Abstract
Aerobic processes require oxygen, and anaerobic processes are typically hindered by it. In many places in the global ocean, oxygen is completely removed at mid‐water depths forming anoxic oxygen minimum zones (A‐OMZs). Within the oxygen gradients linking oxygenated waters with A‐OMZs, there is a transition from aerobic to anaerobic microbial processes. This transition is not sharp and there is an overlap between processes using oxygen and those using other electron acceptors. This review will focus on the oxygen control of aerobic and anaerobic metabolisms and will explore how this overlap impacts both the carbon and nitrogen cycles in A‐OMZ environments. We will discuss new findings on non‐phototrophic microbial processes that produce oxygen, and we focus on how oxygen impacts the loss of fixed nitrogen (as N2) from A‐OMZ waters. There are both physiological and environmental controls on the activities of microbial processes responsible for N2 loss, and the environmental controls are active at extremely low levels of oxygen. Understanding how these controls function will be critical to understanding and predicting how fixed‐nitrogen loss in the oceans will respond to future global warming.
INTRODUCTION
Oxygen typically reaches a minimum concentration at some depth in the marine water column. This minimum results from the aerobic respiration of organic matter produced in, and settling from, the upper photic zone, balanced against oxygen‐rich water derived from high latitudes and circulating in the deep ocean (Wyrtki, 1962). When oxygen levels are reduced to below about 60 μM, these ocean regions are typically called oxygen minimum zones (OMZs). In some regions of the global ocean, patterns of circulation, together with intense upwelling, conspire to reduce oxygen concentrations to levels well below detection with the newest oxygen sensing technologies (<3 to 4 nM) (Revsbech et al., 2011; Thamdrup et al., 2012). We will refer to these as ‘anoxic’ oxygen minimum zones (A‐OMZs), and they are found the Arabian Sea, the North and South Eastern Tropical Pacific and in the Benguela upwelling current. Other regions of the oceans, such as the Cariaco Basin, the Black Sea and various fjords and embayments have bottom waters that are either permanently or periodically anoxic. Such anoxic basins will not be the focus of this review, but they will be mentioned when appropriate.
Sharp oxygen gradients mark the transition between oxygenated waters and the oxygen‐depleted waters of A‐OMZs, and these gradients house fundamental ecological shifts between aerobic and anaerobic metabolic processes (Bristow et al., 2017; Dalsgaard et al., 2014; Jensen et al., 2008; Kalvelage et al., 2011; Ulloa et al., 2012). The aerobic processes we will consider are parts of the C and N cycles and include aerobic respiration, ammonium oxidation and nitrite oxidation, to which we also group oxygenic photosynthesis. The anaerobic processes include nitrate reduction to nitrite, nitrate (and nitrite) reduction to ammonium, denitrification and anammox (Table 1). These microbial processes (together with possibly sulfate reduction; Canfield et al., 2010) are viewed as the most important in determining A‐OMZ ecology and biogeochemical cycling (Dalsgaard et al., 2014; Jensen et al., 2008; Kalvelage et al., 2011; Lam et al., 2009; Ulloa et al., 2012).
TABLE 1.
Metabolisms of interest in A‐OMZs
| Metabolism | Reaction |
|---|---|
| Oxygenic photosynthesis | CO2 + H2O ➔ CH2O + O2 |
| Aerobic respiration | CH2O + O2 ➔ CO2 + H2O |
| Ammonia oxidation | 2NH4 + + 3O2 ➔ 2NO2 − + 2H2O + 4H+ |
| Nitrite oxidation | 2NO2 − + O2 ➔ 2NO3 − |
| Anammox | NO2 − + NH4 + ➔ N2 + 2H2O |
| Nitrate reduction to nitrite | 2NO3 − + CH2O ➔ 2NO2 − + CO2 + H2O |
| Nitrate reduction to ammonium | NO3 − + 2CH2O + 2H+ ➔ NH4 + + 2CO2 + H2O |
| Denitrification | 4NO3 − + 5CH2O + 4H+ ➔ 2 N2 + 5CO2 + 7H2O |
Note: Net reactions are given.
As oxygen concentrations control the transition between aerobic and anaerobic microbial processes, they will also control the nature of elemental cycling, but how? For example, how is carbon mineralization rate impacted by the transition between aerobic and anaerobic respiration? Also, which anaerobic processes dominate carbon mineralization through the transition? The transition between aerobic and anaerobic processes also impacts the cycling of nitrogen. For example, low‐oxygen concentrations will lead to the anaerobic process of nitrate reduction to nitrite (Kalvelage et al., 2013; Lam et al., 2009a) (Table 1). The fate of this nitrite will depend on oxygen, as with sufficient oxygen, the nitrite will be oxidized back to nitrate, forming a cryptic cycle. If this happens, nitrite will not be available to fuel anammox, and thus will not contribute to fixed N loss as N2 from the oceans. Thus, the extent to which low‐oxygen conditions contribute to marine fixed nitrogen loss will depend on the oxygen concentration and relative oxygen sensitivities of the associated aerobic and anaerobic processes (Bristow et al., 2016).
There is growing interest in how oxygen impacts the nitrogen cycle as the oceans have lost some 2% of their oxygen inventory over the last 50 years (Schmidtko et al., 2017; Stramma et al., 2012). This loss has been linked to ocean warming and altered circulation due to climate change (Oschlies et al., 2018; Schmidtko et al., 2017). Further ocean deoxygenation is expecting as the atmosphere and oceans continue to warm (Oschlies, 2021), leading to expanded A‐OMZs and a likely increase in the volume of ocean water where aerobes and anaerobes cohabitate, with possible impacts on elemental cycling and the ocean nutrient (mainly N) economy.
While oxygen contributes to elemental cycling alongoxygen gradients, it may also, paradoxically, contribute to elemental cycling under the ‘anoxic’ conditions of A‐OMZs. For example, photosynthetic oxygen production has been found in the upper reaches of ‘anoxic’ A‐OMZ waters (Garcia‐Robledo et al., 2017), and some microbial aerobic processes have been discovered to occur in the absence of any external supply of oxygen. As examples of the latter case, both the methane oxidizer Candidatus Methylomirabilis oxyfera (Ettwig et al., 2010) and the ammonia oxidizer Nitrosopumilus maritimus (Kraft et al., 2022) produce their own molecular oxygen that they use in aerobic metabolism, with potential impacts on elemental cycling in A‐OMZs.
This review will explore the relationship between oxygen concentrations and aerobic and anaerobic metabolisms of biogeochemical significance in A‐OMZ environments. This review explores how oxygen regulates the transition between aerobic and anaerobic processes, and how this regulation impacts both carbon and nutrient cycling in both A‐OMZs and OMZs. We give attention to how biogeochemical cycling in low‐oxygen environments might be impacted by global warming. By focusing on the oxygen regulation of microbial processes, this contribution complements oxygen‐minimum zone reviews focusing on the nitrogen (Lam & Kuypers, 2011; Ulloa et al., 2012; Wright et al., 2012) and sulfur (Callbeck et al., 2021; van Vliet et al., 2021) cycles, and it follows an earlier review on microbial life at low‐oxygen concentrations (Morris & Schmidt, 2013) and a recent review (Berg et al., 2022) that offers a broad overview of microbial life in low‐oxygen environments.
STRUCTURE OF A‐OMZS
The basic features of A‐OMZ biogeochemistry are shown in Figure 1. This cartoon is modelled after the A‐OMZ of Northern Chile (Canfield et al., 2010), but it reflects features common to most A‐OMZs. These features include oxygenated waters above and below the ‘anoxic’ core depicted by the accumulation of nitrite. Indeed, in A‐OMZ settings, nitrite accumulates only when oxygen is below detection by STOX sensors (Thamdrup et al., 2012). Associated with nitrite accumulation, there is a reduction in the concentrations of nitrate within A‐OMZ waters. Many A‐OMZs also harbour a secondary chlorophyll a maximum in their uppermost layers where oxygen concentrations are below detection and where nitrite has begun to accumulate (Cepeda‐Morales et al., 2009; Garcia‐Robledo et al., 2017; Goericke et al., 2000; Lavin et al., 2010). This secondary chlorophyll a maximum is generated by cyanobacteria of the genus Prochlorococcus.
FIGURE 1.
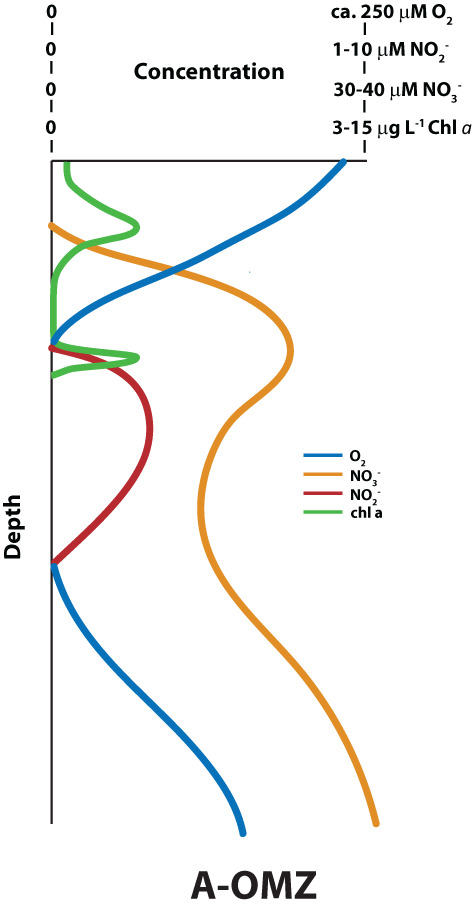
Cartoon showing typical depth distribution of biogeochemical properties in A‐OMZ waters. Typical concentration ranges of chemical species and chl a in A‐OMZs are also given. Source: Figure inspired by Ulloa et al. (2012)
OXYGEN CONTROL OF MICROBIAL METABOLISMS
Terminal oxidases and K m values
Aerobic metabolism in microbes occurs as electrons supplied from an electron donor (e.g. organic matter, ammonium, nitrite) are combined with oxygen at a terminal oxidase enzyme forming water. A number of different terminal oxidases are found, and these can be divided into three broad classes: the heme‐copper oxidases (HCOs), the cytochrome bd‐type oxidases (Degli Esposti et al., 2019; Morris & Schmidt, 2013; Sousa et al., 2012) and the alternative (AOX) oxidases. The HCOs can be further divided into A, B and C class oxidases, with the A class oxidases divided into A1 and A2 types. The cytochrome bd‐type oxidases are found in multiple forms including bd‐1 and cio types (Morris & Schmidt, 2013). The AOX oxidases are not widely distributed among prokaryotes (Stenmark & Nordlund, 2003) and will not be considered further here.
In the context of Michaelis–Menten kinetics, and as reflected in their respective K m values (Table 2), HCO A‐class oxidases and the cytochrome bd cio oxidase have a low affinity for oxygen (K m ranging from 250 to 4300 nM, Table 2), while the HCO‐C class oxidases, as well as the cytochrome bd‐type bd‐1 oxidases, have a high affinity for oxygen (K m ranging from 3 to 300 nM, Table 2). The affinity of the HCO B‐class oxidases has not been explicitly studied, but they are believed to be high‐affinity oxidases based on the environments where a high affinity for oxygen is expected (Morris & Schmidt, 2013).
TABLE 2.
Summary of oxidase enzymes used by prokaryotes and associated K m values a
| Family | HCO | HCO | HCO | |||
|---|---|---|---|---|---|---|
| Class | A | B | C | Cytochrome bd | ||
| Type | A1 | A2 | bd‐1 | cio | ||
| K m (nM) | 600–4300 | 250–3200 | 7–250 | 3–300 | 400–4000 | |
| O2 affinity | Low | Low | High? | High | High | Low |
| References | Arai et al. (2014), Miura et al. (2013), and Ramel et al. (2013) | Arai et al. (2014) | Arai et al. (2014) and Preisig et al. (1996) | Preisig et al. (1996) and Ramel et al. (2013) | Arai et al. (2014)and Miura et al. (2013) and | |
Many microbes have both high‐ and low‐affinity oxidase enzymes, with E. coli as a conspicuous example, containing both the high‐affinity cytochrome bd oxidase and the low‐affinity HCO A‐class cytochrome bo oxidase (D'mello et al., 1996). Indeed, following expectations, E. coli expresses the low‐affinity oxidase under full oxygenation, while under oxygen limitation, the high‐affinity oxidase is preferentially expressed (Cotter et al., 1990).
In A‐OMZ environments, both gene abundance and gene expression data show a dominance of low‐affinity oxidases in environments ranging from full oxygenation to anoxia, but there is a decided increase in the significance of high‐affinity oxidases as oxygen concentrations decrease (Kalvelage et al., 2015). If we turn to K m values, a community level value of 391 nM O2 was obtained from measuring aerobic respiration rates over a wide range of oxygen concentrations (300–10,000 nM) from the low‐oxygen waters of Golfo Dulce, Costa Rica (Garcia‐Robledo et al., 2016). This relatively elevated K m could indicate the co‐expression of both high‐ and low‐affinity oxidase enzymes through the oxygen range tested, with the dominance of one or the other oxidase type depending on the oxygen concentration. In support of this view, separate oxygen draw‐down experiments conducted under oxygen limitation yielded ‘apparent’ K m values of 35–40 nM, and these low K m values could reflect the expression and use of high‐affinity oxidases under oxygen limitation. In a separate study from A‐OMZs of the north and south tropical Pacific, oxygen draw‐down experiments yielded K m values ranging from 18 to 113 nM O2, with typical values of about 100 nM O2 (Tiano et al., 2014). These values are in line with the presence of K m values for high‐affinity oxidases (Table 2), and overall, aerobic heterotrophic microbes are well adapted to their environments with the capacity to use oxygen to very low concentrations.
The same can also be said of nitrifying bacteria. Indeed, ammonium and nitrite oxidation in A‐OMZ waters from Chilean coast were measured down to the 5–33 nM oxygen limits of the experiment (Bristow et al., 2016), with a K m value for ammonium oxidation of 333 nM oxygen, and a K m for nitrite oxidation of 778 nM (Bristow et al., 2016). Careful inspection of the nitrite oxidation kinetic results revealed a best model fit with two simultaneous kinetic responses: one with a K m of under 1 nM, and the other with K m of around 1500 nM. Thus, the nitrifiers involved in nitrite oxidation from the low‐oxygen waters appear to employ both high‐ and low‐affinity oxidase enzymes, with the high‐affinity oxidase displaying an extremely high oxygen affinity. Accordingly, members of the Nitrospina, the most abundant group of nitrite oxidizers in marine environments, encode high‐affinity HCO‐C or HCO‐B type terminal oxidases (Lücker et al., 2013).
We note also that the K m values for nitrogen metabolism could impact the activities of aerobes using both nitrogen and oxygen in their metabolism. Thus, in addition to oxygen, the concentration of ammonium and nitrite could affect the various stages of nitrification in OMZ environments. We will not explicitly explore these K m values and their impact on microbial metabolism, but the microbial affinities for nitrogen substrates will be mentioned where appropriate.
Oxygen limits to aerobic respiration
Michaelis–Menten kinetic analysis reveals when an organism's metabolism becomes oxygen limited, but it does not reveal the lower oxygen limit to metabolism and growth. From an organismal perspective, the oxygen limit to aerobic respiration comes when the energy gained during respiration is insufficient to allow the organism to grow. From an ecological perspective, growth should be sufficiently rapid to balance losses due to death, including predation and viral attack.
We begin with an organismal perspective (see Stolper et al., 2010; Zakem & Follows, 2017). When growth is oxygen limited, as occurs with oxygen concentrations below the K m value, the flux of oxygen into an organism depends on the surface area of the cell and the concentration of oxygen in the environment. If we assume that a cell doubles when accumulating twice its normal carbon content, then the growth rate is given as Stolper et al. (2010):
| (1) |
where μ (d−1) is the growth rate of the organism, [O 2]env is the environmental concentration of oxygen, [O 2]cell is the concentration of oxygen at the cell surface, r (μm) is the radius of the cell, D (μm2 d−1) is the diffusion rate for oxygen, y (mol cell carbon produced/mol O2 respired) is the oxygen efficiency of the organism and q (molC μm−3) is the volumetric carbon content.
For the purpose of an example calculation, we assume [O 2 ]cell = 0, a y value 0.5 as found experimentally for E. coli (Stolper et al., 2010), a q of 1.85 × 10−14 molC μm−3 as typical for E. coli (Stolper et al., 2010) and a D of 1.72 × 108 μm2 d−1 (seawater at 20°C). From these values, we calculate the oxygen concentrations that can sustain a range of growth rates for different cell sizes as shown in Table 3. Different values of y, [O 2]cell, and q will change these results somewhat. For example, y depends on the energy conservation efficiency of the electron transfer chain, as well as the terminal oxidase and the carbon source. However, a reasonable range of these values is unlikely to change the main conclusion that small slowly growing aerobes can grow with oxygen concentrations lower than measurable with currently available oxygen‐sensing technology (see also Stolper et al., 2010). This conclusion is consistent with the ability of E. coli to grow aerobically at oxygen concentrations below the 3 nM detection limit of STOX sensors (Stolper et al., 2010).
TABLE 3.
Calculated oxygen concentrations to sustain growth at different specific
| Specific growth rate d−1 | |||||||
|---|---|---|---|---|---|---|---|
| Diameter (μm) | 1 | 0.5 | 0.2 | 0.1 | 0.05 | 0.02 | 0.01 |
| O2 (nM) | |||||||
| 2 | 103 | 52 | 21 | 10 | 5.2 | 2.1 | 1.0 |
| 1.5 | 58 | 29 | 12 | 5.8 | 2.9 | 1.2 | 0.58 |
| 1 | 26 | 13 | 5.2 | 2.6 | 1.3 | 0.5 | 0.26 |
| 0.8 | 17 | 8.3 | 3.3 | 1.7 | 0.82 | 0.33 | 0.16 |
| 0.6 | 9.3 | 4.6 | 1.9 | 0.93 | 0.47 | 0.18 | 0.093 |
| 0.4 | 4.14 | 2.07 | 0.82 | 0.41 | 0.21 | 0.082 | 0.041 |
Note: Growth rates and different cell sizes.
In nature, however, cells face mortality by cell death, grazing and viral infection, and growth will only be possible if the growth rate of the organism matches or exceeds its rate of removal from the ecosystem. Thus, following Zakem and Follows (2017), we can define a critical O2 concentration, O 2*, where the loss rate, L (d−1), is equal to the growth rate, μ, as expressed in Equation (1), yielding:
| (2) |
In principle, the oxygen level where growth and loss are balanced should set the minimum oxygen concentration in the environment (Zakem & Follows, 2017). If we assume grazing is the most important source of loss, then prokaryote loss rates by grazing in low‐oxygen A‐OMZ settings of 0.06–0.3 d−1 (Medina et al., 2017) should balance rates of cell growth (Table 3). If we focus on prokaryote cells of between 0.6 and 1 μm in diameter, a size that covers most prokaryotes in the water column, growth and grazing is balanced at minimum oxygen levels of between about 0.5 and 5 nM. It is possible that these low‐oxygen levels could impact grazing rates by protists, but the oxygen control of protist grazing is unexplored. Furthermore, anaerobic, grazing protists are found in anoxic environments (e.g. Fenchel et al., 1995), so oxygen concentration may not have a large impact on protist grazing rates.
The analysis above, however, misses the possibility that prokaryotes can grow with a mix of aerobic and anaerobic metabolisms. For example, most microbes capable of nitrate reduction and denitrification are facultative aerobes. Therefore, a microbe may metabolize and grow with oxygen under oxygen‐limiting condition as indicated in Equation (1), but supplement this growth with anaerobic metabolism and thus grow at higher rates than if growing with oxygen alone. These higher growth rates will allow a facultative aerobe to persist in the environment to lower oxygen concentrations than if the organism was a strict aerobe. In this way, oxygen can be drawn down by a facultative aerobe to lower concentrations than expected from Equation (2).
In principle, aerobic autotrophs, like nitrifiers for example, would be subject to the same constraints as outlined in Equation (2), where growth rates must match loss rates for the organism to persist. If aerobic autotrophs lacked alternative anaerobic metabolisms, they might not be able to persist at oxygen levels as low as those for facultative heterotrophic aerobes assuming grazing rates were similar. However, some members of the nitrite‐oxidizing genus Nitrospira, for example, can metabolize organic compounds using nitrate as an electron acceptor (Koch et al., 2015), thus displaying anaerobic metabolic potential and the possibility of surviving under lower oxygen concentrations than if growing as a strict aerobe. Other alternative metabolisms for nitrifiers under anoxic conditions have been recently discovered, and these will be explored in a later section.
Oxygen inhibition of anaerobic metabolism
While oxygen is known to interact with and inhibit many anaerobic organisms, we will focus here on metabolisms of the nitrogen cycle as shown in Table 1. As noted earlier, organisms responsible for nitrate reduction to nitrite and denitrification are facultative anaerobes, so oxygen does not inhibit their anaerobic metabolism, but rather, it offers an energetically favourable alternative. We could imagine that facultative aerobes switch to anaerobic metabolism when oxygen availability limits their metabolic rate relative to the availability of the electron donor (organic matter) driving their metabolism (e.g. Zakem et al., 2020).
In principle, the onset of oxygen limitation by a facultative anaerobic process can be evaluated by looking at its oxygen sensitivity. Thus, for A‐OMZ waters off the coast of Chile, denitrification rates (N2 + N2O production) were reduced to half of their maximum values at 200–300 nM oxygen (Dalsgaard et al., 2014), while for waters of the Bay of Bengal, ‘potential’ rates of denitrification were reduced to half of their maximum rate at oxygen concentrations of about 2000 nM (Bristow et al., 2017). This site‐to‐site variability in the oxygen sensitivity of denitrification could reflect variable contributions from the so‐called ‘aerobic denitrifiers’, who can denitrify at high rates under fully oxygenation conditions (e.g. Robertson et al., 1995). Aerobic denitrifiers apparently defy expectations about how energy limitation during low‐oxygen aerobic respiration triggers the transition to anaerobic metabolism. When aerobic denitrification is observed in pure cultures, however, a surplus of organic carbon is usually present and simultaneous aerobic respiration is the preferred pathway for growth (Chen & Strous, 2013). Therefore, the site‐to‐site variability in denitrification oxygen sensitivity may also be driven by differences in organic carbon availability. Alternatively, denitrification under elevated oxygen concentrations could be sustained by selective environmental adaptations, perhaps driven by dynamic redox fluctuations (Marchant et al., 2017).
The oxygen sensitivity of nitrate reduction to nitrite has been explored for A‐OMZ waters at various sites off the Peruvian coast, and the sensitivity depends very much on sampling site (Kalvelage et al., 2011). At its most sensitive, for water from within anoxic waters of a coastal site of 250 m water depth, rates of nitrate reduction to nitrite were reduced to half‐maximum rates at about 3000 nM O2. At its least sensitive, for shallower slightly oxygenated waters at the same site, nitrate reduction occurred at maximum rates through the whole range of O2 concentrations tested, up to 25,000 nM.
Anammox bacteria are anaerobic autotrophs, and they may be inhibited by oxygen through a reduction in protein function, although this is not certain (Dalsgaard et al., 2014). Several studies have explored the oxygen sensitivity of the anammox processes in A‐OMZ waters. For example, for the A‐OMZ off the coast of Chile, anammox was inhibited to half‐maximum rates at about 900 nM O2, while it persisted at up to 1500 nM O2 (Dalsgaard et al., 2014). Anammox activity in the nitrogenous zone of the Black Sea was much less oxygen sensitive, showing half inhibition at about 10,000 nM O2 and full inhibition at about 13,000 nM (Jensen et al., 2008). Such low‐oxygen sensitivity to anammox was also observed in nitrogenous waters of both the Namibian shelf and coastal Peru (Kalvelage et al., 2011). Overall, the anammox process itself does not appear to very oxygen sensitive. Indeed, the anammox process is conducted in the so‐called anammoxosome, a structurally unique membrane‐bound ‘organelle’ with limited gas permeability that may help protect the anammox process from oxygen (van Niftrik et al., 2004). However, as explored in more detail below, anammox can still be impacted, even at very low‐oxygen concentrations, if oxygen controls the availability of the principal substrates for anammox, nitrite and ammonium.
OXYGEN PRODUCTION IN ANOXIC WATERS
Apart from the oxygen supplied to the ‘anoxic’ core of A‐OMZ waters along gradients by advection and diffusion from above and below, some processes can contribute oxygen directly to the anoxic waters. One of these is oxygenic photosynthesis by cyanobacteria of the genus Prochlorococcus forming a secondary chlorophyll a maximum in the upper reaches of some anoxic A‐OMZ waters (see Figure 1). Such secondary chlorophyll a maxima have been found in the A‐OMZs of the Eastern Tropical North and South Pacific and the Arabian Sea (Cepeda‐Morales et al., 2009; Garcia‐Robledo et al., 2017; Goericke et al., 2000; Lavin et al., 2010).
Experiments monitoring oxygen dynamics in these chlorophyll a‐rich waters demonstrated active photosynthetic oxygen production, where the oxygen, in most cases, was actively consumed generating a cryptic oxygen cycle with no net oxygen accumulation (Garcia‐Robledo et al., 2017). Transcriptomic analysis further revealed active nitrite and ammonium‐oxidizing communities in these ‘anoxic’ waters suggesting that the oxygen produced is actively scavenged by aerobic processes including those of nitrite and ammonium oxidation (Garcia‐Robledo et al., 2017). These observations, together with the discussions above, reveal that both heterotrophic and autotrophic processes of oxygen utilization occur in A‐OMZs at low nM or even sub nM oxygen concentrations.
Such cryptic oxygen cycling may also occur in deeper dark waters, where oxygen production may proceed through nitric oxide (NO) dismutation. Microbial NO‐dismutation was first discovered in the methane‐oxidizing member of the NC10 phylum Candidatus M. oxyfera (Ettwig et al., 2010). Candidatus M. oxyfera reduces nitrite to NO, which then is dismutated to O2 and N2. The produced oxygen is used to oxidize methane to CO2. Recently, an apparently similar NO‐dismutation pathway was discovered in the ammonia‐oxidizing archaeon N. maritimus. In N. maritimus, NO is apparently dismutated to O2 and N2O, and the N2O is then reduced to N2 in an additional enzymatic step (Kraft et al., 2022). The oxygen produced by Nitrosopumilus maritimus is used to oxidize ammonia, its normal metabolism (Kraft et al., 2022). While oxygen production is very tightly coupled to methane oxidation in Candidatus M. oxyfera, oxygen accumulates to about 200 nM in experiments with pure cultures of N. maritimus. This oxygen accumulation indicates that only a portion of the oxygen produced by N. maritimus is used for ammonia oxidation. Therefore, in the environment, trace amounts of oxygen may be available for other aerobic microbes in close proximity to the ammonia‐oxidizing archaea (AOA). AOAs are frequently found in the upper transitional zone of A‐OMZs (Stewart et al., 2012) and methane oxidizers of the NC10 phylum are present and active in the Eastern Tropical North Pacific (ETNP) (Padilla et al., 2016). NO‐dismutation could explain how some ‘aerobic’ organisms can gain energy when no external oxygen is available. NO‐dismutating microbes also stand in competition for nitrite with denitrifiers, anammox bacteria and nitrite oxidizers. Indeed, nitrite reduction to N2 via NO‐dismutation is bioenergetically advantageous over heterotrophic denitrification when the produced oxygen is also consumed by the same organism (Chen & Strous, 2013). Overall, though, the importance of NO dismutation as an O2 source in anoxic A‐OMZ settings will depend on the density of cells that can produce O2 and their actual metabolism in these settings. These are active areas of current research.
TRANSITION FROM AEROBIC TO ANAEROBIC METABOLISM
In this section, we will explore how carbon mineralization rates and mineralization processes are impacted by the transition from oxic to anoxic conditions. As noted earlier, this switch might occur as oxygen concentrations become low enough to limit aerobic metabolism relative to electron donor supply, encouraging a switch to anaerobic metabolism in facultative organisms. For E. coli, the transition between aerobic and anaerobic metabolism is both smooth and gradual, beginning at about 10% air saturation oxygen levels (about 25 μM O2) and continuing to anoxia (Tseng et al., 1996). The nature of this transition has not been explored in natural environments, but there is sufficient information from available process rate measurements to offer some insights.
Our analysis draws from process rate data obtained from the A‐OMZ of northern Chile as reported in references (Babbin et al., 2017; De Brabandere et al., 2014); these data sets are mutually compatible. As reference point for discussion, we calculate the depth distribution of organic carbon mineralization rates as might be expected under oxygenated conditions as determined from the attenuation of organic matter flux in sediment traps. The attenuation is typically modelled with a so‐called ‘Martin’ curve having a mathematical form of:
| (3) |
where Flux Z , is the organic matter flux at any depth, Z, Flux100 is the flux at 100 m, and b is the attenuation coefficient providing the best fit to the sediment trap data. We use a Flux100 for the Equatorial Pacific of 7 mmolC m−2 d−1, a value that matches results from a floating sediment trap deployed off the southern Peruvian margin (Engel et al., 2017) and similar to trap deployments in the Eastern Tropical Southern Pacific (ETSP) (Berelson, 2001). Values for b range mostly from about 0.7–1.2 throughout the global ocean (Berelson, 2001). Using these values for Flux100 and b, rates of carbon mineralization can be calculated as the difference in carbon flux between adjacent depths, assuming that the decrease in flux is due to carbon oxidation. These results are shown in Figure 2, and we interpret them to approximate the depth distribution of carbon oxidation as might be expected for waters of the ETSP.
FIGURE 2.
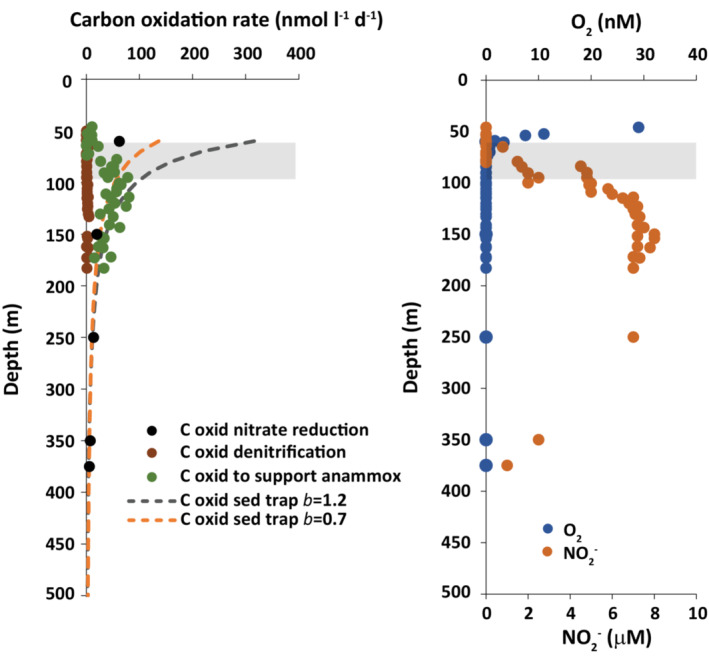
Carbon oxidation rates with depth in A‐OMZ waters from northern Chile/southern Peru together with t depth distributions of oxygen and nitrite. Measured rates of denitrification and nitrate reduction were converted into rates of carbon oxidation. The rates of carbon oxidation required to supply the ammonium needs of anammox are based on measured anammox rates and an assumed 5.8/1 stoichiometry between carbon mineralized to ammonium liberated in A‐OMZ waters. Also shown are expected rates of carbon mineralization based on different models of carbon flux attenuation with depth from sediment traps. See text for details
Overlain on these results is rates of carbon oxidation due to nitrate reduction to nitrite, using the values measured by Babbin et al. (2017) and carbon mineralization due to denitrification with values measured by De Brabandere et al. (2014). Both of these data sets were obtained from about the same region of the ETSP. In addition, we calculate the carbon mineralization rates needed to support the ammonium requirements of anammox bacteria based on the measured rates of N2 production by the anammox process (determined from 15N‐ammonium) as reported by De Brabandere et al. (2014). Ammonium supplies half of the reported total rate of N2 production, and we calculate the carbon oxidation rate required to supply this ammonium using a C/N ratio of 5.8/1. This C/N ratio is that required to reproduce the C/N of settling organic matter in particles captured in the sediment traps deployed by Engel et al. (2017) off the southern Peruvian coast.
From these values, we make several observations. First, denitrification is a minor pathway of carbon mineralization in the A‐OMZ of northern Chile, as already expected due to the low rates typically measured in A‐OMZ waters (De Brabandere et al., 2014; Kalvelage et al., 2011; Lam et al., 2009; Thamdrup et al., 2006). Next, and in contrast, carbon mineralization by nitrate reduction to nitrite generally follows patterns of carbon mineralization as predicted from sediment trap data, except in the oxycline near the top of A‐OMZ waters, where the reported rate is lower than expected. Thus, deeper in the A‐OMZ, nitrate reduction to nitrite appears to be a very important, if not dominant pathway of carbon mineralization (see also Lam et al., 2009). The seemingly low rate of nitrate reduction to nitrite near the bottom of the oxycline could result from a sharing in carbon mineralization between nitrate reduction and aerobic respiration. Alternatively, there could be a suppression of carbon mineralization rates, a kind of ‘metabolic hole’, in the transition from aerobic to anaerobic metabolism. This idea could be tested, but we are unaware of any direct determinations of the carbon mineralization rates in the transition between aerobic and anaerobic metabolism.
Source and fate of ammonium in the upper A‐OMZ and oxygen control of N2O production
Next, we compare the carbon oxidation rates required to support the ammonium requirements for anammox with the predicted rates of carbon mineralization from sediment trap calculations and from measured rates of nitrate reduction to nitrite (Figure 2). Below about 120 m depth, the ammonium requirements for anammox can apparently be supplied by carbon mineralization, and much of this carbon mineralization comes from nitrate reduction to nitrite. It would seem, however, that some additional ammonium might be needed, and this might be supplied by sulfate reduction operating in a cryptic cycle of sulfate reduction and sulfide oxidation (Canfield et al., 2010).
Above about 120 m depth, the situation is different where anammox rates are quite suppressed compared to expected rates of ammonium liberation by organic matter decomposition (Figure 2). This result suggests competition for the ammonium produced by organic matter respiration in this zone. It is possible that oxygen is mixed from overlying waters and is rapidly used by nitrifying microbes, reducing the ammonium available to anammox bacteria. It is also possible, indeed probable, that oxygen produced by photosynthetic cyanobacteria in the secondary Chl a maximum (gray box in Figure 3) is quickly utilized to oxidize ammonium and nitrite. A possible ‘dark’ oxygen source from Nitrososphaerota might also oxidize ammonium. Unrelated to oxygen, some of the ammonium might be incorporated directly by Prochlorococcus during growth. Indeed, cyanobacteria of the deep Chl a maximum in A‐OMZs can uptake ammonium, nitrate and nitrate (Ulloa et al., 2021). To be an important net sink for ammonium, however, Prochlorococcus must quantitatively utilize ammonium over the more available nitrate and nitrite and cells must settle quickly and not recycle ammonium back to the upper A‐OMZ. Further work will be required to fully understand the fate of ammonium in the ‘transitional zone’ of upper A‐OMZ waters.
FIGURE 3.
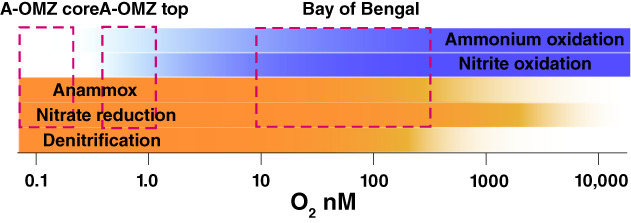
Cartoon showing the oxygen sensitivity of aerobic (blue) and anaerobic (orange) microbial processes. While anammox bacteria can metabolize under low micromolar concentrations of oxygen, microbial ammonium and nitrite oxidation are very efficient at very low‐oxygen concentrations removing these substrates from anammox bacteria at oxygen levels where they would otherwise be active. Thus, in the BoB OMZ, oxygen is sufficiently low that anammox bacteria should be active, but sufficiently high that nitrite and ammonium are effectively oxidized, so there is no anammox activity. In the upper A‐OMZ oxygen concentrations are below detection, and the oxygen range depicted here is an educated guess. Still, anammox activity, while measurable, is restricted due to active ammonium oxidation with oxygen likely coming from oxygenic phototrophic cyanobacteria and possibly N. maritimus. In the A‐OMZ core oxygen concentrations are likely even lower, and an oxygen source is apparently restricted. Here, anammox bacteria operate to their full potential. While physiological possible through a great range of oxygen concentrations, denitrification plays a minor role in both N2 production and carbon mineralization in A‐OMZ environments
Finally, oxygen exerts other types of control on the nitrogen cycle. Indeed, in the oxygen gradients associated with A‐OMZs, N2O, a potent greenhouse gas, is released to the water column through both incomplete nitrification (ammonium oxidation) and denitrification (from both nitrate and nitrite reduction) producing oversaturation and thus the potential for N2O release to the atmosphere. While both nitrification and denitrification are both potential sources for N2O, in the oxygen gradients of A‐OMZ settings, most N2O is apparently released to the water column from denitrification (Ji et al., 2018). Nitrous oxide is released under low‐oxygen conditions of <10 μM O2 to >0 μM O2, but it is not liberated under complete anoxia where nitrification is inhibited and under conditions when denitrification is mostly complete (Ji et al., 2018; Naqvi et al., 2010).
OXYGEN REGULATION OF ANAMMOX
As explored above, when given the necessary substrates, anammox bacteria have a relatively high tolerance for oxygen, performing anammox even in presence of micromolar O2 concentrations. Still, as discussed above, anammox activity in the upper secondary Chl a maximuim of A‐OMZs can be lower‐than‐expected when compared to ammonium production rates, even with undetectable levels of oxygen. In this case, we suggested that ammonium may be removed through nitrification using oxygen from a variety of sources. The nitrifiers utilizing the oxygen, likely members of the Nitrososphaerota (Garcia‐Robledo et al., 2017), have a demonstrated high affinity for ammonium (Martens‐Habbena et al., 2009), and they probably also have a high affinity for oxygen where in oxygen draw‐down experiments, K m values for O2 would be in the low nM range (Kraft et al., 2022). Some of the oxygen produced in and delivered to the zone of the secondary Chl a maximum might be used to oxidize nitrite as well.
In the Bay of Bengal (BoB), anammox activity is also hindered under low‐oxygen concentrations where anammox bacteria should be otherwise physiologically active. Thus, in the BoB OMZ, O2 concentrations are reduced to the range of 10–500 nM (Bristow et al., 2017), but anammox activity was unmeasurable for samples incubated under in situ conditions (Bristow et al., 2017). Still, anammox bacteria were present in the water, and anammox activity was measured when nitrite and ammonium were added to the incubating samples. Nitrite and ammonium are otherwise (mostly) absent from BoB OMZ waters despite their active production by nitrate reduction under low ambient O2 concentrations (Bristow et al., 2017). Thus, in the low‐oxygen waters of the BoB OMZ, both nitrite and ammonium are actively removed by nitrifying microbes, removing them for use by anammox bacteria.
Overall, low‐oxygen conditions can impact anammox by removing key substrates for the process. Substrate limitation occurs at lower oxygen concentrations than the physiological oxygen limit for anammox bacteria. These principles are shown in Figure 3, outlining our best estimates as to the oxygen control of the different aerobic and aerobic processes, and the oxygen levels and environments where various types of anammox inhibition are found, including the Bay of Bengal and the ‘anoxic’ secondary chlorophyll a maximum in A‐OMZs.
CONTROL OF OXYGEN IN OMZS AND A‐OMZS
We argue above that oxygen plays a key role in regulating fixed nitrogen loss from the environment, particularly through anammox, and at oxygen levels well below the physiological constraints of anammox bacteria. In this regard, it matters whether oxygen concentrations are 0.1, 1, or 10 nM (Figure 3), as each bears different consequences for the ultimate regulation of nitrogen loss from the environment. Thus, we might ask what processes set the oxygen concentrations of OMZs and A‐OMZs recognizing the BoB as a conspicuous example of a large basin with low semi‐persistent, but measurable, oxygen concentrations? With continuing ocean deoxygenation, it is possible that low‐oxygen regions like the BoB will develop and expand, and whether they support fixed nitrogen loss will depend critically on the levels of oxygen they attain.
If the sink for oxygen due to organic matter respiration in the marine water column outpaces the source of oxygen from advection and diffusion, anoxia will develop, generating A‐OMZs (Canfield, 2006; Shaffer, 1989). At the same time, there likely exist biological controls and feedbacks regulating how OMZs become A‐OMZs; indeed, some form of oxygen regulation is required to explain the persistence of semi‐stable low‐oxygen concentrations in the BoB (Canfield et al., 2019). We discussed earlier how the balance between growth and death for aerobic microbes could set oxygen levels to low, but measurable, nM levels. However, as also noted, this analysis does not explain the lower oxygen levels attained in A‐OMZs, and it does not consider that microbes might grow by facultative metabolisms.
Thus, we look for other biological controls. Here, the kinetics of aerobic respiration represent a potential control, where oxygen consumption rates are reduced as oxygen concentrations are lowered. If aerobic respiration follows Michaelis–Menten control, then the rate of aerobic respiration as a function of oxygen concentration depends on the K m value. This concept is shown in Figure 4, where results from a simple 3‐box OMZ model show how oxygen concentration responds to upwelling rates with various values of K m (Canfield et al., 2019). Higher upwelling rates generate higher rates of primary production and high fluxes of organic carbon into the OMZ waters (Figure 4A). As expected, higher upwelling rates produce lower oxygen concentrations, but oxygen concentrations stabilize to non‐zero values that depend on the K m value for the aerobic heterotrophs.
FIGURE 4.
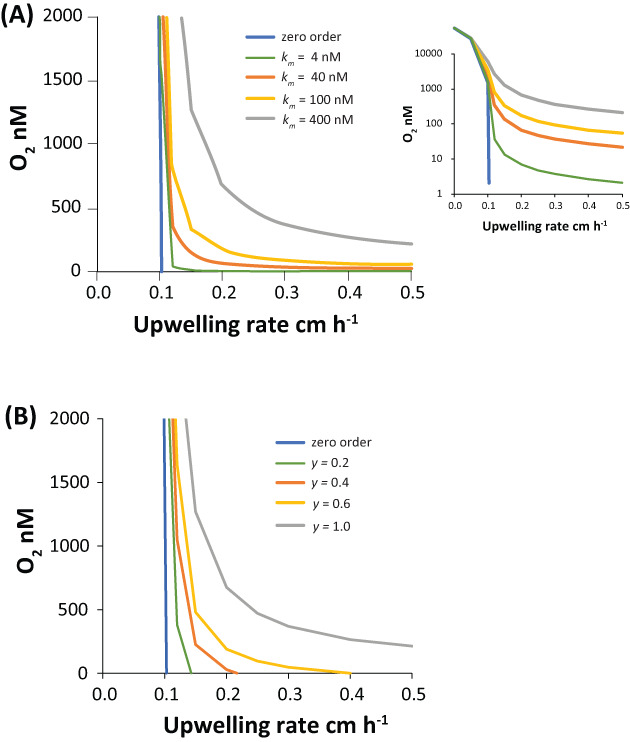
(A) Model results from a 3‐box OMZ model showing the evolution of oxygen in the OMZ box with different values of K m for oxic respiration. Insert shows results on a log scale. (B) As in A, but with variable proportions of the organic matter oxidized with Michaelis–Menten control, with the rest oxidized with zero‐order O2 control; y = 1 reflects full Michaelis–Menten control. K m is set at 0.4 μM O2. See text for details
This simple model generates a stable oxygenated water column, compatible with observations from the BoB, but oxygen concentrations cannot be drawn down to the values attained in A‐OMZs even using the lowest K m values reported for oxidase enzymes (Table 2, Figure 4A). For one, this model misses the oxygen sinks that are generated during the transition to anaerobic metabolism, with the nitrite produced through nitrate reduction as a conspicuous example. If this nitrite is reoxidized with a much lower K m than aerobic respiration, then oxygen concentrations may be drawn down to lower values. This concept was approximated by modelling some portion of the aerobic respiration with zero order kinetics. Such kinetics can be viewed as approximating a very low K m for the second oxygen sink (see Canfield et al., 2019). With this model, a region of oxygen stability is still observed, but oxygen concentrations can be reduced to anoxia (Figure 4B). In this scenario, the low‐oxygen levels of the BoB represent intermediate upwelling rates (and carbon fluxes), while an ‘anoxic’ A‐OMZ would develop under higher carbon fluxes (see Canfield et al., 2019).
Feedbacks involving the nitrogen cycle could also impact the persistence of low‐oxygen conditions in OMZ environments (Canfield et al., 2019). Thus, if denitrification and/or anammox became active at low‐oxygen concentrations, then the subsequent removal of fixed nitrogen through N2 production would limit primary production and the carbon flux into OMZ waters, stabilizing oxygen to levels limiting N2 loss. However, with the low rates of denitrification measured in OMZ waters, and the high oxygen sensitivity for the anammox process, the nitrogen cycle may not be a primary oxygen stabilizing mechanism in OMZ waters. Still, understanding the processes regulating the transition between OMZ to A‐OMZ waters will be critical to correctly predict N2 loss resulting from future ocean deoxygenation, as the processes regulating N2 production function at very low‐oxygen levels.
CONCLUSIONS
This review has focused on how oxygen regulates key microbial processes in OMZs and A‐OMZs. The main conclusions are offered in bullet form:
High‐affinity oxidase enzymes are expressed under low‐oxygen conditions, but the K m values of these enzymes do not predict the oxygen limit to aerobic metabolisms.
The aerobic metabolisms of aerobic respiration and nitrification likely occur at oxygen levels below detection with current oxygen‐sensing technology. This ability to metabolize at ultra‐low oxygen levels likely arises through concurrent aerobic and anaerobic metabolisms in facultative organisms.
Facultative aerobic heterotrophs transition between aerobic and anaerobic metabolisms at low‐oxygen concentrations, but the oxygen levels where this transition occurs and whether this transition is smooth are uncertain.
Nitrate reduction to nitrite is likely the most important anaerobic carbon mineralization process in A‐OMZs, and the rates of this process in the ‘anoxic’ A‐OMZ core appear to follow expectations based on sediment trap carbon dynamics.
The anammox process is suppressed at oxygen concentrations well below the physiological limit for anammox bacteria due to competition, by aerobic processes, for ammonium and nitrite.
In the upper ‘anoxic’ A‐OMZ, anammox rates are lower than expected from rates of ammonia production from carbon mineralization, with ammonium likely oxidized with oxygen produced by oxygenic photosynthesis and maybe also by non‐photosynthetic oxygen production by ammonia‐oxidizing archaea.
In the Bay of Bengal, low, but measurable oxygen concentrations suppress anammox through both ammonium and nitrite limitation.
The persistently low‐oxygen concentrations such as found in the Bay of Bengal likely result from the oxygen control on biological processes such as aerobic respiration and possibly N2 production.
Accurately predicting oxygen concentrations in future global‐warming scenarios is imperative as the impact of low oxygen on N2 production depends critically on the oxygen levels attained.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the very helpful comments of Dave Karl and an anonymous reviewer. The authors also acknowledge financial support from Villum Foundation (grant no. 16518 to Don E. Canfield and grant no. 00025491 to Beate Kraft).
Canfield, D.E. & Kraft, B. (2022) The ‘oxygen’ in oxygen minimum zones. Environmental Microbiology, 24(11), 5332–5344. Available from: 10.1111/1462-2920.16192
Funding information Villum Fonden, Grant/Award Numbers: 16518, 3373223
DATA AVAILABILITY STATEMENT
This review has not generated any new data. Any data referred to in the text is found in the original references.
REFERENCES
- Arai, H. , Kawakami, T. , Osamura, T. , Hirai, T. , Sakai, Y. & Ishii, M. (2014) Enzymatic characterization and in vivo function of five terminal oxidases in Pseudomonas aeruginosa . Journal of Bacteriology, 196, 4206–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbin, A.R. , Peters, B.D. , Mordy, C.W. , Widner, B. , Casciotti, K.L. & Ward, B.B. (2017) Multiple metabolisms constrain the anaerobic nitrite budget in the Eastern Tropical South Pacific. Global Biogeochemical Cycles, 31, 258–271. [Google Scholar]
- Berelson, W.M. (2001) The flux of particulate organic carbon into the ocean interior: a comparison of four U.S. JGOFS gerional studies. Oceanography, 14, 59–67. [Google Scholar]
- Berg, J. , Ahmerkamp, S. , Pjevac, P. , Hausmann, B. , Milucka, J. & Kuypers, M.M. (2022) How low can they go? Aerobic respiration by microorganisms under apparent anoxia. FEMS Microbiology Reviews, 46, fuac006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow, L.A. , Callbeck, C.M. , Larsen, M. , Altabet, M.A. , Dekaezemacker, J. , Forth, M. et al. (2017) N2 production rates limited by nitrite availability in the Bay of Bengal oxygen minimum zone. Nature Geoscience, 10, 24–29. [Google Scholar]
- Bristow, L.A. , Dalsgaard, T. , Tiano, L. , Mills, D.B. , Bertagnolli, A. , Wright, J.J. et al. (2016) Ammonium and nitrite oxidation at nanomolar oxygen concentrations in oxygen minimum zone waters. Proceedings of the National Academy of Sciences of the United States of America, 113, 10601–10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callbeck, C.M. , Canfield, D.E. , Kuypers, M.M. , Yilmaz, P. , Lavik, G. , Thamdrup, B. et al. (2021) Sulfur cycling in oceanic oxygen minimum zones. Limnology and Oceanography, 60, 2360–2392. [Google Scholar]
- Canfield, D.E. (2006) Models of oxic respiration, denitrification and sulfate reduction in zones of coastal upwelling. Geochimica et Cosmochimica Acta, 70, 5753–5765. [Google Scholar]
- Canfield, D.E. , Kraft, B. , Löscher, C.R. , Boyle, R.A. , Thamdrup, B. & Stewart, F.J. (2019) The regulation of oxygen to low concentrations in marine oxygen‐minimum zones. Journal of Marine Research, 77, 297–324. [Google Scholar]
- Canfield, D.E. , Stewart, F.J. , Thamdrup, B. , De Brabandere, L. , Dalsgaard, T. , Delong, E.F. et al. (2010) A cryptic sulfur cycle in oxygen‐minimum‐zone waters off the Chilean coast. Science, 330, 1375–1378. [DOI] [PubMed] [Google Scholar]
- Cepeda‐Morales, J. , Beier, E. , Gaxiola‐Castro, G. , Lavín, M. & Godínez, V. (2009) Effect of the oxygen minimum zone on the second chlorophyll maximum. Ciencias Marinas, 35, 389–403. [Google Scholar]
- Chen, J. & Strous, M. (2013) Denitrification and aerobic respiration, hybrid electron transport chains and co‐evolution. Biochimica et Biophysica Acta (BBA)‐Bioenergetics, 1827, 136–144. [DOI] [PubMed] [Google Scholar]
- Cotter, P. , Chepuri, V. , Gennis, R. & Gunsalus, R. (1990) Cytochrome o (cyoABCDE) and d (cydAB) oxidase gene expression in Escherichia coli is regulated by oxygen, pH, and the fnr gene product. Journal of Bacteriology, 172, 6333–6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard, T. , Stewart, F.J. , Thamdrup, B. , De Brabandere, L. , Revsbech, N.P. , Ulloa, O. et al. (2014) Oxygen at nanomolar levels reversibly suppresses process rates and gene expression in anammox and denitrification in the oxygen minimum zone off northern Chile. MBio, 5, e01966–e01914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brabandere, L. , Canfield, D.E. , Dalsgaard, T. , Friederich, G.E. , Revsbech, N.P. , Ulloa, O. et al. (2014) Vertical partitioning of nitrogen‐loss processes across the oxic‐anoxic interface of an oceanic oxygen minimum zone. Environmental Microbiology, 16, 3041–3054. [DOI] [PubMed] [Google Scholar]
- Degli Esposti, M. , Mentel, M. , Martin, W. & Sousa, F.L. (2019) Oxygen reductases in alphaproteobacterial genomes: physiological evolution from low to high oxygen environments. Frontiers in Microbiology, 10, 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'mello, R. , Hill, S. & Poole, R.K. (1996) The cytochrome bd quinol oxidase in Escherichia coli has an extremely high oxygen affinity and two oxygen‐binding haems: implications for regulation of activity in vivo by oxygen inhibition. Microbiology, 142, 755–763. [DOI] [PubMed] [Google Scholar]
- Engel, A. , Wagner, H. , Le Moigne, F.A. & Wilson, S.T. (2017) Particle export fluxes to the oxygen minimum zone of the eastern tropical North Atlantic. Biogeosciences, 14, 1825–1838. [Google Scholar]
- Ettwig, K.F. , Butler, M.K. , Le Paslier, D. , Pelletier, E. , Mangenot, S. , Kuypers, M.M. et al. (2010) Nitrite‐driven anaerobic methane oxidation by oxygenic bacteria. Nature, 464, 543–548. [DOI] [PubMed] [Google Scholar]
- Fenchel, T. , Bernard, C. , Esteban, G. , Finlay, B.J. , Hansen, P.J. & Iversen, N. (1995) Microbial diversity and activity in a Danish Fjord with anoxic deep water. Ophelia, 43, 45–100. [Google Scholar]
- Garcia‐Robledo, E. , Borisov, S. , Klimant, I. & Revsbech, N.P. (2016) Determination of respiration rates in water with sub‐micromolar oxygen concentrations. Frontiers in Marine Science, 3, 244. [Google Scholar]
- Garcia‐Robledo, E. , Padilla, C.C. , Aldunate, M. , Stewart, F.J. , Ulloa, O. , Paulmier, A. et al. (2017) Cryptic oxygen cycling in anoxic marine zones. Proceedings of the National Academy of Sciences, 114, 8319–8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goericke, R. , Olson, R. & Shalapyonok, A. (2000) A novel niche for Prochlorococcus sp. in low‐light suboxic environments in the Arabian Sea and the Eastern Tropical North Pacific. Deep Sea Research Part I: Oceanographic Research Papers, 47, 1183–1205. [Google Scholar]
- Jensen, M.M. , Kuypers, M.M.M. , Lavik, G. & Thamdrup, B. (2008) Rates and regulation of anaerobic ammonium oxidation and denitrification in the Black Sea. Limnology and Oceanography, 53, 23–36. [Google Scholar]
- Ji, Q. , Buitenhuis, E. , Suntharalingam, P. , Sarmiento, J.L. & Ward, B.B. (2018) Global nitrous oxide production determined by oxygen sensitivity of nitrification and denitrification. Global Biogeochemical Cycles, 32, 1790–1802. [Google Scholar]
- Kalvelage, T. , Jensen, M.M. , Contreras, S. , Revsbech, N.P. , Lam, P. , Gunter, M. et al. (2011) Oxygen sensitivity of anammox and coupled N‐cycle processes in oxygen minimum zones. PLoS One, 6, e29299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalvelage, T. , Lavik, G. , Jensen, M.M. , Revsbech, N.P. , Löscher, C. , Schunck, H. et al. (2015) Aerobic microbial respiration in oceanic oxygen minimum zones. PLoS One, 10, e0133526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalvelage, T. , Lavik, G. , Lam, P. , Contreras, S. , Arteaga, L. , Loscher, C.R. et al. (2013) Nitrogen cycling driven by organic matter export in the South Pacific oxygen minimum zone. Nature Geoscience, 6, 228–234. [Google Scholar]
- Koch, H. , Lücker, S. , Albertsen, M. , Kitzinger, K. , Herbold, C. , Spieck, E. et al. (2015) Expanded metabolic versatility of ubiquitous nitrite‐oxidizing bacteria from the genus Nitrospira. Proceedings of the National Academy of Sciences, 112, 11371–11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft, B. , Jehmlich, N. , Larsen, M. , Bristow, L.A. , Könneke, M. , Thamdrup, B. et al. (2022) Oxygen and nitrogen production by an ammonia‐oxidizing archaeon. Science, 375, 97–100. [DOI] [PubMed] [Google Scholar]
- Lam, P. & Kuypers, M.M.M. (2011) Microbial nitrogen cycling processes in oxygen minimum zones. Annual Review of Marine Science, 3, 317–345. [DOI] [PubMed] [Google Scholar]
- Lam, P. , Lavik, G. , Jensen, M.M. , van de Vossenberg, J. , Schmid, M. , Woebken, D. et al. (2009a) Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proceedings of the National Academy of Sciences of the United States of America, 106, 4752–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin, P. , González, B. , Santibáñez, J.F. , Scanlan, D.J. & Ulloa, O. (2010) Novel lineages of Prochlorococcus thrive within the oxygen minimum zone of the eastern tropical South Pacific. Environmental Microbiology Reports, 2, 728–738. [DOI] [PubMed] [Google Scholar]
- Lücker, S. , Nowka, B. , Rattei, T. , Spieck, E. & Daims, H. (2013) The genome of Nitrospina gracilis illuminates the metabolism and evolution of the major marine nitrite oxidizer. Frontiers in Microbiology, 4, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant, H.K. , Ahmerkamp, S. , Lavik, G. , Tegetmeyer, H.E. , Graf, J. , Klatt, J.M. et al. (2017) Denitrifying community in coastal sediments performs aerobic and anaerobic respiration simultaneously. The ISME Journal, 11, 1799–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens‐Habbena, W. , Berube, P.M. , Urakawa, H. , de la Torre, J.R. & Stahl, D.A. (2009) Ammonia oxidation kinetics determine niche separation of nitrifying archaea and bacteria. Nature, 461, 976–U234. [DOI] [PubMed] [Google Scholar]
- Medina, L.E. , Taylor, C.D. , Pachiadaki, M.G. , Henríquez‐Castillo, C. , Ulloa, O. & Edgcomb, V.P. (2017) A review of protist grazing below the photic zone emphasizing studies of oxygen‐depleted water columns and recent applications of in situ approaches. Frontiers in Marine Science, 4, 105. [Google Scholar]
- Miura, H. , Mogi, T. , Ano, Y. , Migita, C.T. , Matsutani, M. , Yakushi, T. et al. (2013) Cyanide‐insensitive quinol oxidase (CIO) from Gluconobacter oxydans is a unique terminal oxidase subfamily of cytochrome bd. The Journal of Biochemistry, 153, 535–545. [DOI] [PubMed] [Google Scholar]
- Morris, R.L. & Schmidt, T.M. (2013) Shallow breathing: bacterial life at low O2 . Nature Reviews Microbiology, 11, 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi, S. , Bange, H.W. , Farías, L. , Monteiro, P. , Scranton, M. & Zhang, J. (2010) Marine hypoxia/anoxia as a source of CH4 and N2O. Biogeosciences, 7, 2159–2190. [Google Scholar]
- Oschlies, A. (2021) A committed fourfold increase in ocean oxygen loss. Nature Communications, 12, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oschlies, A. , Brandt, P. , Stramma, L. & Schmidtko, S. (2018) Drivers and mechanisms of ocean deoxygenation. Nature Geoscience, 11, 467–473. [Google Scholar]
- Padilla, C.C. , Bristow, L.A. , Sarode, N. , Garcia‐Robledo, E. , Gómez Ramírez, E. , Benson, C.R. et al. (2016) NC10 bacteria in marine oxygen minimum zones. The ISME Journal, 10, 2067–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisig, O. , Zufferey, R. , Thony‐Meyer, L. , Appleby, C.A. & Hennecke, H. (1996) A high‐affinity cbb3‐type cytochrome oxidase terminates the symbiosis‐specific respiratory chain of Bradyrhizobium japonicum . Journal of Bacteriology, 178, 1532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel, F. , Amrani, A. , Pieulle, L. , Lamrabet, O. , Voordouw, G. , Seddiki, N. et al. (2013) Membrane‐bound oxygen reductases of the anaerobic sulfate‐reducing Desulfovibrio vulgaris Hildenborough: roles in oxygen defence and electron link with periplasmic hydrogen oxidation. Microbiology, 159, 2663–2673. [DOI] [PubMed] [Google Scholar]
- Revsbech, N.P. , Thamdrup, B. , Dalsgaard, T. & Canfield, D.E. (2011) Construction of STOX oxygen sensors and their application for determination of O2 concentrations in oxygen minimum zones. In: Klotz, M.G. (Ed.) Methods in enzymology: research on nitrification and related processes, part a, Vol. 486. San Diego: Elsevier Academic Press Inc, pp. 325–341. [DOI] [PubMed] [Google Scholar]
- Robertson, L.A. , Dalsgaard, T. , Revsbech, N.P. & Kuenen, J.G. (1995) Confirmation of aerobic denitrification in batch cultures, using gas chromotography and N‐15 mass spectrometry. FEMS Microbiology Ecology, 18, 113–119. [Google Scholar]
- Schmidtko, S. , Stramma, L. & Visbeck, M. (2017) Decline in global oceanic oxygen content during the past five decades. Nature, 542, 335–339. [DOI] [PubMed] [Google Scholar]
- Shaffer, G. (1989) A model of biogeochemical cycling of phosphorus, nitrogen, oxygen, and sulphur in the ocean: one step toward a global climate model. Journal of Geophysical Research, 94, 1979–2004. [Google Scholar]
- Sousa, F.L. , Alves, R.J. , Ribeiro, M.A. , Pereira‐Leal, R.B. , Teixeira, M. & Pereira, M.M. (2012) The superfamily of heme‐copper oxygen reductases: types and evolutionary considerations. Biochimica et Biophysica Acta, 1817, 629–637. [DOI] [PubMed] [Google Scholar]
- Stenmark, P. & Nordlund, P. (2003) A prokaryotic alternative oxidase present in the bacterium Novosphingobium aromaticivorans . FEBS Letters, 552, 189–192. [DOI] [PubMed] [Google Scholar]
- Stewart, F.J. , Ulloa, O. & DeLong, E.F. (2012) Microbial metatranscriptomics in a permanent marine oxygen minimum zone. Environmental Microbiology, 14, 23–40. [DOI] [PubMed] [Google Scholar]
- Stolper, D.A. , Revsbech, N.P. & Canfield, D.E. (2010) Aerobic growth at nanomolar oxygen concentrations. Proceedings of the National Academy of Sciences of the United States of America, 107, 18755–18760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramma, L. , Prince, E.D. , Schmidtko, S. , Luo, J. , Hoolihan, J.P. , Visbeck, M. et al. (2012) Expansion of oxygen minimum zones may reduce available habitat for tropical pelagic fishes. Nature Climate Change, 2, 33–37. [Google Scholar]
- Thamdrup, B. , Dalsgaard, T. , Jensen, M.M. , Ulloa, O. , Farias, L. & Escribano, R. (2006) Anaerobic ammonium oxidation in the oxygen‐deficient waters off northern Chile. Limnology and Oceanography, 51, 2145–2156. [Google Scholar]
- Thamdrup, B. , Dalsgaard, T. & Revsbech, N.P. (2012) Widespread functional anoxia in the oxygen minimum zone of the eastern South Pacific. Deep‐Sea Research Part I‐Oceanographic Research Papers, 65, 36–45. [Google Scholar]
- Tiano, L. , Garcia‐Robledo, E. , Dalsgaard, T. , Devol, A.H. , Ward, B.B. , Ulloa, O. et al. (2014) Oxygen distribution and aerobic respiration in the north and south eastern tropical Pacific oxygen minimum zones. Deep‐Sea Research Part I‐Oceanographic Research Papers, 94, 173–183. [Google Scholar]
- Tseng, C.P. , Albrecht, J. & Gunsalus, R.P. (1996) Effect of microaerophilic growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, and dmsABC) respiratory pathway genes in Escherichia coli . Journal of Bacteriology, 178, 1094–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa, O. , Canfield, D.E. , DeLong, E.F. , Letelier, R.M. & Stewart, F.J. (2012) Microbial oceanography of anoxic oxygen minimum zones. Proceedings of the National Academy of Sciences of the United States of America, 109, 15996–16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa, O. , Henríquez‐Castillo, C. , Ramírez‐Flandes, S. , Plominsky, A.M. , Murillo, A.A. , Morgan‐Lang, C. et al. (2021) The cyanobacterium Prochlorococcus has divergent light‐harvesting antennae and may have evolved in a low‐oxygen ocean. Proceedings of the National Academy of Sciences, 118, e2025638118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niftrik, L.A. , Fuerst, J.A. , Damsté, J.S.S. , Kuenen, J.G. , Jetten, M.S. & Strous, M. (2004) The anammoxosome: an intracytoplasmic compartment in anammox bacteria. FEMS Microbiology Letters, 233, 7–13. [DOI] [PubMed] [Google Scholar]
- van Vliet, D.M. , von Meijenfeldt, F.B. , Dutilh, B.E. , Villanueva, L. , Sinninghe Damsté, J.S. , Stams, A.J. et al. (2021) The bacterial sulfur cycle in expanding dysoxic and euxinic marine waters. Environmental Microbiology, 23, 2834–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, J.J. , konwar, K.M. & Hallam, S.J. (2012) Microbial ecology of expanding oxygen minimum zones. Microbiology, 10, 381–394. [DOI] [PubMed] [Google Scholar]
- Wyrtki, K. (1962) The oxygen minima in relation to ocean circulation. In: Deep Sea research and oceanographic abstracts. Amsterdam, Netherlands: Elsevier, pp. 11–23. [Google Scholar]
- Zakem, E. & Follows, M. (2017) A theoretical basis for a nanomolar critical oxygen concentration. Limnology and Oceanography, 62, 795–805. [Google Scholar]
- Zakem, E.J. , Mahadevan, A. , Lauderdale, J.M. & Follows, M.J. (2020) Stable aerobic and anaerobic coexistence in anoxic marine zones. The ISME Journal, 14, 288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This review has not generated any new data. Any data referred to in the text is found in the original references.


