Abstract
Obesity and obesity-associated morbidity continues to be a major public health issue worldwide. Dementia is also a major health concern in aging societies and its prevalence has increased rapidly. Many epidemiologic studies have shown an association between obesity and cognitive impairment, but this relationship is not as well established as other comorbidities. Conflicting results related to the age and sex of participants, and the methodology used to define obesity and dementia may account for the uncertainty in whether obesity is a modifiable risk factor for dementia. More recently, sarcopenia and sarcopenic obesity have been reported to be associated with cognitive impairment. In addition, new mediators such as the muscle-myokine-brain axis and gut-microbiota-brain axis have been suggested and are attracting interest. In this review, we summarize recent evidence on the link between obesity and cognitive impairment, especially dementia. In particular, we focus on various metrics of obesity, from body mass index to sarcopenia and sarcopenic obesity.
Keywords: Obesity, Cognitive dysfunction, Dementia, Sarcopenia
INTRODUCTION
According to the 2021 Obesity fact sheet, the prevalence of obesity (body mass index [BMI] ≥25kg/m2) in Korean adults over the age of 20 years in 2019 was 36.3%.1 The prevalence of obesity has steadily increased over the past 11 years.2 Dementia is a major health concern in aging societies and its prevalence has increased rapidly.3 Dementia affects approximately 10.3% of adults over the age of 65 years, according to data from National Health Insurance.4 Alzheimer disease has become one of the top 10 leading causes of death and the associated death rate increased exponentially over the past 20 years in Korea.4 The rapid rise in obesity and dementia is causing a substantial public health burden for both the current aging society and the projected future super-aged society.
Individuals with obesity are at higher risk of type 2 diabetes, hypertension, dyslipidemia, cardiovascular disease, and some cancers, whereas the role of obesity on the risk of dementia remains unclear.5 Although many epidemiologic studies have shown an association between obesity and cognitive impairment since 2003, relatively less attention has been paid to the role of obesity on dementia than other comorbidities.
Previous studies reported inconsistent results regarding the relationship between obesity and cognitive function. Some reported that higher BMI tended to be a risk factor for cognitive impairment,6-8 while others observed a reduced risk of higher BMI in cognitive decline.9,10 The majority of earlier studies used BMI as an assessment tool of obesity. Unfortunately, BMI does not reflect location or amount of body fat nor alterations in body composition. Therefore, measurement of central adiposity indices such as waist circumference (WC) and waist-hip ratio (WHR) have been used to elucidate the association between obesity and cognitive impairment.11
In addition, sarcopenia characterized by loss of muscle mass and strength is aging-related changes in body composition and closely linked to dementia.12-14 Obesity often coexists with poor muscle mass and function, which is called sarcopenic obesity (SO).15,16 The role of sarcopenia and SO on various diseases has been extensively explored. As a similar motivation, there have been increasing studies regarding the role of and mechanistic links between sarcopenia and SO and cognitive impairment.14,17-19
Herein, we present recent evidence on the link between various obesity metrics and cognitive impairment, especially dementia. We discuss data ranging from earlier studies on BMI to very recent research on body weight (Bwt) variability, sarcopenia, and SO.
BMI AND RISK OF COGNITIVE IMPAIRMENT
Mid-life BMI and cognitive impairment
The evidence for the association between higher BMI in mid-life and dementia is relatively consistent. A meta-analysis including 19 studies on 589,649 participants revealed that mid-life obesity (age 35–65 years, BMI ≥30 kg/m2, with long-term follow-up to 42 years) was associated with dementia in late-life (relative risk [RR], 1.33).20 Another meta-analysis of 15 prospective studies with 25,624 participants (follow-up ranging from 3.2 to 36.0 years) showed a J-shaped relationship between mid-life BMI on dementia.21 Underweight, overweight and obesity in mid-life were associated with 1.96, 1.35, and 2.04 times the risk of developing Alzheimer dementia.21 A recent study of 6,582 participants from the English Longitudinal Study of Ageing (age 50 years, mean follow-up period of 11 years) showed that overweight or obese participants were more likely to develop dementia (RR, 1.3).22 This study included apolipoprotein E-e4 (APOE-e4) as one adjustment variable. Previous studies conducted in younger participants at baseline with longer follow-up periods showed an even stronger association between higher BMI and cognitive impairment.23,24
On the other hand, some studies contradict the hypothesis that obesity in mid-life could increase the risk of dementia in old age. A retrospective study of 2 million electronic records of patients from the UK Clinical Practice Research Datalink showed that mid-life obesity (median follow-up of 9.1 years) was associated with substantially reduced dementia risk.9 Very obese subjects (BMI >40 kg/m2) had a 29% lower dementia risk than subjects with normal BMI and the incidence of dementia continued to fall for every increase in BMI category. A cohort of 5,125 adults with normal cognitive function showed that obesity over the age of 45 (BMI ≥25 kg/m2, 6-year follow up) was associated with lower risk of cognitive decline.10 Based on these studies, it seems that relatively shorter follow-up (less than 10 years) may affect the detection of associations between higher BMI in mid-life and cognitive impairment.
Late-life BMI and cognitive impairment
Higher BMI in late-life has been considered as a possible protective factor against dementia in many epidemiological studies. The association between obesity and dementia may be modified by age. A 28-year follow-up in the Whitehall II study examined whether obesity (BMI ≥30 kg/m2) at ages 50, 60, and 70 years was associated with subsequent dementia.25 This study showed a higher risk of dementia for obesity at age 50, but not at ages 60 or 70 years. In a longitudinal study by Sun et al.,26 individuals with higher BMI in late-life experienced a slower decline in cognitive function. Higher late-life BMI was associated with larger hippocampus, entorhinal cortex, and middle temporal lobe volume at baseline. In addition, lower baseline BMI (mean age, 77.9 years) in older adults was related to a faster decline in global cognition.10,27 These findings suggest that the obesity paradox is also observed in the field of cognition and dementia.
Several hypotheses are proposed to explain the obesity paradox in terms of late-life BMI and dementia. The protective role of high BMI on cognitive impairment might be related to changes in body composition with age. Aging is characterized by loss of lean body mass.28 Therefore, low lean body mass can appear as low BMI and high lean body mass as high BMI. If an old person has a high BMI, they are likely to have a high proportion of lean body mass. High BMI suggesting high lean body mass may be involved in reducing the risk of cognitive impairment in an older population.29 In addition, high BMI may also result from increased accumulation of fat in regions other than the abdominal area, such as the legs, which could contribute to a lower risk of cognitive impairment.30 Low BMI may be indicative of malnutrition for older people and malnutrition and cognitive status are closely correlated.28
Nonetheless, there is some evidence that contradicts the above results.7,31-33 In a study by Karlsson et al.,34 higher BMI contributed to a sharp decline in cognitive function in both mid-life and late-life. The study indicated that the detrimental effects of higher BMI persist from mid-life through late-life. West et al.35 reported that long-term adiposity may have an unfavorable impact on the volume of brain regions related to cognitive functioning in older adults with type 2 diabetes.
CENTRAL OBESITY AND RISK OF COGNITIVE IMPAIRMENT
Obesity has traditionally been defined using BMI, but WC or WHR as an indicator of abdominal obesity might be a more sensitive unhealthy adiposity marker than BMI.36 In a prospective evaluation by West and Haan,37 increased central adiposity was associated with a faster rate of cognitive decline during a 5-year follow-up, but BMI was not strongly associated with cognitive scores. In 32 years of longitudinal WHR data from the Prospective Population Study of Women in Sweden,38 a mid-life WHR greater than 0.80 increased risk for dementia approximately two-fold among survivors to age 70 years. A cross-sectional study by Anand et al.39 found that higher visceral adipose tissue (VAT) and total percentage of body fat were significantly associated with reduced cognitive scores among adults with no prior history of clinical cardiovascular disease. For each one-standard-deviation-increase in adiposity equivalent to 36 mL of VAT or a 9.2% increase in body fat, 1-year cognitive aging was reduced. Subjects in the highest quartile of adiposity compared with those in the lowest quartile had a commensurate with 3 years of cognitive aging.
A study by Cho et al.40 investigated whether a positive association exists between WC and dementia according to baseline BMI and WC categories. This study comprised 872,082 participants over the age of 65 who participated in a Korean national health screening examination. WC was significantly associated with increased risk of dementia after adjustment for BMI. Normal weight men and women with abdominal obesity had a prominently increased risk of dementia compared with those without abdominal obesity. WHR per 0.1 increase had 1.39 times higher risk of cognitive impairment in those with BMI >25.3 kg/m2.40 In contrast, in a cohort study of Australians (12,047 men with aged 65-84 years),41 men with WHR ≥0.9 who were overweight had a lower risk of dementia than those with WHR<0.9 who were normal weight.
Older people tend to have more body fat and less lean mass than younger people with a same BMI. Therefore, BMI is a less suitable measurement for total body adiposity in older persons. In addition, BMI does not provide a measure of muscle or of specific fat components such as visceral and subcutaneous fat15 and may not provide the detail necessary to determine whether a person’s weight is healthful.
Only a few studies have analyzed visceral and subcutaneous fat separately as adiposity parameters.42 In the AGES-Reykjavik study (mean age, 76 years),42 there was a decreased probability of dementia per standard deviation increase in subcutaneous fat (odds ratio [OR], 0.72), thigh subcutaneous fat (OR, 0.81), and thigh muscle (OR, 0.63), but not visceral fat. Higher amounts of abdominal and thigh subcutaneous fat were associated with a lower probability of dementia in women only, whereas higher amounts of thigh muscle were associated with a lower probability of dementia in both men and women. Most studies43 revealed an inverse association between increased VAT and cognitive function in both younger and older subjects but a few studies44 showed no relevant associations.
BODY WEIGHT VARIABILITY AND COGNITIVE IMPAIRMENT
Growing evidence has suggested that Bwt variability, weight fluctuations within a specific period, may lead to poorer body-fat distribution, preferential abdominal adiposity, and adverse health outcomes, independent of the extent of obesity.45,46 Although there have been many studies about the association between Bwt variability and health status, very few studies have assessed the effects of Bwt variability on dementia.47-49
A retrospective cohort study of 19,987 participants with a mean age of 73 years using Korean National Health Insurance Service data revealed that high Bwt variability was associated with increased risk of dementia in the elderly.47 Low Bwt variability and obese BMI was associated with decreased risk of dementia. Another study of elderly women demonstrated that instability in Bwt over 5 years between ages 40 and 70 years was associated with increased risk for dementia independently of the direction of weight change.48 In a very recent study,49 participants with significant changes in BMI (increase or decrease of ≥5%) or who had greater variability in BMI, experienced faster cognitive decline. This pattern was consistent irrespective of BMI at baseline. Unstable Bwt can lead to alterations in adipose tissue structure and function that tend to favor abdominal fat accumulation and produce adipokines.45,50,51 In addition, low-grade inflammation may develop in those with higher Bwt variation.52 These mechanisms could negatively affect the brain.
SARCOPENIA/SARCOPENIC OBESITY AND COGNITIVE IMPAIRMENT
Age-related sarcopenia is characterized by decreased muscle mass, decreased muscle strength, and decreased functional performance.12 Weak handgrip strength and lower muscle mass are more prevalent in patients with dementia compared with healthy subjects of the same age.53,54 Epidemiologic evidence suggests that sarcopenia is associated with accelerated cognitive changes and cognitive impairment.19 A recent met-analysis demonstrated that the significant association between sarcopenia and cognitive impairment (OR, 2.2) was independent of the study population, definition of sarcopenia, and degree of cognitive impairment.15 In particular, the association between muscle and cognition may originate from muscle strength or performance versus muscle mass.55,56 Poor muscle performance such as low gait speed and low handgrip strength was found to be significantly associated with cognitive decline.57-62
SO is a condition characterized by co-existence of excess fat mass and low skeletal muscle mass and function.16 In recent decades, greater attention has been paid to SO due to the greater associated disability and morbidity,63 with numerous studies on the role of SO in the association with cognitive dysfunction and dementia.64,65 A very recent study by Someya et al.65 investigated whether SO is associated with cognitive impairment in 1,615 older Japanese participants (aged 65–84 years; mean age, 73.1 years). They divided participants into four groups according to sarcopenia (handgrip strength <28 kg in men and <18 kg in women) and obesity status (BMI ≥25 kg/m2). Only the SO group was independently associated with mild cognitive impairment versus an obese and a control group. In terms of dementia, the SO group had the greatest risk of dementia. Sarcopenia alone was also significantly associated with dementia only in women, and not in men. Obesity alone was not associated with either mild cognitive impairment or dementia. An earlier study by Tolea et al.64 consisted of 353 patients aged 69 years on average divided into three groups: obesity without sarcopenia, SO, and sarcopenia without obesity. The SO group showed a greater degree of cognitive impairment than other groups. Sarcopenia is a more potent predictor of loss of cognitive function than obesity. Strong handgrip strength was associated with less likelihood of developing cognitive impairment compared to weak handgrip strength in obese women (adjusted OR, 0.23). Handgrip strength has been suggested to be a useful marker for predicting future cognitive impairment among obese women. These results are in line with a previous report that showed a stronger association between weak handgrip strength and cognitive impairment in women than in men.66 It remains unclear why sarcopenia or SO is more strongly associated with cognitive impairment in women than in men.
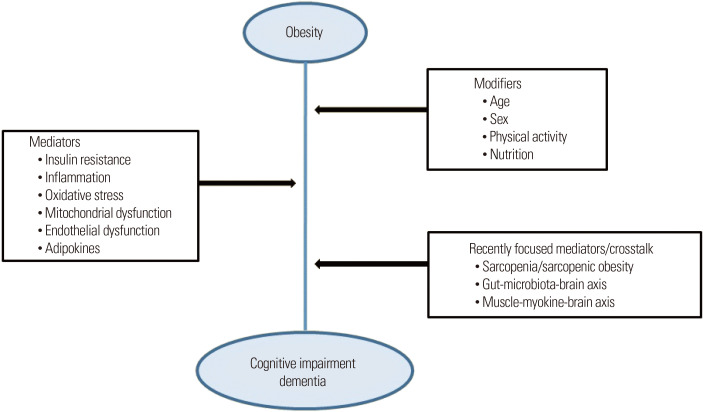
The coexistence of obesity with sarcopenia accelerates loss of muscle mass and function and reduces physical performance through multiple suggested pathogeneses.67 Suggested pathogeneses such as dysfunction of adipose tissue, adipocytokines, oxidative stress, inflammation, insulin resistance, mitochondrial dysfunction, and endothelial dysfunction may contribute to other disorders (Fig. 1).17,63,68,69 Therefore, it is possible that SO can cause significant cognitive impairment compared with obesity or sarcopenia alone.
Figure 1.
Association between obesity and cognitive impairment. The risk of dementia or cognitive impairment is associated with obesity and age-related body composition changes. Insulin resistance, inflammation, oxidative stress, mitochondrial dysfunction, and endothelial dysfunction are commonly related to these conditions. Moreover, the gut-microbiota-brain axis and muscle-myokine-brain axis are recently identified mediators.
However, considering the obesity paradox, obesity could also be a protective factor in older adults with sarcopenia. A recent study by Bahat et al.70 demonstrated that SO was associated with a lower prevalence of impaired functional health status than that of sarcopenia alone. This suggests that obesity might have a protective effect in sarcopenic subjects. Perna et al.71 proposed the existence of two phenotypes: osteosarcopenic visceral obesity and osteosarcopenic subcutaneous obesity. Among these two phenotypes, osteosarcopenic subcutaneous obesity seems to be in line with findings regarding the obesity paradox.
There is evidence that muscle-derived myokines play an important role in regulating muscle mass and function.18,19 Myokine abnormalities may underlie the pathogenesis of age-related diseases such as obesity, sarcopenia, and SO. Myokines act as a mediator between skeletal muscle and the brain, termed the “muscle-brain axis” (Fig. 1).18,19 In addition, the gut microbiota has been suggested to play a part in the mechanistic link between obesity and impaired cognition, termed the “gut-brain axis” (Fig. 1).18,72
LIMITATIONS OF EXISTING STUDIES AND UNRESOLVED ISSUES
Discrepancies between studies may stem from differences in the populations studied (sex and age at baseline, different age criteria for comparison groups) and methodologies applied (parameters of obesity, follow-up period, method of diagnosis for dementia or cognitive impairment, and definition used for sarcopenia or SO). Ascertaining obesity as an independent risk factor for dementia requires careful adjustment of confounding variables, for example, APOE-e4. APOE-e4 is the strongest risk factor gene for Alzheimer disease.73 Nonetheless, many articles did not adjust for that variable. In addition, most studies linking SO to cognitive impairment have been carried out in patients over 65 years of age. Therefore, further studies are needed to evaluate the relationship between SO and cognitive function in younger individuals. Also, mechanisms for the sex differences in the relationship between obesity and cognitive impairment need to be explored. Most of all, intervention studies are needed to reveal whether weight loss or improvement in SO can prevent cognitive impairment.
CONCLUSION
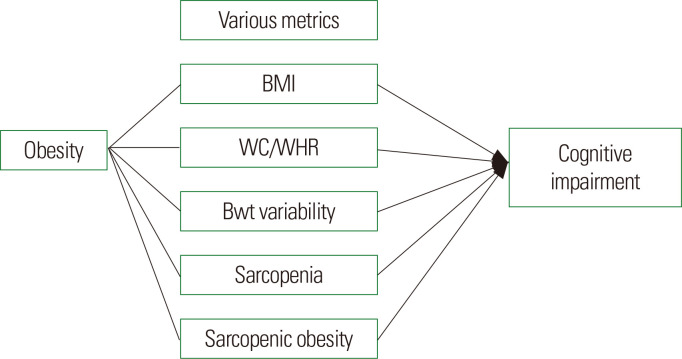
Dementia is a major public health concern. We presented the association between various metrics of obesity and cognitive impairment or dementia by reviewing prior evidence (Fig. 2). Cognitive impairment is an obesity-related comorbidity. However, based on numerous reported studies, the relationship between obesity and cognition is complex and partially modified by age and sex. With the use of various methodologies and study populations, inconsistent results have been reported. Nevertheless, the evidence for the association between higher BMI in mid-life and higher risk of late-life dementia is fairly consistent across various measures of obesity. On the other hand, it is unclear whether the risk of dementia is associated with obesity at older age. Protective effect, namely, obesity paradox or negative effect of obesity on cognition varies with sex and fat components. In the last decade, there has been considerable interest in sarcopenia and SO and related health concerns, particularly cognitive impairment. Among the components of sarcopenia, the association between muscle strength or performance and cognitive impairment showed more consistent results than muscle mass. In addition, there has been increasing evidence for the role of SO compared with obesity or sarcopenia alone in cognitive dysfunction. The high prevalence of cognitive impairment with aging and obesity as a potentially modifiable risk factor warrants further investigation.
Figure 2.
Association between various metrics of obesity and cognitive impairment. The relationship between various obesity indices and cognition is complex. BMI, body mass index; WC, waist circumference; WHR, waist-hip ratio; Bwt, body weight.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Study concept and design: JOM; analysis and interpretation of data: CHJ; drafting of the manuscript: CHJ; critical revision of the manuscript: all authors; and study supervision: JOM.
References
- 1.Korean Society for the Study of Obesity, author. Obesity fact sheet 2021 [Internet] Korean Society for the Study of Obesity; Seoul: 2011. [cited 2022 Dec 10]. Available from: https://www.kosso.or.kr/popup/obesity_fact_sheet.html . [Google Scholar]
- 2.Nam GE, Kim YH, Han K, Jung JH, Rhee EJ, Lee WY, et al. Obesity fact sheet in Korea, 2020: prevalence of obesity by obesity class from 2009 to 2018. J Obes Metab Syndr. 2021;30:141–8. doi: 10.7570/jomes21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, et al. Racial and ethnic estimates of Alzheimer's disease and related dementias in the United States (2015-2060) in adults aged ≥65 years. Alzheimers Dement. 2019;15:17–24. doi: 10.1016/j.jalz.2018.06.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JS, Kang MJ, Lee YJ, Kwak MY, Seo JW, Ko IS. Korean dementia observatory 2021 [Internet] National Medical Center; Seoul: 2022. [cited 2022 Dec 10]. Available from: https://www.nid.or.kr/info/dataroom_view.aspx?bid=243 . [Google Scholar]
- 5.Park JH. Statistics Research Institute; Daejeon: Western disease is on the rise in Korea. [Google Scholar]
- 6.Gunstad J, Lhotsky A, Wendell CR, Ferrucci L, Zonderman AB. Longitudinal examination of obesity and cognitive function: results from the Baltimore longitudinal study of aging. Neuroepidemiology. 2010;34:222–9. doi: 10.1159/000297742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallucci M, Mazzuco S, Ongaro F, Di Giorgi E, Mecocci P, Cesari M, et al. Body mass index, lifestyles, physical performance and cognitive decline: the "Treviso Longeva (TRELONG)" study. J Nutr Health Aging. 2013;17:378–84. doi: 10.1007/s12603-012-0397-1. [DOI] [PubMed] [Google Scholar]
- 8.Besser LM, Gill DP, Monsell SE, Brenowitz W, Meranus DH, Kukull W, et al. Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2014;28:36–43. doi: 10.1097/WAD.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qizilbash N, Gregson J, Johnson ME, Pearce N, Douglas I, Wing K, et al. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3:431–6. doi: 10.1016/S2213-8587(15)00033-9. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Kim Y, Park SM. Body mass index and decline of cognitive function. PLoS One. 2016;11:e0148908. doi: 10.1371/journal.pone.0148908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turcato E, Bosello O, Di Francesco V, Harris TB, Zoico E, Bissoli L, et al. Waist circumference and abdominal sagittal diameter as surrogates of body fat distribution in the elderly: their relation with cardiovascular risk factors. Int J Obes Relat Metab Disord. 2000;24:1005–10. doi: 10.1038/sj.ijo.0801352. [DOI] [PubMed] [Google Scholar]
- 12.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 14.Peng TC, Chen WL, Wu LW, Chang YW, Kao TW. Sarcopenia and cognitive impairment: a systematic review and meta-analysis. Clin Nutr. 2020;39:2695–701. doi: 10.1016/j.clnu.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Ciudin A, Simó-Servat A, Palmas F, Barahona MJ. Sarcopenic obesity: a new challenge in the clinical practice. Endocrinol Diabetes Nutr (Engl Ed) 2020;67:672–81. doi: 10.1016/j.endien.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Donini LM, Busetto L, Bauer JM, Bischoff S, Boirie Y, Cederholm T, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr. 2020;39:2368–88. doi: 10.1016/j.clnu.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Cabett Cipolli G, Sanches Yassuda M, Aprahamian I. Sarcopenia is associated with cognitive impairment in older adults: a systematic review and meta-analysis. J Nutr Health Aging. 2019;23:525–31. doi: 10.1007/s12603-019-1188-8. [DOI] [PubMed] [Google Scholar]
- 18.Bilski J, Pierzchalski P, Szczepanik M, Bonior J, Zoladz JA. Multifactorial mechanism of sarcopenia and sarcopenic obesity: role of physical exercise, microbiota and myokines. Cells. 2022;11:160. doi: 10.3390/cells11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jo D, Yoon G, Kim OY, Song J. A new paradigm in sarcopenia: cognitive impairment caused by imbalanced myokine secretion and vascular dysfunction. Biomed Pharmacother. 2022;147:112636. doi: 10.1016/j.biopha.2022.112636. [DOI] [PubMed] [Google Scholar]
- 20.Albanese E, Launer LJ, Egger M, Prince MJ, Giannakopoulos P, Wolters FJ, et al. Body mass index in midlife and dementia: systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement (Amst) 2017;8:165–78. doi: 10.1016/j.dadm.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12:e426–37. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y, Ajnakina O, Steptoe A, Cadar D. Higher risk of dementia in English older individuals who are overweight or obese. Int J Epidemiol. 2020;49:1353–65. doi: 10.1093/ije/dyaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165:321–6. doi: 10.1001/archinte.165.3.321. [DOI] [PubMed] [Google Scholar]
- 25.Singh-Manoux A, Dugravot A, Shipley M, Brunner EJ, Elbaz A, Sabia S, et al. Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimers Dement. 2018;14:178–86. doi: 10.1016/j.jalz.2017.06.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Z, Wang ZT, Sun FR, Shen XN, Xu W, Ma YH, et al. Late-life obesity is a protective factor for prodromal Alzheimer's disease: a longitudinal study. Aging (Albany NY) 2020;12:2005–17. doi: 10.18632/aging.102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arvanitakis Z, Capuano AW, Bennett DA, Barnes LL. Body mass index and decline in cognitive function in older black and white persons. J Gerontol A Biol Sci Med Sci. 2018;73:198–203. doi: 10.1093/gerona/glx152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338:1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 29.Douketis JD, Paradis G, Keller H, Martineau C. Canadian guidelines for body weight classification in adults: application in clinical practice to screen for overweight and obesity and to assess disease risk. CMAJ. 2005;172:995–8. doi: 10.1503/cmaj.045170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:372–7. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 31.Benito-León J, Mitchell AJ, Hernández-Gallego J, Bermejo-Pareja F. Obesity and impaired cognitive functioning in the elderly: a population-based cross-sectional study (NEDICES) Eur J Neurol. 2013;20:899–906. e76–7. doi: 10.1111/ene.12083. [DOI] [PubMed] [Google Scholar]
- 32.West RK, Ravona-Springer R, Heymann A, Schmeidler J, Leroith D, Koifman K, et al. Waist circumference is correlated with poorer cognition in elderly type 2 diabetes women. Alzheimers Dement. 2016;12:925–9. doi: 10.1016/j.jalz.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danat IM, Clifford A, Partridge M, Zhou W, Bakre AT, Chen A, et al. Impacts of overweight and obesity in older age on the risk of dementia: a systematic literature review and a meta-analysis. J Alzheimers Dis. 2019;70:S87–99. doi: 10.3233/JAD-180763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlsson IK, Gatz M, Arpawong TE, Dahl Aslan AK, Reynolds CA. The dynamic association between body mass index and cognition from midlife through late-life, and the effect of sex and genetic influences. Sci Rep. 2021;11:7206. doi: 10.1038/s41598-021-86667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West RK, Livny A, Ravona-Springer R, Bendlin BB, Heymann A, Leroith D, et al. Higher BMI is associated with smaller regional brain volume in older adults with type 2 diabetes. Diabetologia. 2020;63:2446–51. doi: 10.1007/s00125-020-05264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79:379–84. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 37.West NA, Haan MN. Body adiposity in late life and risk of dementia or cognitive impairment in a longitudinal community-based study. J Gerontol A Biol Sci Med Sci. 2009;64:103–9. doi: 10.1093/gerona/gln006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gustafson DR, Bäckman K, Waern M, Ostling S, Guo X, Zandi P, et al. Adiposity indicators and dementia over 32 years in Sweden. Neurology. 2009;73:1559–66. doi: 10.1212/WNL.0b013e3181c0d4b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anand SS, Friedrich MG, Lee DS, Awadalla P, Després JP, Desai D, et al. Evaluation of adiposity and cognitive function in adults. JAMA Netw Open. 2022;5:e2146324. doi: 10.1001/jamanetworkopen.2021.46324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho GJ, Hwang SY, Lee KM, Choi KM, Baik SH, Kim T, et al. Association between waist circumference and dementia in older persons: a nationwide population-based study. Obesity (Silver Spring) 2019;27:1883–91. doi: 10.1002/oby.22609. [DOI] [PubMed] [Google Scholar]
- 41.Power BD, Alfonso H, Flicker L, Hankey GJ, Yeap BB, Almeida OP. Body adiposity in later life and the incidence of dementia: the health in men study. PLoS One. 2011;6:e17902. doi: 10.1371/journal.pone.0017902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spauwen PJ, Murphy RA, Jónsson PV, Sigurdsson S, Garcia ME, Eiriksdottir G, et al. Associations of fat and muscle tissue with cognitive status in older adults: the AGES-Reykjavik Study. Age Ageing. 2017;46:250–7. doi: 10.1093/ageing/afw219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiba I, Lee S, Bae S, Makino K, Shinkai Y, Shimada H. Visceral fat accumulation is associated with mild cognitive impairment in community-dwelling older Japanese women. J Nutr Health Aging. 2020;24:352–7. doi: 10.1007/s12603-020-1330-7. [DOI] [PubMed] [Google Scholar]
- 44.Hughes TF, Borenstein AR, Schofield E, Wu Y, Larson EB. Association between late-life body mass index and dementia: the Kame Project. Neurology. 2009;72:1741–6. doi: 10.1212/WNL.0b013e3181a60a58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sponholtz TR, van den Heuvel ER, Xanthakis V, Vasan RS. Association of variability in body mass index and metabolic health with cardiometabolic disease risk. J Am Heart Assoc. 2019;8:e010793. doi: 10.1161/JAHA.118.010793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaze AD, Santhanam P, Erqou S, Ahima RS, Bertoni AG, Echouffo-Tcheugui JB. Body weight variability and risk of cardiovascular outcomes and death in the context of weight loss intervention among patients with type 2 diabetes. JAMA Netw Open. 2022;5:e220055. doi: 10.1001/jamanetworkopen.2022.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roh E, Hwang SY, Kim JA, Lee YB, Hong SH, Kim NH, et al. Body weight variability increases dementia risk among older adults: a nationwide population-based cohort study. Front Endocrinol (Lausanne) 2020;11:291. doi: 10.3389/fendo.2020.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ravona-Springer R, Schnaider-Beeri M, Goldbourt U. Body weight variability in midlife and risk for dementia in old age. Neurology. 2013;80:1677–83. doi: 10.1212/WNL.0b013e3182904cee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beeri MS, Tirosh A, Lin HM, Golan S, Boccara E, Sano M, et al. Stability in BMI over time is associated with a better cognitive trajectory in older adults. Alzheimers Dement. 2022;18:2131–9. doi: 10.1002/alz.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cereda E, Malavazos AE, Caccialanza R, Rondanelli M, Fatati G, Barichella M. Weight cycling is associated with body weight excess and abdominal fat accumulation: a cross-sectional study. Clin Nutr. 2011;30:718–23. doi: 10.1016/j.clnu.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 51.Bays HE. Adiposopathy is "sick fat" a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–73. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 52.Tamakoshi K, Yatsuya H, Kondo T, Ishikawa M, Zhang H, Murata C, et al. Long-term body weight variability is associated with elevated C-reactive protein independent of current body mass index among Japanese men. Int J Obes Relat Metab Disord. 2003;27:1059–65. doi: 10.1038/sj.ijo.0802386. [DOI] [PubMed] [Google Scholar]
- 53.De Cock AM, Perkisas S, Verhoeven V, Vandewoude M, Fransen E, Remmen R. The impact of cognitive impairment on the physical ageing process. Aging Clin Exp Res. 2018;30:1297–306. doi: 10.1007/s40520-018-1016-8. [DOI] [PubMed] [Google Scholar]
- 54.Shin HY, Kim SW, Kim JM, Shin IS, Yoon JS. Association of grip strength with dementia in a Korean older population. Int J Geriatr Psychiatry. 2012;27:500–5. doi: 10.1002/gps.2742. [DOI] [PubMed] [Google Scholar]
- 55.Abellan van Kan G. Epidemiology and consequences of sarcopenia. J Nutr Health Aging. 2009;13:708–12. doi: 10.1007/s12603-009-0201-z. [DOI] [PubMed] [Google Scholar]
- 56.Cesari M, Pahor M, Lauretani F, Zamboni V, Bandinelli S, Bernabei R, et al. Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2009;64:377–84. doi: 10.1093/gerona/gln031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol. 2009;66:1339–44. doi: 10.1001/archneurol.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taekema DG, Ling CH, Kurrle SE, Cameron ID, Meskers CG, Blauw GJ, et al. Temporal relationship between handgrip strength and cognitive performance in oldest old people. Age Ageing. 2012;41:506–12. doi: 10.1093/ageing/afs013. [DOI] [PubMed] [Google Scholar]
- 59.Abellan van Kan G, Cesari M, Gillette-Guyonnet S, Dupuy C, Nourhashémi F, Schott AM, et al. Sarcopenia and cognitive impairment in elderly women: results from the EPIDOS cohort. Age Ageing. 2013;42:196–202. doi: 10.1093/ageing/afs173. [DOI] [PubMed] [Google Scholar]
- 60.Chen WL, Peng TC, Sun YS, Yang HF, Liaw FY, Wu LW, et al. Examining the association between quadriceps strength and cognitive performance in the elderly. Medicine (Baltimore) 2015;94:e1335. doi: 10.1097/MD.0000000000001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beeri MS, Leugrans SE, Delbono O, Bennett DA, Buchman AS. Sarcopenia is associated with incident Alzheimer's dementia, mild cognitive impairment, and cognitive decline. J Am Geriatr Soc. 2021;69:1826–35. doi: 10.1111/jgs.17206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai A, Xu W, Sun J, Liu J, Deng X, Wu L, et al. Associations of sarcopenia and its defining components with cognitive function in community-dwelling oldest old. BMC Geriatr. 2021;21:292. doi: 10.1186/s12877-021-02190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hong SH, Choi KM. Sarcopenic obesity, insulin resistance, and their implications in cardiovascular and metabolic consequences. Int J Mol Sci. 2020;21:494. doi: 10.3390/ijms21020494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tolea MI, Chrisphonte S, Galvin JE. Sarcopenic obesity and cognitive performance. Clin Interv Aging. 2018;13:1111–9. doi: 10.2147/CIA.S164113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Someya Y, Tamura Y, Kaga H, Sugimoto D, Kadowaki S, Suzuki R, et al. Sarcopenic obesity is associated with cognitive impairment in community-dwelling older adults: the Bunkyo health study. Clin Nutr. 2022;41:1046–51. doi: 10.1016/j.clnu.2022.03.017. [DOI] [PubMed] [Google Scholar]
- 66.McGrath R, Vincent BM, Hackney KJ, Robinson-Lane SG, Downer B, Clark BC. The longitudinal associations of handgrip strength and cognitive function in aging Americans. J Am Med Dir Assoc. 2020;21:634–9.e1. doi: 10.1016/j.jamda.2019.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14:513–37. doi: 10.1038/s41574-018-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu H, Ballantyne CM. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest. 2017;127:43–54. doi: 10.1172/JCI88880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zamboni M, Gattazzo S, Rossi AP. Myosteatosis: a relevant, yet poorly explored element of sarcopenia. Eur Geriatr Med. 2019;10:5–6. doi: 10.1007/s41999-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 70.Bahat G, Kilic C, Ozkok S, Ozturk S, Karan MA. Associations of sarcopenic obesity versus sarcopenia alone with functionality. Clin Nutr. 2021;40:2851–9. doi: 10.1016/j.clnu.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Perna S, Spadaccini D, Nichetti M, Avanzato I, Faliva MA, Rondanelli M. Osteosarcopenic visceral obesity and osteosarcopenic subcutaneous obesity, two new phenotypes of sarcopenia: prevalence, metabolic profile, and risk factors. J Aging Res. 2018;2018:6147426. doi: 10.1155/2018/6147426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agustí A, García-Pardo MP, López-Almela I, Campillo I, Maes M, Romaní-Pérez M, et al. Interplay between the gut-brain axis, obesity and cognitive function. Front Neurosci. 2018;12:155. doi: 10.3389/fnins.2018.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Husain MA, Laurent B, Plourde M. APOE and Alzheimer's disease: from lipid transport to physiopathology and therapeutics. Front Neurosci. 2021;15:630502. doi: 10.3389/fnins.2021.630502. [DOI] [PMC free article] [PubMed] [Google Scholar]