Abstract
Background and aim of the work:
Bisphenol A (BPA) is a chemical product that is widely used as a plastic precursor. It acts directly on the kidney mitochondria, causing renal dysfunction. N-acetylcysteine is effective in protecting the kidneys from chemical-induced damage. Vitamin E is an antioxidant that protects cells from the damaging effects of free radicals. The aim of this study is to further evaluate and compare NAC and vitamin E to oppose the nephrotoxicity caused by BPA.
Research design and Methods:
Forty-two adult male rats were divided into 7 groups: control, BPA, NAC, vitamin E, BPA plus NAC, BPA plus vitamin E, and combined BPA, NAC and vitamin E. BPA, NAC, vitamin E were given orally at doses of 50 mg/kg, 200 mg/kg, and 1000 mg/kg respectively, for 5 weeks.
Results:
NAC and vitamin E groups showed improved kidney function tests and alleviated BPA-induced oxidative stress; increased GSH and decreased MDA, NO and iNOS levels. NAC and vitamin E significantly attenuated inflammation; decreased NF-κB and increased IL-4, and Nrf2, in addition there was alleviation of renal histopathology. To some extent, vitamin E administration showed significant improvement. Moreover, combined NAC and vitamin E treatment showed more significance than either NAC or vitamin E separate groups.
Conclusions:
This study determined the substantial protective effects of NAC and/or vitamin E in BPA-induced nephrotoxicity through modulation of Nrf2 with subsequent improvement of oxidative stress and inflammation. The alleviation was more significant in combined NAC and vitamin E treatment mainly through their synergistic effect on Nrf2. (www.actabiomedica.it)
Keywords: Bisphenol A, nephrotoxicity, N-acetylcysteine, Vitamin E, Nrf2, iNOS, NF-κB
Introduction
Bisphenol A (BPA), a precursor of polycarbonate plastics and epoxy resins, is widely used in numerous products; thermal receipt paper, metal cans, water pipes, cigarette filters, drinking bowls, sports equipment, medical equipment and plastic toys (1). BPA exposure could occur through ingestion, inhalation and dermal absorption (2). Notably, BPA exposure has increased with coronavirus disease (COVID-19) pandemics as the Infectious Diseases Society of America (IDSA) has included the use of private protecting equipment, consisting of masks and face shields, in its recommendations for simple restrictions, which increase the use of plastic face shields in developing countries (3).
BPA exposure can induce oxidative damage in tissues by enhancing mitochondrial free radical production owing to disrupting the redox status, producing various reactive oxygen species (ROS) (4). BPA targets the kidney mitochondria causing mitochondrial oxidative stress (OS) and whole organ damage (5). Chemical exposure acts as an inflammatory stimulus directly or subsequently to increase of OS resulting in synthesis and secretion of numerous proinflammatory cytokines (6). The transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) controls the expression of genes involved in inflammation (7). Contrary, interleukin 4 (IL-4); a cytokine that functions as a powerful regulator of immunity, acts as an anti-inflammatory due to its ability to down-regulate the production of inflammatory cytokines (8). Nuclear factor erythroid 2-related factor 2 (Nrf2), acts as a main regulator of antioxidant defenses. Nrf2 translocates to the nucleus upon activation by oxidative stress. Overexpression of Nrf2 has been demonstrated to be cytoprotective in tissues (9). Nrf2 plays a central role in controlling the expression of antioxidant genes that ultimately exert anti-inflammatory functions. Nrf2 plays a pivotal role in maintaining intracellular redox homeostasis and regulating inflammation by regulating the heme-oxygenase-1 axis, which is a potent anti-inflammatory target (10).
N-acetylcysteine (NAC), a glutathione precursor, is a well-known drug that is widely used and has a high safety record. It has multiple pleiotropic effects such as antioxidant, anti-inflammatory and anti-apoptotic effects. It also has a protective effect against chemical-related organ damage (11, 12). Additionally, the role of NAC as a mitochondrial protectant for organ maintenance has recently been highlighted (13). Since mitochondrial changes and oxidative stress have been closely linked to BPA toxicity, NAC may be a useful tool for dealing with BPA-induced kidney damage (5).
Vitamin E is a fat-soluble vitamin that has antioxidant and anti-inflammatory properties that protect the cells against the effects of free radicals (14). It has been reported that vitamin E has a nephroprotective effect by alleviating oxidative stress in several experimental animal models (15).
To the best of our knowledge, this research is the first to evaluate and compare the potential protective effect of NAC versus vitamin E and their combination in BPA-induced nephrotoxicity through their influence on Nrf2 gene upregulation with subsequent alleviation of oxidative stress and inflammation mediated renal damage.
Materials and methods
Animals
Forty-two male adult albino rats (average weight 200 ± 20 gm) were used. They were permitted free access to water and food under an ordinary day-night cycle (12 -12 h).
Chemicals used
BPA, NAC and vitamin E were obtained from Sigma-Aldrich (St. Louis, MO, USA). Corn oil was obtained from Elgomhoria Company for Pharmaceuticals (Mansoura, Egypt). Antibodies against Nrf2 and inducible nitric oxide synthase (iNOS) were purchased from Abcam (Egypt).
Study design
After two weeks of adaptation, the rats were separated into 7 groups (6 rats in each group): the 1st group (control group) that given corn oil at a dose of 0.5 millilitre (ml)/kilogram (kg) daily, the 2nd group (BPA group) that received BPA at a dose of 50 milligram (mg)/kg/day dissolved in corn oil (16), the 3rd group (NAC group) that received NAC at a dose of 200 mg/kg/day (17), the 4th group (vitamin E group) that received vitamin E at a dose of 1000 mg/kg/day dissolved in corn oil (18), the 5th group (BPA + NAC) that received BPA at a dose of 50 mg/kg/day plus NAC at a dose of 200 mg/kg/day, the 6th group (BPA + vitamin E) that received BPA at a dose of 50 mg/kg/day plus vitamin E at a dose of 1000 mg/kg/day and the 7th group (BPA + NAC + vitamin E) that received BPA at a dose of 50 mg/kg/day, NAC at a dose of 200 mg/kg/day plus vitamin E at a dose of 1000 mg/kg/day. All chemicals were administered orally via gastric gavage for 5 weeks. Selected doses were chosen upon previous studies of the NAC and vitamin E protective effects against toxin - induced cellular dysfunction (17,18).
Sample collection and processing of specimens
One day after the last dose, the rats were anesthetized with intraperitoneal sodium thiopental (120 mg/kg). Blood samples were obtained from the tail vein to assess renal functions. Kidneys were dissected out. One of the two kidneys was cut sagittal into equal halves. Fresh homogenates from one half were used to assess oxidative stress markers and inflammatory mediators, while the other half was used for estimation of Nrf2 gene expression. The other kidney was handled to prepare paraffin blocks. Sections (3–5 micrometres (µm) thick) were sliced and stained with haematoxylin and eosin (H &E) and periodic acid Schiff (PAS) stains. Also, other slices were immunostained for iNOS and Nrf2 expression.
Assessments of renal function
Blood samples were taken one day after the last dose; to assess serum urea levels, creatinine (Cr) and blood urea nitrogen (BUN) were measured using commercially accessible colorimetric kits from Biodiagnostic Company (Cairo, Egypt) (19).
Preparation of renal tissue homogenate
The renal tissues were washed away and rinsed with ice. These were dabbed lightly between the folds of filter paper and weighed in an analytical scale. 10% of homogenate was arranged in phosphate buffer 0.05 molarity [M], (pH 7) by a polytron homogenizer at 4 Celsius (°C). The homogenates were centrifuged at 10,000 revolutions per minute (rpm) for 20 minutes (min) for the removal of cell debris, intact cells, cell nuclei, erythrocytes and mitochondria. The supernatant was divided and stored at−80 °C (20).
Assessment of renal oxidative stress
Renal homogenates were taken and centrifuged to calculate the levels of malondialdehyde (MDA), nitric oxide (NO) and glutathione (GSH) by using viable colorimetric kits from the Biodiagnostic Company (Cairo, Egypt).
Assessments of renal NF-κB and IL-4
Levels of NF-κB and IL-4 proteins were estimated in renal tissue homogenate using commercial enzyme-linked immunoassay (ELISA) kits (Cloud-Clone Corp., USA) along with the manufacturer’s instructions.
Assessment of renal Nrf2 gene expression
Expression of the Nrf2 gene expression was assessed in renal tissues using real-time quantitative polymerase chain reaction (PCR). Total ribonucleic acid (RNA) was taken out from tissue lysate with Direct-zol RNA Miniprep Plus (Cat# R2072, Zymo Research Corp, USA). Reverse transcription polymerase chain reaction was performed using a Maxima First Strand deoxyribonucleic acid (cDNA) Synthesis Kit (Thermo Fisher Scientific, USA) along with manufacturer’s instructions. The following primers were used: Nrf2 Forward, 5′- AGCAGGACATGGATTTGATT -3′ and reverse, 5′- CTTCTCCTGTTCCTTCTGGA -3′, β-actin forward, 5′- CTAAGGCCAACCGTGAAAAG -3′, and reverse, 5′- GCCTGGATGGCTACGTACA -3′.
Relative quantitation of gene expression was done by an asymmetrical cyanine dye SYBR green master mix (Bioline, UK) and Practical Biosystems™ 7500 real‐time PCR (RT-PCR) apparatus. The program used involved an initial cycle of 2 min at 95°C for polymerase activation, subsequently 40 cycles of initial denaturation at 95°C for 5 second (s), after that an annealing/extension step at 60°C for 30 s. After the RT-PCR run, the data were reported at the cycle threshold (Ct). The relative quantification (RQ) of the target gene was quantified and normalized to housekeeping gene (β-actin) along with the calculation of delta-delta Ct (ΔΔCt). The relative expression levels of mRNAs were considered using the 2−ΔΔCt method (21).
Histopathological examination of renal tissues
Haematoxylin and Eosin stain was performed to study the normal histological structure of the rat kidney and to evaluate the histopathological results among all experimental groups (22). Periodic Acid Schiff (PAS) staining was performed to detect changes in the brush border of the proximal convoluted tubules (PCT) and tubular basement membrane (23).
Immunohistochemical expression of Nrf2 and iNOS
Endogenous peroxidases were blocked with 0.3% H2O2. Antigen retrieval was done using sodium citrate buffer (pH=6) and heating in a microwave for 20 min, and in tris-buffered saline it was blocked with 5% bovine serum albumin. After that, sections were incubated all the night at 4 °C with a primary antibody against Nrf2 and iNOS. The reaction was developed using Avidin/Biotinylated enzyme Complex (ABC) kit along with the manufacturer’s instructions (Abcam, Egypt). Lastly, the sections were counterstained with haematoxylin, dehydrated and mounted with a synthetic resin medium (24).
Image analysis for quantification of the area percentage of positive iNOS and Nrf2 immune reactions
Using a light microscope (Olympus model BX53, Tokyo, Japan) attached to a digital camera (Toucan model BX53, Japan) connected to a computer, images were captured. The renal cortex was examined in four randomly spaced, 5 μm thick sections at a 400x magnification with a 40x lens (area: 0.071mm2). Computerized image analysis was done using Image-j. immune expression was visible as a brownish pigmentation. Utilizing a colour deconvolution plugin and the haematoxylin-3,3’-Diaminobenzidine (HDAB) vector as the chosen colour, three distinct coloured images (green, brown, and blue) were produced. By calculating the area fraction, the DAB images (brown in colour) were calibrated (25).
Statistical analysis
The results were expressed as the mean ± standard deviation (SD). The data were investigated by Statistical Package for the Social Sciences (SPSS) computer software system. Statistical differences were evaluated by one-way ANOVA test followed by post hoc test to distinguish pairwise difference, whereas probability (P) value less than 0.05 was expressed as significant.
Ethical approval
The Institutional Research Board (IRB) approved the study (Code number: R.21.01.1155.R1), Faculty of Medicine, Mansoura University and the study came along with the National Institutes of Health (NIH) and European Union (EU) guidelines for animal care. The animals were housed under veterinary supervision at the Medical Experimental Research Centre (MERC), where the experiment was performed. All efforts were made to diminish the number of animals used and the animal distress.
Results
Renal function assessment results
The BPA group revealed a significant increase in serum creatinine, urea and BUN levels compared to the control group (P < 0.0005, 0.001, 0.001 respectively). NAC administration caused a significant decline in serum creatinine, urea and BUN levels versus the BPA group (P < 0.05). Likewise, vitamin E administration caused a significant decline in the serum creatinine, urea and BUN levels compared to the BPA group (P < 0.001). Moreover, combined NAC and vitamin E treatment showed a significant reduction in serum creatinine, urea and BUN levels versus the BPA group (Figure 1).
Figure 1.
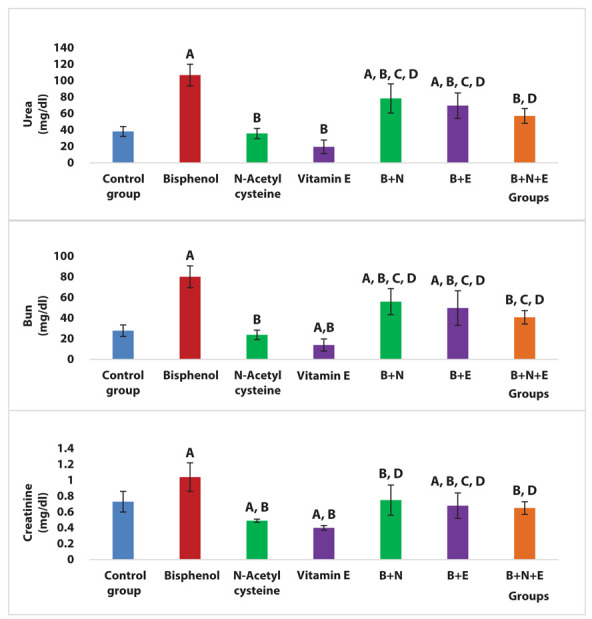
Effects of NAC and vitamin E treatment on serum creatinine, urea and BUN levels, P ≤ 0.05 was significant. A: comparison regarding control group. B: comparison regarding BPA group. C: comparison as the regard NAC group. D: comparison regarding vitamin E group. E: comparison regarding BPA + NAC group. F: comparison regarding BPA + Vitamin E group.
Renal oxidative stress assessment results
There was a significant increase in MDA and NO and a significant decline in GSH levels in the BPA group compared to the control group (P < 0.001). BPA + NAC group revealed a significant reduction in MDA and a significant increase in GSH levels versus BPA group (P < 0.001). Also, BPA + Vitamin E group showed a significant decline in MDA and NO and a significant increase in GSH levels compared to the BPA group (P < 0.001). Moreover, BPA+ NAC + Vitamin E group demonstrated a significant decrease in MDA and NO and a significant increase in GSH levels versus BPA group and BPA + NAC group (P < 0.001, 0.002 respectively) (Figure 2).
Figure 2.
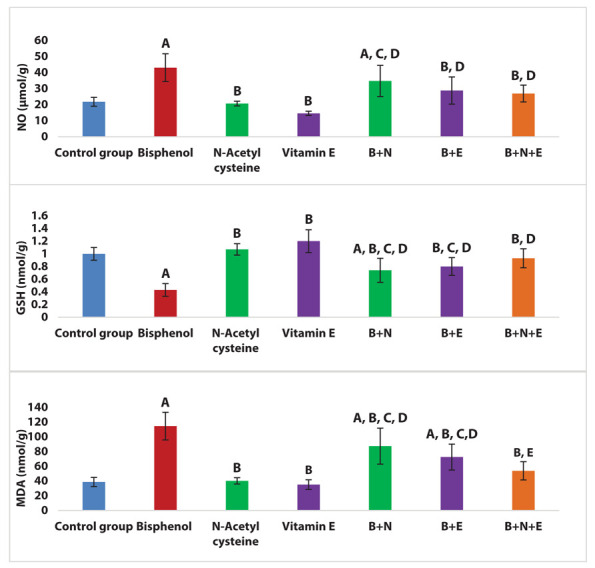
Effects of NAC and vitamin E treatment on renal oxidative stress markers (MDA, NO and GSH), P ≤ 0.05 was significant. A: comparison regarding control group. B: comparison regarding BPA group. C: comparison regarding the NAC group. D: comparison regarding vitamin E group. E: comparison regarding BPA + NAC group. F: comparison regarding BPA + Vitamin E group.
Renal NF-κB and IL-4 assessment results
There was a significant increase in NF-κB and a significant decline in IL-4 levels in the BPA group compared to the control group (P < 0.001). Both BPA + NAC group and BPA + vitamin E group showed a significant reduction in NF-κB and a significant increase in IL-4 levels compared to the BPA group (P < 0.001, 0.001 respectively). Moreover, BPA + vitamin E group showed a significant decline in NF-κB and a significant increase in IL-4 levels compared to the BPA + NAC group (P < 0.001). Additionally, BPA+ NAC + vitamin E group showed a significant reduction in NF-κB and a significant increase in IL-4 levels compared with the BPA group, BPA + NAC group and BPA + vitamin E group (P < 0.001) (Figure 3).
Figure 3.
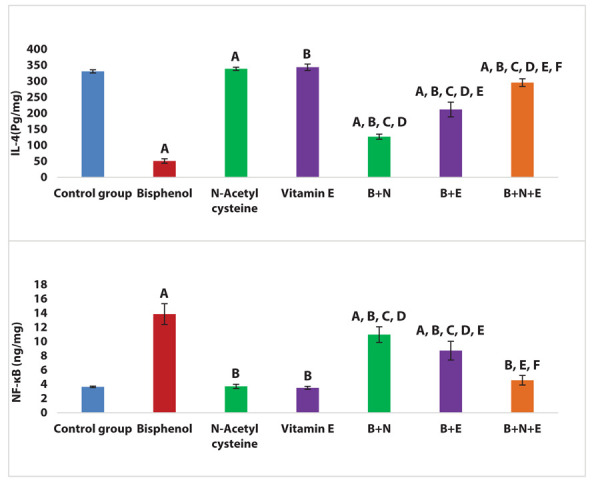
Effects of NAC and vitamin E treatment in renal NF-κB and IL-4, P ≤ 0.05 was significant. A: comparison regarding control group. B: comparison regarding BPA group. C: comparison regarding the NAC group. D: comparison regarding vitamin E group. E: comparison regarding BPA + NAC group. F: comparison regarding BPA + Vitamin E group.
Renal Nrf2 gene expression results
There was a significant reduction in the renal expression of the Nrf2 gene in the BPA group compared to the control group (P < 0.001). Both BPA + NAC group and BPA + vitamin E group showed a significant increase in Nrf2 gene renal expression compared with the BPA group (P < 0.001). Moreover, BPA + Vitamin E group showed a significant increase in renal expression of Nrf2 compared with the BPA + NAC group (P < 0.001). BPA+ NAC + vitamin E group showed a significant increase in renal expression of Nrf2 gene compared with the BPA group, BPA + NAC group and BPA + vitamin E group (P < 0.001) (Figure 4).
Figure 4.
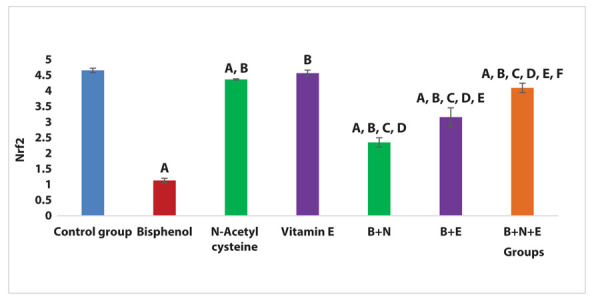
Effects of NAC and vitamin E treatment on renal Nrf2 gene expression, P ≤ 0.05 was significant. A: comparison regarding control group. B: comparison regarding BPA group. C: comparison as the regard NAC group. D: comparison regarding vitamin E group. E: comparison regarding BPA + NAC group. F: comparison regarding BPA + Vitamin E group.
Histopathological examination results
The control, NAC and vitamin E groups showed preserved renal architecture in H & E-stained sections. The glomeruli were bounded by Bowman’s capsule, which consisted of two layers separated by Bowman’s space (Figures 5a, 6a, 6b, respectively). In the BPA group, there was a loss of architecture of some renal tubules, with other tubules that appeared vacuolated. There was a mononuclear cell infiltrate in the renal interstitium with congested renal vessels. Also, hyaline casts appeared in some tubule’s lumens (Figures 5b, 5c, 5d). The administration of NAC or vitamin E with BPA restored the shape of most of the renal tubules and glomeruli. However, some degenerated and vacuolated tubules and mononuclear cellular cell infiltrate were still detected in the interstitium (Figures 6c, 6d). Co-administration of both NAC and vitamin E showed restoration of the renal architecture. However, some vacuolated tubules were still seen (Figures 7a and 7b).
Figure 5.
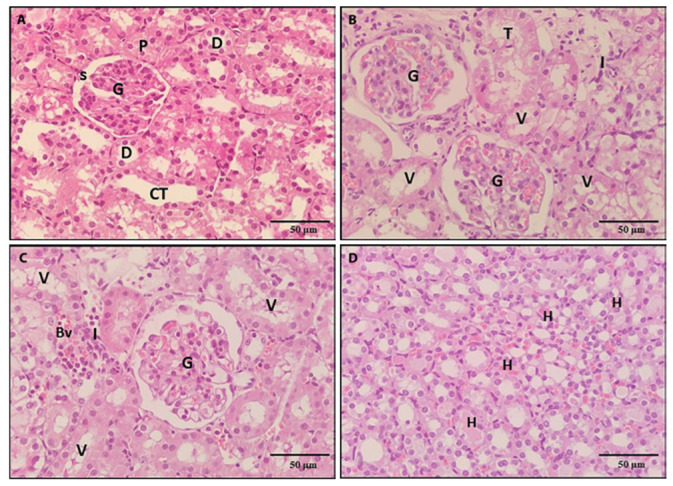
A photomicrograph of a kidney section, 5a: section of a control rat revealed preserved architecture of the proximal convoluted tubules (P), the glomerulus (G), Bowman’s space (S), Distal convoluted tubules (D) and collecting tubules (CT). 5b, 5c, 5d: Sections from rats treated with BPA showing loss of the architecture of some tubules (T) whose cells were completely degenerated. Renal tubules appeared vacuolated (v), mononuclear cells infiltrated in the interstitium (I), dilated congested blood vessels (BV) and hyaline casts (H) in some tubular lumens (H & E x 400).
Figure 6.
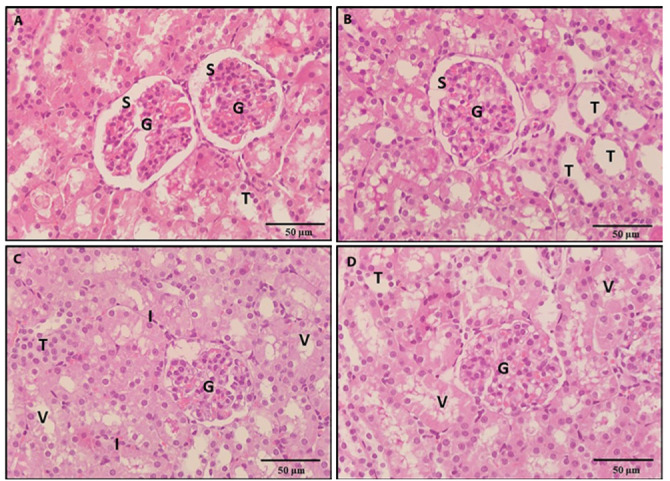
A photomicrograph of a kidney section, 6a: A section of a rat treated with NAC revealed a preserved architecture. 6b: A section of a rat treated with vit E revealed normal kidney histological structure. 6c: A section of a rat treated with BPA and NAC revealed restoration of the shape of most of the renal tubules and glomeruli (G). However, some degenerated (T), vacuolated tubules (v) and mononuclear cellular cell infiltrate (I) in the interstitium were seen. 6d: A section of a rat treated with BPA and vitamin E revealed restoration of the normal shape of the renal structure. However, some vacuolated tubules (v) were seen (H & E x 400).
Figure 7.
A photomicrograph of a kidney section of rats treated with BPA, NAC and vitamin E revealed restoration of the shape of most of the renal tubules (7a, 7b). However, some degenerated (T) and vacuolated tubules (v) were observed (H & E x 400).
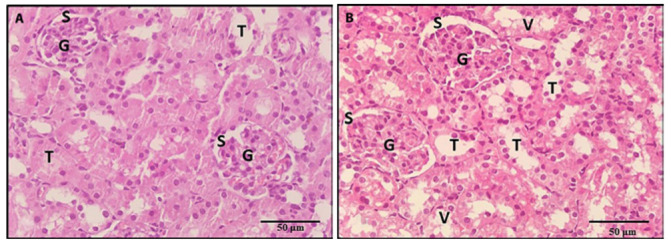
Sections stained with PAS showed, strong positive reaction to PAS in the renal tubular basement membrane, brush border of PCT and the parietal layer of Bowman’s capsule in the control, NAC and vitamin E groups (Figures 8a, 8c, 8d respectively). In the BPA group, there were areas of basement membrane loss of some renal tubules (Figure 8b). Administrations of NAC or vitamin E with BPA produced a positive PAS reaction in Bowman’s capsule parietal layer and basement membrane of most of the renal tubules. However, there was interrupted brush border of some PCT (Figures 9a, 9b). There was a restoration of the strong positive reaction to PAS in the basement membrane of renal tubules, the parietal layer of Bowman’s capsule and the brush border of PCT in co-administrations of NAC and vitamin E with BPA. However, local areas of tubular basement membrane loss were still observed (Figures 9c and 9d).
Figure 8.
A photomicrograph of a kidney section, 8a: Section of a control rat revealed a strong PAS-positive reaction in the renal tubules basement membrane, brush border of PCT and Bowman’s capsule parietal layer (arrows). 8b: Section of a rat treated with BPA revealed an interrupted brush border of PCT (*). There were areas of loss of basement membrane of renal tubules (arrows). 8c, 8d: Sections of rats treated with either NAC or vitamin E revealed a strong PAS-positive reaction in the renal tubules basement membrane (arrows) (PAS x 400).
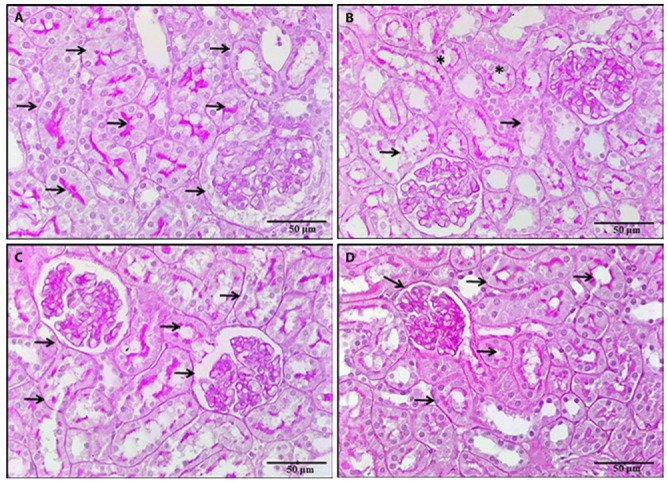
Figure 9.
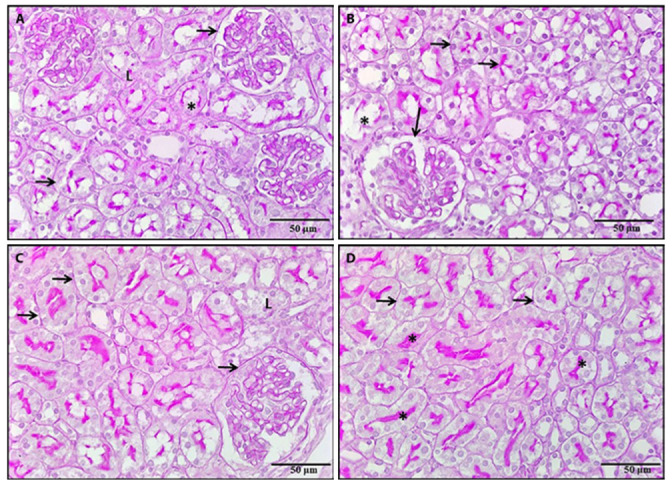
A photomicrograph of a kidney section, 9a: A section of a rat treated with BPA and NAC revealed a positive PAS reaction in the Bowman’s capsule parietal layer and most of the renal tubules basement membrane (arrows). However, there were areas of loss of basement membrane of renal tubules (L) and interrupted brush border of some PCT (*). 9b: A section of rat treated with BPA and vitamin E revealed a positive PAS reaction in the parietal layer of Bowman’s capsule, most of the renal tubules basement membrane and brush border of most PCT (arrows). However, there was an interrupted brush border of some PCT (*). 9c, 9d: Sections of rats treated with BPA, NAC and vitamin E revealed strong positive PAS reactions in the renal tubules basement membrane, the parietal layer of Bowman’s capsule (arrows) and brush border of PCT (*). However, local areas of tubular basement membrane loss were seen (L) (PAS x 400).
Immunohistochemical expression of iNOS results
Renal sections were immunostained with iNOS to determine whether the renoprotective effects of NAC or vitamin E were related to reduced oxidative stress or not. The control, NAC and vitamin E groups showed negative reactions for iNOS (Figures 10a, 10c and 10d). The BPA group revealed strong positive iNOS immunostaining in most of the glomerular cells (Figure 10 b). The administration of NAC or vitamin E with BPA revealed positive iNOS-immunostaining in some of the glomerular cells (Figures 11a and 11b). The group treated with BPA, NAC and vitamin E showed a marked reduction in the number of iNOS-positive glomerular cells (Figures 11c and 11d).
Figure 10.
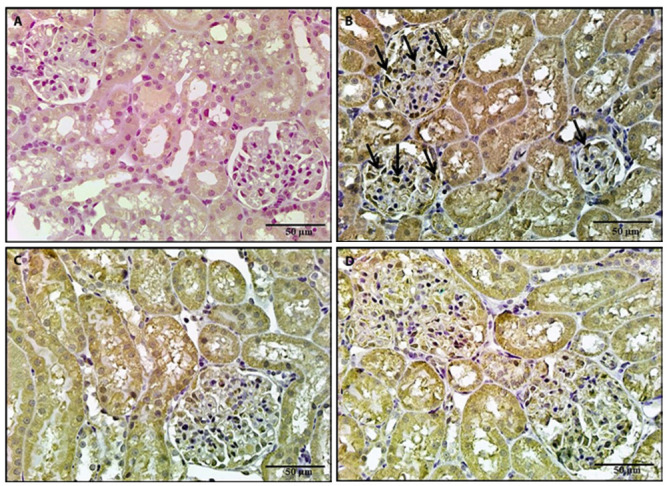
A photomicrograph of a kidney section, 10a: A section of a control rat revealed negative iNOs immunostaining. 10b: A section of a rat treated with BPA revealed positive iNOs immunostaining. 10c, 10d: Sections of rats treated with either NAC or vitamin E revealed negative iNOs immunostaining (iNOS x 400).
Figure 11.
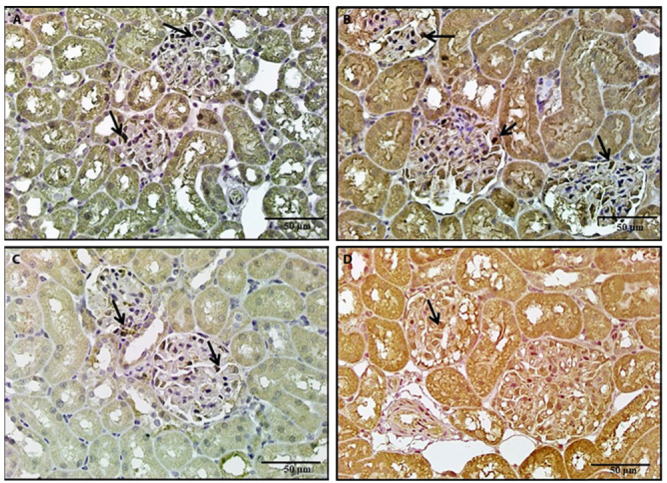
A photomicrograph of a kidney section, 11a: Section of a rat treated with BPA and NAC revealed positive iNOs immunostaining in some of the glomerular cells. 11b: A section of a rat treated with BPA and vitamin E revealed positive iNOs immunostaining in some of the glomerular cells. 11c, 11d: Sections of rats treated with BPA and NAC and vitamin E revealed a marked reduction in iNOs immunostaining (iNOS x 400).
Immunohistochemical expression of Nrf2 results
The control group showed a negative reaction for Nrf2 immunostaining (Figure 12a). Regarding the NAC group and vitamin E group, there was a mild increase in Nrf2 immunostaining in the glomeruli and renal tubules (Figures 12c and 12d). However, there was a weak positive Nrf2 immunostaining in the glomeruli and renal tubules in the BPA group (Figure 12b). The administration of NAC or vitamin E with BPA revealed a moderate increase in Nrf2 immunostaining in the renal tubules and glomeruli (Figures 13a and 13b). The group treated with BPA, NAC and vitamin E showed a marked increase in Nrf2 immunostaining in the glomeruli and renal tubules (Figures 13c and 13d).
Figure 12.
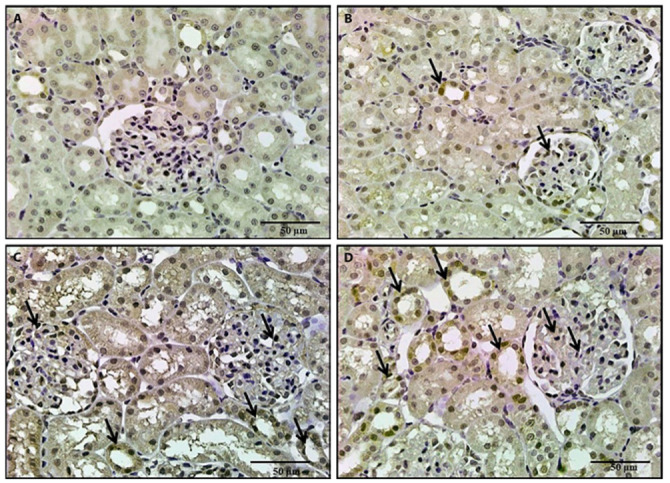
A photomicrograph of a kidney section, 12a: A section of the control rat revealed negative immunostaining. 12b: A section of rat treated with BPA revealed weak positive immunostaining in the glomeruli and renal tubules. 12c, 12d: Sections of rats treated with either NAC or vitamin E revealed mild increase in immunostaining in the glomeruli and renal tubules (Nrf2 x 400).
Figure 13.
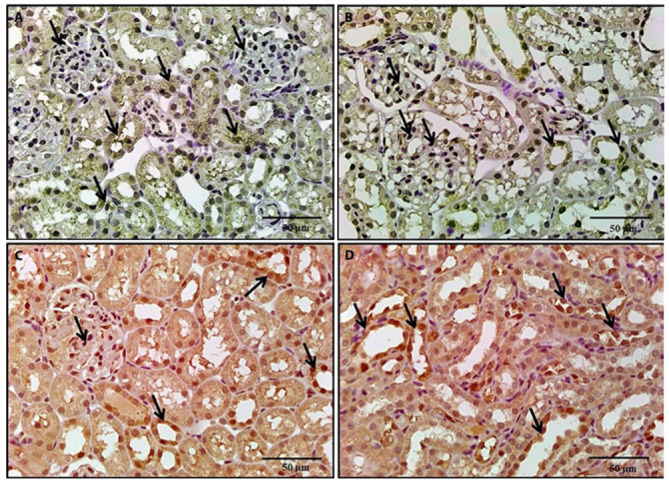
A photomicrograph of a kidney section, 13a: A section of a rat treated with BPA and NAC revealed a moderate increase in immunostaining in renal tubules and glomeruli. 13b: A section of a rat treated with BPA and vitamin E revealed a moderate increase in immunostaining. 13c, 13d: Sections of rats treated with BPA, NAC and vitamin E for revealed a marked increase in renal tubular and glomerular immunostaining (Nrf2 x 400).
Image analysis results of iNOS and NRF2 immune reactions
Regarding the iNOS area percentage of positive immune reaction, there was a significant increase in the BPA group compared to the control, NAC and vitamin E groups (P=0.004, 0.008, 0.007 respectively). Co-administration of NAC with BPA resulted in a significant decrease (P=0.02). Additionally, co-administration of vitamin E with BPA caused more significant decline (P=0.007). Interestingly, combined administration of NAC and vitamin E with BPA resulted in a significant reduction as the regard BPA group (P=0.02) (Figure 14).
Figure 14.
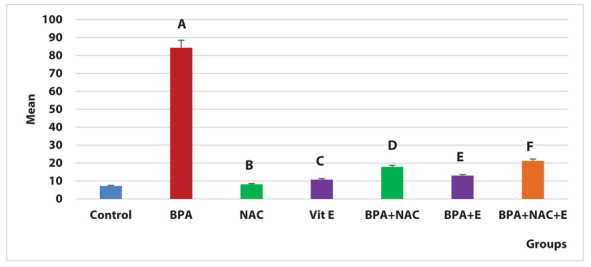
Area percentage of positive iNOS immune reaction in the study groups, A indicates significance between BPA group and control group (P=0.004). B indicates the significance between the BPA group and NAC group (P= 0.008). C indicates significance between the BPA group and vitamin E group (P=0.007). D indicates significance between BPA group and BPA + NAC group (P=0.02). E indicates significance between BPA group and BPA + vitamin E group (P=0.007). F indicates significance between BPA group and BPA + NAC + vitamin E group (P= 0.02).
According to the Nrf2 area percentage of positive immune reactions, BPA group showed a significant decrease compared with the BPA + NAC and BPA + vitamin E groups (P=0.01, 0.02 respectively). The combined administration of NAC and vitamin E with BPA resulted in a significant increase compared with the BPA group (P=0.001) (Figure 15).
Figure 15.
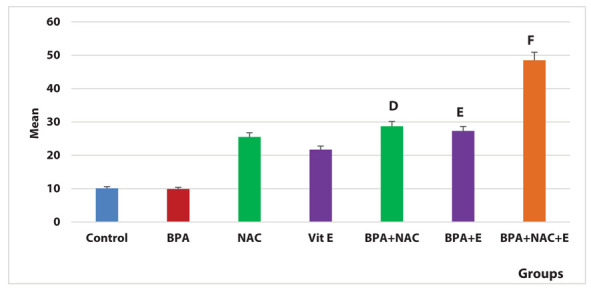
Area percentage of positive Nrf2 immune reaction in the study groups, D indicates significance between BPA group and BPA + NAC group (P=0.01). E indicates significance between BPA group and BPA + vitamin E group (P=0.02). F indicates significance between BPA group and BPA + NAC + vitamin E group (P= 0.001).
Discussion
Bisphenol A (BPA) is a chemical substance of great interest because of its worldwide use (26). BPA exposure, especially in manufacturing workers, has resulted in adverse health effects in multiple organs (27). There is a relationship between high serum BPA levels and increased oxidative stress and inflammatory markers (28).
The current study assessed and compared the renoprotective effects of NAC, vitamin E and their combination in BPA-induced nephrotoxicity in rats. It also explored their underlying mechanisms against BPA-induced nephrotoxicity through modulation of the Nrf2/ NF-κB /ROS molecular pathway.
Results of the current study revealed that there was a deterioration of kidney function determined by significantly elevated serum creatinine, urea and BUN levels in the BPA group compared with the control group. These results reflected that BPA has a negative impact on the glomerular and/or tubular functions resulted in impairment in their ability to excrete waste products. These findings were in accordance with that of Olea-Herrero et al. (29) who explained that BPA can induce podocytopathy with proteinuria by diminishing the synthesis of the proteins involved in the podocyte survival.
NAC administration with BPA caused a significantly reduced serum creatinine, urea and BUN levels compared with the BPA group. These findings were in coherence with those of Peerapanyasut et al. (30) who demonstrated that NAC protected the mitochondria in several experimental models, including BPA-induced neurotoxicity and nephrotoxicity due to its powerful antioxidant, anti-inflammatory and anti-apoptotic effects. Also, this study revealed that vitamin E administration induced a marked reduction in serum creatinine, urea and BUN levels compared with the BPA group. Our results were in agreement with Darwish et al. (31) and Al-Attar (32) who found that vitamin E possesses protective effects against heavy metals-induced nephrotoxicity. Vitamin E protects the kidneys by reducing renal structural damage, inhibiting glomerulosclerosis as well as interacts with lipid radicals, scavenges these free radicals protecting the cell membrane from oxidation and restores the enzymatic antioxidant defenses by elevating the levels of superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) in the blood (33).
Regarding ROS, the current study showed that BPA administration caused a significant increase in renal MDA, NO in addition to, a significantly reduced GSH levels in comparison with the control group. Such results were in agreement with those of Khan et al. (34) and Peerapanyasut et al. (30) who found that BPA-induced functional impairment of the mitochondria in addition to dynamic disruption including increased ROS generation by the mitochondria, decreased mitochondrial membrane potential with mitochondrial swelling and abnormal mitochondrial morphology and quantity.
The administration of NAC with BPA showed a significant reduction in MDA and a significant elevation of GSH level compared to the BPA group. These findings were comparable with Zhang et al. (35) and Small et al. (36) who found that NAC attenuated kidney cortical tubular epithelial cells and achieved protection due to detoxification of ROS and subsequently caused improved mitochondrial membrane destruction. Additionally, Nascimento et al. (37) reported that NAC is a crucial precursor for various endogenous antioxidants responsible for peroxide decomposition. Also, it might have a direct antioxidant effect via acting as free radicals’ scavenger (36). Likewise, combined administration of vitamin E and BPA exhibited a significantly reduced MDA and NO along with significantly elevated GSH levels compared with the BPA group. These findings were consistent with Badgujar et al. (38) who suggested that vitamin E can reduce the lipid peroxidation, retrieve the activity of many antioxidant enzymes and elevate GSH in the cytoplasm, an essential element for ROS removal. Moreover, Abdel-Daim et al. (18) illustrated that vitamin E might neutralize ROS and up-regulate the antioxidant enzyme activity guards the cells against lipid peroxidation, DNA destruction, as well as cell death.
Moving to renal inflammatory and anti-inflammatory mediators, the current study revealed that BPA administration caused a significantly elevated renal NF-κB along with significantly reduced IL-4 levels compared to the control group. The increase in NF-κB level in BPA-induced nephrotoxicity was consistent with Song et al. (39) who concluded that NF-κB pathway might play a pivotal role in renal destruction via the activation of the enhanced complement.
Contrary, BPA and NAC group exhibited a significantly reduced renal NF-κB and significantly increased IL-4 levels in comparison with BPA group. Our results were in consistency with Güntürk et al. (40) who reported that NAC induces cytoplasmic inhibition of NF-kB kinase. Co-administration of vitamin E with BPA also showed a significantly decreased renal NF-κB and a significantly elevated IL-4 levels as compared to both BPA group and BPA + NAC group. These results were in agreement with Glauert (41) who explained that the beneficial role of vit E might be attained via the reduction of oxidative stress or through one of its non-antioxidant functions. The anti-inflammatory activity of vitamin E played a key role in its renoprotection, as proved by Bjelakovic et al. (42).
Inducible nitric oxide synthase is not expressed under normal circumstances; conversely, it up-regulated in appearance of inflammatory conditions in renal epithelium, neutrophils, as well as T lymphocytes (43). In this study, iNOS expression in the BPA group was strong positive in most of the glomerular cells compared to the control group. This result suggested that BPA induced oxidative stress by altering the expression of iNOS. Such finding was in accordance with Chouhan et al. (44) who demonstrated an increase in iNOS expression in the testes of BPA-treated mice. In contrast, iNOS expression decreased after the administration of NAC, vitamin E and their combination. The antioxidant capacity of both NAC and vitamin E has been linked to lower iNOS expression of (37, 18).
For further clarification of the molecular mechanism of BPA - induced nephrotoxicity and the consequent protective effects of NAC and vitamin E, we measured Nrf2 that considered the master regulator of antioxidant defences. Nrf2 was confirmed to reduce ROS production and inhibit the progression of inflammation (45). Bisphenol A produced a significant reduction in the renal expression of Nrf2 in comparison with the control group. Such finding was compatible with Alekhya Sita et al. (46) and Mohammed et al. (47) who reported that BPA nephrotoxicity were related to inflammation and oxidative stress, which alter Nrf2 expression.
In contrast, both BPA + NAC group and BPA + vitamin E group revealed significantly elevated renal expression of Nrf2 in comparison with the BPA group. Interestingly, BPA + vitamin E group revealed a more significant elevation in the renal expression of Nrf2 in comparison with the BPA + NAC group. Nrf2 immunstaining was done for further confirmation; there was a marked decrease in immunostaining of glomeruli and renal tubules in the BPA group and it increased after administration of vitamin E and/or NAC. These results were in coherence with Zhu et al. (48) and Small et al. (36) who proposed that NAC and vitamin E can activate Nrf2 after renal insult. The protective impacts of antioxidant response that was regulated via Nrf2 is essentially due to its transcriptional control via hundreds of antioxidant genes including heme oxygenase-1, which has important cytoprotective, anti-inflammatory and antioxidant properties (49, 50). Vitamin E can promote Nrf2 expression and play a cell protective role in the different pathological conditions (51, 52).
Histopathological examination of H&E and PAS-stained renal sections of the BPA group showed that there were already profound changes in the renal morphology including vacuolation, degeneration of renal tubules, hyaline casts within the tubules, lumen with mononuclear interstitial cellular infiltrate, congestion of renal blood vessels and interruption of the PAS-stained brush border of most of the PCT. These histopathological changes were in agreement with Aslanturk and Uzunhisarcikli (4) who reported that BPA provoked histopathological alterations which were explained by the free radicals generated by BPA, which disrupted the integrity along with the permeability of membranes of various organelles and cells.
Histopathological examination of sections of the BPA treated with NAC, or vitamin E separately or combined demonstrated the restoration of most of the renal tubular shape and preservation of Bowman’s space compared to that of the BPA group. Despite these, some vacuolated tubules and local areas of basement membrane loss were detected. These histopathological findings confirmed that NAC and vitamin E decreased histological damage caused by BPA, but did not completely prevent renal insult. The improving effect of NAC on nephrotoxicity was in accordance with Wang et al. (53) who demonstrated that NAC treatment might prevent renal tubular damage resulted from chronic lead administration, via the antioxidant in addition to chelating abilities. Also, Güntürk et al. (40) detected histological and functional improvement effects of NAC in cisplatin-induced kidney damage. Regarding the vitamin E histological alleviating effect in this study, was in consistency with Khan et al. (54) who detected a protective effect vit E in K+ dichromate-induced nephrotoxicity in rats. Additionally, Kadkhodaee et al. (55) confirmed that vitamin E has protective effects on gentamicin‐induced nephrotoxicity. However, NAC and vitamin E administration protected the renal tissues from the damage effect of BPA in our study, further studies are necessary to fully understand how NAC and vitamin E modulate oxidative stress and inflammation in various experimental circumstances.
Conclusion
Results of the current study highlighted the protective effect of NAC, vitamin E and their combination against BPA-induced nephrotoxicity. Combined NAC and vitamin E administration has obvious protective effects mainly through the synergistic effect on Nrf2 upregulation and suppressing inflammatory mediators. Both agents reduced renal damage, improved renal function and maintained renal architecture. Moreover, vitamin E was more effective than NAC for decreasing lipid peroxidation and attenuating oxidative stress. This suggested that NAC and vitamin E as a free radical scavengers can provide effective protection in animals against oxidative damage and nephrotoxicity induced by BPA.
Conflicts of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
Funding Resources:
None, the authors fully funded the current research.
Authors Contributions:
Eman Abdelrazik and Eman A. E. Farrag designed the study and established the model of the study. Alshimaa Magdy performed and interpreted the biochemical and molecular results. Hend M. Hassan examined the renal tissue specimens and interpreted the histological, immunohistochemical and morphometric results. Data collection and analysis were performed by Zienab Abdallah and Eman A.E. Farrag. All authors wrote, revised the manuscript, and approved the final manuscript.
References
- Alboghobeish S, Mahdavinia M, Zeidooni L, et al. The efficiency of naringin against reproductive toxicity and testicular damages induced by bisphenol A in rats. Iran J Basic Med Sci. 2019;22:315–23. doi: 10.22038/ijbms.2019.29757.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Jonathan N. Endocrine Disrupting Chemicals and Breast Cancer: The Saga of Bisphenol A. In: Zhang X, editor. Estrogen Receptor and Breast Cancer. Cham (Switzerland): Humana Press; 2019. pp. 343–77. [Google Scholar]
- Lynch JB, Davitkov P, Anderson DJ, et al. Infectious Diseases Society of America Guidelines on Infection Prevention for Health Care Personnel Caring for Patients with Suspected or Known COVID-19. Clin Infect Dis. 2020:ciaa1063. doi: 10.1093/cid/ciaa1063. doi: 10.1093/cid/ciaa1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslanturk A, Uzunhisarcikli M. Protective potential of curcumin or taurine on nephrotoxicity caused by bisphenol A. Environ Sci Pollut Res Int. 2020;27:23994–4003. doi: 10.1007/s11356-020-08716-1. [DOI] [PubMed] [Google Scholar]
- Kobroob A, Peerapanyasut W, Chattipakorn N, Wongmekiat O. Damaging Effects of Bisphenol A on the Kidney and the Protection by Melatonin: Emerging Evidences from In Vivo and In Vitro Studies. Oxid Med Cell Longev. 2018:3082438. doi: 10.1155/2018/3082438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid Med Cell Longev. 2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordjour FA, Asiedu E, Larbi A, et al. The role of nuclear factor kappa B (NF-κB) in filarial pathology. J Cell Commun Signal. 2021;15:185–93. doi: 10.1007/s12079-021-00607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. J Immunol. 2012;189:4213–9. doi: 10.4049/jimmunol.1202246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom J, Xu B, Tian X, Chen QM. Nrf2 protects mitochondrial decay by oxidative stress. FASEB J. 2016;30:66–80. doi: 10.1096/fj.14-268904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Buttari B, Panieri E, Profumo E, Saso L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules. 2020;25:5474. doi: 10.3390/molecules25225474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusumano G, Romagnoli J, Liuzzo G, et al. N-Acetylcysteine and High-Dose Atorvastatin Reduce Oxidative Stress in an Ischemia-Reperfusion Model in the Rat Kidney. Transplant Proc. 2015;47:2757–62. doi: 10.1016/j.transproceed.2015.09.035. [DOI] [PubMed] [Google Scholar]
- Aparicio-Trejo OE, Reyes-Fermin LM, Briones-Herrera A, et al. Protective effects of N-acetyl-cysteine in mitochondria bioenergetics, oxidative stress, dynamics and S-glutathionylation alterations in acute kidney damage induced by folic acid. Free Radic Biol Med. 2019;130:379–96. doi: 10.1016/j.freeradbiomed.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Wongjaikam S, Kumfu S, Khamseekaew J, et al. Combined Iron Chelator and Antioxidant Exerted Greater Efficacy on Cardioprotection than Monotherapy in Iron Overloaded Rats. PLoS ONE. 2016;11:e0159414. doi: 10.1371/journal.pone.0159414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi A, Ziamajidi N, Ghafourikhosroshahi A, Abbasalipourkabir R. Effects of vitamin A and vitamin E on attenuation of titanium dioxide nanoparticles-induced toxicity in the liver of male Wistar rats. Mol Biol Rep. 2019;46:2919–32. doi: 10.1007/s11033-019-04752-4. [DOI] [PubMed] [Google Scholar]
- Hayashi D, Yagi K, Song C, et al. Diacylglycerol Kinase alpha is Involved in the Vitamin E-Induced Amelioration of Diabetic Nephropathy in Mice. Sci Rep. 2017;7:2597. doi: 10.1038/s41598-017-02354-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerapanyasut W, Kobroob A, Palee S, Chattipakorn N, Wongmekiat O. N-Acetylcysteine Attenuates the Increasing Severity of Distant Organ Liver Dysfunction after Acute Kidney Injury in Rats Exposed to Bisphenol A. Antioxidants (Basel) 2019;8:497. doi: 10.3390/antiox8100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo El-Atta H, Ahmed D. Testicular dysfunction in malathion induced toxicity in male rats: Protective role of NAC and Silymarin. Mansoura J Forens Med Clin Toxicol. 2020;28:33–45. [Google Scholar]
- Abdel-Daim MM, Abdeen A. Protective effects of rosuvastatin and vitamin E against fipronil-mediated oxidative damage and apoptosis in rat liver and kidney. Food Chem Toxicol. 2018;114:69–77. doi: 10.1016/j.fct.2018.01.055. [DOI] [PubMed] [Google Scholar]
- Valerie Ng. Effects of Disease on Clinical Laboratory Tests. In: Young DS, Friedman RB, editors. Clinical Chemistry. Washington: AACC Press; 2001. pp. 682–3. [Google Scholar]
- Barros AS, Crispim RYG, Cavalcanti JU, et al. Impact of the Chronic Omega-3 Fatty Acids Supplementation in Hemiparkinsonism Model Induced by 6-Hydroxydopamine in Rats. Basic Clin Pharmacol Toxicol. 2017;120:523–31. doi: 10.1111/bcpt.12713. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Bancroft JD, Layton C. Hematoxyline and eosine. In: Suvarna SK, Layton C, Bancroft JD, editors. Bancroft’s Theory and Practice of Histological Techniques. New York, USA: Elsevier; 2019. pp. 126–38. [Google Scholar]
- Layton C, Bancroft JD. Carbohydrates. In: Suvarna SK, Layton C, Bancroft JD, editors. Bancroft’s Theory and Practice of Histological Techniques. New York, USA: Elsevier; 2019. pp. 176–97. [Google Scholar]
- Chen X, Cho D, Yang P. Double staining immunohistochemistry. N Am J Med Sci. 2010;2:241–5. doi: 10.4297/najms.2010.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddleston JM, Aaron JS, Khuon S, Chew T. A guide to accurate reporting in digital image acquisition – can anyone replicate your microscopy data? J Cell Sci. 2021;134:jcs254144. doi: 10.1242/jcs.254144. [DOI] [PubMed] [Google Scholar]
- Rezg R, El-Fazaa S, Gharbi N, Mornagui B. Bisphenol A and human chronic diseases: current evidences, possible mechanisms, and future perspectives. Environ Int. 2014;64:83–90. doi: 10.1016/j.envint.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Hines CJ, Jackson MV, Deddens JA, Clark JC, Ye X, Christianson AL. Urinary bisphenol A (BPA) concentrations among workers in industries that manufacture and use BPA in the USA. Ann Work Expo Health. 2017;61:164–82. doi: 10.1093/annweh/wxw021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch-Panadero E, Mas S, Sanchez-Ospina D. The choice of hemodialysis membrane affects bisphenol-A levels in blood. J Am Soc Nephrol. 2016;27:1566–74. doi: 10.1681/ASN.2015030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olea-Herrero N, Arenas MI, Munoz-Moreno C, et al. Bisphenol-A induces podocytopathy with proteinuria in mice. J Cell Physiol. 2014;229:2057–66. doi: 10.1002/jcp.24665. [DOI] [PubMed] [Google Scholar]
- Peerapanyasut W, Kobroob A, Palee S, Chattipakorn N, Wongmekiat O. Activation of Sirtuin 3 and Maintenance of Mitochondrial Integrity by N-Acetylcysteine Protects Against Bisphenol A-Induced Kidney and Liver Toxicity in Rats. Int J Mol Sci. 2019;20:267. doi: 10.3390/ijms20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwish MA, Abo-Youssef AM, Khalaf MM, Abo-Saif AA, Saleh IG, Abdelghany TM. Vitamin E mitigates cisplatin-induced nephrotoxicity due to reversal of oxidative/nitrosative stress, suppression of inflammation and reduction of total renal platinum accumulation. J Biochem Mol Toxicol. 2017;31:1–9. doi: 10.1002/jbt.21833. [DOI] [PubMed] [Google Scholar]
- Al-Attar AM. Antioxidant effect of vitamin E treatment on some heavy metals-induced renal and testicular injuries in male mice. Saudi J Biol Sci. 2011;18:63–72. doi: 10.1016/j.sjbs.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood N, Hameed A, Hussain T. Vitamin E and Selenium Treatment Alleviates Saline Environment-Induced Oxidative Stress through Enhanced Antioxidants and Growth Performance in Suckling Kids of Beetal Goats. Oxid Med Cell Longev. 2020:4960507. doi: 10.1155/2020/4960507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Beigh S, Chaudhari BP, et al. Mitochondrial dysfunction induced by Bisphenol A is a factor of its hepatotoxicity in rats. Environ Toxicol. 2016;31:1922–34. doi: 10.1002/tox.22193. [DOI] [PubMed] [Google Scholar]
- Zhang F, Lau SS, Monks TJ. The cytoprotective effect of N-acetyl-Lcysteine against ROS-induced cytotoxicity is independent of its ability to enhance glutathione synthesis. Toxicol Sci. 2011;120:87–97. doi: 10.1093/toxsci/kfq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Sanchez WY, Roy SF, et al. N-acetyl-cysteine increases cellular dysfunction in progressive chronic kidney damage after acute kidney injury by dampening endogenous antioxidant responses. Am J Physiol Renal Physiol. 2018;314:F956–68. doi: 10.1152/ajprenal.00057.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento MM, Suliman ME, Silva M, et al. Effect of oral N-acetylcysteine treatment on plasma inflammatory and oxidative stress markers in peritoneal dialysis patients: a placebo-controlled study. Perit Dial Int. 2010;30:336–42. doi: 10.3747/pdi.2009.00073. [DOI] [PubMed] [Google Scholar]
- Badgujar PC, Pawar NN, Chandratre GA, Telang AG, Sharma AK. Fipronil induced oxidative stress in kidney and brain of mice: protective effect of vitamin E and vitamin C. Pestic Biochem Physiol. 2015;118:10–8. doi: 10.1016/j.pestbp.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Song N, Thaiss F, Guo L. NFκB and kidney injury. Frontiers in immunology. 2019;10:815. doi: 10.3389/fimmu.2019.00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güntürk I, Yazici C, Köse SK, Dağli F, Yücel B, Yay AH. The effect of N-acetylcysteine on inflammation and oxidative stress in cisplatin-induced nephrotoxicity: a rat model. Turk J Med Sci. 2019;49:1789–99. doi: 10.3906/sag-1903-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauert HP. Vitamin E and NF‐κB Activation: A Review. Vitam Horm. 2007;76:135–53. doi: 10.1016/S0083-6729(07)76006-5. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Systematic review: primary and secondary prevention of gastrointestinal cancers with antioxidant supplements. Aliment Pharmacol Ther. 2008;28:689–703. doi: 10.1111/j.1365-2036.2008.03785.x. [DOI] [PubMed] [Google Scholar]
- Sedaghat Z, Kadkhodaee M, Seifi B, Salehi E. Inducible and endothelial nitric oxide synthase distribution and expression with hind limb per-conditioning of the rat kidney. Arch Med Sci. 2019;15:1081–91. doi: 10.5114/aoms.2019.85651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouhan S, Yadav SK, Prakash J, et al. Increase in the expression of inducible nitric oxide synthase on exposure to bisphenol A: a possible cause for decline in steroidogenesis in male mice. Environ Toxicol Pharmacol. 2015;39:405–16. doi: 10.1016/j.etap.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Luo JF, Shen XY, Lio CK, et al. Activation of Nrf2/HO-1 Pathway by Nardochinoid C Inhibits Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated Macrophages. Front Pharmacol. 2018;9:911. doi: 10.3389/fphar.2018.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekhya Sita GJ, Gowthami M, Srikanth G, et al. Protective role of luteolin against bisphenol A‐induced renal toxicity through suppressing oxidative stress, inflammation, and upregulating NRF2/ARE/HO‐1 pathway. IUBMB life. 2019;71:1041–7. doi: 10.1002/iub.2066. [DOI] [PubMed] [Google Scholar]
- Mohammed ET, Hashem KS, Ahmed AE, Aly MT, Aleya L, Abdel-Daim MM. Ginger extract ameliorates bisphenol A (BPA)-induced disruption in thyroid hormones synthesis and metabolism: Involvement of Nrf-2/HO-1 pathway. Sci Total Environ. 2020;703:134664. doi: 10.1016/j.scitotenv.2019.134664. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Li J, Wu Z, et al. Acute exposure of ozone induced pulmonary injury and the protective role of vitamin E through the NRF2 pathway in Balb/c mice. Toxicology research. 2016;5:268–77. doi: 10.1039/c5tx00259a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Y, Lambrecht RW, Donohue SE, et al. Role of Bach1 and NRF2 in up‐regulation of the heme oxygenase‐1 gene by cobalt protoporphyrin. The FASEB Journal. 2006;20:2651–3. doi: 10.1096/fj.06-6346fje. [DOI] [PubMed] [Google Scholar]
- Nezu M, Suzuki N. Roles of Nrf2 in Protecting the Kidney from Oxidative Damage. Int J Mol Sci. 2020;21:2951. doi: 10.3390/ijms21082951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Liu Z, Li X, et al. α-Tocopherol is an effective phase II enzyme inducer: protective effects on acrolein-induced oxidative stress and mitochondrial dysfunction in human retinal pigment epithelial cells. J Nutr Biochem. 2010;21:1222–31. doi: 10.1016/j.jnutbio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Fang J, Yin H, Yang Z, et al. Vitamin E protects against cadmium-induced sub-chronic liver injury associated with the inhibition of oxidative stress and activation of NRF2 pathway. Ecotoxicol Environ Saf. 2021;208:111610. doi: 10.1016/j.ecoenv.2020.111610. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang Z, Liu J. Protective effect of N-acetylcysteine on experimental chronic lead nephrotoxicity in immature female rats. Hum Exp Toxicol. 2010;29:581–91. doi: 10.1177/0960327109357270. [DOI] [PubMed] [Google Scholar]
- Khan MR, Siddiqui S, Parveen K, Javed S, Diwakar S, Siddiqui WA. Nephroprotective action of tocotrienol-rich fraction (TRF) from palm oil against potassium dichromate (K 2 Cr 2 O 7)-induced acute renal injury in rats. Chem Biol Interact. 2010;186:228–38. doi: 10.1016/j.cbi.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Kadkhodaee M, Khastar H, Faghihi M, Ghaznavi R, Zahmatkesh M. Effects of co-supplementation of vitamins E and C on gentamicin-induced nephrotoxicity in rat. Exp Physiol. 2005;90:571–6. doi: 10.1113/expphysiol.2004.029728. [DOI] [PubMed] [Google Scholar]


