Abstract
Variation in immune homeostasis, the state in which the immune system is maintained in the absence of stimulation, is highly variable across populations. This variation is attributed to both genetic and environmental factors. However, the identity and function of specific regulators have been difficult to identify in humans. We evaluated homeostatic antibody levels in the serum of the Collaborative Cross (CC) mouse genetic reference population. We found heritable variation in all antibody isotypes and subtypes measured. We identified 4 quantitative trait loci (QTL) associated with 3 IgG subtypes: IgG1, IgG2b, and IgG2c. While 3 of these QTL map to genome regions of known immunological significance (major histocompatibility and immunoglobulin heavy chain locus), Qih1 (associated with variation in IgG1) mapped to a novel locus on Chromosome 18. We further associated this locus with B cell proportions in the spleen and identify Methyl-CpG binding domain protein 1 under this locus as a novel regulator of homeostatic IgG1 levels in the serum and marginal zone B cells (MZB) in the spleen, consistent with a role in MZB differentiation to antibody secreting cells.
Author summary
The baseline immune state is highly variable across populations, which is a function of both environmental and genetics factors. Here, we have used the Collaborative Cross, a genetically diverse mouse population, to study the role of genetic variation on baseline serum antibody concentrations. We identified several regions of the genome that were associated with variation in serum concentrations of multiple antibody subtypes. Most of which are in regions of the genome that have been shown previously to impact immune responses. We also identified a novel region of the genome associated with variation in IgG1 and further identify Methyl-CpG binding domain protein 1 (MBD1) as a regulator of both baseline IgG1 and B cell homeostasis. Given that MBD1 is an epigenetic regulator of differentiation in other contexts, further studies of MBD1 in B cell homeostasis may define previously unknown pathways involved in marginal zone B cell differentiation.
Introduction
Immune homeostasis is the stable state that the immune system maintains in the absence of insult. While the majority of studies on host immunity have focused on the response to specific stimuli (e.g., pathogens, vaccines, allergens, or adjuvants), a growing body of evidence suggests that an individual’s baseline immune status affects subsequent innate or antigen specific immune responses [1–4]. Immune homeostatic parameters are gaining increasing recognition as predictors of clinical outcomes to immunotherapy and vaccination [1,3], and several studies have shown that dysregulation of immune homeostasis can contribute to the development of cancer, autoimmunity, allergies, as well as the progression of immune-related pathology in response to infection [5]. Natural antibodies, which are present before antigen exposure, are a major component of immune homeostasis. These antibodies have been shown to be produced by specific subsets of B cells (e.g., marginal zone B cells and B1 B cells) and provide a first line of defense against infection through low affinity binding to pathogens [6]. Concurrent with our understanding of B cell and antibody biology, there is growing evidence that immune homeostasis is under genetic control [7–10]. However, we still do not fully understand the role that genetic differences between individuals play in driving baseline immunity.
Many studies have shown that humans exhibit significant inter-individual variation in baseline immune phenotypes [3–4,11–12]. However, it has been difficult to identify the genes that contribute to this variation due to a variety of confounding factors. These include difficult to control environmental factors, such as prior microbial and environmental exposures as well as age- dependent changes in immune status that create a highly dynamic immune environment [13]. Rodent models, which allow for greater control of host genetics and environmental exposures, represent an attractive system with which to model and investigate those factors driving the development of various immune homeostatic states. Gene specific knockout mice have been critical to our understanding of the immune system. However, these models represent extreme genetic perturbations, rather than the more subtle effects on gene expression or function more commonly associated with naturally occurring genetic variation in humans.
To better model how natural genetic variation impacts immune homeostasis, we turned to the Collaborative Cross (CC) genetic reference panel (grp) [14]. The CC grp is a large set of recombinant inbred (RI) mouse strains derived from 8 founders: five classical laboratory strains (C57BL/6J (B6), A/J, 129S1/SvImJ (129S1), NOD/ShiLtJ (NOD), and NZO/HlLtJ (NZO)) and three wild-derived strains (PWK/PhJ (PWK), CAST/EiJ (CAST), and WSB/EiJ (WSB)) [14]. These eight founder strains capture >90% of common genetic variation present in laboratory mouse strains and represent the three major subspecies of Mus musculus [15–17]. Importantly, this genetic variation exists across the genome, ensuring no blind spots for genetic mapping analyses. This genetic diversity has resulted in discovery of several genetic loci associated with a variety of biomedically relevant traits [18–24]. We and others have shown that there is extensive variation in splenic T cell populations [7], antibody glycosylation patterns [8], as well as variation in the general immune landscape of the spleen [10], across the CC population.
Here, we investigated how genetic variation impacts one aspect of systemic immune homeostasis: baseline serum antibody levels. We found that serum antibody levels for a variety of immunoglobulin (Ig) isotypes was highly varied across the CC, and we identified four quantitative trait loci (QTL) regulating these phenotypes. Three of the identified QTL mapped to known immunologically relevant regions of the genome, including the major histocompatibility locus and the immunoglobulin heavy chain locus. We also identified a novel locus broadly associated with variation in serum antibody levels, as well as B cell subset proportions in the spleen. Further analysis and independent mutant generation showed that the Methyl-CpG binding domain protein 1 (Mbd1) regulates homeostatic antibody levels and B cell differentiation, providing new insights into the genetic regulation of the humoral immune system and highlighting the utility of forward genetics-based discovery approaches in studying immunity.
Results
Variation in baseline antibody levels in the Collaborative Cross (CC) is largely driven by genetic factors
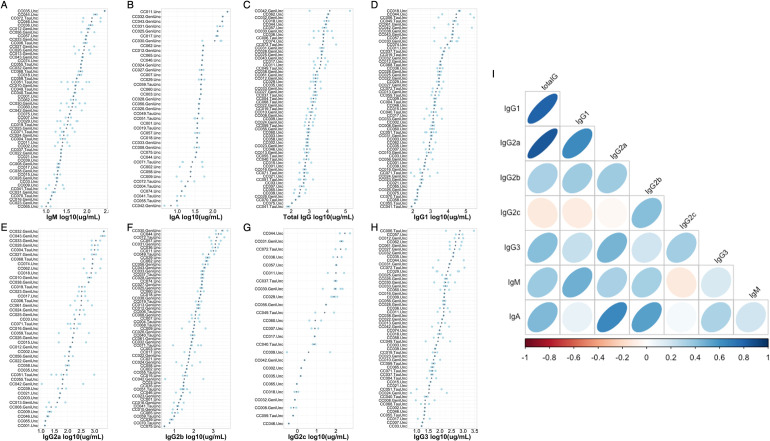
We quantified antibody concentrations in the serum of 117 mice from 58 CC strains (median = 3 mice/strain, 6–8 weeks old) to understand the role of genetic variation on homeostatic antibody levels. Serum from these mice was collected seven days after a non-specific footpad infection of phosphate buffered saline (PBS) with 1% fetal bovine serum (FBS), as these animals were control animals for another study. We assessed the concentrations of total IgA, IgM, and IgG, as well as IgG subtypes (IgG1, IgG2a, IgG2b, IgG2c, IgG3) in these sera by ELISA. We found that antibody concentrations varied greatly (3-50-fold) across these mice (Fig 1 and S1 Table). For example, IgM levels varied from 7.86 ug/mL– 271.39 ug/mL, total IgG levels ranged from 58.76 ug/mL– 173.89 mg/mL, and IgA varied from 4.39 ug/mL– 257.9 ug/mL in this population. Importantly, the variation between strains is much greater than that within strains (median standard deviation within strains is 0.596 ug/mL across all isotypes). We more formally assessed this by estimating the broad sense heritability (proportion of phenotypic variance in our population which can be attributable to genetic differences between individuals) for each of our antibody phenotypes and found that heritability estimates ranged from 0.25–0.66 (Table 1), indicating that genetic differences between strains plays a strong role in impacting this observed phenotypic variation in the CC. To understand the phenotypic relationships between antibody isotypes at homeostasis, we looked at pairwise correlations between all antibody isotypes and subtypes measured in the serum. We found that several antibody isotypes were highly correlated (mean correlation coefficient: 0.406, range: -0.115–0.841). We find that IgG1 levels were highly correlated with total IgG levels (correlation coefficient = 0.805), which was expected given the well characterized prevalence of IgG1 in the makeup of total IgG. However, we found additional interesting relationships among other antibody isotypes. For example, we found that IgA was highly correlated with both IgG2a (correlation coefficient = 0.645) and IgG2b (correlation coefficient = 0.543, Fig 1). These data suggest that there may be common genetic regulation of homeostatic antibody levels.
Fig 1. Variation in baseline antibody levels across CC strains and antibody isotypes.
Total serum antibody levels for (A) IgM, (B) IgA, (C) total IgG, (D) IgG1, (E) IgG2a, (F) IgG2b, (G) IgG2c, and (H) IgG3 were quantified by ELISAs. Each plot is individually ordered so that the strain with the greatest strain mean is at the top of the plot and the strain with the lowest is at the bottom. Grey dots indicate the mean antibody concentration for an individual strain, and light blue dots indicate individual animal measurements. (I) the relationship between individual antibody isotypes and subtypes in the CC strains. Strains not included for a particular isotype (e.g. Panel B. IgA, Panel E. IgG2a, Panel G. IgG2c) had antibody levels that were undetectable for that isotype.
Table 1. Homeostatic antibody serum concentration ranges and broad sense heritability estimates.
| Antibody isotype | Phenotypic range (μg/mL) | Median phenotype value (μg/mL) | Broad sense heritability estimate |
|---|---|---|---|
| Total IgG | 78.09–280910.17 | 1834.49 | 0.488 |
| IgG1 | 58.76–173895.19 | 1498.06 | 0.518 |
| IgG2a | 10.71–2378.67 | 187.10 | 0.462 |
| IgG2b | 3.07–5448.50 | 149.16 | 0.641 |
| IgG2c | 0.29–536.33 | 79.45 | 0.661 |
| IgG3 | 5.61–2906.31 | 101.02 | 0.249 |
| IgM | 7.87–271.39 | 44.22 | 0.561 |
| IgA | 4.39–257.93 | 41.63 | 0.375 |
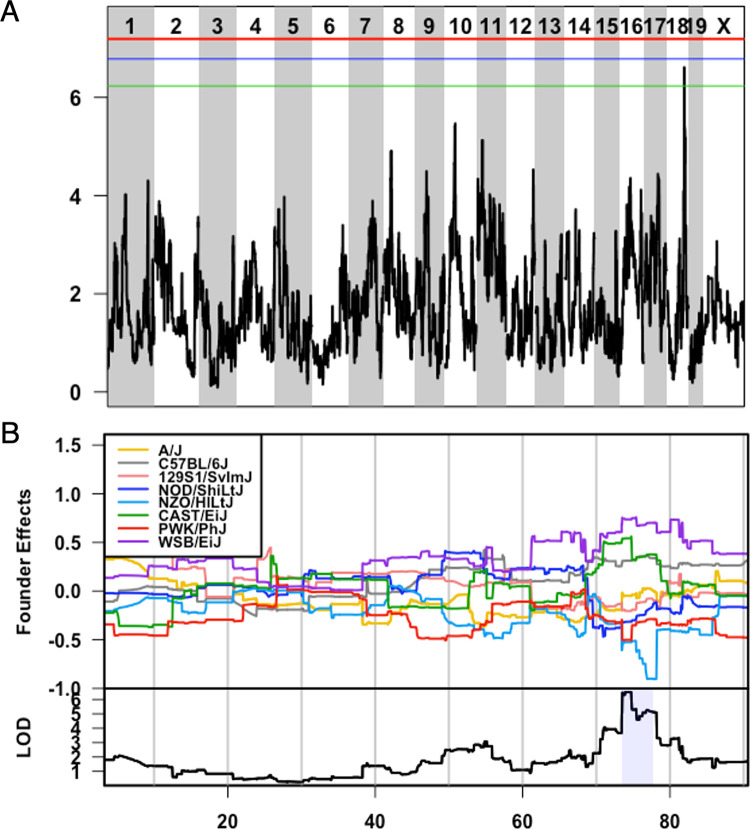
We next conducted genetic mapping to identify polymorphic genome regions associated with antibody level differences between the strains. QTL mapping identified four genome regions associated with variation in antibody levels (Table 2). Qih1, QTL for immune homeostasis 1 (Chromosome 18:73 – 78Mb, genome-wide p < 0.2), was identified for variation in homeostatic IgG1 levels, with the B6, CAST, and WSB haplotypes associated with higher levels of homeostatic IgG1. Qih2 (Chromosome 17: 43.46–44.03Mb, genome-wide p < 0.05, S1 Fig) was identified for variation in homeostatic IgG2c levels, where NOD, B6, CAST, and NZO haplotypes were all associated with higher levels of IgG2c. Additionally for IgG2c, we identified Qih3 (Chromosome. 12:117.73–120.02Mb, genome-wide p < 0.2, S1 Fig), driven by the B6 and NOD haplotypes, which are associated with higher levels of IgG2c. Lastly, we found Qih4 (Chromosome 12: 112.93–115.06Mb, genome-wide p < 0.05, S1 Fig) for variation in IgG2b levels at homeostasis. At this locus, B6 and NOD haplotypes are associated with higher levels of IgG2b. CAST, WSB, A/J, 129S1, and NOD haplotypes are intermediate, and the PWK haplotype is associated with lower levels of IgG2b.
Table 2. QTL identified in homeostatic antibody screen and causal haplotypes driving each QTL.
| QTL Name | Antibody isotype | QTL interval | QTL threshold | Causal haplotypes |
|---|---|---|---|---|
| Qih1 | IgG1 | Chr. 18: 73-78Mb | p < 0.2 | C57BL/6J, WSB/EiJ, CAST/EiJ—high |
|
Qih2
Qih3 |
IgG2c | Chr. 17: 43.4-44Mb Chr. 12: 117.7–120.1Mb |
p < 0.05 p < 0.2 |
C57BL/6J, NOD/ShiLtJ, CAST/EiJ, NZO/HlLtJ—high C57BL/6J, NOD/ShiLtJ–high |
| Qih4 | IgG2b | Chr. 12: 112.9-115Mb | p < 0.05 | C57BL/6J, NOD/ShiLtJ–high PWK/PhJ–low |
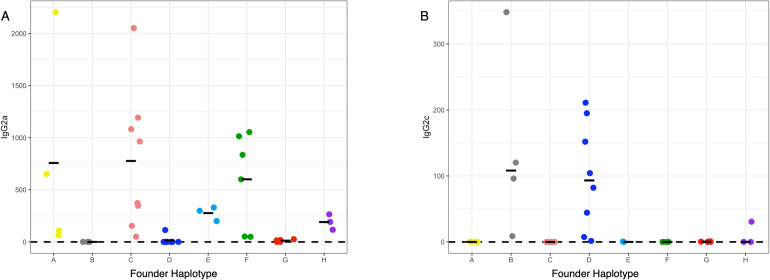
It has long been suggested that expression of IgG2a and IgG2c is mutually exclusive in mouse strains based on the expression of IgG2a in BALB/c mice and IgG2c in B6 and SJL mice [25,26]. However, whether the genes controlling these isotypes were physically linked on the same chromosome, or unique alleles at a locus was not understood. Recent work using isotype specific PCR showed that IgG2a and IgG2c were mutually exclusive in 4 inbred strains of mice: C57BL/6, NMRI, DBA2, and SJL, which the authors took as strong evidence of allelism [26]. Given that we mapped a QTL for IgG2c levels to the heavy chain locus (where the putative allelic variants of IgG2a and IgG2c exist), we examined these phenotypes and genotypes more closely and assessed the relationship between the CC founder haplotypes at this locus, sequence(s) unique to the IgG2a or IgG2c genes, and the CC strains abilities to produce either IgG2a (S2A Fig, IgG2a concentration (ug/mL)) or IgG2c (S2B Fig, IgG2c concentration (ug/mL)). Phenotypically (Fig 2), we found that CC strains with either the B6 or NOD haplotype at the IgH locus expressed IgG2c (> 0.00039 ug/mL, assay limit of detection) and did not express IgG2a, while strains with the other founder haplotypes at the locus expressed IgG2a (> 0.0039 ug/mL, assay limit of detection). While several of the samples containing the 129S1, A/J, NZO, CAST, PWK or WSB haplotypes at the locus were positive for IgG2c, these levels were quite low, and could be consistent with coincidental cross-reactivity of the detection antibodies used. To confirm this relationship more formally with previous analyses in the literature, we queried whole genome sequences of 24 CC strains [27,28] (3 strains with each of the founder haplotypes at IgH) using sequence strings that should exist as unique DNA sequences in the previously proposed IgG2c (CTCAAAGAGTGTCCCCCATGC)or IgG2a (AAGAACTGGGTGGAAAGAAATAGC) alleles [25,26]. We only found evidence for the sequence belonging to IgG2c in the genome sequences of CC strains containing B6 and NOD haplotypes (S2 Table) Similarly, CC strains with the 129S1, A/J, NZO, CAST, PWK and WSB haplotype at the locus only had sequence belonging to IgG2a, further reinforcing our intuition that the low levels of IgG2c detected in some of these strains represent spurious cross-reactivity. As such our data are consistent with, and strengthen the model proposed in [25,26] that IgG2a and IgG2c are, in fact, two alleles of the same gene. Concurrently, our ability to genetically map and associate these phenotypes to a prior implicated causal locus validates our larger genetic mapping approach.
Fig 2. Expression of IgG2a and IgG2c by founder strain haplotype at the heavy chain locus.
The mean value for IgG2a and IgG2c concentration (ug/mL) was determine for each CC strain. Mean values were plotted by the founder strain haplotype at the immunoglobulin heavy chain locus (Chromosome 12: 113 – 120Mb). Black bars indicate the mean concentration for a given founder haplotype and each individual point represents a strain with that founder haplotype at Igh. The y-axis is the concentration for IgG2a or IgG2c in ug/mL and the x-axis is the founder strain haplotype groups. Strains with undetectable concentrations of IgG2a or IgG2c were set to the lower limit of detection of the assay. Limit of detection represented by the dashed black line.
Qih1 broadly impacts homeostatic antibody and splenic B cells
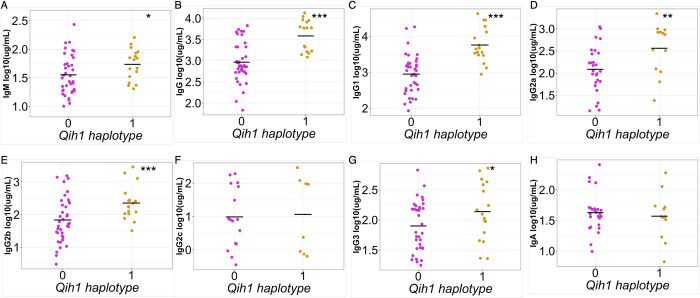
Given the proximity of Qih2, Qih3, and Qih4 to known immunologically relevant genome regions (Qih2 with the major histocompatibility complex (MHC), and Qih3 and 4 with the immunoglobulin heavy chain (IgH) locus), we focused our attention on the novel Qih1 (Fig 3). We first asked whether Qih1 specifically regulated IgG1 levels (the initial trait for which we mapped the locus), or if it was more broadly associated with differences in the levels of other antibody classes and isotypes. We simplified the eight haplotype groups present in the CC into high- and low- response haplotypes at Qih1 (BFH = high, ACDEG = low) using our previously established approach (Noll et al.). We found that there was a significant association between Qih1 haplotype groups and total IgG (p = 1.627e-6, Fig 4B) and IgG2b (p = 0.0024, Fig 4E) levels, as well as marginal associations with IgG2a (p = 0.015, Fig 4D), IgG3 (p = 0.057, Fig 4G), and IgM (p = 0.068, Fig 4A) levels, suggesting that Qih1 has broad effects on antibody levels at homeostasis.
Fig 3. Qih1 identified for variation in homeostatic IgG1 levels is driven by B6, WSB, and CAST haplotypes.
A) LOD plot showing QTL significance (y-axis) across the genome (x-axis) with significance thresholds (genome-wide p-value = 0.05 (red), 0.1 (blue), 0.2 (green)). Qih1 associated allele effects (A/J = yellow, B6 allele = grey, 129S1 allele = pink; NOD allele = dark blue; NZO allele = light blue; CAST = green; PWK = red; WSB = purple) were determined for the associated peak. B) Allele effect plot shows the mean deviation from population-wide mean as on the upper Y-axis for each allele segregating in the CC across the QTL peak region (x-axis positions are megabases on the chromosome) Highlighted region is the QTL confidence interval determined by a 1.5 LOD drop.
Fig 4. Qih1 shows broad effects across antibody isotypes and subtypes.
We assessed the relationship between B6, WSB, and CAST haplotypes (Qih1 haplotype = 1) and serum concentrations of (A) IgM, (B) total IgG, (C) IgG1, (D) IgG2a, (E) IgG2b, (F) IgG2c, (G) IgG3, and (H) IgA. Each point represents the mean value for each CC strain and the mean for each haplotype group on the x-axis is denoted by the grey crossbar. (*p < 0.1, **p < 0.05, ***p < 0.01) p-values determined using nested linear model approach described in the methods.
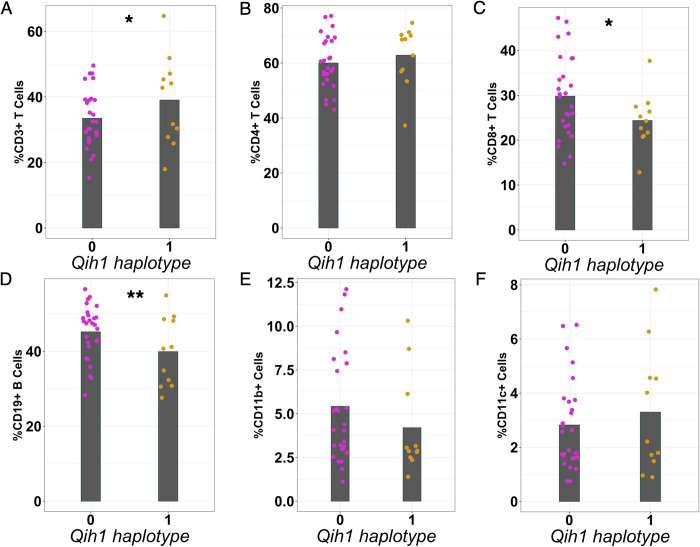
We next addressed whether this genetic regulation of differences in antibody levels was due to intrinsic production differences of antibody on a per cell basis or could be due to the locus controlling the abundance of specific cell populations (e.g., B cells). We took advantage of an independent cohort of 89 mice from 48 CC strains (1–2 mice per strain, S3 Table) [29]. We analyzed splenocytes from these mice for high-level immune populations (e.g., T cells, B cells, dendritic cells, and macrophages) at homeostasis, as these mice had not had any specific immunological perturbations performed on them. As with our original antibody screen, we found that these cell populations were highly variable across animals (2-6-fold differences, S4 Table), and that most of this variation could be attributed to differences between genotypes (heritability of 0.24–0.76, S4 Table). As above, we again assigned these CC strains to either the high or low haplotype groups at Qih1 and assessed the strength of relationships between these Qih1 haplotypes and the measured cell populations. We found an association between Qih1 and total CD19+ B cells in the spleen (p = 0.041, Fig 5D), where CC strains with a high antibody level at Qih1 showed a decrease in the proportion of total splenic B cells. We also found marginal associations between Qih1 and CD3+ T cells (p = 0.088, Fig 5A) and CD8+ T cells (p = 0.078, Fig 5C) in the same direction as the B cell relationship. However, we found no associations with CD4+ T cells (p = 0.414, Fig 5B), CD11b+ cells (p = 0.169, Fig 5E), or CD11c+ cells (p = 0.612, Fig 5F) in this study. These results suggest that Qih1 may broadly regulate multiple aspects of systemic immune homeostasis. However, consistent with its effects on antibody levels, the strongest relationship we observed was between Qih1 haplotype and splenic B cell proportions.
Fig 5. Qih1 shows broad effects on immune cell populations in the spleen.
We assessed the relationship between B6, WSB, and CAST haplotypes (Qih1 haplotype = 1) and (A) CD3+ T cells, (B) CD4+ T cells, (C) CD8+ T cells, (D) CD19+ B cells, (E) CD11b+ cells, and (F) CD11c+ cells in the spleen. Each point represents the mean value for each CC strain and the mean for each haplotype group on the x-axis is denoted by the grey crossbar. (*p < 0.1, **p < 0.05) p-values determined using nested linear model approach described in the methods.
Given the relationship between Qih1 and both antibody levels and the relative abundance of total B cells within the spleen, we next assessed how Qih1 impacts B cell development. We assessed total serum antibody levels and several B cell subsets in the spleen and bone marrow in 67 animals from a selected a set of 12 CC strains (2–8 mice/strain, 6 strains with a B6 haplotype, and 6 strains with contrasting NZO, NOD or PWK haplotypes at Qih1; S3 Table). We analyzed total B220+ B cells, early, immature, and mature B cells in the bone marrow, as well as total B220+, transitional, mature, and B1a (CD5+) B cells in the spleen (S3 Table). Across these B cell populations, we found that there was a trending association between splenic mature B cells and this locus (p = 0.102, S3C Fig), where animals with the B6 haplotype have lower levels. Concurrently, we validated that the B6 haplotype was associated with greater concentrations of both total IgG and IgG1 in the serum (S4 Fig). Taken together, these data suggest that Qih1 may be regulating antibody levels in a B cell intrinsic manner.
MBD1 as a novel regulator of homeostatic antibody levels and splenic B cell subsets
Concurrent with our work investigating the immune mechanisms that Qih1 causes variation in, we used our established QTL candidate analysis pipeline [23] to identify candidate genes underlying the effects of Qih1. Briefly, we first filtered all genes under the locus down to genes expressed in the spleen. Then, we further filtered this gene set down to genes with genetic variants that fit with the allele effects of Qih1. Within the Qih1 locus, there are only 14 protein coding genes, and 10 of these genes had variants specific to the 3 causal haplotypes (in total 12 genes with CAST-private variants, 11 genes with WSB-private variants, and 11 genes with B6-private genetic variants). However, while there were no common variants across all three haplogroups, one gene, Mbd1, contained missense or nonsense (protein sequence) variants that were specific to each of the causal haplotypes (six genes contained CAST-private protein effecting variants, WSB- and B6-private protein effecting variants only occurred in this one gene). As such, we focused our downstream analysis on the role of Methyl-CpG binding domain protein 1 (Mbd1) in regulating baseline antibody and B cell variation.
As noted above, there were no common missense variants in Mbd1 segregating B6, CAST and WSB from the other founder strains. However, all three strains possessed independent missense variants (WSB: rs36715598, SIFT: 0.05; CAST: rs36834535, SIFT: 0.24 and rs222802617, SIFT: 0.09; and B6: rs46321411, SIFT: 1, rs46176119, SIFT: 0, and rs30250376, SIFT: 1). Of note was the excess of non-synonymous differences between B6 and the other common laboratory strain founders of the CC. Therefore, we further investigated the amino acid sequence variation among 29 additional mouse strains and 3 additional species (rats, non-human primates, and humans) (S3 Table). [30,31] Besides the other Clarence Little strains (C57BL/6NJ, C57BL/10J, C57BR/cdJ, C57L/J, C58/J), only ST/bJ, BUB/BnJ (2 variants) and the wild derived ZALENDE/EiJ strains had these ‘B6’ variants. Given the otherwise high level of amino acid conservation in MBD1 across mouse strains, rats, non-human primates, and humans, this indicates that the B6 allele of Mbd1 is both highly evolutionarily derived in this region and likely of a single wild origin that was only introduced into a subset of mouse inbred strains.
MBD1 has been previously shown to be involved in T cell development (consistent with our above observation that there were T cell differences in some of our CC analyses) and autoimmunity [32], neural development [33,34], and adipocyte differentiation [35]. While Mbd1 deficient animals have been previously generated, these mice were initially generated on the 129S4 genetic background [36], and then backcrossed to C57BL/6. Given that 129 (129S1 in the case of the CC) and C57BL/6 had opposing haplotype effects, this makes it difficult to differentiate whether effects on baseline antibody or B cell populations are due to the lack of Mbd1, or other variants in the locus. Therefore, we generated a new Mbd1 knockout (KO) directly on the C57BL/6J genetic background by CRISPR gene editing to directly test whether MBD1 specifically plays a role in regulating homeostatic antibody levels and more broadly, B cell subset differences.
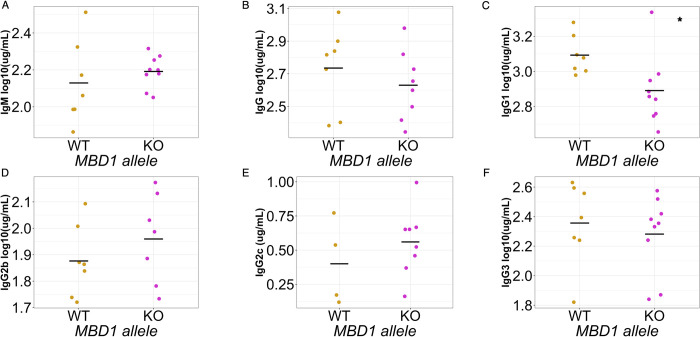
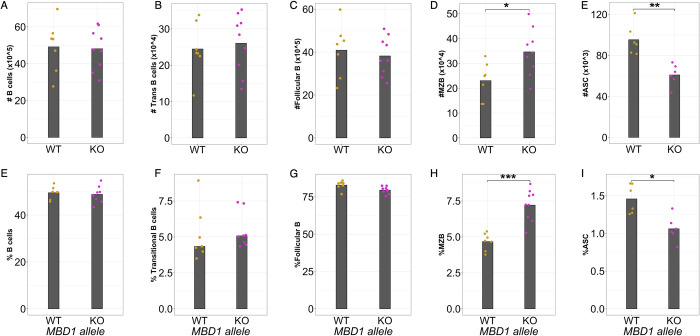
Using a heterozygote-x-heterozygote breeding design, we found that early adult (6-8wk old) Mbd1 KO animals had several antibody and B cell related differences relative to their WT littermates. Specifically, mutant mice had lower levels of IgG1 in the serum compared to WT littermates (Fig 6, recapitulating our observations in the CC). They also had significant increases in marginal zone B cell numbers and proportions (of all B cells) in the spleen, with no differences in total, transitional, or follicular B cells (Fig 7). Given that marginal zone B cells can rapidly differentiate into antibody secreting cells and the inverse relationship between MZBs and antibody levels in our KO mouse, we measured CD138+ antibody secreting cells in the spleen. As expected, we found a decrease in antibody secreting cell abundances in the spleens of Mbd1 KO animals, corresponding to the decrease in antibody and increase in MZBs (Figs 7E, 7J and S5).
Fig 6. MBD1 regulates homeostatic serum IgG1 levels.
Log10 transformed serum antibody concentrations for (A) IgM, (B) total IgG, (C) IgG1, (D) IgG2b, (E) IgG2c, and (F) IgG3. The data shown represent one of two independent experiments, each performed with 6–9 animals from each genotype. Each point represents an individual animal measurement, and the grey crossbar indicates the mean for each genotype. (*p < 0.05) p-values determined using Student’s t test.
Fig 7. MBD1 regulates B cell subsets at homeostasis.
The number and proportion of (A, E) CD19+, B220+ B cells, (B, F) transitional B cells, (C, G) follicular B cells, (D, H) marginal zone B cells, and (E, I) antibody secreting cells were measured in the spleen at baseline The data shown represent one of two independent experiments, each performed with 6–9 animals from each genotype. Each point represents an individual animal measurement, and the grey crossbar indicates the mean for each genotype. (*p < 0.05, **p < 0.01, ***p < 0.001) p-values determined using a Wilcoxon Ranked Sum test.
In a separate cohort of animals, we aged them further to maturity (15–16 weeks old), and assessed IgG1, total IgG and IgM levels. We found that Mbd1 KO animals maintained reduced levels of antibodies (total IgG and IgM, S2 Fig) relative to their wild type littermates through this age. All told, our data in this Mbd1 KO stock are consistent with the haplotype effects on baseline antibody and B cell populations observed in the CC, indicate that MBD1 is a negative regulator of marginal zone B cell differentiation and a suppressor of antibody secretion. Thus, it is likely that MBD1 acts to regulate marginal zone B cell differentiation into an antibody secreting cell, and genetic polymorphisms in the Mbd1 gene contributing to variation in baseline antibody levels and B cell proportions are due to a potential role in this differentiation process.
Discussion
Immune homeostasis is the state in which the immune system is maintained in the absence of specific insults. This balanced state is critical in ensuring an efficient response when challenged, while concurrently limiting immune pathology or unwanted (e.g. allergic) responses. The immune homeostatic state varies greatly in humans [3–4, 11–12] and has been shown to influence responses to immunotherapies and vaccination [1,3]. Therefore, understanding the genetic mechanisms that regulate the homeostatic state is critical to understanding an individual’s potential to respond to pathogen challenge. However, it is challenging to study immune homeostasis in human populations due to environmental factors like prior pathogen exposure, diet [37], or environmental insults [38]–all of which concurrently can perturb the immune system, As such, mouse models have been critical for identifying and understanding genes that regulate immune development and homeostasis [39–43]. We extend this body work by describing variation in homeostatic antibody levels across the Collaborative Cross population and identify four QTL that are associated with variation in three different IgG subtypes (IgG1, IgG2b, IgG2c).
Three of these QTL map to known immunologically important regions, Qih2 (mapped for IgG2c) is located near the major histocompatibility locus, and Qih3 (mapped for IgG2c) and Qih4 (mapped for IgG2b) are located at or near the immunoglobulin heavy chain locus. Both loci have long and well characterized associations with immune responses. Specifically, the MHC locus is overrepresented in genes involved in processing and presentation of foreign peptides to generate adaptive responses. Similarly, the Ig heavy chain locus contains genes necessary for generating the heavy chain component of every antibody isotype. Both regions are gene dense, have copy number variation, as well as gene family birth and loss [30]. As such, it was challenging for us to narrow these down to specific candidates for these loci, however our results point to the highly variable nature of the immune responses across the CC. We also identified a novel locus, Qih1, associated with variation in IgG1 levels at homeostasis, and demonstrated that this locus had broader effects on total antibody levels and splenic immune cell populations. Lastly, we identified a gene underneath Qih1, Mbd1, as a novel regulator of homeostatic antibody and marginal zone B cell differentiation to antibody secreting cells.
Our initial study focused on a variety of circulating antibody isotypes, and we identified loci at or near the major histocompatibility (MHC) locus and the immunoglobulin heavy chain (IgH) locus that are associated with variation in IgG2a/c or IgG2b. These loci validate our approach, as they serve as strong positive controls for regions known to be important for antibody levels. However, they can also inform potential mechanisms driving differences in total homeostatic antibody levels. Qih4 (mapped for IgG2b), located near the IgH locus, would potentially suggest cis-regulatory elements which control expression or regulation of IgG2b alleles. Qih2 (mapped for IgG2c), located near MHC, may indicate a haplotype specific manner of antigen detection and presentation that influences IgG2c expression or relate to the regulation of adaptive immune cell crosstalk, more generally. However, defining the specific mechanism by which Qih2 regulates IgG2c levels requires additional analysis of T cell responses and potentially other aspects of innate immune crosstalk with the adaptive B cell compartment. More broadly, various antibody isotypes and subtypes are important for different aspects of the immune response [44,45]. In our study, we have identified divergent haplotypes across loci regulating antibody levels at homeostasis, which suggests that independent genetic regulation arose from antigenic exposure histories in the evolution of various mouse strains [46].
Our mapping allowed us to return to an observation long known in the literature: that laboratory mice tend to produce either IgG2a or IgG2c, but not both [25]. Additionally, even in outbred mice it was somewhat unclear if these represented two alleles or paralogues closely linked on the same chromosome [25,26]. As described above, we found that CC strains with either B6 or NOD haplotypes at the IgH locus expressed IgG2c and strains with the other founder haplotypes at the locus expressed IgG2a. Concurrently, we took advantage of whole genome sequence data of the CC strains. We identified probe sequences from the original characterization of the IgG2a and IgG2c paralogues and confirmed for strains with a B6 or NOD haplotype at IgH that they not only expressed IgG2c, but that there were only genome sequence reads for the IgG2c gene. Likewise, strains with either A/J, 129S1, NZO, CAST, WSB, or PWK haplotypes at IgH only contained genome sequence reads for the IgG2a gene and not IgG2c. These data indicate that IgG2a and IgG2c indeed represent two distinct alleles in the mouse genome. It has been noted that there are functional differences between IgG2a and IgG2c [47], and these results can help investigators identify relevant mouse strains with specific IgG2 subtypes for functional follow-up to these two alleles.
B cells play a critical role in immune system development and homeostasis. Specific subsets of B cells, B1 and marginal zone B cells, differentiate to antibody secreting cells to produce natural antibodies, which are present before antigen stimulation and provide a first line of defense against infection [48–51]. Natural antibodies are characterized by their broad reactivity and low affinity and are pre-existing or immediately secreted upon stimulation or a ‘light push’ [48]. These antibodies are largely thought to bridge the gap between innate and adaptive immunity and have been studied for their ability to protect against various pathogens [51,52]. Several aspects of natural antibody have been investigated, but it is still largely unclear how these antibodies are regulated and under what contexts they are produced [48,53]. Here we identify a novel genetic regulator, Mbd1, of pre-existing antibody, marginal zone B cell, and antibody secreting cell levels at homeostasis. Our data suggests that MBD1 inhibits marginal zone B cell differentiation to antibody secreting cells at homeostasis, thus regulating pre-existing antibody levels.
MBD1 is a known epigenetic regulator, facilitating chromatin remodeling through various protein-protein interactions, binding directly to methylated DNA, and facilitating transcriptional repression [54,55]. Much of what is known about MBD1’s function comes from studies of neural stem cell differentiation [33] and adipocyte differentiation [35]. In the immune system, previous work has shown that MBD1 facilitates tissue-specific antigen expression through protein-protein interactions with AIRE to promote T cell tolerance [32]. However, MBD1 has not been described as having a role in B cell differentiation. Chromatin modifying complexes and other epigenetic regulators are critical to cellular differentiation, as chromatin accessibility changes over differentiation states allows for proper gene expression. Recent work has highlighted the important role of epigenetic regulators in B cell activation and differentiation. For example, EZH2 [56], LSD1 [57], and DNA methylation [58,59] have all been shown to regulate some aspect antibody secreting cell differentiation. While we do not provide a direct link between MBD1 function and antibody secreting cell differentiation, our data suggests it is likely that MBD1 is regulating chromatin dynamics necessary for altering gene expression profiles that promote antibody secreting cell differentiation. Thus, understanding the role of MBD1 in regulating gene expression profiles necessary for B cell differentiation will be important for defining those genes and regulatory networks that control B cell responses at homeostasis, as well as following infection and activation of adaptive immune responses. serving a comparable role.
Our study encompasses experiments using several cohorts of CC strains with varying ages and experimental designs, as well as a new Mbd1 knockout model on the B6 background. While we find that the B6 allele is consistently associated with greater antibody levels and lower splenic B cell proportions in the CC, we were not able to recapitulate all those phenotypes in our knockout studies. For example, in the CC the B6 haplotype is not only associated with greater levels of IgG1 in the serum but also greater levels of total IgG and marginally associated with greater levels of other antibody subtypes. However, we were only able to recapitulate the impact of MBD1 on IgG1 levels, specifically, in the context of our knockout model. It is not entirely surprising that we are not able to completely recapitulate every phenotype observed in the larger initial screen. In the CC, allelic variants across many genetic backgrounds are averaged, while a knockout represents an extreme abrogation in the context of a single genetic background. In inbred B6 mice, the evolutionarily derived allele of Mbd1 has co-evolved with protein binding partners and regulatory networks, whereas in the CC, those co-evolved networks have been broken apart and alleles are shuffled out of context. Additionally, there are 2 other founder strain haplotypes that were associated with greater antibody levels in the CC, and we do not capture their contributions in our knockout studies. Lastly, we do not map 100% of the genetic regulators of IgG1, as Qih1 only accounts for ~75% of the genetic regulation of IgG1 levels at homeostasis. Although, Qih1/Mbd1 has a large effect, there are other genes that contribute to the regulation of IgG1 and marginal zone B cell differentiation at homeostasis. None the less, we find a consistent role for Qih1/Mbd1 on homeostatic antibody levels and various B cell subsets across our experimental populations. Specifically, we found that a functional B6 Mbd1 allele is consistently associated with greater levels of homeostatic antibody and lower levels of various B cell subsets in the spleen. Interestingly, the association between the B6 allele and lower levels of B cell subsets was not observed in the bone marrow (S3 Fig), suggesting that overall B cell development was intact.
In summary our data provide evidence for strong genetic regulation of homeostatic immunity. We also show the utility of forward genetic screens in diverse mouse populations for identifying novel genes regulating homeostatic immunity. To our knowledge this is the first demonstration that MBD1 may act as a negative regulator of marginal zone B cell differentiation to antibody secreting cells, thereby regulating antibody levels at homeostasis. Additionally, our data further illustrates the role of epigenetic regulators in cellular differentiation and function. Future work to elucidate the specific pathways controlled by MBD1 to regulate marginal zone B cell differentiation could enhance our understanding of marginal zone B cell mediated humoral immunity at homeostasis and in response to pathogen infection.
Methods
Ethics statement
All mouse experiments were conducted under protocols approved by the University of North Carolina at Chapel Hill’s Institutional Animal Care and Use committee, which is AAALAC accredited.
Mice
Collaborative Cross mice
CC mice were obtained from the UNC Systems Genetics Core Facility at the University of North Carolina at Chapel Hill between 2013–2017. All experiments were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee. Mice were sacrificed using isoflurane overdose and terminally bled by cardiac puncture at six to twelve weeks of age depending on the experiment. For the initial baseline antibody screen, experiments were conducted under biosafety level 3 (BSL3) conditions where four to six weeks old female mice were cohoused across strains and allowed to acclimate to the BSL3 for 3–4 days. Mice were then inoculated subcutaneously in the left rear footpad with phosphate-buffered saline (PBS) supplemented with 1% fetal bovine serum (FBS). All other CC studies were conducted in standard animal housing facilities.
Mbd1 knockout mouse
Mbd1 knockout mice were generated at the University of North Carolina at Chapel Hill by the Animal Models Core via CRISPR mutagenesis. Specifically, Cas9 guide RNAs were designed with Benchling software and used to generate a 752bp deletion spanning exons 11 and 13, which resulted in protein ablation. The presence of the knockout or wildtype allele was determined by amplifying across the region using the following primers: forward primer–GCTCACTGAGTAGGGCAAGG, reverse primer–TACGGAGCACACCTTGGCA. Wildtype amplicon: 1262bp. Knockout amplicon: 510bp. We maintained these mice in our colony via 2 generations of backcrossing to C57BL/6J mice (JAX stock #000664) to remove any potential off-target mutations. The stock was thereafter maintained via het-by-het crosses.
ELISA
Total antibody levels were quantified by ELISA. 96-well flat-bottom high-binding plates were coated with anti-Ig antibodies (Southern Biotech) diluted in carbonate buffer for each antibody subtype measured. Serum was diluted in ELISA wash buffer (1x PBS + 0.3% Tween20) with 5% nonfat milk and added to pre-coated plates to incubate in humid storage overnight at 4°C. Plates were washed using ELISA wash buffer and incubated with HRP-conjugated anti-Ig secondary antibodies (Southern Biotech) for 2 hours in humid storage at 4°C. Plates were washed and developed in the dark for 30 minutes at room temperature with citrate substrate buffer, the reaction was stopped with sodium fluoride, and read immediately at 450nm. Standard curves for each antibody subtype (Invitrogen standards) were run with each plate for a specific subtype to determine antibody concentrations.
Sample preparation
Following euthanasia (as described above), blood was collected immediately into serum separator tubes to isolate serum for ELISAs. Serum was aliquoted in 1.7mL Eppendorf snap-cap tubes and stored at -80°C until analyzed by ELISA. Spleens were homogenized using frosted microscope slides and pelleted by centrifugation (1000 RPM/4°C/10 minutes). Spleen homogenates were filtered through 70um mesh, treated with ammonium chloride potassium (ACK) lysing buffer to remove red blood cells, washed, and resuspended in FACS buffer (HBSS + 1–2% FBS). Cell numbers were determined by trypan blue exclusion using a hemocytometer or Countess II automated cell counter.
Flow cytometry
Cells were plated at 1-2x107 per mL in FACS buffer (HBSS + 1–2% FBS) in a 96-well polypropylene round-bottom plate. Cells were centrifuged at 1000 RPM for 4 minutes at 4°C and resuspended in 100uL of fluorochrome-conjugated antibody dilution. Cells were incubated at 4°C for 45 minutes to allow for antibody staining. Following incubation, cells were washed twice with FACS buffer and resuspended in 100uL of FACS buffer. An equal volume of 4% PFA (in PBS) was added to cells to fix, and plates were stored in the dark at 4°C until analyzed of Attune NxT flow cytometer. Data was analyzed using FlowJo software. The following antibodies were used: Live/Dead Fixable Aqua, CD3-PE (145-2C11), CD4-APC/Cy7 (GK1.5), CD8a-PerCP(53–6.7), CD11b-eF450 (M1/70), CD11c-PE/TxRd (N418), CD19-AF647 (6D5), CD45-AF700 (30-F11), CD19-APC/Cy7 (6D5), IgM-PE (ll/41), IgD-FITC(11-26c.2a), CD5-BV421 (53–7.3), CD3-PerCP (145-2C11), CD21/CD35-PE (7E9), IgM-AF594, CD23-PE/Cy7 (B3B4), CD45R-APC (RA3-6B2).
Data processing
Antibody concentrations were determined from a standard curve and log10 transformed to follow a normal distribution. Event counts from flow cytometry gating were used to calculate cell proportions. Using Box-Cox transformation (MASS package in R, version 3.5.1), values were independently transformed for each phenotype to follow a normal distribution. For all phenotypes used for QTL mapping, the average phenotype value was calculated for each Collaborative Cross strain and used to map.
Statistical analysis
Nested linear models
Correlations between identified QTL and immune cell populations and other antibody subtypes were determine by comparing the goodness of model fit of mixed effect linear models using a partial fit F-test. Qih1 haplotype scores were determined by the founder haplotype at the Qih1 peak marker. In both the base and full model, CC Strain is a random effect variable and Qih1 haplotype score is a fixed effect variable. The full model tests whether including information about the haplotype at Qih1 explains more of the phenotypic variation than the CC Strain alone.
Base model: Phenotype ~ CC Strain + error
Full model: Phenotype ~ CC Strain + Qih1 haplotype score + error
Statistical significance for phenotypes compared between wild-type and Mbd1 knockout mice was determined using a Wilcoxon Ranked Sum test.
Broad sense heritability estimates
Heritability calculations were performed as described previously (Noll et al., 2020). Briefly, box-cox transformed phenotype values were used to fit a linear fixed-effect model. The coefficient of genetic determination was calculated as such:
Where MSCC-F1 is the mean square of the CC-F1 and MSε is the mean square of the error using a N = 3 as an average group size, as a measure of broad-sense heritability.
QTL mapping
QTL mapping was performed as previously described [23,24]. Briefly, we used the DOQTL [60] package in the R statistical environment (version 3.5.1). A multiple regression is performed at each marker, assessing the relationship between the phenotype and the haplotype probabilities for each strain. LOD scores are calculated based on the increase in statistical fit compared to a null model, considering only covariates and kinship. To calculate significance thresholds, permutation tests were used to shuffle genotypes and phenotypes without replacement. We determined the 80th, 90th, and 95th percentiles after 500 permutations as cutoffs for suggestive (both p<0.2 and p<0.1) and genome-wide significant (p<0.05). QTL intervals were determined using a 1.5 LOD drop.
Supporting information
(TIFF)
We assessed IgG1, Total IgG, and IgM levels in the serum of 15-16wk old animals. Each point represents an individual animal and the median for each genotype group on the x-axis is denoted by a crossbar. (*p < 0.05)
(TIFF)
We assessed the relationship between B6, WSB, and CAST haplotypes (Qih1 haplotype = 1) and spleen total B220+ B cells (A), transitional (IgMvar, IgD-) B cells (B), mature (IgMvar, IgD+) B cells (C), B1 (CD5+) B cells (D), and bone marrow total B220+ B cells (E), early (IgM-, IgD-)B cells (F), and immature (IgM+, IgD-) B cells (G). Each point represents the mean value for each CC strain and the mean for each haplotype group on the x-axis is denoted by the grey crossbar. (#p < 0.2, *p < 0.1)
(TIFF)
We assessed the relationship between B6, WSB, and CAST haplotypes (Qih1 haplotype = 1) and serum total igG and IgG1 levels. Each point represents the mean value for each CC strain and the mean for each haplotype group on the x-axis is denoted by the grey crossbar.
(TIFF)
(TIFF)
P-values were determined using the cor.test function in R stats package (version 3.5.1).
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We wish to thank the Systems Genetics Core Facility for the maintenance of the Collaborative Cross lines used in these studies. We would also like to thank the UNC Flow Cytometry Core for their help with flow cytometry data acquisition and analysis.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported, in part, by U19AI100625 (to MTH, MTF, and FPMV), P01AI132130 (to FPMV and MTF), R21AI119933 (to MTH), HHMI Gilliam Fellowship (to BKH), and F99AG073570 (to BKH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gnjatic S, Bronte V, Brunet LR, Butler MO, Disis ML, Galon J, et al. Identifying baseline immune-related biomarkers to predict clinical outcome of immunotherapy. J Immunother Cancer. 2017;5: 44. doi: 10.1186/s40425-017-0243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham JB, Swarts JL, Menachery VD, Gralinski LE, Schäfer A, Plante KS, et al. Immune Predictors of Mortality After Ribonucleic Acid Virus Infection. J Infect Dis. 2020;221(6):882–889. doi: 10.1093/infdis/jiz531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.HIPC-CHI Signatures Project Team; HIPC-I Consortium. Multicohort analysis reveals baseline transcriptional predictors of influenza vaccination responses. Sci Immunol. 2017;2(14):eaal4656. doi: 10.1126/sciimmunol.aal4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsang JS, Schwartzberg PL, Kotliarov Y, Biancotto A, Xie Z, Germain RN, et al. Global analyses of human immune variation reveal baseline predictors of postvaccination responses. Cell. 2014;157(2):499–513. doi: 10.1016/j.cell.2014.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crimeen-Irwin B, Scalzo K, Gloster S, Mottram PL, Pleblanski M. Failure of immune homeostasis—the consequences of under and over reactivity. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5(4):413–422. doi: 10.2174/156800805774912980 [DOI] [PubMed] [Google Scholar]
- 6.Palma J, Tokarz-Deptula B, Deptula J, Deptula W. Natural antibodies—facts known and unknown. Cent Eur J Immunol. 2018;43(4):466–475. doi: 10.5114/ceji.2018.81354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham JB, Swarts JL, Mooney M, Choonoo G, Jeng S, Miller DR, et al. Extensive Homeostatic T Cell Phenotypic Variation within the Collaborative Cross. Cell Rep. 2017;21(8):2313–2325. doi: 10.1016/j.celrep.2017.10.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krištić J, Zaytseva OO, Ram R, Nguyen Q, Novokmet M, Vučković F, et al. Profiling and genetic control of the murine immunoglobulin G glycome. Nat Chem Biol. 2018;14(5):516–524. doi: 10.1038/s41589-018-0034-3 [DOI] [PubMed] [Google Scholar]
- 9.Phillippi J, Xie Y, Miller DR, Bell TA, Zhang Z, Lenarcic AB, et al. Using the emerging Collaborative Cross to probe the immune system. Genes Immun. 2014;15(1):38–46. doi: 10.1038/gene.2013.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collin R, Balmer L, Moraham G, Lesage S. Common Heritable Immunological Variations Revealed in Genetically Diverse Inbred Mouse Strains of the Collaborative Cross. J Immunol. 2019;202(3):777–786. doi: 10.4049/jimmunol.1801247 [DOI] [PubMed] [Google Scholar]
- 11.Cassidy JT, Nordby GL, Dodge HJ. Biologic variation of human serum immunoglobulin concentrations: sex-age specific effects. J Chronic Dis. 1974;27(11–12):507–516. doi: 10.1016/0021-9681(74)90026-5 [DOI] [PubMed] [Google Scholar]
- 12.Grundbacher FJ. Heritability estimates and genetic and environmental correlations for the human immunoglobulins G, M, and A. Am J Hum Genet. 1974;26(1):1–12. [PMC free article] [PubMed] [Google Scholar]
- 13.Poon MML, Byington E, Meng W, Kutoba M, Matsumoto R, Grifnoi A, et al. Heterogeneity of human anti-viral immunity shaped by virus, tissue, age, and sex. Cell Rep. 2021;37(9):110071. doi: 10.1016/j.celrep.2021.110071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collaborative Cross Consortium. The genome architecture of the Collaborative Cross mouse genetic reference population. Genetics. 2012;190(2):389–401. doi: 10.1534/genetics.111.132639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Threadgill DW, Miller DR, Churchill GA, de Villena FP. The collaborative cross: a recombinant inbred mouse population for the systems genetic era. ILAR J. 2011;52(1):24–31. doi: 10.1093/ilar.52.1.24 [DOI] [PubMed] [Google Scholar]
- 16.Welsh CE, Miller DR, Manly KF, Wang J, McMillan L, Morahan G, et al. Status and access to the Collaborative Cross population. Mamm Genome. 2012;23(9–10):706–712. doi: 10.1007/s00335-012-9410-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts A, Pardo-Manuel de Villena F, Wang W, McMillan L, Threadgill DW. The polymorphism architecture of mouse genetic resources elucidated using genome-wide resequencing data: implications for QTL discovery and systems genetics. Mamm Genome. 2007;18(6–7):473–481. doi: 10.1007/s00335-007-9045-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferris MT, Aylor DL, Bottomly D, Whitmore AC, Aicher LD, Bell TA, et al. Modeling host genetic regulation of influenza pathogenesis in the collaborative cross. PLoS Pathog. 2013;9(2):e1003196. doi: 10.1371/journal.ppat.1003196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He L, Wang P, Schick SF, Huang A, Jacob P 3rd, Yang X, et al. Genetic background influences the effect of thirdhand smoke exposure on anxiety and memory in Collaborative Cross mice. Sci Rep. 2021;11(1):13285. doi: 10.1038/s41598-021-92702-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham JB, Swarts JL, Leist SR, Schäfer A, Menachery VD, Gralinski LE, et al. Baseline T cell immune phenotypes predict virologic and disease control upon SARS-CoV infection in Collaborative Cross mice. PLoS Pathog. 2021;17(1):e1009287. doi: 10.1371/journal.ppat.1009287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu B, Shorter JR, Williams LH, Bell TA, Hock P, Dalton KA, et al. Collaborative Cross mice reveal extreme epilepsy phenotypes and genetic loci for seizure susceptibility. Epilepsia. 2020;61(9):2010–2021. doi: 10.1111/epi.16617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith CM, Proulx MK, Lai R, Kiristsy MC, Bell TA, Hock P, et al. Functionally Overlapping Variants Control Tuberculosis Susceptibility in Collaborative Cross Mice. mBio. 2019;10(6):e02791–19. doi: 10.1128/mBio.02791-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noll KE, Whitmore AC, West A, McCarthy MK, Morrison CR, Plante KS, et al. Complex Genetic Architecture Underlies Regulation of Influenza-A-Virus-Specific Antibody Responses in the Collaborative Cross. Cell Rep. 2020;31(4):107587. doi: 10.1016/j.celrep.2020.107587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hampton BK, Jensen KL, Whitmore AC, Linnertz CL, Maurizio P, Miller DR, et al. Genetic regulation of homeostatic immune architecture in the lungs of collaborative cross mice. BioRxiv [Preprint]. bioRxiv 2021.04.09.439180: Available from: https://www.biorxiv.org/content/10.1101/2021.04.09.439180v1 doi: 10.1101/2021.04.09.439180 [DOI] [Google Scholar]
- 25.Morgado MG, Cam P, Gris-Liebe C, Cazenave PA, Jouvin-Marche E. Further evidence that BALB/c and C57BL/6 gamma 2a genes originate from two distinct isotypes. EMBO J. 1989;8(11):3245–3251. doi: 10.1002/j.1460-2075.1989.tb08484.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Goldschmidt T, Salter H. Possible allelic structure of IgG2a and IgG2c in mice. Mol Immunol. 2012;50(3):169–171. doi: 10.1016/j.molimm.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 27.Srivastava A, Morgan AP, Najarian ML, Sarsani VK, Sigmon JS, Shorter JS, et al. Genomes of the Mouse Collaborative Cross. Genetics. 2017;206(2):537–556. doi: 10.1534/genetics.116.198838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shorter JR, Najarian ML, Bell TA, Blanchard M, Ferris MT, Hock P, et al. Whole Genome Sequencing and Progress Toward Full Inbreeding of the Mouse Collaborative Cross Population. G3 (Bethesda). 2019;9(5):1303–1311. doi: 10.1534/g3.119.400039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keele GR, Quach BC, Israel JW, Chappell GA, Lewis L, Safi A, et al. Integrative QTL analysis of gene expression and chromatin accessibility identifies multi-tissue patterns of genetic regulation. PLoS Genet. 2020;16(1):e1008537. doi: 10.1371/journal.pgen.1008537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477(7364):289–294. doi: 10.1038/nature10413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doran AG, Wong K, Flint J, Adams DJ, Hunter KW, Keane TM. Deep genome sequencing and variation analysis of 13 inbred mouse strains defines candidate phenotypic alleles, private variation and homozygous truncating mutations. Genome Biol. 2016;17(1):167. doi: 10.1186/s13059-016-1024-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waterfield M, Khan IS, Cortez JT, Fan U, Metzger T, Greer A, et al. The transcriptional regulator Aire coopts the repressive ATF7ip-MBD1 complex for the induction of immunotolerance. Nat Immunol. 2014;15(3):258–265. doi: 10.1038/ni.2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lax E, Sapozhnikov DM. Adult Neural Stem Cell Multipotency and Differentiation Are Directed by the Methyl-CpG-Binding Protein MBD1. J Neurosci. 2017;37(16):4228–4230. doi: 10.1523/JNEUROSCI.0411-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jobe EM, Gao Y, Eisinger BE, Mladucky JK, Giuliani CC, Kelnhofer LE, et al. Methyl-CpG-Binding Protein MBD1 Regulates Neuronal Lineage Commitment through Maintaining Adult Neural Stem Cell Identity. J Neurosci. 2017;37(3):523–536. doi: 10.1523/JNEUROSCI.1075-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumara Y, Nakaki R, Inagaki T, Yoshida A, Kano Y, Kimura H, et al. H3K4/H3K9me3 Bivalent Chromatin Domains Targeted by Lineage-Specific DNA Methylation Pauses Adipocyte Differentiation. Mol Cell. 2015;60(4):584–596. doi: 10.1016/j.molcel.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 36.Zhao X, Ueba T, Christie BR, Barkho B, McConnell MJ, Nakashima K, et al. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc Natl Acad Sci U S A. 2003;100(11):6777–6782. doi: 10.1073/pnas.1131928100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapellos TS, Bonaguro L, Gemünd I, Reusch N, Saglam A, Hinkley ER, et al. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front Immunol. 2019;10:2035. doi: 10.3389/fimmu.2019.02035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smeester L, Bommarito PA, Martin EM, Recio-Vega R, Gonzalez-Corets T, Olivas-Calderon E, et al. Chronic early childhood exposure to arsenic is associated with a TNF-mediated proteomic signaling response. Environ Toxicol Pharmacol. 2017;52:183–187. doi: 10.1016/j.etap.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falk I, Potocnik AJ, Barthlott T, Levelt CN, Elchmann K. Immature T cells in peripheral lymphoid organs of recombinase-activating gene-1/-2-deficient mice. Thymus dependence and responsiveness to anti-CD3 epsilon antibody. J Immunol. 1996;156(4):1362–1368. [PubMed] [Google Scholar]
- 40.Kitamura D, Roes J, Kühn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350(6317):423–426. doi: 10.1038/350423a0 [DOI] [PubMed] [Google Scholar]
- 41.Lansford R, Manis JP, Sonoda E, Rajewsky K, Alt FW. Ig heavy chain class switching in Rag-deficient mice. Int Immunol. 1998;10(3):325–332. doi: 10.1093/intimm/10.3.325 [DOI] [PubMed] [Google Scholar]
- 42.Khattri R, Kasprowicz D, Cox T, Mortrud M, Appleby MW, Brunkow ME, et al. The amount of scurfin protein determines peripheral T cell number and responsiveness. J Immunol. 2001;167(11):6312–6320. doi: 10.4049/jimmunol.167.11.6312 [DOI] [PubMed] [Google Scholar]
- 43.Kasprowicz DJ, Smallwood PS, Tyznik AJ, Ziegler SF. Scurfin (FoxP3) controls T-dependent immune responses in vivo through regulation of CD4+ T cell effector function. J Immunol. 2003;171(3):1216–1223. doi: 10.4049/jimmunol.171.3.1216 [DOI] [PubMed] [Google Scholar]
- 44.Collins AM. IgG subclass co-expression brings harmony to the quartet model of murine IgG function. Immunol Cell Biol. 2016;94(10):949–954. doi: 10.1038/icb.2016.65 [DOI] [PubMed] [Google Scholar]
- 45.Vidarsson G, Dekkers G, Rispen T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith CM, Proulx MK, Olive AJ, Laddy D, Mishra BB, Moss C, et al. Tuberculosis Susceptibility and Vaccine Protection Are Independently Controlled by Host Genotype. mBio. 2016;7(5):e01516–16. doi: 10.1128/mBio.01516-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petruchina I, Tran M, Sadzikava N, Ghochikyan A, Vasilevko V, Agadjanyan MG, et al. Importance of IgG2c isotype in the immune response to beta-amyloid in amyloid precursor protein/transgenic mice. Neurosci Lett. 2003;338(1):5–8. doi: 10.1016/s0304-3940(02)01357-5 [DOI] [PubMed] [Google Scholar]
- 48.Holodick NE, Rodriguez-Zhurbenko N, Hernandez AM. Defining Natural Antibodies. Front Immunol. 2017;8:872. doi: 10.3389/fimmu.2017.00872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou ZH, Zhang Y, Hu YF, Wahl LM, Cisar JO, Notkins AL. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe. 2007;1(1):51–61. doi: 10.1016/j.chom.2007.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subramaniam KS, Datta K, Quintero E, Manix C, Marks MS, Pirofski LA. The absence of serum IgM enhances the susceptibility of mice to pulmonary challenge with Cryptococcus neoformans. J Immunol. 2010;184(10):5755–5767. doi: 10.4049/jimmunol.0901638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. 2007;81(7):3487–3494. doi: 10.1128/JVI.02128-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panda S, Ding JL. Natural antibodies bridge innate and adaptive immunity. J Immunol. 2015;194(1):13–20. doi: 10.4049/jimmunol.1400844 [DOI] [PubMed] [Google Scholar]
- 53.New JS, King RG, Kearney JF. Manipulation of the glycan-specific natural antibody repertoire for immunotherapy. Immunol Rev. 2016;270(1):32–50. doi: 10.1111/imr.12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujita N, Shimotake N, Ohki I, Chiba T, Saya H, Shirakawa M, et al. Mechanism of transcriptional regulation by methyl-CpG binding protein MBD1. Mol Cell Biol. 2000;20(14):5107–5118. doi: 10.1128/MCB.20.14.5107-5118.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ichimura T, Watanabe S, Sakamoto Y, Aoto T, Fujita N, Makao M. Transcriptional repression and heterochromatin formation by MBD1 and MCAF/AM family proteins. J Biol Chem. 2005;280(14):13928–13935. doi: 10.1074/jbc.M413654200 [DOI] [PubMed] [Google Scholar]
- 56.Herviou L, Jourdan M, Martinez A, Cavalli G, Moreaux J. EZH2 is overexpressed in transitional preplasmablasts and is involved in human plasma cell differentiation. Leukemia. 2019;33(8):2047–2060. doi: 10.1038/s41375-019-0392-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haines RR, Barwick BG, Scharer CD, Majumber P, Randall TD, Boss JM. The Histone Demethylase LSD1 Regulates B Cell Proliferation and Plasmablast Differentiation. J Immunol. 2018;201(9):2799–2811. doi: 10.4049/jimmunol.1800952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barwick BG, Scharer CD, Bally APR, Boss JM. Plasma cell differentiation is coupled to division-dependent DNA hypomethylation and gene regulation. Nat Immunol. 2016;17(10):1216–1225. doi: 10.1038/ni.3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barwick BG, Scharer CD, Martinez RJ, Price MJ, Wein AN, Haines RR, et al. B cell activation and plasma cell differentiation are inhibited by de novo DNA methylation. Nat Commun. 2018;9(1):1900. doi: 10.1038/s41467-018-04234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gatti DM, Svenson KL, Shabalin A, Wu L, Valdar W, Simecek P, et al. Quantitative trait locus mapping methods for diversity outbred mice. G3 (Bethesda). 2014;4(9):1623–1633. doi: 10.1534/g3.114.013748 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIFF)
We assessed IgG1, Total IgG, and IgM levels in the serum of 15-16wk old animals. Each point represents an individual animal and the median for each genotype group on the x-axis is denoted by a crossbar. (*p < 0.05)
(TIFF)
We assessed the relationship between B6, WSB, and CAST haplotypes (Qih1 haplotype = 1) and spleen total B220+ B cells (A), transitional (IgMvar, IgD-) B cells (B), mature (IgMvar, IgD+) B cells (C), B1 (CD5+) B cells (D), and bone marrow total B220+ B cells (E), early (IgM-, IgD-)B cells (F), and immature (IgM+, IgD-) B cells (G). Each point represents the mean value for each CC strain and the mean for each haplotype group on the x-axis is denoted by the grey crossbar. (#p < 0.2, *p < 0.1)
(TIFF)
We assessed the relationship between B6, WSB, and CAST haplotypes (Qih1 haplotype = 1) and serum total igG and IgG1 levels. Each point represents the mean value for each CC strain and the mean for each haplotype group on the x-axis is denoted by the grey crossbar.
(TIFF)
(TIFF)
P-values were determined using the cor.test function in R stats package (version 3.5.1).
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.