Abstract
Background and aim:
The Italian data on psoriasis are partial and, in most cases, come from monocentric studies, not representative of the population. Our aim was to conduct a systematic review of the available evidence in order to get the overall picture of the Italian epidemiology (prevalence and incidence); burden of disease (direct and indirect costs, the impact on quality of life); comorbidities; and finally, the patients’ satisfaction and acknowledgement of both the disease and the care services available.
Methods:
This systematic review followed the guidelines of the Cochrane Collaboration and the Prepared Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020. The literature search was conducted on PubMed/Medline and Scopus.
Results:
Out of 387 retrieved articles, 41 were included in the analysis. Psoriasis is a frequent condition in Italy: the prevalence is between 1,8% and 4,8% and the incidence is between 107,742 and 230,62 per 100.000 person-years. The most frequent comorbidities associated to psoriasis are: diabetes, hypertension, obesity, depression, cardiovascular diseases, hypertriglyceridemia, and hypercholesterolemia. The quality of life and severity of the disease are mostly affected by other concurrent diseases, early onset of the disease, low income, and low level of education. The costs of psoriasis were estimated between 500 euro and 15.000 euro depending on the disease’s severity, the treatments used and hospitalization.
Conclusions:
In conclusion, psoriasis is a high-impact chronic disease. It is therefore fundamental to advocate a multidisciplinary approach to obtain a better health outcome, the patients’ management and the cost savings could benefit from it. (www.actabiomedica.it)
Keywords: systematic review, psoriasis, epidemiology, comorbidity, costs, Italy
Introduction
Psoriasis is a chronic, inflammatory, immunological and systemic disease, affecting men and women of all ages and races (1, 2). Despite the genetic predisposition of the psoriasis, several extrinsic (3, 4) (e.g., mechanical stress, environmental pollution, drugs, vaccines, infections, smoke, alcohol) and intrinsic (e.g., metabolic syndrome, obesity, diabetes mellitus, dyslipidemia, hypertension, stress) risk factors play an important role in its occurrence (5-8). Symptomatology may largely vary depending on the severity of the disease; however, itching, pain, burning of the skin (due to red scaly plaques) are the most common, substantially impacting the quality of patients’ life. Moreover many comorbidities can be associated with the disease (9), that is showing an upward trend worldwide (10, 11). Depending on the region, the prevalence varied from 0.09% in the United Republic of Tanzania to 11.4% in Norway (12). Furthermore, psoriatic patients may also be affected from psoriatic arthritis. The main symptoms of psoriatic arthritis are joint pain, stiffness and swelling. In most cases, the skin manifestation of psoriasis precedes the joint manifestation of psoriatic arthritis (13).
Being a chronic and disabling disease, psoriasis causes great physical, emotional, and social burden; that inevitably leads to a considerable use of both healthcare and economic resources. Quality of Life (QoL), in general, is often significantly impaired; disfiguration, disability and loss of productivity are common challenges for people with psoriasis (14). Despite scientific advances, there is still not a unique or defined treatment. Nevertheless, many new pharmacological drugs, especially immunological therapies became available in the last decades (15). However, due to the high costs of clinical trials, the treatments (phototherapy, topical drugs, systemic drugs including immunotherapy) are getting more and more expensive for both patients and the national healthcare system (16). In light of this, and considering that the Italian national healthcare system was funded on universality, equity, and solidarity principles, it is of paramount importance to understand both the national and the regional epidemiology of diseases in order to allocate resources and funding efficiently, especially in a limited financial resource setting (17).
In Italy, psoriasis cases are not compulsory recorded and therefore it is complicated to find incident and prevalent reliable and continuous data over time. As a result, incomplete and not homogeneous data, that often come from only one region or from a specialized clinic are available. Moreover, another research conducted in populations from around the world reported a greater variation of epidemiology (incidence and prevalence), burden and costs for psoriasis and psoriatic arthritis when specific regions of the country were considered (14).
Our aim is therefore to conduct a systematic review of the evidence available in literature to get the overall picture of: the nationwide epidemiology (prevalence and incidence); the burden of disease (direct and indirect costs, the impact on QoL); the prevalence of comorbidities; and finally, the patients’ satisfaction and acknowledgement of both the disease and the care services available.
Materials and methods
This review was conducted in accordance with the Prepared Items for Systematic Reviews and Meta-Analysis (PRISMA) (18). The research protocol was developed in advance, shared and discussed in team and fully approved before starting the review.
PubMed/MEDLINE and Scopus were consulted (the 16th June 2021) using a pre-determined combination of free text and MeSH (Medical Subject Headings) keywords (supplementary Table 1).
Supplementary Table S1.
PubMed search strategy.
| Set | Keywords |
|---|---|
| 1 | (Psoriasis[Title/Abstract] OR “psoriatic arthritis”[Title/Abstract] OR psoriasis[Title/Abstract] OR “pustulosis palmaris et plantaris”[Title/Abstract] OR “palmoplantaris pustulosis”[Title/Abstract] OR “pustular psoriasis of palms and soles”[Title/Abstract] OR psoriasis[MeSH Terms] OR “arthritis, psoriatic”[MeSH Terms]) |
| 2 | (Epidemiology[MeSH Terms] OR Pharmacoepidemiology[MeSH Terms] OR Incidence[MeSH Terms] OR Prevalence[MeSH Terms] OR Health Knowledge, Attitudes, Practice[MeSH Terms] OR Attitude to Health[MeSH Terms] OR Health Education[MeSH Terms] OR Complications [MeSH Subheading] OR “Global Burden of Disease”[MeSH Terms] OR “Cost of Illness”[MeSH Terms] OR “Illness Cost”[Title/Abstract] OR “Illness Costs”[Title/Abstract] OR “Cost of Sickness”[Title/Abstract] OR “Sickness Costs”[Title/Abstract] OR “Sickness Cost”[Title/Abstract] OR “Burden of Illness”[Title/Abstract] OR “Illness Burden”[Title/Abstract] OR “Illness Burdens”[Title/Abstract] OR “Disease Burden”[Title/Abstract] OR “Disease Burdens”[Title/Abstract] OR “Costs of Disease”[Title/Abstract] OR “Disease Cost”[Title/Abstract] OR “Disease Costs”[Title/Abstract] OR “Economic Burden of Disease”[Title/Abstract] OR “Burden Of Disease”[Title/Abstract] OR “Burden Of Diseases”[Title/Abstract] OR “Cost of Disease”[Title/Abstract] OR “Epidemiology”[Title/Abstract] OR “Pharmacoepidemiology”[Title/Abstract] OR “Incidence”[Title/Abstract] OR “Prevalence”[Title/Abstract] OR “Knowledge”[Title/Abstract] OR “Attitude”[Title/Abstract] OR “Practice”[Title/Abstract] OR “Health Attitude”[Title/Abstract] OR “Health Education”[Title/Abstract] OR “Complications”[Title/Abstract] OR “sequelae”[Title/Abstract] OR “sequels”[Title/Abstract]) |
| 3 | Italy[Title/Abstract] |
| Research sets 1,2, and 3 have been combined with the Boolean operator AND | |
Inclusion/exclusion criteria
All the studies that explored the Italian prevalence, incidence, burden of the disease and its direct and indirect costs, and comorbidities were included among the possible outcomes of interest (Table 1). A further outcome of interest was also to explore the patients’ knowledge about the availability of care paths dedicated to them. The inclusion and exclusion criteria have been declined according to the Population, Exposure, Outcome, Study design - Population, Exposure, Outcome and Study design (PEOS)(details in supplementary Table 2).
Table 1.
General characteristics of the included studies, in alphabetical order.
| Author and year of publication | Study design | Country / Region where the study was conducted | Type of psoriasis | Main characteristics of the population | Psoriasis Diagnosis Method | Sample size and gender | Age | Setting | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Altobelli et al, 2007 (41) | Cross-sectional | Italy (21 centers: Ascoli Piceno, Bari, Benevento, Bologna, Brindisi, Brescia, Catania, Catanzaro, Cesena-Forlì, Florence, L’Aquila, Lucca, Milan, Modena, Naples, Palermo, Padua, Prato, Reggio-Calabria, Rome, Verona) | Psoriasis and psoriatic arthritis | Age between 18 and 85 years | Patients entering the public dermatological clinic | 1,954 (F 49%) | 47 | Hospital, 21 dermatological clinics | Loss of working days, comorbidities and satisfaction |
| Augustin et al, 2017 (42) | Cross-sectional | Europe (including Italy) | Moderate to severe chronic psoriasis | Adult population | Self-reported | 357 respondents including 132 Patients (F 49%) | n.a. | Civil society | Comorbidities |
| Bardazzi et al, 2014 (43) | Cross-sectional | Italy (Bologna, Chieti, Catanzaro, Ancona, Modena-Reggio Emilia, Rome, Verona, Brescia, Messina, Bari, Catania, Padua, Pavia) | Psoriasis | Age> 18 years diagnosed with psoriasis | N.s. | n = 207 patients (82 F; 34.2%) | Average age 50.3 ± 14.9 | Hospital, dermatological clinic | Awareness and comorbidities |
| Bardazzi et al, 2016 (44) | Cross-sectional | Italy (Bologna, Chieti, Catanzaro, Ancona, Modena-Reggio Emilia, Rome, Verona, Brescia, Messina, Bari, Catania, Padua, Pavia) | Psoriasis | Age> 18 years with a confirmed diagnosis of psoriasis | N.s. | 300 pz (119 F; 39.9%) | Average age 49.9 | Hospital, dermatological clinic | Awareness, PASI, DLQI and comorbidities |
| Bardazzi et al, 2019 (45) | Cross-sectional | Italy (Bologna) | Plaque psoriasis | Patients aged> 18 with plaque psoriasis at their first visit | Clinical and histological examination conducted by a dermatologist with experience in psoriasis | n= 300 (118 F, 39.3%) | Average age: 49.9 ± 4.8 | Hospital, dermatological clinic | PASI, BSA, DLQI |
| Biondi Oriente et al, 1989 (46) | Cross-sectional | Italy (Naples) | Psoriasis | Patients with psoriasis | Review of medical records | 647 (F 48.6%) | Average age 43.1. range 9-78 years | Hospital, dermatological clinic | Prevalence |
| Brugos-Pol et al, 2016 (16) | Review | Europe (including Italy) | Psoriasis and psoriatic arthritis | Patients with psoriasis and / or psoriatic arthritis | N.a. | 14 studies | N.d. | Studies analyzing the cost of sickness: including information about estimating direct health costs and / or indirect costs (loss of productivity, sick leave, early retirement, occupational rehabilitation) | Costs |
| Caldarola et al, 2019 (47) | Cross-sectional | Italy (Rome, L’Aquila) | Plaque psoriasis | Patients> 18 years old, with plaque psoriasis | N.s. | 560 | 51.6 ± 15.7 | Hospital, dermatological clinic | Skin co-morbidities, PASI and quality of life |
| Carpentieri et al, 2016 (48) | Retrospective cohort | Italy (Bari) | Moderate to severe chronic psoriasis | Males and females; age> 18 years; diagnosis of vulgar psoriasis with or without scalp and / or nail involvement; treatment with traditional therapies | Medical records from 2007 to July 2014 | 100 (F 31%) | 47.0 | Hospital, dermatological clinic | Direct costs, comorbidities and PASI |
| Cimmino et al, 2007 (49) | Review | World (including Italy) | Psoriasis and psoriatic arthritis | Age ≥ 18 years | N.s. | N.d. | N.d. | N.d. | Prevalence and incidence |
| Colombo et al, 2008 (50) | Prospective cohort | Italy (Milan, L’Aquila, Lucca, Lecce) | Chronic moderate to severe plaque psoriasis | Patients with moderate to severe plaque psoriasis; age ≥18 years | Clinical dermatological examination | 150 (F 34%) | 48.3 | Hospital, dermatological clinic | Direct and indirect costs of illness; PASI and DLQI |
| Colombo et al, 2009 (17) | Mathematical simulation model | Italy | Moderate to severe plaque psoriasis | Etanercept 25 mg 2-days / week vs non-systemic therapy. The model is based on 12 weeks of treatment spanning 10 years | N.a. | N.a. | N.a. | N.a. | Direct costs, ICER, QALY |
| Conforti et al, 2020 (51) | Retrospective cohort | Italy (Trieste) | Moderate to severe plaque psoriasis | Caucasian adults | N.s. | 43 (F 30%) | 52 (39-60) years | Hospital, dermatological clinic | Comorbidities |
| Conti et al, 2020 (52) | Prospective cohort | Italy (Modena-Reggio Emilia) | Psoriasis and psoriatic arthritis | Psoriatic Caucasian patients, age ≥ 18 years | N.s. | 219 | 57.22 ± 13.15 years | Hospital, dermatological clinic | Comorbidity and severity |
| De Socio et al, 2018 (53) | Prospective cohort | Italy (Molise) | Psoriatic arthritis | Adults residing in Campobasso with joint or musculoskeletal disorders evaluated by a general practitioner | Rheumatological examination | 409 (F 76%) | 53.8 ± 13.7 | Civil society | Incidence |
| Degli Esposti et al, 2018 (56) | Cross-sectional retrospective | Italy (5 local health authorities, unspecified) | Psoriasis or psoriatic arthritis | Age ≥ 18 years with at least one biologic drug prescription for psoriasis or psoriatic arthritis | Administrative database using ICD-9 | 351 patients (F 49%) | 50.3 ± 13.7 | Civil society | Costs |
| Esposito et al, 2006 (55) | Cross-sectional | Italy | Psoriasis | Patients with psoriasis | N.d. | 2,391 (F 36%) | 49.5 years | Civil society (members of the Association for the Defense of Psoriasis) | Comorbidity, patient satisfaction |
| Esposito et al, 2016 (54) | Cross-sectional | Italy Rome) | Psoriasis | Patients enrolled for psoriatic treatment but lost to follow-up for over> 6 months | Dermatological examination | 75 (F 41%) | 53.4 ± 15.9 years | Hospital, dermatological clinic | Comorbidity, patient satisfaction |
| Feldman et al, 2014 (57) | Review | World (including Italy) | Psoriasis | Adult population | Pubmed and publicly available minutes of national and international conferences | 35 articles | Adults | N.a. | Costs |
| Finzi et al, 1998 (59) | Prospective cohort | Italy | Plaque psoriasis, psoriasis vulgaris (> 80% of sample) | Patients with psoriasis | Psoriasis in patients observed in dermatological centers homogeneously distributed throughout Italy; each center was asked to compile and return a simple report that analyzed the first 10 patients with a new diagnosis of psoriasis every month | 7,992 (F 40%) | 61% of the sample is over 60 years old | Hospital, 104 dermatological clinics | Loss of working days |
| Finzi et al, 2001 (60) | Cross-sectional | Italy | Psoriasis (> 80% plaque psoriasis) | The first 10 psoriatic patients in 104 Italian hospitals | Sample of about 10% of the patients extracted from the AISP study | 793 (F 34%) | 45.3 ± 18.1 | Hospital, dermatological clinic | Hospitalization, days of hospitalization, outpatient visits, costs of therapies |
| Finzi et al, 2007 (58) | Cross-sectional within the PSYCHAE cohort study | Italy | Plaque psoriasis, psoriasis vulgaris (96% of the sample) | Previous or new diagnosis of psoriasis between the ages of 18-65, absence of other major organic diseases and cancer | Dermatological examination | 1,580 (F 43%) | 44 ± 13 years | Hospital, 39 dermatological clinics | Comorbidities |
| Gisondi et al, 2009 (63) | Case-control | Italy (Verona) | Moderate to severe chronic plaque psoriasis | Cases: outpatient patients with psoriasis; controls: people associated by sex and age with skin diseases other than psoriasis | N.s. | 39 cases, 38 controls (F 50%) | 30-60 | Hospital, dermatological clinic | Comorbidities |
| Gisondi et al, 2009 (66) | Case-control | Italy (Verona) | Chronic plaque psoriasis | Cases: adult patients without clinical evidence of malignancy, cirrhosis, other causes of secondary chronic liver disease, who have never taken methotrexate; controls: healthy relatives and health personnel | N.s. | 130 patients (F 31.5%); 260 controls (F 30.8%) | 51.2 ± 13.4 cases; 51.2 ± 8 controls | Hospital, dermatological clinic | Comorbidities, PASI |
| Gisondi et al, 2010 (64) | Case-control | Italy (Verona) | Chronic plaque psoriasis | Patients with chronic plaque psoriasis; control: people associated by sex and age with a diagnosis of skin disease other than psoriasis | N.s. | 234 cases, 234 controls (F 43%) | 30-70 | Hospital, dermatological clinic | Comorbidities |
| Gisondi et al, 2016 (62) | Case-control | Italy (Verona) | Chronic plaque psoriasis | Cases: adult patients without clinical evidence of malignancy, cirrhosis, other causes of secondary chronic liver disease, who have never taken methotrexate; controls: healthy partners of the cases | Dermatological and rheumatological visit | 124 (F 44%); 79 controls (F 46%) | 55 ± 12 cases; 54 ± 15 controls | Hospital, dermatological clinic | Comorbidities, PASI |
| Gisondi et al, 2017 (65) | Cross-sectional | Italy | Psoriasis | Adult family members who have never been diagnosed with psoriasis, not being treated by a doctor at the time of the survey | Self-reported | 359 patients (F 50.7%) | 55.9 | Civil society | Prevalence, comorbidities |
| Gisondi et al, 2014 (61) | Cross-sectional | Italy (Verona) | Chronic plaque psoriasis | Psoriasis patients who are not taking medications known to affect uric acid levels | N.s. | 338 (F 47%) | 51.1 ± 10 | Hospital, dermatological clinic | Comorbidities, PASI |
| Luca et al, 2020 (67) | Cross-sectional | Italy (Rome, Bologna, Catania) | Psoriasis and psoriatic arthritis | Adult outpatient patients who have never been treated with biologics entering a psoriasis treatment center for the first time | N.s. | 179 patients (F 52%) | 47.5 | Hospital, dermatological clinic | Awareness |
| Parisi et al, 2013 (68) | Review | World (including Italy) | Psoriasis | All ages, ages> 18 years | Different for each study included | 1 prevalence study and 1 incidence study | All ages, ages> 18 years | N.a. | Prevalence and incidence |
| Parodi et al, 2014 (69) | Case-control within the hospital | Italy (11 centers: Genoa, Cagliari, Rome, Florence, Jesi, Benevento, Perugia, Catania, Macerata, Trieste, Catanzaro) | Chronic plaque psoriasis for at least 6 months, with no signs and symptoms of guttate psoriasis | Cases: adults, not pregnant, subjects with psoriasis; controls: subjects who come to the clinic for other reasons | N.s. | 390 (F 40%) | 52.9 ± 16.0 | Hospital, 13 dermatological clinics | Comorbidities |
| Pezzolo et al, 2021 (70) | Retrospective cohort | Italy (Bergamo) | Psoriasis | Age ≥ 18 years living in the province of Bergamo with a diagnosis of psoriasis | From administrative databases (hospital discharge forms from public hospitals or accredited private hospitals) | 12,693 (F 54.6%) | Average age 60.8 ± 16.3 | General population | Comorbidity, cause of death |
| Prignano et al, 2018 (71) | Review | Italy | Psoriasis and psoriatic arthritis | Parents and children both suffering from psoriasis or psoriatic arthritis | Different for each study included | 18 studies | All | Internal and outpatient patients | Prevalence and incidence |
| Proietti et al, 2014 (72) | Case-control | Italy (Rome) | Moderate skin psoriasis | Cases: adult outpatients; controls: homogeneous non-psoriatic subjects | PASI | Cases: 26 (F 77%); controls: 27 (F 74%) | Psoriasis: 30.2 ± 1.0 controls: 32.0 ± 1.0 | Hospital, dermatological clinic | Comorbidities |
| Renzi et al, 2006 (73) | Case-control | Italy (Rome) | Psoriasis (all degrees) | Cases: internal and outpatient patients related to the Dermopathic Institute of the Immaculate Conception (IDI-IRCCS), Rome; controls: doctors (dermatologists, internists, hygienists) | N.s. | 402 (203 outpatient patients; 199 internal patients) | 45 ± 15 | Hospital, dermatological clinic | Satisfaction |
| Ricci et al, 2018 (74) | Cross-sectional | Italy (national reference center for skin diseases in Rome) | Psoriasis | All subjects belonging to the vascular surgery department from 1997 to 2013 | Medical Records (Hospital Information System) | 25,956 (F 61%) of which 505 with psoriasis (59.41%) | All ages (<18 years: 8 out of 505) | Hospital | Prevalence |
| Saraceno et al, 2008 (75) | Cross-sectional | Italy | Psoriasis | Representative sample of the Italian population | Self-reported | 4,109 (gender unspecified) | N.d. | Civil society | Prevalence |
| Sardu et al, 2012 (76) | Cross-sectional | Italy (Sardinia) | Psoriasis and psoriatic arthritis | Inhabitants of the southern part of Sardinia | N.s. | 25,885 pz (F 55%) | 15-89 | Civil society | Prevalence |
| Scarpa et al, 1984 (77) | Cross-sectional | Italy (Campania) | Psoriatic arthritis in psoriatic patients | Internal and outpatient patients with psoriasis | N.s. | 180 (F 53%) | 43.8 | Hospital, dermatological clinic | Prevalence |
| Spadonaro et al, 2014 (78) | Prospective cohort | Italy (12 centers not further specified) | Chronic plaque psoriasis | Adult patients who have switched to biologic therapy | N.s. | 178 (F 43%) | 47.7 | Hospital, dermatological clinic | Costs |
| Villacorta et al, 2020 (79) | Cross-sectional multinational retrospective | World (including Italy) | Psoriasis (all degrees) | Adult patients | Patients with clinical diagnosis or prescription of drugs | 138 (F 30%) | 43.1 | Hospital, dermatological clinic | Indirect costs |
Acronyms: BSA: Body Surface Area; DLQI: Dermatology Life Quality Index; F: Female; ICD-9: International Classification of Diseases; ICER: Institute for clinical and economic review; N.a.: Not applicable; N.d.: unavailable; N.s.: not specified; PASI: Psoriasis Area Severity Index; pz: patients; QALY: quality-adjusted life year; USA: United States of America;
Supplementary Table S2.
Inclusion and exclusion criteria according to the acronym PEOS (Population, Exposure, Outcome, Study design - Population, Exposure, Outcome and Study design).
| Detail | |
|---|---|
| Inclusion criteria | P: population = subjects of any age affected by psoriasis E: exposure = psoriasis (in any form) O: outcome = epidemiology (incidence / prevalence), burden (expressed with any measure, for example QUALY, DALY, etc.), costs (direct and indirect) of the disease, progress of complications associated with psoriatic disease, attitudes / knowledge / behavior of psoriatic subjects about the diagnostic-therapeutic (assistance) paths available to them S: study design = observational studies (cohort, case-control, transversal), trials, reviews |
| Exclusion criteria | P: population = subjects not affected by psoriasis or studies in which psoriatic patients are included to evaluate the progress of other diseases E: exposure = other than psoriatic disease O: outcome = efficacy of pharmacological and non-drug therapy, sensitivity / specificity of diagnostic tools, comparison between drugs, comparison between instrumental and non-instrumental diagnostic tests, risk factors, protective factors, attitudes / knowledge / behavior of psoriatic subjects on different aspects with respect to care pathways S: study design = book chapters, letters or comments. |
Studies selection and data extraction
Studies selection was conducted by two authors (L.B. and G.C.) in a two-step process. During the screening process any disagreements between the reviewers were discussed until consensus was reached, or consulting a senior author (V.G.). Data extraction was performed by two authors (L.B. and G.C.) under the supervision of a third author (V.G.), and reported in a standardized ad hoc spreadsheet developed in Excel (for Windows). The spreadsheet was pre-piloted on ten randomly selected articles and modified accordingly.
Results
Studies selection
A total of 387 articles were retrieved; however, 88 articles were immediately removed because duplicate. Further 236 articles were removed, remaining 63 eligible articles. However, at the end of the second screening, 22 articles were removed (based on inclusion/exclusion criteria). A detailed description of exclusion reasons is reported in supplementary Table 3 (19-40). At the end of the screening process, a total of 41 articles were definitively included (16, 17, 41-79) (Figure 1).
Supplementary Table S3.
Detailed description of exclusion reasons.
| References | Number of studies | Reason of exclusion |
|---|---|---|
| (19-26) | 8 | Data from Italy not available or not extractable |
| (27-34) | 8 | Full text not available |
| (35) | 1 | Aggregated data for different rheumatological diseases |
| (36) | 1 | Different outcome |
| (37) | 1 | Not adult population |
| (38) | 1 | Duplication of previus study |
| (39, 40) | 2 | Sub-sample of patients (not general population) |
Figure 1.
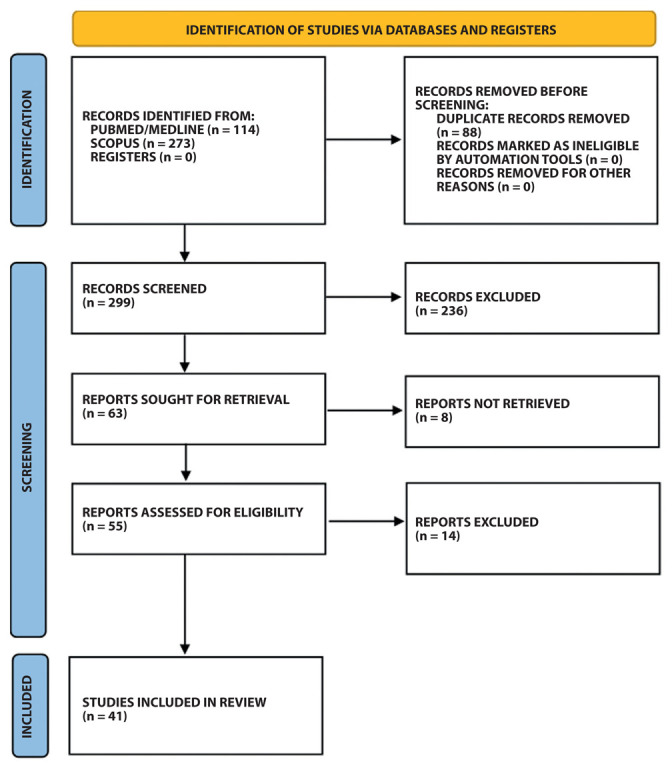
Flow chart of the selection process of the identified articles.
General characteristics of included studies
The first identified work dates back to 1984 (77), while the latest work appears to be published in the course of 2021 (70). Regarding the geographical distribution, some of them (n=5) (16, 42, 49, 68, 79) were international studies in which Italian data were collected and analyzed as well. The remaining articles were representative of the country, because national based (n= 10) (17, 41, 55, 57-60, 71, 74, 75) or because multi-centric studies with at least two regions included (n= 8) (43, 44, 47, 50, 56, 68, 69, 78). The remaining half of the studies consisted of single-center studies (n= 18) (45, 46, 48, 51-54, 61-66, 70, 72, 73, 76, 77). Focusing on the multi-center studies, almost all involved at least one center belonging to a region of northern, central and southern Italy, with the exception of the study conducted by Caldarola et al, (80) which involved two centers, both (Rome and L’Aquila) representative only of central Italy.
Regarding the study design, half of the identified studies are cross-sectional studies (n= 20) (41-47, 54-56, 58, 60-62, 67, 74-77, 79), followed by cohort studies (n=8) (48, 50-53, 59, 70, 78), of which three are retrospective studies (48, 51, 70). The remaining are case-control (n= 7) (62-64, 66, 69, 72, 73), of which one is a hospital based study(69); reviews (n= 5) (16, 49, 57, 68, 71) and, lastly, one mathematical simulation(17).
Regarding the outcomes considered, in most cases the studies evaluated more than one outcome at the same time. Specifically, the prevalence was measured in 9 studies (46, 49, 62, 68, 71, 74-77), the incidence in 4 studies (49, 53, 68, 71), comorbidities were analyzed in 19 studies (41, 42, 47, 48, 51, 52, 54, 55, 58, 61-66, 69, 70, 72, 76), the degree of severity of the disease was measured in 9 studies (41, 42, 47, 48, 51, 52, 54, 55, 58, 61-66, 69, 70, 72), the costs (both direct and indirect) were estimated, also through simulation with mathematical models (17), in 11 studies (16, 17, 41, 48, 50, 56, 57, 59, 60, 78, 79). Lastly, the QoL of patients with psoriasis was evaluated in 4 studies (17, 44, 45, 50); awareness and satisfaction with the level of health assistance received in 7 studies (41, 43, 44, 54, 55, 67, 73).
Prevalence and incidence
A total of 9 studies assessed the prevalence (n=6 for psoriasis; (46, 62, 68, 71, 74, 75); n=4 for psoriatic arthritis and (46, 49, 71, 77). one study combined psoriasis and psoriatic arthritis together (76)). Prevalence widely ranged based on the setting assessed. Indeed, the prevalence of psoriasis among the general population ranged between 4.5% and 4.8% (62), whereas, in the hospital setting (including both inpatient and outpatient individuals) the prevalence ranged between 1.8% and 3.1% (71). Regarding psoriatic arthritis, the prevalence in the general population was assessed in a single study which, however, combined psoriatic arthritis and psoriasis together, reporting a prevalence value of 939 subjects per 100,000 (in women the prevalence is found to be 776 per 100,000; while in men 1,135 per 100,000) (76). In the hospital setting, the prevalence of psoriatic arthritis, among psoriatic patients, ranged between 4.7% and 47.1% (71). Finally, only one study, however rather dated, evaluated the prevalence of the different forms of psoriasis in subjects diagnosed with psoriasis, in particular, 85% were affected by psoriasis vulgaris, 10.5% by eruptive psoriasis, 2.5 % erythrodermal psoriasis, and 2.5% pustular psoriasis (46). Details are reported in supplementary Table 4.
Supplementary Table S4.
Main results of included studies stratified by outcome of interest (excluded comorbidities, reported in Supplementary table 5), and reported in alphabetical order.
| Author, year (Ref) | Type of psoriasys | Prevalence | Incidence | Severity and quality of life | Awareness and satisfaction | Costs and burden |
|---|---|---|---|---|---|---|
| Altobelli et al, 2007 (41) | n.a. | n.a. | 94% of sample was unsatisfied with the overall service, lower number of visits and higher architectonic barriers were lower in the south compared to the north of Italy | working day lost from 1 to 7 was around 40% | ||
| Bardazzi et al, 2014 (43) | n.a. | n.a. | n.a. | Higher level of pts’ awarness of psoriasis was associated with older depressed female, with at least another member of the family affected. | n.a. | |
| Bardazzi et al, 2016 (44) | psoriasis | n.a. | n.a. | PASI score= 4, DLQI score= 6; | Mean awareness: was 61.7 (95% 58.3-65.03); higher level of pts’ awarness of psoriasis was associated with being older, high educated, having at least 1 family member affected, be an internet user, and higher scroe in DLQI. The highest awarness score was reach in patogenesis (80.4%); followed by diagnosis (69.5%), and clinical course (61.3). | n.a. |
| Bardazzi et al, 2019 (45) | plaque psoriasis | n.a. | n.a. | 50% of the sample had a PASI score>10; 50% had a global severity >10 and 25% had a DLQI> 10. The higher value of the three scores was associated with being male, earlier onset of the disease and lower educational and lower income. | n.a. | n.a. |
| Biondi Oriente et al, 1989 (46) | prevalence of psoriasis: 1,86%; of which psoriasis vulgaris 85%, eruptive form 10.5%, erythrodermic 2.5%, pustular form 2.5%, PsA: 21.3% | n.a. | n.a. | n.a. | n.a. | |
| Brugos-Pol et al, 2016 (16) | n.a. | n.a. | n.a. | n.a. | Direct costs of generalized psoriasis (905€/y in 1994); in 2006 generalized psosiasis 8372€/y (direct costs:5690€/y; indirect costs 2682€/y); moderate psoriasis 5226€/y (direct costs 3642€/y; indirect costs 1583€/y) severe psoriasis 11434€/y (direct costs 7683€/y; indirect costs 3751€/y); total cost of psoriatic arthritis in 2008: 1519€/y before biologic agents (BA) therapy (direct costs:943€/y; indirect costs:576€/y) and increase/decrease in mean cost after BA: 4639€/y (direct costs 5052€/y; indirect costs -413€/y) | |
| Caldarola et al, 2019 (47) | plaque psoriasis | n.a. | n.a. | PASI score= 6.9; DLQI score= 9.1; PDI= 28.0. All the scores were significantly higher in those with comorbidities compared to those without. | n.a. | n.a. |
| Carpentieri et al, 2016 (48) | moderate-severe chronic psoriasis | n.a. | n.a. | PASI score before treatment= 10 while after treatment= 0. | n.a. | The total cost per-pt for the 4 cycle of treatment was significantly higher (in each cycle) for cyclosporine compared to tother traditional therapies. Mean cost for cyclosporine per pt per cycle 2000 € vs 500 € for the other traditional treatments. |
| Cimmino et al, 2007 (49) | Prevalence of psoriatic arthritis ranged between 34 and 42%. | Incidence of psoriasis was 107.7/100.000 | n.a. | n.a. | n.a. | |
| Colombo et al, 2008 (50) | moderate-severe chronic plaque psoriasis | n.a. | n.a. | PASI score= 14.7 for moderate and 28,0 for severe. DLQI score >10 in 29.7% of moderate and 40.8% in severe pts. | n.a. | The mean total cost (direct and indirect) per pt was €8,371.61 per year. Direct costs: €5690 (moderate PsO: €3643.02; severe PsO =7683.32€), Indirect costs: (moderate PsO: 1583.02 vs severe PsO: €3751.08); intangible cost: €2681.51 (32%). Direct costs> indirect costs for both forms of PsO |
| Colombo et al, 2009 (17) | moderate-severe chronic plaque psoriasis | n.a. | n.a. | The average QALY per pts over 10 years was similar for moderate and severe pts (6,778 vs 6549). | n.a. | In pts with moderate plaque psoriasis (PASI≥10) the direct cost of intermittent etanercept was 40,051€ for etanercept vs 32,441€ for the non-systemic treatment over 10 years. The costs were higher for sever plaque psoriasis (PASI≥20) [55,959€ vs 50,045€]. The average QALY per pts over 10 years was similar for moderate and severe pts (6,778 vs 6549). The incremental cost-effectiveness ratio (ICER) was 33,216/QALY for the group of pts with moderate psoriasi; and €25,486/QALY for severe psoriasis. |
| Conti et al, 2020 (52) | psoriasis and PsA | n.a. | n.a. | PASI= 13,3 ± 10,0 | n.a. | n.a. |
| De Socio et al, 2018 (53) | incidence of PsA 22,59/100.000pt/year, annual incidence rate 20.14/100.000/year for women and 25.36/100.000/year for men. | n.a. | n.a. | n.a. | ||
| Degli Esposti et al, 2018 (56) | n.a. | n.a. | n.a. | n.a. | Mean annual costs for hospitalization before biological treatment were €217 and €537 for PSO and PsA, respectively, while mean annual cost for concomitant drugs slightly increased after biologics initiation: from €249.8 to €269.4 for PSO and from €331.8 to €346.9 for PsA | |
| Esposito et al, 2006 (55) | n.a. | n.a. | n.a. | Satisfaction on treatment was moderate in 38% of the sample. | n.a. | |
| Esposito et al, 2016 (54) | n.a. | n.a. | n.a. | 44% were unsatisfied with the treatment, 72% required a multidisciplinary consultation. Cardiology: therapeutic adjustment was requested in 33.3% (five of 15) pts; redefined diagnosis in 26.7% (four of 15) cases. Endocrinology: therapeutic adjustment and a redefined diagnosis were needed in 61.1% (11/18) and 33.3% (six of 18) pts. Rheumatology: therapeutic adjustment and a redefined diagnosis were needed in 76.2% (16/21) and 19.0% (four of 21). | n.a. | |
| Feldman et al, 2014 (57) | n.a. | n.a. | n.a. | n.a. | Italian costs are in line with European countries. Specialist visits = 27.74€; day hospitals = 5.03€; Hospitalizations = 759.10€; Topical therapy = 13.63€; Systemic therapy = 99.01€; Medical and pharmacological treatments = 112.73€m, with an annual total cost per pt of 8,372€ | |
| Finzi et al, 1998 (59) | n.a. | n.a. | n.a. | n.a. | Over 90% of cases lost between 1 and 7 working days in the course of the year. | |
| Finzi et al, 2001 (60) | n.a. | n.a. | n.a. | n.a. | Hospedalizations (84%) and systemic therapy (11%) represent the major components of cost. Cost per pt/year: topic therapy (13.63€, systemic therapy: 99,01€, specialist visits 27.74€; day hospital 5.03€, hospitalizations 759,10€, total cost 904.51€. | |
| Gisondi et al 2009 (66) | chronic plaque psoriasis | n.a. | n.a. | PASI score= 12 ± 10 | n.a. | n.a. |
| Gisondi et al 2016 (62) | chronic plaque psoriasis | 4.8% prevalence of psoriasis; | PASI score= 13 ± 10 | |||
| Gisondi et al, 2014 (61) | chronic plaque psoriasis | n.a. | n.a. | PASI score= 15.2 | n.a. | n.a. |
| Luca et al, 2020 (67) | n.a. | n.a. | n.a. | psoriatic pts had a consistent level of knowledge regarding therapy (and biologic treatment) among the three centers. The most frequently sources of information used were media (19%), followed by phyicians (16%), and family (11%). However, physicans were the source most frequently consulted in the north compared to the south of Italy | n.a. | |
| Parisi et al, 2013 (68) | The Italian prevalence was 2.90% (95% CI: 2.39–3.41) among adults | The Italian incidence rate was 230/100,000 person-years among adults. | n.a. | n.a. | n.a. | |
| Prignano et al, 2018 (71) | prevalence of psoriasis ranged between 1.8-3.1%; prevalence of PsA ranged between 4.7–47.1% | incidence of psoriasis was 2.30–3.21 cases per 1000 person-years over the 5-year | n.a. | n.a. | ||
| Renzi et al, 2006 (73) | n.a. | n.a. | n.a. | the overall satisfaction of pts was in 37.5% not completely satisfied, similar proportion also for control group (33.3%) | n.a. | |
| Ricci et al, 2018 (74) | whole population= 1.95%; males= 2.08%; females 1.86% | n.a. | n.a. | n.a. | n.a. | |
| Saraceno et al, 2008 (75) | Prevalence: abruzzo, latium, molise (4,5%); emilia romagna (4%), campania (3,7%), marches, tuscany, umbria (3,4%); friuli, trentino, veneto (2,8%); sicily (2,6%), lombardy (2,4%); Aosta Valley, liguria, piedmont (2.3%), apulia, basilicata, calabria (1,6%), sardinia (0.8%) | n.a. | n.a. | n.a. | n.a. | |
| Sardu et al, 2012 (76) | prevalence 939 psoriasis/PsA per 100.000 (women 776 per 10^5; men 1135 per 10^5) | n.a. | n.a. | n.a. | n.a. | |
| Scarpa et al, 1984 (77) | prevalence of PsA= 34.4% | n.a. | n.a. | n.a. | n.a. | |
| Spadonaro et al, 2014 (78) | n.a. | n.a. | n.a. | n.a. | Direct costs amount to 15,073€ per year per pts for biological treatment compared to 2,166€ per year per pts for standard therapy. The QALY during the first year of treatment with biological drugs was 28,656€/QALY | |
| Villacorta et al, 2020 (79) | n.a. | n.a. | n.a. | n.a. | total work productivity loss (WPL) 25%; work productivity loss due to presenteeism 22.5% |
Acronyms: BSA: Body Surface Area; DLQI: Dermatology Life Quality Index; ICER: Institute for clinical and economic review; n.a.: Not available; PASI: Psoriasis Area Severity Index; pt: patients; PsA: psoriatic arthritis; PsO: psoriasis; QALY: quality-adjusted life year
Incidence of psoriasis was estimated in three of the included studies (49, 68, 71), whilst, only one study assessed the incidence of psoriatic arthritis (53). Values for psoriasis were found to be between 107.7 (49) and 230 (68) per 100,000 per year, while the cumulative rate over the 5-year course was found to be between 2.30-3.21 cases per 1,000 people per year (71). Regarding psoriatic arthritis, the data show an incidence rate of 22.59 per 100,000 patients / year, equally distributed between the two sexes (20.14 per 100,000 / year for women; and of 25.36 for 100,000 / year for men).
Comorbidities
Among the 18 studies (41, 42, 47, 48, 51, 52, 54, 55, 58, 61-66, 69, 70, 72) assessing comorbidities, 3 studies simultaneously reported data for both psoriasis and psoriatic arthritis (41, 42, 52), while the remaining of the studies focused only on psoriasis.
The most frequently studied diseases were diabetes (n = 10), hypertension (n = 8) and obesity (n = 6). Among these, the prevalence of diabetes varied between 4% and 62% of patients with psoriasis and in 30% of patients with psoriatic arthritis, depending on the study considered. Details are reported in supplementary table 5. While the prevalence of hypertension was found to be present in 13% -73% of patients with psoriasis and in 32% of patients with psoriatic arthritis (supplementary table 5). Finally, obesity is present in between 9% and 46.5% of patients with psoriasis and 36% of patients with psoriatic arthritis (supplementary table 5). However, even depression, although less frequently explored, was found to be a co-pathology in 4% -62% of patients with psoriasis and in 30% of patients with psoriatic arthritis, while cardio-vascular diseases, even infrequently studied, show complicate the clinical picture of approximately 7% -86.4% of psoriatic patients and 13.6% of psoriatic arthritis patients (supplementary table 5). Risk factors for cardiovascular diseases such as hypertriglyceridemia, hypercholesterolemia and a high value of the Framingham risk score were also found to be frequently present in patients with psoriasis (from 18% to 38% of the subjects analyzed) (supplementary table 5).
Supplementary Table S5.
Description of the studies that investigated the presence of comorbidities among psoriasis or psoriatic arthritis patients, reported in alphabethical order.
| Author, year (Ref) | Altobelli et al, 2007 (41) | Augustin et al, 2017 (42) | Caldarola et al, 2019 (47) | Carpentieri et al, 2016 (48) | Conforti et al, 2020 (51) | Conti et al, 2020 (52) | Esposito et al, 2006 (55) | Finzi et al, 2007 (58) | Gisondi et al, 2009 (63) | Gisondi et al, 2009 (66) | Gisondi et al, 2010 (64) | Gisondi et al, 2014 (61) | Gisondi et al, 2016 (62) | Gisondi et al, 2017 (65) | Parodi et al, 2014 (69) | Pezzolo et al, 2021 (70) | Proietti et al, 2014 (72) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of psoriasis | PsO | PsA | Pso | PsA | PsO | PsO | PsO | Pso | PsA | PsO | PsO | PsO | PsO | PsO | PsO | PsO | PsO | Pso | PsO | PsO |
| Outcomes | ||||||||||||||||||||
| Depression | 15% | 30% | n.a. | n.a. | n.a. | n.a. | n.a. | 62% | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 4% | n.a. | n.a. | n.a. | ||
| Hypertension | 13% | 32% | n.a. | n.a. | 23% | n.a. | 43.4% | 31% | n.a. | 73% | n.a. | n.a. | 41% | 33% | 19% | n.a. | n.a. | n.a. | ||
| Obesity | 9% | 36% | n.a. | n.a. | 13% | 46.5% | n.a. | 16% | n.a. | n.a. | n.a. | n.a. | 30% | n.a. | 9.1% | n.a. | n.a. | |||
| Diabetes | 7% | 34% | n.a. | n.a. | n.a. | 7% | 15.1% | 15% | n.a. | 18% | n.a. | 18% | 19% | 18% | 9% | 8.5% | n.a. | n.a. | ||
| CV | n.a. | 86.4% | 13.6% | n.a. | n.a. | n.a. | 6,9% | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 7% | n.a. | n.a. | High risk | ||
| Dyslipidemia | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 16% | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |||
| Hypertriglyceridemia | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 38% | n.a. | n.a. | n.a. | n.a. | n.a. | |||
| Hypercholesterolemia | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 18% | n.a. | n.a. | n.a. | n.a. | n.a. | |||
| Hyperuricemia | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 20% | n.a. | n.a. | n.a. | n.a. | n.a. | |||
| Metabolic syndrome | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 30% | n.a. | n.a. | 27% | n.a. | n.a. | |||
| Skin pathology | n.a. | n.a. | 21.1% | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |||
| CKD (low, moderate, high, severe risk) | n.a. | n.a. | n.a. | n.a. | n.a. | 74%; 17.4% 5%; 3.6% | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |||
| Distress | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 54% f | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |||
| NAFLD | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 47% | n.a. | n.a. | n.a. | 44% | n.a. | n.a. | n.a. | n.a. | |||
| Carotid-femoral pulse velocity | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 8.88 ± 1.96 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |||
| PsA | n.a. | n.a. | n.a. | 13% | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |||
| Causes of death | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | Cancers 39%; CV 25%; RD 8% | n.a. | |||
| Scores | ||||||||||||||||||||
| eGFR | n.a. | n.a. | n.a. | n.a. | n.a. | 86.7 ± 23.0 ml/min | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |||
| UACR | n.a. | n.a. | n.a. | n.a. | n.a. | 56.1 ± 299.1 mg/g | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |||
| Framingham risk score | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 11.2 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |||
| Charlson comorbidity index ≥2 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 9.4% | n.a. | |||
CKD: chronic kidney disease; CV: cardiovascular pathologies; eGFR: presumed glomerular filtration rate; NAFLD: Non-alcoholic fatty liver disease; PsA: psoriatic arthritis; PsO: psoriasis; UACR: Urine Albumin-to- Creatinine Ratio, RD: Respiratory diseases
* in this study the prevalence of comorbidities was reported in combined form for PsO and PsA together, without stratification of the results
Severity of disease and quality of life
Disease severity was analyzed in 9 studies (17, 44, 45, 47, 48, 50, 52, 61-63). In all cases, the tool used was the Psoriasis Area Severity Index score (PASI), while only two studies used, in addition to the PASI, other scales, in particular the Body Surface area (BSA) (45) to evaluate skin involvement, and Psoriasis Disability Index score (PDI) (47).
The results of the PASI were reported in the studies in very dissimilar form, so much so that a real comparison was difficult. In fact, in some cases the authors reported the percentage of subjects with a PASI score beyond a certain cut-off, in other cases, the average value was reported, still in others the data referred only to moderate-severe forms of the disease. However, from the data collected it emerges that the PASI score is about 4 among patients with psoriasis (44), however, the data worsens considerably if we focus on moderate or severe forms, where the average score was respectively 14.7 and 28 (50). The value ranged between 6.9 (47) and 15.2 (61) among those with chronic plaque psoriasis. This finding is roughly confirmed in another study which states that 50% of the sample had a PASI score> 10 (45). Finally, the score is around 13 in psoriatic arthritis patients (52). Regarding the other two scales used, BAS and PDI, it was found that in subjects with plaque psoriasis 50% had an overall severity> 10 (45), while the index of psoriasis-related discomfort was equal to 28.0, still among those who were suffering from plaque psoriasis (47).
The perceived QoL was instead measured in 4 studies (17, 44, 45, 50) mainly through two scale: the Dermatology Life Quality Index (DLQI) and the quality-adjusted life-year (QUALY) score. The DLQI score was found to be around 6 for subjects with psoriasis (44), the data worsened in subjects with moderate or severe form, in which the score above 10 was achieved by 29.7% and 40.8% of moderate and severe forms, respectively (50). A score greater than 10 was found in 25% of subjects with plaque psoriasis (45). The only study that evaluated the QoL through the QALY, showed that the QoL of patients with moderate and severe psoriasis was found to be comparable over 10 years of observation (respectively 6.8 vs 6.6) (17).
Regardless of the type of scale used to assess both disease severity and QoL, the studies analyzed showed worse scores among men, early diagnosis, lower income and educational level (45), and lastly, comorbidities (47).
Patient awareness and satisfaction
Awareness about the pathology and care services, and satisfaction with the service provided was measured in all the 7 retrieved studies through the administration of self-completed questionnaires by the interviewees (41, 43, 44, 54, 55, 67, 73). However, questionnaires used were very heterogeneous, making the univocal assessment of the degree of satisfaction and awareness of the patients difficult. However, what emerges is that the degree of satisfaction is generally low with satisfaction percentages that on average do not exceed 40% (73). In particular, the aspect that greatly impacted on the degree of satisfaction is the perceived effectiveness of the therapy (55). However, the reduced satisfaction in terms of therapeutic performance could, at least in part, be due to an inappropriate clinical evaluation (54). Furthermore, over 60% of the subjects required an adjustment of the therapy and an endocrinological re-evaluation; finally, over 70% needed a therapeutic adjustment, and about 20% had to redefine the rheumatological diagnosis (54). Lastly, from a multicenter study, well representative of the northern, central and southern realities of the Italian peninsula, it emerges that the degree of satisfaction regarding the health service received is higher in the northern regions than in the southern ones, in fact, the northern regions show better performance as they guarantee a greater number of outpatient visits, and fewer architectural barriers that guarantee better access to facilities (41).
Regarding the level of awareness of the disease, just over half of the population (≈ 60%) shows a good level of awareness in relation to the diagnosis and clinical course, while the level increases up to 80% when referring to information related to the disease’s pathogenesis (44). A better level of awareness was found to be more frequently associated with women (43), at an advanced age, highly educated, with at least one affected family member (43, 44), able to use internet and with higher DLQI score. Finally, greater awareness was also found to be associated with the type of sources consulted to search information. The most frequently consulted sources were the media (19%), followed by doctors (16%) and other family members (11%) (67). However, in a multicenter study (well representative of the three realities north, center and south of the peninsula), doctors were the most frequently consulted source in the north compared to southern Italy (67).
Costs and burden of psoriasis
Psoriasis and psoriatic arthritis are pathologies with rather high costs, mainly due to the need for hospitalization (responsible for about 80% of the total cost) and the use of systemic therapy (about 20%) (60), especially biological drugs, which probably led to a disproportion of the costs of therapy, rather than the costs associated with hospitalization. However, the above-mentioned costs were measured in a study conducted in early 2000, a period in which biologics were not yet widespread. In fact, a more recent study, from 2014, showed that direct costs amount to € 15,073 / year per patient for biological treatment compared to € 2,166 / year per patient for standard therapy (78). However, the estimated costs of treatment were rather variable, since the costs are closely linked to the degree of severity of the disease and the type of drugs used. For this reason, a progressive increase in costs has been observed over the years, that move from 905 € / year in 1994 (16) (and also found in another study from 2001) (60) as a total cost of generalized psoriasis, to € 8,372 / year in 2006 (direct costs: € 5,690 / year; indirect costs € 2,682 / year) (16). As anticipated, the costs associated with psoriasis are closely related to the level of severity, in fact, studies that have focused on evaluating the direct and indirect costs of the moderate-severe form have shown an average total cost (direct and indirect) per patient with moderate-severe psoriasis of € 8,371.61 per year. In particular, the moderate form reported an estimated total cost of around € 5,000 / year, while the severe form around € 11,000 / year. For both moderate and severe forms, the main cost component has to be attributable to direct costs (16).
Two studies focused on the estimation of costs associated with the use of specific drugs such as cyclosporine (48) and etanercept (17); showing a significant increase in total costs. In particular, the total cost for each patient was significantly higher (in each cycle) for the 4 cycles of cyclosporine treatment compared to other traditional therapies. The average cost of cyclosporine was found to be € 2,000 per patient per cycle compared to € 500 for other traditional treatments (48). The study that evaluated the cost of intermittent treatment with etanercept over 10 years showed an average total cost of € 40,051 versus € 32,441 for non-systemic treatment. Also in this case, costs increased when focusing on moderate to severe forms. However the differences between the treatment with etanercept and the traditional therapy were relatively comparable (55.959 € for Etanercept, against the 50.045 € of traditional therapy), in addition, the incremental cost-effectiveness ratio (ICER) was € 33, 216 / QALY for the group of patients with moderate psoriasis and € 25,486 / QALY for patients with severe psoriasis (17).
Regarding psoriatic arthritis, the total cost in 2008 was found to be 1,519 € / year, before the introduction of biological treatment (direct costs: 943 € / year; indirect costs: 576 € / year). The costs rapidly increased after introduction of biological drugs, reaching € 4,639 / year. The main increase has to be attributable to direct costs which are € 5,052 / year; while indirect costs registered a slight deflection (-413 € / year) (16).
A study carried out a more detailed assessment of the costs of each individual component, obtaining the following values per patient per year: topical therapy (€ 13.63), systemic therapy (99.01 €), specialist visits (€ 27.74), day hospital (€ 5.03), hospitalizations (€ 759.10) with a total cost of € 904.51 (60). Finally, a recent systematic review at the European level compared the data of Italian costs with those of other European countries, finding results that were aligned (57). According to the data collected by the aforementioned review, the Italian costs were found to be: specialist visits = € 27.74; day hospital = € 5.03; hospitalizations = € 759.10; topical therapy = € 13.63; systemic therapy = € 99.01; medical and pharmacological treatment = € 112.73 m, with a total annual cost per patient of € 8,372 (57).
The burden of sickness was also estimated, by counting the days of work lost. This measure has been reported by two studies: the first estimated that about 49% of subjects suffering from psoriatic pathology had experienced a loss of 1-7 working days / year (41), the second estimated a loss of about 25% of total labor productivity, an overlapping percentage (22.5%) as regards the loss of labor productivity due to attendance (59).
Discussion
From the evaluation of the identified articles it emerges that, in the Italian context, the studies concerning epidemiology, burden and costs of disease, QoL, severity, as well as awareness and satisfaction of psoriatic patients, appear to have been published over a rather long period of time, with the first article published in 1984 (77) and the last one published in the first months of 2021 (70). Regarding the geographical distribution, and consequently the representativeness of the entire national territory, this was rather broad as well. Regarding the study design used, most of the studies analyzed were cross-sectional, while only four studies were cohort studies. For this reason, the incidence data were scarce and fragmented, while a more satisfactory overview was obtained with regard to the prevalence and comorbidity data. As for psoriasis, the prevalence among general population was approximately 5%; while in outpatient the prevalence was slightly lower (among 2-3%). Higher values were instead associated with the prevalence of psoriatic arthritis in a hospital setting (5-47%). However, no differences were observed among the regions. The incidence of psoriasis, on the other hand, although referred to a small number of studies, was between 107.742 and 230.62 per 100,000 per year. With regard to psoriatic arthritis, the data show an incidence rate of 22.59 per 100,000 patients / year. Comparing Italian data with those available internationally, it appears that prevalence of psoriasis is extremely variable across the globe with the north part of the world affected the most (81). This might be due to the fact that countries closest to the equator may suffer of underdiagnosis and underreporting, and those more at the north suffer for colder temperatures, which can affect the epidemiology and symptomatology of the psoriasis (82). However, Italian data are comparable with other European studies, according to which in Germany the prevalence of psoriasis was around 2.53%, in Spain it was around 1.43%, and approximately 1.87% in United Kingdom (68).
Several comorbidities affect the psoriatic subjects and patients with psoriatic arthritis. In the following comorbidities occurred the most: diabetes, hypertension, obesity, depression, and cardiovascular diseases. The presence of comorbidities was strongly linked to a lower perceived QoL, as well as to a greater severity of the psoriasis itself. The worst level of QoL was observed among male subjects suffering from plaque psoriasis, subjects whose disease onset was found to be precocious, with a lower income and level of education (45), as well as the greater presence of comorbidities (47). Generally speaking, psoriasis is a disease that highly affects the QoL, especially because many different parts of the body skin can be affected, potentially impacting on the social relationships of the patients (83), both considering intimate and public (with low privacy) situations. Most of the time psoriatic patients feel shamed and perceive the need to hide their disease or their body, highly affecting their self-confidence (84). Our results are in line with international literature, according to which men are more often affected by severe disease, with earlier diagnosis and long disease duration (85).
From the data related to patient awareness and satisfaction, it emerged that the percentages of satisfaction on average do not exceed 40%. In particular, the parameters with the greatest impact on satisfaction concern the effectiveness of the therapy received. This result is in line with previous international publication, according to which a large proportion of individuals expressed dissatisfaction with therapies, and the proportion even increase when focusing on severe psoriasis (86, 87). In fact, based on our results, about 30% of subjects require an adjustment of the therapy over time and about 60% a redefinition of the diagnosis or an endocrinological/rheumatological re-evaluation; emphasizing the importance of a multidisciplinary approach to the disease. However, satisfaction also passes through the quality-of-service delivery (88), and with respect to this, the literature data showed a discrepancy between the northern and southern regions. In fact, patients followed in northern structures expressed greater satisfaction due to easier accessibility to clinics both in terms of hours and in terms of architectural barriers. As far as awareness is concerned, just over half of the interviewees showed to have a good knowledge of the diagnosis and the clinical course, an even greater percentage are aware of the pathogenesis of the disease, especially in women. These data show that much still needs to be done, not only in terms of greater flexibility and reduction of architectural barriers, but also in terms of an integrated and multidisciplinary approach to these types of patients, who require prolonged care, therapeutic reassessment and a diagnostic re-evaluation over the years (89). The most used sources for information on the disease were the media (19%), followed by physicians (16%) and other family members (11%). However, physicians were the most frequently consulted source in the north compared to southern Italy. Therefore, extensive counseling activities, as well as care in the disclosure of correct information also through the most common communication channels currently available (including institutional websites and social media) could represent important allies (90). Indeed, increasing evidence showed that internet, and in general the web, is an increasing source of information, also for rheumatological diseases (91, 92). In light of this, physicians, health institutions and public health experts should monitor social networks and websites so that the information conveyed through the internet could be reliable, understandable and true (90, 93).
Finally, the costs of the disease were taken into account. Hospitalization and systemic therapy make psoriasis and psoriatic arthritis pathologies with rather high costs. In fact, an estimate of over € 15,000 per patient per year is passed for the moderate-severe forms, to about € 500 per patient per year in the less severe forms, which do not require systemic treatment (69). The main determinants of the increase in costs are the severity of the disease and the drug treatment used. In fact, the introduction of biological drugs, although it has had an improvement in the management of these patients, has significantly increased the health costs associated with the care of these subjects (56). In addition to the estimate of direct and indirect costs, the disease burden was estimated by counting the days of work lost, on average about 1-7 days of work lost per year.
The results of this review show a strong lack of knowledge that is still widespread in many areas related to psoriatic disease. First of all, there are very few studies estimating the incidence of the disease in Italy. Furthermore, great heterogeneity emerged in the method of collecting data, which is not always clearly stated. This is a crucial element, since collecting epidemiological data as valid as possible is essential both for the control of the disease and for the development of adequate clinical treatment and intervention planning. Obtaining more reliable data on the incidence and prevalence is important for caring out appropriate health planning. Epidemiological data are, in fact, fundamental to understand the phenomenon and its evolution over time, as well as it allows us to evaluate how much the investments made have actually achieved the desired objectives (94). It is particularly true today, during which limited financial resources are available for the healthcare system (95).
Possible areas of future research should include, on the one hand, the conduct of studies with greater rigor and methodological quality, especially as regards data collection, as well as greater investment in prospective studies, such as to allow for the collection of more evidence on the estimate of the incidence. Furthermore, the development of multicenter studies with a representativeness of the whole national territory appears to be preferable compared to monocentric studies. This would simultaneously allow for greater uniformity of the information collected, concretely allowing greater comparability over time, and between the various Italian regions.
Limitations and strengths of research
The results showed in this review must be read with caution since the methodologies adopted in the individual studies included were rather heterogeneous limiting a direct comparison. In fact, the criteria with which the subjects were recruited were different (for example, different level of severity of the disease or different setting), as well as the way in which psoriasis was diagnosed (in some studies the diagnosis was self-reported, in others, however, it was secondary to clinical / laboratory evaluation). Wide variability was also found in the sampling techniques that included: questionnaires, examinations clinical, combination of questionnaires and clinical examination. The ways in which the various outcomes of interest were measured also differ largely in the various studies. For example, cumulative incidence or punctual incidence. The costs, were estimated as general costs of the disease, costs for the use of some specific drugs, or differentiating direct and indirect costs expressed in euro or in QALY. This difference in methodologies necessarily affected the quality and comparability of the data collected. Moreover, the high heterogeneity both in quality and content of retrieved studies, prevented us from performing a quantitative assessment, as for instance a meta-analysis. However, our study has some important strengths. To the best of our knowledge this is the first systematic review that assessed a broad set of outcomes related to psoriasis and psoriatic arthritis at the Italian level. Actually, previous reviews only focused on one or few outcomes as for instance prevalence (71) or incidence and prevalence (68), but none of them also assessed costs, burden, awareness and comorbidities. Furthermore, most of the previous reviews had not selectively focused on the Italian nation, thus including only a few specific studies from the Italian context (49). Nevertheless, despite the broad approach implemented, a limited number of studies have been retrieved for each endpoint, limiting the possibility to draw strong and definitive conclusions.
Conclusions
Data on psoriasis epidemiology available in Italy are scarce and difficult to compare due to the different study methodologies. The complexity of the disease and the strong impact in terms of quality and severity urgently call for a multidisciplinary approach. In particular, more attention should be paid to subjects with early onset of the disease, with low income and low level of education, since these were found to be the factors most frequently associated with greater severity and worse QoL.
In conclusion, psoriasis appears to be a chronic disease with a strong impact on both the individual and the entire National Health Service. Psoriasis, in fact, in addition to burdening in terms of severity, QoL and lost work days, also has a strong impact in terms of assistance and treatment costs. For this reason, a complete and comprehensive knowledge on psoriasis epidemiology and burden is of paramount importance for improving the QoL and diseases management, as well as reducing the costs that affect both patients and health care service. This data may contribute in a better allocation of limited financial resources.
Acknowledgements:
This research was funded by Altis Omnia Pharma Service srl. The sponsor did not have any role in data collection, data analysis and interpretation, neither in data reporting. The sponsor did not have any role in the decision to public the manuscript.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- Jacobson CC, Kumar S, Kimball AB. Latitude and psoriasis prevalence. J Am Acad Dermatol. 2011;65(4):870–3. doi: 10.1016/j.jaad.2009.05.047. [DOI] [PubMed] [Google Scholar]
- Augustin M, Glaeske G, Radtke MA, Christophers E, Reich K, Schafer I. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol. 2010;162(3):633–6. doi: 10.1111/j.1365-2133.2009.09593.x. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Kishimoto M, Sugai J, Komine M, Ohtsuki M. Risk Factors for the Development of Psoriasis. Int J Mol Sci. 2019;20(18) doi: 10.3390/ijms20184347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Han J, Choi HK, Qureshi AA. Smoking and risk of incident psoriasis among women and men in the United States: a combined analysis. Am J Epidemiol. 2012;175(5):402–13. doi: 10.1093/aje/kwr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:e54. doi: 10.1038/nutd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin AM, Hackett CB, Rogers S, et al. Body mass index, waist circumference and HOMA-IR correlate with the Psoriasis Area and Severity Index in patients with psoriasis receiving phototherapy. Br J Dermatol. 2014;171(2):436–8. doi: 10.1111/bjd.12914. [DOI] [PubMed] [Google Scholar]
- Kumar S, Han J, Li T, Qureshi AA. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27(10):1293–8. doi: 10.1111/jdv.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset L, Halioua B. Stress and psoriasis. Int J Dermatol. 2018;57(10):1165–72. doi: 10.1111/ijd.14032. [DOI] [PubMed] [Google Scholar]
- Nograles KE, Brasington RD, Bowcock AM. New insights into the pathogenesis and genetics of psoriatic arthritis. Nat Clin Pract Rheumatol. 2009;5(2):83–91. doi: 10.1038/ncprheum0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao CG, Zhang GW, Wang GC. Distribution of psoriasis in China: a nationwide screening. Proc Chin Acad Med Sci Peking Union Med Coll. 1987;2(2):59–65. [PubMed] [Google Scholar]
- Yang YC, Cheng YW, Lai CS, Chen W. Prevalence of childhood acne, ephelides, warts, atopic dermatitis, psoriasis, alopecia areata and keloid in Kaohsiung County, Taiwan: a community-based clinical survey. J Eur Acad Dermatol Venereol. 2007;21(5):643–9. doi: 10.1111/j.1468-3083.2006.02036.x. [DOI] [PubMed] [Google Scholar]
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):205–12. doi: 10.1111/jdv.13854. [DOI] [PubMed] [Google Scholar]
- Coates LC, Helliwell PS. Psoriatic arthritis: state of the art review. Clin Med (Lond) 2017;17(1):65–70. doi: 10.7861/clinmedicine.17-1-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Global report on psoriasis. 2016 [Google Scholar]
- Gisondi P, Geat D, Pizzolato M, Girolomoni G. State of the art and pharmacological pipeline of biologics for chronic plaque psoriasis. Curr Opin Pharmacol. 2019;46:90–9. doi: 10.1016/j.coph.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Burgos-Pol R, Martinez-Sesmero JM, Ventura-Cerda JM, Elias I, Caloto MT, Casado MA. The Cost of Psoriasis and Psoriatic Arthritis in 5 European Countries: A Systematic Review. Actas Dermosifiliogr. 2016;107(7):577–90. doi: 10.1016/j.ad.2016.04.018. [DOI] [PubMed] [Google Scholar]
- Colombo GL, Di Matteo S, Peris K, et al. A cost-utility analysis of etanercept for the treatment of moderate-to-severe psoriasis in Italy. Clinicoecon Outcomes Res. 2009;1:53–9. doi: 10.2147/ceor.s7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assessing work-related productivity loss and indirect costs of psoriasis across six countries. The British journal of dermatology. 2020;183(3):e65–e90. doi: 10.1111/bjd.19351. [DOI] [PubMed] [Google Scholar]
- Bewley A, Burrage DM, Ersser SJ, Hansen M, Ward C. Identifying individual psychosocial and adherence support needs in patients with psoriasis: a multinational two-stage qualitative and quantitative study. Journal of the European Academy of Dermatology and Venereology: JEADV. 2014;28(6):763–70. doi: 10.1111/jdv.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb A, Gratacos J, Dikranian A, et al. Treatment patterns, unmet need, and impact on patient-reported outcomes of psoriatic arthritis in the United States and Europe. Rheumatology international. 2019;39(1):121–30. doi: 10.1007/s00296-018-4195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan S, Guyatt V, Franceschetti A, Hautamaki EL. Disease burden and patient reported outcomes among patients with moderate to severe psoriasis: an ethnography study. Psoriasis (Auckland, NZ) 2015;5:1–7. doi: 10.2147/PTT.S74906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Zúñiga MJM, García-Perdomo HA. Systematic review and meta-analysis of the association between psoriasis and metabolic syndrome. Journal of the American Academy of Dermatology. 2017;77(4):657–66.e8. doi: 10.1016/j.jaad.2017.04.1133. [DOI] [PubMed] [Google Scholar]
- Dubertret L, Mrowietz U, Ranki A, et al. European patient perspectives on the impact of psoriasis: the EUROPSO patient membership survey. Br J Dermatol. 2006;155(4):729–36. doi: 10.1111/j.1365-2133.2006.07405.x. [DOI] [PubMed] [Google Scholar]
- Griffiths CEM, Augustin M, Naldi L, et al. Patient-dermatologist agreement in psoriasis severity, symptoms and satisfaction: results from a real-world multinational survey. J Eur Acad Dermatol Venereol. 2018;32(9):1523–9. doi: 10.1111/jdv.14937. [DOI] [PubMed] [Google Scholar]
- Mantovani L, Medaglia M, Piacentini P, et al. Burden of Moderate-to-Severe Plaque Psoriasis and New Therapeutic Approaches (Secukinumab): An Italian Perspective. Dermatol Ther (Heidelb) 2016;6(2):151–67. doi: 10.1007/s13555-016-0114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dini V, Martini P, Bellini M, Bagnoni G, Marsili F, Lancia U. Psoriatic arthritis prevalence in the clinical practice of dermatologists in North-West Tuscany centers of excellence: a screening experience. Giornale italiano di dermatologia e venereologia: organo ufficiale, Societa italiana di dermatologia e sifilografia. 2017;152(1):24–7. doi: 10.23736/S0392-0488.16.05232-9. [DOI] [PubMed] [Google Scholar]
- Gisondi P, Farina S, Giordano MV, Zanoni M, Girolomoni G. Attitude to treatment of patients with psoriasis attending spa center. Giornale italiano di dermatologia e venereologia: organo ufficiale, Societa italiana di dermatologia e sifilografia. 2012;147(5):483–9. [PubMed] [Google Scholar]
- Migkos MP, Somarakis GP, Markatseli TE, Voulgari PV, Drosos AA. Epidemiological characteristics of psoriatic arthritis. Clinical and experimental rheumatology. 2019;37(2):324–32. [PubMed] [Google Scholar]
- Neri L, Miracapillo A. Treatment adherence and real-life effectiveness of topical therapy in patients with mild or moderate psoriasis: uptake of scientific evidence in clinical practice and dermatologists’ preferences for alternative treatment options. Giornale italiano di dermatologia e venereologia: organo ufficiale, Societa italiana di dermatologia e sifilografia. 2015;150(1):19–26. [PubMed] [Google Scholar]
- Sategna-Guidetti C, Bracco E, Marucco E, Bianco L. Psoriasis and Crohn’s disease in Italy. Digestive diseases and sciences. 1988;33(11):1496. doi: 10.1007/BF01537010. [DOI] [PubMed] [Google Scholar]
- Valent F, Tullio A, Errichetti E, Stinco G. The epidemiology of psoriasis in an Italian area: population-based analysis of administrative data. Giornale italiano di dermatologia e venereologia: organo ufficiale, Societa italiana di dermatologia e sifilografia. 2020;155(5):652–7. doi: 10.23736/S0392-0488.18.06020-0. [DOI] [PubMed] [Google Scholar]
- Vena GA, Altomare G, Ayala F, et al. Incidence of psoriasis and association with comorbidities in Italy: a 5-year observational study from a national primary care database. European journal of dermatology: EJD. 2010;20(5):593–8. doi: 10.1684/ejd.2010.1017. [DOI] [PubMed] [Google Scholar]
- Villani A, Cinelli E, Fabbrocini G, Megna M. Psoriasis awareness among general population: preliminary results of an online survey. Italian journal of dermatology and venereology. 2021 doi: 10.23736/S2784-8671.21.06822-X. [DOI] [PubMed] [Google Scholar]
- Giacomelli R, Gorla R, Trotta F, et al. Quality of life and unmet needs in patients with inflammatory arthropathies: results from the multicentre, observational RAPSODIA study. Rheumatology (Oxford, England) 2015;54(5):792–7. doi: 10.1093/rheumatology/keu398. [DOI] [PubMed] [Google Scholar]
- Sampogna F, Gisondi P, Melchi CF, et al. Prevalence of symptoms experienced by patients with different clinical types of psoriasis. Br J Dermatol. 2004;151(3):594–9. doi: 10.1111/j.1365-2133.2004.06093.x. [DOI] [PubMed] [Google Scholar]
- Biondi Oriente C, Scarpa R, Oriente P. Prevalence and clinical features of juvenile psoriatic arthritis in 425 psoriatic patients. Acta Derm Venereol Suppl (Stockh) 1994;186:109–10. doi: 10.2340/00015555186109110. [DOI] [PubMed] [Google Scholar]
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(5):871–81 e1-30. doi: 10.1016/j.jaad.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Balato A, Caiazzo G, Balato N, Napolitano M. group Ls. Psoriatic arthritis onset in psoriatic patients receiving UV phototherapy in Italy. G Ital Dermatol Venereol. 2020;155(6):733–8. doi: 10.23736/S0392-0488.18.06117-5. [DOI] [PubMed] [Google Scholar]
- Morici N, Ferri LA, Alicandro G, et al. Psoriasis and the risk of acute coronary syndrome in the elderly. Int J Cardiol. 2018;273:44–6. doi: 10.1016/j.ijcard.2018.07.134. [DOI] [PubMed] [Google Scholar]
- Altobelli E, Maccarone M, Petrocelli R, et al. Analysis of health care and actual needs of patients with psoriasis: a survey on the Italian population. BMC public health. 2007;7:59. doi: 10.1186/1471-2458-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin M, Vietri J, Tian H, Gilloteau I. Incremental burden of cardiovascular comorbidity and psoriatic arthritis among adults with moderate-to-severe psoriasis in five European countries. Journal of the European Academy of Dermatology and Venereology: JEADV. 2017;31(8):1316–23. doi: 10.1111/jdv.14286. [DOI] [PubMed] [Google Scholar]
- Bardazzi F, Amerio P, Amoruso G, et al. Investigating psoriasis awareness among patients in Italy: validation of a questionnaire. Eur Rev Med Pharmacol Sci. 2014;18(22):3435–52. [PubMed] [Google Scholar]
- Bardazzi F, Amerio P, Amoruso G, et al. Psoriasis awareness among Italian patients: results of a nationwide survey. G Ital Dermatol Venereol. 2016;151(1):1–8. [PubMed] [Google Scholar]
- Bardazzi F, Tengattini V, Rucci P, et al. Socio-economic status and severity of plaque psoriasis: a cross-sectional study in the metropolitan city of Bologna. Eur J Dermatol. 2019;29(2):197–202. doi: 10.1684/ejd.2019.3524. [DOI] [PubMed] [Google Scholar]
- Biondi Oriente C, Scarpa R, Pucino A, Oriente P. Psoriasis and psoriatic arthritis. Dermatological and rheumatological co-operative clinical report. Acta Derm Venereol Suppl (Stockh) 1989;146:69–71. [PubMed] [Google Scholar]
- Caldarola G, De Simone C, Talamonti M, et al. Prevalence of cutaneous comorbidities in psoriatic patients and their impact on quality of life. Eur J Dermatol. 2019;29(2):192–6. doi: 10.1684/ejd.2019.3529. [DOI] [PubMed] [Google Scholar]
- Carpentieri A, Pacello L, De Marco IM, Loiacono A, Picconi O, Loconsole F. Retrospective analysis of the effectiveness and costs of traditional treatments for moderate-to-severe psoriasis: A single-center, Italian study. J Dermatolog Treat. 2016;27(5):399–405. doi: 10.3109/09546634.2015.1133885. [DOI] [PubMed] [Google Scholar]
- Cimmino MA. Epidemiology of psoriasis and psoriatic arthritis. Reumatismo. 2007;(59 Suppl 1):19–24. doi: 10.4081/reumatismo.2007.1s.19. [DOI] [PubMed] [Google Scholar]
- Colombo G, Altomare G, Peris K, et al. Moderate and severe plaque psoriasis: cost-of-illness study in Italy. Ther Clin Risk Manag. 2008;4(2):559–68. doi: 10.2147/tcrm.s2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti C, Currado D, Navarini L, et al. Moderate-to-severe plaque psoriasis, described by PASI>/=10%, can be associated with higher cardiovascular risk according to seven risk algorithms: Results of a 10-year single-center retrospective study and clinical management of psoriatic patients with cardiovascular risk. Dermatol Ther. 2020;33(6):e14451. doi: 10.1111/dth.14451. [DOI] [PubMed] [Google Scholar]
- Conti A, Giovannini L, Mandel VD, et al. Chronic kidney disease in psoriasis: a cohort study. J Dtsch Dermatol Ges. 2020;18(5):438–45. doi: 10.1111/ddg.14087. [DOI] [PubMed] [Google Scholar]
- De Socio A, Perrotta FM, Grasso GM, Lubrano E. Incidence of rheumatoid arthritis, psoriatic arthritis and polymyalgia rheumatica in an inland area of central Italy: results of the CAMPO-RHE study. Postgraduate medicine. 2018;130(1):137–41. doi: 10.1080/00325481.2018.1399774. [DOI] [PubMed] [Google Scholar]
- Esposito M, Faleri S, Babino G, et al. From patients’ needs to treatment outcomes in psoriasis: Results from the ‘pSORRIDI’ experience. J Int Med Res. 2016;44(1 suppl):95–9. doi: 10.1177/0300060515593265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M, Saraceno R, Giunta A, Maccarone M, Chimenti S. An Italian study on psoriasis and depression. Dermatology (Basel, Switzerland) 2006;212(2):123–7. doi: 10.1159/000090652. [DOI] [PubMed] [Google Scholar]
- Degli Esposti L, Perrone V, Sangiorgi D, et al. Analysis of drug utilization and health care resource consumption in patients with psoriasis and psoriatic arthritis before and after treatment with biological therapies. Biologics. 2018;12:151–8. doi: 10.2147/BTT.S168691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman SR, Burudpakdee C, Gala S, Nanavaty M, Mallya UG. The economic burden of psoriasis: a systematic literature review. Expert Rev Pharmacoecon Outcomes Res. 2014;14(5):685–705. doi: 10.1586/14737167.2014.933671. [DOI] [PubMed] [Google Scholar]
- Finzi A, Colombo D, Caputo A, et al. Psychological distress and coping strategies in patients with psoriasis: the PSYCHAE Study. J Eur Acad Dermatol Venereol. 2007;21(9):1161–9. doi: 10.1111/j.1468-3083.2007.02079.x. [DOI] [PubMed] [Google Scholar]
- Finzi AF, Benelli C. A clinical survey of psoriasis in Italy: 1st AISP report. Interdisciplinary Association for the Study of Psoriasis. Journal of the European Academy of Dermatology and Venereology: JEADV. 1998;10(2):125–9. [PubMed] [Google Scholar]
- Finzi AF, Mantovani LG, Belisari A. Italian Association for Studies on P. The cost of hospital-related care of patients with psoriasis in Italy based on the AISP study. Associazione Italiana Studi Psoriasi. Journal of the European Academy of Dermatology and Venereology: JEADV. 2001;15(4):320–4. [PubMed] [Google Scholar]
- Gisondi P. Hyperuricemia in patients with chronic plaque psoriasis. Drug Dev Res. 2014;(75 Suppl 1):S70–2. doi: 10.1002/ddr.21201. [DOI] [PubMed] [Google Scholar]
- Gisondi P, Barba E, Girolomoni G. Non-alcoholic fatty liver disease fibrosis score in patients with psoriasis. J Eur Acad Dermatol Venereol. 2016;30(2):282–7. doi: 10.1111/jdv.13456. [DOI] [PubMed] [Google Scholar]
- Gisondi P, Fantin F, Del Giglio M, et al. Chronic plaque psoriasis is associated with increased arterial stiffness. Dermatology (Basel, Switzerland) 2009;218(2):110–3. doi: 10.1159/000182256. [DOI] [PubMed] [Google Scholar]
- Gisondi P, Farina S, Giordano MV, Girolomoni G. Usefulness of the framingham risk score in patients with chronic psoriasis. Am J Cardiol. 2010;106(12):1754–7. doi: 10.1016/j.amjcard.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Gisondi P, Girolomoni G. Patients’ perspectives in the management of psoriasis: the Italian results of the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey. J Dermatolog Treat. 2017;28(5):390–3. doi: 10.1080/09546634.2016.1255308. [DOI] [PubMed] [Google Scholar]
- Gisondi P, Targher G, Zoppini G, Girolomoni G. Non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. J Hepatol. 2009;51(4):758–64. doi: 10.1016/j.jhep.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Luca M, Musumeci ML, Bardazzi F, et al. Biologic agents perception in patients attending for the first-time to psoriasis centers: a multicenter Italian survey. Giornale italiano di dermatologia e venereologia: organo ufficiale, Societa italiana di dermatologia e sifilografia. 2020;155(2):150–4. doi: 10.23736/S0392-0488.17.05754-6. [DOI] [PubMed] [Google Scholar]
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–85. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- Parodi A, Aste N, Calvieri C, et al. Metabolic syndrome prevalence in psoriasis: a cross-sectional study in the Italian population. American journal of clinical dermatology. 2014;15(4):371–7. doi: 10.1007/s40257-014-0074-8. [DOI] [PubMed] [Google Scholar]
- Pezzolo E, Ciampichini R, Cazzaniga S, Sampietro G, Zucchi A, Naldi L. Psoriasis severity matters when dealing with all-cause mortality in psoriasis patients: a record linkage analysis in Northern Italy. Archives for dermatological research = Archiv fur dermatologische Forschung. 2021;313(4):255–61. doi: 10.1007/s00403-020-02101-1. [DOI] [PubMed] [Google Scholar]
- Prignano F, Rogai V, Cavallucci E, Bitossi A, Hammen V, Cantini F. Epidemiology of Psoriasis and Psoriatic Arthritis in Italy-a Systematic Review. Current rheumatology reports. 2018;20(7):43. doi: 10.1007/s11926-018-0753-1. [DOI] [PubMed] [Google Scholar]
- Proietti I, Raimondi G, Skroza N, et al. Cardiovascular risk in psoriatic patients detected by heart rate variability (HRV) analysis. Drug Dev Res. 2014;(75 Suppl 1):S81–4. doi: 10.1002/ddr.21204. [DOI] [PubMed] [Google Scholar]
- Renzi C, Di Pietro C, Gisondi P, et al. Insufficient knowledge among psoriasis patients can represent a barrier to participation in decision-making. Acta dermato-venereologica. 2006;86(6):528–34. doi: 10.2340/00015555-0145. [DOI] [PubMed] [Google Scholar]
- Ricci F, Panebianco A, Fania L, et al. Age-specific prevalence of psoriasis in an unselected sample of 25,956 Italian hospital inpatients: evidence for selective excess mortality after 70 years of age. Eur J Dermatol. 2018;28(1):103–5. doi: 10.1684/ejd.2017.3177. [DOI] [PubMed] [Google Scholar]
- Saraceno R, Mannheimer R, Chimenti S. Regional distribution of psoriasis in Italy. Journal of the European Academy of Dermatology and Venereology: JEADV. 2008;22(3):324–9. doi: 10.1111/j.1468-3083.2007.02423.x. [DOI] [PubMed] [Google Scholar]
- Sardu C, Cocco E, Mereu A, et al. Population based study of 12 autoimmune diseases in Sardinia, Italy: prevalence and comorbidity. PloS one. 2012;7(3):e32487. doi: 10.1371/journal.pone.0032487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa R, Oriente P, Pucino A, et al. Psoriatic arthritis in psoriatic patients. Br J Rheumatol. 1984;23(4):246–50. doi: 10.1093/rheumatology/23.4.246. [DOI] [PubMed] [Google Scholar]
- Spandonaro F, Ayala F, Berardesca E, et al. The cost effectiveness of biologic therapy for the treatment of chronic plaque psoriasis in real practice settings in Italy. BioDrugs: clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2014;28(3):285–95. doi: 10.1007/s40259-014-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villacorta R, Teeple A, Lee S, Fakharzadeh S, Lucas J, McElligott S. A multinational assessment of work-related productivity loss and indirect costs from a survey of patients with psoriasis. The British journal of dermatology. 2020;183(3):548–58. doi: 10.1111/bjd.18798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarola G, De Simone C, Talamonti M, et al. Prevalence of cutaneous comorbidities in psoriatic patients and their impact on quality of life. Eur J Dermatol. 2019;29(2):192–6. doi: 10.1684/ejd.2019.3529. [DOI] [PubMed] [Google Scholar]
- Danielsen K, Duvetorp A, Iversen L, et al. Prevalence of Psoriasis and Psoriatic Arthritis and Patient Perceptions of Severity in Sweden, Norway and Denmark: Results from the Nordic Patient Survey of Psoriasis and Psoriatic Arthritis. Acta Derm Venereol. 2019;99(1):18–25. doi: 10.2340/00015555-3017. [DOI] [PubMed] [Google Scholar]
- Zheng X, Wang Q, Luo Y, et al. Seasonal Variation of Psoriasis and Its Impact in the Therapeutic Management: A Retrospective Study on Chinese Patients. Clin Cosmet Investig Dermatol. 2021;14:459–65. doi: 10.2147/CCID.S312556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhosle MJ, Kulkarni A, Feldman SR, Balkrishnan R. Quality of life in patients with psoriasis. Health Qual Life Outcomes. 2006;4:35. doi: 10.1186/1477-7525-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SC, Kimball AB, Liewehr DJ, Blauvelt A, Turner ML, Emanuel EJ. Quantifying the harmful effect of psoriasis on health-related quality of life. J Am Acad Dermatol. 2002;47(4):512–8. doi: 10.1067/mjd.2002.122755. [DOI] [PubMed] [Google Scholar]
- Christophers E, Barker JN, Griffiths CE, et al. The risk of psoriatic arthritis remains constant following initial diagnosis of psoriasis among patients seen in European dermatology clinics. Journal of the European Academy of Dermatology and Venereology: JEADV. 2010;24(5):548–54. doi: 10.1111/j.1468-3083.2009.03463.x. [DOI] [PubMed] [Google Scholar]
- Mahler R, Jackson C, Ijacu H. The burden of psoriasis and barriers to satisfactory care: results from a Canadian patient survey. J Cutan Med Surg. 2009;13(6):283–93. doi: 10.2310/7750.2009.08083. [DOI] [PubMed] [Google Scholar]
- Poulin Y, Papp KA, Wasel NR, et al. A Canadian online survey to evaluate awareness and treatment satisfaction in individuals with moderate to severe plaque psoriasis. Int J Dermatol. 2010;49(12):1368–75. doi: 10.1111/j.1365-4632.2010.04660.x. [DOI] [PubMed] [Google Scholar]
- Ismail D, McAteer H, Majeed-Ariss R, McPhee M, Griffiths CEM, Young HS. Research priorities and identification of a health-service delivery model for psoriasis from the UK Psoriasis Priority Setting Partnership. Clin Exp Dermatol. 2021;46(2):276–85. doi: 10.1111/ced.14407. [DOI] [PubMed] [Google Scholar]
- Qureshi AA, Husni ME, Mody E. Psoriatic arthritis and psoriasis: need for a multidisciplinary approach. Semin Cutan Med Surg. 2005;24(1):46–51. doi: 10.1016/j.sder.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Gianfredi V, Odone A, Fiacchini D, Rosselu R, Battista T, Signorelli C. Trust and reputation management, branding, social media management nelle organizzazioni sanitarie: Sfide e opportunity per la comunita igienistica italiana. Journal of Preventive Medicine and Hygiene. 2019;60(3):E108–E9. [Google Scholar]
- Mahroum N, Bragazzi NL, Sharif K, et al. Leveraging Google Trends, Twitter, and Wikipedia to Investigate the Impact of a Celebrity’s Death From Rheumatoid Arthritis. J Clin Rheumatol. 2018;24(4):188–92. doi: 10.1097/RHU.0000000000000692. [DOI] [PubMed] [Google Scholar]
- Gianfredi V, Provenzano S, Santangelo OE. What can internet users’ behaviours reveal about the mental health impacts of the COVID-19 pandemic? A systematic review. Public Health. 2021;198:44–52. doi: 10.1016/j.puhe.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfredi V, Nucci D, Balzarini M, et al. E-Coaching: the DianaWeb study to prevent breast cancer recurrences. Clin Ter. 2020;170(1):e59–e65. doi: 10.7417/CT.2020.2190. [DOI] [PubMed] [Google Scholar]
- Gianfredi V, Balzarini F, Gola M, et al. Leadership in Public Health: Opportunities for Young Generations Within Scientific Associations and the Experience of the “Academy of Young Leaders”. Front Public Health. 2019;7:378. doi: 10.3389/fpubh.2019.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorelli C, Fara GM. COVID-19: Hygiene and Public Health to the front. Acta Biomed. 2020;91(3-S):7–8. doi: 10.23750/abm.v91i3-S.9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


