Summary
Background
A gluten‐free diet reduces symptoms in some patients with irritable bowel syndrome (IBS) through unclear mechanisms.
Aims
To assess the effects of gluten‐free versus gluten‐containing diet on symptoms and the gut microenvironment, and to identify predictors of response to the gluten‐free diet in IBS
Methods
Twenty patients with IBS and 18 healthy controls (HC) followed a gluten‐free diet during two 14‐day intervention periods where they sprinkled either gluten (14 g/day) or rice flour powder over their meals. Primary outcomes included effects of the interventions on IBS symptoms (IBS‐SSS) and bowel habits. Secondary outcomes included effects of gluten‐free diet on faecal microbiota and metabolite profile.
Results
IBS symptoms improved during the gluten‐free (p = 0.02), but not the gluten‐containing period, with no difference between the interventions. IBS patients reported fewer loose stools during the gluten‐free intervention (p = 0.01). Patients with IBS and HC presented distinct metabolite profiles based on the effects of the gluten‐free diet (p < 0.001). True responders (reduced IBS‐SSS by ≥50 solely after gluten‐free period) and non‐responders were discriminated based on the effects of the gluten‐free diet on the microbiota (p < 0.01) and metabolite profiles (p < 0.001). The response to the gluten‐free diet could be predicted by the metabolite profile before the intervention (p < 0.001).
Conclusions
A gluten‐free diet may influence symptoms in a subset of patients with IBS, with a particular effect on bowel habits. A gluten‐free diet seems to impact the gut microenvironment. Responsiveness to the gluten‐free diet may be predicted by the metabolite profile. Clinicaltrials.gov: NCT03869359.
Gluten‐free diet and gut microenvironment in IBS.

1. INTRODUCTION
The majority of irritable bowel syndrome (IBS) patients relate their symptoms to intake of certain foods. 1 The gut microenvironment, where microbiota, food components and the nervous system interact, is suggested to play a key role in gastrointestinal (GI) symptom generation in a subset of IBS patients. 2 Currently, dietary treatments focus on excluding specific food components, for example, gluten. Somewhat conflicting and heterogeneous results have emerged when assessing the gluten‐free diet in IBS. 3 Therefore, the effects of gluten on GI symptoms in IBS patients still remain unclear.
Although a recent study has shown that exclusion diets have an effect on the gut microbiota in IBS, 4 it has not been investigated whether a gluten‐free diet influence the microbiota composition differently in IBS patients and healthy controls (HC). Furthermore, gut microbiota metabolism utilising food components results in a large variety of metabolites, 5 which are suggested to be of importance for gut function and play a role in visceral hypersensitivity in IBS. 5 The effects of the gluten‐free diet on the gut metabolite composition have not been determined in IBS patients.
This study investigated the hypothesis that a gluten‐free diet can reduce GI symptoms in a subset of IBS patients through alterations in the gut microenvironment. Therefore, the primary aim of the study was to assess and compare the efficacy of the gluten‐free and gluten‐containing diets in terms of effects on GI symptoms in IBS patients. Secondary aims were to identify the putative link between gut microenvironment and the diets´ effect on GI symptoms, and to identify potential predictors of clinical response to the gluten‐free diet.
2. MATERIALS AND METHODS
2.1. Participants
Adult sex‐ and age‐matched IBS patients and HC were recruited by public advertisement in university buildings and university‐related social media of the University of Gothenburg, Sweden. The IBS diagnosis (Rome IV) was confirmed by experienced gastroenterologists (M.S. and H.T.) after initial assessment by a trained medical doctor (J.A.). The same data were collected for the whole study population. Exclusion criteria were specific allergy or intolerance to food, severe cardiovascular, hepatic, neurological or psychiatric diseases, other GI disease, diabetes, compliance to a specific diet (including gluten‐free, vegan, low‐carb high‐fat, and low fermentable oligo‐, di‐, monosaccharides and polyols [FODMAP] diet), GI surgery (except for appendectomy or cholecystectomy), use of antibiotics within 1 month before inclusion, and pregnant or lactating females. Participants that already identified gluten as a trigger of their GI symptoms were also excluded. Baseline laboratory tests, including faecal calprotectin, tissue transglutaminase immunoglobulin (Ig) A antibodies, haemoglobin, leucocytes, thrombocytes, C‐reactive protein, sodium, potassium, calcium, albumin, e‐glomerular filtration rate, creatinine, alkaline phosphatase, alanine transaminase, aspartate aminotransferase, thyroxine, thyroid stimulating hormone, haemoglobin A1c, fasting glucose, cholesterol (total), high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, and triglycerides were done to rule out any severe concomitant disease. In addition, the participants reported usage of IBS medication (e.g., anti‐diarrhoeal medication, laxatives and neuromodulators) and probiotics. All participants signed informed consent at the screening visit and before any study related procedures. The study was approved by the regional Ethical Review Board in Gothenburg (Dnr 627‐18) in August 2018, and was carried out at Sahlgrenska University Hospital, Gothenburg, Sweden between October 2018 and November 2019, according to the declaration of Helsinki. All authors had access to the study data and reviewed and approved the final manuscript. Clinicaltrials.gov: NCT03869359.
2.2. Study design
IBS patients and HC were challenged with gluten (14 g/day) and rice flour, both for 2 weeks, while adhering to a strict gluten‐free diet in this single‐centre, double‐blind, randomised, placebo‐controlled, cross‐over trial (Figure 1). A wash‐out period of at least 2 weeks separated the interventions. The participants were on their habitual (gluten‐containing) diet during the screening and wash‐out periods. Randomisation was done by drawing concealed envelops by a nurse not involved in the study. The randomisation code was broken after the analyses of the primary outcomes.
FIGURE 1.
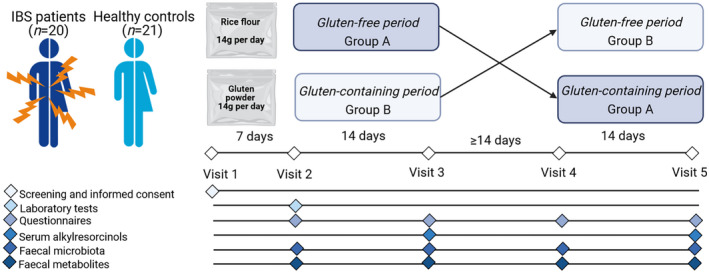
Study design, randomised double‐blind placebo‐controlled crossover trial
2.3. Interventions
The participants were carefully instructed to follow a gluten‐free diet during the interventions, and in order to increase compliance they obtained gluten‐free meal boxes (Table S1) and lists of gluten‐free foods they were allowed to eat. The participants sprinkled powder sachets (gluten and rice flour) over their meals during the interventions. Detailed information of the gluten‐free diet and a list of gluten‐free breakfast options were provided, and the participants recorded all their dietary intake in food diaries. The participants received two meal boxes per day. The participants obtained plastic sachets with vital gluten protein 14 g/day (per 100 g; energy 375 kcal, protein 78.0 g, carbohydrates 7.0 g, fat 1.4 g, fibre 0.5 g, Real Foods) and rice flour 14 g/day, which was low in FODMAPs (per 100 g; energy 375 kcal, protein 7.7 g, carbohydrates 78.0 g, fat 3.6 g, fibre 0.4 g, Doves Farm Foods Ltd). The gluten dose of 14 g/day was chosen in order to be similar to the gluten intake in the general population (10 g/day) and Swedish IBS patients (11 g/day). 6 , 7 The participants were instructed to sprinkle the powder sachets over their meal boxes (twice per day) during the different diet periods. Although the plastic sachets were identical (except marked with ‘A' or ‘B'), the contents differed in colour. To assess adequate blinding, the participants were asked after each intervention whether they thought they received gluten, placebo, or if they could not guess. After both interventions, serum samples were taken and serum alkylresorcinols were analysed using liquid chromatography/mass‐spectrometry (LC–MS) (Food and Nutrition Science, Chalmers Technical University, Gothenburg, Sweden) for assessment of compliance to both gluten‐free and gluten‐containing interventions. Alkylresorcinols are long‐chain phenolic lipids present in gluten‐containing grains, and both epidemiological and intervention studies have shown the potential of alkylresorcinols as a biomarker for both gluten intake and a gluten‐free diet. 7 , 8 , 9
2.4. IBS symptom severity
Before and after the interventions, the participants reported GI symptom severity, using the IBS‐Severity Scoring System (IBS‐SSS). 10 The IBS‐SSS incorporates typical IBS symptoms in five domains scored 0–100 including intensity and frequency of abdominal pain, intensity of abdominal bloating, bowel habit dissatisfaction, and the daily life interference of IBS in general (maximum aggregated score of 500).
2.5. Bowel habits
Bowel habits were documented daily using a stool diary based on the Bristol Stool Form (BSF) scale, 11 which depicts stool types 1–7; 1–2 hard stools, 6–7 loose stools. The data were used to assess the difference in proportions of hard stools, loose stools, and normal stools between the interventions, and to categorise IBS patients into IBS subtypes according to the Rome IV criteria: IBS with constipation (IBS‐C), IBS with diarrhoea (IBS‐D), and IBS with mixed bowel habits (IBS‐M) or unclassified IBS (IBS‐U). 12
2.6. Anxiety, depression and somatic symptoms
Assessments of anxiety and depression were done using the Hospital Anxiety and Depression Scale (HADS), 13 and somatic symptoms were assessed with the Patient Health Questionnaire (PHQ)‐12, 14 which assesses non‐GI somatic symptoms. For both HADS and PHQ‐12 (assessed before and after both interventions), higher scores indicate more severe symptoms.
2.7. Faecal microbiota
Faecal samples (online supporting information) were analysed for microbiota profiles by the commercially available GA‐map Dysbiosis Test (Genetic Analysis AS). 15 The test provides faecal bacterial profiles (16S rRNA analysis), using 48 DNA probes targeting ≥300 bacteria on different taxonomic levels assessed as probe signal intensity. To assess the effects of the interventions, the β‐diversity was estimated by use of the Bray–Curtis dissimilarity index.
2.8. Faecal metabolites
Extracted faecal supernatants were used for untargeted metabolomics, performed by LC–MS (Food and Nutrition Science, Chalmers Technical University, Gothenburg, Sweden). A dataset of >13,000 faecal metabolites was obtained after an analytical workflow (‘Notame’) for untargeted metabolic profiling approaches, utilising LC–MS analysis (online supporting information). 16
2.9. Data analyses and statistics
The first step was to assess the difference after the interventions for the primary outcomes. Here, linear mixed models with unstructured covariance pattern were used with intervention, period and randomisation sequence as fixed factors, and study participant as random factor. Randomisation sequence had no significant effect in any model, therefore, this variable was excluded from all models. Outcomes are presented for intervention. For the primary outcomes, the interventions were also analysed separately (before versus after each intervention period) with linear mixed models. A decrease of ≥50 points in IBS‐SSS defined a responder to treatment, and our definition of a true responder was an IBS patient that solely responded to the gluten‐free intervention. All others were defined as non‐responders. According to this definition, responders solely to the inactive treatment (i.e., gluten‐containing intervention) could also be identified. These patients were defined as placebo‐responders. The second step was to compare the effects of the interventions on the secondary outcomes within groups. Here, both linear mixed models and multivariate analyses, including principal component analysis (PCA) and orthogonal partial least squared discriminant analysis (OPLS‐DA) were used. IBS patients, HC, true responders, and non‐responders, were assessed separately. The third step was to assess effects of the gluten‐free diet while comparing the groups, that is IBS versus HC and true‐responders versus non‐responders, using multivariate analyses. Finally, the fourth step was to assess predictors of response to the gluten‐free diet, where data of true responders and non‐responders were compared before the gluten‐free intervention using multivariate analyses (online supporting information). Data are presented as mean ± standard deviation (SD), frequency (%) or β coefficients with 95% confidence interval (CI). The microbiota and metabolites analyses were also done separately for placebo‐responders. Linear mixed models and univariate analyses were performed using IBM SPSS Statistics, Version 27.0 (IBM). Estimations of Bray–Curtis dissimilarity and PCAs were performed in RStudio (R version 4.0.3) using the vegan and pca3d packages, and OPLS‐DAs were performed using SIMCA Software Version 16.0.2 (Umetrics AB). p ≤ 0.05, and when appropriate after the false discovery rate (FDR) correction (q < 0.05), were considered as statistical significant in all analyses.
2.10. Sample size calculation
The sample size calculation was based on the difference in IBS‐SSS within the same study subject between the diets using paired samples power test (n = [t n‐1 α/2 + t n‐1, β ]2/d 2). A total of 18 pairs (n) was needed to achieve a power of 80% (β) and a level of significance of 0.05 (α), for detecting a mean of the differences of 50 (t) in IBS‐SSS between the pairs, assuming the SD of the differences to be 70 (d = delta/SD).
3. RESULTS
3.1. Participants
Twenty IBS patients and 21 HC were eligible for randomisation to the interventions. All IBS patients and 18 HC completed the study (flow chart: Figure S1). Baseline characteristics are presented in Table 1. None of the participants used IBS medication and one IBS patient used probiotics. The participants had the following outcome on the question whether they thought they had received gluten, placebo or if they did not know: IBS patients: correct answer, 35%; wrong answer, 20%; did not know, 45%; and HC: correct answer, 33%; wrong answer, 11%; did not know, 56%. Higher levels of serum alkylresorcinols were found after the gluten‐containing intervention compared with after the gluten‐free intervention (Table S2), which indicated good dietary compliance.
TABLE 1.
Baseline characteristics
| IBS patients (n = 20) | Healthy controls (n = 21) | |
|---|---|---|
| Age, years, mean ± SD | 25.4 ± 3.2 | 24.6 ± 3.6 |
| Females, % | 90 | 90 |
| BMI, kg/m2, mean ± SD | 24.6 ± 6.6 | 23.6 ± 2.8 |
| Subtype (%) | ||
| IBS‐C | 25 | NA |
| IBS‐D | 40 | |
| IBS‐M | 30 | |
| IBS‐U | 5 | |
| IBS duration, years, mean ± SD | 9.8 ± 5.6 | NA |
| Anti‐tTG antibodies positivity (%) | 0 | 0 |
| Faecal calprotectin, mean ± SD | 22 ± 38 | 22 ± 11 |
| IBS‐SSS total, mean ± SD | 334 ± 63 | 11 ± 16 |
| BSF hard stools, mean % ± SD | 15.6 ± 21.2 | 6.6 ± 9.5 |
| BSF loose stools, mean % ± SD | 22.3 ± 24.0 | 3.2 ± 5.5 |
| BSF normal stools, mean % ± SD | 62.1 ± 24.2 | 90.2 ± 11.6 |
| HADS anxiety, mean ± SD | 7 ± 5 | 4 ± 3 |
| HADS depression, mean ± SD | 3 ± 4 | 1 ± 1 |
| PHQ‐12 somatic symptoms, mean ± SD | 8 ± 4 | 3 ± 2 |
Abbreviations: BMI, body mass index; BSF, Bristol stool form; HADS, Hospital Anxiety and Depression Scale; IBS, irritable bowel syndrome; IBS‐C, IBS with predominant constipation; IBS‐D, IBS with predominant diarrhoea; IBS‐M, IBS with mixed bowel habits; IBS‐SSS, IBS severity scoring system; IBS‐U, IBS with unspecified bowel habits; PHQ, Patient Health Questionnaire; tTG, tissue transglutaminase.
3.2. Step 1: Primary endpoint; comparison of interventions in IBS patients
3.2.1. IBS symptom severity
The effects on IBS symptom severity in IBS patients did not differ between the interventions. However, the overall IBS symptom severity, abdominal pain intensity, and daily life interference improved after the gluten‐free period, but no change in severity of IBS symptoms was found after the gluten‐containing period (Table 2). The flow chart of responses to the interventions and individual changes in IBS‐SSS are presented in Figure 2A,B. Six IBS patients were defined as true responders to the gluten‐free diet, and the remaining 14 IBS patients were defined as non‐responders. Of these non‐responders, five IBS patients were also defined as placebo‐responders.
TABLE 2.
Severity of gastrointestinal symptoms in gluten‐free and gluten‐containing diet interventions
| Gluten‐free diet | Gluten‐containing diet | ||||||
|---|---|---|---|---|---|---|---|
| Before | After | p within a | Before | After | p within a | p between a | |
| IBS (n = 20) | |||||||
| IBS‐SSS total score | 315 ± 63 | 262 ± 111 | 0.02 b | 313 ± 87 | 281 ± 88 | 0.06 | 0.31 |
| Abdominal pain intensity | 59 ± 18 | 45 ± 29 | 0.02 b | 62 ± 19 | 56 ± 19 | 0.11 | 0.07 |
| Abdominal pain frequency | 54 ± 26 | 50 ± 28 | 0.42 | 60 ± 33 | 52 ± 27 | 0.14 | 0.78 |
| Abdominal bloating | 62 ± 32 | 52 ± 34 | 0.16 | 60 ± 24 | 54 ± 36 | 0.45 | 0.68 |
| Bowel habit dissatisfaction | 66 ± 21 | 57 ± 26 | 0.22 | 62 ± 28 | 55 ± 26 | 0.40 | 0.78 |
| Daily life interference | 73 ± 18 | 63 ± 27 | 0.04 | 70 ± 20 | 64 ± 22 | 0.07 | 0.85 |
| HC (n = 21) | |||||||
| IBS‐SSS total score | 12 ± 15 | 9 ± 12 | 0.10 | 9 ± 12 | 9 ± 11 | 0.47 | 0.81 |
| Abdominal pain intensity | 2 ± 5 | 0 ± 1 | 0.18 | 0 ± 0 | 1 ± 3 | 0.32 | 0.47 |
| Abdominal pain frequency | 1 ± 5 | 1 ± 5 | 0.32 | 0 ± 0 | 1 ± 5 | 0.32 | 1.00 |
| Abdominal bloating | 2 ± 6 | 1 ± 3 | 0.07 | 2 ± 6 | 1 ± 3 | 0.59 | 0.54 |
| Bowel habit dissatisfaction | 5 ± 7 | 6 ± 9 | 0.66 | 5 ± 6 | 6 ± 7 | 0.52 | 0.66 |
| Daily life interference | 1 ± 1 | 0 ± 1 | 0.06 | 1 ± 3 | 1 ± 1 | 0.31 | 0.62 |
Note: Data are shown as mean ± SD or frequencies (%), assessed with linear mixed models. p values are main effect for intervention.
Abbreviation: IBS‐SSS, irritable bowel syndrome severity scoring system.
Comparisons were made intention to treat (IBS, n = 20; HC n = 21).
q < 0.05 (false discovery rate correction).
FIGURE 2.
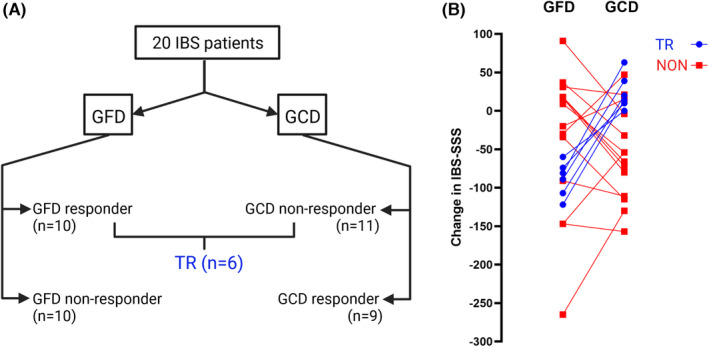
Flow chart of responses to both gluten‐free diet (GFD) and gluten‐containing diet (GCD). (A) Six IBS patients responded to the gluten‐free intervention and did not respond to the gluten‐containing intervention and were identified as true responders (TR), and all others (n = 14) were identified as non‐responders (NON). (B) individual changes in IBS‐SSS during both GFD and GCD.
3.2.2. Bowel habits
The proportion of reported loose stools differed between the interventions. The IBS patients reported fewer loose stools during the gluten‐free intervention, compared with the gluten‐containing intervention (19.2% ± 17.9% vs 27.4% ± 20.9%, p = 0.01, q < 0.05). No differences were observed between the gluten‐free versus gluten‐containing interventions in proportions of reported hard stools (17.0% ± 18.6% vs 12.7% ± 20.8%, p = 0.38) or normal stools (63.8% ± 17.3% vs 59.9% ± 25.2%, p = 0.47). True responders included IBS‐D (n = 4), IBS‐M (n = 1) and IBS‐U (n = 1) subtypes, and non‐responders included IBS‐C (n = 4), IBS‐D (n = 5) and IBS‐M (n = 5) subtypes (p = 0.14).
3.3. Step 2: Secondary endpoints; comparison of interventions within groups
3.3.1. IBS symptoms and bowel habits in HCs
IBS symptoms did not differ between the interventions in HC, and the numerical score remained very low compared to the IBS patients (Table 2). No differences were observed between the gluten‐free vs. gluten‐containing interventions in proportions of hard stools (8.8% ± 12.2% vs 9.6% ± 13.2%, p = 0.68), loose stools (3.0% ± 4.9% vs 4.6% ± 6.5%, p = 0.41), or normal stools (88.2% ± 12.9% vs 85.8% ± 13.6%, p = 0.46).
3.3.2. Anxiety, depression and somatic symptoms
The change in anxiety, depression and somatic symptom severity did not differ between the interventions in IBS patients, HC or non‐responders. However, in true responders, the symptoms of anxiety and depression were more reduced after the gluten‐free compared with the gluten‐containing period (Table S3).
3.3.3. Faecal microbiota
The Bray–Curtis dissimilarity indices did not differ between the interventions analysing all IBS patients (β = 0.002 [−0.031, 0.036], p = 0.63), non‐responders (β = 0.012 [−0.040, 0.063], p = 0.88), HC (β = −0.003 [−0.025, 0.018], p = 0.75), or placebo‐responders (β = −0.078 [−0.364, 0.207], p = 0.97). In true‐responders, the Bray–Curtis dissimilarity indices after the gluten‐free period were lower compared to after the gluten‐containing period (β = −0.030 [−0.041, −0.020], p < 0.001, q < 0.05). PCA (Figure S2) on the change of faecal microbiota composition showed no distinct separation between the interventions and only poor OPLS‐DA models were obtained (Table S4).
3.3.4. Faecal metabolites
PCA (Figure S3) on the change of faecal metabolites showed no distinct separation between the interventions for any of the study groups (i.e., all IBS patients, HC, true responders, non‐responders, or placebo‐responders), and no OPLS‐DA models could be obtained.
3.4. Step 3: Secondary endpoints; comparison of gluten‐free diet between groups
3.4.1. Anxiety, depression and somatic symptoms
The changes in anxiety, depression and severity of somatic symptoms based on the effects of the gluten‐free diet intervention did not differ between IBS patients versus HC or true responders versus non‐responders (Table S5).
3.4.2. Faecal microbiota
PCA of change in faecal microbiota based on the effects of the gluten‐free intervention did not separate IBS patients and HC (Figure 3A), and no OPLS‐DA model could be obtained. However, for true responders versus non‐responders the PCA indicated that the groups could be separated (Figure 3B). After selection of the most discriminant X‐variables, the OPLS‐DA model revealed that the effects of the gluten‐free intervention on change in faecal microbiota differed in true responders versus non‐responders (X‐variables, n = 16). The model had good robustness (R 2 Y = 0.71) and good predictive ability (Q 2 = 0.49) to separate true responders and non‐responders (p = 0.003; Figure 3C). The taxonomic data of the model are presented in Figure S4. PCA of change in faecal microbiota based on the effects of the gluten‐free intervention showed no distinct separation between placebo‐responders and the other patients (Figure S5), and the OPLS‐DA model was poor (R 2 Y = 0.60; Q 2 = 0.28; ΔR 2 Y Q 2 > 0.3).
FIGURE 3.
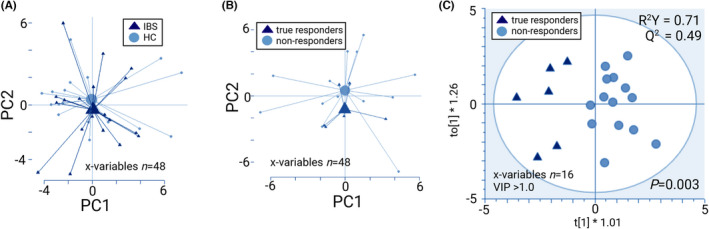
Effects of a 2‐week gluten‐free diet on fold changes (after/before gluten‐free intervention) in faecal microbiota (GA‐map™ dysbiosis test), shown as PCA scatterplots with centroids (A and B) and OPLS‐DA scatterplot (C) Permutation test for (C) showed no overfitting. IBS (n = 20), HC (n = 18), true responders (n = 6) and non‐responders (n = 14).
3.4.3. Faecal metabolites
PCA on change in faecal metabolites of IBS patients versus HC indicated a separation between the groups based on the effects of the gluten‐free intervention (Figure 4A). However, PCA showed no distinct separation of true responders and non‐responders (Figure 4C). After selection of the most discriminant X‐variables, OPLS‐DA models revealed that the effects of the gluten‐free intervention on change in faecal metabolites differed between IBS and HC (X‐variables, n = 29), as well as between true responders and non‐responders (X‐variables, n = 24; Figure 4B–D). The models had good robustness (IBS versus HC, R 2 Y = 0.73; true responders versus non‐responders R 2 Y = 0.82) and good predictive ability (IBS versus HC, Q 2 = 0.66; true responders versus non‐responders Q 2 = 0.79) to separate IBS versus HC and true responders versus non‐responders (p < 0.001 for both). PCA of change in faecal metabolites based on the effects of the gluten‐free intervention showed no distinct separation between placebo‐responders and the other patients (Figure S5), and no OPLS‐DA model could be obtained.
FIGURE 4.
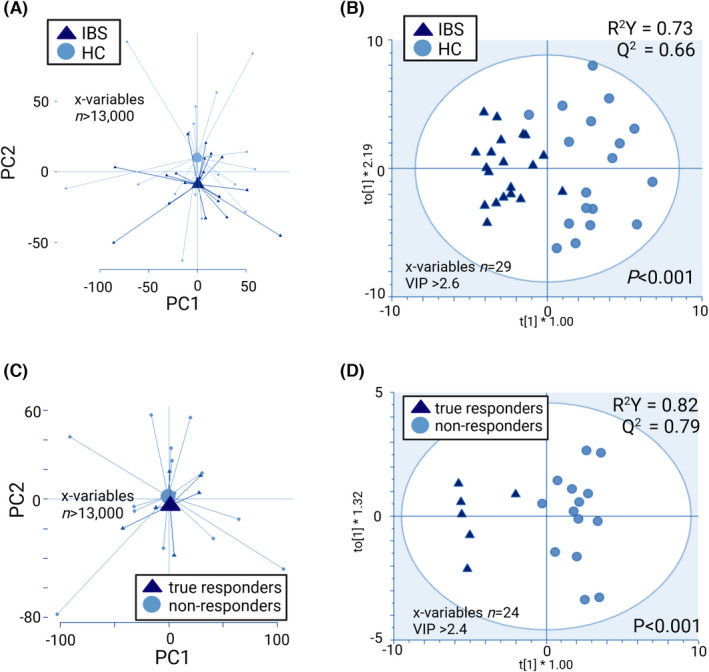
Effects of a 2‐week gluten‐free diet on fold changes (after/before gluten‐free intervention) in metabolites of faecal supernatants (LC–MS), shown as PCA scatterplots with centroids ( A and C) and OPLS‐DA scatterplots (B and D). The R 2 Y value determines goodness of the fit and the Q 2 value represents the predictive ability of the model; R 2 Y ≥ 0.5 and Q 2 ≥ 0.4 are considered satisfactory. Permutation tests for (B) and (D) showed no overfitting. IBS (n = 20), HC (n = 18), true responders (n = 6) and non‐responders (n = 14).
3.5. Step 4: Secondary endpoint; prediction of response to the gluten‐free diet in IBS
PCA on faecal microbiota showed no evidence of separation between true responders and non‐responders before the gluten‐free intervention, and the OPLS‐DA model had poor robustness (R 2 Y = 0.39) and poor predictive ability (Q 2 = 0.12; Figure 5A,B). However, for faecal metabolites, the PCA indicated that true responders and non‐responders could be separated based on their faecal metabolites profile before the gluten‐free intervention (Figure 5C). An OPLS‐DA revealed that the faecal metabolites of true responders and non‐responders differed before the intervention, after selection of the 21 most discriminant variables. The model had a good robustness (R 2Y = 0.81) and high predictive ability (Q 2 = 0.71) (p < 0.001) (Figure 5D). PCA on faecal microbiota and metabolites showed no distinct separation between placebo‐responders and the other patients before the gluten‐free intervention (Figure S5), and no OPLS‐DA models could be obtained.
FIGURE 5.
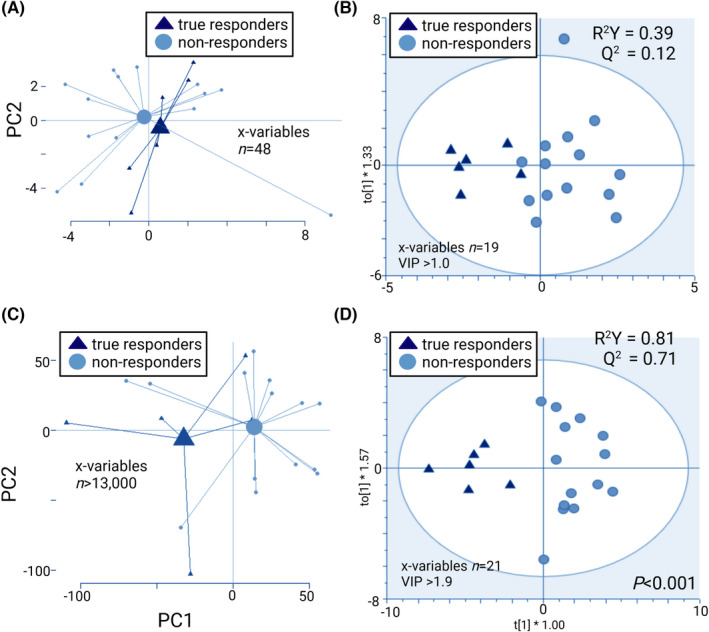
Prediction of response to gluten‐free diet in IBS, assessed before the gluten‐free intervention. Faecal microbiota profiles (GA‐map™ dysbiosis test) shown as PCA scatterplot with centroids (A), and OPLS‐DA scatterplot (B). Metabolite profiles of faecal supernatants (LC–MS) shown as PCA scatterplot with centroids (C), and OPLS‐DA scatterplot (D). The R 2 value determines goodness of the fit and the Q 2 value represents the predictive ability of the model; R 2 Y ≥ 0.5 and Q 2 ≥ 0.4 are considered satisfactory. Permutation test for (D) showed no overfitting. True responders (n = 6) and non‐responders (n = 14).
4. DISCUSSION
This study indicates that gluten might influence symptoms positively in a small subset of IBS patients, with a particular effect on bowel habits. More specifically, although the effect of the gluten‐free versus the gluten‐containing diets on overall IBS symptoms did not differ significantly between the groups, the effect on bowel habits differed between the interventions, with fewer reports of loose stools during the gluten‐free diet. However, the gluten‐free diet improved GI symptoms in a subset of IBS patients, and also had different effects on the faecal metabolite profiles of IBS patients and HC. Moreover, the gluten‐free diet had distinct effects on changes of the faecal microbiota and metabolites profiles in IBS patients who responded favourably to gluten reduction, and the clinical response to a gluten‐free diet may be predicted by the faecal metabolite profile before the intervention.
Previous studies assessing the gluten‐free diet demonstrated heterogeneous findings, and there is yet insufficient evidence to recommend a gluten‐free diet in IBS. 3 The current study shows that a beneficial effect of the gluten‐free diet was only seen in a subset of IBS patients, which could be an explanation for the previous heterogeneous findings. Our data show that the true responders have similar reductions of IBS symptoms to the gluten‐free intervention without a placebo response to the gluten‐containing intervention, whereas the non‐responders constitute a group with heterogeneous IBS symptom responses to both interventions. Regarding the effect on bowel habits, a minor difference between the interventions was observed, with fewer reported loose stools during the gluten‐free intervention. Furthermore, we found that the majority of the true responders were IBS‐D patients, and all IBS‐C patients were non‐responders. Hence, it could be hypothesised that a gluten‐free diet is more effective in IBS‐D, which is in line with results from a previous open label study. 17 Among the true responders, the effects of the gluten‐free diet were not restricted to IBS symptoms and bowel habits, as they also reported reduced anxiety and depression after the gluten‐free, but not after the gluten‐containing intervention. These results are in in line with a recent large open label study, showing decreased levels of anxiety and depression after a gluten‐free diet in IBS patients. 18 However, the mechanisms by which the gluten‐free diet may improve anxiety and depression remain to be determined.
Gluten is the storage protein of wheat grains, and wheat also contains several other components that could aggravate GI symptoms, for example amylase‐trypsin inhibitors (ATIs), and fructan, which is included in the FODMAP concept. 3 In this study, the participants were challenged with vital gluten, which is prepared by a wet milling process. Due to this process, the starch, including fructan, and the majority of ATIs are washed away. 19 During both interventions, the FODMAP content was stable and similar to the reported FODMAP intake in IBS. 20 The participants were challenged with moderate dosages of gluten (14 g/day), which was chosen in order to be similar to the actual gluten intake in the general population (10 g/day) and Swedish IBS patients (11 g/day), 6 , 7 and it was not expected to aggravate GI symptoms to an extent that would risk unblinding or reduce compliance.
We hypothesised that reducing GI symptoms by a gluten‐free diet could be linked to the gut microenvironment in IBS patients. However, no changes in β‐diversity, that is variation of microbial abundances between the interventions, were observed in IBS patients and HC, or non‐responders. However, in true responders the β‐diversity was more similar after the gluten‐free intervention compared with gluten‐containing intervention. Still, this result should be interpreted with caution because the difference between the diets in β‐diversity was, although statistical significant, only minor. Furthermore, within‐group comparisons of changes in faecal microbiota composition showed no differences between the interventions. An explanation for this could be the duration of the interventions, which were relatively short. Individual microbiota composition is known to be stable over time and it may not be expected to change substantially after an intervention of 2 weeks. 21
We have also demonstrated that the gluten‐free intervention influenced faecal microbiota and metabolites differently in true responders and non‐responders. These novel findings suggest that gut microbiota composition and metabolism are linked to the response to the gluten‐free diet in IBS. In addition, no differences were found analysing placebo‐responders versus the other patients. The different effects of the gluten‐free diet on faecal metabolites profiles in IBS and HC, suggest that gut microbial metabolism may react differently to the gluten‐free diet in health and disease.
We investigated if faecal microbiota and metabolite profiles could predict the response to gluten‐free diet in IBS patients. Although true responders and non‐responders could not be separated based on the faecal microbiota profile, they were separated based on their faecal metabolites profile before the intervention. Similar results were found assessing the faecal microbiota profile in IBS, regarding the response to a low FODMAP diet, 22 and recently, a study found that anti‐gliadin immunoglobulin G antibodies may predict the response to the gluten‐free diet. 18 Investigating mechanisms that could be involved in prediction of treatments in IBS are of major importance for moving towards a more individualised treatment approach in IBS. However, these studies, 18 , 22 and also the current study, have small sample sizes and larger studies are needed to validate these findings.
Although the use of serum alkylresorcinols as a biomarker for a gluten‐free diet remains to be validated in larger studies, several studies have shown its potential. 7 , 8 , 9 The findings in our sample that serum alkylresorcinols were lower after the gluten‐free intervention compared with the gluten‐containing intervention support this, and indicates good compliance to the interventions, which we consider as a strength. Another strength of this study is the adequate blinding of the participants and investigators, and confounding factors were minimised by using the randomised crossover design, 23 and the strict in‐ and exclusion criteria. Nocebo and/or placebo responses, which are common in IBS studies, 23 were further minimised due to our definition of ‘true responders’. This definition takes both interventions into account, and reduces the risk of patients with placebo responses in the true responders group. Furthermore, we investigated HCs as a comparator group, and the interventions did not induce any change in primary outcomes in this group. Exclusively validated questionnaires were used, and outcomes were predefined before initiation of the study.
Limitations of this study include the small sample size. Therefore, the results of the secondary outcomes should be interpreted with caution, also taking into account that the IBS patients were divided into true responders and non‐responders for some analyses. Also the large proportion of females and the relatively young age of the cohort could be seen as a limitation of the study. Therefore, our results are representative of young females, but still warrants generalisation to an older population. Further, concerning the faecal microbiota, the GA map Dysbiosis Test 15 analyses a pre‐identified set of bacterial taxa, and 16S gut microbiota sequencing might provide a more detailed understanding of the gut microbiota composition. Still, our chosen method provides absolute abundance of the determined bacteria taxa with high confidence and throughput.
In conclusion, our study indicates that a gluten‐free diet may affect IBS symptoms in general, and bowel habits in particular in a subset of IBS patients. The gluten‐free diet has distinct effect on the gut microenvironment in IBS patients who respond favourably to gluten reduction. Moreover, it seems to be possible to predict the response to the gluten‐free diet by analysing the faecal metabolites profile. These findings suggest that the gut microenvironment may be of importance in the clinical response to the gluten‐free diet in IBS, and future studies should aim to further assess these factors in relation to clinical response to the gluten‐free diet.
AUTHOR CONTRIBUTIONS
Joost P. Algera: Study concept and design; study visits; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content. Maria K. Magnusson: Analysis and interpretation of data; critical revision of the manuscript for important intellectual content. Lena Öhman: Critical revision of the manuscript for important intellectual content. Stine Storsrud: Study concept and design; critical revision of the manuscript for important intellectual content. Magnus Simrén: Study concept and design; obtained funding; critical revision of the manuscript for important intellectual content; study supervision. Hans Törnblom: Study concept and design; obtained funding; critical revision of the manuscript for important intellectual content; study supervision. All authors approved the final version of the manuscript.
FUNDING INFORMATION
This study was funded in full by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF‐agreement (ALFGBG‐726561, 722,331, 875,581), the Swedish Research Council (2018–02566 and 2021–00947), and the Faculty of Medicine at the University of Gothenburg (Dnr DS2019/1843).
AUTHORSHIP
Guarantor of the article: Joost P. Algera.
Supporting information
Data S1
Table S1‐S5‐FigureS1‐S5
ACKNOWLEDGEMENT
Declaration of personal interests: Joost P. Algera; Maria K. Magnusson; Stine Störsrud; and Hans Törnblom: none. Lena Öhman has received financial support for research from Genetic Analysis AS, Biocodex, Danone Nutricia Research and AstraZeneca and served as Consultant/Advisory Board member for Genetic Analysis AS, and as a speaker for Biocodex, Ferring Pharmaceuticals, Takeda, AbbVie and MEDA. Magnus Simrén has received unrestricted research grants from Glycom and Danone Nutricia Research, and served as advisory board member/consultant and/or speaker for Biocodex Glycom, Danone Nutricia Research, Ironwood, Genetic Analysis AS, Kyowa Kirin, Menarini, Arena, Adnovate, Tillotts, Takeda, Alimentary Health, AlfaSigma, Falk Foundation and Shire. Figures were created with Biorender.com.
Algera JP, Magnusson MK, Öhman L, Störsrud S, Simrén M, Törnblom H. Randomised controlled trial: effects of gluten‐free diet on symptoms and the gut microenvironment in irritable bowel syndrome. Aliment Pharmacol Ther. 2022;56:1318–1327. 10.1111/apt.17239
The Handling Editor for this article was Professor Peter Gibson, and it was accepted for publication after full peer‐review.
REFERENCES
- 1. Böhn L, Störsrud S, Törnblom H, Bengtsson U, Simrén M. Self‐reported food‐related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108:634–41. [DOI] [PubMed] [Google Scholar]
- 2. Öhman L, Törnblom H, Simrén M. Crosstalk at the mucosal border: importance of the gut microenvironment in IBS. Nat Rev Gastroenterol Hepatol. 2015;12:36–49. [DOI] [PubMed] [Google Scholar]
- 3. Algera J, Colomier E, Simrén M. The dietary management of patients with irritable bowel syndrome: a narrative review of the existing and emerging evidence. Nutrients. 2019;11:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lenhart A, Dong T, Joshi S, Jaffe N, Choo C, Liu C, et al. Effect of exclusion diets on symptom severity and the gut microbiota in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2022;20(3):e465–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barbara G, Feinle‐Bisset C, Ghoshal UC, Santos J, Vanner S, Vergnolle N, et al. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology. 2016;150:1305–1318.e8. [DOI] [PubMed] [Google Scholar]
- 6. Algera JP, Störsrud S, Lindström A, Simrén M, Törnblom H. Gluten and fructan intake and their associations with gastrointestinal symptoms in irritable bowel syndrome: a food diary study. Clin Nutr. 2021;40:5365–72. [DOI] [PubMed] [Google Scholar]
- 7. Hoppe C, Gøbel R, Kristensen M, Lind MV, Matthiessen J, Christensen T, et al. Intake and sources of gluten in 20‐ to 75‐year‐old Danish adults: a national dietary survey. Eur J Nutr. 2017;56:107–17. [DOI] [PubMed] [Google Scholar]
- 8. Choung RS, Murray JA, Marietta EV, van Dyke CT, Ross AB. Serum alkylresorcinols as biomarkers of dietary gluten exposure in coeliac disease. Aliment Pharmacol Ther. 2017;45:643–52. [DOI] [PubMed] [Google Scholar]
- 9. Ross AB. Present status and perspectives on the use of alkylresorcinols as biomarkers of wholegrain wheat and rye intake. J Nutr Metab. 2012;2012:1, 462967–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. [DOI] [PubMed] [Google Scholar]
- 11. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–4. [DOI] [PubMed] [Google Scholar]
- 12. Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology. 2016;150:1393–407. [DOI] [PubMed] [Google Scholar]
- 13. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- 14. Spiller RC, Humes DJ, Campbell E, Hastings M, Neal KR, Dukes GE, et al. The patient health questionnaire 12 somatic symptom scale as a predictor of symptom severity and consulting behaviour in patients with irritable bowel syndrome and symptomatic diverticular disease. Aliment Pharmacol Ther. 2010;32:811–20. [DOI] [PubMed] [Google Scholar]
- 15. Casèn C, Vebø HC, Sekelja M, Hegge FT, Karlsson MK, Ciemniejewska E, et al. Deviations in human gut microbiota: a novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment Pharmacol Ther. 2015;42:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klåvus A, Kokla M, Noerman S, Koistinen VM, Tuomainen M, Zarei I, et al. “Notame”: workflow for non‐targeted LC–MS metabolic profiling. Metabolites. 2020;10:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aziz I, Trott N, Briggs R, North JR, Hadjivassiliou M, Sanders DS. Efficacy of a gluten‐free diet in subjects with irritable bowel syndrome‐diarrhea unaware of their HLA‐DQ2/8 genotype. Clin Gastroenterol Hepatol. 2016;14:696–703.e1. [DOI] [PubMed] [Google Scholar]
- 18. Pinto‐Sanchez MI, Nardelli A, Borojevic R, de Palma G, Calo NC, McCarville J, et al. Gluten‐free diet reduces symptoms, particularly diarrhea, in patients with irritable bowel syndrome and antigliadin IgG. Clin Gastroenterol Hepatol. 2021;19:2343–2352.e8. [DOI] [PubMed] [Google Scholar]
- 19. Sayaslan A. Wet‐milling of wheat flour: industrial processes and small‐scale test methods. LWT – Food Sci Technol. 2004;37:499–515. [Google Scholar]
- 20. Nybacka S, Störsrud S, Lindqvist HM, Törnblom H, Simrén M, Winkvist A. Habitual FODMAP intake in relation to symptom severity and pattern in patients with irritable bowel syndrome. Nutrients. 2020;13(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Öhman L, Lasson A, Strömbeck A, Isaksson S, Hesselmar M, Simrén M, et al. Fecal microbiota dynamics during disease activity and remission in newly diagnosed and established ulcerative colitis. Sci Rep. 2021;11(1):8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bennet SMP, Böhn L, Störsrud S, Liljebo T, Collin L, Lindfors P, et al. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut. 2018;67(5):872–81. [DOI] [PubMed] [Google Scholar]
- 23. Yao CK, Gibson PR, Shepherd SJ. Design of clinical trials evaluating dietary interventions in patients with functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:748–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1‐S5‐FigureS1‐S5


