Abstract
Background:
The current global monkeypox virus (MPXV) outbreak has disproportionately affected gay, bisexual and other men who have sex with men (GBMSM). Given that many jurisdictions have been faced with limited supplies of MPXV vaccine, we aimed to explore optimal vaccine allocation between 2 linked GBMSM transmission networks over a short-term time horizon, across several epidemic conditions.
Methods:
We constructed a deterministic compartmental MPXV transmission model. We parameterized the model to reflect 2 representative, partially connected GBMSM sexual networks ( cities), using 2022 data from Ontario. We simulated a roll-out of 5000 vaccine doses over 30 days that started 45 days after epidemic seeding with 10 imported cases. Within this model, we varied the relative city (network) sizes, epidemic potentials (R0), between-city mixing and distribution of seed cases between cities. For each combination of varied factors, we identified the allocation of doses between cities that maximized infections averted by day 90.
Results:
Under our modelling assumptions, we found that a limited MPXV vaccine supply could generally avert more early infections when prioritized to networks that were larger, had more initial infections or had greater R0. Greater between-city mixing decreased the influence of initial seed cases and increased the influence of city R0 on optimal allocation. Under mixed conditions (e.g., fewer seed cases but greater R0), optimal allocation required doses shared between cities.
Interpretation:
In the context of the current global MPXV outbreak, we showed that prioritization of a limited supply of vaccines based on network-level factors can help maximize infections averted during an emerging epidemic. Such prioritization should be grounded in an understanding of context-specific risk drivers and should acknowledge potential connectedness of multiple transmission networks.
The emerging outbreak of monkeypox virus (MPXV) worldwide included 1435 cases in Canada as of Oct. 28, 2022.1 A third-generation replication-deficient smallpox vaccine (Imvamune) has been licensed for use against MPXV and related orthopoxviruses in Canada since 2020, for the purpose of national security.2 Shortly after cases were reported in Canadian cities, rapid pre-exposure prophylaxis vaccination efforts were started to help reduce acquisition, infectivity and disease severity among communities disproportionately affected by MPXV, including gay, bisexual and other men who have sex with men (GBMSM).3–6 However, jurisdictions across Canada and beyond were faced with a limited local supply of vaccines during the first few weeks of the MPXV outbreak.
It is well established that prioritizing a limited supply of vaccines to subpopulations with a disproportionately higher transmission risk (i.e., acquisition and/or transmission at the individual level and/or network levels) can maximize infections averted.5–8 Such networks may have different characteristics that shape the epidemic potential within the network itself.9 This potential is often quantified via the basic reproduction number R0, which reflects the expected number of secondary infections generated by a person who is infected in a fully susceptible population.10 A network’s connectedness to other networks further shapes if and how many cases are imported by the time vaccine allocation decisions and rollout begin.11
We sought to explore the optimal allocation of a fixed supply of MPXV vaccine across 2 partially connected transmission networks (reflecting jurisdictions) of GBMSM (reflecting the community with the most cases of MPXV infection currently) under different epidemic conditions. Specifically, we explored differences between 2 jurisdictions in GBMSM population size, epidemic potential (R0), imported or seed cases, and connectedness of the 2 jurisdictions. Our goal was to produce fundamental and generalizable insights into the prioritization of MPXV vaccine in the context of interconnected sexual networks, using jurisdictions (cities) within Ontario as an example, to guide policy-makers in allocating scarce vaccines to maximize infections averted.
Methods
Study design
We constructed a deterministic compartmental model of MPXV transmission. Although stochastic network-based models can capture uncertainty and complex contact patterns better, deterministic compartmental models can estimate expected epidemic dynamics and have smaller data requirements, which are attractive during an emerging epidemic.12 Risk heterogeneity and associated mixing patterns can also be captured in compartmental models via risk-based population stratification.13
Setting and population
The modelled population represents 2 partially connected, sexual transmission networks of GBMSM, although the model captures both sexual and nonsexual transmission. For the purpose of this study, we interpreted the 2 networks as 2 cities ( cities A, B), having a combined GBMSM community size of 100 000 people.
To ground our analyses in a plausible epidemic context in Canada, we used the early MPXV situation in Ontario. The first reported case in Ontario occurred May 20, 2022;14 therefore, we posited possible exposures up to 21 days before in Toronto. Pre-exposure prophylaxis vaccination began June 12, 2022.15 At the time of initial vaccination rollout, about 5000 doses were available in Ontario and decisions were underway about optimal allocation of this limited supply across health units and cities in the province (S.M.: personal communication, 2022).
Model structure and parameterization
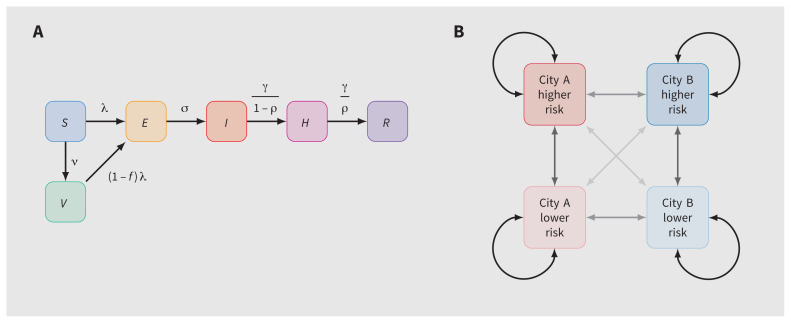
Our model included 6 health states: susceptible, vaccinated, exposed, infectious, isolating and recovered (Figure 1A). Each city was further stratified by levels of sexual risk (higher or lower defined by the numbers of sexual partners) to reflect vaccine prioritization2 and observed differences in the risk of MPXV infection.16 Table 1 summarizes the default model parameters. The definitions of higher and lower levels of sexual risk are outlined in Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221232/tab-related-content.
Figure 1:
Model structure. (A) Health states and transitions. (B) Cities, risk groups and contact networks. Note: E = exposed, H = isolating, I = infectious, R = recovered, risk = risk of monkeypox virus infection or transmission, defined by numbers of sexual partners (definitions in Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221232/tab-related-content), S = susceptible, V = vaccinated. Arrow opacity is qualitatively related to the chance of sexual contact formation from any group to another (higher opacity reflects a greater chance of contact). Rate definitions can be found in Appendix 1, Section A (model details).
Table 1:
Model parameters, including default values and ranges evaluated via grid sweep
| Parameter* | Stratum | Value (range) | Source |
|---|---|---|---|
| Population size | Overall | 100 000 | Wang et al. 202117§ |
| Fraction in city A | 0.50 (0.20–0.80) | § | |
| Fraction at higher risk | City A | 0.10 (0.01–0.50)¶ | Wang et al. 202117§ |
| City B | 0.10 | Wang et al. 202117§ | |
| Contact rate | Close nonsexual, all | 1 | Milwid et al.18§ |
| Sexual, lower risk | 0.01 | Wang et al. 202117§ | |
| Sexual, higher risk, city A | 0.189** (0.10–0.25)¶ | Wang et al. 2021,17§ Endo et al. 202219§ | |
| Sexual, higher risk, city B | 0.189** | Wang et al. 2021,17§ Endo et al. 202219§ | |
| Assortativity† | Cities, all contacts | 0.90 (0.70–1.0) | Armstrong et al. 202020§ |
| Risk, close nonsexual | 0 | § | |
| Risk, sexual | 0.50 | § | |
| Per-contact SAR | Close nonsexual | 0.01†† | Beer and Bhargavi Rao 2019,21 Thornhill et al. 202222 |
| Sexual | 0.90** | Endo et al. 202219§ | |
| Initial infections | Overall | 10 | § |
| Fraction in city A | 0.50 (0–1.0) | § | |
| Duration of period | Latent or incubation | 8 | Thornhill et al. 2022,22 Miura et al. 2022,23 Charniga et al. 2022,24 Guzzetta et al. 202225 |
| Infectious or symptoms | 21 | Thornhill et al. 2022,22 Adler et al. 202226 | |
| Fraction of infectious period isolated | All | 0.50 | Thornhill et al. 2022,22 De Baetselier et al. 202227§ |
| Vaccines available | All | 5000 | § |
| Vaccine effectiveness‡ | All | 0.85 | NACI,2 Fine et al. 1988,28 CDC29 |
| Vaccine prioritization sensitivity | Higher risk | 0.90 | Toronto Public Health3§ |
| Vaccine allocation | City A | 0.50 (0–1.0)‡‡ | – |
Note: CDC = Centers for Disease Control and Prevention, NACI = National Advisory Committee on Immunization, R0 = basic reproduction number (epidemic potential), SAR = secondary attack rate.
All durations are in days; all rates are per day.
Fraction of contacts formed exclusively within group;30 0 implies random mixing between groups and 1 implies no mixing.
Leaky type: partial protection among all vaccinated, not full protection among a fraction vaccinated.
Assumed or representative.
Calculated to fit R0 = 1.5, reflecting prevaccination estimate of monkeypox virus R0 in Ontario14 using EpiNow2.10
Calibrated to fit about 95% incidence via sexual versus close nonsexual contacts.
Optimized parameter.
To parameterize the model, we drew on previous analyses of GBMSM sexual networks in Canada17,20 and emerging MPXV epidemiological data in the context of the current epidemic.22–24,26,31 We calibrated the average numbers of sexual partners among the higher-risk group to obtain city-specific R0 that ranged from 1 to 2. Appendix 1 provides additional details about the model implementation and parameterization.
We initialized all simulations with 10 imported or seed cases in the higher-risk groups, distributed across the 2 cities as described in the Analysis subsection, and across the exposed, infectious and isolating stages proportionally by mean stage duration.
Analysis
We simulated the distribution of 5000 vaccine doses over 30 days, starting 45 days after initial cases were imported (although not necessarily detected). Doses were imperfectly prioritized to the higher-risk group with 90% sensitivity (i.e., 4500 doses reached the higher-risk group and 500 reached the lower-risk group), which reflected early risk-based eligibility criteria in some jurisdictions.3
With this fixed timeline and risk-based prioritization, we explored optimal vaccine allocation between cities A and B over a range of epidemic conditions. For a given set of conditions, we defined the optimal vaccine allocation as that which resulted in the fewest cumulative infections by day 90 in both cities. We identified optimal allocation using the optimize (https://www.rdocumentation.org/packages/stats/versions/3.6.2/topics/optimize) function in R.
We chose this 45-day time horizon and fixed 5000 vaccine doses to reflect a plausible medium-term optimization problem relevant to the early MPXV situation in Ontario. In reality, multiple changing time horizons may require consideration, different numbers of doses may become available and different rates of vaccination may be possible. We aimed to obtain generalizable insights about the relations between specific epidemic conditions and efficient geographic prioritization of a limited supply of vaccines during an outbreak.
As an example of 1 setting we analyzed, we chose parameters representative of a Toronto-like city (A) and another medium-sized Ontario city (B), with GBMSM population sizes of 80 000 and 20 000, respectively,17 and 10% sexual and social network connectivity (ɛc = 0.9).20 We also modelled R0 = 2.0 in city A versus 1.5 in city B, which reflects differences in density of sexual networks as suggested by differential prevalence of bacterial sexually transmitted infections across Ontario cities.32,33 We simulated 100% imported or seed cases in city A, which reflects observed early MPXV case distribution in Ontario.14 We then compared 2 strategies of vaccine allocation by city: proportional to population size and “optimal” (fewest infections by day 90).
Next, we performed an uncertainty analysis of the following epidemic conditions, and identified the optimal vaccine allocation between cities A and B for each combination of conditions: relative size of city A versus city B (1/4 to 4 times); relative epidemic potential in city A (R0 = 1–2 in city A v. fixed R0 = 1.5 in city B), which we adjusted via the sexual activity of the higher-risk group in city A; between-city mixing (0%–30% of all contacts formed randomly between cities); and fraction of imported or seed cases in city A versus city B (0%–100%). We calculated city-specific R0 assuming no between-city mixing.
Ethics approval
Because this study involved the use of publicly available data, approval by a research ethics board was not required.
Results
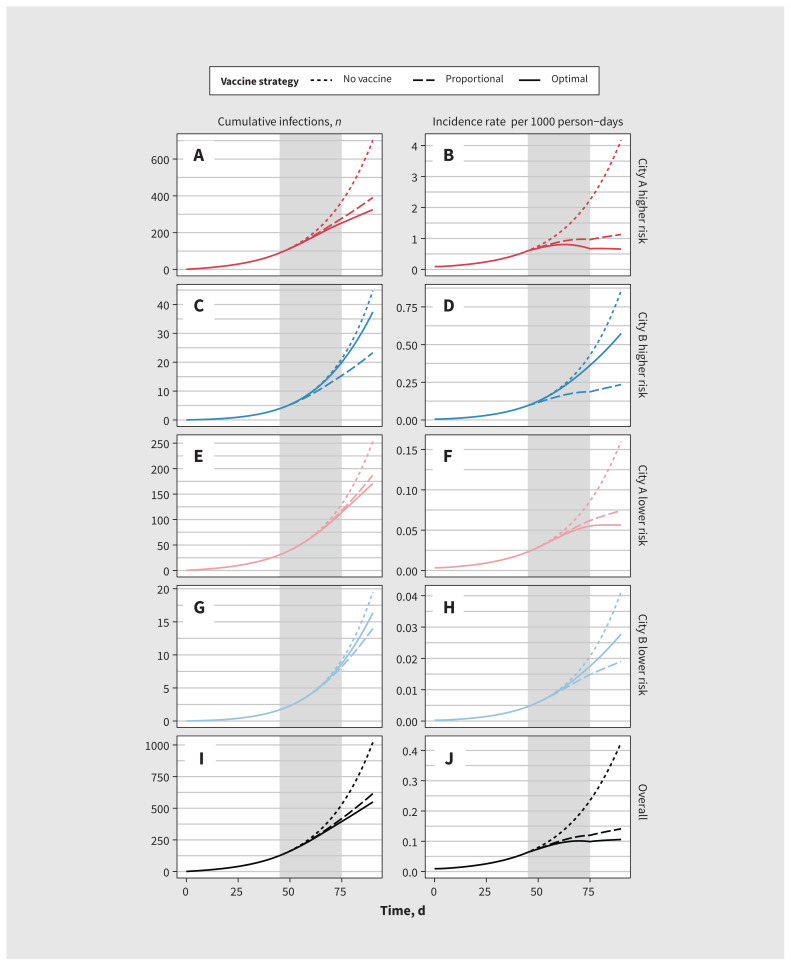
Figure 2 illustrates modelled MPXV incidence and cumulative infections in city A versus city B under different strategies for vaccine allocation. Because of the larger population size, greater epidemic potential (R0) and having all imported or seed cases in city A in this scenario, allocating all 5000 vaccine doses to city A yielded the fewest infections (550; solid line) by day 90 (optimal strategy). Allocating vaccines proportionally to city size yielded 615 infections (broken line), whereas no vaccination yielded 1020 infections (dotted line) (Figure 2I; corresponding incidence rates in Figure 2J).
Figure 2:
Modelled monkeypox virus (MPXV) cumulative infections and incidence rate in 2 cities under 3 different vaccine allocation scenarios. Gray bar indicates the period of vaccine roll-out (days 45–75). City A reflects a Toronto-like city and city B reflects a medium-sized city in Ontario. For vaccine allocation, proportional allocation (to city size) was 75% to city A and 25% to city B, and optimal allocation (most infections averted by day 90) was 100% to city A. Note: Risk = risk of MPXV infection or transmission, defined by numbers of sexual partners (definitions in Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221232/tab-related-content).
Allocating most or all doses to city A allowed infection incidence to rise exponentially in city B (Figure 2D and Figure 2H). However, this approach can still avert more infections overall over shorter time horizons, after which more doses may become available. Appendix 1, Supplemental Figure B.1, illustrates the opposite case (using the default model parameters in Table 1): 2 identical cities with equal seeding, where the optimal allocation is equal between cities.
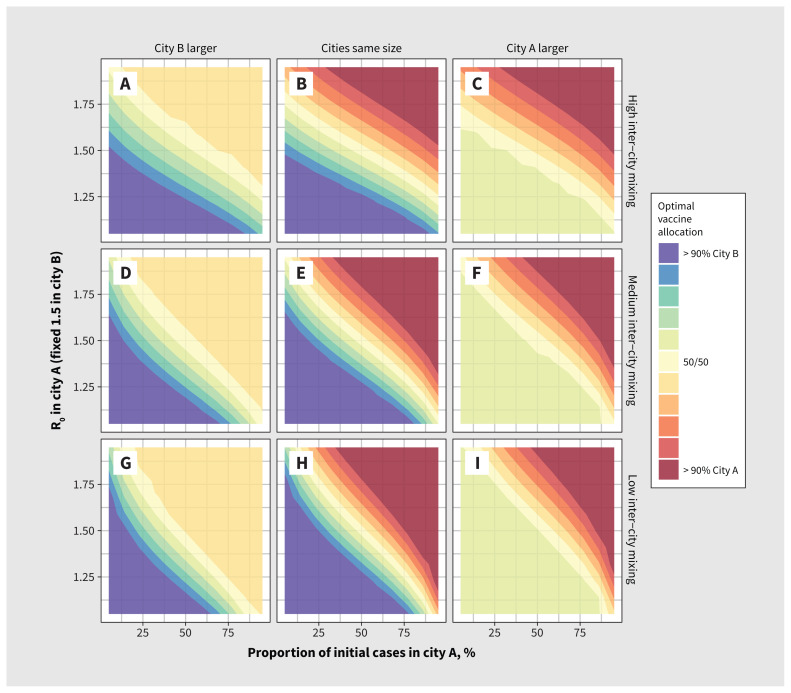
Figure 3 illustrates optimal vaccine allocation between cities A and B across different epidemic conditions. Appendix 1 provides absolute and relative numbers of infections averted under optimal allocation versus no vaccination (Appendix 1, Supplemental Figure B.2, Supplemental Figure B.3) and versus vaccine allocation proportional to city size (Appendix 1, Supplemental Figure B.4, Supplemental Figure B.5), showing under what conditions optimal allocation is most important.
Figure 3:
Optimal monkeypox vaccine allocation between 2 cities under different epidemic conditions. Epidemic potential (R0) in city A varies via the sexual activity among the higher-risk group in city A. We defined optimal vaccine allocation as the fewest cumulative infections by day 90. The larger city is 3 times the size of the other city. We used city assortativity (ɛc) values of 0.80, 0.90 and 0.95 for high, medium and low between-city mixing, respectively.
We found that the strongest determinants of optimal vaccine allocation were relative R0 between cities, the share of seed cases and city size, although the size of the higher-risk group was proportional to city size under our modelling assumptions. Thus, if a larger city had a large R0 and most of the seed cases, it was best to allocate most or all doses to that city in our analysis (solid red or blue corners in Figure 3).
For smaller cities with a large R0 and most of the seed cases, it was sometimes possible to vaccinate the entire higher-risk group; in such instances, the remaining doses were best allocated to the higher-risk group in the other city, yielding the plateaus (solid yellow triangles) in Figure 3 (upper right in panels A, D and G, and lower left in panels C, F and I). The plateaus show how priority populations can change if or after high levels of coverage are achieved in other populations.
When cities with most or all of the seed cases had smaller R0, doses were shared between cities under the optimal allocation strategy (to varying degrees), which suggests that both risk-based (reflecting R0) and proximity-based (reflecting initial cases) prioritization strategies worked together to minimize transmission. In such instances, the other city necessarily had few or no seed cases but a larger R0, to which the same findings apply. These conditions are represented by the yellow diagonal segments in all panels of Figure 3.
Increased levels of mixing between cities mainly acted to reduce the influence of initial seed cases and increase the influence of R0 on optimal allocation of vaccines to each city (shown by the stronger vertical gradients [contours are relatively more horizontal] in Figures 3A, 3B and 3C with more between-city mixing, v. stronger horizontal gradients [contours are relatively more vertical] in Figures 3G, 3H and 3I with less between-city mixing).
Interpretation
We sought to explore how different epidemic conditions could affect optimal allocation of a fixed supply of MPXV vaccine across 2 partially connected transmission networks (e.g., cities or jurisdictions). Under our modelling assumptions, we found that vaccines could generally avert more infections when prioritized to a larger network, a network with more initial infections and a network with greater epidemic potential (R0).
Although our study, for simplicity, focused on 2 partially connected networks, it highlights the importance of measuring outcomes for a population overall, by considering that geographies comprise interconnected networks. That is, while cities across Canada, and globally, feature important within- and between-city differences in size and configuration of transmission networks,34,35 and in access to interventions and services,33,36,37 these cities ultimately remain connected with respect to transmission and cannot be considered in isolation over longer time horizons.20,35,38 We grounded the 2 networks as “cities,” but the implications would hold across geographic scope via vaccine allocation across health units, provinces or even countries.
Within such interconnected settings, our findings are consistent with previous studies that showed that prioritizing limited vaccine supply and/or resources to communities or settings with the highest epidemic potential (shaped by density and other features of the contact network) generally yields the greatest benefit for the population overall.5,6,39 We also identified how key factors, such as number of imported cases and connections between networks, shape efficient early vaccine rollout.
Although our model parameterization reflected GBMSM sexual networks in Ontario, our findings have wider implications for MPXV vaccine rollout globally. The persistent absence of vaccine supply and rollout in regions already endemic for MPXV outbreaks across West and Central Africa, including (although not yet reported) in the context of GBMSM and sexual minorities,40 reflects another failure to uphold principles of equity in global health, paralleling missed opportunities in achieving COVID-19 vaccine equity;41 such failures also undermine efforts to control and mitigate MPXV globally.42
Prioritization based on risk also requires understanding risk. Early MPXV vaccine rollout in Ontario started in Toronto, where cases were already detected, the population size was large and rates of bacterial sexually transmitted infections suggested a potentially denser sexual network and, thus, greater epidemic potential.19 Our model implemented differential R0 between cities via contact rates; however, epidemic potential may also be linked to intervention access, including access to diagnoses and isolation support.36,43 Thus, our findings suggest that characterizing the drivers of epidemic potential across jurisdictions and communities is important, including participatory, community-based surveillance and research into the contexts that lead to disproportionate risks at a network level, not just at an individual level.44,45
Limitations
Our study aimed to provide fundamental and generalizable findings using a broad sensitivity analysis to identify conditions that can shape optimal short-term vaccine allocation with a limited supply. As with any modelling study, our results depended on our modelling assumptions and parameter values; for some of these, limited data were available. We did not evaluate population-level benefits balanced with potential adverse effects, given the existing data on high safety with the smallpox vaccines used in Canada.46,47
We used a simple compartmental model, with only 2 risk groups; future research would benefit from more nuanced representations of risk (e.g., using individual-based sexual network models). Our study also explored only 2 representative GBMSM transmission networks (cities) with a fixed number of doses. Incorporation of the wider population, additional transmission networks or calibration to observed data on cases, service availability and vaccine uptake in specific cities or relevant jurisdictions, could yield more interesting prioritization findings. However, we expect that our findings using these 2 networks would apply across different networks and conditions.
Finally, we restricted our study to a limited vaccine supply with a fixed rollout approach, and future research would benefit from exploring the sensitivity of results to different amounts of finite supply, time–variant vaccination rate and number of imported or seed cases, as well as timing of vaccine availability in relation to epidemic phase.
Conclusion
Strategic prioritization of a limited vaccine supply by network-level risk factors can maximize infections averted over short time horizons in the context of an emerging epidemic, such as the current global MPXV outbreak. Notable factors include the network size, distribution of initial cases, relative epidemic potential within a given network and connectivity between networks. Such epidemic potential is defined not just by possible modes of transmission but by network configuration, access to prevention and care, and by underlying social and structural contexts. Efforts to understand and anticipate epidemic potential across and between different networks before outbreaks occur can support rapid response. Such efforts should be paired with resource prioritization to eliminate existing disparities in health care access and outcomes.
Supplementary Material
Acknowledgements
The authors thank Kristy Yiu (Unity Health Toronto) for research coordination support; Huiting Ma, Linwei Wang, Oliver Gatalo and Ekta Mishra (Unity Health Toronto) for support with conceptualizing and parameterizing the model; and Mackenzie Hamilton (Unity Health Toronto) for her feedback on the manuscript. We also thank Toronto Public Health and members of the MPox Community Mobilization Group for their insights, ongoing engagement and feedback on preliminary results.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Jesse Knight and Sharmistha Mishra conceptualized and designed the study, and drafted the manuscript. Jesse Knight developed the model, conducted the analyses and generated the results. Sharmistha Mishra and Darrell Tan provided key interpretation of the results. Darrell Tan contributed critical review of the manuscript. All authors contributed to addressing revisions, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: The study was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC CGS-D), and the University of Toronto Emerging and Pandemic Infections Consortium (EPIC) MPXV Collaborative Rapid Research Response. No funder had any direct role in the research. Sharmistha Mishra is supported by a Tier 2 Canada Research Chair in Mathematical Modeling and Program Science. Darrell Tan is supported by a Tier 2 Canada Research Chair in HIV Prevention and STI Research.
Data sharing: All analysis code is available at github.com/mishra-lab/mpox-model-compartmental. Figures and numeric results can be obtained directly from this code using R.
References
- 1.Monkeypox epidemiology update. Ottawa: Public Health Agency of Canada; modified 2022 Aug. 30. Available: https://health-infobase.canada.ca/monkeypox/ (accessed 2022 Oct. 30). [Google Scholar]
- 2.Advisory Committee. An Statement (ACS) National Advisory Committee on Immunization (NACI): NACI Rapid Response — updated interim guidance on Imvamune® in the context of ongoing monkeypox outbreaks. Ottawa: Public Health Agency of Canada; 2022. Available: https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/rapid-response-updated-interim-guidance-imvamune-monkeypox-outbreaks.pdf (accessed 2022 Nov. 7). [Google Scholar]
- 3.Monkeypox vaccine fact sheet. Toronto: Toronto Public Health, City of Toronto; 2022. Available: https://www.toronto.ca/wp-content/uploads/2022/07/97ef-MonkeypoxVaccineFactSheet.pdf (accessed 2022 Oct. 30). [Google Scholar]
- 4.Olson I. Quebec expands monkeypox vaccination efforts as virus continues to spread. CBC News 2022. June 14. Available: https://www.cbc.ca/news/canada/montreal/monkeypox-quebec-montreal-vaccine-expansion-1.6488551 (accessed 2022 Oct. 30).
- 5.Garnett GP. Role of herd immunity in determining the effect of vaccines against sexually transmitted disease. J Infect Dis 2005;191(Suppl 1):S97–106. [DOI] [PubMed] [Google Scholar]
- 6.Mishra S, Stall NM, Ma H, et al. A vaccination strategy for Ontario COVID-19 hotspots and essential workers. Version 1.0 Ontario COVID-19 Science Advisory Table. Available: https://covid19-sciencetable.ca/sciencebrief/a-vaccination-strategy-for-ontario-covid-19-hotspots-and-essential-workers/ (accessed 2022 Oct. 30).
- 7.Greenhalgh D. Optimal control of an epidemic by ring vaccination. Commun Stat Stoch Models 1986;2:339–63. [Google Scholar]
- 8.Mylius SD, Hagenaars TJ, Lugnér AK, et al. Optimal allocation of pandemic influenza vaccine depends on age, risk and timing. Vaccine 2008;26:3742–9. [DOI] [PubMed] [Google Scholar]
- 9.Weiss KM, Goodreau SM, Morris M, et al. Egocentric sexual networks of men who have sex with men in the United States: results from the ARTnet study. Epidemics 2020;30:100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbott S, Hellewell J, Sherratt K, et al. EpiNow2: estimate real-time case counts and time-varying epidemiological parameters. EpiForecasts; 2020. Available: https://epiforecasts.io/EpiNow2/ (accessed 2022 Oct. 30).
- 11.Keeling MJ, Eames KTD. Networks and epidemic models. J R Soc Interface 2005;2:295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson LF, Geffen N. A comparison of two mathematical modeling frameworks for evaluating sexually transmitted infection epidemiology. Sex Transm Dis 2016;43:139–46. [DOI] [PubMed] [Google Scholar]
- 13.Garnett GP, Anderson RM. Sexually transmitted diseases and sexual behavior: Insights from mathematical models. J Infect Dis 1996;(Suppl 2):S150–61. [DOI] [PubMed] [Google Scholar]
- 14.Monkeypox in Ontario: May 20, 2022 to October 18, 2022. Toronto: Public Health Ontario; 2022. Available: https://www.publichealthontario.ca/-/media/Documents/M/2022/monkeypox-episummary.pdf (accessed 2022 Oct. 30). [Google Scholar]
- 15.Toronto Public Health holds vaccination clinics to protect community against monkeypox [news release]. Toronto: City of Toronto; 2022. June 11. Available: https://www.toronto.ca/news/toronto-public-health-holds-vaccination-clinics-to-protect-community-against-monkeypox/ (accessed 2022 Oct. 30). [Google Scholar]
- 16.Monkeypox [Q&A]. Geneva: World Health Organization; 2022. Available: https://www.who.int/news-room/questions-and-answers/item/monkeypox (accessed 2022 Oct. 30). [Google Scholar]
- 17.Wang L, Moqueet N, Simkin A, et al. Mathematical modelling of the influence of serosorting on the population-level HIV transmission impact of pre-exposure prophylaxis. AIDS 2021;35:1113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milwid RM, Xia Y, Doyle CM, et al. Past dynamics of HIV transmission among men who have sex with men in Montréal, Canada: a mathematical modeling study. BMC Infect Dis 2022;22:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endo A, Murayama H, Abbott S, et al. Heavy-tailed sexual contact networks and monkeypox epidemiology in the global outbreak. Science 2022;378:90–4. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong E, Coleman T, Lewis NM, et al. Travelling for sex, attending gay-specific venues, and HIV-related sexual risk among men who have sex with men in Ontario, Canada. Can J Hum Sex 2020;29:380–91. [Google Scholar]
- 21.Beer EM, Bhargavi Rao V. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis 2019;13:e0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornhill JP, Barkati S, Walmsley S, et al. SHARE-net Clinical Group. Monkeypox virus infection in humans across 16 countries: April–June 2022. N Engl J Med 2022;387:679–91. [DOI] [PubMed] [Google Scholar]
- 23.Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill 2022;27:2200448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charniga K, Masters NB, Slayton RB, et al. Estimating the incubation period of monkeypox virus during the 2022 multi-national outbreak [preprint]. medRxiv 2022. June 23. doi: 10.1101/2022.06.22.22276713. [DOI] [Google Scholar]
- 25.Guzzetta G, Mammone A, Ferraro F, et al. Early estimates of monkeypox incubation period, generation time, and reproduction number, Italy, May–June 2022. Emerg Infect Dis 2022;28:2078–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adler H, Gould S, Hine P, et al. NHS England High Consequence Infectious Diseases (Airborne) Network. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis 2022;22:1153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Baetselier I, Van Dijck C, Kenyon C, et al.; ITM Monkeypox study group. Retrospective detection of asymptomatic monkeypox virus infections among male sexual health clinic attendees in Belgium. Nat Med 2022. Aug. 12 [Epub ahead of print]. doi: 10.1038/s41591-022-02004-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fine PE, Jezek Z, Grab B, et al. The transmission potential of monkeypox virus in human populations. Int J Epidemiol 1988;17:643–50. [DOI] [PubMed] [Google Scholar]
- 29.Monkeypox and smallpox vaccine guidance. Atlanta: Centers for Disease Control and Prevention; updated 2022 June 2. Available: https://www.cdc.gov/poxvirus/monkeypox/clinicians/smallpox-vaccine.html (accessed 2022 Oct. 30). [Google Scholar]
- 30.Nold A. Heterogeneity in disease-transmission modeling. Math Biosci 1980; 52:227–40. [Google Scholar]
- 31.Multi-jurisdictional monkeypox outbreak 2022: what we know so far. Toronto: Public Health Ontario; 2022. Available: https://www.publichealthontario.ca/-/media/Documents/M/2022/wwksf-multi-jurisdictional-monkeypox-outbreak-2022.pdf (accessed 2022 Oct. 30). [Google Scholar]
- 32.Gesink D, Wang S, Norwood T, et al. Spatial epidemiology of the syphilis epidemic in Toronto, Canada. Sex Transm Dis 2014;41:637–48. [DOI] [PubMed] [Google Scholar]
- 33.Hart TA, Moore DM, Noor SW, et al. Engage Study Team. Prevalence of HIV and sexually transmitted and blood-borne infections, and related preventive and risk behaviours, among gay, bisexual and other men who have sex with men in Montreal, Toronto and Vancouver: results from the Engage Study. Can J Public Health 2021;112:1020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott HM, Irvin R, Wilton L, et al. Sexual behavior and network characteristics and their association with bacterial sexually transmitted infections among Black men who have sex with men in the United States. PLoS One 2015;10:e0146025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gesink D, Wang S, Guimond T, et al. Conceptualizing geosexual archetypes: mapping the sexual travels and egocentric sexual networks of gay and bisexual men in Toronto, Canada. Sex Transm Dis 2018;45:368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millett GA, Peterson JL, Flores SA, et al. Comparisons of disparities and risks of HIV infection in Black and other men who have sex with men in Canada, UK, and USA: a meta-analysis. Lancet 2012;380:341–8. [DOI] [PubMed] [Google Scholar]
- 37.Doran J, Weatherburn P, Hickson F, et al. An update on the performance of STI services for gay and bisexual men across European cities: results from the 2017 European MSM Internet Survey. Sex Transm Infect 2021;97:201–8. [DOI] [PubMed] [Google Scholar]
- 38.Bogoch II, Creatore MI, Cetron MS, et al. Assessment of the potential for international dissemination of Ebola virus via commercial air travel during the 2014 west African outbreak. Lancet 2015;385:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson S-J, Cherutich P, Kilonzo N, et al. Maximising the effect of combination HIV prevention through prioritisation of the people and places in greatest need: a modelling study. Lancet 2014;384:249–56. [DOI] [PubMed] [Google Scholar]
- 40.Manirambona E, Shomuyiwa DO, Musa SS, et al. Monkeypox among men who have sex with men in Africa: the need for testing and vaccination beyond stigma. J Med Virol 2022. Sept. 2 [Epub ahead of print]. doi: 10.1002/jmv.28121. [DOI] [PubMed] [Google Scholar]
- 41.Yamey G, Garcia P, Hassan F, et al. It is not too late to achieve global COVID-19 vaccine equity. BMJ 2022;376:e070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zarocostas J. Monkeypox PHEIC decision hoped to spur the world to act. Lancet 2022;400:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cevik M, Baral SD, Crozier A, et al. Support for self-isolation is critical in COVID-19 response. BMJ 2021;372:n224. [DOI] [PubMed] [Google Scholar]
- 44.Baral S, Logie CH, Grosso A, et al. Modified social ecological model: a tool to guide the assessment of the risks and risk contexts of HIV epidemics. BMC Public Health 2013;13:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cevik M, Baral SD. Networks of SARS-CoV-2 transmission. Science 2021;373:162–3. [DOI] [PubMed] [Google Scholar]
- 46.Frey SE, Winokur PL, Salata RA, et al. Safety and immunogenicity of IMVAMUNE® smallpox vaccine using different strategies for a post event scenario. Vaccine 2013;31:3025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Safety of smallpox vaccines: December 2015. Geneva: World Health Organization. Available: https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/smallpox-vaccines (accessed 2022 Oct. 30). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.