Abstract
Objective
The objective of the study was to evaluate the relationship between a panel of candidate plasma biomarkers and (1) death or severe brain injury on magnetic resonance imaging (MRI) and (2) dysfunctional cerebral pressure autoregulation as a measure of evolving encephalopathy.
Study design
Neonates with moderate-to-severe hypoxic-ischemic encephalopathy (HIE) at 2 level IV neonatal intensive care units were enrolled into this observational study. Patients were treated with therapeutic hypothermia (TH) and monitored with continuous blood pressure monitoring and near-infrared spectroscopy. Cerebral pressure autoregulation was measured by the hemoglobin volume phase (HVP) index; a higher HVP index indicates poorer autoregulation. Serial blood samples were collected during TH and assayed for Tau, glial fibrillary acidic protein, and neurogranin. MRIs were assessed using National Institutes of Child Health and Human Development scores. The relationships between the candidate biomarkers and (1) death or severe brain injury on MRI (defined as a National Institutes of Child Health and Human Development score of ≥ 2B) and (2) autoregulation were evaluated using bivariate and adjusted logistic regression models.
Results
Sixty-two patients were included. Elevated Tau levels on days 2–3 of TH were associated with death or severe injury on MRI (aOR: 1.06, 95% CI: 1.03–1.09; aOR: 1.04, 95% CI: 1.01–1.06, respectively). Higher Tau was also associated with poorer autoregulation (higher HVP index) on the same day (P = .022).
Conclusions
Elevated plasma levels of Tau are associated with death or severe brain injury by MRI and dysfunctional cerebral autoregulation in neonates with HIE. Larger-scale validation of Tau as a biomarker of brain injury in neonates with HIE is warranted.
Therapeutic hypothermia (TH) improves outcomes in neonatal hypoxic-ischemic encephalopathy (HIE), but about 40% of treated patients suffer death or disability.1–4 Serially measured markers of brain injury may help identify patients at risk for poor outcomes as a result of ongoing secondary injury and those who may benefit from adjuvant treatments. Serum biomarkers can be objectively assessed at the bedside, can reflect real-time evolution of neuropathology, and do not require specialized expertise for interpretation such as that needed by other current neurodiagnostic approaches such as magnetic resonance imaging (MRI).
Leading candidates for serum biomarkers include brain-specific proteins Tau and glial fibrillary acidic protein (GFAP). Tau is a microtubule-stabilizing protein5 that is associated with axonal damage6 and neurodegenerative diseases in adults.7,8 In neonatal HIE, Tau is related to brain injury by MRI and 1-year neurodevelopmental outcomes.9–11 GFAP, a cytoskeletal protein found in astrocytes, is also a marker of neurologic injury.12 GFAP is elevated in patients with HIE and is associated with the severity of clinical encephalopathy, brain injury by MRI, and neurodevelopmental outcomes at 18 months.9,13–15 Neurogranin (NRGN) is a brain-specific protein found in excitatory neurons and a more novel candidate brain injury biomarker.7,16,17 Although NRGN has been associated with traumatic brain injury and neurodegeneration in adults,7,18,19 there are few reports in pediatric (including fetal/neonatal) patients.9–11,20–22 A recent study found that elevated serum NRGN is inversely associated with the severity of encephalopathy in neonatal HIE.9
Given that prior biomarker studies in HIE have focused on correlating measures over time with cumulative brain injury assessed after treatment or in later follow-up, we sought to extend the evaluation of candidate plasma biomarkers (Tau, GFAP, and NRGN) by relating serial measures over time with indicators of brain injury that have both spatial (ie, abnormal brain MRI)4,23 and temporal (ie, dysfunctional cerebral autoregulation assessed using the hemoglobin volume phase [HVP] index)24–27 resolution. We hypothesized that elevated plasma Tau, GFAP, and NRGN would be associated with a higher National Institutes of Child Health and Human Development (NICHD) MRI brain injury score or death and correlate with measures of dysfunctional autoregulation over time during TH.
Methods
Neonates with moderate-to-severe HIE treated with hypothermia at 2 level IV neonatal intensive care units (Children’s National Medical Center and Johns Hopkins Hospital) were prospectively enrolled between February 2018 and February 2020 if they had a gestational age ≥35 weeks, had a birth weight of ≥1800 g, had an indwelling arterial line for continuous arterial blood pressure monitoring, and met institutional criteria for TH per the NICHD Neonatal Research Network protocol.4 The parents of all eligible neonates were approached for consent. Exclusions were major congenital anomalies or known/suspected genetic conditions, if the patient was in extremis and died, or if the need for extracorporeal membrane oxygenation was anticipated.
Infants’ encephalopathy levels were assessed at each site using a modified Sarnat score.4,28 Whole-body cooling was conducted according to the NICHD Neonatal Research Network protocol.4 Patients were monitored with continuous video electroencephalogram, continuous blood pressure from an indwelling arterial line, electrocardiogram, and near-infrared spectroscopy (NIRS) through TH and rewarming. Autoregulation and MRI data from this cohort have been reported.29
Cerebral Autoregulation Monitoring
Continuous mean arterial blood pressure (MAP) and bilateral NIRS (ForeSight Elite, Edwards Life Sciences) data (deoxyhemoglobin and oxyhemoglobin signals) were collected by a bedside research laptop and used to calculate total hemoglobin (HbT) as a surrogate for cerebral blood volume. Signals were inspected for artifact in 6-minute epochs using an automated program.24,25 Spectral coherence between MAP and HbT was calculated.26,27 Functional autoregulation is seen when changes in MAP drive changes in HbT with an out-of-phase relationship. Dysfunctional autoregulation is characterized by high coherence between MAP and HbT with an in-phase relationship. The HVP index was calculated as the cosine-transformed phase shift between MAP and HbT at the frequency of maximal coherence as previously described.26,27 The HVP index ranges from −1 to 1, with higher, more positive values indicating dysfunctional autoregulation.
HVP values were summarized over 24-hour periods to coincide with the timeframe of serial plasma biomarker measurement. Multiple summary statistics (minimum, maximum, median, area under the curve [AUC]) were calculated to provide a daily reflection of autoregulatory function. The AUC was calculated from the plot of the HVP over time and included both positive and negative values. A higher AUC indicates greater autoregulatory dysfunction (magnitude) across a longer period of time (duration).
Specimen Collection and Biomarker Determination
Plasma samples were obtained from remnant blood following routine clinical laboratory specimens.9 Excess plasma was stored at −80°C and underwent one thaw-freeze cycle for transfer to study cryovials before assay in bulk. Specimens were collected daily starting from day of life (DOL) 0 to DOL 4 per institutional protocols during TH.
We used a custom duplex enzyme-linked immunosorbent assay (ELISA) to simultaneously measure GFAP and NRGN as previously described.19,30
The lower limits of detection for the GFAP and NRGN as-says were 0.006 and 0.018 ng/mL, and the interassay coefficients of variation were 12% and 24%, respectively. We measured Tau using a commercial ELISA (Meso Scale Diagnostics, LLC, product number N451LAA-1). Plasma samples were diluted 4-fold and assayed according to manufacturer instructions. Samples that registered above the upper limit of detection were diluted 20-fold. The lower limit of detection for this assay was 10.6 pg/mL, the upper limit of detection was 12 500 pg/mL, and the interassay coefficient of variation was 2.2%. To best reflect the peak of injury, if a patient had multiple blood samples available on a given day, we used the sample with the greatest biomarker value for our analysis.
MRI
After rewarming, patients underwent brain MRI during natural sleep at 3–7 days of age using either a 1.5 or 3-T clinical MRI scanner (Skyra, Siemens, or Discovery MR750, GE Healthcare) per clinical standard of care. Two pediatric neuroradiologists with expertise in HIE neuroimaging and interpretation scored the clinical MRI data (including diffusion weighted, T1, and T2 images) from their home institution using the NICHD scoring system. Neuroradiologists were blinded to the autoregulation and biomarker data.4 These neuroradiologists had independently reviewed 10 MRIs and demonstrated acceptable agreement (intraclass correlation coefficient = 0.968).
An NICHD MRI score of 2B or higher was defined as an adverse outcome as this has been predictive of death or an IQ of <70 at 6–7 years of age following HIE in a prior study.23
Statistical Analyses
Data were analyzed using R, version 4.0.3 (www.r-project.org/). Biomarker data were log-transformed for analysis. Left and right values for the HVP index were averaged for analyses after assessing that there was no significant laterality between the 2 sides. We used bivariate and adjusted logistic regression models to assess the relationship between daily levels of candidate biomarkers and severe (≥2B NICHD score) brain injury by MRI or death in the first 2 postnatal weeks and the HVP index. Analyses were adjusted for the study site and severity of encephalopathy. To assess the temporality of the relationship between biomarkers of brain injury and cerebral autoregulation, we tested the association between biomarker values taken on a given day and the HVP index measured on the same day and the prior day (given the possibility that dysfunctional autoregulation may mediate and precede secondary brain injury). We analyzed multiple days of autoregulation (HVP index) per participant and so estimated the association between the HVP index and candidate biomarkers while estimating a random effect at the participant level. Estimates are reported as log ORs with the precision (SE) of the regression coefficient. Our goal was to enroll 90 patients to have adequate power to assess the relationships between the candidate biomarkers and study end points. We did not adjust for multiple comparisons.
Results
Due to the coronavirus disease 2019 pandemic, we ended enrollment after 70 patients. Of the 70 patients enrolled, 62 (89%) had at least one available blood sample and MRI and/or autoregulation data. Two patients had uninterpretable MRI due to motion artifact or incomplete sequence acquisition and were excluded from our analysis of the relationship between biomarkers and MRI injury. Nine patients had inadequate autoregulation data and were excluded from our analysis of the relationship between biomarkers and the HVP index (Figure 1; available at www.jpeds.com). The overall study sample was 60% male, had a mean gestational age of 39 ± 1.6 weeks, and weighed at birth a mean of 3.4 ± 0.6 kg. Twelve (19%) patients presented with severe encephalopathy. The median pH was 7.01 (range: 6.50–7.39) with a median base deficit of 15.0 (0.40–34.0). Median Apgar scores at 1 and 5 minutes were 2 (0–7) and 3 (0–8), respectively. Full study sample characteristics can be found in Table I.
Figure 1.
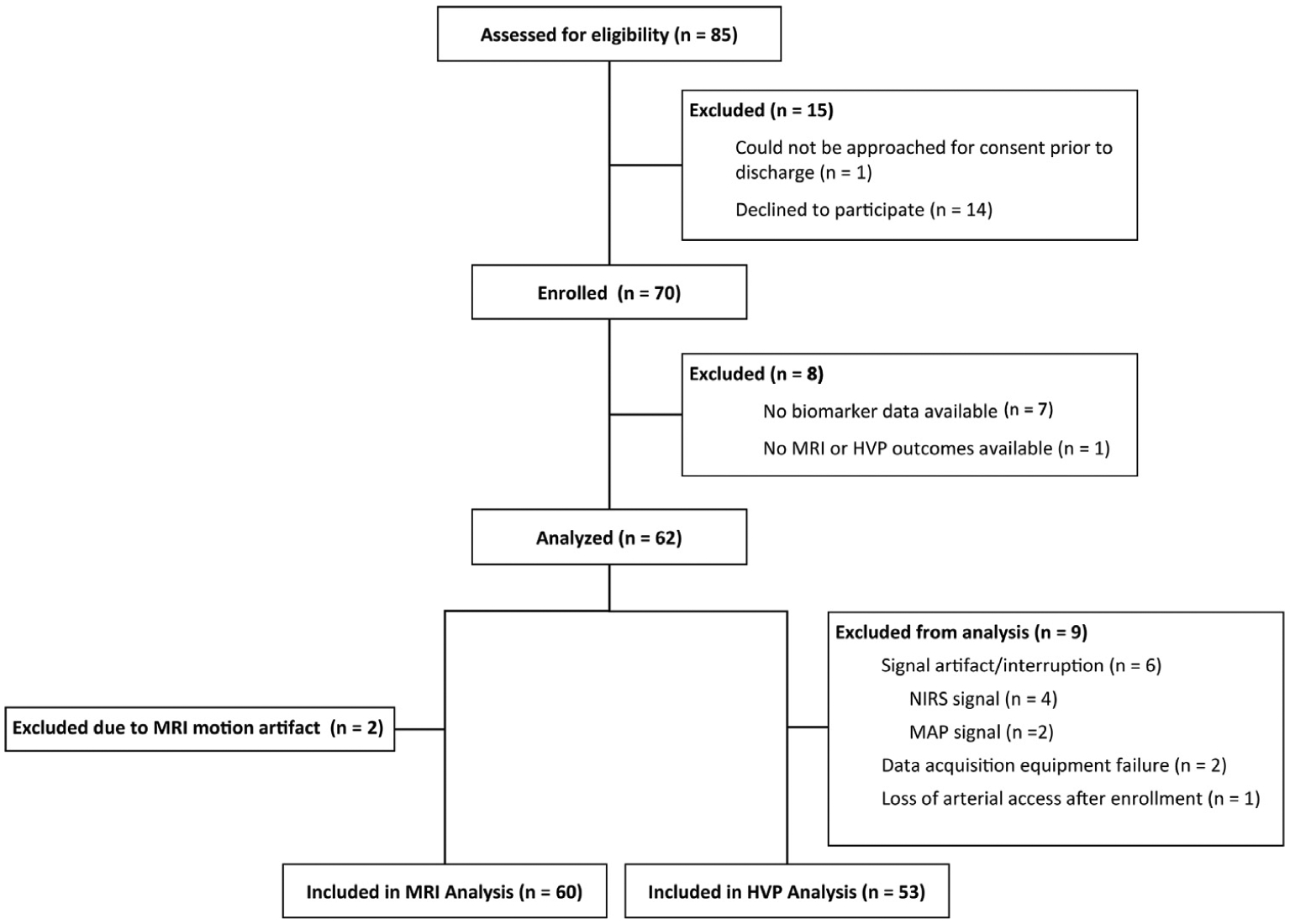
CONSORT diagram of study population.
Table I.
Clinical characteristics of the study cohort (n = 62)
| Variables | N for analysis | N (%) |
|---|---|---|
| Site, n (% Johns Hopkins Hospital) | 62 | 26 (42%) |
| Male sex | 62 | 37 (59%) |
| Ethnicity, n (% Hispanic) | 62 | 12 (19%) |
| Race*, n (%) | 62 | |
| Asian | 2 (3%) | |
| Black or African American | 20 (32%) | |
| White/Caucasian | 34 (55%) | |
| Other | 6 (10%) | |
| 1 min Apgar, median (range) | 61 | 2 (0–7) |
| 5 min Apgar, median (range) | 61 | 3 (0–8) |
| 10 min Apgar, median (range) | 50 | 5 (0–9) |
| pH, median (range) | 62 | 7.01 (6.50–7.39) |
| Base deficit, median (range) | 59 | 15 (0.40–34.0) |
| Gestational age, mean ± SD weeks | 62 | 38.9 ± 1.56 |
| Birth weight, mean ± SD kg | 62 | 3.43 ± 0.57 |
| Encephalopathy grade, n (%) | 62 | |
| Mild | 8 (13%) | |
| Moderate | 42 (68%) | |
| Severe | 12 (19%) | |
| Seizures, n (% yes) | 62 | 18 (29%) |
| NICHD score, n (%) | 60 | |
| 0 | 33 (55%) | |
| 1A | 9 (15%) | |
| 1B | 3 (5%) | |
| 2A | 4 (7%) | |
| 2B | 4 (7%) | |
| 3 | 6 (10%) | |
| Mortality | 1 (2%) |
“Other” includes mixed race Black/White (n = 1); Hispanic, race not specified (n = 4); Non-Hispanic, race not specified.
Maternal race was determined per self-report as documented in the medical record.
Biomarker Association with Brain Injury on MRI or Death
Of the 60 infants with MRI outcomes, 33 had an NICHD score of 0 (no injury), 16 had an NICHD score of 1A-2 A (mild-to-moderate injury), and 10 patients had an NICHD score of 2B or higher (severe injury). One patient died from severe neurologic damage prior to receiving an MRI and was classified in the severe injury group. ORs and aORs for risk of adverse outcome are shown in Table II. Only elevated Tau levels measured on days 2–4 were associated with a greater risk for adverse outcome. This relationship remained significant for Tau on day 2 (aOR: 1.06 for every 1000 pg/mL increase in Tau, 95% CI: 1.03–1.09, P = .001) and day 3 (aOR: 1.04 for every 1000 pg/mL increase in Tau, 95% CI: 1.01–1.06, P = .007) after adjusting for baseline encephalopathy and the study site (Figure 2).
Table II.
Association of biomarkers and death or severe brain injury by MRI (NICHD score ≥2B)
| Timepoints | N babies | Unadjusted | Adjusted* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | aOR | 95% CI | P | ||||
| Lower | Upper | Lower | Upper | ||||||
| Log GFAP | |||||||||
| Day 0 | 48 | 1.00 | 0.98 | 1.01 | .539 | 0.99 | 0.98 | 1.00 | .269 |
| Day 1 | 50 | 1.00 | 0.98 | 1.01 | .567 | 0.99 | 0.98 | 1.01 | .287 |
| Day 2 | 51 | 1.00 | 0.98 | 1.01 | .648 | 1.00 | 0.98 | 1.02 | .857 |
| Day 3 | 45 | 1.00 | 0.98 | 1.01 | .401 | 0.99 | 0.98 | 1.00 | .253 |
| Day 4 | 50 | 1.00 | 0.98 | 1.01 | .474 | 0.99 | 0.98 | 1.00 | .219 |
| Log NRGN | |||||||||
| Day 0 | 48 | 0.98 | 0.87 | 1.09 | .670 | 0.93 | 0.84 | 1.03 | .175 |
| Day 1 | 50 | 0.98 | 0.89 | 1.07 | .627 | 0.94 | 0.86 | 1.03 | .182 |
| Day 2 | 51 | 0.97 | 0.86 | 1.10 | .640 | 1.02 | 0.91 | 1.15 | .693 |
| Day 3 | 45 | 0.97 | 0.91 | 1.04 | .464 | 0.96 | 0.90 | 1.03 | .238 |
| Day 4 | 50 | 0.97 | 0.88 | 1.08 | .609 | 0.94 | 0.86 | 1.04 | .235 |
| Log Tau | |||||||||
| Day 0 | 46 | 1.00 | 0.98 | 1.02 | .780 | 0.99 | 0.97 | 1.01 | .266 |
| Day 1 | 49 | 1.00 | 0.98 | 1.02 | .827 | 0.99 | 0.97 | 1.01 | .278 |
| Day 2 | 51 | 1.07 | 1.04 | 1.10 | .000 | 1.06 | 1.03 | 1.09 | .001 |
| Day 3 | 45 | 1.04 | 1.02 | 1.06 | .000 | 1.04 | 1.01 | 1.06 | .007 |
| Day 4 | 50 | 1.02 | 1.00 | 1.03 | .011 | 1.01 | 1.00 | 1.03 | .156 |
Bolded values denote statistical significance.
Adjusted for baseline encephalopathy (Sarnat score) and site.
Figure 2.
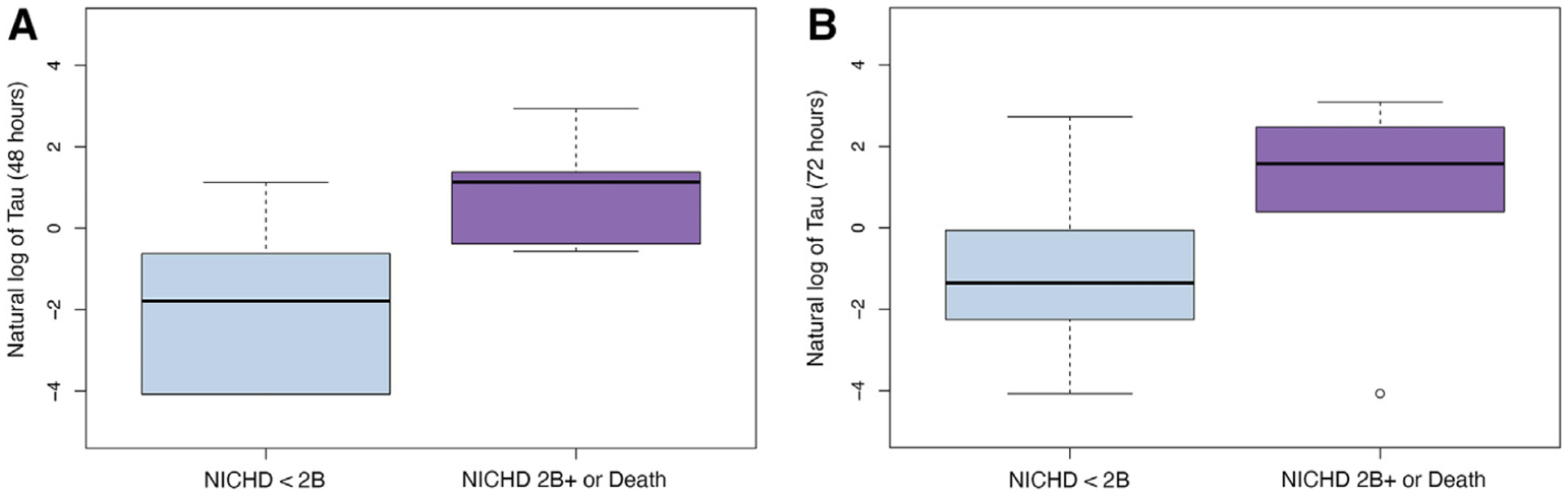
Association of log Tau (pg/mL) with death or severe brain injury by MRI at A, 48 hours (aOR = 1.06, P = .001) and B, 72 hours (aOR = 1.04, P = .007).
Biomarker Association with the HVP Index
When measured on the same day as the HVP index, only Tau was significantly associated with cerebral autoregulation, with an estimated coefficient of 0.0017 (0.0003–0.0031 for a one-point increase of the HVP AUC, P = .022) (HVP AUC, Figure 3). Out of 30 total estimated associations, no other significant associations were observed when assessing the relationship between the HVP index and Tau measured the next day. No relationships were seen between the HVP index and GFAP or NRGN (Table III; available at www.jpeds.com).
Figure 3.
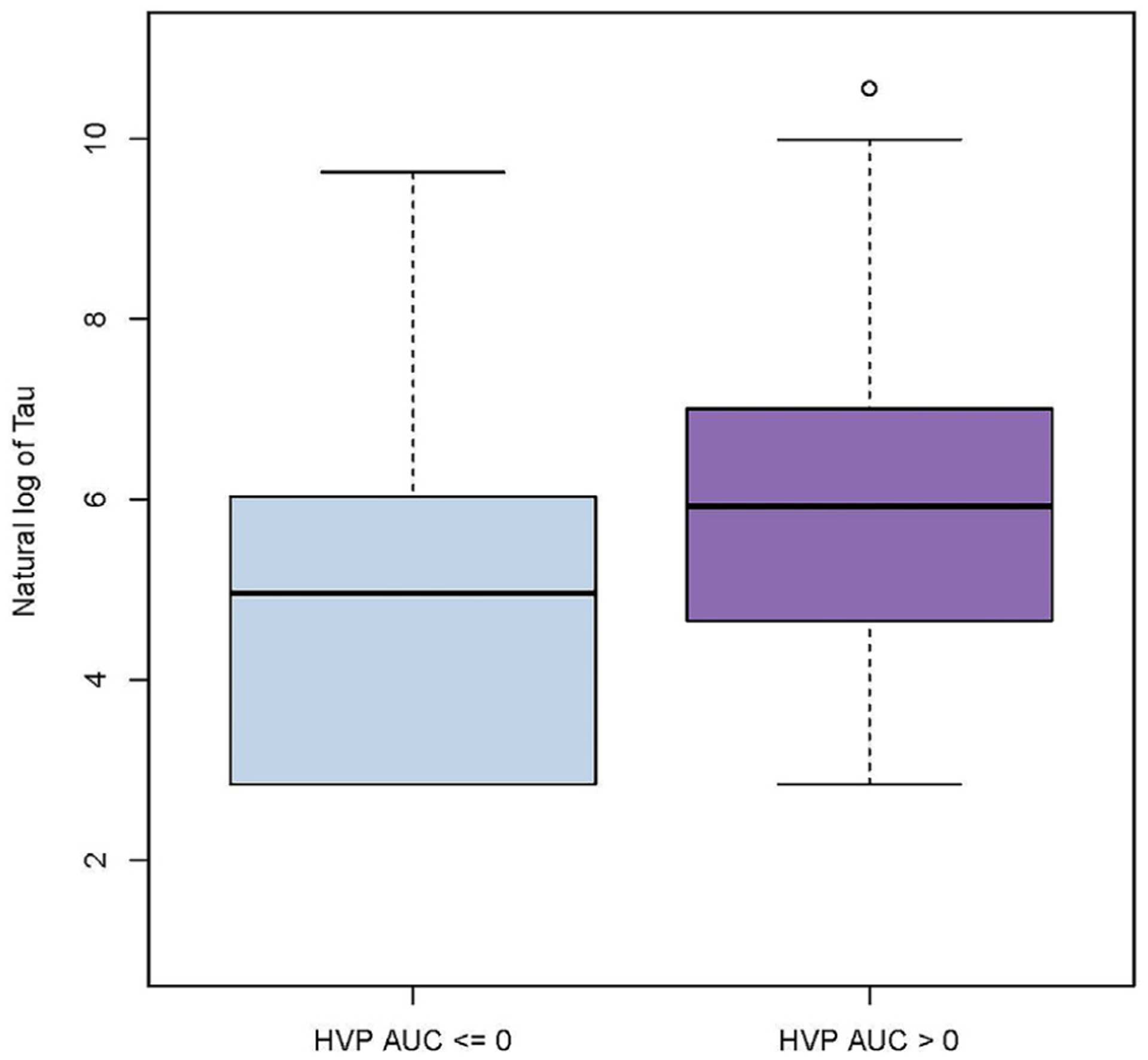
Association of log Tau (pg/mL) with cerebral autoregulation measured by the HVP AUC (β = 0.0017, P = .022).
Table III.
Association of biomarkers and dysfunctional cerebral autoregulation by the HVP index
| Autoregulation metrics | N babies | Unadjusted | Adjusted* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P | β | 95% CI | P | ||||
| Lower | Upper | Lower | Upper | ||||||
| Tau same day | |||||||||
| Mean HVP | 47 | 1.42 | −0.17 | 3.02 | .083 | 1.08 | −0.53 | 2.69 | .192 |
| Median HVP | 47 | 0.59 | −0.29 | 1.47 | .189 | 0.45 | −0.43 | 1.33 | .316 |
| Min HVP | 47 | 0.15 | −4.29 | 4.60 | .946 | −0.20 | −4.62 | 4.22 | .930 |
| Max HVP | 47 | 3.42 | −8.97 | 15.80 | .590 | 3.45 | −8.93 | 15.83 | .586 |
| AUC HVP | 47 | 0.0019 | 0.0005 | 0.0033 | .010 | 0.0017 | 0.0003 | 0.0031 | .022 |
| Tau next day | |||||||||
| Mean HVP | 46 | 1.06 | −0.60 | 2.72 | .214 | 0.82 | −0.80 | 2.44 | .321 |
| Median HVP | 46 | 0.30 | −0.63 | 1.22 | .528 | 0.24 | −0.66 | 1.13 | .606 |
| Min HVP | 46 | −1.05 | −6.98 | 4.88 | .729 | −1.54 | −7.35 | 4.27 | .605 |
| Max HVP | 46 | 2.17 | −9.94 | 14.28 | .726 | 2.57 | −9.28 | 14.42 | .672 |
| AUC HVP | 46 | 0.0006 | −0.0008 | 0.0020 | .430 | 0.0006 | −0.0008 | 0.0019 | .426 |
| GFAP same day | |||||||||
| Mean HVP | 48 | −0.60 | −2.06 | 0.86 | .420 | −0.79 | −2.25 | 0.68 | .294 |
| Median HVP | 48 | −0.39 | −1.18 | 0.39 | .328 | −0.46 | −1.24 | 0.32 | .253 |
| Min HVP | 48 | −2.17 | −5.79 | 1.45 | .243 | −2.28 | −5.90 | 1.33 | .218 |
| Max HVP | 48 | −0.50 | −11.89 | 10.90 | .932 | −0.58 | −11.99 | 10.83 | .921 |
| AUC HVP | 48 | 0.0001 | −0.0012 | 0.0014 | .917 | −0.00003 | −0.0013 | 0.0013 | .966 |
| GFAP next day | |||||||||
| Mean HVP | 46 | 1.02 | −0.46 | 2.49 | .180 | 0.88 | −0.59 | 2.35 | .243 |
| Median HVP | 46 | 0.36 | −0.46 | 1.17 | .391 | 0.31 | −0.50 | 1.12 | .455 |
| Min HVP | 46 | −1.45 | −6.47 | 3.57 | .572 | −1.59 | −6.59 | 3.42 | .536 |
| Max HVP | 46 | 1.59 | −9.24 | 12.42 | .774 | 1.40 | −9.41 | 12.21 | .800 |
| AUC HVP | 46 | 0.0003 | −0.0009 | 0.0015 | .609 | 0.0003 | −0.0009 | 0.0015 | .625 |
| NRGN same day | |||||||||
| Mean HVP | 48 | 0.28 | −0.80 | 1.36 | .612 | 0.18 | −0.91 | 1.27 | .749 |
| Median HVP | 48 | 0.14 | −0.44 | 0.73 | .632 | 0.10 | −0.48 | 0.69 | .730 |
| Min HVP | 48 | −0.68 | −3.37 | 2.02 | .624 | −0.78 | −3.47 | 1.90 | .568 |
| Max HVP | 48 | 1.36 | −7.02 | 9.75 | .750 | 1.96 | −6.43 | 10.36 | .648 |
| AUC HVP | 48 | 0.00092 | −0.00002 | 0.00186 | .056 | 0.0009 | −0.0001 | 0.0018 | .072 |
| NRGN next day | |||||||||
| Mean HVP | 46 | 0.39 | −0.90 | 1.69 | .552 | 0.31 | −0.98 | 1.61 | .636 |
| Median HVP | 46 | 0.19 | −0.53 | 0.90 | .609 | 0.17 | −0.55 | 0.88 | .648 |
| Min HVP | 46 | −1.22 | −5.72 | 3.28 | .596 | −1.59 | −6.09 | 2.91 | .490 |
| Max HVP | 46 | 1.47 | −7.96 | 10.89 | .761 | 2.51 | −6.95 | 11.98 | .604 |
| AUC HVP | 46 | −0.00031 | −0.00138 | 0.00076 | .570 | −0.0003 | −0.0014 | 0.0007 | .557 |
Bolded values denote statistical significance.
Adjusted for baseline encephalopathy (Sarnat score) and site.
Discussion
In this prospective study, elevated Tau levels on DOL 2–3 were associated with a greater risk for death or severe brain injury on MRI. Higher Tau levels also were associated with dysfunctional cerebral autoregulation, as measured by the HVP index. These findings suggest that Tau may serve as a putative blood-based biomarker of evolving encephalopathy as well as a predictor of cumulative brain injury after TH. With ongoing efforts to investigate the safety and efficacy of adjuvant therapies,31,32 there is an emerging need to develop methods and tools which will aid clinicians in identifying patients in need of additional intervention. Our findings support the utility of Tau as not only a marker of future outcome but also as a tool to aid clinicians in real-time identification of at-risk patients.
Although other blood-based biomarkers have been evaluated in HIE,33 we evaluated a selected panel of analytes for evaluation in this study given limited available blood volumes. We included Tau given the high predictive ability we observed in our recent studies in other cohorts of neonates with HIE9–11 and GFAP given that it is one of the only Food and Drug Administration-approved biomarkers of brain injury (www.accessdata.fda.gov/cdrh_docs/reviews/DEN170045.pdf). NRGN was included as a more innovative biomarker with limited study in HIE.
Tau is a microtubule-associated protein in neurons and oligodendrocytes.5 During the initial hypoxic insult, aberrant Tau phosphorylation disrupts microtubule formation and promotes neuronal damage.34 Our data support findings from other studies that also demonstrate an association between elevated Tau during and after TH9,11 and severe brain injury on MRI.9–11 The findings of our current study are consistent with our prior report that showed late Tau measurements after DOL 3 had a predictive value (AUC receiver operating curve = 0.883) that exceeded Tau measurements early in TH.11 However, other reports demonstrated significant associations between Tau measured in the first 48 hours of life and brain injury by MRI.9 Discrepancies in timing may be explained by differences in sampling practices or the impact of limited sample size to detect significant differences across all time points evaluated. It is possible that Tau is most reflective of ongoing secondary injury rather than purely a measure of the initial hypoxic-ischemic insult, contributing to more reliable distinction of outcome groups later in the course of TH. Similar to studies evaluating amplitude integrated electroencephalogram in HIE in which a positive predictive value improves over time,35,36 Tau may similarly reflect early uncertainty in brain injury trajectory that can help define a therapeutic window for neuroprotective interventions. Although the role of Tau in early (<48 hours) prediction of outcome is unclear, the use of Tau in the later stages of hypothermia may provide clinically meaningful information by identifying infants with a poor response to hypothermia, directing adjuvant neuroprotective intervention, and allowing for early reliable prognostication. Larger studies evaluating serial measurements of Tau are needed to validate Tau’s utility as a biomarker. These studies will need to assess the potential role of Tau as a complement to other neuromonitoring tools (eg, amplitude integrated electroencephalogram, NIRS), as well as its value as a stand-alone indicator of brain injury severity in settings where these alternate tools are unavailable.
We evaluated brain injury by MRI as a primary end point using the NICHD scoring system. A recent study reported that the NICHD score had comparably excellent inter-rater reliability and high concordance in classifying severity of brain injury with other commonly used systems.37 Thus, we do not anticipate that our findings would differ based on the selection of MRI scoring methodology.
Although brain MRI provides valuable information for assessing anatomical injury and prognosis for later neurodevelopmental outcome, it does not provide insight into individual responses to treatment in real time. This is a crucial limitation in neonatal HIE where evolution of secondary injury is dynamic, with the peak injury thought to occur around 24 hours following the initial insult.38 Although TH reduces oxidative stress, inflammation, excitotoxicity, and neural cell death,38–45 continued injury may still occur as autoregulatory functions are exhausted and cellular energy demands become decoupled from cerebral blood flow.46–49 Therefore, autoregulatory monitoring may offer insights into evolving secondary injury in infants with HIE.39,50–60
HVP is a relatively novel autoregulation metric that was developed and validated in a piglet model of HIE, where the HVP index performed better than existing autoregulatory indices at distinguishing blood pressure above and below the lower limits of autoregulation.26 The HVP index was correlated with MRI and neurodevelopmental outcomes in a retrospective study of neonatal HIE.27 We reported the association between the HVP index and MRI findings in this prospectively enrolled cohort and described that the predictive ability of the HVP index was time—and possibly temperature treatment phase—dependent.29 In the subset of infants in this study who had autoregulation and biomarker data, the relationship between the HVP index and MRI was significant during hypothermia, with a trend toward significance during normothermia. Given that both the HVP index and circulating biomarkers are dynamic measures hypothesized to reflect evolving injury over time, it is possible that group comparisons at select time points may not accurately reflect the peak of secondary injury among individual patients. Thus, the goal of the current work was to evaluate the temporal association between these 2 putative biomarkers of evolving brain injury to provide insight into their utility as a bedside measure of pathophysiology in real time. However, it remains uncertain whether dysfunctional autoregulation is a mediator (ie, precedes) or indicator (ie, coincides) of brain injury after hypoxia-ischemia. We assessed the temporality of the relationship between the plasma biomarker and HVP levels by varying the time window of HVP relative to biomarker measurement. Tau was associated with the HVP index when the 2 measurements were taken on the same day but not when the HVP index was measured the day before Tau. Although these results suggest that Tau and the HVP index may evolve on a similar time scale, the results could differ if latency could be assessed more granularly (eg, by minutes to hours rather than by day).
The HVP index has advantages over other proposed methods of assessing cerebral oximetry (rSO2) and autoregulation. In our prior piglet study, the HVP index had a higher AUC than the similarly derived rSO2-based metric, although this difference was not statistically significant. That the HVP index performed slightly more optimally in the preclinical setting is important given that this difference is likely underestimated given the multiple factors may influence rSO2 in the clinical setting, including sedation medications, systemic oxygen delivery, and temperature.26 The HVP index addresses limitations of previously described time-domain and frequency-domain methods of autoregulatory signal processing10 and is expected to perform similarly to signal processing approaches combining both time and frequency domains (eg, wavelet processing)61,62 when similar frequency ranges are interrogated.
Because the optimal summary statistic for assessing the HVP index over time is not known, we evaluated multiple methods of summarizing HVP data within a given time window. Greater Tau was related to a higher HVP AUC, reflecting a greater magnitude and duration of dysfunctional autoregulation. We did not identify a relationship between Tau and a maximum HVP index, which indicates the most severe autoregulatory disruption, or a median HVP index, which measures its central tendency and largely excludes peaks of failed autoregulation. Thus, sustained or repeated disruptions to autoregulatory function over time may be more damaging than a single transient but profound disturbance. Given that we used the maximum biomarker level measured within a 24-hour period if multiple specimens were available, future studies will need to assess whether alternative methods to summarize Tau over time will provide different insight into risk for brain injury. This could not be assessed in the current study given that <5% of measures had multiple samples within a 24-hour period. Further work is needed to distinguish the most appropriate summary statistic for assessing the relationship between the HVP index, candidate biomarkers, and brain injury.
Our study did not include an analysis of long-term neurodevelopmental outcomes. However, brain injury assessed by the NICHD MRI score has been shown to be predictive of long-term neurodevelopmental outcomes, with scores ≥2B (ie, the cutoff used in the current study) associated with neurodevelopmental impairment at 18–22 months3 and at 6–7 years of age.23 Elevated levels of Tau have also been correlated with worsened neurodevelopmental outcomes.9–11 Neurodevelopmental follow-up of the current study cohort is ongoing.
We did not observe significant relationships between GFAP and NRGN with MRI or the HVP index. Previous work has shown inconsistent evidence of the value of these analytes as indicators of brain injury in HIE.9–11,13–15 Different specimen handling or processing approaches, analytic methodology, and outcomes may contribute to the variability across studies. Moreover, individual biomarkers peak at different times during treatment.11 It is possible that our sampling approach was not optimized to capture potential relationships between GFAP and NRGN measures and our outcomes. That our data show a trend toward a relationship between higher NRGN and the same-day HVP AUC may warrant further investigation.
Our study has several limitations. We were unable to reach our target sample size due to the onset of the coronavirus disease 2019 pandemic, increasing the likelihood of a type II error. Several patients were excluded from one or both sub-analyses due to missing MRI, autoregulation, or biomarker data. Signal dropout and noise in the autoregulatory data led to missing data points. The use of leftover plasma samples meant that we could not ensure an available blood sample for every patient at each time point and precluded our ability to record exact sample collection times. These missing data points may have introduced some selection bias into our analysis. Our small sample size precluded analysis of validating our method for addressing patients with multiple biomarker samples for a given day. We were unable to assess the temporal relationship between plasma biomarkers and cerebral autoregulation on a more granular level given the clinical protocols for blood specimen collection and because the time between blood sample collection and autoregulation epoch could not always be accurately calculated. Finally, although the MRI is largely predictive of long-term neurodevelopmental outcome, there are some outcomes, such as language outcomes, that are not associated with the NICHD score.37,63
Despite these limitations, we found significant associations between Tau and the outcome measures assessed, suggesting that Tau may be a clinically useful biomarker to identify newborn infants at risk of impaired autoregulation and secondary brain injury due to HIE. Large-scale validation of Tau as a putative biomarker is warranted, such as that planned with analysis of the phase III High-Dose Erythropoietin for Asphyxia and Encephalopathy (HEAL) trial.31
Acknowledgments
This project was funded by the American Heart Association Grant-in-Aid. J.L. also received support from National Institutes of Health grants R01 NS107417 and R01 NS113921. A.M. is a member of The Journal of Pediatrics Editorial Board. J.L. was a paid consultant for Medtronic and is currently a paid consultant for Edwards Life Sciences. The other authors declare no conflicts of interest.
Glossary
- AUC
Area under the curve
- DOL
Day of life
- GFAP
Glial fibrillary protein
- HbT
Total hemoglobin
- HIE
Hypoxic ischemic encephalopathy
- HVP
Hemoglobin volume phase index
- MAP
Mean arterial pressure
- MRI
Magnetic resonance imaging
- NICHD
National Institutes of Child Health and Human Development
- NIRS
Near-infrared spectroscopy
- NRGN
Neurogranin
- TH
Therapeutic hypothermia
Footnotes
Portions of this study were presented as a poster during the Pediatric Academic Societies meeting, April 21–26, 2022, Denver, Colorado.
References
- 1.Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med 2014;371:140–9. [DOI] [PubMed] [Google Scholar]
- 2.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349–58. [DOI] [PubMed] [Google Scholar]
- 3.Shankaran S, Barnes PD, Hintz SR, Laptook AR, Zaterka-Baxter KM, McDonald SA, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 2012;97:F398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005;353:1574–84. [DOI] [PubMed] [Google Scholar]
- 5.Guo T, Noble W, Hanger DP. Roles of tau protein in health and disease. Acta Neuropathol 2017;133:665–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitsch A, Horn C, Kemmling Y, Seipelt M, Hellenbrand U, Stiefel M, et al. Serum tau protein level as a marker of axonal damage in acute ischemic stroke. Eur Neurol 2002;47:45–51. [DOI] [PubMed] [Google Scholar]
- 7.Blennow K A review of fluid biomarkers for Alzheimer’s Disease: moving from CSF to blood. Neurol Ther 2017;6(Suppl 1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blennow K, Zetterberg H. Cerebrospinal fluid biomarkers for Alzheimer’s disease. J Alzheimers Dis 2009;18:413–7. [DOI] [PubMed] [Google Scholar]
- 9.Dietrick B, Molloy E, Massaro AN, Strickland T, Zhu J, Slevin M, et al. Plasma and cerebrospinal fluid candidate biomarkers of neonatal encephalopathy severity and neurodevelopmental outcomes. J Pediatr 2020;226:71–9.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massaro AN, Wu YW, Bammler TK, Comstock B, Mathur A, McKinstry RC, et al. Plasma Biomarkers of Brain Injury in Neonatal Hypoxic-Ischemic Encephalopathy. J Pediatr 2018;194:67–75.e1. [DOI] [PubMed] [Google Scholar]
- 11.McGowan MM, O’Kane AC, Vezina G, Chang T, Bendush N, Glass P, et al. Serial plasma biomarkers of brain injury in infants with neonatal encephalopathy treated with therapeutic hypothermia. Pediatr Res 2021;90:1228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol 2011;93:421–43. [DOI] [PubMed] [Google Scholar]
- 13.Chalak LF, Sanchez PJ, Adams-Huet B, Laptook AR, Heyne RJ, Rosenfeld CR. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J Pediatr 2014;164:468–74.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ennen CS, Huisman TA, Savage WJ, Northington FJ, Jennings JM, Everett AD, et al. Glial fibrillary acidic protein as a biomarker for neonatal hypoxic-ischemic encephalopathy treated with whole-body cooling. Am J Obstet Gynecol 2011;205:251.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massaro AN, Jeromin A, Kadom N, Vezina G, Hayes RL, Wang KK, et al. Serum biomarkers of MRI brain injury in neonatal hypoxic ischemic encephalopathy treated with whole-body hypothermia: a pilot study. Pediatr Crit Care Med 2013;14:310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guadano-Ferraz A, Vinuela A, Oeding G, Bernal J, Rausell E. RC3/neurogranin is expressed in pyramidal neurons of motor and somatosensory cortex in normal and denervated monkeys. J Comp Neurol 2005;493: 554–70. [DOI] [PubMed] [Google Scholar]
- 17.Represa A, Deloulme JC, Sensenbrenner M, Ben-Ari Y, Baudier J. Neurogranin: immunocytochemical localization of a brain-specific protein kinase C substrate. J Neurosci 1990;10:3782–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cevik S, Ozgenc MM, Guneyk A, Evran S, Akkaya E, Calis F, et al. NRGN, S100B and GFAP levels are significantly increased in patients with structural lesions resulting from mild traumatic brain injuries. Clin Neurol Neurosurg 2019;183:105380. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Korley FK, Dai M, Everett AD. Serum neurogranin measurement as a biomarker of acute traumatic brain injury. Clin Biochem 2015;48: 843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goetzl L, Darbinian N, Merabova N. Noninvasive assessment of fetal central nervous system insult: Potential application to prenatal diagnosis. Prenat Diagn 2019;39:609–15. [DOI] [PubMed] [Google Scholar]
- 21.Johannsen J, Weiss D, Daubmann A, Schmitz L, Denecke J. Evaluation of putative CSF biomarkers in paediatric spinal muscular atrophy (SMA) patients before and during treatment with nusinersen. J Cell Mol Med 2021;25:8419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lance EI, Faulcon LM, Fu Z, Yang J, Whyte-Stewart D, Strouse JJ, et al. Proteomic discovery in sickle cell disease: Elevated neurogranin levels in children with sickle cell disease. Proteomics Clin Appl 2021;15:e2100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shankaran S, McDonald SA, Laptook AR, Hintz SR, Barnes PD, Das A, et al. Neonatal magnetic resonance imaging pattern of brain injury as a biomarker of childhood outcomes following a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr 2015;167:987–993 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Govindan RB, Massaro AN, du Plessis A. Ensuring signal quality of cerebral near infrared spectroscopy during continuous longterm monitoring. J Neurosci Methods 2018;309:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuji M, Saul JP, du Plessis A, Eichenwald E, Sobh J, Crocker R, et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 2000;106:625–32. [DOI] [PubMed] [Google Scholar]
- 26.Govindan RB, Brady KM, Massaro AN, Perin J, Jennings JM, DuPlessis AJ, et al. Comparison of Frequency- and Time-Domain Autoregulation and Vasoreactivity Indices in a Piglet Model of Hypoxia-Ischemia and Hypothermia. Dev Neurosci 2019:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massaro AN, Lee JK, Vezina G, Glass P, O’Kane A, Li R, et al. Exploratory assessment of the relationship between hemoglobin volume phase index, magnetic resonance imaging, and functional outcome in neonates with hypoxic-ischemic encephalopathy. Neurocrit Care 2021;35:121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 1976;33:696–705. [DOI] [PubMed] [Google Scholar]
- 29.Chen MW, Lee JK, Vezina G, Tekes A, Perin J, Li R, et al. The utility of cerebral autoregulation indices in detecting severe brain injury varies by cooling treatment phase in neonates with hypoxic-ischemic encephalopathy. Dev Neurosci 2022. 10.1159/000522314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bembea MM, Savage W, Strouse JJ, Schwartz JM, Graham E, Thompson CB, et al. Glial fibrillary acidic protein as a brain injury biomarker in children undergoing extracorporeal membrane oxygenation. Pediatr Crit Care Med 2011;12:572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juul SE, Comstock BA, Heagerty PJ, Mayock DE, Goodman AM, Hauge S, et al. High-dose erythropoietin for asphyxia and encephalopathy (HEAL): A randomized controlled trial - background, aims, and study protocol. Neonatology 2018;113:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu YW, Mathur AM, Chang T, McKinstry RC, Mulkey SB, Mayock DE, et al. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: A Phase II Trial. Pediatrics 2016;137. [DOI] [PubMed] [Google Scholar]
- 33.Murray DM. Biomarkers in neonatal hypoxic-ischemic encephalopathy-Review of the literature to date and future directions for research. Handb Clin Neurol 2019;162:281–93. [DOI] [PubMed] [Google Scholar]
- 34.Wu H, Li Z, Yang X, Liu J, Wang W, Liu G. SBDPs and Tau proteins for diagnosis and hypothermia therapy in neonatal hypoxic ischemic encephalopathy. Exp Ther Med 2017;13:225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sewell EK, Vezina G, Chang T, Tsuchida T, Harris K, Ridore M, et al. Evolution of amplitude-integrated electroencephalogram as a predictor of outcome in term encephalopathic neonates receiving therapeutic hypothermia. Am J Perinatol 2018;35:277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thoresen M, Hellstrom-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics 2010;126:e131–9. [DOI] [PubMed] [Google Scholar]
- 37.Ni Bhroin M, Kelly L, Sweetman D, Aslam S, O’Dea MI, Hurley T, et al. Relationship between MRI scoring systems and neurodevelopmental outcome at two years in infants with neonatal encephalopathy. Pediatr Neurol 2021;126:35–42. [DOI] [PubMed] [Google Scholar]
- 38.Johnston MV, Fatemi A, Wilson MA, Northington F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol 2011;10:372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fellman V, Raivio KO. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res 1997;41:599–606. [DOI] [PubMed] [Google Scholar]
- 40.Northington FJ, Ferriero DM, Flock DL, Martin LJ. Delayed neurodegeneration in neonatal rat thalamus after hypoxia-ischemia is apoptosis. J Neurosci 2001;21:1931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Brien CE, Santos PT, Kulikowicz E, Reyes M, Koehler RC, Martin LJ, et al. Hypoxia-ischemia and hypothermia independently and interactively affect neuronal pathology in neonatal piglets with short-term recovery. Dev Neurosci 2019;41:17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pamenter ME, Lau GY, Richards JG. Effects of cold on murine brain mitochondrial function. PLoS One 2018;13:e0208453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wisnowski JL, Wu TW, Reitman AJ, McLean C, Friedlich P, Vanderbilt D, et al. The effects of therapeutic hypothermia on cerebral metabolism in neonates with hypoxic-ischemic encephalopathy: An in vivo 1H-MR spectroscopy study. J Cereb Blood Flow Metab 2016;36:1075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang T, Li S. Efficacy of different treatment times of mild cerebral hypothermia on oxidative factors and neuroprotective effects in neonatal patients with moderate/severe hypoxic-ischemic encephalopathy. J Int Med Res 2020;48:300060520943770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou KQ, Draghi V, Lear CA, Dean JM, Ashton JL, Hou Y, et al. Protection of axonal integrity with 48 or 72 h of cerebral hypothermia in near-term fetal sheep. Pediatr Res 2020;88:48–56. [DOI] [PubMed] [Google Scholar]
- 46.Basu SK, Kaiser JR, Guffey D, Minard CG, Guillet R, Gunn AJ, et al. Hypoglycaemia and hyperglycaemia are associated with unfavourable outcome in infants with hypoxic ischaemic encephalopathy: a post hoc analysis of the CoolCap Study. Arch Dis Child Fetal Neonatal Ed 2016;101:F149–55. [DOI] [PubMed] [Google Scholar]
- 47.Svedung Wettervik T, Howells T, Hillered L, Rostami E, Lewen A, Enblad P. Autoregulatory or fixed cerebral perfusion pressure targets in traumatic brain injury: determining which is better in an energy metabolic perspective. J Neurotrauma 2021;38:1969–78. [DOI] [PubMed] [Google Scholar]
- 48.Park TS, Gonzales ER, Shah AR, Gidday JM. Hypoglycemia selectively abolishes hypoxic reactivity of pial arterioles in piglets: role of adenosine. Am J Physiol 1995;268(2 Pt 2):H871–8. [DOI] [PubMed] [Google Scholar]
- 49.Chalak LF, Tarumi T,Zhang R. The “neurovascularunit approach” toevaluate mechanisms of dysfunctional autoregulation in asphyxiated newborns in the era of hypothermia therapy. Early Hum Dev 2014;90:687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burton VJ, Gerner G, Cristofalo E, Chung SE, Jennings JM, Parkinson C, et al. A pilot cohort study of cerebral autoregulation and 2-year neurodevelopmental outcomes in neonates with hypoxic-ischemic encephalopathy who received therapeutic hypothermia. BMC Neurol 2015;15: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howlett JA, Northington FJ, Gilmore MM, Tekes A, Huisman TA, Parkinson C, et al. Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy. Pediatr Res 2013;74: 525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Govindan RB, Massaro AN, Andescavage NN, Chang T, du Plessis A. Cerebral pressure passivity in newborns with encephalopathy undergoing therapeutic hypothermia. Front Hum Neurosci 2014;8:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greisen G Effect of cerebral blood flow and cerebrovascular autoregulation on the distribution, type and extent of cerebral injury. Brain Pathol 1992;2:223–8. [DOI] [PubMed] [Google Scholar]
- 54.Lee JK, Brady KM, Chung SE, Jennings JM, Whitaker EE, Aganga D, et al. A pilot study of cerebrovascular reactivity autoregulation after pediatric cardiac arrest. Resuscitation 2014;85:1387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JK, Poretti A, Perin J, Huisman T, Parkinson C, Chavez-Valdez R, et al. Optimizing cerebral autoregulation may decrease neonatal regional hypoxic-ischemic brain injury. Dev Neurosci 2017;39:248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Massaro AN, Govindan RB, Vezina G, Chang T, Andescavage NN, Wang Y, et al. Impaired cerebral autoregulation and brain injury in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. J Neurophysiol 2015;114:818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meek JH, Elwell CE, McCormick DC, Edwards AD, Townsend JP, Stewart AL, et al. Abnormal cerebral haemodynamics in perinatally asphyxiated neonates related to outcome. Arch Dis Child Fetal Neonatal Ed 1999;81:F110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pryds O, Greisen G, Lou H, Friis-Hansen B. Vasoparalysis associated with brain damage in asphyxiated term infants. J Pediatr 1990;117:119–25. [DOI] [PubMed] [Google Scholar]
- 59.Tekes A, Poretti A, Scheurkogel MM, Huisman TA, Howlett JA, Alqahtani E, et al. Apparent diffusion coefficient scalars correlate with near-infrared spectroscopy markers of cerebrovascular autoregulation in neonates cooled for perinatal hypoxic-ischemic injury. AJNR Am J Neuroradiol 2015;36:188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Bel F, Dorrepaal CA, Benders MJ, Zeeuwe PE, van de Bor M, Berger HM. Changes in cerebral hemodynamics and oxygenation in the first 24 hours after birth asphyxia. Pediatrics 1993;92:365–72. [PubMed] [Google Scholar]
- 61.Liu X, Tekes A, Perin J, Chen MW, Soares BP, Massaro AN, et al. Wavelet Autoregulation Monitoring Identifies Blood Pressures Associated With Brain Injury in Neonatal Hypoxic-Ischemic Encephalopathy. Front Neurol 2021;12:662839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian F, Tarumi T, Liu H, Zhang R, Chalak L. Wavelet coherence analysis of dynamic cerebral autoregulation in neonatal hypoxic-ischemic encephalopathy. Neuroimage Clin 2016;11:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weeke LC, Groenendaal F, Mudigonda K, Blennow M, Lequin MH, Meiners LC, et al. A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. J Pediatr 2018;192:33–40.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]


