Keywords: COPD, cotinine, sputum
Abstract
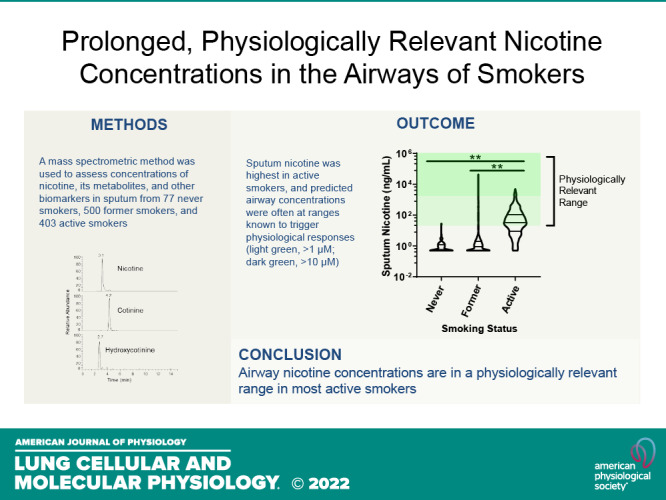
Nicotine from cigarette smoke is a biologically active molecule that has pleiotropic effects in the airway, which could play a role in smoking-induced lung disease. However, whether nicotine and its metabolites reach sustained, physiologically relevant concentrations on airway surfaces of smokers is not well defined. To address these issues, concentrations of nicotine, cotinine, and hydroxycotinine were measured by mass spectrometry (MS) in supernatants of induced sputum obtained from participants in the subpopulations and intermediate outcome measures in COPD study (SPIROMICS), an ongoing observational study that included never smokers, former smokers, and current smokers with and without chronic obstructive pulmonary disease (COPD). A total of 980 sputum supernatants were analyzed from 77 healthy never smokers, 494 former smokers (233 with COPD), and 396 active smokers (151 with COPD). Sputum nicotine, cotinine, and hydroxycotinine concentrations corresponded to self-reported smoking status and were strongly correlated to urine measures. A cutoff of ∼8–10 ng/mL of sputum cotinine distinguished never smokers from active smokers. Accounting for sample dilution during processing, active smokers had airway nicotine concentrations in the 70–850 ng/mL (∼0.5–5 µM) range, and concentrations remained elevated even in current smokers who had not smoked within 24 h. This study demonstrates that airway nicotine and its metabolites are readily measured in sputum supernatants and can serve as biological markers of smoke exposure. In current smokers, nicotine is present at physiologically relevant concentrations for prolonged periods, supporting a contribution to cigarette-induced airway disease.
INTRODUCTION
Nicotine is the major biologically active alkaloid found in cigarette smoke, and its neurophysiological effects underlie its addictive potential (1). The systemic pharmacokinetics of nicotine have been extensively studied, with blood and central nervous system concentrations rapidly increasing with inhalation of cigarette smoke, then falling with an acute half-life of ∼2 h as nicotine is metabolized and excreted (2, 3). An additional component of nicotine clearance with a much longer half-life (11–17 h) has also been identified (2, 4), which likely reflects absorption and delayed release of nicotine by other tissues such as lung, liver, kidney, and spleen (3). Nicotine is extensively metabolized, primarily in the liver, with cotinine and hydroxycotinine the primary metabolites. Cotinine in particular has been widely utilized as a biomarker of smoking since it has a long half-life, and concentrations of cotinine in plasma, saliva, and urine are strongly associated with nicotine exposure (3–5).
Although the impact of smoking on systemic concentrations of nicotine and its metabolites is well understood, much less is known about the airway concentrations of these compounds with cigarette exposure, particularly in chronic smokers. Nicotine concentrations in induced sputum have been reported to be in the ∼50 µM range 5 min after exposure to a conventional cigarette (6) or e-cigarette (7), with significantly lower concentrations of cotinine. However, studies have not assessed the persistence of airway nicotine following acute or chronic exposures. The relevance of airway nicotine has become increasingly apparent, as numerous studies have documented adverse effects of nicotine on epithelia and other cell types within the airway (8–11).
To assess concentrations of nicotine and its metabolites in airway samples in smokers, we applied a mass spectrometric (MS) method to measure nicotine, cotinine, and hydroxycotinine in 980 sputum supernatants collected as part of a multicenter observational study of chronic obstructive pulmonary disease (COPD), the subpopulations and intermediate outcome measures in COPD study (SPIROMICS; ClinicalTrials.gov NCT01969344T4; 12). Analysis of samples from this study allowed us to assess sputum nicotine and its metabolites in active smokers, former smokers, as well as never smoker controls whose smoking history was well documented both by history and through measurement of established biomarkers of cigarette exposure in urine.
METHODS
Subject and Samples
Subjects were part of the SPIROMICS observational study that included a healthy nonsmoking control cohort as well as former or active smokers. All former or active smokers had at least a 20 pack-year history of smoking and included subjects with preserved spirometry as well as subjects with COPD stratified by global initiative for chronic obstructive lung disease (GOLD) status (12). During the study visit, participants completed questionnaires that documented any smoking in the past month, average number of cigarettes smoked per day, as well as the number of cigarettes smoked in the past 24 h, 2 h, and 1/2 h. Sputum was collected and processed using standard methods (13, 14) that included incubations with weight-based volumes of a reducing agent that resulted in an eightfold dilution of sputum contents in the final processed sputum supernatant. Former smokers were defined as those who self-reported abstinence for ≥6 mo with normal spirometry. Sputum values were compared with urine nicotine, cotinine, and hydroxycotinine that were measured as part of SPIROMICS from spot collected, clean catch urine. Selection of the analytic cohort was as previously described (13, 15). The SPIROMICS protocol was approved by the participating universities, and participants provided written informed consent. More information about the study and how to access SPIROMICS data is available at www.spiromics.org.
Nicotine Metabolite and Other Biomarker Measurements
Concentrations of urea, nicotine, cotinine, 3'-hydroxycotinine, adenosine, and adenosine metabolites were measured in sputum supernatants prepared as previously described (13–15) using a MS method (7). Because internal standard for nicotine and its metabolites were not routinely added to all samples at the time of the study, matrix effects and run-to-run variability were controlled by assessing all nicotine metabolites as ratios to the isotopically labeled leucine internal standard, which had a similar column run time. The validity of this approach was assessed in a subset of samples (n = 137) that were prepared with nicotine metabolite internal standards (7). Nicotine metabolite concentrations calculated using the matching internal standards were strongly correlated (r > 0.9) to those calculated using the leucine internal standard for all compounds. This substudy using nicotine metabolite internal standards also confirmed that the reducing agents added during sputum processing did not interfere with the MS method. The lower limit of quantification was calculated as 0.5 ng/mL for all nicotine metabolites based on standard curves and analysis of signal from sample blanks, and a value of 0.5 ng/mL was imputed to all samples with no signal or calculated concentrations below this threshold.
Statistical Analyses
Correlations were performed using linear regression or Spearman correlation depending on the normality of the data. Group comparisons were performed using ANOVA with posttest analysis by Tukey’s multiple comparisons test. Analyses were performed using GraphPad Prism 9.2 (San Diego, CA).
RESULTS
Nicotine, cotinine, and hydroxycotinine were measured in 980 sputum supernatants collected during the initial SPIROMICS study visit, including samples from 77 healthy never smokers (NSs), 500 former smokers (20+ pack/year history, no smoking within 30 days of study visit), and 403 active smokers (20+ pack/year history, active smoking within 30 days of study visit; Table 1). The vast majority of active smokers (376/403, 93%) reported smoking within the 24 h before the study visit, though most (302/403, 75%) denied smoking within 2 h of the visit. Signal was above the detection limits for nicotine in 773 samples, with detectable signal for cotinine in 602 samples and hydroxycotinine in 632 samples.
Table 1.
Study demographics
| Nonsmokers | Former Smokers | Active Smokers | |
|---|---|---|---|
| n | 77 | 500 | 403 |
| Age, yr ± SD | 55.4 ± 10.2 | 66.8 ± 7.5 | 58.7 ± 8.9 |
| Sex, male (%) | 42 (55%) | 186 (37%) | 164 (41%) |
| Race | |||
| White, % | 51 (66%) | 425 (85%) | 251 (62%) |
| African American, % | 21 (27%) | 53 (11%) | 130 (32%) |
| Other, % | 5 (6%) | 22 (4%) | 22 (5%) |
| COPD*, % | 0 (0%) | 349 (70%) | 213 (50%) |
| FEV1, %predicted ± SD | 103 ± 12% | 80 ± 21% | 84 ± 21% |
| Cig smoked per day ± SD | 0 ± 0 | 0 ± 0 | 15.6 ± 10.3 |
*Chronic obstructive pulmonary disease (COPD) defined as global initiative for chronic obstructive lung disease (GOLD) stage 1 or greater. Cig, cigarettes; FEV1, forced expiratory volume in 1 s.
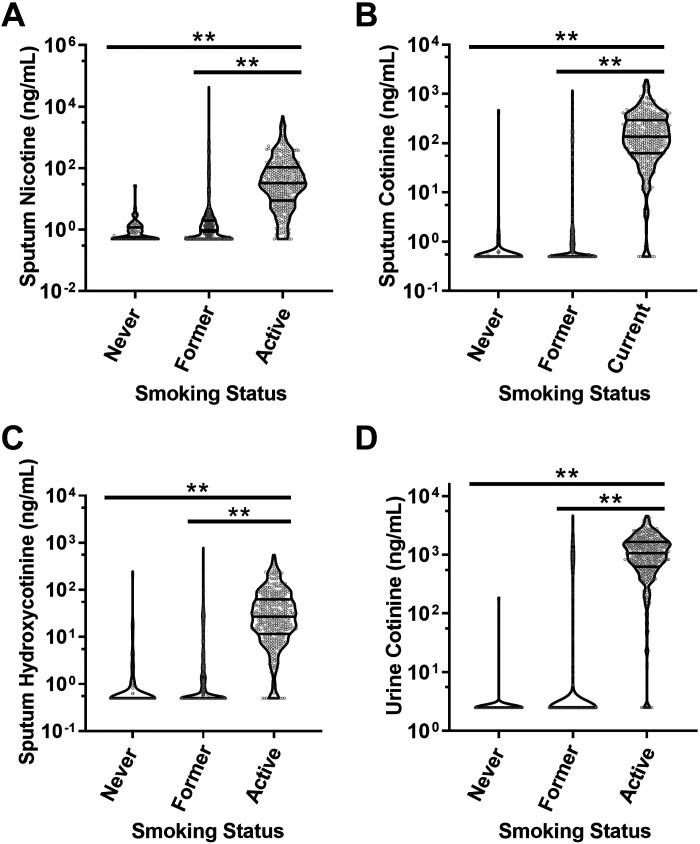
All three sputum nicotine metabolites readily distinguished active smokers from never smokers and former smokers (Fig. 1, A–C). The interquartile range (IQR) of sputum nicotine concentrations in active smokers was 9–107 ng/mL, which translates to airway concentrations of 70–850 ng/mL (0.4–5.3 µM) after taking into account the eightfold sample dilution of sputum during processing (14). Within the active smoking population, 62% had calculated airway nicotine concentrations ≥1 µM (160 ng/mL), whereas 16% had airway nicotine concentrations ≥10 µM (1,600 ng/mL) at the time of sample collection, concentrations commonly used for in vitro studies of nicotine in airway epithelia (16–20). Findings for sputum cotinine were very similar to those for urine cotinine (Fig. 1D), though with lower overall cotinine concentrations in sputum.
Figure 1.
Nicotine metabolites in sputum and urine. A: sputum nicotine was elevated in active smokers relative to never or former smokers. B: sputum cotinine was elevated in active smokers relative to never or former smokers. C: sputum hydroxycotinine was elevated in active smokers relative to never or former smokers. D: urine cotinine as measured through the SPIROMICS core laboratory for comparison. Data are depicted in violin plots as nanograms per milliliter, with lines indicating median and IQR. Note scale differences in A. **P < 0.01 by ANOVA with Tukey’s posttest analysis. IQR, interquartile range.
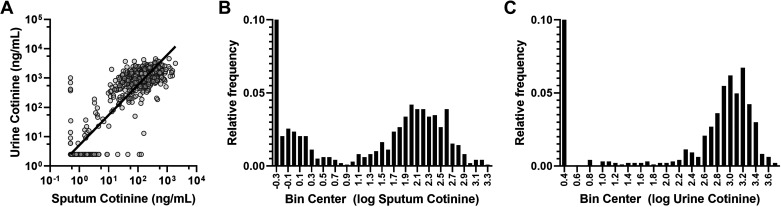
Sputum cotinine was strongly correlated with urine cotinine measured independently as part of the SPIROMICS protocols (12; r = 0.95, P < 0.001, Fig. 2A). Strong correlations were also noted between sputum and urine nicotine (r = 0.81, P < 0.001) and sputum and urine hydroxycotinine (r = 0.87, P < 0.001). Analysis of the frequency distribution of all sputum cotinine values indicated a break point at 8 ng/mL (0.9 log units; Fig. 2B), similar to the 5th percentile value within the active smoker group (10 ng/mL). For urine cotinine, the break point based on frequency distribution was at 100 ng/mL (2.0 log units), with the 5th percentile of the active smoker group at 130 ng/mL (Fig. 2C). A total of nine subjects (<1%) had discordant results in which cotinine concentrations exceeded the break point in one sample type but not the other. Of the five subjects who had substantial urine cotinine (>130 ng/mL) despite undetectable sputum cotinine, one was a former smoker and four were active smokers, though only one reported cigarette smoking within 24 h of the study visit. Of the four subjects with sputum cotinine >8 ng/mL but no urine cotinine, three reported being former smokers though one was an active smoker who reported 10 cigarettes within the past 24 h.
Figure 2.
Sputum vs. urine cotinine. A: strong correlation was observed between sputum and urine cotinine values (r = 0.95, P < 0.0001). B: frequency analysis suggested two populations of sputum cotinine concentrations with a cut-off at 0.8 log units (6 ng/mL). C: frequency analysis of urine cotinine suggested a cut-off between populations of 2.0 log units (100 ng/mL).
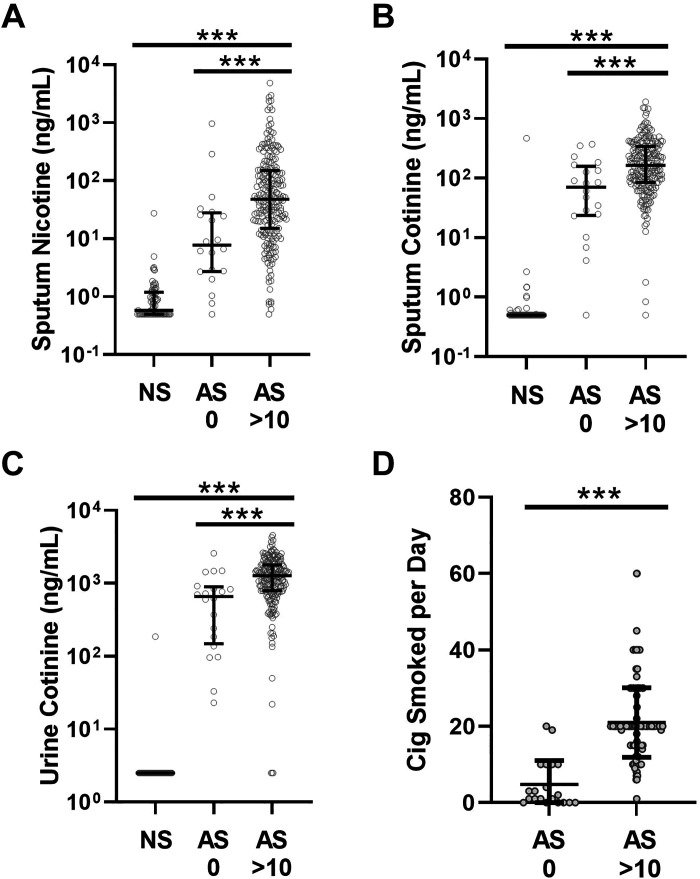
To assess whether airway nicotine persists after acute cigarette smoke exposure, we examined sputum nicotine in the subset of active smokers who did not report active smoking within 24 h of the study visit (AS 0). Despite not smoking for 24 or more hours, median sputum nicotine was 7.8 ng/mL, which translates to airway nicotine concentrations of 63 ng/mL (0.4 µM) with an IQR of 22–220 ng/mL (0.1–1.4 µM) in the AS 0 group when accounting for dilution. These values were higher than those measured in the NS group though lower than the calculated airway concentrations of 390 ng/mL (2.4 µM) with IQR 120–1,200 ng/mL (0.7–7.4 µM) from a control group who smoked heavily in that interval (10 or more cigarettes within 24 h, AS >10; P < 0.001 for both comparisons by Tukey’s posttest after ANOVA; Fig. 3A). Some of the differences between the AS 0 and AS >10 groups reflected lower overall smoking intensity in the AS 0 group, as reflected in lower sputum and urine cotinine (Fig. 3, B and C) and lower reported average cigarettes smoked per day (Fig. 3D).
Figure 3.
Nicotine metabolites and recent smoking. A: sputum nicotine was increased in active smokers who reported smoking >10 cigarettes within 24 h before the study visit (AS >10, n = 227) vs. active smokers who reported no smoking within 24 h before the study visit (AS 0, n = 20). Sputum nicotine was increased in both groups relative to never smokers (NSs). B: similar pattern for sputum cotinine, with increases in both the AS 0 and AS >10 groups relative to NS, with the AS >10 group higher than the AS 0. C: the pattern for urine cotinine was similar to that for sputum cotinine. D: subjects in the AS 0 group reported fewer average cigarettes smoked per day than those in the AS >10 group. ***P < 0.001 by ANOVA with Tukey’s posttest (A–C) or Student’s t test (D). Cig, cigarettes.
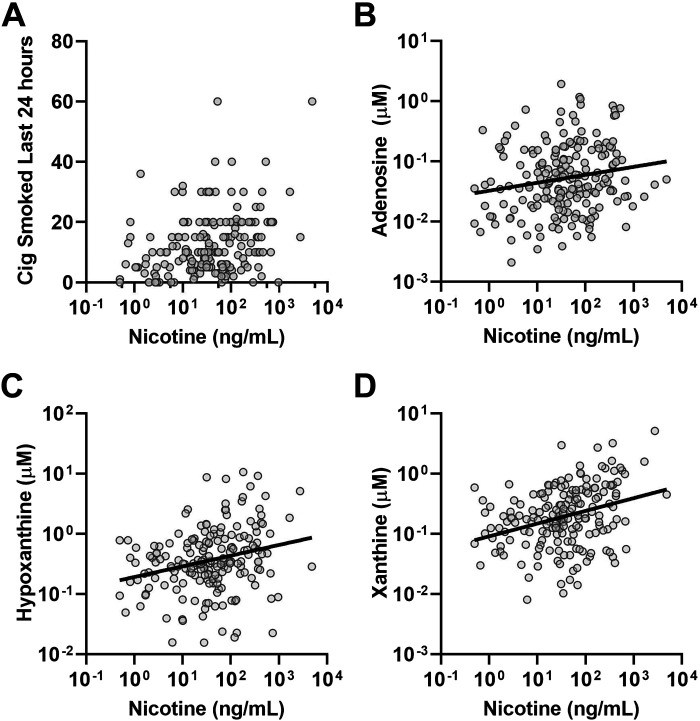
The MS panel utilized in this study also included measurement of a number of metabolites, including those in the adenosine metabolic pathway that have been associated with smoking in prior studies (13, 21). To assess whether the intensity of acute smoking affected these physiological airway responses, we examined the relationship between sputum nicotine as an index of recent smoke exposure and sputum adenosine and its metabolites. To eliminate the chronic disease as a potential confounder, we only examined the subset of active smokers with preserved spirometry (n = 192). In this subset, we observed a modest but statistically significant relationship between sputum nicotine and self-reported number of cigarettes smoked in the prior 24 h (Fig. 4A). Sputum nicotine was significantly correlated with sputum adenosine (Fig. 4B), as well as the adenosine metabolites hypoxanthine and xanthine (Fig. 4, C and D).
Figure 4.
Relationships of sputum nicotine to other measures in active smokers with preserved spirometry. A: a modest but statistically significant correlation was observed between sputum nicotine and reported cigarettes smoked in the 24 h before the study visit (Spearman r = 0.28, P < 0.0001). B–D: weak but statistically significant correlations were noted between sputum nicotine and sputum adenosine (r = 0.18, P < 0.02; B); hypoxanthine (r = 0.26, P < 0.001; C), and xanthine (r = 0.32, P < 0.001; D). Cig, cigarettes.
DISCUSSION
Using a mass spectrometric method, we successfully measured nicotine and its primary metabolites in sputum collected from a large, multicenter study that included former smokers, active smokers, and never-smoking controls. Nicotine metabolites in sputum corresponded very well to self-reported smoking status and were strongly correlated to urine nicotine metabolites measured independently, supporting the overall validity of the method. Sputum nicotine is also correlated to airway adenosine and its metabolites as markers of cigarette smoke exposure.
These findings provide new insights into the role of airway nicotine in active smokers. In particular, our findings suggest that the airway nicotine concentrations typically observed in active smokers are within the range reported to cause adverse physiological effects in human airway epithelia in vitro (8). A meaningful fraction of subjects had airway nicotine concentrations that met or exceeded the 10 µM concentration commonly utilized for in vitro studies (16–18), with even more reaching the low and even submicromolar concentrations of nicotine that have been reported to alter airway cellular signaling and mucus rheology (19, 20).
Although we did not directly measure the pharmacokinetics of airway nicotine, our analyses suggest that airway nicotine remains persistently elevated in active smokers well after acute exposures. As the majority of active smokers denied smoking within 2 h before the study visit, the measured sputum nicotine concentrations are not likely transient increases from acute exposures, Furthermore, sputum nicotine concentrations were elevated even in the small subset of active smokers who denied any cigarette use within 24 h despite this group having much lower overall cigarette exposure. Our findings are most consistent with the presence of a long half-life component of nicotine clearance that has been described in other studies (2, 4). Whether this reflects accumulation and release of nicotine within the airway or replenishment of airway nicotine from systemic sources is unknown. The latter mechanism more likely explains the sputum concentrations of cotinine and hydroxycotinine, since these compounds are formed primarily in the liver and not the lung (3).
Our findings suggest that sputum cotinine can serve as a valid biomarker of tobacco smoke exposure, similar to cotinine measured in blood, urine, or saliva. The cut-point of ∼8–10 ng/mL in processed sputum that effectively distinguished smokers from nonsmokers is similar to the previously reported cut-point of 15 ng/mL in saliva (22), though it should be noted that the sputum cut-point is depressed by sample dilution during processing. Although sputum is a noninvasive sample, collection challenges make it unlikely that sputum would replace saliva or urine as a routine choice for noninvasive smoking biomarker measurement. However, measurement of sputum cotinine could replace separate cotinine measures in studies in which sputum is already being collected for other biomarker measurements. Importantly, we were able to measure cotinine and other relevant metabolites in sputum processed using standardized methods within a large, multicenter observational study (12, 15), demonstrating the feasibility of this approach for other observational or interventional trials.
One limitation of this study is our inability to rule out the possibility that some subjects were obtaining nicotine from a source other than smoking; e.g., from nicotine supplements utilized for smoking cessation. Use of nicotine supplements or electronic nicotine delivery devices was not routinely recorded in SPIROMICS, and it is possible that these contributed to the elevated nicotine metabolite concentrations observed in a small number of subjects who did not report active smoking. We also cannot eliminate the possibility that some subjects misreported their smoking status or intensity. In addition, our MS method detected low concentrations of nicotine and metabolites in many subjects who did not report active smoking or detectable urine cotinine. It is not clear if this represents the ability of MS to detect low-level environmental nicotine exposure; e.g., from secondhand smoke; or if the signal represents background or contaminant peak. As secondhand smoke exposure was not routinely recorded in SPIROMICS, we could not distinguish between these possibilities.
In summary, our analysis demonstrates that active smokers have elevated, physiologically relevant concentrations of nicotine on airway surfaces that persist for prolonged periods even without active smoking. Thus, the pharmacological effects of nicotine on airway epithelia should be considered as a potential contributor to cigarette smoking-induced lung disease. Furthermore, sputum cotinine can be considered as a valid biomarker of nicotine exposure from cigarette smoking and likely other nicotine delivery devices.
DATA AVAILABILITY
Data will be made available from SPIROMICS on request.
GRANTS
This work was supported by SPIROMICS [SPIROMICS was supported by contracts from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI; HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), and grants from the NIH/NHLBI (U01 HL137880 and U24 HL141762), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer Ingelheim Pharmaceuticals, Inc.; Chiesi Farmaceutici S.p.A.; Forest Research Institute, Inc.; GlaxoSmithKline; Grifols Therapeutics, Inc.; Ikaria, Inc.; Novartis Pharmaceuticals Corporation; Nycomed GmbH; ProterixBio; Regeneron Pharmaceuticals, Inc.; Sanofi; Sunovion; Takeda Pharmaceutical Company; and Theravance Biopharma and Mylan]. C.R.E. was supported by Grants R01-HL136961-01S1 and P30-ES10126. R.C.B. was supported by Grants UH3 HL123645, P01 HL108808, P30 DK065988, P01 HL110873, P50 HL107168, and R01 HL136961.
DISCLOSURES
C.B.C. reports personal fees from PulmonX, other from GlaxoSmithKline, personal fees from NUVAIRA, and personal fees from MGC Diagnostics. I.B. has received grants from Amgen, GE Healthcare, Aerogen, Theravance, and Viatris and has received consulting fees from Astra Zeneca, GSK, Theravance, Viatris, Grifols, Inhibrx. L.M.R. is a consultant for the TOPMed Administrative Coordinating Center (through WeStat). R.P.B. reports grants and personal fees from Boehringer Ingelheim, personal fees from Mylan Pharmaceuticals, personal fees from Theravance, grants, personal fees, and nonfinancial support from GSK. A.P.C. reports personal fees from GSK, nonfinancial support from VIDA. J.L.C. reports personal fees from AstraZeneca and CSL Behring. V.E.O. reports personal fees for serving on Independent Data Monitoring Committees for Regeneron and Sanofi. J.M.W. reports grants from Bayer, grants and other from GSK, other from Boehringer Ingelheim, grants and other from Mereo BioPharma, other from PRA. R.C.B. reports receiving fees from Parion Sciences. All disclosures are outside of the submitted work. See grants for full list of support for SPIROMICS. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
C.R.E. conceived and designed research; C.R.E. performed experiments; C.R.E. analyzed data; C.R.E., W.K.O., N.E.A., R.P.B., A.T.H., and R.C.B. interpreted results of experiments; C.R.E. prepared figures; C.R.E. drafted manuscript; C.R.E., W.K.O., N.E.A., A.L.K., C.B.C., I.B., L.M.R., R.P.B., A.P.C., S.P.P., A.T.H., J.L.C., B.R., V.E.O., J.M.W., E.H.-S., S.I.R., and R.C.B. edited and revised manuscript; C.R.E., W.K.O., N.E.A., A.L.K., C.B.C., I.B., L.M.R., R.P.B., A.P.C., S.P.P., A.T.H., J.L.C., B.R., V.E.O., J.M.W., E.H.-S., S.I.R., and R.C.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. More information about the study and how to access SPIROMICS data is at https://www.spiromics.org. We acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Dr. Neil E. Alexis; Dr. Wayne H. Anderson; Dr. Mehrdad Arjomandi; Dr. Igor Barjaktarevic; Dr. R. Graham Barr, DrPH; Lori A. Bateman, MSc; Dr. Surya P. Bhatt; Dr. Eugene R. Bleecker; Dr. Richard C. Boucher; Dr. Russell P. Bowler; Dr. Stephanie A. Christenson; Dr. Alejandro P. Comellas; Dr. Christopher B. Cooper; Dr. David J. Couper; Dr. Gerard J. Criner; Dr. Ronald G. Crystal; Dr. Jeffrey L. Curtis; Dr. Claire M. Doerschuk; Dr. Mark T. Dransfield; Dr. Brad Drummond; Dr. Christine M. Freeman; Dr. Craig Galban; Dr. MeiLan K. Han, MS; Dr. Nadia N. Hansel, MPH; Dr. Annette T. Hastie; Dr. Eric A. Hoffman; Dr. Yvonne Huang; Dr. Robert J. Kaner; Dr. Richard E. Kanner; Dr. Eric C. Kleerup; Dr. Jerry A. Krishnan; Dr. Lisa M. LaVange; Dr. Stephen C. Lazarus; Dr. Fernando J. Martinez, MS; Dr. Deborah A. Meyers; Dr. Wendy C. Moore; Dr. John D. Newell Jr; Dr. Robert Paine, III; Dr. Laura Paulin, MHS; Dr. Stephen P. Peters; Dr. Cheryl Pirozzi; Dr. Nirupama Putcha, MHS; Dr. Elizabeth C. Oelsner, MPH; Dr. Wanda K. O’Neal; Dr. Victor E. Ortega; Dr. Sanjeev Raman; Dr. Stephen I. Rennard; Dr. Donald P Tashkin; Dr. J Michael Wells; Dr. Robert A. Wise; and Dr. Prescott G. Woodruff, MPH. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Dr. Lisa Postow and Lisa Viviano, BSN.
REFERENCES
- 1. Benowitz NL. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addiction. N Engl J Med 319: 1318–1330, 1988. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- 2. Marchand M, Brossard P, Merdjan H, Lama N, Weitkunat R, Lüdicke F. Nicotine population pharmacokinetics in healthy adult smokers: a retrospective analysis. Eur J Drug Metab Pharmacokinet 42: 943–954, 2017. doi: 10.1007/s13318-017-0405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benowitz NL, Hukkanen J, Jacob P 3rd.. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 192: 29–60, 2009. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jacob P 3rd, Yu L, Shulgin AT, Benowitz NL. Minor tobacco alkaloids as biomarkers for tobacco use: comparison of users of cigarettes, smokeless tobacco, cigars, and pipes. Am J Public Health 89: 731–736, 1999. doi: 10.2105/ajph.89.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baltar VT, Xun WW, Chuang S-C, Relton C, Ueland PM, Vollset SE, , et al. Smoking, secondhand smoke, and cotinine levels in a subset of EPIC cohort. Cancer Epidemiol Biomarkers Prev 20: 869–875, 2011. doi: 10.1158/1055-9965.EPI-10-1235. [DOI] [PubMed] [Google Scholar]
- 6. Clunes LA, Bridges A, Alexis N, Tarran R. In vivo versus in vitro airway surface liquid nicotine levels following cigarette smoke exposure. J Anal Toxicol 32: 201–207, 2008. doi: 10.1093/jat/32.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghosh A, Coakley RD, Ghio AJ, Muhlebach MS, Esther CR Jr, Alexis NE, Tarran R. Chronic E-cigarette use increases neutrophil elastase and matrix metalloprotease levels in the lung. Am J Respir Crit Care Med 200: 1392–1401, 2019. doi: 10.1164/rccm.201903-0615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung S, Baumlin N, Dennis JS, Moore R, Salathe SF, Whitney PL, Sabater J, Abraham WM, Kim MD, Salathe M. Electronic cigarette vapor with nicotine causes airway mucociliary dysfunction preferentially via TRPA1 receptors. Am J Respir Crit Care Med 200: 1134–1145, 2019. doi: 10.1164/rccm.201811-2087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crotty Alexander LE, Drummond CA, Hepokoski M, Mathew D, Moshensky A, Willeford A, Das S, Singh P, Yong Z, Lee JH, Vega K, Du A, Shin J, Javier C, Tian J, Brown JH, Breen EC. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am J Physiol Regul Integr Comp Physiol 314: R834–R847, 2018. [Erratum in Am J Physiol Regul Integr Comp Physiol 323: R483, 2022]. doi: 10.1152/ajpregu.00270.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia-Arcos I, Geraghty P, Baumlin N, Campos M, Dabo AJ, Jundi B, Cummins N, Eden E, Grosche A, Salathe M, Foronjy R. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax 71: 1119–1129, 2016. doi: 10.1136/thoraxjnl-2015-208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu Y, Zhang Y, Cardell LO. Nicotine exaggerates LPS-induced airway hyperreactivity via JNK-mediated up-regulation of Toll-like receptor 4. Am J Respir Cell Mol Biol 51: 370–379, 2014. doi: 10.1165/rcmb.2013-0409OC. [DOI] [PubMed] [Google Scholar]
- 12. Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, Kanner R, Kleerup E, Martinez FJ, Woodruff PG, Rennard S; Spiromics Research Group. Design of the subpopulations and intermediate outcomes in COPD study (SPIROMICS). Thorax 69: 491–494, 2014. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Esther CR Jr, O’Neal WK, Anderson WH, Kesimer M, Ceppe A, Doerschuk CM, Alexis NE, Hastie AT, Barr RG, Bowler RP, Wells JM, Oelsner EC, Comellas AP, Tesfaigzi Y, Kim V, Paulin LM, Cooper CB, Han MK, Huang YJ, Labaki WW, Curtis JL, Boucher RC; Subpopulations and Intermediate Outcome Measures in COPD Study. Identification of sputum biomarkers predictive of pulmonary exacerbations in COPD. Chest 161: 1239–1249, 2022. doi: 10.1016/j.chest.2021.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freeman CM, Crudgington S, Stolberg VR, Brown JP, Sonstein J, Alexis NE, Doerschuk CM, Basta PV, Carretta EE, Couper DJ, Hastie AT, Kaner RJ, O'Neal WK, Paine R 3rd, Rennard SI, Shimbo D, Woodruff PG, Zeidler M, Curtis JL. Design of a multi-center immunophenotyping analysis of peripheral blood, sputum and bronchoalveolar lavage fluid in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS). J Transl Med 13: 19, 2015. doi: 10.1186/s12967-014-0374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Esther CR Jr, O’Neal WK, Anderson WH, Kesimer M, Ceppe A, Doerschuk CM, Alexis NE, Hastie AT, Barr RG, Bowler RP, Wells JM, Oelsner EC, Comellas AP, Tesfaigzi Y, Kim V, Paulin LM, Cooper CB, Han MK, Huang YJ, Labaki WW, Curtis JL, Boucher RC; Subpopulations and Intermediate Outcome Measures in COPD Study. Identification of sputum biomarkers predictive of pulmonary exacerbations in chronic obstructive pulmonary disease. Chest 161: 1239–1249, 2022. doi: 10.1016/j.chest.2021.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest 111: 81–90, 2003. doi: 10.1172/JCI200316147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu Y, Cardell LO. Long-term nicotine exposure dampens LPS-induced nerve-mediated airway hyperreactivity in murine airways. Am J Physiol Lung Cell Mol Physiol 313: L516–L523, 2017. doi: 10.1152/ajplung.00222.2016. [DOI] [PubMed] [Google Scholar]
- 18. Li Q, Zhou X, Kolosov VP, Perelman JM. Nicotine suppresses inflammatory factors in HBE16 airway epithelial cells after exposure to cigarette smoke extract and lipopolysaccharide. Transl Res 156: 326–334, 2010. [Erratum in Transl Res 157: 322, 2011]. doi: 10.1016/j.trsl.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 19. Chen EY, Sun A, Chen CS, Mintz AJ, Chin WC. Nicotine alters mucin rheological properties. Am J Physiol Lung Cell Mol Physiol 307: L149–L157, 2014. doi: 10.1152/ajplung.00396.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zou W, Zou Y, Zhao Z, Li B, Ran P. Nicotine-induced epithelial-mesenchymal transition via Wnt/β-catenin signaling in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 304: L199–L209, 2013. doi: 10.1152/ajplung.00094.2012. [DOI] [PubMed] [Google Scholar]
- 21. Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis 148: 91–97, 1993. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- 22. Jarvis MJ, Fidler J, Mindell J, Feyerabend C, West R. Assessing smoking status in children, adolescents and adults: cotinine cut-points revisited. Addiction 103: 1553–1561, 2008. doi: 10.1111/j.1360-0443.2008.02297.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available from SPIROMICS on request.