Abstract
Purpose
Cyclin-dependent kinase 4/6 inhibitors (CDK4/6is), in combination with endocrine therapy (ET), are standard either in the first (1L) or second-line (2L) setting for the treatment of hormone receptor (HR) positive, HER2-negative metastatic breast cancer (MBC). However, the optimal sequencing of treatments after progression on CDK4/6i remains unknown. We performed a single-institution analysis to identify treatments and outcomes after progression on a CDK4/6i.
Methods
We identified patients with HR-positive, HER2-negative MBC prescribed a CDK4/6i in the 1L or 2L settings from December 2014 to February 2018 at Mayo Clinic in Rochester, Minnesota. Outcomes were collected through September 30, 2020.
Results
Palbociclib, in combination with letrozole or fulvestrant, was the most prescribed CDK4/6i. The 1L and 2L CDK4/6i cohorts exhibited comparable overall survival (OS), but progression-free survival (PFS) was longer in the 1L than the 2L cohort [28.2 months (95% CI 19.6–34.9) vs 19.8 months (95% CI 15.7–29.6)]. The most common post-CDK4/6i treatments were PI3K/mTOR inhibitors (PI3K/mTORi), single-agent ET, or chemotherapy. PFS in the 1L CDK4/6i cohort following PI3K/mTORi was 8.5 months (95% CI 5.5 months—NE), single-agent ET was 6.0 months (95% CI 3.3–14.0 months), and chemotherapy PFS was 5.4 months (95% CI 3.3 months—NE).
Conclusions
Following progression on a CDK 4/6i, mPFS was short, with similar PFS times comparing chemotherapy and ET, with slightly longer PFS for targeted strategies (PI3K/mTOR). These results highlight a major need to better understand the mechanisms of CDK4/6i resistance and identify new therapeutic strategies for these patients.
Keywords: CDK 4/6 inhibitor, PI3K/mTOR inhibitor, Subsequent lines of therapy, Metastatic breast cancer
Introduction
Approximately 60–70% of the 150,000 women currently living with metastatic breast cancer (MBC) in the United States have tumors that are hormone receptor (HR)− positive and HER2− negative [1–3]. While endocrine therapy (ET) remains an essential therapeutic option for this population [4], intrinsic or acquired resistance inevitably emerges. Preventing and reversing resistance to ET necessitates the use of effective and optimally sequenced therapies.
Cyclin-dependent 4 and 6 kinases (CDK 4/6) are key regulators of cell-cycle progression [5]. Over the last five years, there have been three Food and Drug Administration (FDA)-approved CDK4/6 inhibitors (CDK4/6i) (palbociclib, ribociclib, and abemaciclib) based on data from phase III randomized clinical trials in the first-line (1L; PALOMA-2, MONALEESA-2, MONALEESA-7, MONARCH-3) [6–9] and second-line (2L; PALOMA-3, MONALEESA-3, MONARCH-2) treatment of advanced breast cancer [10–12]. These studies demonstrated a clinically significant progression-free survival (PFS) benefit when compared to single-agent ET (letrozole or fulvestrant) [8, 9, 13, 14], with updated results highlighting an overall survival (OS) advantage with some CDK4/6i [10–12, 15, 16].
While CDK4/6i have become standard of care as 1L or 2L treatment of HR-positive MBC, the optimal treatment strategy after progression on a CDK4/6i remains uncertain. To determine the prognosis of patients following progression on CDK4/6i based on the type of post-CDK4/6i therapy used, we performed a single-institution retrospective review of patients with HR-positive MBC receiving 1L or 2L CDK4/6i with the goal of describing the prescribing patterns and clinical responses following progression on CDK4/6i-based treatment.
Methods
Patient selection
As part of an Institutional Review Board approved protocol, we utilized the electronic medical record to identify individuals with MBC prescribed palbociclib, ribociclib, or abemaciclib by a medical oncologist in Mayo Clinic (Rochester, MN) between December 2014 and February 2018. We utilized ICD-10 codes and record review to focus on those prescribed 1L or 2L CDK4/6i who received at least 30 days of therapy and attended more than two clinic visits at our institution during treatment. In-class switching of ET (i.e., changing from one aromatase inhibitor to another for toxicity) was not considered a new line of therapy. If a patient switched to a different CDK4/6i (e.g., palbociclib to abemaciclib) or a different class of ET (e.g., letrozole to fulvestrant while remaining on the same CDK4/6i), this was considered a subsequent line of therapy.
Clinical outcomes
Endocrine therapy resistance was classified as primary and secondary, per the 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer [17]. Based on this definition, our 1L cohort was classified as primary ET resistance when metastatic relapse occurred within the first two years of adjuvant ET. Secondary ET resistance was defined as (a) relapse while on adjuvant ET, but after the first 2 years, (b) relapse within 12 months of completing adjuvant ET. In the 2L cohort, primary ET resistance was defined as progression within the first six months of ET for MBC.
Patient data were abstracted from December 1, 2014 through September 30, 2020, and three authors (GMC, KVG, SL) participated in data abstraction. We separately evaluated 1L and 2L cohorts to determine the PFS during and after CDK4/6i therapy. We defined PFS as the time from the date of initiation of CDK4/6i to the date of disease progression that was determined clinically, radiographically, or pathologically. Patients who stopped treatment due to toxicity prior to disease progression were censored at time of therapy discontinuation. We measured OS from the start of CDK4/6i-based therapy until time of death or last follow-up where patients were censored if they were alive or lost to follow-up.
Statistical analyses
Continuous variables were summarized as median and standard deviation (SD) reported with 95% confidence intervals (CI) or 25th/75th percentile (Q1, Q3). In subgroup analyses of the 2L and third-line (3L) setting, samples were too small to calculate an accurate 95% CI and were subsequently denoted as not evaluable (NE), where applicable. All statistical analyses, including Kaplan–Meier survival curves were performed using BlueSky statistics (BlueSky Statistics LLC, Chicago, IL USA).
Results
Patient characteristics
We identified 136 patients who started a CDK4/6i as 1L (n = 91) or 2L (n = 45) therapy for HR-positive MBC. A total 37 patients (27.2%) from both cohorts had a diagnosis of de novo metastatic disease. Of the 99 patients who relapsed following an initial diagnosis of early-stage disease, all underwent previous surgical resection, 21 patients (21.2%) received neoadjuvant chemotherapy, and 52 patients (52.5%) received adjuvant chemotherapy. In patients with relapsed disease, 90 patients (90.9%) had received adjuvant ET with a median treatment duration of 45 months (Q1–Q3 26–60 months) in the 1L cohort and 60 months (Q1–Q3 27.5–60 months) in the 2L cohort.
At the time of diagnosis of metastatic disease, the median age was 59 years in the 1L cohort and 63 years in the 2L cohort. The majority of patients had osseous disease (1L CDK4/6i cohort: 82.4%; 2L CDK4/6i cohort: 77.8%), with few patients with visceral metastases (1L CDK4/6i cohort: 17.6%; 2L CDK4/6i cohort 20%) or brain metastases (1L CDK4/6i cohort 3.3%; 2L CDK4/6i cohort 4.4%). Of the 1L CDK4/6i cohort, 9 (14.1%) were defined as having primary ET resistance and 24 (37.5%) had secondary ET resistance prior to CDK4/6i administration. In contrast, the 2L CDK4/6i cohort had higher rates of primary (n = 10, 35.7%) and secondary ET resistance (n = 10, 35.7%), as the majority of these patients were on ET as 1L treatment for HR-positive MBC (n = 37, 82.2%). Additional demographic details including prior treatment history and ET resistance are given in Table 1.
Table 1.
Patient characteristics in first-line (1L) and second-line (2L) CDK4/6i cohorts
| Total number of patients | 1L CDK4/6i | 2L CDK4/6i | |
|---|---|---|---|
| 91 | 45 | ||
|
| |||
| Age at start of CDK4/6i (years) | Median (Q1–Q3) | 59 (50–66) | 63 (52–71) |
| Deaths, n (%) | 31 (34.1%) | 27 (60%) | |
| Gender, n (%) | Female | 90 (98.9%) | 43 (95.5%) |
| Male | 1 (1.1%) | 2 (4.5%) | |
| M stage at initial diagnosis, n (%) | M0 | 71 (78.0%) | 28 (62.2%) |
| M1 | 20 (22.0%) | 17 (37.8%) | |
| Sites of metastases at diagnosis | Bone | 75 (82.4%) | 35 (77.8%) |
| Liver | 16 (17.6%) | 9 (20%) | |
| Lung | 28 (30.8%) | 6 (13.3%) | |
| Brain | 3 (3.3%) | 2 (4.4%) | |
| Prior curative intent surgery, n (%) | n = 71 | n = 30 | |
| Lumpectomy | 25 (35.2%) | 14 (46.6%)a | |
| Mastectomy | 46 (64.8%) | 16 (53.4%) | |
| Prior chemotherapy, n (%) | n = 71 | n = 28 | |
| Neoadjuvant chemotherapy | 16 (22.5%) | 5 (17.9%) | |
| Adjuvant chemotherapy | 35 (49.3%) | 17 (60.7%) | |
| None | 30 (42.2%) | 6 (21.4%) | |
| Prior adjuvant radiation therapy, n (%) | None | 24 (33.8%) | 7 (25%) |
| Received | 47 (66.2%) | 21 (75%) | |
| Adjuvant ET, n (%) | None | 7 (9.9%) | 2 (5.9%) |
| Received | 64 (90.1%) | 26 (94.1%) | |
| Median Duration of ET, months (Q1–Q3) | 45 (26–60) | 60 (27.5–60) | |
| Type of ET resistanceb | Primary ET resistance | 9 (14.1%) | 10 (35.7%) |
| Secondary ET resistance | 24 (37.5%) | 10 (35.7%) | |
One patient underwent lumpectomy for bone-only metastatic disease after near complete response on ET and another patient underwent mastectomy and found to have metastatic disease shortly after surgery
ET resistance was higher in the 2L CDK4/6i cohort due to the majority of patients having previously been on ET as 1L treatment for metastatic breast cancer
First-line (1L) CDK4/6i cohort outcomes
In the 1L cohort (n = 91), palbociclib + letrozole (81.3%) and palbociclib + fulvestrant (12.1%) were the most commonly prescribed treatments (Table 2). Median follow-up in the 1L cohort was 40.5 months (Q1–Q3 30.3–48.9 months) and median post-CDK4/6i follow-up was 25.5 months (Q1–Q3 12.1–35.3 months). Twenty-three patients (25.3%) remained on 1L treatment and 68 (74.7%) stopped CDK4/6i treatment. Of those who discontinued treatment, 61 (67.0%) progressed, four (4.4%) stopped treatment due to toxicity, and three (3.3%) stopped for other reasons. There were two patients who did not proceed with 2L treatment, one due to patient death and another due to patient preference. The median 1L CDK4/6i PFS was 28.2 months (95% CI 19.6–34.9 months) (Fig. 1A).
Table 2.
CDK4/6i treatment regimens and up to three lines of therapy for management of HR-positive MBC separated based on 1L or 2L CDK4/6i cohort
| First-line treatment |
Second-line treatment |
Third-line treatment |
||||
|---|---|---|---|---|---|---|
| 1L cohort n = 91 (%) | 2L cohort n = 45 (%) | 1L cohort n = 66 (%) | 2L cohort n = 45 (%) | 1L cohort n = 56 (%) | 2L cohort n = 36 (%) | |
|
| ||||||
| CDK4/6i regimens | ||||||
| Palbociclib/letrozole | 74 (81.3) | N/A | 0 (0) | 27 (60) | 1 (1.8) | 1 (2.8) |
| Palbociclib/fulvestrant | 11 (12.1) | N/A | 5 (7.6) | 17 (37.8) | 2 (3.6) | 1 (2.8) |
| Palbociclib/exemestane | 3 (3.3) | N/A | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Palbociclib/anastrozole | 3 (3.3) | N/A | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Abemaciclib | 0 (0) | N/A | 2 (3.0) | 1 (2.2) | 1 (1.8) | 2 (5.6) |
| Single-agent ET | ||||||
| Letrozole | N/A | 14 (31.1) | 3 (5.1) | N/A | 0 (0) | 0 (0) |
| Fulvestrant | N/A | 5 (11.1) | 15 (22.7) | N/A | 8 (14.3) | 4 (11.1) |
| Anastrozole | N/A | 9 (20) | 0 (0) | N/A | 0 (0) | 1 (2.8) |
| Tamoxifen/Endoxifen | N/A | 7 (15.6) | 2 (3.0) | N/A | 1 (1.8) | 2 (5.6) |
| Exemestane | N/A | 2 (4.4) | 2 (3.0) | N/A | 3 (5.4) | 0 (0) |
| PI3K/mTOR inhibitor | ||||||
| Everolimus/exemestane | N/A | 1 (2.2) | 17 (25.8) | N/A | 9 (16.1) | 12 (33.3) |
| Everolimus/fulvestrant | N/A | 0 (0) | 1 (0) | N/A | 2 (3.6) | 0 (0) |
| Everolimus/tamoxifen | N/A | 0 (0) | 0 (0) | N/A | 2 (3.6) | 0 (0) |
| Alpelisib/fulvestrant | N/A | 0 (0) | 4 (6.1) | N/A | 1 (1.8) | 0 (0) |
| Single-agent chemotherapy | ||||||
| Capecitabine | N/A | 0 (0) | 6 (9.1) | N/A | 10 (17.9) | 5 (13.8) |
| Paclitaxel | N/A | 2 (4.4) | 2 (3.0) | N/A | 1 (1.8) | 4 (11.1) |
| Eribulin | N/A | 0 (0) | 3 (4.5) | N/A | 2 (3.6) | 0 (0) |
| Liposomal doxorubicin | N/A | 0 (0) | 0 (0) | N/A | 1 (1.8) | 0 (0) |
| Other | ||||||
| Clinical trial | N/A | 0 (0) | 1 (1.5) | N/A | 7 (12.5) | 3 (8.3) |
| Combination chemo | N/A | 5 (11.1) | 3 (4.5) | N/A | 4 (8) | 1 (2.8) |
| PARP inhibitor | N/A | 0 (0) | 0 (0) | N/A | 1 (1.8) | 0 (0) |
Fig. 1.
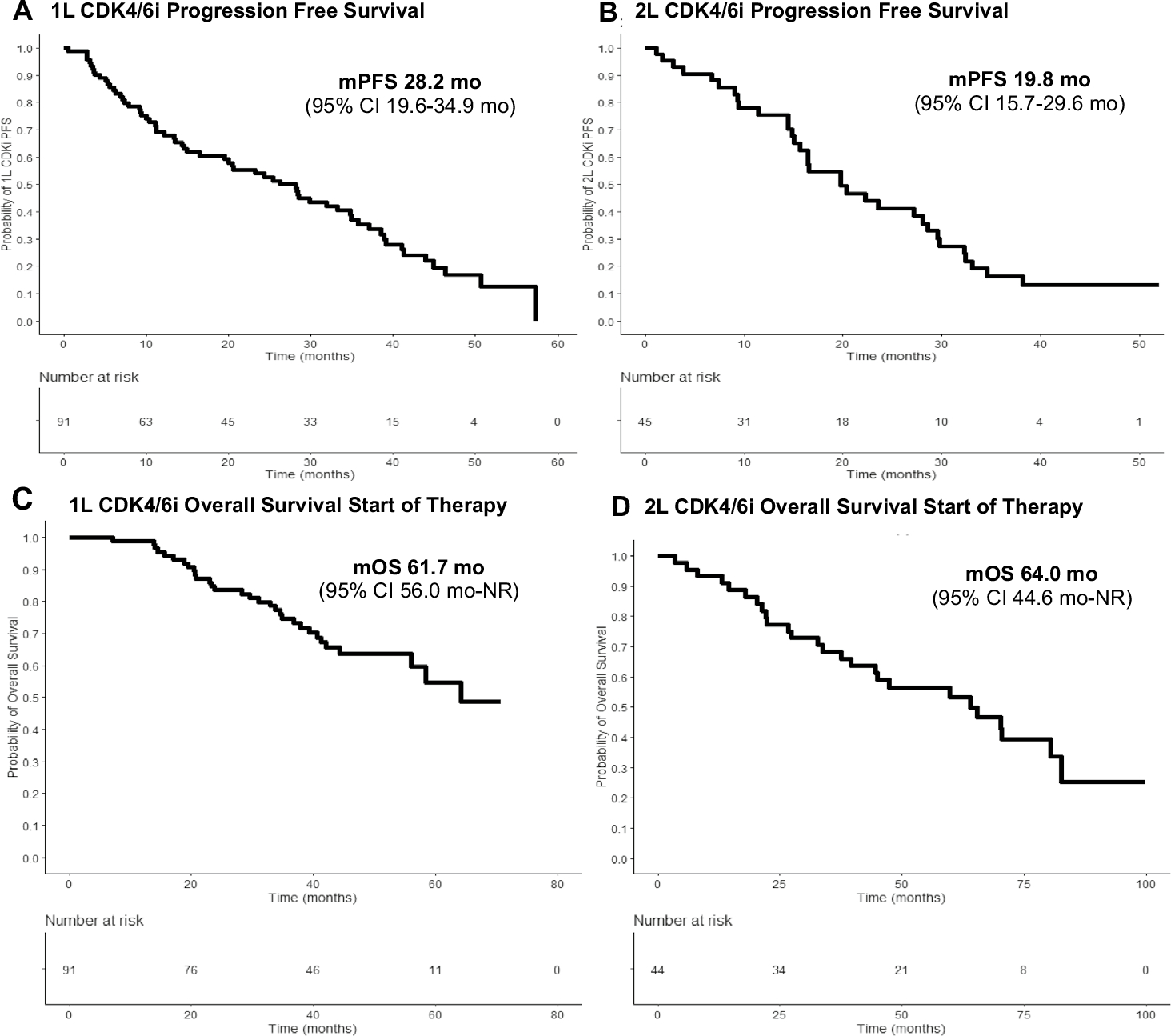
Progression-free survival (PFS) and overall survival curves (OS) for 1L and 2L CDK4/6i cohorts. 1L cohort with A median PFS (mPFS) 28.2 months (95% CI 19.6–34.9 months) and C median OS (mOS) from the start of therapy for metastatic breast cancer (MBC) was 61.7 months (95% CI 56.0 months—NR). mPFS was shorter in the 2L CDK4/6i cohort at 19.8 months (95% CI 15.7–29.6 months), but mOS (D) at the start therapy for MBC was comparable to 1L cohort at 64 months (95% CI 44.6 months—NR)
The most common 2L treatment options after stopping 1L CDK4/6i was PI3K/mTOR inhibitors (PI3K/mTORi) combined with ET (n = 22, 33.3%), single-agent ET (n = 22, 33.3%), and single-agent chemotherapy (n = 11, 16.7%) (Table 2). The median PFS post-1L CDK4/6i for each agent were as follows: PI3K/mTORi, 8.5 months (95% CI 5.5 months—NE); single-agent ET, 6.0 months (6.0 months, 95% CI 3.3–14.0 months); and single-agent chemotherapy, 5.4 months (95% CI 3.3 months—NE) (Fig. 2A). Notably, 5/22 (22.7%) patients treated with PI3K/mTORi stopped therapy due to toxicity. Seven patients (10.6%) either received another CDK4/6i (2/7) or switched classes of ET (5/7), with a median PFS of 26.6 months after 1L CDK4/6i in this subgroup (1.0 month—NE). There were 31 (34.0%) deaths during follow-up and the median OS from the start of therapy for MBC (i.e., start of 1L CDK4/6i) was 61.7 months (95% CI 56.0 months—NR) (Fig. 1C).
Fig. 2.
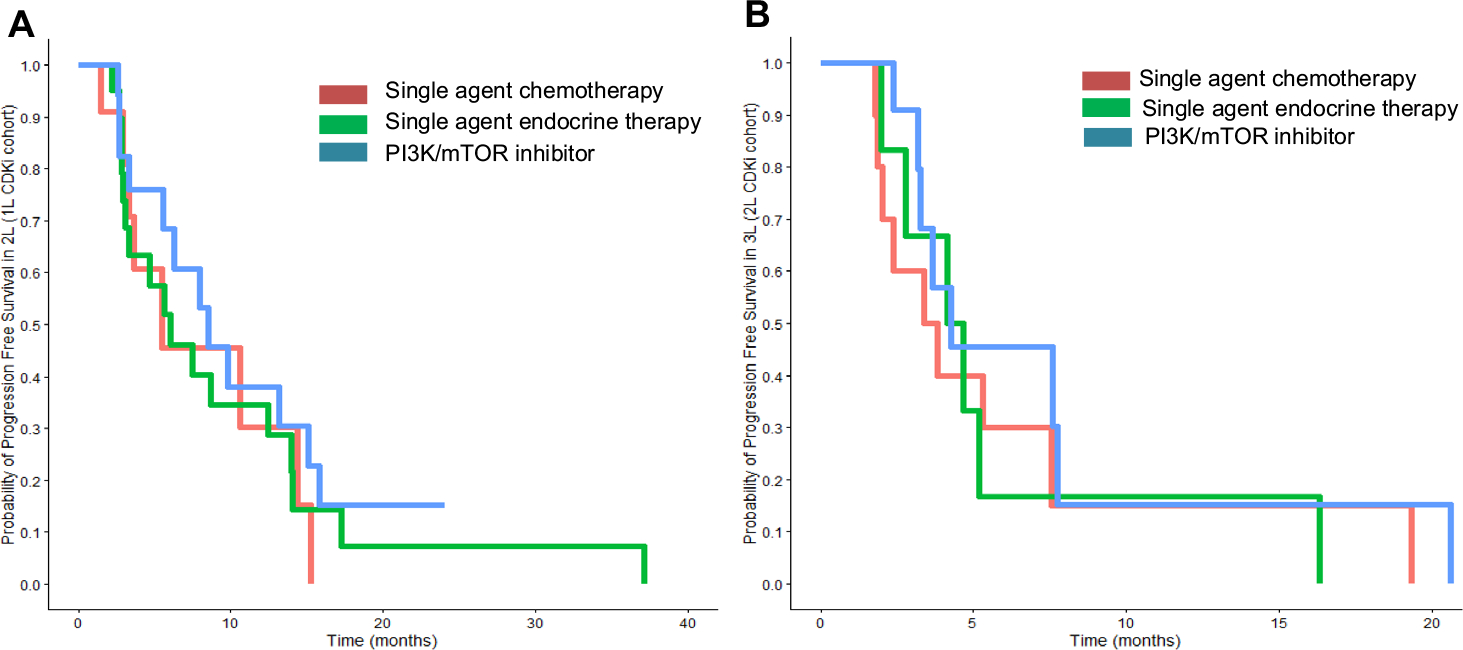
Progression-free survival (PFS) of next line of treatment after progression on CDK4/6i. A 1L CDK4/6i cohort with most commonly prescribed 2L treatments including PI3K/mTOR inhibitors (n = 22, 33.3%, blue), alternative ET (n = 22, 33.3%, green), or single-agent chemotherapy (n = 11, 16.7%, orange). The PFS is longest with PI3K/mTOR inhibitors (8.5 months, 95% CI 5.5 months—NE) followed by single-agent ET (6.0 months, 95% CI 3.3–14.0 months), and single-agent chemotherapy (5.4 months, 95% CI 3.3 months—NE). B The most commonly prescribed subsequent regimens after 2L CDK4/6i included PI3K/mTORi (n = 12, 33.3%, blue), single-agent ET (n = 7, 19.4%, green), and single-agent chemotherapy (n = 9, 25.0%, orange). The 2L CDK4/6i cohort showed the PFS in 3L treatment with PI3K/mTOR inhibitors (4.3 months 95% CI 3.3 months—NE), alternative ET (4.4 months, 95% CI 2.8 months—NE), or single-agent chemotherapy (3.6 months 95% CI 2.0 months—NE) demonstrated similar PFS. Sample sizes were too small in subgroups to determine statistical significance
Second-line (2L) CDK4/6i cohort population
In the 2L cohort (n = 45), the most common prior regimen prescribed for the 1L treatment of metastatic disease was single-agent ET (82.2%) with a median PFS of 13.5 months (95% CI 11.6–28.3 months).
All patients in the 2L cohort were started on a CDK4/6i as 2L therapy, with palbociclib + letrozole (60.0%) and palbociclib + fulvestrant (37.8%) being the most commonly used regimens. Median follow-up in the 2L cohort was 35.8 months (Q1–Q3 18.0–48.4 months) and median post-CDK4/6i follow-up was 16.8 months (Q1–Q3 5.9–32.4 months). A total of 32/45 (71.1%) of patients progressed on 2L CDK4/6i, five (11.1%) remained on 2L CDK4/6i, five (11.1%) stopped due to toxicity, and three (6.7%) stopped for other reasons. On 2L CDK4/6i, the observed PFS was 19.8 months (95% CI 15.7–29.6 months) (Fig. 1B). Subsequent treatment and response data were available in all 36 patients who were post-2L CDK4/6i and included PI3K/mTORi (n = 12, 33.3%), single-agent ET (n = 7, 19.4%), and single-agent chemotherapy (n = 9, 25.0%) (Table 2). Median 3L PFS in the 2L CDK4/6i cohort were noted, with PI3K/mTORi (4.3 months 95% CI 3.3 months—NE), single-agent ET (4.4 months, 95% CI 2.8 months—NE), and single-agent chemotherapy (3.6 months 95% CI 2.0 months—NE) (Fig. 2B). At the end of analysis, 27 patients had died, and the median OS with initiation of any therapy for HR-positive MBC was 64.0 months (95% CI 44.6 months—NR) (Fig. 1D).
Discussion
The use of CDK4/6i in combination with ET as 1L treatment for the management of HR-positive HER2-negative MBC has now become standard of care [4]. However, optimal treatment strategies following progression on CDK4/6i are not well defined. Current options include single-agent ET [4], drugs targeting the PI3K/mTOR pathway such as alpelisib + ET for patients with somatic PIK3CA mutations [18] and everolimus + exemestane [19] or chemotherapy [4]. Our study was a single-institution retrospective cohort study designed to investigate the types of therapies being used in the post-CDK 4/6i setting, as well as the clinical outcomes for patients who progress after 1L or 2L CDK4/6i. Though our study was limited by small sample sizes in subgroup analysis where statistical significance could not be determined for clinical outcomes, we observed that 1L CDK4/6i cohort had improved PFS and OS outcomes compared to 2L CDK4/6i cohort. We reassuringly found that median PFS times in our analysis were quite similar in the 1L setting [28.2 months (95% CI 19.6–34.9 months) compared to clinical trials investigating 1L palbociclib [24.8 months (95% CI 22.1—NR)] [6], while slightly shorter in the 2L setting [19 months (95% CI 15.7–29.6 months)].
Following progression on a CDK4/6i, we found that PI3K/mTORi in combination with ET were the most commonly prescribed 2L or 3L option, followed by single-agent ET and chemotherapy. These data somewhat contrast to a recent large population-based study looking at administrative claims for CDK4/6i-based therapy for treatment of HR-positive HER2-negative MBC, demonstrating that single-agent ET and chemotherapy were the most commonly prescribed regimens following progression on a CDK4/6i with repeat CDK4/6i or everolimus-based regimens less commonly prescribed [20]. This discrepancy is likely due to differing prescribing practices at Mayo Clinic Rochester compared to the community as a large tertiary care center. Overall, we found that the PFS times following progression on a CDK4/6i were short, and ranged from 2.0 to 14.0 months, with shorter PFS times for patients that progressed after receipt of a 2L CDK4/6i. These data did not demonstrate an obvious advantage for one specific post-CDK4/6i-based regimen over another; however, the retrospective nature of this cohort and small sample sizes limits our ability to make comparisons across regimens.
Currently, there are several clinical trials that have evaluated the single-agent antitumor activity of ET after CDK4/6i progression. The Veronica trial was a phase II randomized trial investigating the BCL-2 inhibitor venetoclax combined with fulvestrant compared to fulvestrant monotherapy as 2L or 3L treatment after CDK4/6i progression [21]. There was no statistically significant improvement with the addition of venetoclax, but this study highlighted that single-agent fulvestrant resulted in poor PFS benefit (approximately 1.94 months) after CDK4/6i. Recent data from the EMERALD trial, a phase III randomized control trial of elacestrant, an oral selective estrogen receptor degrader (SERD), compared to fulvestrant or an aromatase inhibitor in patients who had previously progressed on CDK4/6i + ET noted an identical PFS benefit with fulvestrant alone of 1.94 months [22], similar to the Veronica trial.
It has been repeatedly observed that some of the mechanisms that drive resistance to CDK4/6i include mutations in ESR1 [23] and RB1 [24], amplification of CDK4 or CDK6 [25], and Cyclin E [26] and decreased ER expression [27, 28]. Of these mechanisms, the mTOR/AKT/PI3K pathway may contribute to resistance to CDK4/6i and therefore may be a reasonable target after progression on a CDK4/6i [27, 29–32]. In a retrospective cohort study of 23 patients with MBC, those previously treated with prior everolimus in the metastatic setting were significantly less likely to receive a clinical benefit with subsequent treatment with palbociclib + letrozole with a median PFS of 4.2 months [33]. In contrast, Cook et al. [34] demonstrated that patients who received everolimus + exemestane after CDK4/6i or without prior CDK4/6i had similar PFS (3.6 vs 4.2 months) and OS (15.6 vs 11.3 months), inferring that prior use of CDK4/6i does not prevent future benefit to everolimus + exemestane. This is further supported by a larger retrospective study of 622 patients who had received everolimus + exemestane for treatment of HR-positive MBC, wherein 54 patients had received everolimus + exemestane as 2L therapy after progression on a CDK4/6i with median time-to-next treatment being 5.5 months vs 8.3 months in patients who received 1L everolimus + exemestane [35]. However, OS was longer in the cohort of patients who had received CDK4/6i than those who had received 1L ET (OS 59.2 months vs 40.8 months p < 0.01). Additionally, cohort A of the BYLieve trial investigating alpelisib + fulvestrant in patients with PIK3CA mutations who had progressed on CDK4/6i + an aromatase inhibitor reported a median PFS 7.3 months (95% CI 5.6–8.3 months) and median OS was 17.3 months (95% CI 17.2–20.7), with 14% discontinuing treatment due to adverse events [36]. Our data further support the use of PI3K/mTOR inhibition after progression on CDK4/6is, given similar PFS time compared to the BYLieve results. These data provide further support for the use of drugs that target the PI3K/mTOR pathway following progression on CDK4/6i and suggest that further prospective studies should evaluate the antitumor activity of everolimus containing regimens post-CDK4/6i.
There are several limitations to our study including its retrospective design, small sample size, and possible differences in restaging frequency depending on the clinical scenario, which may alter PFS times. Further, we were unable to stratify patients in the PI3K/mTORi cohort based on activating PIK3CA mutations, as there were too few patients to allow for adequate subgroup analysis for those treated with alpelisib + fulvestrant. Additionally, our population primarily received palbociclib-based regimens, which is reflective of prescribing practices at the time at Mayo Clinic Rochester and there are few data regarding the benefit of treatment strategies following abemaciclib or ribociclib. Lastly, the true clinical benefit of PI3K/mTOR is in the 2L setting is likely limited by associated toxicities (e.g., hyperglycemia, diarrhea) and poor tolerance, as demonstrated in our population where just over 20% of patients discontinued due to side effects, suggesting that better management of these toxicities is needed to improve clinical outcomes with these drugs.
In conclusion, our study is one of the largest to date evaluating the prognosis of patients following progression on CDK4/6is. Our data confirm that despite the choice of therapy, the PFS times are modest and generally below 6 months in both the 1L and 2L settings. These sobering data clearly illustrate the need for a better understanding of the mechanisms that drive resistance to CDK4/6i as well as for new targeted therapies. Our findings demonstrating numerically longer PFS times for patients receiving PI3K/mTORi are consistent with the prospective data examining PI3K inhibitors in this setting. Prospective studies will help determine the optimal treatment strategy after CDK4/6i failure, several of which are currently ongoing [30, 37–39].
Funding
This work was funded by Mayo Clinic Clinical and Translational Science (CTSA) grant UL1TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) to KVG. This publication was supported in part by CTSA Grant Number KL2 TR002379 from the NCATS, and the Mayo Clinic Breast Cancer SPORE grant P50 CA116201, Career Enhancement Program, from the NIH to RLF.
Abbreviations
- 1L or 2L or 3L
First line or second line or third line
- CDK4/6(i)
Cyclin-dependent 4/6 kinases (inhibitor)
- ET
Endocrine therapy
- HER2
Human epithelial growth factor receptor 2
- HR
Hormone receptor
- MBC
Metastatic breast cancer
- mTOR
Mammalian target of rapamycin
- NE
Not evaluable
- NR
Not reached
- OS
Overall survival
- PI3K
Phosphatidylinositol 3-kinase
- PI3K/mTORi
Inhibitors of PI3K or mTOR in combination with endocrine therapy
- PFS
Progression-free survival
- Q1
25Th percentile
- Q3
75Th percentile
Footnotes
Declarations
Conflict of interest All authors declare no direct conflicts of interests related to material provided in this manuscript. GMC, SL, KJR, TJH, and PPP have no competing interests. RLF–consulting services for Gilead Sciences, AstraZeneca. Honoraria have been paid to the institution for research activities. COS—Research funding from Eli Lilly, Seattle Genetics, Bavarian Nordic, Minneamrita Therapeutics, Biovica, Nfrence Inc, AACRU, and Sermonix Pharmaceuticals. TCH—Research funding from Takeda Oncology for a phase II clinical trial for metastatic breast cancer. MCL—Research support from Eisai, Exact Sciences, Genentech, Genomic Health, GRAIL, Menarini Silicon Biosystems, Merck, Novartis, Seattle Genetics, Tesaro. MCL also sits on the advisory boards for Astra Zeneca, Celgene, Roche/Genentech, Genomic Health, GRAIL, Ionis, Merck, Pfizer, Seattle Genetics, Syndax. MPG—Personal fees for CME activities from Research to Practice, Clinical Education Alliance, Medscape, personal fees serving as a panelist for a panel discussion from Total Health Conferencing, and personal fees for serving as a moderator for Curio Science. Consulting fees to institution from AstraZeneca, Biovica, Biotheranostics, Blueprint Medicines, Eagle Pharmaceuticals, Lilly, Novartis, Pfizer, Sanofi Genzyme, and Sermonix. Grant funding to institution from Lilly, Pfizer, and Sermonix. MPG is the Erivan K. Haub Family Professor of Cancer Research Honoring Richard F. Emslander, M.D. and receives financial support from the National Cancer Institute under the Mayo Clinic Breast Cancer SPORE (PC50CA116201). KVG—Honoraria to the institution from Novartis. Research funding from Pfizer.
Data availability
All data generated or analyzed during this study are included in this published article. Additional details on data requisition and analysis will be made available at request from the corresponding author.
References
- 1.Mariotto AB, Etzioni R, Hurlbert M, Penberthy L, Mayer M (2017) Estimation of the number of women living with metastatic breast cancer in the united states. Cancer Epidemiol Biomarkers Prev 26:809–815. 10.1158/1055-9965.Epi-16-0889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gong Y, Liu YR, Ji P, Hu X, Shao ZM (2017) Impact of molecular subtypes on metastatic breast cancer patients: a seer population-based study. Sci Rep 7:45411. 10.1038/srep45411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malmgren JA, Mayer M, Atwood MK, Kaplan HG (2018) Differential presentation and survival of de novo and recurrent metastatic breast cancer over time: 1990–2010. Breast Cancer Res Treat 167:579–590. 10.1007/s10549-017-4529-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burstein HJ et al. (2021) Endocrine treatment and targeted therapy for hormone receptor–positive, human epidermal growth factor receptor 2–negative metastatic breast cancer: ASCO guideline update. J Clin Oncol. 10.1200/jco.21.01392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.VanArsdale T, Boshoff C, Arndt KT, Abraham RT (2015) Molecular pathways: targeting the cyclin D-CDK4/6 axis for cancer treatment. Clin Cancer Res 21:2905–2910 [DOI] [PubMed] [Google Scholar]
- 6.Finn RS et al. (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375:1925–1936. 10.1056/NEJMoa1607303 [DOI] [PubMed] [Google Scholar]
- 7.Hortobagyi GN et al. (2016) Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375:1738–1748. 10.1056/NEJMoa1609709 [DOI] [PubMed] [Google Scholar]
- 8.Tripathy D et al. (2018) Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 19:904–915. 10.1016/s1470-2045(18)30292-4 [DOI] [PubMed] [Google Scholar]
- 9.Goetz MP et al. (2017) MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35:3638–3646. 10.1200/jco.2017.75.6155 [DOI] [PubMed] [Google Scholar]
- 10.Turner NC et al. (2018) Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 379:1926–1936. 10.1056/NEJMoa1810527 [DOI] [PubMed] [Google Scholar]
- 11.Slamon DJ et al. (2019) Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med 382:514–524. 10.1056/NEJMoa1911149 [DOI] [PubMed] [Google Scholar]
- 12.Sledge GW Jr et al. (2020) The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: a randomized clinical trial. JAMA Oncol 6:116–124. 10.1001/jamaoncol.2019.4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finn RS et al. (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16:25–35. 10.1016/s1470-2045(14)71159-3 [DOI] [PubMed] [Google Scholar]
- 14.Slamon DJ et al. (2018) Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALESSA-3. J Clin Oncol 36:2465–2472. 10.1200/jco.2018.78.9909 [DOI] [PubMed] [Google Scholar]
- 15.Hortobagyi GN et al. (2021) Overall survival (OS) results from the phase III MONALEESA-2 (ml-2) trial of postmenopausal patients (pts) with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer (ABC) treated with endocrine therapy (ET) & ribociclib (RIB). Ann Oncol 32:S1290–S1291. 10.1016/j.annonc.2021.08.2090 [DOI] [Google Scholar]
- 16.Im S-A et al. (2019) Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 381:307–316. 10.1056/NEJMoa1903765 [DOI] [PubMed] [Google Scholar]
- 17.Cardoso F et al. (2018) 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4)†. Ann Oncol 29:1634–1657. 10.1093/annonc/mdy192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.André F et al. (2019) Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N Engl J Med 380:1929–1940. 10.1056/NEJMoa1813904 [DOI] [PubMed] [Google Scholar]
- 19.Baselga J et al. (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366:520–529. 10.1056/NEJMoa1109653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Princic N et al. (2019) Predictors of systemic therapy sequences following a CDK 4/6 inhibitor-based regimen in post-menopausal women with hormone receptor positive, hEGFR-2 negative metastatic breast cancer. Curr Med Res Opin 35:73–80. 10.1080/03007995.2018.1519500 [DOI] [PubMed] [Google Scholar]
- 21.Lindeman GJ et al. (2021) Results from Veronica: a randomized, phase II study of second-/third-line venetoclax (ven) + fulvestrant (f) versus f alone in estrogen receptor (ER)-positive, HER2-negative, locally advanced, or metastatic breast cancer (la/mbc). J Clin Oncol 39:1004–1004. 10.1200/JCO.2021.39.15_suppl.1004 [DOI] [Google Scholar]
- 22.Bardia A et al. (2021) Elacestrant, an oral selective estrogen receptor degrader (SERD), vs investigator’s choice of endocrine monotherapy for ER+/HER2− advanced/metastatic breast cancer (MBC) following progression on prior endocrine and CDK4/6 inhibitor therapy: results of EMERALD phase 3 trial. J Clin Oncol. 10.1200/JCO.22.00338 [DOI] [Google Scholar]
- 23.Brett JO, Spring LM, Bardia A, Wander SA (2021) ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res 23:85. 10.1186/s13058-021-01462-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thangavel C et al. (2011) Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer 18:333–345. 10.1530/ERC-10-0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finn RS et al. (2020) Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naïve metastatic breast cancer. Clin Cancer Res 26:110–121. 10.1158/1078-0432.Ccr-19-0751 [DOI] [PubMed] [Google Scholar]
- 26.Taylor-Harding B et al. (2015) Cyclin E1 and RTK/RAS signaling drive CDK inhibitor resistance via activation of E2F and ETS. Oncotarget 6:696–714. 10.18632/oncotarget.2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberto M et al. (2021) Cdk4/6 inhibitor treatments in patients with hormone receptor positive, HER2 negative advanced breast cancer: potential molecular mechanisms, clinical implications and future perspectives. Cancers (Basel). 10.3390/cancers13020332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan P, Wang J, Santen RJ, Yue W (2007) Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res 67:1352–1360. 10.1158/0008-5472.Can-06-1020 [DOI] [PubMed] [Google Scholar]
- 29.Basile D et al. (2021) First- and second-line treatment strategies for hormone-receptor (HR)-positive HER2-negative metastatic breast cancer: a real-world study. Breast 57:104–112. 10.1016/j.breast.2021.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bardia A et al. (2021) Phase I/II trial of exemestane, ribociclib, and everolimus in women with HR(+)/HER2(−) advanced breast cancer after progression on CDK4/6 inhibitors (triniti-1). Clin Cancer Res 27:4177–4185. 10.1158/1078-0432.Ccr-20-2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Brien NA et al. (2020) Targeting activated PI3K/mTOR signaling overcomes acquired resistance to CDK4/6-based therapies in preclinical models of hormone receptor-positive breast cancer. Breast Cancer Res 22:89. 10.1186/s13058-020-01320-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark AS, Makhlin I, DeMichele A (2021) Setting the pick: can PI3K inhibitors circumvent CDK4/6 inhibitor resistance? Clin Cancer Res 27:371–373. 10.1158/1078-0432.Ccr-20-3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhakal A et al. (2020) Outcome of everolimus-based therapy in hormone-receptor-positive metastatic breast cancer patients after progression on palbociclib. Breast Cancer (Auckl) 14:1178223420944864–1178223420944864. 10.1177/1178223420944864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook MM et al. (2021) Everolimus plus exemestane treatment in patients with metastatic hormone receptor-positive breast cancer previously treated with CDK4/6 inhibitor therapy. Oncologist 26:101–106. 10.1002/onco.13609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rozenblit M et al. (2021) Patterns of treatment with everolimus exemestane in hormone receptor-positive her2-negative metastatic breast cancer in the era of targeted therapy. Breast Cancer Res 23:14. 10.1186/s13058-021-01394-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rugo HS et al. (2021) Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLIEVE): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol 22:489–498. 10.1016/s1470-2045(21)00034-6 [DOI] [PubMed] [Google Scholar]
- 37.van Ommen-Nijhof A et al. (2018) Selecting the optimal position of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancer - the SONIA study: study protocol for a randomized controlled trial. BMC Cancer 18:1146. 10.1186/s12885-018-4978-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalinsky K et al. (2018) Abstract ot3–05-09: a randomized phase II trial of fulvestrant with or without ribociclib after progression on aromatase inhibition plus cyclin-dependent kinase 4/6 inhibition in patients with unresectable or metastatic hormone receptor positive, her2 negative breast cancer (maintain trial). Cancer Res. 10.1158/1538-7445.Sabcs17-ot3-05-09 [DOI] [Google Scholar]
- 39.Ciruelos E et al. (2021) Abstract ot-13–04: Solti-1716. Targeting non-luminal disease by PAM50 with pembrolizumab + paclitaxel in hormone receptor-positive/HER2-negative advanced/metastatic breast cancer patients who have progressed on or after CDK 4/6 inhibitor treatment (TATEN trial). Cancer Res. 10.1158/1538-7445.Sabcs20-ot-13-04 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. Additional details on data requisition and analysis will be made available at request from the corresponding author.


