Abstract
Identifying the hemodynamic range that best supports cerebral perfusion using near infrared spectroscopy (NIRS) autoregulation monitoring is a potential physiologic marker for neonatal hypoxic-ischemic encephalopathy (HIE) during therapeutic hypothermia. However, an optimal autoregulation monitoring algorithm has not been identified for neonatal clinical medicine. We tested whether the hemoglobin volume phase (HVP), hemoglobin volume (HVx), and pressure passivity index (PPI) identify changes in autoregulation that are associated with brain injury on MRI or death. The HVP measures the phase difference between a NIRS metric of cerebral blood volume, the total hemoglobin (THb), and mean arterial blood pressure (MAP) at the frequency of maximum coherence. The HVx is the correlation coefficient between MAP and THb. The PPI is the percentage of coherent MAP-DHb (difference between oxygenated and deoxygenated hemoglobin, a marker of cerebral blood flow) epochs in a chosen time period. Neonates cooled for HIE were prospectively enrolled in an observational study in two neonatal intensive care units. In analyses adjusted for study site and encephalopathy level, all indices detected relationships between poor autoregulation in the first 6 h after rewarming with a higher injury score on MRI. Only HVx and PPI during hypothermia and the PPI during rewarming identified autoregulatory dysfunction associated with a poor outcome independent of study site and encephalopathy level. Our findings suggest that the accuracy of mathematical autoregulation algorithms in detecting the risk of brain injury or death may depend on temperature and postnatal age. Extending autoregulation monitoring beyond the standard 72 h of therapeutic hypothermia may serve as a method to provide personalized care by assessing the need for and efficacy of future therapies after the hypothermia treatment phase.
Keywords: Cerebral autoregulation, Neonatal hypoxia-ischemia, Hypothermia, Brain injury, MRI, Near infrared spectroscopy
Introduction
Hypoxic-ischemic encephalopathy (HIE) is one of the most common causes of neonatal death worldwide [1]. Therapeutic hypothermia reduces mortality [2] but does not prevent the moderate-to-severe neurologic disabilities that still occur in up to a third of survivors [3, 4]. Adjuvant treatments to hypothermia are urgently needed.
Cardiovascular management during hypothermic treatment of HIE varies considerably with different definitions of hypotension among neonatal intensive care units (NICUs) [5]. Supporting the post-hypoxic brain by maintaining steady cerebral blood flow (CBF) is essential to reducing secondary brain injury. Cerebral autoregulation holds CBF relatively steady across changes in mean arterial blood pressure (MAP) through vasoreactivity. Autoregulatory function during therapeutic hypothermia has been shown to be associated with neurologic outcomes in multiple studies of HIE [6–14]. Despite evidence that autoregulation monitoring could help clarify hemodynamic goals that optimize cerebral vasoreactivity, no autoregulation metric has been proposed in neonatal clinical care medicine.
We developed an autoregulation metric called the hemoglobin volume phase (HVP) index to optimize the signal-to-noise ratio and potentially improve the physiologic accuracy of near infrared spectroscopy (NIRS) autoregulation monitoring. We confirmed that the HVP distinguishes blood pressure above and below the lower limit of autoregulation in a neonatal piglet model of HIE and hypothermia [15] and it may predict clinical neurologic outcome based on a retrospective, single center, pilot study of HIE [7]. Here, we prospectively tested the HVP’s association with severe brain injury by magnetic resonance imaging (MRI) in neonates cooled for HIE in two NICUs. We also tested two established autoregulation indices, the hemoglobin volume index (HVx) [8, 11] and the pressure passivity index (PPI) [6], as comparative metrics to the HVP. The rewarming phase after therapeutic hypothermia is a known risk factor for dysfunctional autoregulation in other populations [16–19]. Therefore, we also sought to evaluate whether these autoregulation metrics changed during cooling treatment phases.
Methods
Neonates diagnosed with HIE and who received therapeutic hypothermia were prospectively enrolled in an observational study at Children’s National Hospital (CNH) and Johns Hopkins Hospital (JHH) NICUs. The investigative review boards (IRB) at both hospitals approved the study protocols (JHH protocol IRB00122513; CNH protocol Pro00008934).
Enrollment Criteria and Clinical Care
All neonates diagnosed with HIE were screened for the study between February 2018 and February 2020. Eligibility criteria included evidence of perinatal asphyxia with pH ≤7.00 or base deficit ≥16 or alternatively a milder acidosis with pH 7.01–7.05 or base deficit 10–16 alongside Apgar score ≤5 or requirement for positive pressure ventilation at 10 min and a sentinel perinatal event; receipt of therapeutic hypothermia; symptoms consistent with moderate-to-severe encephalopathy based on examination by a neonatologist at the study site or referring hospital at time of decision to initiate therapeutic hypothermia; birth weight ≥1,800 g; gestational age ≥35 weeks; and an indwelling arterial line for continuous arterial blood pressure monitoring. The parents of all eligible neonates were approached for study consent. Exclusion criteria included an inability to obtain consent, not having an indwelling arterial line, not receiving hypothermia, or receipt of extracorporeal membrane oxygenation.
All CNH and some JHH neonates were outborn. Outborn neonates were cooled during transport to the CNH and JHH NICUs. An encephalopathy level was assigned to each neonate using the modified Sarnat encephalopathy score [2, 20] by the CNH and JHH neonatologists on admission to the NICU. All neonates received whole-body cooling to a goal core temperature of 33.5 ± 0.5°C for 72 h followed by rewarming by 0.5°C/h over 6 h to a temperature ≥36.5°C (normothermia).
Clinicians followed local clinical protocols that were harmonized between the two sites for blood pressure and seizure management. Dopamine was administered as first-line therapy for hypotension (MAP ≤40), followed by epinephrine or dobutamine and hydrocortisone at the clinicians’ discretion. NIRS regional cerebral oxygen saturations, but not calculated autoregulation indices, were displayed at the bedside for clinicians. Neither NICU had protocolized approaches to blood pressure or ventilatory management based on the NIRS data. Morphine was used as the first line for sedation for all JHH neonates and as needed in CNH neonates. Neonates were monitored with continuous video EEG throughout hypothermia and rewarming and electrographic seizures were initially treated with phenobarbital. Persistent seizures were treated with fosphenytoin, levetiracetam, midazolam, or topiramate at the discretion of the consulting neurologist.
Autoregulation Monitoring
A bedside laptop computer continuously monitored the arterial MAP and bilateral NIRS deoxyhemoglobin (Hb), oxyhemoglobin (HbO2), and regional oxygen saturation (rSO2) at a rate of 6 Hz (Edwards Life Sciences; neonatal probes; Irvine, CA) during hypothermia, rewarming, and the first 6 h of normothermia. The total hemoglobin (THb) was calculated by linearly combining Hb and HbO2 at every sample to obtain a surrogate measure of cerebral blood volume (CBV) [21]. The oxy-deoxyhemoglobin difference (DHb) was calculated by subtracting the Hb from HbO2 to obtain a surrogate measure of CBF [22]. Continuous blood pressure was collected at a rate of 100 Hz at JHH and at 1,000 Hz at CNH. At JHH, the data were acquired using ICM+ software (University of Cambridge, Cambridge Enterprise, Cambridge, UK, https://icmplus.neurosurg.cam.ac.uk). At CNH, the data were collected using custom software developed in MATLAB (Mathworks Inc., Natick, MA, USA).
NIRS and MAP raw data from neonates studied at both sites were exported to MATLAB for offline processing by an investigator blinded to brain MRI and mortality (RG). Averaged signals were up-sampled to 1 Hz for analysis which accounts for the discrepancy between native sample frequencies at each site. Artifacts in the arterial blood pressure tracing, including those from flushes and transducer adjustments, and unreliable NIRS signals were filtered out using an automated program [23, 24]. The remaining signals were divided into consecutive 6-min epochs for calculation of the HVP, HVx, and PPI indices [7, 15].
Summary of Coherence and Phase
When MAP is between the limits of autoregulation (on the autoregulatory plateau) and vasoreactivity is functional, increases in MAP cause cerebral vasoconstriction with a decrease in CBV (THb) to maintain a constant CBF (DHb). This generates coherence between MAP and CBV (THb) with a negative phase shift because MAP and CBV (THb) are out-of-phase [25] (Fig. 1). Of note, this scenario generates no coherence between CBF (DHb) and MAP. When the blood pressure is outside the limits of autoregulation, progressive impairments in vasoreactivity cause pressure passivity as MAP deviates farther from the autoregulatory plateau. For this scenario, both CBV (THb) and CBF (DHb) track MAP passively. Thus, dysfunctional vasoreactivity generates coherent MAP and THb signals with positive phase shift (MAP and THb are in-phase). Furthermore, this scenario generates coherence between DHb and MAP. Coherence and phase are frequency domain metrics.
Fig. 1.
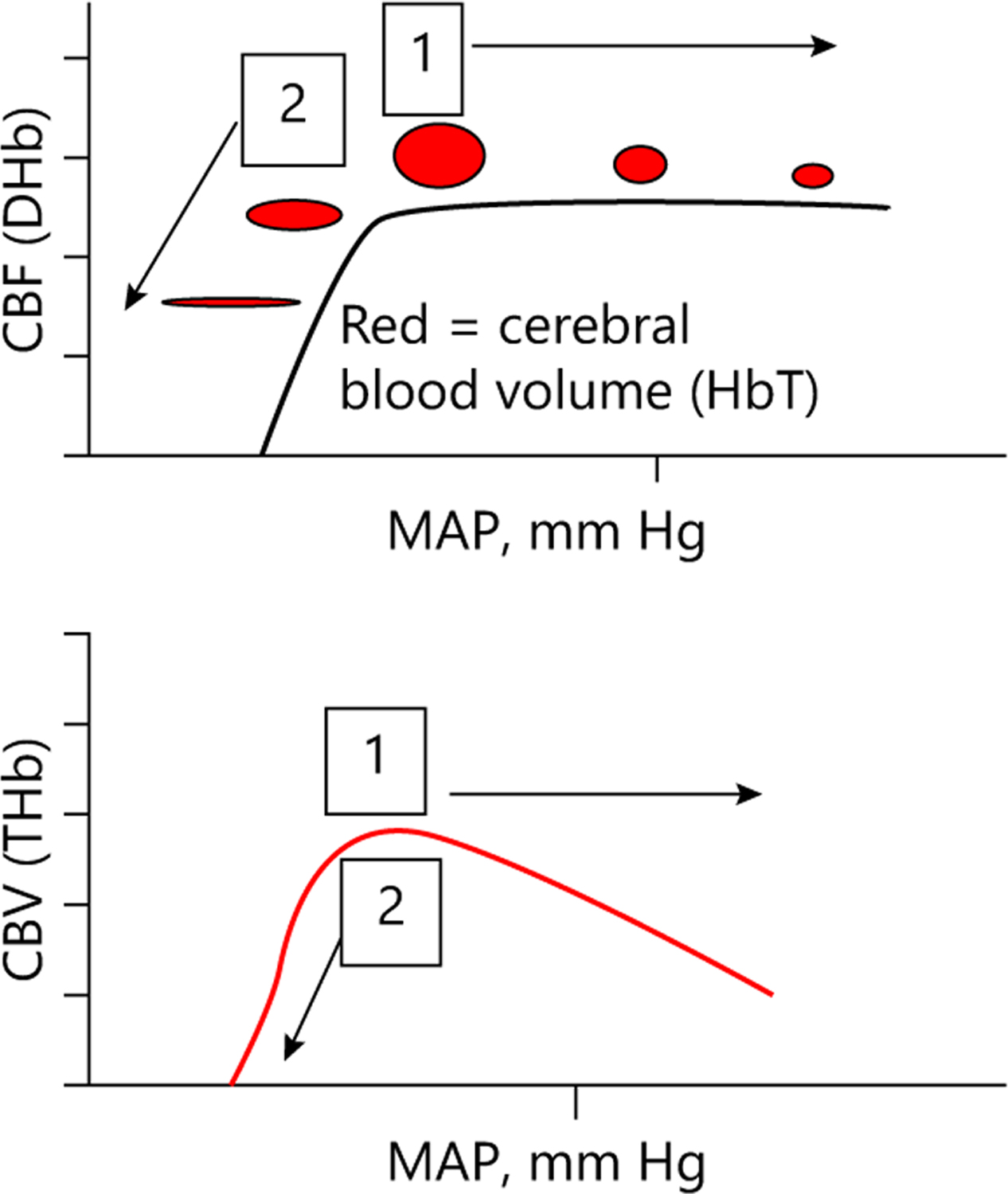
Dynamic relationship between cerebral blood flow (CBF) measured by NIRS oxy-deoxyhemoblobin difference (DHb) versus mean arterial blood pressure (MAP) and cerebral blood volume (CBV) measured by NIRS total hemoglobin (THb) versus MAP. During functional autoregulation (1), MAP and CBV are out-of-phase (moving in opposite directions) and during dysfunctional autoregulation (2), MAP and CBV are in-phase (moving in same direction).
Calculating the Spectral Coherence
Spontaneous autoregulatory fluctuations in MAP and CBF/CBV occur in the time scale of approximately 50–300 s [21, 26]. We therefore calculated coherence between MAP and DHb/THb in the frequency range 0.003–0.02 Hz (50–333 s) where autoregulation is detectable. We estimated coherence using the multivariate autoregressive model whose order was determined by Akaike information criterion [15]. The regression coefficients were estimated using a modified Yule-Walker approach [27, 28] to generate a covariance matrix from the residual errors. The multivariate autoregressive coefficients and error covariance matrix were used to calculate the cross-spectrum between MAP and DHb/THb and the auto (power) spectra of the signals [28]. Spectral coherence was then calculated as the ratio of the square of the magnitude of the cross-spectrum to the product of the auto-spectra of MAP and DHb/THb.
Coherence is a continuous measure that approaches 1 as the signals become increasingly associated. Coherence is zero when the signals are not associated. Statistical significance of coherence was assessed using a data-driven approach as previously reported [15].
Calculating Phase and the HVP
We identified the frequency band with maximum coherence within the frequency range 0.003–0.02 Hz where autoregulation is detectable [21, 26]. Then, the phase shift between MAP and THb was calculated as the argument of the cross-spectrum at the frequency band with maximum coherence. We cosine transformed the phase value to generate the HVP index [15]. HVP is negative and approaches −1 when autoregulatory vasoreactivity is functional. Dysfunctional vasoreactivity causes HVP to be positive and approach +1. We first summarized the HVP as the median within all of hypothermia, 6 h of rewarming, and the first 6 h of normothermia. Given that the optimal summary value for estimating autoregulation metrics over time is unknown, we also performed sensitivity analyses with HVP summarized as the median, mean, area under the curve (AUC), and maximum within each temperature treatment phase window. We required that at least three datapoints were available per 6-h window to calculate a summary value.
Calculating the HVx
We averaged the MAP and THb data in consecutive 10-s epochs. This averaging technique removes high-frequency wave-forms from respiration and pulse. The remaining data represent slow-wave oscillation from autoregulatory vasoreactivity [21]. The HVx was calculated by a Pearson’s correlation coefficient. When vasoreactivity is functional, MAP and THb negatively correlate. This generates a negative HVx that approaches −1 for functional autoregulatory vasoreactivity. Dysfunctional autoregulation causes THb and MAP to correlate, thereby generating a positive HVx that approaches +1 [29]. As with HVP, we analyzed the median, mean, AUC, and maximum within each window.
Calculating the PPI
The PPI was calculated as the percent of epochs where MAP and DHb were significantly coherent [22, 30] in a given window. We confined the analysis to the same frequency band used in the HVP calculation. A higher PPI indicates more autoregulatory dysfunction.
Brain MRI
Post-cooling brain MRIs were obtained in neonates at a target age of 3–7 days using a 1.5 or 3-Tesla scanner at JHH (Skyra, Siemens, Erlangen, Germany) and at CNH (Discovery MR750, GE Healthcare, Milwaukee, WI, USA) per clinical standard of care. Efforts were made to image neonates after feeding or during natural sleep, but infants can receive a dose of midazolam if needed. Two pediatric neuroradiologists who are experienced in neonatal HIE interpretation (GV, AT) used the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network score [31] to grade the diffusion weighted, T1, and T2 images. They were blinded to the autoregulation indices and blood pressure data. The NICHD score is a standardized score with categories of 0: no injury; 1A: minimal cerebral lesions and no injury in the thalamus, basal ganglia, or internal capsule; 1B: more extensive cerebral lesions without injury in the thalamus, basal ganglia, or internal capsule and no infarction; 2A: any injury in thalamus, basal ganglia, or anterior or posterior limb of the internal capsule or watershed infarction; 2B: same criteria as category 2A with additional cerebral lesions; and 3: cerebral hemispheric devastation. A brain injury score of 2B or higher is related to a higher risk of disability, intellectual quotient < 70, or death at 6–7 years of age in HIE [32]. The two neuroradiologists independently scored 10 MRIs to test for agreement. After excellent agreement was confirmed (intraclass correlation coefficient = 0.968), the radiologists interpreted the MRIs from their own institution for analysis.
Statistical Analysis
We analyzed the data using R (www.r-project.org/). Autoregulation indices were assessed for laterality and if no significant differences were observed, the average value between the right and left sides of the brain was used for analyses. Logistic regression tested whether the autoregulation indices from hypothermia, rewarming, and normothermia were associated with the binary outcome of having an MRI brain injury score of 2B or higher or death versus 2A or lower. The analyses were adjusted for Sarnat encephalopathy score (mild, moderate, or severe) and for study site (two levels). For consistency, we considered the encephalopathy score documented by neonatologists upon admission to the study site in our analyses, rather than the qualifying exam that was often performed by providers at the referring hospital. We report the estimates as log odds ratios with the precision (standard error) of the regression coefficient. We planned to enroll a total of 90 patients to have adequate power to assess the relationship of autoregulation metrics and neurologic outcome. Due to the COVID-19 pandemic, the study was stopped after enrolling 70 infants.
Results
Of the 70 infants with HIE that were enrolled (CNH n = 41; JHH n = 29), autoregulation data were available for 55 subjects (CNH n = 31; JHH n = 24). Fifteen enrolled infants were excluded due to signal artifact/interruption (n = 8 NIRS signal, n = 2 MAP signal), data acquisition equipment failure (n = 4), and loss of arterial access after enrollment (n = 1). Clinical characteristics of the cohorts with and without adequate autoregulation data were similar (p > 0.05; data not shown). Two infants had MRIs that were unable to be scored due to significant motion artifact. A total of 53 patients contributed data to the analyses of the association between autoregulation and brain injury by MRI (Fig. 2).
Fig. 2.
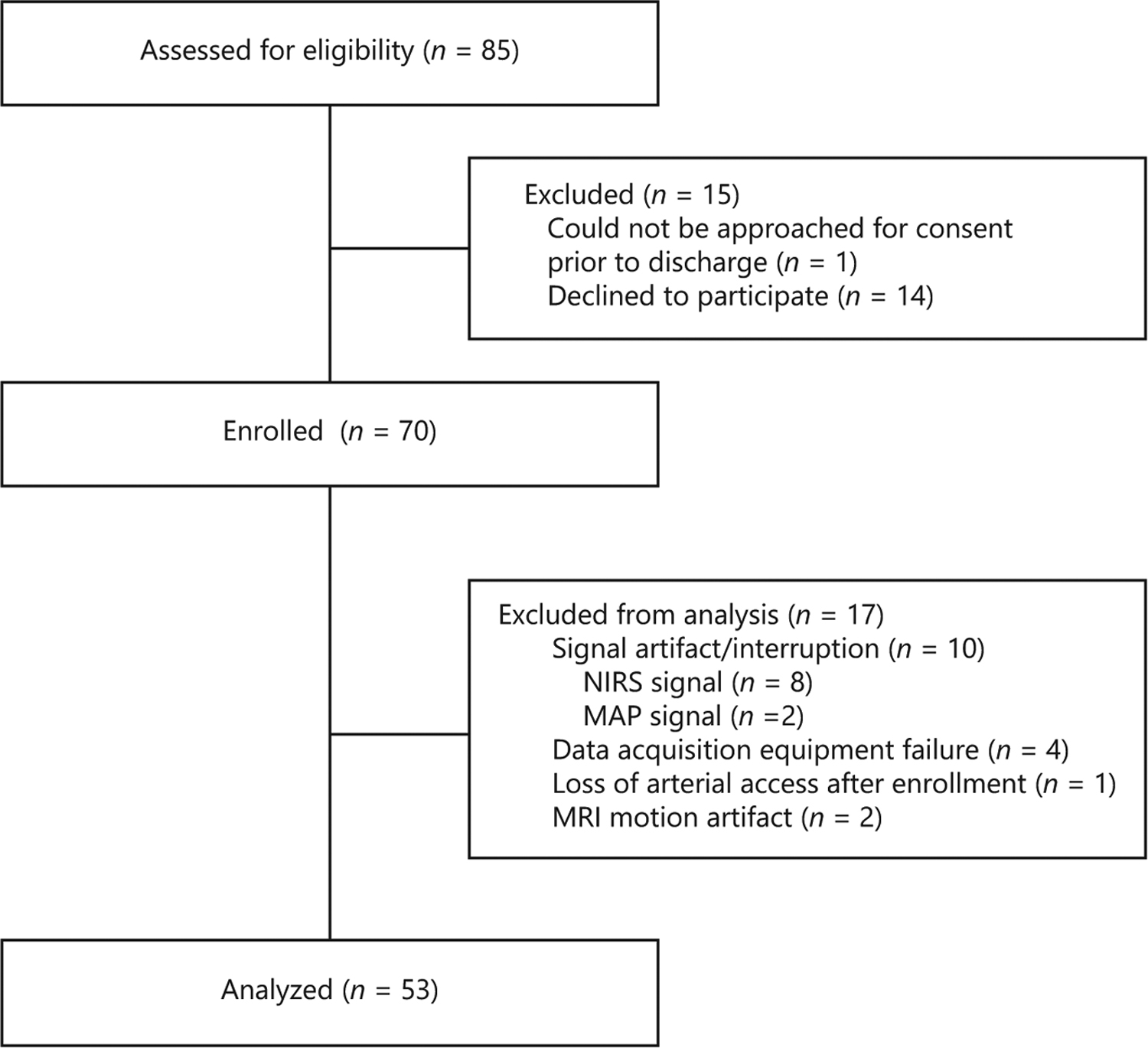
CONSORT diagram showing patient enrollment and exclusion for the study.
The neonates had severe metabolic acidosis consistent with peripartum complications. All infants had moderate-to-severe encephalopathy based on exam at the referring hospital or study site prior to initiation of therapeutic hypothermia. Upon admission to the study site NICU, 93% had persisting moderate or severe encephalopathy and four neonates had mild encephalopathy. One quarter of the cohort had seizures confirmed by EEG. More than 20% had brain injury scores of 2B or higher on MRI or died (Table 1).
Table 1.
Clinical characteristics of the 53 neonates analyzed in the study
| N (%) or mean (SD)^ | |
|---|---|
| Sex (male), n (%) | 31 (58) |
| Ethnicity (Hispanic), n (%) | 12 (21) |
| Race | |
| Asian | 2 (4) |
| Black or African-American | 13 (24) |
| White or Caucasian | 29 (55) |
| Other/unknown | 9 (17) |
| Apgar – 1 mina | 2 (0–6)^ |
| Apgar – 5 mina | 4 (0–7)^ |
| Apgar – 10 minb | 5 (0–9)^ |
| pH | 6.97 (6.5–7.28)^ |
| Base deficitc | 15.7 (5.7) |
| Gestational age, weeks | 38.8 (1.5) |
| Birth weight, kg | 3.4 (0.5) |
| Encephalopathy grade at admission | |
| 1: Mild | 4 (7) |
| 2: Moderate | 38 (72) |
| 3: Severe | 11 (21) |
| Seizures confirmed by EEG | 14 (26) |
| Vasopressor support, n (%) | |
| Any | 28 (53) |
| Dopamine | 27 (51) |
| Epinephrine | 4 (8) |
| Hydrocortisone | 14 (26) |
| Sedation, n (%) | |
| Any | 52 (98) |
| Morphine | 47 (89) |
| Midazolam | 13 (25) |
| Fentanyl | 17 (32) |
| Antiepileptic drug (AED), n (%) | |
| Any | 19 (36) |
| Phenobarbital only | 10 (19) |
| Multiple AEDs | 8 (15) |
| MRI brain injury score or mortality | |
| 0 | 28 (55) |
| 1 (1A) | 6 (11) |
| 2 (1B) | 4 (8) |
| 3 (2A) | 3 (6) |
| 4 (2B) | 5 (9) |
| 5 (3) | 5 (9) |
| 6 (death) | 2 (4) |
Data presented as median (range) where denoted.
Data available for a 52, b 42 and c 50 out of 53 patients.
Only the PPI demonstrated significant laterality (p < 0.05 for all temperature phases), so analyses were performed separately for left and right PPI. HVP and HVx, which did not show consistent differences between the left and right brain, were averaged across sides for the analysis. With the exception of 1 subject with left greater than right injury, no other subjects had laterality on MRI.
All autoregulation indices identified an association between dysfunctional autoregulation and severe brain injury on MRI or death, though these relationships varied by temperature treatment phase. During hypothermia, only the HVP (mean) and left PPI were associated with brain injury or death in the unadjusted analysis. However, after adjusting for the encephalopathy level and study site, only the left PPI and maximum HVx during hypothermia were significantly associated with severe brain injury or death (Table 2).
Table 2.
Associations between autoregulation indices and severe injury (MRI brain injury score ≥2B or death)
| Unadjusted | Adjusteda | |||||||
|---|---|---|---|---|---|---|---|---|
| N b | β | SE | p value | N b | β | SE | p value | |
| Hypothermia | ||||||||
| HVP – median | 52 | 0.297 | 0.175 | 0.095 | 52 | 0.149 | 0.173 | 0.393 |
| HVP – mean | 52 | 0.651 | 0.305 | 0.038 * | 52 | 0.363 | 0.312 | 0.250 |
| HVP – maximum | 52 | 11.865 | 9.508 | 0.218 | 52 | 14.151 | 8.979 | 0.122 |
| HVP-AUC | 52 | 0.0002 | 0.0001 | 0.115 | 52 | 0.0001 | 0.0001 | 0.443 |
| HVx – median | 52 | 0.507 | 0.583 | 0.389 | 52 | 0.283 | 0.552 | 0.611 |
| HVx – mean | 52 | 0.413 | 0.657 | 0.532 | 52 | 0.198 | 0.621 | 0.751 |
| HVx – maximum | 52 | 0.774 | 0.465 | 0.102 | 52 | 1.036 | 0.425 | 0.019 * |
| HVx-AUC | 52 | 0.0001 | 0.0002 | 0.573 | 52 | 0.0001 | 0.0002 | 0.631 |
| PPI (left) | 52 | 0.642 | 0.278 | 0.025 * | 52 | 0.587 | 0.264 | 0.031 * |
| PPI (right) | 52 | 0.159 | 0.291 | 0.588 | 52 | 0.185 | 0.268 | 0.493 |
| Rewarming | ||||||||
| HVP – median | 37 | 0.115 | 0.179 | 0.523 | 37 | 0.058 | 0.166 | 0.729 |
| HVP – mean | 37 | 0.435 | 0.328 | 0.194 | 37 | 0.285 | 0.310 | 0.365 |
| HVP – maximum | 37 | 3.11 | 3.04 | 0.313 | 37 | 0.977 | 2.962 | 0.744 |
| HVP – AUC | 37 | 0.001 | 0.001 | 0.174 | 37 | 0.001 | 0.001 | 0.481 |
| HVx – median | 37 | 1.178 | 0.489 | 0.021 * | 37 | 0.927 | 0.472 | 0.058 |
| HVx – mean | 37 | 1.367 | 0.623 | 0.035 * | 37 | 1.121 | 0.591 | 0.067 |
| HVx – maximum | 37 | 1.14 | 0.45 | 0.015 * | 37 | 0.799 | 0.457 | 0.090 |
| HVx – AUC | 37 | 0.002 | 0.001 | 0.119 | 37 | 0.002 | 0.001 | 0.109 |
| PPI (left) | 37 | 0.78 | 0.29 | 0.011 * | 37 | 0.626 | 0.283 | 0.034 * |
| PPI (right) | 37 | 0.47 | 0.29 | 0.122 | 37 | 0.224 | 0.297 | 0.455 |
| First 6 h of normothermia | ||||||||
| HVP – median | 32 | 0.312 | 0.169 | 0.076 | 32 | 0.334 | 0.173 | 0.063 |
| HVP – mean | 32 | 0.670 | 0.288 | 0.027 * | 32 | 0.662 | 0.292 | 0.031 * |
| HVP – maximum | 32 | 1.93 | 1.78 | 0.285 | 32 | 0.736 | 1.781 | 0.683 |
| HVP – AUC | 32 | 0.002 | 0.001 | 0.049 * | 32 | 0.002 | 0.001 | 0.067 |
| HVx – median | 32 | 1.372 | 0.381 | 0.001 * | 32 | 1.192 | 0.390 | 0.005 * |
| HVx – mean | 32 | 1.505 | 0.477 | 0.004 * | 32 | 1.265 | 0.501 | 0.018 * |
| HVx – maximum | 32 | 1.55 | 0.62 | 0.017 * | 32 | 1.085 | 0.763 | 0.166 |
| HVx – AUC | 32 | 0.005 | 0.001 | 0.002 * | 32 | 0.004 | 0.001 | 0.012 * |
| PPI (left) | 32 | 0.85 | 0.24 | 0.001 * | 32 | 0.807 | 0.240 | 0.002 * |
| PPI (right) | 32 | 0.66 | 0.29 | 0.032 * | 32 | 0.486 | 0.301 | 0.118 |
Right and left measurements were combined for the HVP and HVx because the indices did not differ by side.
p < 0.05. β estimates the log odds ratio. SE, standard error (precision) of β.
The analysis was adjusted for the Sarnat encephalopathy score and study site.
Data were not available for all subjects for all temperature periods due to artifact rejection and/or interruption of NIRS/MAP signal (e.g., clinical repositioning/removal of NIRS, arterial line accessing, etc.).
During rewarming, higher HVx (median, mean, and maximum) and left PPI were associated with severe brain injury on MRI or death in the unadjusted analyses. Only the PPI was related to injury or death after adjusting for the encephalopathy score and study site.
The first 6 h of normothermia showed the most consistent relationships between severe injury and autoregulation metrics. All three indices identified relationships between autoregulation and injury severity in the univariate analyses. The HVP (mean), HVx (median, mean, and AUC), and left PPI were each associated with injury after adjusting for the encephalopathy level and study site.
Discussion
Continuous NIRS blood pressure autoregulation monitoring after neonatal HIE can identify neonates at greatest risk of brain injury or death. Despite years of neonatal autoregulation research [6, 8, 10, 14, 30, 33–35], an optimal autoregulation index has not been integrated into clinical practice. We undertook the current study to examine three candidate autoregulation indices that have shown promise in preclinical [15] and single-center clinical [7–11, 36] HIE studies. Variation in clinical practice, despite efforts to harmonize protocols, could influence the reliability of NIRS autoregulation monitoring. We therefore prospectively studied neonates cooled for HIE at two quaternary NICUs. After adjusting for encephalopathy level and study site, the HVP, HVx, and PPI during the first 6 h of normothermia each identified relationships between dysfunctional autoregulation and brain injury on MRI or death. The utility of these indices varied by temperature. Only HVx and PPI during hypothermia and PPI during rewarming identified autoregulatory disturbances associated with brain injury or death after controlling for encephalopathy level and study site. Thus, the accuracy of mathematical algorithms within different NIRS autoregulation indices could vary by hour of life and temperature treatment phase. Autoregulation monitoring with all three indices after hypothermia and rewarming consistently distinguished infants who died or had significant brain injury by MRI. This indicates that prolonging autoregulation monitoring after therapeutic hypothermia might better identify those infants with more severe brain injury as well as offer an opportunity for precision medicine to direct the ongoing care of neonates after HIE.
Therapeutic hypothermia decreases excitotoxicity [37], oxidative stress [38], mitochondrial dysfunction [39], inflammation [40], and ultimately neural cell death [41] in HIE. However, dysfunctional autoregulation from detrimental mismatches in cerebral oxygen and glucose supply and demand [42, 43] can contribute to continued injury despite hypothermia. Clinicians can potentially mitigate additional injury by adjusting hemodynamic parameters to improve autoregulatory function. This would involve vasoactive medications or intravascular volume support depending upon the clinical situation.
During hypothermia, all three indices detected changes in autoregulation that were associated with severe brain injury on MRI or death. This is consistent with our prior studies [6, 7, 9]. Moreover, the maximum HVx and the PPI identified dysfunctional autoregulation that was associated with outcome independent of encephalopathy level and study site. Given that our study was designed to assess the different temperature treatment phases separately, we evaluated the entire hypothermia period as a whole. Future studies with greater power could evaluate serial and discrete periods of time to refine whether autoregulation monitoring can identify peaks of secondary injury during the hypothermia period from excitotoxicity, oxidative stress, mitochondrial dysfunction, energy failure, and CBF dysregulation [44, 45].
Rewarming is a known risk factor for dysfunctional autoregulation during adult [16] and pediatric [17] hypothermic cardiopulmonary bypass and adult brain trauma [18, 19]. In our study of neonates with HIE, the HVx and PPI confirmed a relationship between dysfunctional autoregulation during rewarming and severe brain injury or death. The PPI specifically detected dysfunctional autoregulation related to outcome after adjusting for the encephalopathy level and study site. Interestingly, the HVP did not identify such a relationship. The reasons for this discrepancy are unclear. Analyzing phase only in highly coherent data may be less important in rewarming. Because HVx incorporates phase [15] and PPI is exclusively based on coherence, both phase and coherence seem important for monitoring autoregulation during rewarming. Neonates were approximately 72–78 h old when rewarming started, and further evolution of brain injury and stopping hypothermia could play critical roles. HVP and HVx are based on THb, which should be minimally affected by shifts in the relative concentrations of oxy- and deoxyhemoblogin as the metabolic rate changes with increasing temperature.
During the first 6 h of normothermia, HVP, HVx, and PPI each identified autoregulatory changes that were associated with severe brain injury on MRI or death. These relationships were significant after controlling for study site and encephalopathy level. Close hemodynamic monitoring is often focused on the therapeutic hypothermia and rewarming phases after neonatal HIE. However, our results suggest that the 6 h normothermia period after rewarming (and potentially longer) is an equally critical period that needs close autoregulation monitoring. This time period may represent a sensitive biomarker for more severe brain injury or offer an opportunity to extend perinatal treatment of HIE in infants who have an ongoing risk of injury. Treatment may include identifying and targeting blood pressures that support autoregulation or using autoregulation monitoring to help measure the effectiveness of adjuvant neuroprotective therapies as they become available (e.g., erythropoietin [46]). Although studies evaluating the extension of hypothermia to optimize the therapeutic effect of cooling did not support a clinical benefit when this approach is applied universally [47], it is possible that an individualized approach to continuing therapeutic hypothermia in infants who have evidence of ongoing secondary injury may be considered. Additional studies should extend the duration of normothermic autoregulation monitoring to better define this potential therapeutic window.
Our study cannot discern whether dysfunctional autoregulation is an indicator or mediator of secondary neural injury after hypoxia-ischemia. Early excitotoxicity, oxidative stress, and mitochondrial dysfunction may contribute to CBF and blood pressure uncoupling. Both hypo- and hyperperfusion from dysfunctional autoregulation can presumably potentiate cellular injury and cell death. Since all our autoregulation indices become positive when blood pressure is below or above the limits of autoregulation, we cannot conclude whether the link between dysfunctional autoregulation and poor outcome were weighted towards hypo- or hypertension. However, prior research indicates that blood pressure below the optimal autoregulatory range is the larger contributor to brain injury [8–11, 14, 36]. While prior approaches have focused on identifying the optimal MAP with most robust autoregulation, optimal MAP cannot be identified in all neonates [11]. Generally, a patient needs to have a relatively wide range of blood pressure to identify optimal MAP during autoregulation monitoring. We previously described the potential advantages of the HVP approach to continuously measure autoregulation and potentially facilitate opportunities for intervention before irreversible brain injury occurs [7]. Further studies are needed to evaluate whether autoregulation monitoring can identify patients with worsening secondary brain injury who may benefit from adjuvant therapies, including interventions that can mitigate cycles of hypoxia and reperfusion from autoregulatory disturbances, to improve outcomes in HIE.
HIE is a global injury and we accordingly did not observe lateral differences for HVP or HVx. Only PPI differed by side, and we analyzed the left and right PPI separately. We observed significant relationships between left PPI and brain injury in unadjusted and adjusted analyses, consistent with the subtle but recognized left hemispheric vulnerability to brain injury [48, 49]. Given that PPI, HVP and HVx are summarized differently over time, it is not surprising that these metrics demonstrate different sensitivity to laterality. Additionally, HIE involves several brain regions that are not directly captured by NIRS. We therefore used an established global brain injury score by MRI as our outcome. The NICHD scoring system has been validated for use in multicenter studies, has excellent inter-rater reliability (and our radiology investigators had high agreement with their scoring), and has been related to school-age neurodevelopmental outcomes [32]. While other scoring systems have been described [50–52], a recent comparative study demonstrated that scores were highly correlated and predictive abilities for motor and cognitive outcomes were comparable across systems [53]. Thus, we do not feel that our results would differ based on the selection of MRI scoring system used in this study.
Our study had several limitations, including a smaller than planned sample size due to early cessation of enrollment related to the COVID-19 pandemic. Having a lower than expected sample size may have increased our risk for type-II error. The sample size was too small to adjust for seizures, vasopressors, sedatives, sex, and multiple comparisons in the analyses. Although we acknowledge the possibility of type-I error given the number of adjusted comparisons made (n = 30), the number of significant associations observed exceeded a reasonably anticipated 5% error rate. Future work will be needed to confirm these findings and to adjust for the potential clinical confounders that are known to affect cerebral oxygenation and autoregulation, including the use of sedatives [54], vasopressors [55, 56], and seizures [54, 57]. Although we enrolled patients from two academic NICUs, clinical practice protocols were generally harmonized between sites and we adjusted for site differences in our analyses. The findings may not be generalizable and our study design was observational. Selection bias may have occurred, including from the necessary exclusion of neonates without an indwelling arterial line. We also had issues with data dropout from artifact rejection and equipment failures (mostly during the early period of the study), although it is reassuring that infants that contributed usable autoregulation data were representative of the overall enrolled cohort. Nonetheless, our data indicate the need to expand NIRS autoregulation monitoring research in larger populations of neonates with HIE and prolong the duration of NIRS monitoring after therapeutic hypothermia. We did not examine long-term neurocognitive outcomes, and these data will be collected in the future.
Conclusion
HVP, HVx, and PPI neuromonitoring after HIE can identify neonates with dysfunctional autoregulation who are at risk of brain injury on MRI or death. HVP did not improve our ability to identify hypothermic autoregulatory changes associated with brain injury or death above that of HVx or PPI. However, all three metrics measured during normothermia distinguished outcome groups in the adjusted analysis. The finding that post-rewarming, normothermic autoregulation is related to brain injury could represent a manifestation of severe HIE injury, an early indicator of significant injury, and a potential opportunity to extend HIE treatment with much needed adjuncts.
Acknowledgment
We thank Srinivas Kota for developing the data acquisition program.
Funding Sources
This project was funded by the American Heart Association Grant-in-Aid (PI: Massaro). J.K.L. also received support from NIH Grants R01 NS107417 and R01 NS113921.
Footnotes
Statement of Ethics
This study was approved by the Institutional Review Board at JHH and CNH (CNH protocol Pro00008934; JHH IRB00122513). Written informed consent to participate in the study was obtained from the parent/legal guardian of all subjects.
Conflict of Interest Statement
Dr. Lee was previously a paid consultant for Medtronic and she is currently a paid consultant for Edwards Life Sciences. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. Medtronic and Edwards Life Sciences had no role in the design of the current study, collection or analysis of the data, interpretation of the results, manuscript writing, or our decision to submit this manuscript for publication.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966): 430–40. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic–ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–84. [DOI] [PubMed] [Google Scholar]
- 3.Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371(2): 140–9. [DOI] [PubMed] [Google Scholar]
- 4.Shankaran S, Barnes PD, Hintz SR, Laptook AR, Zaterka-baxter KM, Mcdonald SA, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2012; 97(6):F398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giesinger RE, Levy PT, Ruoss JL, El Dib M, Mohammad K, Wintermark P, et al. Cardiovascular management following hypoxic–ischemic encephalopathy in North America: need for physiologic consideration. Pediatr Res. 2021;90(3):600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massaro AN, Govindan RB, Vezina G, Chang T, Andescavage NN, Wang Y, et al. Impaired cerebral autoregulation and brain injury in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. J Neurophysiol. 2015;114(2):818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massaro AN, Lee JK, Vezina G, Glass P, O’Kane A, Li R, et al. Exploratory assessment of the relationship between hemoglobin volume phase index, magnetic resonance imaging, and functional outcome in neonates with hypoxic–ischemic encephalopathy. Neurocrit Care. 2021;35(1):121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tekes A, Poretti A, Scheurkogel MM, Huisman TAGM, Howlett JA, Alqahtani E, et al. Apparent diffusion coefficient scalars correlate with near-infrared spectroscopy markers of cerebrovascular autoregulation in neonates cooled for perinatal hypoxic-ischemic injury. Am J Neuroradiol. 2015;36(1):188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrasco M, Perin J, Jennings JM, Parkinson C, Gilmore MM, Chavez-Valdez R, et al. Cerebral autoregulation and conventional and diffusion tensor imaging magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy. Pediatr Neurol. 2018;82:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howlett JA, Northington FJ, Gilmore MM, Tekes A, Huisman TAGM, Parkinson C, et al. Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2013;74(5):525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JK, Poretti A, Perin J, Huisman TAGM, Parkinson C, Chavez-Valdez R, et al. Optimizing cerebral autoregulation may decrease neonatal regional hypoxic-ischemic brain injury. Dev Neurosci. 2017;39(1–4):248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian F, Tarumi T, Liu H, Zhang R, Chalak L. Wavelet coherence analysis of dynamic cerebral autoregulation in neonatal hypoxic-ischemic encephalopathy. NeuroImage Clin. 2016;11:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalak LF, Tian F, Tarumi T, Zhang R. Cerebral hemodynamics in asphyxiated newborns undergoing hypothermia therapy: pilot findings using a multiple-time-scale analysis. Pediatr Neurol. 2016;55:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Tekes A, Perin J, Chen MW, Soares BP, Massaro AN, et al. Wavelet autoregulation monitoring identifies blood pressures associated with brain injury in neonatal hypoxic-ischemic encephalopathy. Front Neurol. 2021;12:662839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Govindan RB, Brady KM, Massaro AN, Perin J, Jennings JM, DuPlessis AJ, et al. Comparison of frequency-and time-domain autoregulation and vasoreactivity indices in a piglet model of hypoxia-ischemia and hypothermia. Dev Neurosci. 2019;40(5–6):547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi B, Brady K, Lee J, Easley B, Panigrahi R, Smielewski P, et al. Impaired autoregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg. 2010;110(2):321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Easley RB, Marino BS, Jennings J, Cassedy AE, Kibler KK, Brady KM, et al. Impaired cerebral autoregulation and elevation in plasma glial fibrillary acidic protein level during cardiopulmonary bypass surgery for CHD. Cardiol Young. 2018;28(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zweifel C, Lavinio A, Steiner LA, Radolovich D, Smielewski P, Timofeev I, et al. Continuous monitoring of cerebrovascular pressure reactivity in patients with head injury. Neurosurg Focus. 2008;25(4):E2. [DOI] [PubMed] [Google Scholar]
- 19.Koizumi H, Suehiro E, Fujiyama Y, Yoneda H, Ishihara H, Nomura S, et al. Effects of brain temperature on cerebrovascular autoregulation during the acute stage of severe traumatic brain injury. Acta Neurochir Suppl. 2016; 122:193–7. [DOI] [PubMed] [Google Scholar]
- 20.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33(10):696–705. [DOI] [PubMed] [Google Scholar]
- 21.Lee JK, Kibler KK, Benni PB, Easley RB, Czosnyka M, Smielewski P, et al. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke. 2009;40(5):1820–6. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji M, Duplessis A, Taylor G, Crocker R, Volpe JJ. Near infrared spectroscopy detects cerebral ischemia during hypotension in piglets. Pediatr Res. 1998;44(4):591–5. [DOI] [PubMed] [Google Scholar]
- 23.Tsuji M, Saul JP, Plessis A, Eichenwald E, Sobh J, Crocker R, et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000;106(4):625–32. [DOI] [PubMed] [Google Scholar]
- 24.Govindan RB, Massaro AN, du Plessis A. Ensuring signal quality of cerebral near infrared spectroscopy during continuous longterm monitoring. J Neurosci Methods. 2018;309: 147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraser CD, Brady KM, Rhee CJ, Easley RB, Kibler K, Smielewski P, et al. The frequency response of cerebral autoregulation. J Appl Physiol. 2013;115(1):52–6. [DOI] [PubMed] [Google Scholar]
- 26.Brady KM, Lee JK, Kibler KK, Smielewski P, Czosnyka M, Easley RB, et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007;38(10):2818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbieri R, Triedman JK, Philip Saul J. Heart rate control and mechanical cardiopulmonary coupling to assess central volume: a systems analysis. Am J Physiol Regul Integr Comp Physiol.. 2002;283(5):R1210–20. [DOI] [PubMed] [Google Scholar]
- 28.Kay SM. Modern spectral estimation: theory and application. Englewood Cliffs, NJ, USA: Prentice Hall; 1988. [Google Scholar]
- 29.Larson AC, Jamrogowicz JL, Kulikowicz E, Wang B, Yang Z-J, Shaffner DH, et al. Cerebrovascular autoregulation after rewarming from hypothermia in a neonatal swine model of asphyxic brain injury. J Appl Physiol. 2013; 115(10):1433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007;61(4):467–73. [DOI] [PubMed] [Google Scholar]
- 31.Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366(22):2085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shankaran S, McDonald SA, Laptook AR, Hintz SR, Barnes PD, Das A, et al. Neonatal magnetic resonance imaging pattern of brain injury as a biomarker of childhood outcomes following a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr. 2015;167(5):987–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chalak LF, Zhang R. New wavelet neurovascular bundle for bedside evaluation of cerebral autoregulation and neurovascular coupling in newborns with hypoxic-ischemic encephalopathy. Dev Neurosci. 2017;39(1–4): 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Traub TM, Grabowski R, Rais-Bahrami K. Pilot study of cerebral and somatic autoregulation using NIRS in preterm neonates. J Neonatal Perinatal Med. 2021;14(3):345–52. [DOI] [PubMed] [Google Scholar]
- 35.Rhee CJ, da Costa CS, Austin T, Brady KM, Czosnyka M, Lee JK. Neonatal cerebrovascular autoregulation. Pediatr Res. 2018;84(5): 602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burton VJ, Gerner G, Cristofalo E, Chung S, Jennings JM, Parkinson C, et al. A pilot cohort study of cerebral autoregulation and 2-year neurodevelopmental outcomes in neonates with hypoxic-ischemic encephalopathy who received therapeutic hypothermia. BMC Neurol. 2015;15:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wisnowski JL, Wu TW, Reitman AJ, McLean C, Friedlich P, Vanderbilt D, et al. The effects of therapeutic hypothermia on cerebral metabolism in neonates with hypoxic-ischemic encephalopathy: an in vivo 1H-MR spectroscopy study. J Cereb Blood Flow Metab. 2016; 36(6):1075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang T, Li S. Efficacy of different treatment times of mild cerebral hypothermia on oxidative factors and neuroprotective effects in neonatal patients with moderate/severe hypoxic-ischemic encephalopathy. J Int Med Res. 2020;48(9):300060520943770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pamenter ME, Lau GY, Richards JG. Effects of cold on murine brain mitochondrial function. PLoS One. 2018;13(12):e0208453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou KQ, Draghi V, Lear CA, Dean JM, Ashton JL, Hou Y, et al. Protection of axonal integrity with 48 or 72 h of cerebral hypothermia in near-term fetal sheep. Pediatr Res. 2020;88(1):48–56. [DOI] [PubMed] [Google Scholar]
- 41.O’Brien CE, Santos PT, Kulikowicz E, Reyes M, Koehler RC, Martin LJ, et al. Hypoxia-ischemia and hypothermia independently and interactively affect neuronal pathology in neonatal piglets with short-term recovery. Dev Neurosci. 2019;41(1–2):17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basu SK, Kaiser JR, Guffey D, Minard CG, Guillet R, Gunn AJ. Hypoglycaemia and hyperglycaemia are associated with unfavourable outcome in infants with hypoxic ischaemic encephalopathy: A post hoc analysis of the CoolCap Study. Arch Dis Child Fetal Neonatal Ed. 2016;101(2):F149–55. [DOI] [PubMed] [Google Scholar]
- 43.Wettervik TS, Howells T, Hillered L, Rostami E, Lewén A, Enblad P. Autoregulatory or fixed cerebral perfusion pressure targets in traumatic brain injury: determining which is better in an energy metabolic perspective. J Neurotrauma. 2021;38(14):1969–78. [DOI] [PubMed] [Google Scholar]
- 44.Johnston MV, Fatemi A, Wilson MA, Northington F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. 2011;10(4):372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wassink G, Gunn ER, Drury PP, Bennet L, Gunn AJ. The mechanisms and treatment of asphyxial encephalopathy. Front Neurosci. 2014;8(40):40–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juul SE, Comstock BA, Heagerty PJ, Mayock DE, Goodman AM, Hauge S, et al. High-dose erythropoietin for asphyxia and encephalopathy (HEAL): a randomized controlled trial-background, aims, and study protocol. Neonatology. 2018;113(4):331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE, et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy a randomized clinical trial. J Am Med Assoc. 2014;312(24):2629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Njiokiktjien C. Differences in vulnerability between the hemispheres in early childhood and adulthood. Fiziol Cheloveka. 2006;32(1): 45–50. [PubMed] [Google Scholar]
- 49.Mullaart RA, Daniëls O, Hopman JC, de Haan AF, Stoelinga GB, Rotteveel JJ. Asymmetry of the cerebral blood flow: an ultrasound Doppler study in preterm newborns. Pediatr Neurol. 1995;13(4):319–22. [DOI] [PubMed] [Google Scholar]
- 50.Trivedi SB, Vesoulis ZA, Rao R, Liao SM, Shimony JS, McKinstry RC, et al. A validated clinical MRI injury scoring system in neonatal hypoxic-ischemic encephalopathy. Pediatr Radiol. 2017;47(11):1491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bednarek N, Mathur A, Inder T, Wilkinson J, Neil J, Shimony J. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology. 2012; 78(18):1420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weeke LC, Groenendaal F, Mudigonda K, Blennow M, Lequin MH, Meiners LC, et al. A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. J Pediatr. 2018;192:33–40.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ní Bhroin M, Kelly L, Sweetman D, Aslam S, O’Dea MI, Hurley T, et al. Relationship between mri scoring systems and neurodevelopmental outcome at two years in infants with neonatal encephalopathy. Pediatr Neurol. 2022;126:35–42. [DOI] [PubMed] [Google Scholar]
- 54.Sokoloff MD, Plegue MA, Chervin RD, Barks JD, Shellhaas RA. Phenobarbital and neonatal seizures affect cerebral oxygen metabolism: A near-infrared spectroscopy study. Pediatr Res. 2015;78(1):91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Curvello V, Hekierski H, Pastor P, Vavilala MS, Armstead WM. Dopamine protects cerebral autoregulation and prevents hippocampal necrosis after traumatic brain injury via block of ERK MAPK in juvenile pigs. Brain Res. 2017;1670:118–24. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 56.Chavez-Valdez R, O’Connor M, Perin J, Reyes M, Armstrong J, Parkinson C, et al. Sex-specific associations between cerebrovascular blood pressure autoregulation and cardiopulmonary injury in neonatal encephalopathy and therapeutic hypothermia. Pediatr Res. 2017;81(5):759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Srinivasakumar P, Zempel J, Wallendorf M, Lawrence R, Inder T, Mathur A. Therapeutic hypothermia in neonatal hypoxic ischemic encephalopathy: electrographic seizures and magnetic resonance imaging evidence of injury. J Pediatr. 2013;163(2):465–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


