Abstract
Objective
The protein leverage hypothesis (PLH) postulates that strong regulation of protein intake drives energy overconsumption and obesity when human diets are diluted by fat and carbohydrates. The two predictions of the PLH are that humans (i) regulate intake to maintain protein within a narrow range and that (ii) energy intake is an inverse function of percentage energy from protein because absolute protein intake is maintained within narrow limits.
Methods
Multidimensional nutritional geometry was used to test the predictions of the PLH using dietary data from the Australian National Nutrition and Physical Activity Survey.
Results
Both predictions of the PLH were confirmed in a population setting: the mean protein intake was 18.4%, and energy intake decreased with increasing energy from protein (L = −0.18, p < 0.0001). It was demonstrated that highly processed discretionary foods are a significant diluent of protein and associated with increased energy but not increased protein intake.
Conclusions
These results support an integrated ecological and mechanistic explanation for obesity, in which low‐protein highly processed foods lead to higher energy intake because of the biological response to macronutrient imbalance driven by a dominant appetite for protein. This study supports a central role for protein in the obesity epidemic, with significant implications for global health.
Study Importance.
What is already known?
Fundamental disagreement exists over the drivers and mechanisms underlying the obesity epidemic. The protein leverage hypothesis (PLH) proposes that in macro‐nutritionally imbalanced food environments, strong human regulation of protein intake drives energy overconsumption and obesity (“protein leverage”) on protein‐dilute highly processed diets. Protein leverage has support from several randomized controlled trials, and here we test the PLH in realistic, ecological settings.
What does this study add?
We show using a large national diet survey that, as predicted by the PLH, macronutrient intake is regulated within narrow limits, energy intake is a negative function of dietary protein concentration, and that the food category principally associated with dilution of dietary protein is highly processed discretionary foods.
How might these results change the direction of research or the focus of clinical practice?
That energy overconsumption is driven by a strong human protein appetite interacting with imbalanced food environments highlights the importance of interventions focused on food environments. Methodologically, our analysis addresses the controversy over diet recall data by showing that a phenomenon established in randomized controlled trials (protein leverage) is also detectable in dietary surveillance data, thus establishing both causality (in experimental settings) and relevance (in population settings) of protein leverage.
INTRODUCTION
With an estimated 11 million premature deaths and 255 million disability‐adjusted life years lost annually because of suboptimal nutrition, there is an urgent need to understand the factors that influence human diets and their health consequences [1]. A substantial amount of research has been directed at the problem at multiple levels, from the physiological pathways by which diet influences health, to the cognitive mechanisms driving food choice and the roles of food environments in influencing diets. Yet the problem continues to grow with no apparent solution in sight. New approaches are needed to understand and improve human diets.
Human nutrition science may benefit from theory and approaches from the field that applies evolutionary and ecological frameworks to the study of animal nutrition, nutritional ecology [2]. “Ecological Social Models” in public health research provide a framework for examining the interactive effects of personal and environmental factors on behavior and health [3]. In this respect, these models share important equivalences with nutritional ecology models, which conceptualize diets as a link within a system that comprises the animal and its environment. Key parameters of nutritional ecology models include constraints and other influences on dietary intake set by the environment, the strategies the animal deploys to deal with these constraints and influences, and the consequences for both the animal and the environment of the interaction [2]. To deal with the complexity of nutritional interactions between animals and food environments, which involve many nutrients and other food components (e.g., fiber, toxins), as well as several levels in the dietary hierarchy (e.g., foods, meals, diets), a multidimensional modeling framework called nutritional geometry was developed in nutritional ecology [2].
Nutritional geometry studies conducted on many species, both in the laboratory and in the field, have identified the interaction of nutrient‐specific appetites to be a powerful determinant of how animals respond to the opportunities and constraints set by nutritional environments and the consequences of these responses. Specific appetites for the macronutrients protein, fat, and carbohydrates, as well as for some micronutrients [4], have proven to be a central mechanism that guides the selection of diets that provide the required levels of multiple nutrients simultaneously, i.e., balanced diets. When caused to eat nutritionally imbalanced diets (e.g., experimentally or because of natural fluctuations in food availability), however, animals are unable to achieve the target intakes for all nutrients and are confronted with a trade‐off between undereating some nutrients and overeating others. In many species studied, several nonhuman primates included, protein intake is regulated more strongly than fat and carbohydrates, and consequently protein intake remains relatively invariant while the intakes of fat and carbohydrates (and total energy) vary more widely with variation in dietary macronutrient balance [5, 6].
This pattern of macronutrient regulation, termed “protein prioritization,” underpins a novel hypothesis for human obesity, the protein leverage hypothesis (PLH). According to the PLH, fat, carbohydrates, and total energy overconsumption is a passive outcome of humans strongly regulating protein intake within narrow boundaries when dietary protein is diluted by fat and carbohydrates [5, 7]. There now exists significant experimental evidence in support of the key components of the PLH, namely that nutrient‐specific appetites interact to direct dietary intake toward a particular dietary macronutrient balance, and with macro‐nutritionally imbalanced diets, protein regulation dominates fat and carbohydrates. Campbell et al. [8] demonstrated under randomized controlled conditions that adult human subjects consistently selected a diet of 14.7% protein when the options ranged from 10% to 25% protein, confirming the results of an earlier small‐scale study [9]. Several randomized controlled trials in which humans have been experimentally constrained to imbalanced diets have demonstrated the protein prioritization pattern of macronutrient regulation and confirmed that it leads to energy overconsumption on protein‐dilute diets [5, 6]. One study also found a negative relationship between change in body weight during the trial and the percentage contribution of protein to energy intake [8]. The two clinical trials that have failed to show increased intake on low‐protein diets [10, 11] used experimental diets that were very low in protein (5%), beyond the regulatory limits of the system [5].
An important priority is to determine whether protein leverage plays a role in driving energy overconsumption and obesity in free‐living humans, and if so, what the ecological causes of dietary protein dilution are. Studies have demonstrated that percentage energy from protein in American diets has decreased coincident with the rise of obesity, as evidenced both in data from the National Health and Nutrition Examination Survey (NHANES) and the Food and Agriculture Organization Food Balance Sheets [12, 13]. A retrospective study of a cohort of youth likewise found the predicted negative relationship between dietary percentage protein and energy consumption. One analysis of the NHANES data highlighted a category of highly processed foods, ultraprocessed foods, as a likely diluent of protein in American diets [14]. This is consistent with an experimental study that found inpatient adults exposed to ultraprocessed diets ingested more carbohydrates, fat, and total energy than those on unprocessed diets and gained weight during the 14‐day trial, whereas protein intake did not differ across the diets [15]. However, there has been no integrated study testing in the same population data for macronutrient balancing, protein leveraging on imbalanced protein‐dilute diets, or the ecological cause of dietary protein dilution.
We applied nutritional geometry to perform an integrated test of key predictions of the PLH using diet surveillance population data. Using the Australian Health Survey data: (i) respondents regulate daily macronutrient intake toward a range of approximately 15% to 25% energy from protein (macronutrient balancing); (ii) daily dietary protein intake is more constant across the survey respondents than daily carbohydrate and fat intakes (protein prioritization pattern of macronutrient regulation), and therefore daily energy intake is an inverse function of dietary percentage protein (protein leverage); and (iii) highly processed industrial foods are generally low in protein relative to fat and carbohydrates and thus predispose to dietary protein dilution and excess energy intake via protein leverage. All predictions were supported, with important implications for nutritional research and for focusing policy and other measures for transforming obesogenic food environments.
METHODS
Respondents and dietary data collection
This study used data from the cross‐sectional 2011 to 2012 National Nutrition and Physical Activity Survey (NNPAS), undertaken by the Australian Bureau of Statistics (ABS). The survey was designed to collect national benchmark data on nutrition and physical activity of the Australian population using a multistaged area sample of private households. It was conducted between May 2011 and June 2012 in adults and children aged 2 years and older. Ethics approval for the survey was granted by the Australian Government Department of Health and Aging Departmental Ethics Committee in 2011. Further details about the scope and the methodology of the survey are available from the NNPAS Users' Guide by the ABS [16].
Details on data collection of the 24‐hour recall used to assess diets and definitions of all outcomes, exposures, predictors, potential confounders, and effect modifiers are provided in online Supporting Information Methods.
Data analysis
To test the prediction that people regulate the balance of macronutrients eaten over the time scale of a single day, the day interview was divided into Eating Period 1 (EP1) (≥00:00 to <11:00), EP2 (≥11:00 to <16:00), and EP3 (≥16:00) (Supporting Information Figure S1). Respondents' proportion of energy intake from protein in EP1 was categorized as below the Acceptable Macronutrient Distribution Range (AMDR) range (<15%), within the AMDR (15%–25%), or above the AMDR (>25%). Comparisons between time points were assessed using repeated measures ANOVA with between‐subject factors as adjustments. A sensitivity analysis was conducted to adjust for potential confounding from regression to the mean, adjusting for baseline protein energy at EP1 (percentage) minus the baseline mean protein energy at EP1 (percentage) [17]. This did not alter our conclusions.
Estimated regression coefficients for change in energy and food intakes with the proportion of energy from protein at EP1 across different eating periods and between tertiles of discretionary food were identified using generalized linear regression analysis. The analysis was adjusted for sex, age, country of birth (Australia, English‐speaking countries, or other countries), energy intake versus basal metabolic rate, physical activity level as defined by the ABS (High, Moderate, Low, Sedentary [very low], Sedentary [no exercise], or Not Stated) [16], and season of interview. All regressions were computed in SAS 9.4 (SAS Institute Inc.).
Ratios between dietary macronutrients reported by the respondents were analyzed and displayed in the right‐angled mixture triangle surface plots [2]. Mixture models, also known as Scheffe' polynomials, were fitted to the data with macronutrients as repeated measures over total energy intake or individual nutrient intake. To prepare graphs and run associated tests, R packages required xtable, mgcv, sp, lattice, ellipse, survival, nlme, mixexp, plyr, ggplot2, scales, directlabels, and lsmeans. Data analysis and graphics were performed using R software [31].
To test for protein prioritization, power regression was conducted (SPSS Statistics version 25, IBM Corp.) for modeling dietary percentage energy from protein to total macronutrient energy intake for the specified macronutrients (p) [5, 12]. In this test, if protein intake (P) remains constant and nonprotein energy varies exponentially with changing dietary proportion of energy from protein (p), the exponent (L) in the equation Pp L takes a value of −1. In the case of partial protein prioritization, absolute energy intake from protein increases with increased percentage energy from protein, but to a lesser degree than total energy intake decreases (−1 < L < 0), indicating that factors other than protein have an influence on the variance in total energy intake. If L > 0, then total energy intake increases with an increase in percentage energy from protein.
Where stated, the NNPAS weights were applied to data to provide estimates for the Australian population accounting for nonresponse and the complex survey design including replicate weights. For all tests, p < 0.05 was considered statistically significant.
RESULTS
Respondents and timing of food intake
The study population included 9341 adults (≥19 years) with a mean age of 46.3 years. The mean (SE) energy intake was 8671 kJ (52.2) and the mean (SE) percentage energy from protein was 18.4% (0.1%), from carbohydrates 43.5% (0.2%), from fat 30.9% (0.1%), from fiber 2.2 (0.1%), and from alcohol 4.3% (0.1%) (Table 1). Respondents' energy intake in relation to time of consumption is plotted in Supporting Information Figure S1. Energy intake accumulated within the three peak eating periods around 8:00 am, 1:00 pm, and 7:00 pm, with small snack intakes in between. EP1 was the smallest eating period of the recorded day with the least amount of energy and foods consumed, whereas EP3 was the largest.
TABLE 1.
Characteristics and macronutrient intakes of participants in the National Nutrition and Physical Activity Survey
| Value | |
|---|---|
| Male gender, % | 49.4 |
| Age (y), % | |
| 18–50 | 58.4 |
| 51–70 | 29.9 |
| 71+ | 11.7 |
| Country of birth, % | |
| Australia | 68.8 |
| Canada, Ireland, NZ, South Africa, UK, US | 11.6 |
| Other | 19.6 |
| SEIFA, % | |
| Lowest—quintile 1 | 18.1 |
| Middle—quintile 2–3 | 59.7 |
| Highest—quintile 5 | 22.2 |
| Tertiary education, % | |
| Not known | 1.5 |
| No tertiary education | 38.2 |
| Vocational college | 34.9 |
| University | 25.3 |
| BMI, % | |
| Underweight (<18.5 kg/m2) | 1.8 |
| Normal (≥18.5 to <25 kg/m2) | 35.5 |
| Overweight (≥25.0 to <30.0 kg/m2) | 36.4 |
| Obesity (≥30.0 kg/m2) | 26.3 |
| Energy reporting status, % | |
| Low energy (energy intake: basal metabolic rate ratio <0.87) | 16.8 |
| Unknown | 14.0 |
| Plausible | 69.2 |
| Energy and macronutrients, mean | |
| Energy (kJ) | 8671.6 |
| Protein (% total energy) | 18.4 |
| Carbohydrates (% total energy) | 43.5 |
| Fat (% total energy) | 30.9 |
| Fiber (% total energy) | 2.2 |
| Alcohol (% total energy) | 4.3 |
Note: Survey weights applied.
Abbreviations: NZ, New Zealand; SEIFA, Socio‐Economic Indexes for Areas.
Macronutrient balancing
The cumulative change of macronutrient proportions over the day is shown in Figure 1, with respondents separated by percentage energy from protein below, within, or above the AMDR at EP1. The figure shows that respondents categorized according to whether above, below, or within the AMDR at EP1 tracked across diet space toward a common position by the end of the day (EP3), as predicted by the macronutrient balancing model. As predicted, respondents with percentage energy from protein below the AMDR at EP1 increased the ratio of protein in subsequent eating periods, those who started within the AMDR for protein maintained this, and respondents with percentage energy from protein above the AMDR at EP1 showed a decline through the day (Figure 1). There was, nonetheless, a statistically significant difference between groups for cumulative intake to EP3 (i.e., total intake across the day) (F 2,4 = 860.0, p < 0.0001). This suggests that the compensation through the day was not complete for those with a lower proportion of energy at the start of the day. Participants with a higher proportion of energy from protein at the start of the day (i.e., EP1) had lower daily energy intake (−18.6 [5.7], p = 0.0017, Table 2).
FIGURE 1.
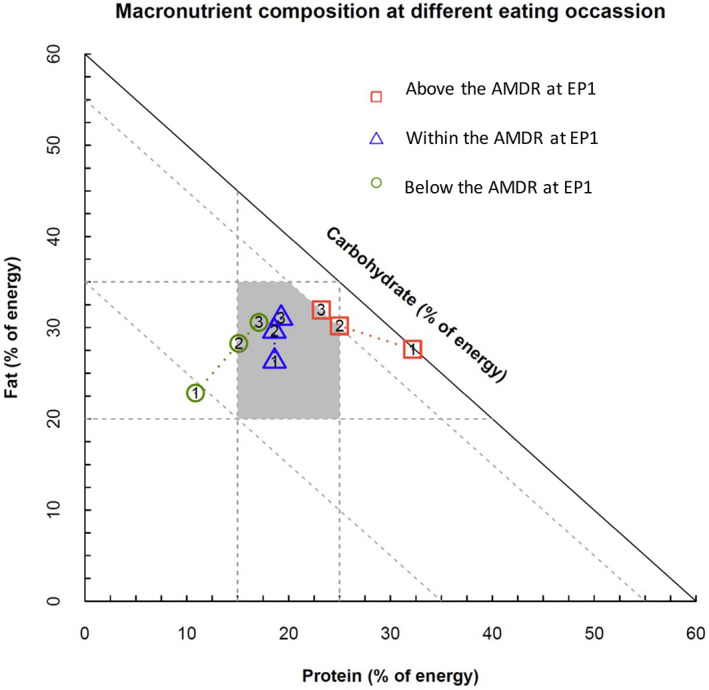
Cumulative proportion of macronutrients from Eating Period 1 (EP1) to EP3 by reported protein density below, within, or above the AMDR at EP1. The day was divided into three intervals; EP1 between midnight and 11 am; EP2 between 11:00 am and 4:00 pm; and EP3 after 4:00 pm, indicated as 1, 2, 3 on the figure. Positions at 3 indicate the macronutrient proportions over the day, that is, from the start of the day to the end of the day. Shaded polygon area: Acceptable Macronutrient Distribution Range (AMDR) for Australians and New Zealanders [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 2.
Estimated regression coefficients for change in energy and food intakes with the proportion of energy from protein at different eating periods
| EP | Dietary component | Estimate (SE) | p value a |
|---|---|---|---|
| EP1 (midnight to 11 am) | Energy (kJ) | 7.8 (3.3) | 0.0208 |
| Meat and alternatives (g) | 1.9 (0.1) | <0.0001 | |
| Grain (g) | −0.2 (0.2) | 0.9136 | |
| Vegetables (g) | 0.04 (0.1) | 0.7313 | |
| Fruit (g) | −2.7 (0.2) | <0.0001 | |
| Dairy products (g) | 5.9 (0.4) | <0.0001 | |
| Discretionary (servings) b | −0.03 (0.0) | <0.0001 | |
| EP2 (11 am to 4 pm) | Energy (kJ) | −18.4 (4.0) | <0.0001 |
| Meat and alternatives (g) | 0.1 (0.2) | 0.5789 | |
| Grain (g) | −0.9 (0.1) | <0.0001 | |
| Vegetables (g) | −0.2 (0.2) | 0.2835 | |
| Fruit (g) | 0.1 (0.3) | 0.7468 | |
| Dairy products (g) | 0.03 (0.2) | 0.8626 | |
| Discretionary (servings) | −0.02 (0.0) | 0.0006 | |
| EP3 (4 pm to midnight) | Energy (kJ) | −8.0 (4.0) | 0.0507 |
| Meat and alternatives (g) | 0.2 (0.2) | 0.4440 | |
| Grain (g) | −0.5 (0.2) | 0.0214 | |
| Vegetables (g) | −0.6 (0.3) | 0.0409 | |
| Fruit (g) | −1.0 (0.4) | 0.0153 | |
| Dairy products (g) | 0.02 (0.3) | 0.9381 | |
| Discretionary (servings) | −0.01 (0.0) | 0.0227 | |
| Total (midnight to midnight) | Energy (kJ) | −18.6 (5.7) | 0.0017 |
| Meat and alternatives (g) | 11.0 (0.4) | <0.0001 | |
| Grain (g) | −1.3 (0.3) | 0.0002 | |
| Vegetables (g) | −0.2 (0.3) | 0.8420 | |
| Fruit (g) | −3.2 (0.4) | <0.0001 | |
| Dairy products (g) | 6.0 (0.5) | <0.0001 | |
| Discretionary (servings) | −0.1 (0.0) | <0.0001 |
Abbreviation: EP, eating period.
Generalized linear model adjusted for gender, age, country of birth, energy intake vs. basal metabolic rate, physical activity level, and season of interview. Survey weights applied.
1 serving = 600 kJ.
Protein leverage
Figure 2A–G plots the relationships between the proportion of energy from macronutrients and absolute intakes of various dietary components. The combined intakes of carbohydrates and fat (panel A) and total energy (panel B) increased with decreasing proportion of macronutrient energy from protein, as predicted by the PLH. The same relationships were observed in the sensitivity analysis using the first and the second day of the survey (Supporting Information Figure S2). Increasing energy intake on low‐protein diets was due both to carbohydrates (panel C) and fat (panel D) intakes being high on low‐protein diets. As would be expected, dietary energy density was highest for diets with a high proportional fat content (panel E), corresponding with high absolute fat intake (panel D). Because fat has twice the energy density of carbohydrates and protein, energy density per se might thus contribute to high energy intake. It cannot, however, on its own explain the observed variation in energy intake, because both the dry weight of food (panel F) and carbohydrates eaten (panel C) were high in regions where energy density was low. In contrast, intakes of all components except protein increased with decreasing dietary protein, as expected under the protein leverage model. Table 3 shows that all these relationships were highly significant, and the strength of leverage (the L value) varied among components. For none was leverage complete (indicated by L = −1), with the strongest leverage occurring for carbohydrates (L = −0.59) and the weakest being for dry weight intake (L = −0.14). Incomplete protein leverage indicates that in addition to the intake of nonprotein dietary components decreasing with increasing dietary percentage protein, absolute protein intake increased but to a lesser extent [5], as shown in Figure 2G.
FIGURE 2.
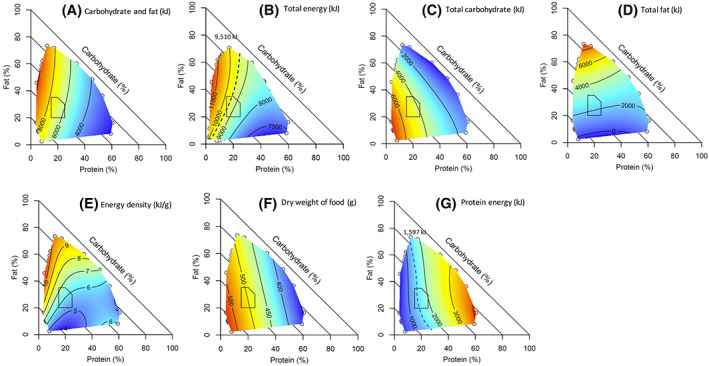
Surface plots showing the relationship between daily dietary macronutrient distributions and energy intake from different dietary components for adults. (A) Carbohydrates and fat (kJ). (B) Total energy (kJ). (C) Total carbohydrates (kJ). (D) Total fat (kJ). (E) Energy density (kJ/g). (F) Dry weight of food (g). (G) Protein energy (kJ). For any point on the colored surface, the point represents the average energy for that contribution of protein (%E), fat (%E), and carbohydrates (%E) from the dietary component. As percentage energy from protein increases along the x axis, total energy decreases (red to blue), and total protein increases (blue to red). Carbohydrates (%E) are deterministically implied as the proportion from macronutrients = 100%, and the value is shown as diagonal lines with slope = −1. The polygon represents the Australian/New Zealand Acceptable Macronutrient Distribution Range (AMDR). The dashed line in panel (B) represents the estimated energy requirements based on the basal metabolic rate of the average adult from the survey assuming equilibrium and a physical activity level of 1.4 (9510 kJ); the data indicate that diets within 15%–20% energy from protein correspond to equilibrium energy intake, whereas dietary protein densities below and above this level are associated with positive and negative energy balance, respectively. The dashed contour in panel (F) represents an approximate average protein requirement for the survey population (1597 kJ), based on an average person's weight of 78.3 kg (95% CI: 77.8–78.8) and the maximum population‐safe requirements estimated by Elango et al. (1.2 g/kg) [18] [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 3.
The exponent (L) from power regression testing protein prioritization of adults in the NNPAS
| Protein range | Dry weight (g) | Total energy | Fat | Carbohydrates | ||||
|---|---|---|---|---|---|---|---|---|
| L | p | L | p | L | p | L | p | |
| Full range of protein, %E | −0.14 | <0.0001 | −0.18 | <0.0001 | −0.20 | <0.0001 | −0.59 | <0.0001 |
| 10%–30% energy a | −0.16 | <0.0001 | −0.20 | <0.0001 | −0.22 | <0.0001 | −0.58 | <0.0001 |
Note: Survey weight applied. An exponent of −1 indicates complete protein prioritization where absolute protein intake remains constant, and carbohydrates and fat differ with the proportion of dietary protein intake.
Abbreviations: NNPAS, National Nutrition and Physical Activity Survey; %E, percentage energy.
10%–30% protein indicates the usual variation in human protein intake.
Superimposed on Figure 2B,G are contours representing the population‐level recommendations for energy and protein intakes [18], respectively. The contours delineate the dietary macronutrient compositions that the data predict to be associated with positive (to the left) and negative (to the right) energy balance and negative (to the left) and positive (to the right) protein balance.
Food macronutrient composition and protein leverage
Figure 3 shows the macronutrient composition of foods from the five food groups and discretionary foods in the Australian food nutrient database. Five food group foods predominantly have a protein content of greater than 15% of energy, whereas discretionary foods are comparatively protein dilute with <15% of energy from protein and variable carbohydrate and fat content. Dietary dilution of protein was evident for participants consuming more discretionary foods relative to five food group foods, as evidenced by the fact that percentage energy from protein decreased with increasing discretionary food intake (protein dilution) (Figure 4A). In contrast, intake of five food group foods decreased with decreasing percentage energy from protein and peaked with high fat intake (Figure 4B). Because the PLH concerns the role of dietary percentage protein in influencing the intakes of other dietary components, we also compared the dietary intakes for participants with different percentage energy from protein (Supporting Information Table S1). The distribution of dietary protein as a proportion of total energy is shown in Supporting Information Figure S3. Participants with a lower proportion of energy from protein (below the AMDR) consumed more discretionary foods and less of the five food groups (Supporting Information Table S1). Similarly, those with lower energy from protein at EP1 also had an overall poorer diet quality at each mealtime, consuming more discretionary foods and less meat and alternatives (Table 2).
FIGURE 3.
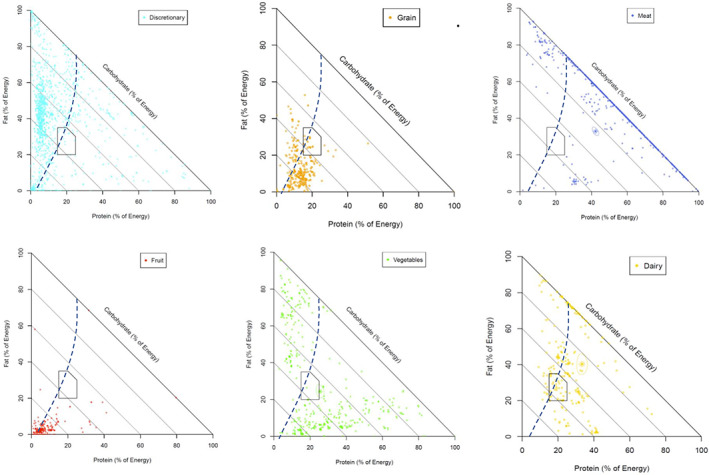
Macronutrient composition for discretionary foods and the five food groups. Percentage energy from protein, carbohydrates, and fat for discretionary foods and the five food groups as consumed by participants in the National Nutrition and Physical Activity Survey (2011–2012) including from left to right, top to bottom: discretionary foods; grains and cereals; meat including poultry, fish, eggs, tofu, nuts, seeds, legumes, and beans; fruit; vegetables; and dairy products including milk, yogurt, cheese, and/or alternatives. The circled data points indicate the mean nutrient composition of the food group. The dashed line represents the estimated energy requirements based on the basal metabolic rate ratio of the average adult from the NNPAS assuming equilibrium and a physical activity level of 1.4 (9510 kJ). Polygon area: Acceptable Macronutrient Distribution Range (AMDR) for Australians and New Zealanders [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
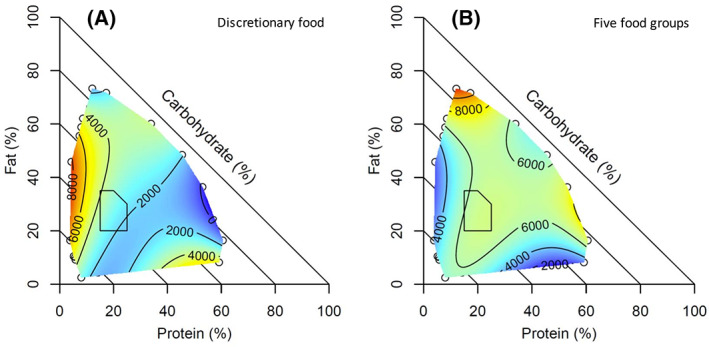
Surface plots showing the relationship between daily dietary macronutrients and total daily energy (kJ) from (A) discretionary foods and (B) five food group foods plotted on percentage energy from macronutrients (lower intake represented with cooler colors, i.e., blue, and higher intake represented with warmer colors, i.e., red). Polygon area: Acceptable Macronutrient Distribution Range (AMDR) for Australians and New Zealanders [Color figure can be viewed at wileyonlinelibrary.com]
Figure 5 shows the dietary macronutrient composition for respondents separated into low, intermediate, or high levels (tertiles) of discretionary food intake. Total energy intake increased with increasing discretionary food intake and it was 7638 kJ and 9772 kJ for the lowest and highest tertile consumers, respectively. Absolute protein intake was maintained almost constant at ~1500 kJ across all tertiles of discretionary foods. Those with the greater consumption of five food groups foods (i.e., lowest percentage energy from discretionary foods) had higher percentage energy from protein, lower nonprotein energy, and the lowest total energy intake. In comparison, those who consumed the most discretionary foods had the lowest percentage energy from protein and higher absolute nonprotein energy and total energy intakes.
FIGURE 5.
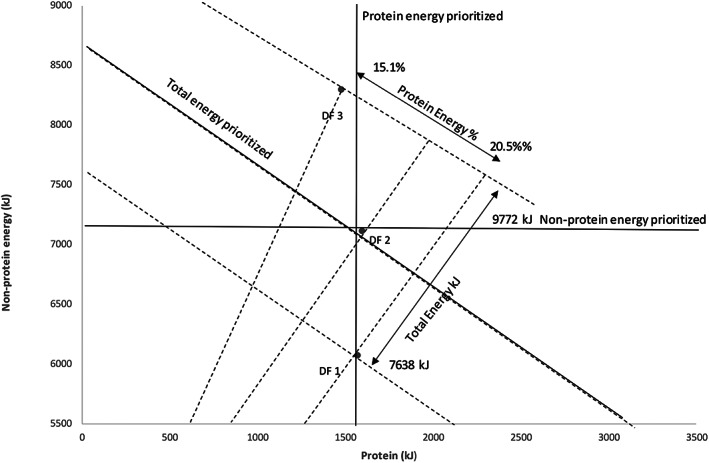
Protein and nonprotein energy intakes by respondent group of discretionary food intake. If the respondents prioritized total energy intake, regardless of its macronutrient source, the data would align along a negative‐sloped diagonal representing constant energy intake (x + y = constant); if nonprotein energy was prioritized, the data would align along a horizontal line (y = constant); and if protein was prioritized, the data would align along a vertical line (x = constant). The analysis shows that the respondents maintained absolute protein intake relatively tightly, with nonprotein energy intake varying more passively with dietary macronutrient ratios. Protein density decreased with larger intake of discretionary foods (ranging from 20.5% for respondents categorized as discretionary food consumption tertile 1 to 15.1% for respondents from tertile 3), and total energy intake increased (from 7638 kJ for tertile 1 to 9772 kJ for tertile 3). Data are adjusted for age, sex, Socio‐Economic Indexes for Areas, country of birth, energy intake to basal metabolic rate ratio.
DISCUSSION
The persistent rise of obesity and diabetes despite immense research effort to find solutions has stimulated robust debate around the relative merits of different kinds of evidence used in nutritional research [19]. A key finding from our analysis of dietary surveillance data is that absolute energy intake varied inversely with dietary percentage energy from protein, as predicted (our prediction ii), if a strong human appetite for protein drove the overconsumption of fat and carbohydrates in protein‐dilute diets (protein leverage). This on its own is not definitive evidence for the PLH, because there are alternative plausible explanations. For example, the hyperpalatability of aggressively marketed low‐protein, energy‐dense, industrially processed foods could account for the correlation independent of protein appetite or indeed for the opposite reason to PLH, namely that excess energy intake is driven not by a strong appetite for protein but strong appetites for fat and carbohydrates. Additionally, relationships in population data could be affected by the degree of variance and covariance among estimated measures of nutrient and energy intakes [20].
Although such alternative explanations may contribute to patterns seen in population data, many sources of evidence independently point to a dominant protein appetite interacting with dietary protein dilution as a driver of energy overconsumption [5]. In addition to several randomized controlled trials in human diets, this has been observed in experiments in laboratory animals and in nonhuman primates in the wild, including our closest living relatives, chimpanzees (reviewed in [4, 5]). In laboratory model systems, the mechanisms of protein appetite are increasingly understood, both in invertebrates [21, 22] and mammals, in which it has been shown that fibroblast growth factor 21 is the circulating signal of low protein status in humans and rodents, acting in the brain to stimulate protein appetite [23, 24].
Our analysis of daily dietary trajectories (Figure 1) is also consistent with a specific appetite for protein driving the regulation of protein intake (our prediction i), which is the key mechanistic component of protein leverage [5, 7]. Respondents who reported proportional protein intakes lower or higher than the AMDR range of 15% to 25% at subsequent eating periods showed a compensatory intake of higher and lower protein, respectively, whereas those who started within the AMDR range remained there throughout the day. As with the protein leverage effect, this too is potentially subject to confounds but it has independently been demonstrated in randomized controlled trials. In one study, participants consistently selected a diet of approximately 15% energy from protein [8], a value that corresponds closely with results of a recent comparative analysis using national survey data which showed consistency of protein intake at approximately 15% of energy across US demographic groups as well as 13 countries with gross domestic products >$10,000 per capita per annum [25]. A recent experimental study demonstrated that higher protein intake reduced subsequent protein intake and that it was regulated meal by meal, supporting our hypothesis [26].
There is thus significant evidence that, firstly, humans regulate the percentage of dietary energy contributed by protein to within a relatively narrow range and, secondly, that low‐protein diets are associated with increased energy consumption via protein leverage. This set of observations raises important questions concerning how and why the diet balancing mechanisms are overridden to cause humans to select protein‐dilute diets in obesogenic food environments. In addressing this question, it is important to bear in mind that macronutrient balancing and ingestion of low‐protein diets that lead to energy hyperphagia are not mutually exclusive, because homeostatic regulation can be significant but incomplete owing to other factors that influence dietary intake. A potential illustration of this is our finding that respondents whose diets at the first eating period were below the AMDR and above the AMDR both compensated by increasing and decreasing proportional protein intake, respectively, at subsequent eating periods, which as discussed previously is consistent with nutrient balancing. That homeostatic response was, however, incomplete, as the cumulative intakes over the full day of the two groups remained lower and higher than the average, respectively, despite the compensatory response. This finding raises the possibility that even transient diversions from a macronutrient balanced diet could have lasting and cumulative effects on energy intake, suggesting an important avenue for experimental research. It also raises the ecological question of which factors might be associated with the ingestion, either transient or sustained, of protein‐dilute diets.
A first step toward addressing that question is identifying the categories of foods associated with dietary protein dilution. As predicted (prediction iii), our analysis implicates highly processed discretionary foods as a likely cause of protein dilution. This category of foods clustered disproportionately within the low‐protein region of macronutrient space (Figure 3), and their contribution to the daily diet correlated positively with total fat, carbohydrates, and total energy intakes. That there was, in contrast, no effect of these foods on absolute protein intake is consistent with the mechanism through which processed foods translate into excess energy intake being protein leverage. The same pattern was observed independently in an analysis of the US NHANES diet data [14]. This result is also consistent with a recent randomized controlled trial that found that inpatients who were provided ultraprocessed diets showed no difference in absolute protein intake relative to a control group on an unprocessed diet, but they ingested significantly more fat, carbohydrates, and total energy and gained more weight during the 14‐day trial [15].
Several factors have been identified that predispose to the consumption of highly processed industrial foods, including their hyperpalatability, relatively cheap price, convenience, aggressive marketing, their ubiquity in food environments, and corporate political activity interfering with public health policy [27]. A particularly insidious proposed mechanism is the “protein decoy effect,” in which homeostatic protein seeking responses are diverted by cheap, abundant, fat‐ and carbohydrate‐rich, umami‐flavored, savory snack foods, which exacerbate rather than ameliorate the protein deficiency they are selected to redress [28].
Our analysis thus suggests a model in which characteristics of industrial manufactured foods such as their low cost and hyperpalatability influence the selection of these foods over whole‐food alternatives, with the result that their high fat and carbohydrate content dilutes dietary protein. This triggers a combination of protein seeking and compensatory intake, in which fat and carbohydrates are overingested as a homeostatic response to maintain protein intake at the target level in the face of a protein‐dilute diet (protein leverage). The effect is exacerbated by other dimensions of industrial food manufacturing, including their high energy density due to low‐fiber and umami‐flavored protein decoys subverting the selection of high‐protein alternatives.
As noted earlier, like any analysis of population data, our study is susceptible to confounds and artifacts. However, there are several factors that suggest the results and conclusions are credible. First, our study was strongly prediction driven and not based on a posteriori rationalization of statistically significant patterns. Second, all our key predictions (macronutrient balancing, protein leverage, and the role of highly processed foods) are consistent with results of previous randomized controlled trials and mechanistic evidence. Third, many of the results have been observed separately in other population studies and other contexts. Finally, the results fit into a coherent model proposing a plausible mechanism to explain the well‐established association between ultraprocessed foods and energy overconsumption, obesity, and poor health [29].
We stress that our findings should not be interpreted as an indictment of low‐protein diets per se or as an endorsement of habitual diets that exceed the recommended proportion of dietary energy from protein. High‐protein diets were predicted by our analysis (Figure 2B), and demonstrated in many other studies, to be associated with low energy intake, and they could play an important role in weight loss [30]. However, these diets were also associated with excess protein intake (Figure 2G), and several sources of evidence suggest that chronic exposure to high protein intake (especially when paired with low carbohydrates) accelerate the rate of aging (notably during middle and early late‐life) and reduce health span [31, 32], an effect that might be associated particularly with animal‐derived proteins [33]. Conversely, several populations with exceptionally good health and long life‐spans, such as the Traditional Okinawan, Blue Zone Mediterranean, and Kitivan Islander populations, have diets with low proportional protein, in the order of 10% [2].
Congruently, despite low protein levels, these populations do not have a high incidence of obesity, which seemingly contradicts the prediction of our model. A key difference, however, is that in obesogenic food environments such as Australia and the US [14], protein is diluted by industrially manufactured foods that are low in fiber and high in refined carbohydrates [34]. In contrast, traditional low‐protein diets are rich in whole foods, and the principal diluent of protein is complex carbohydrates, including resistant starches, derived from fiber‐rich plant foods [35]. It is likely that, in these diets, the satiating effect of fiber mitigates reduced satiation of low protein, and emerging evidence suggests that complex carbohydrates are metabolically healthier and less obesogenic than refined simple carbohydrates [36]. On close inspection, our analysis is consistent with this. Figure 2B shows that when dietary percentage protein was low, energy hyperphagia was particularly pronounced midway up the fat axis, in the region corresponding to the composition of processed discretionary foods (Figure 3A), compared with the region closer to the origin corresponding to high‐carbohydrate plant foods (grains, fruit, and some vegetables; Figure 3). Indeed, in this latter region, diets with protein content of 10% intersected with the energy equilibrium line, suggesting that these diets would not be associated with energy overconsumption.
In addition to its direct relevance for understanding the causes of obesity, our study addresses an important issue regarding the use of evidence in nutrition science. Nutritional epidemiology has been criticized on several grounds, including the accuracy of dietary measures, bias, its correlative nature, and its vulnerability to confounds, even considered by some as “pseudoscience” [37]. Others have noted that many of these criticisms arise from a misunderstanding of the role of diet surveillance data, and alternative sources of evidence, such as randomized controlled trials and meta‐analyses, often considered the gold standard in evidence, introduce problems of their own. Randomized controlled trials have been criticized for framing diets within a drug trial paradigm, which is inappropriate because nutritional exposures are substantially more complex than pharmaceutical treatments [38]. Meta‐analysis has been criticized based on variation in the design, context, and populations across nutritional studies and as being “weighted averages of expert opinions” [39]. Our analysis supports the view that observational and experimental evidence should not be regarded as competing but as complementary sources of evidence, and the greatest confidence is provided when there is congruence in the results from several sources [40, 41, 42]. This approach is common in the evolutionary and ecological sciences, where direct evidence can be difficult to obtain, and related views have previously been expressed for human nutrition science [43, 44, 45].
Our application of an ecological approach to analyze observational diet surveillance data shows tight congruence with experimental studies and other sources of evidence. While this provides supporting evidence for protein leverage, its primary value is that it suggests the protein leverage mechanism is not only real (as established in experimental studies and corroborated here), but also relevant in free‐living context; that is, it directly tests the PLH [5]. Together, causation and relevance provide a strong foundation for evidence‐based nutrition.
AUTHOR CONTRIBUTIONS
Conceptualization: Zhixian Sui, Sean C P Coogan, David Raubenheimer. Methodology: Amanda Grech, Zhixian Sui, Anna Rangan, Stephen J Simpson, Sean C P Coogan, David Raubenheimer. Investigation: Amanda Grech, Zhixian Sui, Anna Rangan, Stephen J Simpson, Sean C P Coogan, David Raubenheimer. Visualization: Amanda Grech, Zhixian Sui. Funding acquisition: Anna Rangan, Stephen J Simpson, David Raubenheimer. Supervision: Anna Rangan, David Raubenheimer. Writing (original draft): Zhixian Sui, Sean C P Coogan. Writing (review and editing): Amanda Grech, Zhixian Sui, Anna Rangan, Stephen J Simpson, Sean C P Coogan, David Raubenheimer.
FUNDING INFORMATION
This research was funded by grants from the National Health and Medical Research Council (NHMRC) Nutrition and Complexity Program Grant (GNT1149976) and by Meat and Livestock Australia. The funding bodies had no input into the results presented in the current analysis.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
Supporting information
Figure S1. Reported average energy intake throughout the day (whole hour).
Figure S2. Surface plots showing the relationship between daily dietary macronutrient distributions and total energy intake for plausible reporters, aged 19 years and over, using the average of two 24‐hour recalls from the National Nutrition and Physical Activity Survey, 2011–2012 (n = 4271).
Figure S3. Frequency of percentage energy from protein (%E) for adults from the National Nutrition and Physical Activity Survey.
Table S1. Macronutrients and food intakes by respondents reported protein density below, within, or above the AMDR.
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
The authors thank the Australian Bureau of Statistics for providing access to the Australian National Nutrition and Physical Activity Survey data set. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Grech A, Sui Z, Rangan A, Simpson SJ, Coogan SCP, Raubenheimer D. Macronutrient (im)balance drives energy intake in an obesogenic food environment: An ecological analysis. Obesity (Silver Spring). 2022;30(11):2156‐2166. doi: 10.1002/oby.23578
Funding information The Meat and Livestock Association; The National Health and Medical Research Council, Australia, Nutrition and Complexity Program Grant, Grant/Award Number: GNT1149976
Contributor Information
Amanda Grech, Email: amanda.grech@sydney.edu.au.
David Raubenheimer, Email: david.raubenheimer@sydney.edu.au.
DATA AVAILABILITY STATEMENT
All data are available upon request from the Australian Bureau of Statistics. Code and materials used in the analysis are available from the researchers.
REFERENCES
- 1.GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393:1958‐1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raubenheimer D, Simpson SJ. Nutritional ecology and human health. Annu Rev Nutr. 2016;36:603‐626. [DOI] [PubMed] [Google Scholar]
- 3. Lang T, Rayner G. Overcoming policy cacophony on obesity: an ecological public health framework for policymakers. Obes Rev. 2007;8(suppl 1):165‐181. [DOI] [PubMed] [Google Scholar]
- 4. Simpson SJ, Raubenheimer D. The Nature of Nutrition: A Unifying Framework From Animal Adaptation to Human Obesity. Princeton University Press; 2012. [Google Scholar]
- 5. Raubenheimer D, Simpson SJ. Protein leverage: theoretical foundations and ten points of clarification. Obesity (Silver Spring). 2019;27:1225‐1238. [DOI] [PubMed] [Google Scholar]
- 6. Gosby AK, Conigrave AD, Lau NS, et al. Testing protein leverage in lean humans: a randomised controlled experimental study. PloS One. 2011;6:e25929. doi: 10.1371/journal.pone.0025929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simpson SJ, Raubenheimer D. Obesity: the protein leverage hypothesis. Obes Rev. 2005;6:133‐142. [DOI] [PubMed] [Google Scholar]
- 8. Campbell CP, Raubenheimer D, Badaloo AV, et al. Developmental contributions to macronutrient selection: a randomized controlled trial in adult survivors of malnutrition. Evol Med Public Health. 2016;1:158‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simpson SJ, Batley R, Raubenheimer D. Geometric analysis of macronutrient intake in humans: the power of protein? Appetite. 2003;41:123‐140. [DOI] [PubMed] [Google Scholar]
- 10. Martens E, Lemmens S, Westerterp‐Plantenga M. No difference in protein leverage affecting energy intake between soy and whey protein [Experimental Biology abstract]. FASEB J. 2013;27(suppl 1):1075.8. [Google Scholar]
- 11. Martens EA, Lemmens SG, Westerterp‐Plantenga MS. Protein leverage affects energy intake of high‐protein diets in humans. Am J Clin Nutr. 2013;97:86‐93. [DOI] [PubMed] [Google Scholar]
- 12. Hall KD. The potential role of protein leverage in the US obesity epidemic. Obesity (Silver Spring). 2019;27(8):1222‐1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yancy WS Jr, Wang CC, Maciejewski ML. Trends in energy and macronutrient intakes by weight status over four decades. Public Health Nutr. 2014;17:256‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steele EM, Raubenheimer D, Simpson SJ, Baraldi LG, Monteiro CA. Ultra‐processed foods, protein leverage and energy intake in the USA. Public Health Nutr. 2018;21:114‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hall KD, Ayuketah A, Brychta R, et al. Ultra‐processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30:67‐77.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Australian Bureau of Statistics . Australian Health Survey: Users' Guide, 2011–13. Australian Government Publishing Service, Australian Bureau of Statistics; 2013. [Google Scholar]
- 17. Barnett AG, Van Der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34:215‐220. [DOI] [PubMed] [Google Scholar]
- 18. Elango R, Humayun MA, Ball RO, Pencharz PB. Evidence that protein requirements have been significantly underestimated. Curr Opin Clin Nutr Metab Care. 2010;13:52‐57. [DOI] [PubMed] [Google Scholar]
- 19. Satija A, Yu E, Willett WC, Hu FB. Understanding nutritional epidemiology and its role in policy. Adv Nutr. 2015;6:5‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park Y, Dodd KW, Kipnis V, et al. Comparison of self‐reported dietary intakes from the automated self‐administered 24‐h recall, 4‐d food records, and food‐frequency questionnaires against recovery biomarkers. Am J Clin Nutr. 2018;107:80‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Münch D, Ezra‐Nevo G, Francisco AP, Tastekin I, Ribeiro C. Nutrient homeostasis ‐ translating internal states to behavior. Curr Opin Neurobiol. 2020;60:67‐75. [DOI] [PubMed] [Google Scholar]
- 22. Kim B, Kanai MI, Oh Y, et al. Response of the microbiome‐gut‐brain axis in drosophila to amino acid deficit. Nature. 2021;593:570‐574. [DOI] [PubMed] [Google Scholar]
- 23. Hill CM, Qualls‐Creekmore E, Berthoud HR, et al. FGF21 and the physiological regulation of macronutrient preference. Endocrinology. 2020;161:bqaa019. doi: 10.1210/endocr/bqaa019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hill CM, Laeger T, Dehner M, et al. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Cell Rep. 2019;27:2934‐2947.e2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lieberman HR, Fulgoni VL III, Agarwal S, Pasiakos SM, Berryman CE. Protein intake is more stable than carbohydrate or fat intake across various US demographic groups and international populations. Am J Clin Nutr. 2020;112:180‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cabeza de Baca T, Piaggi P, Gluck ME, Krakoff J, Votruba SB. Meal‐to‐meal and day‐to‐day macronutrient variation in an ad libitum vending food paradigm. Appetite. 2022;171:105944. doi: 10.1016/j.appet.2022.105944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fardet A, Lakhssassi S, Briffaz A. Beyond nutrient‐based food indices: a data mining approach to search for a quantitative holistic index reflecting the degree of food processing and including physicochemical properties. Food Funct. 2018;9:561‐572. [DOI] [PubMed] [Google Scholar]
- 28. Simpson SJ, Raubenheimer D. Perspective: tricks of the trade. Nature. 2014;508:S66. doi: 10.1038/508S66a [DOI] [PubMed] [Google Scholar]
- 29. Pagliai G, Dinu M, Madarena MP, Bonaccio M, Iacoviello L, Sofi F. Consumption of ultra‐processed foods and health status: a systematic review and meta‐analysis. Br J Nutr. 2021;125:308‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clifton PM, Keogh JB, Noakes M. Long‐term effects of a high‐protein weight‐loss diet. Am J Clin Nutr. 2008;87:23‐29. [DOI] [PubMed] [Google Scholar]
- 31. Senior AM, Solon‐Biet SM, Cogger VC, et al. Dietary macronutrient content, age‐specific mortality and lifespan. Proc Biol Sci. 2019;286:20190393. doi: 10.1098/rspb.2019.0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Solon‐Biet SM, McMahon AC, Ballard JWO, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum‐fed mice. Cell Metab. 2014;19:418‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang J, Liao LM, Weinstein SJ, et al. Association between plant and animal protein intake and overall and cause‐specific mortality. JAMA Intern Med. 2020;180:1173‐1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baker Machado P, Santos T, Sievert K, et al. Ultra‐processed foods and the nutrition transition: global, regional and national trends, food systems transformations and political economy drivers. Obes Rev. 2020;21:e13126. doi: 10.1098/rspb.2019.0393 [DOI] [PubMed] [Google Scholar]
- 35. Raubenheimer D, Gosby AK, Simpson SJ. Integrating nutrients, foods, diets, and appetites with obesity and cardiometabolic health. Obesity (Silver Spring). 2015;23:1741‐1742. [DOI] [PubMed] [Google Scholar]
- 36. Wali JA, Raubenheimer D, Senior AM, Le Couteur DG, Simpson SJ. Cardio‐metabolic consequences of dietary carbohydrates: reconciling contradictions using nutritional geometry. Cardiovasc Res. 2020;117:386‐401. [DOI] [PubMed] [Google Scholar]
- 37. Mitka M. Do flawed data on caloric intake from NHANES present problems for researchers and policy makers? JAMA. 2013;310:2137‐2138. [DOI] [PubMed] [Google Scholar]
- 38. Ludwig DS, Ebbeling CB, Heymsfield SB. Improving the quality of dietary research. JAMA. 2019;322:1549‐1550. [DOI] [PubMed] [Google Scholar]
- 39. Barnard ND, Willett WC, Ding EL. The misuse of meta‐analysis in nutrition research. JAMA. 2017;318:1435‐1436. [DOI] [PubMed] [Google Scholar]
- 40. Biglan A, Johansson M, Van Ryzin M, Embry D. Scaling up and scaling out: consilience and the evolution of more nurturing societies. Clin Psychol Rev. 2020;81:101893. doi: 10.1016/j.cpr.2020.101893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brittan G, Bandyopadhyay PS. Ecology, evidence, and objectivity: in search of a bias‐free methodology. Front Ecol Evol. 2019;7:399. doi: 10.1016/j.cpr.2020.101893 [DOI] [Google Scholar]
- 42. Ruse M. Darwin's debt to philosophy: an examination of the influence of the philosophical ideas of John FW Herschel and William Whewell on the development of Charles Darwin's theory of evolution. Stud Hist Philos Sci. 1975;6:159‐181. [DOI] [PubMed] [Google Scholar]
- 43. Alpers DH, Bier DM, Carpenter KJ, et al. History and impact of nutritional epidemiology. Adv Nutr. 2014;5:534‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mozaffarian D, Forouhi NG. Dietary guidelines and health—is nutrition science up to the task? BMJ. 2018;360:k822. doi: 10.1136/bmj.k822 [DOI] [PubMed] [Google Scholar]
- 45. Brown AW, Aslibekyan S, Bier D, et al. Toward more rigorous and informative nutritional epidemiology: the rational space between dismissal and defense of the status quo [published online October 22, 2021]. Crit Rev Food Sci Nutr. doi: 10.1080/10408398.2021.1985427 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Reported average energy intake throughout the day (whole hour).
Figure S2. Surface plots showing the relationship between daily dietary macronutrient distributions and total energy intake for plausible reporters, aged 19 years and over, using the average of two 24‐hour recalls from the National Nutrition and Physical Activity Survey, 2011–2012 (n = 4271).
Figure S3. Frequency of percentage energy from protein (%E) for adults from the National Nutrition and Physical Activity Survey.
Table S1. Macronutrients and food intakes by respondents reported protein density below, within, or above the AMDR.
Appendix S1. Supporting Information.
Data Availability Statement
All data are available upon request from the Australian Bureau of Statistics. Code and materials used in the analysis are available from the researchers.


