Abstract
Sex‐based differences in physiological traits may be influenced by both evolutionary and environmental factors. Here we used male and female flies from >80 Drosophila species reared under common conditions to examine variance in a number of physiological traits including size, starvation, desiccation and thermal tolerance. Sex‐based differences for desiccation and starvation resistance were comparable in magnitude to those for size, with females tending to be relatively more resistant than males. In contrast thermal resistance showed low divergence between the sexes. Phylogenetic signal was detected for measures of divergence between the sexes, such that species from the Sophophora clade showed larger differences between the sexes than species from the Drosophila clade. We also found that sex‐based differences in desiccation resistance, body size and starvation resistance were weakly associated with climate (annual mean temperature/precipitation seasonality) but the direction and association with environment depended on phylogenetic position. The results suggest that divergence between the sexes can be linked to environmental factors, while an association with phylogeny suggests sex‐based differences persist over long evolutionary time‐frames.
Keywords: body size, physiology, sex‐specific variation, stress resistance
Sex‐specific adaptation resulting in sex‐based differences (sexual dimorphism in morphological traits) is widespread, and a predominant factor contributing to within species diversity. Yet we have little understanding of how, and why, sex‐based differences evolve. Using Drosophila species as our study system we find evolutionary history and ecological divergence (climatic selection) are important drivers of sex‐based differences in body size and physiological traits but little evidence for correlated trait evolution.

1. INTRODUCTION
Divergence in traits between the sexes is common across many organisms and can reflect opposing selection acting on each sex, although a shared genome means there are likely to be limits in the extent to which sex‐based difference can evolve (Lande, 1980). Sexual size dimorphism is by far the best characterized of the sexually dimorphic traits (Fairbairn et al., 2007; Rohner et al., 2018), but physiological traits can also vary between the sexes (Han & Dingemanse, 2017; Lasne et al., 2018). Sexual selection is often invoked as the primary driver of morphological divergence between the sexes; however, natural selection (sex‐based ecological divergence) may also drive sex‐based differences (Darwin, 1871; Shine, 1989) and could be particularly important for physiological traits as they are frequently linked to species distributions (Addo‐Bediako et al., 2000; Kellermann, Loeschcke, et al., 2012; Kellermann, Overgaard, et al., 2012).
Linking sex‐based differences in traits to environmental conditions provides one way of testing whether ecologically divergent optima (spatial selection) might contribute to differences between the sexes (Endler, 1977). Sex‐specific selection on physiological traits could occur if different climatic niches are utilized by males and females (Shine, 1989). This could arise if, hypothetically, males were active for longer while searching for females and subsequently exposed to a wider range of environmental conditions in comparison to females that tended to remain close to oviposition sites. There is some evidence for sex‐based size dimorphism varying with latitude and environment in Drosophila and lizards, which has been interpreted in terms of spatial selection (Blanckenhorn et al., 2006; Chelini et al., 2021; Lasne et al., 2018; Rohner et al., 2018). However, very little is known about how the sexes differ in physiological traits and how this varies across space. A study on Drosophila melanogaster found evidence for sex‐based differences in physiology but no evidence for spatially varying selection (Lasne et al., 2018). Studies in lizards have found that traits such as running speed show sex‐based differences, which could be driven by niche differentiation between the sexes (Chelini et al., 2021; Lailvaux, 2007). If physiological differences between the sexes is driven by differences in habitat use this could have implications for the evolution of physiological traits under climate change (Pottier et al., 2021).
Sex‐based differences in physiological traits and body size may arise due to pleiotropic correlations between traits (Bonduriansky & Chenoweth, 2009). Physiological traits are often correlated with body size, because the mechanisms that underpin physiological traits can be closely linked to size. For example, smaller individuals have a greater surface area to volume ratio, which, in turn, influences desiccation resistance, with smaller individuals losing proportionally more water and subsequently having lower desiccation resistance (Chown et al., 2011). Similarly, larger individuals are likely to have greater energy stores and subsequently greater starvation resistance since metabolic rate typically increases with an allometric factor of less than one (Chippindale et al., 1996; Schmidt‐Nielsen, 1984). This is further supported by studies showing that body size can partly account for variation in starvation and desiccation tolerance in ectotherms (Chown et al., 2011; Gillooly et al., 2001; Harshman et al., 1999). Consequently, we may observe sex‐based differences in physiological traits simply because of sexual dimorphism in body size or vice‐versa (Bonduriansky & Chenoweth, 2009; Fairbairn & Roff, 2006), and we would expect males (the smaller sex) to be less resistant.
Despite decades of research, the main direct and indirect drivers of the evolution of sex‐based differences remain unclear but are likely to be diverse even within one group such as spiders and frogs (Kuntner & Coddington, 2020; Pincheira‐Donoso et al., 2021). Most research has primarily focused on morphological divergence between the sexes despite evidence for sex‐based differences in other traits. In the present study we examined the extent of sex‐based difference in physiological traits for over 80 Drosophila species. We first considered how sex‐based differences vary across traits and among species. Given previous studies have shown that the evolution of sexual dimorphism in body size is linked to the phylogeny (Rohner et al., 2018; Sztepanacz & Houle, 2021), we analysed the extent to which variation in sex‐based differences in physiology and body size are linked to species evolutionary history. We then investigated if environmental factors are associated with sex‐based differences by asking whether divergence between the sexes differed across environments. It is difficult to generate concrete a‐priori hypotheses about how the environment might select for divergence between the sexes given so few studies have examined physiological traits in this context. Based on theory we might expect greater divergence between the sexes in more variable environments (Connallon & Hall, 2016). But it is hard to predict whether females vs males will be the more resistant sex given that both selection to locate mates (in males) or oviposition sites (in females) might favour greater resistance. Finally, we examined whether correlations with body size drive divergence between the sexes in physiological traits. If correlations were an important mechanism driving sex‐based divergence in physiological traits we expected species that show sex‐based differences in body size to also show sex‐based differences in physiological traits, where the largest sex would also be the most resistant.
2. MATERIALS AND METHODS
We characterized sex‐based differences in Drosophila species for body size (80 species, measured as body mass) and physiological traits ‐ desiccation resistance (96), starvation resistance (85), cold resistance (101) and heat resistance (94) from previously published trait estimates, details of which can be found in the source references (Kellermann et al., 2013; Kellermann, Loeschcke, et al., 2012; Kellermann, Overgaard, et al., 2012; Messamah et al., 2017).
Briefly, all traits were estimated in the same laboratory, using the same rearing conditions. Drosophila species were maintained for a minimum of 2 generations at 20°C under 12:12 light. Most species were maintained on a Leeds medium (oats, sugar and yeast based; Kellermann, Loeschcke, et al., 2012). Because a common garden design was used to estimate body size and physiological traits, our estimates of sex‐based differences in these traits reflect genetic rather than environmental differences.
2.1. Body mass
Body mass data were taken from two papers (Kellermann et al., 2013; Messamah et al., 2017). Body mass in one paper (Kellermann et al., 2013) was calculated as the average wet mass from ten individuals of each sex, while in the other paper (Messamah et al., 2017) wet mass was estimated as the mass of three groups of 10 individuals per sex. Mass was then divided by 10 to give an estimate of individual wet mass and then averaged across the three replicates. For both studies, weight was measured to 6 decimal places using a Sartorius MC5 micro‐balance (Sartorius Gottingen). Body mass is a commonly used metric to estimate size in Drosophila and typically correlates well with patterns established through other measures such as wing and thorax length (Hallas et al., 2002; Robertson, 1960).
2.2. Desiccation resistance
Ten individuals of each sex were placed individually into empty vials (5 ml), secured with gauze and placed into a tank containing silica gel, which reduces the relative humidity (RH) to 5%–10%. Individual flies were scored until no movement was observed (Kellermann, Loeschcke, et al., 2012).
2.3. Starvation resistance
Ten individuals of each sex were placed individually into 50 ml vials containing 2 ml of agar (to avoid dehydration stress) and scored every 12 h until mortality was observed (Kellermann et al., 2013).
2.4. Cold and heat resistance
Cold and heat resistance were measured as lower and upper thermal limits, respectively, via a ramping method (Kellermann, Loeschcke, et al., 2012; Kellermann, Overgaard, et al., 2012). Ten flies of each sex were placed individually into a 5 ml vial and placed into a water bath initially set to 20°C. The temperature of the water bath was increased (upper limits)/decreased (lower limits) at a rate of 0.1°C/min and the temperature at which flies were knocked down was recorded (Kellermann, Loeschcke, et al., 2012; Kellermann, Overgaard, et al., 2012). Cold and heat data were analysed as minutes to knockdown rather than temperature to avoid issues with scale when calculating the sexual dimorphism index (described below).
2.5. Climate data
For each species climate data were taken from previously published studies (Kellermann, Loeschcke, et al., 2012). For additional species previously not published in Kellermann, Overgaard, et al. (2012), climate data were extracted using the same method. Distributional data (GPS co‐ordinates) were collated from the TaxoDros website (http://www.taxodros.uzh.ch) and environmental data extracted, for each distribution point from the WorldClim data set (www.worldclim.org; Hijmans et al., 2005). The environmental data were averaged so that each species had a single value for each environmental variable. To ensure averages were not biased by outlier data points and that they were representative of overall differences in the environments where species were found, we also calculated the median and mode of the environmental variables for each species and found these to all be strongly correlated with each other and the average measure (r 2 > 0.93). We focused on seven environmental variables related to temperature and precipitation: annual mean temperature (AMT), lowest temperature of the coldest month (T MIN), highest temperature of the warmest month (T MAX), annual precipitation (P ANN), precipitation of the driest month (P DRY), precipitation of the wettest month (P WET) and precipitation of the warmest month (P WARM).
2.6. Data analyses
2.6.1. Sex‐based differences
To first determine whether there was trait divergence between the sexes for each species, which would provide evidence for sex‐based differences/sexual dimorphism, we performed separate species ANOVAs on the raw trait values (Table 1) testing for a sex effect. Where these analyses provided evidence for sex‐based differences, we then explored patterns of sex‐based differences across the phylogeny. We did so using the framework applied to studying sexual dimorphism in morphological traits that uses the sexual dimorphism index (SDI) to quantify sex‐based differences in traits and to make our analysis comparable across different trait classes. Note, we use SDI only in the context of asking how does divergence between the sexes vary across the phylogeny, we did not include SDI as a response variable in any of our initial analyses testing for sex differences across traits because ratios when used as a response variable can generate spurious relationships with predictor variables (Curran‐Everett, 2013). Therefore, we computed the commonly used sexual dimorphism index (SDI; bigger sex/smaller sex)‐1 (Lovich & Gibbons, 1992) for body size and physiological traits only for the phylogenetic comparisons. In this case because females were on average the larger and generally more resistant sex, SDI was calculated as SDI = , but because there is variation across species in which sex is larger/resistant and to show the directionality of SDI, we allowed SDI to be both negative and positive.
TABLE 1.
Two‐way contingency table comparing the number of individuals that show significant and positive sex‐ based differences (F > M) to those that show significantly negative sex‐based differences (F < M) or no difference between the sexes (F = M) across trait pairs
| Body size | |||
| F > M | F < M/F = M | ||
| Desiccation | F > M | 32 | 0 |
| F < M/F = M | 26 | 5 | |
| Body size | |||
| Starvation | F > M | 40 | 3 |
| F < M/F = M | 11 | 1 | |
| Desiccation | |||
| Starvation | F > M | 33 | 35 |
| F < M/F = M | 5 | 15 |
Note: Significant departures from expected, denoting significant associations between SDI of trait pairs, in bold.
All phylogenetic analyses were performed in R using the ape v 5.5 and phytools v 0.7‐90 packages (Revell, 2012; Team, 2014). To visualize patterns of SDI evolution across the phylogeny, we performed an ancestral trait reconstruction for continuous characters using a maximum likelihood approach with fastANC and contMAP in the phytools package (Revell, 2012), assuming a Brownian model of evolution. Because this phylogeny included species that we previously had characterized for body size and physiological traits but were unable to model within a phylogenetic framework, the current data set includes more species than were originally analysed in the Kellermann, Overgaard, et al. (2012) studies. For cold and heat resistance, we found SDI did not vary much across the species (see Section 3 below) and did not consider them further with respect to phylogenetic patterns of variation or individual species one‐way ANOVA's. We estimated the degree of phylogenetic association for SDI by calculating phylogenetic signal, where a ʎ = 1 suggests strong phylogenetic signal and ʎ = 0 suggests no association between SDI and phylogeny (Pagel, 1999).
2.6.2. Association between climate and the sexes
To examine whether sex‐based differences in traits varied across environments (sex × environment interaction), we performed a mixed‐effects linear model using the lme4 package (Bates et al., 2014). Species were considered random effects while sex and environment were considered fixed effects. Effect sizes for each model, marginal and conditional R 2 (R 2 m and R 2 c, respectively) were calculated using the MuMIn package (Barton, 2022). The interaction term sex × environment was included in the model to test whether divergence between the sexes depends on the environment. The significance of the interaction term was assessed both with an ANOVA and by comparing model AICs with and without the interaction term (Tables S2 and S3). We focused on a single temperature and precipitation variable, annual mean temperature and precipitation seasonality, respectively, as we tended to find high correlation among different temperature and precipitation variables, meaning slightly different measures of temperature/precipitation tell you the same thing. Temperature and precipitation are also known to shape Drosophila species distributions (Kellermann, Loeschcke, et al., 2012; Kellermann, Overgaard, et al., 2012). Drosophila mojavensis was excluded from the environmental analysis for desiccation resistance as this desert species represented an outlier (Table S6, Figure S1 for analysis with D. mojavensis).
To examine whether species, which showed sex‐based differences in one trait (based on the separate species ANOVAs) were more likely to have sex‐based differences in another trait, which would then suggest a degree of correlation between sex‐based differences, we considered three categories: females were significantly larger/more resistant than males (F > M) and males were significantly larger/more resistant than females (F < M) or females and males were equal (F = M). However, because there were very few species in the F < M category, we pooled the last two categories for analysis. Species were placed into these categories based on the results from individual species one‐way ANOVAs, if we found a significant effect of sex at the single species level, we looked at the means to determine the direction of the sex difference and then categorized the species as F < M or F > M. Species with non‐significant sex effect terms were classified as F = M. We then created two‐way contingency tables for pairs of traits based on these categories (body size vs. desiccation resistance, body size vs. starvation resistance and desiccation resistance vs. starvation resistance). Goodness of fit was assessed using Fisher's exact test. We followed this approach rather than using correlations because of issues in correlating SDIs from different traits, which would produce spurious trait associations (see above).
3. RESULTS
3.1. How do sex‐based differences vary across traits?
To explore how sex‐based differences varied across traits, we calculated the commonly used sexual dimorphism index, SDI (Figure 1). The greatest differences between the sexes was observed for the physiological traits desiccation and starvation resistance and for body size (trait variance [σ 2]: desiccation σ 2 = 0.06, starvation σ 2 = 0.09 and body size σ 2 = 0.04; Figure 1). SDI tended to be lower for the physiological traits cold and heat resistance; there was little variation across the species and sex‐based differences were small for these traits (cold and heat σ 2 = 0.001; Figure 1). For desiccation resistance, starvation resistance and body size, sex‐based differences were predominately female‐biased, although some species showed either no difference between the sexes or male biased SDI. The extent of male biased SDI was lower than the extent of female‐biased SDI. That is, where males were the larger/more resistant sex, the extent of the differences between the sexes was smaller.
FIGURE 1.
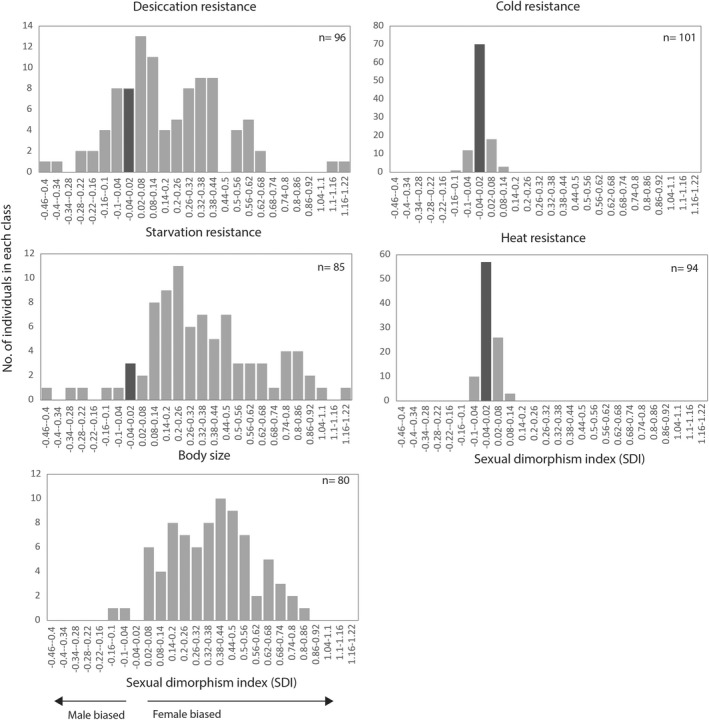
Variation in sexual dimorphism index (SDI) across body size and physiological traits for the Drosophila species. The dark shaded bar represents SDI of approximately 0, while the arrows indicate whether SDI is female or male biased
For all traits, sex‐based differences tended to be female‐biased as evident by positive values of SDI (Figure 1). The body size of most species was sexually dimorphic, with females on average 22% larger than males and only nine of 80 species showing no evidence for sexual dimorphism (one‐way ANOVA's Table S7 and Figure 2b). This was followed by starvation resistance where females were on average 24% more resistant than males and with 17 of 85 species showing no difference between the sexes (Table S7 and Figure 2b). Finally, for desiccation resistance females were on average 8% more resistant than males and approximately half the species showed no evidence of sex‐based differences (Table S7 and Figure 2b). Significant phylogenetic signal was detected for SDI for all traits (ʎ = 0.20–0.54; Figure 2b), suggesting evolutionary history may influence the degree of sex‐based differences across the species. This was further evident when we mapped SDI onto the phylogeny where we found that species from the Sophophora genus were characterized by larger sex‐based differences resulting in a larger average SDI for the three traits examined (Figure 2b,c).
FIGURE 2.
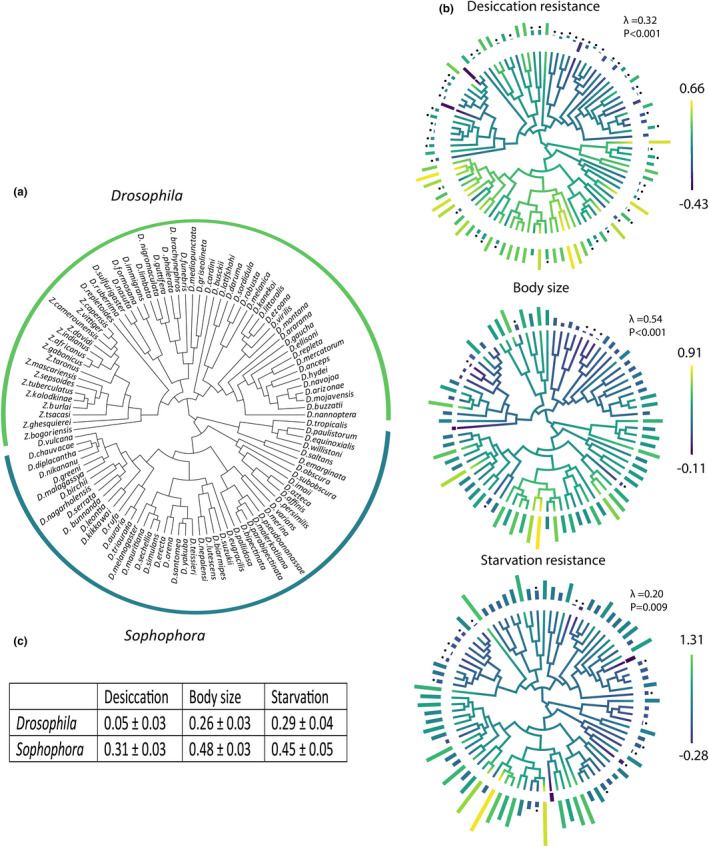
Phylogenetic analysis of sex‐based differences in desiccation resistance, starvation resistance and body size. (a) Phylogenetic hypothesis of the Drosophila species examined and their sub‐groups. (b) Sex‐based differences mapped onto the phylogeny via ancestral trait reconstruction for continuous characters for traits desiccation resistance (top), body size (middle) and starvation resistance (bottom). Bars represent the average SDI for each trait, with dots above the bars indicating a significant difference between the sexes when analysed with a one‐way ANOVA. (c) Trait SDI means and their standard errors split by the two subgenera Drosophila and Sophophora
3.2. Sex‐based differences and associations with environment
Here we examined whether climate is associated with sex‐based differences in physiology by looking for sex × environment interactions on the raw trait values rather than using the SDI. A significant sex × environment interaction provides support for the hypothesis that females and males are divergent because of different ecological optima. The climate variables examined have previously been shown to be strong drivers of physiological traits in Drosophila (Kellermann, Loeschcke, et al., 2012; Kellermann, Overgaard, et al., 2012). Because of the phylogenetic signal observed in the phylogenetic analysis above, we split the analysis by the Sophophora and Drosophila subgenera. We found that much of the phylogenetic signal detected in SDI was no longer significant once we split species into the subgenera (Table S1).
Overall, we found that our random (species) and fixed (sex and environment) effects (R 2 c) could explain 62%–90% of the variation in our traits, with our fixed effects (R 2 m) accounting for 22%–54% of the variation. Unsurprisingly, differences between species contributed the largest amount of variation observed in our traits. Nonetheless, in most instances, sex, environment and their interactions can explain an appreciable amount of the variation as well (Tables S4 and S5). Furthermore, we have focused on models with a significant environment term as we are interested in how sexes diverge in traits that vary significantly with the environment.
For annual mean temperature, we found a significant sex × environment interaction for both desiccation resistance and body size in the Sophophora genus (Figure 3). Divergence between the sexes increased in warmer environments for desiccation resistance (R 2 m = 0.22) while the opposite was true for body size where divergence between the sexes became larger in cooler environments (R 2 m = 0.54; Figure 3). Similar to desiccation resistance, in the Sophophora genus, divergence between the sexes increased in warmer environments for starvation resistance (R 2 m = 0.17), while in the Drosophila genus the reverse was true with divergence between the sexes increasing in colder environments (R 2 m = 0.27; Figure 3). When we considered precipitation seasonality rather than mean annual temperature we found a significant sex × environment interaction for starvation resistance in the Sophophora genus (R 2 m = 0.19; Figure 4). The divergence between the sexes was such that greater precipitation seasonality was linked to greater divergence between the sexes (Figure 4). For the Drosophila genus we found a significant environment × sex interaction for starvation resistance (R 2 m = 0.07, R 2 m = 0.13, respectively; Figure 4). For starvation resistance we found precipitation seasonality to be a strong driver of divergence between the sexes, but unlike the Sophophora genus we found environments with less precipitation seasonality drove divergence between the sexes. Opposing patterns of divergence between the sexes and environment in the Sophophora and Drosophila genus suggests the evolution of sexual dimorphism depends on both evolutionary history and environment. Full model outputs including model estimates and the R 2 c can be found in Supporting Information (Tables S4 and S5).
FIGURE 3.
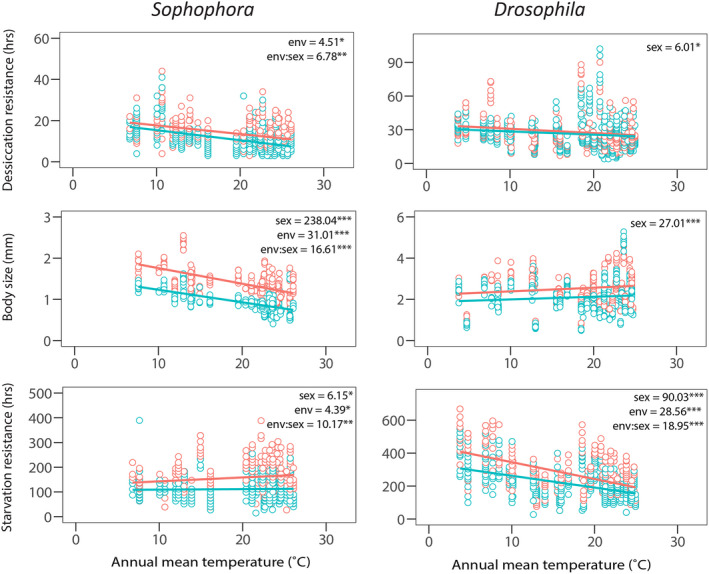
The relationship between annual mean temperature and traits for each sex. Association between traits and annual mean temperature for the two subgenera Sophophora and Drosophila for traits desiccation resistance, body size and starvation resistance with females = orange and males = blue (individual data points plotted). The χ 2 for significant model terms are given for each trait from the mixed‐effects model, where *p < 0.05, **p < 0.01 and ***p < 0.001
FIGURE 4.
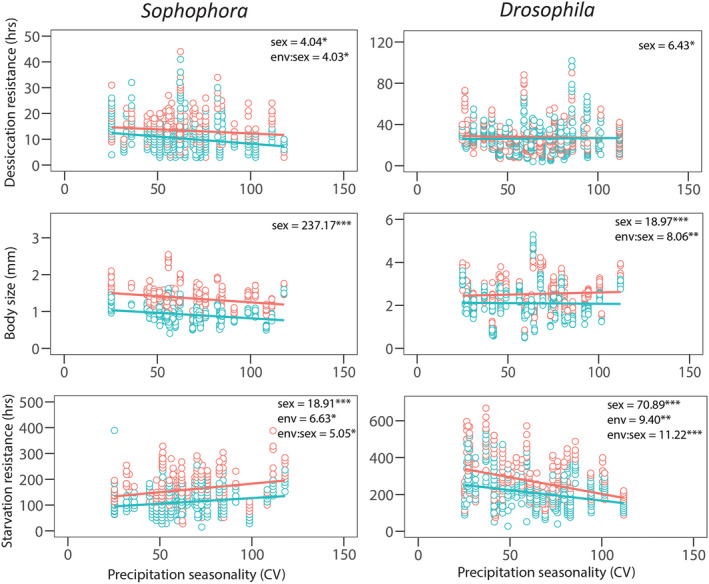
The relationship between precipitation seasonality and traits for each sex. Association between desiccation and starvation resistance and precipitation seasonality for the Sophophora and Drosophila genus with females = orange and males = blue (individual data points plotted). The χ 2 for significant model terms are given for each trait from the mixed‐effects model where *p < 0.05, **p < 0.01 and ***p < 0.001
3.3. Are there associations between sex‐based differences in physiology and body size?
Directly correlating sex‐based differences between traits can cause spurious relationships when using ratios as estimates of SDI (Curran‐Everett, 2013). Instead we ask to what extent do species that show sexual dimorphism in body size also show sex‐based differences in desiccation and starvation resistance. Using two‐way contingency tables we find the number of individuals showing F > M or F < M/F = M between body size and starvation resistance and desiccation resistance and starvation resistance did not differ from expected numbers and hence no significant association was detected for these trait pairs (Table 1). We did, however, find a significant association between body size and desiccation resistance (p = 0.02), such that species that exhibited female‐biased sex‐based differences in body size tended to also exhibit female‐biased sex‐based differences in desiccation resistance.
4. DISCUSSION
In the present study of 80–95 Drosophila species, we found sex‐based differences for physiological traits desiccation and starvation resistance in a number of species comparable to that seen for sexual dimorphism in body size, but no evidence for sex‐based differences in cold and heat resistance. We test two main hypotheses for why we might observe sex‐based differences in physiological traits, the first being that differences between the sexes could arise if physiological traits differ in their ecological optima (Endler, 1977; Shine, 1989). Alternatively, if physiological traits are correlated with traits under sexual/natural selection, sex‐based differences could arise as a consequence of selection on one but not both traits (Bonduriansky & Chenoweth, 2009). Here we found some evidence that variation in sex‐based differences was linked to climatic variables (annual mean temperature and precipitation seasonality) for desiccation resistance, body size and starvation resistance, consistent with the first hypothesis. However, how sex‐based differences diverged across environments depended on whether the Sophophora or the Drosophila genus was examined. Species that showed significant sex‐based differences in physiological traits (mostly F > M) were not necessarily more likely to show sex‐based differences in body size. The size bias almost always was in the direction of females being larger than the males (Figure 2), while for physiological traits there were fewer species that showed significant sex‐based differences and the extent of the differences between the sexes tended to be smaller (i.e. female and male trait values were closer to each other).
Phylogenetic signal in SDI was detected for all traits, but more so for body size. This result is in line with other studies on Drosophila showing strong phylogenetic signal in body size (Rohner et al., 2018; Sztepanacz & Houle, 2021) and other traits (Kellermann, Loeschcke, et al., 2012). SDI appeared to differ between the two Drosophila subgenera Drosophila and Sophophora, such that species from the Sophophora subgenus were more likely to show significant sex effects and a greater degree of divergence between the sexes. Similar evolutionary divergence between the two subgenera has been detected in another study on size dimorphism in Drosophila (Sztepanacz & Houle, 2021). Finding an association between phylogeny and sex‐based differences is perhaps not surprising given that the evolution of sex‐specific optima is thought to occur rapidly and persist across quite a long evolutionary timeframe (Connallon & Hall, 2016). One reason that the extent of sex‐based differences may persist through speciation events is that sex‐based differences involve complex trade‐offs between survival and reproduction acting across different levels of biological organization (Acerenza, 2016; Gidaszewski et al., 2009). Perturbing these complex trade‐offs could have large fitness consequences that limit shifts in the extent of sex‐based differences through evolutionary time. Nevertheless, the presence of persistent sex‐based differences over evolutionary time may have consequences for the capacity for these traits to rapidly shift under changing environments, particularly if climate change results in a shift in the sex‐specific optima, and if antagonistic sexual selection is strong and environmentally sensitive (Chenoweth et al., 2008; Connallon & Hall, 2016; Ketola et al., 2012; Lande, 1980).
We found some evidence suggesting that climatic factors shape divergence between the sexes with respect to desiccation resistance, body size and starvation resistance. The association between sex‐based divergence and environment depended on evolutionary history. Within the Sophophora clade annual mean temperature and precipitation seasonality were linked to divergence between the sexes. The direction of divergence tended to be trait dependent. For starvation resistance, divergence between the sexes was highest in warm and highly seasonal precipitation environments. Selection for female and male trait optima could be in different directions promoting divergence between the sexes in these types of environments. However, in the Drosophila genus, we found the opposite tendency such that in highly seasonal precipitation environments there was less divergence between the sexes. This result in the Drosophila genus is in agreement with the theory that environmental variability could align the direction of selection on males and females resulting in less divergence between the sexes in variable environments (Connallon & Hall, 2018). At least with the current data set we have noticed a tendency for species within the Sophophora genus to exist in more tropical climates while the Drosophila genus occupy more cooler and drier environments and there are likely different selective drivers in these different types of habitats. This could be one reason why we see annual mean temperature and precipitation seasonality select for opposite patterns between the sexes in the Sophohora and Drosophila clades. Climate has been linked to the evolution of sexual size dimorphism in a number of organisms (Lima et al., 2017; Littleford‐Colquhoun et al., 2019). But similar to (Rohner et al., 2018), who found divergence between females and males was highly clade specific; we found divergence between the sexes depended on the environment (sex × environment interactions), and the direction of the relationship with the environment depended on the genus. Even where we found evidence for sex × environment interactions, divergence between the sexes tended to be small, perhaps reflecting a shared genome, which may constrain evolutionary divergence between the sexes (Bonduriansky & Chenoweth, 2009; Lande, 1980).
Given that there was no strong tendency for species with sexual dimorphism in desiccation and starvation resistance to show dimorphism in body size, it is unclear if some of the observed sex‐based differences/sexual dimorphism was caused by pleiotropy (Bonduriansky & Chenoweth, 2009). Larger body size might be expected to increase desiccation and starvation resistance but there are also many other traits affecting physiological trait variation (Chown et al., 2011; Hoffmann & Harshman, 1999). It is possible that other correlated traits may drive divergence between the sexes. Cuticular hydrocarbons for example, are linked to courtship behaviour in many Drosophila species, thought to play a role in desiccation resistance and are known to vary between the sexes in some Drosophila species and display sexually antagonistic sexual selection (Chenoweth et al., 2008; Jallon & David, 1987; Jezovit et al., 2017).
In conclusion, the extent of divergence between the sexes varied across Drosophila species and traits. For heat and cold resistance, we found little evidence of divergence between the sexes, which could mean these traits are not linked to sexually selected traits or have the same optima and, therefore, do not experience antagonistic selection between the sexes. The evolution of sex‐based differences in desiccation resistance, body size and starvation resistance was at least in part mediated by climate, but depended on a species' evolutionary history. Sex differences in effect size were small compared to body mass apart from starvation resistance. The deeply rooted phylogenetic signatures may be because adaptive sex‐based differences will arise quickly but are slow to decay over evolutionary time (Connallon & Hall, 2018), but could also suggest that different selective processes are acting across species clades. Further mechanistic studies are needed to determine whether phylogenetic patterns represent constraints in the evolution of sex‐based differences or adaptation to local environments.
Funding information
This work was supported by the DECRA (VK) and DP (AA, CMS and VK) schemes from the Australian Research Council.
ACKNOWLEDGEMENTS
We thank the reviewers for their comments which greatly improved the manuscript. We thank Janneke Wit, Pernille Sarup, Anny Bang and Doth Andersen for technical assistance. Financial support was provided by the Australian Research council through their Fellowship (VK) and Discovery Project schemes (VK, CMS, AAH), the Science and Industry and Endowment Fund (CMS and AAH), Monash University through their supporting diversity schemes (VK) and the Danish Research Council (JO). Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/jeb.14104.
Supporting information
Table S1. Phylogenetic signal as estimated as ʎ for traits desiccation resistance, body size and starvation resistance for the two subgenera Sophophora and Drosophila.
Table S2. Akaike Information Criterion for the mixed‐effects models examining the effects of annual mean temperature (AMT) and sex on traits desiccation resistance, body size and starvation resistance for the Sophophora and Drosophila genus.
Table S3. Akaike Information Criterion for the mixed‐effects models examining the effects of precipitation seasonality (P season) and sex on traits desiccation resistance, body size and starvation resistance for the Sophophora and Drosophila genus.
Table S4. Results from a mixed‐effects model for the two subgenera Sophophora and Drosophila for desiccation resistance, body size and starvation resistance examining whether the sexes differ in traits and whether the relationship between traits and annual mean temperature changes with sex.
Table S5. Results from a mixed‐effects model for the two subgenera Sophophora and Drosophila for desiccation resistance, body size and starvation resistance examining whether the sexes differ in traits and whether the relationship between traits and precipitation seasonality changes with sex.
Table S6. Results from a mixed‐effects model with a single species outlier removed from the Drosophila genus for desiccation resistance examining whether the sexes differ in traits and whether the relationship between traits and precipitation seasonality changes with sex.
Table S7. One‐way ANOVA’s testing whether the sexes differ in their means for traits desiccation resistance, body size and starvation resistance.
Figure S1. The relationship between environment and traits for each sex.
Kellermann, V. , Overgaard, J. , Sgrò, C. M. , & Hoffmann, A. A. (2022). Phylogenetic and environmental patterns of sex differentiation in physiological traits across Drosophila species. Journal of Evolutionary Biology, 35, 1548–1557. 10.1111/jeb.14104
DATA AVAILABILITY STATEMENT
Full data sets including R code can be downloaded from dryad: https://doi.org/10.5061/dryad.x0k6djhn5.
REFERENCES
- Acerenza, L. (2016). Constraints, trade‐offs and the currency of fitness. Journal of Molecular Evolution, 82, 117–127. [DOI] [PubMed] [Google Scholar]
- Addo‐Bediako, A. , Chown, S. L. , & Gaston, K. J. (2000). Thermal tolerance, climatic variability and latitude. Proceedings of the Royal Society B: Biological Sciences, 267, 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton, K. (2022). MuMIn: Multi‐model inference. R package version 1.46.0.
- Bates, D. , Maechler, M. , Bolker, B. M. , & Walker, S. (2014). lme4: Linear mixed effects models using Eigen and S4. J Stat Software.
- Blanckenhorn, W. U. , Stillwell, R. C. , Young, K. A. , Fox, C. W. , & Ashton, K. G. (2006). When Rensch meets Bergmann: Does sexual size dimorphism change systematically with latitude? Evolution, 60, 2004–2011. [PubMed] [Google Scholar]
- Bonduriansky, R. , & Chenoweth, S. F. (2009). Intralocus sexual conflict. Trends in Ecology & Evolution, 24, 280–288. [DOI] [PubMed] [Google Scholar]
- Chelini, M. C. , Brock, K. , Yeager, J. , & Edwards, D. L. (2021). Environmental drivers of sexual dimorphism in a lizard with alternative mating strategies. Journal of Evolutionary Biology, 34, 1241–1255. [DOI] [PubMed] [Google Scholar]
- Chenoweth, S. F. , Rundle, H. D. , & Blows, M. W. (2008). Genetic constraints and the evolution of display trait sexual dimorphism by natural and sexual selection. The American Naturalist, 171, 22–34. [DOI] [PubMed] [Google Scholar]
- Chippindale, A. K. , Chu, T. J. F. , & Rose, M. R. (1996). Complex trade‐offs and the evolution of starvation resistance in Drosophila melanogaster . Evolution, 50, 753–766. [DOI] [PubMed] [Google Scholar]
- Chown, S. L. , Sorensen, J. G. , & Terblanche, J. S. (2011). Water loss in insects: An environmental change perspective. Journal of Insect Physiology, 57, 1070–1084. [DOI] [PubMed] [Google Scholar]
- Connallon, T. , & Hall, M. D. (2016). Genetic correlations and sex‐specific adaptation in changing environments. Evolution, 70, 2186–2198. [DOI] [PubMed] [Google Scholar]
- Connallon, T. , & Hall, M. D. (2018). Environmental changes and sexually antagonisitic selection, eLS. John Wiley & Sons, Ltd. [Google Scholar]
- Curran‐Everett, D. (2013). Explorations in statistics: The analysis of ratios and normalized data. Advances in Physiology Education, 37, 213–219. [DOI] [PubMed] [Google Scholar]
- Darwin, C. R. (1871). The descent of man, and selection in relation to sex. J. Murray. [Google Scholar]
- Endler, J. A. (1977). Geographic variation, speciation and clines. Monographs in Population Biology, 10, 1–246. [PubMed] [Google Scholar]
- Fairbairn, D. J. , Blanckenhorn, W. U. , & Szekely, T. (2007). Sex, size, and gender roles. Evoltuionary studies of sexual size dimorphism. Oxford University Press. [Google Scholar]
- Fairbairn, D. J. , & Roff, D. A. (2006). The quantitative genetics of sexual dimorphism: Assessing the importance of sex‐linkage. Heredity, 97, 319–328. [DOI] [PubMed] [Google Scholar]
- Gidaszewski, N. A. , Baylac, M. , & Klingenberg, C. P. (2009). Evolution of sexual dimorphism of wing shape in the Drosophila melanogaster subgroup. BMC Evolutionary Biology, 9, 110. 10.1186/1471-2148-9-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly, J. F. , Brown, J. H. , West, G. B. , Savage, V. M. , & Charnov, E. L. (2001). Effects of size and temperature on metabolic rate. Science, 293, 2248–2251. [DOI] [PubMed] [Google Scholar]
- Hallas, R. , Schiffer, M. , & Hoffmann, A. A. (2002). Clinal variation in Drosophila serrata for stress resistance and body size. Genetical Research, 79, 141–148. [DOI] [PubMed] [Google Scholar]
- Han, C. S. , & Dingemanse, N. J. (2017). Sex‐dependent expression of behavioural genetic architectures and the evolution of sexual dimorphism. Proceedings of the Royal Society B: Biological Sciences, 284, 9. 10.1098/rspb.2017.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman, L. G. , Hoffmann, A. A. , & Clark, A. G. (1999). Selection for starvation resistance in Drosophila melanogaster: Physiological correlates, enzyme activities and multiple stress responses. Journal of Evolutionary Biology, 12, 370–379. [Google Scholar]
- Hijmans, R. J. , Cameron, S. E. , Parra, J. L. , Jones, P. G. , & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25, 1965–1978. [Google Scholar]
- Hoffmann, A. A. , & Harshman, L. G. (1999). Desiccation and starvation resistance in Drosophila: Patterns of variation at the species, population and intrapopulation levels. Heredity, 83, 637–643. [DOI] [PubMed] [Google Scholar]
- Jallon, J. , & David, J. R. (1987). Variations in cuticular hydrocarbons among the eight species of the Drosophila melanogaster subgroup. Evolution, 41, 294–302. [DOI] [PubMed] [Google Scholar]
- Jezovit, J. A. , Levine, J. D. , & Schneider, J. (2017). Phylogeny, environment and sexual communication across the Drosophila genus. The Journal of Experimental Biology, 220, 42–52. [DOI] [PubMed] [Google Scholar]
- Kellermann, V. , Loeschcke, V. , Hoffmann, A. A. , Kristensen, T. N. , Fløjgaard, C. , David, J. R. , & Overgaard, J. (2012). Phylogenetic constraints in key functional traits behind species' climate niches: Patterns of desiccation and cold resistance across 95 Drosophila species. Evolution, 66, 3377–3389. [DOI] [PubMed] [Google Scholar]
- Kellermann, V. , Overgaard, J. , Hoffmann, A. A. , Flojgaard, C. , Svenning, J. C. , & Loeschcke, V. (2012). Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proceedings of the National Academy of Sciences of the United States of America, 109, 16228–16233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermann, V. , Overgaard, J. , Loeschcke, V. , Kristensen, T. N. , & Hoffmann, A. A. (2013). Trait associations across evolutionary time within a Drosophila phylogeny: Correlated selection or genetic constraint? PLoS One, 8(9), e72072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketola, T. , Kristensen, T. N. , Kellermann, V. , & Loeschcke, V. (2012). Can the evolution of sexual dimorphism be triggered by developmental temperatures? Journal of Evolutionary Biology, 25, 847–855. [DOI] [PubMed] [Google Scholar]
- Kuntner, M. , & Coddington, J. A. (2020). Sexual size dimorphism: Evolution and perils of extreme phenotypes in spiders. Annual Review of Entomology, 65, 57–80. [DOI] [PubMed] [Google Scholar]
- Lailvaux, S. P. (2007). Interactive effects of sex and temperature on locomotion in reptiles. Integrative and Comparative Biology, 47, 189–199. [DOI] [PubMed] [Google Scholar]
- Lande, R. (1980). Sexual dimorphism, sexual selection and adaptation in polygenic characters. Evolution, 34, 292–305. [DOI] [PubMed] [Google Scholar]
- Lasne, C. , Hangartner, S. B. , Connallon, T. , & Soro, C. M. (2018). Cross‐sex genetic correlations and the evolution of sex‐specific local adaptation: Insights from classical trait clines in Drosophila melanogaster . Evolution, 72, 1317–1327. [DOI] [PubMed] [Google Scholar]
- Lima, P. A. , Bidau, C. J. , Alencar, C. , & Molina, W. F. (2017). Latitudinal influence on the sexual dimorphism of the marine fish Bathygobius soporator (Gobiidae: Teleostei). Evolutionary Biology, 44, 374–385. [Google Scholar]
- Littleford‐Colquhoun, B. L. , Clemente, C. , Thompson, G. , Cristescu, R. H. , Peterson, N. , Strickland, K. , Stuart‐Fox, D. , & Frere, C. H. (2019). How sexual and natural selection shape sexual size dimorphism: Evidence from multiple evolutionary scales. Functional Ecology, 33, 1446–1458. [Google Scholar]
- Lovich, J. E. , & Gibbons, J. W. (1992). A review of the techniques for quantifying sexual size dimorphism. Growth, Development, and Aging, 56, 269–281. [PubMed] [Google Scholar]
- Messamah, B. , Kellermann, V. , Malte, H. , Loeschcke, V. , & Overgaard, J. (2017). Metabolic cold adaptation contributes little to the interspecific variation in metabolic rates of 65 species of Drosophilidae . Journal of Insect Physiology, 98, 309–316. [DOI] [PubMed] [Google Scholar]
- Pagel, M. (1999). Inferring the historical patterns of biological evolution. Nature, 401, 877–884. [DOI] [PubMed] [Google Scholar]
- Pincheira‐Donoso, D. , Harvey, L. P. , Grattarola, F. , Jara, M. , Cotter, S. C. , Tregenza, T. , & Hodgson, D. J. (2021). The multiple origins of sexual size dimorphism in global amphibians. Global Ecology and Biogeography, 30, 443–458. [Google Scholar]
- Pottier, P. , Burke, S. , Drobniak, S. M. , Lagisz, M. , & Nakagawa, S. (2021). Sexual (in)equality? A meta‐analysis of sex differences in thermal acclimation capacity across ectotherms. Functional Ecology, 35, 2663–2678. [Google Scholar]
- Revell, L. J. (2012). phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223. [Google Scholar]
- Robertson, F. W. (1960). The ecological genetics of growth in Drosophila 1. Body size and developmental time on different diets. Genetical Research, 1, 288–304. [DOI] [PubMed] [Google Scholar]
- Rohner, P. T. , Pitnick, S. , Blanckenhorn, W. U. , Snook, R. R. , Bachli, G. , & Lupold, S. (2018). Interrelations of global macroecological patterns in wing and thorax size, sexual size dimorphism, and range size of the Drosophilidae . Ecography, 41, 1707–1717. [Google Scholar]
- Schmidt‐Nielsen, K. (1984). Scaling: Why is animal size so important? Cambridge University Press. [Google Scholar]
- Shine, R. (1989). Ecological causes for the evolution of sexual dimorphism – A review of the evidence. The Quarterly Review of Biology, 64, 419–461. [DOI] [PubMed] [Google Scholar]
- Sztepanacz, J. L. , & Houle, D. (2021). Allometry constrains the evolution of sexual dimorphism in Drosophila across 33 million years of divergence. Evolution, 75, 1117–1131. [DOI] [PubMed] [Google Scholar]
- Team, R. C. (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R‐project.org/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Phylogenetic signal as estimated as ʎ for traits desiccation resistance, body size and starvation resistance for the two subgenera Sophophora and Drosophila.
Table S2. Akaike Information Criterion for the mixed‐effects models examining the effects of annual mean temperature (AMT) and sex on traits desiccation resistance, body size and starvation resistance for the Sophophora and Drosophila genus.
Table S3. Akaike Information Criterion for the mixed‐effects models examining the effects of precipitation seasonality (P season) and sex on traits desiccation resistance, body size and starvation resistance for the Sophophora and Drosophila genus.
Table S4. Results from a mixed‐effects model for the two subgenera Sophophora and Drosophila for desiccation resistance, body size and starvation resistance examining whether the sexes differ in traits and whether the relationship between traits and annual mean temperature changes with sex.
Table S5. Results from a mixed‐effects model for the two subgenera Sophophora and Drosophila for desiccation resistance, body size and starvation resistance examining whether the sexes differ in traits and whether the relationship between traits and precipitation seasonality changes with sex.
Table S6. Results from a mixed‐effects model with a single species outlier removed from the Drosophila genus for desiccation resistance examining whether the sexes differ in traits and whether the relationship between traits and precipitation seasonality changes with sex.
Table S7. One‐way ANOVA’s testing whether the sexes differ in their means for traits desiccation resistance, body size and starvation resistance.
Figure S1. The relationship between environment and traits for each sex.
Data Availability Statement
Full data sets including R code can be downloaded from dryad: https://doi.org/10.5061/dryad.x0k6djhn5.


