ABSTRACT
Objectives
To evaluate and compare the diagnostic test accuracy (DTA) of three‐dimensional transvaginal ultrasound (3D‐TVS) and magnetic resonance imaging (MRI) for deep myometrial infiltration (DMI) and cervical invasion for preoperative staging and surgery planning in patients with endometrial cancer (EC).
Methods
This systematic review and meta‐analysis investigated the DTA of MRI and 3D‐TVS for DMI and cervical invasion in patients with EC. A literature search was performed using MEDLINE, Scopus, EMBASE, ScienceDirect, The Cochrane library, ClinicalTrials.gov, Cochrane Central Register of Controlled Trials, EU Clinical Trials Register and World Health Organization International Clinical Trials Registry Platform to identify relevant studies published between January 2000 and December 2021. Study quality was assessed using the Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) tool.
Results
Five studies, including a total of 450 patients, were included in the systematic review. All five studies compared the DTA of 3D‐TVS vs MRI for DMI, and three studies compared the DTA of 3D‐TVS vs MRI for cervical invasion. Pooled sensitivity, positive likelihood ratio and negative likelihood ratio for detecting DMI using 3D‐TVS were 77% (95% CI, 66–85%), 4.57 and 0.31, respectively. The respective values for detecting DMI on MRI were 80% (95% CI, 73–86%), 4.22 and 0.24. Bivariate metaregression indicated a similar DTA of 3D‐TVS and MRI (P = 0.80) for the correct identification of DMI. Pooled ln diagnostic odds ratio for detecting cervical invasion was 3.11 (95% CI, 2.09–4.14) for 3D‐TVS and 2.36 (95% CI, 0.90–3.83) for MRI. The risk of bias was low for most of the four domains assessed in QUADAS‐2.
Conclusion
3D‐TVS demonstrated good diagnostic accuracy in terms of sensitivity and specificity for the evaluation of DMI and cervical invasion, with results comparable with those of MRI. Thus, we confirmed the potential role of 3D‐TVS in the preoperative staging and surgery planning in patients with EC. © 2022 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: 3D transvaginal ultrasound, cervical invasion, deep myometrial invasion, endometrial cancer, meta‐analysis
Short abstract
This article's abstract has been translated into Spanish and Chinese. Follow the links from the abstract to view the translations.
RESUMEN
Ecografía transvaginal tridimensional frente a imagen por resonancia magnética para la estadificación preoperatoria de la invasión profunda del miometrio y del cuello uterino en pacientes con cáncer de endometrio: revisión sistemática y metaanálisis
Objetivo Evaluar y comparar la precisión de la prueba diagnóstica (PPD) de la ecografía transvaginal tridimensional (ETV‐3D) y la imagen por resonancia magnética (IRM) para la infiltración miometrial profunda (IMP) y la invasión cervical para la estadificación preoperatoria y la planificación de la cirugía en pacientes con cáncer de endometrio (CE).
Métodos Esta revisión sistemática y metaanálisis investigó la PPD de la IRM y la ETV‐3D para la IMP y la invasión cervical en pacientes con CE. Se realizó una búsqueda bibliográfica en MEDLINE, Scopus, EMBASE, ScienceDirect, The Cochrane Library, ClinicalTrials.gov, el Registro Central Cochrane de Ensayos Controlados, el Registro de Ensayos Clínicos de la UE y la Plataforma del Registro Internacional de Ensayos Clínicos de la Organización Mundial de la Salud para identificar los estudios pertinentes publicados entre enero de 2000 y diciembre de 2021. La calidad de los estudios se evaluó mediante la herramienta de Evaluación de Calidad de los Estudios de Precisión Diagnóstica‐2 (QUADAS‐2, por sus siglas en inglés).
Resultados En la revisión sistemática se incluyeron cinco estudios con un total de 450 pacientes. Los cinco estudios compararon la PPD de la ETV‐3D frente a la IRM para la IMP, y tres estudios compararon la PPD de la ETV‐3D frente a la IRM para la invasión cervical. La sensibilidad combinada, el cociente de verosimilitud positivo y el cociente de verosimilitud negativo para detectar la IMP mediante la ETV‐3D fueron del 77% (IC 95%, 66–85%), 4,57 y 0,31, respectivamente. Los valores respectivos para detectar la IMP en la IRM fueron del 80% (IC 95%, 73–86%), 4,22 y 0,24. La metaregresión bivariante indicó una PPD similar de la ETV‐3D y la IRM (P=0,80) para la identificación correcta de la IMD. La razón de momios diagnóstica combinada para detectar la invasión cervical fue de 3,11 (IC 95%, 2,09–4,14) para la ETV‐3D y de 2,36 (IC 95%, 0,90–3,83) para la IRM. El riesgo de sesgo fue bajo para la mayoría de los cuatro dominios evaluados en QUADAS‐2.
Conclusiones La ETV‐3D demostró una buena precisión diagnóstica en términos de sensibilidad y especificidad para la evaluación de la IMD y la invasión cervical, con resultados comparables a los de la IRM. Por tanto, se confirmó el papel potencial de la ETV‐3D en la estadificación preoperatoria y la planificación de la cirugía en pacientes con CE.
摘要
子宫内膜癌患者深肌层浸润和宫颈浸润术前分期的三维经阴道超声与磁共振成像:系统回顾和荟萃分析
目的 评价和比较用于子宫内膜癌(EC)患者术前分期和手术计划的三维经阴道超声(3D‐TVS)和磁共振成像(MRI)对子宫深肌层浸润(DMI)和宫颈浸润的诊断测试准确性(DTA)。
方法 本系统回顾和荟萃分析研究了MRI和3D‐TVS对EC患者DMI和宫颈浸润的诊断测试准确性。使用MEDLINE、Scopus、EMBASE、ScienceDirect、The Cochrane library、ClinicalTrials.gov、Cochrane Central Register of Controlled Trials、EU Clinical Trials Register和世界卫生组织国际临床试验注册平台进行了文献检索,以确定2000年1月至2021年12月期间发表的相关研究。研究质量采用诊断准确性研究质量评价工具‐2(QUADAS‐2)进行了评估。
结果 五项研究被纳入系统回顾,共计450名患者。所有五项研究都比较了3D‐TVS与MRI对DMI的诊断测试准确性,三项研究比较了3D‐TVS与MRI对宫颈浸润的诊断测试准确性。使用3D‐TVS检测DMI的集合敏感性、阳性似然比和阴性似然比分别为77%(95%CI,66‐85%)、4.57和0.31。通过MRI检测DMI的相应数值分别为80%(95%CI,73‐86%)、4.22和0.24。双变量元回归显示3D‐TVS和MRI对正确发现DMI的诊断测试准确性相似(P=0.80)。3D‐TVS检测宫颈浸润的集合诊断优势比为3.11(95%CI,2.09‐4.14),MRI为2.36(95%CI,0.90‐3.83)。通过QUADAS‐2评估的四个领域中,大多数的偏倚风险都很低。
结论 3D‐TVS在评估DMI和宫颈浸润的敏感性和特异性方面表现出良好的诊断准确性,其结果与MRI的结果相当。因此,我们证实了3D‐TVS在EC患者的术前分期和手术计划中的潜在作用。
CONTRIBUTION —
What are the novel findings of this work?
Our systematic review and meta‐analysis comparing the diagnostic test accuracy (DTA) of three‐dimensional transvaginal ultrasound (3D‐TVS) and magnetic resonance imaging (MRI) for deep myometrial invasion (DMI) and cervical invasion demonstrated good performance of 3D‐TVS, confirming its value for preoperative staging and surgery planning in patients with endometrial cancer.
What are the clinical implications of this work?
This is the first systematic review to compare the DTA of 3D‐TVS and MRI in the assessment of DMI and cervical invasion in patients with endometrial cancer. Given the importance of preoperative staging, accurate imaging assessment is essential for adequate surgical planning. The DTA of 3D‐TVS was comparable with that of MRI, indicating that both techniques may be used for the evaluation of DMI and cervical invasion.
INTRODUCTION
Endometrial cancer (EC) is the most common gynecological malignancy in Europe, and it represents the sixth most frequent cancer in women worldwide 1 , 2 , 3 . Office hysteroscopy‐guided endometrial sampling is the preferred tool for the histological diagnosis of EC 4 , 5 .
According to the European Society of Gynaecological Oncology (ESGO), the European Society for Radiotherapy and Oncology (ESTRO) and the European Society of Pathology (ESP) guidelines 6 , total hysterectomy with bilateral salpingo‐oophorectomy represents the standard method of surgical staging. With regard to lymph‐node status, the guidelines recommend sentinel lymph‐node biopsy in patients with low‐ or intermediate‐risk disease and systematic pelvic/para‐aortic lymphadenectomy in those with high‐risk disease. To determine the correct surgical management, it is fundamental to assess the following risk factors: (1) tumor grade and molecular classification; (2) lymphovascular space invasion; (3) non‐endometrioid histology; (4) cervical invasion; (5) deep myometrial invasion (DMI); (6) lymph‐node involvement and distant metastasis 6 . The first three risk factors can be identified only on histology. Other factors can be identified using cross‐sectional imaging modalities, including computed tomography (CT) or positron emission tomography to assess for lymph node involvement and distant metastasis, magnetic resonance imaging (MRI) to assess for DMI, cervical invasion and lymph node metastasis and transvaginal sonography (TVS) to assess for DMI and cervical invasion 7 , 8 , 9 , 10 , 11 .
MRI has demonstrated high specificity for the preoperative assessment of DMI, cervical invasion and lymph‐node metastasis owing to its good performance in soft tissue evaluation 8 , 9 . The diagnostic test accuracy (DTA) of TVS for detecting DMI is similar to that of MRI 6 . Preoperative TVS assessment for DMI and cervical invasion is best performed by an expert sonographer, because the assessment of these medical professionals shows greater agreement with the results of histopathology and better interobserver reproducibility than does that of gynecologists 6 .
In recent years, the potential of three‐dimensional TVS (3D‐TVS) to replace MRI has been studied. Studies comparing 3D‐TVS and MRI have shown similar DTA in assessing DMI and cervical invasion 12 , 13 , 14 , 15 . A meta‐analysis comparing two‐dimensional TVS (2D‐TVS) and MRI for the preoperative detection of myometrial infiltration has been published 11 , but no meta‐analysis has compared the DTA of MRI with that of 3D‐TVS in preoperative staging. Furthermore, ESGO/ESTRO/ESP guidelines do not recommend the use of intraoperative frozen section for DMI because of its poor reproducibility and interference with adequate pathological processing 6 . Therefore, although the correct assessment of DMI and cervical invasion is a fundamental step for preoperative staging and surgical planning, there is no consensus regarding the best diagnostic approach.
In this systematic review and meta‐analysis, we compared the DTA of 3D‐TVS and MRI in the assessment for DMI and cervical invasion for preoperative staging and surgery planning in patients with EC.
METHODS
Study design and eligibility criteria
This systematic review and meta‐analysis investigated the DTA of MRI and 3D‐TVS for DMI and cervical invasion in patients with EC, using final histological assessment of hysterectomy specimens as the reference standard. The report was written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses and the Synthesizing Evidence from Diagnostic Accuracy Tests guidelines 16 . The protocol did not require registration.
Observational prospective or retrospective studies comparing the DTA of 3D‐TVS and MRI for DMI and cervical invasion in patients with EC were included. Narrative or systematic reviews, case reports, case series and conference abstracts were excluded from the analysis. Only studies in which all included patients underwent surgery were considered eligible. Eligibility criteria for studies were as follows: study population comprising patients with a diagnosis of EC; 3D‐TVS and MRI used as index tests; histological examination after surgery used as the reference standard; evaluation of DTA of TVS vs MRI for DMI and cervical invasion; study conducted at a tertiary center hospital; and sufficient information available to produce a 2 × 2 table for the calculation of sensitivity and specificity. No language restrictions were applied. DMI was diagnosed if myometrial invasion was ≥ 50% 17 . Cervical invasion was defined as positive when the tumor invaded cervical stroma but did not extend beyond the uterus 17 .
Study selection and data extraction
A literature search of relevant studies published between January 2000 and December 2021 was conducted using the following electronic databases: MEDLINE, Scopus, EMBASE, ScienceDirect, The Cochrane library, ClinicalTrials.gov, Cochrane Central Register of Controlled Trials, EU Clinical Trials Register and World Health Organization International Clinical Trials Registry Platform. The strategy for electronic search was as follows: Endometrial cancer [MeSH] OR uterine cancer [MeSH] OR myometrial invasion OR cervical invasion AND three‐dimensional transvaginal sonography OR 3D transvaginal sonography OR transvaginal sonography OR magnetic resonance imaging.
Two authors (G.S. and M.N.) independently screened the records for inclusion in this review and extracted all relevant data. A manual search of references of the included studies was also performed to ensure that relevant studies were not missed. Any disagreement was resolved by consensus. Data collection included general information about each study and relevant outcome measures, including the number of true‐positive, false‐positive, true‐negative and false‐negative cases.
Quality assessment
As recommended by The Cochrane Collaboration 18 , quality assessment was conducted using the Quality Assessment of Diagnostic Accuracy Studies‐2 tool 19 , which includes four domains: (1) patient selection, (2) index test, (3) reference standard and (4) flow and timing. Each domain was assessed in terms of risk of bias, and the first three domains were assessed in terms of concerns regarding applicability. Each item was rated as having low, high or unclear risk. Three authors (A.V., M.N. and F.C.) independently evaluated the methodological quality using a standard form with quality assessment criteria and a flow diagram. Any disagreement was resolved by consensus.
Statistical analysis
Statistical analysis was performed using the mada 20 package in R version 4.1 (R Foundation for Statistical Computing, Vienna, Austria) 21 ; P< 0.05 was considered to indicate statistical significance. Given that the sensitivity and specificity of a diagnostic test are interrelated, the bivariate approach was favored over the univariate approach in the meta‐analysis of DTA 22 , 23 . The bivariate approach estimates a bivariate normal model for the logit‐transformed pairs of sensitivity and false‐positive rate (FPR), using a linear mixed model with random effects 23 , 24 . DTA was compared between the two imaging methods using hierarchical summary receiver‐operating‐characteristics (HSROC) curves and metaregression. Post‐test probabilities were calculated and plotted on Fagan nomograms, which were constructed using positive (LR+) and negative (LR−) likelihood ratios. LR+ and LR– were used to characterize the clinical utility of a test and estimate the post‐test probability of disease.
In the meta‐analysis of DTA, heterogeneity is anticipated, but univariate measures of heterogeneity, such as the I 2 statistic, may not be appropriate. Hence, the observed heterogeneity was evaluated graphically by the scatter of points and from the prediction ellipse 18 .
The DTAs of MRI and TVS were investigated for both DMI and cervical invasion. If the number of studies assessing each specific outcome was ≥ 5, it was considered adequate for the application of the bivariate approach, which has been recommended over the univariate approach for the meta‐analysis of DTA, where appropriate 18 . The bivariate approach allows the estimation of summary measures of sensitivity and specificity, which are more informative for the reader. If the analysis included fewer than five studies, the univariate approach to the meta‐analysis of DTA was adopted. Since pooled sensitivity or specificity can be misleading, the diagnostic odds ratio (DOR) was pooled for descriptive purposes 18 .
Of note, the limited number of included studies did not allow meaningful investigation of the publication bias or the performance of sensitivity analysis, subgroup analysis and metaregression with stratifying factors 18 , 25 .
RESULTS
Study selection and characteristics
The electronic search yielded a total of 220 citations. After the removal of 111 duplicate records, 109 references remained. Of these, 99 records were excluded following title/abstract screening, as they were not relevant to the review. The full text of the 10 remaining manuscripts was examined, and five of these were excluded because they did not meet the inclusion criteria (four studies used only 3D‐TVS and one study did not provide sufficient data to obtain a 2 × 2 table). Thus, five studies were included in the systematic review and meta‐analysis 26 , 27 , 28 , 29 , 30 ; a flowchart summarizing study identification and selection is shown in Figure S1.
The included studies were published between 2012 and 2019 and included a total of 450 patients 26 , 27 , 28 , 29 , 30 . Two studies were prospective 26 , 27 and three were retrospective 28 , 29 , 30 . All five studies compared 3D‐TVS with MRI for DMI, while only three studies compared the DTA of 3D‐TVS and MRI for cervical invasion 27 , 28 , 29 (Table 1). All studies evaluated the histological grade of the tumor and reported its histological type (endometrioid vs non‐endometrioid). On definitive histology, 138 (30.7%) women in our population had DMI (myometrial invasion ≥ 50%) and 40 (12.0%) had cervical invasion.
Table 1.
Characteristics of studies comparing three‐dimensional transvaginal ultrasound (3D‐TVS) with magnetic resonance imaging for detection of deep myometrial invasion (DMI) and cervical invasion (CI) in patients with endometrial cancer (EC) included in systematic review and meta‐analysis
| Study | Study period | Study design | n | Patients' age (years) (mean (range)) | Inclusion/exclusion criteria | DMI (≥ 50%) (n) | CI (n) | Pathologist blinded |
|---|---|---|---|---|---|---|---|---|
| Saarelainen (2012) 26 | 2008–2009 | Prosp | 20 | 68.0 (59–81) | Included all cases with EC diagnosis | 12 | NE | Yes |
| Christensen (2016) 28 | 2008–2011 | Retro | 110 | 65.5 (32–85) | Included cases with endometrial hyperplasia with atypia and EC; excluded cases with Stage 3–4, serous, clear‐cell or Grade 3 carcinomas | 34 | 9 | Yes |
| Rodríguez‐Trujillo (2016) 30 | 2012–2015 | Retro | 98 | NS | Excluded cases with advanced EC | 34 | NE | Yes |
| Yildirim (2018) 27 | 2013–2016 | Prosp | 40 | 61.1 (41–80) | Excluded cases with advanced EC | 17 | 7 | NS |
| Yang (2019) 29 | 2016–2017 | Retro | 182 | 54.1 (32–78) | Included all patients with EC diagnosis | 33 | 9 | Yes |
Only first author of each study is given. The reference standard was definitive histology for all studies. NE, not evaluated; NS, not specified; Prosp, prospective; Retro, retrospective.
Three studies specified the experience of the examiners for both techniques 26 , 27 , 28 , while two studies specified the examiner's experience only for one of the techniques 29 , 30 . In the study by Saarelainen et al. 26 , 3D‐TVS was performed by examiners with 2 years' experience in 3D ultrasound, and MRI assessment was performed by two radiologists experienced in oncological MRI. In the study by Christensen et al. 28 , 3D‐TVS examination was performed by senior doctors, and the radiologist had more than 5 years' experience in pelvic MRI for the evaluation of myometrial involvement. Similarly, in the study by Yildirim et al. 27 , 3D‐TVS investigators had more than 4 years of experience, and the radiologist was experienced in abdominal radiology. In the study by Yang et al. 29 , all radiological diagnoses were made by three senior radiologist doctors, while the experience of the examiners performing 3D‐TVS was not specified. In the study by Rodríguez‐Trujillo et al. 30 , 3D‐TVS was performed by a single gynecologist experienced in gynecological ultrasound, but the authors did not specify the experience of the radiologist.
Index test evaluation
3D‐TVS
In three studies, 3D‐TVS examiners compared the most suspicious area for myometrial invasion with the endometrial–myometrial junction in the adjacent and opposite sides of the uterus 27 , 29 , 30 . Marked asymmetry was considered indicative of DMI (≥ 50% infiltration); if myometrial thickness of the two myometrial walls was similar, infiltration was considered to be superficial (< 50%). Saarelainen et al. 26 assessed the myometrium in sagittal, transverse and coronal planes on 3D‐TVS, and the maximum depth of myometrial invasion was estimated in all planes based on the subjective impression of the examiner. Christensen et al. 28 used a formula to categorize myometrial invasion as deep (≥ 50%) or superficial (< 50%).
For cervical invasion on 3D‐TVS, diagnosis was based on the subjective identification of tumor invasion by the examiner in the study by Yildirim et al. 27 . In the study by Yang et al. 29 , cervical invasion was diagnosed on 3D‐TVS if the cervix was destroyed by the tumor or the cervical canal was enlarged owing to growth of tumor tissue. A vaginal probe was also used to apply pressure onto the cervix, and a lack of movement was considered to indicate cervical involvement. Finally, in the study by Christensen et al. 28 , cervical involvement was diagnosed on 3D‐TVS when the borders of the tumor extended to the uterine cervix or there was disruption of normal echogenicity of the cervical stroma.
MRI
In four studies, the diagnostic criteria for DMI on MRI were based on the evaluation of the junctional zone 26 , 27 , 28 , 29 . If the low signal intensity, circular endometrial–myometrial junction was irregular or incomplete, the myometrium was considered to be infiltrated; if signal intensity of the tumor on T2‐weighted imaging was greater than half compared with the maximum thickness of the myometrium, it was considered indicative of DMI. In the study by Rodríguez‐Trujillo et al. 30 , the extent of myometrial infiltration on MRI was estimated subjectively by the examiner according to their impression of depth of invasion (≥ 50% or < 50%) during virtual navigation using the same method as for 3D‐TVS examination.
Yildirim et al. 27 defined cervical invasion as positive if the examiner suspected tumor invasion on subjective assessment. In the study by Christensen et al. 28 , cervical involvement was defined as present when the tumor borders extended to the uterine cervix or there was disruption of the normal low‐signal cervical stroma. Yang et al. 29 did not provide a clear definition of cervical invasion.
Diagnostic performance of 3D‐TVS vs MRI
All five studies evaluated the DTA of 3D‐TVS and MRI for the identification of DMI 26 , 27 , 28 , 29 , 30 . Pooled sensitivity was 77% (95% CI, 66–85%) for 3D‐TVS and 80% (95% CI, 73–86%) for MRI. Pooled FPR was 18% (95% CI, 11–29%) for 3D‐TVS and 19% (95% CI, 11–30%) for MRI. HSROC curves for 3D‐TVS and MRI are shown in Figure 1. Bivariate metaregression indicated a similar DTA of 3D‐TVS and MRI (P = 0.80) for the correct identification of myometrial invasion. Heterogeneity is presented graphically by the prediction ellipses in Figure 1.
Figure 1.
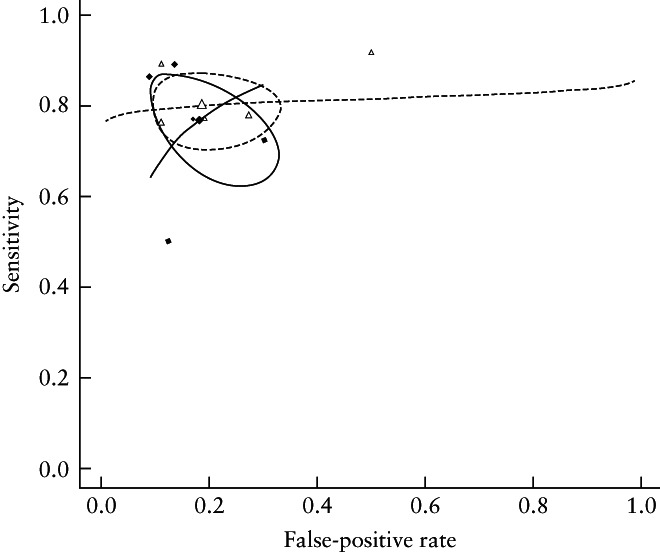
Summary receiver‐operating‐characteristics curves showing diagnostic test accuracy of transvaginal ultrasound ( ) and magnetic resonance imaging (
) and magnetic resonance imaging ( ) for deep myometrial invasion. Confidence region of summary estimate for each approach is represented by circle.
) for deep myometrial invasion. Confidence region of summary estimate for each approach is represented by circle.
Pooled ln DOR was 2.84 (95% CI, 1.88–3.81) for 3D‐TVS and 3.07 (95% CI, 2.37–3.78) for MRI. LR+ and LR– for the detection of DMI using 3D‐TVS were 4.57 and 0.31, respectively. LR+ and LR– for the detection of DMI using MRI were 4.22 and 0.24, respectively. Fagan nomograms suggested that a positive test on 3D‐TVS and MRI increased the pretest probability of DMI from 40% 15 to 74% and 74%, respectively, while a negative test on 3D‐TVS and MRI decreased the pretest probability from 40% to 16% and 14%, respectively (Figure 2).
Figure 2.
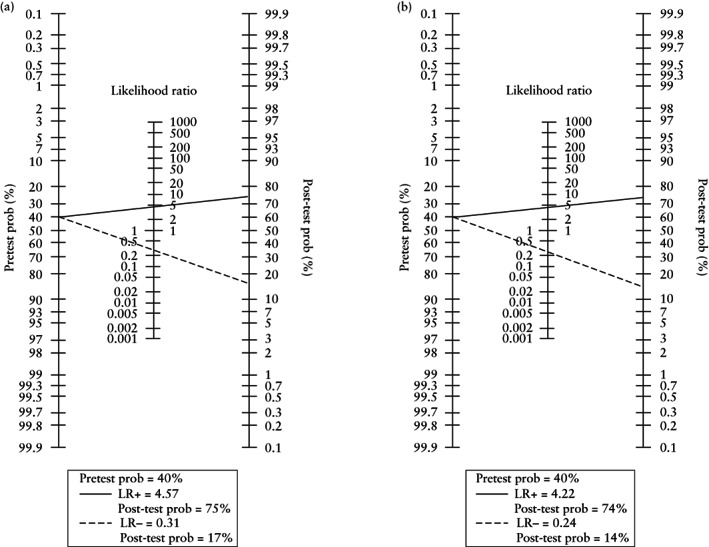
Fagan plots demonstrating post‐test probability (prob) of deep myometrial invasion in patients with endometrial cancer following a positive ( ) or negative (
) or negative ( ) result on three‐dimensional transvaginal ultrasound (a) or magnetic resonance imaging (b). LR+, positive likelihood ratio; LR−, negative likelihood ratio.
) result on three‐dimensional transvaginal ultrasound (a) or magnetic resonance imaging (b). LR+, positive likelihood ratio; LR−, negative likelihood ratio.
Three studies 25 , 26 , 27 evaluated the DTA of 3D‐TVS and MRI for the identification of cervical invasion. Using the univariate approach, we found a pooled ln DOR of 3.11 (95% CI, 2.09–4.14) for 3D‐TVS and 2.36 (95% CI, 0.90–3.83) for MRI.
Quality assessment
Risk of bias
For the patient selection domain, the overall quality of the studies was high, and all studies provided a specific description of the inclusion strategy. The inclusion of
patients with hyperplasia with atypia was considered to increase the risk of overestimating the accuracy
of MRI and 3D‐TVS for myometrial invasion ≥ 50%, considering the premalignant definition of this diagnosis. Three studies did not include patients with advanced EC 27 , 28 , 30 . Christensen et al. 28 included patients with endometrial hyperplasia with atypia on preoperative histology and excluded women with known Stage 3–4, serous, clear‐cell or Grade 3 carcinomas based on endometrial samples; the study was considered to have a high risk of bias in patient selection. Rodríguez‐Trujillo et al. 30 included patients diagnosed with early‐stage endometrial cancer, with the tumor confined to the corpus uteri, and Yildirim et al. 27 excluded patients who had clinically advanced EC. Both studies were considered to have a low risk of bias. The studies by Saarelainen et al. and Yang et al. included all patients with a diagnosis of EC 26 , 29 , and were considered to have an unclear risk of bias.
For the index test domain, the studies by Yang et al. 29 and Rodríguez‐Trujillo et al. 30 were judged to have an unclear risk of bias because they did not report clearly whether examiners were blinded to histological results and did not detail their experience in abdominal radiology or gynecological 3D‐TVS. Moreover, Yang et al. 29 did not explain clearly the method adopted to assess cervical involvement on MRI. In the other three studies, the index test was performed by an examiner who was blinded to the pathology report 26 , 27 , 28 . These studies provided an adequate description of procedures to define DMI (≥ 50% of myometrial thickness in any of the three spatial orthogonal planes) and cervical invasion 26 , 27 , 28 . In all three studies, the examiners had significant experience in gynecological ultrasound and radiological imaging. Thus, these studies were considered to have a low risk of bias.
For the reference standard domain, all studies used the final histopathological diagnosis of myometrial and cervical invasion on hysterectomy specimens as the reference standard. Yildirim et al. 27 did not report whether the pathologist was blinded to 3D‐TVS or MRI findings, making the risk of bias unclear. In the other four studies, the pathologist was blinded, thus we consider these studies to have a low risk of bias 26 , 28 , 29 , 30 . In all the studies, histological samples were reviewed by a pathologist specializing in gynecological pathology.
Finally, for the flow and timing domain, two studies did not report the time interval between the index and reference tests and were considered to have an unclear risk of bias 29 , 30 . In other studies, the authors specified the time interval between the index and reference tests. In the study by Yildirim et al. 27 , patients underwent surgery within 2 months after biopsy and underwent MRI and 3D‐TVS within 15 days before surgery. In the study by Christensen et al. 28 , all women underwent surgery within 21 days after ultrasound and MRI examination. In the study by Saarelainen et al. 26 , MRI examination was performed 1–17 days and ultrasound examination 24 h prior to the operation. These three studies were considered to have a low risk of bias. Table S1 summarizes the risk of bias of the included studies.
Applicability concerns
For the patient selection domain, the quality of included studies was high, and all were considered to have low applicability concerns 26 , 27 , 28 , 29 , 30 . For the index test domain, four studies were judged to be of good quality (low concerns regarding applicability) because they described adequately the methodology of the index test 26 , 27 , 28 , 30 . In contrast, Yang et al. 29 did not describe clearly the methods adopted to evaluate cervical invasion on MRI. Finally, for the reference standard domain, all studies used histology as the reference test; therefore, all studies were considered to be of good quality (low concerns regarding applicability). Table S1 summarizes the applicability of the included studies.
DISCUSSION
Synthesis of results and comparison with existing literature
To our knowledge, this is the first meta‐analysis to compare 3D‐TVS with MRI for the diagnosis of DMI and cervical invasion in patients with EC. We found that 3D‐TVS and MRI had comparable DTA for the diagnosis of DMI with low between‐study heterogeneity. Using the univariate approach, we found comparable DOR for 3D‐TVS and MRI for cervical invasion (CIs overlapping). These results confirmed the potential value of 3D‐TVS for staging EC in terms of DMI and cervical invasion. In addition, compared with MRI, 3D‐TVS is a less expensive technology, widely available in most settings and without contraindications for its application. It could be performed during the first oncological visit, reducing costs, time of preoperative diagnostic work‐up and patients' anxiety 11 , 31 , 32 , 33 , 34 .
The prognosis for patients with EC is improved when the diagnosis is made at an early stage (survival rates: from 95% in Stage I to 17% in Stage IV) 6 . Therefore, an accurate presurgical staging method is fundamental to prognosis and surgery planning 6 . MRI and 2D‐TVS are the most commonly used techniques for the preoperative assessment of cervical invasion and DMI 6 , 8 , 9 , 11 , 35 , 36 . In large meta‐analyses, MRI showed a pooled sensitivity of up to 90% and a pooled specificity of up to 89% for detecting DMI 36 , 37 . No difference was found between contrast‐enhanced MRI and diffusion‐weighted MRI 30 , 36 , 37 , 38 . For cervical invasion, all studies reported low sensitivity (up to 55%) and high specificity (up to 95%) of MRI 39 , 40 , 41 .
A meta‐analysis including 2773 patients evaluated the diagnostic performance of 2D‐TVS for the preoperative assessment of DMI and found a pooled sensitivity of 82% and a pooled specificity of 81% 42 , which are lower than values reported for MRI. In that study, subjective impression and objective measurements resulted in comparable DTA. Unfortunately, the authors did not distinguish between advanced and early‐stage EC, possibly overestimating the DTA 42 . Indeed, the most recent study including only low‐grade and preoperative early‐stage EC found a lower sensitivity of 69% and specificity of 87% for 2D‐TVS and respective values of 51% and 91% for MRI 43 .
Alcázar et al. 11 performed a meta‐analysis comparing 2D‐TVS and MRI (560 patients included). The authors showed that MRI had a higher sensitivity and similar specificity (83% and 82%, respectively) compared with 2D‐TVS (75% and 82%, respectively) for detecting DMI. However, the observed difference was not statistically significant, and the authors concluded that 2D‐TVS may be used in clinical settings with limited resources.
In recent years, clinicians have proposed 3D‐TVS as a new ultrasound technique for the preoperative assessment of patients with EC. This method introduces several advantages because three different axes and the coronal view may add valuable information about DMI in the uterine cornua. Moreover, 3D rendering techniques may increase the contrast between tissues, resulting in enhancement of tissue demarcation 12 . One of the first prospective studies on the use of 3D‐TVS demonstrated that subjective impression of DMI had an overall sensitivity of 92.6% and a specificity of 82.3% 12 . Subsequently, Ergenoglu et al. 13 reported that 3D‐TVS had a sensitivity of 100% and a specificity of 88% for the diagnosis of DMI.
Furthermore, two objective methods for the diagnosis of DMI using 3D‐TVS have been proposed. Alcázar et al. 12 proposed virtual navigation to measure the shortest myometrial tumor‐free distance to the serosa, while Mascilini et al. 44 proposed to evaluate the tumor /uterine volume ratio. However, the literature on this topic is limited, and the same studies demonstrated that subjective and objective assessments of DMI were comparable 12 , 44 .
A recent meta‐analysis, including patients that underwent 3D‐TVS without head‐to‐head comparison with MRI, indicated a sensitivity and specificity of about 84% and 81%, respectively, considering subjective impression as the reference technique. Despite the different methodological limitations, such as the use of the univariate approach and the small numbers of patients and studies included, these data support the potential value of 3D‐TVS in the detection of DMI 15 .
As mentioned, the literature on the use of 3D‐TVS for detecting cervical invasion is limited. The few studies that compared the sensitivity and specificity of MRI and 3D‐TVS for detecting cervical invasion showed no significant differences. However, the positive predictive value of 3D‐TVS has been reported to be significantly higher than that of MRI. In all studies, we found that the sensitivity of MRI and 3D‐TVS for cervical invasion was inferior to that for DMI. On account of high specificity (> 90%), these techniques are superior for excluding non‐invasive cervical cancer 27 , 28 , 29 , 32 .
Strengths and limitations
The main limitation of this meta‐analysis is the small number of studies and patients included. The bivariate approach, which is the recommended procedure for DTA meta‐analysis, was applicable only for DMI (five or more studies included). The bivariate approach was not appropriate for the meta‐analysis of studies evaluating cervical invasion (only three studies included), for which only the univariate approach with DOR was used.
Nevertheless, we must emphasize that this is the first meta‐analysis to compare the DTA between 3D‐TVS and MRI for the preoperative staging of EC focusing specifically on DMI and cervical invasion. Rigorous methodology, application of the bivariate approach for the evaluation of DMI and low between‐study heterogeneity represent the strengths of our study. Finally, we found an overall moderate‐to‐good quality of the studies when considering the definition of the index test and reference standard.
Conclusions
In conclusion, our findings demonstrate good DTA of 3D‐TVS for DMI in patients with EC, showing this technique to be comparable with MRI in terms of sensitivity and specificity. For cervical invasion, although 3D‐TVS appeared to perform better than MRI, we cannot give specific recommendations because the literature on this topic is limited. Certainly, there are some topics that need to be investigated further, including the introduction of an objective method for the evaluation of DMI by 3D‐TVS and a learning curve that would maximize the test performance. In our opinion, the application of 3D‐TVS for the preoperative assessment of DMI in patients with EC has high clinical potential that should be investigated further and validated in large prospective clinical trials.
Supporting information
Figure S1 Flowchart summarizing inclusion in systematic review and meta‐analysis of studies comparing diagnostic test accuracy of three‐dimensional transvaginal ultrasound vs magnetic resonance imaging for deep myometrial and cervical invasion in patients with endometrial cancer.
Table S1 Quality assessment (risk of bias and concerns about applicability) for all studies included in the meta‐analysis according to Quality Assessment of Diagnostic Accuracy Studies criteria
ACKNOWLEDGMENTS
We thank all members of the research team of the gynecology and obstetrics unit of the University of Padua. Open Access Funding was provided by Universita degli Studi di Padova within the CRUI‐CARE Agreement.
This article's abstract has been translated into Spanish and Chinese. Follow the links from the abstract to view the translations.
DATA AVAILABILITY STATEMENT
Data sharing not applicable.
REFERENCES
- 1. Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007; 18: 581–592. [DOI] [PubMed] [Google Scholar]
- 2. Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW, Birch C, Berkowitz RS, Muto MG, Crum CP. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol 2007; 31: 161–169. [DOI] [PubMed] [Google Scholar]
- 3. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 4. Saccardi C, Spagnol G, Bonaldo G, Marchetti M, Tozzi R, Noventa M. New Light on Endometrial Thickness as a Risk Factor of Cancer: What Do Clinicians Need to Know? Cancer Manag Res 2022; 14: 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saccardi C, Vitagliano A, Marchetti M, Lo Turco A, Tosatto S, Palumbo M, De Lorenzo LS, Vitale SG, Scioscia M, Noventa M. Endometrial Cancer Risk Prediction According to Indication of Diagnostic Hysteroscopy in Post‐Menopausal Women. Diagnostics (Basel) 2020; 10: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Concin N, Matias‐Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, Ledermann J, Bosse T, Chargari C, Fagotti A, Fotopoulou C, Gonzalez Martin A, Lax S, Lorusso D, Marth C, Morice P, Nout RA, O'Donnell D, Querleu D, Raspollini MR, Sehouli J, Sturdza A, Taylor A, Westermann A, Wimberger P, Colombo N, Planchamp F, Creutzberg CL. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer 2021; 31: 12–39. [DOI] [PubMed] [Google Scholar]
- 7. Bollineni VR, Ytre‐Hauge S, Bollineni‐Balabay O, Salvesen HB, Haldorsen IS. High Diagnostic Value of 18F‐FDG PET/CT in Endometrial Cancer: Systematic Review and Meta‐Analysis of the Literature. J Nucl Med 2016; 57: 879–885. [DOI] [PubMed] [Google Scholar]
- 8. Lee Y‐J, Moon MH, Sung CK, Chun YK, Lee YH. MR assessment of myometrial invasion in women with endometrial cancer: discrepancy between T2‐weighted imaging and contrast‐enhanced T1‐weighted imaging. Abdom Radiol (NY) 2016; 41: 127–135. [DOI] [PubMed] [Google Scholar]
- 9. Arnaiz J, Muñoz A‐B, Verna V, Gonzalez‐Rodilla I, Schneider J. Magnetic Resonance Imaging for the Pre‐Surgical Assessment of Endometrial Cancer: Results in a Routine Clinical Setting, Outside Dedicated Trials; a Cross‐sectional Study. Anticancer Res 2016; 36: 1891–1894. [PubMed] [Google Scholar]
- 10. Bogani G, Gostout BS, Dowdy SC, Multinu F, Casarin J, Cliby WA, Frigerio L, Kim B, Weaver AL, Glaser GE, Mariani A. Clinical Utility of Preoperative Computed Tomography in Patients With Endometrial Cancer. Int J Gynecol Cancer 2017; 27: 1685–1693. [DOI] [PubMed] [Google Scholar]
- 11. Alcázar JL, Gastón B, Navarro B, Salas R, Aranda J, Guerriero S. Transvaginal ultrasound versus magnetic resonance imaging for preoperative assessment of myometrial infiltration in patients with endometrial cancer: a systematic review and meta‐analysis. J Gynecol Oncol 2017; 28: e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alcázar JL, Galván R, Albela S, Martinez S, Pahisa J, Jurado M, López‐García G. Assessing myometrial infiltration by endometrial cancer: uterine virtual navigation with three‐dimensional US. Radiology 2009; 250: 776–783. [DOI] [PubMed] [Google Scholar]
- 13. Ergenoglu M, Akman L, Terek MC, Sanhal CY, Yeniel O, Cilengiroglu OV, Ozsaran AA, Dikmen Y, Zekioglu O. The prediction of myometrial infiltration by three‐dimensional ultrasonography in patients with endometrial carcinoma: a validation study from Ege University Hospital. Med Ultrason 2016; 18: 201–206. [DOI] [PubMed] [Google Scholar]
- 14. Eriksson LS, Lindqvist PG, Flöter Rådestad A, Dueholm M, Fischerova D, Franchi D, Jokubkiene L, Leone FP, Savelli L, Sladkevicius P, Testa AC, Van den Bosch T, Ameye L, Epstein E. Transvaginal ultrasound assessment of myometrial and cervical stromal invasion in women with endometrial cancer: interobserver reproducibility among ultrasound experts and gynecologists. Ultrasound Obstet Gynecol 2015; 45: 476–482. [DOI] [PubMed] [Google Scholar]
- 15. Costas T, Belda R, Alcazar JL. Transvaginal three‐dimensional ultrasound for preoperative assessment of myometrial invasion in patients with endometrial cancer: a systematic review and meta‐analysis. Med Ultrason 2022; 24: 77–84. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 2009; 105: 103–104. [DOI] [PubMed] [Google Scholar]
- 18. Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and Presenting Results. In Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0, Deeks JJ, Bossuyt PM, Gatsonis C (eds). The Cochrane Collaboration: London, UK, 2010; Available online: http://srdta.cochrane.org/ [accessed 1 January 2019]. [Google Scholar]
- 19. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS‐2 Group. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–536. [DOI] [PubMed] [Google Scholar]
- 20. Doebler P (2017). mada: Meta‐Analysis of Diagnostic Accuracy. R package version 0.5.8. https://CRAN.R‐project.org/package=mada. [Google Scholar]
- 21. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- 22. Reitsma J, Glas A, Rutjes A, Scholten R, Bossuyt P, Zwinderman A. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005; 58: 982–990. [DOI] [PubMed] [Google Scholar]
- 23. Kambeitz J, Kambeitz‐Ilankovic L, Leucht S, Wood S, Davatzikos C, Malchow B, Falkai P, Koutsouleris N. Detecting neuroimaging biomarkers for schizophrenia: a meta‐analysis of multivariate pattern recognition studies. Neuropsychopharmacology 2015; 40: 1742–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arends LR, Hamza TH, van Houwelingen JC, Heijenbrok‐Kal MH, Hunink MGM, Stijnen T. Bivariate random effects meta‐analysis of ROC Curves. Med Decis Making 2008; 28: 621–638. [DOI] [PubMed] [Google Scholar]
- 25. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005; 58: 882–893. [DOI] [PubMed] [Google Scholar]
- 26. Saarelainen SK, Kööbi L, Järvenpää R, Laurila M, Mäenpää JU. The preoperative assessment of deep myometrial invasion by three‐dimensional ultrasound versus MRI in endometrial carcinoma. Acta Obstet Gynecol Scand 2012; 91: 983–990. [DOI] [PubMed] [Google Scholar]
- 27. Yildirim N, Saatli B, Kose S, Sancar C, Ulukus C, Koyuncuoglu M, Saygili U, Obuz F. Predictability of myometrial, lower uterine segment and cervical invasion with 3D transvaginal ultrasonography and magnetic resonance imaging in endometrial cancer patients: a prospective cohort study. Med Ultrason 2018; 20: 348–354. [DOI] [PubMed] [Google Scholar]
- 28. Christensen JW, Dueholm M, Hansen ES, Marinovskij E, Lundorf E, Ørtoft G. Assessment of myometrial invasion in endometrial cancer using three‐dimensional ultrasound and magnetic resonance imaging. Acta Obstet Gynecol Scand 2016; 95: 55–64. [DOI] [PubMed] [Google Scholar]
- 29. Yang T, Tian S, Li Y, Tian X, Wang W, Zhao J, Pei M, Zhao M, Wang L, Quan S, Yang X. Magnetic Resonance Imaging (MRI) and Three‐Dimensional Transvaginal Ultrasonography Scanning for Preoperative Assessment of High Risk in Women with Endometrial Cancer. Med Sci Monit 2019; 25: 2024–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodríguez‐Trujillo A, Martínez‐Serrano MJ, Martínez‐Román S, Martí C, Buñesch L, Nicolau C, Pahisa J. Preoperative Assessment of Myometrial Invasion in Endometrial Cancer by 3D Ultrasound and Diffusion‐Weighted Magnetic Resonance Imaging: A Comparative Study. Int J Gynecol Cancer 2016; 26: 1105–1110. [DOI] [PubMed] [Google Scholar]
- 31. Jantarasaengaram S, Praditphol N, Tansathit T, Vipupinyo C, Vairojanavong K. Three‐dimensional ultrasound with volume contrast imaging for preoperative assessment of myometrial invasion and cervical involvement in women with endometrial cancer. Ultrasound Obstet Gynecol 2014; 43: 569–574. [DOI] [PubMed] [Google Scholar]
- 32. Sanjuán A, Escaramís G, Ayuso JR, Román SM, Torné A, Ordi J, Lejárcegui JA, Pahisa J. Role of magnetic resonance imaging and cause of pitfalls in detecting myometrial invasion and cervical involvement in endometrial cancer. Arch Gynecol Obstet 2008; 278: 535–539. [DOI] [PubMed] [Google Scholar]
- 33. SGO Clinical Practice Endometrial Cancer Working Group ; Burke WM, Orr J, Leitao M, Salom E, Gehrig P, Olawaiye AB, Brewer M, Boruta D, Villella J, Herzog T, Abu Shahin F, Society of Gynecologic Oncology Clinical Practice Committee . Endometrial cancer: a review and current management strategies: part I. Gynecol Oncol 2014; 134: 385–392. [DOI] [PubMed] [Google Scholar]
- 34. Sundar S, Balega J, Crosbie E, Drake A, Edmondson R, Fotopoulou C, Gallos I, Ganesan R, Gupta J, Johnson N, Kitson S, Mackintosh M, Martin‐Hirsch P, Miles T, Rafii S, Reed N, Rolland P, Singh K, Sivalingam V, Walther A. BGCS uterine cancer guidelines: Recommendations for practice. Eur J Obstet Gynecol Reprod Biol 2017; 213: 71–97. [DOI] [PubMed] [Google Scholar]
- 35. American Cancer Society . Survival rates for endometrial cancer. Available from: https://www.cancer.org/cancer/ endometrial‐cancer/detection‐diagnosis‐staging/survivalrates.html. [Accessed November 2020].
- 36. Luomaranta A, Leminen A, Loukovaara M. Magnetic resonance imaging in the assessment of high‐risk features of endometrial carcinoma. A meta‐analysis. Int J Gynecol Cancer 2015; 25: 837–842. [DOI] [PubMed] [Google Scholar]
- 37. Das SK, Niu XK, Wang JL, Zeng LC, Wang WX, Bhetuwal A, Yang HF. Usefulness of DWI in preoperative assessment of deep myometrial invasion in patients with endometrial carcinoma: a systematic review and meta‐analysis. Cancer Imaging 2014; 14: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Andreano A, Rechichi G, Rebora P, Sironi S, Valsecchi MG, Galimberti S. MR diffusion imaging for preoperative staging of myometrial invasion in patients with endometrial cancer: a systematic review and meta‐analysis. Eur Radiol 2014; 24: 1327–1338. [DOI] [PubMed] [Google Scholar]
- 39. Sanjuán A, Cobo T, Pahisa J, Escaramís G, Ordi J, Ayuso JR, Garcia S, Hernández S, Torné A, Martínez Román S, Lejárcegui JA, Vanrell JA. Preoperative and intraoperative assessment of myometrial invasion and histologic grade in endometrial cancer: role of magnetic resonance imaging and frozen section. Int J Gynecol Cancer 2006; 16: 385–390. [DOI] [PubMed] [Google Scholar]
- 40. Undurraga M, Petignat P, Pelte MF, Jacob S, Dubuisson JB, Loubeyre B. Magnetic resonance imaging to identify risk of lymph node metastasis in patients with endometrial cancer. Int J Gynaecol Obstet 2009; 104: 233–235. [DOI] [PubMed] [Google Scholar]
- 41. Cicinelli E, Marinaccio M, Barba B, Tinelli R, Colafiglio G, Pedote P, Rossi C, Pinto V. Reliability of diagnostic fluid hysteroscopy in the assessment of cervical invasion by endometrial carcinoma: a comparative study with transvaginal sonography and MRI. Gynecol Oncol 2008; 111: 55–61. [DOI] [PubMed] [Google Scholar]
- 42. Alcázar JL, Orozco R, Martinez‐Astorquiza Corral T, Juez L, Utrilla‐Layna J, Mínguez JA, Jurado M. Transvaginal ultrasound for preoperative assessment of myometrial invasion in patients with endometrial cancer: a systematic review and meta‐analysis. Ultrasound Obstet Gynecol 2015; 46: 405–413. [DOI] [PubMed] [Google Scholar]
- 43. Cubo‐Abert M, Díaz‐Feijoo B, Bradbury M, Rodríguez‐Mías NL, Vera M, Pérez‐Hoyos S, Gómez‐Cabeza JJ, Gil‐Moreno A. Diagnostic performance of transvaginal ultrasound and magnetic resonance imaging for preoperative evaluation of low‐grade endometrioid endometrial carcinoma: prospective comparative study. Ultrasound Obstet Gynecol 2021; 58: 469–475. [DOI] [PubMed] [Google Scholar]
- 44. Mascilini F, Testa AC, Van Holsbeke C, Ameye L, Timmerman D, Epstein E. Evaluating myometrial and cervical invasion in women with endometrial cancer: comparing subjective assessment with objective measurement techniques. Ultrasound Obstet Gynecol 2013; 42: 353–358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Flowchart summarizing inclusion in systematic review and meta‐analysis of studies comparing diagnostic test accuracy of three‐dimensional transvaginal ultrasound vs magnetic resonance imaging for deep myometrial and cervical invasion in patients with endometrial cancer.
Table S1 Quality assessment (risk of bias and concerns about applicability) for all studies included in the meta‐analysis according to Quality Assessment of Diagnostic Accuracy Studies criteria
Data Availability Statement
Data sharing not applicable.


