Abstract
The Coronavirus Disease 2019 (Covid-19) pandemic had dramatic effect on mental health, causing long-term psychiatricmorbidity. At present, there are no randomized trials reporting the effect of physical exercise on individuals with post- Covid-19 condition are available. The aim of this review was to summarize the evidence regarding the evidence on exercise as a treatment for anxiety and depression symptoms secondary to chronic diseases, which may be generalized to individuals suffering from the post- Covid-19 condition.
Trials were included if they reported the effects of physical exercise programs on anxiety or depression symptoms in adults, either healthy or affected by chronic diseases. Outcomes were changes of anxiety or depression severity after an exercise-based intervention.
Of the 2161 RCTs identified, eight out of 15 studies were included. Exercise was associated with greater improvements of depressive (SMD = −0.169; 95 % CI −0.302 at −0.003; p = 0.013) and anxiety symptoms (SMD = −0.263, 95 % CI −0.418 at −0.109; p = 0.001), compared with control interventions.
Supervised exercise programs were effective against symptoms of anxiety or depression among individuals with chronich illnesses. Pending specific clinical trials, exercise may be considered for adoption among patients with the post Covid-19 condition.
Keywords: Post-Covid-19 condition, Physical activity, Anxiety, Depression, Exercise
1. Introduction
Mental disorders are the leading cause of disability worldwide, affecting >340 million people (World Health Organization, 2017). People with mental health problem have a significantly higher risk of developing physical health problems than the general population (Crone et al., 2004). Physical comorbidities affected about 65 % of people with depressive disorders (The economic cost of serious mental illness and comorbidities in Australia and New Zealand, 2016), commonly including obesity, type 2 diabetes, metabolic syndrome, and cardiovascular disease (Vancampfort et al., 2015). This scenario has further worsened since February 2020 with the spread of the COVID 19 pandemic. More than 560 million cases of COVID-19infection, including 6.361.157 deaths have been recorded worldwide (WHO Coronavirus (COVID-19) Dashboard, n.d.). While most of the infected people heal within few weeks, several people develop persistent symptoms, regardless of age and other comorbidities. This condition has been referred to as “Long COVID” (E. Maxwell Living With COVID19: A Dynamic Review of the Evidence Around Ongoing COVID19 Symptoms (Often Called Long COVID), n.d.) and was subsequently defined by World Health Organization (WHO) as post-Covid-19 condition. The Post-Covid-19 condition occurs more frequently in those with a history of SARS CoV-2 infection and has its onset, about 3 months since the onset of COVID-19. Symptoms usually last for 2 months include fatigue, shortness of breath, cognitive dysfunction. The Post-Covid-19 condition has a strong impact on everyday functioning. Symptoms may be new onset following initial recovery from an acute COVID-19 episode or persist from the initial illness. Symptoms may also fluctuate or relapse over time (A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus, 2021). It has been estimated that the proportion of people who had COVID-19 that go on to experience post-COVID syndrome is 13.3 % (persistence of symptoms for one months or longer) to 2.5 % (three months or longer) reaching a prevalence of 30 % (6 months or longer) among patients who were hospitalized (CDC, 2022).
The pandemic is exacting a heavy toll on mental health: it causes long term psychiatric symptoms including post-traumatic stress disorder (PTSD), depression, anxiety, and obsessive-compulsive symptoms even after the recovery from the acute infection (Tomasoni et al., 2021; Mazza et al., 2020; Chamberlain et al., 2021; Taquet et al., 2021; Uzunova et al., 2021).
Moreover, Huang C. et al reported that COVID-19 survivors were mainly troubled with fatigue or muscle weakness, sleep difficulties, and anxiety or depression at 6 months after acute infection (Huang et al., 2021).
In sum, there is a clear need for effective treatments to improve both physical and mental health (Stubbs et al., 2018; Firth et al., 2019), and multicomponent lifestyle interventions incorporating a combination of physical exercise and diet (Schuch et al., 2016). The benefits of exercise for psycho-physical well-being are increasingly recognized and result in a significant reduction in the rate of morbidity and mortality (Richardson et al., 2005; Zhou et al., 2021).
To date, there is a lack of RCTs that investigates the effectiveness of exercise for control of anxiety and depression in subjects suffering from post-Covid-19 condition. Currently, a trial is evaluating whether breathing exercises and singing can improve breathing abnormalities in post-COVID syndrome (clinical.trial.gov identification number NCT04810065) another a trial is evaluating whether physical exercise can alleviate post-SARS-CoV2 fatigue (NCT04841759).
Therefore, the aim of this systematic review is to collect the evidence present in literature regarding the use and effectiveness of exercise programs in the management of primary anxiety and depression or associated to other chronic diseases. This may be useful to understand if such intervention can be successfully adapted to a population affected by post-Covid-19 condition.
2. Materials and method
The systematic review with meta-analysis was planned and conducted in accordance with PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Metanalyses) and the recommendations of the Cochrane Collaboration.
2.1. Study selection/inclusion criteria
2.1.1. Types of studies
Randomized controlled trials (RCTs).
2.1.2. Types of participants
Studies of adult aged 18 years or older, both male and female. Will be included in the Meta-analysis RCTs carried out on healthy subjects or affected by musculoskeletal, metabolic, cardiovascular, neurological or psychopathological disorders. RCTs carried out on subjects with total impediment to exercising due to physical or mental impairment clinically diagnosed will be excluded.
2.1.3. Types of intervention
Any form of exercise as it of aerobic nature, against resistance or calisthenics exercise supervised or home-based. Recreational activities focused on exercise, counseling services and monitoring of exercise through wearable devices were also considered eligible type of interventions.
2.1.4. Control group
Studies comparing the intervention with the normal habits, usual care and cognitive behavioral therapy or other types of activity with no significant physical effort were considered admissible. Studies with significantly different control groups for age, pathology, M/F ratio or socioeconomic conditions compared to the intervention group were excluded.
2.2. Types of outcome measures
We included RCTs that reported objectives directly or indirectly evaluation of exercise capacity even through the administration of validated questionnaires. For the inclusion, RCTs must reported objectives measures of at least one of these outcomes:
-
1.
depression symptoms measured by self-assessment scales such as Beck Depression Inventory (Beck et al., 1961), or scales administered by medical personnel, as Hamilton Rating Scale for Depression (Hamilton, 1960), or any other validated scale considered specific;
-
2.
anxiety symptoms, measured using self-reporting scales such as Beck Anxiety Inventory (Beck et al., 1961), or scales administered by medical personnel such as Hamilton Anxiety Scale (Hamilton, 1960), or any other validated scale considered specific.
2.3. Search methods
The research was conducted on article published before April 1, 2021 on Medline (via PubMed), Embase and Cochrane CENTRAL for Randomized Controlled Trials (RCTs) online database. The literature search was constructed around search terms for “Exercise” and search terms for “Mental health”. For PubMed the following search strategy was used: ((exercise[MeSH Terms]) OR (sedentary behavior[MeSH Terms])) AND (mental health[MeSH Terms]) NOT (child [MeSH Terms]) NOT (adolescents [MeSH Terms]). The search strategy was adapted for each database, as necessary.
Abstracts identified during the literature search were screened by two authors independently. Potentially eligible articles were read in full by two review authors to determine whether they met the eligibility criteria. Disagreements were discussed with a third review author until consensus was reached. If necessary, additional information was obtained from the study authors contacted via e-mail.
2.4. Data extraction and management
Data on patients (age, gender, diagnosis of both control and intervention group), interventions (type, frequency, intensity, duration), control intervention (type, frequency and duration), outcomes characteristics (type of scale) and results (mean and standard deviation of both control and intervention group before and after intervention or control condition) were extracted from two authors independently using an a priori developed data extraction form. Discrepancies were discussed with a third review author until consensus was reached. If necessary, the study authors were contacted for additional information.
2.5. Data analysis
The effects of the exercise on control were analyzed in two separate meta-analysis on depressive symptoms or anxiety symptoms. If a study examined multiple intervention groups based on exercise, for the purposes of statistical analysis, they were considered as several divided studies compared to the same control group (sedentary/inactive). If a single study presented outcome measures for both depressive and anxiety symptoms, for analysis statistics, they were considered as two studies with the same population and intervention but analyzed in their respective meta-analysis. Duplicate citations were filtered using the Zotero software. Meta-analyses were conducted using software MedCalc® Statistical Software version 20.008 (MedCalc Software Ltd., Ostend, Belgium) and Review Manager (RevMan) Version 5.4. Since a limited number of studies was expected to be eligible and random effects tests are regarded as only approximate if the number of studies is small, a fixed effects model was used (Hedges and Vevea, 1998). For continuous outcomes, standardized mean differences (SMD) with 95 % confidence intervals (CIs) were calculated as the difference in means between groups divided by the pooled standard deviation (Cohen, 1988). Where no standard deviations were available, attempts were made to obtain the missing data from the trial authors by email. A negative SMD indicates beneficial effects of exercise compared to the control intervention for all outcomes. Cohen's categories were used to evaluate the magnitude of the overall effect size with (1) SMD = 0.2 to 0.5: small; (2) SMD = 0.5 to 0.8: medium, and (3) SMD > 0.8: large effect sizes (Cohen, 1988).
Statistical heterogeneity between studies was analyzed using the Cochran's Q test and I2 statistics. The I2 is a measure of how much variance between studies can be attributed to differences between studies rather than chance. A value of 0 % indicates no observed heterogeneity, and larger values show increasing heterogeneity. The magnitude of heterogeneity was categorized as (1) I 2 = 0–24 %: low heterogeneity; I 2 = 25–49 %: moderate heterogeneity; I 2 = 50–74 %: substantial heterogeneity; and I 2 = 75–100 %: considerable heterogeneity (Higgins et al., 2003). The level of significant heterogeneity was set at P ≤ 0.05.
Risk of bias was assessed by two authors independently using the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2), this tool assesses risk of bias on the following domain: bias arising from the randomization process, Bias due to deviations from intended interventions, Bias due to missing outcome data, Bias in measurement of the outcome, Bias in selection of the reported result. For each criterion, risk of bias was assessed as (1) low risk of bias, (2) some concerns, (3) high risk of bias. Conflicts of opinion were discussed with a third review author until consensus is reached. If necessary, additional information was retrieved from the study authors. The study was judged to be at “Low risk” if all domains had a “Low risk” rating, the study was judged “some concerns” if at least one domain was judged to be “Some concerns” but no domain was judged as“ high risk”. The study was instead judged as “High Risk of Bias” if it presented at least one domain judged as “High Risk”.
3. Results
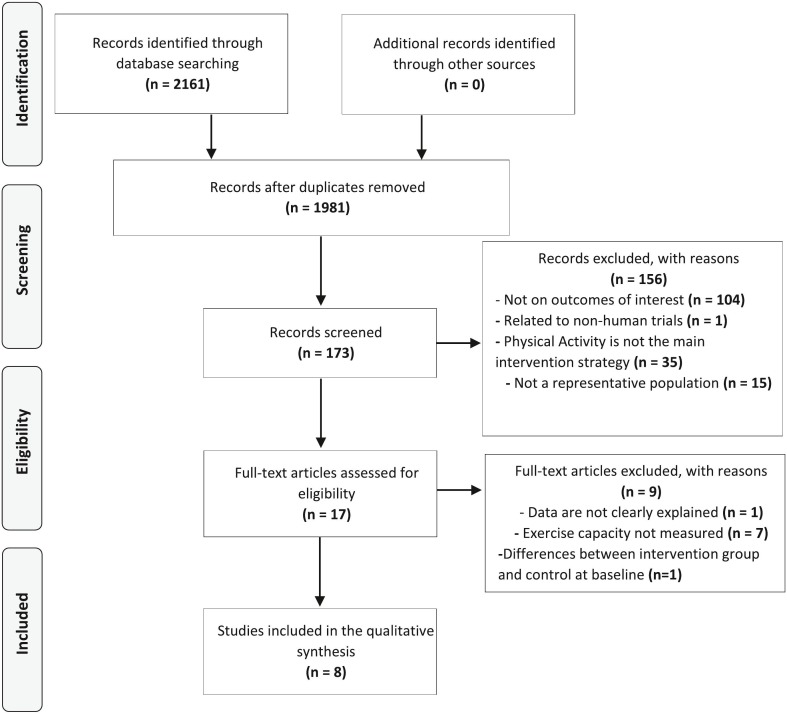
Two thousand and eight records were returned through the literature search. After exclusion of duplicates, 1981 records were screened and 1808 records were excluded because they were not RCTs, they were not in English, or the full text was not available. Out of the 17 full texts assessed for eligibility, 9 articles were excluded because data were not explained, or exercise capacity was not measured. Finally, eight RCTs were included in Meta-analysis (Fig. 1 ) (Gondoh et al., 2009; Imayama et al., 2011; Telenius et al., 2015; Malmberg Gavelin et al., 2018; Lucibello et al., 2020; Chien et al., 2011; Armstrong and Edwards, 2004; Bouaziz et al., 2019).
Fig. 1.
Identification and selection of studies for the review. Adapted from Moher et al. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial.
3.1. Setting of included studies
Of the eight RCTs included two were held in Asia (Gondoh et al., 2009; Chien et al., 2011), three in Europe (Telenius et al., 2015; Malmberg Gavelin et al., 2018; Bouaziz et al., 2019), one in the United States (Imayama et al., 2011), one in Canada (Lucibello et al., 2020), and one in Australia (Armstrong and Edwards, 2004). Patients were recruited via mail (Imayama et al., 2011), through university-related services (Imayama et al., 2011; Lucibello et al., 2020), from care facilities (Telenius et al., 2015), hospitals (Chien et al., 2011; Armstrong and Edwards, 2004), or through voluntary members (Bouaziz et al., 2019). Two studies included healthy participants (Gondoh et al., 2009; Lucibello et al., 2020), two included subjects with mental disorders such as stress-related exhaustion (Malmberg Gavelin et al., 2018) and dementia (Telenius et al., 2015), one included obese women (Imayama et al., 2011), one included patients with chronic heart failure (Chien et al., 2011), one included women affected by post-partum depression (Armstrong and Edwards, 2004), and one had a population of subjects with various chronic diseases including dysmetabolism, hypertension and musculoskeletal pathologies (Bouaziz et al., 2019).
3.2. Participant characteristics
3.2.1. Depressive disorder
872 subjects were examined, 471 from intervention groups (average age of 52.0 years of which 84 % female, 50 % obese, 20 % with dementia, 10 % with stress-related exhaustion, 9 % healthy, 5 % with heart failure, 3 % dysmetabolic, 2 % hypertensive, 2 % with postpartum depression and 1 % with musculoskeletal diseases) and 401 belonging to the control group (average age 48 of which 84 % female, 44 % obese, 21 % with dementia 10 % with stress related exhaustion, 9 % healthy, 7 % with heart failure, 4 % dysmetabolic, 3 % hypertensive and 2 % with postpartum depression). Trial and population characteristics related to depressive disorder are shown in Table 1 .
Table 1.
Characteristics of the studies included in the metanalysis.
| Author, year | Population | Groups (n) | Duration | PA intervention | Assessment | Outcome: anxiety and depression |
|---|---|---|---|---|---|---|
| Imayama et al., 2011 | 204 subjects Mean age: 57.7 ± 4.5 Sex: female (100 %) Diagnosis: obesity |
PA (117) Control, sedentary (87) |
12 months | PA sessions: aerobic training 70/85 % HRmax Frequency: 45 min, 5×/week |
EXC: maximal treadmill test (VO2max) Anxiety and depression: BSI-18 |
BSI-18 anxiety (points) after treatment: PA + diet group from 44.2 ± 6.8 to 43.5 ± 6.4 (p = 0.15). Control group from 45.3 ± 7 to 45.3 ± 8.7. BSI-18 depression (points) after treatment: PA + diet group decreased from 48.3 ± 8.7 to 46.2 ± 8.2. Control group decreased from 48 ± 9 to 48.4 ± 9.6 |
| Imayama et al., 2011 | 204 subjects Mean age: 57.7 ± 4.5 Sex: female (100 %) Diagnosis: obesity |
PA + Diet (117) Control, sedentary (87) |
12 months | PA sessions: aerobic training 70/85 % HRmax Frequency: 45 min, 5×/week |
EXC: maximal treadmill test (VO2max) Anxiety and depression: BSI-18 |
BSI-18 anxiety (points) after treatment: PA group from 43.5 ± 6.1 to 43 ± 6.9 (p = 0.14). Control group from 45.3 ± 7 to 45.3 ± 8.7. BSI-18 depression (points) after treatment: PA group decreased from 48.3 ± 9.4 to 48.2 ± 9.8. Control group decreased from 48 ± 9 to 48.4 ± 9.6 |
| Malmberg Gavelin et al., 2018 | 88 subjects Mean age: 43 ± 7.5 Sex: female (88 %) Diagnosis: stress-related exhaustion disorder |
PA (47) CBT (41) |
3 months | PA sessions: indoor Cycling 70/85 % HRmax Frequency: 40 min, 3×/week |
EXC: submaximal test (VO2max) Anxiety and depression: Hospital Anxiety and Depression scale |
HAD anxiety (points) after treatment: PA group from 9.19 ± 3.83 to 7.67 ± 4.73 (p = 0.14). Control group from 9.41 ± 3.82 to 7.18 ± 4.30. HAD depression (points) after treatment: PA group decreased from 6.81 ± 3.79 to 4.82 ± 3.55. Control group decreased from 7.26 ± 3.48 to 5.25 ± 4.12 |
| Bouaziz et al., 2019 | 60 subjects Mean age: 73.5 ± 3 Sex: female (73 %) Diagnosis: overweight, dyslipidemia, diabetes, hypertension |
PA (30) Control, sedentary (30) |
2 months | PA sessions: HIIT cycle ergometer 4 min VT/1 min 40 % VT Frequency: 30 min, 2×/week |
EXC: 6-MWT Anxiety and depression: GADS |
GADS depression subscore; −42.8 % in IATP-R group while +26.3 % in sedentary group. No significant difference in anxiety subscore |
| Lucibello et al., 2020 | 46 subjects Mean age: 19.5 ± 1.5 Sex: female (64 %) Diagnosis: healthy subjects |
PA (25) Control, sedentary (21) |
2 months | PA sessions: HIIT Cycle ergometer 95 % HRmax 1 min, 30 % HRmax 1 min Frequency: 30 min, 2×/week |
EXC: submaximal test (VO2max) Anxiety and depression: 21-Item Beck Anxiety and Depression Inventory |
BDI-II anxiety (points) after treatment: Exercise group decreased from 9.8 ± 8.1 to 4.6 ± 5.8. Control group decreased from 10.6 ± 7.2 to 6 ± 8.7. BDI-II depression (points) after treatment: Exercise group decreased from 13.3 ± 7.3 to 9.6 ± 9. Control group decreased from 14.3 ± 10 to 9.4 ± 10.8 |
| Chien et al., 2011 | 51 subjects Mean age: 58 ± 16 Sex: female (22 %) Diagnosis: chronic heart failure |
PA (24) Control, sedentary (27) |
2 months | PA sessions: home-based walking activity, moderate intensity Frequency: 30 min, 3×/week |
EXC: 6-MWT Anxiety and depression: Hospital Anxiety and Depression scale |
HADS anxiety (points) after treatment: Exercise group decreased from 2.9 ± 3 to 2.2 ± 2.4. Control group decreased from 3.2 ± 3.8 to 1.7 ± 1.7. HADS depression (points) after treatment: Exercise group decreased from 3.7 ± 3.1 to 2.9 ± 3.3. Control group decreased from 3.9 ± 3.1 to 2.5 ± 2.8 |
| Armstrong and Edwards, 2004 | 19 subjects Sex: female (100 %) Diagnosis: post-partum depression |
PA (9) Social Support (10) |
3 months | PA sessions: pram-walking 60–75 % HRmax Frequency: 60 min, 2×/week for 3 months |
EXC: submaximal test (VO2max) Depression: EPDS |
EDPS score after treatment: Exercise group decreased significantly from 17.25 ± 4 to 6.33 ± 3.67 (p < 0.05). Social support group decreased from 17.17 ± 4.45 to 13.33 ± 7.66 (p > 0.5) |
| Gondoh et al., 2009 | 30 subjects Mean age: 21 ± 2.4 Sex: female (63 %) Diagnosis: healthy subjects |
PA (15) Control, sedentary (15) |
5 months | PA sessions: aerobic dance Frequency: 60 min, 2×/week |
EXC: submaximal test (VO2max) Depression: CES-D |
CES-D score after treatment: Training group decreased significantly from 15.5 ± 8.9 to 10.3 ± 6.9 (p = 0.012). Control group from 15.9 ± 11.3 to 14.3 ± 9.2 |
| Telenius et al., 2015 | 170 subjects Mean age: 87 ± 7 Sex: female (74 %) Diagnosis: dementia |
PA (87) Control, recreational activities (83) |
3 months | PA sessions: Strengthening exercise 12 RM + balance exercise Frequency: 60 min, 2×/week |
EXC: 30-s CST Depression: Cornell Scale for depression and dementia |
CSDD anxiety (points) after treatment: Exercise group from 4.9 ± 4.8 to 4.8 ± 3.80 . Control group from 4.9 ± 4.2 to 5.1 ± 4. |
List of abbreviations: BDI-II = Beck Depression Inventory; BSI = Brief Symptom Inventory; CBT = cognitive behavioral therapy; CES-D = center for epidemiologic studies depression scale; CSDD = Cornell Scala for Depression CST = chair stand test; EPDS = Edinburgh postpartum depression scale; EXC = exercise capacity; HADS = Hospital Anxiety and Depression Scale; HIIT = high intensity interval training; HR = heart rate; IATP-R = Interval Aerobic Training Programme with Active Recovery bouts; GADS = Global Anxiety and Depression Scale; PA = physical activity; RM = repetition maximum; 6-MWT = Six-minute walking test.
3.2.2. Anxiety disorder
653 subjects were examined, 360 belonging to the intervention groups (average age of 47.53 years of which 87.3 % female, 66 % obese 13 % with stress related exhaustion, 7 % with heart failure and 7 % healthy, 3 % hypertensive, 3 % dysmetabolic and 1 % with musculoskeletal diseases) and 293 belonging to the control group (average age 47 yrs. of which 87.7 % female, 60 % obese, 14 % with stress-related exhaustion, 9 % with heart failure, 7 % healthy, 5 % hypertensive, 4 % dysmetabolic and 1 % with skeletal muscle pathologies). Trial and population characteristics related to anxiety disorder are shown in Table 1.
3.3. Intervention characteristics
Of the eight RCTs included, one based the intervention on aerobic dance program (Gondoh et al., 2009), one on aerobic training protocol (Imayama et al., 2011), one on strength exercise program combined with balance exercises (Telenius et al., 2015), one on indoor aerobic cycling training (Malmberg Gavelin et al., 2018), two on High Intensity Interval Training (HIIT) protocol on the bike ergometer (Lucibello et al., 2020; Bouaziz et al., 2019), one proposed a home-based walking program (Chien et al., 2011), and one a pram-walking program (Armstrong and Edwards, 2004).
3.4. Control characteristics
In the eight RCTs included the control groups were enrolled in cognitive-behavioral therapy (Malmberg Gavelin et al., 2018), five studies required to maintain their habits after making sure that it was an inactive population (Gondoh et al., 2009; Imayama et al., 2011; Lucibello et al., 2020; Chien et al., 2011; Bouaziz et al., 2019), in one study the control group performed recreational activity without physical effort (Telenius et al., 2015), and in one study they were enrolled in a social support group (Armstrong and Edwards, 2004).
3.5. Outcome measures
The evaluation of symptoms of anxiety was based in one study on the 21-item Beck Anxiety Inventor (Lucibello et al., 2020), one on the Anxiety Brief Symptom Inventory (BSI-18) (Imayama et al., 2011), two on the Hospital Anxiety and Depression scale (Malmberg Gavelin et al., 2018; Chien et al., 2011), and one on the Global Anxiety and Depression Sub score (GADS) (Bouaziz et al., 2019).
Depressive symptoms were assessed with the following scales: one study used the Depression Brief Symptom Inventory (BSI-18) (Imayama et al., 2011), one the 21-item Beck Depression Inventory-II (Lucibello et al., 2020), one the Cornell Scale for depression and dementia (Telenius et al., 2015), one used the Center for Epidemiologic Studies Depression scale (CES-D) (Gondoh et al., 2009), two used the Hospital Anxiety and Depression scale (Malmberg Gavelin et al., 2018; Chien et al., 2011), one used the Edinburgh Postpartum Depression Scale EPDS (Armstrong and Edwards, 2004), and one used the Global Anxiety and Depression Sub score (GADS) (Bouaziz et al., 2019).
Exercise capacity was evaluated with submaximal tests for the indirect estimation of the maximum consumption of oxygen (VO2 max) (Gondoh et al., 2009; Malmberg Gavelin et al., 2018; Armstrong and Edwards, 2004; Bouaziz et al., 2019), by a maximal treadmill cap-test for the direct evaluation of maximum oxygen consumption (Imayama et al., 2011), by the 30-s chair test (Telenius et al., 2015), or by the 6-Minute Walking Test (6-MWT) (Lucibello et al., 2020; Chien et al., 2011).
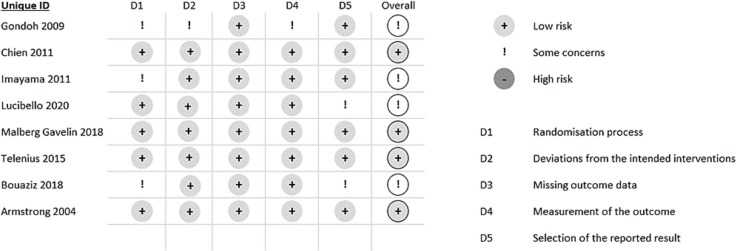
3.6. Risk of bias in individual studies
Four RCTs reported an overall low risk of bias (Gondoh et al., 2009; Malmberg Gavelin et al., 2018; Lucibello et al., 2020; Armstrong and Edwards, 2004), while the other four reported some criticalities in the overall judge (Imayama et al., 2011; Telenius et al., 2015; Chien et al., 2011; Bouaziz et al., 2019). Three studies (Gondoh et al., 2009; Imayama et al., 2011; Armstrong and Edwards, 2004) reported criticality in the D1 domain regarding the randomization process as the methods of allocation have not been clearly explained. One RCT (Gondoh et al., 2009) presented criticality in the D2 domain regarding deviation from intervention intentions as there was no information on the blinding of the operator who deliver the intervention. One study (Gondoh et al., 2009) presented criticisms in the D4 domain concerning the detection of outcome measures as it was not possible to determine whether the operators involved in this task were blind. Two studies (Lucibello et al., 2020; Bouaziz et al., 2019) have reported criticalities in the domain D5 regarding the selection of the reported results as it was not possible to establish whether the operator who carried out the statistical analysis was aware of the data relating to participation in the intervention or control group. These analyses are reported in Fig. 2 .
Fig. 2.
Assessment of risk of bias. Adapted from Sterne et al. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2).
3.7. Analyses of the overall effect
3.7.1. Depression
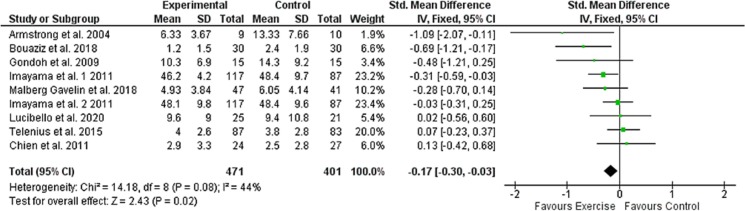
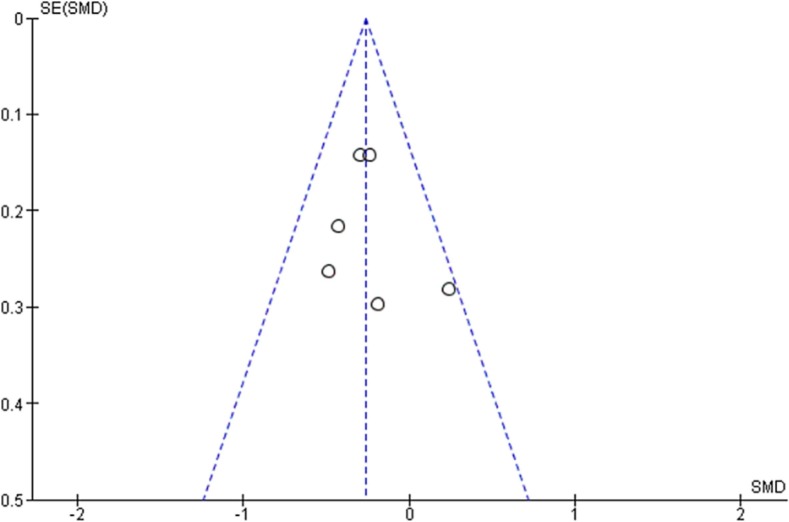
Meta-analyses revealed a small but statistically significant effect of exercise on the severity of depressive symptoms compared to the control group activity (SMD = −0.169; 95 % CI −0.302; a −0.003; p = 0.013; Fig. 3 ). The analysis also reveals no significant heterogeneity between the studies considered (I2 = 46.06 %; p = 0.0625). Considering the substantial homogeneity of the studies included in this systematic review, a fixed-effect model for the assessment of the total effect of the intervention was used. The funnel diagram (Fig. 4 ) revealed no risk of publication bias and further confirmation was given by Egger's (Intercept = −1.69; 95 % CI −4.47 to 1.07; p = 0.19) and Begg's tests (Kendall's Tau = −0.33; p = 0.21). Based on Cohen's categories, the overall effect size is considered small. Three RCTs (Imayama et al., 2011; Chien et al., 2011; Armstrong and Edwards, 2004) showed a significant improvement of the intervention group compared to the control group, two RCTs (Gondoh et al., 2009; Malmberg Gavelin et al., 2018) showed an improvement of the intervention group compared to control although not statistically significant. Three RCTs (Imayama et al., 2011; Telenius et al., 2015; Lucibello et al., 2020) showed no differences in depressive symptoms between the intervention group and the control group, while an RCT (Chien et al., 2011) showed an improvement in the control group compared to the intervention, even if not statistically significant. Of the three RCTs that showed a significant superiority of the intervention over the control group, two proposed a protocol based on aerobic exercise (Imayama et al., 2011; Armstrong and Edwards, 2004) and one proposed a protocol of HIIT (Bouaziz et al., 2019).
Fig. 3.
Effects of exercise versus control conditions on depressive symptoms, showing Standardized mean difference estimates of effect size with 95 % CIs and relative weight (% weight) for each trial.
Fig. 6.
Funnel Plot of effect of physical exercise on depression.
3.7.2. Anxiety
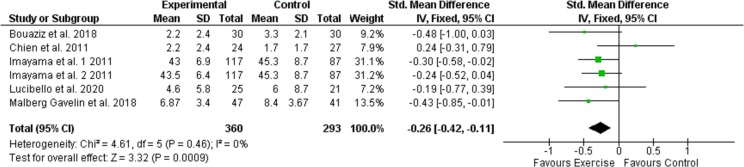
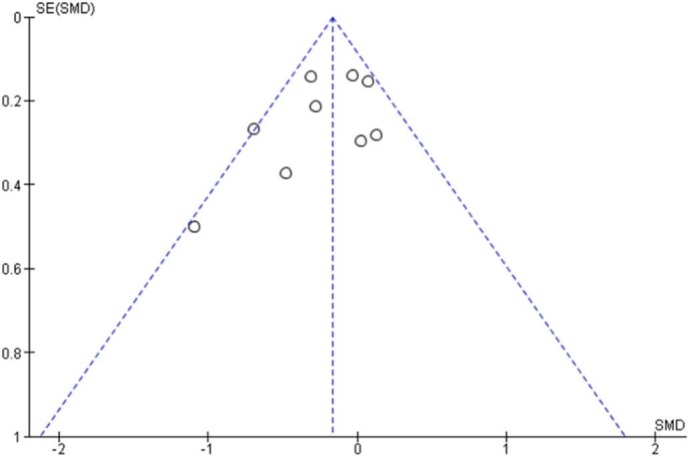
Also, for anxiety symptoms, the meta-analyses revealed small but significative evidence of the effectiveness of exercise interventions compared to the control group results (SMD = −0.263, 95 % CI −0.418 a −0.109; p = 0.001; Fig. 5 ). Considering the substantial homogeneity of the studies under consideration, a fixed-effect model for the assessment of the total effect of the intervention was applied. The funnel diagram (Fig. 6 ) revealed no risk of publication bias, further confirmation was given by Egger's (Intercept = 0.69; 95 % CI −3.34 to 4.74; P = 0.65) and Begg's tests (Kendall's Tau = 0.067; p = 0.85) Based on Cohen's categories, the overall effect size was considered small. Two RCTs (Imayama et al., 2011; Malmberg Gavelin et al., 2018) showed a significant improvement in the intervention group compared to controls. Three RCTs (Imayama et al., 2011; Lucibello et al., 2020; Bouaziz et al., 2019) showed an improvement of the intervention group compared to control although there is no statistical significance while an RCT (Chien et al., 2011) showed a positive effect of the control group compared to exercise even in the absence of significance statistic. The improvement of the outcome relative to the symptom of anxiety in study who based intervention on physical exercise of aerobic type is significative already with a frequency of three weekly sessions maintained for a period of three months. Modest evidence regards the use of HIIT protocols. Two studies (Lucibello et al., 2020; Bouaziz et al., 2019) showed a greater improvement of anxiety symptoms in the intervention group compared to control while not reaching statistical significance.
Fig. 4.
Effects of exercise versus control conditions on anxiety symptoms, showing Standardized mean difference estimates of effect size with 95 % CIs and relative weight (% weight) for each trial.
Fig. 5.
Funnel Plot of effect of physical exercise on anxiety.
4. Discussion
This systematic review evaluated and synthesized the available evidence on exercise for depression and anxiety in different population of adult and elderly subjects. Exercise was more effective than control interventions to reduce the severity of anxiety and depression, thus may be a useful strategy for patients with Post-Covid-19 condition. Exercise is defined as type of physical activity that is planned and structured towards improving physical fitness. The potential mechanisms involved in the development of the neuropsychiatric manifestations of COVID-19 seems to be mainly related to the systemic immune-inflammatory response and to the psychological stressors induced by SARS-CoV-2 infection (Troyer et al., 2020).
In the same way, systemic inflammation has been linked to multiple chronic conditions included in the present study (e.g. obesity, heart diseases and cognitive impairment) (Pawelec et al., 2014).
The practice of physical exercises acts as a modulator of the immune system, during and after physical exercise, pro- and anti-inflammatory cytokines are released, lymphocyte circulation increases, as well as cell recruitment (Pawelec et al., 2014).
The literature suggested that exercise can lead numerous interdependent changes in the brain to produce an environment that is protective against depression (da Silveira et al., 2021).
The analysis of the eight studies included in this revision suggests that people with primary symptoms of anxiety and depression or secondary to other chronic diseases, enrolled in a supervised exercise program significantly improves their mental health conditions compared to intervention based on traditional treatment or absence of exercise.
The largest effect size for depressive symptoms was observed in Armstrong et al.'s study in which was examined the effect of exercise on women affected by post-partum depression (Armstrong and Edwards, 2004).
This suggests that the effect of exercise is greater in the control and reduction of depression symptoms especially if administered to a population with a diagnosis of depressive disorder. The improvement of the outcome relative to the depressive symptom in who has done physical exercise of aerobic type is significative even with a frequency of two weekly sessions maintained for a period of three months. Promising results were observed in one study who have proposed an aerobic dance program (Gondoh et al., 2009). This study does not reach the statistical significance because of the amplitude of the standard deviation but presents a moderate effect size favorable to the intervention (SMD = −0,479). This suggests that activities with high psychosocial involvement such as aerobic dance, although not improving the functional parameter probably due to poor customization of physical exercise, can instead improve depressive symptoms.
R anxiety symptoms, the only RCT that did not show an improvement in anxiety symptoms in the intervention group compared to control was the study in which a home- based walking protocol was used (Chien et al., 2011). This study showed a significant improvement in functional outcome (6MWT) but did not improve anxiety symptoms. This suggests that supervised exercise, frequently associated with more opportunities of social interactions, is more effective in the improvement of anxiety symptoms.
4.1. Agreement with previous systematic reviews
The results obtained in the present review are in agreement with previous reviews that examined the association between depressive symptoms and exercise used as the main intervention (Morres et al., 2019; Rethorst et al., 2009). Similar results have been observed also regarding the anxiety disorder where previous reviews have demonstrated the efficacy intervention exercise based compared to control groups in the treatment of anxiety disorder (Stubbs et al., 2017).
4.2. Limitations of this study and future implications for research
The present meta-analysis considered a small number of studies due to the exclusion criteria applied. However, specific tests were carried out (described in the subject and methods chapter) to exclude publication bias. Despite no statistical heterogeneity has been detected in any of the two metanalysis, another limit of this review can be identified in the heterogeneity of the study population and type of interventions. Although the purpose of our study is to demonstrate that the most frequent psychiatric symptoms secondary to various chronic diseases, including Covid-19 infection, improve following adapted exercise programs, we did not include specific studies on post-Covid-19 condition patients because at present there is not any randomized control trial on this topic related to exercise. It is therefore desirable that the increasing cases of patients with psychopathological symptoms associated with post-Covid-19 condition increases the number of RCTs considering the association between physical exercise and mental health making the type of intervention more homogeneous.
4.3. Implications for clinical practice
The benefits of regular physical exercise on different aspects of human health are widely known (Mandini et al., 2018a; Mandini et al., 2018b). More recently evidence suggests that exercise-based programs have also positive effects in the control of anxiety and depression in both healthy and due to chronic disease, including obesity, stress related exhaustion, chronic heart failure, dementia, diabetes, and hypertension. The positive effects of exercise on psychopathological symptoms associated with others chronic pathologies, allow us to suppose that the enrolment of post-Covid-19 condition patients in exercise programs, supervised by exercise physiologists, may be useful in the maintenance or improvement of their mental health.
Various guidelines have included recommendations for treating and management of post-Covid-19 condition in however, these undoubtedly have to evolve as new evidence comes to light (WHO Coronavirus (COVID-19) Dashboard, n.d.; Overview | COVID-19 Rapid Guideline: Managing the Long-term Effects of COVID-19 | Guidance | NICE, n.d.; CDC to Release Long COVID Guidance. US News & World Report, n.d.; Information on COVID-19 Treatment, Prevention and Research. COVID-19 Treatment Guidelines, n.d.; ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic, n.d.).
Greater understanding of the pathogenesis, risk factors, symptoms, and methods of treating post-Covid-19 condition is required to reduce the strain and demand on people with the condition and the healthcare systems that will endeavor to support them. It is desirable that in future studies examine the adherence and effect of exercise program in these patients, in order to develop guidelines for exercise appropriate to the treatment and control of psychopathological symptoms related to Covid-19 infections.
Funding
The study was conducted by the University of Ferrara, Italy.
CRediT authorship contribution statement
AR, TP and SM had access to all of the data and take full responsibility for the integrity of the data and the accuracy of the data analysis. AR and SM performed statistical analyses. TP and SM drafted the manuscript. SM, VZ, AR, TP contributed with acquisition of the data. GG and GM contributed with conceptual design. VZ contributed with analyses and interpretation of the results. All authors contributed in drafting the article or reviewing it for significant intellectual content. All authors gave final approval for the version to be submitted.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgments
None.
References
- A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus, 2021. 2021. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (6 October) [DOI] [PMC free article] [PubMed]
- Armstrong K., Edwards H. The effectiveness of a pram-walking exercise programme in reducing depressive symptomatology for postnatal women. Int. J. Nurs. Pract. 2004;10(4):177–194. doi: 10.1111/j.1440-172X.2004.00478.x. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bouaziz W., Schmitt E., Vogel T., et al. Effects of a short-term interval aerobic training programme with active recovery bouts (IATP-R) on cognitive and mental health, functional performance and quality of life: a randomised controlled trial in sedentary seniors. Int. J. Clin. Pract. 2019;73(1) doi: 10.1111/ijcp.13219. [DOI] [PubMed] [Google Scholar]
- CDC . Centers for Disease Control and Prevention; 2022. Post-COVID Conditions.https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html Published July 11. [Google Scholar]
- CDC to Release Long COVID Guidance. US News & World Report. www.usnews.com/news/health-news/articles/2021-05-07/cdc-to-release-clinical-guidance-on-identifying-managing-long-covid
- Chamberlain S.R., Grant J.E., Trender W., Hellyer P., Hampshire A. Post-traumatic stress disorder symptoms in COVID-19 survivors: online population survey. BJPsych Open. 2021;7(2) doi: 10.1192/bjo.2021.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien C.L., Lee C.M., Wu Y.W., Wu Y.T. Home-based exercise improves the quality of life and physical function but not the psychological status of people with chronic heart failure: a randomised trial. J. Physiother. 2011;57(3):157–163. doi: 10.1016/S1836-9553(11)70036-4. [DOI] [PubMed] [Google Scholar]
- Cohen J. Academic Press; 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- Crone D., Heaney L., Herbert R., Morgan J., Johnston L., Macpherson R. A comparison of lifestyle behaviour and health perceptions of people with severe mental illness and the general population. J. Public Ment. Health. 2004;3:19–25. doi: 10.1108/17465729200400025. [DOI] [Google Scholar]
- da Silveira M.P., da Silva Fagundes K.K., Bizuti M.R., Starck É., Rossi R.C., de Resende e Silva D.T. Physical exercise as a tool to help the immune system against COVID-19: an integrative review of the current literature. Clin Exp Med. 2021;21(1):15–28. doi: 10.1007/s10238-020-00650-3. Published July 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E. Maxwell Living With COVID19: A Dynamic Review of the Evidence Around Ongoing COVID19 Symptoms (Often Called Long COVID). n.d.
- ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance
- Firth J., Siddiqi N., Koyanagi A., et al. The lancet psychiatry commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry. 2019;6(8):675–712. doi: 10.1016/S2215-0366(19)30132-4. [DOI] [PubMed] [Google Scholar]
- Gondoh Y., Sensui H., Kinomura S., et al. Effects of aerobic exercise training on brain structure and psychological well-being in young adults. J. Sports Med. Phys. Fitness. 2009;49(2):129–135. [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges L.V., Vevea J.L. Fixed- and random-effects models in meta-analysis. Psychol. Methods. 1998;3(4):486–504. doi: 10.1037/1082-989X.3.4.486. [DOI] [Google Scholar]
- Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., et al. 6-Month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet Lond. Engl. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayama I., Alfano C.M., Kong A., et al. Dietary weight loss and exercise interventions effects on quality of life in overweight/obese postmenopausal women: a randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2011;8:118. doi: 10.1186/1479-5868-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Information on COVID-19 Treatment, Prevention and Research. COVID-19 Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/
- Lucibello K.M., Paolucci E.M., Graham J.D., Heisz J.J. A randomized control trial investigating high-intensity interval training and mental health: a novel non-responder phenotype related to anxiety in young adults. Ment. Health Phys. Act. 2020;18 doi: 10.1016/j.mhpa.2020.100327. [DOI] [Google Scholar]
- Malmberg Gavelin H., Eskilsson T., Boraxbekk C.J., Josefsson M., Stigsdotter Neely A., Slunga Järvholm L. Rehabilitation for improved cognition in patients with stress-related exhaustion disorder: RECO - a randomized clinical trial. Stress. 2018;21(4):279–291. doi: 10.1080/10253890.2018.1461833. [DOI] [PubMed] [Google Scholar]
- Mandini S., Conconi F., Mori E., Myers J., Grazzi G., Mazzoni G. Walking and hypertension: greater reductions in subjects with higher baseline systolic blood pressure following six months of guided walking. PeerJ. 2018;6 doi: 10.7717/peerj.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandini S., Collini G., Grazzi G., Lavezzi E., Mazzoni G., Conconi F. Reduction in risk factors for cardiovascular diseases and long-lasting walking habit in sedentary male and female subjects following 1 year of guided walking. Sport Sci. Health. 2018;14(1):121–126. doi: 10.1007/s11332-017-0412-3. [DOI] [Google Scholar]
- Mazza M.G., De Lorenzo R., Conte C., et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morres I.D., Hatzigeorgiadis A., Stathi A., et al. Aerobic exercise for adult patients with major depressive disorder in mental health services: a systematic review and meta-analysis. Depress Anxiety. 2019;36(1):39–53. doi: 10.1002/da.22842. [DOI] [PubMed] [Google Scholar]
- Overview | COVID-19 Rapid Guideline: Managing the Long-term Effects of COVID-19 | Guidance | NICE. https://www.nice.org.uk/guidance/ng188 [PubMed]
- Pawelec G., Goldeck D., Derhovanessian E. Inflammation, ageing and chronic disease. Curr. Opin. Immunol. 2014;29:23–28. doi: 10.1016/j.coi.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Rethorst C.D., Wipfli B.M., Landers D.M. The antidepressive effects of exercise: a meta-analysis of randomized trials. Sports Med. 2009;39(6):491–511. doi: 10.2165/00007256-200939060-00004. [DOI] [PubMed] [Google Scholar]
- Richardson C.R., Faulkner G., McDevitt J., Skrinar G.S., Hutchinson D.S., Piette J.D. Integrating physical activity into mental health services for persons with serious mental illness. Psychiatr. Serv. 2005;56(3):324–331. doi: 10.1176/appi.ps.56.3.324. [DOI] [PubMed] [Google Scholar]
- Schuch F.B., Vancampfort D., Richards J., Rosenbaum S., Ward P.B., Stubbs B. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016;77:42–51. doi: 10.1016/j.jpsychires.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Stubbs B., Vancampfort D., Rosenbaum S., et al. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: a meta-analysis. Psychiatry Res. 2017;249:102–108. doi: 10.1016/j.psychres.2016.12.020. [DOI] [PubMed] [Google Scholar]
- Stubbs B., Vancampfort D., Hallgren M., et al. EPA guidance on physical activity as a treatment for severe mental illness: a meta-review of the evidence and position statement from the european psychiatric association (EPA), supported by the International Organization of Physical Therapists in mental health (IOPTMH) Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2018;54:124–144. doi: 10.1016/j.eurpsy.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8(2):130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenius E.W., Engedal K., Bergland A. Long-term effects of a 12 weeks high-intensity functional exercise program on physical function and mental health in nursing home residents with dementia: a single blinded randomized controlled trial. BMC Geriatr. 2015;15:158. doi: 10.1186/s12877-015-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The economic cost of serious mental illness and comorbidities in Australia and New Zealand . VU Research Repository. Victoria University; Melbourne Australia: 2016. The economic cost of serious mental illness and comorbidities in Australia and New Zealand.https://www.ranzcp.org/Files/Publications/RANZCP-Serious-Mental-Illness.aspx [Google Scholar]
- Tomasoni D., Bai F., Castoldi R., et al. Anxiety and depression symptoms after virological clearance of COVID-19: a cross-sectional study in MilanItaly. J Med Virol. 2021;93(2):1175–1179. doi: 10.1002/jmv.26459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova G., Pallanti S., Hollander E. Presentation and management of anxiety in individuals with acute symptomatic or asymptomatic COVID-19 infection, and in the post-COVID-19 recovery phase. Int. J. Psychiatry Clin. Pract. 2021;25(2):115–131. doi: 10.1080/13651501.2021.1887264. [DOI] [PubMed] [Google Scholar]
- Vancampfort D., Stubbs B., Mitchell A.J., et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry Off J World Psychiatr Assoc WPA. 2015;14(3):339–347. doi: 10.1002/wps.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int
- World Health Organization . Global Health Estimates (No. WHO/MSD/MER/2017.2) World Health Organization; 2017. Depression and other common mental disorders. [Google Scholar]
- Zhou X., Liao S., Qi L., Wang R. Physical activity and its association with cognitive function in middle- and older-aged Chinese: Evidence from China Health and Retirement Longitudinal Study, 2015. Eur J Sport Sci. 2021:1–11. doi: 10.1080/17461391.2021.1897164. Published online March 22. [DOI] [PubMed] [Google Scholar]