1. Introduction
Nicotinic acetylcholine receptors (nAChRs) are pentameric ligand-gated ion channels involved in the signal transduction of nicotinic-based signals throughout the peripheral and central nervous system (CNS). They may come in heteromeric form encompassing various combinations of α- and β-subunits or homomeric form expressing only α-subunits, with the most prevalent nAChR subtypes being the heteromeric α4β2* and homomeric α7 receptors. The most abundant nAChR in CNS is α4β2* [the * denotes that these nAChRs can contain other α and β subunits as well, reviewed in (Gotti et al., 2006)]. Based on previous preclinical studies, various α4β2* nAChR agonists have antinociceptive effects for a vast range of pain rodent models from acute to chronic and even in inflammatory states (Bannon et al., 1998; Boyce et al., 2000; Damaj et al., 1998, 1999; Decker et al., 2004; Kesingland et al., 2000; Lawand et al., 1999; Lynch et al., 2005; Nirogi et al., 2013; Rowbotham et al., 2009; Zhang et al., 2012). However, agonists alone are limited in their use. This is due to their relatively low functional selectivity amongst the different nAChR subtypes, rapid desensitization, and numerous side effects (Nirogi et al., 2013; Zhang et al., 2012).
Nicotine is an alkaloid acting at multiple nAChRs subtypes including α4β2* nAChRs. Significant pain relief induced by nicotine has been observed in both human and rodents, especially through the α4β2* nAChR subtype (Ditre et al., 2016; Jin et al., 2014; Zhu et al., 2011). This is supported in knockout mice studies where mice who lack either α4 or β2 genes have reduced nicotinic antinociceptive properties (Marubio et al., 1999). However, the short half-life and side effects of nicotine limit its usability as a treatment for pain (Nirogi et al., 2013). On the other hand, nicotine-induced antinociception still provides enormous insight into fundamental mechanisms on α4β2* nAChRs in pain and further understanding into the development of therapeutic treatments for pain.
An alternative way to take advantage of the α4β2* nAChR’s role in analgesia is targeting an allosteric site with allosteric ligands that increase the response of the native agonist without activating the receptor themselves. This alternative is known as positive allosteric modulators (PAMs), and these compounds have already been shown to have the potential to improve cognitive function while also decreasing pain by enhancing agonist responses at lower or non-effective doses of nicotine or agonist concentrations. These compounds also potentially reduce the side-effects of the orthosteric ligand (Lee et al., 2011; Moerke et al., 2016; Mohler et al., 2014; Nirogi et al., 2013; Pandya and Yakel, 2011; Rode et al., 2012). Combining an α4β2* nAChR PAM with a full orthosteric agonist may therefore provide a viable strategy in therapeutic drug development for pain treatment.
Desformylflustrabromine (dFBr) is a nAChR PAM that was shown to selectively act as an α4β2* nAChR PAM (Weltzin and Schulte, 2015, 2010). We therefore investigated the effects of dFBr on nicotine’s antinociceptive properties using an experimental neuropathic model of pain in mice.
2. Methods
2. 1. Animals
Male adult (8–10 weeks of age) ICR mice obtained from Harlan Laboratories (Indianapolis, IN) were used throughout the study. Mice were housed in a 21°C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care–approved animal care facility. They were housed in groups of four and had free access to food and water. The rooms were on a 12-hour light/dark cycle. All experiments were performed during the light cycle, and the study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. All studies were carried out in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. Animals were sacrificed via CO2 following by cervical dislocation after the experiments finished, unless noted otherwise. Any subjects that subsequently showed behavioral disturbances unrelated to the pain induction procedure were excluded from further behavioral testing.
2.2. Drugs
(−)-Nicotine hydrogen tartrate [(−)-1-methyl-2-(3- pyridyl) pyrrolidine (+)-bitartrate] was purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). dFBr was obtained from Tocris Biosciences (Minneapolis, MN). Dihydro-β-erythroidine hydrobromide (DHβE) and morphine was provided as part of the drug supply program from the National Institute for Drug Abuse (Rockville, MD). All drugs were dissolved in physiologic saline (0.9% sodium chloride) and freshly prepared solutions injected subcutaneous (s.c.) at a total volume of 1 ml/100 g body weight, unless noted otherwise. All doses are expressed as the free base of the drug. The range of doses was selected similar based on previous studies with dFBr in mice (Liu, 2013; Mitra et al., 2017; Moerke et al., 2016).
2.3. Chronic constrictive nerve injury (CCI)-induced neuropathic pain model
Animals were anesthetized using 4% isoflurane and maintained with 2% isoflurane in oxygen using a face mask and a vaporizer (VetEquip Inc, Pleasanton, CA). An incision was made just below the hip bone, parallel to the sciatic nerve. The left common sciatic nerve was exposed at the level proximal to the sciatic trifurcation and a nerve segment 3–5 mm long was separated from surrounding connective tissue. Two loose ligatures with 5–0 silk suture were made around the nerve with a 1.0–1.5 mm interval between each of them. Muscles were closed with suture thread and the wound with wound clips. The CCI procedure results in ipsilateral allodynic responses; neuropathic pain behavior continues for a minimum of 2 months (Bagdas et al., 2015a). For group of sham procedure, same operation protocol used without ligating of sciatic nerve. All animals were randomly assigned to CCI or sham surgeries. Drugs were injected between 14–28 days after CCI surgery and the animals tested for changes in mechanical thresholds before any drug testing. Animal placement into drug administration was randomized and a minimum 72 h washout period was imposed between each test day with a within subject design. Each mouse was used max for three times.
2.4. Evaluation of mechanical allodynia
Mechanical thresholds were determined according to the method of Chaplan et al. (Chaplan et al., 1994) and as modified in Bagdas et al (2015). A series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) with logarithmically incremental stiffness ranging from 2.83 to 5.07 expressed as dsLog 10 of [10 £ force in (mg)] were applied to the paw with a modified up-down method (Dixon, 1965). The mechanical threshold was expressed as gram, indicating the force of the Von Frey hair to which the animal reacted (paw withdrawn, licking, or shaking). All behavioral testing on animals was performed in a blinded manner.
In the first set of experiments, mice received various doses of nicotine (0.1, 0.5, 1 and 1.5 mg/kg) or saline and mechanical paw withdrawal thresholds were determined at 5, 10, 15 and 20 min after injection. In the second set of experiments, mice were pretreated with either saline or different doses of dFBr (1, 3, 6 and 9 mg/kg) 15 min before nicotine (0.5 mg/kg) or its vehicle in the test. In addition, different nicotine doses (0.1, 0.25 and 0.5 mg/kg) were also tested with a dose of 9 mg/kg dFBr in combination studies. In a third set of experiments, mice were tested in combination of DHβE (2 mg/kg), dFBr (9mg/kg) and nicotine (0.5 mg/kg). Pretreatment times were chosen based on previous studies (Bagdas et al., 2015a; Walters et al., 2006) and preliminary results from a pilot study with dFBr (Table 1). Drugs are injected 15 min interval and mechanical paw withdrawal thresholds were examined at 5, 10, 15 and 30 min after nicotine or saline injection. Additionally, mice were injected various doses of morphine (0.32, 1, 3.2 and 6.4 mg/kg) or saline as a fourth set of experiments. The effect of dFBr (9 mg/kg) on morphine (1 mg/kg)-evoked antiallodynia was also tested. For this part of study, animals were tested in von Frey test at 15, 30, 60 and 120 min after last injection.
Table 1.
Effects of desformylflustrabromine on mechanical thresholds in CCI-performed mice.
| After injection (min) | |||||||
|---|---|---|---|---|---|---|---|
| Dose of dFBr | Baseline | 0 | 15 | 30 | 60 | 120 | 240 |
| 0 mg/kg (saline) | 2.306 ± 0.265 | 0.055 ± 0.08 | 0.097 ± 0.62 | 0.105 ± 0.061 | 0.056 ± 0.023 | 0.056 ± 0.023 | - |
| 1 mg/kg | 2.836 ± 0.355 | 0.047 ± 0.013 | 0.034 ± 0.012 | 0.072 ± 0.040 | 0.040 ± 0.013 | 0.146 ± 0.058 | - |
| 6 mg/kg | 2.477 ± 0.375 | 0.023 ± 0.040 | 0.175 ± 0.105 | 0.067 ± 0.023 | 0.030 ± 0.08 | 0.050 ± 0.016 | - |
| 9 mg/kg | 2.567 ± 0.318 | 0.159 ± 0.039 | 0.256 ± 0.044 | 0.144 ± 0.011 | 0.041 ± 0.008 | 0.148 ± 0.080 | 0.161 ± 0.021 |
A range of doses of desformylflustrabromine (dFBr), an α4β2* nAChR PAM, on nicotine treatment of CCI mice were tested using von Frey test. For this reason, dFBr (1, 6, and 9 mg/kg) or vehicle (0.9% saline) was injected subcutaneously. Mechanical thresholds were determined 15, 30, 60, 120 and 240 min after dFBr or saline injection. Data reflect mean ± SEM, n = 6–8 mice/group. BL: Baseline
2.5. Motor coordination
The effects of drugs on motor coordination were measured using the rotarod test (IITC Inc. Life Science, Woodland Hills, CA) as previously described (Freitas et al., 2013a). Percent impairment was calculated as follows: % impairment = ((180 - test time) / 180) * 100. For this series of experiments, mice were pretreated with either vehicle or dF-Br (9 mg/kg, s.c.) 15 min before nicotine (0.5, 1, 1.5 mg/kg, s.c.) or its vehicle in the test. Another set of experiments were done by using CCI-operated mice after 2 weeks of surgery. Animals received dFBr (9 mg/kg, s.c.) or saline, and 15 minutes later mice were given an injection of nicotine (1.5 mg/kg) or saline as vehicle. Mice were placed on the rotarod 5 min after last injection.
2.6. Locomotor activity
Mice were placed into individual Omnitech (Columbus, OH) photocell activity cages (28 × 16.5 cm). Interruptions of the photocell beams (two banks of eight cells each) were recorded for the next 30 min. Data were expressed as the number of photocell interruptions. In the last series of experiments, mice were pretreated with either vehicle or dF-Br (9 mg/kg, s.c.) 15 min before nicotine (0.5, 1, 1.5 mg/kg, s.c.) or its vehicle in the test.
2.7. Statistical Analysis
The data obtained were analyzed using GraphPad Prism software, version 6.0 (GraphPad Software, Inc., La Jolla, CA) and expressed as the mean ± S.E.M. Statistical analysis was done using the 2-way repeated measure analysis of variance test (ANOVA), followed by the post hoc Sidak test. The 2-way ANOVA was used to analyze the differences in motor coordination. The P values < 0.05 were considered significant. For the percentage reversal of mechanical allodynia analysis, the test thresholds of post drug administration (time point: 10 min) were compared and calculated according to the following equation: Percentage reversal effect = [1-(baseline value – value at 10 min)/baseline value] × 100. ED50 (effective dose 50%) values with 95% confidence limits (CL) were calculated by unweighted least-squares linear regression as described by Tallarida and Murray (Tallarida and Murray, 1986).
3. Results
3.1. Nicotine dose-dependently attenuates CCI-induced neuropathic pain
The antiallodynic effects of nicotine, a non-selective nAChR agonist, were studied in the CCI-induced neuropathic pain model. Nicotine dose-dependently and time-dependently reversed CCI-induced allodynia [Fdose(4,20) = 15.23, P < 0.001, Ftime (5,25) = 199.8, P < 0.001 and Fdosextime(20,100) = 4.688, P < 0.001; Fig 1A]. Nicotine also showed antinociceptive responses in sham-treated mice [Fdose(4,20) = 12.45, P < 0.001, Ftime (5,25) = 21.64, P < 0.001 and Fdosextime(20,100) = 8.405, P < 0.001; Fig 1B]. Significant antinociceptive effects of nicotine was seen by starting 5 min after injection at high doses of nicotine (1 and 1.5 mg/kg) but not low doses (0.1 and 0.5 mg/kg) in both CCI- and sham-operated mice. In addition, ED50 value (± CL), calculated based on the percentage of reversal at 10 min after nicotine administration in CCI-treated mice is 0.90 (0.63 – 1.25) mg/kg (Fig 1C).
Figure 1. Effects of nicotine administration on CCI-induced neuropathic pain.
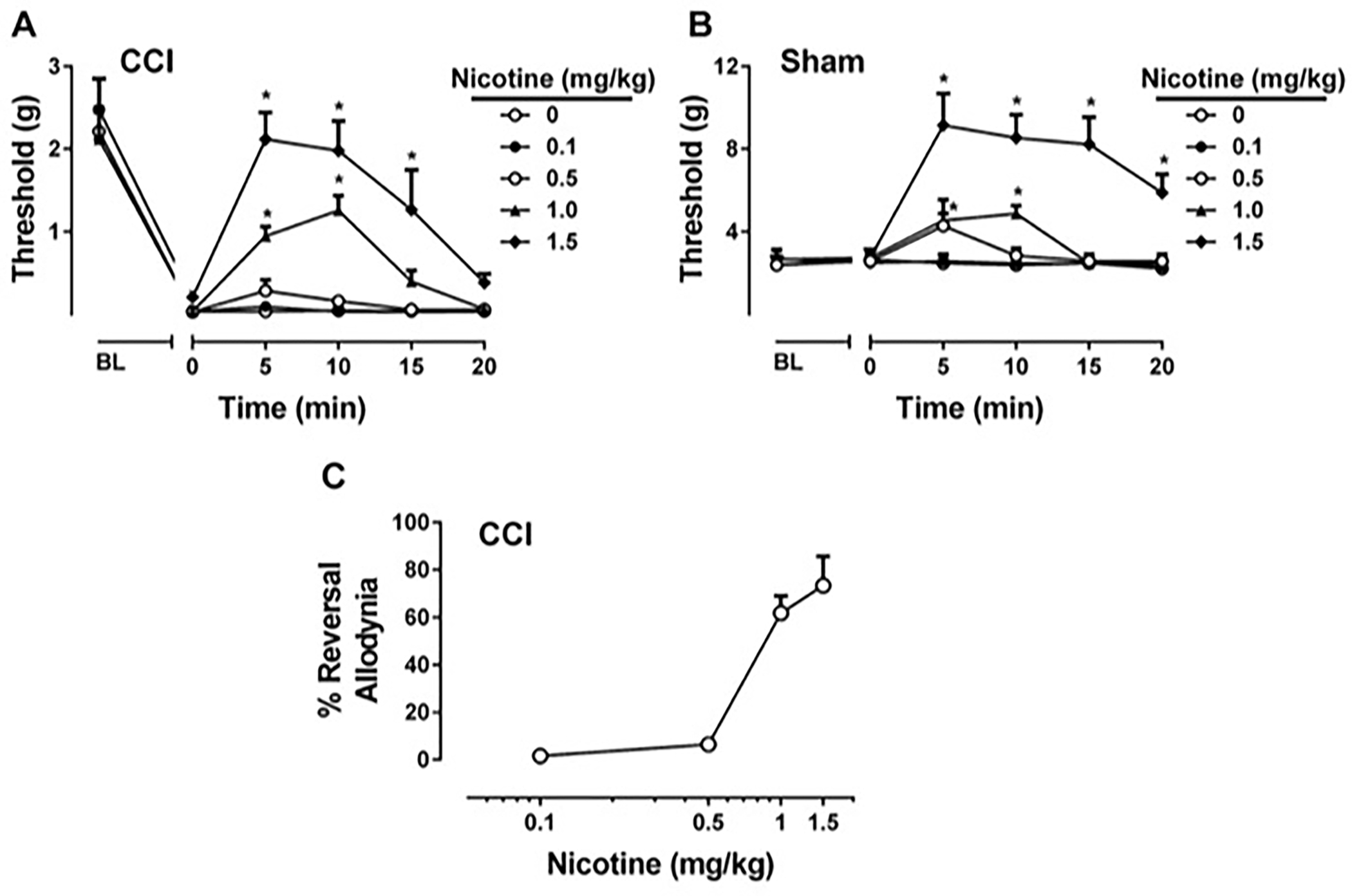
Effects of nicotine treatment of CCI mice (A), and sham mice (B) were tested using von Frey test. Nicotine treatment of CCI mice was presented as percentage of the nociceptive threshold measured before surgery, and were calculated on the 10th min of treatment (C). Nicotine (0.1, 0.5, 1, and 1.5 mg/kg) or vehicle (0.9% saline) was injected subcutaneously. * P < 0.05 vs vehicle. Data reflect mean ± SEM, n = 6 mice/group. BL: Baseline
3.2. dFBr dose-dependently potentiates nicotine-evoked antinociceptive effects
We then determined the effects of dFBr, an α4β2* nAChR PAM, on nicotine-evoked antiallodynia in CCI-induced neuropathic pain model. To test possible potentiation, different doses of dFBr (1, 3, 6, 9 mg/kg) were tested in combination with a low ineffective dose of nicotine (0.5 mg/kg). As shown in Fig. 2A, dFBr pretreatment increased nicotine-induced mechanical threshold values which indicates that dFBr potentiated the antiallodynic effects of nicotine. Two-way repeated measure RM ANOVA of the mechanical threshold values yielded a significant effect of treatment [Ftreatment(6,30) = 20.3500, P < 0.001], time [Ftime (5,25) = 159.00, P < 0.001] and interaction [Ftreatmentxtime(30,150) = 3.90, P < 0.001; Fig 2A]. As expected, nicotine failed to induce a significant effect of antiallodynia (P > 0.05) and dFBR did not alter mechanical thresholds on its own (P > 0.05). Furthermore, Sidak post hoc test revealed a significant potentiation effect by dFBr which seen at the doses 9 mg/kg (P < 0.001) and 6 mg/kg (P < 0.001) after 5 min of nicotine administration (Fig 2A). In addition, potentiation effect induced by dFBr at the highest dose (9 mg/kg) lasted 20 min. The α4β2* nAChR PAM dFBr at the highest dose in this study did not change mechanical thresholds by itself and potentiated the antinociceptive effects of nicotine in sham mice [Ftreatment(6,30) = 3.863, P < 0.01; Ftime (5,25) = 2.815, P < 0.05; and Ftreatmentxtime(20,100) = 2.78, P < 0.001; Fig 2B].
Figure 2. Effect of desformylflustrabromine on nicotine-induced antinociception.
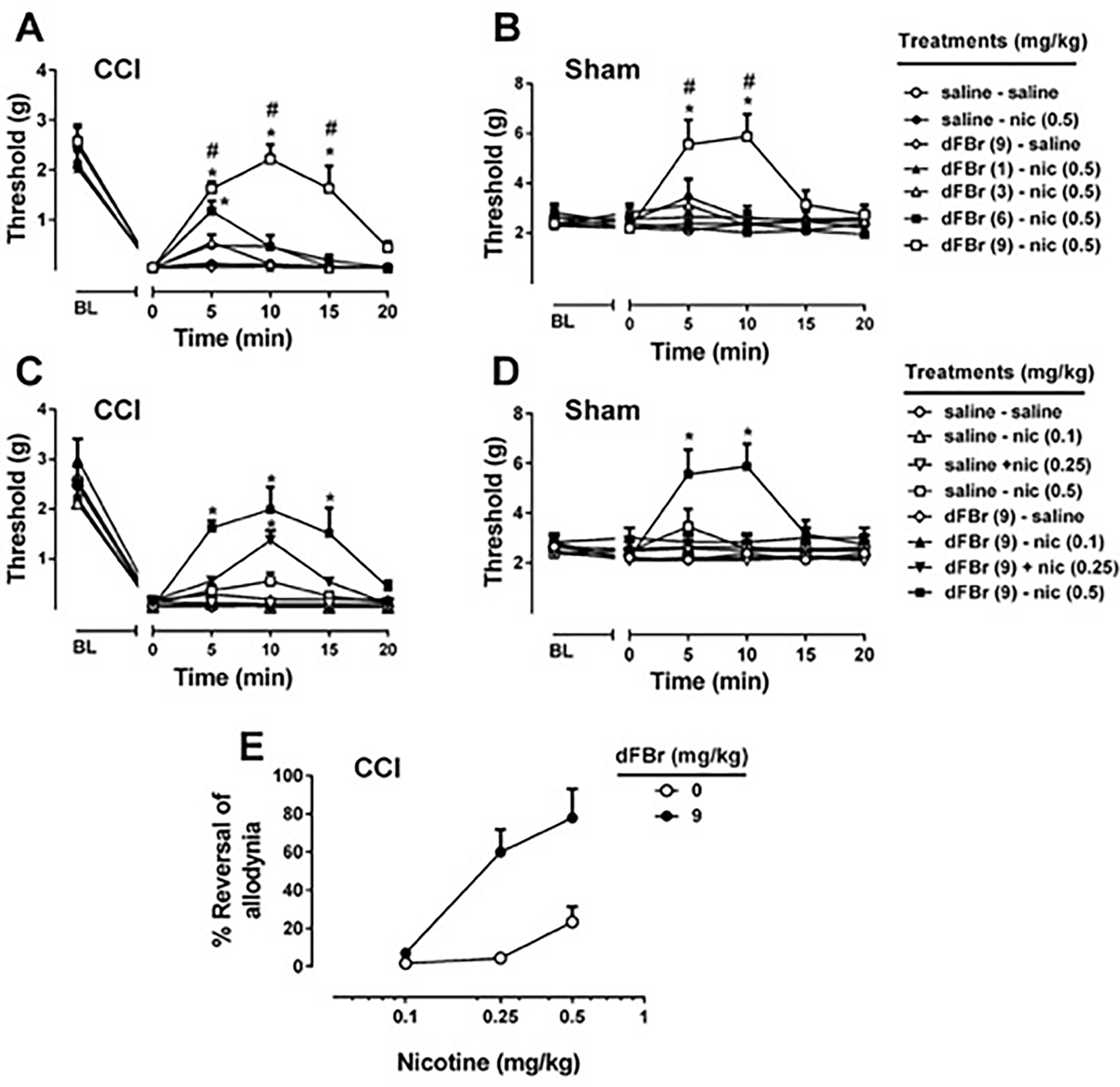
A range of doses of desformylflustrabromine (dFBr), an α4β2* nAChR PAM, on nicotine treatment of CCI mice (A), and sham mice (B) were tested using von Frey test. For this reason, dFBr (1, 3, 6, and 9 mg/kg) or vehicle (0.9% saline) was injected subcutaneously 15 min before nicotine (0.5 mg/kg) or vehicle (0.9% saline) treatment. The potentiation effect of dFBr was also investigated with different doses of nicotine in CCI (C) and sham (D) mice. The highest dose of dFBr (9 mg/kg) or vehicle (0.9% saline) was injected subcutaneously 15 min before nicotine (0.1, 0.25, and 0.5 mg/kg) or vehicle (0.9% saline) treatment. The percentage of reversal (E) was calculated at 10 min after nicotine administration in CCI-treated mice. * P < 0.05 vs saline-saline treatment. # P < 0.05 vs saline-nicotine treatment. Data reflect mean ± SEM, n = 6 mice/group. BL: Baseline
To understand the effect of dFBr on the potency of nicotine-evoked antiallodynia, mice were pretreated with dFBr (at the highest dose of this study, 9 mg/kg, pretreatment time 15 min) with a various nicotine doses (0.1, 0.25 and 0.5 mg/kg, s.c.). Two-way ANOVA revealed significant effect of treatment, time and interaction in CCI [Ftreatment(7,35) = 10.34, P < 0.001; Ftime (5,25) = 261.4, P < 0.001; and Ftreatmentxtime(35,175) = 3.919, P < 0.001; Fig 2C] and sham [Ftreatment(7,35) = 2.416, P < 0.05; Ftime (5,25) = 2.561, P = 0.0529; and Ftreatmentxtime(35,175) = 4.118, P < 0.001; Fig 2D]. Post hoc test using the Sidak correction revealed dFBr, at the dose of 9 mg/kg, was able to potentiate 0.25 and 0.5 mg/kg dose of nicotine in CCI mice but not lower dose (0.1mg/kg). ED50 value for nicotine antiallodynic effect in combination with dFBr, calculated based on the percentage of reversal at 10 min after nicotine administration in CCI-treated mice, was determined to be 0.24 (0.18 – 0.33) mg/kg (Fig 2E).
3.3. Effects of dFBr on nicotine is β2-nAChR-dependent
We next evaluated the possible role of α4β2* nAChRs in the antinociceptive effects of dFBr on nicotine responses. To test this, mice were pretreated with DHβE, a selective β2* nAChR antagonist, prior to nicotine administration. Two way repeated measure ANOVA revealed significant effects for treatment and time in both CCI [Ftreatment(4,20) = 13.00, P < 0.001; Ftime (5,25) = 64.06, P < 0.001; and Ftreatmentxtime(20,100) = 7.03, P < 0.001; Fig 3A] and sham [Ftreatment(4,20) = 2.16, P = 0.11; Ftime (5,25) = 25.43, P < 0.001; and Ftreatmentxtime(20,100) = 3.23, P < 0.001; Fig 3B] mice. As seen Fig 3A and 3B, dFBr (9 mg/kg) potentiated the effects of nicotine (0.5 mg/kg) as expected (P<0.05) and DHβE totally reversed the antinociceptive effects of combination in both CCI and sham mice (P<0.05).
Figure 3. Effects of dihydro-β-erythroidine on desformylflustrabromine-induced potentiation of antinociceptive effects of nicotine.
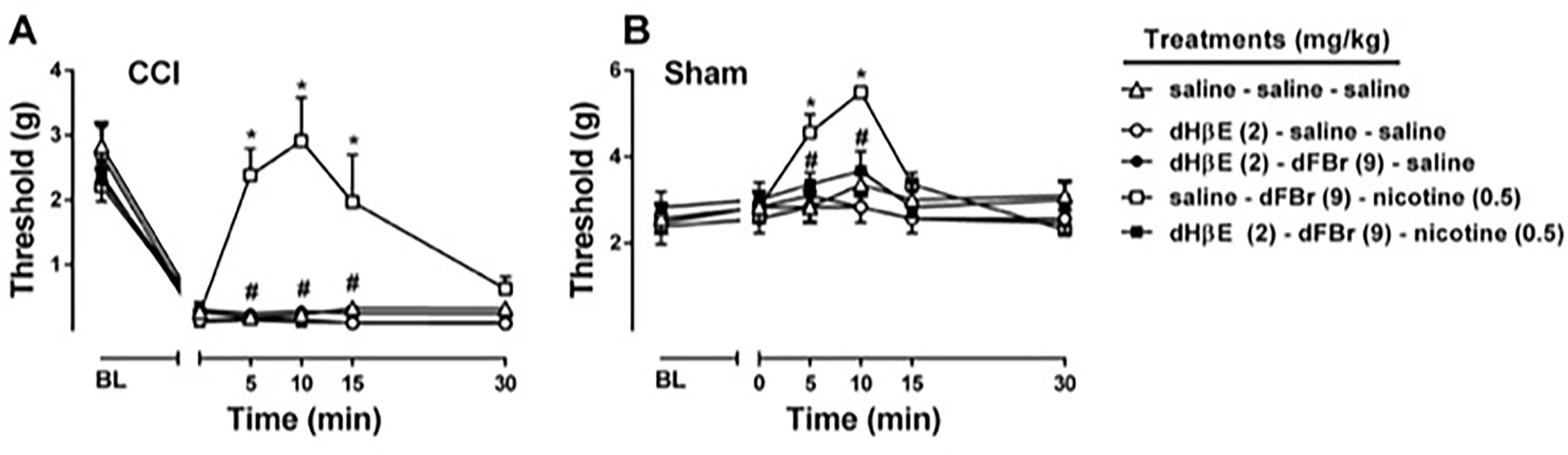
Effects of dihydro-β-erythroidine (DHβE), competitive α4β4 and α4β2* nAChR antagonist, on the combination of desformylflustrabromine (dFBr), an α4β2* nAChR PAM, with nicotine treatment of CCI mice (A), and sham mice (B) were tested using von Frey test. DHβE (2 mg/kg) or vehicle (0.9% saline) was administered and after 15 min dFBr (9 mg/kg) was injected. Following 15 min after dFBr injection, nicotine (0.5 mg/kg) or vehicle (0.9% saline) was injected subcutaneously. * P < 0.05 vs saline-saline-saline treatment. # P < 0.05 vs saline-dFBr-nicotine. Data reflect mean ± SEM, n = 6 mice/group. BL: Baseline
3.4. dFBr has no effect on morphine-induced antinociceptive behavior in CCI-induced neuropathic pain
Morphine significantly showed antiallodynic effects in a dose-dependent manner in CCI model [Fdose(4,20) = 11.38, P < 0.001; Ftime (5,25) = 87.45, P < 0.001; and Fdosextime(20,100) = 7.84, P < 0.001; Fig 4A]. We then tested a low dose of morphine (1 mg/kg) in presence of dFBr (9 mg/kg) treatment to understand whether dFBr potentiates antiallodynic properties of morphine. Morphine with or without dFBr showed significant antiallodynic effects [Ftreatment(3,15) = 7.86, P < 0.01; Ftime (5,25) = 78.40, P < 0.001; and Ftreatmentxtime(15,75) = 2.78, P < 0.01; Fig 4B]. Post hoc test using the Sidak correction revealed, in contrast to nicotine, dFBr failed to potentiate the effect of morphine (P > 0.05), which indicates the effects of dFBr was selective to nicotine. As seen in Fig 4C, the area under curves which were obtained from Fig 4B also proved morphine by itself or in the presence of dFBr showed antiallodynic effects [Ftreatment (3,20) = 8.478, P < 0.001]. There was no significant difference between dFBr + morphine and saline + morphine groups.
Figure 4. Effect of dFBr on morphine-induced antiallodynia in CCI-induced neuropathic pain.
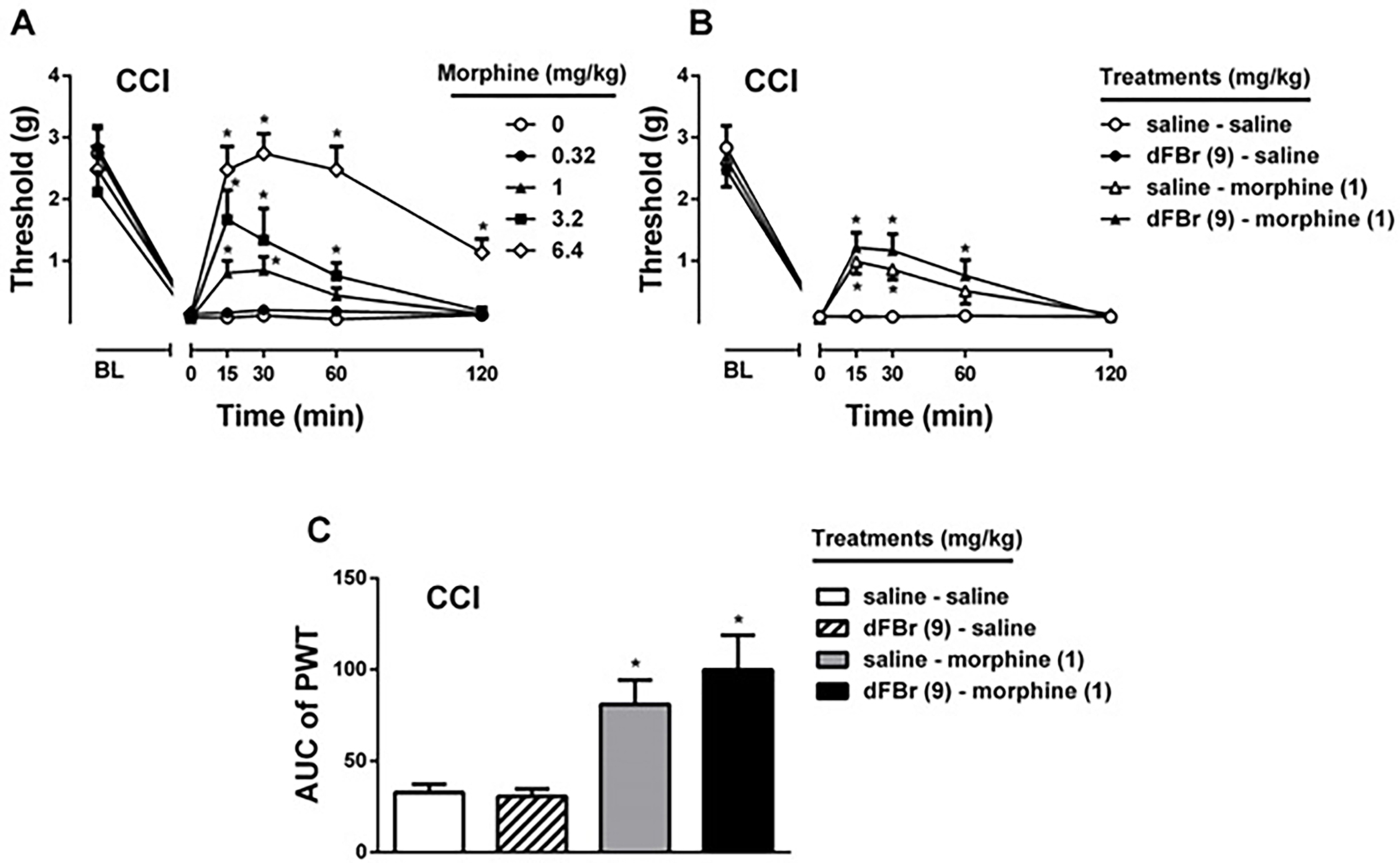
Effects of morphine treatment of CCI mice (A) was tested using von Frey test. Morphine (0.32, 1, 3.2 and 6.4 mg/kg) or vehicle (0.9% saline) was injected subcutaneously. Possible potentiation effect of dFBr (9 mg/kg, subcutaneously) on morphine (B) was tested using a low dose (1 mg/kg) of morphine in von Frey test. In addition to mechanical paw withdrawal threshold (B) and Area under curve (AUC) of threshold values (C) were shown for combination study. * P < 0.05 vs saline itself treatment. Data reflect mean ± SEM, n = 6 mice/group. BL: Baseline
3.5. dFBr reverses nicotine-induced motor impairment and locomotor depression
To test possible impact of dFBr on nicotine-induced motor impairment, we used dFBr and nicotine combination in rotarod test. As seen in Fig 5A, nicotine (0.25, 0.5, 1, and 1.5 mg/kg) alone resulted in motor impairment in naïve mice in a dose-dependent manner starting at the 0.5 mg/kg dose [Fnicotine treatment(4,50) = 9.31, P < 0.001]. At the highest effective dose of dFBr (9 mg/kg) in the CCI model, naïve mice treated with dFBr alone did not show significant changes in motor coordination using the rotarod test (P > 0.05). Furthermore, when mice were treated with combination of dFBr and nicotine no disruption of motor coordination of the animals were observed (P > 0.05). dFBr pretreatment significantly reversed nicotine-induced motor impairment behavior at all doses [FdFBr treatment(1,50) = 37.39, P < 0.001]. The two way ANOVA revealed a significant interaction between dFBr and nicotine treatments [Finteraction(4,50) = 5.57, P < 0.001]. Similar results were seen with dFBr and nicotine at the highest doses on motor impairment in CCI-operated mice after 2 weeks of surgery. While nicotine (1.5 mg/kg, s.c.) itself induced a significant motor impairment, dFBr (9 mg/kg, s.c.) pretreatment blocked the effect of nicotine [Fnicotine treatment(1,20) = 701.3, P < 0.001; FdFBr treatment(1,20) = 506.5, P < 0.001; Finteraction(1,20) = 584.1, P < 0.001; Table 2].
Figure 5: Impact of dFBr on nicotine-induced motor impairment and locomotor depression.
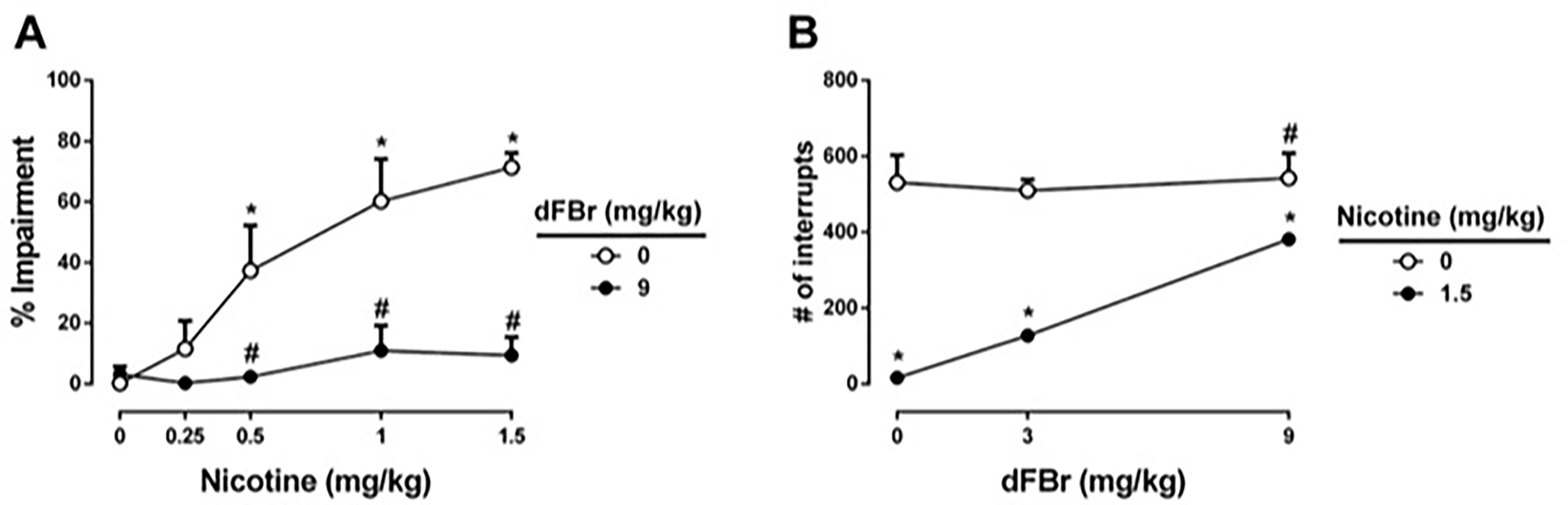
Effects of dFBr on possible nicotine-induced motor impairment (A) and locomotor depression (B) were tested. Mice received dFBr (3 or 9 mg/kg, subcutaneously) or saline, and 15 minutes later mice were given a subcutaneous injection of nicotine (0.25, 0.5, 1 or 1.5 mg/kg) or saline as vehicle. Mice were placed on the rotarod or placed into photocell activity cages 5 min after last injection. * P < 0.05 vs saline-saline treatment. # P < 0.05 vs saline-nicotine treatment. Data reflect mean ± SEM, n = 6 mice/group, as time to fall in % impairment for each group.
Table 2.
Effects of desformylflustrabromine on motor coordination in CCI-performed mice.
| Dose of nicotine | Saline | dFBr (9 mg/kg) |
|---|---|---|
| 0 mg/kg (saline) | 2.050 ± 0.846 | 4.433 ± 1.377 |
| 1.5 mg/kg | 74.633 ± 1.794 * | 7.750 ± 1.544 # |
Desformylflustrabromine (dFBr) and nicotine were tested for possible motor impairment in CCI-operated mice after 2 weeks of surgery. Mice received dFBr (9 mg/kg, subcutaneously) or saline, and 15 min later mice were given a subcutaneous injection of nicotine (1.5 mg/kg) or saline as vehicle. Mice were placed on the rotarod 5 min after last injection. Data reflect mean ± SEM, n = 6 mice/group, as time to fall in % impairment for each group.
P < 0.05 vs saline-saline treatment.
P < 0.05 vs saline-nicotine treatment.
Fig 5B shows the effects of dFBr on nicotine-evoked locomotor depression. dFbr had no significant effect on locomotor activity on its own (P > 0.05). However, nicotine (1.5 mg/kg), the highest dose in this study, alone evoked a large decrease in locomotor activity [Fnicotine treatment(1,30) = 103.5, P < 0.001]. dFBr pretreatment significantly reversed nicotine-induced locomotor suppression behavior [FdFBr treatment(2,30) = 10.75, P < 0.01]. The two way ANOVA revealed a significant interaction between dFBr and nicotine treatments [Finteraction(2,30) = 8.822, P < 0.001].
4. Discussion
In our study, we report for the first time the effects of dFBr pharmacological interaction with nicotine in a CCl-induced neuropathic pain model. Nicotine alone significantly increased the mechanical paw withdrawal thresholds in CCl and sham mice at doses of 1.0 and 1.5 mg/kg, but failed to at 0.1, and 0.5 mg/kg (Fig. 1). When dFBr administered alone, it failed to show any effect on mechanical paw withdrawal thresholds at any of the doses tested. However, dFBr dose-dependently potentiated effects of nicotine (0.5 mg/kg). A low dose of nicotine (0.25 mg/kg) was also potentiated by dFBr (9 mg/kg) in CCI mice (Fig. 2). The potentiation effects of dFBr on nicotine-evoked antinociception was blocked by DHβE, which indicates that the effect of dFBr is primarily mediated by β2*-nAChR subtypes (Fig. 3). When co-administered with morphine rather than nicotine, dFBr showed no effect on paw withdrawal thresholds evoked by morphine which suggests the effect of dFBr is selective for nAChRs but not opioidergic receptors (Fig. 4). Motor coordination of mice was tested to investigate any motor impairment effects induced by dFBr. There were significant reversal effects of motor impairment induced by nicotine observed during the rotarod test (Fig. 5). Collectively, the present study results suggest that dFBr potentiates nicotine’s antiallodynic effects in a dose- and time-dependent manner in neuropathic pain model without affecting motor coordination.
In the current study, dFBr potentiated the effects of nicotine in mouse model of neuropathic pain through α4β2* nAChRs. Our results are consistent with previous reports (Lee et al., 2011; Rode et al., 2012; Zhu et al., 2011) which shows that positive allosteric modulation of α4β2* nAChR with compounds such as NS206 and NS9283 can lead to an enhancement of the antinociceptive properties of nicotinic agonists in chronic inflammatory and neuropathic pain models. In the present study, dFBr did not alter mechanical thresholds on its own. This lack of effect is consistent with other α4β2* PAMs such as NS9283 which lacked anti-allodynic activity on its own in chronic pain models (Rode et al., 2012; Zhu et al., 2011), suggesting the absence of a β2* nAChR-mediated endogenous antinociceptive cholinergic tone in neuropathic pain. This is different from α7 nAChR PAMs which were shown to be active when given alone in these models (Bagdas et al., 2015b, 2016; Damaj et al., 2014; Freitas et al., 2013). Moreover, when evaluated in combination with morphine, dFBr (9 mg/kg) had no significant effect on morphine-induced antiallodynic response, suggesting behavioral selectivity of dFBr for potentiating nicotine-induced antinociceptive effects.
It has been shown that allosteric modulation of nicotine’s activity on different behavioral tests may provide a useful tool to understand α4β2* nAChR function and lower side effects (Grupe et al., 2014; Liu, 2013; Maurer et al., 2016; Moerke et al., 2016; Mohler et al., 2014). In the present study, we demonstrated that co-administration of α4β2* nAChR PAM dFBr enhanced nicotine induced anti-allodynia. In addition, we tested possible enhancements on nicotine-induced motor impairment and locomotor depression by dFBr. Surprisingly, dFBr blocked the negative effects of nicotine on motor coordination and locomotor activity, which suggests that dFBr, decreases possible adverse effects of nicotine. Consistent with our results, other studies also showed both inhibitory and enhancement outcomes by using α4β2* PAMs in the presence of an agonist (Lee et al., 2011; Rode et al., 2012; Zhu et al., 2011). It has been suggested that the stoichiometry of α4β2 receptors gives the possibility for two different receptor classes. The low sensitivity (LS) receptors (α4)3(β2)2 and the high sensitivity (HS) receptors (α4)2(β2)3, refer to the relative affinity for ACh (Moroni et al., 2006; Nelson et al., 2003; Rode et al., 2012). Effecting LS or HS receptors may lead to different outcomes and represent different mechanisms in vivo (Rode et al., 2012; Zhang et al., 2012). Recent data showed that dFBr preferentially enhanced LS receptors (Weltzin and Schulte, 2015). However, it is not clear that this preferential selectivity of dFBr to different stoichiometry of α4β2* receptors, plays a role in its differential activities on nicotine’s pharmacological responses. This dichotomy in dFBr’s effects on nicotine may also results from the bell shaped concentration curve of dFBr on expressed α4β2 nAChRs function, where the drug at higher concentrations blocks cholinergic functional responses (Weltzin and Schulte, 2010). Finally, the inhibitory effect may be derived from the possibility that these two pharmacological effects of nicotine (allodynia and motor activity) are mediated by different α4β2* nAChR subtypes. For example, nicotine’s antiallodynic properties has been reported to be partially mediated by α5-containing nAChR subtype such as α4α5β2* in a chronic neuropathic model (Bagdas et al., 2015a). Further investigation of dFBr on the function of the different α4β2* nAChR subtypes is needed to understand the potency and/or therapeutic window of α4β2* nAChR PAMs on the different behavioral and pharmacological effects of nicotine and nicotinic agonists.
In the current study, we demonstrated that administration of dFBr, α4β2* PAM, failed to reduce pain behavior on its own. However, the combination of dFBr with nicotine significantly reversed neuropathic pain behavior dose- and time-dependently without motor impairment, confirming the dissociation of analgesic activity and adverse effects. Thus, the present results suggest that allosteric modulation of α4β2* nAChR may provide new strategies in chronic neuropathic pain.
Funding Sources:
This study was supported by NIH grants [R01-CA206028] and [DA 005274] to MID.
Abbreviations:
- dFBr
desformylflustrabromine
- PAM
positive allosteric modulator
- nAChRs
nicotinic acetylcholine receptors
- CCI
chronic constriction nerve injury
- DhβE
dihydro-β-erythroidine
- AUC
area under the curve
Footnotes
Conflicts of interest: None declared.
References
- Bagdas D, AlSharari SD, Freitas K, Tracy M, Damaj MI (2015a). The role of alpha5 nicotinic acetylcholine receptors in mouse models of chronic inflammatory and neuropathic pain. Biochem Pharmacol 97, 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Targowska-Duda KM, López JJ, Perez EG, Arias HR, Damaj MI (2015b). The Antinociceptive and Antiinflammatory Properties of 3-furan-2-yl-N-p-tolyl-acrylamide, a Positive Allosteric Modulator of α7 Nicotinic Acetylcholine Receptors in Mice. Anesth Analg 121, 1369–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Wilkerson JL, Kulkarni A, Toma W, AlSharari S, Gul Z, Lichtman AH, Papke RL, Thakur GA, Damaj MI (2016). The α7 nicotinic receptor dual allosteric agonist and positive allosteric modulator GAT107 reverses nociception in mouse models of inflammatory and neuropathic pain. Br J Pharmacol 2506–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon AW, Decker MW, Kim DJB, Campbell JE, Arneric SP (1998). ABT-594, a novel cholinergic channel modulator, is efficacious in nerve ligation and diabetic neuropathy models of neuropathic pain. Brain Res 801, 158–163. [DOI] [PubMed] [Google Scholar]
- Boyce S, Webb JK, Shepheard SL, Russell MGN, Hill RG, Rupniak NMJ (2000). Analgesic and toxic effects of ABT-594 resemble epibatidine and nicotine in rats. Pain 85, 443–450. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Fei-Yin M, Dukat M, Glassco W, Glennon R.a, Martin BR. (1998). Antinociceptive responses to nicotinic acetylcholine receptor ligands after systemic and intrathecal administration in mice. J Pharmacol Exp Ther 284, 1058–1065. [PubMed] [Google Scholar]
- Damaj MI, Freitas K, Bagdas D, Flood P (2014). Nicotinic Receptors as Targets for Novel Analgesics and Anti-inflammatory Drugs. In Nicotinic Receptors, Lester RAJ, ed. (New York, NY: Springer New York; ), pp. 239–254. [Google Scholar]
- Damaj MI, Glassco W, Aceto MD, Martin BR (1999). Antinociceptive and pharmacological effects of metanicotine, a selective nicotinic agonist. J Pharmacol Exp Ther 291, 390–398. [PubMed] [Google Scholar]
- Decker MW, Rueter LE, Bitner RS (2004). Nicotinic acetylcholine receptor agonists: a potential new class of analgesics. Curr Top Med Chem 4, 369–384. [DOI] [PubMed] [Google Scholar]
- Ditre JW, Heckman BW, Zale EL, Kosiba JD, Maisto SA (2016). Acute analgesic effects of nicotine and tobacco in humans. Pain 157, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas K, Ghosh S, Ivy Carroll F, Lichtman AH, Imad Damaj M (2013). Effects of alpha 7 positive allosteric modulators in murine inflammatory and chronic neuropathic pain models. Neuropharmacology 65, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F (2006). Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci 27, 482–491. [DOI] [PubMed] [Google Scholar]
- Grupe M, Grunnet M, Laursen B, Bastlund JF (2014). Neuropharmacological modulation of the P3-like event-related potential in a rat two-tone auditory discrimination task with modafinil and NS9283, a positive allosteric modulator of α4β2 nAChRs. Neuropharmacology 79, 444–455. [DOI] [PubMed] [Google Scholar]
- Jin Z, Khan P, Shin Y, Wang J, Lin L, Cameron MD, Lindstrom JM, Kenny PJ, Kamenecka TM (2014). Synthesis and activity of substituted heteroaromatics as positive allosteric modulators for α4β2α5 nicotinic acetylcholine receptors. Bioorg Med Chem Lett 24, 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesingland AC, Gentry CT, Panesar MS, Bowes MA, Vernier JM, Cube R, Walker K, Urban L (2000). Analgesic profile of the nicotinic acetylcholine receptor agonists, (+)-epibatidine and ABT-594 in models of persistent inflammatory and neuropathic pain. Pain 86, 113–118. [DOI] [PubMed] [Google Scholar]
- Lawand NB, Lu Y, Westlund KN (1999). Nicotinic cholinergic receptors: potential targets for inflammatory pain relief. Pain 80, 291–299. [DOI] [PubMed] [Google Scholar]
- Lee CH, Zhu C, Malysz J, Campbell T, Shaughnessy T, Honore P, Polakowski J, Gopalakrishnan M (2011). α4β2 neuronal nicotinic receptor positive allosteric modulation: An approach for improving the therapeutic index of α4β2 nAChR agonists in pain. Biochem Pharmacol 82, 959–966. [DOI] [PubMed] [Google Scholar]
- Liu X (2013). Positive allosteric modulation of α4β2 nicotinic acetylcholine receptors as a new approach to smoking reduction: Evidence from a rat model of nicotine self-administration. Psychopharmacology (Berl) 230, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JJ, Wade CL, Mikusa JP, Decker MW, Honore P (2005). ABT-594 (a nicotinic acetylcholine agonist): Anti-allodynia in a rat chemotherapy-induced pain model. Eur J Pharmacol 509, 43–48. [DOI] [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Léna C, Le Novère N, de Kerchove d’Exaerde M, Huchet M, Damaj MI, Changeux J (1999). Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature 398, 805–810. [DOI] [PubMed] [Google Scholar]
- Maurer JJ, Sandager-Nielsen K, Schmidt HD (2016). Attenuation of nicotine taking and seeking in rats by the stoichiometry-selective alpha4beta2 nicotinic acetylcholine receptor positive allosteric modulator NS9283. Psychopharmacology (Berl) 1–10. [DOI] [PubMed] [Google Scholar]
- Mitra S, Mucha M, Khatri SN, Glenon R, Schulte MK, Bult-Ito A (2017). Attenuation of Compulsive-Like Behavior Through Positive Allosteric Modulation of α4β2 Nicotinic Acetylcholine Receptors in Non-Induced Compulsive-Like Mice. Front Behav Neurosci 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerke MJ, de Moura FB, Koek W, McMahon LR (2016). Effects of nicotine in combination with drugs described as positive allosteric nicotinic acetylcholine receptor modulators in vitro: discriminative stimulus and hypothermic effects in mice. Eur J Pharmacol 786, 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler EG, Franklin SR, Rueter LE (2014). Discriminative-stimulus effects of NS9283, a nicotinic α4β2*positive allosteric modulator, in nicotine-discriminating rats. Psychopharmacology (Berl) 231, 67–74. [DOI] [PubMed] [Google Scholar]
- Moroni M, Zwart R, Sher E, Cassels BK, B.I. (2006). Alpha4 Beta2 Nicotinic Receptors With High and Low Acetylcholine Sensitivity: Pharmacology,Stoichiometry, and Sensitivity To Long-Term Exposure To Nicotine. Mol Pharmacol 70, 755–768. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J (2003). Alternate Stoichiometries of alpha 4beta 2 Nicotinic Acetylcholine Receptors. Mol Pharmacol 63, 332–341. [DOI] [PubMed] [Google Scholar]
- Nirogi R, Goura V, Abraham R, Jayarajan P (2013). α4β2* Neuronal Nicotinic Receptor Ligands (Agonist, Partial Agonist and Positive Allosteric Modulators) As Therapeutic Prospects for Pain. Eur J Pharmacol 712, 22–29. [DOI] [PubMed] [Google Scholar]
- Olsen JA, Kastrup JS, Peters D, Gajhede M, Balle T, Ahring PK (2013). Two distinct allosteric binding sites at α4β2 nicotinic acetylcholine receptors revealed by NS206 and NS9283 give unique insights to binding activity-associated linkage at cys-loop receptors. J Biol Chem 288, 35997–36006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya A, Yakel JL (2011). Allosteric modulators of the α4β2 subtype of neuronal nicotinic acetylcholine receptors. Biochem Pharmacol 82, 952–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode F, Munro G, Holst D, Nielsen E, Troelsen KB, Timmermann DB, Ronn LCB, Grunnet M (2012). Positive allosteric modulation of α4β2 nAChR agonist induced behaviour. Brain Res 1458, 67–75. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC, Rachel Duan W, Thomas J, Nothaft W, Backonja MM (2009). A randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of ABT-594 in patients with diabetic peripheral neuropathic pain. Pain 146, 245–252. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB (1986). Manual of Pharmacologic Calculations (New York, NY: Springer New York; ). [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI (2006). The β2 but not α7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 184, 339–344. [DOI] [PubMed] [Google Scholar]
- Weltzin MM, Schulte MK (2015). Desformylflustrabromine Modulates alpha4beta2 Neuronal Nicotinic Acetylcholine Receptor High- and Low-Sensitivity Isoforms at Allosteric Clefts Containing the beta2 Subunit. J Pharmacol Exp Ther 354, 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xiao Y.De, Jordan KG, Hammond PS, Van Dyke KM, Mazurov A. a., Speake JD, Lippiello PM, James JW, Letchworth SR, Bencherif M, Hauser (2012). Analgesic effects mediated by neuronal nicotinic acetylcholine receptor agonists: Correlation with desensitization of α4β2* receptors. Eur J Pharm Sci 47, 813–823. [DOI] [PubMed] [Google Scholar]
- Zhu CZ, Chin C-L, Rustay NR, Zhong C, Mikusa J, Chandran P, Salyers A, Gomez E, Simler G, Lewis LG, Gauvin D, Baker S, Pai M, Tovcimak A, Brown J, Komater V, Fox GB, Decker MW, Jacobson PB, Gopalakrishnan M, Lee C-H, Honore P (2011). Potentiation of analgesic efficacy but not side effects: Co-administration of an α4β2 neuronal nicotinic acetylcholine receptor agonist and its positive allosteric modulator in experimental models of pain in rats. Biochem Pharmacol 82, 967–976. [DOI] [PubMed] [Google Scholar]


