Abstract
Circulatory support with extracorporeal membrane oxygenation (ECMO) is being increasingly used in several critical situations but evidence of its impact on outcomes is inconsistent. Understanding of the specific indications and appropriate timing of implantation of this technology might lead to improved results. Indeed, the line between success and futility may be sometimes very thin when facing a patient in critical condition. New techniques with lighter, simpler and effective devices are being developed. Hence, ECMO has become an accessible technology that is being increasingly used outside of the operating room by heart failure specialists, critical care cardiologists and intensivists. Proper timing of utilization and choice of device may lead to better outcomes. We herein aim to improve this knowledge gap by conducting a literature review to provide simple information, evidence-based indications and a practical approach for cardiologists who may encounter acutely ill adult patients that may be ECMO candidates.
This article is part of the Emerging concepts in heart failure management and treatment Special Issue: https://www.drugsincontext.com/special_issues/emerging-concepts-in-heart-failure-management-and-treatment
Keywords: cardiac arrest, cardiac arrhythmias, cardiogenic shock, extracorporeal membrane oxygenation, heart failure, heart transplantation, pulmonary embolism, myocardial infarction, myocarditis
Introduction
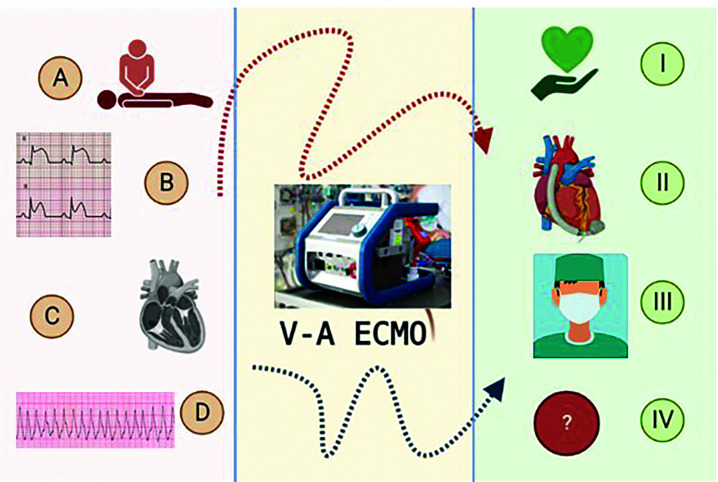
Extracorporeal membrane oxygenation (ECMO) devices were developed in the 1960s, primarily for use in cardiac surgery. Their initial use outside of the operating room was marked by many complications and technical problems, which dropped the initial enthusiasm toward more routine use.1 Over time, improvements in cannula design and coatings, the development of more efficient membranes, and refinement in implantation techniques gradually earned this technology its place in the intensive care unit, initially in paediatric patients. Good initial results in children motivated its use in adults, particularly patients with acute respiratory diseases.1 In the last 20 years, thanks to several encouraging results from case series and meta-analyses, the use of ECMO in cardiology has increased dramatically. Currently, the Society for Extracorporeal Life Support (ELSO) recommends its use for selected patients in cardiogenic shock (CS) or refractory cardiorespiratory arrest and for arrhythmic storm. The goal of treatment can be as a bridge to recovery, decision, transplantation or durable mechanical support2 (Figure 1).
Figure 1. Venoarterial ECMO as a bridge.
Left panel – Clinical scenarios: (A) In refractory cardiac arrest (extracorporeal cardiopulmonary resuscitation); (B) in cardiogenic shock associated with acute myocardial infarction; (C) in the setting of acutely decompensated chronic heart failure, myocarditis, etc.; or (D) in arrhythmic storm. Right panel – Goals of support: (I) RECOVERY; (II) durable left ventricular assist device; (III) cardiac transplantation, or (IV) undetermined (bridge to decision, candidacy).
The objectives of this review are to understand the basic functioning of a venoarterial ECMO (VA-ECMO), its indications, its haemodynamic impacts and the expected complications, and to provide an update on the new devices available and the foreseeable future for this type of technology.
Methods
We performed a literature search using the MeSH terms “extracorporeal membrane oxygenation” with the following terms: “heart failure”, “cardiac arrest”, “cardiogenic shock”, “heart transplantation”, “pulmonary embolism”, “myocarditis”, “cardiac arrhythmias” and “myocardial infarction”. We reviewed all articles published in the last 5 years as well as their references for relevance and duplication, leaving 75 articles. This review is based on these manuscripts, together with our clinical experience.
Review
The concepts of ECMO support
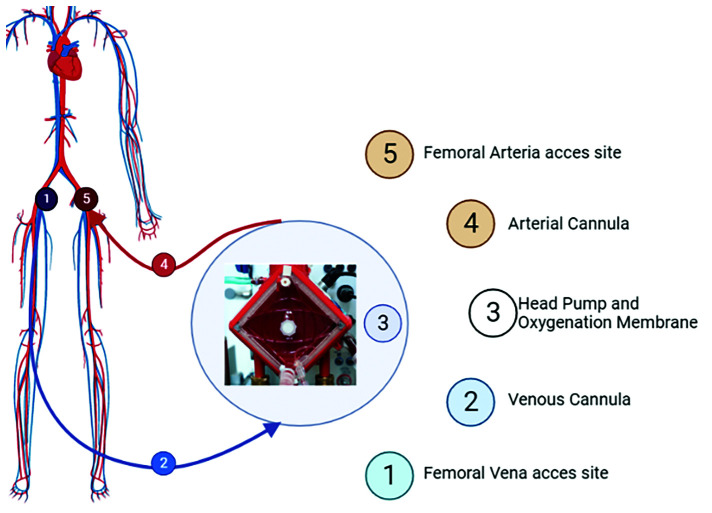
ECMO is a cardiopulmonary bypass machine that can be used as respiratory or cardiorespiratory support. Typically, it is composed of a centrifugal pump with the adjunct of a gas exchange system. The device should provide adequate cardiac output and gas exchange (oxygenation and CO2 extraction) to deliver nutriments and perfusion to the body. It can be implanted centrally, in the operating room or peripherally, usually via the femoral vasculature (Figure 2).
Figure 2. Peripheral VA-ECMO.
Extracorporeal membrane oxygenation (ECMO) is a cardiopulmonary bypass device that allows circulatory and respiratory supports. Using a centrifugal pump with a gas exchange system, venoarterial (VA)-ECMO permits adequate cardiac output, degree of oxygenation and CO2 extraction. The most frequently used access site is the femoral artery.
In its veno-venous configuration (VV-ECMO), the blood is drained from a large calibre vein (usually the femoral), pumped through an extracorporeal membrane responsible for gas exchange, and returned to the patient through another venous cannula, providing respiratory support. This modality is widely used in acute hypoxaemic and hypercapnic respiratory failure (e.g. viral lung diseases such as influenza, COVID-19). The factors determining the extent of oxygenation provided include the FiO2 selected, blood flow through the ECMO and the patient’s native lung function; the amount of CO2 extraction is inversely proportional to the gas flow through the membrane.3
In patients with CS, both circulatory support and adequate gas exchange are required. Consequently, blood is drained as for VV-ECMO support but is returned to the patient’s arterial system, typically retrograde through the femoral artery, a configuration called VA-ECMO. It can generate a cardiac output up to 4–6 l/min, providing adequate tissue perfusion in the absence of adequate biventricular function. In the VA-ECMO configuration, retrograde flow from the femoral artery increases aortic pressure and left ventricular (LV) afterload, which may worsen the native LV function of the already failing ventricle. In situations of cardiac dysfunction but with some residual contractility and coexisting respiratory failure, oxygenated blood from the membrane mixes with oxygen-poor blood ejecting from the LV. As a result, there may be a decrease in the oxygen supply to the proximal branches of the aortic arch and poor oxygenation may occur at the coronary and cerebral levels. This syndrome, characterized by differential hypoxaemia, is called the Harlequin syndrome and can lead to major neurological consequences, more pronounced left ventricular dysfunction and, therefore, ineffective ECMO support. Early diagnosis is key and arterial oxygen saturation in the right upper extremity and cerebral oxygenation should be monitored continuously; optimization of mechanical ventilation parameters may also be helpful. To overcome this situation, options include modifying the cannulas’ configuration for a venous-arterial-venous ECMO (triple cannulation strategy using an internal jugular vein or femoral vein) or venous-venous-arterial ECMO (in cases of right ventricular (RV) dysfunction), changing the arterial femoral inflow cannula toward an axillary return, decreasing ECMO flow, or converting to a central cannulation.4
Approach strategy and cannulation in VA-ECMO
The ideal cannulation should provide the simplest, least traumatic and most durable access for optimal support with reduced likelihood of complications, keeping in mind that the amount of blood flow through the ECMO depends more on the drainage from the venous cannula than on the size of the arterial cannula. Venous cannulation should be performed using the Seldinger technique with 19–25 Fr cannulas with peripheral access, either jugular or femoral.
The most frequently used return site is the common femoral artery because it does not require a surgical cut-down and has a low rate of bleeding complications. From this approach, a distal perfusion cannula is sited into the femoral superficial artery for distal perfusion, lowering the risk of lower ipsilateral extremity ischaemia. Axillar approach permits for greater mobilization, enhanced supra-aortic oxygenated flow, and less complications such as limb ischaemia and embolism.5
The choice of size for the arterial cannula depends on two aspects: the diameter of the artery and the flow required for circulatory support. They usually range from 15 to 23 Fr. The formula used to define its dimension is5: maximal size that will be accommodated by the vessel in Fr is equal to the arterial diameter in millimetres times three.
Types of pumps
There are two distinct types of pumping system:6 (1) roller pumps, which provide a servo-regulation that is safe, especially for prolonged use; however, they are generally larger, heavier and are more prone to mechanical failure. (2) Centrifugal pumps, in which the rotating pump head generates flow and pressure. They were initially associated with haemolysis, venous line thrombosis and generation of empty space caused by increased suction pressure. Limitations in the speed settings and improvements in the pumps and circuits of the newer generations have solved these problems in most cases. This type of ECMO is simple to assemble, lightweight and portable.
Miniaturized ECMO
Due to the wide availability of ECMO teams, many patients can now receive temporary circulatory support in community hospitals, or even in the field, and then be transferred to tertiary care centres for consideration of advanced therapies. In case of such emergencies, providing fast, easy, and safe support is essential to protect/recover end-organ function before CS progresses toward a cardio-metabolic one with multiorgan failure; ‘time to support’ has therefore become a key metric of success.7 Technological development led to the advent of such ‘miniaturized’ ECMO, which are simpler and more versatile than the traditional cardiopulmonary bypass machine, allowing patients to be quickly supported outside of the operating theatre (e.g. catheterization laboratory, cardiac intensive care units, emergency department, and even out-of-hospital cardiopulmonary resuscitation (CPR)).8–10
Cardiohelp TM (Maquet Cardiopulmonary AG, Hirrlingen, Germany)
Cardiohelp is the most widely used portable device. It consists of a series of disposable accessories that include percutaneous access cannulas (HLS© cannulas) of shorter length, specifically designed with depth markers to guide insertion, coated with a biocompatible surface to decrease the rate of bleeding complications.11 The kit also includes connectors, a Rotassist© centrifugal pump and an advance 7.0 gas exchanger. It weighs approximatively 10 kg, which makes it easily transportable and usable in many different settings. Other advantages include its high safety profile, intuitive touch screen with advanced options.12 This small, biocompatible circuit has been developed for shorter duration support and has been shown to be equally effective than its larger competitors during the first 24 hours of support, with efficient gas exchange and similar complication rates.10,13 In addition, it exhibited similar intermediate-term outcomes compared with other larger ECMO systems such as the Centrimag (Abbot), Rotaflow (Getinge), Dideco ECC.05 (Sorin) and the Deltastream system with Hilite 700 lp + DP3 pump head (Medos Medizintechnik AG).12
TandemLife (LivaNova, Houston Texas, USA)
TandemHeart is a percutaneous device combining a centrifugal pump and two cannulas; first, a suction cannula is positioned in the left atrium through a trans-septal puncture via a venous access; this oxygenated blood is drained by a 17-Fr diameter cannula to the TandemLife pump head and is returned to the femoral artery cannula (Figure 3). It was developed for the treatment of refractory CS, a scenario in which it has been shown to improve cardiac index and decrease pulmonary pressure but does not have any proven impact on mortality.14,15 It may also be used in presence of left-sided mechanical valves (aortic valve replacement/mitral valve replacement), when Impella is contraindicated.
Figure 3. Contemporary, commercially available percutaneous left ventricular (upper panel) and right ventricular (lower panel) mechanical support devices.
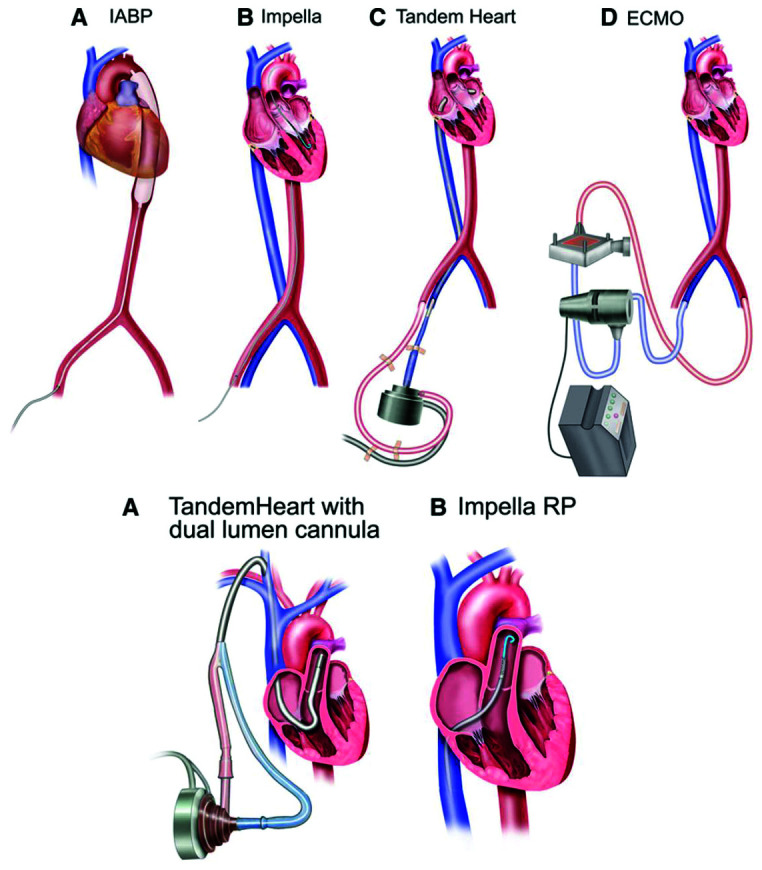
ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump.
Reproduced with permission from Mandawat A, Rao SV. Percutaneous mechanical circulatory support devices in cardiogenic shock. Circ Cardiovasc Interv. 2017;10:e004337. https://doi.org/10.1161/CIRCINTERVENTIONS.116.004337
Interestingly, it may be combined with an ECMO system (TandemLung Oxygenator), allowing for biventricular support and gas exchange without the need for a transseptal puncture. This system has a 0.9% saline reservoir that allows rapid priming of the pump by the nursing staff. A small study of five patients in CS after myocardial infarction (MI) supported by an intra- aortic balloon pump (IABP) demonstrated haemodynamic improvements, providing a cardiac support up to 4 l/minute but the mortality rate remained over 60%,15,16 emphasizing once again the importance of early mechanical support in these patients in CS, before the advent of cardiometabolic shock and irreversible multiorgan failure.
The device can also be configured for right ventricular support, using a ProtekDuo© cannula in the pulmonary artery: blood is extracted in the right atrium and ‘pushed’ into the pulmonary circulation. The main advantages of the TandemHeart reside in its rapidity of donning, the simplicity of the system, and its ability to increase cardiac output.17
Impella and Breethe (ABIOMED, Danvers, USA)
This is an axial flow device that unloads the LV and circulates the blood from the LV into the Aorta across the aortic valve, thus decreasing LV and pulmonary pressures whilst increasing cardiac output (Figure 3). Three versions are available: LP 2.5 L, CP 3.5 L and LP 5.0. The use of Impella© in CS has gained popularity in the last decade; initially used exclusively for LV failure, the advent of the Impella RP for RV support offers the possibility to support the RV alone or in a biventricular combination (called Bi-Pella). A case series of 20 patients using the Bi-Pella configuration showed the feasibility to improved cardiac output and decreased pulmonary artery pressure but the mortality remains high (50%). Factors associated with poor outcomes included higher doses of inotropes and lactate preimplantation and higher indices of RV elevated afterload such as higher pulmonary artery pressure (PAP), pulmonary vascular resistance (PVR), pulmonary artery (PA) compliance and PA elastance,18 all associated with delayed support, re-emphasizing the importance of minimizing the door-to-support interval. Other groups have reported improvements in haemodynamics and successful explant of the device.19,20
Breethe Oxy 1© is an extracorporeal circulatory pump with an oxygenation membrane meant to be combined with an Impella. It weighs 21 kg, has a user-friendly touch screen, improved biocompatibility and lower haemolysis index when compared with similar pumps such as RotaFlow and Centrimag.21
Strategies for LV unloading under ECMO support
Due to its intrinsic mode of action (retrograde flow into the arterial circulation), patients on ECMO support face a marked increase in LV afterload, with a reciprocal increase in left atrial pressure leading to pulmonary oedema, reduced native LV ejection, LV distension and/or LV thrombosis.22 Although ventricular function may improve under support, through correction of hypoxaemia, acidosis and improvement in coronary perfusion, it is often necessary to overcome the deleterious haemodynamic consequences of ECMO on the LV;23,24 two strategies – passive or active/invasive – have been proposed.25
Passive strategies
Inotropic agents
β-Adrenergic or phosphodiesterase inhibitors can improve myocardial contractility and alleviate at least partially the increased LV wall stress induced by ECMO. They should nevertheless be used with caution, as they can contribute to increased oxygen consumption and cause arrhythmias.
Vasodilation and establishing an euvolaemic state
Peripheral vascular resistance can be reduced with vasodilators, such as nitroprusside, whilst euvolaemia can be achieved with diuretics and/or continuous haemofiltration, or haemodialysis in case of acute kidney injury, which is commonly encountered in situations requiring ECMO.
High PEEP ventilation
Positive end-expiratory pressure (PEEP) over 10 cmH2O is suggested to prevent ventilation/perfusion mismatch due to atelectasis, especially in presence of lower tidal volumes, keeping in mind the potential deleterious haemodynamic impacts of high levels of PEEP. Indeed, increasing intra-thoracic pressure and pulmonary vascular resistances in patients with RV dysfunction can further decrease the cardiac output. Nonetheless, in patients with predominantly LV failure, high levels of PEEP reduce trans-mural left ventricular gradient and improves LV ejection, which is helpful in presence of pulmonary oedema.26 The ELSO recommend PEEP levels between 5 and 15 cmH2O, individualized according to each patient’s circulatory characteristics.27
Active/invasive strategies
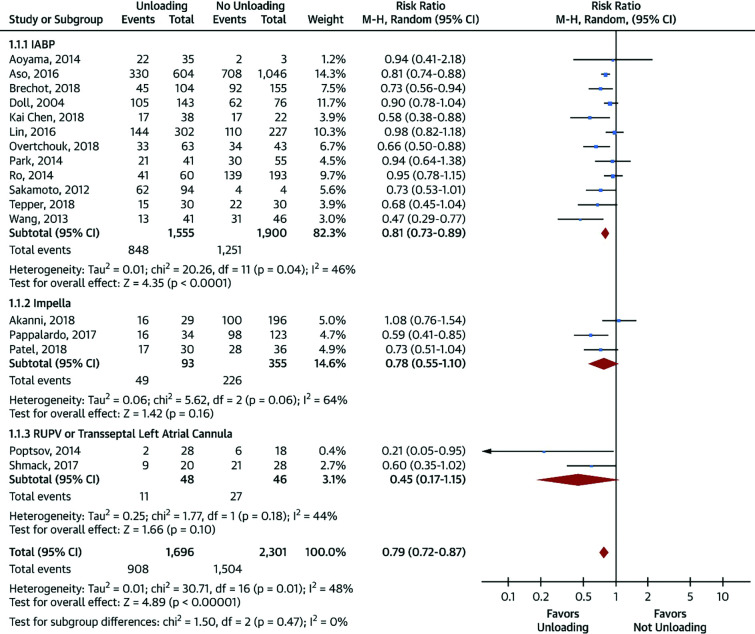
We will briefly describe the most common unloading strategies used in clinical practice. A thorough review of these strategies can be found elsewhere.24 A comparison of the different unloading strategies is described in Figure 4.
Figure 4. Meta-analysis of mortality in patients treated with venoarterial extracorporeal membrane oxygenation (VA-ECMO) with versus without left ventricular unloading.
Reproduced with permission from Russo JJ, Aleksova N, Pitcher I, et al. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol. 2019;73(6):654–662. https://doi.org/10.1016/j.jacc.2018.10.085
Intra-aortic balloon pump
The use of an IABP may unload the LV under ECMO support by both decreasing the LV end-diastolic pressure and improving coronary perfusion. Whilst this combination has been initially controversial, its advantages in terms of haemodynamic improvement, weaning rate and survival has been demonstrated.28 The optimal candidates for such a strategy seems to be patients in CS post-cardiotomy or after MI, potentially because of improved coronary perfusion.29
Impella
This continuous flow device decreases LV and pulmonary pressures and improves cardiac output, thereby providing better LV unloading and coronary perfusion than IABP, even in presence of arrhythmias. This strategy has been shown in a multicentre cohort study of 686 patients to improve survival in comparison with VA-ECMO alone but was associated with higher rate of complications.30 The main limitations are cost and the impossibility to be used in case of severe aortic regurgitation or aortic valve stenosis/calcification.
Central decompression cannula
LV decompression can also be achieved by surgically inserting a cannula into the pulmonary artery, left atrium, or LV to directly drain blood into the ECMO circuit and reduce LV diastolic pressure. It may therefore useful for patients with post-cardiotomy shock on central ECMO but can also be performed less invasively using an anterior mini-thoracotomy for LA drainage or a transapical approach to directly drain the LV.31 Implanting a ‘central’ venous cannula into the left atrium or pulmonary artery to drain into the ECMO circuit can also be performed percutaneously.32 This convenient approach is associated with improved survival compared to VA-ECMO alone.33
Interatrial septostomy
Finally, another alternative approach would be the creation of an atrial septal defect to unload the left atrium (LA); the consequential increased blood flow on the right-side caused by the generation of this left-to-right shunt can then be drained in the ECMO circuit by the venous cannula present in the RA. This approach is effective for LA decompression, leads to LV improvement, and is associated with reduced need for inotropes and higher rates of successful ECMO weaning.34
The choice of one decompression modality over the other depends on the cardiovascular substrate, the possibility of myocardial recovery and patient characteristics (such as RV function, valvular disease, or presence of mechanical valves), together with local expertise, availability and cost.
In a meta-analysis of 3997 patients (Figure 4),24 the use of ventricular unloading strategies (IABP, percutaneous mechanical circulatory support (MCS) or interatrial septostomy) compared with VA-ECMO alone was associated with lower mortality (RR 0.79; 95% CI 0.72–0.87; p<0.00001), without any statistically significant differences in terms of bleeding, limb ischaemia, renal replacement therapy, stroke, or multiorgan failure. Unfortunately, important information related to mortality that may confound these findings, such as the aetiology of CS or time to support, are not provided in the publication. However, there was a higher level of haemolysis when LV unloading strategies were used (RR 2.15; 95% CI 1.49–3.11; p<0.0001).
ECMO in frequent scenarios
The different clinical scenarios are illustrated in Figure 1.
Refractory cardiac arrest
The use of ECMO in the context of refractory cardiorespiratory arrest (CA) is called extracorporeal CPR (ECPR) and provides circulatory support in the absence of return of spontaneous circulation (ROSC) during CPR manoeuvres. Most of the current evidence comparing the standard approach with ECPR comes from single-centre retrospective studies, prospective observational registries and meta-analyses of case series.
Data from in-hospital CA are compelling, with clear benefits of ECMO35,36 in terms of survival rate to discharge versus usual CPR (HR 0.51; 95% CI 0.35–0.74; p<0.0001), 30 days (HR 0.47; 95% CI 0.28–0.77; p=0.003) and 1 year survival (HR 0.53; 95% CI 0.33–0.83; p=0.006).35
One meta-analysis of 3098 patients (708 ECPR versus 2390 CPR)37 showed an absolute increase of 30-day survival of 13% with the use of ECPR (95% CI 6–20; p<0.001) compared with patients in which extracorporeal life support was not used (number needed to treat (NNT) 7.7) and a higher rate of favourable neurological outcome at 30 days (absolute risk difference 14%; 95% CI 7–20%; p<0.0001; NNT 7.1). The overall long-term survival rate was low in this refractory CA cohort but markedly better in the ECPR group (28.7% versus 15.9%; absolute difference 15%; 95% CI 11–20%; p<0.0001; NNT 6.7). Patients in the ECPR group were younger, more likely to have had an acute MI and undergo percutaneous coronary intervention (PCI); nonetheless, propensity-matched analysis, including 5 studies and 438 patients (219 in both groups), showed similar results. Neurological consequences according to both the Pittsburgh and modified Glasgow scales also favoured ECPR, with fewer sequelae.37 These retrospective analyses have obviously many biases but suggest that ECPR during refractory CA should be considered in carefully selected patients.
Out-of-hospital CA (OHCA) is usually associated with delayed initiation of CPR and prolonged resuscitation efforts and, therefore, benefit of ECPR is less clear. Initial reports showed similar low survival (15%) and higher rates of neurological and device-related complications compared with conventional CPR.38 More recently, registry data failed to show any difference in survival (ECPR 8.4% versus CRP 8.6%; p=0.41) with propensity-matched scores. Nevertheless, improved survival was seen in those with an initial shockable rhythm, transient ROSC during resuscitation and ECMO implantation before hospitalization,39 highlighting the even more crucial role of OHCA patient selection for ECPR candidacy.
There are few prospective studies in this situation. In a single-centre, open-label, randomized trial of 30 patients with OHCA and refractory ventricular fibrillation (without ROSC after three shocks), on automated CPR and with an estimated transfer time shorter than 30 minutes within the Lund University Cardiac Arrest System, the use of ECPR was associated with an absolute increase in survival to hospital discharge of 36% compared with CRP only (43% and 7%, respectively), with a posteriori probability of ECMO superiority of 0.9861. The study was stopped prematurely by ethic committee due to the excess benefits of an early ECMO strategy.40
Another single-centre randomized trial of 256 OHCA patients showed a trend toward improved survival with minimal or no neurological impairment at 180 days (31.5% and 22.0% for ECPR and CPR, respectively, absolute difference of +9.5%, 95% CI –1.3 to 20.1; p=0.09) and similar trend in the rate of cardiac recovery at 30 days (43.5% and 34.1% for ECPR and CPR, respectively; absolute difference 9.4%, 95% CI −2.5 to 21; p=0.12).41
ECPR provides robust circulatory support, rapid hypothermia and neurological protection that facilitates procedures, such as primary angioplasty or pulmonary thrombectomy, to treat the initial cause of CA.35,36 Giving priority to prompt mechanical circulatory support to improve tissue perfusion and halt the progression toward a cardio-metabolic shock, over restoring the coronary circulation, requires a change in mindset, especially in ST elevation myocardial infarction-associated shock and CA but is mandatory to improve outcomes.
Criteria associated with improved prognosis with ECPR include patients who presented refractory CA for more than 10 minutes despite the use of CPR with no-flow times less than 5 minutes; prompt ECMO initiation; and short implantation time, defined as the arrival of the implant team in less than 10 minutes during the day and up to 30 minutes at night, with a short cannulation time between 10 and 15 minutes.1,35,36
A recent report from the Canadian Cardiovascular Society revealed that most of cardiac transplantation centres have developed detailed ECMO protocols, which is not the case in hospitals with only vascular surgery on-site. The identification of their key elements would be essential in the development of practice manuals to contribute to a standardized national protocol of extracorporeal life support for optimal patient care.42 Such criteria for consideration of ECPR candidacy are presented in Box 1.
Box 1. Suggested criteria for extracorporeal CPR candidacy consideration .
Age under 65 years
Shockable initial rhythm
Witnessed cardiac arrest
Cardiopulmonary resuscitation (CPR) initiation in less than 5 minutes
No return of spontaneous circulation after 15 minutes of regular CPR
Serum lactates below 12 mmol/l
Reproduced with permission from Fagan A, Grunau B, Caddell A, et al. CEPP: Canadian Extracorporeal Life Support (ECLS) protocol project. CJC Open. 2022;4(6): 520–531. https://doi.org/10.1016/j.cjco.2022.02.005
ECMO in CS
CS is a critical haemodynamic situation characterized by hypoperfusion and signs of tissue damage, due to acute ischaemic or non-ischaemic event or disease progression, with or without a history of chronic heart failure. It is associated with a very high but variable mortality depending on its aetiology and definition. In order to harmonize definitions and compare outcomes, the Society for Cardiac Angiography (SCAI) has developed a common classification.43
The paucity of good quality randomized-controlled trials is noteworthy. For example, the classic pillars of pharmacological therapies (inotropes, vasopressors) are associated with poor survival and controversial evidence at best and they have been associated with increased oxygen demand, myocardial ischaemia, arrhythmias and, in certain circumstances, higher mortality.37 Similar controversy still existed with the use of IABP,38 suggesting that re-establishing circulation should be performed early to avoid the evolution from a purely circulatory problem toward a multisystemic inflammatory process or cardio-metabolic shock. The concept of ‘door-to-balloon’, which allowed a change in mortality in MI, has been translated to a ‘door-to-support’ concept in patients with CS as no device-based strategy has improved survival without changing the system of referral.7,44
ECMO can be use in transition from the SCAI shock stage C (Classic CS) to D (Deteriorating) and even in stage E (Extremis).43 The selection of candidates for support, the timing and the choice of devices in CS are subjects of heated debates. Early mechanical circulatory support implies assuming possible unnecessary complications. On the other hand, in patients with signs of irreversible multiorgan dysfunction, ECMO may be futile.37,43 The underlying diagnosis and individual risks must also be considered to plan the trajectory of care: is there a long term or ‘B’ plan such as cardiac transplantation, durable ventricular assist device, arrhythmia ablation (ventricular tachycardia, treatment of atrial fibrillation addressing the pulmonary veins or the auriculo-ventricular junction), or is the goal recovery? Hence, anticipating the duration of support and the type of strategy are essential in the thinking process for ECMO use as part of a global bundle of care, with early involvement of the cardiac transplantation team. Use of temporary MCS does not save lives per se but a timely and appropriate use of support can.
Initiation of MCS in CS should be performed after a short (undefined) course of inotropes. The choice of device may vary according to individual sites, experience and availability but ECMO should usually be preferred in presence of biventricular dysfunction, as defined by Cardiac Index lower than 2.2 l/min/m2, Wedge over 15 mmHg, right atrial pressure-to-Wedge ratio over 0.8, pulmonary artery pulse index <1.85, and cardiac power output lower than 0.6 and/or the presence refractory ventricular arrhythmias (ventricular tachycardia or ventricular fibrillation). Biochemical criteria45,46 include serum lactates >5 mmol (with an upper limit of 12 mmol/l) and/or the incapacity to clear the lactates, pH <7.20, mixed venous saturation <50%, central venous oxygen saturation (ScVO2) <55%, hypoxia/hypercapnia and >50% drop in glomerular filtration rate.43
CS as a complication of MI
CS is the leading cause of death in patients with MI. Whilst the mortality in all-comers MI is below 5%, overall mortality in MI-associated CS (MI-CS) remains high, around 40–70%, and has not changed in the last two decades despite treatment with inotropes, vasopressors and diuretics.47
The use of IABP has been the mainstay of MCS in MI-CS for over two decades but its use declined after the IABP shock II study showed no improvement in 30-day survival.47–49 The use of Impella 2.5 has shown interesting initial results. In the USPella study, patients in CS had higher survival to discharge (65.1% versus 40.7%, p=0.003) if the Impella was implanted before PCI.50 The National CS initiative51 was a single-arm prospective study using the same inclusion criteria as the IABP SHOCK trial and evaluated the use of Impella (mostly CP Impella) in the early stages of MI-CS (pre-PCI), and showed a survival rate of 72%, a dramatic improvement over the results of the previous studies such as SHOCK (53%),47 IABP SHOCK (60%)48 and Culprit SHOCK (49%).52
Currently, no randomized-controlled study has been published on ECMO use in MI-CS. A meta-analysis of the International Society of Heart and Lung Transplantation (ISHLT), including 80 studies and 7774 patients undergoing ECMO support for AMI-CS, showed a 30-day mortality rate of 60%.53 Many factors may account for this poor prognosis as mortality in AMI-CS does not depend solely on the establishment of mechanical circulatory assistance. Predictors of poor outcomes include advanced age, sub-optimal angioplasty results (TIMI 2 flow), presence of profound shock (SCAI stage E) at the time of implant, end-stage heart failure, CPR secondary to asystole and late timing of support. All these factors illustrated very high-risk patients for whom decision to undergo temporary MCS or not should be taken promptly, on an individual basis by a Heart team.
CS resulting from myocarditis
Patients who develop refractory CS as a complication of myocarditis may benefit from early temporary MCS and ECMO (in cases needing biventricular support) as a bridge to recovery, left ventricular assist device (LVAD) or transplantation. Transfer to a transplantation centre should be considered early on for those eligible. The advantages of this strategy over the primary implant of a durable LVAD are two-fold. First, it allows for the possibility of myocardial recovery before deciding on durable support. Second, even if the patient becomes an LVAD candidate, the implantation will be performed with much lower surgical risk in INTERMACS 3, stable on inotropes, versus the crash and burn INTERMACS 1 profile.54 Recovery rates of ventricular function with ECMO have been reported to be between 60% and 80%,53 depending on the aetiology (higher for lymphocytic myocarditis, lower for giant cells myocarditis or sarcoidosis in the absence of specific treatment, with the lowest for acute decompensation of chronic myocarditis). In multicentre studies, a survival rate between 57% and 66% has been demonstrated with the use of ECMO in CS associated with myocarditis.55
CS in transplantation candidates
ECMO has the advantages of being mobile, easily deployable and suitable for emergencies. In patients with end-stage heart disease, it may serve as a bridge to definitive therapies. Although durable LVADs are currently the most widely used option as bridge to transplantation in the United States, the results of patients with INTERMACS I profile are poor.54 Hence, temporary MCS should be used to stabilize the patient first, allowing recovery from end-organ dysfunction but also triaging between patients at high risk of dying and survivors, who will then undergo durable LVAD implantation at a much lower risk, a strategy called bridge-to-bridge. This explains the higher short-term and medium-term mortalities in patients undergoing ECMO compared with those with durable LVAD.56,57
UNOS changed its priority system for organ allocation in 2018 and gave patients undergoing ECMO the highest priority for cardiac transplantation.56 This has translated in an increase in VA-ECMO implantation (14 patients in 2010 and 107 in 2020), a lower waitlist time for those supported (5 days versus 31 days; p<0.001) and higher incidence of transplantation (81.5% versus 43% at 1 year; p<0.001). Consequently, as the new system is a faster pathway to transplantation, a lower incidence of cardiac recovery was described (1.5% versus 7.9% at 1 year; p<0.001).58
CS after cardiac surgery
Primary graft dysfunction
is one of the major causes of death after heart transplantation and occurs in up to 24% of patients. The use of ECMO in this situation is associated with a weaning rate of 68%, hospital discharge of 50% but long-term survival thereafter similar to the patients with normal cardiac function after transplantation.59
Post-cardiotomy shock
remains the main indication of VA-ECMO, mostly by failure to wean-off from cardiopulmonary bypass after cardiac surgery, in the presence of LV, biventricular or respiratory failure. Complications of ECMO in this setting are higher than for other situations, with 30% of patients experiencing neurological complications, bleeding rate of 90% and variable survival, ranging from 16% to 52%;60 the reported mortality was 15% in a publication from the ELSO registry in 2017.61 Patients undergoing coronary revascularization combined with aortic valve replacement seem to be more at risk, possibly due to the combination of a hypertrophic myocardium coupled with underlying ischaemia in these patients.62
Drug-induced CS
Many medications can lead to severe cardiac injury/depression causing drug-induced CS. In this setting, ECMO can provide circulatory and respiratory support but not toxin removal per se. According to a retrospective analysis of 104 adults from the ELSO registry, the most frequent classes of drugs involved are cardiovascular (47%), opioids (6.7%), cocaine (4%) and antidepressants (4%), with various cardiac depressor agents accounting for another 23%. Survival to discharge was 52.9%, with a median duration of VA-ECMO of 68 hours (IQR 48–113). Non-survivors showed persistent acidosis at 24-hours after VA-ECMO cannulation compared to survivors both in terms of lower pH (pH 7.42 (IQR 7.35–7.46) versus 7.30 (7.21–7.44); p=0.003) and bicarbonate levels (HCO3: 24 mmol/L (IQR 20–26) versus 20 (IQR 16–24); p=0.005). Renal replacement therapy (50.9%) and arrhythmia (26.3%) were the most frequently reported complications.63
Pulmonary embolism
A massive pulmonary embolism (PE) is defined by circulatory collapse, RV dysfunction and hypoxaemia. VA-ECMO can provide haemodynamic support by off-loading the RV and providing cardiopulmonary bypass, which then facilitates mechanical or pharmacological thrombolysis. Although there are no prospective, multicentre studies, many publications showed improved survival rate (though variable) with the use of ECMO.64–66
By contrast, a recent meta-analysis of 1138 patients receiving VA-ECMO support compared with a control group of 809 patients showed no differences in short-term survival (RR 0.91, 95% CI 0.71–1.16).67 Risk factors for a lesser survival were age >60 years (RR 0.72, 95% CI 0.52–0.99; p<0.05) and the presence of CA before or during VA-ECMO initiation (RR 0.88, 95% CI 0.77–1.01; p=0.06), whilst the use of surgical embolectomy was associated with improved survival (RR 1.96, 95%CI 1.39–2.76; p<0.01). Although CA due to a massive PE is a very extreme entity, with a survival rate of only 34%, it remains higher than previously reported with the use of thrombolysis only (16%).68 Therefore, the current ESC guidelines (2019) recommend consideration for ECMO in patients with PE and refractory circulatory collapse or cardiac arrest, in association with surgical or catheter-guided embolectomy (class IIb, level of evidence C).69
Patients with PE-associated shock supported by ECMO have higher rate of complications and increased bleeding risk, especially if previously treated with thrombolytics. On the other hand, anticoagulation may facilitate reperfusion in the pulmonary circulation and reduce the incidence of long-term pulmonary arterial hypertension, therefore potentially reducing the need for embolectomy and its potential associated supplementary risk.70
ECMO complications
Arterial dissection, pseudoaneurysm and retroperitoneal bleeding are the most common vascular complications related to cannulation and occur in 7–14% of patients. The treatment of arterial dissection depends on the presence of signs of ischaemia and varies from conservative strategies to the placement of a stent and replacing the arterial cannula into another access. Large pseudoaneurysms and those associated with an arteriovenous communication need chirurgical intervention. Retroperitoneal bleeding can occur in the presence of anticoagulation, even with minimal vascular injuries; a decreasing haematocrit, increasing lactates level and haemodynamic deterioration are clues suggestive of this condition. A CT scan should be performed to confirm the diagnosis, with management including transfusion, reversal of anticoagulation and, in refractory cases, endovascular embolization.71,72 On the other hand, the presence of thrombi within the circuit is associated with stroke and poor neurological outcomes.
The potential benefits of ECMO must always be weighed against its possible complications. The balance between thrombosis and anticoagulation is possibly the most delicate issue during ECMO support. New types of coated cannulas allow for full reversal of anticoagulation after cardiac surgery, without requirement for intravenous heparin therapy for up to 24 hours. Whilst anticoagulation would be required for longer-term support, the optimal agent and dosage have not yet been determined; targeting a partial thromboplastin time between 70 and 90 seconds73 has been associated with fewer haemorrhagic complications but there is currently no standardized protocol. Finally, ECMO support is associated with many other complications. The types and rates of these complications in the 14,580 patients included in the ELSO registry have been reported (Table 1).74
Table 1.
ECMO complications.
| ECMO complications | Rate (%) |
|---|---|
| Circuit components clots | 9.2 |
| Haemolysis | 3.4 |
| Cannulation site bleeding | 12.5 |
| Surgical site bleeding | 14 |
| Cardiac tamponade | 4.4 |
| Pulmonary haemorrhage | 2.3 |
| Central nervous system haemorrhage | 1.4 |
| CNS infarction | 3.5 |
| Renal replacement therapy required | 29.6 |
| Culture-proven infection | 7.6 |
| Limb ischemia | 5.3 |
| Limb amputation | 0.7 |
Reproduced with permission from Lorusso R, Shekar K, MacLaren G, et al. ELSO interim guidelines for venoarterial extracorporeal membrane oxygenation in adult cardiac patients. ASAIO J. 2021;67(8):827–844. https://doi.org/10.1097/MAT.0000000000001510
Conclusions
VA-ECMO as a mechanical circulatory support coupled with an oxygenation membrane is a versatile tool for critical patients. It is mobile, easily deployable and associated with a rapid haemodynamic response. Complications are frequent, so careful selection of patients is necessary to avoid indiscriminate and futile use.
ECMO can provide adequate organ and tissue perfusion in the presence of inadequate native cardiac output but does not by itself improve ventricular function and may even decrease it, by increasing the afterload to a failing LV. The outcomes vary according to the characteristics of the patient, the cardiovascular substrate, the possibility of recovery, the supportive treatments, the metabolic factors present at the time of implantation and the impact on circulation during circulatory support. The most important factor for good outcomes is the interval of time spent in CS before MCS support.
Whilst the optimal door-to-support time remains to be defined, applying clinical judgement according to the different scenarios herein presented might be useful. The candidates for ECMO support consideration are extremely sick and MCS is often their last option, despite the low survival rate. A multidisciplinary approach with early involvement of the heart team, including cardiac transplantation, development of protocols and strict selection of patients is the mainstay of a successful CS programme, evaluating all therapeutic options (including MCS) according to the patient’s specificity, goals and preferences (when available).
New smaller devices have revolutionized the way of ECMO is deployed. With percutaneous approaches, user-friendly software and new circuits, the use of this strategy is becoming less complex and safer. The evidence supporting the use of ECMO comes mostly from retrospective, single-centre, or meta-analysis studies. More prospective, randomized studies are necessary to better understand its use in different situations, accepting that they would be difficult to conduct given the absence of alternatives for physicians facing acutely and severely ill patients.
Acknowledgements
None.
Footnotes
Contributions: FS prepared the manuscript. AB and AD guided the orientation of the manuscript. AB, SVP, EK and AD reviewed the manuscript. AD and EK made reviews and editions related to language and writing. All authors take responsibility for the integrity of the work as a whole and have given their approval for this version to be published. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2022/12/dic.2022-7-7-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2023 Swedzky F, Barbagelata A, Perrone S, Kaplinsky E, Ducharme A. https://doi.org/10.7573/dic.2022-7-7. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Funding declaration: There was no funding associated with the preparation of this article.
References
- 1.Guglin M, Zucker MJ, Bazan VM, et al. Venoarterial ECMO for adults. J Am Coll Cardiol. 2019;73(6):698–716. doi: 10.1016/j.jacc.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 2.Richardson AC, Tonna JE, Nanjayya V, et al. Extracorporeal cardiopulmonary resuscitation in adults. Interim guideline consensus statement from the extracorporeal life support organization. ASAIO J. 2021;67(3):221–228. doi: 10.1097/MAT.0000000000001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol. 2014;63(25):2769–2778. doi: 10.1016/j.jacc.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 4.Zarragoikoetxea I, Pajares A, Moreno I, et al. Documento de consenso SEDAR/SECCE sobre el manejo de ECMO. Cir Cardiovasc. 2021;28(6):332–352. doi: 10.1016/j.circv.2021.06.006. [DOI] [Google Scholar]
- 5.Pavlushkov E, Berman M, Valchanov K. Cannulation techniques for extracorporeal life support. Ann Transl Med. 2017;5(4):70. doi: 10.21037/atm.2016.11.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lequier L, Horton SB, McMullan DM, Bartlett RH. Extracorporeal membrane oxygenation circuitry. Pediatr Crit Care Med. 2013;14:S7–S12. doi: 10.1097/PCC.0b013e318292dd10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapur NK, Davila CD. Timing, timing, timing: the emerging concept of the ‘door to support’ time for cardiogenic shock. Eur Heart J. 2017;38(47):3532–3534. doi: 10.1093/eurheartj/ehx406. [DOI] [PubMed] [Google Scholar]
- 8.Merkle J, Djorjevic I, Sabashnikov A, et al. Mobile ECMO – A divine technology or bridge to nowhere? Expert Rev Med Devices. 2017;14(10):821–831. doi: 10.1080/17434440.2017.1376583. [DOI] [PubMed] [Google Scholar]
- 9.Brasseur A, Scolletta S, Lorusso R, Taccone FS. Hybrid extracorporeal membrane oxygenation. J Thorac Dis. 2018;10(S5):S707–S715. doi: 10.21037/jtd.2018.03.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arlt M, Philipp A, Voelkel S, et al. Hand-held minimised extracorporeal membrane oxygenation: a new bridge to recovery in patients with out-of-centre cardiogenic shock. Eur J Cardiothorac Surg. 2011;40(3):689–694. doi: 10.1016/j.ejcts.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 11.Martucci G, Panarello G, Occhipinti G, et al. Impact of cannula design on packed red blood cell transfusions: technical advancement to improve outcomes in extracorporeal membrane oxygenation. J Thorac Dis. 2018;10(10):5813–5821. doi: 10.21037/jtd.2018.09.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alwardt CM, Wilson DS, Alore ML, Lanza LA, Devaleria PA, Pajaro OE. Performance and safety of an integrated portable extracorporeal life support system for adults. J Extra Corpor Technol. 2015;47(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- 13.Palanzo DA, El-Banayosy A, Stephenson E, Brehm C, Kunselman A, Pae WE. Comparison of hemolysis between CentriMag and RotaFlow rotary blood pumps during extracorporeal membrane oxygenation: CentriMag and ROTAFLOW rotary blood pumps. Artif Organs. 2013;37(9):E162–E166. doi: 10.1111/aor.12158. [DOI] [PubMed] [Google Scholar]
- 14.Herlihy JP, Loyalka P, Jayaraman G, Kar B, Gregoric ID. Extracorporeal membrane oxygenation using the TandemHeart System’s catheters. Tex Heart Inst J. 2009;36(4):337–341. [PMC free article] [PubMed] [Google Scholar]
- 15.Li YW, Rosenblum WD, Gass AL, Weiss MB, Aronow WS. Combination use of a TandemHeart with an extracorporeal oxygenator in the treatment of five patients with refractory cardiogenic shock after acute myocardial infarction. Am J Ther. 2013;20(2):213–218. doi: 10.1097/MJT.0b013e3182068db7. [DOI] [PubMed] [Google Scholar]
- 16.Vijayakumar N, Badheka A, Chegondi M, Mclennan D. Successful use of Protek Duo cannula to provide veno-venous extra-corporeal membrane oxygenation and right ventricular support for acute respiratory distress syndrome in an adolescent with complex congenital heart disease. Perfusion. 2021;36(2):200–203. doi: 10.1177/0267659120923880. [DOI] [PubMed] [Google Scholar]
- 17.Ravichandran AK, Baran DA, Stelling K, Cowger JA, Salerno CT. Outcomes with the Tandem Protek Duo dual-lumen percutaneous right ventricular assist device. ASAIO J. 2018;64(4):570–572. doi: 10.1097/MAT.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 18.Kuchibhotla S, Esposito ML, Breton C, et al. Acute biventricular mechanical circulatory support for cardiogenic shock. J Am Heart Assoc. 2017;6(10):e006670. doi: 10.1161/JAHA.117.006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu CY, Hättasch R, Praeger D, et al. Percutaneous biventricular Impella support in therapy-refractory cardiogenic shock. Heart Lung. 2018;47(3):250–252. doi: 10.1016/j.hrtlng.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Puerto E, Martín-Asenjo R, Maruri R, Domínguez-Pérez L, Arribas Ynsaurriaga F, Bueno H. First experience of percutaneous Bi-Pella in Spain. Rev Esp Cardiol Engl Ed. 2021;74(8):719–721. doi: 10.1016/j.rec.2021.01.015. [DOI] [PubMed] [Google Scholar]
- 21.He G, Zhang J, Shah A, et al. Flow characteristics and hemolytic performance of the new Breethe centrifugal blood pump in comparison with the CentriMag and Rotaflow pumps. Int J Artif Organs. 2021;44(11):829–837. doi: 10.1177/03913988211041635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of mechanical circulatory support. J Am Coll Cardiol. 2015;66(23):2663–2674. doi: 10.1016/j.jacc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Yan S, Cai L, Zhang Q. Does VA-ECMO plus impella work in refractory cardiogenic shock? JACC Heart Fail. 2019;7(4):364. doi: 10.1016/j.jchf.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Russo JJ, Aleksova N, Pitcher I, et al. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol. 2019;73(6):654–662. doi: 10.1016/j.jacc.2018.10.085. [DOI] [PubMed] [Google Scholar]
- 25.Ricarte Bratti JP, Cavayas YA, Noly PE, Serri K, Lamarche Y. Modalities of left ventricle decompression during VA-ECMO therapy. Membranes. 2021;11(3):209. doi: 10.3390/membranes11030209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt M, Pellegrino V, Combes A, Scheinkestel C, Cooper D, Hodgson C. Mechanical ventilation during extracorporeal membrane oxygenation. Crit Care. 2014;18(1):203. doi: 10.1186/cc13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Extracorporeal Life Support Organization. ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support Version 1.4. Ann Arbor, MI, USA: Aug, 2017. [Accessed September 1, 2022]. https://www.elso.org/portals/0/elso%20guidelines%20general%20all%20ecls%20version%201_4.pdf . [Google Scholar]
- 28.Kowalewski M, Malvindi PG, Zieliński K, et al. Left ventricle unloading with veno-arterial extracorporeal membrane oxygenation for cardiogenic shock. Systematic review and meta-analysis. J Clin Med. 2020;9(4):1039. doi: 10.3390/jcm9041039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madershahian N, Wippermann J, Liakopoulos O, et al. The acute effect of IABP-induced pulsatility on coronary vascular resistance and graft flow in critical ill patients during ECMO. J Cardiovasc Surg. 2011;52(3):411–418. [PubMed] [Google Scholar]
- 30.Schrage B, Becher PM, Bernhardt A, et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter Cohort study. Circulation. 2020;142(22):2095–2106. doi: 10.1161/CIRCULATIONAHA.120.048792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weymann A, Schmack B, Sabashnikov A, et al. Central extracorporeal life support with left ventricular decompression for the treatment of refractory cardiogenic shock and lung failure. J Cardiothorac Surg. 2014;9(1):60. doi: 10.1186/1749-8090-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avalli L, Maggioni E, Sangalli F, Favini G, Formica F, Fumagalli R. Percutaneous left-heart decompression during extracorporeal membrane oxygenation: an alternative to surgical and transeptal venting in adult patients. ASAIO J. 2011;57(1):38–40. doi: 10.1097/MAT.0b013e3181fe5d0b. [DOI] [PubMed] [Google Scholar]
- 33.Alhussein M, Osten M, Horlick E, et al. Percutaneous left atrial decompression in adults with refractory cardiogenic shock supported with veno-arterial extracorporeal membrane oxygenation. J Card Surg. 2017;32(6):396–401. doi: 10.1111/jocs.13146. [DOI] [PubMed] [Google Scholar]
- 34.Alhussein M, Osten M, Horlick E, et al. Percutaneous left atrial decompression in adults with refractory cardiogenic shock supported with veno-arterial extracorporeal membrane oxygenation. J Card Surg. 2017;32(6):396–401. doi: 10.1111/jocs.13146. [DOI] [PubMed] [Google Scholar]
- 35.Chen YS, Lin JW, Yu HY, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet. 2008;372(9638):554–561. doi: 10.1016/S0140-6736(08)60958-7. [DOI] [PubMed] [Google Scholar]
- 36.Shin TG, Choi JH, Jo IJ, et al. Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: a comparison with conventional cardiopulmonary resuscitation. Crit Care Med. 2011;39(1):1–7. doi: 10.1097/CCM.0b013e3181feb339. [DOI] [PubMed] [Google Scholar]
- 37.Ouweneel DM, Schotborgh JV, Limpens J, et al. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med. 2016;42(12):1922–1934. doi: 10.1007/s00134-016-4536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson NJ, Acker M, Hsu CH, et al. Extracorporeal life support as rescue strategy for out-of-hospital and emergency department cardiac arrest. Resuscitation. 2014;85(11):1527–1532. doi: 10.1016/j.resuscitation.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 39.Bougouin W, Dumas F, Lamhaut L, et al. Extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: a registry study. Eur Heart J. 2020;41(21):1961–1971. doi: 10.1093/eurheartj/ehz753. [DOI] [PubMed] [Google Scholar]
- 40.Yannopoulos D, Bartos J, Raveendran G, et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet. 2020;396(10265):1807–1816. doi: 10.1016/S0140-6736(20)32338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belohlavek J, Smalcova J, Rob D, et al. Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2022;327(8):737. doi: 10.1001/jama.2022.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fagan A, Grunau B, Caddell A, et al. CEPP: Canadian Extracorporeal Life Support (ECLS) protocol project. CJC Open. 2022;4(6):520–531. doi: 10.1016/j.cjco.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94(1):29–37. doi: 10.1002/ccd.28329. [DOI] [PubMed] [Google Scholar]
- 44.Esposito ML, Kapur NK. Acute mechanical circulatory support for cardiogenic shock: the ‘door to support’ time. F1000Research. 2017;6:737. doi: 10.12688/f1000research.11150.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang L, Fan Y, Lin R, He W. Blood lactate as a reliable marker for mortality of pediatric refractory cardiogenic shock requiring extracorporeal membrane oxygenation. Pediatr Cardiol. 2019;40(3):602–609. doi: 10.1007/s00246-018-2033-2. [DOI] [PubMed] [Google Scholar]
- 46.Scolari FL, Schneider D, Fogazzi DV, et al. Association between serum lactate levels and mortality in patients with cardiogenic shock receiving mechanical circulatory support: a multicenter retrospective cohort study. BMC Cardiovasc Disord. 2020;20(1):496. doi: 10.1186/s12872-020-01785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fincke R, Hochman JS, Lowe AM, et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol. 2004;44(2):340–348. doi: 10.1016/j.jacc.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 48.Prondzinsky R, Lemm H, Swyter M, et al. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med. 2010;38(1):152–160. doi: 10.1097/CCM.0b013e3181b78671. [DOI] [PubMed] [Google Scholar]
- 49.Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 50.O’Neill WW, Schreiber T, Wohns DHW, et al. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella registry. J Intervent Cardiol. 2014;27(1):1–11. doi: 10.1111/joic.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basir MB, Kapur NK, Patel K, et al. Improved outcomes associated with the use of shock protocols: updates from the national cardiogenic shock initiative. Catheter Cardiovasc Interv. 2019;93(7):1173–1183. doi: 10.1002/ccd.28307. [DOI] [PubMed] [Google Scholar]
- 52.Thiele H, Akin I, Sandri M, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377(25):2419–2432. doi: 10.1056/NEJMoa1710261. [DOI] [PubMed] [Google Scholar]
- 53.Alba AC, Foroutan F, Buchan TA, et al. Mortality in patients with cardiogenic shock supported with VA ECMO: a systematic review and meta-analysis evaluating the impact of etiology on 29,289 patients. J Heart Lung Transplant. 2021;40(4):260–268. doi: 10.1016/j.healun.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Pozzi M, Banfi C, Grinberg D, et al. Veno-arterial extracorporeal membrane oxygenation for cardiogenic shock due to myocarditis in adult patients. J Thorac Dis. 2016;8(7):E495–E502. doi: 10.21037/jtd.2016.06.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Venkataraman S, Bhardwaj A, Belford PM, Morris BN, Zhao DX, Vallabhajosyula S. Veno-arterial extracorporeal membrane oxygenation in patients with fulminant myocarditis: a review of contemporary literature. Medicina. 2022;58(2):215. doi: 10.3390/medicina58020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fukuhara S, Takeda K, Kurlansky PA, Naka Y, Takayama H. Extracorporeal membrane oxygenation as a direct bridge to heart transplantation in adults. J Thorac Cardiovasc Surg. 2018;155(4):1607–1618e6. doi: 10.1016/j.jtcvs.2017.10.152. [DOI] [PubMed] [Google Scholar]
- 57.Parhar KK, Fedak PWM. Bridging to heart transplant with extracorporeal membrane oxygenation: good or VAD? J Thorac Cardiovasc Surg. 2018;155(4):1619–1620. doi: 10.1016/j.jtcvs.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 58.Topkara VK, Sayer GT, Clerkin KJ, et al. Recovery with temporary mechanical circulatory support while waitlisted for heart transplantation. J Am Coll Cardiol. 2022;79(9):900–913. doi: 10.1016/j.jacc.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Alessandro C, Aubert S, Golmard JL, et al. Extra-corporeal membrane oxygenation temporary support for early graft failure after cardiac transplantation. Eur J Cardiothorac Surg. 2009;37(2):343–349. doi: 10.1016/j.ejcts.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 60.Lorusso R, Raffa GM, Alenizy K, et al. Structured review of post-cardiotomy extracorporeal membrane oxygenation: part 1 – Adult patients. J Heart Lung Transplant. 2019;38(11):1125–1143. doi: 10.1016/j.healun.2019.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whitman GJR. Extracorporeal membrane oxygenation for the treatment of postcardiotomy shock. J Thorac Cardiovasc Surg. 2017;153(1):95–101. doi: 10.1016/j.jtcvs.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 62.Doll N, Kiaii B, Borger M, et al. Five-year results of 219 consecutive patients treated with extracorporeal membrane oxygenation for refractory postoperative cardiogenic shock. Ann Thorac Surg. 2004;77(1):151–157. doi: 10.1016/S0003-4975(03)01329-8. [DOI] [PubMed] [Google Scholar]
- 63.Weiner L, Mazzeffi MA, Hines EQ, Gordon D, Herr DL, Kim HK. Clinical utility of venoarterial-extracorporeal membrane oxygenation (VA-ECMO) in patients with drug-induced cardiogenic shock: a retrospective study of the Extracorporeal Life Support Organizations’ ECMO case registry. Clin Toxicol. 2020;58(7):705–710. doi: 10.1080/15563650.2019.1676896. [DOI] [PubMed] [Google Scholar]
- 64.Giraud R, Laurencet M, Assouline B, De Charrière A, Banfi C, Bendjelid K. Can VA-ECMO be used as an adequate treatment in massive pulmonary embolism? J Clin Med. 2021;10(15):3376. doi: 10.3390/jcm10153376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Munakata R, Yamamoto T, Hosokawa Y, et al. Massive pulmonary embolism requiring extracorporeal life support treated with catheter-based interventions. Int Heart J. 2012;53(6):370–374. doi: 10.1536/ihj.53.370. [DOI] [PubMed] [Google Scholar]
- 66.Maggio P, Hemmila M, Haft J, Bartlett R. Extracorporeal life support for massive pulmonary embolism. J Trauma Inj Infect Crit Care. 2007;62(3):570–576. doi: 10.1097/TA.0b013e318031cd0c. [DOI] [PubMed] [Google Scholar]
- 67.Karami M, Mandigers L, Miranda DDR, et al. Survival of patients with acute pulmonary embolism treated with venoarterial extracorporeal membrane oxygenation: a systematic review and meta-analysis. J Crit Care. 2021;64:245–254. doi: 10.1016/j.jcrc.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Javaudin F, Lascarrou JB, Le Bastard Q, et al. Thrombolysis during resuscitation for out-of-hospital cardiac arrest caused by pulmonary embolism increases 30-day survival. Chest. 2019;156(6):1167–1175. doi: 10.1016/j.chest.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 69.Guía ESC. 2019 para el diagnóstico y tratamiento de la embolia pulmonar aguda. Rev Esp Cardiol. 2020;73(6):497.e1–497.e58. doi: 10.1016/j.recesp.2019.12.030. [DOI] [Google Scholar]
- 70.Corsi F, Lebreton G, Bréchot N, et al. Life-threatening massive pulmonary embolism rescued by venoarterial-extracorporeal membrane oxygenation. Crit Care. 2017;21(1):76. doi: 10.1186/s13054-017-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pillai AK, Bhatti Z, Bosserman AJ, Mathew MC, Vaidehi K, Kalva SP. Management of vascular complications of extra-corporeal membrane oxygenation. Cardiovasc Diagn Ther. 2018;8(3):372–377. doi: 10.21037/cdt.2018.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mondie C, Maguire NJ, Rentea RM. StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. [Accessed September 1, 2022]. Retroperitoneal hematoma. http://www.ncbi.nlm.nih.gov/books/NBK558928/ [PubMed] [Google Scholar]
- 73.Thomas J, Kostousov V, Teruya J. Bleeding and thrombotic complications in the use of extracorporeal membrane oxygenation. Semin Thromb Hemost. 2018;44(01):020–029. doi: 10.1055/s-0037-1606179. [DOI] [PubMed] [Google Scholar]
- 74.Lorusso R, Shekar K, MacLaren G, et al. ELSO interim guidelines for venoarterial extracorporeal membrane oxygenation in adult cardiac patients. ASAIO J. 2021;67(8):827–844. doi: 10.1097/MAT.0000000000001510. [DOI] [PubMed] [Google Scholar]