Abstract
The aggregation substance (AS) surface protein from Enterococcus faecalis has been implicated as an important virulence factor for the development of infective endocarditis. To evaluate the role of antibodies specific for Asc10 (the AS protein from the conjugative plasmid pCF10) in protective immunity to infective endocarditis, an N-terminal region of Asc10 lacking the signal peptide and predicted to be surface exposed (amino acids 44 to 331; AS44–331) was cloned with a C-terminal histidine tag translational fusion and expressed from Escherichia coli. N-terminal amino acid sequencing of the purified protein revealed the correct sequence, and rabbit polyclonal antisera raised against AS44–331 reacted specifically to Asc10 expressed from E. faecalis OG1SSp, but not to other proteins as judged by Western blot analysis. Using these antisera, flow cytometry analysis demonstrated that antibodies to AS44–331 bound to a surface-exposed region of Asc10. Furthermore, antibodies specific for AS44–331 were opsonic for E. faecalis expressing Asc10 in vitro but not for cells that did not express Asc10. New Zealand White rabbits immunized with AS44–331 were challenged intravenously with E. faecalis cells constitutively expressing Asc10 in the rabbit model of experimental endocarditis. Highly immune animals did not show significant differences in clearance of organisms from the blood or spleen or in formation of vegetations on the aortic valve, in comparison with nonimmune animals. Although in vivo expression of Asc10 was demonstrated by immunohistochemistry, these experiments provide evidence that immunity to Asc10 does not play a role in protection from experimental infective endocarditis due to E. faecalis and may have important implications for the development of immunological approaches to combat enterococcal endocarditis.
Infective endocarditis is a microbial infection of the endothelial lining of the heart that typically occurs on damaged or prosthetic heart valves (41). Enterococcal endocarditis was reported as early as 1906 (3), and these organisms are now considered the third-most-common cause of infective endocarditis, causing up to 20% of all bacterial endocarditis cases (2, 25, 30, 41). The characteristic lesion seen with infective endocarditis is termed the vegetation, which is composed in part of fibrin and platelets attached to the underlying endothelium (25). During infection, bacteria in vegetations may grow to reach densities of 109 to 1010 cells per gram, and the organisms may become metabolically dormant, causing resistance to the bactericidal activity of β-lactam and glycopeptide antibiotics (25). Furthermore, the vegetation is thought to exclude or hinder host defenses from clearing bacteria. This infection can lead to deformity and destruction of the heart valve leaflets, rupture of the chordae tendineae, or dysfunction of prosthetic valves, and significant damage may cause congestive heart failure leading to death.
The current antimicrobial therapy recommended for enterococcal endocarditis generally requires the synergistic activity of a cell wall-active agent and an aminoglycoside (reviewed in reference 25). A major complication and contributor to poor clinical outcome of enterococcal endocarditis is the high incidence of multiple-antibiotic resistance that can be either intrinsic or carried on conjugative plasmids and transposons. Due to their ability to acquire high-level resistance to clinically used antibiotics such as the aminoglycosides, β-lactams, and glycopeptides and their ability to disseminate these multiple-antibiotic-resistant traits, the enterococci have received notable attention (for reviews, see references 11, 13, 23, and 31–33). Strains now exist that are resistant to all clinically used antibiotics, including vancomycin, often considered a drug of last resort. It is recognized that treatment failure is not uncommon, and surgical removal of the infected valve may be the only curative treatment in some cases (15). For these reasons, it is important to evaluate novel strategies to combat enterococcal infections.
The enterococcal aggregation substance (AS) is a large (137-kDa) surface-expressed protein encoded by pheromone-responsive, conjugative plasmids that is necessary for the formation of large-cell aggregates during gene transfer between donor and recipient cells (39). Various lines of evidence support the function of AS as a virulence factor. This protein promotes adherence to cultured pig kidney tubular cells (26) and internalization into cultured intestinal epithelial cells (37). Furthermore, AS appears to promote opsonin-independent binding to polymorphonuclear leukocytes (PMNs), likely through interaction with complement receptor 3 (CR3) and other receptors (53), and also increases survival, once internalized (40). Similarly, AS was recently shown to promote adherence, uptake, and survival within human macrophages (47).
The AS protein is apparently multifunctional, and although enterococci possess subtle virulence factors that are not easily identified, most studies suggest that AS is a significant virulence factor for the development of infective endocarditis (reviewed in reference 29). Studies of Enterococcus faecalis containing the pheromone-responsive plasmid pAD1 (which also encodes a cytolysin) showed that when AS was present alone, it contributed to an increase in the size of the observed vegetations (7). Results from our laboratory with E. faecalis containing variants of pCF10 (45) also supported these findings in that AS both increased the size of vegetations and increased mortality. However, Berti et al. (5) used a rat endocarditis model with pAD1 derivatives and concluded that AS had no significant influence on the virulence of the organism. Inducible expression of AS alone in a heterologous host (Lactococcus lactis) has provided evidence for increased binding to fibrin as well as elevated cell surface hydrophobicity (20). Both of these factors could in part explain the contribution of AS to endocarditis where fibrin is a major component of heart vegetations, and increased cell hydrophobicity has been linked to an elevated adherence of bacteria (24). While the involvement of AS in the virulence of endocarditis seems established, the actual mechanisms by which this multifunctional protein contributes to disease appear to be complex.
In conjunction with the above evidence, the AS surface protein should represent a good target for vaccine development due to its high incidence in clinical isolates (8, 51) and its likely involvement as a virulence factor in several model systems. This study was designed to investigate the role of host antibodies to AS in protective immunity against endocarditis. In the rabbit model of experimental endocarditis, we used a highly purified, surface-exposed recombinant region of Asc10 (the AS protein from the conjugative plasmid pCF10) to immunize rabbits, followed by challenge with Asc10-expressing E. faecalis. Our data indicate that the presence of specific, high-titer opsonic antibodies to Asc10 is not protective in this disease model, and these findings may have larger implications for the development of novel immunological therapies for enterococcal endocarditis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Enterococci were routinely grown at 37°C in Todd-Hewitt (TH) broth (Difco Laboratories, Inc., Detroit, Mich.) or beef heart medium (46) containing 5% glucose phosphate buffer (0.33 M glucose, 0.5 M NaHCO3, 0.68 M NaCl, 0.12 M Na2HPO4, and 0.027 M l-glutamine) prior to animal experiments. Escherichia coli was grown in Luria-Bertani broth (42). For preparation of solid media, agar was added to a final concentration of 1.5%. Strains were stored at −86°C in their appropriate broth, supplemented with 20% (vol/vol) glycerol. Antibiotics were added as selective agents when appropriate: for enterococci, erythromycin (5 μg/ml), chloramphenicol (20 μg/ml), streptomycin (1,000 μg/ml), and rifampin (200 μg/ml); for E. coli, erythromycin (100 μg/ml) and kanamycin (50 μg/ml).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| XL1 Blue | Cloning strain | Stratagene |
| BL21(DE3) | Expression host | Novagen |
| E. faecalis | ||
| OG1SSp | Plasmid-free parent strain; chromosomal Strr Specr | 10 |
| OG1RF | Plasmid-free parent strain; chromosomal Rifr Fusr | 10 |
| Plasmids | ||
| pCF10 | Pheromone-inducible conjugative plasmid containing wild-type prgB | 9 |
| pET28b | Knr; cloning vector | Novagen |
| pINY1801 | Cmr; fragment from pCF10 cloned in shuttle vector pWM401; confers constitutive expression of Asc10 | 50 |
| pMSP3535 | Emr; nisin-inducible expression vector | 6 |
| pMSP7517 | Emr; pMSP3535::prgB; contains full-length prgB under control of the nisin-inducible promoter | 20 |
| pMSP7513n | Knr; pET28b::orf prgB1–331 | This study |
| pJKM81 | Knr; pET28b::prgB1–331 | This study |
| pJKM82 | Knr; pET28b::prgB44–331 | This study |
Cmr, chloramphenicol resistant; Emr, erythromycin resistant; Knr, kanamycin resistant; Specr, spectinomycin resistant; Strr, streptomycin resistant; Rifr, rifampin resistant; Fusr, fusidic acid resistant.
Cloning, expression, and purification of an N-terminal region of Asc10.
Standard methods were used for restriction enzyme digestion, ligation, agarose gel electrophoresis, and E. coli transformation (42). PCRs were done with Taq or Pfu DNA polymerases using standard protocols. Clones were sequenced to confirm that PCR did not incorporate random mutations.
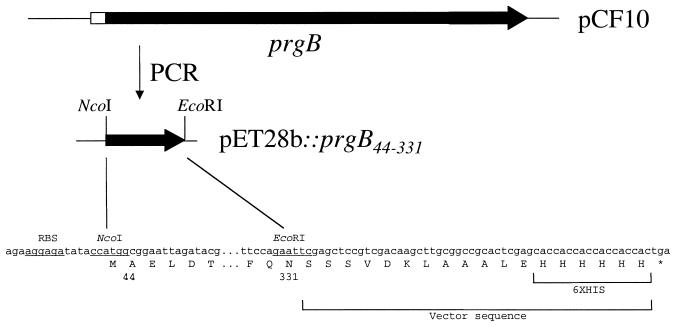
For initial expression of an N-terminal piece of the Asc10 protein, a first plasmid (pMSP7513n) was constructed in pET28b (Table 1) encoding residues 1 through 331 of Asc10 that also contained a short upstream open reading frame that was thought to be necessary for efficient expression of Asc10. Failure to detect significant amounts of protein from this construct when induced in E. coli BL21(DE3) resulted in our constructing a second clone of the same Asc10 fragment without the upstream open reading frame (pJKM81). To construct this plasmid, primer PrgB(NcoI) (5′-TGATCCATGGAGGAGGATGATACATG-3′) and the T7 terminator primer (Promega) were used to amplify this coding region from pMSP7513n, and the product was cloned with NcoI (underlined in the primer) and EcoRI (naturally occurring in the prgB gene which encodes Asc10) into pET28b. Again, very small amounts of the recombinant Asc10 protein were produced from E. coli BL21(DE3). A third construct lacking coding sequences for the signal peptide (coding for amino acids 44 to 331 [AS44–331]) was similarly amplified using primer JKM60 (5′-GCGCCATGGCGGAATTAGATACGCAA-3′) and the T7 terminator primer and cloned into pET28b to create pJKM82 (Fig. 1). This construct resulted in expression of significant amounts of the recombinant AS44–331 protein (Fig. 2).
FIG. 1.
Diagrammatic representation of AS44–331 cloning. Single-strand DNA sequence encoding amino acids 44 to 331 of AS (prgB) from pCF10 was amplified by PCR and cloned into pET28b. Shown is the region of prgB used for cloning and the location of the six-His sequence added to AS44–331. Underlined regions indicate the ribosomal binding site (RBS) and restriction enzymes used for cloning.
FIG. 2.
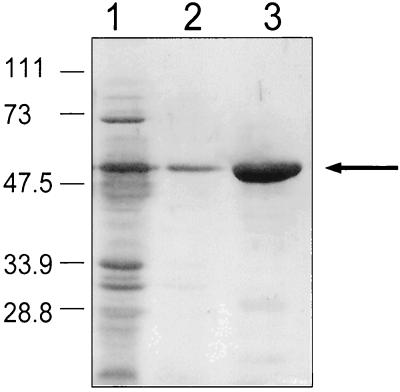
SDS-PAGE analysis of purified AS44–331 protein. Purification was achieved with a nickel column with E. coli BL21(DE3) containing pJKM82 as described in Materials and Methods. Lane 1, cell extract fall through in binding buffer; lane 2, wash buffer run through the nickel column; lane 3, elution of AS44–331 from the nickel column. The band represents approximately 10 μg of protein. Numbers on the left refer to molecular masses in kilodaltons.
For purification of recombinant AS44–331 protein, cultures were grown in dialyzed beef heart medium containing 1% glucose phosphate buffer at 37°C and induced with 0.2 mM isopropyl β-d-thiogalactoside (Sigma Chemical Co., St. Louis, Mo.) when the optical density at 600 nm was approximately 0.5. After 3 to 4 h, cells were pelleted, resuspended in binding buffer (500 μM imidazole, 500 mM NaCl, 20 mM Tris-HCl [pH 7.9]), and lysed by sonication. Recombinant AS44–331 was purified on a nickel column (His·Bind kit; Novagen, Madison, Wis.) following the manufacturer's recommendations (Fig. 2).
SDS-PAGE, Western blot analysis, and determination of the amino acid sequence of recombinant AS44–331.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was done as described by Laemmli (27) using a Mini-PROTEAN II apparatus (Bio-Rad, Hercules, Calif.). Electroblotting was done using a wet-blot system (Mini-PROTEAN II; Bio-Rad) with Sequi-Blot polyvinylidene difluoride membranes (Bio-Rad) and developed according to standard protocols (42). Purified AS44–331 was electroblotted onto a Sequi-Blot polyvinylidene difluoride membrane, and the N-terminal amino acid sequence was determined by the Mayo Protein Core Facility (Mayo Clinic, Rochester, Minn.) by automated Edman degradation.
Flow cytometry analysis.
The plasmid pMSP7517 (Table 1) contains the full-length prgB under transcriptional control of the nisin-inducible promoter (20). E. faecalis OG1SSp containing pMSP7517 and OG1RF containing pMSP7517 were subcultured at 1% in TH broth and grown at 37°C for 3 h. Expression of prgB from pMSP7517 was induced with 25 ng of nisin (Sigma)/ml, and strains were grown for a further 1.5 h. Cells (100 μl) were resuspended in 500 μl of phosphate-buffered saline (PBS) (0.005 M NaPO4, 0.15 M NaCl [pH 7.2]) containing 5% milk solids (PBSM) and incubated at room temperature for 30 min as an initial blocking step. Cells were washed three times with PBS and resuspended in 100 μl of PBSM containing either polyclonal anti-AS44–331 antibodies or anti-AS monoclonal antibodies that are known to bind to surface-exposed regions of wild-type Asc10 (38). Both were used at a 1:1,000 dilution and incubated for 1 h. Cells were washed three times with PBS and resuspended in 100 μl of PBSM containing either anti-rabbit or anti-mouse fluorescein isothiocyanate conjugate (each, 1:1,000 dilution) (Sigma) and incubated for 30 min. Cells were washed three times with PBS and resuspended in fluorescence-activated cell sorter buffer. Fluorescence was read with a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.), and fluorescence plots were analyzed using CellQuest software (Becton Dickinson).
Opsonization assay.
E. faecalis strain OG1SSp was subcultured at 1% from an overnight culture and grown for 3 h. Expression of Asc10 was induced by the addition of 25 ng of nisin/ml to strains containing pMSP7517. Control cultures contained pMSP3535 and were treated identically. All strains were grown for a further 1.5 h. Cells were diluted 10−3, and 100 μl of cells was mixed with freshly drawn, EDTA-anticoagulated whole rabbit blood containing 10% (100 μl) of AS44–331-specific or control normal rabbit serum. The mixture was incubated at 37°C with rotation, and viable bacterial counts were determined by plating on TH agar at 0 and 120 min.
Experimental endocarditis.
Healthy New Zealand White rabbits were obtained from Birchwood Farms (Red Wing, Minn.). Rabbits were immunized with 25 μg of purified AS44–331 emulsified in Freund's incomplete adjuvant at 2-week intervals, for a total of three immunizations. Control animals were also included that did not receive immunizations. Preimmune and postimmune (week 7) sera were obtained for all rabbits, and anti-AS44–331 titers were determined by indirect enzyme immunosorbent assay (ELISA) with 1 μg of AS44–331 to coat each well. Anti-rabbit immunoglobulin G horseradish peroxidase conjugate (Sigma) was used as the secondary antibody. All serum titers were determined in parallel.
Animals were then challenged using the rabbit model of infective endocarditis as previously described (45). Briefly, animals were anesthetized with ketamine (25 mg/kg of body weight) and xylazine (20 mg/kg of body weight) for the duration of the surgery. To induce the valve damage that is required for vegetation formation, a catheter (outside diameter, 1.27 mm; Becton Dickinson, Sparks, Md.) was inserted down the left carotid artery and placed over the aortic valve for 2 h. The catheter was then removed, the left carotid artery was ligated, and the incision was closed with sutures. Following surgery, rabbits were challenged intravenously via the marginal ear vein with 2 ml of E. faecalis OG1SSp(pINY1801) from an overnight stationary-phase culture for all experiments. Plasmid pINY1801 contains a cloned fragment of pCF10 that lacks the negative control region and constitutively expresses Asc10 (50). Due to the constitutive aggregation phenotype of this strain, cells were briefly sonicated immediately prior to intravenous injection. Bacterial plate counts were determined for all cultures to evaluate the number of challenge organisms administered, and all animals received from 1.5 × 109 to 2.5 × 109 CFU/rabbit. Within 10 min after the organisms were administered, peripheral blood was drawn from the marginal ear vein, and quantitative blood counts were determined on TH medium. Quantitative blood cultures were also taken each day. Animals were sacrificed on day 3 with a lethal injection of pentobarbitol, and hearts were resected. Vegetations were aseptically harvested, weighed, and homogenized in PBS, and total bacterial counts were determined. Spleens were also weighed and homogenized in PBS, and bacterial counts were determined.
Immunohistochemistry.
To determine if AS was expressed in vivo, we performed the endocarditis experiment on a separate rabbit, and the vegetation was harvested, fixed in S.T.F. fixative (Streck Laboratories, La Vista, Neb.), and imbedded in paraffin, and 5-μm sections were prepared with a microtome. Slides were deparaffinized by being heated to 55°C for 1 h, washed twice in xylene, and hydrated by consecutive washes through ethanol gradients (100, 80, and 70% and H2O). The slides were blocked with TNB buffer (0.01 M Tris, 0.15 M NaCl, 0.5% blocking reagent [NEN Life Science Products, Boston, Mass.] [pH 8]) containing 1.5% horse serum. Sections were incubated with anti-AS44–331 polyclonal rabbit antisera (1:100 dilution) in TNB buffer containing 1.5% horse serum. Control sections were stained similarly with preimmune serum from the same rabbit. The secondary antibody was anti-rabbit horseradish peroxidase conjugate (1:100 dilution) (Sigma) in TNB buffer containing 1.5% horse serum. Sections were developed with 3,3-diaminobenzidine as chromogen (Vector Laboratories, Burlingame, Calif.), counterstained with hematoxylin (Sigma), dehydrated by successive washes through ethanol, and viewed under light microscopy. All wash steps were done with TNT buffer (0.01 M Tris-HCl, 0.15 M NaCl, 0.05% Tween 20 [pH 8]).
Statistical analysis.
ELISA titers were determined as the reciprocal of the dilution that produced a reading at an optical density at 490 nm of 0.1 or greater that was arbitrarily defined as the endpoint titer. The Student t test was used to determine the significance of killing for the opsonization assays.
RESULTS
Cloning of AS44–331.
To generate antibodies specific for Asc10, a hydrophilic region of this protein predicted to be surface exposed was cloned with a C-terminal 6 × His tag (Fig. 1) and overexpressed from E. coli. Highly purified AS33–441 was obtained with a nickel column (Fig. 2). Curiously, the purified protein eluted from the column ran at an apparent molecular mass of approximately 58 kDa by SDS-PAGE while the predicted molecular mass was only 32 kDa. The N-terminal amino acid sequence of purified protein was determined and shown to be Ala-Glu-Leu-Asp-Thr-Gln-Pro-Gly-Ser-Thr. This sequence matched amino acids 44 to 53 of Asc10, indicating that this protein was in fact AS44–331. Furthermore, this protein band reacted with anti-6 × His antibodies in a Western blot, indicating the C terminus of the protein was also correct (data not shown). Lastly, a similar clone of the same fragment containing an N-terminal 6 × His tag had the same apparent molecular mass as AS44–331 as judged by SDS-PAGE (data not shown).
Antibodies raised against AS44–331 are specific for wild-type AS.
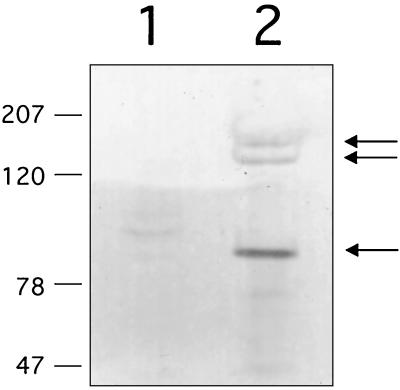
AS44–331 purified from E. coli was used as an immunogen to prevent cross-reactivity with other enterococcal proteins. Immunization of a New Zealand White rabbit yielded antisera that were specific for wild-type Asc10. This was shown by comparison of surface extracts from nisin-induced and uninduced cultures of E. faecalis containing pMSP7517. This plasmid contains wild-type prgB under control of the nisin promoter such that the only difference in surface extracts from induced and uninduced cultures is expression of Asc10. As seen by Western blot analysis, antibodies raised against AS33–441 were highly specific for wild-type Asc10 (Fig. 3). Furthermore, the ability to invoke antibodies specific for wild-type Asc10 confirmed the identity of this protein. Importantly, these antibodies did not significantly cross-react with other enterococcal proteins (Fig. 3).
FIG. 3.
Western blot analysis of surface extracts of E. faecalis OG1SSp(pMSP7517) with antiserum raised against recombinant AS44–331. Wild-type AS rapidly degrades when released from the cell, and multiple bands are common. Lane 1, uninduced culture (Asc10 negative); lane 2, Asc10 induced with 25 ng of nisin/ml. Arrows indicate bands corresponding to fragments of the Asc10 protein.
Amino acids 44 to 331 of AS are surface exposed.
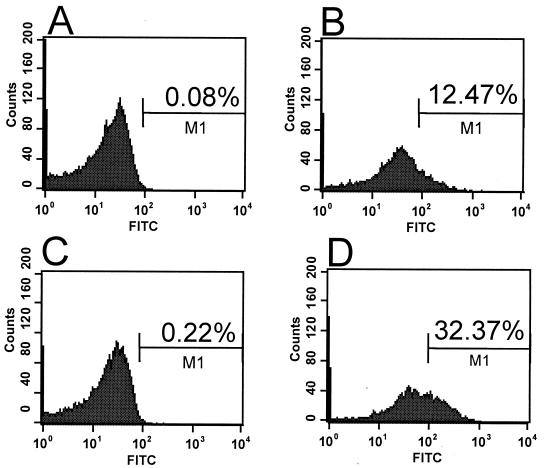
Surface localization of the cloned region (amino acids 44 to 331) of Asc10 was analyzed by flow cytometry. E. faecalis OG1SSp containing pMSP7517 was used for these experiments. Polyclonal antisera raised against AS44–331 bound to the surface of E. faecalis expressing Asc10 but not to cells that did not express Asc10 (Fig. 4). As a positive control for these experiments, a mouse anti-Asc10 monoclonal antibody (49) was used that has been demonstrated to bind to surface-exposed Asc10 by immunogold labeling and scanning electron microscopy (38). In both experiments, cells expressing Asc10 showed increased fluorescence, indicating that the anti-AS44–331 antibodies were binding to surface-exposed Asc10. Essentially identical results were also found when E. faecalis OG1RF containing pMSP7517 was used (data not shown). These experiments clearly showed that antibodies raised against the AS44–331 protein bind to wild-type, surface-exposed Asc10.
FIG. 4.
Antibodies raised against the recombinant AS44–331 protein bind to the surface on E. faecalis cells only when Asc10 is expressed. Surface localization of the AS44–331 fragment was determined by flow cytometry analysis of intact E. faecalis OG1SSp(pMSP7517) cells. In experiments shown in panels A and C, Asc10 is not expressed. Expression of AS was induced (B and D) by the addition of 25 ng of nisin/ml to cultures. Panels A and B represent binding of a monoclonal antibody known to recognize surface-exposed Asc10, and panels C and D represent polyclonal antiserum raised against recombinant AS44–331. Secondary antibodies were labeled with fluorescein isothiocyanate. The percentage of gated populations is shown. Similar results were seen with E. faecalis OG1RF containing pMSP7517 (data not shown).
Antibodies to AS44–331 are opsonic for Asc10-expressing E. faecalis.
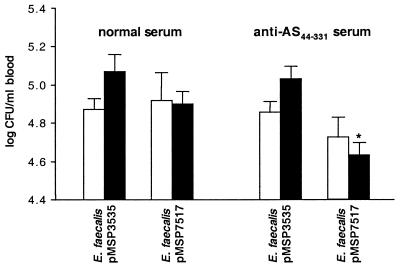
Antibodies raised against AS44–331 were tested for their ability to promote killing through opsonization of Asc10-expressing E. faecalis in whole rabbit blood. As seen in Fig. 5, E. faecalis OG1SSp expressing Asc10 was more efficiently killed in whole rabbit blood containing specific AS44–331 antisera than in blood containing normal rabbit serum at 120 min. Also, strains that did not express Asc10 (those containing pMSP3535) were not opsonized by normal or AS44–331-specific antisera, indicating that opsonic activity was specific for the Asc10 protein. The killing effect seen for Asc10-expressing E. faecalis OG1SSp in whole blood containing anti-AS44–331 antisera was not due to antiserum-induced bacterial clumping because brief sonication of the samples did not increase bacterial counts (data not shown).
FIG. 5.
Opsonization for intracellular killing of E. faecalis strains OG1SSp by AS44–331-specific antiserum. Strains containing pMSP7517 express Asc10, and control strains contain pMSP3535. Open bars represent bacterial counts at 0 min, and solid bars represent counts at 120 min. Data represent the average of five independent experiments. Error bars, SEM. ∗, P < 0.05 by the Student t test when compared with all other bacterial counts at 120 min. There was no significant difference among bacterial counts at 0 min.
Antibodies to Asc10 are not protective in experimental endocarditis.
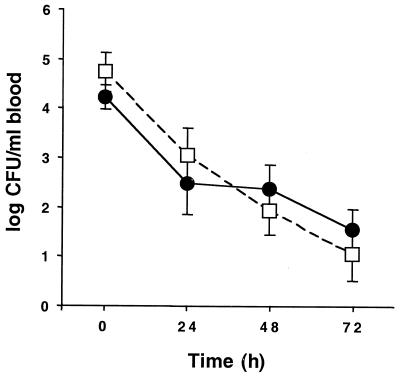
The purified AS44–331 protein was used to immunize healthy New Zealand White rabbits to generate high-titer antibodies specific for Asc10. A total of seven rabbits were used in each group. Compared with preimmune titers (a geometric mean of titers of 44 ± 21 [standard error of the mean, or SEM] for immunized rabbits and 32 ± 28 for control rabbits that were not immunized), antibody titers from immunized animals specific for AS44–331 were substantially increased to 3,446 ± 558 for immunized rabbits, versus 36 ± 21 for controls. These antibody titers were determined by ELISA from samples obtained 1 week after the third immunization. All animals were then challenged in the rabbit model of infective endocarditis with E. faecalis OG1SSp containing pINY1801. Blood counts were determined daily over the course of the experiment and are shown in Fig. 6. Bacteria were rapidly cleared from the blood, and counts were generally very low by day 3. There were no significant differences in counts from each day between the two groups of rabbits.
FIG. 6.
Bacteremia in AS44–331-immune (●) and nonimmune (□) New Zealand White rabbits challenged with E. faecalis OG1SSp containing pINY1801 over 72 h. For each rabbit, approximately 2 × 109 CFU of stationary-phase E. faecalis OG1SSp containing pINY1801 was initially administered by the marginal ear vein. Blood samples were drawn daily and plated on TH agar to determine counts. Data are the averages for seven rabbits per group. Error bars, SEM.
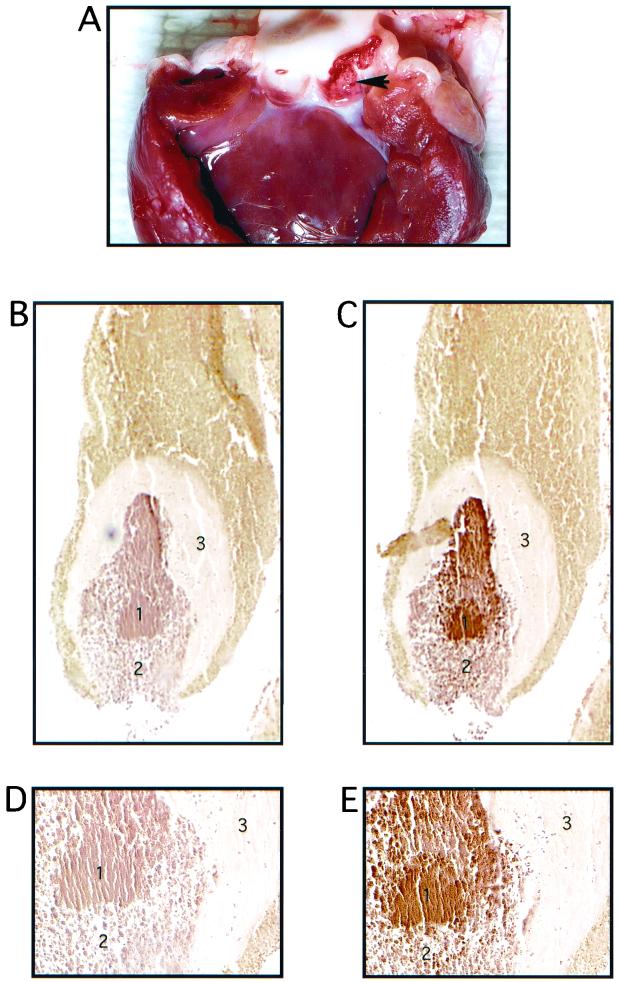
After 72 h, vegetation formation was significant in both groups of animals, despite the low numbers of bacteria in the blood at the time animals were euthanized. An example of a vegetation is shown in Fig. 7A. The vegetation typically formed within the concave surface of one of the aortic valve cusps. In this model, the vegetation will eventually grow to occlude the aortic valve, leading to mortality.
FIG. 7.
Vegetation formation on rabbit heart valve tissue by E. faecalis OG1SSp containing pINY1801 and demonstration of in vivo expression of Asc10. A typical vegetation formation on the rabbit aortic valve is shown in panel A. The arrowhead indicates the vegetation located within one of the aortic valves. Panels B and C (magnification, ×40) and D and E (magnification, ×200) represent consecutive 5-μm sections of a vegetation stained with preimmune serum (B and D) and AS44–331-immune serum (C and E). The secondary antibody was anti-rabbit horseradish peroxidase conjugate, and sections were developed with 3,3-diaminobenzidine as chromogen (brown color). Regions of the vegetation are identified as follows: a mass of E. faecalis cells (1) bordered by an influx of immune cells (2) and surrounded by a platelet/fibrin layer (3). Slides were counterstained with hematoxlyin.
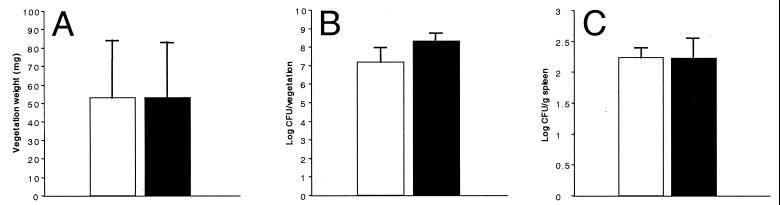
Vegetation material was first aseptically harvested and weighed; although significant variation was seen for vegetation weight, overall results were strikingly similar (Fig. 8A). Also, when total bacteria from each vegetation were counted, slightly higher numbers were obtained from animals immune to AS44–331; but again these were not significantly different compared with counts obtained from nonimmune animals (Fig. 8B). Spleens were also aseptically harvested, weighed, and homogenized, and bacterial plate counts were determined. Although spleens were enlarged for animals in both groups, there were no significant differences in spleen weight (not shown) or total bacteria counts (Fig. 8C).
FIG. 8.
Comparison between AS44–331-immune (solid bars) and nonimmune (open bars) New Zealand White rabbits challenged with E. faecalis OG1SSp containing pINY1801. Immune animals were immunized with 25 μg of purified AS44–331 protein in Freund's incomplete adjuvant at 2-week intervals for a total of three immunizations. After 72 h, hearts were resected, vegetations were weighed (A) and homogenized, and bacterial counts were quantitatively determined (B). Spleens were also aseptically harvested, weighed, and homogenized, and bacterial counts were quantitatively determined (C). Data represent the average for seven rabbits per group. Error bars, SEM.
In a separate experiment, endocarditis was induced in a single rabbit with E. faecalis OG1SSp(pINY1801). The vegetation from this rabbit was harvested for evaluation of in vivo expression of Asc10 by immunohistochemistry techniques. Paraffin-imbedded sections (5 μm) were stained with normal rabbit serum (Fig. 7B and D) or rabbit serum hyperimmune to AS44–331 (Fig. 7C and E). Specific staining of the bacteria contained within the endocarditis infection is clearly seen in Fig. 7C and E, but not in Fig. 7B and D, indicating that Asc10 was expressed in vivo inside the vegetation.
DISCUSSION
Considering that a crucial step in the development of endocarditis is the initial colonization of endothelial lesions by bacteria, we believe it may be prudent to target individual bacterial surface factors involved in adherence to damaged tissue. This study was initiated to evaluate the role of active immunity to Asc10, an enterococcal surface protein and virulence factor, for protection against infective endocarditis. To our knowledge, this study represents the first attempt to develop an immunological approach to protect against enterococcal endocarditis. The AS protein may represent an important target for preventing the initial development of this disease, in that this protein is known to promote binding to kidney tubular cells (26) and internalization into cultured intestinal epithelial cells (37). However, promotion of binding to damaged endothelial tissue by AS has not been shown.
The production of antibodies specific for AS was initiated with the purification of a recombinant N-terminal fragment that contained amino acids 44 to 331 of wild-type Asc10. As a group, the AS proteins show a high degree of homology (21) and, overall, the region of Asc10 cloned in this study shares 65% identity with characterized AS proteins from the conjugative plasmids pAD1 (17), pPD1 (16), and pHKK701 (19). If this approach was successful in protecting against enterococcal endocarditis, this region provided cross-protection against enterococci expressing other AS proteins (21). Although strains of E. faecalis are apparently more virulent and more frequently isolated from infections, the development of drug resistance is more common with Enterococcus faecium. A recent study of blood isolates revealed that 63% of E. faecalis isolates contained the AS gene, compared to 13% of E. faecium isolates (14). However, the role of AS in the virulence of E. faecium has not been studied. One exception would be AS373, which shows little homology to canonical AS and a lack of Arg-Gly-Asp (RGD) motifs (34) that may be important for adhesion by binding to host cell integrins (53), although this remains to be formally shown for AS proteins. The AS44–331 fragment does not contain either of the RGD sequences, and it may be appropriate in future work to generate recombinant fragments containing both of these motifs.
Analysis of antisera specific for AS44–331 using flow cytometry with intact cells expressing wild-type Asc10 from E. faecalis conclusively demonstrated that epitopes located within residues 44 to 331 are surface exposed and accessible to antibody. This result was also important to further define the membrane topology of Asc10. Only two major regions of Asc10 (not including the signal peptide) have potential membrane-spanning regions. From this analysis, it is likely that residues 44 through 510 are located outside the cytoplasmic membrane because predicted membrane-spanning segments are not located within this region. This result was also consistent with previous work that demonstrated this region is surface exposed (21).
Various studies have shown promise that immunological approaches could provide some degree of protection against infective endocarditis. For example, immunization with killed whole cells of Streptococcus mutans and Streptococcus sanguis (12), Streptococcus pneumoniae (1), nutritionally variant streptococci (52), Pseudomonas aeruginosa (4), and Candida albicans (43) all had protective effects in the rabbit model of infective endocarditis. In contrast, however, immunization with whole killed cells of Staphylococcus aureus was not protective in this model (18). More-defined vaccines, staphylococcal capsular polysaccharide adhesin from Staphylococcus epidermidis (48), fibronectin-binding protein from S. aureus (44), FimA from Streptococcus parasanguinis (54), and a capsular-protein conjugate vaccine from S. aureus (28), were all protective in animal models of infective endocarditis. Collectively, it appears that the critical target for developing therapeutic strategies to combat infective endocarditis may be the inhibition of initial adherence to valve tissue. This is consistent with the results shown in Fig. 7, where the vegetation is surrounded by a fibrous layer that likely excludes or inhibits antibody binding to the bacterial cells.
When rabbits were immunized with the AS44–331 fragment, despite demonstrated high antibody titers, no significant protection was observed when rabbits were challenged in the endocarditis model as assessed by quantitative blood cultures, vegetation weight, and bacterial colonization of the vegetation, as well as bacterial counts in spleen. Antibody titers in immune animals were approximately 2 orders of magnitude higher than titers in nonimmune animals, indicating that low antibody titers were not likely the reason for the lack of protection. This was somewhat surprising, as opsonization by Asc10-specific antibodies was expected to allow for enhanced clearing of bacteria. Although opsonic activity was not dramatic, this decrease is similar to the results of previous opsonization experiments with enriched human leukocytes (22) or purified PMNs (40). Opsonization may have further been decreased by the ability of AS to protect against killing (40, 47, 53). In our hands, however, increased survival of Asc10-expressing cells was not seen, although we used whole rabbit blood. Also, nisin-inducible plasmids are very efficient for the expression of proteins, and the high levels of Asc10 may have impaired growth due to energy requirements. Regardless, a significant difference was seen between normal and AS44–331 immune serum on AS-expressing cells.
The inability to demonstrate protection may have important implications for the development of immunological approaches to combat enterococcal endocarditis. Failure to protect may be due to early initial binding of bacteria to damaged tissue, followed by adherence of platelets and fibrin, that protects enterococci from immune clearance by preventing antibody deposition. Although this hypothesis seems attractive, it does not entirely fit our present data. Blood cultures taken each day from immune and nonimmune rabbits did not show enhanced clearance of bacteria, which would imply that antibodies were not opsonic in vivo. Previous work had established that challenge with numbers of E. faecalis lower than 2 × 107 enterococci did not result in vegetation formation after 72 h (45). However, it is plausible that in these earlier experiments vegetations had formed that were not yet large enough to be visualized after the 3-day period and that very few cells are actually necessary to form the vegetation. Perhaps if lower numbers of bacteria were used and the incubation period of the model was extended, protection would be seen.
Other plausible explanations could be that AS is not involved in early establishment of the vegetation and that its primary role in virulence is immune-system evasion. It has been shown that AS-expressing E. faecalis adhered better to PMNs (40) and macrophages (47), and despite an increased uptake, AS-expressing E. faecalis was not killed as effectively as bacteria lacking AS. In macrophages, the oxidative burst was significantly reduced (47), whereas in PMNs the maturation of the phagosomes appeared to be impaired (40), resulting in increased survival of AS-bearing cells of both types. It was stressed that E. faecalis lacking AS also bound to PMNs in a CR3-independent pathway, although in lower numbers (40). This emphasizes that several factors in E. faecalis work together to cause PMN binding. The advantage AS provided in these associations was dependent on the mechanism by which the bacteria entered the host cells. The CR3 receptor apparently played a pivotal role in both interactions, allowing E. faecalis to enter each type of cell by an alternative, nonopsonic route that did not trigger the normal arsenal of antimicrobial measures. The study involving the macrophages (47) also provided evidence that the N-terminal RGD sequence and the surrounding region were essential for this interaction, such that mutant proteins lacking this sequence did not bind efficiently to macrophages. It is possible that Asc10 provided sufficient protection in vivo from killing for both Asc10-immune and control animals through this mechanism.
It remains formally possible that expression of the enterococcal capsule was induced in vivo, providing protection against antibody deposition. Antibodies against the enterococcus capsule have been successfully employed to decrease systemic infection in a mouse model (22). Although the antibodies were very efficient against a number of clinical isolates, other isolates were not opsonized by these antibodies so that only a certain percentage of strains could be successfully treated. A similar situation would be faced with antibodies against AS, since only a certain percentage of clinical isolates contains pheromone plasmids and therefore AS proteins. A high degree of homology at the protein level would likely produce antibody effective against the wide variety of sex-pheromone plasmids. The involvement of a certain region of AS in the interaction with macrophages suggests that antibodies against that region of the protein could be more effective in blocking the RGD sequences and subsequently the interaction with immune cell surface receptors, preventing internalization of E. faecalis by the CR3-dependent pathway it apparently uses to gain access to immune cells without triggering their defense mechanisms. Finally, it is possible that antibodies to AS or other surface proteins function to aggregate bacteria at the vegetation and actually intensify the infection. In this study, immune animals had higher, but not significantly different, numbers of bacteria per vegetation, supporting this possibility.
Although clinical isolates of E. faecalis are more likely to possess pheromone-responsive plasmids, and thus express AS proteins, there are a significant number of endocarditis isolates that do not contain these plasmids (8). Furthermore, because experimental endocarditis can be induced with strains that lack AS (45), this protein is clearly not the only factor involved in the pathogenesis of enterococcal endocarditis (reviewed in reference 29). For example, the recently described Ace protein has been shown to bind to extracellular matrix proteins such as collagen type IV, found in cardiac tissue (35), and nearly all patients with E. faecalis endocarditis had antibodies to this protein (36). It is possible that the Ace protein compensates for a blocked, absent, or nonfunctional AS protein. Clearly, the molecular pathogenesis of enterococcal endocarditis is complex, and further careful analysis will provide a better understanding of factors involved in this disease and will ultimately lead to better clinical therapies.
ACKNOWLEDGMENTS
This work was supported by grant HL51987 to G.M.D. and P.M.S. from the National Institutes of Health.
We gratefully acknowledge Diane Maher and Peter Southern (Department of Microbiology, University of Minnesota) for excellent advice and assistance with the immunohistochemistry experiments. We thank Ben Madden (Mayo Clinic, Rochester, Minn.) for protein sequencing and Timothy Leonard for assistance with photography.
REFERENCES
- 1.Adler S W, II, Selinger D S, Reed W P. Effect of immunization on the genesis of pneumococcal endocarditis in rabbits. Infect Immun. 1981;34:55–61. doi: 10.1128/iai.34.1.55-61.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almirante B, Tornos M P, Gurgui M, Pujol M, Miro J M. Prognosis of enterococcal endocarditis. Rev Infect Dis. 1991;13:1248–1249. doi: 10.1093/clinids/13.6.1248. [DOI] [PubMed] [Google Scholar]
- 3.Andrewes F W, Horder T J. A study of the streptococci pathogenic for man. Lancet. 1906;ii:708–713. [Google Scholar]
- 4.Archer G L, Johnston J L. Effect of type-specific active immunization on the development and progression of experimental Pseudomonas aeruginosa endocarditis. Infect Immun. 1979;24:167–173. doi: 10.1128/iai.24.1.167-173.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berti M, Candiani G, Kaufhold A, Muscholl A, Wirth R. Does aggregation substance of Enterococcus faecalis contribute to development of endocarditis? Infection. 1998;26:48–53. doi: 10.1007/BF02768756. [DOI] [PubMed] [Google Scholar]
- 6.Bryan E M, Bae T, Kleerebezem M, Dunny G M. Improved vectors for nisin-controlled expression in gram-positive bacteria. Plasmid. 2000;44:183–190. doi: 10.1006/plas.2000.1484. [DOI] [PubMed] [Google Scholar]
- 7.Chow J W, Thal L A, Perri M B, Vazquez J A, Donabedian S M, Clewell D B, Zervos M J. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1993;37:2474–2477. doi: 10.1128/aac.37.11.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coque T M, Patterson J E, Steckelberg J M, Murray B E. Incidence of hemolysin, gelatinase, and aggregation substance among enterococci isolated from patients with endocarditis and other infections and from feces of hospitalized and community-based persons. J Infect Dis. 1995;171:1223–1229. doi: 10.1093/infdis/171.5.1223. [DOI] [PubMed] [Google Scholar]
- 9.Dunny G, Funk C, Adsit J. Direct stimulation of the transfer of antibiotic resistance by sex pheromones in Streptococcus faecalis. Plasmid. 1981;6:270–278. doi: 10.1016/0147-619x(81)90035-4. [DOI] [PubMed] [Google Scholar]
- 10.Dunny G M, Craig R A, Carron R L, Clewell D B. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid. 1979;2:454–465. doi: 10.1016/0147-619x(79)90029-5. [DOI] [PubMed] [Google Scholar]
- 11.Dunny G M, Leonard B A, Hedberg P J. Pheromone-inducible conjugation in Enterococcus faecalis: interbacterial and host-parasite chemical communication. J Bacteriol. 1995;177:871–876. doi: 10.1128/jb.177.4.871-876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durack D T, Gilliland B C, Petersdorf R G. Effect of immunization on susceptibility to experimental Streptococcus mutans and Streptococcus sanguis endocarditis. Infect Immun. 1978;22:52–56. doi: 10.1128/iai.22.1.52-56.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eliopoulos G M. Vancomycin-resistant enterococci. Mechanism and clinical relevance. Infect Dis Clin North Am. 1997;11:851–865. doi: 10.1016/s0891-5520(05)70393-7. [DOI] [PubMed] [Google Scholar]
- 14.Elsner H A, Sobottka I, Mack D, Claussen M, Laufs R, Wirth R. Virulence factors of Enterococcus faecalis and Enterococcus faecium blood culture isolates. Eur J Clin Microbiol Infect Dis. 2000;19:39–42. doi: 10.1007/s100960050007. [DOI] [PubMed] [Google Scholar]
- 15.Francioli P. Antibiotic treatment of streptococcal and enterococcal endocarditis: an overview. Eur Heart J. 1995;16(Suppl B):75–79. doi: 10.1093/eurheartj/16.suppl_b.75. [DOI] [PubMed] [Google Scholar]
- 16.Galli D, Friesenegger A, Wirth R. Transcriptional control of sex-pheromone-inducible genes on plasmid pAD1 of Enterococcus faecalis and sequence analysis of a third structural gene for (pPD1-encoded) aggregation substance. Mol Microbiol. 1992;6:1297–1308. doi: 10.1111/j.1365-2958.1992.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 17.Galli D, Lottspeich F, Wirth R. Sequence analysis of Enterococcus faecalis aggregation substance encoded by the sex pheromone plasmid pAD1. Mol Microbiol. 1990;4:895–904. doi: 10.1111/j.1365-2958.1990.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg D P, Ward J I, Bayer A S. Influence of Staphylococcus aureus antibody on experimental endocarditis in rabbits. Infect Immun. 1987;55:3030–3034. doi: 10.1128/iai.55.12.3030-3034.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heaton P M, Discotto L F, Pucci M J, Handwerger S. Mobilization of vancomycin resistance by transposon-mediated fusion of a VanA plasmid with an Enterococcus faecium sex pheromone-response plasmid. Gene. 1996;171:9–17. doi: 10.1016/0378-1119(96)00022-4. [DOI] [PubMed] [Google Scholar]
- 20.Hirt H, Erlandsen S L, Dunny G M. Heterologous inducible expression of Enterococcus faecalis pCF10 aggregation substance Asc10 in Lactococcus lactis and Streptococcus gordonii contributes to cell hydrophobicity and adhesion to fibrin. J Bacteriol. 2000;182:2299–2306. doi: 10.1128/jb.182.8.2299-2306.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirt H, Wanner G, Galli D, Wirth R. Biochemical, immunological and ultrastructural characterization of aggregation substances encoded by Enterococcus faecalis sex-pheromone plasmids. Eur J Biochem. 1993;211:711–716. doi: 10.1111/j.1432-1033.1993.tb17600.x. [DOI] [PubMed] [Google Scholar]
- 22.Huebner J, Quaas A, Krueger W A, Goldmann D A, Pier G B. Prophylactic and therapeutic efficacy of antibodies to a capsular polysaccharide shared among vancomycin-sensitive and -resistant enterococci. Infect Immun. 2000;68:4631–4636. doi: 10.1128/iai.68.8.4631-4636.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huycke M M, Sahm D F, Gilmore M S. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg Infect Dis. 1998;4:239–249. doi: 10.3201/eid0402.980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkinson H F, Lamont R J. Streptococcal adhesion and colonization. Crit Rev Oral Biol Med. 1997;8:175–200. doi: 10.1177/10454411970080020601. [DOI] [PubMed] [Google Scholar]
- 25.Karchmer A W. Infective endocarditis. In: Root R K, Waldvogel F, Corey L, Stamm W, editors. Clinical infectious disease. New York, N.Y: Oxford University Press, Inc.; 1999. pp. 621–635. [Google Scholar]
- 26.Kreft B, Marre R, Schramm U, Wirth R. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun. 1992;60:25–30. doi: 10.1128/iai.60.1.25-30.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Lee J C, Park J S, Shepherd S E, Carey V, Fattom A. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect Immun. 1997;65:4146–4151. doi: 10.1128/iai.65.10.4146-4151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormick J K, Hirt H, Dunny G M, Schlievert P M. Pathogenic mechanisms of enterococcal endocarditis. Curr Infect Dis Rep. 2000;2:315–321. doi: 10.1007/s11908-000-0009-9. [DOI] [PubMed] [Google Scholar]
- 30.Megran D W. Enterococcal endocarditis. Clin Infect Dis. 1992;15:63–71. doi: 10.1093/clinids/15.1.63. [DOI] [PubMed] [Google Scholar]
- 31.Moellering R C., Jr The specter of glycopeptide resistance: current trends and future considerations. Am J Med. 1998;104:3S–6S. doi: 10.1016/s0002-9343(98)00148-x. [DOI] [PubMed] [Google Scholar]
- 32.Mundy L M, Sahm D F, Gilmore M. Relationships between enterococcal virulence and antimicrobial resistance. Clin Microbiol Rev. 2000;13:513–522. doi: 10.1128/cmr.13.4.513-522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray B E. The life and times of the Enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muscholl-Silberhorn A. Cloning and functional analysis of asa373, a novel adhesin unrelated to the other sex pheromone plasmid-encoded aggregation substances of Enterococcus faecalis. Mol Microbiol. 1999;34:620–630. doi: 10.1046/j.1365-2958.1999.01631.x. [DOI] [PubMed] [Google Scholar]
- 35.Nallapareddy S R, Qin X, Weinstock G M, Hook M, Murray B E. Enterococcus faecalis adhesin, Ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infect Immun. 2000;68:5218–5224. doi: 10.1128/iai.68.9.5218-5224.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nallapareddy S R, Singh K V, Duh R W, Weinstock G M, Murray B E. Diversity of ace, a gene encoding a microbial surface component recognizing adhesive matrix molecules, from different strains of Enterococcus faecalis and evidence for production of Ace during human infections. Infect Immun. 2000;68:5210–5217. doi: 10.1128/iai.68.9.5210-5217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olmsted S B, Dunny G M, Erlandsen S L, Wells C L. A plasmid-encoded surface protein on Enterococcus faecalis augments its internalization by cultured intestinal epithelial cells. J Infect Dis. 1994;170:1549–1556. doi: 10.1093/infdis/170.6.1549. [DOI] [PubMed] [Google Scholar]
- 38.Olmsted S B, Erlandsen S L, Dunny G M, Wells C L. High-resolution visualization by field emission scanning electron microscopy of Enterococcus faecalis surface proteins encoded by the pheromone-inducible conjugative plasmid pCF10. J Bacteriol. 1993;175:6229–6237. doi: 10.1128/jb.175.19.6229-6237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olmsted S B, Kao S M, van Putte L J, Gallo J C, Dunny G M. Role of the pheromone-inducible surface protein Asc10 in mating aggregate formation and conjugal transfer of the Enterococcus faecalis plasmid pCF10. J Bacteriol. 1991;173:7665–7672. doi: 10.1128/jb.173.23.7665-7672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rakita R M, Vanek N N, Jacques-Palaz K, Mee M, Mariscalco M M, Dunny G M, Snuggs M, Van Winkle W B, Simon S I. Enterococcus faecalis bearing aggregation substance is resistant to killing by human neutrophils despite phagocytosis and neutrophil activation. Infect Immun. 1999;67:6067–6075. doi: 10.1128/iai.67.11.6067-6075.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice L B, Calderwood S B, Eliopoulos G M, Farber B F, Karchmer A W. Enterococcal endocarditis: a comparison of prosthetic and native valve disease. Rev Infect Dis. 1991;13:1–7. doi: 10.1093/clinids/13.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 43.Scheld W M, Calderone R A, Brodeur J P, Sande M A. Influence of preformed antibody on the pathogenesis of experimental Candida albicans endocarditis. Infect Immun. 1983;40:950–955. doi: 10.1128/iai.40.3.950-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schennings T, Heimdahl A, Coster K, Flock J I. Immunization with fibronectin binding protein from Staphylococcus aureus protects against experimental endocarditis in rats. Microb Pathog. 1993;15:227–236. doi: 10.1006/mpat.1993.1073. [DOI] [PubMed] [Google Scholar]
- 45.Schlievert P M, Gahr P J, Assimacopoulos A P, Dinges M M, Stoehr J A, Harmala J W, Hirt H, Dunny G M. Aggregation and binding substances enhance pathogenicity in rabbit models of Enterococcus faecalis endocarditis. Infect Immun. 1998;66:218–223. doi: 10.1128/iai.66.1.218-223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlievert P M, Shands K N, Dan B B, Schmid G P, Nishimura R D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981;143:509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- 47.Süβmuth S D, Muscholl-Silberhorn A, Wirth R, Susa M, Marre R, Rozdzinski E. Aggregation substance promotes adherence, phagocytosis, and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst. Infect Immun. 2000;68:4900–4906. doi: 10.1128/iai.68.9.4900-4906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeda S, Pier G B, Kojima Y, Tojo M, Muller E, Tosteson T, Goldmann D A. Protection against endocarditis due to Staphylococcus epidermidis by immunization with capsular polysaccharide/adhesin. Circulation. 1991;84:2539–2546. doi: 10.1161/01.cir.84.6.2539. [DOI] [PubMed] [Google Scholar]
- 49.Tortorello M, Adsit J, Krug D, Antczak D, Dunny G. Monoclonal antibodies to cell surface antigens involved in sex pheromone induced mating in Streptococcus faecalis. J Gen Microbiol. 1986;132:857–864. doi: 10.1099/00221287-132-4-857. [DOI] [PubMed] [Google Scholar]
- 50.Trotter K M, Dunny G M. Mutants of Enterococcus faecalis deficient as recipients in mating with donors carrying pheromone-inducible plasmids. Plasmid. 1990;24:57–67. doi: 10.1016/0147-619x(90)90025-8. [DOI] [PubMed] [Google Scholar]
- 51.Valdivia E, Martin-Sanchez I, Quirantes R, Martinez-Bueno M, Galvez A, Maqueda M. Incidence of antibiotic resistance and sex pheromone response among enterococci isolated from clinical human samples and from municipal waste water. J Appl Bacteriol. 1996;81:538–544. doi: 10.1111/j.1365-2672.1996.tb03544.x. [DOI] [PubMed] [Google Scholar]
- 52.van de Rijn I. Role of culture conditions and immunization in experimental nutritionally variant streptococcal endocarditis. Infect Immun. 1985;50:641–646. doi: 10.1128/iai.50.3.641-646.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanek N N, Simon S I, Jacques-Palaz K, Mariscalco M M, Dunny G M, Rakita R M. Enterococcus faecalis aggregation substance promotes opsonin-independent binding to human neutrophils via a complement receptor type 3-mediated mechanism. FEMS Immunol Med Microbiol. 1999;26:49–60. doi: 10.1111/j.1574-695X.1999.tb01371.x. [DOI] [PubMed] [Google Scholar]
- 54.Viscount H B, Munro C L, Burnette-Curley D, Peterson D L, Macrina F L. Immunization with FimA protects against Streptococcus parasanguis endocarditis in rats. Infect Immun. 1997;65:994–1002. doi: 10.1128/iai.65.3.994-1002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]