported,130,131 and it is expected that increased use and technological improvement will reduce production time.21
The majority of concerns expressed in the reviewed studies were related to patient-specific implants and surgical guides. Two important drawbacks of patient-specific prostheses were the possibility of defect/implant mismatch, and the difficulty of implant removal due to bone-tissue ingrowth. The custom form of the prostheses demands complete commitment toward the preoperative resection plan, which is not always feasible due to unexpected differences between computer-reproduced and actual anatomy.31,42 Very limited possibilities of intraoperative implant modification may cause the need to modify the operative procedure.42–44 To avoid this, some authors chose to prepare several sizes of implants for intraoperative selection.16,30,32,33,46
However, patient-specific devices are considered “custom” by the FDA, and they can only be produced in fewer than 5 units per year.1 In instances when several iterations of the same device are produced for a single patient, the unused devices must be returned to the manufacturer and/or destroyed, which has to be certified by the physician.132
The second drawback regarding implants refers specifically to their porous structure that facilitates osseointegration. Although good incorporation is a desirable outcome, it may complicate implant removal in the case of hardware failure, requiring additional bone resection around the implant.63,133 On the other hand, Wei et al.45 reported that due to bone ingrowth into a sacral endoprosthesis, instrument failure at 8 months of follow-up did not impair the quality of life and ambulation.
Regarding patient-specific cutting/drill guides, one of the main problems reported was the need for soft tissues to be completely removed to make the guides fit the boney anatomy, which can increase intraoperative blood loss and operation time.78,83 Any slight relative activity during operation, unplanned breakage of bony landmarks, or unexpected anatomical features can cause discrepancies between the presurgical simulation and the actual procedure,83,88 and may even prevent the surgical procedure from being performed.86 Finally, with resection guides, the depth of osteotomy can be difficult to estimate, potentially leading to extensive cartilage damage.68
Regulatory aspects of 3D printing of medical devices
At present, all medical devices, including those that are 3D printed, must conform to the same regulatory frameworks to be used legally. The regulations vary across different countries and were reviewed in previous papers for the United States,11,134 the European Union (EU),11,134 Japan,134 and Australia.11 Nevertheless, the potentially personalized nature and decentralized manufacturing of 3D-printed devices present unique legislative challenges, especially in cases that do not allow for lengthy processes of obtaining regulatory approval.
For example, the standard FDA approval of Class III medical devices is a lengthy process that can take 3–7 years, and it requires preclinical laboratory and animal testing, and clinical trials.1 This can present substantial barriers especially when rare, life-threatening, or severely debilitating medical conditions need to be treated urgently. For these cases, specific pathways are established for expanded access to unapproved medical devices.1
In the United States, the majority of 3D-printed medical devices are cleared by the FDA under the emergency circumstances or via the conventional 510 (k) pathway,2 including many of those produced by the medical companies listed in this review. The expanded access programs for rapid approval of products that have not yet received FDA approval include Emergency Use, Compassionate Use, Continued Access, and Treatment Investigational Device Exemption.134 Companies such as Ossis offer individual applications for patients to receive a compassionate use approved medical device.135
In the EU, exceptional use of non-CE marked medical devices can be authorized according to the MDR on a case-by-case basis at the request of a medical consultant and/or device manufacturer.13 Both the United States and EU regulations also include a specific exemption for custom-made devices.1,11
Most of the studies that reported the use of approved devices outsourced their design and fabrication to established medical companies, and in only three cases, the approval was acquired via compassionate or emergency use exemption.19,43,62 Almost three-quarters of the studies did not detail regulatory aspects, or only described obtaining permission to use the device from an internal review board.
Interestingly, the use of custom device exemption was only reported in one European study.30 In the FDA regulations, such an exemption applies to devices that are designed to treat individual patients' unique pathology/physiology, and thus they necessarily deviate from an otherwise applicable performance standard such that investigations would be impractical. They are also not generally available for commercial distribution from a manufacturer, importer, or distributor.1 It has been acknowledged in previous papers that the clinical and regulatory issues regarding custom 3D-printed medical devices were complex and evolving, especially to prevent their uncontrolled use and human experimentation.1,136
Limitations
Some studies of other medical devices were excluded from this review, as they did not meet our inclusion criteria regarding the data provided on 3D-printing technology or patient testing, or because they employed indirect AM. Also excluded from this study are papers addressing anatomical modeling for surgical planning, which is now quite well reported in the literature. The authors also note that there might be inconsistencies regarding the employed materials and technologies in the previous studies that we were unable to clarify.
Conclusions
The use of 3D-printed medical devices in the direct treatment of patients has increased considerably since 2015. This review identified 110 papers reporting on 140 medical devices, the technological aspects of their fabrication, and their use and clinical outcomes in several medical fields. Metal and nonmetal 3D printing in orthopedics and orthopedic oncology were the most common applications, and this was led by established companies with regulatory procedures. There is an emerging trend toward the use of nonmetal 3D printing in medicine for patient-specific and precision devices in neurosurgery and oncology.
Regarding implantable devices, the most used material was titanium alloy and the most commonly chosen 3D-printing technology EBM. For surgical guides and instruments, polymer-based materials were most often used, notably medical-grade PA with SLS, and PLA with FDM. Noninvasive devices that did not require sterilization were mostly printed by using FDM, and the choice of material depended on the device's specific use.
Several advantages of 3D-printed medical devices were consistently reported across the reviewed studies, including the possibility of solving rare, unconventional medical problems, increased procedure ease and accuracy, reduced duration, and improved outcomes. Among the most commonly reported drawbacks of 3D printing were high cost and relatively long manufacturing times, particularly the latter regarding the management of progressive diseases and emergencies. For patient-specific prostheses and surgical guides, the most frequent concern was that the actual procedure needed to precisely match the preoperative plan, which cannot always be achieved due to unpredictable intraoperative situations.
Approximately a quarter of the studies outsourced the device design and fabrication to certified medical device companies, and in almost three-quarters, permission to use the device was obtained from an internal review board. There is a need to further support rapid regulatory and ethics review approaches to optimize the potential for use of 3D printing in medicine, in particular for time-critical clinical challenges.
Acknowledgments
None other than the funding bodies that are detailed next.
Authorship Confirmation Statement
T.K. performed the systematic search, extracted the data, and led to the writing of the article. A.S. was the second reviewer and contributed to the study design and article writing. K.J.O.S. contributed to the study design and the writing of the article. C.M. contributed to the analysis of the literature and the writing of the article from a manufacturing perspective. C.P.D. contributed to the analysis of the literature and the writing of the article from a medical perspective. L.W.O.S. contributed to the study design, oversight of the search and analysis, the structure of the article, and the writing.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This publication has emanated from research supported by Science Foundation Ireland (SFI) under Grant Numbers SFI 16/RC/3918 and SFI 20/COV/0031, co-funded by the European Regional Development Fund.
References
- 1. Van Norman GA. Expanded patient access to investigational new devices: Review of emergency and nonemergency expanded use, custom, and 3D-printed devices. JACC Basic Transl Sci 2018;3:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liaw C-Y, Guvendiren M. Current and emerging applications of 3D printing in medicine. Biofabrication 2017;9:024102. [DOI] [PubMed] [Google Scholar]
- 3. Culmone C, Smit G, Breedveld P. Additive manufacturing of medical instruments: A state-of-the-art review. Addit Manuf 2019;27:461–473. [Google Scholar]
- 4. Zhou H, Bhaduri SB. 12 - 3D printing in the research and development of medical devices. In: Yang L, Bhaduri SB, Webster TJ, eds. Biomaterials in Translational Medicine. Academic Press, 2019, pp. 269–289. [Google Scholar]
- 5. Luo H, Meyer-Szary J, Wang Z, et al. Three-dimensional printing in cardiology: Current applications and future challenges. Cardiol J 2017;24:436–444. [DOI] [PubMed] [Google Scholar]
- 6. Emelogu A, Marufuzzaman M, Thompson SM, et al. Additive manufacturing of biomedical implants: A feasibility assessment via supply-chain cost analysis. Addit Manuf 2016;11:97–113. [Google Scholar]
- 7. Imširović A, Kumnova G.. Utilizing 3D Printing to Provide Customized Joysticks. Gothenburg, Sweden: Chalmers University of Technology, 2017. [Google Scholar]
- 8. Holmström J, Partanen J, Tuomi J, et al. Rapid manufacturing in the spare parts supply chain. J Manuf Technol Manag 2010;21:687–697. [Google Scholar]
- 9. Slotwinski JA. Additive manufacturing: Overview and NDE challenges. AIP Conference Proceedings 2014; 1581:1173–1177. [Google Scholar]
- 10. Rayna T, Striukova L. From rapid prototyping to home fabrication: How 3D printing is changing business model innovation. Technol Forecast Soc Change 2016;102:214–224. [Google Scholar]
- 11. Horst A, McDonald F. Uncertain but not unregulated: medical product regulation in the light of three-dimensional printed medical products. 3D Print Addit Manuf 2020;7:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. FDA. Technical Considerations for Additive Manufactured Medical Devices; Guidance for Industry and Food and Drug Administration Staff. Washington: Federal Information & News Dispatch, LLC, 2017; p. 57462. [Google Scholar]
- 13. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC (Text with EEA relevance.) Official Journal of the European Union, L 117, 2017, pp. 1–175. [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009;339:332–336. [PMC free article] [PubMed] [Google Scholar]
- 15. Thompson RG. 3.2 - Anatomics 3D-printed titanium implants from head to heel. In: Froes FH, Qian M, eds. Titanium in Medical and Dental Applications. Woodhead Publishing, 2018, pp. 225–237. [Google Scholar]
- 16. Lador R, Regev G, Salame K, et al. Use of 3-dimensional printing technology in complex spine surgeries. World Neurosurg 2020;133:e327–e341. [DOI] [PubMed] [Google Scholar]
- 17. Zhao Y, Moran K, Yewondwossen M, et al. Clinical applications of 3-dimensional printing in radiation therapy. Med Dosim 2017;42:150–155. [DOI] [PubMed] [Google Scholar]
- 18. Jovičić MŠ, Vuletić F, Ribičić T, et al. Implementation of the three-dimensional printing technology in treatment of bone tumours: A case series. Int Orthop 2020. [Epub ahead of print]; DOI: 10.1007/s00264-020-04787-4. [DOI] [PubMed] [Google Scholar]
- 19. Morrison RJ, Hollister SJ, Niedner MF, et al. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci Transl Med 2015;7:285ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oswald N, Senanayake E, Naidu B, et al. Chest wall mechanics in vivo with a new custom-made three-dimensional–printed sternal prosthesis. Ann Thorac Surg 2018;105:1272–1276. [DOI] [PubMed] [Google Scholar]
- 21. Tran MD, Varzaly JA, Chan JCY, et al. Novel sternal reconstruction with custom three-dimensional–printed titanium PoreStar prosthesis. Innovations 2018;13:309–311. [DOI] [PubMed] [Google Scholar]
- 22. Les AS, Ohye RG, Filbrun AG, et al. 3D-printed, externally-implanted, bioresorbable airway splints for severe tracheobronchomalacia. Laryngoscope 2019;129:1763–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang W-F, Choi WS, Leung YY, et al. Three-dimensional printing of patient-specific surgical plates in head and neck reconstruction: A prospective pilot study. Oral Oncol 2018;78:31–36. [DOI] [PubMed] [Google Scholar]
- 24. Melville JC, Manis CS, Shum JW, et al. Single-unit 3D-printed titanium reconstruction plate for maxillary reconstruction: The evolution of surgical reconstruction for maxillary defects—A case report and review of current techniques. J Oral Maxillofac Surg 2019;77:874-e1. [DOI] [PubMed] [Google Scholar]
- 25. Ackland D, Robinson D, Lee PVS, et al. Design and clinical outcome of a novel 3D-printed prosthetic joint replacement for the human temporomandibular joint. Clin Biomech (Bristol, Avon) 2018;56:52–60. [DOI] [PubMed] [Google Scholar]
- 26. Zou Y, Yang Y, Han Q, et al. Novel exploration of customized 3D printed shoulder prosthesis in revision of total shoulder arthroplasty: A case report. Medicine (Baltimore) 2018;97:e13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu N, Li S, Liu Y, et al. Novel exploration of 3D printed personalized total elbow arthroplasty to solve the severe bone defect after internal fixation failure of comminuted distal humerus fracture: A case report. Medicine (Baltimore) 2020;99:e21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amelot A, Colman M, Loret J-E. Vertebral body replacement using patient-specific three–dimensional-printed polymer implants in cervical spondylotic myelopathy: An encouraging preliminary report. Spine J 2018;18:892–899. [DOI] [PubMed] [Google Scholar]
- 29. Mobbs RJ, Coughlan M, Thompson R, et al. The utility of 3D printing for surgical planning and patient-specific implant design for complex spinal pathologies: Case report. J Neurosurg Spine 2017;26:513–518. [DOI] [PubMed] [Google Scholar]
- 30. Willemsen K, Nizak R, Noordmans HJ, et al. Challenges in the design and regulatory approval of 3D-printed surgical implants: A two-case series. Lancet Digit Health 2019;1:e163–e171. [DOI] [PubMed] [Google Scholar]
- 31. Girolami M, Boriani S, Bandiera S, et al. Biomimetic 3D-printed custom-made prosthesis for anterior column reconstruction in the thoracolumbar spine: A tailored option following en bloc resection for spinal tumors: Preliminary results on a case-series of 13 patients. Eur Spine J 2018;27:3073–3083. [DOI] [PubMed] [Google Scholar]
- 32. Wei F, Li Z, Liu Z, et al. Upper cervical spine reconstruction using customized 3D-printed vertebral body in 9 patients with primary tumors involving C2. Ann Transl Med 2020;8:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li X, Wang Y, Zhao Y, et al. Multilevel 3D printing implant for reconstructing cervical spine with metastatic papillary thyroid carcinoma. Spine (Phila Pa 1976) 2017;42:E1326–E1330. [DOI] [PubMed] [Google Scholar]
- 34. Xu N, Wei F, Liu X, et al. Reconstruction of the upper cervical spine using a personalized 3D-printed vertebral body in an adolescent with ewing sarcoma. Spine (Phila Pa 1976) 2016;41:E50–E54. [DOI] [PubMed] [Google Scholar]
- 35. Hunn SAM, Koefman AJ, Hunn AWM. 3D-printed titanium prosthetic reconstruction of the C2 vertebra: Techniques and outcomes of three consecutive cases. Spine (Phila Pa 1976) 2020;45:667–672. [DOI] [PubMed] [Google Scholar]
- 36. Thayaparan GK, Owbridge MG, Thompson RG, et al. Designing patient-specific 3D printed devices for posterior atlantoaxial transarticular fixation surgery. J Clin Neurosci 2018;56:192–198. [DOI] [PubMed] [Google Scholar]
- 37. Thayaparan GK, Owbridge MG, Thompson RG, et al. Designing patient-specific solutions using biomodelling and 3D-printing for revision lumbar spine surgery. Eur Spine J 2018;28(S2):18–24. [DOI] [PubMed] [Google Scholar]
- 38. Patel V, Kovalsky D, Craig Meyer S, et al. Prospective trial of sacroiliac joint fusion using 3D-printed triangular titanium implants. Med Devices (Auckl) 2020;13:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Siu TL, Rogers JM, Lin K, et al. Custom-made titanium 3-dimensional printed interbody cages for treatment of osteoporotic fracture–related spinal deformity. World Neurosurg 2018;111:1–5. [DOI] [PubMed] [Google Scholar]
- 40. Chung SS, Lee KJ, Kwon YB, et al. Characteristics and efficacy of a new 3-dimensional printed mesh structure titanium alloy spacer for posterior lumbar interbody fusion. Orthopedics 2017;40:e880–e885. [DOI] [PubMed] [Google Scholar]
- 41. Thayaparan GK, Owbridge MG, Thompson RG, et al. Patient-specific processes for occipitocervical fixation using biomodelling and additive manufacturing. J Clin Neurosci 2020;71:251–256. [DOI] [PubMed] [Google Scholar]
- 42. Chin BZ, Ji T, Tang X, et al. Three-level lumbar en bloc spondylectomy with three-dimensional−printed vertebrae reconstruction for recurrent giant cell tumor. World Neurosurg 2019;129:531–537.e1. [DOI] [PubMed] [Google Scholar]
- 43. Chatain GP, Finn M. Compassionate use of a custom 3D-printed sacral implant for revision of failing sacrectomy: Case report. J Neurosurg Spine 2020;1(aop):1–6. [DOI] [PubMed] [Google Scholar]
- 44. Kim D, Lim J-Y, Shim K-W, et al. Sacral reconstruction with a 3D-printed implant after hemisacrectomy in a patient with sacral osteosarcoma: 1-year follow-up result. Yonsei Med J 2017;58:453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wei R, Guo W, Ji T, et al. One-step reconstruction with a 3D-printed, custom-made prosthesis after total en bloc sacrectomy: A technical note. Eur Spine J 2016;26:1902–1909. [DOI] [PubMed] [Google Scholar]
- 46. Wei R, Guo W, Yang R, et al. Reconstruction of the pelvic ring after total en bloc sacrectomy using a 3D-printed sacral endoprosthesis with re-establishment of spinopelvic stability: A retrospective comparative study. Bone Joint J 2019;101 B:880–888. [DOI] [PubMed] [Google Scholar]
- 47. Lv ZR, Li ZF, Yang ZP, et al. One-step reconstruction with a novel suspended, modular, and 3D-printed total sacral implant resection of sacral giant cell tumor with preservation of bilateral S1–3 nerve roots via a posterior-only approach. Orthop Surg 2020;12:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li Z, Chen G, Xiang Y, et al. Treatment of massive iliac chondrosarcoma with personalized three-dimensional printed tantalum implant: A case report and literature review. J Int Med Res 2020;48:0300060520959508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Citak M, Kochsiek L, Gehrke T, et al. Preliminary results of a 3D-printed acetabular component in the management of extensive defects. Hip Int 2018;28:266–271. [DOI] [PubMed] [Google Scholar]
- 50. Chen X, Xu L, Wang Y, et al. Image-guided installation of 3D-printed patient-specific implant and its application in pelvic tumor resection and reconstruction surgery. Comput Methods Programs Biomed 2016;125:66–78. [DOI] [PubMed] [Google Scholar]
- 51. Liu X, Liu Y, Lu W, et al. Combined application of modified three-dimensional printed anatomic templates and customized cutting blocks in pelvic reconstruction after pelvic tumor resection. J Arthroplasty 2019;34:338–345.e1. [DOI] [PubMed] [Google Scholar]
- 52. Kieser DC, Ailabouni R, Kieser SCJ, et al. The use of an Ossis custom 3D-printed tri-flanged acetabular implant for major bone loss: Minimum 2-year follow-up. Hip Int 2018;28:668–674. [DOI] [PubMed] [Google Scholar]
- 53. Wong KC, Kumta SM, Gee NVL, et al. One-step reconstruction with a 3D-printed, biomechanically evaluated custom implant after complex pelvic tumor resection. Comput Aided Surg 2015;20:14–23. [DOI] [PubMed] [Google Scholar]
- 54. Wang B, Hao Y, Pu F, et al. Computer-aided designed, three dimensional-printed hemipelvic prosthesis for peri-acetabular malignant bone tumour. Int Orthop 2018;42:687–694. [DOI] [PubMed] [Google Scholar]
- 55. Zhang Y, Zhang L, Sun R, et al. A new 3D printed titanium metal trabecular bone reconstruction system for early osteonecrosis of the femoral head. Medicine (Baltimore) 2018;97:e11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Luo W, Huang L, Liu H, et al. Customized knee prosthesis in treatment of giant cell tumors of the proximal tibia: Application of 3-dimensional printing technology in surgical design. Med Sci Monit 2017;23:1691–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lu M, Li Y, Luo Y, et al. Uncemented three-dimensional-printed prosthetic reconstruction for massive bone defects of the proximal tibia. World J Surg Oncol 2018;16:47–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu W, Shao Z, Rai S, et al. Three-dimensional-printed intercalary prosthesis for the reconstruction of large bone defect after joint-preserving tumor resection. J Surg Oncol 2020;121:570–577. [DOI] [PubMed] [Google Scholar]
- 59. Sultan AA, Mahmood B, Samuel LT, et al. Cementless 3D printed highly porous titanium-coated baseplate total knee arthroplasty: Survivorship and outcomes at 2-year minimum follow-up. J Knee Surg 2020;33:279–283. [DOI] [PubMed] [Google Scholar]
- 60. Faldini C, Mazzotti AA-O, Belvedere C, et al. A new ligament-compatible patient-specific 3D-printed implant and instrumentation for total ankle arthroplasty: From biomechanical studies to clinical cases. J Orthop Traumatol 2020;21:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Patel H, Kinmon K. Revision of failed total ankle replacement with a custom 3-dimensional printed talar component with a titanium truss cage: A case presentation. J Foot Ankle Surg 2019;58:1006–1009. [DOI] [PubMed] [Google Scholar]
- 62. Hsu AR, Ellington JK. Patient-specific 3-dimensional printed titanium truss cage with tibiotalocalcaneal arthrodesis for salvage of persistent distal tibia nonunion. Foot Ankle Spec 2015;8:483–489. [DOI] [PubMed] [Google Scholar]
- 63. Dekker TJ, Steele JR, Federer AE, et al. Use of patient-specific 3D-printed titanium implants for complex foot and ankle limb salvage, deformity correction, and arthrodesis procedures. Foot Ankle Int 2018;39:916–921. [DOI] [PubMed] [Google Scholar]
- 64. Yang JC-S, Chen C-F, Luo C-A, et al. Clinical experience using a 3D-printed patient-specific instrument for medial opening wedge high tibial osteotomy. Biomed Res Int 2018;2018:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gorbatov RO, Malyshev EE, Romanov AD, Karyakin NN. Total knee arthroplasty using virtual prototyping and additive manufacturing. Sovremennye Tehnologii v Medicine [Modern Technologies in Medicine] 2018;10:146. [Google Scholar]
- 66. Park JW, Kang HG, Lim KM, et al. Bone tumor resection guide using three-dimensional printing for limb salvage surgery. J Surg Oncol 2018;118:898–905. [DOI] [PubMed] [Google Scholar]
- 67. Gemalmaz HC, Sarıyılmaz K, Ozkunt O, et al. Postoperative mechanical alignment analysis of total knee replacement patients operated with 3D printed patient specific instruments: A prospective cohort study. Acta Orthop Traumatol Turc 2019;53:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rosseels W, Herteleer M, Sermon A, et al. Corrective osteotomies using patient-specific 3D-printed guides: A critical appraisal. Eur J Trauma Emerg Surg 2018;45:299–307. [DOI] [PubMed] [Google Scholar]
- 69. Nizam I, Batra AV. Accuracy of bone resection in total knee arthroplasty using CT assisted-3D printed patient specific cutting guides. SICOT-J 2018;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhou F, Xue F, Zhang S. The application of 3D printing patient specific instrumentation model in total knee arthroplasty. Saudi J Biol Sci 2020;27:1217–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gauci M-O, Chelli M, Fernandez J, et al. Patient-specific three-dimensional-printed instrumentation for radius lengthening osteotomy by a volar approach in epiphysiodesis sequelae: A case report. J Orthop Case Rep 2020;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gómez-Palomo JM, Estades-Rubio FJ, Meschian-Coretti S, et al. Internal hemipelvectomy and reconstruction assisted by 3D printing technology using premade intraoperative cutting and placement guides in a patient with pelvic sarcoma: A case report. JBJS Case Connect 2019;9:e0060. [DOI] [PubMed] [Google Scholar]
- 73. Sun ML, Zhang Y, Peng Y, et al. Accuracy of a novel 3D-printed patient-specific intramedullary guide to control femoral component rotation in total knee arthroplasty. Orthop Surg 2020;12:429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sun M, Zhang Y, Peng Y, et al. Gait analysis after total knee arthroplasty assisted by 3D-printed personalized guide. Biomed Res Int 2020;2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Arnal-Burró J, Pérez-Mañanes R, Gallo-del-Valle E, et al. Three dimensional-printed patient-specific cutting guides for femoral varization osteotomy: Do it yourself. Knee 2017;24:1359–1368. [DOI] [PubMed] [Google Scholar]
- 76. Zavattero E, Fasolis M, Novaresio A, et al. The shape of things to come: In-hospital three-dimensional printing for mandibular reconstruction using fibula free flap. Laryngoscope 2020;130:E811–E816. [DOI] [PubMed] [Google Scholar]
- 77. Shen Z, Wang H, Duan Y, et al. Application of 3D printed osteotomy guide plate-assisted total knee arthroplasty in treatment of valgus knee deformity. J Orthop Surg Res 2019;14:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Garg B, Gupta M, Singh M, et al. Outcome and safety analysis of 3D-printed patient-specific pedicle screw jigs for complex spinal deformities: A comparative study. Spine J 2019;19:56–64. [DOI] [PubMed] [Google Scholar]
- 79. Huang H, Hsieh M-F, Zhang G, et al. Improved accuracy of 3D-printed navigational template during complicated tibial plateau fracture surgery. Australas Phys Eng Sci Med 2015;38:109–117. [DOI] [PubMed] [Google Scholar]
- 80. Naddeo F, Fontana C, Naddeo A, et al. Novel design for a customized, 3D-printed surgical template for thoracic spinal arthrodesis. Int J Med Robot 2019;15:e2005. [DOI] [PubMed] [Google Scholar]
- 81. Naddeo F, Cataldo E, Narciso N, et al. “In vivo” validation of 3D-printed innovative surgical template for lumbar spinal arthrodesis. Appl Sci 2020;10:5977. [Google Scholar]
- 82. Yang F, Yao S, Chen K-F, et al. A novel patient-specific three-dimensional-printed external template to guide iliosacral screw insertion: A retrospective study. BMC Musculoskelet Disord 2018;19:397–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Deng T, Jiang M, Lei Q, et al. The accuracy and the safety of individualized 3D printing screws insertion templates for cervical screw insertion. Comput Assist Surg (Abingdon) 2016;21:143–149. [DOI] [PubMed] [Google Scholar]
- 84. Liu K, Zhang Q, Li X, et al. Preliminary application of a multi-level 3D printing drill guide template for pedicle screw placement in severe and rigid scoliosis. Eur Spine J 2016;26:1684–1689. [DOI] [PubMed] [Google Scholar]
- 85. Marengo N, Matsukawa K, Monticelli M, et al. Cortical bone trajectory screw placement accuracy with a patient-matched 3-dimensional printed guide in lumbar spinal surgery: A clinical study. World Neurosurg 2019;130:e98–e104. [DOI] [PubMed] [Google Scholar]
- 86. Luo M, Wang W, Yang N, et al. Does Three-dimensional printing plus pedicle guider technology in severe congenital scoliosis facilitate accurate and efficient pedicle screw placement? Clin Orthop Relat Res 2019;477:1904–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Takemoto M, Fujibayashi S, Ota E, et al. Additive-manufactured patient-specific titanium templates for thoracic pedicle screw placement: Novel design with reduced contact area. Eur Spine J 2016;25:1698–1705. [DOI] [PubMed] [Google Scholar]
- 88. Cecchinato R, Berjano P, Zerbi A, et al. Pedicle screw insertion with patient-specific 3D-printed guides based on low-dose CT scan is more accurate than free-hand technique in spine deformity patients: A prospective, randomized clinical trial. Eur Spine J 2019;28:1712–1723. [DOI] [PubMed] [Google Scholar]
- 89. Bigeleisen PE. Design and production of an articulating needle guide for ultrasound-guided needle block manufactured with a three-dimensional printer: Technical communication. A&A Pract 2017;8:272–275. [DOI] [PubMed] [Google Scholar]
- 90. Wan S-X, Meng F-B, Zhang J, et al. Experimental study and preliminary clinical application of mini-invasive percutaneous internal screw fixation for scaphoid fracture under the guidance of a 3D-printed guide plate. Curr Med Sci 2019;39:990–996. [DOI] [PubMed] [Google Scholar]
- 91. Zhang L, Wang L, Kadeer X, et al. Accuracy of a 3-dimensionally printed navigational template for localizing small pulmonary nodules: A noninferiority randomized clinical trial. JAMA surg 2019;154:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cui Z, Wang Z, Ye G, et al. A novel three-dimensional printed guiding device for electrode implantation of sacral neuromodulation. Colorectal Dis 2018;20:O26–O29. [DOI] [PubMed] [Google Scholar]
- 93. Tao H, Xiaodan Y, Ying X, et al. Therapeutic value of 3-D printing template-assisted 125I-seed implantation in the treatment of malignant liver tumors. Onco Targets Ther 2017;10:3277–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pakzaban P. A 3-Dimensional-printed spine localizer: Introducing the concept of online dissemination of novel surgical instruments. Neurospine 2018;15:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang Q, Guo W, Liu Y, et al. Application of a 3D-printed navigation mold in puncture drainage for brainstem hemorrhage. J Surg Res 2020;245:99–106. [DOI] [PubMed] [Google Scholar]
- 96. Garcia-Sevilla M, Moreta-Martinez R, Garcia-Mato D, et al. Surgical navigation for palate carcinoma resection using a noninvasive 3D-printed reference frame. Int J Comput Assist Radiol Surg 2020;15:S135–S136. [Google Scholar]
- 97. Duan XJ, Fan HQ, Wang FY, et al. Application of 3D-printed customized guides in subtalar joint arthrodesis. Orthop Surg 2019;11:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mishra A, Verma T, Rajkumar, et al. 3D printed patient-specific acetabular jig for cup placement in total hip arthroplasty. Indian J Orthop 2020;54:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Huang W, Lu J, Chen K-M, et al. Preliminary application of 3D-printed coplanar template for iodine-125 seed implantation therapy in patients with advanced pancreatic cancer. World J Gastroenterol 2018;24:5280–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang J, Zhang P, Wu L, et al. Application of an individualized and reassemblable 3D printing navigation template for accurate puncture during sacral neuromodulation. Neurourol Urodyn 2018;37:2776–2781. [DOI] [PubMed] [Google Scholar]
- 101. Yen C-I, Zelken JA, Chang C-S, et al. Computer-aided design and three-dimensional printing improves symmetry in heminasal reconstruction outcomes. J Plast Reconstr Aesthet Surg 2019;72:1198–1206. [DOI] [PubMed] [Google Scholar]
- 102. Zhou JMS, Pan BMD, Yang QMD, et al. Three-dimensional autologous cartilage framework fabrication assisted by new additive manufactured ear-shaped templates for microtia reconstruction. J Plast Reconstr Aesthet Surg 2016;69:1436–1444. [DOI] [PubMed] [Google Scholar]
- 103. Verweij JP, Moin DA, Mensink G, et al. Autotransplantation of premolars with a 3-dimensional printed titanium replica of the donor tooth functioning as a surgical guide: Proof of concept. J Oral Maxillofac Surg 2016;74:1114–1119. [DOI] [PubMed] [Google Scholar]
- 104. Bohl MA, Xu DS, Cavallo C, et al. The barrow innovation center case series: A novel 3-dimensional–printed retractor for use with electromagnetic neuronavigation systems. World Neurosurg 2018;116:e1075–e1078. [DOI] [PubMed] [Google Scholar]
- 105. Ko WJ, Song GW, Hong SP, et al. Novel 3D-printing technique for caps to enable tailored therapeutic endoscopy. Dig Endosc 2016;28:131–138. [DOI] [PubMed] [Google Scholar]
- 106. Xu Z-Y, Ren H-J, Huang J-J, et al. Application of a 3D-printed “fistula stent” in plugging enteroatmospheric fistula with open abdomen: A case report. World J Gastroenterol 2019;25:1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Baltz GC, Chi PCM, Wong PF, et al. Development and validation of a 3D-printed bolus cap for total scalp irradiation. J Appl Clin Med Phys 2019;20:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Robar JL, Moran K, Allan J, et al. Intrapatient study comparing 3D printed bolus versus standard vinyl gel sheet bolus for postmastectomy chest wall radiation therapy. Pract Radiat Oncol 2018;8:221–229. [DOI] [PubMed] [Google Scholar]
- 109. Sasaki DK, McGeachy P, Alpuche Aviles JE, et al. A modern mold room: Meshing 3D surface scanning, digital design, and 3D printing with bolus fabrication. J Appl Clin Med Phys 2019;20:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Buonamici F, Furferi R, Governi L, et al. A practical methodology for computer-aided design of custom 3D printable casts for wrist fractures. Vis Comput 2019;36:375–390. [Google Scholar]
- 111. Li H, Qiao F, Li D, et al. Personalized human factor and ergonomics: Usability design of 3D printed patient-specific fracture external fixator. In: International Conference on Applied Human Factors and Ergonomics. Cham: Springer, 2019; pp. 119–128. [Google Scholar]
- 112. Qiao F, Li D, Jin Z, et al. A novel combination of computer-assisted reduction technique and three dimensional printed patient-specific external fixator for treatment of tibial fractures. Int Orthop 2016;40:835–841. [DOI] [PubMed] [Google Scholar]
- 113. Xu R, Wang Z, Ren Z, et al. Comparative study of the effects of customized 3D printed insole and prefabricated insole on plantar pressure and comfort in patients with symptomatic flatfoot. Med Sci Monit 2019;25:3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Xu R, Wang Z, Ma T, et al. Effect of 3D printing individualized ankle-foot orthosis on plantar biomechanics and pain in patients with plantar fasciitis: A randomized controlled trial. Med Sci Monit 2019;25:1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wang K, Shi Y, He W, et al. The research on 3D printing fingerboard and the initial application on cerebral stroke patient's hand spasm. Biomed Eng Online 2018;17:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zheng Y, Liu G, Yu L, et al. Effects of a 3D-printed orthosis compared to a low-temperature thermoplastic plate orthosis on wrist flexor spasticity in chronic hemiparetic stroke patients: A randomized controlled trial. Clin Rehabil 2019;34:026921551988517-204. [DOI] [PubMed] [Google Scholar]
- 117. Yoo H-J, Lee S, Kim J, et al. Development of 3D-printed myoelectric hand orthosis for patients with spinal cord injury. J Neuroeng Rehabil 2019;16:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Huang T-Y, Pan L-LH, Yang W-W, et al. biomechanical evaluation of three-dimensional printed dynamic hand device for patients with chronic stroke. IEEE Trans Neural Syst Rehabil Eng 2019;27:1246–1252. [DOI] [PubMed] [Google Scholar]
- 119. Zuniga J. 3D printed antibacterial prostheses. Appl Sci 2018;8:1651. [Google Scholar]
- 120. Eggbeer D, Bibb R, Evans P, et al. Evaluation of direct and indirect additive manufacture of maxillofacial prostheses. Proc Inst Mech Eng H 2012;226:718–728. [DOI] [PubMed] [Google Scholar]
- 121. Xepapadeas AB, Weise C, Frank K, et al. Technical note on introducing a digital workflow for newborns with craniofacial anomalies based on intraoral scans—Part I: 3D printed and milled palatal stimulation plate for trisomy 21. BMC Oral Health 2020;20:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ruzza A, Parekh M, Ferrari S, et al. Preloaded donor corneal lenticules in a new validated 3D printed smart storage glide for Descemet stripping automated endothelial keratoplasty. Br J Ophthalmol 2015;99:1388–1395. [DOI] [PubMed] [Google Scholar]
- 123. Williams E, Bond K, Isles N, et al. Pandemic printing: A novel 3D-printed swab for detecting SARS-CoV-2. Med J Aust 2020;213:276–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gemalmaz HC, Sarıyılmaz K, Ozkunt O, et al. A new osteotomy for the prevention of prominent lateral condyle after cubitus varus correctional surgery-made possible by a 3D printed patient specific osteotomy guide: A case report. Int J Surg Case Rep 2017;41:438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Fousová M, Vojtěch D, Kubásek J.. 3D printing of biomedical titanium alloy. In: Proceedings 25th Anniversary International Conference on Metallurgy and Materials (May 25th-27th, Brno, Czech Republic), 2016, pp. 1564–1569. [Google Scholar]
- 126. Calignano F, Galati M, Iuliano L, et al. Design of additively manufactured structures for biomedical applications: A review of the additive manufacturing processes applied to the biomedical sector. J Healthc Eng 2019;2019:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Fan D, Li Y, Wang X, et al. Progressive 3D printing technology and its application in medical materials. Front Pharmacol 2020;11:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. De Beer N, Bloem N. An economic cost model for patient-specific intervertebral disc implants. 2011. [Google Scholar]
- 129. Spetzger U, Frasca M, König SA. Surgical planning, manufacturing and implantation of an individualized cervical fusion titanium cage using patient-specific data. Eur Spine J 2016;25:2239–2246. [DOI] [PubMed] [Google Scholar]
- 130. O'Sullivan KJ, O'Sullivan AG, Power N, et al. Use of 3D printing to create a bespoke repair of a Percutaneous Endoscopic Gastrostomy (PEG) tube in patient unfit for surgical replacement. BMJ Innov 2018;4:29–31. [Google Scholar]
- 131. Dunne CP, O'Sullivan KJ, O'Sullivan L, et al. Foreseeing the microbiology of bespoke 3D-printed medical devices. J Hosp Infect 2018;99:237–238. [DOI] [PubMed] [Google Scholar]
- 132. U.S. Food and Drug Administration. Custom Device Exemption - Guidance for Industry and Food and Drug Administration Staff. 2014. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/custom-device-exemption. (last accessed October 8, 2020).
- 133. Suska F, Kjeller G, Tarnow P, et al. Electron beam melting manufacturing technology for individually manufactured jaw prosthesis: A case report. J Oral Maxillofac Surg 2016;74:1706-e1. [DOI] [PubMed] [Google Scholar]
- 134. Tsuyuki K, Yano K, Watanabe N, et al. Compassionate use of drugs and medical devices in the United States, the European Union and Japan. Regen Ther 2016;4:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. OSSIS Ltd. OSSIS Products. https://www.ossis.com/products (last accessed October 8, 2020).
- 136. Morrison RJ, Kashlan KN, Flanangan CL, et al. Regulatory considerations in the design and manufacturing of implantable 3D-printed medical devices. Clin Transl Sci 2015;8:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]