ABSTRACT
The SARS-CoV-2 pandemic still represents a threat for immunosuppressed and hematological malignancy (HM) bearing patients, causing increased morbidity and mortality. Given the low anti-SARSCoV-2 IgG titers post-vaccination, the COVID-19 threat prompted the prophylactic use of engineered anti-SARS-CoV-2 monoclonal antibodies. In addition, potential clinical significance of T cell responses has been overlooked during the first waves of the pandemic, calling for additional in-depth studies. We reported that the polarity and the repertoire of T cell immune responses govern the susceptibility to SARS-CoV-2 infection in health care workers and solid cancer patients. Here, we longitudinally analyzed humoral and cellular immune responses at each BNT162b2 mRNA vaccine injection in 47 HM patients under therapy. Only one-third of HM, mostly multiple myeloma (MM) bearing patients, could mount S1-RBD-specific IgG responses following BNT162b2 mRNA vaccines. This vaccine elicited a S1-RBD-specific Th1 immune response in about 20% patients, mostly in MM and Hodgkin lymphoma, while exacerbating Th2 responses in the 10% cases that presented this recognition pattern at baseline (mostly rituximab-treated patients). Performing a third booster barely improved the percentage of patients developing an S1-RBD-specific Th1 immunity and failed to seroconvert additional HM patients. Finally, 16 patients were infected with SARS-CoV-2, of whom 6 developed a severe infection. Only S1-RBD-specific Th1 responses were associated with protection against SARS-CoV2 infection, while Th2 responses or anti-S1-RBD IgG titers failed to correlate with protection. These findings herald the paramount relevance of vaccine-induced Th1 immune responses in hematological malignancies.
KEYWORDS: COVID-19, vaccination, T cell
Introduction
Patients with hematologic malignancies (HM) have been severely hit by the COVID-19 pandemic caused by the severe acute respiratory syndrome coronavirus 2 of the genus Betacoronavirus (SARS-CoV-2). The SARS-CoV-2-related mortality in this population is consistently reported at about 30 %.1–3 These are rates observed before the vaccination era and in the context of wild-type and alpha variants of SARS-CoV-2.
We recently demonstrated that individuals susceptible to SARS-CoV-2 infection exhibit a deficit in the T helper 1 (Th1) peptide repertoire affecting the receptor-binding domain (RBD) of the spike protein.4 Therefore, mounting a Th1 memory response following vaccination is probably crucial to ensure efficient protection against SARS-CoV-2 infection. However, much of the focus in vaccine response has focused on the capacity of antiviral vaccines to elicit neutralizing antibodies, with less emphasis on their potential to induce T cell differentiation and activation.
Several reports exploring the humoral responses following mRNA vaccination in immunocompromised patients heralded low IgG anti-CoV-2 titers.5–8 Given the poor prognosis of SARS-CoV-2 infected HM patients, immunization modalities have evolved toward a reinforced priming-boost regimen, starting from two priming injections up to three priming doses with a “booster” recall injection (for a total of 4 doses). However, most of these works failed to deeply investigate T cell responses and their clinical significance during mRNA vaccination.
In this study, we performed a longitudinal monitoring of BNT162b2 vaccine-induced SARS-CoV-2 specific humoral and Th1/Th2 T cell responses in 47 HM patients during the course of their cancer therapy with the aim to identify the most reliable immunological correlate with protection against COVID-19. We found that BNT162b2 vaccine was poorly immunogenic in HM patients, with less than one-third of patients developing S1-RBD-specific IgG, Th1 or Th2 responses. While the third priming dose did not increase the percentage or titers of seroconversion, it slightly tended to augment the magnitude of Th1 cell responses. Patients failing to mount a humoral or cellular immune response following the three priming doses did not benefit from the booster dose. S1-RBD-specific Th1 immunity but not Th2 nor humoral immune responses represented the most suitable correlate of protection against SARS-CoV-2 infection in HM patients.
Methods
Patient and cohort characteristics
Forty-seven patients diagnosed with a variety of HM were included (Table 1) in the ONCOVID – Cohort 6 – clinical trial (ethic protocol number EudraCT: 2020–001250-21, https://clinicaltrials.gov/ct2/show/NCT04341207), between March 5, 2021, and April 22, 2021. This study was conducted after written informed consent in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki. These patients were all actively treated for their HM disease following standard-of-care, receiving either chemotherapy, anti-CD20 therapy alone or in association with chemotherapy, anti-CD38 therapy, or immunosuppressive drugs post allogeneic hematopoietic stem cell transplantation. Patients received a minimum of 2 priming BNT162b2 injections. A third priming injection and a booster injection (5 months minimum after the last priming dose) were systematically offered to all patients. Visit 1 (V1) corresponds to the day of the first priming vaccine dose. V2, V3, and V4 are, respectively, the second, the third priming dose, and a consultation without immunization 1 month after the previous one. Finally, V5 corresponds to a consultation 1 month after the booster immunization dose. Blood samples were collected in heparinized tubes at five different time points (illustrated in Figure 1b). We longitudinally monitored the occurrence of SARS-CoV-2 infection by asking at each hospital visit or by telephone, on a monthly basis, whether the patient had been infected with SARS-CoV-2, as assessed by the PCR routine test or an antigenic test. The last follow-up took place on August 17, 2022.
Table 1.
Patient characteristics in the two groups based on SARS-CoV-2 non-infected and infected patients with hematological malignancies. Comparisons between SARS-CoV-2 infected versus uninfected patients were performed using unpaired Student t-tests and Fisher exact tests, respectively, for quantitative and qualitative variables.
| No CoV-2 infection. n = 31 |
CoV-2 infection. n = 16 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | n | (%) | n | (%) | n | (%) | p value | |
| Gender | ||||||||
| Female | 24 | 51.1 | 15 | 48.4 | 9 | 56.3 | 0.760 | |
| Male | 23 | 48.9 | 16 | 51.6 | 7 | 43.7 | ||
| Age (years). mean ± SEM | 63.3 ± 17.1 | 66.9 ± 13.9 | 57.5 ± 19.8 | 0.013 | ||||
| range (18–92) | ||||||||
| Number of anti CoV-2 vaccine shots. mean ± SEM | range (2–4) | 3.4 ± 0.6 | 3 ± 0.7 | 3 ± 0.7 | 0.723 | |||
| Anti CoV-2 vaccination boost* | 34 | 72.3 | 21 | 67.7 | 13 | 81.3 | 0.494 | |
| Hematological malignancies | 0.940 | |||||||
| B cell non Hodgkin lymphoma | 21 | 44.8 | 13 | 42 | 8 | 50 | ||
| Hodgkin lymphoma | 8 | 17.0 | 5 | 16.1 | 3 | 18.7 | ||
| Multiple myeloma | 8 | 17.0 | 5 | 16.1 | 3 | 18.7 | ||
| Acute myeloid leukemia | 5 | 10.6 | 4 | 12.9 | 1 | 6.3 | ||
| Others | 5 | 10.6 | 4 | 12.9 | 1 | 6.3 | ||
| Ongoing therapy | 0.736 | |||||||
| Chemotherapy | 16 | 34.0 | 11 | 35.5 | 5 | 31.3 | ||
| Chemotherapy + Rituximab | 15 | 31.9 | 10 | 32.2 | 5 | 31.3 | ||
| Daratumumab based regimen | 7 | 14.9 | 4 | 12.9 | 3 | 18.7 | ||
| Rituximab alone | 6 | 12.8 | 3 | 9.7 | 3 | 18.7 | ||
| Others | 3 | 6.4 | 3 | 9.7 | 0 | 0 | ||
| Cancer status at vaccination | Controlled disease | 39 | 82.9 | 26 | 83.9 | 13 | 81.3 | 0.821 |
| Uncontrolled disease | 8 | 17.1 | 5 | 16.1 | 3 | 18.7 | ||
| Known COVID19 infection before vaccination | 3 | 6.4 | 1 | 3.2 | 2 | 12.5 | 0.264 | |
| CoV-2 infection characteristics | ||||||||
| time from last vaccine injection, month. mean ± SEM | 2.59 ± 2.70 | |||||||
| delta CoV-2 | 6 | 37.5 | ||||||
| omicron CoV-2 | 10 | 62.5 | ||||||
Figure 1.
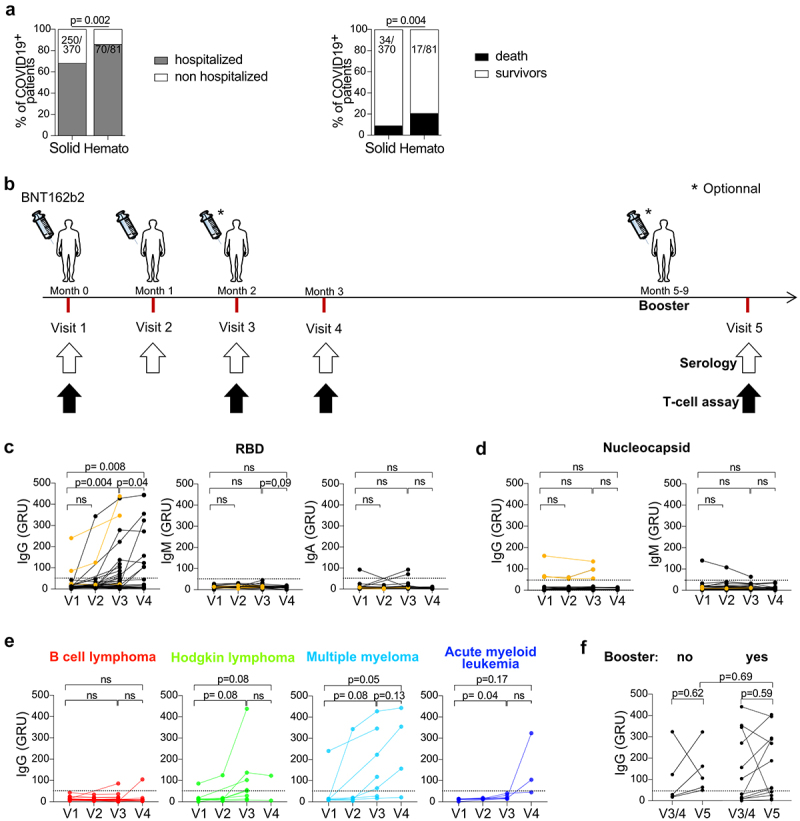
Antibody responses after BNT162b2 injections in HM patients. a. SARS-CoV-2 related hospitalization rates and mortality rates observed in Gustave Roussy Cancer Campus from March 2020 to February 2021, prior to vaccination, in patients with solid tumors and hematologic malignancies. b. Vaccination schedule and sample collection. Study participants received two or three priming doses of BNT162b2 on months 0, 1 and 2 and a booster dose at least 5 months after the last priming dose. Sera and PBMC were obtained on month 0 (pre-priming), 1, 2 and 3 and one month after the booster dose. c. Anti-CoV-2 S1-RBD IgG, IgM and IgA levels were measured in the sera during the priming dose injections. d. Anti-CoV-2 nucleocapsid (NC) IgG and IgM levels were measured in the sera. The three HM patients previously infected with SARS-CoV-2 are depicted in orange. e-f. Anti-CoV-2 S1-RBD IgG levels are shown according to HM type (e) and booster dose (f) (yes, n = 12, no, n = 5). Chi2 test analysis was performed for a. Paired student t-tests were performed for c-f.
GRU: Genalyte Reactive Unit.
Anti-SARS-CoV-2 immunoglobulin measurements
Serologic analyses of SARS-CoV-2–specific IgA, IgM, and IgG antibodies were measured in 173 serum samples from 47 patients with the Maverick SARS-CoV-2 Multi-Antigen Serology Panel (Genalyte) according to the manufacturer’s instructions. The Maverick SARS-CoV-2 Multi-Antigen Serology Panel (Genalyte) is able to detect antibodies to SARS-CoV-2 antigens: nucleocapsid (NC) and Spike S1-RBD in a multiplex format based on photonic ring resonance technology. Results are given as Genalyte Reactive Unit (GRU). Fifty GRU are equivalents to 250 Binding antibody units (BAU).
Isolation of PBMCs from fresh peripheral blood
Venous blood samples (20 mL) were collected in heparinized tubes (BD Vacutainer LH 170 U.I.). Less than 8 hours after the blood collection, peripheral blood mononuclear cells (PBMCs) were freshly isolated by the lymphocyte separation medium (Eurobio Scientific) followed by a Ficoll Hypaque density gradient centrifugation according to the manufacturer’s instructions (Leucosep tubes, Greiner; Biocoll, Bio&SELL). PBMCs were aliquoted in 1 mL of cryopreservation medium (CryoStor, STEMCELLS Technologies) in cryovials (two cryovials per patient) and stored in liquid nitrogen.
In vitro stimulation assay with single RBD wild-type and mutated peptides
Lyophilized peptides were dissolved in sterile water and used at 2 ug/mL in RPMI 1640 glutamax media (GIBCO) supplemented with 1% penicillin/streptomycin (GIBCO). Single peptides (24 in total, described in Table S1) were plated in duplicates in 96-well round-bottom TPP-treated culture plates. A pool of wild-type SARS-CoV-2 RBD peptides and a pool of mutated SARS-CoV-2 RBD peptides were plated in duplicate. Peptide plates were then stored at −80°C until use. The day of the experiment, peptide plates were thawed at room temperature. Frozen PBMCs were thawed, washed, and resuspended in RPMI 1640 media (GIBCO). Viability and count were evaluated using a Vi-Cell XR Cell Counter (Beckman Coulter). PBMCs were then plated in RPMI 1640 glutamax media (GIBCO) supplemented with 1% penicillin/streptomycin (GIBCO), with 200 UI/mL rhIL2 (Miltenyi) and 200 UI/mL rhIL15 (Miltenyi) at a cell density of 10 × 103 cells and incubated with each peptide or each pool of peptide at 37°C in 5% CO2. PBMCs were stimulated with 60 ng/mL OKT-3 antibody (Thermo Fisher Scientific, clone OKT3) as positive controls, and PBMCs alone served as negative controls. After 6 hours, 20 uL of human AB serum was added to each well and plates were incubated at 37°C in 5% CO2 for 6 additional days. On day 7, supernatants were harvested and frozen at −80°C. The concentration of IFNγ and IL-5 in the culture supernatant was determined using a commercial ELISA kit (BioLegend: ELISA MAX Deluxe Set Human IFNγ (cat. #430104), ELISA MAX Deluxe Set Human IL5 (cat. #430404)).
Accelerated semi-automatic whole blood peptide-based assay using VIDAS9
Fresh blood collected in heparinized tubes was stimulated for 22 hours at 37°C under 5% CO2 with peptide pools spanning distinctive genomic sequences of the wild-type or mutated RBD sequence (Supplementary Table 2) diluted in IFA solution (bioMérieux). The IFA solution was used as a negative control, and a mitogen (MIT) was used as a positive control. We used 15-mer peptides covering the wild-type or mutated RBD region (Supplementary Table 2). The concentration of IFNγ in the supernatant was measured using the VIDAS automated platform (VIDAS IFNγ RUO, bioMérieux). The positivity range was 0.08 to 0.3 IU/mL, and IFA positivity thresholds were defined at 0.08 IU/ml. The IFNγ response was defined as positive when the IFNγ concentration of the test was above threshold and the negative control was below threshold. All positive controls were ≥1 IU/mL.
Statistical analyses
Group comparisons were performed using nonparametric test with the Wilcoxon–Mann–Whitney test for independent samples and the Wilcoxon signed rank test for paired samples. The comparison of categorical data was performed using the Fisher's exact test. All hypothesis tests were two-sided and considered statistically significant when p < .05. Graphical illustrations were drawn using the standard GraphPad Prism visualization.
Results
BNT162b2 mRNA vaccination in a cohort of HM patient
As reported worldwide across various hospitals,1–3,10 we observed a high rate of moderate or severe SARS-CoV-2 infections among cancer patients at the Gustave Roussy Cancer Campus, Villejuif, France, during the first wave of the pandemic. Indeed, patients with hematological malignancies (HM) had significantly more severe SARS-CoV-2 infections than patients with solid tumors. From March 2020 to February 2021 prior to vaccination, we retrospectively observed an 86% (70/81) SARS-CoV-2-related hospitalization rate in HM patients, compared with 68% (250/370) in solid tumor bearing patients and a significantly higher SARS-CoV-2-related 30-day-mortality rate in HM patients than in solid tumor patients (21%, 17/81, versus 9%, 34/370, Figure 1a).
To better understand the potential causality link between the vaccination introduction and the reduction of mortality after February 2021, we conducted a longitudinal translational research study including 47 patients diagnosed with HM and undergoing anti-cancer treatments, to characterize humoral and T cell responses elicited by the BNT162b2 mRNA vaccine and we prospectively recorded the incidence and severity of SARS-CoV-2 infection in the subsequent waves of the COVID-19 pandemic (up to August 2022) (Figure 1b). Patient characteristics are described in Table 1. In the context of the vaccination campaign, two priming doses of BNT162b2 mRNA vaccine were administered 28 days apart. In accordance with the recommendations of the French Health Authorities, a third priming dose 28 d apart from the last one was offered to HM patients. Twenty patients (42.6%) received two priming doses and 27 patients (57.4%) received three priming doses. A minimum of 5 months later, 34 patients (72.3%) received a booster injection.
We prospectively collected the PBMCs and sera at each priming dose and 1 month after the last dose. We were able to collect PBMCs and sera from 12 patients 1 month after the booster dose, and concomitantly, we also harvested samples from 5 patients who did not receive a booster dose (Figure 1b, visit 5). In parallel, the incidence of SARS-CoV-2 infection and its severity were monitored throughout the study (up to 18 months after initial inclusions).
Humoral responses following BNT162b2 vaccination are impaired in most HM patients
To characterize the B cell response elicited by mRNA vaccination in our cohort of HM patients, we measured anti-SARS-CoV-2 (CoV-2) IgG, IgM, and IgA antibody titers at each visit (Figure 1b). To distinguish the humoral response elicited by mRNA vaccination (encoding the spike protein, including its receptor-binding domain S1-RBD) from that post-SARS-CoV-2 infection (giving rise to potential responses directed against the structural proteins such as the nucleocapsid (NC)), we specifically monitored Ig titers directed against CoV-2 S1-RBD and NC, the latter being increased only after viral infection. BNT162b2 administration led to a significant increase of anti-CoV-2 S1-RBD IgG titers, with no effect on anti-CoV-2 S1-RBD IgM and IgA titers (Figure 1c). In addition, anti-CoV-2 S1-RBD IgG titers were significantly higher after three priming doses than after two priming doses (Figure 1c, left panel). However, the number of patients who developed a robust immune response (defined as >50 GRU, which is equivalent to 250 BAU) did not increase significantly after the third priming injection. These results suggest that the third priming injection provides only a quantitative increase in anti-CoV-2 S1-RBD IgG in HM patients. HM patients who failed to develop a humoral response after two priming injections did not benefit from a third priming injection. As a positive control of viral encounter, the three HM patients who had been infected with SARS-CoV-2 prior to vaccination had high titers of anti-CoV-2 NC IgG at baseline (V1), but low titers of anti-CoV-2 NC IgA and IgM that were not affected by BNT162b2 injections (Figure 1d). Strikingly, we observed that only patients with multiple myeloma were able to achieve a robust anti-CoV-2 S1-RBD IgG response after priming doses of BNT162b2, in contrast to patients with B-cell non-Hodgkin lymphoma, Hodgkin lymphoma, and acute myeloid leukemia (Figure 1e).
We obtained sera from 17 patients at visit 5, of whom 12 patients received a booster dose and 5 did not. We did not observe a significant increase of anti-CoV-2 S1-RBD IgG titers between patients who received the booster and those who did not (figure 1f). In addition, patients who did not mount a humoral response after the priming doses also did not develop a humoral response after the booster dose (figure 1f).
Altogether, only multiple myeloma could mount S1-RBD-specific IgG responses following BNT162b2 mRNA vaccines, while most of the other HM patients appeared refractory, even after four inoculations of the vaccine.
S1-RBD-specific Th1 dominate over Th2 responses after BNT162b2 vaccines in HM patients
To further dissect cellular responses following BNT162b2 injections in HM patients, we developed an in vitro assay, as previously described.4 Fifteen-mer non-overlapping amino acid sequences covering the SARS-CoV-2 S1-RBD domain, as well as positive controls (OKT3) and negative controls (PBMC alone and SARS-CoV-2 nucleocapsid 8), were plated in duplicate in 96 well plates. The selected amino acid sequences included peptides from the wild-type SARS-CoV-2 Wuhan strain and from SARS-CoV-2 variants of concern (alpha to omicron). The complete list of peptides is described in Table S1. Additionally, a pool of wild-type peptides and a pool of their mutant counterparts were plated to measure the overall response to S1-RBD protein. After a 7 d in vitro stimulation of PBMC with each peptide supplemented with IL-2 and IL-15, we monitored IFNγ, and IL-5, proxies for Th1 and Th2 responses, respectively, to examine the polarization of S1-RBD specific T-cell responses (Figure 2a).
Figure 2.
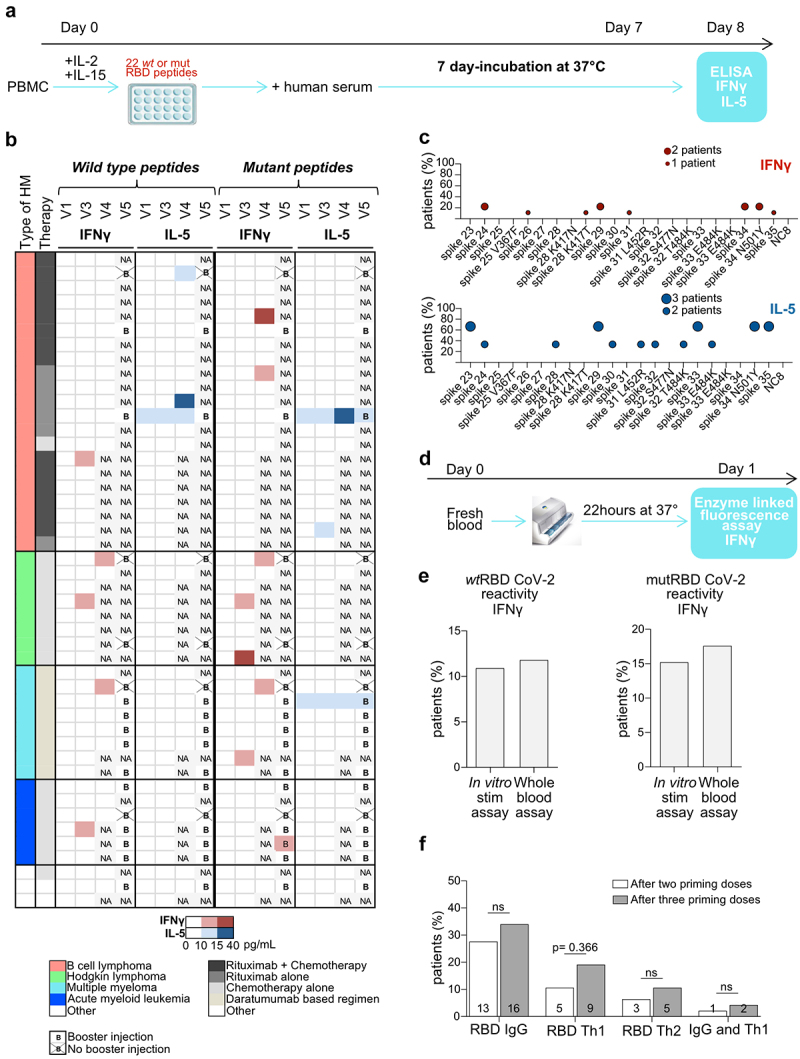
T cell responses after BNT162b2 injections in patients with HM. a. Experimental setting for the in vitro stimulation (IVS) assay based on 22 peptides. b. Heatmap representing cytokine release (IFNγ (red) and IL5 (blue)) detected in IVS assay (a) from PBMC drawn at visits 1, 3, 4 and 5 and stimulated with a pool of wild-type or mutant S1-RBD peptides (Table S1). Results are shown according to HM type, therapy, time and intensity of the response (the color gradient is proportional to the quantity of IFNγ or IL-5 release). c. Idem as in B but showing the reactivity to single peptide contained in the pool as a percentage of reactive patients who developed a S1-RBD specific IFNγ (red) or IL-5 (blue) cytokine release for wild-type and mutant epitopes. d. Experimental setting for the whole-blood automatic stimulation assay. e. Head-on comparison of paired specimen analyzed with the classical (A) or the automatic Vidas assay (d). The percentage of patients who developed IFNγ reactivity to wild-type and mutant peptides using the two different techniques is depicted. The threshold for IFNγ positivity is ≥ 10 pg/mL for in vitro stimulation assay and ≥ 0.08 UI/mL for whole blood assay. f. Analysis of the effects of the booster on humoral and cellular immune responses directed against S1-RBD, by the percentages of positive patients. Student t-test or ANOVA Fischer exact method.
The T cell secretory pattern specific to S1-RBD was more geared toward Th1 (IFNγ) than Th2 (IL-5) production (Figure 2b). Indeed, IFNγ and IL-5 were secreted in 10/46 (22%) and 5/46 patients (11%), respectively. Only three priming doses resulted in a significant increase in IFNγ release against both wild-type or mutated epitopes (Figure S1A-B). With the exception of one patient, the booster dose did not trigger an S1-RBD specific Th1 cell response if it was not initially observed after the priming doses (Figure 2b). In contrast to Th1 responses, IL-5 reactivity was already detectable at baseline (in the absence of prior history of COVID-19) in 2/5 reactive individuals and was acquired or exacerbated during immunization in 4 additional cases (Figure 2b, Figure S1C). Of note, patients with Hodgkin lymphoma (3/8, 37%), multiple myeloma (3/8, 37%), or acute myeloid leukemia (2/6, 33%) tended to mount S1-RBD-specific Th1 responses more efficiently than the B-cell non-Hodgkin lymphoma patients (3/21,14%). Interestingly, all (but one) Th2 responses were obtained from rituximab-treated patients. Moreover, the breadth of peptide recognition coverage was very narrow for the Th1 specificity. Indeed, IFNγ production was directed toward one or two wild-type epitope(s) for responder patients, with poor cross-reactivity with mutated counterparts (Figure 2c). In contrast, the peptide repertoire leading to IL-5 release was somewhat broader and more cross-reactive with mutated epitopes than the Th1 repertoire (Figure 2c).
Strikingly, no Th1 or Th2 memory response could be observed in the three patients who had been previously infected with SARS-CoV-2 before vaccination, despite the presence of high titers of anti-CoV-2 nucleocapsid IgG at baseline.
To further confirm these findings, we reanalyzed wild-type and mutant RBD-specific T-cell reactivities after vaccination (V2 or V3) in 17 HM patients, using a simple 22-h whole-blood stimulation assay allowing the quantitative measurement of IFNγ against two pools of wild-type or mutant S1-RBD peptides using the enzyme-linked fluorescent assay technique in an automated platform (VIDAS IFNγ9, Figure 2d, Table S2). We observed approximately 11% and 15% of IFNγ reactivities against the pool of wild-type and mutated S1-RBD peptides, respectively. Overall, the percentage of IFNγ reactivities against wild-type or mutant S1-RBD peptides remained low whatever the techniques performed.
Of note, only 2% of HM patients developed both anti-S1-RBD IgG and S1-RBD-specific T cell response after two priming doses, increasing to 4% after three priming doses (figure 2f). Interestingly, there was no correlation between lymphocyte counts at baseline or anti-S1-RBD IgG titers and peptide-specific cytokine release (Figure S1C-D). Of note, IFNγ reactivity to the positive control (OKT3) was preserved for almost all B-cell lymphoma patients (20/21), 6/8 Hodgkin lymphoma patients, and 7/8 multiple myeloma patients. However, T cell capability to produce IFNγ in response to OKT3 was reduced in acute myeloid leukemia patients (3/6 patients) (Figure S1E). Patients’ IL-5 release to OKT3 was less prominent than secretion of IFNγ.
Altogether, using two different assays, we showed that the BNT162b2 mRNA vaccine was capable of triggering Th1 immune responses in only 20% of HM patients, mostly Hodgkin lymphoma and multiple myeloma bearing patients with little cross-reactivity against the corresponding mutant epitopes of the VOC. Baseline S1-RBD-specific Th2 responses were mostly found in rituximab-treated B cell lymphoma patients.
Th1 responses are associated with protection against SARS-CoV-2 infection
To determine the clinical significance of these humoral and T-cell responses monitored during the vaccination course, we asked (during a hospital visit or by telephone) to all patients on a monthly basis, for up to 18 months after inclusion, whether they were infected with SARS-CoV-2. Sixteen patients (34%) ultimately developed SARS-CoV-2 infection after full vaccination (Figure 3a), within a mean time of 2.6 months following the last vaccine injection (Table 1). These patients were infected with delta VOC (37.5%) or omicron VOC (62.5%). Of these, 10 (21%) developed mild infection, and 6 (13%) developed severe infection including one patient who died of SARS-CoV-2 infection (Figure 3b). Strikingly, none of the patients who were infected with SARS-CoV-2 had developed S1-RBD-specific Th1 response after vaccination (Figure 3c). The presence of anti-S1-RBD IgG humoral response was not protective against SARS-CoV-2 infection (Figure 3c). Post-vaccine S1-RBD Th1 response appears to be the most suitable surrogate for protective immunity against SARS-CoV-2 infection and morbidity, in contrast to humoral responses. Strikingly, the unique patient who presented a post-vaccine S1-RBD Th2 response and got infected with SARS-CoV-2, had a very severe infection requiring intensive care management, supporting our previous study showing that a disbalance between type 1 and 2 cytokine release was associated with high susceptibility to COVID-19.7 Despite the low number of severe SARS-CoV-2 infection, we could undoubtedly identify that anti-S1-RBD IgG failed to protect against severe SARS-CoV-2 infection, highlighting the necessity to develop a S1-RBD-specific Th1 immunity after vaccination to be protected against SARS-CoV-2 infection and morbidity.
Figure 3.
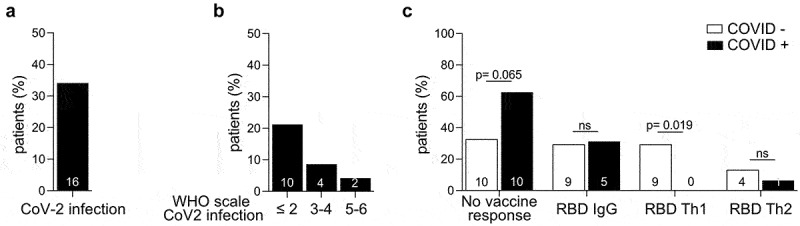
Th1 immunity directed against S1-RBD is protective against COVID-19 in HM patients. a-b. Percentages of HM patients diagnosed with COVID-19 during the prospective study and severity of the disease, according to the WHO scale is depicted. Grade 1: asymptomatic, grade 2: symptomatic without hospitalization, grade 3: symptomatic with hospitalization but without oxygen mask, 4: symptomatic with hospitalization and with oxygen mask, 5: symptomatic with hospitalization in ICU, 6: death. c. Patients were stratified according to their ability to develop a S1-RBD-specific IgG, Th1 or Th2 response (defined respectively as anti-CoV-2 RBD IgG > 50 GRU, IFNγ ≥ 10 pg/mL or IL-5 ≥ 10 pg/mL), after 2 or 3 priming doses. Of note, no patient developed simultaneously S1-RBD specific IgG and Th2 responses. ANOVA Fisher exact method: *p < .05.
Discussion
Here, we report that only one-third of HM patients developed anti-S1-RBD IgG, mainly multiple myeloma patients. This result was expected and is consistent with previous studies.5,6,11–15 Administration of a third priming dose did not significantly increase the number of seroconverted patients. It has been reported that anti-CD20 therapy skewed vaccine responses, compromising circulating follicular helper T (TFH) cell responses while preserving CD8 T cell induction and Th1 cell priming.16 In our cohort, we did observe a lack of humoral response in B cell lymphoma (treated with anti-CD20), as expected, but we did not notice a compensatory IFNγ T cell response. In contrast, B-cell lymphoma patients mounted a S1-RBD-specific Th1 immunity less frequently than Hodgkin lymphoma, multiple myeloma, or acute myeloid leukemia, despite subnormal IFNγ release upon TCR cross-linking (OKT3 stimulation). In fact, despite their small numbers, anti-CD20-treated individuals exhibited exacerbated S1-RBD-specific Th2 responses following immunization.
Of note, we only explored T cell responses against the S1-RBD amino-acid sequences since this T cell repertoire was the only clinically significant one for the protection against COVID-19 in the first waves of the pandemic.4 Hence, we showed that the vaccine elicited a S1-RBD T cell response in approximately 30% of patients. This T cell response was predominantly oriented toward a Th1 response. We could not predict the amplitude of vaccine-induced Th1 responses based on the patient lymphocyte count at baseline nor IgG titers. Finally, the wild-type S1-RBD Th1 immunity was poorly cross-reactive to the VOC-related S1-RBD peptides. Of note, almost all patients responded to TCR cross-linking, suggesting that the lack of immunization against S1-RBD could not be assigned to therapy-induced T cell anergy. Th1 cells can produce IFNγ, IL-2, and TNF and promote macrophage activation, antibody-dependent cell cytotoxicity, delayed type hypersensitivity, and opsonizing and complement-fixing IgG2a antibody production.17 Indeed, none of the 16 patients who were infected with SARS-CoV-2 in our longitudinal cohort succeeded in mounting the canonical S1-RBD specific Th1 response despite vaccination while harboring high titers of anti-S1-RBD IgG. Automated screening of Th1 reactivities is currently under development9,18 and may identify patients in critical need of prophylactic anti-SARS-CoV-2 targeted measures. Of note, none of the patients have received Omicron-specific boosters at the time of the study since these vaccines were not available. This booster should be systematically offered to all hematological patients.
Given the impact of distinct adjuvants on the epigenetic marks of antigen presenting cells,19 one might postulate that alternate vaccination strategies could be more suitable than mRNA nanoparticles to reinstate T cell immunity, depending on the HM subtype. The BioNtech/Pfizer vaccine was shown to activate CD8 + T cell response in a type I interferon-dependent MDA5 signaling20 that might be altered in distinct HM diseases. Such vaccination alternatives have been proposed, including CoVac-1, a peptide-based vaccine candidate endowed with safety and efficacy for Th1 responses.21,22 A phase I–II clinical trial assessing the safety and immunogenicity of SARS-CoV-2 derived multi-peptide vaccine in combination with the TLR1/2 ligand XS15 in adults with congenital or acquired B-cell/antibody deficiency is ongoing (NCT04954469) and may provide important information. Moreover, as the strategy of mRNA vaccines develops exponentially23 with new targets such as influenza- and cytomegalo-viruses, it is of crucial importance to understand the bases of their immunogenicity in immunocompromised individuals, who are likely to be the primary target of these future vaccines.
Supplementary Material
Acknowledgments
LZ laboratory was supported by MALAKOFF HUMANIS, the French Ministry of Health & Solidarity, Association pour la recherche sur le cancer (ARC); Agnès b., Seerave Foundation, the Germano-French ANR Ileobiome - 19-CE15-0029-01 and H2020 ONCOBIOME N°825410, RHU5 “ANR-21-RHUS-0017” IMMUNOLIFE”; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE), the European Union Horizon 2020 ONCOBIOME: Project Number: 825410, Project Acronym: ONCOBIOME, Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001), The Organization for Partnerships in Leukemia. AC was supported by MALAKOFF HUMANIS.
Funding Statement
The author(s) reported that there is no funding associated with the work featured in this article.
Disclosure statement
LZ and CB designed research and wrote the paper. CB, YH, CT, AC, RB, PL, CF, IL, MM performed research, and analyzed the data. EDS and MM provided critical expertise and reagents. AT, CCL, SDB, CF, AB, LD, JL, AM, and VR managed the patient’s information and data. All authors provided critical revision of the manuscript and had final approval of the manuscript for publication.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/2162402X.2022.2163785
References
- 1.Pagano L, Salmanton-García J, Marchesi F, Busca A, Corradini P, Hoenigl M, Klimko N, Koehler P, Pagliuca A, Passamonti F, et al. COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA). J Hematol Oncol. 2021;14(1):168. doi: 10.1186/s13045-021-01177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bange EM, Han NA, Wileyto P, Wileyto P, Kim JY, Gouma S, Robinson J, Greenplate AR, Hwee MA, Porterfield F, Owoyemi O, Naik K.. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med. 2021. Jul;27(7):1280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goubet A-G, Dubuisson A, Geraud A, Danlos F-X, Terrisse S, Silva CAC, Drubay D, Touri L, Picard M, Mazzenga M, et al. Prolonged SARS-CoV-2 RNA virus shedding and lymphopenia are hallmarks of COVID-19 in cancer patients with poor prognosis. Cell Death Differ. 2021;28(12):3297–3315. doi: 10.1038/s41418-021-00817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahrner J-E, Lahmar I, Goubet A-G, Haddad Y, Carrier A, Mazzenga M, Drubay D, Alves Costa Silva C, de Sousa E, Thelemaque C, et al. The polarity and specificity of antiviral t lymphocyte responses determine susceptibility to SARS-CoV-2 infection in patients with cancer and healthy individuals. Cancer Discov. 2022;12(4):958–983. doi: 10.1158/2159-8290.CD-21-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teh JSK, Coussement J, Neoh ZCF, Spelman T, Lazarakis S, Slavin MA, Teh BW. Immunogenicity of COVID-19 vaccines in patients with hematologic malignancies: a systematic review and meta-analysis. Blood Adv. 2022;6(7):2014–2034. doi: 10.1182/bloodadvances.2021006333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agha ME, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to coronavirus disease 2019 messenger RNA vaccines in patients with hematologic malignancies: a need for vigilance in the postmasking era. Open Forum Infect Dis. 2021;8(7):ofab353. doi: 10.1093/ofid/ofab353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung DJ, Shah GL, Devlin SM, Ramanathan LV, Doddi S, Pessin MS, Hoover E, Marcello LT, Young JC, Boutemine SR, et al. Disease- and therapy-specific impact on humoral immune responses to COVID-19 vaccination in hematologic malignancies. Blood Cancer Discov. 2021;2(6):568–576. doi: 10.1158/2643-3230.BCD-21-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031–1033. doi: 10.1016/j.ccell.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mouton W, Compagnon C, Saker K, Daniel S, Lacoux X, Pozzetto B, Oriol G, Djebali S, Berthier F, Marvel J, Walzer T. A novel whole-blood stimulation assay to detect and quantify memory T-cells in COVID-19 patients. medRxiv. 2021;21253202. [Google Scholar]
- 10.Chari A, Samur MK, Martinez-Lopez J, Cook G, Biran N, Yong K, Hungria V, Engelhardt M, Gay F, García Feria A, et al. Clinical features associated with COVID-19 outcome in multiple myeloma: first results from the international myeloma society data set. Blood. 2020;136(26):3033–3040. doi: 10.1182/blood.2020008150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergman P, Blennow O, Hansson L, Mielke S, Nowak P, Chen P, Söderdahl G, Österborg A, Smith CIE, Wullimann D, et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine. 2021;74:103705. doi: 10.1016/j.ebiom.2021.103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thakkar A, Gonzalez-Lugo JD, Goradia N, Gali R, Shapiro LC, Pradhan K, Rahman S, Kim SY, Ko B, Sica RA, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39(8):1081–1090.e2. doi: 10.1016/j.ccell.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maneikis K, Šablauskas K, Ringelevičiūtė U, Vaitekėnaitė V, Čekauskienė R, Kryžauskaitė L, Naumovas D, Banys V, Pečeliūnas V, Beinortas T, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8(8):e583–e592. doi: 10.1016/S2352-3026(21)00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benda M, Mutschlechner B, Ulmer H, Grabher C, Severgnini L, Volgger A, Reimann P, Lang T, Atzl M, Huynh M, et al. Serological SARS-CoV-2 antibody response, potential predictive markers and safety of BNT162b2 mRNA COVID-19 vaccine in haematological and oncological patients. Br J Haematol. 2021;195(4):523–531. doi: 10.1111/bjh.17743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, Morales M, Ziv T, Shorer Arbel Y, Scarfò L, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apostolidis SA, Kakara M, Painter MM, Goel RR, Mathew D, Lenzi K, Rezk A, Patterson KR, Espinoza DA, Kadri JC, Markowitz DM. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 18.Jaganathan S, Stieber F, Rao SN, Nikolayevskyy V, Manissero D, Allen N, Boyle J, Howard J. Preliminary evaluation of QuantiFERON SARS-CoV-2 and QIAreach Anti-SARS-CoV-2 total test in recently vaccinated individuals. Infect Dis Ther. 2021;10(4):2765–2776. doi: 10.1007/s40121-021-00521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee A, Wimmers F, Pulendran B. Epigenetic adjuvants: durable reprogramming of the innate immune system with adjuvants. Curr Opin Immunol. 2022;77:102189. doi: 10.1016/j.coi.2022.102189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Lee A, Grigoryan L, Arunachalam PS, Scott MKD, Trisal M, Wimmers F, Sanyal M, Weidenbacher PA, Feng Y, et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat Immunol. 2022;23(4):543–555. doi: 10.1038/s41590-022-01163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heitmann JS, Bilich T, Tandler C, Nelde A, Maringer Y, Marconato M, Reusch J, Jäger S, Denk M, Richter M, et al. A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity. Nature. 2022;601(7894):617–622. doi: 10.1038/s41586-021-04232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pardieck IN, van der Sluis TC, van der Gracht ETI, Veerkamp DMB, Behr FM, van Duikeren S, Beyrend G, Rip J, Nadafi R, Beyranvand Nejad E, et al. A third vaccination with a single T cell epitope confers protection in a murine model of SARS-CoV-2 infection. Nat Commun. 2022;13(1):3966. doi: 10.1038/s41467-022-31721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar A, Blum J, Thanh Le T, Havelange N, Magini D, Yoon IK. The mRNA vaccine development landscape for infectious diseases. Nat Rev Drug Discov. 2022;21(5):333–334. doi: 10.1038/d41573-022-00035-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


