ABSTRACT
Osteoporosis carries a high medical, economic, and societal burden principally because of the risk of severe fractures. The objective of this cost‐of‐illness study was to describe health resource utilization and associated costs in all patients aged ≥50 years hospitalized for a severe osteoporotic fracture over a 6‐year period (2009 to 2014) in France. Data were extracted from the French national healthcare database (SNDS) on all health care resource utilization between the index date (date of hospitalization for first fracture during the enrollment period) and study end (December 31, 2016) or until the patient died. Costing was restricted to direct costs and determined from the payer perspective. Variables related to costs were identified through multivariate logistic regression analysis. A total of 356,895 patients were included (median follow‐up 39.1 months). In the year after the index fracture, 36,622 patients (10.5%) were rehospitalized for a fracture‐related reason. Only 18,474 (5.3%) underwent bone densitometry and 58,220 (16.7%) received a specific treatment. The total annual per capita osteoporosis‐related cost in the year after the index severe osteoporotic fracture was €18,040 (from €8598 for multiple ribs to €21,085 for hip fracture) of which €17,905 was incurred by fracture‐related costs. The cost incurred by management of osteoporosis was €135. Over years 2 to 5, the mean annual per capita costs of fracture treatment (€806, mostly attributable to the treatment of refractures) continued to dominate those of osteoporosis management (€99). Total annual cost of care was €1260 million (year 2014). Variables associated with higher cost were older age, male sex, site of fracture, a history of prior osteoporotic fracture, and the number of refracture events. The 5‐year cost of severe osteoporotic fractures to the French health care system is high and mostly attributable to the treatment of refractures. Improved fracture prevention measures in patients with osteoporosis is crucial to reduce the economic burden of the disease. © 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: HEALTH ECONOMICS, OSTEOPOROSIS, GENERAL POPULATION STUDIES, THERAPEUTICS, FRACTURE PREVENTION
Introduction
Information on the current economic burden of severe osteoporotic fractures in France is limited. In a European‐wide survey of the medical management, epidemiology, and economic burden of osteoporosis conducted by the International Osteoporosis Foundation (IOF), it was estimated that around 380,000 new severe osteoporotic fractures occurred in France in 2010.( 1 ) We have recently performed a study in the French national healthcare database (Système National des Données de Santé [SNDS]) with the goal of identifying and characterizing all patients hospitalized in France for a severe osteoporotic fracture over a 6‐year period.( 2 ) In our study, the prevalence of severe osteoporotic fractures was higher than the IOF estimate, with around half a million individuals identified, corresponding to 83,000 new fractures a year and a crude annual incidence rate of ~3.6 cases/1000 in the population ≥50 years of age.( 2 )
Currently, most of the available primary data on the cost of osteoporosis in France are restricted to hospitalization and rehabilitation costs for specific fracture types.( 3 , 4 , 5 ) An overview of the burden of fragility fractures in six European countries, including France, estimated the mean cost in the year after fracture to be €12,856 for hip fractures and €3205 for vertebral fractures, with a direct cost of incident fracture in 2017 reported to be €3748 million.( 6 ) These cost estimates are lower than those measured directly in the International Costs and Utilities Related Osteoporotic Fractures Study (ICURO), a recent prospective microcosting study conducted in France,( 7 ) in which total direct and indirect costs over 18 months (including initial fracture‐related costs and follow‐up costs) were €23,926 for hip fractures (€15,951 per annum) and €14,561 for vertebral fractures (€9707 per annum). The inconsistency between the findings of these different studies emphasizes the need for a more comprehensive evaluation of the economic burden related to osteoporosis and fracture management. Administrative health insurance databases are an important resource for conducting such outcomes research in general and cost‐of‐illness studies in particular.( 8 ) They allow real‐world disease management to be described faithfully and, in countries with comprehensive national health insurance coverage, such as France, with exhaustive patient identification, thus minimizing the risk of selection bias or recall bias and providing high statistical power for analysis. In this respect, our study in the SNDS provides an opportunity to collect exhaustive data on health care resource utilization and costs in a large sample of individuals hospitalized with severe osteoporotic fractures. The objective of this study was to describe health resource utilization and associated costs collected in the FRACTOS study in all patients aged ≥50 years hospitalized for a severe osteoporotic fracture over a 6‐year period (2009 to 2014) in France.
Subjects and Methods
This cost of illness study uses data from the FRACTOS study, a retrospective cohort study performed within the SNDS database, the French national health care database.( 9 , 10 ) The SNDS database covers all reimbursed health care expenditures in both the public and private sectors for >99% of the French population. The scope of the study was patients with severe osteoporotic fractures defined as fractures of the hip, pelvis, proximal humerus, vertebrae, or multiple ribs and requiring hospitalization. The rationale for the choice of these fracture sites is that they are associated with an elevated mortality risk.( 11 , 12 ) However, vertebral or rib fractures that did not require an overnight hospital stay were de facto excluded.
The study cohort was composed of all patients ≥50 years hospitalized for management of a severe osteoporotic fracture over a 6‐year period between January 1, 2009, and December 31, 2014. The design and methodology of the FRACTOS study have been described in detail previously( 2 ) and are summarized briefly below.
In the present analysis, we followed all patients from the date of the first hospitalization for a severe osteoporotic fracture during the inclusion period (the index date up to the study end on December 31, 2016) or until the patient died (a maximum period of 8 years). For patients experiencing multiple fracture events, the first event documented was considered to be the index fracture, and subsequent events were considered refractures (recurrent fractures). All health care resource utilization over this post‐index period was documented. The cost analysis was performed from the perspective of the payer and covered all health care resource expenditures reimbursed by the national health insurance. Accrued costs were evaluated at the horizons of 1 and 5 years.
Participants
All patients aged ≥50 years hospitalized for a severe osteoporotic fracture during the inclusion period were enrolled. Severe osteoporotic fractures were identified from the ICD‐10 disease classification codes in the hospital discharge summaries. These codes are listed in Supplemental Table S1. Patients with fractures at multiple sites and those with osteoporosis with current pathological fracture (ICD‐10 code M80) were excluded because the cost could not be assigned unequivocally to a specific index fracture.
Patients with other medical conditions that could account for the fracture or its care were ineligible. These included Paget's disease, any cancer, infectious arthritis, planned surgical interventions (including elective orthopedic surgery or interventions involving care of a prosthesis), or identified traumatic injury. However, patients treated with glucocorticoids could be included. The corresponding ICD‐10 codes are listed in Supplemental Table S1. In addition, patients who changed their insurance regimen during the data collection period were excluded because exhaustive documentation of health care resource utilization throughout the period could not be guaranteed.
Data extraction
At the time of the index hospitalization, age, sex, health insurance status, and municipality of residence were documented. During this post‐index period, information was collected on all hospital and community health care resource utilization until the end of the follow‐up period. This included data on inpatient or outpatient visits, rehabilitation care, home care, consultations with general practitioners (GPs) or community‐based specialists such as rheumatologists, surgeon visits, paramedical care (for example, nurse visits, physiotherapy or occupational therapy), investigations (for example, X‐rays, dual‐energy X‐ray absorptiometry [bone densitometry], or blood tests), medical devices, medications, and reimbursed medical transplantation. It should be noted that for consultations in hospitals, the specialty of the physician consulted is not identified in the SNDS. In addition, data on physiotherapy sessions were only available for the number of outpatient physiotherapy sessions in the community once the patient has been discharged from hospital. Refracture events at any site were documented throughout the post‐index period.
The database was also searched backward for 3 years before 2009 (pre‐index period) to collect historical data on confounding pathologies that might also account for the fracture and to document any previous severe osteoporotic fractures that may have occurred before the enrollment period. Patients with confounding pathologies were excluded (see above) but not those with previous fractures. Any delivery of specific or non‐specific osteoporosis treatments during the pre‐index period was also documented.
Medications considered as specific anti‐osteoporotic medications were those available at the time of the study, namely bisphosphonates (etidronate, alendronate, risedronate, ibandronate, or zoledronic acid), raloxifene, teriparatide, denosumab, strontium ranelate, and hormone replacement therapy (HRT). Strontium ranelate was not evaluated because it was no longer reimbursed (and thus no longer documented in the SNDS) in 2015 and withdrawn from the market 2 years later. Prescriptions for calcium or vitamin D, which are not considered to be specific anti‐osteoporosis treatments, were also documented. A full list of health care resources documented is provided in Supplemental Table S2.
Derived variables
Osteoporotic treatment duration was determined as the time between the first documented delivery of medication and the end of the theoretical treatment period covered by the last delivery (or until the end of the study or until the patient died) without treatment interruption. A treatment was considered to be interrupted if the interval between the theoretical end of a treatment and the next treatment dispensation was >90 days.
Health care resource utilization
Health care consumption was quantified by type of expense and by year of follow‐up. Consumption was divided into three categories, namely consumption related to the fracture itself, consumption related to management of osteoporosis, and unrelated consumption (including consumption related to comorbidities). Resources entering into the first two categories are listed in Table 1. All other items were considered unrelated health care consumption, and these data are not presented in the present report.
Table 1.
Healthcare Resource Utilization by Fracture Site Over the 12‐Month Period After the Index Hospitalization
| Hip (n = 208,102) | Proximal humerus (n = 52,468) | Vertebra (n = 31,979) | Pelvis (n = 38,051) | Multiple ribs (n = 17,184) | Total (N = 347,784) | |
|---|---|---|---|---|---|---|
| Related to the fracture itself | ||||||
| Hospitalization for severe osteoporotic fracture | 208,102 (100%) | 52,468 (100%) | 31,979 (100%) | 38,051 (100%) | 17,184 (100%) | 347,784 (100%) |
| Index hospitalization | 208,102 (100%) | 52,468 (100%) | 31,979 (100%) | 38,051 (100%) | 17,184 (100%) | 347,784 (100%) |
| Readmission | 20,872 (10.0%) | 5290 (10.1%) | 4405 (13.8%) | 4972 (13.1%) | 1083 (6.3%) | 36,622 (10.5%) |
| Follow‐up and rehabilitation care | 116,386 (55.9%) | 15,577 (29.7%) | 6821 (21.3%) | 20,409 (53.6%) | 2127 (12.4%) | 161,320 (46.4%) |
| Home care | 851 (0.4%) | 80 (0.2%) | 94 (0.3%) | 152 (0.4%) | 6 (<0.1%) | 1183 (0.3%) |
| Surgeon visit a | 63,110 (30.3%) | 18,526 (35.3%) | 5421 (17.0%) | 6290 (16;5%) | 1397 (8.1%) | 94,744 (27.2%) |
| Outpatient physiotherapy session b | 100,262 (48.2%) | 37,054 (70.6%) | 13,199 (41.3%) | 14,519 (38.2%) | 3333 (19.4%) | 168,367 (48.4%) |
| No. of sessions per patient (mean ± SD) | 8.5 ± 12.7 | 13.9 ± 13.5 | 5.5 ± 9.4 | 5.8 ± 10.6 | 2.5 ± 6.7 | 8.5 ± 12.4 |
| Median [IQR] | 0 [0–14] | 12 [0–22] | 0 [0–9] | 0 [0–9] | 0 [0–0] | 0 [0–15] |
| X‐ray sessions c | 188,313 (90.5%) | 47,978 (91.4%) | 25,190 (78.8%) | 30,116 (79.1%) | 12,624 (73.5%) | 304,221 (87.5%) |
| Medical devices | 9640 (4.6%) | 842 (1.6%) | 622 (1.9%) | 1168 (3.1%) | 244 (1.4%) | 12,516 (3.6%) |
| Related to management of osteoporosis | ||||||
| Community rheumatologist consultation | 12,539 (6.0%) | 4357 (8.3%) | 4420 (13.8%) | 3662 (9.6%) | 1335 (7.8%) | 26,313 (7.6%) |
| GP consultation for prescription of an osteoporosis treatment | 79,989 (38.4%) | 21,127 (40.3%) | 14,412 (45.1%) | 18,179 (47.8%) | 4859 (28.3%) | 138,566 (39.8%) |
| Dentist visit | 42,079 (20.2%) | 15,375 (29.3%) | 11,626 (35.4%) | 10,301 (27.1%) | 5497 (32.0%) | 84,878 (24.4%) |
| Bone densitometry | 8851 (4.3%) | 2938 (5.6%) | 4137 (12.9%) | 2117 (5.6%) | 431 (2.5%) | 18,474 (5.3%) |
| Blood tests | 155,971 (74.9%) | 40,117 (76.5%) | 24,324 (76.1%) | 29,290 (77.0%) | 12,590 (73.3%) | 262,310 (75.4%) |
| Specific osteoporosis medication delivered during year | 31,385 (15.1%) | 8127 (15.5%) | 8250 (25.8%) | 8683 (22.8%) | 1775 (10.3%) | 58,220 (16.7%) |
IQR = interquartile range; SD = standard deviation; GP = general practitioner.
Data are provided for the 356,895 patients of the analysis population. Unless otherwise specified, data are presented as the number (%) of patients using each health care resource item during the 12 months after the index hospitalization. Definition of items as in Supplemental Table S2.
Within the 2 months after the index hospitalization for a severe osteoporotic fracture.
Within the 3 months after the index hospitalization for a severe osteoporotic fracture.
Within the 12 months after the index hospitalization for a severe osteoporotic fracture.
Costing
Costing was restricted to direct costs and was determined from the perspective of the payer (national health insurance). The proportion of cost reimbursed by national health insurance varies according to the type of expense. For example, medications delivered in the community are reimbursed at 15%, 30%, or 65%, depending on the benefit that the medication provides, whereas imaging procedures are reimbursed at 70% to 100% and surgery at 100%. Certain patients are eligible for full reimbursement on the grounds of long‐term illness or low income; these patients were identified and the full cost of their care was taken into account in the analysis.
Costs to the payer for medical procedures, consultations, and medications were attributed from official French national tariffs for medical procedures, health care consultations, and medication defined by the Economic Committee for Medicinal Products and are expressed in 2018 Euros, adjusted on the basis of the consumer price index. Costs of hospitalization by type of hospital stay for public hospitals and private clinics were determined based on hospital expenditure reimbursed to the hospital. Each hospital stay was identified by a diagnosis‐related group based on the reason for hospitalization described in the hospital discharge summary. Each diagnosis‐related group is attributed a specific fixed tariff covering all estimated costs of hospitalization, including medical procedures, drugs, and medical devices delivered in hospital during hospital stay. Each discharge summary also provides a severity qualifier (ranging from 1 to 4) for the stay, which is taken into account in the cost tariffication of the stay. Some expensive drugs and innovative medical technologies (for example, immunotherapies and cancer‐targeted therapies) specified on a nominative list are costed and reimbursed apart, and these costs were also integrated. Annual per capita costs were determined by type of expenditure and overall. Specific costs of health care consumption related to the fracture itself and of consumption related to management of osteoporosis (as defined in Supplemental Table S3) were determined.
Statistical analysis
Data on health care resource utilization were analyzed for the follow‐up population, consisting of all members of the analysis population with at least 1 day of follow‐up after the index hospitalization. Two time horizons were considered, namely the year after the index hospitalization and the 5 years after the index hospitalization. For the latter, only those patients for whom 5 full years of follow‐up were available were considered.
Health care consumption is presented as the number (and percentage) of patients using the resource at least once. For each individual patient, all costs engendered were consolidated by type of expenditure. These individual patient costs were aggregated across the cohort to generate a per capita cost for each type of expenditure. All costs are presented as mean with standard deviation (SD) (and, where appropriate, median with interquartile range [IQR]) per capita costs. Persistence with treatments taken after the fracture was evaluated from the time the treatment was started using Kaplan–Meier survival analysis.
A generalized linear model with an identity link function and a gamma distribution was performed to identify variables potentially associated with total cost over 5 years as a continuous variable. This analysis was performed on the patient population for whom 5 complete years of data were available. Independent categorical variables tested in the model were age (by class), sex, site of the index fracture, number of refractures (0, 1, or >1), and fracture history before the index fracture. Associations with cost were presented as risk ratios (RR) with their Wald 95% confidence intervals (95% CI). All analyses were performed using SAS software version 16.2 (SAS Institute, Cary, NC, USA).
Ethics
The study was conducted in accordance with relevant international and French regulatory requirements. Use of the SNDS database for this type of study is regulated by the National Health Data Agency (Institut National des Données de Santé). The FRACTOS study was authorized by the CEREES (the French expert ethical committee for health technology studies and evaluations) in February 2018 and by the French national data protection agency (Commission Nationale de l'Informatique et des Libertés) in March 2018.
Results
Study population
Overall, 560,499 patients with at least one hospitalization for severe osteoporotic fracture were identified in the SNDS database (General Regimen) from 2009 to 2014, of whom 356,895 (63.7%) fulfilled the eligibility criteria. The principal reasons for exclusion were the presence of a confounding pathology or being younger than 50 years (Fig. 1). Notably, 93,259 patients were ineligible because they had a documented diagnostic code for a malignant neoplasm before or after the index date from 2006 to 2016 inclusive. Data on health care consumption was available for 347,784 patients (97.4%) during the first year of follow‐up and for 136,929 (38.4%) during 5 years of follow‐up (Fig. 1). The median duration of follow‐up was 39.1 months (IQR 21.8–60.5). The numbers of patients retained in the study, deceased, or refractured by year of follow‐up are presented in Supplemental Table S4.
Fig. 1.
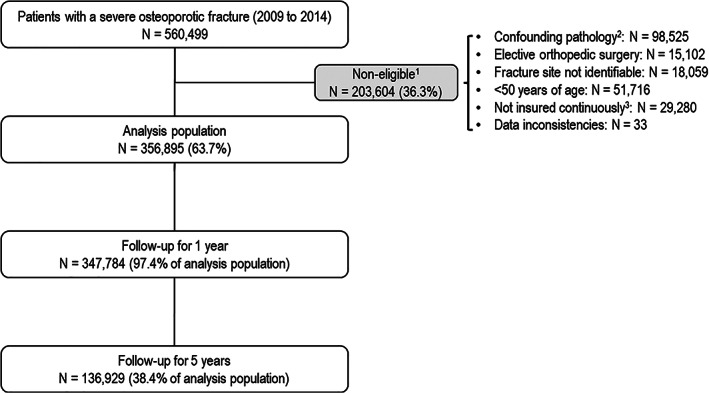
Patient flow chart. 1Reasons for non‐eligibility are not mutually exclusive and individual patients may thus have more than one reason for exclusion. A total of 203,604 patients were not eligible for the study. The sum of the number of individual patients with a specific reason for exclusion (confounding pathology for 98,525 patients, <50 years of age for 51,716 patients, etc., as listed in the right‐hand column) is 212,715 patients. In consequence, 9111 patients (212,715 − 203,604) present more than one reason for non‐eligibility. 2History of Paget's disease, cancer, infectious arthritis, or bone fragility secondary to malignant disease or to surgical interventions. 3Covered by more than one Health Insurance Regimen during the study period. Percentages are calculated with respect to the preceding line.
The characteristics of the analysis population have been described previously( 2 ) and are provided for information in Supplemental Table S5. Index hospitalizations were mostly related to hip fractures, which accounted for 60% of all index hospitalizations. Four percent of patients had been previously hospitalized for a fracture in the 3 years preceding the index hospitalization, and 17.0% had been prescribed a specific osteoporosis treatment at least once before the index fracture. The mean age ± SD of the analysis population was 78.8 ± 12.0 years, being around a decade older in patients with hip or pelvis fractures. Three‐quarters of the patients were women (74.5%), except for multiple rib fractures, which most frequently occurred in men.
Health care resource utilization
In the year after the index fracture, all patients had been hospitalized for a severe osteoporotic fracture at least once (by definition) and 36,222 (10.5%) had been rehospitalized during the same year (Table 1). Rehospitalizations were motivated either by a new fracture, irrespective of site or severity, which occurred in 19,856 (5.6%) of these patients (Supplemental Table S4) or by postsurgical management of the original fracture.
After a fracture‐related hospitalization (index fracture or refracture), 46.4% of patients (n = 161,320) were discharged to a rehabilitation unit within the year after the index fracture. This was most frequently the case for patients with hip (55.9% of patients) or pelvis fractures (53.6%) compared with patients with vertebral (21.3%) or multiple rib fractures (12.4%) (Table 1). A similar overall proportion of patients (48.4%) participated in physiotherapy sessions within 3 months of their hospitalization. This proportion varied considerably between fracture sites, being lowest for multiple rib fractures (19.4%) and highest for proximal humerus fractures (70.6%) (Table 1). The mean annual number of physiotherapy sessions also varied according to the fracture site, from 2.5 ± 6.7 for multiple rib fractures to 13.9 ± 13.5 for proximal humerus fractures (Table 1).
In subsequent years, the proportion of patients undergoing a fracture‐related hospitalization decreased to a plateau of around 4% per year for the remainder of the follow‐up period (Fig. 2).
Fig. 2.
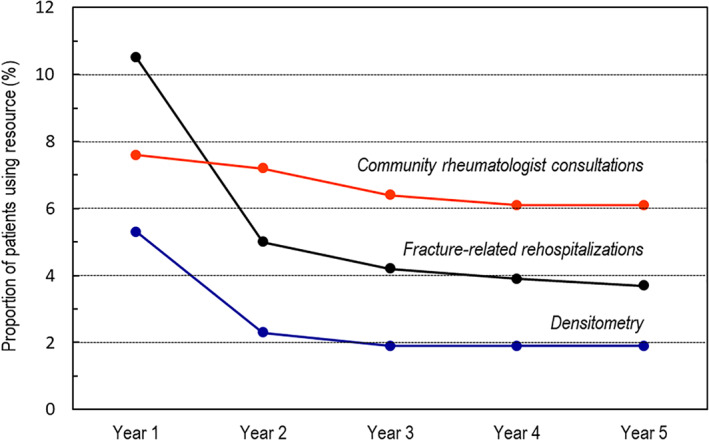
Variation of fracture‐related rehospitalization and osteoporosis management rates over time.
Regarding specific osteoporosis management during the first 12 months of follow‐up period, 7.6% of patients consulted a community‐based rheumatologist; this proportion ranged from 6.0% for patients with a hip fracture to 13.8% for those with vertebral fractures (Table 1). Overall, 18,474 patients (5.3%) underwent bone densitometry during this period. This rate was similar between fracture types, with the exception of patients with vertebral fractures, of whom 12.9% underwent bone densitometry (Table 1). In the following years, the proportion of patients undergoing densitometry decreased to 1.9% from year 3 onward (Fig. 2).
Visits to GPs at which a prescription was made for a specific osteoporosis treatment or calcium or vitamin D supplementation were identified. These consultations were made by 138,566 patients (39.8%) of in the year after the index hospitalization (Table 1). During this period, 58,220 patients (16.7%) received a specific osteoporosis treatment delivered from a community pharmacist, most frequently patients with vertebral fractures (25.8%). Of these treated patients, 21,228 (36.5%) had never been delivered a specific osteoporosis treatment during the pre‐index period and were thus considered treatment‐naïve. The specific treatments delivered to these treatment‐naïve patients were most frequently bisphosphonates (in 83.0% of patients initiating a specific treatment; Supplemental Table S6), principally alendronate (n = 11,697; 36.2% of newly treated patients). In addition, 4707 patients received strontium ranelate (14.6%) and 3709 received HRT (11.5%). The other specific treatments were each used by less than 1000 patients (6%). In contrast, calcium or vitamin D supplementation (or both) was prescribed to 86.4% of these newly treated patients (n = 74,858).
For treatment‐naïve patients delivered a specific osteoporosis treatment after the index hospitalization, the median treatment persistence with the first‐line treatment was 13 weeks after the first delivery. A Kaplan–Meier persistence curve is presented in Fig. 3. The proportion of patients continuously under treatment was 49.0% (95% CI 48.6% to 49.3%) at year 1, 31.7% (31.3% to 32.0%) at year 2, and 12.9% (12.6% to 13.2%) at year 5.
Fig. 3.
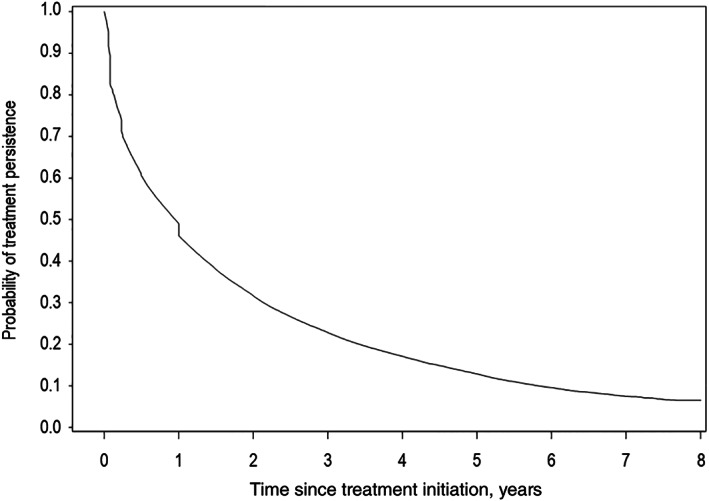
Treatment persistence with first‐line specific osteoporosis treatments.
Cost
During the year after the index hospitalization for a severe osteoporotic fracture, the total annual per capita osteoporosis‐related cost to public health insurance of these patients was €18,040, of which the majority (€17,905) was incurred by treatment of the fracture (ranging from €8474 for multiple rib fractures to €20,964 for hip fractures) (Table 2). Most of this expenditure corresponded to the cost of hospitalization (Table 3). In comparison, the costs incurred by management of osteoporosis were much lower, ranging from €122 for hip fractures to €213 for vertebral fractures. The principal contributors to these management costs were GP consultations for prescription of osteoporosis medication (37.0% of management costs), medication (28.9%), and blood tests (25.2%) (Table 3). This mean per capita cost of management of osteoporosis was relatively similar across fracture types, with the exception of vertebral fractures, which incurred higher management costs, principally attributable to a higher expenditure on osteoporosis medication. Patients with vertebral fractures also incurred higher costs (around twofold higher than for hip fracture) for bone densitometry and for community‐based rheumatologist visits (data not shown), although these cost items contribute only marginally to the total cost. Per capita costs of management of osteoporosis were lower in men than in women (€106 versus €146), the difference being accounted for principally by lower spending on medication (Table 3). In contrast, hospitalization costs were higher for men than for women (Table 3).
Table 2.
Mean and Median Costs of Patients Hospitalized for Severe Osteoporotic Fractures (2018 €)
| Hip (n = 215,672) | Proximal humerus (n = 52,922) | Vertebra (n = 32,231) | Pelvis (n = 38,620) | Multiple ribs (n = 17,450) | Total (N = 356,895) | |
|---|---|---|---|---|---|---|
| Year 1: annual per capita costs | ||||||
| Total cost | ||||||
| Mean ± SD | 21,085 ± 17,740 | 12,559 ± 17,313 | 12,935 ± 15,600 | 17,074 ± 17,284 | 8598 ± 10,090 | 18,040 ± 17,653 |
| Median [IQR] | 16,342 [9,283‐27,339] | 6731 [2727–16,263] | 7895 [3573–16,077] | 12,044 [5370–22,649] | 4968 [1745–10,992] | 13,038 [6056–24,054] |
| Related to the fracture itself | ||||||
| Mean ± SD | 20,964 ± 17,750 | 12,423 ± 17,319 | 12,723 ± 15,600 | 16,921 ± 17,291 | 8474 ± 10,922 | 17,905 ± 17,664 |
| Median [IQR] | 16,207 [9,157–27,219] | 6591 [2570–16,122] | 7682 [3359–15,824] | 11,860 [5220–22,525] | 4852 [1597–10,873] | 12,901 [5904–23,921] |
| Related to osteoporosis management | ||||||
| Mean ± SD | 122 ± 218 | 136 ± 196 | 213 ± 463 | 153 ± 309 | 125 ± 182 | 135 ± 258 |
| Median [IQR] | 65 [14–154] | 88 [34–173] | 108 [46–225] | 80 [26–184] | 85 [33–158] | 75 [20–167] |
| Years 2 to 5: annual per capita costs | ||||||
| Total cost | ||||||
| Mean ± SD | 933 ± 3356 | 814 ± 3006 | 724 ± 2972 | 1152 ± 3900 | 637 ± 2817 | 901 ± 3307 |
| Median [IQR] | 67 [16–214] | 82 [31–198] | 88 [36–215] | 82 [26–256] | 71 [28–154] | 74 [22–210] |
| Related to the fracture itself a | ||||||
| Mean ± SD | 845 ± 3348 | 716 ± 2999 | 595 ± 2955 | 1048 ± 3886 | 550 ± 2811 | 806 ± 3298 |
| Median [IQR] | 0 [0–22] | 0 [0–19] | 0 [0–18] | 0 [0–26] | 0 [0–13] | 0 [0–20] |
| Related to osteoporosis management | ||||||
| Mean ± SD | 88 ± 165 | 98 ± 157 | 129 ± 225 | 105 ± 225 | 87 ± 126 | 96 ± 177 |
| Median [IQR] | 43 [11–108] | 60 [22–122] | 70 [29–143] | 55 [18–125] | 57 [22–109] | 50 [15–116] |
IQR = interquartile range; SD = standard deviation.
Data are provided for the 347,784 patients of the analysis population who completed 12 months of follow‐up.
Including both costs of refracture events and of complications of the initial fracture.
Table 3.
Mean Per Capita Costs of Specific Health Care Consumption (Year 1)
| Item | Mean per capita cost (2018 €) | ||
|---|---|---|---|
| Men (n = 91,137) | Women (n = 265,758) | Total (N = 356,895) | |
| Related to the fracture itself | |||
| Mean ± SD | 16,836 ± 17,690 | 18,271 ± 17,640 | 17,905 ± 17,664 |
| Median [IQR] | 11,330 [4697–22,777] | 13,433 [6426–24,284] | 12,901 [5904–23,921] |
| Hospitalization | 11,017 (65.4%) | 10,733 (58.7%) | 10,806 (60.3%) |
| Follow‐up and rehabilitation care | 5499 (32.7%) | 7171 (39.2%) | 6744 (37.7%) |
| Home care | 43 (0.3%) | 34 (0.2%) | 36 (0.2%) |
| Surgeon visits | 3 (<0.1%) | 3 (<0.1%) | 3 (<0.1%) |
| Outpatient physiotherapy sessions | 93 (0.6%) | 134 (0.7%) | 124 (0.7%) |
| X‐ray sessions | 30 (0.2%) | 38 (0.2%) | 36 (0.2%) |
| Medical devices | 27 (0.2%) | 26 (0.1%) | 26 (0.1%) |
| Transportation for any of the above | 125 (0.7%) | 133 (0.7%) | 131 (0.7%) |
| Related to management of osteoporosis | |||
| Mean ± SD | 106 ± 198 | 146 ± 275 | 135 ± 258 |
| Median [IQR] | 66 [15–134] | 79 [22–181] | 75 [20–167] |
| Community rheumatologist consultation | 1 (0.9%) | 2 (1.4%) | 2 (1.5%) |
| GP visit for osteoporosis medication | 54 (50.9%) | 49 (33.6%) | 50 (37.0%) |
| Dentist visit | 1 (0.9%) | 1 (0.7%) | 1 (0.7%) |
| Bone densitometry | <1 (0.4%) | 1 (0.7%) | 1 (0.7%) |
| Blood tests | 29 (27.4%) | 36 (24.7%) | 34 (25.2%) |
| Osteoporosis medication | 12 (11.3%) | 49 (33.6%) | 39 (28.9%) |
| Transportation for any of the above | 8 (7.5%) | 8 (5.5%) | 8 (5.9%) |
SD = standard deviation; IQR = interquartile range; GP = general practitioner.
Data are provided for the 356,895 patients of the analysis population. Data are presented as mean € ± SD and median € (IQR) for the per capita costs at year 1 for the global costs related to the fracture itself and to management of osteoporosis. For subdivisions of these costs, data are presented as mean per capita cost in € and the percentage of the global cost it accounted for.
Over the following years, the costs of both fracture treatment and osteoporosis management decreased (Fig. 4). The mean annual per capita cost of fracture treatment in subsequent years of follow‐up was €806, and these costs were incurred principally as a result of a small number of refracture events and of hospital readmissions for care or complications of the original fracture treatment. Although the absolute costs incurred by management of osteoporosis decreased progressively in the years after the index hospitalization (from a mean per capita cost of €135 in year 1 to €99 in year 5), the cost structure remained essentially the same over time.
Fig. 4.
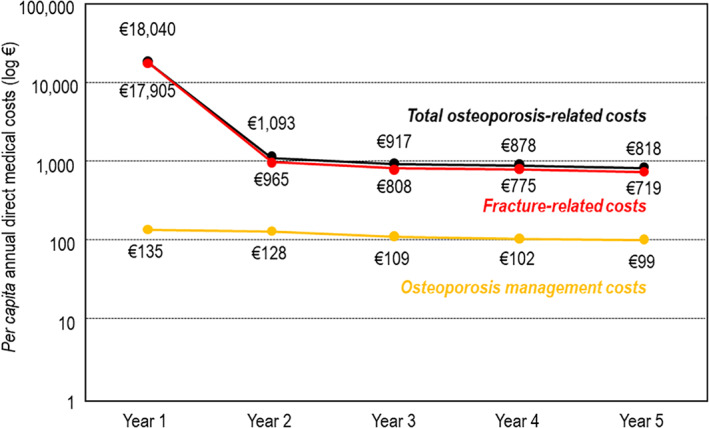
Variation of cost of osteoporosis over time. Costs (y axis) are presented on a logarithmic scale for clarity because of the large variation in the different costs presented.
By multiplying the mean per capita cost by the total number of patients with severe osteoporotic fractures, the total cost to national health insurance of osteoporosis‐related health care management can be estimated at €1260 million for the year 2014. This included €1230 million for fracture‐related costs and €29 million for the costs of osteoporosis management.
Variables associated with cost
In the multivariate analysis, all variables evaluated were significantly associated with the mean total 5‐year cost of severe osteoporotic fractures. Variables associated with higher cost were older age, male sex, site of fracture (highest cost for hip fractures and lowest cost for multiple rib fractures), and a history of osteoporotic fracture (Fig. 5).
Fig. 5.
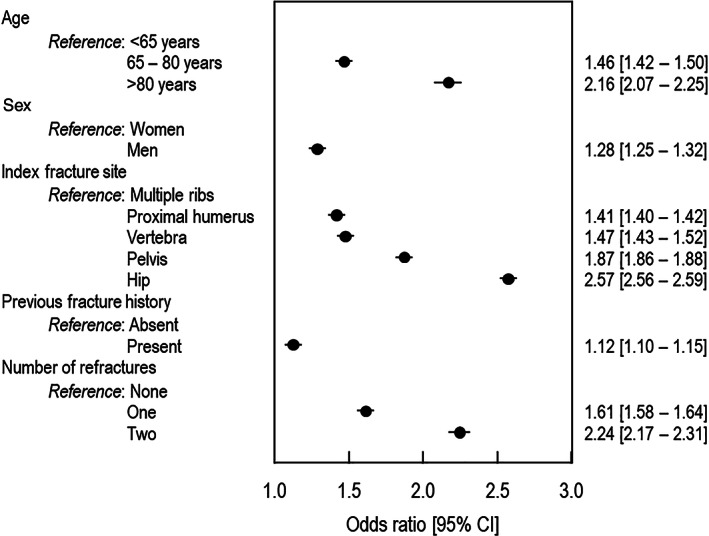
Variables associated with the mean per capita cost of osteoporosis over the 5‐year period.
Discussion
This study provides an estimate of the total direct medical costs associated with severe osteoporotic fractures in France. With an annual per capita cost of fracture and osteoporosis management of €18,040 in the year after the fracture and, on average, more than 75,000 severe osteoporotic fractures requiring hospitalization occurring annually, ( 2 ) the total direct annual medical cost of these fractures to the French public health system was estimated to have been around €1.26 billion in 2014. This is higher than the reported direct medical cost of management of acute coronary artery disease in France (€770 million in 2013), comparable to those of management of chronic heart failure (€1.2 billion in 2013) and somewhat lower than those of acute stroke (€3.5 billion in 2013).( 13 )
The cost findings can also be compared with a previous study of the cost of osteoporosis in the French National Hospital Database (PMSI) in 2008, which included women aged ≥50 years, principally with hip fracture.( 4 ) This earlier study reported a lower overall cost for hospitalization (€415 million) and for follow‐up and rehabilitation stays (€331 million). In addition, mean annual per capita costs of hospitalization were lower (€6127) than in FRACTOS, although rehabilitation costs were higher (€10,546).( 4 ) This difference can be explained, at least in part, by the inclusion of patients with osteoporosis but without fracture, in the study of Maravic and colleagues.( 4 ) In 2001, median inpatient per capita costs for hip fracture was €8727 and an overall cost to the health system for all severe osteoporotic fractures was €715 to €763 million,( 5 ) around half the cost reported in the present study.
Our data are more consistent with recently published data from the ICUROS study, which reported total costs over 18 months after index fracture of €23,926 for hip fracture and €14,561 for vertebral fracture.( 7 ) The FRACTOS and ICUROS studies were performed contemporaneously but used two quite different approaches. ICUROS was a micro‐costing study in which costs are documented prospectively over 18 months in a relatively small number of patients with fragility fractures (n = 431) recruited from six specialist osteoporosis departments in university hospitals. Our FRACTOS study was a retrospective study evaluating, in principle, all patients hospitalized for an osteoporotic fracture in France (N = 356,895) over a follow‐up period of 2 to 8 years. Because of the way the data were collected, ICUROS should capture osteoporosis‐related costs more accurately, but the representativeness of the findings for all patients with osteoporotic fractures in France may be questionable because the study was performed only in six tertiary centers. In contrast, the FRACTOS study documents in principle all patients with severe osteoporotic fractures in France and thus should capture all reimbursed osteoporosis care. On the other hand, because we do not have access to the medical records in FRACTOS, we had to make assumptions about which costs were attributable to osteoporosis (for example, we assumed that all visits to rheumatologists were related to osteoporosis). The two approaches are thus complementary, and the fact that they provide consistent findings provides strong evidence that the real cost burden of osteoporosis and related fractures in France is much higher than described in previous estimates, ( 1 , 6 ) which, in the absence of recent reliable data, were based on extrapolations from fragmented data from different sources.
Within the year after the index hospitalization, the fracture‐related costs dominated expenditure on osteoporosis, accounting for >95% of costs. Compared with the cost of acute fracture care, the per capita cost of osteoporosis management was much lower (€122 to €213 in the year after the fracture, depending on the fracture site). The cost of osteoporosis management is intrinsically lower than that of fracture management, as care can be provided in the community without the need for hospitalization. However, the low level of expenditure is also explained by the low number of patients who are being managed for their osteoporosis per se. For example, in the year after hospitalization for a severe osteoporotic fracture, only 8% of patients consulted a community rheumatologist, 5% underwent bone densitometry, and 17% received a specific osteoporosis treatment. Although 40% consulted a GP in relation to their osteoporosis (as identified through prescription and delivery of a specific or non‐specific osteoporosis treatment), in most cases this only resulted in a prescription for calcium or vitamin D supplementation. These findings indicate that the quality of osteoporosis management after a fracture in terms of assessment and treatment of osteoporosis is low. Such a treatment gap with respect to recommended practice( 14 ) has been observed in many other health systems.( 15 , 16 , 17 , 18 , 19 , 20 )
In France, densitometry has been fully reimbursed since 2006, is recommended in osteoporosis practice guidelines, and is widely available. In fact, France has the highest number of densitometry machines per head of population in Europe. Similarly, osteoporosis treatments are fully reimbursed. The low numbers of patients experiencing fractures to whom densitometry is offered and treatments prescribed has been a consistent finding of studies in France for the last 20 years.( 21 , 22 ) The reason for this possibly lies in the segmentation of care in France, such that when a patient is discharged from hospital after a fracture, there is little communication between the orthopedic surgery department and the general practitioner, who is responsible for prescribing densitometry and osteoporosis treatments.( 23 ) Establishment of more Fracture Liaison Services in France (there are currently only 30) for osteoporosis care, which have been shown to be a cost‐effective way to reduce refracture rates and their associated expenditure in other countries such as the United Kingdom,( 24 , 25 ) may help improve this situation.( 26 ) The treatment gap in osteoporosis care in France is well recognized and has recently prompted initiatives on the part of the health authorities to improve this as part of the “Ma santé 2022, un engagement collectif” program.
A novel feature of our study was the longitudinal follow‐up of costs. This demonstrated that, over 5 years, the fracture‐related costs continued to dominate the economic burden of osteoporosis. In the years after the index fracture, acute fracture care cost around €900 a year on average compared with around €110 for osteoporosis management (which includes the costs of medication). This cost was principally incurred as a result of refractures. Osteoporosis management costs remained low and actually declined over the follow‐up period.
It is well established that osteoporotic fractures frequently cluster in time( 27 , 28 ) and that the risk of refracture can be reduced by specific osteoporotic treatment.( 29 ) From a strictly economic standpoint, although more intensive osteoporosis management would involve additional expenditure outlay in the short term, it may be expected that, in theory, this would be compensated by important savings in the long term due to prevention of costs associated with refracture. An increase in the proportion of patients actively managed for their osteoporosis as well as more active management in terms of prescription of specific treatments and regular follow‐up with densitometry would both be expected to reduce the economic burden of osteoporotic fractures. We cannot address this question directly because we do not have data on the relative risk of refracture in patients who were treated compared with untreated patients in this population. A dedicated cost‐effectiveness study of osteoporosis treatment in the French context would be necessary. A recent modeling study has suggested that such a treatment paradigm would be cost‐effective in several European countries, including France, although this was based on extrapolations from rather old data.( 30 ) Dedicated cost‐effectiveness studies have been performed for some of these treatments in other European countries, such as denosumab in Sweden,( 31 ) Spain,( 32 ) and Belgium( 33 ) and zoledronic acid in Norway, Finland, and the Netherlands( 34 ); these have generally concluded that the treatments are cost‐effective, particularly in high‐risk patients. A study evaluating cost‐effectiveness in France is currently underway.
We also performed a multivariate analysis of variables potentially influencing cost. Older age is associated with higher cost, which would be expected as these patients are more fragile and certain fracture sites, notably the hip and pelvis, which are associated with reduced mobility, also carry a higher cost. Mobility impairment has also been identified in the ICUROS study as a predictor of increased costs.( 7 ) In addition to these unsurprising associations, a history of osteoporotic fracture is also associated with higher cost. This observation is consistent with the longitudinal data demonstrating that the high cost of osteoporosis is driven by repeated fracture events and further emphasizes the importance of measures to reduce fracture risk in patients with osteoporosis.
The study has certain limitations, most of which are intrinsic to the nature of the database. All severe osteoporotic fractures may not have been captured, for example, because fractures that did not require hospitalization, such as certain vertebral and multiple rib fractures, will not have been documented. In addition, around one‐third of the ~500,000 patients originally identified were excluded from the analysis, principally because the site of the fracture could not be identified because the patient had a confounding pathology that precluded definite attribution of the fracture to osteoporosis or because the patient was not continuously insured during the follow‐up period. It should also be noted that wrist and forearm fractures, which are not considered to be severe osteoporotic fractures in the French osteoporosis practice guidelines, ( 12 ) were not evaluated, and the cost of these fractures are thus not included. Patients with fractures at multiple sites were also excluded. For these reasons, the cost estimates reported in the study are likely to underestimate the real total cost of these fractures. Moreover, the notion of a severe osteoporotic fracture is not specifically documented in the hospital discharge summary and the attribution of this diagnosis was made operationally by excluding patients with alternative explanations for fractures (such as Paget's disease or accidents). For this reason, certain cases of severe osteoporotic fracture may have been wrongly excluded, which may also contribute to an underestimate of the real burden of osteoporosis fracture. It is also not possible to identify consultations with specialists in hospital, so the proportion of patients consulting rheumatologists, which is only based on community‐based physicians, is probably underestimated. In addition, we did not capture costs generated from consultations from other medical specialties such as gynecologists or geriatricians, who may also contribute to osteoporosis care, or costs that were not reimbursed, such as over‐the‐counter purchase of calcium or vitamin D supplements. Finally, the specific osteoporosis‐related costs were estimated from expenditures considered a priori as likely to be associated with acute fracture care or osteoporosis management. However, the degree of imprecision is expected to be limited given that the management of osteoporosis and osteoporotic fractures is relatively specific. It should also be noted that the end of the follow‐up period was in 2016, and it is possible that costs will have evolved since this time. However, we do not consider that structural changes in osteoporosis care in France have occurred since 2016 that would have a major impact on cost.
In conclusion, the FRACTOS study has shown that the cost of severe osteoporotic fractures to the French health care system is much higher than previously estimated in earlier insurance database studies. Over time, most of the long‐term cost is associated with treatment of refractures, and a relatively low proportion of patients are treated for osteoporosis. Improved fracture prevention measures in patients with osteoporosis are crucial to reduce the economic burden of the disease.
Disclosures
AC and LP are employees of UCB Pharma SA. GB and CC are employees of IQVIA, and FM was an employee of IQVIA at the time of the study, a contract research agency responsible for the operational management of the study, funded by UCB Pharma SA. CR has received grants or honoraria from Alexion, Amgen, Regeneron, and UCB. TT has received consultancy/speaker's fees from Amgen, Arrow, Biogen, Chugai, Expanscience, Grunenthal, Jansen, LCA, Lilly, MSD, Nordic, Novartis, Pfizer, Sanofi, Thuasne, Theramex, TEVA, and UCB and financial support or fees for research activities from Bone Therapeutics, Chugai, and UCB. JP has received honoraria from Amgen, MSD, Eli Lilly, Novartis, Pfizer, and UCB. FT is head of the Centre de Pharmacoépidémiologie (Cephepi) of the Assistance Publique—Hôpitaux de Paris and of the Clinical Research Unit of Pitié‐Salpêtrière hospital. Both of these institutions have received unrestricted research funding and grants for the research projects handled and fees for consultant activities from a large number of pharmaceutical companies that have contributed indiscriminately to the salaries of its employees. FT is not employed by these institutions and has not receive any personal remuneration from these companies.
Author Contributions
Thierry Thomas: Conceptualization; methodology; project administration; supervision; validation. Florence Tubach: Conceptualization; methodology; project administration; supervision; validation. Geoffray Bizouard: Conceptualization; data curation; methodology; project administration; supervision. Anne Crochard: Conceptualization; methodology; project administration; supervision. Frédérique Maurel: Conceptualization; data curation; methodology; project administration; supervision. Laure Perrin: Conceptualization; methodology; project administration; supervision. Cédric Collin: Conceptualization; data curation; methodology; project administration; supervision. Christian Roux: Conceptualization; methodology; project administration; supervision; visualization. Julien Paccou: Conceptualization; methodology; project administration; supervision; validation.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4720.
Supporting information
Appendix S1. Supporting information
Acknowledgments
The authors thank Prof Claude Le Pen (Laboratoire d'Économie et de Gestion des Organizations de Santé, Université Paris‐Dauphine), who sadly passed away before the development of this article, for his contributions to the work. Writing and editorial assistance was provided by Adam Doble, SARL Foxymed (Paris, France), funded by UCB. This study was funded by UCB Pharma and Amgen, Inc.
Authors’ roles: Conceptualization, methodology, project administration, and supervision: all authors. Data curation: GB, FM, and CC. Validation: TT, FT, and JP. Visualization: CR.
Data Availability Statement
The study was performed in the SNDS database, which managed by the French national health insurance fund, the CNAMTS (Caisse nationale de l'assurance maladie des travailleurs salariés). Access to the database to institutions who meet the criteria for access to confidential data is permitted. Applications should be made to the National Health Data Institute (INDS; 19 rue Arthur Croquette, 94220 Charenton‐le‐Pont, Telephone: +33 1 45 18 43 90; Email: contact@indsante.fr; Website: https://www.indsante.fr/fr), which is responsible for access to all health data in France and is a one‐stop‐shop window for access to the SNDS database.
References
- 1. Hernlund E, Svedbom A, Ivergard M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roux C, Thomas T, Paccou J, et al. Refracture and mortality following hospitalization for severe osteoporotic fractures: the Fractos study. JBMR Plus. 2021;5(7):e10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levy P, Levy E, Audran M, Cohen‐Solal M, Fardellone P, Le Parc JM. The cost of osteoporosis in men: the French situation. Bone. 2002;30(4):631‐636. [DOI] [PubMed] [Google Scholar]
- 4. Maravic M, Jouaneton B, Vainchtock A, Tochon V. Economic burden of osteoporosis in women: data from the 2008 French hospital database (PMSI). Clin Exp Rheumatol. 2012;30(2):222‐227. [PubMed] [Google Scholar]
- 5. Maravic M, Le Bihan C, Landais P, Fardellone P. Incidence and cost of osteoporotic fractures in France during 2001. A methodological approach by the national hospital database. Osteoporos Int. 2005;16(12):1475‐1480. [DOI] [PubMed] [Google Scholar]
- 6. Borgstrom F, Karlsson L, Ortsater G, et al. Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos. 2020;15(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coassy A, Svedbom A, Locrelle H, et al. Costs of patient management over 18 months following a hip, clinical vertebral, distal forearm, or proximal humerus fragility fracture in France—results from the ICUROS study. Osteoporos Int. 2022;33(3):625‐635. [DOI] [PubMed] [Google Scholar]
- 8. Berdai D, Thomas‐Delecourt F, Szwarcensztein K, et al. Requests for post‐registration studies (PRS), patients follow‐up in actual practice: changes in the role of databases. Therapie. 2018;73(1):13‐24. [DOI] [PubMed] [Google Scholar]
- 9. Bezin J, Duong M, Lassalle R, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26(8):954‐962. [DOI] [PubMed] [Google Scholar]
- 10. Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the Systeme National d'Information Interregimes de l'Assurance Maladie (SNIIRAM) to the Systeme National des Donnees de Sante (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(Suppl 4):S149‐S167. [DOI] [PubMed] [Google Scholar]
- 11. Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low‐trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301(5):513‐521. [DOI] [PubMed] [Google Scholar]
- 12. Briot K, Roux C, Thomas T, et al. 2018 update of French recommendations on the management of postmenopausal osteoporosis. Joint Bone Spine. 2018;85(5):519‐530. [DOI] [PubMed] [Google Scholar]
- 13. Tuppin P, Riviere S, Rigault A, et al. Prevalence and economic burden of cardiovascular diseases in France in 2013 according to the national health insurance scheme database. Arch Cardiovasc Dis. 2016;109(6–7):399‐411. [DOI] [PubMed] [Google Scholar]
- 14. Kanis JA, Cooper C, Rizzoli R, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eliasaf A, Amitai A, Maram Edry M, Yosselson Superstine S, Rotman PP. Compliance, persistence, and preferences regarding osteoporosis treatment during active therapy or drug holiday. J Clin Pharmacol. 2016;56(11):1416‐1422. [DOI] [PubMed] [Google Scholar]
- 16. Malle O, Borgstroem F, Fahrleitner‐Pammer A, Svedbom A, Dimai SV, Dimai HP. Mind the gap: incidence of osteoporosis treatment after an osteoporotic fracture—results of the Austrian branch of the International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS). Bone. 2021;142:115071. [DOI] [PubMed] [Google Scholar]
- 17. Cheung MY, Ho AW, Wong SH. Post‐fracture care gap: a retrospective population‐based analysis of Hong Kong from 2009 to 2012. Hong Kong Med J. 2018;24(6):579‐583. [DOI] [PubMed] [Google Scholar]
- 18. Prieto‐Alhambra D, Reyes C, Sainz MS, et al. In‐hospital care, complications, and 4‐month mortality following a hip or proximal femur fracture: the Spanish Registry of Osteoporotic Femur Fractures prospective cohort study. Arch Osteoporos. 2018;13(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leslie WD, Giangregorio LM, Yogendran M, et al. A population‐based analysis of the post‐fracture care gap 1996‐2008: the situation is not improving. Osteoporos Int. 2012;23(5):1623‐1629. [DOI] [PubMed] [Google Scholar]
- 20. Barton DW, Behrend CJ, Carmouche JJ. Rates of osteoporosis screening and treatment following vertebral fracture. Spine J. 2019;19(3):411‐417. [DOI] [PubMed] [Google Scholar]
- 21. Cortet B, Chauvin P, Feron JM, et al. Fragility fractures in France: epidemiology, characteristics and quality of life (the EPIFRACT study). Arch Osteoporos. 2020;15(1):46. [DOI] [PubMed] [Google Scholar]
- 22. Lespessailles E, Gabach P, Buchon D, et al. Decline in BMD testing: a nationwide study in France. J Bone Miner Res. 2015;30(S1):427. [Google Scholar]
- 23. Launois R, Cabout E, Benamouzig D, et al. Barriers and expectations for patients in post‐osteoporotic fracture care in France: the EFFEL study. Value Health. 2022;25(4):571‐581. [DOI] [PubMed] [Google Scholar]
- 24. McLellan AR, Wolowacz SE, Zimovetz EA, et al. Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost‐effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos Int. 2011;22(7):2083‐2098. [DOI] [PubMed] [Google Scholar]
- 25. Leal J, Gray AM, Hawley S, et al. Cost‐effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: a population‐based study. J Bone Miner Res. 2017;32(2):203‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Briot K. Fracture liaison services. Curr Opin Rheumatol. 2017;29(4):416‐421. [DOI] [PubMed] [Google Scholar]
- 27. Kanis JA, Johnell O, De Laet C, et al. A meta‐analysis of previous fracture and subsequent fracture risk. Bone. 2004;35(2):375‐382. [DOI] [PubMed] [Google Scholar]
- 28. Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15(4):721‐739. [DOI] [PubMed] [Google Scholar]
- 29. Saito T, Sterbenz JM, Malay S, Zhong L, MacEachern MP, Chung KC. Effectiveness of anti‐osteoporotic drugs to prevent secondary fragility fractures: systematic review and meta‐analysis. Osteoporos Int. 2017;28(12):3289‐3300. [DOI] [PubMed] [Google Scholar]
- 30. Svedbom A, Hadji P, Hernlund E, et al. Cost‐effectiveness of pharmacological fracture prevention for osteoporosis as prescribed in clinical practice in France, Germany, Italy, Spain, and the United Kingdom. Osteoporos Int. 2019;30(9):1745‐1754. [DOI] [PubMed] [Google Scholar]
- 31. Jonsson B, Strom O, Eisman JA, et al. Cost‐effectiveness of denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int. 2011;22(3):967‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Darba J, Kaskens L, Sorio Vilela F, Lothgren M. Cost‐utility of denosumab for the treatment of postmenopausal osteoporosis in Spain. Clinicoecon Outcomes Res. 2015;7:105‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hiligsmann M, Reginster JY. Potential cost‐effectiveness of denosumab for the treatment of postmenopausal osteoporotic women. Bone. 2010;47(1):34‐40. [DOI] [PubMed] [Google Scholar]
- 34. Akehurst R, Brereton N, Ariely R, et al. The cost effectiveness of zoledronic acid 5 mg for the management of postmenopausal osteoporosis in women with prior fractures: evidence from Finland, Norway and The Netherlands. J Med Econ. 2011;14(1):53‐64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information
Data Availability Statement
The study was performed in the SNDS database, which managed by the French national health insurance fund, the CNAMTS (Caisse nationale de l'assurance maladie des travailleurs salariés). Access to the database to institutions who meet the criteria for access to confidential data is permitted. Applications should be made to the National Health Data Institute (INDS; 19 rue Arthur Croquette, 94220 Charenton‐le‐Pont, Telephone: +33 1 45 18 43 90; Email: contact@indsante.fr; Website: https://www.indsante.fr/fr), which is responsible for access to all health data in France and is a one‐stop‐shop window for access to the SNDS database.


