Abstract
Background and aim:
Vitamin D (VD) reduces interferon-gamma (IFN-γ) production and prevents nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation, impacting the inhibition of the autoimmunity process such as autoimmune thyroiditis (AITD). Children with Down syndrome (DS) are reported to have a higher risk of autoimmunity and lower VD levels than non-DS. Therefore, this study aimed to evaluate VD levels in Indonesian DS children and their relationship with marker of AITD.
Methods:
This study was conducted on DS children at Dr Soetomo Hospital between February 2021-June 2022. Socio-demographic status, amount of milk, fish and meat consumption, and duration of sun exposure were obtained using a self-report questionnaire. Thyroid hormone (TSH and FT4), thyroid antibody (TPO-Ab and Tg-Ab), 25 (OH)D, IFN-γ, and NF-κB levels were measured using ELISA.
Results:
Of the 80 participants, 53.75% had sufficient (50.829±17.713 ng/ml) and 46.25% had non-sufficient (20.606±5.974 ng/ml) VD levels. Daily milk consumption, meat and fish consumption were risk factors contributing to VD levels in multivariate analysis [p=0.003, OR=1.007(1.003–1.012); p=0.004, OR=1.816(1.209– 2.728), respectively]. Participants with sufficient VD had significantly higher TPO-Ab (p=0.007) and Tg-Ab (p=0.016). Mean of VD levels were significantly negatively correlated with IFN-γ levels (r =-0.262, p=0.037) and positively correlated with TPO-Ab (r= 0.432, p=1x10-5,) and Tg-Ab (r= 0.375, p=0.001).
Conclusions:
Majority of subjects had sufficient VD levels. VD suppresses IFN-γ, but is unable to affect NF-κB levels, presumably causing high levels of TPO-Ab and Tg-Ab in sufficient VD patient. (www.actabiomedica.it)
Keywords: Vitamin D, thyroid autoantibodies, IFN-γ, NF-κB, Down syndrome, Indonesia
Introduction
Vitamin D (VD) or 25-hydroxy-VD (25(OH)D) is a secosteroid with pleiotropic roles in various physiological processes. VD’s role in the immune modulation response appears to have immunomodulating actions in autoimmune diseases (1). VD’s influence on autoimmune thyroid disease (AITD) has been widely studied. Most existing data support a relationship between VD deficiency and an increased tendency to develop higher titers of AITD-related antibodies (2-4). The severity of 25-hydroxy VD deficiency was associated with onset of Hashimoto’s thyroiditis (HT) and thyroid antibody levels (5). Several VD modulates autoimmunity through specific enhancement of the innate immune system and inhibition of adaptive immune responses through the VD receptor (VDR) that is expressed in all immune cells (6,7). VD administration in the early phase of cell differentiation reduces interferon-gamma (IFN-γ) production, inducing type 2 T helper cells (Th2) and GATA binding protein 3 (GATA3), inhibiting the autoimmune process (8). It has been shown that VD down-regulates nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in lymphocytes (9) and prevents NF-κB activation in monocytes, regulating the expression of cellular factors contributing to reduced NF-κB DNA binding of NF-κB (9-11). NF-κB is a well-known transcription factor of proinflammatory mediators that plays a crucial role in establishing immune tolerance, including central tolerance and the peripheral function of regulatory T cells. Therefore, defective or deregulated NF-κB activation may contribute to autoimmunity and inflammation (12).
Down syndrome (DS) is a genetic disorder caused by trisomy for all or part of chromosome 21, leading to an excess of phenotypic-influencing genes in the DS critical regions (DSCRs), including immune system defects that increase autoimmunity risk such as autoimmune thyroiditis (AITD) (13). AITD in the primary forms of autoimmune hypothyroid (Hashimoto thyroiditis [HT]) or autoimmune hyperthyroid (Grave’s disease [GD]) is more common at a younger age in DS than in non-DS children (14). HT is a form of autoimmune hypothyroidism with a high proportion of Th1 cells, which secrete proinflammatory cytokines such as IFN-γ (15). Study in non DS patients with HT reported increased of IFN-γ serum levels, expression of intrathyroidal IFN-γ, and high levels of IFN-γ production by clones derived from infiltrating lymphocytes (16-19) and also level of NF-κB1 protein as a risk factor for HT disease by directly modulating interleukin-6 (IL-6) serum levels (20). The worldwide prevalence of VD deficiency is relatively high even in countries with plenty of sunlight, ranging from 1%–95%, depending on the threshold used to define it (21-27). While Indonesia is located on the equator and has year-round sun exposure, shifts in lifestyle and sedentary activities and a lack of VD-fortified foods increase VD deficiency risk. Most Indonesian children have VD insufficiency (28,29) and lower VD levels than Malaysia, Vietnam, and Thailand (30). However, the latest study by Pulungan et al. found that Indonesian children mostly had sufficient VD levels (31). Several studies have found lower VD levels in DS than in non-DS children (32-34). Another study showed that DS children with regular sun exposure do not require VD supplementation (35). However, DS children spend more time indoors and are less physically active (36). Until recently, there have been no reports on VD levels in DS children in Indonesia as well as no published study that evaluated the role of VD and marker of thyroid autoimmune in DS population.
Therefore, this study aimed to investigate the VD profile in Indonesian DS children and their correlation with marker in thyroid autoimmune.
Patients and methods
Study participants
This cross-sectional study enrolled DS aged 1-month to 18 years at the Pediatric Outpatient Clinic of Child Health of the Dr Soetomo General Hospital in Surabaya, Indonesia, between February 2021 and June 2022. This study was approved by the Institutional Review Board of Dr Soetomo General Hospital (0397/KEPK/III/2022). The parents of participants provided informed consent. Participants were enrolled using a consecutive sampling method. We excluded participants with acute medical conditions, allergies, malignancy, micronutrient deficiency, or who had taken VD supplements before the study. DS children with severe malnutrition-based growth curves were also excluded. DS was diagnosed based on karyotyping. Thyroid function was detailed as hypothyroidism, hyperthyroidism and euthyroid based on normal value of free thyroxine (FT4) and thyroid-stimulating hormone (TSH) (37). Diagnosis of AITD was based on the positivity of thyroid antibodies, namely thyroid peroxidase antibody (TPO-Ab) and Thyroglobulin antibody (Tg-Ab). If there was at least one positive antibody marker with normal or impaired thyroid function, the diagnosis of AITD had been established (38). Sex, age, sociodemographic status, amount and type of milk, fish and meat consumption, and sun exposure duration were obtained using a self-report questionnaire completed by the parents. Total sun exposure was calculated as minutes per week based on the average time of daily sun exposure. The amount of milk consumption was calculated as mL per day, while fish and meat consumption was calculated as slices per week.
Five millilitres of blood were drawn from each participant. Plasma samples were tested for their 25(OH)D, TSH, FT4, IFN-γ, NF-κB, anti–thyroid peroxidase antibody (TPO-Ab), and anti-thyroglobulin antibody (Tg-Ab) levels. In addition to the examination of circulating 25(OH)D, TSH, FT4, and IFN- which are commonly performed, the examination of NFkB levels uses many methods. Several studies already had evaluated NFkB level in plasma and serum (39,40).
Measurement of VD levels
VD levels were measured using an enzyme-linked immunosorbent assay (ELISA) method (25-hydroxy VD ELISA kit; catalog number (Cat. No) CAN-VD-510 from DBC Canada). The plasma concentration of 25-hydroxy vitamin D (25(OH)D) is considered to be the primary indicator of vitamin D status. The Pediatric Endocrine Society defines normal 25(OH)D levels as 30–100 ng/mL, insufficiency as 21–29 ng/mL, and deficiency as <20 ng/mL (41). In this study, we categorized participants into sufficient and nonsufficient (deficient/insufficient) groups.
Measurement of TSH and FT4 levels
FT4 levels were measured using free thyroxine (FT4) ELISA; Cat. No CAN-FT4-4340, while TSH levels were measured using thyroid stimulating hormone (TSH) ELISA; Cat. No CAN-TSH-4080 by from DBC-Diagnostics Biochem Canada Inc. The reference range values for pediatric care by Sperling et al (42).
Measurement of IFN-γ and NF-κB levels
ELISA methods were used to measure IFN-γ (Human Interferon γ ELISA kit; Cat. No. E0105Hu from BT Lab) and NF-κB (Human Nuclear Factor-Kappa B LAB kit; Cat. No. E0690Hu from BT Lab) levels.
Measurement of TPO-Ab and Tg-Ab levels
ELISA methods were used to measure TPO-Ab (TPO-Ab ELISA kit; Cat. No. DE7580 from Demeditec Diagnostics GmbH) and Tg-Ab (Tg-Ab ELISA kit; Cat. No. DE7590 from Demeditec Diagnostics GmbH). Interpretation of autoimmune thyroid markers was based on manufacturer specified cutoff values of >75 IU/mL (positive), 50–75 IU/mL (borderline), and <50 IU/mL (negative) for TPO-Ab and >150 IU/mL (positive),100–150 IU/mL (intermediate), and <100 IU/mL (negative) for Tg-Ab, respectively.
Statistical analysis
The data were examined for normality with the Kolmogorov-Smirnov test and homogeneity with the Levene test. Subjects were categorized into two groups, VD sufficient and non-sufficient groups. Descriptive analysis was performed to describe the demographic characteristic of DS patients based on the 25(OH)D adequacy status. Factors affecting VD levels were analyzed with univariate and multivariate analysis. Group different tests were conducted using the Chi-square test between VD sufficient and non-sufficient categories. Levels difference of 25(OH)D, IFN-γ, and NF-κB were analyzed with the T-test and Man Whitney test. The association between 25(OH)D and IFN-γ, NF-κB, were analyzed with the spearman correlation test. Variables were considered statistically significant with a P-value <0.05. The analysis of data was conducted using the SPSS version 25 (IBM Co., New York, USA).
Results
This study enrolled 80 DS participant. Among of them, 14/80 (17.3%) children are deficient, 23/80 (28.4%) are insufficient, and 43/80 (53.1%) are sufficient of 25-hydroxy-VD. After categorized participants into sufficient and non-sufficient (deficient/insufficient) groups, we had 37/80 (46.25%) participant as non-sufficient versus 43/80 (53.75%) participant as sufficient VD. Mean level of 25(OH) D was 36.85 ± 20.322 ng/mL (Table 1).
Table 1.
Demographic characteristics of DS participants.
| Characteristic | VD Non-Sufficient (n= 37) | VD Sufficient (n=43) | p-value |
|---|---|---|---|
| Vitamin D level (ng/mL; mean ± SD) | 20.606±5.974 | 50.829±17.713 | 1x10-5 T |
| Age at the time of enrollment (months; Median (Min – Max) | 46.162 ± 50.72 | 25.465 ± 21.473 | 0.64 M |
| Body mass index (BMI-SDS; mean ± SD) | 15.601 ± 3.065 | 14.634 ± 3.237 | 0.175 T |
| Sex (n, %) Male Female |
19 (38.8%) 18 (58.1%) |
30 (61.2%) 13 (41.9%) |
0.092 C |
| History of prematurity/low birth weight (n, %) NO YES |
35 (50%) 2 (20%) |
35 (50%) 8 (80%) |
0.097 C |
| Mother’s level of education (n, %) Elementary school Junior high school Senior high school Bachelor (higher education) |
1 (25%) 1 (25%) 18 (42.9%) 17 (56.7%) |
3 (75%) 3 (75%) 24 (57.1%) 13 (43.3%) |
0.398 C |
| Associated congenital malformation (n, %) Congenital heart defect Hirschsprung’s disease None |
6 (30%) 0 (0%) 31 (52.5%) |
14 (70%) 1 (100%) 28 (47.5%) |
0.141 C |
| Thyroid function (n, %) Hypothyroid Hyperthyroid Euthyroid |
23 (45.1%) 1 (20%) 13 (54.2%) |
28 (54.9%) 4 (80%) 11 (45.8%) |
0.365 C |
| Duration of levothyroxine therapy (months, Median (Min – Max)) | 15 (10 – 63) | 15 (0 – 58) | 0.740 M |
| Daily sun exposure(hours/week); Median (Min – Max)) | 15 (5 – 100) | 35 (15 -240) | 1x10-5 M |
| Daily Milk consumption (cc/day); Median (Min – Max)) | 200 (0 -800) | 500 (180 -2100) | 1x10-5 M |
| Meat and fish consumption (slice/week); Median (Min – Max)) | 2 (1-8) | 7 (2-8) | 1x10-5 M |
Key: *significant with p < 0.05; T Independent t-test; C Chi-square test; M Mann–Whitney test.
We evaluated several factors that could potentially influence VD adequacy in the sufficient and non-sufficient groups (Table 2) and also comparisons of average levels of thyroid autoantibodies against TPO-Ab and Tg-Ab and of inflammatory markers IFN-γ and NF-κB between sufficient and non-sufficient VD patients (Figure 1).
Table 2.
Univariate and Multivariate Analysis for Risk Factor Contributing for Vitamin D levels.
| No | Variable | Bivariate | Multivariate | ||
|---|---|---|---|---|---|
| p-value | OR 95% CI | p-value | OR 95% CI | ||
| 1 | Age | 0.026* | 0.985 (0.972 – 0.998) | 0.057 | 0.972 (0.943 – 1.001) |
| 2 | BMI | 0.970 | 0.998 (0.890 – 1.119) | - | - |
| 3 | Daily sun exposure (hours/week) | 0.003* | 1.049 (1.017 – 1.082) | 0.108 | 1.048 (0.990 – 1.110) |
| 4 | Daily milk consumption (cc/day) | 1x10-5 * | 1.011 (1.006 – 1.016) | 0.003* | 1.007 (1.003 – 1.012) |
| 5 | Meat and fish consumption (slice/week) | 1x10-5 * | 1.056 (1.528 – 2.768) | 0.004* | 1.816 (1.209 – 2.728) |
Key: *, significant at p<0.05; CI, confidence interval; OR, odds ratio.
Figure 1.
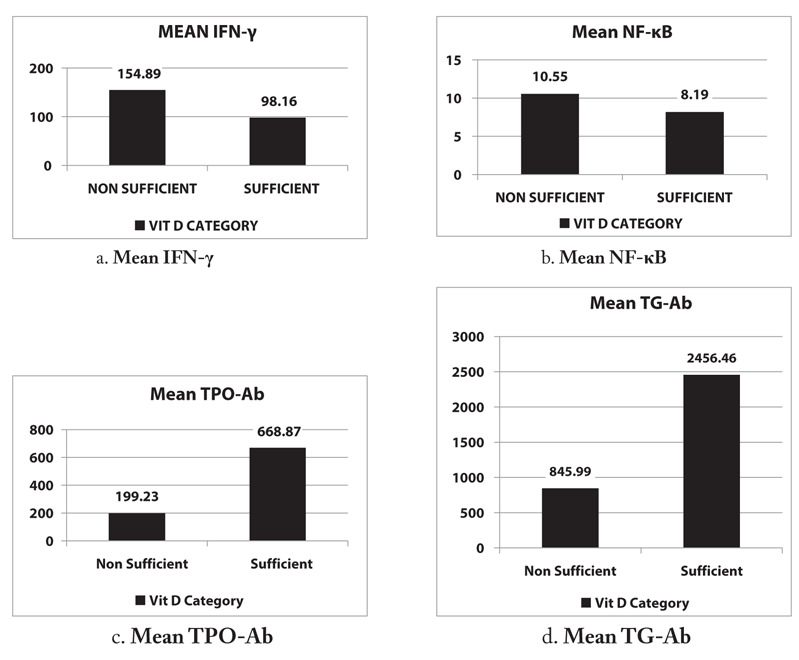
Average levels of IFN-γ, NF-κB, TPO-Ab, and Tg-Ab in sufficient and nonsufficient VD participants. Mean (A) IFN-γ, (B) NF-κB, (C) TPO-Ab, and (D) Tg-Ab levels.
The IFN-γ and NF-κB levels were desriptively higher in the nonsufficient group than in the sufficient group but not significantly different (p=0.218 and p=0.556, respectively). Conversely, TPO-Ab and Tg-Ab levels were significantly higher in the sufficient group than in the non-sufficient group (p=0.007 and p=0.016, respectively).
In our study, mean level of 25(OH)D was significantly negatively correlated with IFN-γ and significantly positively correlated with TPO-Ab and Tg-Ab (Table 3).
Table 3.
The correlation 25(OH)D with IFN-γ, NFκB, TPO-Ab, Tg-Ab.
| Variabel | Mean level of 25(OH)D | |
|---|---|---|
| p-value | Coeficient Correlation (r) | |
| IFN-γ (ng/mL; mean ± SD) | 0.037* | -0.262 |
| NFκB (ng/mL; mean ± SD) | 0.175 | -0.172 |
| TPO-Ab (IU/mL; mean ± SD) | 1x10-5* | 0.432 |
| Tg-Ab (IU/mL; mean ± SD) | 0.001* | 0.375 |
Key: *, Sig p<0.05.
Discussion
This cross-sectional study showed that VD sufficiency was more prevalent than VD insufficiency in Indonesian DS children, with a mean level of 36.85 ± 20.322 ng/mL. Our finding was similar to El-Hawary et al., who found their mean VD level to be 30.65 ± 20.64, but only 40% were in the sufficient VD category (34). However, Stagi et al. reported a very high prevalence of VD deficiency in DS children with a mean VD level of 14.34 ± 8.31 (33).
The majority of our participants had thyroid disorders with the most common form being hypothyroidism in 51/80 (63.75%) children, while 24/80 (30.00%) children being euthyroid. Our data showed no significant difference in VD status in each group based on thyroid function. Thyroid dysfunction in DS children is reported in 28%-54% of patients with a frequency that increases with age (43). The effect of 25(OH)D level on thyroid functions is still controversial. Adequacy of serum 25(OH)D level is required for the maintenance of euthyroid functions (44). A large population-based study reported that increased 25(OH)D levels were associated with decreased circulating TSH among younger individuals (45), while a cross-sectional study in 2869 in previously healthy children aged 6–24 months found that serum 25(OH)D levels had no significant correlation with TSH, FT3 and FT4 levels, however, VD deficiency was associated with hypothyroidism (44).
Our study found that daily milk consumption and meat and fish consumption were risk factors contributing to VD levels in VD sufficiency. These data were similar to a large Canadian study of 1311 children that found that increased cow’s milk consumption was associated with increased 25(OH)D levels. Many children in South East Asia (SEA) consume <1% of the recommended daily VD intake (46,47), and the availability of natural VD-rich food sources in the region is limited, with low and infrequent consumption of meat and dairy products (48,49). However, studies have shown that it takes at least two cups (500 mL) of cow’s milk per day to maintain adequate VD levels (50).
We found lower IFN-γ levels in patients in the sufficient VD group and a significant negative correlation between VD and IFN-γ levels, meaning that high levels of vitamin D in our patients were associated with lower IFN-γ levels. Study in-vitro showed vitamin D inhibited IFN-γ production and increased IL-10 production by peripheral blood mononuclear cells (PBMCs) derived from healthy individuals (51). Several other in vitro studies using PBMC culture and intracellular cytokine staining obtained similar results (52-54). VD administration in the early phase of cell differentiation reduces interferon-gamma (IFN-γ) production, inducing type 2 T helper cells (Th2) and GATA binding protein 3 (GATA3), inhibiting the autoimmune process (8). Unfortunately, Ragab et al, in the same study, found no correlation between serum vitamin D levels and IFN-γ or IL-10 cytokine concentration produced in the culture supernatant. However, the correlation between circulating level of VD and IFN-γ in our study was consistent with what He et al reported in their study (53). They found that the IFN-γ concentrations were significantly higher in the vitamin D sufficient athletes.
Defective or deregulated NF-κB activation may contribute to autoimmunity (12). Descriptively, our VD sufficient group had lower NF-κB levels than our non-sufficient group. VD and its VDR complex can interact with transcription factors such as NF-κB. VD was able to downregulate NF-κB levels in lymphocytes, preventing NF-κB activation in monocytes and regulating the expression of cellular factors contributing to reduced NF-κB DNA binding (9-11). However, our study found no significant correlation between VD and NF-κB levels in plasma. An adult study also reported no significant effect of VD on NF-κB activity in obese and overweight individuals with VD deficiency and Diabetes Mellitus (55,56).
TPO-Ab and Tg-Ab are two types of thyroid autoantibodies, the levels of which are positively associated with thyroid inflammation and hypothyroidism severity (57). Studies in non-DS children showed that VD deficiency was associated with an increased risk of autoimmune disease (7). Surprisingly, we found a significant correlation between mean levels of 25(OH)D with anti-TPO-Ab and Tg-Ab levels, indicating that high VD levels are significantly associated with high autoantibody levels. Many adult studies report lower levels of 25-hydroxy VD was correlated with thyroid autoantibodies such as TPO-Ab (58–60) and Tg-Ab (61). Few studies have reported an association between AITD and VD levels with inconclusive results. However, our conflicting results were consistent with those of Yasmeh et al. and Efframidis et al. on the adult population (62,63). While Goswami et al. found only a weak correlation between 25(OH)D and anti-TPO-Ab levels (64), an absence of correlation between 25(OH)D, anti-TPO-Ab, and anti-Tg-Ab levels was also observed in two population-based studies in Thailand and China (45,65). A pediatric study also reported no correlation between 25-hydroxy VD and anti-TPO-Ab levels (66).
VD plays a small but significant role in AITD pathogenesis, which may be more apparent when combined with other factors (2). In addition, the most important factors in AITD development in children are genetic factors, while non-genetic factors only play a role in 20% (67), suggesting that VD’s role in AITD is smaller in children than in adults (68). We hypothesize that VD suppresses proinflammatory cytokine IFN-γ production by Th1 cells, an important activation mechanism in AITD, but is unable to affect NF-κB activity, which play a major role in proinflammatory cytokine IL-6 production, allowing the autoimmunity process to continue, leading to a very high levels of thyroid autoantibodies such as TPO-Ab and Tg-Ab.
A major strength of this study is its first-ever exploration of the association between VD and several marker of thyroid autoimmune in DS children, a population at high risk of autoimmunity. This study provides basic data on the VD adequacy in DS children in Indonesia, a country with abundant exposure to sunlight but lower natural VD-rich food daily intake. In addition, its results provide a different perspective on the role of VD levels in autoimmunity development processes, especially AITD in children with special conditions such as DS.
This study has several weaknesses. Firstly, its cross-sectional design could not determine whether adequate VD levels in participants with positive thyroid autoimmune markers had existed before examination or were improved at the time of examination. Secondly, this was a single-centre study, and its findings might not represent all DS children. Therefore, further studies with larger sample sizes, better designs, more global scopes, and longer evaluations of VD levels are needed to provide more uniform and consistent data on the role of VD in DS children.
Conclusions
Our study has shown that children with DS had a sufficient VD level. Higher VD levels in DS children in our study are presumably due to daily milk consumption equal to 500 cc daily and daily intake of 1 slice meat and fish. Higher VD levels are significantly correlated with lower IFN-γ levels but not with NF-κB levels. Since the higher TPO-Ab and Tg-Ab levels were correlated with VD levels, further case-control studies with larger sample sizes are needed to provide more consistent data on the role of VD on AITD development in DS children.
Acknowledgments:
The authors wish to thank the endocrine team of Dr. Soetomo Hospital, Surabaya, Indonesia.
Ethical Approval:
The Clinical Research Unit of Dr. Soetomo Hospital in Surabaya, Indonesia, approved this study and assigned it the ethical number 0397/KEPK/III/2022. All participants in this study were following the Declaration of Helsinki. All participant’s parents or legal guardians signed the informed consent form.
Conflict of Interest:
Each author declares no commercial associations (e.g consultancies, stock ownership, equity interest, patient/licensing arrangemen etc.) that might pose a conflict of interest in connection with the submitted article.
Author Contribution:
YH, AE, and BS conceptualized, concept the methodology and software used, analysed data, interpreted results, prepare, and wrote the initial draft of the manuscript. BS and NR analysed data, interpreted results, assisted in drafting the manuscript. NR and MF critically reviewed, edited the manuscript, guided manuscript writing. BS and AE collected data, prepared the figure and tables, analysed data. All author agreed and give final approval to the submitted manuscript.
References
- Umar M, Sastry KS, Chouchane AI. Role of vitamin D beyond the skeletal function: A review of the molecular and clinical studies. Int J Mol Sci. 2018;19(6):1–28. doi: 10.3390/ijms19061618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira IH, Rodrigues D, Paiva I. Vitamin d and autoimmune thyroid disease— cause, consequence, or a vicious cycle? Nutrients. 2020;12(9):1–20. doi: 10.3390/nu12092791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao G, Zhu Y, Fang L. Correlation Between Hashimoto’s Thyroiditis–Related Thyroid Hormone Levels and 25-Hydroxyvitamin D. Front Endocrinol (Lausanne) 2020;11(February):1–7. doi: 10.3389/fendo.2020.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YY, Chung YJ. Vitamin D supplementation does not prevent the recurrence of Graves’ disease. Sci Rep. 2020;10(1):1–7. doi: 10.1038/s41598-019-55107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt NC, Karbek B, Ucan B, et al. The association between severity of vitamin D deficiency and Hashimoto’s thyroiditis. Endocr Pract. 2013;19(3):479–84. doi: 10.4158/EP12376.OR. [DOI] [PubMed] [Google Scholar]
- Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94(1):26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankers W, Colin EM, van Hamburg JP, Lubberts E. Vitamin D in Autoimmunity: Molecular Mechanisms and Therapeutic Potential. Front Immunol. 2017;7:1–26. doi: 10.3389/fimmu.2016.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloka S, Silva C, Wang J, Yong VW. Predominance of Th2 polarization by Vitamin D through a STAT6-dependent mechanism. J Neuroinflammation. 2011;8:1–10. doi: 10.1186/1742-2094-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XP, Bellido T, Manolagas SC. Down-regulation of NF-κB protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1995;92(24):10990–4. doi: 10.1073/pnas.92.24.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stio M, Martinesi M, Bruni S, et al. The Vitamin D analogue TX 527 blocks NF-κB activation in peripheral blood mononuclear cells of patients with Crohn’s disease. J Steroid Biochem Mol Biol. 2007;103(1):51–60. doi: 10.1016/j.jsbmb.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Harant H, Wolff B, Lindley IJD. 1a,25-Dihydroxyvitamin D3 decrease DNA binding of nuclear factor-kB in human fibroblast. FEBS lett. 1999;436(3):329–34. doi: 10.1016/s0014-5793(98)01153-3. [DOI] [PubMed] [Google Scholar]
- Sun S-C, Chang J-H, Jin J. Regulation of NF-κB in Autoimmunity. Trends Immunol. 2013;34(6):282–9. doi: 10.1016/j.it.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner KJ. Molecular Basis of Pharmacotherapies for Cognition in Down Syndrome. Trends Pharmacol Sci. 2010;31(2):1–16. doi: 10.1016/j.tips.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi F, Giaccherino R. Endocrine Autoimmunity in Down’s Syndrome. Front Horm Res. 2017;48:133–46. doi: 10.1159/000452912. [DOI] [PubMed] [Google Scholar]
- Karanikas G, Schuetz M, Wahl K, et al. Relation of anti-TPO autoantibody titre and T-lymphocyte cytokine production patterns in Hashimoto’s thyroiditis. Clin Endocrinol (Oxf) 2005;63(2):191–6. doi: 10.1111/j.1365-2265.2005.02324.x. [DOI] [PubMed] [Google Scholar]
- Drugarin D, Negru S, Koreck A, Zosin I, Cristea C. The pattern of a T(H)1 cytokine in autoimmune thyroiditis. Immunol Lett. 2000;71(2):73–7. doi: 10.1016/s0165-2478(99)00156-x. [DOI] [PubMed] [Google Scholar]
- Hamilton F, Balck M, Farquharson MA, Stewart C, Foulis AK. Spatial correlation between thyroid epithelial cells expressing class II MHC molecules and interferon‐gamma‐containing lymphocytes in human thyroid autoimmune disease. Clin Exp Immunol. 1991;83(1):64–8. doi: 10.1111/j.1365-2249.1991.tb05589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti S, del Prete GF, Mastromauro C, et al. The Autoimmune Infiltrate of Basedow’s Disease: Analysis at Clonal Level and Comparison with Hashimoto’s Thyroiditis. Exp Clin Endocrinol Diabetes. 1991;97(2):139–46. doi: 10.1055/s-0029-1211053. [DOI] [PubMed] [Google Scholar]
- Roura-Mir C, Catálfamo M, Sospedra M, Alcalde L, Pujol-Borrell R, Jaraquemada D. Single-cell analysis of intrathyroidal lymphocytes shows differential cytokine expression in Hashimoto’s and Graves’ disease. Eur J Immunol. 1997;27(12):3290–302. doi: 10.1002/eji.1830271228. [DOI] [PubMed] [Google Scholar]
- Koc A, Batar B, Celik O, Onaran I, Tasan E, Sultuybek GK. Polymorphism of the NFKB1 affects the serum inflammatory levels of IL-6 in Hashimoto thyroiditis in a Turkish population. Immunobiology. 2014;219(7):531–6. doi: 10.1016/j.imbio.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Rovner AJ, Brien KO. O. Hypovitaminosis D Among Healthy Children in the United States. Arch Pediatr Adolesc Med. 2008;162(6):513–9. doi: 10.1001/archpedi.162.6.513. [DOI] [PubMed] [Google Scholar]
- Bener A, Al-Ali M, Hoffmann GF. Vitamin D deficiency in healthy children in a sunny country: Associated factors. Int J Food Sci Nutr. 2009;60(Suppl. 5):60–70. doi: 10.1080/09637480802400487. [DOI] [PubMed] [Google Scholar]
- Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001-2004. Pediatrics. 2009;124(3):1–18. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Absoud M, Cummins C, Lim MJ, Wassmer E, Shaw N. Prevalence and predictors of vitamin D insufficiency in children: A great britain population based study. PLoS One. 2011;6(7):6–11. doi: 10.1371/journal.pone.0022179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor GL, Chee WSS, Shariff ZM, et al. High prevalence of vitamin D insufficiency and its association with BMI-for-age among primary school children in Kuala Lumpur, Malaysia. BMC Public Health. 2011;11(1):95. doi: 10.1186/1471-2458-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voortman T, Van den Hooven EH, Heijboer AC, Hofman A, Jaddoe VWV, Franco OH. Vitamin D deficiency in school-age children is associated with sociodemographic and lifestyle factors. J Nutr. 2015;145(4):791–8. doi: 10.3945/jn.114.208280. [DOI] [PubMed] [Google Scholar]
- Roh YE, Kim BR, Choi WB, et al. Vitamin D deficiency in children aged 6 to 12 years: Single center’s experience in busan. Ann Pediatr Endocrinol Metab. 2016;21(3):149–54. doi: 10.6065/apem.2016.21.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soesanti F, Pulungan A, Tridjaja B, Batubara JR. Vitamin D profile in healthy children aged 7-12 years old in Indonesia. Int J Pediatr Endocrinol. 2013;2013(S1):P167. [Google Scholar]
- Ernawati F, Budiman B. Current Vitamin D Satus of Indonesian Children Age 2 - 12,9 Years Old. Gizi Indones. 2015;38(1):73–80. [Google Scholar]
- Koon Poh B, Rojroongwasinkul N, Khanh Le Nguyen B, et al. 25-Hydroxy-Vitamin D Demography and the Risk of Vitamin D insufficiency in the South East Asian Nutrition Surveys (SEANUTS) Asia Pac J Clin Nutr. 2016;25(3):538–48. doi: 10.6133/apjcn.092015.02. [DOI] [PubMed] [Google Scholar]
- Pulungan A, Soesanti F, Tridjaja B, Batubara J. Vitamin D insufficiency and its contributing factors in primary school-aged children in Indonesia, a sun-rich country. Ann Pediatr Endocrinol Metab. 2021;26(2):92–8. doi: 10.6065/apem.2040132.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubillaga P, Garrido A, Mugica I, Ansa J, Zabalza R, Emparanza JI. Effect of vitamin D and calcium supplementation on bone turnover in institutionalized adults with Down’s Syndrome. Eur J Clin Nutr. 2006;60(5):605–9. doi: 10.1038/sj.ejcn.1602357. [DOI] [PubMed] [Google Scholar]
- Stagi S, Lapi E, Romano S, et al. Determinants of vitamin d levels in children and adolescents with Down syndrome. Int J Endocrinol. 2015;2015:1–11. doi: 10.1155/2015/896758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hawary MM, El-Shafie SM, El-Awady H, Ragab T, Nabile R. Assessment of serum level of vitamin D in infants and children with Down syndrome. Middle East J Med Genet. 2019;7:2090–8571. [Google Scholar]
- Del Arco C, Riancho JA, Luzuriaga C, Gonzalez-Macias J, Florez J. Vitamin D status in children with Down’s syndrome. J Intellect Disabil Res. 1992;36(3):251–7. doi: 10.1111/j.1365-2788.1992.tb00512.x. [DOI] [PubMed] [Google Scholar]
- Valtuena J, Gonzalez-Gross M, Huybrechts I, et al. Factors Associated with Vitamin D Deficiency in European Adolescents: The HELENA Study. J Nutr Sci Vitaminol. 2013;59:161–71. doi: 10.3177/jnsv.59.161. [DOI] [PubMed] [Google Scholar]
- Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379(9821):1142–54. doi: 10.1016/S0140-6736(11)60276-6. [DOI] [PubMed] [Google Scholar]
- Nicholson LB, Wong FS, Ewins DL, et al. Susceptibility to autoimmune thyroiditis in Down’s syndrome is associated with the major histocompatibility class II DQA 0301 allele. Clin Endocrinol. 1994;41(3):381–3. doi: 10.1111/j.1365-2265.1994.tb02561.x. [DOI] [PubMed] [Google Scholar]
- Ismail S, Mayah W, El Battia H, et al. Plasma nuclear factor kappa B and serum peroxiredoxin 3 in early diagnosis of hepatocellular carcinoma. Asian Pacific J Cancer Prev. 2015;16(4):1657–63. doi: 10.7314/apjcp.2015.16.4.1657. [DOI] [PubMed] [Google Scholar]
- Budiutari NN, Dachlan YP, Nugraha J. Overview of Nuclear Factor-Kb (Nf-Kb) and Non-Structural Protein (1Ns1) in Patients With Dengue Fever in Premier Hospital, Surabaya. Indones J Trop Infect Dis. 2019;7(5):109. [Google Scholar]
- Chang SW, Lee HC. Vitamin D and health - The missing vitamin in humans. Pediatr Neonatol. 2019;60(3):237–44. doi: 10.1016/j.pedneo.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Sperling MA. Sperling Pediatric Endocrinology. 5th ed. Elsevier; 2020. [Google Scholar]
- Szeliga K, Antosz A, Skrzynska K, Kalina-Faska B, Januszek-Trzciakowska A, Gawlik A. Subclinical Hypothyroidism as the Most Common Thyroid Dysfunction Status in Children With Down’s Syndrome. Front Endocrinol (Lausanne) 2022;12(January) doi: 10.3389/fendo.2021.782865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Wu CY, Deng YH, Wu JL. Associations between serum 25-hydroxyvitamin d levels and thyroid function parameters in previously healthy children aged 6 to 24 months. Risk Manag Healthc Policy. 2020;13:1647–53. doi: 10.2147/RMHP.S269640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chailurkit LO, Aekplakorn W, Ongphiphadhanakul B. High vitamin D status in younger individuals is associated with low circulatingthyrotropin. Thyroid. 2013;23(1):25–30. doi: 10.1089/thy.2012.0001. [DOI] [PubMed] [Google Scholar]
- Poh BK, Ng BK, Siti Haslinda MD, et al. Nutritional status and dietary intakes of children aged 6 months to 12 years: Findings of the Nutrition Survey of Malaysian Children (SEANUTS Malaysia) Br J Nutr. 2013;110(SUPPL.3):521–35. doi: 10.1017/S0007114513002092. [DOI] [PubMed] [Google Scholar]
- Laillou A, Wieringa F, Tran TN, et al. Hypovitaminosis D and Mild Hypocalcaemia Are Highly Prevalent among Young Vietnamese Children and Women and Related to Low Dietary Intake. PLoS One. 2013;8(5):1–10. doi: 10.1371/journal.pone.0063979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senaprom S, Yamborisut U, Rojroongwasinkul N, et al. Factors associated with vitamin D status among Thai children aged 3-13 years. Southeast Asian J Trop Med Public Health. 2016;47(2):277–86. [PubMed] [Google Scholar]
- Neufingerl N, Djuwita R, Otten-Hofman A, et al. Generating fatty acid and vitamin D composition data of Indonesian foods. J Food Compos Anal. 2016;50:36–48. [Google Scholar]
- Maguire JL, Lebovic G, Kandasamy S, et al. The relationship between cow’s milk and stores of vitamin D and iron in early childhood. Pediatrics. 2013;131(1):e144–51. doi: 10.1542/peds.2012-1793. [DOI] [PubMed] [Google Scholar]
- Ragab D, Soliman D, Samaha D, Yassin A. Vitamin D status and its modulatory effect on interferon gamma and interleukin-10 production by peripheral blood mononuclear cells in culture. Cytokine. 2016;85:5–10. doi: 10.1016/j.cyto.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Rigby WFC, Denome S, Fanger MW. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J Clin Invest. 1987;79(6):1659–64. doi: 10.1172/JCI113004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C-S, Fraser WD, Gleeson M. Influence of Vitamin D Metabolites on Plasma Cytokine Concentrations in Endurance Sport Athletes and on Multiantigen Stimulated Cytokine Production by Whole Blood and Peripheral Blood Mononuclear Cell Cultures. ISRN Nutr. 2014;2014:1–9. doi: 10.1155/2014/820524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HFJ, O’Garra A. 1α,25-Dihydroxyvitamin D3 Has a Direct Effect on Naive CD4 + T Cells to Enhance the Development of Th2 Cells. J Immunol. 2001;167(9):4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- Mousa A, Naderpoor N, Johnson J, et al. Effect of Vitamin D supplementation on inflammation and nuclear factor kappa-B activity in overweight/obese adults: A randomized placebo-controlled trial. Sci Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-15264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani HH, Hosseini ES, Nikzad H, et al. The effects of vitamin D supplementation on signaling pathway of inflammation and oxidative stress in diabetic hemodialysis: A randomized, double-blind, placebo-controlled trial. Front Pharmacol. 2018;9:1–8. doi: 10.3389/fphar.2018.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyzik A, Grywalska E, Matyjaszek-Matuszek B, Roliński J. Immune disorders in Hashimoto’s thyroiditis: What do we know so far? J Immunol Res. 2015;2015 doi: 10.1155/2015/979167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovinazzo S, Vicchio TM, Certo R, et al. Vitamin D receptor gene polymorphisms/haplotypes and serum 25(OH)D3 levels in Hashimoto’s thyroiditis. Endocrine. 2017;55(2):599–606. doi: 10.1007/s12020-016-0942-5. [DOI] [PubMed] [Google Scholar]
- Sayki Arslan M, Topaloglu O, Ucan B, et al. Isolated vitamin D deficiency is not associated with nonthyroidal illness syndrome, but with thyroid autoimmunity. Sci World J. 2015;2015 doi: 10.1155/2015/239815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DY, Kim KJ, Kim D, Hwang S, Lee EJ. Low serum vitamin D is associated with anti-thyroid peroxidase antibody in autoimmune thyroiditis. Yonsei Med J. 2014;55(2):476–81. doi: 10.3349/ymj.2014.55.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zynat J, Guo Y, et al. Low Serum Vitamin D Is Associated with Anti-Thyroid-Globulin Antibody in Female Individuals. Int J Endocrinol. 2015;2015:285–90. doi: 10.1155/2015/285290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmeh J, Farpour F, Rizzo V, Kheradnam S, Sachmechi I. Hashimoto thyroiditis not associated with Vitamin D deficiency. Endocr Pract. 2016;22(7):809–13. doi: 10.4158/EP15934.OR. [DOI] [PubMed] [Google Scholar]
- Effraimidis G, Badenhoop K, Tijssen JGP, Wiersinga WM. Vitamin D deficiency is not associated with early stages of thyroid autoimmunity. Eur J Endocrinol. 2012;167(1):43–8. doi: 10.1530/EJE-12-0048. [DOI] [PubMed] [Google Scholar]
- Ke W, Sun T, Zhang Y, et al. 25-hydroxyvitamin D serum level in Hashimoto’s thyroiditis, but not Graves’ disease is relatively deficient. Endocr J. 2017;64(6):581–7. doi: 10.1507/endocrj.EJ16-0547. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wang Z, Sun M, et al. Association of high vitamin D status with low circulating thyroid-stimulating hormone independent of thyroid hormone levels in middle-aged and elderly males. Int J Endocrinol. 2014;2014:1–7. doi: 10.1155/2014/631819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönmezgöz E, Ozer S, Yilmaz R, Önder Y, Bütün I, Bilge S. Hypovitaminosis D in Children with Hashimoto’s Thyroiditis. Rev Med Chil. 2016;144(5):611–6. doi: 10.4067/S0034-98872016000500009. [DOI] [PubMed] [Google Scholar]
- Brix TH, Kyvik KO, Christensen K, Hegedüs L. Evidence for a major role of heredity in Graves’ disease: A population-based study of two Danish twin cohorts. J Clin Endocrinol Metab. 2001;86(2):930–4. doi: 10.1210/jcem.86.2.7242. [DOI] [PubMed] [Google Scholar]
- Shin DH, Baek IC, Kim HJ, et al. HLA alleles, especially amino-acid signatures of HLA-DPB1, might contribute to the molecular pathogenesis of early-onset autoimmune thyroid disease. PLoS One. 2019;14(5):1–12. doi: 10.1371/journal.pone.0216941. [DOI] [PMC free article] [PubMed] [Google Scholar]


