IMPORTANCE:
Aerosolized prostacyclins are frequently used in patients with severe acute respiratory distress syndrome and refractory hypoxia. Previous studies have shown improvement in oxygenation with use of pulmonary vasodilators such as iloprost and epoprostenol; however, there is no head-to-head comparison between these agents.
OBJECTIVES:
To compare the effects of inhaled epoprostenol and inhaled iloprost in critically ill patients with refractory hypoxia.
DESIGN, SETTING, AND PARTICIPANTS:
We performed a retrospective cohort analysis of patients admitted to the ICUs at the University of Oklahoma Health Sciences Center between 2015 and 2018. Adult patients who received aerosolized epoprostenol or iloprost for more than 4 hours were included in the analysis.
MAIN OUTCOMES AND MEASURES:
The primary endpoint measured was to compare the change in Pao2/Fio2 ratio between patients treated with iloprost compared with epoprostenol. Secondary outcomes measured were 90-day in-hospital mortality and improvement in vasopressor requirements.
RESULTS:
A total of 126 patients were included in the study, 95 of whom received iloprost (75%) and 31 patients (25%) received epoprostenol. There were significant improvements in Pao2/Fio2 ratio in both the iloprost and epoprostenol group. Patients in the epoprostenol group appeared to have a higher 90-day mortality compared with the iloprost group. However, our study was not powered to detect a mortality difference and this finding likely represents a sicker population in the epoprostenol group and prescription bias. The use of iloprost was associated with higher vasopressor requirements in the first 12 hours of administration, an association was not observed in the epoprostenol group.
CONCLUSIONS AND RELEVANCE:
In this retrospective cohort analysis, use of both pulmonary vasodilators was associated with similar improvement in gas exchange. The mortality difference observed likely represents difference in severity of illness. Further studies are needed to corroborate these findings.
Keywords: acute respiratory distress syndrome, aerosolized prostacyclins, epoprostenol, iloprost, refractory hypoxia
KEY POINTS
Question: We aim to compare the effects of inhaled epoprostenol and inhaled iloprost in critically ill patients with refractory hypoxia.
Findings: This retrospective study shows that use of both iloprost and epoprostenol are associated with similar improvement in oxygenation. We do observe a mortality difference with higher mortality in epoprostenol group; however, this likely represents a sicker population in the epoprostenol group and prescription bias.
Meanings: With the current COVID-19 pandemic and increased use of aerosolized prostacyclins for refractory hypoxia, our findings emerge at a crucial time and reflect upon the effects of different aerosolized prostacyclins in critically ill patients. As both agents have similar improvements in gas exchange, iloprost may be a better option in patients with refractory hypoxia given the need for continuous nebulization with epoprostenol, which can be a nuisance for transport and imaging.
Acute respiratory distress syndrome (ARDS) is a severe and rapidly progressive form of respiratory failure, with an estimated occurrence rate in the United States of about 40 cases per 100,000 persons (1). ARDS accounts for 10% of admissions to ICU and for 23% of the total number of patients requiring mechanical ventilation. Hospital mortality is around 40–45% in patients with severe ARDS (2). Patients who survive ARDS are found to have long-term disabilities including exercise limitation, decreased physical quality of life and psychologic sequalae (3). Furthermore, ARDS has a significant burden on healthcare system, with a mean ICU cost of 74,000 U.S. dollars per patient (4).
ARDS can be further classified into pulmonary ARDS (ARDSp) and extrapulmonary ARDS (ARDSexp) based on pathophysiology (5). In ARDSp, there is a direct injury to the alveolar epithelium, while in extrapulmonary ARDS, there is indirect insult that leads to release of inflammatory cytokines from the vascular endothelium. Notable examples of ARDSexp include lung contusion, burn, and pancreatitis. Despite difference in pathophysiology and etiologies, prognosis of ARDSp and ARDSexp remains similar (6).
Management of ARDS is primarily focused on improving gas exchange and providing supportive care while the underlying lung injury is addressed. Mechanical ventilation is the cornerstone of supportive care of patients with ARDS (7). Refractory hypoxia refers to the presence of persistent hypoxemia despite optimal level of inspired oxygen and has been defined in literature as Pao2/Fio2 less than 100 mm Hg on a Fio2 of 0.8–1.0 with positive end-expiratory pressure (PEEP) of greater than 15 cm H2O or with plateau pressures greater than 30 cm H2O for more than 12 hours despite lung-protective ventilation (8, 9). Management of refractory hypoxia remains a clinical challenge for intensivists, and commonly used rescue strategies include prone positioning, neuromuscular blockade, extracorporeal membrane oxygenation (ECMO), and pulmonary vasodilators.
The management strategy for ARDSexp including lung contusion is also supportive therapy (10). However, there are lack of data on effects of pulmonary vasodilators in such patients.
Epoprostenol and iloprost are commonly used inhaled pulmonary vasodilators for refractory hypoxia. Epoprostenol has a half-life of 3–5 minutes and as such must be used as a continuous nebulization (11). This becomes particularly challenging during transport of patients especially for imaging. Some formulations of epoprostenol hydrolyze at neutral pH and must be diluted in an alkaline solution (12). Iloprost, on the contrary, has a half-life of 30 minutes and can be used as an inhalation every 2 to 4 hours (13).
Previous studies have shown improvements in oxygenation and gas exchange with the use of aerosolized prostacyclins, although without differences in survival (14–16). However, there are limited data comparing the efficacy of different pulmonary vasodilators in the setting of ARDS, such as iloprost or epoprostenol.
We aim to compare the effectiveness of iloprost and epoprostenol in patients with hypoxemic respiratory failure and refractory hypoxia in regard to clinical outcomes and survival.
MATERIALS AND METHODS
Population and Setting
We performed a retrospective cohort analysis of patients admitted to all ICUs of the University of Oklahoma Health Sciences Center between the years 2015–2018. Patients were included in the analysis if they were older than 18 years old, had hypoxemic respiratory failure requiring mechanical ventilation, and had received either aerosolized iloprost or aerosolized epoprostenol for at least 4 hours. Patients were excluded if they had received more than one class of pulmonary vasodilators at any point during their hospital stay to avoid data contamination. For patients that had more than one admission to an ICU, only the first admission was included in the analysis.
During the study period at our institute, the protocol for dosing and monitoring of aerosolized prostacyclins was respiratory therapist driven. However, the decision on when to initiate an aerosolized prostacyclin and the choice of agent to use was dependent on the clinical judgment of the treating physician. Flolan and Ventavis were used as formulations for epoprostenol and iloprost, respectively. Prostacyclins were administered via a Aerogen nebulization system. Flolan was prepared in a sterile diluent (pH~12). Iloprost was administered as an inhalation every 2 to 4 hours per respiratory therapist protocol. Information regarding the administration of these agents is available in the Online Data Supplement (http://links.lww.com/CCX/B124).
Ethics Committee Approval
The study was approved by the University of Oklahoma Health Sciences Center Institutional Review Board on May 28, 2015 (Protocol number 9246). Given the retrospective nature of the study, informed consent was waived. Procedures were followed in accordance with ethical standards and with Helsinki Declaration of 1975.
Data Collection, Definitions, and Outcomes
Data were collected from the electronic medical record system, including demographics, body mass index (BMI), arterial blood gases, vasopressor requirements, serum lactate level, liver function tests, fluid balance, need for prone positioning, neuromuscular blockade, PEEP as well as tidal volume and mode of the mechanical ventilation. The data for neuromuscular blockade, tidal volume and proning were collected only after initiation of prostacyclin, which may affect interpretation of results. The Sequential Organ Failure Assessment (SOFA) score and the Acute Physiology and Chronic Health Evaluation (APACHE II) score were used as an index to describe the severity of illness. The definition of ARDS was based on clinical documentation of providers. The Berlin criteria was not strictly used to define ARDS; however, all patients had hypoxia and diffuse bilateral lung opacities. The etiology of hypoxia was classified as ARDSp or ARDSexp from lung contusion. The ratio Pao2/Fio2 was used as marker of respiratory failure severity.
Data were also collected longitudinally for vasopressor requirements, lactate levels and Pao2/Fio2, with interval variability based each individual case, but rounded to 4-hour intervals up to 48 hours. Since patients may receive different vasopressors over time, the norepinephrine equivalent dose was used for standardization. Briefly, norepinephrine equivalent dose = norepinephrine dose (µg/min) + epinephrine dose (µg/min) + (phenylephrine dose [µg/min] ÷ 10) + (dopamine dose [µg/kg/min] ÷ 2); if on vasopressin, (0.1 × actual weight in kg) was added to norepinephrine equivalent dose (17).
In patients that had a 2D transthoracic echocardiogram during the index admission, right ventricular function was assessed qualitatively. Presence of right ventricular dilation or right ventricular systolic dysfunction was documented. Since the data were heterogeneous, the timing of echocardiogram was variable and could be before intubation, after intubation and after initiation of prostacyclin.
The primary outcome of the study was to compare the change in Pao2/Fio2 between patients treated with iloprost and epoprostenol. Other outcomes of interest included in-hospital mortality truncated to 90 days and change over time in vasopressor requirements (norepinephrine equivalent dose).
Statistical Analysis
Continuous data are reported as median (interquartile range). Categorical variables are reported as counts and percentages. For changes over time in Pao2/Fio2 and changes in vasopressor requirements (norepinephrine equivalent dose), linear mixed models were used for repeated measures analysis. Model terms included hours as a categorical variable, study agent (iloprost or epoprostenol), and the interaction hours-study agent. The interaction term was dropped from the model if its p value was less than 0.1.
A p value of less than or equal to 0.05 was considered statistically significant. SAS Version 9.4 (SAS, Cary, NC) was used for analysis.
Mortality was defined as all-cause mortality, with data truncated at 90 days. Mortality was recorded with the date of patient’s death (or date of transition to palliative measures). Survival was evaluated using Kaplan-Meier analysis and log-rank test. A Cox proportional hazard model was used for multivariate analysis. Covariates were included in the model if the p value was less than or equal to 0.10 in the univariate analysis. Covariates were evaluated for collinearity. Proportional hazard model assumptions were assessed with Grambsch & Therneau “ZPH” test and the supremum test for proportional hazard.
RESULTS
Demographics and Clinical Characteristics of Study Population
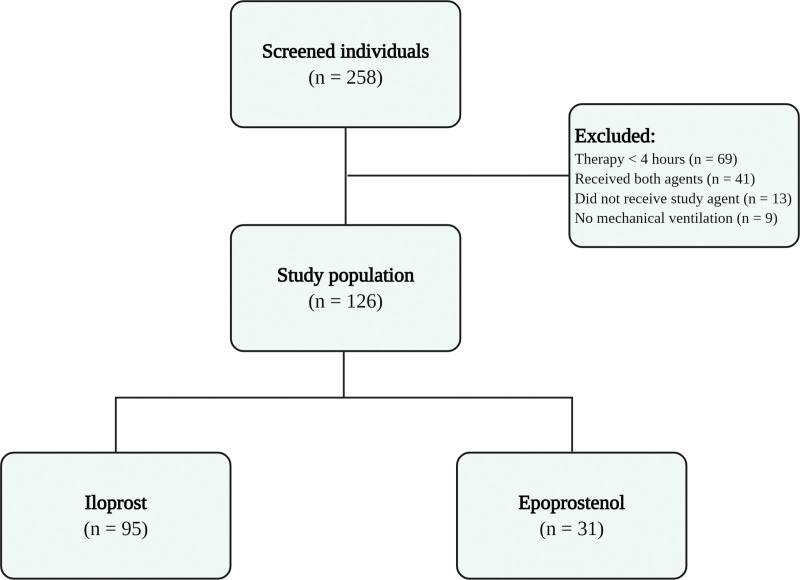
Two-hundred fifty-eight individuals were screened, and 132 patients were excluded from the study based on the above-mentioned exclusion criteria. Ultimately, 126 patients were included in the study for analysis (Fig. 1). The median age of study participants was 56 years (37–64 yr), and 35% of patients were female. ARDSp was deemed the etiology of respiratory failure in 48% of the study population. An echocardiogram was performed in 97 patients; there was evidence of right ventricular dilation or systolic dysfunction in 31% of these patients. Right ventricular dysfunction was based only on qualitative echocardiographic assessment and right ventricular dilation.
Figure 1.
Study flow chart.
Fifty-six percent of participants received a tidal volume of 6–8 mL/kg ideal body weight (IBW); up to 32% patients received more than 8 mL/kg IBW. The median PEEP was 10 cm H2O (6–10 cm H2O). Neuromuscular blockade was administered in 56% of study population and prone positioning was used only in 23% of patients. Cisatracurium was the agent used for neuromuscular blockage and was administered as a bolus followed by infusion. Since the data collection of the study, steps have been taken to improve adherence to lung-protective ventilation and prone positioning, which include education of providers/staff across different ICUs.
Comparison of Baseline Characteristics Between Study Groups
Of the 126 patients included in the analysis, iloprost was administered in 95 patients (75%) and epoprostenol was used in 31 patients (25%) (Fig. 1). Both groups were similar with regards to baseline characteristics including age, sex at birth, APACHE II and SOFA scores, etiology of respiratory failure, tidal volume, PEEP, use of neuromuscular blockade, and prone positioning (Table 1) Patients in the iloprost group had lower aspartate aminotransferase levels and a lower 24-hour fluid balance prior to initiation of prostacyclin. Of note, patients in the iloprost group had higher baseline Pao2/Fio2 ratio compared with the epoprostenol group (median Pao2/Fio2 of 95.36 [74.78–133.11] and 74.44 [56–108.57], in the iloprost and epoprostenol groups, respectively) (Table 1).
TABLE 1.
Comparison of Baseline Demographic and Clinical Characteristics
| Demographic Characteristic | Iloprost (n = 95) | Epoprostenol (n = 31) | p a |
|---|---|---|---|
| Age, yr | 57 (41–64) | 52 (37–63) | 0.3288 |
| Female sex, n (%) | 35 (36.8) | 9 (29) | 0.5178b |
| Body mass index, kg/m2 | 29.8 (25–35.5) | 31 (26.9–34.1) | 0.5493 |
| Acute Physiology and Chronic Health Evaluation II | 20 (16–24) | 20 (15–27) | 0.5260 |
| Sequential Organ Failure Assessment | 7 (6–9) | 8 (7–10) | 0.0659 |
| Charlson Comorbidity Index | 2 (1–4) | 1 (0–3) | 0.3712 |
| Service, n (%) | 0.6926 | ||
| Trauma ICU | 46 (48.4) | 17 (54.8) | |
| Medical ICU | 22 (23.2) | 5 (16.1) | |
| Other ICU | 27 (28.4) | 9 (29.1) | |
| Acute respiratory distress syndrome (pulmonary), n (%) | 42 (44.2) | 17 (54.8) | 0.4074b |
| Right ventricular dysfunction, n (%) (n = 97) | 23 (30.3) | 7 (33.3) | 0.7944b |
| Creatinine, mg/dL (n = 125) | 0.8 (0.6–1.5) | 1 (0.7–1.9) | 0.2333 |
| Aspartate aminotransferase, international units/L (n = 95) | 44 (27–96) | 100.5 (55.5–195.5) | 0.0015 |
| Lactic acid, mmol/L (n = 62) | 2 (1.2–6.1) | 2.1 (1.1–9.1) | 0.7060 |
| Pao2/Fio2 (n = 123) | 95.36 (74.78–133.11) | 74.44 (56–108.57) | 0.0273 |
| Tidal volume, n (%) (n = 89) | 0.2979 | ||
| TV < 6 mL/kg IBW | 5 (7.35) | 4 (19.05) | |
| TV 6–8 mL/kg IBW | 40 (58.82) | 11 (52.38) | |
| TV > 8 mL/kg IBW | 23 (33.82) | 6 (28.57) | |
| Positive end-expiratory pressure (n = 120) | 10 (7–12) | 10 (7.5–10) | 0.3023 |
| Neuromuscular blockade, n (%) | 46 (48.42) | 21 (67.74) | 0.0612 |
| Prone positioning, n (%) | 21 (22.11) | 9 (29.03) | 0.4317 |
| Use of diuretics, n (%) | 41 (43.16) | 12 (38.71) | 0.6631 |
| Renal replacement therapy, n (%) | 20 (21.28) | 6 (19.35) | 0.8192 |
| Fluid balance (mL) (n = 124) | 1,418 (-32.7 to 2,547.9) | 2,530.6 (1,247–4,833.4) | 0.0012 |
| Need for vasopressors, n (%) | 40 (42.11) | 15 (48.39) | 0.5403 |
| Number of vasopressors (n = 55) | 1 (1–2) | 1 (1–2) | 0.8284 |
| Dose of vasopressorsc (µg/min) (n = 55) | 15 (7–30) | 20 (10–63) | 0.3202 |
| Duration of therapy, hr | 93 (25–161) | 72 (26–168) | 0.8321 |
| Timing between mechanical ventilation initiation to prostacyclin, d (n = 123) | 3 (1.0–6.5) | 2 (1.0–6.0) | 0.4125 |
| Timing between admission date to initiation to prostacyclin, d | 4 (2.0–10.0) | 4 (2.0–7.0) | 0.8736 |
| Duration of mechanical ventilation, d (n = 117) | 13 (6.0–25.0) | 11 (4.0–22.0) | 0.5603 |
IBW = ideal body weight, TV = tidal volume.
p values calculated using Wilcoxon signed-rank test for continuous variables or χ2 test for categorical variables, unless otherwise noted.
Fisher exact test used.
Calculated as equivalent dose of norepinephrine—see Text for details.
Continues variables are expressed as median (interquartile range), categorical variables are expressed as n (%). Total number of observations is 126 unless otherwise noted.
Gas Exchange
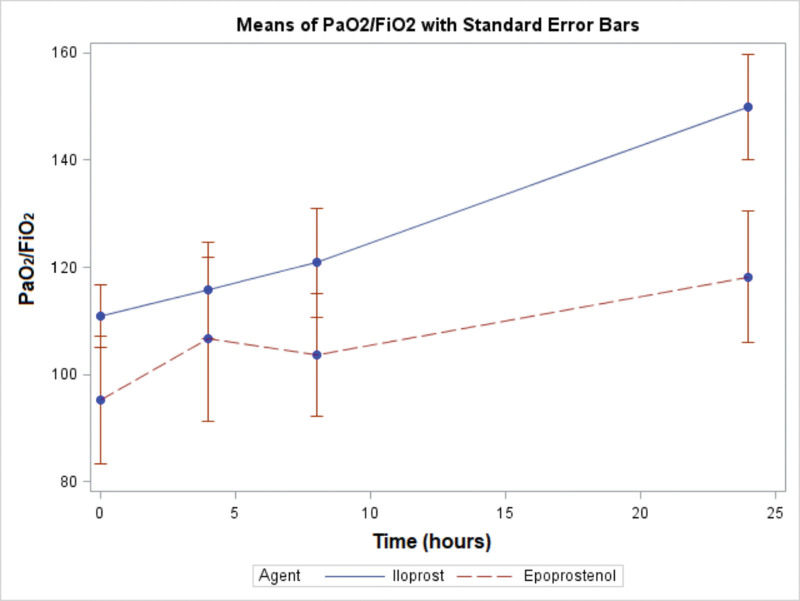
Both agents were associated with improvements in Pao2/Fio2 ratios from baseline. The Pao2/Fio2 was numerally higher for the iloprost group than the epoprostenol group at all time points (Fig. 2). The linear mixed model showed there was no significant interaction between agents and hours (p = 0.945). Across agents, Pao2/Fio2 increased over time (p < 0.001), with no significant difference between the two agents (p = 0.092).
Figure 2.
Changes in oxygenation between study groups. The figure shows changes in Pao2/Fio2 over time. The bars indicate the se. Both agents were associated with improvements in Pao2/Fio2 from baseline, and the magnitude of improvement did not differ between groups. Pao2/Fio2 was numerically higher at each time point in the iloprost group, although the difference was not statistically significant.
Clinical Outcomes and Mortality
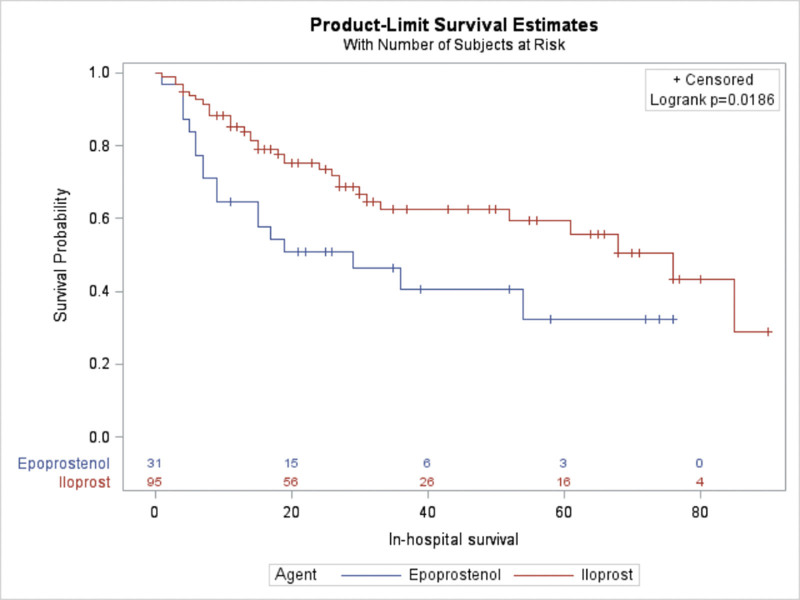
The univariate analysis of mortality showed that the use of epoprostenol was associated with higher 90-day mortality compared with the iloprost group. However, this finding should be interpreted with caution as the study was underpowered to detect mortality difference. The unadjusted hazard ratio (HR) for the association between epoprostenol use and mortality was 1.96 (95% CI, 1.103–3.485). Of note, this association remained significant in the multivariate Cox proportional hazards model (adjusted for age, presence of shock, SOFA score, ICU service, and BMI), with a HR 3.123 (95% CI, 1.604–6.083; p = 0.0008) (Table 2). Supplemental Table 1 (http://links.lww.com/CCX/B124) displays the HRs for all components of the multivariate model. We further performed multivariate analysis (model 2; Supplemental Table 2, http://links.lww.com/CCX/B124) adjusted for age, Pao2/Fio2 ratio, presence of shock, SOFA score, ICU service, BMI, and missing data. While association of higher mortality with epoprostenol remained statistically significant (HR, 3.045 [95% CI, 1.554–5.966; p = 0.0012]), we believe this likely does not reflect clinical significance as explained in detail later. The median in-hospital survival was 76 days for iloprost, compared with 29 days for epoprostenol (log-rank test p = 0.0186) (Fig. 3).
TABLE 2.
Univariate and Multivariate Cox Proportional Hazards Model for 90-Day Mortality
| Type of Analysis | Univariate Analysis | Multivariable Analysisa | ||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p |
| Epoprostenol use (compared with iloprost) | 1.96 (1.103–3.485) | 0.0219 | 3.123 (1.604–6.083) | 0.0008 |
Model adjusted for age, presence of shock, Sequential Organ Failure Assessment score, ICU service, and body mass index.
Figure 3.
Survival comparison between study groups. Kaplan-Meier figure displaying a comparison of 90-d in hospital survival for patients with severe hypoxemic respiratory failure that received either iloprost or epoprostenol. Log-rank test p = 0.0186.
Vasopressor Requirements and Liberation From Mechanical Ventilation
In the analysis of vasopressor requirements, an interaction between study agent and time was found (p = 0.018). Figure S1 (http://links.lww.com/CCX/B124) shows that the norepinephrine equivalent dose was numerically higher for the iloprost group for the first 12 hours, and then it was numerically lower after hour 12, although this difference was not statistically significant. Time to liberation from mechanical ventilation was not statistically different between both groups (Fig. S2, http://links.lww.com/CCX/B124).
DISCUSSION
Our study showed that the use of both medications was associated with an improvement in Pao2/Fio2 ratio, although there was no significant difference in the magnitude of improvement between agents. An initial increase in vasopressor requirement was observed in the iloprost group during the first 12 hours; however, the pressor requirements decreased in the ensuing hours. Epoprostenol was associated with higher 90-day mortality compared with iloprost. This association remained statistically significant after adjusting for confounders such as age, severity of illness, ICU service, and BMI. However, given the significant prescription bias in the study and low sample size, this finding should be interpreted with caution.
ARDS is characterized by ventilation-perfusion mismatch and increased shunting leading to hypoxemia (18). Management of ARDS is mainly supportive involving mechanical ventilation and treatment of the underlying etiology. There are only a few interventions that have shown to improve mortality outcomes in patients with ARDS. Lung-protective ventilation, which refers to a low tidal volume ventilation (4–6 mL/kg of IBW) and a plateau pressure less than 30 mm Hg, is associated with improvement in survival (19). Prone positioning with subsequent improvement of ventilation/perfusion matching and decreasing shunt is also associated with improved survival (20).
Interventions for patients with severe respiratory failure include prone positioning (20), conservative fluid strategy (21), and neuromuscular blockade (22, 23). Of these interventions, only prone positioning has been shown to be associated with improvement in survival (20). Refractory hypoxia is defined as a Pao2 of less than 60 mm Hg, despite an Fio2 of 0.8–1.0, and PEEP greater than 10–20 cm H2O for more than 12–24 hours (24). Refractory hypoxia is associated with increased in-hospital mortality, with a two-fold increase in the adjusted odds of death when compared with ARDS patients without refractory hypoxia (24, 25).
ECMO has emerged as an important rescue therapy for refractory hypoxemia (26). However, at the time of the study, ECMO was not available at our institute.
Aerosolized pulmonary vasodilators are another frequently used therapeutic approach in patients with refractory hypoxia. Inhaled selective pulmonary vasodilators decrease pulmonary vascular resistance and act preferentially on well-ventilated areas decreasing ventilation-perfusion mismatch in addition to exhibiting anti-proliferative and anti-inflammatory properties (27). The most commonly used pulmonary vasodilators include inhaled nitric oxide (iNO) and the aerosolized prostacyclins, epoprostenol, and iloprost.
The use of pulmonary vasodilators in patients with severe ARDS and refractory hypoxia is associated with improvements in oxygenation and gas exchange (28). A meta-analysis of 25 studies showed that the use of pulmonary vasodilators in ARDS is associated with significant improvement in absolute Pao2, Pao2/Fio2 ratio, and pulmonary artery pressures (27). However, most of the studies included were observational. There is a limited number of randomized controlled trials studying the effects of these agents on oxygenation, hospital stay, and mortality. Dahlem et al (29) showed improvement in oxygenation with the use of epoprostenol without any significant adverse events when compared with placebo. Investigators from the University of Oklahoma have previously described the effects of nebulized iloprost in 20 patients with ARDS, with improvement in oxygenation without adverse effect on hemodynamics (14). None of these trials were designed for assessment of survival or ICU length of stay. Adhikari et al (30) performed a systemic review of 12 randomized controlled trials studying the effects of iNO in patients with ARDS and found short-term benefits in oxygenation but no improvement in survival.
There are limited data comparing the different pulmonary vasodilators. A study performed by Zwissler et al (31) comparing iNO and epoprostenol did not show any major difference in efficacy. Prostacyclins are postulated to enhance the cyclic adenosine monophosphate axis with resultant increase in surfactant production, an effect not associated with iNO use (32). Furthermore, prostacyclins also exert an anti-inflammatory effect by suppression of tumor necrosis factor alpha synthesis, which may potentiate their benefit in ARDS (33). Particular concerns with use of iNO are renal toxicity (34) and high-cost expenditure associated with its use (16).
To date, there has been no head-to-head comparison between the aerosolized prostacyclins. Our study is the first to compare the clinical response of iloprost and epoprostenol, in patients with hypoxemic respiratory failure requiring mechanical ventilation.
Our study shows that both iloprost and epoprostenol use were associated with improvements in gas exchange, as reflected by improvements in the Pao2/Fio2 ratio; this improvement was numerically higher with iloprost than the improvement seen with epoprostenol, although the difference did not reach statistical significance. Given its longer half-life, iloprost could theoretically have a higher risk of systemic adverse events such as hypotension (35), although no changes in the total norepinephrine equivalent dose (surrogate of the severity of hemodynamic instability) were noted in our study when comparing iloprost and epoprostenol. This is in accordance with the previous study performed at our institution (14).
Our study shows that the use of epoprostenol was associated with higher mortality rates, compared with iloprost use, in both the univariate and multivariate analysis (after adjusting for age, BMI, shock, and ICU service). This difference likely stems from the difference in management strategies and various confounders. As the decision to initiate inhaled pulmonary vasodilator was nonprotocolized and physician based, it is possible that sicker patients were started on continuous epoprostenol by providers compared with interval dosing of iloprost.
An important aspect to consider for the future, could be the role of right ventricular dysfunction in ARDS, and a possible different response to pulmonary vasodilators could also be hypothesized. However, we could not formally test for a differential response in each group due to the relatively low number of patients that had an echocardiogram in our cohort. Previous studies have shown that presence of right ventricular injury is associated with higher mortality in patients with ARDS (36). Interventions such as prone positioning have been postulated to improve right ventricular performance in such patients (37).
Since both the agents improved oxygenation in a similar manner and epoprostenol administration requires continuous administration, iloprost may be a more suitable option in patients with refractory hypoxia, specially if transport or imaging is anticipated.
These results of our study are important because it is the first study comparing clinical outcomes between aerosolized prostacyclins. Although iloprost has been extensively studied in the pulmonary hypertension patient population, there are limited data on the outcomes of patients with ARDS treated with iloprost.
Our study has several limitations. First of all, given its retrospective nature, it is not possible to fully control for biases such as prescription bias (meaning, the medical providers decided to prescribe one agent vs the other). In fact, the epoprostenol group had lower oxygenation at baseline, and it could have reflected a sicker population (although SOFA and APACHE II scores were similar). Other limitations of our study include the relatively small sample size and that the data belongs to a single institution. Another limitation is that data for some of the other important outcomes including rates of tracheostomy and adverse events such as bleeding, bronchospasm, and thrombocytopenia were not available.
Despite these limitations, to increase the strength and significance of our study findings, we decided to exclude patients who could have received more than one study agent during their hospital stay to minimize contamination.
CONCLUSIONS
This study further adds to our knowledge about the effects of aerosolized prostacyclins in critically ill patients with respiratory failure. We show that in patients with hypoxemic respiratory failure on mechanical ventilation, both iloprost and epoprostenol improve gas exchange with no significant difference in the magnitude of improvement between agents. The mortality was higher in those treated with aerosolized epoprostenol compared with inhaled iloprost; however, our study was underpowered to detect such survival difference. Further studies are needed to corroborate these findings, given the high frequency of use of pulmonary vasodilators.
Supplementary Material
Footnotes
Drs. Hussain and Jaliawala contributed equally to this article.
Dr. Bernardo serves on the scientific advisory board for Janssen Pharmaceuticals. The remaining authors have disclosed that they do not have any potential conflicts of interest.
All authors reviewed and revised the article. Drs. Hussain and Jaliawala had full access to all the data in the study and takes responsibility for its integrity and the data analysis. Dr. Bernardo contributed to study design, data analysis, and article writing. Drs. Hussain and Jaliawala contributed to data generation and article writing. Dr. Zhao contributed to data analysis and article editing. Dr. Jaliawala contributed to data generation and article editing. Dr. Tsui contributed to study design, data analysis, and article editing. Dr. Brown contributed to study design, data analysis, and article editing. Dr. Hussain is the guarantor of the content of the article.
Presented, in part, at the American Thoracic Society International Conference, Dallas, TX, May 21, 2019.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Li G, Malinchoc M, Cartin-Ceba R, et al. : Eight-year trend of acute respiratory distress syndrome: A population-based study in Olmsted County, Minnesota. Am J Respir Crit Care Med 2011; 183:59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators: Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
- 3.Herridge MS, Tansy CM, Matte A, et al. : Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364:1293–1304 [DOI] [PubMed] [Google Scholar]
- 4.Cheung AM, Tansey CM, Tomlinson G, et al. : Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 2006; 174:538–544 [DOI] [PubMed] [Google Scholar]
- 5.Pelosi P, D’Onofrio D, Chiumello D, et al. : Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl 2003; 42:48s–56s [DOI] [PubMed] [Google Scholar]
- 6.Anan K, Kawamura K, Suga M, et al. : Clinical differences between pulmonary and extrapulmonary acute respiratory distress syndrome: A retrospective cohort study of prospectively collected data in Japan. J Thorac Dis 2018; 10:5796–5803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battaglini D, Sottano M, Ball L, et al. : Ten golden rules for individualized mechanical ventilation in acute respiratory distress syndrome. J Intensive Med 2021; 1:42–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villar J, Kacmarek RM: Rescue strategies for refractory hypoxemia: A critical appraisal. Med Rep 2009; 1:91–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esan A, Hess D, Raoof S, et al. : Severe hypoxemic respiratory failure: Part 1: Ventilatory strategies. Chest 2010; 137:1203–1216 [DOI] [PubMed] [Google Scholar]
- 10.Ganie FA, Lone H, Lone GN, et al. : Lung contusion: A clinico-pathological entity with unpredictable clinical course. Bull Esmerg Trauma 2013; 1:7–16 [PMC free article] [PubMed] [Google Scholar]
- 11.Hill NS, Preston IR, Roberts KE: Inhaled therapies for pulmonary hypertension. Respir Care 2015; 60:794–802; discussion 802–805 [DOI] [PubMed] [Google Scholar]
- 12.Glaxo Smith Kline. FLOLAN - Prescribing Information. Available at: https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Flolan/pdf/FLOLAN-PI-PIL.PDF. Accessed September 24, 2021.
- 13.Mubarak KK: A review of prostaglandin analogs in the management of patients with pulmonary arterial hypertension. Respir Med 2010; 104:9–21 [DOI] [PubMed] [Google Scholar]
- 14.Sawheny E, Ellis AL, Kinasewitz GT: Iloprost improves gas exchange in patients with pulmonary hypertension and ARDS. Chest 2013; 144:55–62 [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui S, Salahuddin N, Zubair S, et al. : Use of inhaled PGE1 to improve diastolic dysfunction, pulmonary hypertension and hypoxia in ARDS—a randomized clinical trial. Open J Anesth 2013; 3:109–115 [Google Scholar]
- 16.Torbic H, Szumita PM, Anger KE, et al. : Inhaled epoprostenol vs inhaled nitric oxide for refractory hypoxemia in critically ill patients. J Crit Care 2013; 28:844–848 [DOI] [PubMed] [Google Scholar]
- 17.Khanna A, English SW, Wang XS, et al. : ATHOS-3 investigators: Angiotensin II for the treatment of vasodilatory shock. N Engl J Med 2017; 377:419–430 [DOI] [PubMed] [Google Scholar]
- 18.Radermacher P, Maggiore SM, Mercat A: Fifty years of research in ARDS. Gas exchange in acute respiratory distress syndrome. Am J Respir Crit Care Med 2017; 196:964–984 [DOI] [PubMed] [Google Scholar]
- 19.Brower RG, Matthay MA, Morris A, et al. ; Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308 [DOI] [PubMed] [Google Scholar]
- 20.Guérin C, Reignier J, Richard JC, et al. : Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168 [DOI] [PubMed] [Google Scholar]
- 21.Network AR, Wheeler AP, Bernard GR, et al. : Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575 [DOI] [PubMed] [Google Scholar]
- 22.Papazian L, Forel JM, Gacouin A, et al. : Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–1116 [DOI] [PubMed] [Google Scholar]
- 23.Moss M, Huang DT, Brower RG, et al. ; National Heart, Lung, and Blood Institute PETAL Clinical Trials Network: Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med 2019; 380:1997–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claar DD, Hyzy RC: Refractory hypoxemia and acute respiratory distress syndrome adjunctive therapies: An open question? Ann Am Thorac Soc 2017; 14:1768–1769 [DOI] [PubMed] [Google Scholar]
- 25.Duan EH, Adhikari NK, D’Aragon F, et al. : Management of acute respiratory distress syndrome and refractory hypoxemia. A multicenter observational study. Ann Am Thorac Soc 2017; 14:1818–1826 [DOI] [PubMed] [Google Scholar]
- 26.Peek GJ, Mugford M, Tiruvoipati R, et al. : Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicenter randomized controlled trial. Lancet 2009; 374:1351–1363 [DOI] [PubMed] [Google Scholar]
- 27.Fuller BM, Mohr NM, Skrupky L, et al. : The use of inhaled prostaglandins in patients with ARDS: A systematic review and meta-analysis. Chest 2015; 147:1510–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siobal MS, Kallet RH, Pittet JF, et al. : Description and evaluation of a delivery system for aerosolized prostacyclin. Resp Care 2003; 48:742–753 [PubMed] [Google Scholar]
- 29.Dahlem P, van Aalderen WM, de Neef M, et al. : Randomized controlled trial of aerosolized prostacyclin therapy in children with acute lung injury. Crit Care Med 2004; 32:1055–1060 [DOI] [PubMed] [Google Scholar]
- 30.Adhikari NK, Burns KE, Friedrich JO, et al. : Effect of nitric oxide on oxygenation and mortality in acute lung injury: Systematic review and meta-analysis. BMJ 2007; 334:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zwissler B, Kemming G, Habler O, et al. : Inhaled prostacyclin (PGI2) versus inhaled nitric oxide in adult respiratory distress syndrome. Am J Respir Crit Care Med 1996; 154:1671–1677 [DOI] [PubMed] [Google Scholar]
- 32.Rose F, Zwick K, Ghofrani H, et al. : Prostacyclin enhances stretch-induced surfactant secretion in alveolar epithelial type II cells. Am J Respir Crit Care Med 1999; 160:846–851 [DOI] [PubMed] [Google Scholar]
- 33.Eisenhut T, Sinha B, Grottrup-Wolfers E, et al. : Prostacyclin analogs suppress the synthesis of tumor necrosis factor-alpha in LPS-stimulated human peripheral blood mononuclear cells. Immunopharmacology 1999; 26:259–264 [DOI] [PubMed] [Google Scholar]
- 34.Ruan SY, Huang TM, Wu HY, et al. : Inhaled nitric oxide therapy and risk of renal dysfunction: A systematic review and meta-analysis of randomized trials. Crit Care 2015; 19:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Searcy RJ, Morales JR, Ferreira JA, et al. : The role of inhaled prostacyclin in treating acute respiratory distress syndrome. Ther Adv Res Dis 2015; 9:302–312 [DOI] [PubMed] [Google Scholar]
- 36.Sato R, Dugar S, Cheungpasitporn W, et al. : The impact of right ventricular injury on the mortality in patients with acute respiratory distress syndrome: A systematic review and meta-analysis. Crit Care 2021; 25:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hussain ST, Bernardo RJ: Acute impact of prone positioning on the right ventricle in COVID-19–associated acute respiratory distress syndrome. Circ Heart Fail 2022; 15:e009197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.