BACKGROUND:
The exact relation between anastomotic height after rectal cancer surgery and postoperative bowel function problems has not been investigated in the long term, resulting in ineffective treatment.
OBJECTIVE:
The goal of this study was to determine the effect of anastomotic height on long-term bowel function and generic quality of life.
DESIGN:
This was a multicenter, cross-sectional study.
SETTINGS:
Seven hospitals in the north of the Netherlands participated.
PATIENTS:
All patients who underwent rectal cancer surgery between 2009 and 2015 in participating hospitals received the validated Defecation and Fecal Continence and Short-Form 36 questionnaires. Deceased patients, patients with a permanent stoma or an anastomosis >15 cm from the anal verge, patients with intellectual disability, and patients living abroad were excluded.
MAIN OUTCOME MEASURES:
Primary outcomes were constipation (Rome IV), fecal incontinence (Rome IV), and major low anterior resection syndrome. Secondary outcomes were the generic quality of life scores.
RESULTS:
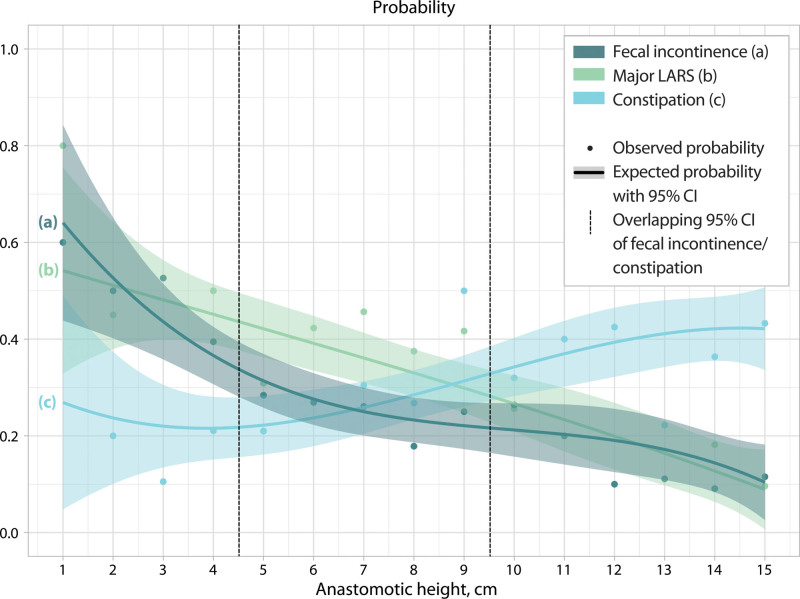
The study population (n = 630) had a median follow-up of 58.0 months. In multivariable analysis, constipation (OR = 1.08; 95% CI, 1.02-1.15; p = 0.011), fecal incontinence (OR = 0.91; 95% CI, 0.84-0.97; p = 0.006), and major low anterior resection syndrome (OR = 0.93; 95% CI, 0.87-0.99; p = 0.027), were significantly associated with anastomotic height. The curves illustrating the probability of constipation and fecal incontinence crossed at an anastomotic height of 7 cm, with 95% CIs overlapping between 4.5 and 9.5 cm. There was no relation between quality-of-life scores and anastomotic height.
LIMITATIONS:
The study is limited by its cross-sectional design.
CONCLUSIONS:
This study might serve as a guide for the clinician to effectively screen and treat fecal incontinence and constipation during patient follow-up after rectal cancer surgery. More attention should be paid to fecal incontinence in patients with an anastomosis below 4.5 cm and toward constipation in patients with an anastomosis above 9.5 cm. See Video Abstract at http://links.lww.com/DCR/B858.
LA ALTURA ANASTOMÓTICA ES UN INDICADOR VALIOSO DE LA FUNCIÓN INTESTINAL A LARGO PLAZO DESPUÉS DE LA CIRUGÍA PARA EL CÁNCER DE RECTO
ANTECEDENTES:
La relación exacta entre la altura anastomótica después de la cirugía de cáncer de recto y los problemas posoperatorios de la función intestinal no se ha investigado a largo plazo, lo que causa un tratamiento ineficaz.
OBJETIVO:
Determinar el efecto de la altura anastomótica sobre la función intestinal a largo plazo y la calidad de vida genérica.
DISEÑO:
Estudio multicéntrico transversal.
DISEÑO DEL ESTUDIO:
Participaron siete hospitales holandeses en el norte de los Países Bajos.
PACIENTES:
Todos los pacientes que se sometieron a cirugía de cáncer de recto entre 2009 y 2015 en los hospitales participantes recibieron los cuestionarios validados de Defecación y Continencia Fecal y Short-Form 36. Se excluyeron pacientes fallecidos, pacientes con estoma permanente o anastomosis > 15 cm del borde anal, discapacidad intelectual o residentes en el extranjero.
PRINCIPALES MEDIDAS DE RESULTADO:
Los resultados primarios fueron estreñimiento (Roma IV), incontinencia fecal (Roma IV) y síndrome de resección anterior baja mayor. Los resultados secundarios fueron las puntuaciones genéricas de calidad de vida.
RESULTADOS:
La población de estudio (N = 630) tuvo una mediana de seguimiento de 58.0 meses. En el análisis multivariable el estreñimiento (OR = 1,08, IC del 95%, 1,02-1,15, p = 0,011), incontinencia fecal (OR = 0,91, IC del 95%, 0,84–0,97, p = 0,006) y síndrome de resección anterior baja mayor (OR = 0,93, IC del 95%, 0,87–0,99, p = 0,027) se asociaron significativamente con la altura anastomótica. Las curvas que ilustran la probabilidad de estreñimiento e incontinencia fecal se cruzaron a una altura anastomótica de 7 cm, con IC del 95% superpuestos entre 4,5 y 9,5 cm. No hubo relación entre las puntuaciones de calidad de vida y la altura anastomótica.
LIMITACIONES:
El estudio está limitado por su diseño transversal.
CONCLUSIONES:
Este estudio podría servir como una guía para que el médico evalúe y trate eficazmente la incontinencia fecal y el estreñimiento durante el seguimiento de los pacientes después de la cirugía de cáncer de recto. Se debe prestar más atención a la incontinencia fecal en pacientes con anastomosis por debajo de 4,5 cm y al estreñimiento en pacientes con anastomosis por encima de 9,5 cm. Consulte Video Resumen en http://links.lww.com/DCR/B858. (Traducción—Dr. Yazmin Berrones-Medina)
Keywords: Anastomosis, Bowel dysfunction, Follow-up, Postoperative, Rectal cancer
Annually, more than 700,000 people worldwide receive a diagnosis of rectal cancer.1 During the past decade, the 5-year survival rate of rectal cancer has improved considerably.2 Consequently, optimal long-term bowel function has become an important health issue to a growing number of people.
Recent systematic reviews showed that years after surgery, 35% of patients suffer from fecal incontinence and 41% from major low anterior resection syndrome (LARS).3,4 Nevertheless, many clinicians still neglect to assess postoperative bowel function comprehensively during regular follow-up visits.5
An association between bowel function problems and anastomotic height after surgery for rectal cancer was suggested years ago.6–9 Regression analyses in more recent studies showed an increase in fecal incontinence or major LARS with anastomotic heights categorized below 3,10 6,11 7,12 and 1013 cm from the anal verge. Other studies advocated that anastomotic height neither influences fecal incontinence14,15 nor the LARS score.16,17 Why the association between anastomotic height and different bowel function problems is not universally acknowledged can be attributed to the random categorization of anastomotic height and the short follow-up time in most studies. To date, the exact relation between anastomotic heights and different validated bowel function scores has not been investigated long-term. This information, however, would guide the clinician during the follow-up of patients after surgery for rectal cancer. Given the profound impact on daily life of bowel function problems, we hypothesize that not only bowel function but also generic quality of life varies along with differences in anastomotic height.
Our primary aim was therefore to determine the effect of anastomotic heights on long-term bowel function and generic quality of life after surgery for rectal or rectosigmoid cancer.
MATERIALS AND METHODS
Study Design and Participants
This cross-sectional study was performed between October 2017 and December 2019 in 7 hospitals in the north of the Netherlands. Patients who had undergone a low anterior resection or anterior resection for rectal or rectosigmoid cancer between 2009 and 2015 were identified from the mandatory Dutch ColoRectal Audit (DCRA) registry. A low anterior resection or anterior resection was defined as a total mesorectal excision with coloanal or colorectal anastomosis <15 cm from the anal verge.18 Only patients without a permanent stoma or a previous primary colorectal tumor were included. Finally, deceased patients, intellectually disabled patients, patients <18 years at the time of surgery, and patients with an unknown or foreign residential address were excluded. Patients who gave their written informed consent were asked to complete the validated Defecation and Fecal Continence (DeFeC) and the Short-Form (SF) 36 questionnaires.19–21 The questionnaires were completed either digitally or on paper, depending on the patient’s preference. The study was conducted in accordance with the Medical Ethical Review Board of the University Medical Center Groningen (approval code: METc 2017/245).
Outcome Measures
The primary outcomes were constipation, fecal incontinence, and major LARS, which were assessed by the DeFeC questionnaire. It includes different commonly used scoring systems for bowel functions. Constipation was defined according to the Rome IV criteria,22 including at least 2 of the following symptoms: straining, hard or lumpy stool, sense of obstruction, incomplete defection, manual facilitation of defecation, and/or less than 3 stools per week. Additionally, as described in the criteria, patients were only classified as constipated if loose stools were rarely present without laxatives. Fecal incontinence was also defined according to the Rome IV criteria23 and described as recurrent uncontrolled passage of stools at least several times per month. The following fecal incontinence–associated symptoms were analyzed separately: soiling (loss of some feces), urge incontinence (inability to reach the toilet in time), solid incontinence (loss of much solid feces without urge), and liquid incontinence (loss of watery stool or diarrhea). Major LARS was defined according to the LARS score, incorporating flatus and liquid incontinence, altered stool frequency, clustering, and urgency. The definition of major LARS is a LARS score between 30 to 42 points.5
The secondary outcome was generic quality of life according to the SF-36 questionnaire, which comprises 36 questions in 8 domains. With these questions, a Likert scale can be calculated between 0-100.20 A higher score indicates better quality of life.
Definitions
Clinical and perioperative variables were verified by one investigator screening the medical records. Anastomotic height was calculated as centimeters from anastomosis to the anal verge and was obtained from the surgical reports. If not mentioned there, the first postoperative endoscopy report was screened. Follow-up time was calculated as the difference between the date of completing the questionnaires and the time of primary surgery or reversal of the temporary stoma. Severity of comorbidity at surgery was classified according to the Charlson Comorbidity Index (CCI).24 The European Perioperative Clinical Outcome definitions were used to define postoperative complications.25 The pathological tumor stage was reported. Neoadjuvant radiotherapy was categorized into short-course (25 Gy in 5 fractions) and long-course radiotherapy (45-50.4 Gy in 25 to 28 fractions). Patients without lymphatic involvement but with >5 mm extramural invasion also received radiotherapy. In most cases, long-course neoadjuvant radiotherapy was combined with chemotherapy.
Statistical Analysis
Continuous variables were expressed as mean (SD) or median (IQR). Either one-way ANOVA, Mann-Whitney, or Kruskal-Wallis tests were used. Categorical variables were presented as number (%) with Pearson’s χ2 tests. Univariable and multivariable binary logistic regression analyses were used to determine the association between anastomotic height and constipation, fecal incontinence, and major LARS. Covariates for the multivariable models were selected based on the a priori possibility of confounding and/or tendency toward statistical significance in univariable analysis (p < 0.10). Potential interactions were assessed. Cubic spline regression analysis was used for probability figures. Spearman’s coefficient test was used for correlations, where rho <0.3 was interpreted as negligible. A p of <0.05 was considered statistically significant. Analyses were performed with IBM SPSS Statistics, Version 23.0 (Armonk, NY, USA) and the probability figures were generated using R, Version 3.6.3 (R Foundation of Statistical Computing, Vienna, Austria). The illustration was created with Biorender.com. The results were reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines of the EQUATOR network.26
RESULTS
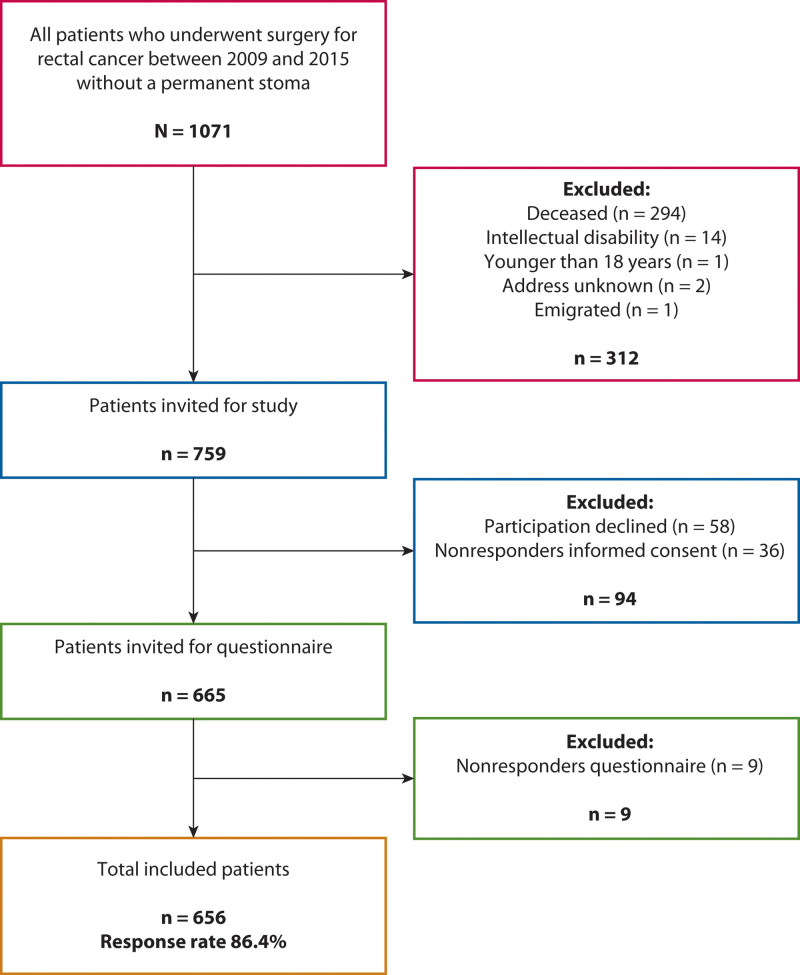
Between 2009 and 2015, 1071 patients underwent surgery for rectal or rectosigmoid cancer without construction of a permanent stoma, of whom 759 were eligible for inclusion. A total of 656 patients (86.3%) completed the questionnaires (Fig. 1). We excluded 26 patients because of missing data on anastomotic height. All patient characteristics of the included study population are presented in Table 1. Overall median anastomotic height was 8.0 cm (IQR 5.0-12.0) from the anal verge and median follow-up was 58.0 (IQR 39.0-79.0) months. Dropout analysis only revealed a significantly older age in the nonresponders (67.0 vs 65.0 years; p = 0.025) (Supplemental Digital Content 1 at http://links.lww.com/DCR/B859).
FIGURE 1.
Flow chart of inclusion and exclusion.
TABLE 1.
Patient characteristics
| Variables | Total (N = 630) |
|---|---|
| Anastomotic height (cm) a | 8.0 (5.0–12.0) |
| Basic characteristics, n (%) | |
| Male patients | 389 (61.7) |
| Age at surgery (years) a | 65.0 (58.0–69.0) |
| Follow-up (months) a,b | 58.0 (39.0–79.0) |
| BMI at surgery (kg/m2) c | 26.1 (3.8) |
| ASA score at surgery | |
| I | 192 (31.3) |
| II | 355 (57.8) |
| III | 64 (10.4) |
| IV | 3 (0.5) |
| Charlson comorbidity index at surgery a | 2.0 (2.0–2.0) |
| Previous lower abdominal surgery | 158 (25.1) |
| Previous upper abdominal surgery | 55 (8.7) |
| Smoking | |
| No | 467 (81.8) |
| Yes | 87 (15.2) |
| Recently quit | 17 (3.0) |
| Oncologic characteristics, n (%) | |
| Tumor stage (UICC) | |
| I | 229 (36.4) |
| II | 202 (32.1) |
| III | 180 (28.6) |
| IV | 18 (2.9) |
| Distant metastasis | |
| No | 572 (90.8) |
| Liver | 35 (5.6) |
| Lung | 14 (2.2) |
| Multiple locations | 9 (1.4) |
| Neoadjuvant and adjuvant treatment, n (%) | |
| Radiotherapy | |
| No | 314 (50.3) |
| Neoadjuvant short course | 185 (29.6) |
| Neoadjuvant long course | 118 (18.9) |
| On pelvic region for other reason | 7 (1.1) |
| Adjuvant chemotherapy | 114 (18.4) |
| Years since last radiotherapy a | 6.0 (4.0–8.0) |
| Years since last chemotherapy a | 5.0 (3.0–7.0) |
| Surgical characteristics, n (%) | |
| Setting | |
| Elective | 569 (96.4) |
| Emergency | 22 (3.6) |
| Surgical approach | |
| Open | 226 (35.9) |
| Laparoscopic | 351 (55.8) |
| Conversion | 52 (8.3) |
| Method of anastomosis | |
| Hand-sewn | 30 (4.8) |
| Stapled | 589 (95.2) |
| Reconstruction | |
| Side-to-end | 418 (77.6) |
| Side-to-side | 18 (3.3) |
| End-to-end | 103 (19.1) |
| Temporary stoma | 320 (50.8) |
| Postoperative characteristics, n (%) | |
| Anastomotic leakage | 37 (5.9) |
| Reoperation | 32 (5.1) |
| Overall other types of complications | |
| No | 445 (70.6) |
| One complication | 136 (21.6) |
| More complications | 49 (7.8) |
Values are expressed as median (IQR).
Time between completing the questionnaires and the primary surgery or the reversal of the temporary stoma.
Values are expressed as mean (SD).
ASA = American Society of Anesthesiologists; BMI = body mass index; UICC = Union for International Cancer Control.
Univariable and Multivariable Analysis of Bowel Function Problems
Constipation, fecal incontinence, and major LARS were prevalent in 31.0%, 24.5%, and 30.0% of the study population. Univariable analysis showed increased odds for constipation along with higher anastomotic heights, while fecal incontinence and major LARS were associated with lower anastomotic heights (p < 0.001 for all variables, Supplemental Digital Content 2 at http://links.lww.com/DCR/B860). The univariable associations of constipation, fecal incontinence, and major LARS with different patient characteristics can be found in Supplemental Digital Content 2 http://links.lww.com/DCR/B860. Type of anastomosis did not show a significantly different association with constipation, fecal incontinence, or major LARS. A stapled anastomosis showed a significantly increased association with major LARS (OR = 6.46; 95% CI, 1.52–27.4; p = 0.011). A temporary stoma was significantly associated with less constipation (OR = 0.65; 95% CI, 0.46–0.92; p = 0.014) but more fecal incontinence (OR = 2.52; 95% CI, 1.72–3.69; p < 0.001) and more major LARS (OR = 3.56; 95% CI, 2.46–5.16; p < 0.001).
In multivariable analysis, constipation, fecal incontinence, and major LARS all remained significantly associated with anastomotic heights, when theoretically adjusted for sex, age, and follow-up time, and mathematically for all other variables tending toward significance in univariable analysis (Table 2). Constipation was associated with higher anastomotic heights (OR = 1.08; 95% CI, 1.02–1.15; p = 0.011). Apart from an increased American Society of Anesthesiologists (ASA) score at surgery (OR = 1.43; 95% CI, 1.05–1.95; p = 0.022), no other variables were independently associated with constipation in multivariable analysis.
TABLE 2.
Multivariable logistic regression of constipation, fecal incontinence, and major LARS
| Variables | Constipation | Fecal incontinence | Major LARS | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Anastomotic height, cm | 1.08 (1.02–1.15) | 0.011* | 0.91 (0.85–0.98) | 0.010* | 0.93 (0.87–0.99) | 0.032* |
| Sex | ||||||
| Male | Reference | Reference | Reference | |||
| Female | 1.22 (0.84–1.77) | 0.302 | 0.74 (0.48–1.14) | 0.169 | 0.79 (0.51–1.21) | 0.279 |
| Age at surgery, y | 0.99 (0.97–1.01) | 0.250 | 1.00 (0.98–1.02) | 0.976 | 1.00 (0.98–1.02) | 0.987 |
| Follow-up, mo | 1.00 (0.99–1.01) | 0.776 | 1.00 (0.99–1.01) | 0.836 | 1.00 (0.99–1.01) | 0.908 |
| ASA score at surgery | 1.43 (1.05–1.95) | 0.022* | 0.81 (0.57–1.14) | 0.228 | ||
| Previous lower abdominal surgery | ||||||
| No | Reference | |||||
| Yes | 1.47 (0.94–2.30) | 0.094 | ||||
| Radiotherapy | ||||||
| No | Reference | Reference | Reference | |||
| Neoadjuvant short-course | 0.93 (0.55–1.57) | 0.791 | 2.60 (1.49–4.54) | 0.001** | 3.41 (2.00–5.86) | <0.001** |
| Neoadjuvant long-course | 0.88 (0.47–1.65) | 0.699 | 2.64 (1.40–4.97) | 0.003** | 2.02 (1.09–3.73) | 0.025* |
| Adjuvant chemotherapy | ||||||
| No | Reference | Reference | ||||
| Yes | 1.24 (0.77–1.99) | 0.383 | 0.82 (0.44–1.51) | 0.513 | ||
| Setting | ||||||
| Elective | Reference | |||||
| Emergency | 0.40 (0.05–3.27) | 0.395 | ||||
| Surgical approach | ||||||
| Open | Reference | Reference | Reference | |||
| Laparoscopic | 1.49 (0.99–2.26) | 0.058 | 0.79 (0.51–1.22) | 0.288 | 0.85 (0.55–1.33) | 0.481 |
| Conversion | 0.97 (0.46–2.05) | 0.940 | 0.84 (0.38–1.84) | 0.667 | 1.04 (0.49–2.20) | 0.914 |
| Method of anastomosis | ||||||
| Hand-sewn | Reference | |||||
| Stapled | 2.70 (0.57–12.87) | 0.212 | ||||
| Temporary stoma | ||||||
| No | Reference | Reference | Reference | |||
| Yes | 1.08 (0.67–1.75) | 0.749 | 0.94 (0.56–1.61) | 0.843 | 1.57 (0.93–2.64) | 0.091 |
| Anastomotic leakage | ||||||
| No | Reference | Reference | ||||
| Yes | 2.17 (1.03–4.56) | 0.041* | 1.31 (0.60–2.87) | 0.497 | ||
Statistical significance of p < 0.05.
** Statistical significance of p < 0.005.
LARS = low anterior resection syndrome.
In contrast to constipation, fecal incontinence was associated with lower anastomotic heights (OR = 0.91; 95% CI, 0.85–0.98; p = 0.010). The likelihood of fecal incontinence was almost triple for both short-course and long-course neoadjuvant radiotherapy (OR = 2.60; 95% CI, 1.49–4.54; p = 0.001 and OR = 2.64; 95% CI, 1.40–4.97; p = 0.003). Likewise, anastomotic leakage (OR = 2.17; 95% CI, 1.03–4.56; p = 0.041) was also independently associated with increased odds of fecal incontinence. Usage of a temporary stoma was no longer significantly associated with fecal incontinence.
Finally, multivariable analysis confirmed that major LARS was associated with lower anastomotic heights (OR = 0.93; 95% CI, 0.87–0.99; p = 0.032). Also, short-course and long-course neoadjuvant radiotherapy were associated with increased odds of major LARS (OR = 3.41; 95% CI, 2.00–5.86; p < 0.001 and OR = 2.02; 95% CI, 1.09–3.73; p = 0.025). Neither method of anastomosis nor usage of a temporary stoma remained significantly associated with major LARS.
Probability of Bowel Function Problems
In accordance with the results of the univariable and multivariable analyses, the probability of constipation increased along with higher anastomotic heights, while the probability of fecal incontinence decreased (Fig. 2). The curves indicating the probability of constipation and fecal incontinence crossed at an anastomotic height of 7 cm. Given the overlapping 95% CIs of the probability of constipation and fecal incontinence at an anastomotic height between 4.5 cm to 9.5 cm, 3 functional groups can be distinguished according to anastomotic height (Fig. 2). The probability of major LARS showed an almost linear decline along with anastomotic height, reaching from 0.54 to 0.09.
FIGURE 2.
The probability of constipation, fecal incontinence, and major LARS according to anastomotic height. LARS = low anterior resection syndrome.
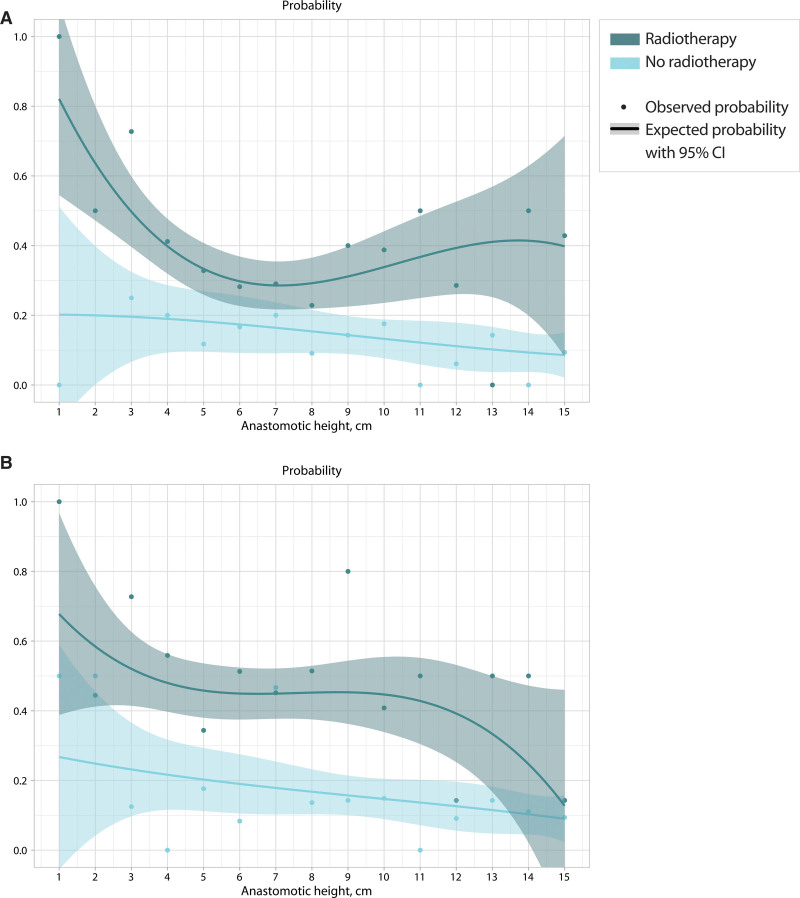
Because both fecal incontinence and major LARS showed significant associations with neoadjuvant radiotherapy, the same probability analysis was performed separately for patients with and without neoadjuvant radiotherapy (Fig. 3). As is shown in this figure, the combination of neoadjuvant radiotherapy and lower anastomotic heights resulted in a peak in the probability of both fecal incontinence (Fig. 3A) and major LARS (Fig. 3B).
FIGURE 3.
The impact of neoadjuvant radiotherapy on the probability of fecal incontinence and major LARS according to anastomotic height. A, Fecal incontinence. B, Major LARS. LARS = low anterior resection syndrome.
Pattern of Bowel Symptoms According to Anastomotic Height
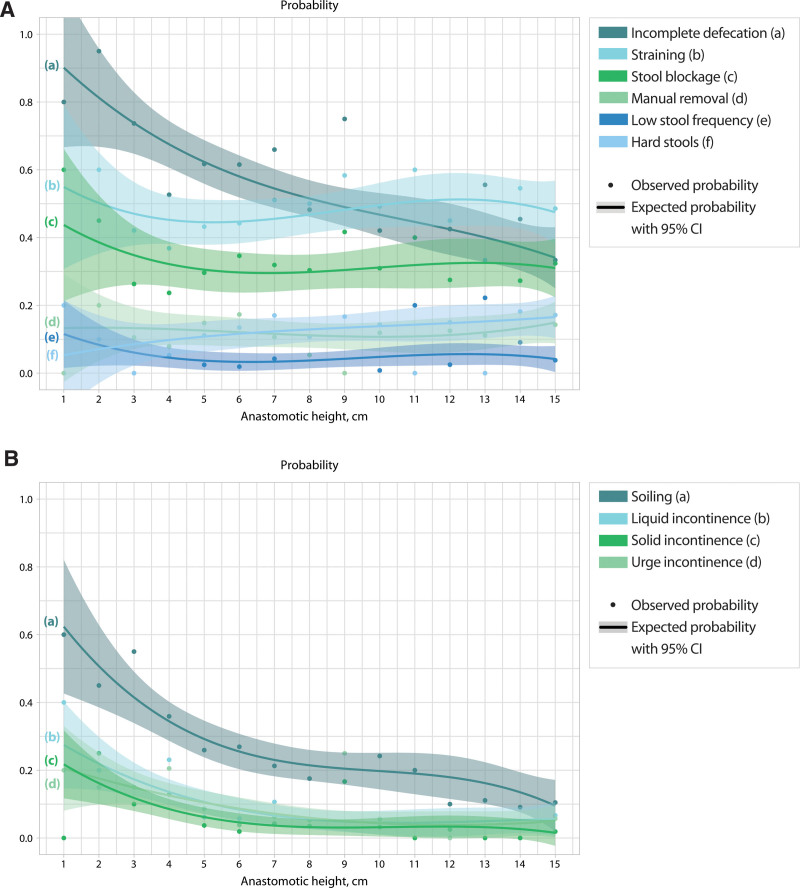
Of all constipation-associated symptoms, the probability of incomplete defecation was highest in patients with an anastomotic height below 9.5 cm (Fig. 4A). Soiling showed the highest probability among all fecal incontinence–associated symptoms (Fig. 4B).
FIGURE 4.
The probability of constipation and fecal incontinence-associated symptoms according to anastomotic height. A, Constipation-associated symptoms. B, Fecal incontinence-associated symptoms. LARS = low anterior resection syndrome.
Use of Defecation Treatment
Both antidiarrheals and colonic irrigations were used more frequently in patients with a lower anastomosis (OR = 0.81; 95% CI, 0.71–0.91; p < 0.001 and OR = 0.81; 95% CI, 0.67–0.97; p = 0.022, Supplemental Digital Content 3 at http://links.lww.com/DCR/B861). The use of laxatives and enemas to treat constipation did not significantly differ along with anastomotic height. Antidiarrheals or colonic irrigations were given to 17.9% of the patients with fecal incontinence. Of the constipated patients, 37.8% were treated with a laxative or enema.
Quality of Life
Only the physical functioning and bodily pain domains significantly correlated with anastomotic heights, but both correlations were negligible (rho, -0.115; p = 0.004 and rho, -0.081; p = 0.045, Supplemental Digital Content 4 at http://links.lww.com/DCR/B862).
DISCUSSION
This study is the first to demonstrate the exact relation between anastomotic height and the probability of constipation, fecal incontinence, and major LARS in the long term. Although anastomotic height cannot be controlled in oncological surgery, the screening of postoperative bowel function according to anastomotic height can be improved.
In accordance with clinical experience, constipation was more frequent in patients with higher anastomotic heights, while fecal incontinence and major LARS were associated with lower anastomotic heights. These findings withstood multivariable analysis in which we adjusted for sex, age, and follow-up time. Most studies corroborate our findings of no significant association of both sex10,11,16,17,27–29 and age10,11,13,17,28,29 with constipation, fecal incontinence, or major LARS. Regarding follow-up time, longitudinal studies showed no significant improvements of bowel function after 1-year follow-up.28,30 Therefore, our study confirms the persistent nature of bowel function problems after surgery for rectal or rectosigmoid cancer, which indicates that clinicians should screen for and treat these problems.
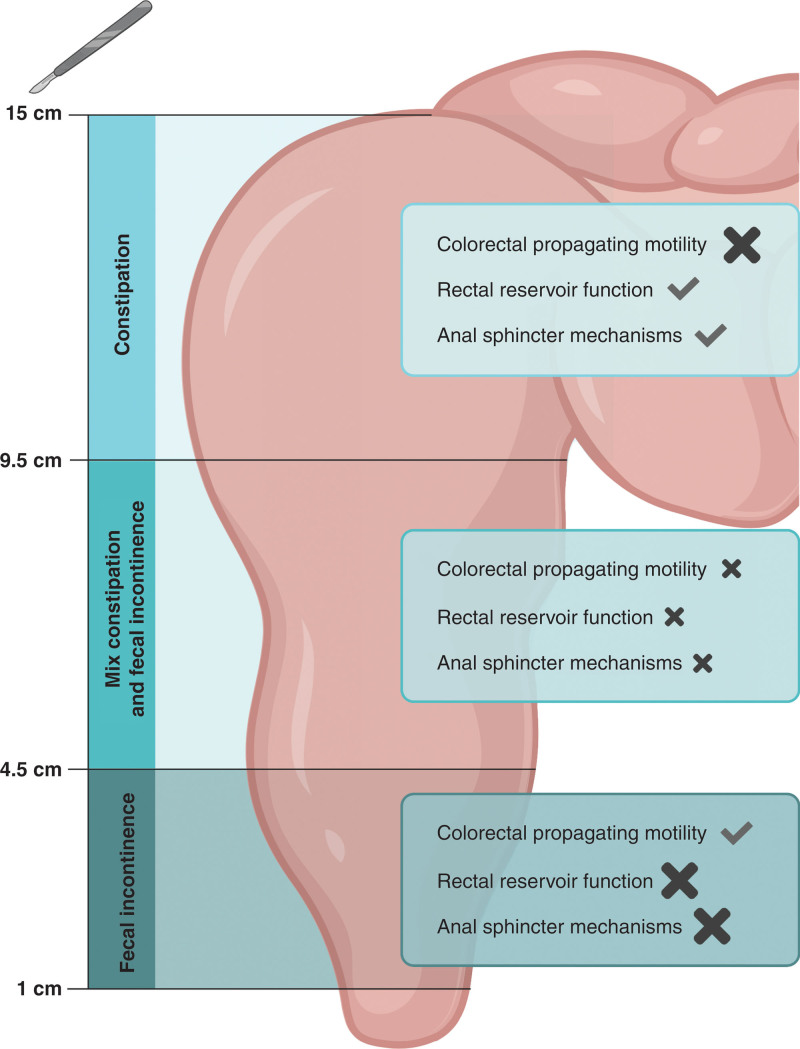
Until now, only 1 long-term study analyzed anastomotic height as a continuous variable, and it also reported increased odds of major LARS along with lower anastomotic heights in multivariable regression.29 The varying pattern of bowel function problems according to varying anastomotic heights clearly indicates a multifactorial pathophysiology of the postoperative bowel function problems. Pathophysiological research on rectal cancer patients showed indications of surgical damage to at least 3 factors: innervation of anal sphincter mechanisms,8,31,32 rectal reservoir function,15,31,33 and colorectal propagating motility.34 A balance between these three factors hypothetically explains the clinical pattern of bowel function problems along with anastomotic height (Fig. 5). At first, in patients with an anastomosis above 9.5 cm, specifically, colorectal propagating motility might be surgically damaged, which is clinically illustrated by the high probability of constipation in these patients. Second, in patients with an anastomosis between 4.5 cm and 9.5 cm, light surgical damage to all 3 factors can be expected, given the fact that the resection was close to the sigmoid, considerable rectal length was removed, and surgery was performed deep into the pelvis, which might have damaged the innervation of the anal sphincter mechanisms. Some constipation might therefore be counterbalanced by some fecal incontinence, which coincides with the clinical picture of acceptable rates of both constipation and fecal incontinence. Finally, in patients with an anastomosis below 4.5 cm‚ the lack of rectal reservoir function and damage to anal sphincter mechanisms will dominate,6,8 resulting in an increased probability of fecal incontinence. Remarkably, our results showed that apart from fecal incontinence, the probability of incomplete defecation was also extremely high in patients with low anastomoses, as was described previously.7 This might be caused by surgical disruption of a specific neural pathway in the anorectal region, but further pathophysiological research is required in this field.
FIGURE 5.
Proposed pathophysiological balance of surgical damage after resection with different anastomotic heights. Check mark indicates no damage, a large cross indicates severe damage, and a small cross indicates light damage.
The probability of major LARS showed an almost linear decline along with anastomotic height, mainly corresponding to the pattern of fecal incontinence. Although the LARS score helped to diminish the heterogeneity of bowel function scores in research about surgery for rectal cancer, the score heavily underestimates constipation-associated symptoms.35 Our findings underscore the substantial presence of constipation in patients who had undergone surgery for rectal or rectosigmoid cancer, which cannot be captured by solely focusing on fecal incontinence–associated symptoms.
Despite the increased probability of constipation with increasing anastomotic height, the use of laxatives or enemas did not vary along with the anastomotic height. Interestingly, only 38% of constipated patients received laxatives or enemas and only 18% of patients with fecal incontinence received antidiarrheals or colonic irrigations. The current study therefore illustrates how both postoperative fecal incontinence and constipation are undertreated.
Apart from surgical damage, radiotherapy has been frequently shown to increase both fecal incontinence15,36,37 and major LARS11,17,27,29,38 in the long term. This issue is reinforced by our findings‚ which showed that both short-course and long-course neoadjuvant radiotherapy almost tripled the likelihood of fecal incontinence and major LARS, especially in combination with low anastomotic heights; findings that are also supported by others.11,36,39 Radiotherapy-related problems had mainly been attributed to ischemia and fibrosis of the irradiated tissue, resulting in additional loss of rectal capacity and damage to the internal anal sphincter.15,33,40 Notably, the time since the last radiotherapy is neither associated with constipation, fecal incontinence, nor major LARS‚ indicating that improvement with time of radiotherapy-related problems does not seem to persist in the long term.33 Other independent significant predictors in the multivariate models in this study were the ASA score and anastomotic leakage. Constipation was associated with a higher ASA score at surgery, which might reflect a general effect of comorbidities on bowel motility. The likelihood of fecal incontinence was also doubled after the occurrence of anastomotic leakage, although the significance was borderline. Other long-term reports evaluating this association presented contradictory conclusions,11,16,27 which is probably caused by the small number of events and the lack of a uniform definition of anastomotic leakage.
Although this study shows a strong association between bowel function problems and anastomotic height, both physical and mental generic quality of life scores were not related to anastomotic height. Previous research found an association between generic quality of life and fecal continence or worse LARS scores after surgery for rectal cancer.12,41 It might be that patients with high anastomoses are equally bothered by constipation when compared to patients with low anastomoses‚ who mainly suffer from fecal incontinence. Furthermore, the long follow-up time in this study may also have played a role, as coping with bowel function problems may improve.41
This study is strengthened by the high response rate (86.3%), large study population, and long follow-up time. Moreover, simultaneous collection of different validated bowel function scores enhances interstudy comparisons. Nonetheless, there are some limitations. First, given the cross-sectional study design, we were unable to assert whether bowel function problems already existed preoperatively and how they developed over time. To rule out this theoretically confounding effect as much as possible, we adjusted for follow-up time in all multivariable models. Second, some clinical data were missing in the medical records, but data regarding anastomotic height, our main variable, were only lacking in fewer than 4% of cases. Third, anastomotic height was obtained retrospectively from the surgery or endoscopic reports. Lastly, the exclusion of deceased patients and patients with a permanent stoma and the slightly higher age of the nonresponders could have led to selection bias. This would, however, only strengthen the conclusion of this study, because bowel function problems were already prevalent in the included “healthier” patients.
CONCLUSION
This study revealed that anastomotic height is valuable as a predictor of whether patients will suffer from constipation and/or fecal incontinence after surgery for rectal or rectosigmoid cancer in the long term. To enable effective screening and treatment, more attention should be drawn toward fecal incontinence in patients with an anastomosis below 4.5 cm. In patients with an anastomosis above 9.5 cm, the focus should be on constipation. Patients with an anastomosis between 4.5 cm and 9.5 cm should be screened and treated for both fecal incontinence and constipation. The detrimental effects of neoadjuvant radiotherapy on long-term continence should also be heeded, especially in patients with the lowest anastomoses. The fact that generic quality of life was comparable between patients with different anastomotic heights might indicate that they are bothered in equal measure by constipation and fecal incontinence. This study might serve as a guide for the clinician to effectively screen and treat fecal incontinence and constipation during the follow-up of patients after surgery for rectal and rectosigmoid cancer.
ACKNOWLEDGMENTS
The authors thank RoQua staff members I.A.M. ten Vaarwerk and E. Visser for processing the data of the questionnaires. We also acknowledge T. van Wulfften Palthe, Ph.D., for correcting the English manuscript. Finally, we thank the patients for participating in this study.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML and PDF versions of this article on the journal’s website (www.dcrjournal.com).
Funding/Support: None reported.
Financial Disclosures: None reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Scheer AS, Boushey RP, Liang S, Doucette S, O’Connor AM, Moher D. The long-term gastrointestinal functional outcomes following curative anterior resection in adults with rectal cancer: a systematic review and meta-analysis. Dis Colon Rectum. 2011;54:1589–1597. [DOI] [PubMed] [Google Scholar]
- 4.Croese AD, Lonie JM, Trollope AF, Vangaveti VN, Ho YH. A meta-analysis of the prevalence of low anterior resection syndrome and systematic review of risk factors. Int J Surg. 2018;56:234–241. [DOI] [PubMed] [Google Scholar]
- 5.Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012;255:922–928. [DOI] [PubMed] [Google Scholar]
- 6.Matzel KE, Stadelmaier U, Muehldorfer S, Hohenberger W. Continence after colorectal reconstruction following resection: impact of level of anastomosis. Int J Colorectal Dis. 1997;12:82–87. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen OO, Petersen IK, Christiansen J. Anorectal function following low anterior resection. Colorectal Dis. 2003;5:258–261. [DOI] [PubMed] [Google Scholar]
- 8.Lee SJ, Park YS. Serial evaluation of anorectal function following low anterior resection of the rectum. Int J Colorectal Dis. 1998;13:241–246. [DOI] [PubMed] [Google Scholar]
- 9.Ho YH, Wong J, Goh HS. Level of anastomosis and anorectal manometry in predicting function following anterior resection for adenocarcinoma. Int J Colorectal Dis. 1993;8:170–174. [DOI] [PubMed] [Google Scholar]
- 10.Denost Q, Laurent C, Capdepont M, Zerbib F, Rullier E. Risk factors for fecal incontinence after intersphincteric resection for rectal cancer. Dis Colon Rectum. 2011;54:963–968. [DOI] [PubMed] [Google Scholar]
- 11.Qin Q, Huang B, Cao W, et al. Bowel dysfunction after low anterior resection with neoadjuvant chemoradiotherapy or chemotherapy alone for rectal cancer: a cross-sectional study from China. Dis Colon Rectum. 2017;60:697–705. [DOI] [PubMed] [Google Scholar]
- 12.Keane C, O’Grady G, Bissett I, Woodfield J. Comparison of bowel dysfunction between colorectal cancer survivors and a non-operative non-cancer control group. Colorectal Dis. 2020;22:806–813. [DOI] [PubMed] [Google Scholar]
- 13.van Heinsbergen M, Janssen-Heijnen ML, Leijtens JW, Slooter GD, Konsten JL. Bowel dysfunction after sigmoid resection underestimated: multicentre study on quality of life after surgery for carcinoma of the rectum and sigmoid. Eur J Surg Oncol. 2018;44:1261–1267. [DOI] [PubMed] [Google Scholar]
- 14.Jehle EC, Haehnel T, Starlinger MJ, Becker HD. Level of the anastomosis does not influence functional outcome after anterior rectal resection for rectal cancer. Am J Surg. 1995;169:147–52; discussion 152. [DOI] [PubMed] [Google Scholar]
- 15.Pollack J, Holm T, Cedermark B, Holmström B, Mellgren A. Long-term effect of preoperative radiation therapy on anorectal function. Dis Colon Rectum. 2006;49:345–352. [DOI] [PubMed] [Google Scholar]
- 16.Gadan S, Floodeen H, Lindgren R, Matthiessen P. Does a defunctioning stoma impair anorectal function after low anterior resection of the rectum for cancer? a 12-year follow-up of a randomized multicenter trial. Dis Colon Rectum. 2017;60:800–806. [DOI] [PubMed] [Google Scholar]
- 17.Dulskas A, Kavaliauskas P, Pilipavicius L, Jodinskas M, Mikalonis M, Samalavicius NE. Long-term bowel dysfunction following low anterior resection. Sci Rep. 2020;10:11882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DʼSouza N, de Neree Tot Babberich MPM, d’Hoore A, et al. Definition of the rectum: an International, expert-based Delphi consensus. Ann Surg. 2019;270:955–959. [DOI] [PubMed] [Google Scholar]
- 19.Meinds RJ, Timmerman MEW, van Meegdenburg MM, Trzpis M, Broens PMA. Reproducibility, feasibility and validity of the Groningen Defecation and Fecal Continence questionnaires. Scand J Gastroenterol. 2018;53:790–796. [DOI] [PubMed] [Google Scholar]
- 20.Ware J, Snoww KK, Kosinski MA, Gandek BG. SF-36 Health Survey: Manual and Interpretation Guide. Boston: MA: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 21.Aaronson NK, Muller M, Cohen PD, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51:1055–1068. [DOI] [PubMed] [Google Scholar]
- 22.Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology. 2016;S0016-5085(16)00222-5. [DOI] [PubMed] [Google Scholar]
- 23.Rao SS, Bharucha AE, Chiarioni G, et al. Functional anorectal disorders. Gastroenterology. 2016;S0016-5085(16)00175-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 25.Jammer I, Wickboldt N, Sander M, et al.; European Society of Anaesthesiology (ESA) and the European Society of Intensive Care Medicine (ESICM); European Society of Anaesthesiology; European Society of Intensive Care Medicine. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol. 2015;32:88–105. [DOI] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 2007;85:867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen TY, Wiltink LM, Nout RA, et al. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clin Colorectal Cancer. 2015;14:106–114. [DOI] [PubMed] [Google Scholar]
- 28.Sandberg S, Asplund D, Bisgaard T, et al. Low anterior resection syndrome in a Scandinavian population of patients with rectal cancer: a longitudinal follow-up within the QoLiRECT study. Colorectal Dis. 2020;22:1367–1378. [DOI] [PubMed] [Google Scholar]
- 29.Sun W, Dou R, Chen J, et al. Impact of long-course neoadjuvant radiation on postoperative low anterior resection syndrome and quality of life in rectal cancer: post hoc analysis of a randomized controlled trial. Ann Surg Oncol. 2019;26:746–755. [DOI] [PubMed] [Google Scholar]
- 30.Floodeen H, Lindgren R, Hallböök O, Matthiessen P. Evaluation of long-term anorectal function after low anterior resection: a 5-year follow-up of a randomized multicenter trial. Dis Colon Rectum. 2014;57:1162–1168. [DOI] [PubMed] [Google Scholar]
- 31.Jiang JK, Lin JK. Anorectal dysfunction following low anterior resection for rectal carcinoma: a comparison between handsewn and stapled anastomosis. Colorectal Dis. 1999;1:73–79. [DOI] [PubMed] [Google Scholar]
- 32.Machado M, Nygren J, Goldman S, Ljungqvist O. Functional and physiologic assessment of the colonic reservoir or side-to-end anastomosis after low anterior resection for rectal cancer: a two-year follow-up. Dis Colon Rectum. 2005;48:29–36. [DOI] [PubMed] [Google Scholar]
- 33.van Duijvendijk P, Slors JF, Taat CW, et al. Prospective evaluation of anorectal function after total mesorectal excision for rectal carcinoma with or without preoperative radiotherapy. Am J Gastroenterol. 2002;97:2282–2289. [DOI] [PubMed] [Google Scholar]
- 34.Koda K, Saito N, Seike K, Shimizu K, Kosugi C, Miyazaki M. Denervation of the neorectum as a potential cause of defecatory disorder following low anterior resection for rectal cancer. Dis Colon Rectum. 2005;48:210–217. [DOI] [PubMed] [Google Scholar]
- 35.Ribas Y, Aguilar F, Jovell-Fernández E, Cayetano L, Navarro-Luna A, Muñoz-Duyos A. Clinical application of the LARS score: results from a pilot study. Int J Colorectal Dis. 2017;32:409–418. [DOI] [PubMed] [Google Scholar]
- 36.Peeters KC, van de Velde CJ, Leer JW, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients–a Dutch colorectal cancer group study. J Clin Oncol. 2005;23:6199–6206. [DOI] [PubMed] [Google Scholar]
- 37.Downing A, Glaser AW, Finan PJ, et al. Functional outcomes and health-related quality of life after curative treatment for rectal cancer: a population-level study in England. Int J Radiat Oncol Biol Phys. 2019;103:1132–1142. [DOI] [PubMed] [Google Scholar]
- 38.Jimenez-Gomez LM, Espin-Basany E, Trenti L, et al. Factors associated with low anterior resection syndrome after surgical treatment of rectal cancer. Colorectal Dis. 2017;Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Kupsch J, Jackisch T, Matzel KE, et al. Outcome of bowel function following anterior resection for rectal cancer-an analysis using the low anterior resection syndrome (LARS) score. Int J Colorectal Dis. 2018;33:787–798. [DOI] [PubMed] [Google Scholar]
- 40.Theisen J, Kauer WK, Nekarda H, Schmid L, Stein HJ, Siewert JR. Neoadjuvant radiochemotherapy for patients with locally advanced rectal cancer leads to impairment of the anal sphincter. J Gastrointest Surg. 2006;10:309–314. [DOI] [PubMed] [Google Scholar]
- 41.Zutshi M, Hull T, Shedda S, Lavery I, Hammel J. Gender differences in mortality, quality of life and function after restorative procedures for rectal cancer. Colorectal Dis. 2013;15:66–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.