Abstract
Non‐adherence to medications is a critical challenge in the management of people with chronic kidney disease (CKD). This review explores the complexities of adherence in this population, the unique barriers and enablers of good adherence behaviours, and the role of emerging digital health technologies in bridging the gap between evidence‐based treatment plans and the real‐world standard of care. We present the current evidence supporting the use of digital health interventions among CKD populations, identifying the key research questions that remain unanswered, and providing practical strategies for clinicians to support medication adherence in a digital age.
Keywords: chronic kidney disease (CKD), digital health, eHealth, medication adherence
Summary at a glance
This manuscript reviews the current evidence for the use of digital health technologies to enhance medication adherence in chronic kidney disease populations. Practical strategies and considerations for integrating technologies into clinical practice are provided.
1. INTRODUCTION
Chronic kidney disease (CKD) poses a major risk to the wellbeing of those affected, with a risk of impaired quality of life and reduced life expectancy, and imposes a formidable economic burden on health care systems caring for such individuals. A variety of medications are used in the management of CKD to reduce the progression to kidney failure, to ameliorate the high risk of cardiovascular complications, to address symptoms that can deleteriously affect quality of life, and to manage common co‐morbid conditions such as diabetes and elevated blood pressure. 1 , 2 , 3 People with CKD are often prescribed complex medication regimens and are tasked with understanding and taking these medications appropriately, often for the remainder of their life. 2 Unsurprisingly, adherence to medications, defined as the extent to which patients take medications as prescribed by their health care providers, 4 is suboptimal and a significant clinical challenge. Lower levels of medication adherence are associated with increased rates of hospital admissions, poorer health outcomes, increased morbidity and mortality and increased health system costs. 5 It is therefore a major public health priority to explore innovative and evidence‐based strategies to improve medication adherence, and digital health developments provide a promising new avenue for change.
2. MEDICATION ADHERENCE
Despite the rapid advancement in medication development, medication non‐adherence remains at an estimated 40%–60% in developed countries among people with chronic disease. 6 , 7 , 8 , 9 Traditionally, medication adherence has been viewed as static and dichotomous (i.e., a person is adherent or non‐adherent). 7 However, medication adherence is dynamic and varies in response to factors such as disease activity, treatment methods and psychosocial factors. 7 , 10 Adherence has been conceptualized into three core components: initiation, implementation and persistence. 7 , 10 A fourth concept, primary non‐adherence, where a patient is prescribed a medication but never fills a prescription, is differentiated from these other components of adherence (Table 1). While non‐adherence may reflect an individual's autonomy in self‐management, or their decision not to consent to an ongoing treatment based upon their own interpretation of the risk/benefit profile, 11 it remains an important surrogate marker for a variety of patient‐important health outcomes. 5
TABLE 1.
| Component of adherence | Description | Potential role for digital technology |
|---|---|---|
| Primary non‐adherence | When a patient never fills a prescription. A large retrospective study estimated that 28% of patients never filled a new prescription, with highest primary non‐adherence rates found for diabetic, anti‐hypertensives and lipid‐lowering drugs 53 |
Can be monitored through electronic prescribing with linkage to pharmacy dispensation data Minimal research into digital health strategies to improve primary non‐adherence |
| Initiation | When a patient begins taking a medication |
Can be monitored through electronic pill boxes or other monitoring systems Minimal research into digital health strategies to improve initiation |
| Implementation (or execution) | When a patient takes a medication, often measured as the proportion of prescribed pills that are taken in a given time frame | Some demonstrated efficacy of text message programs, smartphone applications, and the use of the ‘internet of things’ to provide education and reminders that boost implementation and persistence of medication‐taking |
| Persistence |
When a patient continues to take a medication continuously over longer intervals There is some evidence to suggest that approximately 40% of patients discontinue medications within 1 year 10 |
|
| Discontinuation | When a patient stops taking a medication | Minimal research into digital health strategies to reduce discontinuation rates |
3. MEDICATION ADHERENCE IN CKD
Chronic kidney disease poses challenges for optimizing medication adherence as people with advanced CKD require an average of 10 regular daily medications. 2 Adherence can be significantly complicated by the complexity of the medication schedule, medication‐related symptoms, and psychosocial burdens which may be disease‐related or concomitant. 1 , 12 , 13 Table 2 highlights the various reported adherence rates in CKD populations. Medication adherence rates vary significantly between studies, in part due to differing definitions and measures, 14 with objective measures such as pill counts and prescription refills tending to indicate worse adherence rates than patient self‐report by validated questionnaires, or less specific measures such as serum phosphate levels. 14 Nonetheless, it is clear that medication non‐adherence is a key issue in CKD management.
TABLE 2.
Medication adherence in various CKD populations
| Chronic kidney disease |
| Approximately 30% of people are non‐adherent to blood pressure lowering medications 14 , 15 resulting in an estimated 57% increased risk for progression to ESKD, and a 45% and 126% increased risk of cardiovascular events and death, respectively 15 , 16 |
| Dialysis |
|
Similar to CKD populations, approximately 38% of people are non‐adherent to blood pressure lowering medications 2 Non‐adherence rates for phosphate binders average 50% |
| Transplant |
| Estimated non‐adherence to immunosuppression estimated to be 36% among kidney transplant recipients (compared to 7–15% in other transplant populations) 17 resulting in 1.5 times the risk of acute rejection and 65% higher risk of graft loss 18 |
3.1. Risk factors for medication non‐adherence in CKD
There are well‐established risk factors for suboptimal medication‐taking behaviours, including demographic (e.g., younger age, male), cultural (e.g., non‐Caucasian ethnicity) and psychosocial (e.g., forgetfulness, depression, anxiety, lower socioeconomic status, lower level of education, lack of social support and poor treatment engagement) factors in people with CKD. 1 , 2 , 3 , 12 , 19 , 20 , 21 Forgetting medications remains the most significant patient‐reported reason for sub‐optimal adherence, 3 , 4 raising the possibility of medication reminders as an intervention strategy. The relative importance of individual predictors of non‐adherence appears to vary across different clinical settings or stages of CKD. For example, while men show higher rates of non‐adherence in predialysis CKD and transplant, this is not the case in dialysis, where men and women show similar rates of non‐adherence to phosphate binders. 2 , 3 , 12 Similarly, while low education levels in people with a functioning kidney transplant predicts poor adherence, this is not seen in the dialysis‐dependent populations. The reasons for such differences are not clear, and further evaluation is required.
The impact of out‐of‐pocket cost on treatment adherence is significant and widespread, affecting those in both high‐ and low‐middle income countries, but with a disproportionate impact upon low socioeconomic households and the uninsured. 22 Out‐of‐pocket costs are associated with poorer medication adherence, skipping of dialysis sessions, and forgoing of treatment altogether, leading to worse patient outcomes. 22
Health literacy (HL), reflecting the cognitive and social skills that determine a person's motivation and ability to access, understand and use information to promote and maintain good health, can also significantly influence adherence. 23 In CKD, poor HL is estimated to affect approximately 25% of the population, and is associated with medication non‐adherence, more rapid deterioration in kidney function, and higher mortality rates. 23
Qualitative interview studies with people with CKD have described numerous enablers and barriers of appropriate medication‐taking (Figure 1). 24 , 25 , 26 Factors that may support appropriate medication‐taking include people's perception that medications can improve their future health and symptom burden. Medications that could slow disease progression are often prioritized. Patients reported personalized strategies, stable and established routines, and clinician rapport helped to maintain better adherence. However, lack of education regarding medication benefits may lead to patients changing or ceasing their medications, particularly those with adverse side effects – ‘some medicines make me dizzy…it is a problem as I get no support at home’. 25
FIGURE 1.
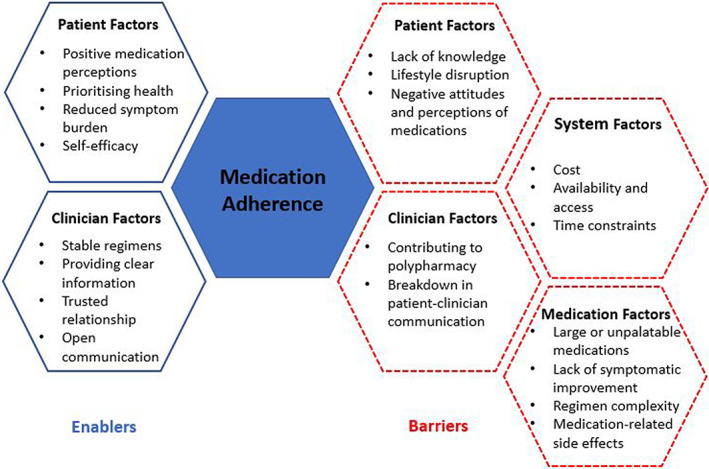
Authors' synthesis of barriers and enablers reported by patients with CKD through qualitative studies 24 , 25 , 26
4. METHODS TO IMPROVE MEDICATION ADHERENCE IN CKD
Improving medication‐taking requires a multidimensional approach, exploring patient factors (knowledge, beliefs about consequences, behavioural regulation, memory and decision‐making processes), environmental factors (access, cost) and health system factors (patient‐clinician communication). 2 , 14 , 27 Examples of interventions that have improved medication adherence in CKD have included cost subsidies for phosphate binders and multidisciplinary team patient education. 28 However, such interventions may be time‐consuming and costly. The introduction of technology and digital health and its incorporation into medication management may provide new and revolutionary methods in addressing key components of the adherence constructs.
5. USING DIGITAL TECHNOLOGIES TO IMPROVE MEDICATION ADHERENCE IN CKD
Over recent decades, the rapid evolution of digital technology has resulted in unprecedented opportunities to connect with patients and promote improvements in health behaviours such as medication adherence. These approaches differ widely in mode and style, but can be grouped under the concept of digital health or eHealth, defined broadly as ‘health services and information delivered or enhanced through the Internet and related technologies’. 29 This umbrella term includes interventions as diverse as text message programs and mobile phone applications (together known as mHealth 30 ), the ‘internet of things’, and social media engagement. They can revolve around education, behavioural counselling, self‐monitoring, reminder systems, clinical decision aids, or combinations of purposes. 31
The potential of digital health interventions lies in the ability to provide access to education and support regardless of geographical location, reduce the patient‐borne costs related to travel and clinic appointments and reduce clinician burden and time. Digital interventions may target modifiable patient‐factors and behaviours, however not all factors associated with poor medication adherence can be targeted by digital interventions. While there are evolving opportunities, many questions remain to be answered, including which patients are most likely to benefit, which technologies are most likely to yield results, and how they should be applied in a way that is patient‐centred, sustainable, and cost‐effective.
5.1. Electronic prescribing
The use of electronic prescribing, rather than providing patients with a paper script that could be misplaced, may be associated with an improvement in primary adherence of 10%. 5 More sophisticated electronic prescription software can also receive dispensation data from pharmacies, 5 which could help to monitor for primary non‐adherence, later non‐adherence, or discontinuation. The clinician could then discuss any adherence barriers during the next office visit.
5.2. Mobile phone text messaging
Mobile phone text messaging is a promising eHealth medication adherence intervention, with an emerging body of work supporting efficacy in facilitating consistent medication use in chronic disease populations. A meta‐analysis of 16 randomized controlled trials, including several chronic disease populations such as people with elevated blood pressure and asthma, suggested that text messaging programs were associated with a doubling of the odds of medication adherence (odds ratio [OR] 2.11; 95% confidence interval [CI] 1.52–2.93; p < 0.001) from an assumed baseline of 50%, and resulted in an estimated absolute increase in adherence of 17.8% 32 These interventions primarily provide medication reminders, which help to address forgetfulness as a key adherence barrier. 3 They may also be used to provide education to address the negative attitudes towards medications and to support self‐efficacy and self‐management, although the effect of text messaging on facilitating knowledge and self‐management in chronic disease remains unclear and is likely context‐dependent. 33 Whether text message interventions can improve, or take into account, factors such as HL remains unknown. Text message programs have the added benefit of being low‐cost interventions using ubiquitous technology, 33 which could be particularly advantageous in developing countries where there may be less access to face‐to‐face education and support.
There are few examples of text message programs rigorously assessed in people with CKD or kidney transplants, and these have been limited by small sample size and short duration. 31 A study of 34 adolescents and young adults with CKD found improvements in electronically monitored adherence with the use of a 8‐week program of education and support text messages (p = 0.04). 34 There was no significant change in self‐efficacy scores. In the TAKE‐IT randomized trial, 169 adolescents or young adults with kidney transplants received regular text messages, e‐mails and/or visual cue dose reminders, and third monthly face‐to‐face coaching sessions 12 months, resulting in significantly greater odds of taking medications at or near the prescribed time (OR 1.74; 95% CI 1.21–2.50). 35 The intervention was based upon a self‐management model, although self‐management confidence was not assessed.
Designing a text message intervention requires many strategic decisions that could influence the efficacy and acceptability. Given the limited number of clinical trials in this space, there is scant evidence to guide decisions about design features, including how best to adapt text messaging interventions to specific clinical contexts. Text message personalisation, such as inclusion of the participant's name, may improve engagement. 32 Programs have shown benefits regardless of whether the messages were unidirectional or two‐way communication. 32 The frequency of messaging also requires careful consideration, with the majority of interventions in chronic disease populations involving daily messaging. 32 In a study of people discharged from hospital with elevated blood pressure, 94% of participants who chose to receive text medication reminders opted for a daily frequency, and 97% of those who chose to receive informational texts selected a thrice‐weekly frequency. 36 The optimum strategy may vary according to the clinical setting and the recipient's own preferences. Lastly, the appropriate duration of text message interventions is unclear, and is likely context‐dependent; simple text message education programs may only require a short program, while some individuals could potentially benefit from receiving text message adherence reminders indefinitely. There is a need for randomized assessment of whether user‐determined customisation of intervention duration and frequency may help to better service an individual's needs. 32
5.3. Smart phone applications
Smartphone applications, or ‘apps’, also provide potential opportunities for enhanced adherence, and a host of different applications have been developed, 37 although few have been evaluated in clinical trials. Among people with chronic disease, such applications have been associated with small improvements in medication adherence. 38 Producing an application of ongoing utility is, however, challenging. In the iNephro Project, 11 688 smartphone users with chronic health conditions such as CKD downloaded the 'Medication Plan’ application which allowed creation of a medication list, and the sending of ‘push‐notification’ alerts to remind users about taking their medications. 39 The use of the intervention declined over the first 2 months, and by 12 months only 1% of users were regularly using the application. The effect upon adherence was not measured.
For individuals with advanced CKD, Ong and colleagues designed a smartphone application to facilitate medication management, blood pressure monitoring, symptom assessment, and tracking of laboratory results. 40 Across 45 participants, 127 discrepancies in home medication use were identified, including patients taking medications that were not prescribed, were prescribed at a different dose, or had been previously ceased. While these discrepancies could reflect patient autonomy and self‐management leading to evolving treatment strategies, differences from the documented care plans may increase the risk of communication breakdown and subsequent errors in shared decision‐making and management. Medication adherence data was not reported, although the intervention was associated with a 3.5 mmHg improvement in systolic blood pressure control (95% CI −1.8 to −5.0 mmHg). The majority of participants (~77%) expressed interest in continuing use of the application after the 6‐month study period, and most reported that they felt more confident and in control of their CKD management after the intervention, reflecting improved self‐efficacy.
A smartphone application designed to improve general medication adherence among 138 Korean individuals who had received kidney transplants did not, however result in significant improvements over 6 months. 42 The use of the application, which provided medication reminders, an educational video about the importance of immunosuppressive therapies, and adherence tracking, declined to only 11.5% by 3 months, with the difficulty in engaging patients likely diminishing any intervention effect. There was a trend towards higher adherence rates among those individuals who engaged with the application beyond the first month. The authors suggested that better engagement might be achieved with improved personalisation and feedback, and through introducing the intervention at a critical juncture such as soon after receiving a kidney transplant. There may also be a discrepancy between clinician and patient opinions about what constitutes a helpful application, which could contribute to low engagement; in a systematic assessment of available kidney‐specific smartphone applications, nephrologists' and patients' assessment of application quality were poorly correlated. 37 There is a clear need for consumer engagement throughout the design and development of interventions and for further research to identify strategies and/or design features to improve participant engagement.
5.4. The ‘Internet of things’
The ‘Internet of things’ is a broad term for the range of ‘smart’ devices that use an Internet connection or near‐field communication technology to form an integrated network. 43 Innovation is progressing rapidly in this field, with the potential to support and improve adherence. For example, McGillicuddy and colleagues developed an application that received data from an electronic medication dispenser and a sphygmomanometer to generate context‐specific text messages to promote adherence, and demonstrated significant improvements in both adherence and clinic‐measured blood pressure. 41 Post‐intervention surveys suggested high levels of satisfaction with the program.
In another study involving electronic pill boxes, 120 people with kidney transplants were all provided sophisticated pill boxes with three variations in function. 44 In the control group, the pill boxes monitored adherence. In the second group, the pill bottles also emitted a chime and illuminated a light to indicate when a dose was due, and patients could also set customized medication reminders by texts, telephone calls, alarms and/or e‐mails. 44 In the third group, there was the addition of a fortnightly notification sent to the treating physician when adherence dropped below 90%. Adherence in the second half of the 180‐day intervention period was 24% higher with the pill bottle alerts and customized reminders compared to the control group (78% vs. 55%, 95% CI for difference 10%–38%) and 33% higher in the group that also had physician notifications (88% vs. 55%, 95% CI for difference 21%–46%). Interestingly, almost all participants self‐reported perfect or near‐perfect adherence, reflecting the accuracy of recall versus contemporaneously assessed objective measures for medication adherence.
The use of the ‘Internet of things’ for adherence continues to evolve along with new technological advances, with developments as advanced as a smartwatch application that uses gyroscopic movement sensors and deep machine learning to recognize the body movements that suggest that a medication is being taken. 43 Significant further research is needed to explore the feasibility of such technology in patient care.
5.5. Other digital health strategies
There is a wide range of other eHealth strategies with the potential to improve kidney health self‐management and adherence. The very language of digital communication is evolving, with growing use of emojis as standardized international symbols that can be integrated into digital health interventions and may aid patient understanding and engagement. 45 , 46 Engagement in treatments may also be improved through the use of ubiquitous social media services. A Renal Health Instagram platform based in Brazil has more than 8500 ‘followers’, with users expressing that the service is very useful for sharing their experiences, as well as learning about their disease management. 47 Similarly, communities of people with kidney disease have developed on Facebook, which has been seen to provide opportunities to share information and experiences, and to foster emotional and social support. 48 Interestingly, a study seeking to establish the potential of Twitter for boosting health engagement randomized 611 patients with elevated blood pressure to either a control group or to health information disseminated twice weekly. While the intervention was deemed feasible, it unfortunately did not improve participants' blood pressure control or their measured level of health engagement. 49 These programs together highlight the scope for future patient engagement initiatives in the social media domain.
5.6. Challenges of digital integration
There are challenges and risks of eHealth interventions that require consideration and mitigation at the patient, clinician and health system levels. 50 At a patient level, privacy is a key concern, with a need for careful data management protocols to prevent breaches of confidentiality. 51 It is also possible that interventions could pose a small risk of harm, as generic health messaging may not be sufficiently tailored to individual patients, and often needs to be expressed with few words and limited context, introducing the possibility of safety issues. Patients without access to the required technology, or with lower digital health literacy, may also be disadvantaged by the growing use of digital health technology, contributing to a form of health inequality known as the ‘digital divide’. 46
Stakeholder engagement has suggested several concerns that may reduce clinicians' uptake of digital health interventions, including the lack of official endorsement of applications by expert bodies, a paucity of clinical trial evidence to support the efficacy and safety of different digital health strategies, and a lack of clinician familiarity with specific interventions. 52
At a healthcare system level, the translation of digital health interventions from the research setting into routine clinical practice has been slow. 30 Identified barriers include the need for further evidence of efficacy for patient outcomes, the cost of technologies and the infrastructure needed to support their widespread use, and competing healthcare system priorities. 30 Further research into maximizing the cost‐effectiveness and sustainability of these interventions may be beneficial in encouraging further uptake.
The authors' recommendations for practical strategies to improve adherence with the use of digital interventions are outlined in Box 1.
BOX 1.
Authors' recommendations for clinicians for enhancement of adherence in clinical practice
|
5.7. Limitations of existing digital health literature
There are significant gaps in the current evidence base for digital health technologies to support adherence. There is a need for more consistent use of adherence definitions and measurements to enable meaningful synthesis and evaluation of interventions. This includes a need to distinguish between the components of adherence discussed in Table 1; most digital health interventions have included participants already receiving long‐term treatments, and assess outcomes pertinent to treatment implementation and persistence, without providing an analysis of the rates of primary non‐adherence, initiation or discontinuation. As a result, the impact of digital health technology on these outcomes remains unclear.
There is a paucity of literature regarding specific design features of digital interventions to promote engagement, uptake and retention of users, including those with lower levels of digital health literacy. A deeper understanding of the appropriate, or optimal, use of behavioural change techniques within these digital health interventions may also significantly advance this field. Future research is needed to establish whether the benefit of the interventions is sustained over time, as none of the aforementioned studies assessed adherence beyond the end of the intervention period.
There is also a need to extend upon existing studies to confirm efficacy in different settings and to provide reassurance about generalisability. Most of the discussed studies involve relatively small numbers of participants, and the majority of interventions have been tested in developed nations, such that the feasibility and merits of the interventions may not apply to other settings including low‐resource countries. A detailed exploration is needed to understand the infrastructure, training and support that would be required to implement digital interventions in a variety of geographic and sociocultural settings so that access to digital health platforms can help to improve, rather than exacerbate, global health inequality.
Further research is also needed to explore the persistent barriers to non‐adherence for individuals already engaging with digital health interventions. The majority of interventions address the adherence barrier of forgetfulness, or the need for education and support to improve medication attitudes, self‐management, and self‐efficacy, although few studies directly assess these outcomes and their relationship to overall adherence. Such interventions do not address other adherence barriers such as cost, medication complexity, the stability of medication regimens, or side effects. While this may be an inherent limitation of digital health strategies, it is important to investigate the key remaining barriers in populations already using digital health platforms, and to explore whether digital health innovation could help to target a wider range of adherence barriers than what has previously been attempted.
6. CONCLUSION
Medication adherence remains a key challenge in improving health outcomes, and people with chronic kidney disease must often manage particularly complex medication regimens that pose a further barrier to adherence. Digital health technologies are tools that may hold promise in supporting patients in their adherence behaviours, although the existing studies generally involve small numbers of participants, short duration of follow‐up, and only address a subset of adherence barriers. Much further work is needed to identify the most effective digital health interventions in different clinical settings, to determine the sustainability of benefits, to demonstrate implementation on a large scale, and to understand how to integrate their use into routine patient care.
CONFLICT OF INTEREST
None of the authors have conflicts of interest to declare.
ACKNOWLEDGEMENT
DVO received support through the NHMRC Clinical Trials Centre Postgraduate Research Scholarship and subsequently the Royal Australasian College of Physicians Jacquot Research Entry Scholarship, as well as the Australian Government Research Training Program Scholarships. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
O'Hara DV, Yi TW, Lee VW, Jardine M, Dawson J. Digital health technologies to support medication adherence in chronic kidney disease. Nephrology. 2022;27(12):917‐924. doi: 10.1111/nep.14113
Daniel V. O'Hara and Tae Won Yi contributed equally to this work.
REFERENCES
- 1. Sousa H, Ribeiro O, Paúl C, et al. Social support and treatment adherence in patients with end‐stage renal disease: a systematic review. Semin Dial. 2019;32(6):562‐574. [DOI] [PubMed] [Google Scholar]
- 2. Ghimire S, Castelino RL, Lioufas NM, Peterson GM, Zaidi STR. Nonadherence to medication therapy in haemodialysis patients: a systematic review. PLoS One. 2015;10(12):e0144119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seng JJB, Tan JY, Yeam CT, Htay H, Foo WYM. Factors affecting medication adherence among pre‐dialysis chronic kidney disease patients: a systematic review and meta‐analysis of literature. Int Urol Nephrol. 2020;52(5):903‐916. [DOI] [PubMed] [Google Scholar]
- 4. Osterberg L, Blaschke T. Adherence to medication. New Eng J Med. 2005;353(5):487‐497. [DOI] [PubMed] [Google Scholar]
- 5. Neiman AB, Ruppar T, Ho M, et al. Improving medication adherence for chronic disease management—innovations and opportunities. Am J Transplant. 2018;18(2):514‐517. [DOI] [PubMed] [Google Scholar]
- 6. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86:304‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stirratt MJ, Curtis JR, Danila MI, Hansen R, Miller MJ, Gakumo CA. Advancing the science and practice of medication adherence. J Gen Intern Med. 2018;33(2):216‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28(4):437‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. KDIGO . Clinical practice guideline for the Care of Kidney Transplant Recipients. Am J Transplant. 2009;9(s3):S1‐S155. [DOI] [PubMed] [Google Scholar]
- 10. Blaschke TF, Osterberg L, Vrijens B, Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Ann Rev Pharmacol Toxicol. 2012;52(1):275‐301. [DOI] [PubMed] [Google Scholar]
- 11. Sandman L, Granger BB, Ekman I, Munthe C. Adherence, shared decision‐making and patient autonomy. Med Health Care Philos. 2012;15(2):115‐127. [DOI] [PubMed] [Google Scholar]
- 12. Gokoel SRM, Gombert‐Handoko KB, Zwart TC, van der Boog PJM, Moes D, de Fijter JW. Medication non‐adherence after kidney transplantation: a critical appraisal and systematic review. Transplant Rev. 2020;34(1):100511. [DOI] [PubMed] [Google Scholar]
- 13. Murali KM, Mullan J, Roodenrys S, Hassan HC, Lambert K, Lonergan M. Strategies to improve dietary, fluid, dialysis or medication adherence in patients with end stage kidney disease on dialysis: a systematic review and meta‐analysis of randomized intervention trials. PLoS One. 2019;14(1):e0211479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burnier M, Pruijm M, Wuerzner G, Santschi V. Drug adherence in chronic kidney diseases and dialysis. Nephrol Dial Transplant. 2015;30(1):39‐44. [DOI] [PubMed] [Google Scholar]
- 15. Santoro A, Perrone V, Giacomini E, et al. Association between hyperkalemia, RAASi non‐adherence and outcomes in chronic kidney disease. J Nephrol. 2021;35(2):463‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Provenzano M, Minutolo R, Chiodini P, et al. Competing‐risk analysis of death and end stage kidney disease by Hyperkalaemia status in non‐dialysis chronic kidney disease patients receiving stable nephrology care. J Clin Med. 2018;7(12):499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dew MA, Dimartini AF, De Vito DA, et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83(7):858‐873. [DOI] [PubMed] [Google Scholar]
- 18. Taber DJ, Fleming JN, Fominaya CE, et al. The impact of health care appointment non‐adherence on graft outcomes in kidney transplantation. Am J Nephrol. 2017;45(1):91‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Constantiner M, Cukor D. Barriers to immunosuppressive medication adherence in high‐risk adult renal transplant recipients. Dial Transplant. 2011;40(2):60‐66. [Google Scholar]
- 20. Karamanidou C, Clatworthy J, Weinman J, Horne R. A systematic review of the prevalence and determinants of nonadherence to phosphate binding medication in patients with end‐stage renal disease. BMC Nephrol. 2008;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Belaiche S, Décaudin B, Dharancy S, Noel C, Odou P, Hazzan M. Factors relevant to medication non‐adherence in kidney transplant: a systematic review. Int J Clin Med. 2017;39(3):582‐593. [DOI] [PubMed] [Google Scholar]
- 22. Dodd R, Palagyi A, Guild L, Jha V, Jan S. The impact of out‐of‐pocket costs on treatment commencement and adherence in chronic kidney disease: a systematic review. Health Policy Plan. 2018;33(9):1047‐1054. [DOI] [PubMed] [Google Scholar]
- 23. Boonstra MD, Reijneveld SA, Foitzik EM, Westerhuis R, Navis G, de Winter AF. How to tackle health literacy problems in chronic kidney disease patients?: a systematic review to identify promising intervention targets and strategies. Nephrol Dial Transplant. 2020;36(7):1207‐1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Evangelidis N, Sautenet B, Manera KE, et al. Perspectives on blood pressure by patients on haemo‐ and peritoneal dialysis. Nephrol Ther. 2021;26(1):62‐69. [DOI] [PubMed] [Google Scholar]
- 25. Ghimire S, Castelino RL, Jose MD, Zaidi STR. Medication adherence perspectives in haemodialysis patients: a qualitative study. BMC Nephrol. 2017;18(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McKillop G, Joy J. Patients' experience and perceptions of polypharmacy in chronic kidney disease and its impact on adherent behaviour. J Ren Care. 2013;39(4):200‐207. [DOI] [PubMed] [Google Scholar]
- 27. Tesfaye WH, Erku D, Mekonnen A, et al. Medication non‐adherence in chronic kidney disease: a mixed‐methods review and synthesis using the theoretical domains framework and the behavioural change wheel. J Nephrol. 2021;34(4):1091‐1125. [DOI] [PubMed] [Google Scholar]
- 28. Murali KM, Lonergan M. Breaking the adherence barriers: strategies to improve treatment adherence in dialysis patients. Semin Dial. 2020;33(6):475‐485. [DOI] [PubMed] [Google Scholar]
- 29. Eysenbach G. What is e‐health? J Med Internet Res. 2001;3(2):E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. WHO Global Observatory for eHealth . mHealth: New Horizons for Health through Mobile Technologies: Second Global Survey on eHealth. World Health Organization; 2011. [Google Scholar]
- 31. Stevenson JK, Campbell ZC, Webster AC, et al. eHealth interventions for people with chronic kidney disease. Cochrane Database Syst Rev. 2019;8(8):Cd012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thakkar J, Kurup R, Laba T‐L, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta‐analysis. J Am Med Assoc Intern Med. 2016;176(3):340‐349. [DOI] [PubMed] [Google Scholar]
- 33. de Jongh T, Gurol‐Urganci I, Vodopivec‐Jamsek V, et al. Mobile phone messaging for facilitating self‐management of long‐term illnesses. Cochrane Database Syst Rev. 2012;12(12):Cd007459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eaton C, Comer M, Pruette C, Psoter K, Riekert K. Text messaging adherence intervention for adolescents and young adults with chronic kidney disease: pilot randomized controlled trial and stakeholder interviews. J Med Internet Res. 2020;22(8):e19861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Foster BJ, Pai ALH, Zelikovsky N, et al. A randomized trial of a multicomponent intervention to promote medication adherence: the teen adherence in kidney transplant effectiveness of intervention trial (TAKE‐IT). Am J Kidney Dis. 2018;72(1):30‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nelson LA, Spieker AJ, Kripalani S, et al. User preferences for and engagement with text messages to support antihypertensive medication adherence: findings from a pilot study evaluating an emergency department‐based behavioral intervention. Patient Educ Couns. 2021;105(6):606‐1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singh K, Diamantidis CJ, Ramani S, et al. Patients' and nephrologists' evaluation of patient‐facing smartphone apps for CKD. Clin J Am Soc Nephrol. 2019;14(4):523‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peng Y, Wang H, Fang Q, et al. Effectiveness of mobile applications on medication adherence in adults with chronic diseases: a systematic review and meta‐analysis. J Manag Care Spec Pharm. 2020;26(4):550‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Becker S, Kribben A, Meister S, Diamantidis CJ, Unger N, Mitchell A. User profiles of a smartphone application to support drug adherence‐experiences from the iNephro project. PLoS One. 2013;8(10):e78547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ong SW, Jassal SV, Miller JA, et al. Integrating a smartphone‐based self‐management system into usual care of advanced CKD. Clin J Am Soc Nephrol. 2016;11(6):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McGillicuddy JW, Gregoski MJ, Weiland AK, et al. Mobile health medication adherence and blood pressure control in renal transplant recipients: a proof‐of‐concept randomized controlled trial. JMIR Res Protoc. 2013;2(2):e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Han A, Min SI, Ahn S, et al. Mobile medication manager application to improve adherence with immunosuppressive therapy in renal transplant recipients: a randomized controlled trial. PLoS One. 2019;14(11):e0224595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fozoonmayeh D, Le HV, Wittfoth E, et al. A scalable smartwatch‐based medication intake detection system using distributed machine learning. J Med Syst. 2020;44(4):76. [DOI] [PubMed] [Google Scholar]
- 44. Reese PP, Bloom RD, Trofe‐Clark J, et al. Automated reminders and physician notification to promote immunosuppression adherence among kidney transplant recipients: a randomized trial. Am J Kidney Dis. 2017;69(3):400‐409. [DOI] [PubMed] [Google Scholar]
- 45. Stonbraker S, Porras T, Schnall R. Patient preferences for visualization of longitudinal patient‐reported outcomes data. J Am Med Inform Assoc. 2020;27(2):212‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lai D, Lee J, He S. Emoji for the medical community—challenges and opportunities. JAMA. 2021;326(9):795‐796. [DOI] [PubMed] [Google Scholar]
- 47. da Silva Junior GB, Askari M, Dourado DXC, de Oliveira JGR, de Vasconcelos Filho JE. The renal health Instagram: an analysis of comments. Stud Health Technol Inform. 2020;270:781‐785. [DOI] [PubMed] [Google Scholar]
- 48. Ahmed S, Haines‐Saah RJ, Afzal AR, et al. User‐driven conversations about dialysis through Facebook: a qualitative thematic analysis. Nephrol Ther. 2017;22(4):301‐307. [DOI] [PubMed] [Google Scholar]
- 49. Mancheno C, Asch DA, Klinger EV, et al. Effect of posting on social media on systolic blood pressure and management of hypertension: a randomized controlled trial. Am Heart J. 2021;10(19):e020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dawson J, Lambert K, Campbell KL, Kelly JT. Incorporating digital platforms into nutritional care in chronic kidney disease. Semin Dial. 2021. [DOI] [PubMed] [Google Scholar]
- 51. Hussein WF, Bennett PN, Pace S, et al. The mobile health readiness of people receiving in‐center hemodialysis and home dialysis. Clin J Am Soc Nephrol. 2020;16(1):98‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Leigh S, Ashall‐Payne L. The role of health‐care providers in mHealth adoption. Lancet Digital Health. 2019;1(2):e58‐e59. [DOI] [PubMed] [Google Scholar]
- 53. Fischer MA, Stedman MR, Lii J, et al. Primary medication non‐adherence: analysis of 195,930 electronic prescriptions. J Gen Intern Med. 2010;25(4):284‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Talevski J, Wong Shee A, Rasmussen B, Kemp G, Beauchamp A. Teach‐back: a systematic review of implementation and impacts. PLoS One. 2020;15(4):e0231350. [DOI] [PMC free article] [PubMed] [Google Scholar]


