Abstract
Background
Intracardiac echocardiography (ICE) is frequently used to guide electrophysiology procedures. The novel automated algorithm Cartosoundfam is a model‐based algorithm which reconstructs a 3D anatomy of the left atrium (LA) based on a set of 2D intracardiac echocardiography (ICE) frames, without the need to manually annotate ultrasound (US) contours.
Objective
The aim of this study was to determine the feasibility of the Cartosoundfam module in routine clinical setting.
Methods
We included 16 patients undergoing LA mapping/catheter ablation. Two‐dimensional US frames were acquired from the right atrium (RA) and the right ventricular outflow tract. The Cartosoundfam map was validated in two steps: (1) identification of anatomical structures (pulmonary veins [PV] and LA body and appendage) by alignment of the ablation catheter to the automated map; and (2) analysis of the automated lesion tags (Visitag) location in relation to the PV antrum of the Cartosoundfam map in nine patients with paroxysmal atrial fibrillation (AF) undergoing first time pulmonary vein isolation (PVI).
Results
Mean 2D US frames per patient were 29 ± 6 and acquisition time was 16 ± 4 min. All anatomical structures were correctly identified in all patients (step 1). In the step 2 validation, the median distance to the map was 2.0 (IQR: 2.4) mm and the majority of the Visitags were classified as satisfactory (69%) but all PV segments had some Visitags classified as unsatisfactory.
Conclusion
The automated ICE‐based algorithm correctly identified the LA anatomical structures in all patients with a 69% anatomical accuracy of the Visitags alignments to the PV antrum segments.
Keywords: atrial fibrillation, catheter ablation, intracardiac echocardiography, novel technologies, pulmonary vein isolation
1. INTRODUCTION
Standard two‐dimensional (2D), phased‐array intracardiac echocardiography (ICE) is a valuable tool in interventional cardiology procedures such as catheter ablation of cardiac arrhythmias. It has a wide range of applications, including real‐time identification of relevant anatomic structures, assessing accurate placement of mapping and ablation catheters, limiting radiation exposure and recognizing complications early. 1 Specifically for atrial fibrillation (AF) ablation, ICE provides assessment of left atrial appendage (LAA) thrombosis, transeptal punction guidance, esophageal course and proper tissue‐catheter contact on particular structures such as the left atrial ridge. 2 Furthermore, 2D ultrasound (US) image frames can be used for the reconstruction of a 3D anatomical map with the Cartosound module (Biosense Webster, Inc., CA, USA) by using the Soundstar ICE‐catheter (Biosense Webster, Inc.), which contains a magnetic sensor in the tip that provides intracardiac US image planes, including their location and orientation in the Carto mapping system (Biosense Webster). The Cartosound map can subsequently be integrated with an anatomical map collected with a mapping catheter (Fast anatomical mapping, FAM, Biosense Webster) or used as a standalone anatomy map in the Carto mapping system. Even though the Cartosound map may provide increased anatomical accuracy over a FAM map and reduce procedural time, it depends on accurate and reproducible manual annotation of the US frames contours by an experienced technician. Hence, there is a need for an ICE‐based automated anatomical map software with good anatomical accuracy without the need of an experienced technician, especially for common standardized ablation procedures such as pulmonary vein isolation.
The Cartosoundfam module (Biosense Webster) is a novel pre‐commercial model‐based algorithm (available in the future Carto 3 version 8 release), based on machine learning methodology, which provides auto segmentation of the LA with its anatomical structures based on a series of selected 2D US frames acquired from the right atrium (RA) and right ventricular outflow tract (RVOT). The potential advantages of the Cartosoundfam module are shortening of mapping time and more accurate delineation of the anatomy boundaries compared to traditional FAM, without depending on manual annotation by an experienced technician. The purpose of this feasibility study was to determine the feasibility and safety of this novel automated ICE‐based algorithm during mapping and ablation of the left atrium (LA).
2. METHODS
We included 16 patients undergoing ICE‐guided mapping and/or catheter ablation of the LA (Table 1). All data collection was approved by the regional ethics committee and patient data were collected in accordance with the institutional ethics guidelines.
TABLE 1.
Patient demographics
| Age (years) | 63.9 ± 9.3 |
|---|---|
| Male (No, %) | 14 (87.5) |
| BMI (kg/m2) mean ± SD | 26.7 ± 3.2 |
| Hypertension (No, %) | 6 (37.5) |
| Diabetes (No, %) | 1 (6.3) |
| Stroke (No, %) | 2 (12.5) |
| CHA2DS2VASc score (IQR) | 2.0 (2.0) |
| Left ventricular ejection fraction (%, IQR) | 55 (4.8) |
| PLAX (mm) mean ± SD | 39.6 ± 2.9 |
| LAVI (mL/m2) mean ± SD | 37.71 ± 10.2 |
| General anesthesia (No, %) | 7 (43.8) |
| Procedure duration (min) mean ± SD | 162.5 ± 44.7 |
Abbreviations: BMI, body mass index; IQR, interquartile range; LAVI, left atrial volume index; PLAX , parasternal long axis; SD, standard deviation.
The procedures were performed, under conscious sedation or general anesthesia, according to conventional and local standards as described previously. 3 Briefly, a Soundstar 8F ICE catheter was utilized by two operators (FA and ND), with extensive experience with the use of ICE during electrophysiological (EP) procedures, and advanced from the right or left femoral vein through a 9F short sheath and positioned in right cardiac chambers. The ICE images were displayed through the Cartosound module via a General Electric Vivid iq (Boston, MA, USA) ultrasound machine.
The Cartosoundfam was run on a Carto workstation (EMT version). The workflow consisted of advancing the ICE catheter first from the home view to the superior vena cava (SVC) – RA junction and the rotated clockwise until the right pulmonary artery – right superior pulmonary vein (RSPV) could be observed in the same view. From here the ICE catheter was rotated counterclockwise to acquire 2D US frames of the right pulmonary veins (RPV), posterior LA wall and eventually the left pulmonary veins (LPV). The ICE catheter was then advanced into the RVOT and continuously rotated clockwise to collect 2D US frames of the anterior LA wall, LAA, and posterior LA wall. All US images were acquired in real‐time during catheter manipulation by the operator and processed in the Carto system by an experienced technician. As recommended by the manufacturer, a minimum of two US frames per anatomical structure (LAA and pulmonary veins) were recorded in the end‐expiratory phase during isovolumic relaxation (end of T‐wave in lead II, III or aVF of the 12‐lead electrocardiographic recording). After selection of suitable US frames, by the operator and technician, the Cartosoundfam module was then executed in order to obtain the LA anatomy (Figure 1). Manual annotation or labeling of the different anatomical LA structures were therefore not necessary. Subsequently, a single ICE‐guided transeptal puncture with a Brockenbrough needle was performed in all cases. Pulmonary vein isolation (PVI) was performed with a SmartTouch (Biosense Webster) according to CLOSE protocol 4 in nine patients with paroxysmal AF (40 W throughout, 3 mm lesion radius, 3 mm for 8 s stability criteria, aiming for 5−20 g mean contact force and interlesion distance of 3−4 mm). There were no changes to the standard PVI approach during the EP procedures due of the use of the Cartosoundfam module.
FIGURE 1.
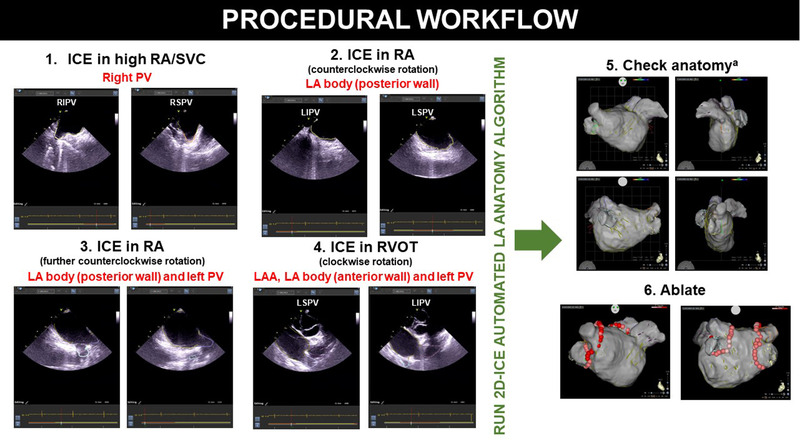
Procedural workflow overview of the Cartosoundfam algorithm. Abbreviations: ICE,= intracardiac echocardiography; LA, left atrium; LAA, left atrial appendage; PV, pulmonary vein; RA, right atrium; RVOT, right ventricular outflow tract. aConfirm anatomy accuracy with ablation‐ or mapping‐catheter and erase/add anatomy if necessary [Colour figure can be viewed at wileyonlinelibrary.com]
The Cartosoundfam map was validated in two steps: (1) identification of anatomical structures (PV and LA appendage and body) by alignment of the ablation catheter to the map; and (2) offline analysis of PV antral anatomical accuracy by analysis of the relationship with the automated lesion tags (Visitag module, Biosense Webster) and the PV antrum of the automated map in nine patients with paroxysmal AF undergoing first time PVI. Pulmonary vein pairs were divided into six segments (roof, anterior superior, anterior inferior, floor, posterior inferior, posterior superior; total of 12 segments per patient) and were classified, according to the distance from Visitag center to the map surface. Therefore, zero distance indicated that the ablation tag was located perfectly on the map surface and a positive value indicated some deviation from the surface. The ablation tags were represented by a sphere with an arbitrary radius, conventionally 3 mm, and all the distances reported in this study were computed from the center of the ablation tag. If the reported distance was less than the radius, the Visitag was still touching the reconstruction surface and was providing a good visual assistance during the procedure. If the distance was larger than the Visitag radius, this indicated that the Visitag was either floating above the surface or buried inside the anatomical map. As a result, the Visitags were classified as satisfactory and unsatisfactory if they deviated ≤3 mm or > 3 mm from the map surface, respectively.
2.1. Statistical analysis
Normality was evaluated using the Kolmogorov‐Smirnov and Shapiro‐Wilk tests for sample sizes of ≤50 and > 50, respectively. Continuous variables are expressed as means ± standard deviation (SD) or as medians with interquartile range (IQR) for skewed or non‐normal data. For correlation of small sample sizes, the Spearman's rho was used. The statistical analyses were performed using SPSS software (version 25.0; SPSS, Inc., Chicago, IL, USA).
3. RESULTS
Sixteen patients were included in the study, the majority male with few cardiovascular risk factors, slightly dilated LA, and general anesthesia was used in almost half of the cases (Table 1). In all patients the ICE catheter was advanced to the heart and placed in SVC, RA and RVOT under ICE guidance alone. Mean 2D US frames per patient was 29 ± 6 and acquisition time was 16 ± 4 min. A total of 65 ± 12 contours per patient (LA: 28 ± 5; LAA: 9 ± 3; LIPV: 8 ± 3; LSPV: 8 ± 3; RIPV: 6 ± 3; RSPV: 6 ± 2) were automatically identified by the Cartosoundfam module (Table 2). Patient examples of the automated annotation of the 2D US frames and LA anatomy processing is shown in Figure 2A,B.
TABLE 2.
Cartosoundfam acquisition characteristics
| Contours per anatomical structure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Arrhythmia type | Ablation target | 2D US frames acquisition (min) | 2D US frames | Total contours | LA | LAA | LIPV | LSPV | RIPV | RSPV |
| 1 | Parox AF | PVI | 16 | 32 | 62 | 32 | 5 | 9 | 7 | 7 | 1 |
| 2 | Parox AF | PVI | 22 | 36 | 75 | 32 | 6 | 6 | 10 | 14 | 7 |
| 3 | Parox AF | PVI | 17 | 33 | 65 | 26 | 8 | 8 | 8 | 9 | 6 |
| 4 | Parox AF | PVI | 19 | 28 | 63 | 27 | 8 | 7 | 12 | 5 | 4 |
| 5 | Parox AF | PVI | 18 | 29 | 75 | 26 | 9 | 9 | 6 | 10 | 9 |
| 6 | Parox AF | PVI | 21 | 23 | 55 | 23 | 7 | 5 | 5 | 8 | 7 |
| 7 | Parox AF | PVI | 18 | 34 | 79 | 34 | 12 | 12 | 9 | 5 | 7 |
| 8 | Parox AF | PVI | 18 | 29 | 68 | 29 | 10 | 10 | 6 | 7 | 6 |
| 9 | Parox AF | PVI | 11 | 22 | 55 | 22 | 9 | 7 | 9 | 4 | 4 |
| 10 | Pers AF | PVI | 15 | 22 | 54 | 21 | 9 | 8 | 8 | 3 | 5 |
| 11 | VT | PMPap | 12 | 32 | 85 | 32 | 14 | 16 | 12 | 7 | 4 |
| 12 | Parox AF | Redo PVI | 14 | 24 | 56 | 24 | 9 | 7 | 5 | 7 | 4 |
| 13 | AT | AT | 17 | 26 | 47 | 26 | 9 | 4 | 8 | 3 | 7 |
| 14 | WPW | Left AP | 11 | 38 | 83 | 38 | 11 | 7 | 13 | 6 | 8 |
| 15 | Pers AF | Redo PVI | 10 | 21 | 45 | 21 | 8 | 5 | 5 | 4 | 2 |
| 16 | Parox AF | PVI | 16 | 36 | 69 | 30 | 14 | 3 | 10 | 3 | 9 |
Abbreviations: AF, atrial fibrillation; AP , accessory pathway; AT, atrial tachycardia; LA, left atrium; LAA, left atrial appendage; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; parox , paroxysmal; pers, persistent; PMPap, posteromedial papillary muscle; PVI, pulmonary vein isolation; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; US, ultrasound; VT, ventricular tachycardia; WPW, Wolff‐Parkinson‐White.
FIGURE 2.
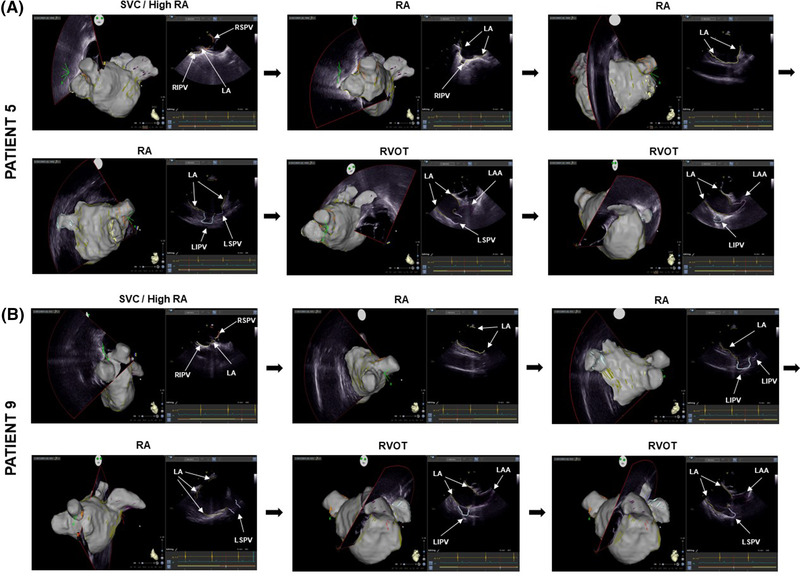
Cartosoundfam map with corresponding ultrasound (US) frames and automatically annotated anatomy contours of patient 5 (A) and 9 (B): dark green = left atrium; orange = right superior pulmonary vein (PV); yellow = right inferior PV; light blue = left inferior PV; light purple = left superior PV; dark purple = left atrial appendage. Abbreviations: RA, right atrium; RVOT, right ventricular outflow tract; SVC, superior vena cava. The images are arranged according to the procedural workflow order indicated in Figure 1 [Colour figure can be viewed at wileyonlinelibrary.com]
In the step 1 validation, all anatomical structures were correctly identified in the whole study group by the Cartosoundfam module. The step 2 validation included a total of 108 PV segments and 611 Visitags (85 Visitags were excluded for presenting forces < 5 or > 20 g). The median force was 10.5 (IQR: 6.6) g and median distance to the map was 2.0 (IQR: 2.4) mm. The majority of the Visitags were classified as satisfactory (68.7%), but all PV segments had some Visitags classified as unsatisfactory. PV segments with the highest degree of satisfactory and unsatisfactory Visitag classification were left floor and right anterior superior PV, respectively (Table 3 and Figure 3). The satisfactory Visitag range per patient was 56–78%. There was no correlation between the procedure chronological order and the Visitag classification (p = .47). Finally, in all cases, the PV segment anatomical discrepancy was corrected with standard anatomy corroboration with the ablation catheter in ≤2 min. Catheter ablation was successfully performed directly on the Cartosoundfam map, which included additional FAM of the PV antra collected by the ablation catheter when necessary (PVI ablation time 1524 [IQR: 251] s, number of lesions of 84 [IQR: 23], procedure time 165 ± 42 min). No complications occurred.
TABLE 3.
Cartosoundfam PV anatomy map validation of patients undergoing first time PVI
| No Visitags | Force (g) | Visitag distance to anatomy surface (mm) | Satisfactory anatomya (%) | |
|---|---|---|---|---|
| Right side | ||||
| Anterior RSPV | 74 | 14.1 (5.4) | 2.8 (4.2) | 51.4 |
| Anterior RIPV | 47 | 14.5 (4.8) | 1.3 (2.0) | 85.1 |
| Floor | 32 | 13.4 (4.8) | 1.6 (1.6) | 78.1 |
| Posterior RIPV | 62 | 10.8 (3.3) | 2.3 (2.4) | 58.1 |
| Posterior RSPV | 51 | 10.6 (5.7) | 2.2 (2.7) | 66.7 |
| Roof | 42 | 10.9 (5.3) | 2.7 (2.7) | 57.1 |
| Left side | ||||
| Anterior LSPV | 96 | 8.9 (4.7) | 1.8 (2.1) | 76.0 |
| Anterior LIPV | 46 | 7.4 (2.7) | 1.9 (2.1) | 73.9 |
| Floor | 24 | 8.1 (4.7) | 1.7 (1.3) | 91.7 |
| Posterior LIPV | 41 | 9.5 (3.5) | 2.1 (2.1) | 73.2 |
| Posterior LSPV | 52 | 9.6 (3.8) | 1.8 (2.3) | 71.2 |
| Roof | 44 | 9.9 (4.0) | 2.2 (4.4) | 61.4 |
| All | 611 | 10.5 (6.6) | 2.0 (2.4) | 68.7 |
Values are expressed as absolute numbers, median (interquartile range), and percentages as indicated.
Abbreviations: LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; PV, pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
aAccepting an error of ≤ 3 mm from Visitag center to anatomy surface.
FIGURE 3.
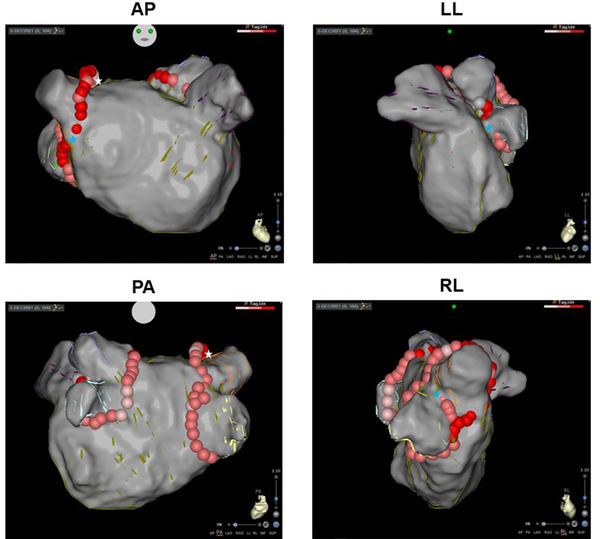
Cartosoundfam map with Visitag lesions in patient 5 who underwent pulmonary vein (PV) isolation. Minor anatomy corrections were subsequently carried out after aligning the ablation catheter along the PV antra (white star = insufficient anatomy; blue star = excessive anatomy). Abbreviations: AP, anteroposterior; LL, left lateral; PA, posteroanterior; RL, right lateral [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
The main findings of this study were that the Cartosoundfam module (available in the future Carto 3 version 8 release) can (a) correctly identify all anatomical structures (LA body, LAA, and PV) in all patients; and (b) provide acceptable anatomical PV antrum accuracy. Therefore, our initial results support the feasibility of using the Cartosoundfam module in routine clinical practice.
In many electrophysiology laboratories, especially in the United States, ICE is nowadays a standard tool in AF ablation procedures. Since the initial part of an AF ablation consists of reconstructing an accurate LA map, typically with the mapping catheter, it would be of interest to automate this process and make it less dependent on the level of the assisting technician as well as to reduce procedural time. The development of the CartoSoundFAM module for the next version (Carto 3 v8) of the Carto system, which automates the contour recognition of the LA anatomical structures and produces an automated anatomical map without the need for manual contour annotation, may help in this regard.
As with any procedural technical aspect it is crucial to establish a workflow. First, the order of scanning the anatomical structures to avoid having to go back and recollect missing anatomical regions needs to be established if the operator does not have previous experience with Cartosound in the LA. Second, care should be taken when manipulating the ICE catheter in order to optimize the 2D US image as much as possible. Finally, it seems reasonable to collect more than the minimum ≥2 frames per anatomical structure recommended by the manufacturer in order to increase the likelihood of anatomy accuracy by the algorithm. In our study, we collected a mean of seven frames per PV and despite being first‐time users of the Cartosoundfam module the mean acquisition time could be considered acceptable (16 min). The algorithm identified all anatomical structures in all patients with reasonably good PV segment anatomy accuracy (69% of all segments), as judged by the Visitag closeness to the anatomy shell. As a result, in all PVI cases, ablation could be carried out directly on the automated LA anatomy after relatively little anatomy correction with the ablation catheter. Consequently, for operators who routinely use ICE for AF ablation procedures, the Cartosoundfam map could be obtained during the routine LA anatomy screening and will subsequently obviate the need for a traditional FAM map with a mapping catheter (unless there is need for electrical substrate characterization of the atria).
5. LIMITATIONS
We have not compared the anatomical accuracy of the Cartosoundfam map with a traditional multipolar catheter mapping or against CT/MR imaging. However, we do believe that using Visitag as a surrogate for PV antral anatomical boundary is of clinical value, given that it is what practically matters to the operator, that is, a good alignment with the Visitags and the anatomical shell for performing an effective and safe PVI. Nonetheless, a potential error with Visitag location is due to LA wall tending by the ablation or mapping catheter, however, only Visitags with a force of 5–20 g were included in the analysis which should minimize this issue. Future head‐to‐head comparison with merging of the FAM reconstruction to a CT imaging will hopefully provide results on this (NCT04600245). The use of the Cartosoundfam module is dependent on previous ICE experience and may not be suitable for novice ICE users. However, it may obviate the need for an experienced technician, as it does not depend on correct manual annotation. Another limitation is that the machine learning methodology which Cartosoundfam is based on, can only process images from RA and the RVOT and may have difficulties with unusual anatomical variances. Of note, in this study all patients presented standard LA anatomies consisting of four separate PVs. Increasingly, ICE operators are placing the ICE catheter in the LA for better image resolution of the same chamber, which is therefore incompatible with the Cartosoundfam module. We used a relatively high number of contours per anatomical structure, and we can therefore not be certain that the algorithm will perform similarly with if only the minimum recommended number (≥2) of contours had been collected. Finally, the Cartosoundfam is a pre‐commercial module, which will be available in the future Carto 3 version 8 release and therefore its widespread clinical application is limited, and further external validation is currently difficult.
6. CONCLUSION
The automated ICE‐based algorithm correctly identified the LA anatomical structures in all patients with an acceptable anatomical accuracy of the Visitags alignments to the PV antrum segments. More work is needed to compare its accuracy and effectiveness to other commonly used anatomical reconstruction techniques.
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST
Finn Åkerström, Nikola Drca, Mats Jensen‐Urstad, and Frieder Braunschweig have served as paid speakers and consultants to Biosense Webster.
ACKNOWLEDGMENTS
The authors would like to thank Linus Holmström and Olivier Clair‐Legrand for their excellent clinical technical support and Guy Wekselman, Noha El‐Zehiry, and Glenn Bugauisan for technical support with the offline data analysis.
Akerström F, Drca N, Jensen‐Urstad M, Braunschweig F. Feasibility of a novel algorithm for automated reconstruction of the left atrial anatomy based on intracardiac echocardiography. Pacing Clin Electrophysiol. 2022;45:1288–1294. 10.1111/pace.14599
REFERENCES
- 1. Kim SS, Hijazi ZM, Lang RM, Knight BP. The use of intracardiac echocardiography and other intracardiac imaging tools to guide noncoronary cardiac interventions. J Am Coll Cardiol. 2009; 53: 2117‐2128. [DOI] [PubMed] [Google Scholar]
- 2. Gianni C, Sanchez JE, Della Rocca DG, et al. Intracardiac echocardiography to guide catheter ablation of atrial fibrillation. Card Electrophysiol Clin. 2021; 13: 303‐311. [DOI] [PubMed] [Google Scholar]
- 3. Akerstrom F, Bastani H, Insulander P, Schwieler J, Arias MA, Jensen‐Urstad M. Comparison of regular atrial tachycardia incidence after circumferential radiofrequency versus cryoballoon pulmonary vein isolation in real‐life practice. J Cardiovasc Electrophysiol. 2014; 25: 948‐952. [DOI] [PubMed] [Google Scholar]
- 4. Taghji P, El Haddad M, Phlips T, et al. Evaluation of a strategy aiming to enclose the pulmonary veins with contiguous and optimized radiofrequency lesions in paroxysmal atrial fibrillation: a pilot study. JACC Clin Electrophysiol. 2018; 4: 99‐108. [DOI] [PubMed] [Google Scholar]


