Abstract
The nitric oxide (NO)–soluble guanylate cyclase (sGC)–cyclic guanosine monophosphate (cGMP) pathway is dysregulated in patients with heart failure (HF) resulting in myocardial and vascular dysfunction that contributes to its progression. Vericiguat is a novel direct sGC stimulator that targets in at least two ways the NO–sGC–cGMP pathway with the subsequent restoration of cGMP activity. The VICTORIA trial assessed the effects of vericiguat (versus placebo) in 5050 patients with chronic HF (NYHA class II–IV), left ventricular ejection fraction (LVEF) <45%, elevated natriuretic peptide levels and a recent HF decompensation (hospitalized or outpatient intravenous diuretics). After a median follow-up of 10.8 months, a lower risk (10% reduction) of the primary combined outcome (cardiovascular death or HF hospitalization) was achieved (HR 0.90, 95% CI 0.83–0.98; p=0.02). The composite endpoint was driven by HF hospitalizations (HR 0.9, 95% CI 0.81–1.00; p=0.048) whilst CV death reduction was not statistically significant on its own. The target dose was achieved in 89% of patients treated with vericiguat, and no significant differences were observed in the rates of syncope or hypotension. The VICTORIA trial showed that vericiguat was safe, well tolerated and without need of laboratory testing. The aim of this review is to provide comprehensive information about vericiguat in terms of its differential mechanism of action and clinical data particularly focused on the VICTORIA trial. A comparison is also made with DAPA-HF and EMPEROR-Reduced considering that, in all these contemporary trials, a new study medication was added to the standard triple HF therapy. This is a relevant issue because the VICTORIA trial had a significant but less powerful effect than DAPA-HF and EMPEROR-Reduced on HF outcomes in a setting of more severe disease, higher event rate and shorter follow-up. In addition, relevant data on other previous studies are also provided in both HF with reduced LVEF (SOCRATES-Reduced) and HF with preserved LVEF (SOCRATES-Preserved and VITALITY-Preserved).
This article is part of the Emerging concepts in heart failure management and treatment Special Issue: https://www.drugsincontext.com/special_issues/emerging-concepts-in-heart-failure-management-and-treatment
Keywords: guanylate cyclase stimulators, heart failure, vericiguat, VICTORIA trial
Introduction
Heart failure (HF) remains a major public health concern with an approximate global prevalence of more than 64 million patients and a persistent increase in its incidence mainly due to increasingly longer life expectancy.1 In this context, acutely decompensated HF represents the most frequent cause of hospitalization in individuals older than 65 years, which results in about 1 million hospitalizations yearly only in the United States.2,3 This entity is also associated with a high rate of readmission (10% at 90 days) whilst its overall mortality (since initial diagnosis) has been estimated between 30% and 50% at 1 and 5 years, respectively.4,5
Although hospitalization and survival rates have improved in the last decade, mortality and morbidity due to HF continue to be significant. For example, in the PARADIGM-HF study (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure), the composite of death from cardiovascular (CV) causes or HF hospitalization (HFH) was 21.8% in the sacubitril/valsartan arm (27 months of follow-up).6 More recently, in the DAPA-HF trial (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), the primary endpoint (composite of death from CV causes or worsening HF) reached 16.8% in the dapagliflozin group (median follow-up 18.2 months)7 whilst, in the EMPEROR-Reduced trial (Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure), a similar primary outcome (combined CV death or hospitalization for worsening HF) affected 19.4% of the active arm (median follow-up 16 months).8
Despite significant positive results of these trials, a persistent necessity for novel therapies remains and therefore, in this context, the introduction of vericiguat could be relevant. The VICTORIA trial (Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction) assessed the clinical efficacy of vericiguat, a novel oral soluble guanylate cyclase (sGC) stimulator, versus placebo in patients with chronic HF and reduced ejection fraction (HFrEF), resulting in a significant 10% reduction (33.6% versus 37.8%) of its combined primary endpoint, which was death from CV causes or first HFH (median follow-up 10.8 months).9 In the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure, vericiguat was awarded a class 2B recommendation (‘usefulness’/‘may be considered’) and a level of evidence B (data derived from a single randomized clinical trial). Therefore, vericiguat was recommended for patients in New York Heart Association (NYHA) functional class II–IV who had worsening HF (on guideline-directed medical therapy (GDMT)) to reduce the risk of CV mortality or HFH.10 In the same direction, the 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure assigned vericiguat to a class 2b recommendation (‘weak’/‘may be considered’) and a level of evidence BR (‘moderate quality’/evidence based on only one randomized controlled trial). Therefore, vericiguat should be considered in selected patients with HFrEF at high risk and recent worsening of HF (already on GDMT) to reduce HFH and CV death.11
The aim of this review is to reassess the existing evidence regarding vericiguat (differential mechanism of action and relevant clinical data) with the intention of examining its place in therapy precisely within the recent introduction of sodium–glucose cotransporter 2 inhibitors (SGLT2is) as a GDMT for HFrEF.10,11
Guanylate cyclase pathway
In the CV system, nitric oxide (NO) is synthesized by endothelial nitric oxide synthase (eNOS) from l-arginine after being activated by an increase of intra-cytoplasmic calcium levels (phosphorylation or via calcium/calmodulin).12 eNOS was originally described in the endothelial cells of large vessels but has also been identified in the endothelium of coronary arteries, endocardial cells, cardiomyocytes and specialized cardiac conduction cells, amongst others.13 NO rapidly diffuses in two main directions: to the bloodstream where it binds to the haem moiety of haemoglobin (to be transported) and to the haem moiety of the sGC enzyme in neighbouring vascular smooth muscle cells. sGC promotes the dephosphorylation of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP), which acts as a second messenger by activating protein kinase G (downstream effectors). Phosphorylated substrates of protein kinase G are widely present in several cardiovascular and renal cells (cardiomyocytes, fibroblasts, endothelial cells, platelets, renin-secreting juxtaglomerular cells, etc.). NO exerts a capital role by controlling vascular tone and blood pressure (smooth muscle relaxation), preventing platelet aggregation, and protecting endothelial integrity because it exerts anti-inflammatory, antithrombotic and vasodilator effects. Furthermore, it improves coronary blood flow and diastolic relaxation whilst protecting against inflammation, fibrosis, hypertrophy and apoptosis of cardiomyocytes in response to cardiac injury.12–14 The bioavailability of NO can be severely compromised in different diseases such as atherosclerosis, hypertension, diabetes mellitus, coronary artery disease, ischaemia-reperfusion injury and vascular aging.14–16 In this context, HF represents another case of limited NO availability because it is characterized by an associated increase in oxidative stress, release of pro-apoptotic factors, reduced endothelial synthesis of NO, excessive elimination of NO and an altered reaction of sGC that results in a deficient cGMP state with subsequent negative impact over cardiac, vascular and kidney functions.12,17
Pharmacodynamics and pharmacokinetics of vericiguat
Vericiguat is an oral agent that directly raises cGMP availability by increasing sGC sensitivity to endogenous NO (stabilizing their mutual binding) and, indirectly, via a NO-independent pathway (Figure 1). This has a favourable impact on decongestion, afterload reduction, coronary blood flow improvement, platelet activation inhibition, CV inflammation and remodelling attenuation, kidney protection, and diuresis and natriuresis promotion.18 Vericiguat is a class II (Biopharmaceutics Classification System) low-soluble and highly permeable agent with a single daily dosage (up-titrated from 2.5 to 10 mg in the VICTORIA trial). Its water solubility is low (1.6 L/hour in healthy volunteers, 1.3 L/hour in patients with HFrEF) whilst its intestinal permeability is high, allowing recovery of >98% of the administered dose. Once ingested with food, vericiguat exhibits elevated bioavailability and a reduced variability with a median time of <2.5 hours to reach its maximum plasmatic concentration (half-life: 18–22 hours).19 Plasma protein binding of vericiguat is around 98% (mainly albumin) and is not altered by renal or hepatic insufficiency; glucuronidation via uridine diphosphate-glucuronosyltransferase 1A9 and 1A1 represents its main metabolic pathway and practically has no effects over the major cytochrome P450s system. In healthy individuals, 53.1% and 45.2% of the ingested dose is eliminated via diuresis and faeces, respectively.19
Figure 1. Cardiovascular effects of vericiguat.
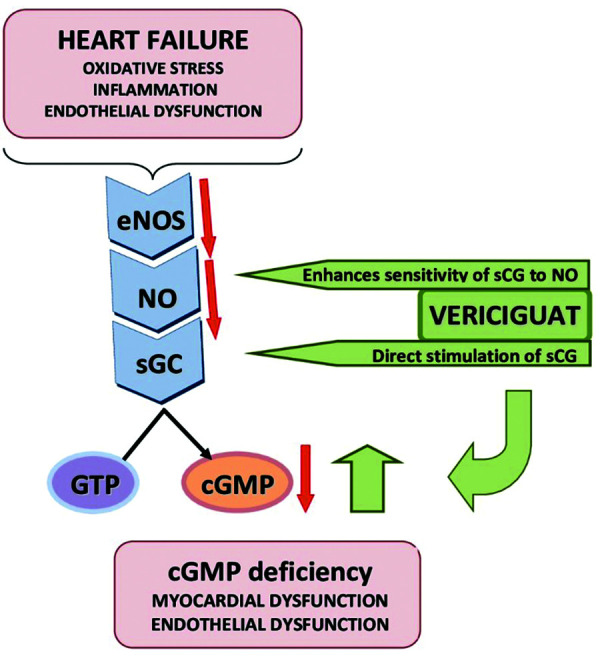
In a heart failure setting, an increased oxidative stress, inflammation and endothelial dysfunction reduce nitric oxide (NO) bioavailability, which results in soluble guanylate cyclase (sGC) deficiency with a subsequent reduction in cyclic guanosine monophosphate (cGMP) synthesis (orange arrows). A lower sGC activity is associated with coronary microvascular dysfunction, altered diastolic relaxation, inflammation, fibrosis, hypertrophy and apoptosis of cardiomyocytes in response to cardiac injury.12–17 Vericiguat directly stimulates sGC (independent of NO involvement) and it also sensitizes this enzyme to endogenous NO by the stabilization of its binding site. Consequently, by restoring cGMP deficiency (green arrows), myocardial function and vascular tone improve.18
Vericiguat is a poorly soluble and highly permeable agent that, once ingested, requires a median time of <2.5 hours to reach its maximum plasma concentration (half-life 18–22 hours). It is approximately 98% bound to plasma proteins (essentially albumin) and is not affected by renal or hepatic insufficiency. In healthy subjects, 53.1% and 45.2% of the administered dose is excreted in the urine and faeces, respectively.19
eNOS, endothelial nitric oxide synthase; GTP, guanosine triphosphate.
Vericiguat in HFrEF
In the clinical field of HFrEF, vericiguat has been studied in the phase II, SOCRATES-Reduced study20 and in the pivotal phase III VICTORIA trial.9
SOCRATES-reduced
This trial was a dose-finding study that enrolled 456 patients with LVEF <45% and a recent (within 4 weeks) episode of HF decompensation (HFH or outpatient visit requiring intravenous diuretics), congestive signs, elevated natriuretic peptides levels, brain natriuretic peptide (BNP) ≥300 ng/L (atrial fibrillation: ≥500 ng/L), or N-terminal pro-B-type natriuretic peptide (NT-proBNP) ≥1000 ng/L (atrial fibrillation ≥1600 ng/L).20 SOCRATES-Reduced was not designed for clinical outcomes because its primary endpoint was the change (baseline to week 12) in log-transformed level of NT-proBNP. Its study population was randomized to receive placebo (n=92) or one of four daily vericiguat doses: 1.25 mg (n=91), 2.5 mg (n=91), 5 mg (n=91) or 10 mg (n=91).
In an initial analysis, the variation of the main outcome was not statistically significant between the whole pooled vericiguat group versus the placebo arm (p=0.15) but, in a secondary evaluation (using linear regression analysis), a clear dose–response relationship was observed (reaching a statistical significance) in the vericiguat 10 mg arm (p=0.0483).20 In addition, there was a favourable trend but statistically non-significant (p=0.72) towards the reduction of the composite CV death and HFH in the vericiguat 5 and 10-mg groups (versus placebo): 19.6% (placebo), 18.7% (1.25-mg group/p=0.97), 19.8% (2.5-mg group/p=1.01), 12.1% (5-mg group/p=0.63) and 11% (10-mg group/p=0.633). Positively, a slight but significant increase (p<0.05) of LVEF (versus placebo) was observed in the 10-mg group (+3.7% versus +1.5%). In general, vericiguat was well tolerated and no significant changes were observed on blood pressure or heart rate.20
VICTORIA trial
The phase III VICTORIA trial was an event-driven study that evaluated, in terms of efficacy, the incorporation of vericiguat or placebo to GDMT in patients with HFrEF (not taking nitrates) with a pre-estimated trial duration time of 18 months. Main inclusion criteria were LVEF <45%, NYHA functional class II–IV, previous HFH or use of outpatient intravenous diuretics for HF within 6 months before randomization, and elevated levels of BNP or NT-proBNP (≥1000 ng/L in sinus rhythm/≥1600 ng/L in atrial fibrillation).9 Table 1 shows the baseline demographic characteristics of the vericiguat group compared to the active arms of two other contemporary studies (DAPA-HF and EMPEROR-Reduced) in which the study medication was also added to GDMT.7–9 PARADIGM-HF, which is another relatively recent landmark trial (2014), was not included for the comparison because its study medication (sacubitril/valsartan) represented a replacement treatment (not an addition).6
Table 1.
Comparison of baseline demographical parameters between DAPA-HF, EMPEROR-Reduced and VICTORIA trial.
| Characteristics (intervention arm) | DAPA-HF (n=4744) | EMPEROR-Reduced (n=3730) | VICTORIA (n=5050) |
|---|---|---|---|
| Patients included (n) | 2373 | 1863 | 2526 |
| Study medication | Dapagliflozin | Empagliflozin | Vericiguat |
| Administration timeframe | Once/daily | Once/daily | Once/daily |
| Comparator | Placebo | Placebo | placebo |
| Follow-up (months) | 18.2 | 16.0 | 10.8 |
| Age (y) | 66.2 | 67.2 | 67.5 |
| Female (%) | 23.8 | 23.5 | 24.0 |
| NYHA functional class III (%) | 31.5 | 24.4 | 40 |
| NYHA functional class IV (%) | 0.8 | 0.5 | 1.4 |
| CAD (%) | 55.5 | 52.8 | 59.8 |
| LVEF (%) | 31.2 | 27.7 | 29 |
| NT-proBNP (ng/L) | 1428 | 1887 | 2803 |
| eGFR (mL/minute/1.73 m2) | 66.0 | 61.8 | 61.3 |
| Atrial fibrillation (%) | 38.6 | 35.6 | 43.5 |
| Previous HFH (%) | 47.4 | 31.0 | 84.2 |
| IV diuretic for HF (no HFH) | NA | NA | 15.8 |
| Sacubitril/valsartan (%) | 10.5 | 18.3 | 14.3 |
| ICD (%) | 26.2 | 31.0 | 27.6 |
| CRT (%) | 8 | 11.8 | 14.7 |
Main demographic characteristics of the intervention arms of DAPA-HF study, EMPEROR-Reduced study and VICTORIA trial.7–9
CAD, coronary artery disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HFH, heart failure hospitalization; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association.
The primary endpoint was a combination of CV death or first HFH whilst the secondary outcomes were the components of the primary outcome, first and subsequent HFH, a composite of death from any cause or first HFH, and death from any cause. In total, 5050 patients were randomized to receive (1:1 ratio) placebo or vericiguat with a target dose of 10 mg once daily; its median follow-up was 10.8 months. The combined primary endpoint of CV death or HFH occurred in 35.5% of the vericiguat group compared with 38.5% of the placebo group (HR 0.90, 95% CI 0.82–0.98; p=0.02) and it represented a 10% relative difference reduction, an annualized absolute risk reduction of 4.2% and a number needed to treat of 24.9 However, this positive result was basically based on the reduction in HFH in the vericiguat arm (27.4% versus 29.6% placebo; HR 0.90, 95% CI 0.81–1.00; p=0.048) without a significant difference in the secondary endpoint of death from CV causes (16.4% vericiguat versus 17.5% placebo; HR 0.93, 95% CI 0.81–1.06; p=0.269). Overall death was comparable in both arms (20.3% vericiguat versus 21.2% placebo) and serious adverse events were also balanced between both groups (32.8% vericiguat versus 34.8% placebo).9 Table 2 summarizes the main outcomes between the vericiguat group and the active arms of its contemporaries DAPA-HF and EMPEROR-Reduced studies. In all three cases, the study medication was added to GDMT.7–9
Table 2.
Comparison of main outcomes between DAPA-HF, EMPEROR-Reduced and VICTORIA trial.
| Outcome | DAPA-HF (n=4744) | EMPEROR-Reduced (n=3730) | VICTORIA (n=5050) |
|---|---|---|---|
| Follow-up (months) | 18.2 | 16.0 | 10.8 |
| Primary outcomes rate: active/placebo (%) | |||
| Combined (CV death + HFH) | 16.3/21.2 | 19.4/24.7 | 33.6/37.8 |
| HFH | 10.0/13.7 | 10.7/15.5 | 27.4/29.6 |
| CV death | 9.6/11.5 | 7.6/8.1 | 8.2/8.9 |
| HR (95% CI) | |||
| Combined (CV death + HFH) | 0.74 (0.65–0.85) | 0.75 (0.65–0.86) | 0.90 (0.82–0.98) |
| HFH | 0.70 (0.59–0.83) | 0.69 (0.59–0.81) | 0.90 (0.81–1.00) |
| CV death | 0.82 (0.69–0.98) | 0.92 (0.75–1.12) | 0.93 (0.81–1.06) |
| AER: active/placebo (%) | |||
| Combined (CV death + HFH) | 11.6/15.6 | 15.8/21.0 | 33.6/37.8 |
| HFH | 6.9/9.8 | 10.7/15.5 | 25.9/29.1 |
| CV death | 6.5/7.9 | 7.6/8.1 | 12.9/13.9 |
| ARR (%) | |||
| Combined (CV death + HFH) | 4.0 | 5.2 | 4.2 |
| HFH | 2.9 | 4.8 | 2.7 |
| CV death | 1.4 | 0.6 | 1.0 |
| NNT | 25.0 | 19.2 | 23.8 |
Vericiguat had a favourable safety profile (89% of patients reached target dose) and, in this context, symptomatic hypotension (9.1% versus 7.9%; p=0.121) and syncope (4.0% versus 3.5%; p=0.303) affected more patients in the vericiguat arm compared to placebo. More cases of anaemia were reported in the vericiguat arm (7.6 versus 5.7%), though severe cases were uncommon (1.6 versus 0.9%). A mean haemoglobin change of −0.38 ± 1.27 g/dL with vericiguat and −0.14 ± 1.30 in the placebo group occurred in the first 16 weeks of the study.9 It should be noted that, in the VICTORIA trial, anaemia was defined as haemoglobin <13.0 g/dL in men and <12.0 g/dL in women, which meant that, at baseline, 35.7% of patients (n=1719) had anaemia.9 The aetiology of this anaemia is not clear and was linked to haemodilution (fluid overload) and a decreased erythropoietin production. At week 16, 1643 patients had anaemia, of whom 284 were new cases for vericiguat and 219 for placebo (p<0.001). After week 16, there were no further decreases in haemoglobin during the rest of the study. The benefit of vericiguat was independent of haemoglobin values, though there was a slight decrease in haemoglobin concentrations up to week 16 (this did not imply less clinical efficacy).21
The positive effects of vericiguat (primary outcome) were consistent in all prespecified subgroups (age, race, index events, NYHA functional class, LVEF, renal function use of sacubitril/valsartan) (Table 3) except for patients with the highest quartile of baseline NT-proBNP (>5314 ng/L), in whom a loss of benefit was observed.9 However, a post hoc analysis raised the positive effects of vericiguat to randomization values of NT-proBNP up to 8000 ng/L.22 In the case of vericiguat and coronary artery disease (CAD), an additional analysis showed that its pre-existence was associated with more CV death and HFH in comparison with patients with HF without CAD. In the VICTORIA trial, a total of 2704 patients (58.3%) had CAD (history of myocardial infarction, coronary angioplasty or coronary surgical revascularization) and they were older, more commonly male, had diabetes, with a lower glomerular filtration rate (eGFR) and more had implantable cardioverter defibrillators than those without CAD (all p<0.0001). Consequently, the primary combined outcome (CV death/HFH) was more present in patients with CAD versus patients without CAD (40.6 versus 30.1/100 patient-years; adjusted HR 1.23; p<0.001) with a higher overall mortality (17.9% versus 12.7%; adjusted HR 1.32; p<0.001).23 With respect to vericiguat and renal function, the VICTORIA trial included patients with an eGFR of up to 15 mL/minute/1.73 m2.9 A post hoc analysis revealed that there were no differences between both arms (vericiguat/placebo) regarding renal function trajectories (baseline serum creatinine and weeks 16, 32 and 48 measurements). In addition, the beneficial effect of vericiguat was not influenced by the baseline eGFR (interaction p=0.48); therefore, in consequence, vericiguat represents an added value for patients with HF, in whom renal failure is frequent.24 Finally, a total of 47% of patients had a history of atrial fibrillation in the VICTORIA trial and individuals with atrial fibrillation had a higher risk of CV death than those without (HR 1.25, 95% CI 1.01–1.47). The benefit of vericiguat in both the primary combined outcome and its components was independent of its presence and, during the whole study, new-onset atrial fibrillation occurred in only 6.1% of patients without atrial fibrillation at baseline.25
Table 3.
Subgroup analysis of the primary endpoint in VICTORIA trial.
| Subgroup | Hazard ratio | 95% CI |
|---|---|---|
| All patients | 0.90 | 0.82–0.92 |
| Sex | ||
| Male | 0.90 | 0.81–1.00 |
| Female | 0.88 | 0.73–1.08 |
| Age (years) | ||
| <65 | 0.81 | 0.70–0.95 |
| >65 | 0.94 | 0.84–1.06 |
| <75 | 0.84 | 0.75–0.94 |
| >75 | 1.04 | 0.88–1.21 |
| Race | ||
| White | 0.91 | 0.81–1.02 |
| Asian | 0.91 | 0.75–1.11 |
| Black | 0.85 | 0.56–1.28 |
| Other | 0.80 | 0.57–1.11 |
| HF decompensation events | ||
| Intravenous diuretics (previous 3 months) | 0.78 | 0.60–1.02 |
| HFH (previous 3 months) | 0.93 | 0.84–1.04 |
| HFH (previous 3–6 months) | 0.85 | 0.67–1.07 |
| NYHA functional class | ||
| I–II | 0.91 | 0.80–1.04 |
| III–IV | 0.87 | 0.77–0.99 |
| LVEF (%) | ||
| <35% | 0.88 | 0.79–0.97 |
| ≥35% | 0.96 | 0.81–1.14 |
| <40% | 0.88 | 0.80–0.97 |
| ≥40% | 1.05 | 0.81–1.36 |
| eGFR (mL/minute/1.73 m 2) | ||
| ≤30 | 1.06 | 0.83–1.34 |
| >30 to ≤60 | 0.84 | 0.73–0.96 |
| >60 | 0.92 | 0.80–1.07 |
| Sacubitril/valsartan use at baseline | ||
| Yes | 0.88 | 0.70–1.11 |
| No | 0.90 | 0.81–0.99 |
| NT-proBNP level (pg/mL) | ||
| Quartile 1: ≤1.556 | 0.78 | 0.62–0.99 |
| Quartile 2: 1.556–2.816 | 0.73 | 0.60–0.90 |
| Quartile 3: 2816–5314 | 0.82 | 0.69–0.99 |
| Quartile 4: >5314 | 1.16 | 0.99–1.35 |
eGFR, estimated glomerular filtration rate; HF, heart failure; HFH, heart failure hospitalization; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association.
Table adapted from the VICTORIA trial.9
Vericiguat in HFpEF
Although this review is focused on the therapeutic place of vericiguat in HFrEF, some relevant data of clinical studies in HF with preserved LVEF (HFpEF) are briefly provided. In this setting, vericiguat was evaluated in two phase II studies but without encouraging results: SOCRATES-Preserved26 and VITALITY-Preserved.27
SOCRATES-Preserved was a randomized, double-blind, placebo-controlled, multicentre, dose-establishing study that included 477 patients with worsening HFpEF; a total of 93 patients were randomized to the placebo arm and 384 to vericiguat in four arms (96–1.25, 95–2.5, 95–5 and 96–10 mg). Vericiguat was well tolerated but it could not significantly modify (versus placebo) either NT-proBNP levels or left atrial volume (12 weeks follow-up) whilst the prespecified exploratory endpoint of quality of life was improved in the vericiguat 10-mg arm.26 VITALITY-Preserved was also a double-blind, placebo-controlled, multicentre trial of patients with HFpEF with a recent decompensation (n=789). Patients were randomized (1:1:1) to receive vericiguat, up-titrated to 15 mg (n=264) or 10 mg (n=263) daily oral dosages, compared with placebo (n=262). Unfortunately, vericiguat in both dosages was not able to improve physical capacity (6-minute walking test) and a quality-of-life score.27
Strengths and limitations of the VICTORIA trial
Considering the results of the VICTORIA trial,9 vericiguat has obtained a secondary place in therapy in both European and American guidelines to be used to reduce the risk of HFH and CV death in patients with HFrEF (already on GDMT) with a recent episode of decompensation.10,11 In this context, wide use of vericiguat in clinical practice does not seem expected, particularly when the optimal GDMT has recently evolved into four therapeutic pillars10,11 that are not reflected in the population of the VICTORIA trial.9 In any case, as vericiguat is the first sGC stimulator introduced in the therapeutic armamentarium of HF, there are some important points to highlight:
– The VICTORIA trial was a multicentre, randomized double-blind, placebo-controlled study that included more patients (n=5050) than the DAPA-HF (n=4744) and EMPEROR-Reduced (n=3730) studies (two recent studies chosen for comparisons).7–9 In all these cases, a fourth type of agent was administered to patients with HF already on GDMT (Table 1) and this represents a major difference (comparative analysis) with the PARADIGM-HF trial (n=8442) in which sacubitril/valsartan was a switching therapy (instead angiotensin-converting enzyme inhibitors).6
– The VICTORIA trial showed that vericiguat has a satisfactory safety profile, is well tolerated, improves HF outcomes and requires only once daily administration without renal function or electrolyte control, therapeutic drug monitoring, etc.9
– The inclusion criteria of the VICTORIA trial were stricter than those of the DAPA-HF study and EMPEROR-Reduced studies because its entire population had been hospitalized in the previous 6 months (most within the past 3 months) or required intravenous diuretics (outpatients).7–9
– In consequence, patients in the VICTORIA trial had more severe disease than those included in the DAPA-HF or EMPEROR-Reduced studies as verified by the advanced NYHA functional class (41.4% III/IV), higher NT-proBNP levels (2803 ng/L), a relevant presence of atrial fibrillation (43.5%) or resynchronization therapy (14.7%) and, obviously, a higher event rate.7–9
– In this sicker population, vericiguat achieved a 10% relative difference (versus placebo) in the primary composite outcome at a median follow-up of 10.8 months (driven by HFH reduction), implying a reduction in the absolute event rate of 4.2 events per 100 patient-years, which was similar to or even better than the rate observed in the DAPA-HF study (4.0 events per 100 patient-years) or the EMPEROR-Reduced study (5.2 events per 100 patient-years).7–9
– In this context, the number needed to treat to prevent an event was lower in the VICTORIA trial (23.8) than in the DAPA-HF study (25) but higher than in the EMPEROR-Reduced study (19.2).7–9
– Even though vericiguat did not reduce overall mortality, whilst dapagliflozin (DAPA-HF study) and empagliflozin (EMPEROR-Reduced) did, it should be considered that the VICTORIA trial had a relatively short follow-up (10.8 months), which was associated with a very high event rate (combined primary outcome) in the control arm; events per 100 patient-years were 37.8 in the VICTORIA trial versus 15.6 in the DAPA-HF and 21.0 in EMPEROR-Reduced studies.7–9
– It should be highlighted that the results of the previously mentioned SOCRATES-Reduced study reinforce the use of vericiguat in patients with more severe HF. As in the VICTORIA trial, the population of patients with HF incorporated in SOCRATES (n=456/mean age 68 years) was sicker than that included in other recent studies. A total of 78% of patients had had a previous HFH whilst the remaining 22% had required outpatient use of intravenous diuretics (mean LVEF was 29.6% and median baseline NT-proBNP level was 3076 pg/m). As described, only vericiguat titration up to 10 mg/day (highest dose) was associated with a drop in NT-proBNP levels, a slightly LVEF increase, and a lower number of worsening HF episodes.20
– As limitations, it could be pointed out that Black patients were underrepresented (4.9%) in the VICTORIA trial because most of the included patients were white (64.1%) or Asian (22.4%).
– In addition, only 58.7% of patients (vericiguat arm) were on HF triple therapy; a small proportion were administered sacubitril/valsartan (14.3%) or an SGLT2i (48.5% of patients had diabetes mellitus at baseline in the active arm).9
Discussion
As previously mentioned in the introduction, even patients receiving a GDMT continue to have a significant rate of CV events, meaning that there are remaining therapeutic needs to be met.6–8 The VICTORIA trial is, in our opinion, an important study because it successfully introduced the first-in-class sGC stimulator in the clinical scenario of HF and, particularly, in a setting of patients at high risk9 who are not represented in the latest HF trials.6–8 In individuals with a recent worsening HF event, vericiguat provided a significant novel addition to usual treatment by improving HF outcomes with a satisfactory safety profile. A strict comparison between the VICTORIA trial and the recent clinical studies with SGLT2i (DAPA-HF and EMPEROR-Reduced) with different entry criteria and patients with less severe HF, would not be completely accurate (Table 1).7–9
A recent (2021) network meta-analysis comparing phase III trial treatment arms that included sacubitril/valsartan, SGLT2i and vericiguat (against their respective placebo arms) revealed that SGLT2i therapy was not significantly associated with a reduced risk of CV death or HFH compared to sacubitril/valsartan (HR 0.92, 95% CI 0.81–1.05) or vericiguat (HR 0.83, 95% CI 0.73–0.94). In the same direction, a non-significant effect of SGLT2i on CV mortality versus sacubitril/valsartan (HR 1.04, 95% CI 0.88–1.24) and vericiguat (HR 0.88, 95% CI 0.63–1.22) was also detected. However, SGLT2i therapy showed its most important effect (versus placebo) on HFH (HR 0.69, 95% CI 0.62–0.77) where it was greater than that of vericiguat (HR 0.77, 95% CI 0.66–0.89) but lower than sacubitril/valsartan (HR 0.87, 95% CI 0.75–1.02).28 Therefore, and as a relevant conclusion of this meta-analysis for our review, vericiguat (apart from sacubitril/valsartan) was associated with a significantly lower risk of the combined outcome of CV death or HFH or CV death alone compared to SGLT2i.28 In another and more recent network meta-analysis of randomized controlled trials, Pagnesi et al.29 compared all the new therapies for patients with HFrEF, including SGLT2i, vericiguat and omecamtiv mecarbil (versus placebo). In this case, they found that SGLT2is were the most effective therapy for all the analysed endpoints, whilst vericiguat and omecamtiv mecarbil occupied the second and third position, respectively. The primary endpoint (CV death or HFH) was significantly reduced by SGLT2i therapy (HR 0.77; 95% CI 0.71–0.83) followed by vericiguat (HR 0.84, 95% CI 0.75–0.93) and omecamtiv mecarbil (RR 0.80, 95% CI 0.72–0.88). With respect to the secondary endpoints of CV death and overall mortality, SGLT2i treatment was found to be no better than vericiguat but better than omecamtiv mecarbil (with no significant difference between vericiguat and omecamtiv mecarbil). Finally, in the case of HFH, SGLT2i therapy was again superior to vericiguat and omecamtiv mecarbil but no difference was found between them.29 The authors stated that the probability of each treatment being the best was:
– CV death or HFH: SGLT2i 77.24%, vericiguat 15.92%, omecamtiv mecarbil 6.55%, placebo 0.29%
– CV death: SGLT2i 61.14%, vericiguat 25.89%, omecamtiv mecarbil 11.48%, placebo 1.49%
– Overall mortality: SGLT2i 64.97%, vericiguat 28.40%, omecamtiv mecarbil 11.48%, placebo 3.66%
– HFH: SGLT2i 78.21%, vericiguat 19.12%, omecamtiv mecarbil 2.19%, placebo 0.48%
In consequence and considering this analysis, SGLT2is are the best therapy to reduce outcomes in patients with HFrEF treated with standard triple therapy followed by vericiguat.29 Finally, in another very recent systematic review and network meta-analysis of drug therapy for HFrEF, Tromp et al.30 showed that the combination of an angiotensin receptor-neprilysin inhibitor plus beta-blocker plus mineralocorticoid receptor antagonist plus SGLT2i was the most effective compared to other types of double, triple and quadruple combinations. In this case, this scheme was able to extend the life of a 70-year-old patient by an additional 5 years (2.5–7.5) as it proved to be the most effective in reducing all-cause death (HR 0, 39, 95% CI 0.31–0.49), the composite outcome of CV death or HFH (HR 0.36, 95% CI 0.29–0.46), and CV mortality (HR 0.33; 95% CI 0.26–0.43). The second most effective combination was angiotensin receptor-neprilysin inhibitor plus beta-blocker plus mineralocorticoid receptor antagonist plus vericiguat with reductions of all-cause death (HR 0.41, 95% CI 0.32–0.53), the composite outcome of CV death or HFH (HR 0.43, 95% CI 0.34–0.55), and CV mortality (HR 0.35, 95% CI 0.26–0.47).30 Therefore, and as a conclusion of these three meta-analyses, the therapeutic position of vericiguat in patients with HF is clearly reinforced.28–30
As it was previously mentioned, the VICTORIA trial, which was an event-driven trial, required only 10.8 months of follow-up to reach the pre-established total number of events in comparison with the abovementioned studies, which needed a more extended duration (Table 2).7–9 Likely, this relatively short follow-up period was enough to reduce hospitalizations but insufficient in terms of mortality reduction; in any case, the VICTORIA trial showed a premature separation (month 4) of the event curves (vericiguat versus placebo), which was maintained until the end of the study.9 On the other hand, the VICTORIA trial has left some points to be clarified such as the efficacy of vericiguat in more stable patients (less events rate) or its potential synergy in patients already taking a complete GDMT, including sacubitril/valsartan and SGLT2i (not reflected in VICTORIA trial demography). In addition, its apparent lack of efficacy in patients exhibiting NT-proBNP levels of >8000 ng/L might suggest that its clinical introduction in a decompensated setting should be postponed at least until a lower peptide value can be reached.22
Conclusion
In patients with HFrEF and a recent acute decompensation episode, the VICTORIA trial showed clinical benefits from the innovative strategy of increasing sGC activity by adding vericiguat to GDMT. This can reflect an evolution of HF therapeutics because patients enrolled in this trial were sicker than those included in other recent studies; nevertheless, both European and American guidelines assign vericiguat a weak place in therapy. It should be underscored that, unfortunately, vericiguat was introduced at a time when essential treatment of HF was migrating from three different therapeutic families to four. However, we believe that vericiguat would be a realistic alternative for patients with frequent HFH episodes or at high risk of recurrent HFH after discharge and to prevent new admissions in individuals already taking the recent optimized GDMT. Other important groups are individuals who receive intermittent intravenous diuretics or inotropes (outpatients) or those who cannot take other usual HF therapies due to renal insufficiency.
Acknowledgements
None.
Footnotes
Contributions: EK wrote the manuscript. SP and AB reviewed the manuscript and made tables and the figure. All authors take responsibility for the integrity of the work as a whole and give their approval for this version to be published. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2022/08/dic.2022-5-5-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2023 Kaplinsky E, Perrone S, Barbagelata A. https://doi.org/10.7573/dic.2022-5-5. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/emerging-concepts-in-heart-failure-management-and-treatment-focus-on-vericiguat
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Storrow AB, Jenkins CA, Self WH, et al. The burden of acute heart failure on U.S. emergency departments. JACC Heart Fail. 2014;2:269–277. doi: 10.1016/j.jchf.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene SJ, Fonarow GC, Vaduganathan M, et al. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol. 2015;12:220–229. doi: 10.1038/nrcardio.2015.14. [DOI] [PubMed] [Google Scholar]
- 5.Mamas MA, Sperrin M, Watson MC, et al. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur J Heart Fail. 2017;19:1095–1104. doi: 10.1002/ejhf.822. [DOI] [PubMed] [Google Scholar]
- 6.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–904. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 7.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 8.Packer M, Anker SD, Butler J, et al. EMPEROR-Reduced trial investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong PW, Pieske B, Anstrom KJ, et al. VICTORIA study group. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883–1893. doi: 10.1056/NEJMoa1915928. [DOI] [PubMed] [Google Scholar]
- 10.McDonagh TA, Metra M, Adamo M, et al. ESC scientific document group. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 11.Writing Committee Members; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Card Fail. 2022;28(5):E1–E167. doi: 10.1016/j.cardfail.2022.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Munzel T, Feil R, Mulsch A, et al. Physiology and pathophysiology of vascular signaling controlled by guanosine 3′,5′-cyclic monophosphate-dependent protein kinase. Circulation. 2003;108:2172–2183. doi: 10.1161/01.CIR.0000094403.78467.C3. [DOI] [PubMed] [Google Scholar]
- 13.Searles CD. The nitric oxide pathway and oxidative stress in heart failure. Congest Heart Fail. 2002;8:142–147. doi: 10.1111/j.1527-5299.2002.00715.x. [DOI] [PubMed] [Google Scholar]
- 14.Kraehling JR, Sessa WC. Contemporary approaches to modulating the nitric oxide-cGMP pathway in cardiovascular disease. Circ Res. 2017;120:1174–1182. doi: 10.1161/CIRCRESAHA.117.303776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burley DS, Ferdinandy P, Baxter GF. Cyclic GMP and protein kinase-G in myocardial ischaemia-reperfusion: opportunities and obstacles for survival signaling. Br J Pharmacol. 2007;6:855–869. doi: 10.1038/sj.bjp.0707409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golshiri K, Ataei Ataabadi E, Portilla Fernandez EC, Jan Danser AH, Roks AJM. The importance of the nitric oxide-cGMP pathway in age-related cardiovascular disease: focus on phosphodiesterase-1 and soluble guanylate cyclase. Basic Clin Pharmacol Toxicol. 2020;127:67–80. doi: 10.1111/bcpt.13319. [DOI] [PubMed] [Google Scholar]
- 17.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evgenov OV, Pacher P, Schmidt PM, Haskó G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boettcher M, Gerisch M, Lobmeyer M, et al. Metabolism and pharmacokinetic drug-drug interaction profile of vericiguat, a soluble guanylate cyclase stimulator: results from preclinical and phase I healthy volunteer studies. Clin Pharmacokinet. 2020;59:1407–1418. doi: 10.1007/s40262-020-00895-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gheorghiade M, Greene SJ, Butler J, et al. SOCRATES-REDUCED investigators and coordinators. effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: The SOCRATES-REDUCED randomized trial. JAMA. 2015;314:2251–2262. doi: 10.1001/jama.2015.15734. [DOI] [PubMed] [Google Scholar]
- 21.Ezekowitz JA, Zheng Y, Cohen-Solal A, et al. Hemoglobin and clinical outcomes in the VerICiguaT global study in patients with heart failure and reduced ejection fraction (VICTORIA) Circulation. 2021;144:1489–1499. doi: 10.1161/CIRCULATIONAHA.121.056797. [DOI] [PubMed] [Google Scholar]
- 22.Ezekowitz JA, O’Connor CM, Troughton RW, et al. N-terminal Pro-B-type natriuretic peptide and clinical outcomes: vericiguat heart failure with reduced ejection fraction study. JACC Heart Fail. 2020;8:931–939. doi: 10.1016/j.jchf.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Saldarriaga C, Atar D, Stebbins A, et al. VICTORIA Study Group. Vericiguat in patients with coronary artery disease and heart failure with reduced ejection fraction. Eur J Heart Fail. 2022;24(5):782–790. doi: 10.1002/ejhf.2468. [DOI] [PubMed] [Google Scholar]
- 24.Voors AA, Mulder H, Reyes E, et al. VICTORIA study group. Renal function and the effects of vericiguat in patients with worsening heart failure with reduced ejection fraction: insights from the VICTORIA (Vericiguat Global Study in Subjects with HFrEF) trial. Eur J Heart Fail. 2021;23:1313–1321. doi: 10.1002/ejhf.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponikowski P, Alemayehu W, Oto A, et al. Vericiguat in patients with atrial fibrillation and heart failure with reduced ejection fraction: insights from the VICTORIA trial. Eur J Heart Fail. 2021;23:1300–1312. doi: 10.1002/ejhf.2285. [DOI] [PubMed] [Google Scholar]
- 26.Pieske B, Maggioni AP, Lam CSP, et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J. 2017;38:1119–1127. doi: 10.1093/eurheartj/ehw593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong PW, Lam CSP, Anstrom KJ, et al. VITALITY-HFpEF Study Group. Effect of Vericiguat vs Placebo on quality of life in patients with heart failure and preserved ejection fraction: the VITALITY-HFpEF randomized clinical trial. JAMA. 2020;324:1512–1521. doi: 10.1001/jama.2020.15922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aimo A, Pateras K, Stamatelopoulos K, et al. Relative efficacy of sacubitril-valsartan, vericiguat, and SGLT2 inhibitors in heart failure with reduced ejection fraction: a systematic review and network meta-analysis. Cardiovasc Drugs Ther. 2021;35(5):1067–1076. doi: 10.1007/s10557-020-07099-2. [DOI] [PubMed] [Google Scholar]
- 29.Pagnesi M, Baldetti L, Aimo A, et al. Prognostic benefit of new drugs for HFrEF: a systematic review and network meta-analysis. J Clin Med. 2022;11(2):348. doi: 10.3390/jcm11020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tromp J, Ouwerkerk W, van Veldhuisen DJ, et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2022;10(2):73–84. doi: 10.1016/j.jchf.2021.09.004. [DOI] [PubMed] [Google Scholar]

