The increase in cross‐sectional imaging across medical disciplines has led to a rise in incidentally‐detected renal tumours worldwide [1]. Contemporary imaging cannot reliably distinguish benign from malignant tumours. Renal tumour biopsy is a relatively safe, albeit invasive test with good diagnostic accuracy [2]; however, there is a lack of consensus on its role in the renal tumour diagnostic paradigm [3]. Surgical resection of renal tumours remains the current standard of care, which is considered both diagnostic and therapeutic. However, up to 30% of renal tumours in surgical series are found to be benign [4]. Surgery, with its associated risks, morbidity and costs may represent significant overtreatment of benign tumours [5]. The radiotracer 99mTc‐sestamibi (MIBI) combined with single‐photon emission computed tomography/computed tomography (SPECT/CT) (MIBI‐kidney) is an emerging imaging test for non‐invasive characterisation of renal tumours [6].
Biological differences in sestamibi radiotracer handling by the tumour cells results in clinically indolent oncocytoma‐chromophobe spectrum tumours appearing tracer avid on MIBI‐kidney, while clear cell and papillary RCC are relatively photopenic [7]. MIBI‐kidney therefore has the potential to address the unmet clinical need for a non‐invasive diagnostic tool to risk‐stratify renal tumours.
We previously reported findings with examples of radiology images in the first two UK patients to undergo MIBI‐kidney in this setting [8]. We now report results from a pilot diagnostic accuracy study of MIBI‐kidney against a reference standard of histopathology from surgical specimens or tumour biopsy in what is, to our knowledge, the first UK trial of MIBI‐kidney.
The trial was granted ethical approval (Research Ethics Committee [REC] Ref 20/YH/0279) and registered on a clinical trials registry (ISRCTN 23 705 289). From May–October 2021, patients with local renal tumours pending surgery or biopsy, and patients on surveillance for biopsy‐confirmed oncocytoma were recruited from the Royal Free Hospital Specialist Centre for Kidney Cancer. Participants with biopsy‐confirmed oncocytoma were recruited consecutively at follow‐up appointments, while those with indeterminate lesions were purposively sampled to achieve a diverse study population and refine inclusion criteria, e.g., tumour size, stage, concomitant chronic kidney disease, personal history of other primary malignancy. Participants underwent MIBI‐kidney according to a previously described protocol [8].
Baseline cross‐sectional imaging of the index renal tumour were pseudonymised, and tumour boundaries annotated by a radiologist.
Two independent and experienced nuclear medicine physicians with access to the annotated cross‐sectional imaging reviewed the MIBI‐kidney studies on two separate occasions. The second review of imaging was performed after a 2‐month interval, with studies presented in a different order and assigned a new pseudonymised identification. Nuclear medicine physicians recorded an overall qualitative assessment of tumours as avid, photopenic or indeterminate. A quantitative measure of relative maximum uptake ratio between the tumour and ipsilateral renal parenchyma was also performed. Between first and second review, reporting nuclear medicine clinicians discussed and refined their methods for measurement of quantitative data within the nuclear imaging software (Hermes Medical Solutions). Inter‐ and intra‐rater reliability of the qualitative assessment was assessed using Gwet's first‐order coefficient (AC1) and Cohen's kappa, and quantitative assessment using Cronbach's alpha.
Biopsy and surgical specimens were reviewed by an experienced uropathologist blinded to the result of the MIBI‐kidney. Oncocytomas were defined as benign, and all RCC subtypes considered malignant.
A total of 20 patients were included; median (interquartile range [IQR]) age 66 (55–70) years and 60% were male. The median (IQR) tumour size was 4.5 (3.5–6.1) cm. Clinical stage was T1a in 40%, T1b in 45%, T2a in 5% and T3a in 10%.
Histopathology results were available for 19 patients (six biopsy, 13 surgical pathology). The final patient commenced active surveillance without a histological diagnosis after two non‐diagnostic attempts at biopsy, with tumour size unchanged on imaging at the 6‐month follow‐up.
Summary results are demonstrated in Fig. 1. Eight MIBI‐kidney scans were reported as avid (six oncocytomas, one oncocytic RCC and one chromophobe RCC), and 12 photopenic (of which 11 had evaluable histology: nine clear cell RCC, two papillary RCC). No tumours were reported as indeterminate on MIBI‐kidney imaging. Inter‐ and intra‐rater agreement for qualitative reporting of MIBI‐kidney images as avid or photopenic was excellent at 100% (Gwet's AC1 1.0, κ 1.0). Inter‐rater assessment for quantitative assessment of relative maximum uptake ratio was acceptable for first review (Cronbach's α 0.76) and excellent for second review (Cronbach's α 0.97). Intra‐rater reliability was acceptable for reader 1 (Cronbach's α 0.70) and excellent for reader 2 (Cronbach's α 0.99).
Fig. 1.
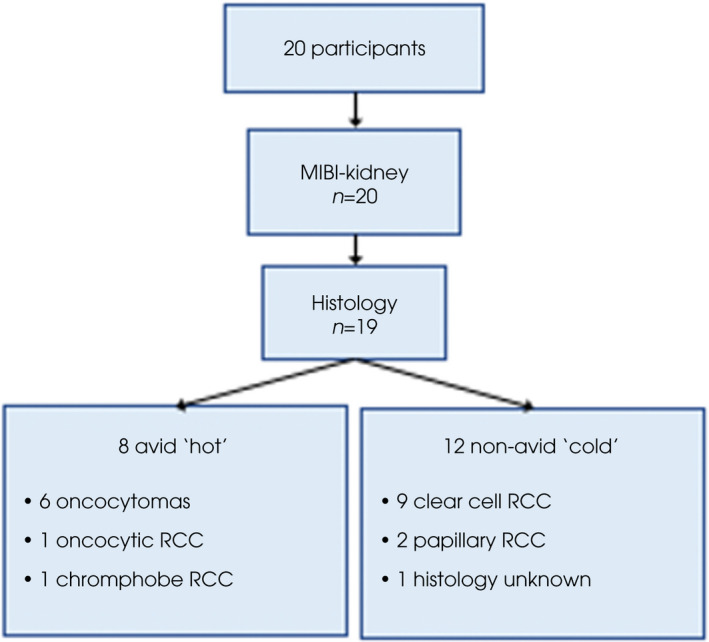
Study flow chart representing MIBI‐kidney and histopathology results.
The sensitivity and specificity of MIBI‐kidney to detect oncocytic‐chromophobe spectrum tumours from other RCCs was 100% (95% CI 74%–100%) and 100% (95% CI 63%–100%), respectively. Sensitivity and specificity to detect benign vs malignant tumours was 100% (95% CI 54%–100%) and 85.7% (95% CI 57%–98%), respectively.
This study was intended as a pump‐priming pilot and as such is limited by small sample size. Additionally, participant selection bias in order to refine inclusion/exclusion criteria for future work may have resulted in over‐estimation of diagnostic accuracy.
The MIBI‐kidney is a promising diagnostic tool for the risk stratification of renal tumours. The major limitation of MIBI‐kidney is the potential avid uptake by chromophobe RCC, and thus an inability to differentiate between chromophobe RCC and benign oncocytomas. Given the favourable prognosis of a cT1 chromophobe RCC, this differentiation may not be clinically relevant or necessary in a patient with comorbidities, pre‐existing kidney disease or otherwise keen to avoid surgery. However, a confirmatory biopsy will still have a role and may be warranted in otherwise fit patients for whom the distinction will impact on the treatment decision process.
This UK‐first MIBI‐kidney trial adds to the growing evidence base for non‐invasive risk‐stratification of indeterminate renal tumours using this test. Our future work will include a multicentre feasibility study to assess barriers and facilitators of set‐up at additional sites, development of training materials to interpret MIBI‐kidney and a health economic evaluation of MIBI‐kidney in the diagnostic pathway for renal tumours in the NHS.
Funding
Royal Free Charity, St Peter's Trust, Royal College of Surgeons of England.
Disclosure of Interests
None.
Abbreviations
- AC1
Gwet's first‐order coefficient
- IQR
interquartile range
- MIBI‐kidney
99mTc‐sestamibi combined with single‐photon emission computed tomography/computed tomography of the kidney
Acknowledgements
Hannah Warren receives salary support from The Urology Foundation and the Pan London Cancer Alliance (Royal Marsden Partners, North Central London Cancer Alliance, North East London Cancer Alliance, South East London Cancer Alliance and the NIHR BRCs). We thank the patients who have participated in this pilot study.
References
- 1. Ervik M, Lam F, Laversanne M, Ferlay J, Bray F. Global cancer observatory: cancer over time. Int Agency Res Cancer 2021. Available at: https://gco.iarc.fr/overtime. Accessed 15 July 2022 [Google Scholar]
- 2. Marconi L, Dabestani S, Lam TB et al. Systematic review and meta‐analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol 2016; 69: 660–73 [DOI] [PubMed] [Google Scholar]
- 3. Kutikov A, Smaldone MC, Uzzo RG, Haifler M, Bratslavsky G, Leibovich BC. Renal mass biopsy: always, sometimes, or never? Eur Urol 2016; 70: 403–6 [DOI] [PubMed] [Google Scholar]
- 4. Kim JH, Li S, Khandwala Y, Chung KJ, Park HK, Chung BI. Association of Prevalence of benign pathologic findings after partial nephrectomy with preoperative imaging patterns in the United States from 2007 to 2014. JAMA Surg 2019; 154: 225–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernando A, Fowler S, Brien TO. Nephron‐sparing surgery across a nation – Outcomes from the British Association of Urological Surgeons 2012 national partial nephrectomy audit. BJU Int 2016; 117: 874–82 [DOI] [PubMed] [Google Scholar]
- 6. Wilson MP, Katlariwala P, Murad MH, Abele J, Mcinnes MDF, Low G. Diagnostic accuracy of 99mTc – sestamibi SPECT/CT for detecting renal oncocytomas and other benign renal lesions: a systematic review and meta – analysis. Abdom Radiol 2020; 45: 2532–41 [DOI] [PubMed] [Google Scholar]
- 7. Rowe SP, Gorin MA, Solnes LB et al. Correlation of 99m Tc‐sestamibi uptake in renal masses with mitochondrial content and multi‐drug resistance pump expression. EJNMMI Res 2017; 7: 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller J, Campain N, Boydell A et al. ‘Case of the month’ from the specialist Centre for Kidney Cancer, Royal Free London Hospital, UK: 99m Tc‐sestamibi SPECT‐CT to differentiate renal cell carcinoma from benign oncocytoma. BJU Int 2022; 129: 28–31 [DOI] [PubMed] [Google Scholar]


