Abstract
Pseudomonas aeruginosa is an opportunistic pathogen which causes sight-threatening corneal infections in humans. The purpose of this study was to evaluate various immunization routes that may provide protection against Pseudomonas keratitis and to define the molecular mechanisms involved in the protection. Sprague-Dawley rats (10 to 12 weeks old) were immunized using paraformaldehyde-killed P. aeruginosa (strain 6206) via oral, nasal, and intra-Peyer's patch (IPP) routes followed by an ocular topical booster dose. Scratched corneas were challenged with an infective dose of P. aeruginosa. Following clinical examination, eyes were enucleated for histology, polymorphonuclear leukocyte (PMN) quantitation, bacterial count, enzyme-linked immunosorbent assay, and RNase protection assay. PMN infiltration was higher early (4 h) during the infection in immunized rats than in nonimmunized rats. Later during the infection, the number of PMNs diminished in immunized rats while in nonimmunized animals the number of PMNs continued to increase. Bacteria were cleared much faster from immunized groups than from the nonimmunized group, and the nasally immunized group had the most efficacious response among the immunized groups. Nasal and IPP immunization groups had increased cytokine expression of interleukin-2 (IL-2) and IL-5 and differed from each other for IL-6. All three immunized groups had significantly reduced IL-1β levels when compared with the nonimmunized rats and a significantly altered profile for CINC-1 expression. This study has shown that the route of immunization modulates the inflammatory response to ocular P. aeruginosa infection, thus affecting the severity of keratitis and adverse pathology, with nasal immunization being the most effective.
Corneal ulceration as a result of bacterial infection is a potentially devastating disease which may lead to permanent scarring of the cornea and loss of visual acuity or vision. The pathogenesis is considered to be multifactorial and includes numerous bacterial proteases, toxins, and other virulence factors as well as mediators produced by a host's own inflammatory responses (17, 32). Pseudomonas aeruginosa is a frequently isolated pathogen from bacterial keratitis and accounts for 70% of soft contact lens-associated cases (31). Once infection is initiated it is often difficult to control because of its progressive nature and/or the possible resistance to antibiotics of the infecting bacteria. Even if the infection responds to antibiotics, inflammation can persist. Polymorphonuclear leukocytes (PMNs) are the major inflammatory cells that migrate into the corneal stroma early after the onset of infection (16). Although PMNs are required for the removal of viable bacteria from the tissue, their continued presence may lead to extensive corneal damage.
Protective mechanisms against bacterial infection may include recruitment of phagocytic cells, specific B- and T-cell responses, and the presence of antigen-specific antibodies. Previous studies using passive transfer of monoclonal antibodies to outer membrane proteins of P. aeruginosa and immune sera produced during corneal infection have shown that passive immunization can provide partial protection against infection (26, 38). Similarly, active immunization with lipopolysaccaride and elastase can protect the cornea to some degree against bacterial infection (19). Immunization via nonocular routes (subcutaneous and intraperitoneal) with peptide antigens of herpes simplex virus has been shown to protect mice against corneal challenge with herpes simplex virus (14). These studies suggest that considerable protection can be achieved by manipulating the formulation of vaccines and immunization routes and schedules. However, effector mechanisms of immunity against P. aeruginosa infection in the eye remain poorly understood. Thus, understanding effector mechanisms can help in designing strategies for better management of sight-threatening corneal inflammation.
Cytokines play an important role in inflammatory and immune responses. They have both beneficial and detrimental influences. Various cytokines have been shown to enhance immunoglobulin A (IgA) antibody responses, especially the immunosuppressive cytokines interleukin-4 (IL-4), IL-10, and transforming growth factor beta (7). IL-5 and IL-6 induce IgA-committed B cells to terminally differentiate into IgA plasma cells (3). Synthesis and secretion of the secretory component is stimulated by tumor necrosis factor alpha and -beta, IL-1α, and IL-1β (15). On the other hand, proinflammatory cytokines produced during bacterial infection regulate PMN recruitment by inducing chemokines. Recent studies have shown that IL-1β and macrophage inflammatory protein 2 (murine IL-8 homolog) are major cytokines involved in the direct and indirect recruitment of PMNs (18, 29). Incorneal infections with P. aeruginosa, the host's own inflammatory response is primarily derived from stimulated PMNs (32), and the inappropriate production of inflammatory cytokines possibly contributes to corneal damage. Effective immunization should protect the host not only by facilitating effective removal of bacteria but also by controlling the inflammatory process through appropriate cytokine expression and release.
The purpose of this study was to evaluate the various routes (ocular topical [OT], oral, nasal, and intra-Peyer's patch [IPP]) that can provide significant protection against P. aeruginosa keratitis. Further, we attempted to define the mechanisms involved in protection against acute bacterial ocular infections.
MATERIALS AND METHODS
Animal model.
Sprague-Dawley (inbred) rats of 10 to 12 weeks of age were used in this study. Eye swabs were taken from each rat for bacteriological culture prior to the study, and rats that were not carrying P. aeruginosa were used. Baseline measurements of corneal integrity that included slit lamp biomicroscopy were performed on all rats.
Bacterial strain and growth conditions.
The cytotoxic strain 6206 of P. aeruginosa was used. Strain 6206 was isolated from a human corneal ulcer and classified as a cytotoxic strain on the basis of its interaction with corneal epithelial cells in vitro (8). Bacteria were grown in 10 ml of tryptone soy broth (Oxoid Ltd., Sydney, Australia) overnight at 37°C, harvested and washed three times in sterile phosphate-buffered saline (PBS), and resuspended in PBS prior to use.
Vaccine.
Vaccine was prepared by exposing P. aeruginosa strain 6206 (2 × 1010 CFU/ml) to 1% (wt/vol) paraformaldehyde (Sigma Chemical Co., Sydney, Australia) in PBS (pH 7.4) for 2 h at 37°C. After incubation, bacteria were washed three times in sterile PBS. For oral, nasal, and OT immunization, paraformaldehyde-killed bacteria were suspended in PBS to a concentration of 2 × 1010 CFU/ml. Paraformaldehyde-killed bacteria emulsified at a 1:1 ratio with incomplete Freund's adjuvant (Pierce, Sydney, Australia) were used to immunize rats via their intestinal Peyer's patches.
Immunization.
The primary mucosal immunization protocols were described elsewhere (9). In this study the following four immunization schedules were included: (i) combined IPP-OT immunization, (ii) combined oral-OT immunization, (iii) combined nasal-OT immunization, and (iv) OT immunization only. The OT immunization was included because local booster doses have been shown to be necessary for an optimal response in other systems (36). For each immunization group, 16 rats (3 animals for histology, 3 for enzyme-linked immunosorbent assays [ELISAs] and bacterial counts, 3 for PMN quantitation, 3 for lymphocyte proliferation assay [mesentric lymph nodes] and antigen-specific antibody detection [blood and tears], and 4 for mRNA quantitation) were used at each time point. Test groups were anesthetized by inhalation of isoflurane (Cenvet, Sydney, Australia).
(i) Peyer's patch immunization.
The delivery procedure involved performing a laparotomy to expose the small intestine and delivery of a small volume (50 μl) of the inoculum subserosally to each Peyer's patch. The incision was closed by suturing the abdominal wall and skin.
(ii) Oral immunization.
Daily doses (2 × 1010 CFU/ml) of paraformaldehyde-killed bacteria suspended in PBS were administered on days 1 to 5 and then days 10 to 14 in a 0.5-ml volume via an infant feeding tube.
(iii) Nasal immunization.
Killed bacteria (2 × 1010 CFU/ml) suspended in PBS were administered on days 1 to 3 and then days 7 to 10 intranasally in a volume of 0.2 ml.
(iv) OT booster dose.
The tear fluid was blotted from the corner of the eye, and 5 μl of vaccine (paraformaldehyde-killed bacteria suspended in PBS) was delivered onto the corneal surface on the 7th day after completion of oral and nasal immunization and 14 days after IPP immunization.
Animal infection.
After completion of the immunization schedule and 7 days postbooster, rats were anesthetized and the left and right corneas were scratched using a 26-gauge needle. Left scratched corneas were challenged topically with 2 × 106 live bacteria (P. aeruginosa strain 6206) in a 5-μl dose, while the right eyes served as scratch controls.
Clinical examination.
Anesthetized animals were examined at 4, 8, and 24 h and 3, 5, and 7 days postinfection using a slit lamp biomicroscope to grade the severity of infection. The following anterior segment variables were assessed: (i) corneal infiltrate density, grades 0 to 4, where 0 corresponds to none, 1 corresponds to very slight (iris detail visible), 2 corresponds to slight (iris detail partly obscured), 3 corresponds to moderate (iris detail not visible), and 4 corresponds to severe (opaque); (ii) depth of infiltrates, 0 to 100%, where 100% means the full corneal thickness shows infiltrates; (iii) extent of infiltrates, 0 to 100%, where 100% corresponds to full corneal coverage; (iv) epithelial defect size, 0 to 4 mm, where 4.0 mm means full epithelial loss; (v) epithelial defect depth, 0 to 100%, where 100% means a defect involving the full epithelial thickness; and (vi) edema severity, 0 to 4, where 0 corresponds to none, 1 corresponds to very slight, 2 corresponds to slight, 3 corresponds to moderate, and 4 corresponds to severe. The anterior chamber reaction was graded on the basis of cells (grades 0 to 4), flare (grades 0 to 4), fibrinotic membrane presence or absence, hypopyon presence or absence, and hyphema presence or absence. A composite corneal disease score was derived from the sum of the first five variables and a maximum total corneal score would be the total of each grade for each variable (i.e., 20).
Antigen-specific IgG and IgA detection by ELISA.
Animals were examined for an antibody response for 3 weeks after immunization. Eye wash or blood samples were collected each week to monitor the effect of the vaccine. Rats were bled from the lateral tail vein once per week after immunization to detect IgG in serum. Tears were collected by washing eyes with 20 μl of PBS (pH 7.4) to detect ocular IgA. Specific antibody to P. aeruginosa was measured by ELISA. Bacterial antigen was prepared from bacteria grown overnight on 10 nutrient agar plates. Cells were collected, washed, and resuspended in 5 ml of PBS. Suspended bacteria were sonicated using a small probe assembly. Sonication (Branson Sonifier 250; Branson Ultrasonics Corp., Danbury, Conn.) was performed with the amplitude set at 6 μ for 3 cycles of 30 s each on ice. Sonicated bacteria were centrifuged at 10,000 × g for 15 min, and the supernatant was used as a crude polyvalent antigen. ELISA plates were coated by adding 100 μl of polyvalent antigen diluted 1:1,000 in carbonate and bicarbonate buffer (pH 9.6) and were incubated at 4°C overnight. After completion of incubation, plates were washed and blocked with blocking buffer (5% skim milk and 0.05% Tween 20). Diluted serum or eye wash (control sera, 1:100; immune sera, 1:1000; control eye wash, 1:10; and immune eye wash, 1:100) was added in 100-μl volumes. IgG and IgA were probed using peroxidase-conjugated goat anti-rat IgA and IgG (Pharmingen, Sydney, Australia). Antibody present in samples was detected by adding color substrate tetramethyl benzidine, and the reaction was detected at A450.
Bacterial enumeration.
Clearance of P. aeruginosa from infected corneas was monitored by assessing the number of viable bacteria in whole eye homogenates at 4, 8, and 24 h and 3, 5, and 7 days postinfection. Small aliquots (20 μl in duplicate) of serial dilutions were plated onto nutrient agar plates. Plates were incubated for 18 h at 37°C. Results were expressed as the mean CFU/cornea ± standard errors of the means (SEMs).
PMN quantitation.
Samples were assayed for myeloperoxidase (MPO) activity as previously described (13). Briefly, the whole eye collected at various time points (4, 8, and 24 h and 3, 5, and 7 days) was homogenized in 1 ml of hexadecyl trimethylammonium bromide (HTAB) buffer (0.5% HTAB in 50 mM phosphate buffer, pH 6.0) and sonicated for 10 s in an ice bath. The samples were freeze-thawed three times and centrifuged at 8000 × g for 20 min. Supernatant (0.1 ml) was mixed with 2.9 ml of 50 mM phosphate buffer (pH 6.0) containing 0.167 mg of O-dianisidine hydrochloride per ml and 0.0005% hydrogen peroxide. The change in absorbance at 460 nm was monitored continuously for 5 min in a spectrophotometer (Unicam; Selby Bioscientific, Sydney, Australia). One unit of MPO activity was determined to be equivalent to approximately 2 × 105 PMNs/ml (4).
Lymphocyte proliferation assay.
The lymphocyte proliferation assay was performed as described by Kyd et al. (21). Briefly, lymphocytes were obtained by passing mesenteric lymph nodes through a steel sieve and washing them in cold, sterile PBS supplemented with calcium, magnesium (CSL Biosciences, Sydney, Australia), 5% fetal calf serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 0.25 μg of amphotericin B/ml (CSL Biosciences). Viable cells were counted by trypan blue exclusion. Cells were resuspended in culture medium RPMI 1640 (CSL Biosciences) containing HEPES (pH 7.2), 5 × 10−5 M β-mercaptoethanol (ICN, Sydney, Australia), 2 mM l-glutamine, 5% fetal calf serum, and penicillin, streptomycin, and amphotericin B (as described above) at a final concentration of 106 cells/ml. Polyvalent antigen was diluted in culture medium in a 10-fold dilution series and filter sterilized. The cell suspension and antigen were cultured in triplicate in a final volume of 0.2 ml/well. Lymphocyte proliferation was determined by [3H]thymidine (Amersham Australia, Sydney, Australia) incorporation for the last 8 h of a 4-day culture by counting radioactivity in a scintillation counter. Results were calculated by subtraction of the background counts (radioactivity) from the geometric means (counts) of triplicate wells.
Histopathology of rat corneas.
Rats were sacrificed at 4, 8, and 24 h and 3, 5, and 7 days postinfection and corneas were fixed in 2.5% (vol/vol) glutaraldehyde in 0.1 M sodium cacodylate (pH 7.4) at 4°C for 4 h. Fixed tissues were washed three times with PBS and dehydrated in graded ethanol (30, 50, 70, and 90%). Tissues were left at least 1 day in the infiltrating solution (90% ethanol and historesin at a 1:1 ratio) before they were embedded in Historesin Plus (Leica, Sydney, Australia). Sections of 3 μm in thickness (Leica RM 2155) were stained with toluidine blue and examined under a light microscope for the presence of infiltrating leukocytes and epithelial defects.
RNA purification and RNase protection assay.
RNA was extracted from whole rat eyes collected at different time points in Tri-solution (Sigma-Aldrich, Sydney, Australia). RNA was isolated using standard methods of phenol-chloroform extraction and ethanol precipitation from homogenized eyes. Concentration was detected by measuring the absorbance at 260 nm. Various cytokines were detected using a multiprobe RNase protection assay (Pharmingen). Briefly, a mixture of 32P-labeled antisense riboprobe was generated from a cytokine template. Total RNA isolated from whole rat eyes was hybridized with 32P-labeled riboprobe at 56°C overnight. After completion of hybridization, the samples were digested with T1 nuclease and proteinase K. Protected fragments were purified by phenol-chloroform extraction followed by ethanol precipitation. Protected hybridized RNA samples were air dried and reconstituted in 2 μl of loading buffer, and the samples were resolved on a 4.5% polyacrylamide sequencing gel. After completion, the gel was transferred onto filter paper, dried, and exposed to X-ray film (Kodak X-omat; Sigma-Aldrich) overnight at −70°C. Film was then developed and bands were identified by comparing molecular weights to a cytokine template (rCK-1). Relative quantities were determined using Multi-analyst software (Bio-Rad, Sydney, Australia).
Cytokine and chemokine protein detection by ELISA.
Cytokine levels were measured in ocular homogenates of challenged eyes of immunized and nonimmunized animals at different time points using commercially available ELISA kits (R & D Systems, Minneapolis, Minn.). Samples for ELISA were prepared by homogenizing the whole rat eye in sterile PBS. Homogenates were centrifuged at 1,800 × g for 20 min at 4°C. The resulting supernatants were used to quantitate CINC-1 (human IL-8 homolog), IL-1β, IL-6, IL-4, IL-10, and IL-2 proteins. Samples diluted 1:5 in the sample diluting buffer were added in duplicate wells. Samples were analyzed following the manufacturer's instructions. The lower detection limit ranged between 5 and 20 pg/ml for different cytokines.
Statistical analysis.
Statistical analysis of data was performed by using one way analysis of variance tests to assess the differences in cytokine gene and protein expression in the corneas of immunized and nonimmunized animals infected with P. aeruginosa. In addition, Pearson's correlations were sought between bacterial clearance and/or PMN recruitment and the levels of cytokines. Mean differences were considered significant when P was ≤0.05.
RESULTS
Clinical Examination. (i) Nonimmunized animals.
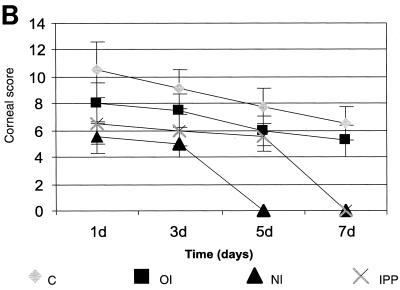
Control nonimmunized rats challenged with P. aeruginosa strain 6206 developed a predominantly edematous response at 24 h postchallenge. A single peripheral ring infiltrate covered 50 to 75% (grade 3) of the corneal diameter, and 75% (grade 3) of the stroma was involved, with moderate to severe density (grade 3.5). Ulceration involved up to 25% (grade 1) of the corneal epithelial thickness. The anterior chamber reaction was moderate, and there was moderate conjunctival redness. The composite corneal score for the severity of disease was 10.5 ± 2.1 (Fig. 1). At 7 days postchallenge, the severity (6.5 ± 1.2) of the disease was reduced.
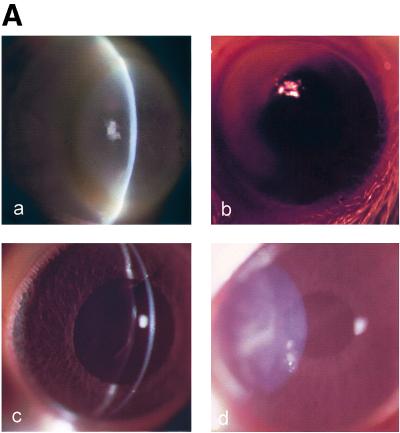
FIG. 1.
(A) Clinical examination of nonimmunized and immunized rat corneas inoculated with cytotoxic P. aeruginosa strain 6206. Panels: a, nonimmunized rat corneas showing densely packed infiltrates that appeared as a ring in the periphery and mid-periphery of the cornea at 24 h postchallenge; b, orally immunized rat corneas (50%) showing dense infiltrates in the periphery of the cornea, with fewer infiltrates in the central cornea; c, nasally immunized rat corneas (25%) showing few focal infiltrates but diffuse infiltrates all over the anterior corneal stroma; d, IPP-immunized rat corneas (25%) showing dense infiltrates in the mid-periphery of the corneal stroma. (B) Composite clinical scores for nonimmunized and immunized rat corneas challenged with cytotoxic P. aeruginosa strain 6206 at various time points. Mean differences were considered significant when P was ≤0.05. C, nonimmunized; OI, orally immunized; NI, nasally immunized; IPP, IPP immunized.
(ii) Oral immunization.
The corneas of 25 to 50% of the immunized animals were clear at 24 h postchallenge. Infected corneas showed complete or incomplete ring infiltrates at the periphery, with moderate densities (grade 3). Infiltrates involved 40 to 50% (grades 2 to 2.5) of the stromal thickness and 50% of the corneal diameter (grades 2 to 2.5), with overlying epithelial defects. There was a mild to moderate anterior chamber response and some hypopyon was seen. The composite score for the severity of the disease was 8.0 ± 1.5. At 7 days postchallenge, the severity of the disease was reduced (5.2 ± 1.2) (Fig. 1).
(iii) Nasal immunization.
At 24 h postchallenge, 75% of the animals showed clear, healthy corneas and infected animals showed a few focal and diffuse infiltrates and no epithelial defects. The composite score for the severity of disease was 5.5 ± 1.2. At 7 days postchallenge, the corneas of nasally immunized rats appeared normal (Fig. 1).
(iv) IPP immunization.
The corneas of most IPP-immunized animals (50 to 75%) were normal 24 h after challenge with strain 6206. Infected corneas were edematous, a few focal stromal infiltrates covered 25% (grades 1.5 to 2.0) of the corneal diameter, and 40% of infected corneas had stromal involvement (grades 2 to 2.5) with mild densities (grades 2 to 2.5). There was no epithelial defect present. In these animals an anterior chamber examination revealed a fibrinous reaction (grades 2 to 3). The composite score for the severity of disease was 6.5 ± 1.5. At 7 days postchallenge, the corneas appeared normal (Fig. 1).
Histological examination. (i) Nonimmunized (control) animals.
There was massive PMN infiltration streaming from the limbus and conjunctiva to the mid-periphery (densely packed) of the corneal stroma and fewer PMNs in the central cornea at 24 h postchallenge with strain 6206 in nonimmunized animals. The PMNs were lined up at the Descemet's membrane. Bacteria could be seen at the wound site and throughout the stroma. The epithelial defect was moderate (Fig. 2). At 7 days postchallenge, the infiltrates could be seen throughout the corneal stroma but the density was much less compared to that at 24 h postchallenge. New vessel growth was evident, and the epithelium was healed completely.
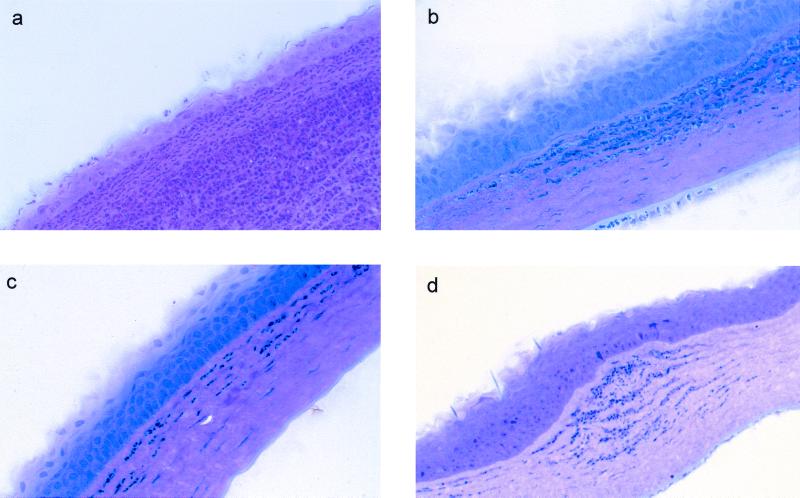
FIG. 2.
Histological examination at 24 h postchallenge of nonimmunized and immunized rat corneas inoculated with cytotoxic P. aeruginosa strain 6206. Panels: a, corneas of nonimmunized rats showing massive PMN infiltration streaming through the limbus and conjunctiva into the mid-periphery of the corneal stroma, with fewer PMNs in the central cornea, bacteria that could be seen throughout the corneal stroma, and thinned epithelium in the periphery and mid-periphery of the cornea; b, corneas of orally immunized rats (50%) showing infiltrates in the periphery of the cornea, with fewer PMNs in the central cornea and healed epithelium at the original scratch site; c, corneas of nasally immunized rats (25%) showing diffuse infiltrates all over the corneal stroma, with the scratch site being healed completely and with no epithelial defect seen at 24 h post challenge; d, IPP-immunized rat corneas (25%) showing few patches of focal infiltrates in the mid-periphery of the corneal stroma and diffuse infiltrates in the periphery of the cornea, with bacteria that could be seen near focal infiltrates in the corneal stroma.
(ii) Oral immunization.
The corneas of immunized rats that developed infection (50 to 75%) after challenge with strain 6206 showed PMN infiltration, with PMN streaming from the limbus to the periphery of the corneal stroma. Bacteria could be seen at the wound site and anterior stroma. A moderate epithelial defect was present (Fig. 2). At 7 days postchallenge, the infiltrates were still present in diffuse and focal patches and bacteria could not be seen in the corneal stroma.
(iii) Nasal immunization.
The corneas of intranasally immunized rats showed diffuse infiltration throughout the corneal stroma. The epithelium was intact (Fig. 2). At 7 days postchallenge, the corneal histology appeared normal.
(iv) IPP immunization.
Immunized animals (25 to 50%) challenged with strain 6206 showed focal patches of infiltration in the stroma at 24 h postchallenge. Infected corneas were edematous, and no epithelial defect was present (Fig. 2). At 7 days postchallenge, very few infiltrates were seen in the corneal stroma.
Evidence for the presence of antigen-specific antibody in tear fluid and serum of immunized rats.
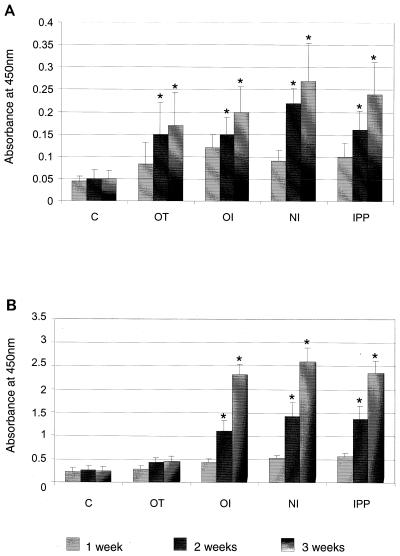
The antibody response following immunization was measured by ELISA. Antigen-specific IgA was measured every week for 3 weeks after OT, nasal, oral, and IPP immunization. All immunization routes elicited significantly higher levels (P < 0.0001) of antigen-specific IgA antibodies in tear fluid than control nonimmunized rats. The most vigorous response was seen 3 weeks after immunization. There was no significant difference found in IgA antibody levels between the immunization groups (Fig. 3A). Antigen-specific IgG antibodies in serum were present in significantly higher (P < 0.0001) levels in nasally, orally, and IPP-immunized groups compared to OT-immunized and control nonimmunized rats. The peak response was seen 3 weeks after immunization in all immunized groups. There was no significant difference in IgG antibody levels found between the nasally, orally, and IPP-immunized groups (Fig. 3B).
FIG. 3.
P. aeruginosa-specific IgA and IgG antibodies in tears and serum of nonimmunized (C) and immunized animals (immunized with paraformaldehyde-killed whole bacteria) at 1, 2, and 3 weeks after immunization. (A) Antigen-specific IgA antibodies in tears measured by ELISA. (B) Antigen-specific IgG antibodies in serum. Each value represents the mean ± SEM for samples from three rats in each group. The ELISA titer corresponds to the absorbance at 405 nm. Mean differences were considered significant (∗) when P was ≤0.05. OI, orally immunized; NI, nasally immunized; IPP, IPP immunized.
Evidence for rapid bacterial clearance in immunized groups.
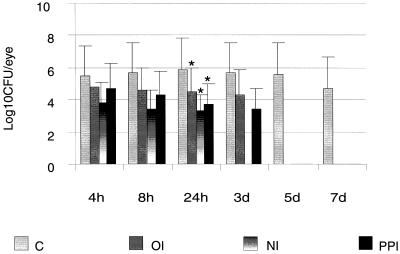
Viable counts of the infected eye from immunized and nonimmunized animals were performed at 4 h postchallenge and continued for up to 7 days. All immunized groups showed rapid clearance of bacteria. Significantly lower numbers of bacterial cells were present in nasally (P = 0.03), IPP- (P = 0.045), and orally (P = 0.048) immunized animals at 24 h postchallenge than in nonimmunized animals. Bacteria could not be recovered from nasally immunized groups by day 3, and by day 5 all immunized groups lacked recoverable bacteria. Bacterial cells could not be cultured from clinically clear corneas of IPP- orally, and nasally immunized rats (Fig. 4).
FIG. 4.
Bacterial number in whole eye of nonimmunized (C) and immunized rats challenged with cytotoxic P. aeruginosa strain 6206 at 4, 8, and 24 h and 3, 5, and 7 days postchallenge. For each time point three individual eyes were used for each group. Results are presented as mean log10 number of CFU ± SEM/eye. Mean differences were considered significant (∗) when P was ≤0.05. OI, orally immunized; NI, nasally immunized; IPP, IPP immunized.
Effect of immunization on PMN infiltration.
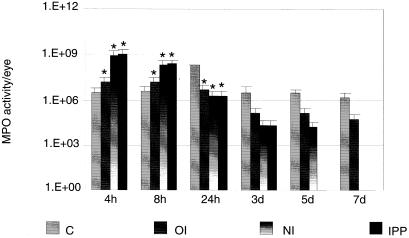
The MPO activity in experimental groups was calculated by subtracting the MPO activity of the normal eye (21 × 103 ± 4.6 × 103). Comparison of MPO activity in immunized and nonimmunized animals showed significantly higher (nasal, P = 0.03; IPP, P = 0.035; oral, P = 0.05) infiltration levels of PMNs in all three immunized groups at 4 h post challenge which were significantly diminished (nasal, P < 0.01; IPP, P < 0.001, P < 0.001) at 24 h post challenge compared to nonimmunized rats. The levels of PMNs in nonimmunized rats peaked at 24 h postchallenge and remained elevated for up to 7 days (Fig. 5).
FIG. 5.
MPO activity in whole eyes of nonimmunized (C) and immunized rats challenged with cytotoxic P. aeruginosa strain 6206 at 4, 8, and 24 h and 3, 5, and 7 days postchallenge. Three individual eyes were used for each group at each time point. Results are reported as mean MPO activity ± SEM/eye. Mean differences were considered significant (∗) when P was ≤0.05. OI, orally immunized; NI, nasally immunized; IPP, IPP immunized.
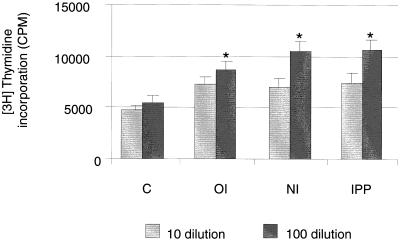
Enhanced antigen-specific lymphocyte proliferation in immunized animals.
Lymphocytes isolated from mesenteric lymph nodes from immunized and nonimmunized rats were cultured with killed bacteria (at 1:10 and 1:100 antigen dilutions) to assess the levels of antigen-specific lymphocyte responses. Antigen-specific proliferation was significantly higher (1:100 dilution, P < 0.0001) in immunized groups than in nonimmunized rats. Lymphocytes isolated from nasally and IPP-immunized animals showed significantly higher (P < 0.001) proliferation in the presence of killed bacteria than in orally immunized animals (Fig. 6).
FIG. 6.
P. aeruginosa-specific proliferation of lymphocytes isolated from MLN of nonimmunized (C) and immunized rats. Mesenteric lymph nodes from immunized rats were collected at 7 days post-booster immunization. Results are reported as means ± SEMs of triplicate cultures for each of three rats per group at two concentrations (1:10 and 1:100). Mean differences were considered significant (∗) when P was ≤0.05. OI, orally immunized; NI, nasally immunized; IPP, IPP immunized.
Differential profile of cytokine mRNA expression in immunized groups.
The rCK-1 template with multiple probes (IL-1β, IL-4, IL-5, IL-6, IL-2, and IL-10) was used to detect mRNA in immunized and nonimmunized groups. Immunized groups showed differential mRNA expression compared to the nonimmunized control group.
(i) Nonimmunized (control) rats.
Various cytokines were present in the corneas of immunized and control rats infected with strain 6206. Transcripts of IL-1β and IL-4 were highly upregulated, while IL-6 was upregulated to a lesser extent, at 24 h postchallenge compared to immunized groups. IL-10 was present in significantly lower (P < 0.0003) levels than in immunized groups. Transcripts of IL-2 and IL-5 were not detected at any time points.
(ii) Oral immunization.
There was upregulation of IL-1β mRNA expression at 24 h postchallenge compared to other immunized (nasal and IPP) groups. IL-4 and IL-10 mRNA showed similar expression patterns to those of other immunized animals. Similar to controls, IL-2 and IL-5 mRNAs were not detected.
(iii) Nasal immunization.
The expression profile of cytokine mRNA in nasally immunized rats differed from those of both nonimmunized and orally immunized animals. Transcripts of IL-2, IL-5, and IL-10 were upregulated, while IL-1β and IL-4 mRNA were expressed at significantly lower (IL-1β, P < 0.0002; IL-4, P < 0.003) levels at 24 h postchallenge than in control nonimmunized animals. Expression of IL-6 mRNA was below the detection limit at any time point.
(iv) IPP immunization.
Rats immunized through IPP had a similar pattern of cytokine mRNA expression to those that were immunized nasally, except for IL-6 expression. IPP-immunized rats showed increased expression of IL-2, IL-5, and IL-10 mRNA and decreased expression of IL-1β, IL-4, and IL-6 at 24 h postchallenge compared to controls. Unlike nasally immunized rats, IPP-immunized animals expressed both IL-5 and IL-6 mRNA (Fig. 7).
FIG. 7.
Kinetics and levels of cytokine mRNA expression quantified by RNase protection assay in whole rat eyes of nonimmunized (C) and immunized rats challenged with cytotoxic P. aeruginosa strain 6206. The graphs show means ± SEMs from three experiments, with five animals in each group at each time point, normalized with two housekeeping genes (L32 and GAPDH). Mean differences were considered significant (∗) when P was ≤0.05. OI, orally immunized; NI, nasally immunized; IPP, IPP immunized.
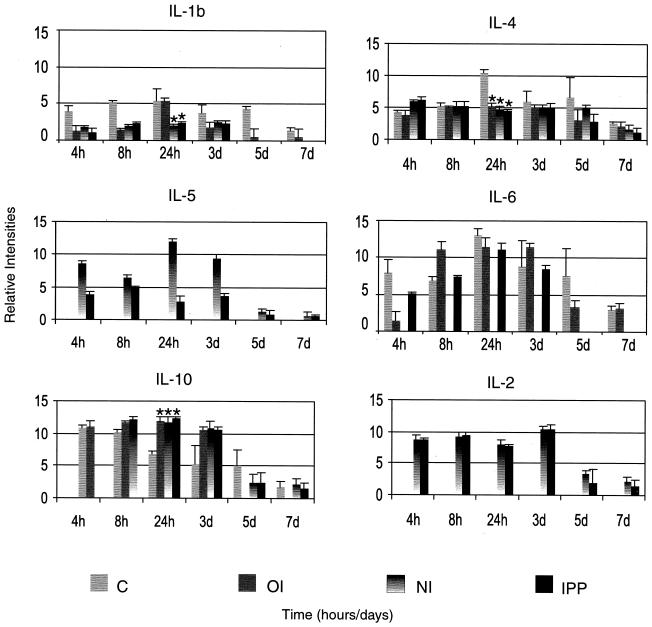
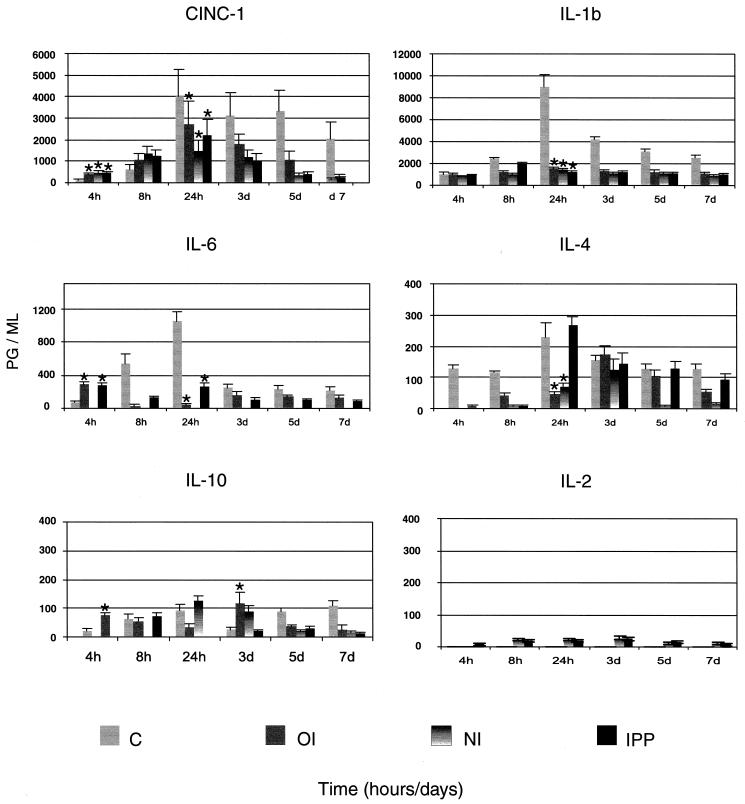
Effect of immunization on cytokine protein secretion.
The protein levels were not determined for all cytokines (those probed for mRNA) due to the limited availability of reagents for rats.
(i) Nonimmunized controls.
In nonimmunized rats, neutrophil chemoattractant CINC-1 protein levels were significantly lower (P < 0.04) early (4 h) during the infection and were significantly higher (P < 0.03) later (24 h) during the infection than those of immunized rats. Expression of CINC-1 protein remained high up to 7 days postinfection. The amount of IL-1β protein gradually increased and peaked at 24 h postchallenge and remained high up to 7 days postinfection. IL-6 protein also peaked at 24 h, diminished drastically at 3 days postinfection, and remained low up to 7 days postchallenge. Nonimmunized rats showed high levels of IL-4 protein which peaked at 24 h (P < 0.03) postchallenge and remained high up to 7 days postinfection.
(ii) Oral immunization.
Expression of CINC-1 protein was significantly higher (P < 0.04) early during the infection (4 and 8 h) and significantly lower (P = 0.013) by 24 h postinfection than that of nonimmunized control rats. IL-1β protein levels were low throughout the period of infection compared to those of nonimmunized rats. The levels of IL-6 protein were significantly higher (P < 0.033) in orally immunized rats at 4 h postchallenge than those of nonimmunized rats. The pattern of protein expression was reversed at 8 h postinfection, with IL-6 protein levels increasing dramatically in nonimmunized animals. IL-10 and IL-4 proteins showed a biphasic pattern, with the first peak appearing at 4 to 8 h and the second at 3 days postinfection.
(iii) Nasal immunization.
The protein secretion pattern of CINC-1 and IL-1β was similar to those of orally and IPP-immunized rats. IL-6 protein was below the limit of detection. IL-4 and IL-10 proteins were present late during the infection. IL-2 protein was present at most time points but at very low levels.
(iv) IPP immunization.
The pattern of CINC-1 protein secretion was the same as in orally or nasally immunized rats. IL-1β and IL-6 levels were low throughout the period of infection compared to nonimmunized rats, except for the levels of IL-6 at 4 h (P < 0.033). IL-4 protein was highly upregulated at 24 h postinfection and diminished thereafter. Unlike in nasally immunized rats, IL-10 protein showed a biphasic pattern peaking very early (4 to 8 h) and late (5 days) during the infection (Fig. 8). IL-2 protein was present at all time points at very low levels.
FIG. 8.
Kinetics and levels of cytokine protein secretion quantified by ELISA in whole rat eyes of nonimmunized (C) and immunized rats challenged with cytotoxic P. aeruginosa strain 6206. The results are presented as means ± SEMs from three experiments, with three animals in each group at each time point. Mean differences were considered significant (∗) when P was ≤0.05.
DISCUSSION
Our study showed that the route of immunization affects the severity and persistence of microbial keratitis. Immunization has the potential to modulate the inflammatory response to an infection. This modulation includes the production of chemical signals, cytokines and chemokines, with recruitment and activation of cells involved in clearing the infection (29). This study has demonstrated that immunization changes the kinetics of PMN infiltration, with immune groups having more rapid recruitment and resolution of PMNs in the cornea than the nonimmune group. Associated with this was a more rapid clearance of bacteria, differences in the levels of cytokines expressed and produced, and reduced adverse pathology. In particular, the IPP and intranasal immunization regimes with an OT boost provided the best protection from corneal ulceration.
CINC-1 is a potent activator and attractant of neutrophils (27). Increased CINC-1 levels were detected earlier (4 to 8 h) postinfection in immunized rats than in nonimmunized rats, with all groups peaking at 24 h postchallenge. However, despite the earlier increased production of CINC-1 in the immunized groups, the peak levels of CINC-1 were significantly lower in the immunized groups and also decreased far more rapidly. The changes in the CINC-1 levels corresponded to the recruitment and resolution profiles of the PMNs. The rate of PMN recruitment in other disease settings has been associated with early bacterial clearance, such as enhanced respiratory clearance of nontypeable Haemophilus influenzae following mucosal immunization (5, 10). Persistence of PMNs in the nonimmune animals during the later stages of infection may contribute to corneal scarring and perforation.
For the PMN response to be beneficial rather than detrimental, a rapid resolution of PMN infiltration must occur. Immunization of rats against P. aeruginosa corneal infection achieved a rapid resolution of PMN infiltrates. In addition to the modulation of CINC-1 levels, there were reduced levels of the proinflammatory cytokines (IL-1β and IL-6) and similar or higher levels of the cytokines associated with immunosuppressive or IgA antibody responses (IL-10 and IL-4). Balanced expression of proinflammatory and anti-inflammatory cytokines in the immunized animals compared to the overwhelming proinflammatory cytokine response in the nonimmune group may control inflammation by regulating not only inflammatory cell recruitment but also IgA secretion.
Clearance of bacteria from the ocular surface is presumed to involve the combined actions of PMNs and secretory IgA. Immunization induced significantly elevated levels of antigen-specific IgA in tears and IgG in serum. The role of antigen-specific antibodies in protection against corneal infection is controversial, with correlation between the presence of antibody and protection not always being clearly defined. A recent (24) study has shown that secretory IgA can significantly inhibit P. aeruginosa binding to wounded mouse cornea in vitro, thereby protecting against keratitis. One of the mechanisms by which IgA antibodies may prevent bacterial colonization is by specifically interacting with bacterial adhesins required for binding to mucosal tissue (24). IgA is capable of potentiating the function of innate antibacterial factors and interacting with mucosal phagocytic cells and lymphocytes (25). Oral immunization with Acanthamoeba spp. antigen mixed with cholera toxin induces the production of parasite-specific IgA in mucosal secretions and prevents corneal infection (23). Although antigen-specific IgG antibody appears to be important for opsonophagocytosis (34), a correlation between the presence of opsonizing antibodies and protection in vivo has not been clearly determined to be an essential mechanism of effective immunity (33, 35). There is also evidence that suggests that systemically derived IgG may also be capable of conferring protection in the cornea (28). In addition to measuring significant titers of antigen-specific IgA in tears, we have demonstrated the presence of a group of IgA-enhancing Th2-type cytokines (IL-4, IL-5, IL-6, and IL-10) which may provide an environment for preferential immunoglobulin class switching for IgA in the eye.
Previous studies using a rat model for pulmonary P. aeruginosa infection have shown that mucosal immunization significantly alters the profile of inflammatory cytokines produced in response to infection (5). Other evidence also suggests that nasal and IPP immunization with mucosal adjuvant induces dominant Th2 responses in nasal-associated lymphoid tissue and Peyer's patches (12, 39). This study has shown that the route of immunization changes the profile of cytokine expression during P. aeruginosa corneal infection, with the most significant differences appearing in the nasal and IPP immunization groups. Expression of IL-2 and IL-5 were especially altered, with nasally immunized rats expressing high levels of IL-5 and baseline levels of IL-6 mRNA, with corresponding baseline levels of IL-6 protein. In contrast, orally immunized rats showed no IL-5 expression but had high IL-6 expression and secretion, while IPP immunization resulted in the upregulation of both IL-5 and IL-6. IL-5 and IL-6 are known to differentially influence the B-1 and B-2 lineage of plasma cells (2). Collectively, the data suggest that nasally immunized animals may be producing IgA plasma cells of B-1 lineage, which are IL-5 dependent and IL-6 independent (2), whereas orally immunized animals may be producing predominantly cells of B-2 lineage. B-1 cells are physically and functionally unique B cells producing antibodies to bacterial antigens such as lipopolysaccharide and phosphocholine (1). B-1 cells mainly reside in mucosal effector tissues, while conventional IgA+ B-2 cells reside in mucosal inductive sites (39). Nasal-associated lymphoid tissue functions as a primary inductive site for IgA antibody in tears by contributing triggered IgA-committed B cells to the lacrimal gland (22). A recent study (30) has shown that a high frequency of IgA-committed B-1 cells occurs in the lacrimal gland (an effector site).
A role for T cells and cytokines produced by activated T cells in protection from ocular bacterial infections has not been demonstrated previously. Nasally and IPP-immunized rats induced antigen-specific lymphocyte responses, providing evidence that an antigen-specific T-lymphocyte response was induced by immunization and that these lymphocytes migrated from the site of immunization. Immunologically specific T cells recruit neutrophils in an antigen-dependent and dose-dependent fashion (6). Cytokines released by activated T cells may direct the activity of nonspecific effector cells (21, 37). All of these studies have shown the involvement of T cells and cytokines in respiratory disease models. Evidence that supports the relevance of a CD4+ Th1- versus Th2-type immune response was presented in a study that used a mouse P. aeruginosa keratitis model. Data from this study suggest that Th2-responsive mice regulate inflammatory cellular infiltrates more efficiently by downregulating the inflammatory response, which in turn results in less corneal stromal damage (11, 20). Further studies are required to define the importance of a T-cell response in protection against ocular infection.
This study has demonstrated that the immunization route modulates the inflammatory response to ocular P. aeruginosa infection, thus affecting the severity of keratitis and adverse pathology. The results show that immunization affects the rate of bacterial clearance and alters the profile of cytokines produced in response to ocular infection, with nasal immunization resulting in the most significant level of protection. The results suggest that the degree of protection afforded by immunization may depend upon the rapid recruitment of PMNs, the induction of antigen-specific IgA, and the balanced production of proinflammatory and immunosuppressive cytokines and that T-cell responses may influence these events.
ACKNOWLEDGMENTS
This research was partly supported by the Australian Federal Government through the Cooperative Research Centres Program.
We thank Reg Wong for excellent statistical analysis, Wen Wang for technical assistance, Denise Lawler and Robyn Lawler for helping with animal experiments, and Philip Julian and Carol Woollcott for their help in preparing illustrations.
REFERENCES
- 1.Aramaki M, Nagasawa T, Koeshi T, Ishikawa I. Presence of activated B-1 cells in chronic inflamed gingival tissue. J Clin Immunol. 1998;18:421–429. doi: 10.1023/a:1023234823783. [DOI] [PubMed] [Google Scholar]
- 2.Bao S, Beagley K W, Murray A M, Caristo V, Matthaei K I, Young I G, Husband A J. Intestinal IgA plasma cells of the B1 lineage are IL-5 dependent. Immunology. 1998;94:181–188. doi: 10.1046/j.1365-2567.1998.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beagley K W, Eldridge J H, Kiyono H, Lee F. Interleukins and IgA synthesis: human and murine IL-6 induce high rate of IgA secretion in IgA-committed B-cells. J Exp Med. 1989;169:2133–2148. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley P P, Christensen R D, Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982;60:618–625. [PubMed] [Google Scholar]
- 5.Buret A, Dunkley M L, Pang G, Clancy R L, Cripps A W. Pulmonary immunity to Pseudomonas aeruginosa in intestinally immunized rats: role of alveolar macrophages, tumor necrosis factor, and interleukin-la. Infect Immun. 1994;62:5335–5343. doi: 10.1128/iai.62.12.5335-5343.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell P A. The neutrophil, a professional killer of bacteria, may be controlled by T cells. Clin Exp Immunol. 1990;79:141–143. doi: 10.1111/j.1365-2249.1990.tb05169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Challacombe S J, Tomasi T B., Jr Systemic tolerance and secretory immunity after oral immunization. J Exp Med. 1980;152:1459–1472. doi: 10.1084/jem.152.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole N, Willcox M D P, Fleiszig S M J, Stapleton F, Bao S, Tout S, Husband A. Different strains of Pseudomonas aeruginosa isolated from ocular infections or inflammation display distinct corneal pathologies in an animal model. Curr Eye Res. 1998;17:730–735. [PubMed] [Google Scholar]
- 9.Cripps A W, Dunkley M L, Clancy R L. Mucosal and systemic immunizations with killed Pseudomonas aeruginosa protect against acute respiratory infection in rats. Infect Immun. 1994;62:1427–1436. doi: 10.1128/iai.62.4.1427-1436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foxwell A R C, Kyd J M, Cripps A W. Characterization of the immunological response in the clearance of nontypable Haemophilus influenzae from the lung. Immunol Cell Biol. 1998;76:323–331. doi: 10.1046/j.1440-1711.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 11.Hazlett L D, McClellan S, Kwon B, Barrett R. Increased severity of Pseudomonas aeruginosa corneal infection in strains of mice designated as Th1 versus Th2 responsive. J Immunol. 2000;41:805–810. [PubMed] [Google Scholar]
- 12.Hiroi T, Yanagita M, Iijima H, Iwatani K, Yoshida T, Takatsu K, Kiyono H. Deficiency of IL-5 receptor a-chain selectively influences the development of the common mucosal immune system independent IgA-producing B-1 cells in mucosa-associated tissue. J Immunol. 1999;162:821–828. [PubMed] [Google Scholar]
- 13.Hobden J A, Masinick S A, Barrett R P, Hazlett L D. Proinflammatory cytokine deficiency and pathogenesis of Pseudomonas aeruginosa keratitis in aged mice. Infect Immun. 1997;65:2754–2758. doi: 10.1128/iai.65.7.2754-2758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue Y, Shimomura Y, Manabe R. Herpes simplex virus glycoprotein D: protective immunity against murine herpetic keratitis. Investig Ophthalmol Vis Sci. 1992;31:411–418. [PubMed] [Google Scholar]
- 15.Kelleher R S, Hann L E, Edwards J A. Endocrine, neural and immune control of secretory component output by lacrimal gland acinar cells. J Immunol. 1991;146:3405–3412. [PubMed] [Google Scholar]
- 16.Kernacki K A, Berk R S. Characterization of the inflammatory response induced by corneal infection induced by Pseudomonas aeruginosa. J Ocul Pharmacol. 1994;10:281–288. doi: 10.1089/jop.1994.10.281. [DOI] [PubMed] [Google Scholar]
- 17.Kernacki K A, Hobden J A, Hazlett L D, Friedman R, Berk R S. In vivo bacterial protease production during Pseudomonas aeruginosa corneal infection. Investig Ophthalmol Vis Sci. 1995;36:1371–1379. [PubMed] [Google Scholar]
- 18.Kernacki K A, Barrett R P, Hobden J A, Hazlett L D. Macrophage inflammatory protein-2 is a mediator of polymorphonuclear neutrophil influx in ocular bacterial infection. J Immunol. 2000;164:1037–1045. doi: 10.4049/jimmunol.164.2.1037. [DOI] [PubMed] [Google Scholar]
- 19.Kreger A S, Lyerly D M, Hazlett L D, Berk R S. Immunization against experimental Pseudomonas aeruginosa and Serratia marcescens keratitis. Vaccination with lipopolysaccharide endotoxins and proteases. Investig Ophthalmol Vis Sci. 1986;27:932–939. [PubMed] [Google Scholar]
- 20.Kwon B, Hazlett L D. Association of CD4+ T cell-dependent keratitis with genetic susceptibility to Pseudomonas aeruginosa ocular infection. J Immunol. 1997;159:6283–6290. [PubMed] [Google Scholar]
- 21.Kyd J M, Dunkley M L, Cripps A W. Enhanced respiratory clearance of nontypable Haemophilus influenzae following mucosal immunization with P6 in rat model. Infect Immun. 1995;63:2931–2940. doi: 10.1128/iai.63.8.2931-2940.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lather D M R, Gill R F, Montgomery P C. Inductive pathways leading to rat tear IgA antibody responses. Investig Ophthalmol Vis Sci. 1998;39:1005–1011. [PubMed] [Google Scholar]
- 23.Leher H F, Alizadeh H, Taylor W M, Shea A S, Silvany R S, Klink F V, Jager M J, Niederkorn J Y. Role of mucosal IgA in the resistance to Acanthamoeba keratitis. Investig Ophthalmol Vis Sci. 1998;39:2666–2673. [PubMed] [Google Scholar]
- 24.Masinick S A, Montgomery C P, Montgomery P C, Hazlett L D. Secretory IgA inhibits Pseudomonas aeruginosa binding to cornea and protects against keratitis. Investig Ophthalmol Vis Sci. 1997;38:910–918. [PubMed] [Google Scholar]
- 25.McGhee J R, Kiyono H. New perspective in vaccine development: mucosal immunity to infections. Infect Agents Dis. 1993;2:55–73. [PubMed] [Google Scholar]
- 26.Moon M M, Hazlett L D, Hancock R E, Berk R S, Barrett R. Monoclonal antibodies provided protection against ocular Pseudomonas aeruginosa infection. Investig Ophthalmol Vis Sci. 1988;29:1277–1284. [PubMed] [Google Scholar]
- 27.Mulligan M S, Jones M L, Bolanowski M A, Baganoff M P, Deppeler W L, Meyers D M, Ryan U S, Ward P A. Inhibition of lung inflammatory reactions in rats by an anti-human IL-8 antibody. J Immunol. 1993;150:5585–5595. [PubMed] [Google Scholar]
- 28.Preston M J, Gereceker A A, Koles N L, Pollack M, Pier G B. Prophylactic and therapeutic efficacy of immunoglobulin G antibodies to Pseudomonas aeruginosa lipopolysaccharide against murine experimental corneal infection. Investig Ophthalmol Vis Sci. 1997;38:1418–1425. [PubMed] [Google Scholar]
- 29.Rudner X L, Kernacki K A, Barrett R P, Hazlett L D. Prolonged elevation of IL-1 in Pseudomonas aeruginosa ocular infection regulates macrophage-inflammatory protein-2 production, polymorphonuclear neutrophil persistence, and corneal perforation. J Immunol. 2000;164:6576–6582. doi: 10.4049/jimmunol.164.12.6576. [DOI] [PubMed] [Google Scholar]
- 30.Saitoh-Inagawa W, Takachika H, Yanagita M, Iijima H, Uchio E, Ohno S, Aoki K, Kiyono H. Unique characteristics of lacrimal gland as a part of mucosal immune network: high frequency of IgA-committed B-1 cells and NK1.1+ ab T cells. Investig Ophthalmol Vis Sci. 2000;41:138–144. [PubMed] [Google Scholar]
- 31.Schein O D, Ormerod L D, Barraquer E. Microbiology of contact lens associated keratitis. Cornea. 1989;8:281–285. [PubMed] [Google Scholar]
- 32.Steuhl K P, Doring G, Thiel H J. The significance of bacterial and host factors in corneal infections caused by Pseudomonas aeruginosa. Fortschr Ophthalmol. 1989;86:283–286. [PubMed] [Google Scholar]
- 33.Troelstra A, Vogel L, van Alphen L, Eijk P, Jansen H, Dankert J. Opsonic antibodies to outer membrane protein P2 of nonencapsulated Haemophilus influenzae are strain specific. Infect Immun. 1994;62:779–784. doi: 10.1128/iai.62.3.779-784.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verhoef J, Visser M R. Neutrophil phagocytosis and killing: normal function and microbial evasion. In: Abramson J S, Wheeler J G, editors. The neutrophil. New York, N.Y: Oxford University Press; 1993. pp. 108–137. [Google Scholar]
- 35.Wallace F J, Clancy R L, Cripps A W. An animal model demonstration of enhanced clearance of non-typable Haemophilus influenzae from respiratory tract after antigen stimulation of gut associated lymphoid tissue. Am Rev Respir Dis. 1989;140:311–316. doi: 10.1164/ajrccm/140.2.311. [DOI] [PubMed] [Google Scholar]
- 36.Wallace F J, Cripps A W, Clancy R L, Husband A J, Witt C S. A role for intestinal T lymphocytes in bronchus mucosal immunity. Immunology. 1991;74:68–73. [PMC free article] [PubMed] [Google Scholar]
- 37.Wallace F J, Witt C S, Clancy R L, Cripps A W, Husband A J. Protection against non-typable Haemophilus influenzae following sensitization of gut lymphoid tissue: role of specific antibody and phagocytes. Immunol Cell Biol. 1995;73:258–265. doi: 10.1038/icb.1995.42. [DOI] [PubMed] [Google Scholar]
- 38.Welsh N H, Rauch A J, Graffin S L. Topical immunotherapy for Pseudomonas keratitis in rabbits: use of anti-lipopolysaccharide plasma. Br J Ophthalmol. 1984;68:828–832. doi: 10.1136/bjo.68.11.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanagita M, Hiroi T, Kitagaki N, Hamada S, Ito H, Shimauchi H, Murakami H, Kiyono H. NALT immunity: fimbriae-specific Th1 and Th2 cell regulated IgA responses for the inhibition of bacterial attachment to epithelial cells and subsequent inflammatory cytokines. J Immunol. 1999;162:3559–3565. [PubMed] [Google Scholar]