Abstract
Environmental stresses can compromise the interactions of plants with beneficial microbes. In the present review, experimental results showing that stresses negatively affect the abundance and/or functionality of plant beneficial microbes are summarized. It is proposed that the environmental interference of these plant−microbe interactions is explained by the stress‐mediated induction of plant signalling pathways associated with defence hormones and reactive oxygen species. These plant responses are recognized to regulate beneficial microbes within plants. The direct negative effect of stresses on microbes may also contribute to the environmental regulation of these plant mutualisms. It is also posited that, in stress situations, beneficial microbes harbour mechanisms that contribute to maintain the mutualistic associations. Beneficial microbes produce effector proteins and increase the antioxidant levels in plants that counteract the detrimental effects of plant stress responses on them. In addition, they deliver specific stress‐protective mechanisms that assist to their plant hosts to mitigate the negative effects of stresses. Our study contributes to understanding how environmental stresses affect plant−microbe interactions and highlights why beneficial microbes can still deliver benefits to plants in stressful environments.
Keywords: abiotic and biotic stresses, antioxidants, effectors, endophytes, mycorrhizae, phytohormones, reactive nitrogen species, reactive oxygen species
SUMMARY STATEMENT
Here, it is proposed that the environmental interference of plant‐microbe interactions is explained by the stress‐mediated induction of certain plant signalling pathways and that specific mechanisms harboured by beneficial microbes assist in maintaining the mutualisms in stress situations.
1. INTRODUCTION
Plants are constantly challenged by a broad range of biotic and abiotic environmental stressors. Biotic stressors include pathogens, herbivores and competitors, while abiotic stressors include drought, salinity, heat and nutrient scarcity (Suzuki et al., 2014). Environmental stressors typically affect the plant physiology and metabolism, which can reduce the growth and reproduction of plants (Branco et al., 2022). Furthermore, stressors often compromise the association of plants with beneficial microbes, which can also limit plant fitness (Kiers et al., 2010; Rudgers et al., 2020). Plant beneficial microbes confer to their hosts with mechanisms that efficiently mitigate the detrimental effects of stresses (Bastías et al., 2022). Foliar fungal endophytes of genus Epichloë endow to plants with specialized metabolites (mainly alkaloids) that increase the levels of resistance against herbivores (Schardl et al., 2004). They also stimulate certain plant responses that enhance the resistance/tolerance to pathogens, drought and other stresses (Card et al., 2021; Decunta et al., 2021). Similarly, root fungal endophytes trigger a wealth of molecular processes in the hosts, including calcium signalling and the production of osmolytes like proline or soluble sugars, that increase the plant tolerance to abiotic and biotic stresses (Giauque et al., 2019; Hereme et al., 2020; Lata et al., 2018; Vadassery & Oelmüller, 2009). Mycorrhizal fungi enhance the host acquisition of nutrients (such as phosphorus and nitrogen) and water that results in net benefits for plants specially growing in poor soils (Bennett & Groten, 2022). Furthermore, they stimulate host immune responses that increases the resistance levels of plants against pathogens and insect herbivores (Pieterse et al., 2014; Pozo et al., 2015).
Most of the research regarding stresses and plant−microbe interactions has been focused on investigating microbial stress‐protective traits. However, stressors can compromise the associations of plants with beneficial microbes, and the mechanisms explaining this negative effect of stresses on plants have been scantly considered in the specialized literature. In this review, firstly, published results showing that environmental stresses compromised plant−microbe interactions were summarized. The study compilation was focused on distinct groups of beneficial microbes of plants: foliar endophytes, root endophytes and mycorrhizal fungi (mainly arbuscular mycorrhizae). Secondly, the potential mechanisms by which the stressors would interfere plant−microbe interactions were identified. It is proposed that beneficial microbes are affected by the plant responses triggered by the stresses (i.e., plant immunity and oxidative stress) and by the stress itself. Thirdly, putative mechanisms that beneficial microbes would use to counteract the plant stress responses and to alleviate the negative effects of stresses on plants and microbes were described. These microbial‐derived mechanisms may contribute to maintain the mutualism between the plant and microbe and to enhance the performance of plants in stress situations.
2. PLANT−MICROBE INTERACTIONS ARE COMPROMISED IN STRESS SITUATIONS
2.1. Environmental interference of foliar endophytes
Environmental stress can compromise the associations between plants and beneficial foliar endophytes. Within foliar tissues, endophyte fungi can be locally or systemically distributed. They extend hyphae along intercellular spaces of plant hosts where they obtain nutrients and carbohydrates from the apoplast (Christensen et al., 2008; Christensen & Voisey, 2007). These endophytes are transmitted vertically via plant seed, horizontally via contagious spread of symbionts and some species simultaneously transmit both vertically and horizontally (Rodriguez et al., 2009). Vertically transmitted endophytes form mutualistic relationship with plants and generally reach high prevalence in plant populations. A remarkable example of this are endophytes of genus Epichloë that form persistent associations with grasses of subfamily Pooideae (Gundel et al., 2011; Schardl et al., 2004).
Stresses can interfere with symbioses between plants and foliar endophytes by altering the magnitude of the benefits provided by symbionts (e.g., herbivory protection and plant growth promotion) (Bastías et al., 2021; Schardl et al., 2004). Elevated ozone levels reduced the Epichloë endophyte‐derived resistance to insects in Lolium multiflorum plants (Ueno et al., 2016). More severe was the effect of UV‐B radiation on Epichloë‐derived benefits. Elevated UV‐B levels completely supressed the endophyte‐based herbivore resistance in Festuca pratensis plants. Even worse was the effect of UV‐A radiation on the endophyte‐based herbivore resistance. Elevated UV‐A levels made the endophyte‐symbiotic plants more susceptible to insect herbivores than their nonsymbiotic counterparts (McLeod et al., 2001). Similarly to the UV‐B radiation, low temperature also suppressed the endophyte‐based resistance to insects in symbiotic L. multiflorum plants (Hennessy et al., 2016). Under drought stress, Festuca arundinacea plants associated with endophytes were more susceptible to insect herbivores than their nonsymbiotic counterparts in situations of water restriction (the opposite occurred under high water availability) (Bultman & Bell, 2003). Drought also supressed the disease protection conferred by endophytes against phytopathogens in Lolium perenne plants (i.e., Bipolaris sorokiniana) (Li et al., 2020). Stress can also affect plant growth stimulation conferred by endophytes to their hosts (Bastías et al., 2021). For example, drought supressed the endophyte‐mediated growth promotion in L. perenne and Festuca sinensis plants (Marks & Clay, 2007; Xu et al., 2021). Furthermore, the endophyte‐based resistance to insects was reduced by the presence of mycorrhizal fungi that presumably competed for resources in L. perenne plants (Vicari et al., 2002). Interestingly, the detrimental effect of stresses on the endophyte‐derived benefits can be transmitted intergenerationally. Daughter symbiotic plants of L. multiflorum produced from mothers that were exposed to ozone exhibited lower levels of resistance to insects compared to mother plants that were not exposed to the stress (Bubica Bustos et al., 2020).
The stress‐mediated compromise of Epichloë‐derived benefits may be explained by the reduction in concentrations of endophyte‐derived antiherbivore alkaloids and alteration in fungal mycelial biomass within plant tissues. Elevated carbon dioxide levels reduced the concentrations of endophytic alkaloids in F. arundinacea plants (Brosi et al., 2011; Ryan, Rasmussen et al., 2014). Similarly, decreased contents of endophyte‐derived alkaloid were exhibited by endophyte‐symbiotic L. perenne plants simultaneously associated with mycorrhizal fungi. In this experiment, the mycorrhizal treatment also reduced the mycelial biomass of Epichloë endophytes within plant tissues (Liu et al., 2011). Certain experimental results suggest that stresses inhibit the performance of endophyte‐symbiotic plants. Under drought conditions, endophyte‐symbiotic L. perenne plants accumulated less biomass and produced less seeds than nonsymbiotic plants (Cheplick et al., 2000; Hesse et al., 2003). Similarly, plants of the same species associated with endophytes exhibited lower biomass than nonsymbiotic plants during the recovery period post drought (Cheplick, 2004). Furthermore, lower regrowth rate following a treatment of simulated folivory was documented in certain genotypes of L. perenne plants associated with endophytes compared to their nonsymbiotic counterparts (Cheplick, 1998).
2.2. Environmental interference of root endophytes
As with the foliar endophytes, environmental stresses can interfere with symbioses between plants and beneficial root endophytes. Most root endophyte fungi grow in the apoplast of epidermal and cortex cells, without entering the central cylinder of their host plants' root. They form loose hyphal networks that invaginate the plasma membranes of the plant cells. In contrast to mycorrhizal fungi, most root endophyte fungi, like for instance Serendipita indica, do not induce the formation of differentiated plant or fungal structures, when penetrating and colonizing the cells of their host plants (Weiß et al., 2016). At present, there is only very little known about the mechanisms by which plants and microbes steer their interactions under ever‐changing environmental conditions. However, there is mounting evidence that plants have to tightly control their root microbiota to maintain their fitness (Wolinska et al., 2021). A striking example of this are the major changes observed in root‐associated fungal communities in Triticum aestivum plants under drought stress (Salamon et al., 2020). Another study from the same group pointed into the same direction, highlighting that drought stress indirectly affected the plant−fungal interactions in roots of T. aestivum (subspecies vulgare and spelta), while still promoting plant growth and several physiological parameters, including photosynthetic activity, electron transport rate and water use efficiency (Ratajczak et al., 2020).
Multiple studies have demonstrated that environmental stresses contribute to control the colonization of roots by endophytes. For example, soils with high levels of both cupper (Cu) and lead (Pb) negatively affected the colonization of S. indica fungi on roots of Ocimum basilicum plants, while individual treatments with either Cu or Pb showed no negative impact on the colonization (Sabra et al., 2018). Noteworthy in this context is the additional finding that the simultaneous infection of O. basilicum roots with S. indica and the arbuscular mycorrhiza fungus Rhizophagus irregularis reduced the colonization of plant roots by S. indica when grown in presence of Pb alone. Furthermore interesting is the observation that the co‐colonization of roots with both fungi could significantly stimulate the mycorrhization with R. irregularis under Cu and combined Cu and Pb stress, although it remains to be remarked that the abundance of mycorrhizae was generally low under heavy metal stress. Furthermore, a recent study provided comprehensive evidence that a number of climate change‐related abiotic stresses, including drought and mechanical stress (through an increased compactness of the soil), had a negative effect on the root colonization of S. indica on Zea mays plants (Hosseini et al., 2018). The study also highlighted that, plants inoculated with this root endophyte fungus still performed better when exposed to combined drought and mechanical stress, even though the fungal load was diminished. This would imply that host plants may possess mechanisms to maintain a necessary level of symbiosis to gain the benefits under stress, without putting their own survival into jeopardy. In the context of soil compactness, a recent study reported that compacted soils reduced the free diffusion of plant‐produced ethylene which, in turn, accumulated in the rhizosphere and the root tissue, where it restricted root growth (Pandey et al., 2021). Another work on the root colonization of Arabidopsis plants with S. indica demonstrated that ethylene signalling and ethylene‐targeted transcription factors were essential to establish the plant−fungal interaction (Camehl et al., 2010). Overall, there is currently very limited insights into climate change‐mediated abiotic stresses in the rhizosphere (Fonseca de Lima et al., 2021), and much more work is needed to obtain a deeper understanding of the intricacies of plant−fungal interactions under these conditions.
Multiple experimental results suggest that distinct root endophytes enhance the tolerance of plants to salt stress (e.g., S. indica, Fusarium culmorum, Talaromyces minioluteus, Penicillium murcianum fungi) (González‐Teuber et al., 2022; Pérez‐Alonso et al., 2020; Rodriguez et al., 2008). However, there are few studies that empirically evaluate whether salt stress exerts negative effects on the symbiosis between plants and fungal root endophytes. One of these studies documented a significant reduction in the colonization rate of Oryza sativa roots by S. indica when symbiotic plants were subjected to salt stress (Jogawat et al., 2016).
Depending on the given host plant—root endophyte combination and the specific environmental stress applied, the beneficial effect of the symbiont can be considerably reduced. To give just a few examples, severe drought stress significantly reduced the S. indica‐mediated root growth promotion on Z. mays plants (Zhang et al., 2018), while high salt conditions minimized the root growth promoting effect of Aspergillus aculeatus endophytes on Cynodon dactylon plants (Xie et al., 2017). However, there is only scarce information on scenarios in which the interaction of beneficial root endophytes with their host plants converts into a burden for the plant, drastically hampering its fitness. In this context, it has been reported that several endophytes possess a considerable phenotypic plasticity, which allows them to switch between endophytic and necrotrophic lifestyles (Delaye et al., 2013). This could possibly be taken as an additional indication for the intimate control of the symbiotic relationship of the interacting organisms.
2.3. Environmental interference of mycorrhizal fungi
Similar to foliar and root endophytes, most studies regarding stresses and plant‐mycorrhizal associations have described the stress‐protective traits that these fungi confer to their hosts (Balestrini et al., 2018; Porcel et al., 2012; Rivero et al., 2018). However, multiple evidence suggest that abiotic and biotic stresses compromise these plant−microbe interactions.
Drought can reduce the colonization of arbuscular mycorrhizal fungi (although mycorrhizal plants generally perform better than their nonsymbiotic counterparts) (Augé, 2001; Balestrini et al., 2018; Chitarra et al., 2016). This reduction in mycorrhizal root colonization is explained in part by the negative effect of the stress on the development of the fungus in soil and rhizosphere. For instance, drought (and other stresses as well) inhibited the spore germination and elongation of germinative hyphae of mycorrhizal fungi in soil (Lenoir et al., 2016). The stress‐mediated alteration in the plant metabolisms and/or development (e.g., by reducing root size) also contributes to the detrimental effect of drought on the mycorrhizal root colonization (Lenoir et al., 2016; Millar & Bennett, 2016). For example, drought reduced the ability of mycorrhizal fungi to promote the expression of plant genes involved in the transport of nutrients between the plant and fungus in Solanum lycopersicum (e.g., transporters of phosphate, ammonium, peptides, amino acids) (Balestrini et al., 2019). The decreased expression of these plant genes may be explained by a reduced abundance of mycorrhizal arbuscules in plant roots (Chitarra et al., 2016).
Soil nutrients, such as phosphorus and nitrogen, are known to be involved in the regulation of plant‐mycorrhizal symbioses. The addition of phosphorus and nitrogen in soil decreased the diversity (and abundance) of mycorrhizal fungal species in plants (Ma et al., 2021). This reduced diversity of fungi has been explained by a shortage in the allocation of carbon‐based compounds towards mycorrhizae because plant hosts apparently rely less on the fungus to obtain nutrients from the soil (Branco et al., 2022). At physiological level, high nutrient contents in soil down‐regulated the expression of plant genes that facilitate the mycorrhizal root colonization (e.g., phosphate transporters) and promote the development of arbuscules in roots (i.e., half‐size ATP‐binding cassete transporters) (Breuillin et al., 2010; Wang et al., 2017). Furthermore, high phosphorus levels repressed the expression of plant genes involved in the biosynthesis of strigolactones that stimulate the growth and branching of mycorrhizal hyphae. Low strigolactone levels in plants normally decrease the germination of mycorrhizal fungi (López‐Ráez, 2016; Wang et al., 2017). The pH is another soil aspect that control plant‐mycorrhizal symbioses. Soils with acidic pH reduced the abundance of mycorrhizal fungal arbuscules (and impeded their development) in roots of S. lycopersicum plants. The disrupted transfer of lipids between the plant and fungus seemed to explain the negative effect of this stress on the symbiosis (Feng et al., 2020; Liu et al., 2020).
Insect herbivory compromises the abundance and diversity of arbuscular mycorrhizal species in plants (Frew, 2022; Shi et al., 2022). The presence of the phloem‐feeding aphid Acyrthosiphon pisum decreased the colonization of mycorrhizal fungi in Vicia faba plants (Babikova et al., 2014). Similarly, a treatment of simulated folivory reduced the abundance of mycorrhizal arbuscules in Medicago sativa plants. In this experiment, the reduction in arbuscule abundance was associated with limited availability of photosynthates in plant tissues (Saravesi et al., 2014).
3. PLANT BENEFICIAL MICROBES ARE AFFECTED BY ENVIRONMENTAL STRESSES: MECHANISTIC PERSPECTIVES
Plants respond to environmental stresses by inducing signalling cascades that are governed by phytohormones, reactive oxygen species (ROS) and other signalling molecules (Figure 1). The signalling cascades converge in the activation and/or repression of master regulators that control the expression of phytohormone‐ and ROS‐responsive transcription factors (Devireddy et al., 2021; Zhang et al., 2022). These transcription factors regulate the expression of plant genes encoding for proteins involved in the adjustment of plant phenotypes to stresses (Kranner et al., 2010; Pozo et al., 2015). Although phytohormone, ROS and other signalling pathways are critical for plants to adequate their phenotypes to the environmental context, the induction of some of these signalling pathways can compromise the presence and/or functionality of beneficial microbes (Foo et al., 2013; Xu et al., 2018) (Figure 1).
Figure 1.

Beneficial microbes are affected by plant stress responses. Plants are challenged by distinct abiotic and/or biotic stresses. They perceive stresses by cell membrane receptors including receptor like kinases (e.g., BAK1). Activated receptors coordinate multiple responses in plant cells, such as calcium (Ca2+) fluxes and phosphorylation of proteins, that stimulate the biosynthesis of signalling molecules including hormones salicylic acid (SA), jasmonic acid (JA) and ethylene (ET), reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Pozo et al., 2015). Hormones and ROS orchestrate signalling cascades that modulate master regulators (e.g., NPR1, JAZ, EIN3) of the expression of key stress‐responsible transcription factors (e.g., TGA, MYC, ERF1). The activation of these transcription factors, during the processing of the stress signalling, leads to massive transcriptome changes that modulate the phenotype of plants in response to the stress. We propose that plant SA‐, JA‐, ET‐ and ROS‐associated stress responses can negatively affect beneficial microbes (e.g., foliar endophytes, root endophytes, mycorrhizal fungi). This is because these responses typically are associated with production of antimicrobial proteins (e.g., glucanases, chitinases), changes in growth of microbes within plant tissues, reinforcement of plant cell walls, oxidative stress and senescence of plant tissues (i.e., programmed cell death). The list of molecules is focused on defence‐related hormonal and ROS signalling pathways and is not exhaustive (roles of these molecules are explained in the main text). Arrows indicate positive regulation and truncated lines inhibition or negative regulation. Red connectors denote direct effects of stresses on plants. BAK1, BOTRYTIS‐INDUCED KINASE1; EIN3, ET‐INSENSITIVE 3; ERF1, ET RESPONSE FACTOR 1; JAZ, JASMONATE ZIM DOMAIN; MYC, MYC‐type basic helix‐loop‐helix transcription factor; NPR1, NONEXPRESSOR OF PR GENES 1; TGA, TGACG sequence‐specific binding. [Color figure can be viewed at wileyonlinelibrary.com]
3.1. Defence‐related phytohormones interfere plant−microbe interactions
In addition to their major roles in plant stress responses, defence‐related phytohormones salicylic acid, jasmonic acid and ethylene are master regulators of the interaction of plants with beneficial symbionts (Pieterse et al., 2014). These hormones are activated following the plant perception of multiple abiotic/biotic stresses and also beneficial microbes (Broekaert et al., 2006; Khan et al., 2015; Per et al., 2018; Pieterse et al., 2014). The perception is carried out by specific receptors located in plant cell membranes such as receptor‐like kinases and histidine kinases that recognize specific signals in the stressors (e.g., microbe‐associated molecular patterns) (Osakabe et al., 2013). During the recognition, plants increase calcium (Ca2+) contents in cells, accumulate ROS in the apoplast, and phosphorylate distinct mitogen‐associated protein kinases. These early responses terminate by inducing proteins that regulate the production and accumulation of salicylic acid, jasmonic acid and/or ethylene hormones (Saijo et al., 2018). The accumulation of defence‐related phytohormones also occur in distant tissues as result of proteins that systemically distribute defence signals (e.g., pipecolic acid, methyl salicylate) (Pieterse et al., 2014). High salicylic acid levels in cells induce activities of NONEXPRESSOR OF PR GENES 1 proteins that in turn induce TGACG sequence‐specific binding transcription factors. These transcription factors regulate the expression of salicylic acid‐responsive genes (Li et al., 2019). In case of jasmonic acid, the accumulation of this hormone in cells triggers the conjugation with amino acids that produces the active form, the jasmonoyl‐isoleucine. This conjugated hormone induces the ubiquitination of JASMONATE ZIM DOMAIN (JAZ) proteins by the coronatine insensitive 1 containing the skp1‐cullin 1‐f‐box ubiquitin ligase complex. Ubiquitinated JAZ are degraded via 26‐S proteasomes. JAZ proteins are repressors of transcription factors that induce the expression of jasmonic acid‐responsive genes (e.g., MYC‐type basic helix‐loop‐helix transcription factor). Therefore, the degradation of JAZ proteins by proteasomes promotes the expression of jasmonic acid‐related response genes (Ballaré, 2014; Li et al., 2019). The accumulation of ethylene in cells stimulates its binding with specific intracellular receptors (e.g., ethylene‐receptor 1/2), and this binding prevents the proteosome‐mediated degradation of ETHYLENE‐INSENSITIVE (EIN) 2/3 and EIN3‐LIKE 1 proteins. These depressed proteins activate several transcription factors that regulate the expression of the gene ETHYLENE‐RESPONSE FACTOR (ERF) 1 (and other genes) encoding for a protein that activates ethylene‐responsive genes (Broekaert et al., 2006). The ERF1 transcription factor is also activated by jasmonic acid (Lorenzo et al., 2003).
Multiple lines of evidence show that the activities of defence‐related hormone signalling pathways (i.e., salicylic acid, jasmonic acid and ethylene) negatively affect plant beneficial microbes. For example, endophyte‐symbiotic L. multiflorum and F. arundinacea plants treated with salicylic acid or methyl jasmonate (an activator of jasmonic acid‐related defence responses) reduced the concentration of fungal‐derived alkaloids and promoted susceptibility of symbiotic plants against insect herbivores (Bastías et al., 2018a, 2018b; Simons et al., 2008). Similarly, the endophyte‐mediated growth promotion in Achnatherum sibiricum plants was erased when symbiotic plants were exposed to methyl jasmonate (Qin et al., 2019). Furthermore, the colonization of roots by arbuscular mycorrhizal fungi is affected by plant defence hormones (Foo et al., 2013). Plants of Nicotiana tabacum with enhanced salicylic acid levels showed reduced mycorrhization (Herrera Medina et al., 2003). Reduced colonizations of arbuscular mycorrhizal fungi were also documented in ethylene‐exposed Pisum sativum plants and ethylene‐overproducing S. lycopersicum plants (Geil et al., 2001; Torres de Los Santos et al., 2011; Zsögön et al., 2008). Similar outcomes have been documented in root endophytes. The overexpression of the plant ERF1 transcription factor reduced the root colonization and also eliminated the benefits of the endophyte S. indica in A. thaliana plants (Camehl et al., 2010). Further experimental results suggested that the activation of jasmonic acid and ethylene plant signalling pathways reduced the root colonization of S. indica endophytes in A. thaliana and Dimocarpus longan plants (Cheng et al., 2022; Khatabi et al., 2012).
Beneficial fungal microbes may be regulated by the action of certain proteins of response to defence‐related hormonal signalling pathways. These proteins include β−1,3‐glucanases, chitinases and pathogenesis‐related enzymes that degrade fungal cell walls via hydrolysis of structural components (e.g., glucans) and callose synthase enzymes that block the spread of the fungal mycelia in plants tissues via the reinforcement of plant cell walls (Dupont et al., 2015; Kou et al., 2021; Redkar et al., 2022). Furthermore, defence‐related hormones could control the abundance of beneficial microbes in plant tissues by activating senescence responses (= programmed cell death) in infection sites (Bernacki et al., 2021; Brodersen et al., 2005).
3.2. ROS interfere plant−microbe interactions
ROS, and other free radicals, also regulate the interaction of plants with beneficial symbionts (Calcagno et al., 2012; Tanaka et al., 2006; Wawra et al., 2016). ROS are normally produced in chloroplasts, peroxisomes and mitochondria organelles as by‐products of the metabolism (Miller et al., 2010). These molecules are formed by transferring electrons with high‐energy to molecular oxygen and include hydrogen peroxide, singlet oxygen and superoxide radical. At high levels, ROS cause oxidative damage to DNA, lipids and proteins that can lead to cell death (Raja et al., 2017). ROS contents in cells rapidly augment in presence of stressors (Huang et al., 2019). This abrupt increment in ROS levels is due, in part, to the action of the enzyme nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. NADPH oxidases belong to the respiratory burst oxidase homolog (RBOH) family in plants, and RBOH‐derived ROS can act as signalling molecules (Miller et al., 2010). RBOH enzymes are located in plasma membranes and transfer electrons from cytosolic NADPH or nicotinamide adenine dinucleotide to apoplastic molecular oxygens which leads to the generation of superoxide radicals that can be converted to hydrogen peroxide by the superoxide dismutase enzyme (Sukuzi et al., 2012). RBOHs possess several regions of regulation including phosphorylation sites, Ca2+‐binding EF‐hand sites and phosphatidic acid‐binding sites (Kadota et al., 2015). RBOHs are normally induced by phosphorylation upon the perception of stressors. For instance, the enzyme RBOHD is phosphorylated by the receptor‐like kinase receptor BOTRYTIS‐INDUCED KINASE 1 in presence of pathogens. This RBOHD phosphorylation causes a ROS‐mediated induction of defences in A. thaliana (Kadota et al., 2015).
The plant production of ROS in response to stress may compromise beneficial microbes. ROS can cause oxidate damage in microbial cells, potentially leading to cell death (Kadota et al., 2015). In Z. mays, the accumulation of ROS in the cytoplasm of plant cells containing mycorrhizal fungi apparently promoted the degradation of fungal arbuscules (Fester & Hause, 2005). Furthermore, ROS alter the development of fungal hyphae within plant tissues which reduces the fitness of their hosts (Kayano et al., 2018). Symbiotic L. perenne plants with reduced ROS levels showed stunted (and sometime lethal) phenotypes due to an unrestricted growth of Epichloë endophytes within plant tissues. This plant phenotype was associated with endophytes that exhibited mutations in the NoxA gene which encodes for a ROS‐producer NADPH oxidase (Tanaka et al., 2006). Moreover, stunted phenotypes have also been documented in L. perenne plants associated with endophytes with mutations in genes that regulate the activity of fungal NADPH oxidases (Kayano et al., 2018). Finally, ROS‐mediated responses also strengthen plant cell walls (via cross‐linking of glycoproteins and callose deposition) that could potentially restrict the mycelial dissemination of beneficial fungi within plant tissues (Kadota et al., 2015).
It is worth mentioning that environmental stresses can affect plant−microbe interactions by mechanisms not related to plant stress responses. For instance, environmental stresses directly inhibit the development of beneficial microbes in soil and reduce the plant production of strigolactones (e.g., Lenoir et al., 2016; López‐Ráez, 2016; Ryan, Rasmussen et al., 2014). This direct effect of stresses is particularly relevant in those plant−microbe interactions that the microbe has to colonize the plant (Branco et al., 2022; Lenoir et al., 2016; Nivedita et al., 2021).
4. BENEFICIAL MICROBES HARBOUR MECHANISMS OF PROTECTION AGAINST ENVIRONMENTAL STRESSES
Several experimental results show that, even when stresses compromise the performance of plant beneficial microbes, these organisms still deliver benefits to their plant hosts. For instance, while nitrogen limitation in soil reduced the concentration of endophyte‐derived antiherbivore alkaloids in F. arundinacea, symbiotic plants still were more resistant to aphids than their nonsymbiotic counterparts (Ryan, Rasmussen et al., 2014; Ryan, Shukla et al., 2014). Beneficial microbes harbour mechanisms that counteract plant stress responses and protect hosts from stresses (Figure 2). These mechanisms may contribute to maintain the mutualistic associations in stress situations.
Figure 2.
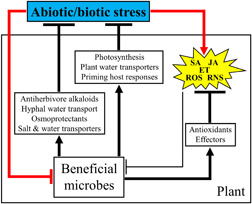
Beneficial microbes counteract plant stress responses and protect hosts from stresses. Abiotic and biotic stresses induce stress responses in plants that are governed by signalling molecules including salicylic acid (SA), jasmonic acid (JA), ethylene (ET), reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Pozo et al., 2015). Plant beneficial symbionts are affected directly by abiotic/biotic stresses or indirectly by plant stress responses (i.e., SA‐, JA‐, ET‐, ROS‐ and RNS‐associated responses). These symbionts protect themselves from plant stress responses by the production of antioxidants that reduce ROS‐ and RNS‐derived oxidative damage and fungal effectors that efficiently repress defensive hormone‐ and ROS‐associated host responses. Additionally, plant beneficial symbionts protect from stresses by producing stress‐protective mechanisms (e.g., antiherbivore alkaloids, osmoprotectant molecules) and/or inducing plant tolerance/resistance mechanisms against stresses (e.g., photosynthesis, priming of plant immune responses). The symbionts' mechanisms that counteract plant stress responses or protect plants from stresses are described in the main text. Arrows indicate positive regulation and truncated lines inhibition or negative regulation. Red connectors denote direct effects of stresses on plants and beneficial microbes. The plant regulation of beneficial microbes is represented with a thinner truncated line since plant stress responses may be attenuated by the microbial production of effectors and increase in antioxidant levels in plants. [Color figure can be viewed at wileyonlinelibrary.com]
Plant beneficial microbes counteract plant stress responses by secreting effector proteins that reduce or prevent the induction of defence‐related hormonal signalling pathways (Hassing et al., 2019). Plant JA‐related defence responses were repressed by Laccaria bicolor mycorrhizal fungi that produced the effector mycorrhizal induced small secreted protein (MISSP) 7. The defence suppression occurred since the fungal MISSP7 stabilized and prevented the degradation of plant JAZ proteins that are repressors of jasmonic acid‐responsive genes (Plett et al., 2014). Similarly, plant ethylene‐related defence responses were inhibited by the arbuscular mycorrhizal fungus R. irregularis that produced the effector secreted protein (SP) 7. In this case, the inhibition of the plant defence was since the SP7 interfered with the plant transcription factor ERF19 (Kloppholz et al., 2011). Plant immune responses were also repressed by the fungus R. irregularis that produced the effector nuclear localized effector 1 that prevented the mono‐ubiquitination of the plant histone 2B. Mono‐ubiquitinated histone 2B proteins regulate salicylic acid and jasmonic acid/ethylene plant signalling pathways (Wang et al., 2021). Additionally, crinkler (CRN) 1 is another effector produced by R. irregularis that control plant immune responses. While the specific mechanism of action of CRN1 is unknown, a reduced expression of this fungal effector decreased the size and abundance of mycorrhizal arbuscules in roots of Medicago truncatula plants (Voß et al., 2018).
Plant beneficial microbes also secrete effectors that counteract ROS‐associated plant stress responses. The root endophyte S. indica inhibited ROS‐derived plant immune responses by producing the effector fungal glucan‐binding 1 that altered the cell wall composition of the fungus. This remodelled cell wall would bypass the plant recognition of the fungus, avoiding the plant production of ROS and the associated immune response (Wawra et al., 2016). Furthermore, beneficial microbes stimulate the formation of antioxidants in plants that efficiently neutralize ROS (Balestrini et al., 2018; Hamilton et al., 2012; Li et al., 2021; Noctor & Foyer, 1998). Antioxidants with major roles in plants include catalase, flavonoids, peroxidases, proline and superoxide dismutase (Noctor et al., 2018). For instance, the presence of Epichloë endophytes promoted the growth of Elymus dahuricus plants under drought which was associated with increased antioxidant contents and reduced oxidative damage in plants (Zhang & Nan, 2007). Similarly, endophytes of the same genus in L. perenne plants challenged by distinct phytopathogens increased antioxidant concentrations (i.e., peroxidases, proline and superoxide dismutase) and decreased the levels of oxidative damage in plants (Ma et al., 2015). The symbiosis of Colobanthus quitensis with the beneficial root endophyte fungi Penicillium brevicompactum and P. chrysogenum triggered the formation flavonoid antioxidants, including quercetin, which increased the protection of host plants against highly damaging UV‐B radiation (Barrera et al., 2020). The increased drought tolerance in mycorrhizal‐symbiotic S. lycopersicum plants was associated with enhanced concentrations of proline antioxidants and reduced accumulation of hydrogen peroxide compounds (Chitarra et al., 2016). In the same plant species, the presence of mycorrhizal fungi alleviated the negative effects of combined drought and heat stresses by increasing the activities of antioxidant enzymes that reduced the peroxidation of lipids and accumulation of hydrogen peroxide in plant tissues (Duc et al., 2018).
Beneficial microbes confer to plant hosts multiple mechanisms of protection against stresses. We briefly summarized some of these mechanisms since there are several comprehensive reviews regarding this topic (e.g., Branco et al., 2022; Pérez‐Alonso et al., 2020; Schardl et al., 2004). Foliar Epichloë endophytes confer bioactive alkaloids to plant hosts that increase the plant resistance to herbivores (Bastias et al., 2017). These endophytes also produce mannitol, a sugar alcohol with osmotic and antioxidant effects, that would increase the plant tolerance to drought (Nagabhyru et al., 2013; Rasmussen et al., 2008). Arbuscular mycorrhizal fungi increase the plant tolerance to drought by the direct transport of water from soil to plant root via a hyphal extracytoplasmic pathway (Kakouridis et al., 2022). Root endophytes enhance the plant tolerance to salinity by the action of several fungal salt transporter proteins (e.g., ENA ATPases) that efficiently reduce the accumulation of sodium within root cells. Additionally, the up‐regulation of the fungal high osmolarity glycerol gene, encoding a mitogen‐activated protein kinase involved in osmoregulation, increased the salt stress tolerance in O. sativa plants (Jogawat et al., 2016; Nivedita et al., 2021). Beneficial microbes also mitigate the negative effects of stresses by promoting stress‐protective responses in their plant hosts. For example, foliar Epichloë endophytes induced plant salicylic acid and jasmonic acid signalling pathways that were correlated with enhanced resistance of host plants to Blumeria graminis and Curvularia lunata phytopathogens (Kou et al., 2021; Shi et al., 2020). The fungal root endophyte Thermomyces lanuginosus was reported to improve the heat stress tolerance of Cucumis sativus plants by affecting photosynthetic parameters, water use efficiency and inducing antioxidant activities (Ali et al., 2018). In addition to the direct transport of water from soil, under drought situations, arbuscular mycorrhizal fungi increased the expression of plant (and fungal) genes encoding for aquaporins in roots of S. lycopersicum plants. The enhanced expression of aquaporin genes improved the hydraulic conductivity and cytoplasm‐to‐cytoplasm water flow in symbiotic plants (Chitarra et al., 2016; Quiroga et al., 2019). Furthermore, mycorrhizal fungi can stimulate a ‘prime state’ of plant responses to stresses (Pozo et al., 2015). In this state, plant responses are faster, stronger and/or more sustained upon the stress (Martinez‐Medina et al., 2016). For example, a priming of jasmonic acid‐related responses induced by the mycorrhizal fungus Funneliformis mosseae increased the level of resistance of S. lycopersicum plants against Helicoverpa arimigera caterpillars (Song et al., 2013). Similarly, the mycorrhizal‐based primed accumulation of antiherbivore compounds in the same plant species increased the resistance of plants against Spodoptera frugiperda caterpillars (Rivero et al., 2021). Under salinity stress, S. lycopersicum plants showed a primed accumulation of salt‐protective compounds including plant catechins and B6 vitamers in presence of mycorrhizal fungi (Rivero et al., 2018).
5. CONCLUSION AND FUTURE PERSPECTIVES
In the present review, several experimental results showing that environmental stresses compromised plant−microbe interactions were highlighted. It was proposed that this environmental interference of plant−microbe interactions is explained by the stress‐mediated induction of salicylic acid, jasmonic acid, ethylene, ROS and other signalling pathways (Figure 1). These plant signalling pathways are recognized to regulate beneficial microbes (e.g., Kloppholz et al., 2011; Plett et al., 2014; Wawra et al., 2016). The direct effects of stresses on microbes may also explain the environmental interference of plant−microbe associations (Figure 2). Further experiments manipulating plant stress responses will be critical for determining the relative importance of direct and plant‐mediated effects of stressors in compromising plant−microbe interactions. For instance, mutant plants for hormone (i.e., salicylic acid, jasmonic acid, ethylene) and/or ROS production are useful tools for altering the relationship between direct and plant‐mediated effects of stressors on beneficial microbes (e.g., Jayakannan et al., 2015; Nadarajah, 2020). Beneficial microbes harbour mechanisms that counteract plant stress responses. They produced effector proteins that reduced or prevented the induction of plant hormonal and ROS signalling pathways (Hassing et al., 2019; Wawra et al., 2016). In addition, they increased the antioxidant levels in plants that reduced the oxidative damage caused by ROS bursts in stress situations (Hamilton et al., 2012). Beneficial microbes also confer to their hosts effective stress‐protective mechanisms (Figure 2). Both microbial counter‐defences to plant stress responses and microbial‐derived stress‐protective mechanisms might contribute to maintain the mutualisms. Further research manipulating beneficial microbes will be essential to determine the contribution of the distinct microbial‐derived mechanisms of mutualism protection in stress situations. For example, mutant Epichloë endophytes with disrupted alkaloid production would increase the relative importance of the microbial mechanisms of counter‐defences over the stress‐protective ones in relation to the mutualism maintenance (e.g., Miller et al., 2022). Furthermore, considering that plant responses to stresses depend on the complexity of the environment (Song et al., 2022; Suzuki et al., 2014), it would be valuable in further investigations to determine whether the microbial mechanisms of stress protection can still maintain the mutualisms in contrasting stress scenarios (e.g., chronic vs. sporadic, severe vs. mild). Our work contributes to understand how environmental stresses affect plant−microbe interactions and highlights why beneficial microbes still deliver benefits to plants under stressful environments.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
We thank Qianhe Liu, Zac Beechey‐Gradwell, three anonymous reviewers and the journal editor for critical revision of the manuscript. D. A. B. acknowledges the research support provided by the Endeavour Fund from the New Zealand Ministry of Business, Innovation and Employment (MBIE), contract number LVLX1702. R. B. acknowledges the research support provided by the project PRIMA–OPTIMUS PRIME (Italian MUR DD 16302/2021). S. P. obtained financial support by grant PID2020‐119441RB‐I00 funded by MCIN/AEI/10.13039/501100011033 and, as appropriate, by ‘ERDF A way of making Europe’, by the ‘European Union’ or by the ‘European Union NextGenerationEU/PRTR’. P. E. G. research activities are supported by the Agencia Nacional de Investigaciones Argentina (ANPCyT) PICT‐2018‐01593 and the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT‐2021‐1210908). Open access publishing facilitated by AgResearch Ltd, as part of the Wiley ‐ AgResearch Ltd agreement via the Council of Australian University Librarians.
Bastías, D.A. , Balestrini, R. , Pollmann, S. & Gundel, P.E. (2022) Environmental interference of plant−microbe interactions. Plant, Cell & Environment, 45, 3387–3398. 10.1111/pce.14455
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- Ali, A.H. , Abdelrahman, M. , Radwan, U. , El‐Zayat, S. & El‐Sayed, M.A. (2018) Effect of Thermomyces fungal endophyte isolated from extreme hot desert‐adapted plant on heat stress tolerance of cucumber. Applied Soil Ecology, 124, 155–162. [Google Scholar]
- Augé, R.M. (2001) Water relations, drought and vesicular‐arbuscular mycorrhizal symbiosis. Mycorrhiza, 11, 3–42. [Google Scholar]
- Babikova, Z. , Gilbert, L. , Randall, K.C. , Bruce, T.J.A. , Pickett, J.A. & Johnson, D. (2014) Increasing phosphorus supply is not the mechanism by which arbuscular mycorrhiza increase attractiveness of bean (Vicia faba) to aphids. Journal of Experimental Botany, 65, 5231–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrini, R. , Chitarra, W. , Antoniou, C. , Ruocco, M. & Fotopoulos, V. (2018) Improvement of plant performance under water deficit with the employment of biological and chemical priming agents. The Journal of Agricultural Science, 156, 680–688. [Google Scholar]
- Balestrini, R. , Rosso, L.C. , Veronico, P. , Melillo, M.T. , De Luca, F. , Fanelli, E. et al. (2019) Transcriptomic responses to water deficit and nematode infection in mycorrhizal tomato roots. Frontiers in Microbiology, 10, 1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré, C.L. (2014) Light regulation of plant defense. Annual Review of Plant Biology, 65, 335–363. [DOI] [PubMed] [Google Scholar]
- Barrera, A. , Hereme, R. , Ruiz‐Lara, S. , Larrondo, L.F. , Gundel, P.E. , Pollmann, S. et al. (2020) Fungal endophytes enhance the photoprotective mechanisms and photochemical efficiency in the Antarctic Colobanthus quitensis (Kunth) Bartl. exposed to UV‐B radiation. Frontiers in Ecology and Evolution, 8, 122. [Google Scholar]
- Bastías, D.A. , Applegate, E.R. , Johnson, L.J. & Card, S.D. (2022) Factors controlling the effects of mutualistic bacteria on plants associated with fungi. Ecology Letters, 25, 1879–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastías, D.A. , Gianoli, E. & Gundel, P.E. (2021) Fungal endophytes can eliminate the plant growth–defence trade‐off. New Phytologist, 230, 2105–2113. [DOI] [PubMed] [Google Scholar]
- Bastias, D.A. , Martínez‐Ghersa, M.A. , Ballaré, C.L. & Gundel, P.E. (2017) Epichloë fungal endophytes and plant defenses: not just alkaloids. Trends in Plant Science, 22, 939–948. [DOI] [PubMed] [Google Scholar]
- Bastías, D.A. , Martínez‐Ghersa, M.A. , Newman, J.A. , Card, S.D. , Mace, W.J. & Gundel, P.E. (2018a) The plant hormone salicylic acid interacts with the mechanism of anti‐herbivory conferred by fungal endophytes in grasses. Plant, Cell & Environment, 41, 395–405. [DOI] [PubMed] [Google Scholar]
- Bastías, D.A. , Martínez‐Ghersa, M.A. , Newman, J.A. , Card, S.D. , Mace, W.J. & Gundel, P.E. (2018b) Jasmonic acid regulation of the anti‐herbivory mechanism conferred by fungal endophytes in grasses. Journal of Ecology, 106, 2365–2379. [Google Scholar]
- Bennett, A.E. & Groten, K. (2022) The costs and benefits of plant–arbuscular mycorrhizal fungal interactions. Annual Review of Plant Biology, 73, 649–672. [DOI] [PubMed] [Google Scholar]
- Bernacki, M.J. , Rusaczonek, A. , Czarnocka, W. & Karpiński, S. (2021) Salicylic acid accumulation controlled by LSD1 is essential in triggering cell death in response to abiotic stress. Cells, 10, 962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco, S. , Schauster, A. , Liao, H.‐L. & Ruytinx, J. (2022) Mechanisms of stress tolerance and their effects on the ecology and evolution of mycorrhizal fungi. New Phytologist, 235, 2158–2175. [DOI] [PubMed] [Google Scholar]
- Breuillin, F. , Schramm, J. , Hajirezaei, M. , Ahkami, A. , Favre, P. , Druege, U. et al. (2010) Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning. The Plant Journal, 64, 1002–1017. [DOI] [PubMed] [Google Scholar]
- Brodersen, P. , Malinovsky, F.G. , Hématy, K. , Newman, M.‐A. & Mundy, J. (2005) The role of salicylic acid in the induction of cell death in Arabidopsis acd11. Plant Physiology, 138, 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekaert, W.F. , Delauré, S.L. , De Bolle, M.F.C. & Cammue, B.P.A. (2006) The role of ethylene in host‐pathogen interactions. Annual Review of Phytopathology, 44, 393–416. [DOI] [PubMed] [Google Scholar]
- Brosi, G.B. , McCulley, R.L. , Bush, L.P. , Nelson, J.A. , Classen, A.T. & Norby, R.J. (2011) Effects of multiple climate change factors on the tall fescue–fungal endophyte symbiosis: infection frequency and tissue chemistry. New Phytologist, 189, 797–805. [DOI] [PubMed] [Google Scholar]
- Bubica Bustos, L.M. , Ueno, A.C. , Di Leo, T.D. , Crocco, C.D. , Martínez‐Ghersa, M.A. , Molina‐Montenegro, M.A. et al. (2020) Maternal exposure to ozone modulates the endophyte‐conferred resistance to aphids in Lolium multiflorum plants. Insects, 11, 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultman, T.L. & Bell, G.D. (2003) Interaction between fungal endophytes and environmental stressors influences plant resistance to insects. Oikos, 103, 182–190. [Google Scholar]
- Calcagno, C. , Novero, M. , Genre, A. , Bonfante, P. & Lanfranco, L. (2012) The exudate from an arbuscular mycorrhizal fungus induces nitric oxide accumulation in Medicago truncatula roots. Mycorrhiza, 22, 259–269. [DOI] [PubMed] [Google Scholar]
- Camehl, I. , Sherameti, I. , Venus, Y. , Bethke, G. , Varma, A. , Lee, J. et al. (2010) Ethylene signalling and ethylene‐targeted transcription factors are required to balance beneficial and nonbeneficial traits in the symbiosis between the endophytic fungus Piriformospora indica and Arabidopsis thaliana . New Phytologist, 185, 1062–1073. [DOI] [PubMed] [Google Scholar]
- Card, S.D. , Bastías, D.A. & Caradus, J.R. (2021) Antagonism to plant pathogens by Epichloë fungal endophytes—a review. Plants, 10, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, C. , Li, D. , Wang, B. , Liao, B. , Qu, P. , Liu, W. et al. (2022) Piriformospora indica colonization promotes the root growth of Dimocarpus longan seedlings. Scientia Horticulturae, 301, 111137. [Google Scholar]
- Cheplick, G.P. (1998) Genotypic variation in the regrowth of Lolium perenne following clipping: effects of nutrients and endophytic fungi. Functional Ecology, 12, 176–184. [Google Scholar]
- Cheplick, G.P. (2004) Recovery from drought stress in Lolium perenne (Poaceae): are fungal endophytes detrimental. American Journal of Botany, 91, 1960–1968. [DOI] [PubMed] [Google Scholar]
- Cheplick, G.P. , Perera, A. & Koulouris, K. (2000) Effect of drought on the growth of Lolium perenne genotypes with and without fungal endophytes. Functional Ecology, 14, 657–667. [Google Scholar]
- Chitarra, W. , Pagliarani, C. , Maserti, B. , Lumini, E. , Siciliano, I. , Cascone, P. et al. (2016) Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiology, 171, 1009–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, M.J. , Bennett, R.J. , Ansari, H.A. , Koga, H. , Johnson, R.D. , Bryan, G.T. et al. (2008) Epichloë endophytes grow by intercalary hyphal extension in elongating grass leaves. Fungal Genetics and Biology, 45, 84–93. [DOI] [PubMed] [Google Scholar]
- Christensen, M.J. & Voisey, C.R. (2007) The biology of the endophyte/grass partnership. 6th International Symposium on Fungal Endophytes of Grasses Grasslands and Practice Series, 13, 123–133. [Google Scholar]
- Decunta, F.A. , Pérez, L.I. , Malinowski, D.P. , Molina‐Montenegro, M.A. & Gundel, P.E. (2021) A systematic review on the effects of Epichloë fungal endophytes on drought tolerance in cool‐season grasses. Frontiers in Plant Science, 12, 644731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaye, L. , García‐Guzmán, G. & Heil, M. (2013) Endophytes versus biotrophic and necrotrophic pathogens—are fungal lifestyles evolutionarily stable traits. Fungal Diversity, 60, 125–135. [Google Scholar]
- Devireddy, A.R. , Zandalinas, S.I. , Fichman, Y. & Mittler, R. (2021) Integration of reactive oxygen species and hormone signaling during abiotic stress. The Plant Journal, 105, 459–476. [DOI] [PubMed] [Google Scholar]
- Duc, N.H. , Csintalan, Z. & Posta, K. (2018) Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiology and Biochemistry, 132, 297–307. [DOI] [PubMed] [Google Scholar]
- Dupont, P.Y. , Eaton, C.J. , Wargent, J.J. , Fechtner, S. , Solomon, P. , Schmid, J. et al. (2015) Fungal endophyte infection of ryegrass reprograms host metabolism and alters development. New Phytologist, 208, 1227–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Z. , Liu, X. , Zhu, H. & Yao, Q. (2020) Responses of arbuscular mycorrhizal symbiosis to abiotic stress: a lipid‐centric perspective. Frontiers in Plant Science, 11, 578919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fester, T. & Hause, G. (2005) Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza, 15, 373–379. [DOI] [PubMed] [Google Scholar]
- Fonseca de Lima, C.F. , Kleine‐Vehn, J. , De Smet, I. & Feraru, E. (2021) Getting to the root of belowground high temperature responses in plants. Journal of Experimental Botany, 72, 7404–7413. [DOI] [PubMed] [Google Scholar]
- Foo, E. , Ross, J.J. , Jones, W.T. & Reid, J.B. (2013) Plant hormones in arbuscular mycorrhizal symbioses: an emerging role for gibberellins. Annals of Botany, 111, 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew, A. (2022) Root herbivory reduces species richness and alters community structure of root‐colonising arbuscular mycorrhizal fungi. Soil Biology and Biochemistry, 171, 108723. [Google Scholar]
- Geil, R.D. , Peterson, L.R. & Guinel, F.C. (2001) Morphological alterations of pea (Pisum sativum cv. sparkle) arbuscular mycorrhizas as a result of exogenous ethylene treatment. Mycorrhiza, 11, 137–143. [DOI] [PubMed] [Google Scholar]
- Giauque, H. , Connor, E.W. & Hawkes, C.V. (2019) Endophyte traits relevant to stress tolerance, resource use and habitat of origin predict effects on host plants. New Phytologist, 221, 2239–2249. [DOI] [PubMed] [Google Scholar]
- González‐Teuber, M. , Contreras, R.A. , Zúñiga, G.E. , Barrera, D. & Bascuñán‐Godoy, L. (2022) Synergistic association with root endophytic fungi improves morpho‐physiological and biochemical responses of Chenopodium quinoa to salt stress. Frontiers in Ecology and Evolution, 9, 787318. [Google Scholar]
- Gundel, P.E. , Rudgers, J.A. & Ghersa, C.M. (2011) Incorporating the process of vertical transmission into understanding of host–symbiont dynamics. Oikos, 120, 1121–1128. [Google Scholar]
- Hamilton, C.E. , Gundel, P.E. , Helander, M. & Saikkonen, K. (2012) Endophytic mediation of reactive oxygen species and antioxidant activity in plants: a review. Fungal Diversity, 54, 1–10. [Google Scholar]
- Hassing, B. , Winter, D. , Becker, Y. , Mesarich, C.H. , Eaton, C.J. & Scott, B. (2019) Analysis of Epichloë festucae small secreted proteins in the interaction with Lolium perenne . PLoS One, 14, e0209463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy, L.M. , Popay, A.J. , Finch, S.C. , Clearwater, M.J. & Cave, V.M. (2016) Temperature and plant genotype alter alkaloid concentrations in ryegrass infected with an Epichloë endophyte and this affects an insect herbivore. Frontiers in Plant Science, 7, 1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereme, R. , Morales‐Navarro, S. , Ballesteros, G. , Barrera, A. , Ramos, P. , Gundel, P.E. et al. (2020) Fungal endophytes exert positive effects on Colobanthus quitensis under water stress but neutral under a projected climate change scenario in Antarctic. Frontiers in Microbiology, 11, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera Medina, M.J. , Gagnon, H. , Piché, Y. , Ocampo, J.A. , García Garrido, J.M. & Vierheilig, H. (2003) Root colonization by arbuscular mycorrhizal fungi is affected by the salicylic acid content of the plant. Plant Science, 164, 993–998. [Google Scholar]
- Hesse, U. , Schöberlein, W. , Wittenmayer, L. , Förster, K. , Warnstorff, K. , Diepenbrock, W. et al. (2003) Effects of Neotyphodium endophytes on growth, reproduction and drought‐stress tolerance of three Lolium perenne L. genotypes. Grass and Forage Science, 58, 407–415. [Google Scholar]
- Hosseini, F. , Mosaddeghi, M.R. , Dexter, A.R. & Sepehri, M. (2018) Maize water status and physiological traits as affected by root endophytic fungus Piriformospora indica under combined drought and mechanical stresses. Planta, 247, 1229–1245. [DOI] [PubMed] [Google Scholar]
- Huang, H. , Ullah, F. , Zhou, D.‐X. , Yi, M. & Zhao, Y. (2019) Mechanisms of ROS regulation of plant development and stress responses. Frontiers in Plant Science, 10, 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakannan, M. , Bose, J. , Babourina, O. , Shabala, S. , Massart, A. , Poschenrieder, C. et al. (2015) The NPR1‐dependent salicylic acid signalling pathway is pivotal for enhanced salt and oxidative stress tolerance in Arabidopsis . Journal of Experimental Botany, 66, 1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogawat, A. , Vadassery, J. , Verma, N. , Oelmüller, R. , Dua, M. , Nevo, E. et al. (2016) PiHOG1, a stress regulator MAP kinase from the root endophyte fungus Piriformospora indica, confers salinity stress tolerance in rice plants. Scientific Reports, 6, 36765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota, Y. , Shirasu, K. & Zipfel, C. (2015) Regulation of the NADPH oxidase RBOHD during plant immunity. Plant and Cell Physiology, 56, 1472–1480. [DOI] [PubMed] [Google Scholar]
- Kakouridis, A. , Hagen, J.A. , Kan, M.P. , Mambelli, S. , Feldman, L.J. , Herman, D.J. et al. (2022) Routes to roots: direct evidence of water transport by arbuscular mycorrhizal fungi to host plants. New Phytologist, 236, 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayano, Y. , Tanaka, A. & Takemoto, D. (2018) Two closely related Rho GTPases, Cdc42 and RacA, of the en‐dophytic fungus Epichloë festucae have contrasting roles for ROS production and symbiotic infection synchronized with the host plant. PLoS Pathogens, 14, e1006840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M.I.R. , Fatma, M. , Per, T.S. , Anjum, N.A. & Khan, N.A. (2015) Salicylic acid‐induced abiotic stress tolerance and underlying mechanisms in plants. Frontiers in Plant Science, 6, 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatabi, B. , Molitor, A. , Lindermayr, C. , Pfiffi, S. , Durner, J. , von Wettstein, D. et al. (2012) Ethylene supports colonization of plant roots by the mutualistic fungus Piriformospora indica . PLoS One, 7, e35502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiers, E.T. , Palmer, T.M. , Ives, A.R. , Bruno, J.F. & Bronstein, J.L. (2010) Mutualisms in a changing world: an evolutionary perspective. Ecology Letters, 13, 1459–1474. [DOI] [PubMed] [Google Scholar]
- Kloppholz, S. , Kuhn, H. & Requena, N. (2011) A secreted fungal effector of Glomus intraradices promotes symbiotic biotrophy. Current Biology, 21, 1204–1209. [DOI] [PubMed] [Google Scholar]
- Kou, M.‐Z. , Bastías, D.A. , Christensen, M.J. , Zhong, R. , Nan, Z.‐B. & Zhang, X.‐X. (2021) The plant salicylic acid signalling pathway regulates the infection of a biotrophic pathogen in grasses associated with an Epichloë endophyte. Journal of Fungi, 7, 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranner, I. , Minibayeva, F.V. , Beckett, R.P. & Seal, C.E. (2010) What is stress? Concepts, definitions and applications in seed science. New Phytologist, 188, 655–673. [DOI] [PubMed] [Google Scholar]
- Lata, R. , Chowdhury, S. , Gond, S.K. & White, Jr., J.F. (2018) Induction of abiotic stress tolerance in plants by endophytic microbes. Letters in Applied Microbiology, 66, 268–276. [DOI] [PubMed] [Google Scholar]
- Lenoir, I. , Fontaine, J. & Lounès‐Hadj Sahraoui, A. (2016) Arbuscular mycorrhizal fungal responses to abiotic stresses: a review. Phytochemistry, 123, 4–15. [DOI] [PubMed] [Google Scholar]
- Li, D. , Bodjrenou, D.M. , Zhang, S. , Wang, B. , Pan, H. , Yeh, K.‐W. et al. (2021) The endophytic fungus Piriformospora indica reprograms banana to cold resistance. International Journal of Molecular Sciences, 22, 4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. , Duan, T. & Li, Y. (2020) Effects of the fungal endophyte Epichloë festucae var. lolii on growth and physiological responses of perennial ryegrass cv. Fairway to combined drought and pathogen stresses. Microorganisms, 8, 1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N. , Han, X. , Feng, D. , Yuan, D. & Huang, L.‐J. (2019) Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: do we understand what they are whispering. International Journal of Molecular Sciences, 20, 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q. , Parsons, A.J. , Xue, H. , Fraser, K. , Ryan, G.D. , Newman, J.A. et al. (2011) Competition between foliar Neotyphodium lolii endophytes and mycorrhizal Glomus spp. fungi in Lolium perenne depends on resource supply and host carbohydrate content. Functional Ecology, 25, 910–920. [Google Scholar]
- Liu, X. , Feng, Z. , Zhao, Z. , Zhu, H. & Yao, Q. (2020) Acidic soil inhibits the functionality of arbuscular mycorrhizal fungi by reducing arbuscule formation in tomato roots. Soil Science and Plant Nutrition, 66, 275–284. [Google Scholar]
- López‐Ráez, J.A. (2016) How drought and salinity affect arbuscular mycorrhizal symbiosis and strigolactone biosynthesis. Planta, 243, 1375–1385. [DOI] [PubMed] [Google Scholar]
- Lorenzo, O. , Piqueras, R. , Sánchez‐Serrano, J.J. & Solano, R. (2003) Ethylene response factor1 integrates signals from ethylene and jasmonate pathways in plant defense. The Plant Cell, 15, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, M. , Christensen, M.J. & Nan, Z. (2015) Effects of the endophyte Epichloë festucae var. lolii of perennial ryegrass (Lolium perenne) on indicators of oxidative stress from pathogenic fungi during seed germination and seedling growth. European Journal of Plant Pathology, 141, 571–583. [Google Scholar]
- Ma, X. , Geng, Q. , Zhang, H. , Bian, C. , Chen, H.Y.H. , Jiang, D. et al. (2021) Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity and ecosystem multifunctionality. New Phytologist, 229, 2957–2969. [DOI] [PubMed] [Google Scholar]
- Marks, S. & Clay, K. (2007) Low resource availability differentially affects the growth of host grasses infected by fungal endophytes. International Journal of Plant Sciences, 168, 1269–1277. [Google Scholar]
- Martinez‐Medina, A. , Flors, V. , Heil, M. , Mauch‐Mani, B. , Pieterse, C.M.J. , Pozo, M.J. et al. (2016) Recognizing plant defense priming. Trends in Plant Science, 21, 818–822. [DOI] [PubMed] [Google Scholar]
- McLeod, A.R. , Rey, A. , Newsham, K.K. , Lewis, G.C. & Wolferstan, P. (2001) Effects of elevated ultraviolet radiation and endophytic fungi on plant growth and insect feeding in Lolium perenne, Festuca rubra, F. arundinacea and F. pratensis . Journal of Photochemistry and Photobiology, 62, 97–107. [DOI] [PubMed] [Google Scholar]
- Millar, N.S. & Bennett, A.E. (2016) Stressed out symbiotes: hypotheses for the influence of abiotic stress on arbuscular mycorrhizal fungi. Oecologia, 182, 625–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, G. , Suzuki, N. , Ciftci‐Yilmaz, S. & Mittler, R. (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell & Environment, 33, 453–467. [DOI] [PubMed] [Google Scholar]
- Miller, T.A. , Hudson, D.A. , Johnson, R.D. , Singh, J.S. , Mace, W.J. , Forester, N.T. et al. (2022) Dissection of the epoxyjanthitrem pathway in Epichloë sp. LpTG‐3 strain AR37 by CRISPR gene editing. Frontiers in Fungal Biology, 3, 944234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah, K.K. (2020) ROS homeostasis in abiotic stress tolerance in plants. International Journal of Molecular Sciences, 21, 5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagabhyru, P. , Dinkins, R.D. , Wood, C.L. , Bacon, C.W. & Schardl, C.L. (2013) Tall fescue endophyte effects on tolerance to water‐deficit stress. BMC Plant Biology, 13, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivedita, R.A. , Ramchiary, N. & Abdin, M.Z. (2021) A high‐throughput RNA‐Seq approach to elucidate the transcriptional response of Piriformospora indica to high salt stress. Scientific Reports, 11, 4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor, G. & Foyer, C.H. (1998) Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology, 49, 249–279. [DOI] [PubMed] [Google Scholar]
- Noctor, G. , Reichheld, J.‐P. & Foyer, C.H. (2018) ROS‐related redox regulation and signaling in plants. Seminars in Cell & Developmental Biology, 80, 3–12. [DOI] [PubMed] [Google Scholar]
- Osakabe, Y. , Yamaguchi‐Shinozaki, K. , Shinozaki, K. & Tran, L.‐S.P. (2013) Sensing the environment: key roles of membrane‐localized kinases in plant perception and response to abiotic stress. Journal of Experimental Botany, 64, 445–458. [DOI] [PubMed] [Google Scholar]
- Pandey, B.K. , Huang, G. , Bhosale, R. , Hartman, S. , Sturrock, C.J. , Jose, L. et al. (2021) Plant roots sense soil compaction through restricted ethylene diffusion. Science, 371, 276–280. [DOI] [PubMed] [Google Scholar]
- Pérez‐Alonso, M.‐M. , Guerrero‐Galán, C. , Scholz, S.S. , Kiba, T. , Sakakibara, H. , Ludwig‐Müller, J. et al. (2020) Harnessing symbiotic plant–fungus interactions to unleash hidden forces from extreme plant ecosystems. Journal of Experimental Botany, 71, 3865–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Per, T.S. , Khan, M.I.R. , Anjum, N.A. , Masood, A. , Hussain, S.J. & Khan, N.A. (2018) Jasmonates in plants under abiotic stresses: crosstalk with other phytohormones matters. Environmental and Experimental Botany, 145, 104–120. [Google Scholar]
- Pieterse, C.M.J. , Zamioudis, C. , Berendsen, R.L. , Weller, D.M. , Van Wees, S.C.M. & Bakker, P.A.H.M. (2014) Induced systemic resistance by beneficial microbes. Annual Review of Phytopathology, 52, 347–375. [DOI] [PubMed] [Google Scholar]
- Plett, J.M. , Daguerre, Y. , Wittulsky, S. , Vayssières, A. , Deveau, A. , Melton, S.J. et al. (2014) Effector MiSSP7 of the mutualistic fungus Laccaria bicolor stabilizes the Populus JAZ6 protein and represses jasmonic acid (JA) responsive genes. Proceedings of the National Academy of Sciences, 111, 8299–8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcel, R. , Aroca, R. & Ruiz‐Lozano, J.M. (2012) Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agronomy for Sustainable Development, 32, 181–200. [Google Scholar]
- Pozo, M.J. , López‐Ráez, J.A. , Azcón‐Aguilar, C. & García‐Garrido, J.M. (2015) Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytologist, 205, 1431–1436. [DOI] [PubMed] [Google Scholar]
- Qin, J. , Wu, M. , Liu, H. , Gao, Y. & Ren, A. (2019) Endophyte infection and methyl jasmonate treatment increased the resistance of Achnatherum sibiricum to insect herbivores independently. Toxins, 11, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga, G. , Erice, G. , Ding, L. , Chaumont, F. , Aroca, R. & Ruiz‐Lozano, J.M. (2019) The arbuscular mycorrhizal symbiosis regulates aquaporins activity and improves root cell water permeability in maize plants subjected to water stress. Plant, Cell & Environment, 42, 2274–2290. [DOI] [PubMed] [Google Scholar]
- Raja, V. , Majeed, U. , Kang, H. , Andrabi, K.I. & John, R. (2017) Abiotic stress: interplay between ROS, hormones and MAPKs. Environmental and Experimental Botany, 137, 142–157. [Google Scholar]
- Rasmussen, S. , Parsons, A.J. , Fraser, K. , Xue, H. & Newman, J.A. (2008) Metabolic profiles of Lolium perenne are differentially affected by nitrogen supply, carbohydrate content, and fungal endophyte infection. Plant Physiology, 146, 1440–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak, K. , Sulewska, H. , Błaszczyk, L. , Basińska‐Barczak, A. , Mikołajczak, K. , Salamon, S. et al. (2020) Growth and photosynthetic activity of selected spelt varieties (Triticum aestivum ssp. spelta L.) cultivated under drought conditions with different endophytic core microbiomes. International Journal of Molecular Sciences, 21, 7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redkar, A. , Sabale, M. , Zuccaro, A. & Di Pietro, A. (2022) Determinants of endophytic and pathogenic lifestyle in root colonizing fungi. Current Opinion in Plant Biology, 67, 102226. [DOI] [PubMed] [Google Scholar]
- Rivero, J. , Álvarez, D. , Flors, V. , Azcón‐Aguilar, C. & Pozo, M.J. (2018) Root metabolic plasticity underlies functional diversity in mycorrhiza‐enhanced stress tolerance in tomato. New Phytologist, 220, 1322–1336. [DOI] [PubMed] [Google Scholar]
- Rivero, J. , Lidoy, J. , Llopis‐Giménez, Á. , Herrero, S. , Flors, V. & Pozo, M.J. (2021) Mycorrhizal symbiosis primes the accumulation of antiherbivore compounds and enhances herbivore mortality in tomato. Journal of Experimental Botany, 72, 5038–5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, R.J. , Henson, J. , Van Volkenburgh, E. , Hoy, M. , Wright, L. , Beckwith, F. et al. (2008) Stress tolerance in plants via habitat‐adapted symbiosis. The ISME journal, 2, 404–416. [DOI] [PubMed] [Google Scholar]
- Rodriguez, R.J. , White, J.F. , Arnold, A.E. & Redman, R.S. (2009) Fungal endophytes: diversity and functional roles. New Phytologist, 182, 314–330. [DOI] [PubMed] [Google Scholar]
- Rudgers, J.A. , Afkhami, M.E. , Bell‐Dereske, L. , Chung, Y.A. , Crawford, K.M. , Kivlin, S.N. et al. (2020) Climate disruption of plant‐microbe interactions. Annual Review of Ecology, Evolution, and Systematics, 51, 561–586. [Google Scholar]
- Ryan, G.D. , Rasmussen, S. , Xue, H. , Parsons, A.J. & Newman, J.A. (2014) Metabolite analysis of the effects of elevated CO2 and nitrogen fertilization on the association between tall fescue (Schedonorus arundinaceus) and its fungal symbiont Neotyphodium coenophialum . Plant, Cell & Environment, 37, 204–212. [DOI] [PubMed] [Google Scholar]
- Ryan, G.D. , Shukla, K. , Rasmussen, S. , Shelp, B.J. & Newman, J.A. (2014) Phloem phytochemistry and aphid responses to elevated CO2, nitrogen fertilization and endophyte infection. Agricultural and Forest Entomology, 16, 273–283. [Google Scholar]
- Sabra, M. , Aboulnasr, A. , Franken, P. , Perreca, E. , Wright, L.P. & Camehl, I. (2018) Beneficial root endophytic fungi increase growth and quality parameters of sweet basil in heavy metal contaminated soil. Frontiers in Plant Science, 9, 1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo, Y. , Loo, E.P. & Yasuda, S. (2018) Pattern recognition receptors and signaling in plant–microbe interactions. The Plant Journal, 93, 592–613. [DOI] [PubMed] [Google Scholar]
- Salamon, S. , Mikołajczak, K. , Błaszczyk, L. , Ratajczak, K. & Sulewska, H. (2020) Changes in root‐associated fungal communities in Triticum aestivum ssp. spelta L. and Triticum aestivum ssp. vulgare L. under drought stress and in various soil processing. PLoS One, 15, e0240037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravesi, K. , Ruotsalainen, A.L. & Cahill, J.F. (2014) Contrasting impacts of defoliation on root colonization by arbuscular mycorrhizal and dark septate endophytic fungi of Medicago sativa . Mycorrhiza, 24, 239–245. [DOI] [PubMed] [Google Scholar]
- Schardl, C.L. , Leuchtmann, A. & Spiering, M.J. (2004) Symbioses of grasses with seedborne fungal endophytes. Annual review of plant biology, 55, 315–340. [DOI] [PubMed] [Google Scholar]
- Shi, X. , Qin, T. , Liu, H. , Wu, M. , Li, J. , Shi, Y. et al. (2020) Endophytic fungi activated similar defense strategies of Achnatherum sibiricum host to different trophic types of pathogens. Frontiers in Microbiology, 11, 1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. , Zhang, K. , Ma, T. , Zhang, Z. , Li, P. , Xing, Z. et al. (2022) Foliar herbivory reduces rhizosphere fungal diversity and destabilizes the co‐occurrence network. Frontiers in Microbiology, 13, 846332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, L. , Bultman, T.L. & Sullivan, T.J. (2008) Effects of methyl jasmonate and an endophytic fungus on plant resistance to insect herbivores. Journal of Chemical Ecology, 34, 1511–1517. [DOI] [PubMed] [Google Scholar]
- Song, Y. , Sterck, F. , Sass‐Klaassen, U. , Li, C. & Poorter, L. (2022) Growth resilience of conifer species decreases with early, long‐lasting and intense droughts but cannot be explained by hydraulic traits. Journal of Ecology, 110, 2088–2104. [Google Scholar]
- Song, Y.Y. , Ye, M. , Li, C.Y. , Wang, R.L. , Wei, X.C. , Luo, S.M. et al. (2013) Priming of anti‐herbivore defense in tomato by arbuscular mycorrhizal fungus and involvement of the jasmonate pathway. Journal of Chemical Ecology, 39, 1036–1044. [DOI] [PubMed] [Google Scholar]
- Sukuzi, N. , Koussevitzky, S. , Mittler, R. & Miller, G. (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant, Cell & Environment, 35, 259–270. [DOI] [PubMed] [Google Scholar]
- Suzuki, N. , Rivero, R.M. , Shulaev, V. , Blumwald, E. & Mittler, R. (2014) Abiotic and biotic stress combinations. New Phytologist, 203, 32–43. [DOI] [PubMed] [Google Scholar]
- Tanaka, A. , Christensen, M.J. , Takemoto, D. , Park, P. & Scott, B. (2006) Reactive oxygen species play a role in regulating a fungus–perennial ryegrass mutualistic interaction. The Plant Cell Online, 18, 1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres de Los Santos, R. , Vierheilig, H. , Ocampo, J.A. & García Garrido, J.M. (2011) Altered pattern of arbuscular mycorrhizal formation in tomato ethylene mutants. Plant Signaling & Behavior, 6, 755–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno, A.C. , Gundel, P.E. , Omacini, M. , Ghersa, C.M. , Bush, L.P. & Martínez‐Ghersa, M.A. (2016) Mutualism effectiveness of a fungal endophyte in an annual grass is impaired by ozone. Functional Ecology, 30, 226–232. [Google Scholar]
- Vadassery, J. & Oelmüller, R. (2009) Calcium signaling in pathogenic and beneficial plant microbe interactions. Plant Signaling & Behavior, 4, 1024–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari, M. , Hatcher, P.E. & Ayres, P.G. (2002) Combined effect of foliar and mycorrhizal endophytes on an insect herbivore. Ecology, 83, 2452–2464. [Google Scholar]
- Voß, S. , Betz, R. , Heidt, S. , Corradi, N. & Requena, N. (2018) RiCRN1, a crinkler effector from the arbuscular mycorrhizal fungus Rhizophagus irregularis, functions in arbuscule development. Frontiers in Microbiology, 9, 2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P. , Jiang, H. , Boeren, S. , Dings, H. , Kulikova, O. , Bisseling, T. et al. (2021) A nuclear‐targeted effector of Rhizophagus irregularis interferes with histone 2B mono‐ubiquitination to promote arbuscular mycorrhization. New Phytologist, 230, 1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Shi, J. , Xie, Q. , Jiang, Y. , Yu, N. & Wang, E. (2017) Nutrient exchange and regulation in arbuscular mycorrhizal symbiosis. Molecular Plant, 10, 1147–1158. [DOI] [PubMed] [Google Scholar]
- Wawra, S. , Fesel, P. , Widmer, H. , Timm, M. , Seibel, J. , Leson, L. et al. (2016) The fungal‐specific β‐glucan‐binding lectin FGB1 alters cell‐wall composition and suppresses glucan‐triggered immunity in plants. Nature Communications, 7, 13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiß, M. , Waller, F. , Zuccaro, A. & Selosse, M.‐A. (2016) Sebacinales—one thousand and one interactions with land plants. New Phytologist, 211, 20–40. [DOI] [PubMed] [Google Scholar]
- Wolinska, K.W. , Vannier, N. , Thiergart, T. , Pickel, B. , Gremmen, S. , Piasecka, A. et al. (2021) Tryptophan metabolism and bacterial commensals prevent fungal dysbiosis in Arabidopsis roots. Proceedings of the National Academy of Sciences, 118, e2111521118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Y. , Han, S. , Li, X. , Amombo, E. & Fu, J. (2017) Amelioration of salt stress on bermudagrass by the fungus Aspergillus aculeatus . Molecular Plant‐Microbe Interactions, 30, 245–254. [DOI] [PubMed] [Google Scholar]
- Xu, L. , Wu, C. , Oelmüller, R. & Zhang, W. (2018) Role of phytohormones in Piriformospora indica‐induced growth promotion and stress tolerance in plants: more questions than answers. Frontiers in Microbiology, 9, 1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, W. , Li, M. , Lin, W. , Nan, Z. & Tian, P. (2021) Effects of Epichloë sinensis endophyte and host ecotype on physiology of Festuca sinensis under different soil moisture conditions. Plants, 10, 1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Zhu, J. , Gong, Z. & Zhu, J.‐K. (2022) Abiotic stress responses in plants. Nature Reviews Genetics, 23, 104–119. [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Wang, J. , Xu, L. , Wang, A. , Huang, L. , Du, H. et al. (2018) Drought stress responses in maize are diminished by Piriformospora indica . Plant Signaling & Behavior, 13, e1414121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y.P. & Nan, Z.B. (2007) Growth and anti‐oxidative systems changes in Elymus dahuricus is affected by Neotyphodium endophyte under contrasting water availability. Journal of Agronomy and Crop Science, 193, 377–386. [Google Scholar]
- Zsögön, A. , Lambais, M.R. , Benedito, V.A. , Figueira, A.V.D.O. & Peres, L.E.P. (2008) Reduced arbuscular mycorrhizal colonization in tomato ethylene mutants. Scientia Agricola, 65, 259–267. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


