Abstract
Background
Otitis media with effusion (OME) is common and may cause hearing loss with associated developmental delay. Treatment remains controversial.
Objectives
To examine the evidence for treating children with hearing loss associated with OME with systemic or topical intranasal steroids.
Search methods
We searched the Cochrane ENT Group Trials Register; CENTRAL; PubMed; EMBASE; CINAHL; Web of Science; BIOSIS Previews; Cambridge Scientific Abstracts; mRCT and additional sources for published and unpublished trials. The date of the most recent search was 26 August 2010.
Selection criteria
Randomised controlled trials of oral and topical intranasal steroids, either alone or in combination with another agent such as an oral antibiotic. We excluded publications in abstract form only; uncontrolled, non‐randomised or retrospective studies; and studies reporting outcomes by ears (rather than children).
Data collection and analysis
The authors independently extracted data from the published reports using standardised data extraction forms and methods. We assessed the quality of the included studies using the Cochrane 'Risk of bias' tool. We expressed dichotomous results as a risk ratio (RR) and continuous data as weighted mean difference (WMD), both with 95% confidence intervals (CI). Where feasible we pooled studies using a random‐effects model and performed tests for heterogeneity between studies. In trials with a cross‐over design, we did not use post cross‐over treatment data.
Main results
We included 12 medium to high‐quality studies with a total of 945 participants. No study documented hearing loss associated with OME prior to randomisation. The follow‐up period was generally limited, with only one study of intranasal steroid reporting outcome data beyond six months. There was no evidence of benefit from steroid treatment (oral or topical) in terms of hearing loss associated with OME. Pooled data using a fixed‐effect model for OME resolution at short‐term follow up (< 1 month) showed a significant effect of oral steroids compared to control (RR 4.48; 95% CI 1.52 to 13.23; Chi² 2.75, df = 2, P = 0.25; I² = 27%). Oral steroids plus antibiotic also resulted in an improvement in OME resolution compared to placebo plus antibiotic at less than one month follow up, using a random‐effects model (RR 1.99; 95% CI 1.14 to 3.49; five trials, 409 children). However, there was significant heterogeneity between studies (P < 0.01, I² = 69%). There was no evidence of beneficial effect on OME resolution at greater than one month follow up with oral steroids (used alone or with antibiotics) or intranasal steroids (used alone or with antibiotics) at any follow‐up period. There was also no evidence of benefit from steroid treatment (oral or topical) in terms of symptoms.
Authors' conclusions
While oral steroids, especially when used in combination with an oral antibiotic, lead to a quicker resolution of OME in the short term, there is no evidence of longer‐term benefit and no evidence that they relieve symptoms of hearing loss. We found no evidence of benefit from treatment of OME with topical intranasal steroids, alone or in combination with an antibiotic, either at short or longer‐term follow up.
Plain language summary
Oral or topical nasal steroids for hearing loss associated with otitis media with effusion (glue ear) in children
Glue ear (otitis media with effusion ‐ OME) is sticky fluid in the middle ear that does not cause pain or fever but can reduce hearing. Steroid drugs (taken orally or as nose spray) are sometimes used to try to speed up the resolution of effusion and so prevent hearing loss. Other treatment options include oral antibiotics and other medicines, or surgical procedures such as grommets (ventilation tubes). This review of trials found that oral steroids (especially when used in combination with antibiotics) speeded up the resolution of OME in the short term. However, there was no long‐term evidence to show lasting benefit or improved hearing. There was no evidence that using steroid drugs as a nose spray benefited children with OME.
Summary of findings
Background
Description of the condition
This is one of a number of reviews of treatment options for patients with OME prepared within the Cochrane Ear, Nose and Throat Disorders Group.
Otitis media with effusion (OME), or 'glue ear', is characterised by an accumulation of fluid in the middle ear, in the absence of acute inflammation. It is an important and common problem. OME is the commonest cause of acquired hearing loss in childhood and may negatively effect language development (Haggard 1991; Lous 1995a; Lous 1995b). OME is also associated with anxiety/depression and attention disorders and so may affect both children, their families and peers (Gouma 2010). OME in early childhood has been shown to affect IQ, behaviour and reading into late teens (Bennett 2001). The reason why the condition develops is uncertain, but low‐grade infection, poor Eustachian tube function, allergy and adenoidal infection or hypertrophy have all been implicated (Chantzi 2005). OME often resolves spontaneously and return to normal middle ear function often occurs within three months (MRC Multi‐Centre Otitis Media Study Group 2001).
Prevalence
OME has a prevalence of about 20% at around two years of age with another peak at six years (Zielhuis 1990). The prevalence of recurrent otitis media may be increasing (Lanphear 1997) and visits to the doctor for otitis media and otitis media with effusion have risen (from 9.9 million in 1975 to 24.5 million in 1990 in the US) (Schappert 1992). Overall, the prognosis for OME is good, with over 50% of OME episodes resolving spontaneously within three months and 95% within one year. However, 30% to 40% of children have recurrent OME episodes and 5% of preschool children aged five years have persistent (longer than three months) bilateral hearing loss associated with OME (NICE 2008). Although the diagnosis of OME in primary care has increased over the last decade, the number of grommet operations performed in England and Wales fell from 43,300 in 1994‐1995 to 25,442 in 2009‐2010, primarily as a result of the ‘watchful waiting’ strategy (HES 2010).
Cost
The total annual cost of treating children under five for OME is over GBP 5000 million (EUR 4800) annually in the US (Gates 1996). The insertion of grommets (ventilation or tympanostomy tubes) is the second commonest surgical procedure in children, costing USD 1200 million (EUR 1100) annually in the US (Gates 1996). The full cost of ventilation tube insertion is estimated to be GBP 1208 in the UK and hearing aid treatment for OME is estimated to cost GBP 752 (NICE 2008). OME is a common reason for prescribing antibiotics that contributes to the growing problem of bacterial resistance.
Diagnosis
The recommended technique for diagnosing otitis media with effusion is impedance audiometry (tympanometry) in combination with otomicroscopy or pneumatic otoscopy (Bluestone 1988). OME is deemed to be present when the tympanometry results in a flat curve (relative gradient less than 0.1, type B), when mobility of the tympanic membrane is absent or reduced, or fluid or air bubbles are evident behind the ear drum. However, not all children with effusions suffer significant hearing loss. The positive predictive value for hearing loss of 25 dB or more of tympanometry characteristic of OME ranges from 49.0% to 66.4% (Dempster 1991; Kazanas 1994; MRC Multi‐Centre Otitis Media Study Group 1999). Tympanometry is therefore a surrogate measure for hearing loss associated with OME, and intervention decisions should ideally be made when hearing loss is documented (MRC Multi‐Centre Otitis Media Study Group 1999).
Description of the intervention
Many patients with OME require no specific treatment. The most common medical treatment options include the use of decongestants, mucolytics, steroids, antihistamines, antibiotics and auto‐inflation. Surgical treatment options include grommet insertion, myringotomy (tympanocentesis, i.e. surgical incision of the ear drum, with or without aspiration of fluid from the middle ear cavity) and adenoidectomy. The optimal treatment strategy remains controversial, there being wide international variability in clinical practice. The UK National Institute for Health and Clinical Excellence (NICE) recommends active observation for three months before intervention is considered (NICE 2008).
How the intervention might work
There is in vitro and animal model evidence that steroids modulate effusions (Baggett 1997; Tan 1997; Haddard 1998; Yaman 2008). It is hypothesised that steroids could clear effusions by: (a) stabilising membrane phospholipid breakdown and thus preventing the formation of arachidonic acid and associated inflammatory mediators; (b) shrinking peri‐tubal lymphoid tissue; (c) enhancing secretion of Eustachian tube surfactant; and (d) reducing middle ear fluid viscosity (Rosenfeld 1992; Ducharme 2003).
Topical intranasal steroids
Topical intranasal steroids may be safer than systemic preparations because the glucocorticoid is rapidly degraded in the nasal mucosa to less active metabolites and any unchanged drug that is absorbed is metabolised in the first pass through the liver (Tracy 1998). Systemic adverse effects are therefore less likely, while the desired anti‐inflammatory effects may be similar.
Oral steroids
Systemic steroids, however, may be able to gain access to the middle ear, while topical intranasal steroids would not be expected to reach the middle ear but may modulate Eustachian tube function. Clinicians may be concerned about using systemic steroids for what may be a self‐limiting condition. However, short courses of oral corticosteroids are widely used to treat acute asthma in children and are usually well‐tolerated. Side effects such as dyspepsia and behavioural disturbances are infrequent and resolve on drug withdrawal. There have been case reports of disseminated varicella infection in a child receiving short‐term steroids for asthma (Wu 2008). Although long‐term or frequent courses of oral steroids are associated with important adverse effects, repeated short courses of prednisolone (median of four courses in a year) in children with asthma were shown to be safe and not associated with any lasting effects on bone metabolism or mineralisation or adrenal function (Ducharme 2003). The National Institutes of Health (NIH) in the US advises that children who have not had chickenpox and periodically take oral corticosteroids should receive the varicella vaccine after they have been steroid‐free for at least one month (NIH 2007).
Why it is important to do this review
Antibiotics, topical or systemic antihistamines, topical or systemic decongestants, topical or systemic corticosteroids, homeopathy, cranial osteopathy, acupuncture, dietary modification (including probiotics) and immunostimulants are not recommend for treating OME (Griffin 2006; NICE 2008). Standard treatments for OME, such as hearing aids and ventilation tube insertion, are not trouble‐free. Ventilation tube insertion involves a general anaesthetic. It is associated with an improvement in the mean hearing levels of 4 to 10 dB in children with bilateral tubes during the first six months of follow up but this diminishes with time (Browning 2010). Hearing aids can be uncomfortable and be associated with bullying and perceived stigma (Dengerink 1984). An alternative treatment, cheap and effective within primary care, would reduce secondary care referral and represent and important opportunity for cost saving to the health service and parents.
The effect of systemic and topical intranasal steroids on otitis media with effusion in children has been the subject of randomised and controlled clinical trials. At least three meta‐analyses have addressed the topic and have found evidence for a 'steroid effect' (Nuss 1990; Rosenfeld 1991; Stool 1994). All concluded that steroids cleared effusions more quickly in the short term. However, trials were often small and results of individual trials were contradictory. Nuss et al included trials published in abstract form only, whose quality could not be adequately assessed. One trial (Podoshin 1990) was not published when Rosenfeld and colleagues conducted their meta‐analysis. Stool et al pooled a study of topical steroids with studies of systemic steroids. Since these three meta‐analyses, two randomised controlled trials have examined the effect of topical intranasal steroids in OME (Tracy 1998; Williamson 2009) and at least three other relevant trials involving oral steroids have been published (Rosenfeld 1995; Hemlin 1997; Mandel 2002). Existing systematic reviews have not examined topical steroid treatment separately. This systematic review updates existing studies and includes separate consideration of topical intranasal steroids.
Objectives
To determine the beneficial and harmful effects of treatment with steroids (both oral and topical intranasal) for children with hearing loss associated with otitis media with effusion. Our a priori hypothesis was that steroid treatment (oral or topical intranasal), either alone or in combination with another agent, is effective in treating the hearing loss associated with OME and in resolving effusions in children.
The primary outcomes sought were changes in hearing. Secondary outcomes were effect on effusions and adverse effects of treatment. As a minimum requirement, we expected studies to report short‐term effects on effusions identified by a combination of pneumo‐otoscopy or otomicroscopy and tympanometry.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials of oral and topical intranasal steroids. We included randomised studies that used non‐intervention controls where blinding of outcome assessment was adequate. We excluded publications that were only available in abstract form since adequate appraisal was not possible; uncontrolled, non‐randomised or retrospective studies; and studies reporting outcomes only with ears as the unit of analysis (rather than children) since observations made on the ears of a single child cannot be regarded as independent. In addition, we considered that if a 'single‐ear view' of the whole child could not be extracted from the information provided, studies might be poor in other ways. Williams has shown that studies reporting outcomes by ear have larger effect sizes than studies using the child as the unit of analysis (Williams 1993). We made attempts to contact the authors of studies published only in abstract form for a full study report, and included these where obtained. We excluded studies (or data from arms of studies) comparing treatment with steroid plus additional treatment to treatment with placebo plus placebo because it was not possible to identify the 'steroid effect' from such data.
Types of participants
The focus was on studies of children up to the age of 12 years, and we report when older subjects were included. The age of the patients is pertinent in respect of both the natural history of the disease process and the measurable outcomes (see below).
We divided the studies into subgroups according to the following ways of assessing exposure:
1. The diagnosis of otitis media with effusion defined by:
A: Air‐bone gap of 10 dB or more plus two or more of:
otomicroscopy
pneumatic otoscopy
tympanometry (type B and C2)
B: Two or more of:
otomicroscopy
pneumatic otoscopy
tympanometry (type B and C2)
C: One of otoscopy alone or tympanometry (type B and C2)
D: Poorly or not defined
2. Significant hearing loss defined by:
A: Pure‐tone audiometry hearing loss of more than 20 dB at two or more times within three months (for example, mean of 500, 1000 and 2000 Hz hearing loss bilaterally)
B: Defined, but less strict than A
C: Uncertain or not defined
Types of interventions
Systemic or topical intranasal steroids compared with control (placebo or non‐intervention control). We included additional treatments such as antibiotics so long as they were identical in the treatment and in the control groups. We grouped studies according to the comparisons made: (1) oral steroid versus control; (2) oral steroid plus additional treatment versus control plus identical additional treatment; (3) topical intranasal steroid versus control; and (4) topical intranasal steroid plus additional treatment versus control plus identical additional treatment.
Types of outcome measures
Primary outcomes
Differences in hearing level.
Degree of conductive hearing loss (assessment of air‐bone gaps).
Secondary outcomes
Duration of hearing loss.
Presence or absence of fluid in the middle ear cavity: short (< one month), intermediate (one to < six months) and longer term (≥ six months).
Symptom score.
Developmental outcomes, such as language development and behaviour.
Possible adverse effects (for example, evidence of immunosuppression, abdominal pain, atrophy of the nasal mucosa, epistaxis, changes in behaviour).
Cost‐effectiveness data.
N.B. Where a study reported results at multiple follow‐up periods, we only used the data relating to the longest follow‐up period (or closest to the upper limit for short, intermediate and long) in the analysis. We considered duration of follow up, where feasible, from randomisation not treatment completion.
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials. There were no language, publication year or publication status restrictions. The date of the last search was 26 August 2010, following a previous update search in March 2005.
Electronic searches
For the update of this review we searched: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 3); PubMed; EMBASE; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; BIOSIS Previews; CNKI; ISRCTN; ClinicalTrials.gov; ICTRP and Google.
We modelled subject strategies for databases on the search strategy designed for CENTRAL. Although the strategy was specific to otitis media with effusion, we did not restrict it to children nor did we attempt to exclude acute otitis media in case this excluded material of relevance. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2, Box 6.4.b. (Handbook 2009)). Search strategies for the major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned reference lists of identified studies for further trials. We searched PubMed, TRIPdatabase, NHS Evidence ‐ ENT and Audiology, and Google to retrieve existing systematic reviews possibly relevant to this systematic review, in order to search their reference lists for additional trials. We sought abstracts from conference proceedings via the Cochrane Ear, Nose and Throat Disorders Group Trials Register. In previous searches in 2005, we also wrote to experts asking about knowledge of additional studies for the review.
Data collection and analysis
Selection of studies
We carried out searches independently. We assessed the full text of all studies loosely meeting the inclusion criteria independently and resolved differences of opinion regarding inclusion by consensus, or by using a third party in the face of ongoing disagreement.
Data extraction and management
We independently extracted data from the published reports using standardised data extraction forms. Disagreement was resolved by consensus or referral to a third party after returning to the original publication. For each trial, we documented the following aspects:
methods (including methods of allocation, blinding, study structure);
participants (including ages, setting, inclusion criteria, prospective or retrospective documentation of effusions prior to allocation, method of diagnosis);
interventions (including dosages, duration, care in matching placebo and active treatment); and
outcomes (including definitions of cure, hearing and language assessments and adverse effects of treatment).
In studies that provided data for various definitions of cure, we used data for the strictest definition. In studies with a cross‐over design, where patients who did not have OME resolution at follow up were given the alternative treatment, we did not use data from the cross‐over treatment period. Similarly, in multi‐arm studies (e.g. steroid versus antibiotic versus non‐steroidal anti‐inflammatory versus control), we only used data for the steroid‐treated and control groups.
Assessment of risk of bias in included studies
We independently assessed the risk of bias of the included studies using the scheme described in the Cochrane Handbook for Systematic Review of Interventions (Handbook 2011). This involved assessing studies for:
selection bias (presence or absence of adequate sequence generation and allocation concealment);
performance bias (presence or absence of blinding of patients and outcome assessors);
attrition bias (losses to follow up); and
detection bias (quality of outcome assessment and selective reporting of results).
We used the Cochrane ‘Risk of bias’ tool in RevMan 5.1 (RevMan 2011), which involved describing each of these domains as reported in the trial and then assigning a judgement about the adequacy of each entry. This involved making a prespecified judgement of 'high', 'low' or 'unclear' risk of bias.
Data synthesis
We used the statistical methods for dichotomous outcomes described by Yusuf et al (Yusuf 1985). Results were expressed as a risk ratio (RR) for achieving the outcome in question at a given point in time together with the 95% confidence interval (CI) for this estimate. We decided to use RRs rather than odds ratios (ORs) as they are easier to interpret and consistent with general intuition (Davies 1998). We presented continuous data using the weighted mean difference (and 95% CI). We pooled studies using a random‐effects method. We were interested in the broad perspective of whether steroid treatment (systemic or intranasal and with or without antibiotics) is beneficial for treating OME in children. We were therefore interested in the average effect of the intervention across clinically diverse studies, which represented the different mix of participants and implementations of the interventions that we wanted our results to be generalisable to. We assessed statistical heterogeneity between studies using the Chi² test, the I² statistic and visual inspection of the forest plots. When this was present we tried to investigate the reasons for the heterogeneity (including methodological factors or outcome assessment), but the small number of included studies precluded the use meta‐regression or subgroup analyses. When considerable heterogeneity (i.e. I² ranged from 75% to 100%) (Deeks 2008) was present and there was inconsistency in the direction of the results then pooling was not considered appropriate (the P value of the Chi² test and width of the CIs were also taken into consideration when making this interpretation).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
We updated the searches for the review in September 2009 and August 2010. The searches retrieved a total of 45 references; we considered four to be potentially relevant and obtained the full‐text papers. Following review of the full text we included one new study in the review (Williamson 2009) and excluded a further three (Ortega del Alamo 2005; Cengel 2006; Choung 2008).
Included studies
Nine studies involved assessment of oral steroids (Schwartz 1980; Niederman 1984; Macknin 1985; Lambert 1986; Berman 1990; Giebink 1990; Podoshin 1990; Hemlin 1997; Mandel 2002) and three studies involved assessment of topical intranasal steroids (Shapiro 1982; Tracy 1998; Williamson 2009). One study was open, comparing children treated with steroids to non‐intervention controls (Giebink 1990).
Description of participants
Most studies (both of oral and topical intranasal steroids) documented effusions by a combination of pneumatic otoscopy and tympanometry (1B). No study documented hearing loss from OME two or more times in the three months prior to study entry (2A). Only one study required documented hearing loss for all children prior to study entry (Macknin 1985). Three studies included children older than 12 years: two studies included children aged up to 14 years (Niederman 1984; Macknin 1985) and one study included children up to 15 years (Lambert 1986).
Description of interventions
For oral steroids, six studies evaluated the use of prednisolone (Schwartz 1980; Lambert 1986; Berman 1990; Giebink 1990; Podoshin 1990; Mandel 2002) at doses ranging from one (Schwartz 1980; Giebink 1990; Podoshin 1990; Mandel 2002) to up to two (Berman 1990) mg/kg/day for a duration of seven (Berman 1990) to 14 days (Lambert 1986; Giebink 1990; Podoshin 1990). Two studies used dexamethasone 0.15 mg/kg in tapering doses for two weeks (Niederman 1984; Macknin 1985). One study evaluated the use of a single dose of betamethasone (6 mg) given on day 10 of the antibiotic treatment (Hemlin 1997).
For topical intranasal steroids, one study used dexamethasone (one spray in each nostril three times a day) for three weeks (Shapiro 1982), one study used beclomethasone (two sprays in each nostril twice a day) for 12 weeks (Tracy 1998), and one study used mometasone furoate (one spray in each nostril once a day) for three months (Williamson 2009).
Primary outcome measures
Audiometry
Six included studies provided audiometry data at follow up (Macknin 1985; Lambert 1986; Berman 1990; Podoshin 1990; Mandel 2002; Williamson 2009), but only three provided data that could be used in this review (numbers of children with partial or no improvement on audiometric evaluation by Podoshin 1990; numbers of children improving their hearing loss by at least 10 dB by Macknin 1985 and pass/fail of audiometry sweep at 25 dB HL by Williamson 2009). (The definition of hearing loss was not clear for Podoshin 1990, but this was the only study included in the analysis for steroid plus antibiotic). Audiometry data in the remaining studies used ears as the unit of analysis. One study (Williamson 2009) also reported median number of days with hearing loss (for the purpose of this review median has been assumed to be the same as the mean).
Secondary outcome measures
Presence of OME
All 12 studies provided data on the resolution of OME at follow up.
Symptoms and language development
Only two studies used symptoms as an outcome (Tracy 1998; Williamson 2009). One (Tracy 1998), a study of topical intranasal steroid plus antibiotic versus placebo plus antibiotic, reported on symptoms in the form of a scale that was considered as continuous data. The validation of the symptom scale was not described. The second (Williamson 2009), which compared intranasal steroid with placebo, collected data on seven symptoms using diaries but did not report the findings. The study also used a condition‐specific functional scale (OM8‐30) developed by the Medical Research Council (MRC). However, most of the results relating to this scale were reported as unusable box plots (based on medians and inter quartile ranges) and P values relating to non‐significant findings.
Only one study (Williamson 2009) reported effects on language or other aspects of development, which was one of the domains of the OM8‐30 used. However, the results were not presented in a usable format.
Adverse effects
Adverse effects of steroid treatment were reported in five studies of oral therapy (Niederman 1984; Macknin 1985; Giebink 1990; Hemlin 1997; Mandel 2002) and three studies of topical therapy (Shapiro 1982; Tracy 1998; Williamson 2009).
Cost‐effectiveness data
Only one study (Williamson 2009) included an economic evaluation. The main outcome measure was the incremental cost per quality‐adjusted life‐year (QALY) gained for topical steroids compared with placebo. There was no evidence that intranasal steroids are a cost‐effective use of UK National Health Service (NHS) resources for treating OME. Children receiving topical steroids accrued slightly, but not statistically significant, higher costs (incremental cost/child: £11 pound, 95% CI ‐199 to 222) and non‐statistically significantly fewer QALYs (incremental QALY gain/child: ‐0.0166, 95% CI ‐0.0652 to 0.0320) than those receiving placebo. Topical steroids had a 24.19% probability of being cost‐effective at a GBP 20,000 per QALY gained threshold, a 23.82% probability of being more effective and a 46.25% probability of being less costly.
General
In cases in which the same data apparently appear twice (Schwartz 1980 and Schwartz 1981; Giebink 1988 and Giebink 1990), these data are used only once in this review.
Studies fell into four categories:
oral steroids versus control;
oral steroids plus antibiotic versus control plus antibiotic;
topical intranasal steroid versus control; and
topical intranasal steroid plus antibiotic versus control plus antibiotic or antibiotic alone.
The longest follow up was for nine months (Williamson 2009).
Excluded studies
We excluded a total of 10 studies for the following reasons: published in only abstract form (Woodhead 1986; Heary 1990); non‐randomised (Persico 1978; Rosenfeld 1995); used ears instead of patients as the unit of analysis (Cengel 2006); compared antibiotic plus steroid with non placebo control (Schwartz 1981); compared antibiotic plus steroid with placebo plus placebo (Berman 1987; Daly 1991); compared antibiotic plus steroid with antibiotic only and did not report blind outcome assessment (Choung 2008); and did not evaluate steroid (Ortega del Alamo 2005).
Risk of bias in included studies
The overall quality of the included studies was generally fairly high (see Figure 1, 'Risk of bias' graph and Figure 2, 'Risk of bias' summary) although methodological issues were poorly reported. Most studies were described as double‐blind, randomised controlled trials, but very few (4/12) reported their method of sequence generation. It was not possible to judge whether an adequate method was used to conceal allocation for two of the nine studies that evaluated oral steroids (Lambert 1986; Mandel 2002) and one of the three studies that evaluated topical steroids (Shapiro 1982), although both used a placebo control; the remaining studies used adequate allocation concealment. Most studies did not include drop‐outs or missing data in their analyses, with loss to follow up being greater than 10% in three studies (Niederman 1984; Berman 1990; Williamson 2009) (range 15% to 24%). Two further studies (Schwartz 1981; Lambert 1986) reported the number of participants included in the analyses, but it was unclear if this was the same as the number of participants initially randomised.
1.
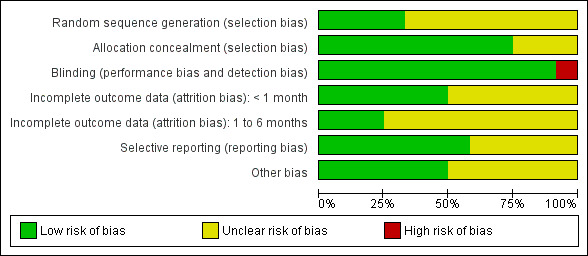
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
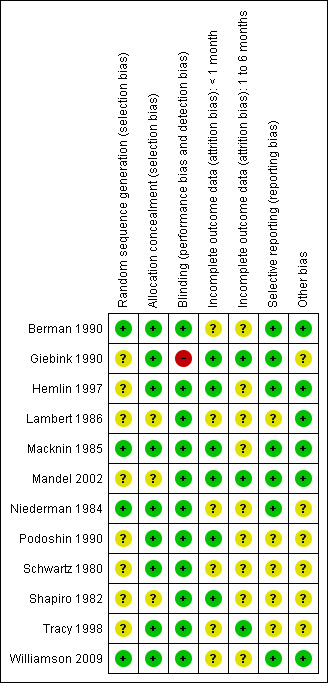
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Oral steroids
In the study by Podoshin (oral steroid plus antibiotic versus placebo plus antibiotic) the reporting of outcomes were confusing: 'success' is defined as normal otoscopy, tympanometry and closure of the air‐bone gap, yet outcomes for 'complete improvement' are given according to audiometry and tympanometry separately (Podoshin 1990).
Giebink and colleagues used an open design because treatment regimens differed and placebo control was not feasible (Giebink 1990). However, treatment allocation was remote (concealed) and objective outcome measures used. Percentages of children followed up (rather than absolute numbers) were reported.
Topical intranasal steroids
In the trial of topical intranasal steroids plus antibiotic versus control plus antibiotic, Tracy and colleagues pooled data for children treated with antibiotics plus placebo and children treated with antibiotics alone (Tracy 1998). These groups had similar outcomes, therefore we present these pooled data.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Summary of findings: oral steroids versus control.
| Oral steroids compared with inactive control for otitis media with effusion in children | ||||||
|
Patient or population: Children with otitis media with effusion Settings: Hospital (secondary or tertiary care) Intervention: Oral steroids for the treatment of OME Comparison: Inactive control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Inactive control | Oral steroids | |||||
|
Hearing loss [6 weeks] |
Medium‐risk population | RR 1.09 (0.8 to 1.49) | 44 (1) | +++O moderate | Single RCT with small sample size | |
| 739 per 1000 | 806 per 1000 (591 to 1101) | |||||
|
OME resolution at short‐term follow up (2 weeks) [< 1 month] |
Medium‐risk population | RR 3.80 (0.93 to 15.52) | 108 (3) | +++O moderate1 | ||
| 50 per 1000 | 190 per 1000 (47 to 776) | |||||
|
OME resolution at intermediate‐term follow up (4 to 6 weeks) [1 to < 6 months] |
Medium‐risk population | RR 1.54 (0.76 to 3.14) | 106 (3) | +++O moderate2 | ||
| 111 per 1000 | 169 per 1000 (84 to 345) | |||||
|
OME resolution at long‐term follow up [≥ 6 months] |
See comment | See comment | Not estimable | See comment | See comment | No studies reported long‐term outcome data |
| Symptoms | See comment | See comment | Not estimable | See comment | See comment | No studies reported data on symptoms |
|
Adverse effects [4 to 6 weeks] |
See comment | See comment | Not estimable | 143 (3) | See comment3 | There were no serious adverse effects3 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OME: otitis media with effusion; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias in studies low, but overall sample size small and wide confidence interval.
2 Risk of bias fairly low, but overall sample size is small.
3 Two studies reported that there were no significant adverse effects, but did not give actual data (or description of adverse effects). One study only reported on haematological complications. The study also reported that there was a transient drop in serum cortisol levels in 14 steroid‐treated patients.
Summary of findings 2. Summary of findings: oral steroids plus antibiotic versus control plus antibiotic.
| Oral steroids plus antibiotics compared with inactive control plus antibiotics for otitis media with effusion in children | ||||||
|
Patient or population: Children with otitis media with effusion Settings: Hospital (secondary or tertiary care) Intervention: Oral steroids plus antibiotics for the treatment of OME Comparison: Inactive control plus antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Inactive control plus antibiotics | Oral steroids plus antibiotics | |||||
|
Hearing loss [2 months]1 |
Medium‐risk population | RR 1.01 (0.73 to 1.40) | 99 (1)2 | +++O moderate3 | ||
| 591 per 1000 | 596 per 1000 (431 to 826) | |||||
|
OME resolution at short‐term follow up (7 to 28 days) [< 1 month] |
Medium‐risk population | RR 1.99 (1.14 to 3.49) | 5 (409) | ++++ high | ||
| 500 per 1000 | 999 per 1000 (570 to 1745) | |||||
|
OME resolution at intermediate‐term follow up (1 to 2 months) [1 to < 6 months] |
Medium‐risk population | RR1.44 (0.97 to 2.13) | 2 (231) | ++++ high | ||
| 261 per 1000 | 381 per 1000 (257 to 564) | |||||
|
OME resolution at long‐term follow up [≥ 6 months] |
See comment | See comment | Not estimable | See comment | See comment | No studies reported long‐term outcome data |
| Symptoms | See comment | See comment | Not estimable | See comment | See comment | No studies reported data on symptoms |
|
Adverse effects [2 weeks to 6 months] |
Medium‐risk population | RR 1.50 (0.81 to 2.78) | 255 (2) | ++OO low4 | There were no serious adverse effects5 | |
| 178 per 1000 | 267 per 1000 (144 to 495) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OME: otitis media with effusion; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Partial or no improvement on audiometry evaluation, definition of hearing loss not clear.
2 Three studies that reported audiometry data used ears as the unit of allocation and were therefore not included in the analysis.
3 Single RCT.
4 Severity of adverse effects not reported in one study, but the intervention was discontinued due to adverse effects in five participants (two in steroid group and three in control). For this study, treatments were administered in two phases (four‐arm study analysed as two‐arm) and adverse effects reported separately for both phases; data from end of first phase (completion of two‐week steroid treatment) used in meta‐analysis (during which no patient discontinued treatment due to adverse effects). Follow up in the second study was six months, but treatment failures were not followed up beyond two to 11 days.
5 Adverse effects were mainly mild to moderate and included dermatitis, diarrhoea, loose stools, vomiting, stomach pain and gastroenteritis.
Summary of findings 3. Summary of findings: intranasal steroids versus control.
| Intranasal (topical) steroids compared with inactive control for otitis media with effusion in children | ||||||
|
Patient or population: Children with otitis media with effusion Settings: Hospital or general practice (primary care) Intervention: Intranasal steroids for the treatment of OME Comparison: Inactive control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Inactive control | Intranasal steroids | |||||
|
Median days with hearing loss [3 months] |
The median days with hearing loss for the control group was 4 | The median days with hearing loss in the intervention groups was the same at 4 | 200 [1] | +++O moderate1 | ||
|
Hearing loss (Audiometry failing) [9 months]2 |
Medium‐risk population | RR 1.17 (0.87 to 1.58) | 141 [1] | +++O moderate3 | ||
| 507 per 1000 | 593 per 1000 (441 to 801) | |||||
|
OME resolution at short‐term follow up (< 1 month) [3 weeks] |
Medium‐risk population | RR 0.64 (0.31 to 1.31) | 44 (1) | +++O moderate4 | ||
| 522 per 1000 | 334 per 1000 (162 to 684) | |||||
|
OME resolution at intermediate‐term follow up (3 months) [1 to < 6 months ] |
Medium‐risk population | RR 1.11 (0.85 to 1.46) | 172 (1) | +++O moderate3 | ||
| 523 per 1000 | 577 per 1000 (442 to759) | |||||
|
OME resolution at long‐term follow up (9 months) [≥ 6 months] |
Medium‐risk population | RR 0.85 0.65 to 1.11) | 144 (1) | +++O moderate3 | ||
| 653 per 1000 | 555 per 1000 (424 to 725) | |||||
| Symptoms | See comment | See comment | Not estimable | See comment | See comment | No studies reported data on symptoms |
|
Adverse effects [3 months] |
Medium‐risk population | RR 1.26 (0.80 to 1.99) | 231
(2)5 See comment |
++OO low6 | There were no serious adverse effects in 2 studies (1 not included in analysis)5 | |
| 267 per 1000 | 336 per 1000 (214 to 531) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OME: otitis media with effusion; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Single well‐conducted RCT.
2 Fail on more than two frequencies in both ears at 0.5, 1, 2, 3, 4 kHz using hand‐held audiometers at 25 dB HL.
3 Single well‐conducted RCT with 35%, 21% or 34% lost to follow up, respectively.
4 Single RCT with small sample size.
5 Adverse effects were relatively minor and included in analysis were cough, dry throat, epistaxis and nasal stinging. One study reported that there were no adverse reactions or pathological nasal mucosal changes (actual numbers not stated). The study also reported that there was a transient drop in serum cortisol levels in 2 steroid‐treated patients.
6 Adverse effects poorly reported in one RCT and data only available for 40% of participants.
Summary of findings 4. Summary of findings: intranasal steroids plus oral antibiotic versus control plus antibiotic.
| Intranasal (topical) steroids plus oral antibiotics compared with inactive control plus oral antibiotics for otitis media with effusion in children | ||||||
|
Patient or population: Children with otitis media with effusion Settings: Hospital (secondary or tertiary care) Intervention: Intranasal steroids plus oral antibiotics for the treatment of OME Comparison: Inactive control plus antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Inactive control plus antibiotics | Intranasal steroids plus oral antibiotics | |||||
| Hearing loss | See comment | See comment | Not estimable | See comment | See comment | No studies reported data on hearing loss |
|
OME resolution at short‐term follow up [< 1 month] |
See comment | See comment | Not estimable | See comment | See comment | No studies reported data on short‐term follow up |
|
OME resolution at intermediate‐term follow up (3 months) [1 to < 6 months] |
Medium‐risk population | RR 1.26 (0.54 to 2.96) | 59 (1) | +++O moderate1 | ||
| 25 per 1000 | 32 per 1000 (14 to 74) | |||||
|
OME resolution at long‐term follow up [≥ 6 months] |
See comment | See comment | Not estimable | See comment | See comment | No studies reported data on short‐term follow up |
|
Symptom score [3 months] |
The mean symptom score for the control group was 12.4 mm | The mean symptom score in the intervention group was 4.5 mm lower | 39 [1] | +++O moderate1 | ||
|
Adverse effects [3 months] |
See comment | See comment | Not estimable | 59 (1) | See comment2 | There were no significant side effects |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OME: otitis media with effusion; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Single RCT with small sample size.
2 One study reported that other than transient nasal stinging and epistaxis no significant side effects (that required discontinuation of treatment) were reported (actual numbers not stated).
Overall the numbers of studies for each comparison were small (ranged from one to five). The number of participants available for each comparison ranged from 15 to 409.
Oral steroids versus control
Three included studies provided data on a total of 108 patients randomised to treatment with oral steroids versus placebo (Niederman 1984; Macknin 1985) or no‐treatment control (Giebink 1990).
Primary outcomes
Only one study included usable data on our primary outcome measure, hearing loss (Macknin 1985). The results did not show benefit from using oral steroids compared with placebo, in terms of hearing not improved by at least 10 dB in either ear at six weeks follow up (risk ratio (RR) 1.09; 95% confidence interval (CI) 0.80 to 1.49, 44 children) (Analysis 1.1).
1.1. Analysis.
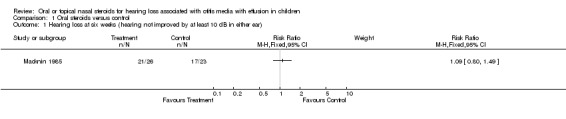
Comparison 1 Oral steroids versus control, Outcome 1 Hearing loss at six weeks (hearing not improved by at least 10 dB in either ear).
Secondary outcomes
Two studies reported data on OME resolution, for which the findings did not show a significant effect of steroids compared to placebo or no intervention control at short‐term follow up (two weeks) (RR 3.80; 95% CI 0.93 to 15.52, three trials, 108 children) (Analysis 1.2) and intermediate‐term follow up (four to six weeks) (RR 1.54; 95% CI 0.76 to 3.14, three trials, 106 children) (Analysis 1.3). These findings were based on a random‐effects model. The summary effect size at short‐term follow up is large with a wide CI that only just crossed zero. When analysing the same data using a fixed‐effect model, the results showed oral steroids to be significantly better than the control (RR 4.48; 95% CI 1.52 to 13.23). Although the studies were clinically heterogeneous, statistical between‐study heterogeneity for this comparison was fairly low (Chi² 2.75, df = 2, P = 0.25; I² = 27%).
1.2. Analysis.
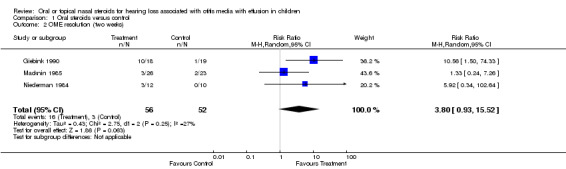
Comparison 1 Oral steroids versus control, Outcome 2 OME resolution (two weeks).
1.3. Analysis.
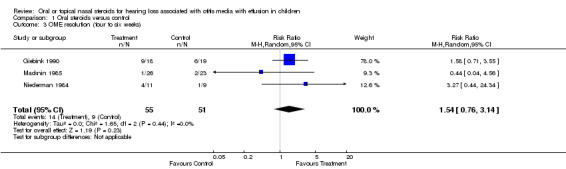
Comparison 1 Oral steroids versus control, Outcome 3 OME resolution (four to six weeks).
Oral steroids plus antibiotics versus control plus antibiotics
Six included studies provided data on a total of 508 patients randomised to treatment with oral steroids plus antibiotic versus placebo plus antibiotic.
Primary outcomes
Four studies (Lambert 1986; Berman 1990; Podoshin 1990; Mandel 2002) included audiometry data, but only one reported data that could be used in the analysis (Podoshin 1990). There was no significant difference between the intervention groups in terms of the number of participants with partial or no improvement in hearing loss (RR 1.01; 95% CI 0.73 to 1.40; 99 children) at two months follow up (Analysis 2.1).
2.1. Analysis.

Comparison 2 Oral steroids plus antibiotic versus control plus antibiotic, Outcome 1 Hearing loss at two months (at least some conductive hearing loss).
Secondary outcomes
The RR for OME resolution at short‐term follow up (seven to 28 days) was 1.99 (95% CI 1.14 to 3.49, five trials, 409 children) in favour of the steroid group (Schwartz 1980; Lambert 1986; Berman 1990; Hemlin 1997; Mandel 2002) (Analysis 2.2). However, there was significant heterogeneity between these studies (P < 0.01, I² = 69%). One study reported inconsistent results that were in favour of the control group, but the summary effect estimate was very close to zero (RR 0.88; 95% CI 0.51 to 1.50) and therefore not considered to be inconsistent enough to prevent pooling. There was no clear reason as to why this study would report inconsistent findings, although the study was poorly reported and included slightly older children, ranging from two to 15 years (mean six years). Removing this potential outlier resulted in homogeneity between the remaining studies (Lambert 1986) (Tau² = 0.00; Chi² = 2.86, df = 3 (P = 0.41); I² = 0%) and an increase in the effect size and its precision (RR 2.39; 95% CI 1.69 to 3.37, four trials, 349 children). Two studies (Podoshin 1990; Mandel 2002) reported data on intermediate follow up for which there was a non‐significant benefit in favour of the steroid group for OME resolution at one to two months (RR 1.44; 95% CI 0.97 to 2.13, two trials, 231 children) (Analysis 2.3). Only one study (Hemlin 1997) reported long‐term (six months) outcome data on resolution of OME but only provided this data for a subset of 15 (13%) patients who were considered cured at prior follow‐up visits.
2.2. Analysis.
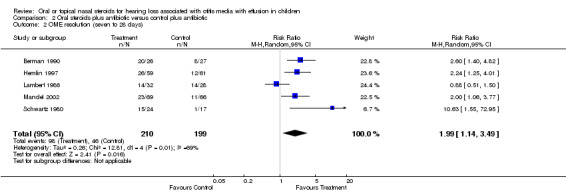
Comparison 2 Oral steroids plus antibiotic versus control plus antibiotic, Outcome 2 OME resolution (seven to 28 days).
2.3. Analysis.
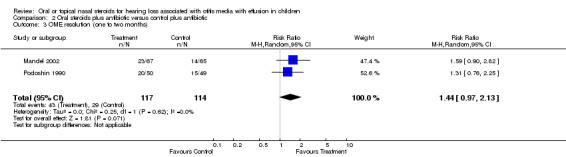
Comparison 2 Oral steroids plus antibiotic versus control plus antibiotic, Outcome 3 OME resolution (one to two months).
Topical intranasal steroids versus control
Two included studies provided data on a total of 238 patients randomised to treatment with intranasal steroids versus placebo (Shapiro 1982; Williamson 2009).
Primary outcomes
One study (Williamson 2009) included audiometry data. There was no important difference between the groups for median days hearing loss (weighted mean difference (WMD) 0.0; 95% CI ‐4.51 to 4.51, 200 children) at three months follow up (Analysis 3.2) or the number of participants failing the audiometry test (fail on more than two frequencies in both ears at 0.5, 1, 2, 3 and 4 kHz using hand‐held audiometers at 25 dB HL) (RR 1.17; 95% CI 0.87 to 1.58; 141 children) at nine months follow up (Analysis 3.1).
3.2. Analysis.
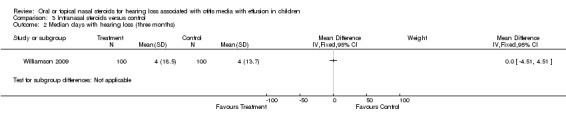
Comparison 3 Intranasal steroids versus control, Outcome 2 Median days with hearing loss (three months).
3.1. Analysis.

Comparison 3 Intranasal steroids versus control, Outcome 1 Audiometry failing at nine months (audiometry sweep at 25 dB HL, fail on more than two out of five frequencies in both ears).
Secondary outcomes
There was no evidence of improvement in OME resolution with the use of intranasal steroid at short‐term follow up (three weeks) (RR 0.64; 95% CI 0.31 to 1.31, one trial, 44 children) (Analysis 3.3) (Shapiro 1982). There was also no important difference between the intervention groups for OME resolution at intermediate follow up (three months) (RR 1.11; 95% CI 0.85 to 1.46, one trial, 172 children) (Analysis 3.4) (Williamson 2009) or long‐term follow up (nine months) (RR 0.85; 95% CI 0.65 to 1.11, one trial, 144 children) (Analysis 3.5) (Williamson 2009).
3.3. Analysis.
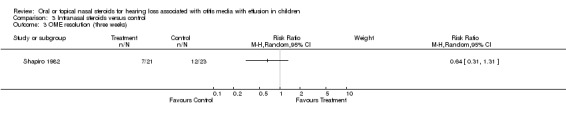
Comparison 3 Intranasal steroids versus control, Outcome 3 OME resolution (three weeks).
3.4. Analysis.
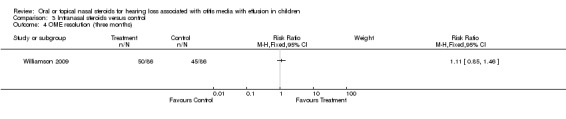
Comparison 3 Intranasal steroids versus control, Outcome 4 OME resolution (three months).
3.5. Analysis.
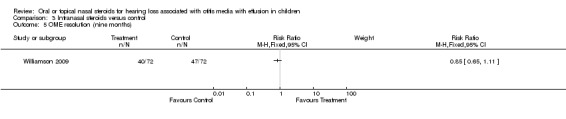
Comparison 3 Intranasal steroids versus control, Outcome 5 OME resolution (nine months).
Topical intranasal steroids plus antibiotics versus control plus antibiotics
There was only one study that compared topical intranasal steroids plus antibiotics with placebo plus antibiotics (Tracy 1998).
Primary outcomes
No study of topical intranasal steroids included audiometry data.
Secondary outcomes
One study (Tracy 1998) reported data on OME resolution at intermediate follow up, for which there was no important difference between the steroid and the control group at three months (RR 1.26; 95% CI 0.54 to 2.96, 53 children) (Analysis 4.1). The study also included data on a symptom score used after three months, which also showed no overall benefit of using steroid (WMD ‐4.5; 95% CI ‐10.28 to 1.28, 39 children) (Analysis 4.2).
4.1. Analysis.
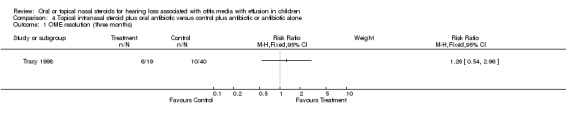
Comparison 4 Topical intranasal steroid plus oral antibiotic versus control plus antibiotic or antibiotic alone, Outcome 1 OME resolution (three months).
4.2. Analysis.
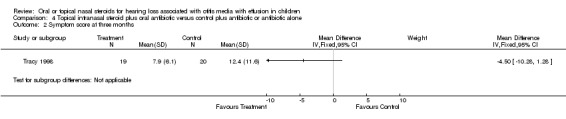
Comparison 4 Topical intranasal steroid plus oral antibiotic versus control plus antibiotic or antibiotic alone, Outcome 2 Symptom score at three months.
Adverse effects
Only four studies (Giebink 1990; Hemlin 1997; Mandel 2002; Williamson 2009) reported the actual number and type of adverse effects experienced; one (Giebink 1990) only included haematological adverse effects. There were no significant differences between oral steroid plus antibiotic and oral placebo plus antibiotic (RR 1.34; 95% CI 0.84 to 2.14, two trials, 255 children) (Analysis 2.4) (Hemlin 1997; Mandel 2002) or intranasal steroid and placebo (RR 1.26; 95% CI 0.80 to 1.99, 172 children) (Analysis 3.6) (Williamson 2009) for adverse effects. In the studies by Shapiro and Giebink transient drops in cortisol levels were measured in steroid‐treated patients. Other studies mentioned the following mild to moderate adverse effects: vomiting, diarrhoea, stomach ache, gastroenteritis, dermatitis, increased appetite, hyperactivity, rash transient nasal stinging epistaxis, nosebleed, dry throat and cough.
2.4. Analysis.
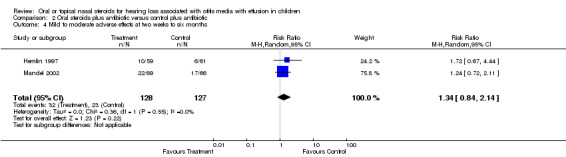
Comparison 2 Oral steroids plus antibiotic versus control plus antibiotic, Outcome 4 Mild to moderate adverse effects at two weeks to six months.
3.6. Analysis.
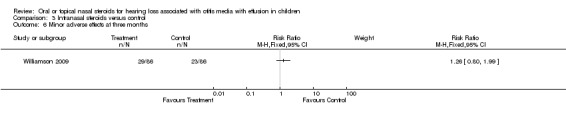
Comparison 3 Intranasal steroids versus control, Outcome 6 Minor adverse effects at three months.
Only one study (Mandel 2002) reported that some patients had to discontinue treatment due to adverse effects, although none appear to be associated with steroid treatment. Treatment was divided into two phases, an initial two‐week period of steroid plus antibiotic versus placebo plus antibiotic, and then a second two‐week phase that evaluated prolonged antibiotic treatment. During phase I placebo was discontinued due to adverse effects in two participants and in phase II amoxicillin or placebo was discontinued due to adverse effects in three participants (two in the steroid and one in the placebo + antibiotic group). The actual severity of the adverse effects was not reported, but could be considered potentially serious. (Only data on adverse effects from phase I that included the steroid treatment are included in the meta‐analysis). No serious or lasting adverse effects were reported in the remaining four studies on oral steroids mentioning adverse effects (Niederman 1984; Macknin 1985; Giebink 1990; Hemlin 1997; Mandel 2002) or in the three studies on topical intranasal steroids (Shapiro 1982; Tracy 1998; Williamson 2009).
General
Our objectives were to assess whether the average effect of steroids for OME is beneficial, which is a much broader perspective than that of individual studies that assess the use of a specific steroid intervention in a narrow patient population. We also wanted to able to generalise the findings to a range of scenarios. As such our review included a clinically diverse set of studies that differed in terms of pharmacological interventions including the steroid formulation, steroid dose, duration of steroid treatment and concomitant antibiotic; and study population including age, setting, proportion with bilateral OME and duration of OME at study entry. However, we deemed the studies to have enough in common for it to make sense to synthesise the data, and statistical heterogeneity was not considerable.
Ten comparisons across the study groups included only one study, so they offer no new information. Overall, seven comparisons favoured steroids, six favoured controls and two favoured neither. However, the confidence intervals were generally wide and included unity for all but one comparison. There were a small number of studies in many of the comparisons, therefore risk of bias was not used in sensitivity analyses.
Discussion
Oral steroids
There was no evidence of the beneficial effect of oral steroids (with or without antibiotics) on hearing loss associated with otitis media with effusion (OME). However, this outcome was only evaluated by two studies of small (oral steroids) to moderate size (oral steroids plus antibiotics). One study also included patients with unilateral hearing loss and the other did not state how many had bilateral or unilateral hearing loss. Only one study required documented hearing loss for all children prior to study entry.
Children treated with a steroid used together with an antibiotic were twice as likely to have improved resolution of their effusion as those treated with antibiotics plus placebo in the short term (up to two weeks post‐randomisation: risk ratio (RR) 1.99; 95% confidence interval (CI) 1.14 to 3.49). However, the beneficial effect of steroids was no longer statistically significant (RR 1.44; 95% CI 0.97 to 2.13) at intermediate follow up (up to two months after randomisation), although the meta‐analysis only included two studies (231 children).
Oral steroids, when used alone, also appear to have a beneficial effect on the resolution of OME in the short term. Using a random‐effects model, children who were treated with oral steroids were over three times more likely to have an improved resolution of effusion than those treated with placebo, but the confidence interval was wide and included unity (RR 3.80; 95% CI 0.93 to 15.52). However, when a fixed‐effect model was used to pool the same data the findings became statistically significant. Although the clinical heterogeneity between studies means that a random‐effects model was appropriate, there was no statistical heterogeneity present and the findings of the fixed‐effect model demonstrates the potential effectiveness of oral steroids for OME resolution in the short term. However, the beneficial effect of oral steroids, as with the steroids plus antibiotics, was no longer statistically significant (RR 1.54; 95% CI 0.76 to 3.14) at intermediate follow up (up to six weeks after randomisation) but the meta‐analysis was based on fewer than 100 children (three studies).
Few data were available for longer‐term outcomes. One study (Hemlin 1997) reported six‐month data on OME resolution, but only provided these data for a subset of 15 (13%) patients who were considered cured at prior follow‐up visits. No study assessed the effect of steroids on hearing or speech in the longer term.
Data concerning adverse effects were provided in five studies. No lasting or serious adverse effects were reported. However, the numbers of children involved are insufficient to judge safety in pragmatic settings and no long‐term data were available. Furthermore, we only included randomised controlled trials in our review, which is not the optimum study design for evaluating adverse effects, especially if they are uncommon or long‐term.
Topical nasal steroids
Given concern about treating what is often a self‐limiting condition with systemic steroids, we were particularly interested to examine evidence for the effectiveness of topical intranasal steroids. The two studies we included of topical intranasal steroid and the one study of topical intranasal steroids in combination with an antibiotic showed no benefit in the short, intermediate or long term.
Only one study (Williamson 2009), which compared topical intranasal steroid with placebo, reported on hearing loss, for which there was no benefit. The same study also reported long‐term data on speech and language, which was part of a subscale of the condition‐specific outcome scale OM8‐30. However, the findings were reported in a format we could not use for this review and so could not be included in our analyses.
All three studies mentioned adverse effects. No lasting or serious adverse effects were found.
General
No study documented hearing loss prospectively prior to study entry. Seven studies included audiometry data in their outcomes, but hearing loss results of only three studies (Macknin 1985; Podoshin 1990; Williamson 2009) could be used in this review. Steroid treatment did not improve hearing loss. Only two studies attempted to measure the effect of steroids on subjective symptoms.
Comparing our results to previous reviews, Nuss and Berman (Nuss 1990) included studies published in abstract form only (Heary 1990) and a non‐randomised, open study (Persico 1978). They concluded that combination therapy (oral steroids plus antibiotics) is worth considering in children with OME persisting beyond eight weeks prior to surgical intervention. Rosenfeld and colleagues (Rosenfeld 1991) performed a meta‐analysis of six randomised trials, and concluded that children receiving oral steroids for seven to 14 days were three times more likely than control subjects to have both ears free of effusion at the end of therapy (95% CI 2.2 to 4.1). They also found significant heterogeneity between studies. Three of the studies involved treatment with oral steroid plus antibiotic (odds ratio (OR) favouring steroid plus antibiotic treatment 2.8; 95% CI 2.0 to 4.0) and three oral steroid alone (OR favouring steroids 3.7; 95% CI 2.0 to 6.7). These authors concluded that despite favourable ORs for short‐term resolution of effusions, they could not recommend the use of steroids for OME until more is known about which children are most likely to derive benefit. The difference between these reviews and the results we obtained are accounted for by the inclusion of a study published after their analysis was done (Hemlin 1997). We considered clinical effectiveness. Berman and colleagues performed a meta‐analysis of clinical effectiveness to establish cost‐effectiveness of treatment for OME (Berman 1994). In comparing steroid plus antibiotic, they included two trials that did not meet our inclusion criteria (Berman 1987; Daly 1991). They concluded that steroid plus antibiotic was the most cost‐effective intervention to clear bilateral middle ear effusion for children at a first follow‐up visit six weeks after diagnosis of acute otitis media. However, they did not consider risks, adverse effects and parental preferences. The meta‐analysis for the Agency for Health Care Policy and Research (AHCPR) used Bayesian statistical methods (Stool 1994). Based on the same studies we included for oral steroids compared to control, they found a mean difference in OME at two weeks of 18.4% (95% CI ‐3.4% to 38.6%) favouring steroid therapy. By four to six weeks the difference in improvement was 4.5% (95% CI ‐11.7% to 20.6%). Comparing oral steroid plus antibiotic to antibiotic, they included the trial by Podoshin (Podoshin 1990) but the trial by Hemlin (Hemlin 1997) was not yet published. They found a mean improvement for antibiotic steroid combination compared to antibiotic alone of 25.1% (95% CI ‐1.3% to 49%) that was not statistically significant. They also did a comparison of steroids plus antibiotic compared to control and included a trial of topical nasal steroids in this comparison; they found a mean improvement of OME due to steroid plus antibiotic therapy of 21.4% (95% CI ‐1.4% to 42.6%) that was not statistically significant. We did not carry out this comparison since treatment arms differed more than by steroid alone. The AHCPR recommended that steroids should not be used for OME. The UK National Institute for Health and Clinical Excellence (NICE) recommends against intranasal steroid treatment for OME (NICE 2008).
Authors' conclusions
Implications for practice.
There is no evidence of beneficial effect from steroids (oral or topical) on hearing loss associated with OME. There is some evidence demonstrating short‐term improvement of OME from oral steroids, especially when used in combination with antibiotics. However, we found no evidence for lasting beneficial effect on effusions from oral or topical intranasal steroid treatment, and no short‐term benefit from intranasal steroids.
Implications for research.
OME may be present without significant hearing loss and there is a high rate of spontaneous recovery from the hearing loss associated with OME, so future studies should document hearing loss associated with OME for a period prior to beginning study treatment and at follow up. In the absence of evidence that unilateral OME influences language development, the practice of either treating in routine clinical practice or entering children with unilateral OME into treatment research studies is questionable. Follow up should be longer and ideally include symptom, or quality of life, and hearing assessments. Audiometry data should be presented not as mean hearing levels for groups but as numbers of children with defined levels of hearing loss in their best hearing ear. Data should not be presented with ears as the unit of analysis since observations on the different ears of the same child cannot be regarded as independent. Assessors of outcomes should be blinded to the treatment allocation. Improvement should be clearly defined, for example authors should present data for children with bilateral OME resolving in one but not both ears. Analysis should be on the basis of intention‐to‐treat. A short course of oral steroids followed by longer‐term topical intranasal steroids has so far not been evaluated.
What's new
| Date | Event | Description |
|---|---|---|
| 23 November 2010 | New citation required and conclusions have changed | We included one new study (Williamson 2009) and excluded three further studies (Ortega del Alamo 2005; Cengel 2006; Choung 2008). The conclusions regarding the use of oral steroid at short‐term follow up remain the same, but the evidence for the effectiveness of oral steroid plus antibiotic therapy is stronger than that of the use of oral steroid alone. There is also imperfect evidence suggesting that the use of oral steroid plus antibiotic may be effective at intermediate follow up. We no longer conclude that there is evidence of short‐term improvement with topical intranasal steroid with or without antibiotics. The conclusions regarding long‐term outcomes remain the same. We have adopted the Cochrane 'Risk of bias' tool for assessment of study quality (which involved reassessing the studies included in the previous version of the review). We have also incorporated 'Summary of findings' tables, used the I² statistic to assess heterogeneity and changed the analysis method for dichotomous outcomes from odds ratio (OR) using a fixed‐effect method to risk ratio (RR) using a random‐effects method. The authorship of the review has also changed. |
| 23 November 2010 | New search has been performed | We ran new full searches in August 2010. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 16 March 2010 | Amended | Correction to analyses headings and labels following feedback (unpublished). |
| 26 October 2008 | Amended | Converted to new review format. |
| 17 May 2006 | New citation required and conclusions have changed | Substantive amendment. |
| 16 August 2002 | New search has been performed | Substantive amendment. |
Acknowledgements
Chantal L Thomas was a former lead author of the review and conducted the 2006 major update (Thomas 2006), carrying out searching for trials, quality assessment of trials, data extraction and data analysis.
We wish to thank Dr Christine Clar, Dr Martin Burton and Ms Jenny Bellorini for editorial help, and Gemma Sandberg for conducting the update searches. In addition to peer reviewers, Dr Arne Ohlsson provided valuable feedback. We are grateful to Paul Harnett and Dr Richard Martin of the University of Bristol for identifying and bringing to our attention an error in our data entry for the study by Giebink and colleagues (Giebink 1990) in a previous version of this review.
Appendices
Appendix 1. Search strategies
| CENTRAL | PubMed | EMBASE (Ovid) |
| # 1 OTITIS MEDIA WITH EFFUSION single term (MeSH) # 2 glue ear # 3 otitis media NEAR effusion* or middle ear NEAR effusion* # 4 eustachian tube NEAR effusion* OR eustachian tube NEAR dysfunction* # 5 nonsuppurative otitis OR non suppurative otitis # 6 tympanitis OR serous otitis OR secretory otitis OR otitis serosa.TI,AB. # 7 mucoid NEAR otitis OR mucous NEAR otitis OR seromucoid NEAR otitis # 8 mucoid NEAR middle ear OR mucous NEAR middle ear OR seromucoid NEAR middle ear # 9 adhesive NEAR otitis OR exudative NEAR otitis # 10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 # 11 STEROIDS explode all trees (MeSH) # 12 HYDROXYCORTICOSTEROIDS explode all trees (MeSH) # 13 GLUCOCORTICOIDS explode all trees (MeSH) # 14 ANTI‐INFLAMMATORY AGENTS STEROIDS explode all trees (MeSH) # 15 GLUCOCORTICOSTEROIDS SYNTHETIC explode all trees (MeSH) # 16 steroid* OR corticosteroid* or glucocorticoid* # 17 beclomethasone OR betamethasone # 18 budesonide # 19 cortisone # 20 dexamethasone # 21 flunisolide # 22 fluticasone # 23 fludrocortisone # 24 hydrocortisone OR cortisol # 25 methylprednisolone # 26 mometasone # 27 prednisolone # 28 prednisone # 29 triamcinolone # 30 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 # 31 #10 AND #30 | #1 "OTITIS MEDIA WITH EFFUSION" [Mesh] #2 "glue ear" [tiab] OR ("otitis media" [tiab] AND effusion* [tiab]) OR ("middle ear" [tiab] AND effusion* [tiab]) OR ("eustachian tube" [tiab] AND effusion [tiab]) OR ("Eustachian tube" [tiab] AND dysfunction* [tiab]) OR "nonsuppurative otitis" [tiab] OR "non suppurrative otitis" [tiab] OR tympanitis [tiab] OR "serous otitis" [tiab] OR "secretory otitis" [tiab] OR "otitis serosa" [tiab] OR (mucoid [tiab] AND otitis [tiab]) OR (mucous [tiab] AND otitis [tiab]) OR (seromucoid [tiab] AND otitis [tiab]) OR (mucoid [tiab] AND "middle ear" [tiab] OR (mucous [tiab] AND "middle ear" [tiab]) OR (seromucoid [tiab] AND middle [tiab]) #3 #1 OR #2 #4 "STEROIDS" [Mesh] OR "HYDROXYCORTICOSTEROIDS" [Mesh] OR "GLUCOCORTICOIDS" [Mesh] OR "ANTI‐INFLAMMATORY AGENTS STEROIDS" [Mesh] OR "GLUCOCORTICOSTEROIDS SYNTHETIC" [Mesh] #5 steroid* [tiab] OR corticosteroid* [tiab] OR glucocorticoid* [tiab] OR beclomethasone [tiab] OR betamethasone [tiab] OR budesonide [tiab] OR cortisone [tiab] OR dexamethasone [tiab] OR flunisolide [tiab] OR fluticasone [tiab] OR fludrocortisone [tiab] OR hydrocortisone [tiab] OR cortisol [tiab] OR methylprednisolone [tiab] OR mometasone [tiab] OR prednisolone [tiab] OR prednisone [tiab] OR triamcinolone [tiab] #6 #4 OR #5 #7 #3 AND #6 | 1 mucoid otitis media/ 2 ((glue adj ear) or ((otitis adj media) and effusion*) or ((middle adj ear) and effusion*) or ((eustachian adj tube) and effusion) or ((Eustachian adj tube) and dysfunction*) or (nonsuppurative adj otitis) or (non adj suppurrative adj otitis) or tympanitis or (serous adj otitis) or (secretory adj otitis) or (otitis adj serosa) or (mucoid and otitis) or (mucous and otitis) or (seromucoid and otitis) or ((mucoid and middle) adj ear) or ((mucous and middle) adj ear) or (seromucoid and middle)).tw. 3 secretory otitis media/ or serous otitis media/ 4 1 or 3 or 2 5 exp CORTICOSTEROID/ 6 (steroid* or corticosteroid* or glucocorticoid* or beclomethasone or betamethasone or budesonide or cortisone or dexamethasone or flunisolide or fluticasone or fludrocortisone or hydrocortisone or cortisol or methylprednisolone or mometasone or prednisolone or prednisone or triamcinolone).tw. 7 6 or 5 8 4 and 7 |
| Web of Science | BIOSIS Previews/ CAB Abstracts (Ovid) | CINAHL |
| #1 TS=((glue adj ear) or ((otitis adj media) and effusion*) or ((middle adj ear) and effusion*) or ((eustachian adj tube) and effusion) or ((Eustachian adj tube) and dysfunction*) or (nonsuppurative adj otitis) or (non adj suppurrative adj otitis) or tympanitis or (serous adj otitis) or (secretory adj otitis) or (otitis adj serosa) or (mucoid and otitis) or (mucous and otitis) or (seromucoid and otitis) or ((mucoid and middle) adj ear) or ((mucous and middle) adj ear) or (seromucoid and middle)) #2 TS=(steroid* or corticosteroid* or glucocorticoid* or beclomethasone or betamethasone or budesonide or cortisone or dexamethasone or flunisolide or fluticasone or fludrocortisone or hydrocortisone or cortisol or methylprednisolone or mometasone or prednisolone or prednisone or triamcinolone) #3 #1 AND #2 | 1 ((glue adj ear) or ((otitis adj media) and effusion*) or ((middle adj ear) and effusion*) or ((eustachian adj tube) and effusion) or ((Eustachian adj tube) and dysfunction*) or (nonsuppurative adj otitis) or (non adj suppurrative adj otitis) or tympanitis or (serous adj otitis) or (secretory adj otitis) or (otitis adj serosa) or (mucoid and otitis) or (mucous and otitis) or (seromucoid and otitis) or ((mucoid and middle) adj ear) or ((mucous and middle) adj ear) or (seromucoid and middle)).tw. 2 (steroid* or corticosteroid* or glucocorticoid* or beclomethasone or betamethasone or budesonide or cortisone or dexamethasone or flunisolide or fluticasone or fludrocortisone or hydrocortisone or cortisol or methylprednisolone or mometasone or prednisolone or prednisone or triamcinolone).tw. | S1 (MH "Otitis Media with Effusion") S2 TX glue AND ear S3 TX ( middle OR otitis OR eustachian ) and TX ( effusion OR serous OR secretory OR nonsuppurative OR mucoid ) S4 S1 or S2 or S3 S5 TX steroid* or corticosteroid* or glucocorticoid* or beclomethasone or betamethasone or budesonide or cortisone or dexamethasone or flunisolide or fluticasone or fludrocortisone or hydrocortisone or cortisol or methylprednisolone or mometasone or prednisolone or prednisone or triamcinolone S6 (MH "Steroids") OR (MH " GLUCOCORTICOIDS") S7 S5 or S6 S8 S4 and S7 |
Data and analyses
Comparison 1. Oral steroids versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hearing loss at six weeks (hearing not improved by at least 10 dB in either ear) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 OME resolution (two weeks) | 3 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 3.80 [0.93, 15.52] |
| 3 OME resolution (four to six weeks) | 3 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.76, 3.14] |
Comparison 2. Oral steroids plus antibiotic versus control plus antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hearing loss at two months (at least some conductive hearing loss) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 OME resolution (seven to 28 days) | 5 | 409 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [1.14, 3.49] |
| 3 OME resolution (one to two months) | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.97, 2.13] |
| 4 Mild to moderate adverse effects at two weeks to six months | 2 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.84, 2.14] |
Comparison 3. Intranasal steroids versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Audiometry failing at nine months (audiometry sweep at 25 dB HL, fail on more than two out of five frequencies in both ears) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Median days with hearing loss (three months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 OME resolution (three weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4 OME resolution (three months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5 OME resolution (nine months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Minor adverse effects at three months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 4. Topical intranasal steroid plus oral antibiotic versus control plus antibiotic or antibiotic alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 OME resolution (three months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Symptom score at three months | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Berman 1990.
| Methods | Randomised, placebo‐controlled trial (patients who failed to respond at 2 weeks crossed over to alternative treatment) | |
| Participants | 68 children aged 6 months to 5.4 years recruited from the otitis clinic Effusion present for at least 6 weeks and all had received at least 2 courses of antibiotics Diagnosis (see 'Types of participants'): 1B, 2B 77% bilateral effusions |
|
| Interventions | Antibiotic (trimethoprim‐sulfamethoxazole) for 30 days plus prednisone 0.5 to 1.0 mg/kg/dose/BD for first 7 days (n = 26) versus antibiotic plus placebo (n = 27) | |
| Outcomes | Pneumo‐otoscopy and tympanometry resolution and/or speech threshold no greater than a 15 dB hearing loss on audiometry Follow up at 2 weeks (4‐week data not used due to cross‐over design) Audiometry data used ear as unit of analysis |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned from a random numbers table provided by statistician" |
| Allocation concealment (selection bias) | Low risk | Quote: "investigator treating patient was blinded to the randomisation scheme" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Unclear if outcome assessors were blind, but study reported as double‐blind |
| Incomplete outcome data (attrition bias) < 1 month | Unclear risk | 22% of patients who did not return for follow up were excluded from analysis (9 in prednisone group, 6 in placebo group; reasons not stated) |
| Incomplete outcome data (attrition bias) 1 to 6 months | Unclear risk | — |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | — |
Giebink 1990.
| Methods | Randomised controlled trial, 4 parallel groups, open design | |
| Participants | 76 children aged 10 months to 7.9 years. Participants identified by screening medical records of 16,609 consecutive children examined following a recent episode of OME. Participants had continuous OME for at least 8 weeks, 3 or more episodes within previous 18 months and completed a course of antibiotic therapy for most recent acute OM Diagnosis: 1B, 2C 60% bilateral |
|
| Interventions | Prednisone 1 mg/kg in tapering dose for 2 weeks (n = 18) versus no treatment controls (n = 19) (versus prednisone plus trimethoprim‐sulfamethoxazole (n = 20) versus ibuprofen (n = 15)) | |
| Outcomes | Pneumo‐otoscopy and tympanometry resolution at 2 and 4 weeks after entry There were no significant haematologic complications of medical treatment. Transient drop in serum cortisol in 14/17 treated with prednisone. Patients who were considered treatment failure (at 4 weeks or beyond) were referred for surgical evaluation and discontinued the study. 78% were followed up for at least 4 months and 35% for 12 months. Survival analyses performed on time from randomisation to clearance and time from clearance to relapse, but no data reported only Kaplan‐Meier survival curves presented for illustrative purposes and therefore could not be included in our review Comparative audiometry data used ear as unit of analysis and was based on only 29 (38%) patients who had an audiometric evaluation (39 patients remained in the study at 12 months); 24 had hearing loss |
|
| Notes | Not placebo‐controlled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | Quote: "all medications dispensed by pharmacist outside of the clinical area" Comment: allocation considered blind as it appears to have been remote |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "blinded protocol was not used because the drug protocols were different; however, objective outcome determinations were used" Quote: [Audiometric examinations were performed at the end of follow up (12 months)] "'examiners were unaware of the patient’s initial treatment assignment" Nothing is reported for the 2 to 4‐week assessment |
| Incomplete outcome data (attrition bias) < 1 month | Low risk | 4/76 (5%) participants excluded, group allocation not stated; 1 due to refusal to take steroid, 2 requiring additional treatment (1 for acute OM) and 1 met the exclusion criteria. Possible further 3 excluded from steroid group in analysis; inconsistencies in reporting. Outcome measures given as percentages, so actual numbers followed up not clear (although drop‐outs would still be less than 10%). |
| Incomplete outcome data (attrition bias) 1 to 6 months | Low risk | 4/76 (5%) participants excluded, reasons as above. Possible further 3 excluded from steroid group in analysis; inconsistencies in reporting (although drop‐outs would still be less than 10%) |
| Selective reporting (reporting bias) | Low risk | Poor reporting of results. Outcome measures given as percentages and unclear if number of participants in intervention group was 18 or 15, although this is unlikely to affect the findings of our review. Study follow up was up to 12 months, but useable data only presented for 2 and 4 weeks follow up. |
| Other bias | Unclear risk | Some imbalance in baseline characteristics. Intervention group tended to be younger, had more bilateral OME, greater prior episodes and more likely to have had prior surgery than control group. However, the sample size was small and the inconsistency may be due to chance. Multivariate analysis indicated that none of these factors were significant covariates for any of the outcomes (results not reported). |
Hemlin 1997.
| Methods | Randomised, placebo‐controlled trial; 3 parallel groups with random allocation in ratio of 3:3:1 | |
| Participants | 142 children aged from 2 to 12 years seen in the Department of Otolaryngology Effusion present for at least 3 months Diagnosis: 1B, 2C 84% bilateral |
|
| Interventions | Antibiotic for 10 days (cefixime) plus single dose of betamethasone 6 mg on day 10 (n = 59) versus antibiotic plus placebo (n = 61) (versus placebo only (n = 20)) | |
| Outcomes | Otomicroscopy and tympanometry resolution Cure defined as 1 of 2 affected ears clear or both clear after 1 ear affected 2 to 11 days after completion of treatment (treatment failures were not followed up beyond this) Follow up also at 6 weeks and 6 months after completion of treatment, but for patients with OME resolution at preceding follow up only Adverse effects classified as possibly or probably treatment‐related included dermatitis, diarrhoea, loose stools, vomiting, stomach pain and gastroenteritis. 65% were mild and remainder moderate; none were serious. A further 8 participants experienced adverse effects that were deemed not related to treatment, group allocation not stated. |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | Quote: "The drugs were dispensed double blind by a double‐dummy technique" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | — |
| Incomplete outcome data (attrition bias) < 1 month | Low risk | 1 patient from cefixime plus placebo group (adverse event) and 1 from cefixime plus betamethasone group (withdrawal before starting treatment) excluded from analysis |
| Incomplete outcome data (attrition bias) 1 to 6 months | Unclear risk | — |
| Selective reporting (reporting bias) | Low risk | Follow‐up data were not reported for participants who did not have resolution 2 to 11 days after completing treatment. Longer‐term follow up therefore only considers relapse rates for a subgroup of patients. |
| Other bias | Low risk | — |
Lambert 1986.
| Methods | Randomised, placebo‐controlled trial (patients who failed to respond at 2 weeks crossed over to alternative treatment) | |
| Participants | 60 children aged from 2 to 15 years completed the study. Participants were recruited from the otolaryngology clinic. Effusion present for at least 2 months Diagnosis: 1A, 2C 72% bilateral |
|
| Interventions | Antibiotic (amoxycillin) plus prednisone 1.5 mg/kg daily in tapering doses (n = 32) versus antibiotic plus placebo (n = 28) for 2 weeks | |
| Outcomes | Resolution defined by otoscopy and tympanometry Improvement defined by better hearing on pure tone audiometry Not clear if resolution referred to clearing of both ears when bilateral OME at entry Measured 7 to 10 days after completion of treatment Audiometry data used ear as unit of analysis and present outcomes as ears resolving with each treatment |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Although the study was (double‐blind) placebo‐controlled it is not clear if the person enrolling patients was blind to treatment allocation and control truly indistinguishable from intervention Quote: "placebo, which consisted of a cherry‐flavoured lactose syrup, was given in the same manner" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Unclear if outcome assessors were blind, but study reported as double‐blind |
| Incomplete outcome data (attrition bias) < 1 month | Unclear risk | 60 participants included in analysis, but not clear if more were randomised |
| Incomplete outcome data (attrition bias) 1 to 6 months | Unclear risk | — |
| Selective reporting (reporting bias) | Unclear risk | Poor reporting of results |
| Other bias | Low risk | — |
Macknin 1985.
| Methods | Randomised, placebo‐controlled trial; randomisation to strata based on history (recent acute versus non acute otitis) and age. Tympanometry 'blinded'. | |
| Participants | 49 children aged from 6 months to 14 years. Participants were recruited from a hospital‐based paediatric group practice. Participants enrolled 6 weeks after initial presentation with acute OM (after completing antibiotic therapy for 10 days) or 3 weeks after initial presentation with non acute OME Diagnosis: 1A, 2B 67% bilateral |
|
| Interventions | Dexamethasone 0.15 mg/kg in tapering doses for 2 weeks (n = 26) versus placebo (n = 23) | |
| Outcomes | Audiometry, tympanometry and pneumo‐otoscopy resolution Improved hearing by at least 10 dB in 1 or both ears Outcomes measured at 2 and 6 weeks after entering study There were no statistically significant differences (Chi2 or Fisher’s exact test) in the complications between the intervention groups rates as determined by daily phone reports and 19‐symtpom checklist (actual data not reported). Severity of symptoms was not stated but none discontinued treatment due to adverse effects. |
|
| Notes | Code broken early (and study terminated) because remission rates less than expected spontaneously | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers used |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled study with randomisation performed by pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blind outcome assessors not reported but "double blind" |
| Incomplete outcome data (attrition bias) < 1 month | Low risk | Data presented for 49 participants, but 6 were taken off treatment during study (mainly due to acute OME) of which only 2 were included in analysis; study size therefore assumed to be 53 and loss to follow up 8%; 2 participants in each group |
| Incomplete outcome data (attrition bias) 1 to 6 months | Unclear risk | — |
| Selective reporting (reporting bias) | Low risk | Study terminated early due to concern that steroid was impairing resolution. Likely to result in lack of power rather than bias. |
| Other bias | Low risk | — |
Mandel 2002.
| Methods | Randomised, placebo‐controlled trial; 4 parallel groups, randomisation to strata based on age, laterality and duration of effusion | |
| Participants | 144 children aged from 1 to 9 years seen in the OME research centre at a children's hospital Effusion present for at least 2 months Diagnosis: 1B, 2C 70% bilateral |
|
| Interventions | Prednisolone 0.5 mg/kg, twice a day for 10 days (max 30 mg), then once a day for 4 days (max 15 mg), plus antibiotic for either 28 days or 14 days (with placebo for 14 days) (n = 69) versus placebo plus antibiotic for either 28 days or 14 days (n = 66) | |
| Outcomes | Audiometry, tympanometry and pneumatic otoscopy Absence of OME and improved hearing Data on hearing status, in terms of speech recognition threshold (SRT) and pure tone average (PTA), were reported separately for both right and left ear. Data on speech awareness threshold (SAT) were only reported for 26/144 (18%) participants (aged 12 to 29 months). Possible side effects included vomiting, diarrhoea, increased appetite, hyperactivity and rash. Data presented separately for first 2 weeks of treatment (steroid treatment; included in the meta‐analysis) and second 2 weeks of treatment (prolonged antibiotic treatment; 7/67 (10%) in steroid group and 9/65 (14%) in control). During week 1 to 2 steroid or placebo were discontinued due to adverse effects in 2 participants (placebo) and in week 3 to 4 amoxicillin or placebo was discontinued due to adverse effects in 3 participants (2 in steroid and 1 in placebo + antibiotic). Allergy and immunological testing Outcomes measured at 2 and 4 weeks after entry |
|
| Notes | Only 77% of the 188 participants required by sample size estimates were recruited Participants with OME at 4 weeks after entry were discharged. Participants who had no middle ear effusion at 4 weeks were monitored for recurrence for up to 16 weeks after entry. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomised in a double‐blind manner" Comment: it is not clear if the person enrolling patients were blind to treatment allocation, but placebos were reported to have been supplied by the same pharmaceutical company as interventions and likely to be identical |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Unclear if outcome assessors were blind, but study reported as double‐blind |
| Incomplete outcome data (attrition bias) < 1 month | Low risk | Analyses were done on the basis of the intention‐to‐treat principle (analysed according to group allocation) but missing data excluded. Loss to follow up was fairly low (6%) and comparable between groups: intervention 4/73, control 5/71; reason not stated. |
| Incomplete outcome data (attrition bias) 1 to 6 months | Low risk | Analyses were done on the basis of the intention‐to‐treat principle (analysed according to group allocation) but missing data excluded. Loss to follow up was fairly low (8%) and comparable between groups: intervention 6/73, control 6/71; reason not stated. |
| Selective reporting (reporting bias) | Low risk | Follow‐up data were not reported for participants who did not have resolution after completing treatment. Longer‐term follow up therefore only considers relapse rates for a subgroup of patients. |
| Other bias | Low risk | More participants in steroid group were enrolled in winter but this is unlikely to have influenced the findings |
Niederman 1984.
| Methods | Randomised, placebo‐controlled trial | |
| Participants | 26 children aged from 2 to 14 years seen in the hospital and medical centre‐based Ambulatory Care Clinics Effusion present for 8 weeks Diagnosis: 1B, 2C 77% bilateral |
|
| Interventions | Dexamethasone 0.15 mg/kg tapering over 2 weeks (n = 12) versus placebo (n = 10) | |
| Outcomes | Pneumo‐otoscopy and tympanometry resolution in both ears Outcomes measured at 2 and 5 weeks after study entry No significant side effects observed |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised using a list of preselected random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "[Interventions] were identical". "All medications were dispensed by the hospital pharmacist in a double‐blind design". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Unclear if outcome assessors were blind, but study reported as double‐blind |
| Incomplete outcome data (attrition bias) < 1 month | Unclear risk | 4/26 (15%) participants excluded from analyses, group allocation not stated; due to non‐compliance with medication or follow‐up visits |
| Incomplete outcome data (attrition bias) 1 to 6 months | Unclear risk | Missing data for 30% (6/26), group allocation not stated; due to non‐compliance with medication or follow‐up visits |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Unclear risk | Some baseline imbalance, children slightly older, more bilateral and longer duration of OME in placebo group. Small sample size therefore could be due to chance. |
Podoshin 1990.
| Methods | Randomised, placebo‐controlled trial; 3 parallel groups | |
| Participants | 150 children aged between 3 and 8 years seen in the Department of Otolaryngology Previously untreated OME, present for at least 2 months Diagnosis: 1B, 2B; children also had audiometry at entry Not stated how many bilateral |
|
| Interventions | Antibiotic (amoxycillin) plus prednisone 1 mg/kg daily in tapering doses (n = 50) for 2 weeks versus antibiotic plus placebo (n = 49) (vs placebo alone (n = 37)) | |
| Outcomes | Audiometry and tympanometry improvement. Criteria for complete, partial or no improvement reported in terms of audiometric and tympanometric evaluations combined, but results reported separately for audiometry and tympanometry. Tympanometry data (complete) used for meta‐analyses for OME resolution and audiometry data (complete) used to represent hearing loss (complete assumed to be based on closure of air‐born gap and partial some conductive hearing loss). Follow up at 2 months after entry |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled with capsules being prepared by pharmacy for each child entered and then given to clinician; enrolment appears to have been done at outpatients |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Unclear if outcome assessors were blind, but study reported as double‐blind |
| Incomplete outcome data (attrition bias) < 1 month | Low risk | Not analysed by intention‐to‐treat; 14 (9%) participants were excluded from the analyses, group allocation not stated |
| Incomplete outcome data (attrition bias) 1 to 6 months | Unclear risk | — |
| Selective reporting (reporting bias) | Unclear risk | Poor reporting of results. Confusion about outcomes; 'success' defined as normal otoscopy, tympanometry and closure of air‐bone gap. No absolute values for audiometry data. Yet outcomes for 'complete improvement' are given according to audiometry and tympanometry separately (tympanometry data used in the review). |
| Other bias | Unclear risk | Few baseline characteristics reported |
Schwartz 1980.
| Methods | Randomised, placebo‐controlled trial (patients who failed to respond at 1 week crossed over to alternative treatment) | |
| Participants | 41 children aged 1.2 to 10 years seen by 2 paediatricians in separate private clinics Effusions present for at least 3 weeks despite previous antibiotics and/or decongestants Diagnosis: 1B, 2C 48% bilateral |
|
| Interventions | Antibiotic (sulfisoxazole) plus prednisone 1 mg/kg/day tapering over 7 days (n = 24) versus antibiotic plus placebo (n = 17) | |
| Outcomes | Tympanometry and pneumo‐otoscopy clearing of effusions Follow up at 1 week after entry (data for the period after participants cross‐over have not been included) Not clear whether 'cleared' refers to clearing in all affected ears |
|
| Notes | Not clear why numbers in treatment groups unbalanced. Small sample size therefore likely due to chance, but the number of participants enrolled and randomised not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not state |
| Allocation concealment (selection bias) | Low risk | Quote: "Pulverized prednisone tables or lactose powder packed in unmarked gelatin capsules and placed in identical coded vials by a registered pharmacist"; patients are assumed to have been enrolled in the clinic |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Unclear if outcome assessors were blind, but study reported as double‐blind |
| Incomplete outcome data (attrition bias) < 1 month | Unclear risk | 41 participants included in study, but not clear if only 41 were randomised |
| Incomplete outcome data (attrition bias) 1 to 6 months | Unclear risk | — |
| Selective reporting (reporting bias) | Unclear risk | Poor reporting |
| Other bias | Unclear risk | Baseline characteristics not reported |
Shapiro 1982.
| Methods | Randomised, placebo‐controlled trial | |
| Participants | 45 children aged from 2 to 10 years. Study conducted at the Children's Orthopedic Hospital and Medical Centre. All had persistent Eustachian tube dysfunction (documented with abnormal tympanometry) due to allergic rhinitis which failed to respond to 4 weeks of oral antihistamine and decongestants Diagnosis: 1B, 2C 61% bilateral |
|
| Interventions | Aerosolised dexamethasone 1 spray in each nostril 3 times a day for 3 weeks (n = 21) versus aerosolised placebo (n = 24) | |
| Outcomes | Improvement in middle ear pressure and gradient (data on ear pressure used in meta‐analyses) Physician assessment of mobility and position of tympanic membrane and presence of middle ear fluid Follow up at 3 weeks after completing treatment Transient drop in serum cortisol levels in 2 dexamethasone‐treated patients; no adverse reactions, no pathological nasal mucosal changes |
|
| Notes | All children had allergic rhinitis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Although the study was placebo‐controlled it is not clear if the person enrolling patients was blind to treatment allocation. Treatment "randomly assigned", no further information given. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled study, although unclear if outcome assessors were blind Quote: "At the conclusion of the study the code was broken" |
| Incomplete outcome data (attrition bias) < 1 month | Low risk | Loss to follow up 2%; 1 participant in placebo group excluded from analysis |
| Incomplete outcome data (attrition bias) 1 to 6 months | Unclear risk | — |
| Selective reporting (reporting bias) | Unclear risk | No values reported for physician‐rated mobility and position of tympanic membrane and presence of middle ear fluid. No difference between groups reported but no P value given (or test reported). |
| Other bias | Unclear risk | Groups reported to be similar for OME severity at baseline, no other characteristics reported |
Tracy 1998.
| Methods | Randomised, placebo‐controlled trial; 3 parallel groups | |
| Participants | 61 children aged from 3 to 11 years recruited from a military‐dependent population referred to the Medical Centre‐based paediatric Chronic Ear Clinic Participants had persistent middle ear effusion for at least 3 months and a minimum of 3 episodes of acute OM within past 6 months or 4 in past year Diagnosis: 1B, 2C Not clear how many bilateral |
|
| Interventions | Antibiotic (amoxycillin) plus aqueous intra‐nasal beclomethasone 2 sprays BD in each nostril both for 12 weeks (n = 19) versus antibiotic plus placebo (n = 20) (versus antibiotic alone (n = 20)) | |
| Outcomes | Tympanic pressures scores, otoscopic examination scores, symptom scores and OME resolution. For patient symptoms both a mean total score (using a visual analogue scale, range 0 to 100) and a mean score for 4 ear‐associated symptoms were reported, but only the former is included in our meta‐analyses. OME resolution was reported separately according to middle ear pressure criteria and otoscopic examination score criteria (the former was used in the meta‐analysis). Antibiotic plus placebo and antibiotic alone groups pooled for the outcomes relating to resolution of OME Follow up at 4, 8 and 12 weeks after entry Other than transient nasal stinging and epistaxis no significant side effects (that required discontinuation of treatment) were reported (numbers not stated) |
|
| Notes | Validation of symptom score not described No differences in outcome between atopic and non‐atopic subjects |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | Quote: "Preparation and dispensing of drugs as well as randomization of patients to each arm of the study was accomplished independently by a clinical pharmacologist" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Unclear if outcome assessors were blind, but study reported as double‐blind |
| Incomplete outcome data (attrition bias) < 1 month | Unclear risk | — |
| Incomplete outcome data (attrition bias) 1 to 6 months | Low risk | 2 participants (3%) were lost to follow up due to families moving away (group allocation not stated) and excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | OME resolution data reported as percentages. Mean tympanic membrane pressure and median otoscopic exam score were reported in graphical format only and data were presented separately for left and right ear; number of participants with unilateral and bilateral OME not stated. |
| Other bias | Unclear risk | More participants in the placebo group (45%) had a smoker present at home than in the intervention group (16%) |
Williamson 2009.
| Methods | Randomised, placebo‐controlled trial; randomisation using blocks of 4 | |
| Participants | 217 children aged 4 to 11 years presenting to the GP with 1 or more episodes of otitis media or ear‐related problems in previous 12 months. 72 (33%) received active monitoring for 3 months prior to randomisation. Diagnosis: 1C (tympanometry type B/B or B/C2), 2C (pure‐tone audiometry was not used as an inclusion criterion, because of poor validity in young children and hearing threshold not known to be an effect modifier) 100% bilateral |
|
| Interventions | Topical nasal steroid mometasone furoate (50 mcg) (n = 105) versus placebo (n = 112) used once daily for 3 months | |
| Outcomes | Primary: proportion of children cleared of OME assessed by tympanometry (to type C1 or A in one or more ears) at 1 month after entry Secondary: OME clearance at 3 and 9 months after entry; disease‐specific functional status (OM8‐30 score), hearing difficulty, days with earache (within 3 months), adverse effects, health utilities, resources and costs Adverse effects were relatively minor and included cough, dry throat, epistaxis and nasal stinging. At 1 month 43 (55%) participants experienced adverse effects in the intervention group and 35 (45%) in the control. |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence used |
| Allocation concealment (selection bias) | Low risk | Drug supplier conducted randomisation and labelled the drugs, which were numbered in auditable sequence |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, research nurse and outcome assessors were blind. Success of blinding (children and parents) evaluated and maintained until analysis phase. |
| Incomplete outcome data (attrition bias) < 1 month | Unclear risk | — |
| Incomplete outcome data (attrition bias) 1 to 6 months | Unclear risk | Analysis of primary outcome based on intention‐to‐treat (analysed according to group allocation). Missing data assumed to be missing at random and not included in analyses. Loss to follow up was fairly high: 11% at 1 month (data not used in our meta‐analysis), 21% at 3 months, 34% at 9 months. Missing data assumed to be missing at random and not included in analyses. |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | — |
BD: twice a day OM: otitis media OME: otitis media with effusion
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berman 1987 | ALLOCATION
Randomly assigned, double‐blinded PARTICIPANTS Patients with middle ear effusion of 2 months or longer INTERVENTIONS Compared antibiotic plus steroid with placebo plus placebo |
| Cengel 2006 | ALLOCATION
Quasi‐randomised trial, with every second child enrolled into treatment or control group. Families that did not want to their child to be in the group that they were allocated were allowed to change groups. ANALYSIS OME resolution using ears as unit of analysis rather than children |
| Choung 2008 | ALLOCATION
Randomised trial PARTICIPANTS 84 children with OME diagnosed at a tertiary hospital aged 5 months to 12 years INTERVENTIONS Included 5 comparison groups, but did not use placebo: antibiotics (n = 16) versus antibiotics plus steroid (prednisolone) (n = 18) versus antibiotics plus antihistamines (n = 15) versus antibiotics plus steroids plus antihistamines (n = 17) versus mucolytics (n = 17). Blind outcome assessment was not reported and allocation of treatment does not appear to have been concealed. Quote: "after obtaining consent from parents or guardians, we consecutively and randomly prescribed....". No further information was provided on method of randomisation and allocation concealment. |
| Daly 1991 | ALLOCATION
Stratified, allocation scheme unclear PARTICIPANTS Children aged 6 months to 8 years with preceding acute otitis media or OME identified through screening hospital notes of children being examined for an ear recheck and meeting specific inclusion criteria INTERVENTIONS Compared antibiotic plus steroid with placebo plus placebo |
| Heary 1990 | Paper in abstract form only ‐ no full publication |
| Ortega del Alamo 2005 | ALLOCATION:
Quasi‐randomised (children recruited in a consecutive sequence, alternating the treatment groups to which they were assigned) PARTICIPANTS: 62 children aged 2 to 8 years with a diagnosis of OME INTERVENTIONS Compared antibiotics treatment with or without associated AM3; AM3 is the active principle for Inmunoferon®, an immunomodulatory drug |
| Persico 1978 | ALLOCATION Patients not randomised, open study |
| Rosenfeld 1995 | ALLOCATION Patients not randomised (allocation according to carer preference) |
| Schwartz 1981 | ALLOCATION
Double‐blinded, cross‐over, randomly‐assigned PARTICIPANTS Children aged 14 months to 10 years with persistent OME for 3 weeks or more, despite antimicrobial and/or decongestant therapy INTERVENTIONS Compared antibiotic plus steroid with non‐placebo control |
| Woodhead 1986 | Paper in abstract form only ‐ no full publication |
OME: otitis media with effusion
Differences between protocol and review
We adopted the Cochrane 'Risk of bias' method for assessing study quality (Handbook 2011) at the 2010 update. We also added 'Summary of findings' tables; used the I² statistic to assess heterogeneity; and changed the analysis method for dichotomous outcomes from odds ratio (OR) to risk ratio (RR).
Contributions of authors
Christopher C Butler: protocol development (with advice from C Clar), searching for trials, quality assessment of trials, data extraction and development of final review.
Judith van der Voort: searching for trials, quality assessment of trials, data extraction, data analysis and development of final review.
Ruth A Lewis: quality assessment of trials, data extraction, data analysis and development of updated review. Corresponding author.
Sharon A Simpson: quality assessment of trials, data extraction, data analysis and development of updated review.
Sources of support
Internal sources
-
Department of Primary Care and Public Health, Cardiff University, UK.
Supports the salaries of Butler, Simpson and Lewis.
-
Wales School of Primary Care Research, UK.
Chris Butler has administrative support provided by the School.
External sources
-
NHS Wales Office for Research and Development for Health and Social, UK.
now called The National Institute For Social Care and Health Research All‐Wales (NISCHR)
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Berman 1990 {published data only}
- Berman S, Grose K, Nuss R, Huber‐Navin C, Roark R, Gabbard SA, et al. Management of chronic middle ear effusion with prednisone combined with trimethoprim‐sulfamethoxazole. Pediatric Infectious Disease Journal 1990;9(8):533‐8. [DOI] [PubMed] [Google Scholar]
Giebink 1990 {published data only}
- Giebink GC, Batalden PB, Le CT, Russ JN, Knox JK, Anderson RS, et al. Randomized controlled trial comparing trimethoprim‐sulfamethoxazole, prednisone, ibuprofen, and no treatment in chronic otitis media with effusion. Lim DJ, Bluestone CD, Klein JO, Nelson JD, editor(s). Recent Advances in Otitis Media: IVth International Symposium on Otitis Media with Effusion. Philadelphia: B.C. Decker Company, 1988:240‐4.
- Giebink GS, Batalden PB, Le CT, Lassman FM, Buran DJ, Seltz AE. A controlled trial comparing three treatments for chronic otitis media with effusion. Pediatric Infectious Disease Journal 1990;9(1):33‐40. [DOI] [PubMed] [Google Scholar]
Hemlin 1997 {published data only}
- Hemlin C, Carenfelt C, Papatziamos G. Single dose of betamethasone in combined medical treatment of secretory otitis media. Annals of Otology Rhinology and Laryngology 1997;106:359‐63. [DOI] [PubMed] [Google Scholar]
Lambert 1986 {published data only}
- Lambert PR. Oral steroid therapy for chronic middle ear perfusion: a double‐blind crossover study. Otolaryngology ‐ Head and Neck Surgery 1986;95:193‐9. [DOI] [PubMed] [Google Scholar]
Macknin 1985 {published data only}
- Maknin ML, Jones PK. Oral dexamethasone for treatment of persistent middle ear effusion. Pediatrics 1985;75:329‐35. [PubMed] [Google Scholar]
Mandel 2002 {published data only}
- Mandel EM, Casselbrant ML, Rockette HE, Fireman P, Kurs‐Lasky M, Bluestone CD. Systemic steroid for chronic otitis media with effusion in children. Pediatrics 2002;110(6):1071‐80. [DOI] [PubMed] [Google Scholar]
Niederman 1984 {published data only}
- Niederman LG, Walter‐Bucholtz V, Jabalay T. A comparative trial of steroids versus placebos for treatment of chronic otitis media with effusion. Recent Advances in Otitis Media with Effusion. Philadelphia: B.C. Decker Inc, 1984:273‐5.
Podoshin 1990 {published data only}
- Podoshin L, Fradis M, Ben‐David Y, Faraggi D. The efficacy of oral steroids in the treatment of persistent otitis media with effusion. Archives of Otolaryngology ‐ Head and Neck Surgery 1990;116:1404‐6. [DOI] [PubMed] [Google Scholar]
Schwartz 1980 {published data only}
- Schwartz RH, Puglese J, Schwartz DM. Use of a short course of prednisone for treating middle ear effusion; a double‐blind crossover study. Annals of Otology, Rhinology and Laryngology 1980;89(Suppl 68):296‐300. [DOI] [PubMed] [Google Scholar]
Shapiro 1982 {published data only}
- Shapiro GG, Bierman CW, Furukuwa CT, Pierson WE, Berman R, Donaldson J, et al. Treatment of persistent Eustachian tube dysfunction with aerosolised nasal dexamethasone phosphate versus placebo. Annals of Allergy 1982;49:81‐5. [PubMed] [Google Scholar]
Tracy 1998 {published data only}
- Tracy JM, Demain JG, Hoffman KM, Goetz DW. Intranasal beclomethasone as an adjunct to treatment of chronic middle ear effusion. Annals of Allergy, Asthma and Immunology 1998;80:198‐206. [DOI] [PubMed] [Google Scholar]
Williamson 2009 {published data only}
- Williamson I, Benge S, Barton S, Petrou S, Letley L, Fasey N, et al. A double‐blind randomised placebo‐controlled trial of topical nasal steroids in 4‐11 year old children with persistent bilateral otitis media with effusion (OME) in primary care. Health Technology Assessment 2009;13(37):1‐144. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Berman 1987 {published data only}
- Berman S, Grose K, Zerbe GO. Medical management of chronic middle‐ear effusion. American Journal of Disease in Children 1987;141:690‐4. [DOI] [PubMed] [Google Scholar]
Cengel 2006 {published data only}
- Cengel S, Akyol MU. The role of topical nasal steroids in the treatment of children with otitis media with effusion and/or adenoid hypertrophy. International Journal of Pediatric Otorhinolaryngology 2006;70:639‐45. [DOI] [PubMed] [Google Scholar]
Choung 2008 {published data only}
- Choung Y‐H, Shin YR, Choi SJ, Park K, Park HY, Lee JB, et al. Management for the children with otitis media with effusion in the tertiary hospital. Clinical and Experimental Otorhinolaryngology 2008;1(4):201‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daly 1991 {published data only}
- Daly K, Giebink GS, Batalden PB, Anderson RS, Le CT, Lundgren B. Resolution of otitis media with effusion with the use of a stepped treatment regimen of trimethoprim‐sulfamethoxazole and prednisone. Pediatric Infectious Disease Journal 1991;7(7):500‐6. [DOI] [PubMed] [Google Scholar]
Heary 1990 {published data only}
- Heary C, Hokanson J, Ury H, Chang C, Coplan B, Hall M. Lack of efficacy of short‐term prednisone, trimethoprim‐sulfamethoxazole, alone or combined, in persistent otitis media with effusion: season of entry as a possible determinant of outcome. American Journal of Disease in Childhood 1990;144:420. [Google Scholar]
Ortega del Alamo 2005 {published data only}
- Ortega del Alamo P, Rivera Rodríguez T, Sanz Fernández R. The effect of AM3 in the resolution of otitis media with effusion (OME) in paediatric patients. Acta Otorrinolaringologica Espanola 2005;56(1):1‐5. [DOI] [PubMed] [Google Scholar]
Persico 1978 {published data only}
- Persico M, Podoshin L, Fradis M. Otitis media with effusion: a steroid and antibiotic therapeutic trial before surgery. Annals of Otology, Rhinology and Laryngology 1978;87:191‐5. [DOI] [PubMed] [Google Scholar]
Rosenfeld 1995 {published data only}
- Rosenfeld RM. Nonsurgical management of surgical otitis media with effusion. Journal of Laryngology and Otology 1995;109:811‐6. [DOI] [PubMed] [Google Scholar]
Schwartz 1981 {published data only}
- Schwartz RH. Otitis media with effusion: results of treatment with a short course of oral prednisone or intranasal beclomethasone. Otolaryngology ‐ Head and Neck Surgery 1981;89:386‐91. [DOI] [PubMed] [Google Scholar]
Woodhead 1986 {published data only}
- Woodhead JC, Milavetz G, Dusdieker LB, Booth BM, Wilmoth RN. Prednisone treatment of otitis media with effusion. American Journal of Disease in Childhood 1986;140:318. [Google Scholar]
Additional references
Baggett 1997
- Baggett HC, Prazma J, Rose AS, Lane AP, Pillsbury HC. The role of glucocorticoids in endotoxin‐ mediated otitis media with effusion. Archives of Otolaryngology ‐ Head and Neck Surgery 1997;123:41‐6. [DOI] [PubMed] [Google Scholar]
Bennett 2001
- Bennett KE, Haggard MP, Silva PA, Stewart, IA. Behaviour and developmental effects of otitis media with effusion in the teens. Archives of Disease in Childhood 2001;85:91‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berman 1994
- Berman S, Roark R, Luckey D. Theoretical cost effectiveness of management options for children with persisting middle ear effusion. Pediatrics 1994;93:353‐63. [PubMed] [Google Scholar]
Bluestone 1988
- Bluestone CD, Klein JO. Otitis media in infants and children. Philadelphia: W.B. Saunders Company, 1988. [Google Scholar]
Browning 2010
- Browning GG, Rovers MM, Williamson I, Lous J, Burton MJ. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD001801.pub3] [DOI] [PubMed] [Google Scholar]
Chantzi 2005
- Chantzi FM, Bairmis T, Papadopoulos NG, Kafetzis DA. Otitis media with effusion: an effort to understand and clarify the uncertainties. Expert Review of Anti‐infective Therapy 2005;3(1):117‐29. [DOI] [PubMed] [Google Scholar]
Davies 1998
- Davies HTO, Crombie IK, Tavakoli M. When can odds ratios mislead?. BMJ 1998;316(7136):989‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2008
- Deeks JJ, Higgins JPT, Altman DG (editors). Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, 2008. [Google Scholar]
Dempster 1991
- Dempster JH, MacKenzie K. Tympanometry in the detection of hearing impairments associated with otitis media with effusion. Clinical Otolaryngology and Allied Sciences 1991;16:157‐9. [DOI] [PubMed] [Google Scholar]
Dengerink 1984
- Dengerink JE, Porter JB. Childrens attitudes towards peers wearing hearing aids. Language, Speech and Hearing Services in Schools 1984;205‐9:15. [Google Scholar]
Ducharme 2003
- Ducharme FM, Chabot G, Polychronakos C, Glorieux F, Mazer B. Safety profile of frequent short courses of oral glucocorticoids in acute pediatric asthma: impact on bone metabolism, bone density, and adrenal function. Pediatrics 2003;111(2):376‐83. [DOI] [PubMed] [Google Scholar]
Gates 1996
- Gates GA. Cost effectiveness considerations in otitis media treatment. Otolaryngology ‐ Head and Neck Surgery 1996;114:525‐30. [DOI] [PubMed] [Google Scholar]
Gouma 2010
Griffin 2006
- Griffin G, Flynn CA, Bailey RE, Schultz JK. Antihistamines and/or decongestants for otitis media with effusion (OME) in children. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003423.pub2] [DOI] [PubMed] [Google Scholar]
Haddard 1998
- Haddad J, Egusa K, Takoudes TG. Effects of 21‐aminosteroid U‐74389G on acute otitis media in a guinea pig model. Otolaryngology ‐ Head and Neck Surgery 1998;118:44‐8. [DOI] [PubMed] [Google Scholar]
Haggard 1991
- Haggard M, Hughes E. Screening children's hearing: a review of the literature and the implications for otitis media. London: HMSO 1991.
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
HES 2010
- Hospital Episode Statistics (HES online). http://www.hesonline.nhs.uk/ (accessed 23 February 2010).
Kazanas 1994
- Kazanas SG, Maw R. Tympanometry, stapedius reflex and hearing impairment in children with otitis media with effusion. Acta Oto‐Laryngologica 1994;114:410‐4. [DOI] [PubMed] [Google Scholar]
Lanphear 1997
- Lanphear BP, Byrd RS, Auinger P, Hall CB. Increasing prevalence of recurrent otitis media among children in the United States. Pediatrics 1997;99:468(a). [DOI] [PubMed] [Google Scholar]
Lous 1995a
- Lous J. Otitis media and reading achievement: a review. International Journal of Pediatric Otorhinolaryngology 1995;32:105‐21. [DOI] [PubMed] [Google Scholar]
Lous 1995b
- Lous J. Secretory otitis media in schoolchildren: is screening for secretory otitis media advisable?. Danish Medical Bulletin 1995;42(1):71‐99. [PubMed] [Google Scholar]
MRC Multi‐Centre Otitis Media Study Group 1999
- MRC Multi‐Centre Otitis Media Study Group. Sensitivity, specificity and predictive value of tympanometry in predicting a hearing impairment in otitis media with effusion. Clinical Otolaryngology and Allied Sciences 1999;24(4):294‐300. [DOI] [PubMed] [Google Scholar]
MRC Multi‐Centre Otitis Media Study Group 2001
- MRC Multi‐Centre Otitis Media Study Group. Risk Factors for Persistence of Bilateral Otitis Media with Effusion. Clinical Otolaryngology 2001;26:147‐56. [DOI] [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence. NICE Guideline 60: Surgical management of otitis media with effusion in children. London: National Institute for Health and Clinical Excellence, February 2008 (www.nice.org.uk/nicemedia/pdf/CG60NICEguideline.pdf). [Google Scholar]
NIH 2007
- EPR 3. Expert panel report 3: Guidelines for the Diagnosis and Management of Asthma. NIH Publication No. 07‐4051. Bethesda, MD: U.S. Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program, 2007 (available at http://www.nhlbi.nih.gov/guidelines/asthma/asthmafullrpt.pdf).
Nuss 1990
- Nuss R, Berman S. Medical management of persistent middle ear effusion. American Journal of Asthma and Allergy for Pediatricians 1990;4:17‐22. [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rosenfeld 1991
- Rosenfeld RM, Mandel EM, Bluestone CD. Systemic steroids for otitis media with effusion in children. Archives of Otolaryngology ‐ Head and Neck Surgery 1991;117:984‐9. [DOI] [PubMed] [Google Scholar]
Rosenfeld 1992
- Rosenfeld RM. New concepts for steroid use in otitis media with effusion. Clinical Pediatrics 1992;31(10):615‐21. [DOI] [PubMed] [Google Scholar]
Schappert 1992
- Schappert SM. Office Visits for Otitis Media: United States, 1975‐90. Advance data from vital and health statistics; no 214. Hyattsville, Maryland: National Center for Health Statistics, 1992. [PubMed] [Google Scholar]
Stool 1994
- Stool SE, Berg AO, Berman S, et al. Otitis media with effusion in young children. Clinical practice guideline. Number 12. Agency for Health Care Policy and Research, Public Health Services, US Department of Health and Human Services 1994.
Tan 1997
- Tan G, Escoubet B, Abbeele G, Friedlander P, Huy TB, Herman P. Modulation of middle ear epithelial function by steroids: clinical relevance. Acta Otolaryngologica (Stockholm) 1997;117:284‐8. [DOI] [PubMed] [Google Scholar]
Williams 1993
- Williams RL, Chalmers TC, Stange KC, Chalmers FT, Bowlin SJ. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. JAMA 1993;270:1344‐51. [PubMed] [Google Scholar]
Wu 2008
- Wu CT, Tsai SC, Lin JJ, Hsia SH. Disseminated varicella infection in a child receiving short‐term steroids for asthma. Pediatric Dermatology 2008;25(4):484‐6. [DOI] [PubMed] [Google Scholar]
Yaman 2008
- Yaman H, Ozturk K, Uyar Y, Gurbilek M. Effectiveness of corticosteroids in otitis media with effusion: an experimental study. Journal of Laryngology and Otology 2008;122(1):25‐30. [DOI] [PubMed] [Google Scholar]
Yusuf 1985
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Progress in Cardiovascular Diseases 1985;27:335‐71. [DOI] [PubMed] [Google Scholar]
Zielhuis 1990
- Zielhuis GA, Rach GH, Bosch AV, Broek PV. The prevalence of otitis media with effusion: a critical review of the literature. Clinical Otolaryngology 1990;15:283‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Butler 2001
- Butler CC, Voort JH. Steroids for otitis media with effusion: a systematic review. Archives of Pediatrics and Adolescent Medicine 2001;155(6):641‐7. [DOI] [PubMed] [Google Scholar]
Butler 2002
- Butler CC, Voort JH. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD001935.pub2] [DOI] [PubMed] [Google Scholar]
Thomas 2006
- Thomas CL, Simpson S, Butler CC, Voort JH. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001935.pub2] [DOI] [PubMed] [Google Scholar]


