Abstract
The burden of nonalcoholic fatty liver disease (NAFLD) is rising globally. Cardiovascular disease is the leading cause of death in patients with NAFLD. Nearly half of individuals with NAFLD have coronary heart disease, and more than a third have carotid artery atherosclerosis. Individuals with NAFLD are at a substantially higher risk of fatal and nonfatal cardiovascular events. NAFLD and cardiovascular disease share multiple common disease mechanisms, such as systemic inflammation, insulin resistance, genetic risk variants, and gut microbial dysbiosis. In this review, we discuss the epidemiology of cardiovascular disease in NAFLD, and highlight common risk factors. In addition, we examine recent advances evaluating the shared disease mechanisms between NAFLD and cardiovascular disease. In conclusion, multidisciplinary collaborations are required to further our understanding of the complex relationship between NAFLD and cardiovascular disease and potentially identify therapeutic targets.
Keywords: nonalcoholic fatty liver disease, pathogenesis, atherosclerosis, coronary artery disease
Graphical Abstract
One-quarter of the world’s population has nonalcoholic fatty liver disease (NAFLD).1,2 NAFLD encompasses nonalcoholic fatty liver, the benign, nonprogressive form, and nonalcoholic steatohepatitis (NASH), the progressive, inflammatory form that can progress to advanced fibrosis, cirrhosis, and hepatocellular carcinoma.3–8 With the obesity pandemic, NAFLD cases are projected to exceed 100 million in the U.S. over the next decade.9
NAFLD is strongly associated with obesity, insulin resistance, dyslipidemia, the metabolic syndrome, and cardiovascular disease.10–16 Cardiovascular disease is the leading cause of death in patients with NAFLD, accounting for nearly a third of total mortality.17–19 Even though causality has not been established, multiple studies have demonstrated that the presence and severity of NAFLD is an independent predictor for the development of cardiovascular disease and events.12,13,20–24 Although cardiovascular disease and NAFLD share many risk factors, an individual with NAFLD is estimated to have a 57 to 69% increased risk of cardiovascular disease, independent of known common risk factors.12,25,26 These data suggest that either NASH itself promotes cardiovascular disease, or that there are additional unidentified common risk factors. In this review, we discuss the prevalence, risk factors, and potential shared mechanisms of cardiovascular disease and NAFLD.
Prevalence of Coronary Heart Disease in NAFLD
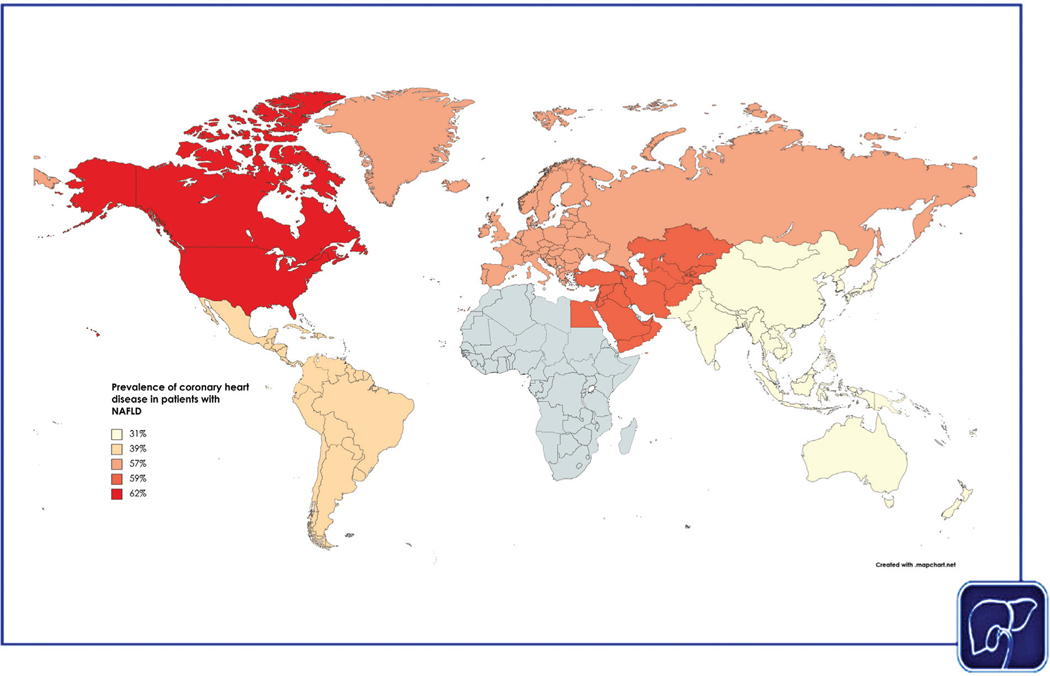
A recent meta-analysis of 38 studies determined that the pooled prevalence of coronary heart disease (a continuum ranging from coronary artery atherosclerosis to coronary artery disease) among patients with NAFLD was 45%.27 The prevalence of coronary heart disease by region ranged from 31% in Asia to 62% in North America (Fig. 1). Patients with NAFLD had increased odds of coronary heart disease (pooled odds ratio [OR] 1.3) compared with patients without NAFLD. In addition, the prevalence of coronary heart disease was higher among NAFLD patients with moderate or severe steatosis versus those with mild steatosis (38% vs. 30%). Among patients with NAFLD who had symptoms suggestive of ischemic heart disease, the prevalence of clinical coronary artery disease was 55%, and the presence of NAFLD was associated with increased odds of clinical coronary artery disease (OR 2.2). These findings are consistent with an older meta-analysis of 16 studies, which determined that patients with NAFLD had a higher odds of fatal and/or nonfatal cardiovascular events (including ischemic heart disease and stroke) compared with patients without NAFLD.12 In the older meta-analysis, the higher risk of cardiovascular events in NAFLD persisted even after sensitivity analyses that excluded studies with participants with diabetes, hypertension, and myocardial infarction. By contrast, a matched cohort study of population-based, electronic primary health care databases from Italy, the Netherlands, Spain, and the U.K. compared patients with a recorded diagnosis of NAFLD or NASH versus patients without a recorded diagnosis of NAFLD or NASH and did not find a significant association between NAFLD and the risk of cardiovascular events.28 However, the prevalence of NAFLD in these databases were lower than expected (0.6% in 2007 to 1.9% in 2014), which suggested possible misclassification and underdiagnosis of NAFLD in the control group.
Fig. 1.
Estimated global prevalence of coronary heart disease, by World Health Organization (WHO) region.
Taken together, these data suggest that coronary heart disease is common among patients with NAFLD and is more prevalent among those with moderate to severe steatosis. Screening for coronary heart disease among patients with NAFLD may be guided by the severity of hepatic steatosis, and identification of such patients may allow for intensive risk factor management and prevention of ischemic events.
Prevalence of Carotid Atherosclerosis and Stroke in NAFLD
A recent meta-analysis of 30 studies determined that the prevalence of carotid atherosclerosis among patients with NAFLD was 35%.29 By region, the prevalence of carotid atherosclerosis among patients with NAFLD was lowest in the Middle East (19%) and highest in Europe (45%). The presence of NAFLD was associated with higher odds of carotid atherosclerosis (OR 3.3). The authors determined that the prevalence of stroke among NAFLD patients was 5%. Patients with NAFLD had higher odds (OR 1.9) of developing stroke compared with non-NAFLD controls. The degree of steatosis was associated with incremental odds of carotid atherosclerosis and stroke. These findings suggest that targeted screening for carotid atherosclerosis among patients with NAFLD may be guided by the severity of hepatic steatosis. The associations between NAFLD and other cardiovascular disease are summarized in Table 1.
Table 1.
Selected meta-analyses that examined the association between nonalcoholic fatty liver disease (NAFLD) and cardiovascular disease
| Author, publication year | Cardiovascular disease | Number of included studies | Main findings | Strength of association |
|---|---|---|---|---|
| Toh et al,27 2021 | Coronary heart disease | 38 | The pooled prevalence of coronary heart disease was 44.6% (95% CI, 36.0–53.6%) among 67,070 patients with NAFLD with an odds ratio of 1.33 (95% CI, 1.21–1.45%; p < 0.0001), compared with patients without NAFLD | Moderate to strong |
| Tang et al,29 2022 | Carotid atherosclerosis and stroke | 30 | 35.02% (CI, 27.36–43.53%) of patients with NAFLD had carotid atherosclerosis, with an odds ratio of 3.20 (95% CI, 2.37–4.32; p < 0.0001) compared with patients without NAFLD. The prevalence of stroke was 5.04% (95% CI, 2.74–9.09%) among patients with NAFLD with an odds ratio of 1.88 (95% CI, 1.23–2.88; p = 0.02) compared with patients without NAFLD | Moderate to strong |
| Yong et al,106 2022 | Echocardiographic-derived cardiac function and structural characteristics | 41 | Patients with NAFLD had poorer systolic indices with lower ejection fraction (mean difference: −0.693; 95% CI: −1.112 to −0.274; p = 0.001), and worse diastolic indices with higher E/e’ (mean difference: 1.575; 95% CI: 0.924–2.227; p < 0.001) compared with non-NAFLD patients The presence of NAFLD was associated with increased left ventricular mass (mean difference: 34.484; 95% CI: 26.236–42.732; p < 0.001) and epicardial adipose thickness (mean difference:0.1343; 95%CI: 0.055–0.214; p = 0.001) | Moderate to strong |
| Mantovani et al,107 2019 | Atrial fibrillation | 9 | NAFLD was associated with an increased risk of prevalent atrial fibrillation (odds ratio 2.07, 95% CI 1.38–3.10) | Moderate |
| Ciardullo et al,108 2022 | Hypertension | 11 | The presence of NAFLD was associated with an increased risk of incident hypertension (hazard ratio 1.66; 95% confidence interval [CI], 1.38–2.01; p < 0.001) | Moderate |
Abbreviations: CI, confidence interval; NAFLD, nonalcoholic fatty liver disease.
Potential Shared Mechanisms of Disease between NAFLD and Cardiovascular Disease
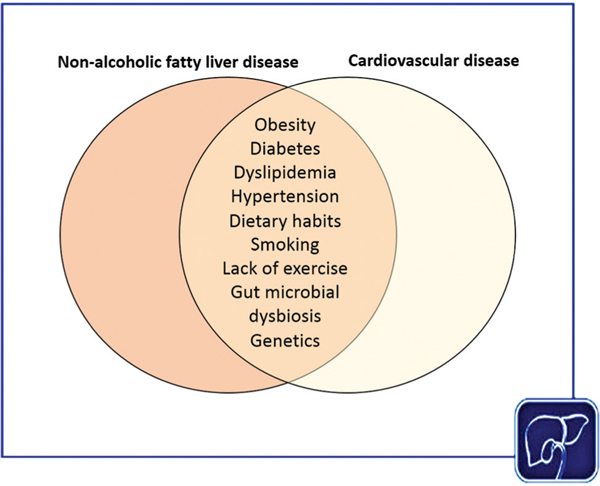
The shared risk factors between NAFLD and cardiovascular disease are summarized in Fig. 2. The above-mentioned meta-analysis of NAFLD and prevalence of coronary heart disease determined that age, hypertension, and diabetes were significantly associated with coronary heart disease in NAFLD.27 Other established shared risk factors include dyslipidemia, insulin resistance, metabolic syndrome, dietary habits, smoking, and the lack of physical exercise.10,30–34 A substantial proportion of patients with NAFLD and cardiovascular disease harbor the metabolic syndrome.35,36 A study utilizing systems biology modeling demonstrated substantial overlap in disease mechanisms between NAFLD and the metabolic syndrome and suggested that multitarget therapeutic approaches may be a better strategy to treat NASH.34 In addition, case–control studies have demonstrated that the presence of NAFLD was associated with increased levels of serum biomarkers of atherosclerosis.37 A meta-analysis determined that patients with NAFLD have a 57 to 69% increased risk of cardiovascular disease, independent of known common risk factors, suggesting that either NASH may be an independent cause of cardiovascular disease, or there may be additional unidentified risk factors.12 However, it is possible that this association may have confounded by undiagnosed confounders in the individual studies, such as diabetes or obesity. Due to the dynamic bidirectional relationship between NAFLD, shared metabolic risk factors, and cardiovascular disease, isolating the specific contribution of NAFLD to cardiovascular disease is challenging. In the following segment, we discuss several putative shared mechanisms of disease between NAFLD and cardiovascular disease.
Fig. 2.
Established shared risk factors between nonalcoholic fatty liver disease (NAFLD) and cardiovascular disease.
NAFLD and Low-Grade Systemic Inflammation
NAFLD causes systemic inflammation through multiple complex interactions between the gut microbiome, liver, and adipose tissue.3,38 NASH is associated with the activation of the inflammasome of multiple cell types, including both resident and infiltrating cells.3,38,39 Free fatty acids induce hepatocytes to secrete tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-8, inducing systemic inflammation.40–42 In a study of 2,482 participants from the Framingham Heart Study, the presence of liver fat was independently associated with increased serum concentration of systemic markers of inflammation, including C-reactive protein (CRP), urinary isoprostanes, IL-6, intercellular adhesion molecule 1, and P-selectin, after adjustment for body mass index and other components of the metabolic syndrome.43
In a study of 3,876 participants from the Multi-Ethnic Study of Atherosclerosis, participants with NAFLD had higher serum concentrations of markers of inflammation, including LpPLA2 activity, high-sensitivity CRP, and IL-6.44 In this study, IL-6 was independently associated with the presence and severity of subclinical atherosclerosis, defined by coronary artery calcium.
Systemic inflammation is associated with the development of incident cardiovascular disease.45 Low-grade systemic inflammation secondary to NAFLD leads to the release of proinflammatory cytokines and may promote the development of atherogenic cardiovascular disease by inducing endothelial dysfunction and enhancing plaque formation.46–49 Inflammation is a key determinant that links risk factors for atherosclerosis to the development of coronary artery disease, and further mechanistic studies are required to characterize the relationship between NAFLD, systemic inflammation, and cardiovascular disease.50
NAFLD and Epicardial Adipose Tissue
NAFLD is associated with increased epicardial adipose tissue, and a higher epicardial fat thickness is associated with more severe liver fibrosis.51,52 Epicardial adipose tissue has close proximity with the coronary arteries and myocardium, shares a common microcirculation with the myocardium, and secretes proinflammatory cytokines, such as IL-6 and TNF-α that promote atherogenesis and heart failure by inducing infiltration of the intima and fibrosis.53,54 In addition, the inflammatory cytokines secreted by the epicardial adipose tissue may also contribute to the activation of hepatic stellate cells and induce liver fibrosis.55 Atherosclerotic plaques are more prevalent in regions of coronary arteries surrounded by epicardial adipose tissue, adding weight to a possible link between ectopic fat in the epicardium and coronary artery disease.54 In a study of 147 patients with biopsy-confirmed NAFLD, epicardial fat, morphological, and functional cardiac alterations were more pronounced among patients with severe fibrosis.52 In our opinion, these data suggest that NASH and cardiovascular disease may share synergistic mechanisms that arise from a systemic proinflammatory, proatherogenic, and profibrogenic environment maintained by ectopic adipose tissue. More studies are required to validate this hypothesis.
Insulin Resistance in NAFLD and Cardiovascular Disease
Dysregulated glucose metabolism and insulin resistance are key drivers of both NAFLD and cardiovascular disease. Skeletal muscle insulin resistance diverts glucose away from skeletal muscle glycogen synthesis and into the liver.56,57 The increased delivery of glucose to the liver, along with hyperinsulinemia, stimulates sterol regulatory element-binding protein 1c (SREBP1c), which promotes increased expression of key hepatic enzymes that regulate de novo lipogenesis, resulting in increased very-low-density lipoprotein production, hypertriglyceridemia, and NAFLD.3,58 Hyperinsulinemia further triggers hepatic gluconeogenesis, further increasing insulin levels, thus promoting a vicious cycle of dysregulated glucose metabolism.
Insulin resistance is a strong predictor of atherosclerotic cardiovascular disease.59,60 High levels of insulin accelerate the atherosclerotic process by several mechanisms, including stimulating de novo lipogenesis leading to increased low-density lipoprotein (LDL) synthesis, promoting vascular smooth muscle cell growth and proliferation, increasing collagen synthesis and enhancing LDL cholesterol (LDL-C) transport into arterial smooth muscle cells.61–65 These data reaffirm the key role of insulin resistance in the pathogenesis of both NAFLD and cardiovascular disease.
Endothelial Dysfunction in NAFLD and Cardiovascular Disease
Endothelial dysfunction is the starting point of atherogenesis.66 An Italian study assessed endothelial function by measuring the vasodilatory response of the brachial artery in response to ischemia.67 The study compared 52 patients with NAFLD versus 28 matched controls and determined that NAFLD was independently associated with increased endothelial dysfunction after adjustment for confounders. A study from Taiwan determined that patients with NAFLD had substantially reduced circulating bone marrow-derived endothelial progenitor cell levels and function compared with matched controls.68 The findings of this Taiwanese study suggested that attenuated endothelial repair capacity may contribute to atherosclerotic disease progression and increased cardiovascular risk in NAFLD patients. In addition, patients with NAFLD are known to have higher levels of homocysteine, which further promotes atherogenesis by inducing oxidative stress and endothelial dysfunction.69–71 In a case–control study of patients with NASH, simple steatosis, and controls, the presence of NASH was associated with a higher expression of mediators of atherogenesis and endothelial damage (ACE, CSF2, IL1A, LIF, MMP1, and TGFβ1).72 These emerging data suggest that endothelial dysfunction may play an important role in the development of cardiovascular disease among individuals with NAFLD.
PPARs in NAFLD and Cardiovascular Disease
Peroxisome proliferator-activated receptors (PPARs) are nuclear receptors and comprise three isoforms (PPARα, PPARβ/δ, and PPARγ).73 PPARs are centrally involved in the regulation of genes that influence the metabolism of triglycerides, glucose, lipogenesis, fatty acid transport, inflammation, fibrosis, and atherosclerosis.74,75 A randomized, placebo-controlled phase 2 trial of saroglitazar, a dual PPAR-α/γ agonist in patients with NAFLD/NASH, determined that saroglitazar was associated with reductions in alanine aminotransferase, liver fat content, atherogenic lipids, and lipotoxic lipid species.76 These data suggest that saroglitazar has the potential to improve both NAFLD and cardiovascular disease, but a phase 3 trial, which is ongoing, is required to confirm this (Clinical-Trials.gov NCT04193982). The NATIVE trial was a phase 2b, randomized, placebo-controlled trial of a pan-PPAR (lanifibranor) agonist for the treatment of NASH.77 In this study, participants who received 1200 mg of lanifibranor daily had a greater proportion with a decrease in at least two points in the Steatosis, Activity, Fibrosis score compared with those who received placebo (55% vs. 33%, p = 0.007). These data prompted the initiation of a phase 3 study which is ongoing (Clinical-Trials.gov NCT04849728). By contrast, a randomized, phase 2 trial of selective PPAR-α modulator (SPPARMα), pemafibrate, in patients with NAFLD did not meet the primary endpoint of reduction in liver fat content, although it was associated with reduction in liver fibrosis and LDL-C.78 A randomized trial of pioglitazone, a PPAR-γ/PPAR-α agonist, in patients with NASH and diabetes or prediabetes, determined that long-term treatment with pioglitazone was associated with NASH resolution and improvements in insulin resistance and lipid profile.79 Taken together, these data suggest that targeting the PPAR isoforms may be a potential strategy for treating both NAFLD and cardiovascular disease.
Gut Microbial Dysbiosis and Systemic Inflammation
NAFLD is associated with disruption of intestinal enterocyte intercellular tight junctions, resulting in increasing gut permeability and translocation of gut bacteria and lipopolysaccharides.80,81 A prospective study conducted using whole-genome shotgun sequencing of deoxyribonucleic acid extracted from the stool of participants with biopsy-confirmed NAFLD determined that advanced fibrosis was associated with an increased abundance of proinflammatory Gram-negative Proteobacteria (including Escherichia coli).82 Another study evaluated 16S gut-microbiome compositions of 203 well-characterized participants, including 26 with NAFLD cirrhosis, and similarly found that participants with NAFLD and advanced liver disease were enriched in Gram-negative bacterium such as Enterobacteriaceae and Gallibacterium.83 In a metagenome-wide association study of participants with atherosclerotic cardiovascular disease compared with healthy controls, Gram-negative bacteria (including E. coli) were elevated among participants with atherosclerotic cardiovascular disease.84 Likewise, a case–control study sequencing the 16S rRNA V4 tag compared patients with large artery atherosclerotic ischemic stroke and transient ischemic attacks against healthy controls and identified increased Gram-negative bacteria (including Enterobacteriaceae and Proteobacteria) among cases.85 These data suggest that gut microbial dysbiosis in patients with NAFLD contributes to systemic inflammation and may potentiate cardiovascular disease.47,86–88
Genetic Determinants of NAFLD and Cardiovascular Disease
Multiple studies have shown that hepatic steatosis and fibrosis are heritable traits.89,90 The most well-studied single-nucleotide polymorphism (SNP) in NAFLD is the patatin-like phospholipase domain-containing 3 (PNPLA3) c.444 C > G SNP, which encodes the I148M variant and has been strongly linked to an increased risk of liver-related mortality in NAFLD.91–93 PNPLA3 is a multifunctional enzyme implicated in the regulation of lipids and retinyl ester activity.94 The TM6SF2 rs58542926 C > T polymorphism codes for an E to K substitution at position 167 that results in loss of function and is associated with reduced hepatic TM6SF2 messenger ribonucleic acid (RNA) and protein expression, higher levels of alanine transaminase, and lower levels of LDL-C.95,96 A large exome-wide association study of plasma lipids in more than 300,000 participants determined that variants in PNPLA3 and TM6SF2 were associated with higher liver fat and a greater risk for diabetes, but lower blood lipid levels and a lower risk for coronary artery disease.97 A meta-analysis of 10 studies (101,326 individuals) determined that carriers of the TM6SF2 variant had lower levels of total cholesterol, LDL, and triglycerides, along with a higher risk of NAFLD.98 Another study that included 60,801 cases with coronary artery disease and 123,504 controls determined that the TM6SF2 variant was protective against coronary artery disease, while the cardioprotective effect of the PNPLA3 was only modest.99 Similarly, the impact of the TM6SF2 E167K variant was assessed in 1,819 Swedish individuals and was found to be associated with a lower incidence of cardiovascular events.100 A prospective study of 270 patients undergoing coronary angiography determined that the PNPLA3 variant was associated with lower LDL-C and a lower risk of coronary heart disease after adjusting for confounders.101 Taken together, these data indicate that variants in PNPLA3 and TM6SF2 are associated with an increased risk of NAFLD, but a reduced risk of coronary artery disease, possibly due to lower serum lipid levels.
Implications for Future Drug Development
Continued research into the shared mechanisms and distinctions, between NAFLD and cardiovascular disease may help tailor NAFLD drug development toward the treatment of both NAFLD and cardiovascular disease and avoid adding to cardiovascular risk. The challenges faced with the use of the first-generation steroidal farnesoid X receptor (FXR) agonist obeticholic acid in the treatment of patients with NASH are an example of how the interplay between NAFLD and cardiovascular disease may influence side effect profiles. The phase 2b, randomized, placebo-controlled trial of obeticholic acid performed in patients with NASH determined that obeticholic acid improved the histological features of NASH, but was associated with increases in total serum cholesterol and LDL-C, and a decrease in high-density lipoprotein (HDL) cholesterol.102 Similarly, the interim analysis of the phase 3 trial of obeticholic acid showed that LDL-C increased in the initial months after treatment initiation but fell with time and approached baseline by month 18.103 The impact of obeticholic acid on LDL is related to an FXR-specific effect, which downregulates an LDL-C receptor and increases transfer cholesteryl ester transfer protein, leading to increased LDL-C and reduced HDL cholesterol. Future drug developmental efforts in NASH should be guided by greater efforts to understand the complex interactions between NAFLD and cardiovascular disease.
Future Directions
The formation of cross-disciplinary collaborations and consortiums with prospective studies are essential to untangle the complex relationship between NAFLD and cardiovascular disease. In addition, as analytical technologies progress and allow for a deeper understanding of the complex pathways between NAFLD and cardiovascular disease, these may give rise to the identification of shared therapeutic targets. As an example, single-cell RNA sequencing technologies are currently enabling characterization of the molecular phenotypes of the various cell types of normal and diseased tissues. These methods have recently been applied to examine the resident nonparenchymal cell types in normal versus cirrhotic human livers104 and the composition of cells within human atherosclerotic lesions.105 In each case, striking disease-specific variation was observed both in cellular population structure and in gene expression within specific cell types. There are hints of dysregulation of common pathways that may account for these findings, but analysis of these types of data has not yet begun in a systematic manner. Importantly, application of single-cell RNA sequencing technologies will provide an important additional tool in analyzing responses to therapeutic interventions in preclinical models.
Conclusion
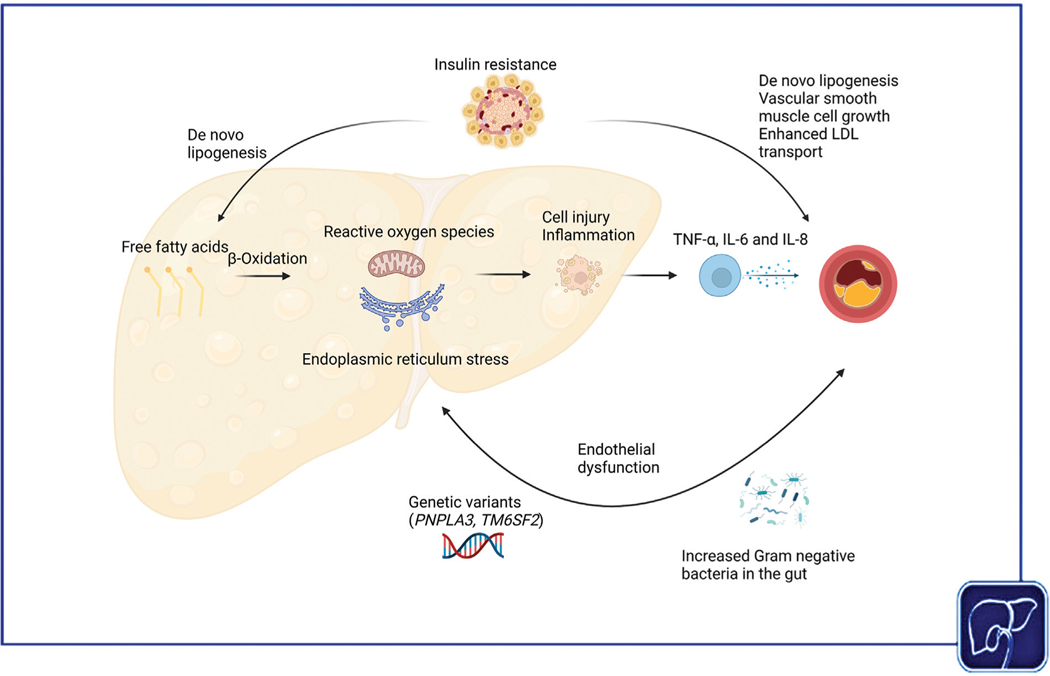
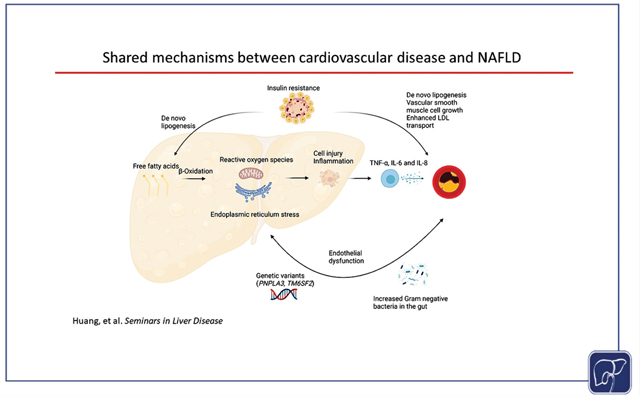
There is a high prevalence of cardiovascular disease in NAFLD, and patients with NAFLD are at increased risk of cardiovascular-related morbidity and mortality. NAFLD and cardiovascular disease share many common disease mechanisms, including low-grade systemic inflammation, endothelial dysfunction, insulin resistance, gut microbial dysbiosis, and genetic risk alleles (Fig. 3). Multidisciplinary collaborations are required to further our understanding of the complex relationship between NAFLD and cardiovascular disease.
Fig. 3.
Putative shared disease mechanisms between cardiovascular disease and nonalcoholic fatty liver disease (NAFLD). In NAFLD, there is increased production of proinflammatory cytokines, which promotes atherosclerosis. Individuals with NAFLD and cardiovascular disease have endothelial dysfunction, which may be mediated by early atherosclerosis. Insulin resistance is a key driver of both NAFLD and cardiovascular disease. With more advanced hepatic and cardiovascular disease, there is increased abundance of proinflammatory Gram-negative bacteria in both NAFLD and cardiovascular disease. Genetic variants in PNPLA3 and TM6SF2 promote hepatic steatosis and advanced fibrosis but reduce systemic lipid levels and may be cardioprotective.
Funding
R.L. receives funding support from NIAAA (U01AA029019), NIEHS (5P42ES010337), NCATS (5UL1TR001442), NIDDK (U01DK130190, U01DK061734, R01DK106419, P30DK120515, R01DK121378, R01DK124318), NHLBI (P01HL147835), and DOD PRCRP (W81XWH-18-2-0026).
Conflict of Interest
R.L. serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals, and Viking Therapeutics. In addition, his institutions received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes, and Terns Pharmaceuticals. Co-founder of LipoNexus Inc.
D.H. receives funding support from Singapore Ministry of Health’s National Medical Research Council under its NMRC Research Training Fellowship (MOH-000595-01). In addition, he has served as an advisory board member for Eisai.
J.L.W. holds patents related to the use of oxidation specific antibodies held by UCSD. He is cofounder of Oxitope and Kleanthi Diagnostic and is a consultant to Ionis Pharmaceuticals.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64(01):73–84 [DOI] [PubMed] [Google Scholar]
- 2.Le MH, Yeo YH, Li X, et al. Global NAFLD prevalence - a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2021; S1542-3565(21)01280–5 [DOI] [PubMed] [Google Scholar]
- 3.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021;184(10):2537–2564 [DOI] [PubMed] [Google Scholar]
- 4.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10(11):686–690 [DOI] [PubMed] [Google Scholar]
- 5.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021;18(04):223–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan DJH, Ng CH, Lin SY, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol 2022;23(04):521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang DQ, Fowler KJ, Liau J, et al. Comparative efficacy of an optimal exam between ultrasound versus abbreviated MRI for HCC screening in NAFLD cirrhosis: a prospective study. Aliment Pharmacol Ther 2022;55(07):820–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab 2022;34(07):969–977.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67(01):123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu Rev Pathol 2016;11:451–496 [DOI] [PubMed] [Google Scholar]
- 11.Caussy C, Aubin A, Loomba R. The relationship between type 2 diabetes, NAFLD, and cardiovascular risk. Curr Diab Rep 2021;21(05):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol 2016;65(03):589–600 [DOI] [PubMed] [Google Scholar]
- 13.Sinn DH, Kang D, Chang Y, et al. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: a retrospective cohort study. Gut 2017;66(02):323–329 [DOI] [PubMed] [Google Scholar]
- 14.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66(06):1138–1153 [DOI] [PubMed] [Google Scholar]
- 15.Lonardo A, Sookoian S, Pirola CJ, Targher G. Non-alcoholic fatty liver disease and risk of cardiovascular disease. Metabolism 2016;65(08):1136–1150 [DOI] [PubMed] [Google Scholar]
- 16.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363(14):1341–1350 [DOI] [PubMed] [Google Scholar]
- 17.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129(01):113–121 [DOI] [PubMed] [Google Scholar]
- 18.Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44(04):865–873 [DOI] [PubMed] [Google Scholar]
- 19.Younossi ZM, Stepanova M, Rafiq N, et al. Nonalcoholic steatofibrosis independently predicts mortality in nonalcoholic fatty liver disease. Hepatol Commun 2017;1(05):421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pais R, Giral P, Khan JF, et al. ; LIDO Study Group. Fatty liver is an independent predictor of early carotid atherosclerosis. J Hepatol 2016;65(01):95–102 [DOI] [PubMed] [Google Scholar]
- 21.Puchner SB, Lu MT, Mayrhofer T, et al. High-risk coronary plaque at coronary CT angiography is associated with non-alcoholic fatty liver disease, independent of coronary plaque and stenosis burden: results from the ROMICAT II trial. Radiology 2015;274(03):693–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JG, Jung J, Verma KK, et al. Liver stiffness by magnetic resonance elastography is associated with increased risk of cardiovascular disease in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2021;53(09):1030–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanWagner LB, Ning H, Lewis CE, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: the Coronary Artery Risk Development in Young Adults Study. Atherosclerosis 2014;235(02):599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol 2008;49(04):600–607 [DOI] [PubMed] [Google Scholar]
- 25.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24(07):908–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Targher G, Bertolini L, Padovani R, et al. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care 2006;29(06):1325–1330 [DOI] [PubMed] [Google Scholar]
- 27.Toh JZK, Pan XH, Tay PWL, et al. A meta-analysis on the global prevalence, risk factors and screening of coronary heart disease in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2021:S1542–3565(21)01035–1 [DOI] [PubMed]
- 28.Alexander M, Loomis AK, van der Lei J, et al. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. BMJ 2019;367:l5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang ASP, Chan KE, Quek J, et al. NAFLD increases risk of carotid atherosclerosis and ischemic stroke. An updated meta-analysis with 135,602 individuals. Korean J Hepatol 2022;28(3):483–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15(01):11–20 [DOI] [PubMed] [Google Scholar]
- 31.Leslie T, Pawloski L, Kallman-Price J, et al. Survey of health status, nutrition and geography of food selection of chronic liver disease patients. Ann Hepatol 2014;13(05):533–540 [PubMed] [Google Scholar]
- 32.Gerber L, Otgonsuren M, Mishra A, et al. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population-based study. Aliment Pharmacol Ther 2012;36(08):772–781 [DOI] [PubMed] [Google Scholar]
- 33.Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus mechanisms, management, and clinical considerations. Circulation 2016;133(24):2459–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sookoian S, Pirola CJ. Review article: shared disease mechanisms between non-alcoholic fatty liver disease and metabolic syndrome - translating knowledge from systems biology to the bedside. Aliment Pharmacol Ther 2019;49(05):516–527 [DOI] [PubMed] [Google Scholar]
- 35.Salari N, Doulatyari PK, Daneshkhah A, et al. The prevalence of metabolic syndrome in cardiovascular patients in Iran: a systematic review and meta-analysis. Diabetol Metab Syndr 2020;12(01):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yki-Järvinen H.Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2014;2(11):901–910 [DOI] [PubMed] [Google Scholar]
- 37.Sookoian S, Castaño GO, Burgueño AL, et al. Circulating levels and hepatic expression of molecular mediators of atherosclerosis in nonalcoholic fatty liver disease. Atherosclerosis 2010;209(02): 585–591 [DOI] [PubMed] [Google Scholar]
- 38.Anstee QM, Mantovani A, Tilg H, Targher G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2018;15(07):425–439 [DOI] [PubMed] [Google Scholar]
- 39.Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol 2017;17(05):306–321 [DOI] [PubMed] [Google Scholar]
- 40.Feldstein AE, Werneburg NW, Canbay A, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology 2004;40(01):185–194 [DOI] [PubMed] [Google Scholar]
- 41.Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol 2008;103(06):1372–1379 [DOI] [PubMed] [Google Scholar]
- 42.Chávez-Tapia NC, Rosso N, Uribe M, Bojalil R, Tiribelli C. Kinetics of the inflammatory response induced by free fatty acid accumulation in hepatocytes. Ann Hepatol 2013;13(01):113–120 [PubMed] [Google Scholar]
- 43.Fricker ZP, Pedley A, Massaro JM, et al. Liver fat is associated with markers of inflammation and oxidative stress in analysis of data from the Framingham Heart Study. Clin Gastroenterol Hepatol 2019;17(06):1157–1164.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon TG, Trejo MEP, McClelland R, et al. Circulating interleukin-6 is a biomarker for coronary atherosclerosis in nonalcoholic fatty liver disease: results from the Multi-Ethnic Study of Atherosclerosis. Int J Cardiol 2018;259:198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaptoge S, Di Angelantonio E, Pennells L, et al. ; Emerging Risk Factors Collaboration. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med 2012;367(14):1310–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dusi V, Ghidoni A, Ravera A, De Ferrari GM, Calvillo L. Chemokines and heart disease: a network connecting cardiovascular biology to immune and autonomic nervous systems. Mediators Inflamm 2016;2016:5902947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;73(16):2089–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010;464(7293):1357–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bäck M, Yurdagul A Jr, Tabas I, Öörni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol 2019;16(07):389–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Libby P, Ridker PM, Hansson GKLeducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009;54(23):2129–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petta S, Argano C, Colomba D, et al. Epicardial fat, cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease: association with the severity of liver disease. J Hepatol 2015;62(04):928–933 [DOI] [PubMed] [Google Scholar]
- 52.Liu B, Li Y, Li Y, et al. Association of epicardial adipose tissue with non-alcoholic fatty liver disease: a meta-analysis. Hepatol Int 2019;13(06):757–765 [DOI] [PubMed] [Google Scholar]
- 53.Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol 2018;71(20):2360–2372 [DOI] [PubMed] [Google Scholar]
- 54.Madonna R, Massaro M, Scoditti E, Pescetelli I, De Caterina R. The epicardial adipose tissue and the coronary arteries: dangerous liaisons. Cardiovasc Res 2019;115(06):1013–1025 [DOI] [PubMed] [Google Scholar]
- 55.Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab 2011;22(11):450–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perseghin G, Price TB, Petersen KF, et al. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 1996;335(18):1357–1362 [DOI] [PubMed] [Google Scholar]
- 57.Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A 2007;104(31):12587–12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flannery C, Dufour S, Rabøl R, Shulman GI, Petersen KF. Skeletal muscle insulin resistance promotes increased hepatic de novo lipogenesis, hyperlipidemia, and hepatic steatosis in the elderly. Diabetes 2012;61(11):2711–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howard G, O’Leary DH, Zaccaro D, et al. ; The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Insulin sensitivity and atherosclerosis. Circulation 1996;93(10):1809–1817 [DOI] [PubMed] [Google Scholar]
- 60.Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLoS One 2012;7(12):e52036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scherer T, Lindtner C, O’Hare J, et al. Insulin regulates hepatic triglyceride secretion and lipid content via signaling in the brain. Diabetes 2016;65(06):1511–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cusi K, Maezono K, Osman A, et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest 2000;105(03):311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lindsey JB, House JA, Kennedy KF, Marso SP. Diabetes duration is associated with increased thin-cap fibroatheroma detected by intravascular ultrasound with virtual histology. Circ Cardiovasc Interv 2009;2(06):543–548 [DOI] [PubMed] [Google Scholar]
- 64.Porter KE, Riches K. The vascular smooth muscle cell: a therapeutic target in Type 2 diabetes? Clin Sci (Lond) 2013;125(04):167–182 [DOI] [PubMed] [Google Scholar]
- 65.Di Pino A, DeFronzo RA. Insulin resistance and atherosclerosis: implications for insulin-sensitizing agents. Endocr Rev 2019;40(06):1447–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004;109(23, Suppl 1):III27–III32 [DOI] [PubMed] [Google Scholar]
- 67.Villanova N, Moscatiello S, Ramilli S, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology 2005;42(02):473–480 [DOI] [PubMed] [Google Scholar]
- 68.Chiang C-H, Huang P-H, Chung F-P, et al. Decreased circulating endothelial progenitor cell levels and function in patients with nonalcoholic fatty liver disease. PLoS One 2012;7(02):e31799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gulsen M, Yesilova Z, Bagci S, et al. Elevated plasma homocysteine concentrations as a predictor of steatohepatitis in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol 2005;20(09):1448–1455 [DOI] [PubMed] [Google Scholar]
- 70.Guthikonda S, Haynes WG. Homocysteine: role and implications in atherosclerosis. Curr Atheroscler Rep 2006;8(02):100–106 [DOI] [PubMed] [Google Scholar]
- 71.McCully KS. Homocysteine and the pathogenesis of atherosclerosis. Expert Rev Clin Pharmacol 2015;8(02):211–219 [DOI] [PubMed] [Google Scholar]
- 72.Sookoian S, Gianotti TF, Rosselli MS, Burgueño AL, Castaño GO, Pirola CJ. Liver transcriptional profile of atherosclerosis-related genes in human nonalcoholic fatty liver disease. Atherosclerosis 2011;218(02):378–385 [DOI] [PubMed] [Google Scholar]
- 73.Michalik L, Auwerx J, Berger JP, et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev 2006;58(04):726–741 [DOI] [PubMed] [Google Scholar]
- 74.Han L, Shen WJ, Bittner S, Kraemer FB, Azhar S. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-β/δ and PPAR-γ. Future Cardiol 2017;13 (03):279–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sookoian S, Rosselli MS, Gemma C, et al. Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: impact of liver methylation of the peroxisome proliferator-activated receptor γ coactivator 1α promoter. Hepatology 2010;52(06):1992–2000 [DOI] [PubMed] [Google Scholar]
- 76.Gawrieh S, Noureddin M, Loo N, et al. Saroglitazar, a PPAR-α/γ agonist, for treatment of NAFLD: a randomized controlled double-blind phase 2 trial. Hepatology 2021;74(04):1809–1824 [DOI] [PubMed] [Google Scholar]
- 77.Francque SM, Bedossa P, Ratziu V, et al. ; NATIVE Study Group. A randomized, controlled trial of the Pan-PPAR agonist lanifibranor in NASH. N Engl J Med 2021;385(17):1547–1558 [DOI] [PubMed] [Google Scholar]
- 78.Nakajima A, Eguchi Y, Yoneda M, et al. Randomised clinical trial: pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2021;54(10):1263–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med 2016;165(05):305–315 [DOI] [PubMed] [Google Scholar]
- 80.Sharpton SR, Ajmera V, Loomba R. Emerging role of the gut microbiome in nonalcoholic fatty liver disease: from composition to function. Clin Gastroenterol Hepatol 2019;17(02):296–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009;49(06):1877–1887 [DOI] [PubMed] [Google Scholar]
- 82.Loomba R, Seguritan V, Li W, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab 2017;25(05):1054–1062.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caussy C, Tripathi A, Humphrey G, et al. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat Commun 2019;10(01):1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 2017;8(01):845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yin J, Liao SX, He Y, et al. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc 2015;4(11):e002699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int 2017;16(04):375–381 [DOI] [PubMed] [Google Scholar]
- 87.Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016;63 (03):764–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aron-Wisnewsky J, Vigliotti C, Witjes J, et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol 2020;17(05): 279–297 [DOI] [PubMed] [Google Scholar]
- 89.Loomba R, Schork N, Chen CH, et al. ; Genetics of NAFLD in Twins Consortium. Heritability of hepatic fibrosis and steatosis based on a prospective twin study. Gastroenterology 2015;149(07):1784–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eslam M, George J. Genetic contributions to NAFLD: leveraging shared genetics to uncover systems biology. Nat Rev Gastroenterol Hepatol 2020;17(01):40–52 [DOI] [PubMed] [Google Scholar]
- 91.Stender S, Loomba R. PNPLA3 genotype and risk of liver and all-cause mortality. Hepatology 2020;71(03):777–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Unalp-Arida A, Ruhl CE. Patatin-like phospholipase domain-containing protein 3 I148M and liver fat and fibrosis scores predict liver disease mortality in the U.S. population. Hepatology 2020;71(03):820–834 [DOI] [PubMed] [Google Scholar]
- 93.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40(12):1461–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bruschi FV, Claudel T, Tardelli M, et al. The PNPLA3 I148M variant modulates the fibrogenic phenotype of human hepatic stellate cells. Hepatology 2017;65(06):1875–1890 [DOI] [PubMed] [Google Scholar]
- 95.Eslam M, Mangia A, Berg T, et al. ; International Liver Disease Genetics Consortium. Diverse impacts of the rs58542926 E167K variant in TM6SF2 on viral and metabolic liver disease phenotypes. Hepatology 2016;64(01):34–46 [DOI] [PubMed] [Google Scholar]
- 96.Kozlitina J, Smagris E, Stender S, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2014;46(04):352–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu DJ, Peloso GM, Yu H, et al. ; Charge Diabetes Working Group; EPIC-InterAct Consortium; EPIC-CVD Consortium; GOLD Consortium; VA Million Veteran Program. Exome-wide association study of plasma lipids in >300,000 individuals. Nat Genet 2017;49(12):1758–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pirola CJ, Sookoian S. The dual and opposite role of the TM6SF2-rs58542926 variant in protecting against cardiovascular disease and conferring risk for nonalcoholic fatty liver: a meta-analysis. Hepatology 2015;62(06):1742–1756 [DOI] [PubMed] [Google Scholar]
- 99.Simons N, Isaacs A, Koek GH, Kuč S, Schaper NC, Brouwers MCGJ. PNPLA3, TM6SF2, and MBOAT7 genotypes and coronary artery disease. Gastroenterology 2017;152(04):912–913 [DOI] [PubMed] [Google Scholar]
- 100.Dongiovanni P, Petta S, Maglio C, et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology 2015; 61(02):506–514 [DOI] [PubMed] [Google Scholar]
- 101.Rüschenbaum S, Schwarzkopf K, Friedrich-Rust M, et al. Patatin-like phospholipase domain containing 3 variants differentially impact metabolic traits in individuals at high risk for cardiovascular events. Hepatol Commun 2018;2(07):798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. ; NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385(9972):956–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Younossi ZM, Ratziu V, Loomba R, et al. ; REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019;394(10215):2184–2196 [DOI] [PubMed] [Google Scholar]
- 104.Ramachandran P, Dobie R, Wilson-Kanamori JR, et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 2019;575(7783):512–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Depuydt MAC, Prange KHM, Slenders L, et al. Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ Res 2020;127(11):1437–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yong JN, Ng CH, Lee CW, et al. Non-alcoholic fatty liver disease association with structural heart, systolic and diastolic dysfunction: a meta-analysis. Hepatol Int 2022;16(02):269–281 [DOI] [PubMed] [Google Scholar]
- 107.Mantovani A, Dauriz M, Sandri D, et al. Association between non-alcoholic fatty liver disease and risk of atrial fibrillation in adult individuals: an updated meta-analysis. Liver Int 2019;39(04):758–769 [DOI] [PubMed] [Google Scholar]
- 108.Ciardullo S, Grassi G, Mancia G, Perseghin G. Nonalcoholic fatty liver disease and risk of incident hypertension: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2022;34(04):365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]