Abstract
Enteropathogenic Escherichia coli (EPEC) is a human pathogen that attaches to intestinal epithelial cells and causes chronic watery diarrhea. A close relative, enterohemorrhagic E. coli (EHEC), causes severe bloody diarrhea and hemolytic-uremic syndrome. Both pathogens insert a protein, Tir, into the host cell plasma membrane where it binds intimin, the outer membrane ligand of EPEC and EHEC. This interaction triggers a cascade of signaling events within the host cell and ultimately leads to the formation of an actin-rich pedestal upon which the pathogen resides. Pedestal formation is critical in mediating EPEC- and EHEC-induced diarrhea, yet very little is known about its composition and organization. In EPEC, pedestal formation requires Tir tyrosine 474 phosphorylation. In EHEC Tir is not tyrosine phosphorylated, yet the pedestals appear similar. The composition of the EPEC and EHEC pedestals was analyzed by examining numerous cytoskeletal, signaling, and adapter proteins. Of the 25 proteins examined, only two, calpactin and CD44, were recruited to the site of bacterial attachment independently of Tir. Several others, including ezrin, talin, gelsolin, and tropomyosin, were recruited to the site of EPEC attachment independently of Tir tyrosine 474 phosphorylation but required Tir in the host membrane. The remaining proteins were recruited to the pedestal in a manner dependent on Tir tyrosine phosphorylation or were not recruited at all. Differences were also found between the EPEC and EHEC pedestals: the adapter proteins Grb2 and CrkII were recruited to the EPEC pedestal but were absent in the EHEC pedestal. These results demonstrate that although EPEC and EHEC recruit similar cytoskeletal proteins, there are also significant differences in pedestal composition.
Enteropathogenic Escherichia coli (EPEC) is a gram-negative pathogen that causes chronic, watery diarrhea in humans, primarily young children and infants (25). It belongs to a family of pathogens that cause focused actin accumulation beneath the site of bacterial attachment. Another member of this family is enterohemorrhagic E. coli (EHEC), the causative agent of hemolytic-uremic syndrome (often referred to as “hamburger disease”). EPEC attaches to the host intestinal epithelial cell in clusters, or microcolonies, in a process referred to as localized adherence. EHEC, however, does not form microcolonies during infection.
Following initial adherence to the epithelial cells, EPEC and EHEC secrete virulence factors, Esps (E. coli-secreted proteins), via a specialized type III secretion system (18). Several of the Esps are delivered directly into the host cell, including EspB, EspD, and Tir (22, 42, 43). The Esps and the secretion system are encoded in a chromosomal pathogenicity island called the locus of enterocyte effacement (28). Secretion of the EPEC and EHEC virulence factors leads to effacement of the microvillus structure and reorganization of the actin cytoskeleton to form a pedestal-like structure, the attaching and effacing (AE) lesion (23, 30, 39). AE lesion formation is critical in mediating diarrhea production in the host, but its exact role in disease is unknown. It may lead to a loss in absorptive intestinal surfaces, resulting in ionic imbalance and diarrhea production.
Among the bacterial factors delivered to the host cell is a protein called the translocated intimin receptor (Tir). EPEC Tir appears as a 90-kDa tyrosine-phosphorylated protein in the host cell plasma membrane, where it functions as the receptor for the EPEC outer membrane ligand, intimin (22). EHEC Tir is phosphorylated, presumably on serine and threonine residues, but not on tyrosine residues (8). Tir-intimin interactions lead to intimate bacterial attachment to the host cell and AE lesions.
Tir is comprised of two transmembrane domains, an extracellular intimin binding domain and two intracellular domains corresponding to the N and C termini (7, 21). The C terminus of EPEC Tir contains several tyrosine residues, one of which (tyrosine 474) is essential in pedestal formation but not in Tir delivery to the host membrane (21). EHEC Tir is not tyrosine phosphorylated but still rearranges actin to form a functional pedestal.
Upon Tir insertion in the host cell plasma membrane, several cytoskeletal proteins are recruited to the site of EPEC attachment. These include α-actinin, ezrin, cortactin, talin, fimbrin, vasodilator-stimulated phosphoprotein (VASP), villin, neural-Wiskott-Aldrich syndrome protein (N-WASP), and the actin-related protein 2 and 3 (Arp2/3) complex (1, 5, 11, 14, 20, 40). N-WASP and the Arp2/3 complex are essential for pedestal formation (20). α-Actinin has recently been shown to bind Tir directly at its N terminus independently of Tir phosphorylation (14) and may function to link Tir directly to the actin cytoskeleton. Little, however, is known about the cytoskeletal composition of the EHEC pedestal. Cortactin, α-actinin, and actin are the only proteins to date shown to be specifically recruited to the EHEC pedestal (5, 17).
In this study, 25 cytoskeletal, signaling, and adapter proteins were screened for recruitment to EPEC and EHEC pedestals, and the specific role of Tir and its tyrosine phosphorylation was also examined. A comparison of the EPEC and EHEC pedestal composition illustrates that although the pedestals are similar, there are also significant differences.
MATERIALS AND METHODS
Cell culture and bacterial growth.
HeLa cells, a human epithelial cell line (CCL2; American Type Culture Collection), were grown in Dulbecco's minimal Eagle medium (DMEM) supplemented with 10% fetal calf serum at 37°C in a humidified atmosphere with 5% CO2. The EPEC strains used in this study were E2348/69, Δtir (E2348/69 with a tir deletion), and Δtir complemented with pACYC184tirY474F. The EHEC strain used was 86-24 (serotype O157:H7). All EPEC and EHEC strains were grown in Luria-Bertani broth at 37°C in overnight cultures without shaking.
Immunofluorescence.
HeLa cells were seeded onto 12-mm-diameter coverslips at a density of 2 × 104 cells/ml. The following day, they were infected with 1 μl of an overnight EPEC culture (for 3 h) or EHEC (for 4 h) per ml in DMEM at 37°C and 5% CO2. The DMEM was then changed, and the remaining adherent bacteria were allowed to infect for two more hours. Following infection, the coverslips were washed three times with phosphate-buffered saline (PBS) and fixed with 2.5% paraformaldehyde. The coverslips were washed extensively after fixing, and the cells were permeabilized with 0.5% Triton X-100 in PBS. Following permeabilization, the cells were washed with 0.1% Triton X-100 in PBS, blocked with 10% normal goat serum in PBS, and then probed with either monoclonal Tir (2A8), paxillin (Transduction Labs), VASP (Transduction Labs), gelsolin (Sigma), Shp1 (Transduction Labs), ezrin (Sigma), talin (Sigma), p130cas (Sigma), cortactin (Upstate Biotechnology), zyxin (a kind gift from J. Wehland), calpactin (Signal Transduction Labs), tropomyosin (Sigma), vinculin (Sigma), or focal adhesion kinase (FAK) (Transduction Labs) or polyclonal CD44 (Transduction labs), lipoma-preferred partner (LPP) (a kind gift from R. Golsteyn), N-WASP (a kind gift from Jeffery Peterson), Arp3 (a kind gift from Edith Gouin), actin depolymerizing factor (ADF/cofilin) (Cytoskeleton Inc.), CrkII (Santa Cruz), Grb2 (Santa Cruz), or Shc (Upstate Biotechnology). Following the primary antibody, the cells were washed extensively with 0.1% Triton X-100 in PBS and probed with Alexa dye-conjugated antibodies, Alexa-conjugated phalloidin to detect actin (Molecular Probes), and 4′,6′-diamidino-2-phenylindole (DAPI) (1 μg/ml; Sigma) to stain the bacterial and host cell DNA. The coverslips were mounted in Mowiol (Aldrich) and viewed at 350, 488, and 594 nm on a Zeiss Axiophot epifluorescence microscope.
RESULTS
EPEC recruits several cytoskeletal and signaling proteins to the pedestal.
HeLa cells infected with EPEC formed elongated pedestals ranging between 1 and 3 μm in length. They were prepared for immunofluorescence and probed for cytoskeletal and signaling proteins. Of the proteins tested, the following were found to be in the pedestal: CrkII, Grb2, ADF/cofilin, LPP, p130cas, Shc, gelsolin, CD44, calpactin, zyxin, and vinculin (Fig. 1A to K). Other host proteins that were not recruited include β1 and α5 integrin, pp60src, FAK, and Shp (Fig. 1 and data not shown).
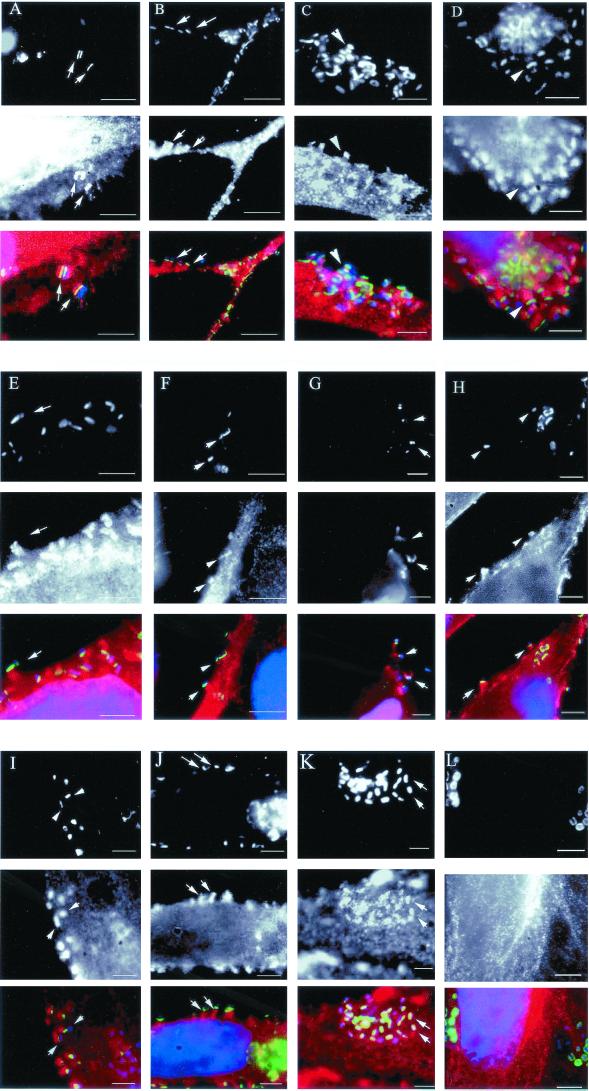
FIG. 1.
Cytoskeletal proteins recruited to the EPEC pedestal. HeLa cells were infected with EPEC for 5 h. Upon Tir translocation to the cells (A to K, top panels), numerous cytoskeletal proteins were recruited to the pedestal (A to I, middle panels), including CrkII (A), Grb2 (B), cofilin (C), LPP (D), p130cas (E), Shc (F), gelsolin (G), CD44 (H), calpactin (I), zyxin (J), and vinculin (K). In contrast, pp60src (L) was not recruited to the pedestal. The bottom panels represent a merger of Tir (green), the cytoskeletal protein (red), and DAPI-stained EPEC (blue). Bars, 5 μm. Arrows denote recruitment of cytoskeletal proteins to EPEC pedestals.
CD44 and calpactin are recruited independently of Tir delivery.
HeLa cells were infected with EPECΔtir, which delivers Esps to the host cell but is incapable of forming pedestals due to the absence of Tir. Of all the proteins tested, only CD44 and calpactin were localized to the site of bacterial adherence, indicating that these proteins are recruited independently of Tir (Fig. 2A to D). All other proteins had a staining pattern similar to that for uninfected cells (data not shown). To address the function of CD44 in the pedestal, CD44-deficient Swiss 3T3 fibroblasts were infected with EPEC for 5 h and prepared for immunofluorescence. Elongated EPEC pedestals were formed in the absence of CD44 as determined by actin and Tir staining and were indistinguishable from those formed in CD44-containing cells (data not shown).
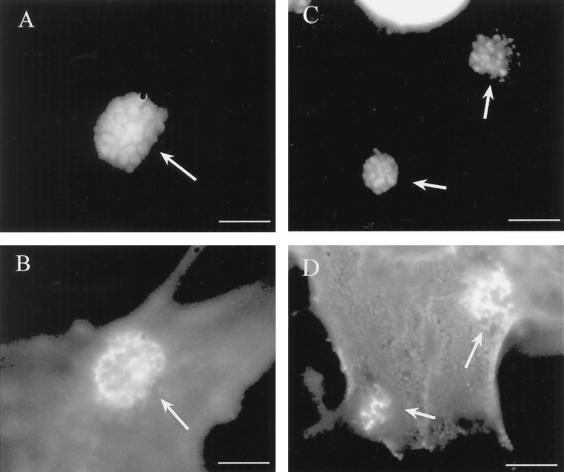
FIG. 2.
CD44 and calpactin are recruited to the site of EPEC adherence independently of Tir translocation. HeLa cells were infected with EPECΔtir for 5 h. EPECΔtir microcolonies were labeled by DAPI (A, C). CD44 (B) and calpactin (D) are recruited beneath EPECΔtir in a honeycomb pattern. Bars, 5 μm. Arrows denote sites of CD44 or calpactin recruitment beneath adherent EPEC microcolonies.
Gelsolin, tropomyosin, ezrin, α-actinin, and talin are recruited to EPEC independently of Tir tyrosine phosphorylation.
HeLa cells were infected with EPECΔtir/tirY474F, an EPEC strain capable of delivering Tir to the host but lacking the tyrosine residue that is phosphorylated in the host cell. Phosphorylation of EPEC Tir tyrosine 474 is critical for pedestal formation. Of the proteins tested, only gelsolin, tropomyosin, ezrin, and talin were recruited to the site of EPEC adherence without Tir tyrosine phosphorylation (Fig. 3A to D). A previous study has shown α-actinin to have a similar recruitment pattern independent of Tir tyrosine phosphorylation (14). The other proteins had a staining similar to that of uninfected cells, as seen with CrkII staining (Fig. 3F). α-Actinin has been shown to directly bind the N terminus of Tir, but its role in pedestal formation was unknown (13, 14). Overexpression of α-actinin in EPEC-infected HeLa cells resulted in a twofold increase in pedestal length over that of untransfected cells or cells transfected with vector alone (data not shown).
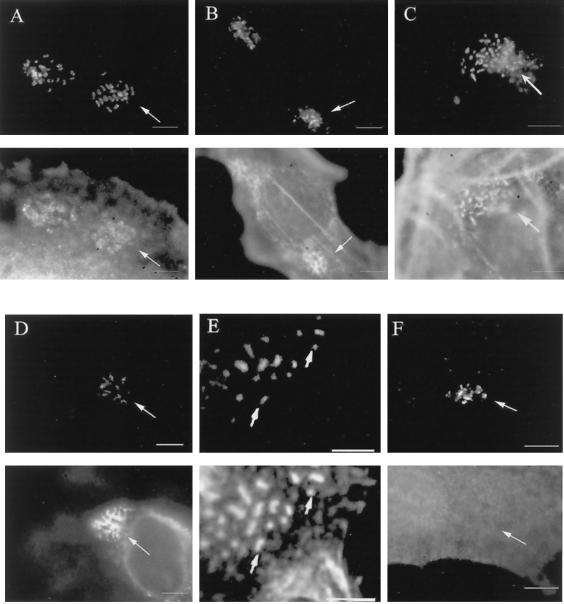
FIG. 3.
Talin, tropomyosin, gelsolin, ezrin, and α-actinin are recruited to the site of EPEC adherence independently of Tir tyrosine phosphorylation. HeLa cells were infected with EPECΔtir/tirY474F for 5 h. Upon TirY474F translocation to the cells (A to F, top panels), talin (A, bottom panel), tropomyosin (B, bottom panel), gelsolin (C, bottom panel), ezrin (D, bottom panel), and α-actinin (E, bottom panel) are recruited to the site of EPEC adherence. All other proteins were not recruited, as exemplified by CrkII (F, bottom panel). Bars, 5 μm. Arrows denote sites of recruitment of cytoskeletal proteins with TirY474F beneath adherent EPEC.
EHEC does not recruit the adapter protein CrkII or Grb2 to its pedestal.
HeLa cells were infected with EHEC, and pedestals were examined for recruitment of cytoskeletal and signaling proteins. Of the proteins tested, only CrkII and Grb2 differed, being recruited to EPEC but not EHEC pedestals (Fig. 4). All other proteins were recruited similarly beneath both EPEC and EHEC, indicating the similar but not identical cytoskeletal composition of these pedestals.
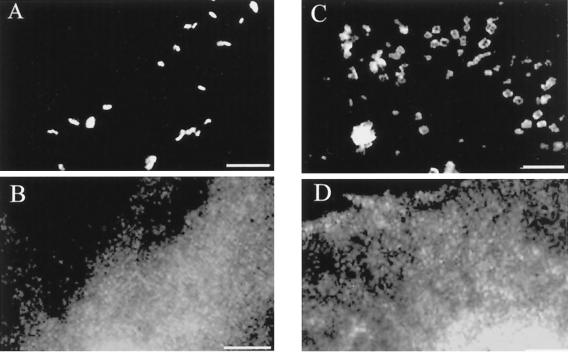
FIG. 4.
EHEC does not recruit the adapter proteins CrkII and Grb2 to the EHEC pedestal. HeLa cells were infected with EHEC for 6 h. Upon translocation of Tir to the cell (A, C), CrkII (B) and Grb2 (D) were not rearranged as seen during EPEC infection (see Fig. 1). Bars, 5 μm.
DISCUSSION
Since the initial survey of cytoskeletal proteins recruited to the site of EPEC attachment was performed (11), our knowledge of EPEC and the cytoskeleton has expanded significantly. The identification of Tir, a bacterial protein, as the receptor for EPEC intimate adherence has allowed researchers to dissect pedestal formation even further through genetic manipulation of Tir (22). Elongated pedestal formation is not only dependent on EPEC Tir but on its tyrosine phosphorylation by a currently unidentified kinase. Additionally, the discovery that EHEC Tir is not tyrosine phosphorylated warrants comparison of these two pedestals (8, 9).
Pathogenic E. coli may be used as a model system to study signaling to the actin cytoskeleton across the plasma membrane in response to external stimuli. Indeed, there are many parallels between EPEC pedestal formation and the formation of focal adhesions. Focal adhesions are found at sites of eukaryotic cell attachment to the extracellular matrix (ECM). This attachment is mediated through a family of integral membrane proteins called integrins, which link the ECM to the cytoskeleton. Many of the cytoskeletal and signaling proteins that were examined in this study are also involved in focal adhesion formation (Table 1). Both α5 and β1 integrins were screened in this study, but they were not found in the EPEC or EHEC pedestal. This was not unexpected, as a previous report suggested that β1 integrins play no role in EPEC infection (26). However, it is interesting that so many focal adhesion proteins were localized to the pedestal in the absence of β1 integrins. This suggests that Tir might function much like an integrin. There are several lines of evidence that support this hypothesis. First, both Tir and β1 integrin span the plasma membrane and, upon binding of their extracellular ligand, signal to the actin cytoskeleton. Secondly, Tir binds α-actinin and talin directly, as do β1 integrins (13, 14, 34). This interaction occurs at the N terminus of Tir independently of Tir tyrosine phosphorylation. Intimin also binds β1 integrins directly, although the function of this interaction is unclear (12). The Tir intimin binding area (or intimin binding domain) is homologous to the ECM binding domain of integrins (21), which may explain why intimin can bind β1 integrins. Additionally, there is a high degree of homology between Yersinia invasins and E. coli intimins (29). Yersinia invasin binds β1 integrin with a very high affinity during invasion of host epithelial cells (16), much like intimin binding to Tir (27).
TABLE 1.
Host proteins characterized in E. coli pedestala
| Protein | Presence in EPEC pedestals | Presence in EHEC pedestals | Tir dependence | Tir PY dependence | Location in pedestal | Reference or source |
|---|---|---|---|---|---|---|
| Actin-associated proteins | ||||||
| Arp2/3 | + | + | + | + | Tip and length | This study; 20 |
| α-Actinin | + | + | + | − | Tip and length | 14 |
| Calpactin | + | + | − | − | Tip and length | This study |
| Cofilin | + | + | + | − | Tip and length | This study |
| Cortactin | + | + | − | − | Tip | This study; 5 |
| Ezrin | + | + | + | − | Along length | This study; 11 |
| Gelsolin | + | + | + | − | Tip and length | This study |
| LPP | + | + | + | + | Along length | This study |
| N-WASP | + | + | + | + | Tip | This study; 20 |
| Paxillin | − | − | − | − | − | This study |
| p130cas | + | + | + | + | Tip and length | This study |
| Talin | + | + | + | − | Along length | This study; 11 |
| Tropomyosin | + | + | + | − | Along length | This study; 40 |
| VASP | + | + | + | + | Tip | This study; 14 |
| Vinculin | + | + | + | + | Along length | This study; 11 |
| Zyxin | + | + | + | + | Along length | This study |
| Membrane proteins | ||||||
| α5 integrin | − | − | − | − | − | This study |
| β1 integrin | − | − | − | − | − | This study; 26 |
| CD44 | + | + | − | − | Tip | This study |
| Adapter proteins | ||||||
| CrkII | + | − | + | + | Tip and length | This study |
| Grb2 | + | − | + | + | Along length | This study |
| Shc | + | + | + | + | Along length | This study |
| Kinases/phosphatases | ||||||
| pp60src | − | − | − | − | − | This study |
| Shp | − | − | − | − | − | This study |
| FAK | − | − | − | − | − | This study |
+, protein recruitment to the site of EPEC or EHEC adherence. −, no rearrangement of the cytoskeletal protein during EPEC/EHEC infection as determined by immunofluorescence.
In addition to α-actinin and talin, several other proteins found in focal adhesions are recruited to the EPEC pedestal, including zyxin, LPP, vinculin, VASP, and p130cas. α-Actinin and talin mediate direct linkages with β1 integrins and actin (14, 34, 36). Both also function to bind and cross-link actin (19). Vinculin does not directly bind to integrins but links actin filaments to integrins through other proteins, such as talin (4). VASP targets profilin and F-actin to the site of focal adhesions (37). To date, profilin has not been seen in the pedestal either by indirect immunofluorescence or by green fluorescent protein-profilin transfections (D. L. Goosney, unpublished observation). Zyxin and its homologue, LPP, function in focal adhesions and cell-cell contacts (35, 38). Both shuttle between the cytoplasm and the nucleus and have transcriptional activation capacity (32, 35). The primary difference between them in cultured epithelial cells is that zyxin colocalizes with stress fibers as well as contact sites, whereas LPP is found only in the sites of contact (35). Both proteins bind and presumably target VASP to focal adhesions.
p130cas is a multidomained adapter-type protein found in focal adhesions. In addition to being a substrate for FAK and src, it can interact with the adapter molecule Crk (3, 15). It is surprising that pp60src and FAK were not localized to the pedestal during EPEC or EHEC infection. This could be simply due to problems detecting the proteins with the antibodies available or could suggest that another signaling pathway is being used by the pathogens to initiate pedestal formation. Alternatively, Tir tyrosine phosphorylation may be occurring only transiently; therefore, recruitment of the kinase may be missed in this screening.
Small GTP-binding proteins are essential for focal adhesion and stress fiber formation. Rho activation during adhesion results in activation of phosphatidylinositol 3-kinase (31, 44), which plays a role in restructuring the focal adhesion. Several reports indicate that EPEC does not use the small GTPases during pedestal formation (2, 10). Interestingly, N-WASP is recruited to EPEC pedestals via its GTPase binding domain (20). Deletion of this domain results in lack of focusing of N-WASP at the tip. Preliminary studies indicate that a compactin- and ToxB-insensitive GTPase, Chp, or Chp-like protein may be the protein responsible for directing pedestal formation, as it localizes to the tip of the pedestal (20).
Several other proteins were recruited to the pedestal in addition to those normally found in focal adhesions—CD44, calpactin, ezrin, ADF/cofilin, gelsolin, Shc, CrkII, Grb2, and tropomyosin—illustrating differences between the two structures. CD44 is a membrane receptor for hyaluronic acid, whereas calpactin (p11) acts together with annexin II at the plasma membrane to function in membrane fusion and host cell exocytosis. Both CD44 and the annexin II-p11 complex colocalize in lipid rafts (33), which act to concentrate signaling proteins in various cellular functions, including signal transduction and protein sorting. Although CD44 and calpactin are recruited independently of Tir, they may be brought to the site of EPEC attachment through other Esps or unknown bacterial factors and may function in earlier signaling events during EPEC infection. CD44 itself does not function in pedestal formation, as EPEC-infected CD44-deficient cell lines still form an elongated pedestal. It is tempting to speculate that CD44 and calpactin may function in lipid rafts during initial EPEC signaling.
That CD44 was recruited but is not functional in the pedestal is an important caveat. It demonstrates that not every protein recruited to the site of EPEC attachment is necessarily functional in pedestal formation. Such proteins may function in other aspects of the infection process (for example, host recognition or signaling to the nucleus).
Once Tir is delivered to the host cell, it is tyrosine phosphorylated in EPEC but not in EHEC. We addressed the role of EPEC Tir tyrosine phosphorylation by characterizing cytoskeletal proteins recruited to the site of bacterial attachment independently of this phosphorylation event. It has been previously shown that α-actinin and talin bind to the N terminus of Tir independently of its tyrosine phosphorylation in the cell (13, 14; Goosney, unpublished). In this study, we show that ezrin, talin, gelsolin, and tropomyosin are also recruited in the absence of tyrosine phosphorylation. α-Actinin, talin, and ezrin are all proteins that link the actin cytoskeleton to the plasma membrane. As mentioned previously, talin and α-actinin link to integrins in focal adhesions and bind Tir directly in pedestals. Ezrin links the cytoskeleton to the plasma membrane in structures such as microvilli and microspikes. These three proteins may be involved in meditating a stable anchor from EPEC to the host cell cytoskeleton.
Gelsolin is a Ca2+-sensitive, F-actin-severing protein that also caps barbed ends of actin filaments and functions to increase free pointed ends (41). Gelsolin may be recruited early to the site of EPEC attachment to provide EPEC with a source of free-end filaments from which to build the pedestal. Tropomyosin is an actin binding protein that can be targeted to sites of active actin rearrangements by gelsolin (24). The wide range of proteins recruited either dependently or independently of Tir tyrosine phosphorylation suggests that Tir may have more than one function in the host cell—recruiting proteins that serve to stabilize Tir interactions with the cytoskeleton and recruiting those that can provide new actin filaments for pedestal formation.
Given the differences in recruitment between phosphorylated and unphosphorylated Tir, we also investigated the composition of the EHEC pedestal. EHEC triggers an elongated pedestal similar to that of EPEC but without Tir tyrosine phosphorylation. All proteins were recruited and localized to the tip or the length of the pedestal in the same manner as EPEC, with the exception of the adapter proteins Grb2 and CrkII. Adapter proteins mediate protein-protein interactions through their multiple SH2 and SH3 binding domains. Grb2 and CrkII were recruited along the length of the EPEC pedestal but were not in the EHEC pedestal. Both are comprised of two SH3 domains and one SH2 domain, which allow them to form multimeric complexes involved in signaling and cytoskeletal rearrangements. Grb2 was recently identified as an activator of the N-WASP–Arp2/3 cascade (6). It is tempting to speculate that EPEC uses one of these adapters to recruit N-WASP and Arp2/3 to the pedestal.
This is the first reported difference between EPEC and EHEC pedestals and indicates that the role of tyrosine phosphorylation may be related to recruitment of adapter proteins to the site of bacterial adherence. EHEC may build a slightly different pedestal independent of known host adapters, perhaps by delivering its own bacterial adapter to the host cell. Alternatively, one or more adapters may interact with the tyrosine-phosphorylated residue of EPEC to initiate pedestal formation, whereas this interaction is bypassed in EHEC pedestals.
The results presented here provide significant insight into how the EPEC and EHEC pedestals are formed during infection. CD44 and calpactin were recruited independently of Tir in the host cell, suggesting that they are recruited before pedestal formation occurs. Other proteins, including α-actinin, ezrin, talin, gelsolin, and tropomyosin, are recruited to the site of EPEC attachment independently of Tir tyrosine phosphorylation and may link Tir directly to the cytoskeleton. Tir tyrosine phosphorylation may then recruit additional factors, such as N-WASP and the Arp2/3 complex, which would target actin-polymerizing machinery to the plasma membrane, initiating full pedestal formation. Although EHEC recruited most of the same proteins as EPEC, there were some key differences, namely in the adapter proteins Grb2 and CrkII. EPEC may require these adapter proteins to bind tyrosine-phosphorylated Tir and recruit additional factors to the pedestal, whereas EHEC may bypass this step as it does not require tyrosine phosphorylation of Tir. The similarities of both pedestals with focal adhesions suggest that they are a useful tool for characterizing localized actin rearrangements at the plasma membrane.
ACKNOWLEDGMENTS
We are grateful to J. Wehland for the anti-zyxin antibody, Roy Golsteyn for the anti-LPP antibody, Edith Gouin for the Arp3 antibody, Jeff Petterson for the N-WASP antibody, and Samantha Gruenheid for critical reading of the manuscript.
This work was supported by a Natural Sciences and Engineering Research Council Scholarship to D.L.G. and a Canadian Institute of Health Research Operating Grant and Howard Hughes International Research Fellowship to B.B.F. B.B.F. is a CIHR Scientist.
REFERENCES
- 1.Adam T, Arpin M, Prevost M C, Gounon P, Sansonetti P J. Cytoskeletal rearrangements and the functional role of T-plastin during entry of Shigella flexneri into HeLa cells. J Cell Biol. 1995;129:367–381. doi: 10.1083/jcb.129.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Ami G, Ozeri V, Hanski E, Hofmann F, Aktories K, Hahn K M, Bokoch G M, Rosenshine I. Agents that inhibit Rho, Rac, and Cdc42 do not block formation of actin pedestals in HeLa cells infected with enteropathogenic Escherichia coli. Infect Immun. 1998;66:1755–1758. doi: 10.1128/iai.66.4.1755-1758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnham R M, Harte M T, Richardson A, Parsons J T, Bouton A H. The identification of p130cas-binding proteins and their role in cellular transformation. Oncogene. 1996;12:2467–2472. [PubMed] [Google Scholar]
- 4.Burridge K, Mangeat P. An interaction between vinculin and talin. Nature. 1984;308:744–746. doi: 10.1038/308744a0. [DOI] [PubMed] [Google Scholar]
- 5.Cantarelli V V, Takahashi A, Akeda Y, Nagayama K, Honda T. Interaction of enteropathogenic or enterohemorrhagic Escherichia coli with HeLa cells results in translocation of cortactin to the bacterial adherence site. Infect Immun. 2000;68:382–386. doi: 10.1128/iai.68.1.382-386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlier M F, Nioche P, Broutin-L'Hermite I, Boujemaa R, Le Clainche C, Egile C, Garbay C, Ducruix A, Sansonetti P, Pantaloni D. GRB2 links signaling to actin assembly by enhancing interaction of neural Wiskott-Aldrich syndrome protein (N-WASp) with actin-related protein (ARP2/3) complex. J Biol Chem. 2000;275:21946–21952. doi: 10.1074/jbc.M000687200. [DOI] [PubMed] [Google Scholar]
- 7.de Grado M, Abe A, Gauthier A, Steele-Mortimer O, DeVinney R, Finlay B B. Identification of the intimin-binding domain of Tir of enteropathogenic Escherichia coli. Cell Microbiol. 1999;1:7–19. doi: 10.1046/j.1462-5822.1999.00001.x. [DOI] [PubMed] [Google Scholar]
- 8.Deibel C, Kramer S, Chakraborty T, Ebel F. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol Microbiol. 1998;28:463–474. doi: 10.1046/j.1365-2958.1998.00798.x. [DOI] [PubMed] [Google Scholar]
- 9.DeVinney R, Stein M, Reinscheid D, Abe A, Ruschkowski S, Finlay B B. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect Immun. 1999;67:2389–2398. doi: 10.1128/iai.67.5.2389-2398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebel F, von Eichel-Streiber C, Rohde M, Chakraborty T. Small GTP-binding proteins of the Rho- and Ras-subfamilies are not involved in the actin rearrangements induced by attaching and effacing Escherichia coli. FEMS Microbiol Lett. 1998;163:107–112. doi: 10.1111/j.1574-6968.1998.tb13033.x. [DOI] [PubMed] [Google Scholar]
- 11.Finlay B B, Rosenshine I, Donnenberg M S, Kaper J B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frankel G, Lider O, Hershkoviz R, Mould A P, Kachalsky S G, Candy D C A, Cahalon L, Humphries M J, Dougan G. The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to beta1 integrins. J Biol Chem. 1996;271:20359–20364. doi: 10.1074/jbc.271.34.20359. [DOI] [PubMed] [Google Scholar]
- 13.Freeman N L, Zurawski D V, Chowrashi P, Ayoob J C, Huang L, Mittal B, Sanger J M, Sanger J W. Interaction of the enteropathogenic Escherichia coli protein, translocated intimin receptor (Tir), with focal adhesion proteins. Cell Motil Cytoskelet. 2000;47:307–318. doi: 10.1002/1097-0169(200012)47:4<307::AID-CM5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 14.Goosney D, DeVinney R, Pfuetzner R, Frey E, Strynadka N C, Finlay B B. Enteropathogenic E. coli translocated intimin receptor, Tir, interacts directly with alpha-actinin. Curr Biol. 2000;10:735–738. doi: 10.1016/s0960-9822(00)00543-1. [DOI] [PubMed] [Google Scholar]
- 15.Harte T M, Hildebrand J D, Burnham M R, Bouton A H, Parsons J T. p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J Biol Chem. 1996;271:13649–13655. doi: 10.1074/jbc.271.23.13649. [DOI] [PubMed] [Google Scholar]
- 16.Isberg R R, Leong J M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 17.Ismaili A, Philpott D J, Dytoc M T, Soni R, Ratnam S, Sherman P M. Alpha-actinin accumulation in epithelial cells infected with attaching and effacing gastrointestinal pathogens. J Infect Dis. 1995;172:1393–1396. doi: 10.1093/infdis/172.5.1393. [DOI] [PubMed] [Google Scholar]
- 18.Jarvis K G, Giron J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jockusch B M, Isenberg G. Interaction of alpha-actinin and vinculin with actin: opposite effects on filament network formation. Proc Natl Acad Sci USA. 1981;78:3005–3009. doi: 10.1073/pnas.78.5.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalman D, Weiner O D, Goosney D L, Sedat J W, Finlay B B, Abo A, Bishop J M. Enteropathogenic E. coli acts through WASP and Arp2/3 complex to form actin pedestals. Nat Cell Biol. 1999;1:389–391. doi: 10.1038/14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenny B. Phosphorylation of tyrosine 474 of the enteropathogenic Escherichia coli (EPEC) Tir receptor molecule is essential for actin nucleating activity and is preceded by additional host modifications. Mol Microbiol. 1999;31:1229–1241. doi: 10.1046/j.1365-2958.1999.01265.x. [DOI] [PubMed] [Google Scholar]
- 22.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 23.Knutton S, Lloyd D R, McNeish A S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987;55:69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koepf E K, Burtnick L D. Interaction of plasma gelsolin with tropomyosin. FEBS Lett. 1992;309:56–58. doi: 10.1016/0014-5793(92)80738-3. [DOI] [PubMed] [Google Scholar]
- 25.Levine M M, Edelman R. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol Rev. 1984;6:31–51. doi: 10.1093/oxfordjournals.epirev.a036274. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Magoun L, Leong J M. β1-chain integrins are not essential for intimin-mediated host cell attachment and enteropathogenic Escherichia coli-induced actin condensation. Infect Immun. 1999;67:2045–2049. doi: 10.1128/iai.67.4.2045-2049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo Y, Frey E A, Pfuetzner R A, Creagh A L, Knoechel D G, Haynes C A, Finlay B B, Strynadka N C. Crystal structure of enteropathogenic Escherichia coli intimin-receptor complex. Nature. 2000;405:1073–1077. doi: 10.1038/35016618. [DOI] [PubMed] [Google Scholar]
- 28.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGraw E A, Li J, Selander R K, Whittam T S. Molecular evolution and mosaic structure of alpha, beta, and gamma intimins of pathogenic Escherichia coli. Mol Biol Evol. 1999;16:12–22. doi: 10.1093/oxfordjournals.molbev.a026032. [DOI] [PubMed] [Google Scholar]
- 30.Moon H W, Whipp S C, Argenzio R A, Levine M M, Giannella R A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami H, Iwashita T, Asai N, Iwata Y, Narumiya S, Takahashi M. Rho-dependent and -independent tyrosine phosphorylation of focal adhesion kinase, paxillin and p130Cas mediated by Ret kinase. Oncogene. 1999;18:1975–1982. doi: 10.1038/sj.onc.1202514. [DOI] [PubMed] [Google Scholar]
- 32.Nix D A, Beckerle M C. Nuclear-cytoplasmic shuttling of the focal contact protein, zyxin: a potential mechanism for communication between sites of cell adhesion and the nucleus. J Cell Biol. 1997;138:1139–1147. doi: 10.1083/jcb.138.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliferenko S, Paiha K, Harder T, Gerke V, Schwarzler C, Schwarz H, Beug H, Gunthert U, Huber L A. Analysis of CD44-containing lipid rafts: recruitment of annexin II and stabilization by the actin cytoskeleton. J Cell Biol. 1999;146:843–854. doi: 10.1083/jcb.146.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otey C A, Pavalko F M, Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990;111:721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petit M M, Fradelizi J, Golsteyn R M, Ayoubi T A, Menichi B, Louvard D, Van de Ven W J, Friederich E. LPP, an actin cytoskeleton protein related to zyxin, harbors a nuclear export signal and transcriptional activation capacity. Mol Biol Cell. 2000;11:117–129. doi: 10.1091/mbc.11.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rees D J, Ades S E, Singer S J, Hynes R O. Sequence and domain structure of talin. Nature. 1990;347:685–689. doi: 10.1038/347685a0. [DOI] [PubMed] [Google Scholar]
- 37.Reinhard M, Giehl K, Abel K, Haffner C, Jarchau T, Hoppe V, Jockusch B M, Walter U. The proline-rich focal adhesion and microfilament protein VASP is a ligand for profilins. EMBO J. 1995;14:1583–1589. doi: 10.1002/j.1460-2075.1995.tb07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reinhard M, Jouvenal K, Tripier D, Walter U. Identification, purification, and characterization of a zyxin-related protein that binds the focal adhesion and microfilament protein VASP (vasodilator-stimulated phosphoprotein) Proc Natl Acad Sci USA. 1995;92:7956–7960. doi: 10.1073/pnas.92.17.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 40.Sanger J M, Chang R, Ashton F, Kaper J B, Sanger J W. Novel form of actin-based motility transports bacteria on the surfaces of infected cells. Cell Motil Cytoskelet. 1996;34:279–287. doi: 10.1002/(SICI)1097-0169(1996)34:4<279::AID-CM3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Southwick F S. Gelsolin and ADF/cofilin enhance the actin dynamics of motile cells. Proc Natl Acad Sci USA. 2000;97:6936–6938. doi: 10.1073/pnas.97.13.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor K A, O'Connell C B, Luther P W, Donnenberg M S. The EspB protein of enteropathogenic Escherichia coli is targeted to the cytoplasm of infected HeLa cells. Infect Immun. 1998;66:5501–5507. doi: 10.1128/iai.66.11.5501-5507.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wachter C, Beinke C, Mattes M, Schmidt M A. Insertion of EspD into epithelial target cell membranes by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1999;31:1695–1707. doi: 10.1046/j.1365-2958.1999.01303.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, King W G, Dillon S, Hall A, Feig L, Rittenhouse S E. Activation of platelet phosphatidylinositide 3-kinase requires the small GTP-binding protein Rho. J Biol Chem. 1993;268:22251–22254. [PubMed] [Google Scholar]