Keywords: COVID, inflammation, lung, vagus nerve, VNS
Abstract
It is becoming increasingly appreciated that the nervous and immune systems communicate bidirectionally to regulate immunological outcomes in a variety of organs including the lung. Activation of neuronal signaling can be induced by inflammation, tissue damage, or pathogens to evoke or reduce immune cell activation in what has been termed a neuroimmune reflex. In the periphery, these reflexes include the cholinergic anti-inflammatory pathway, sympathetic reflex, and sensory nociceptor-immune cell pathways. Continual advances in neuroimmunology in peripheral organ systems have fueled small-scale clinical trials that have yielded encouraging results for a range of immunopathologies such as rheumatoid arthritis. Despite these successes, several limitations should give clinical investigators pause in the application of neural stimulation as a therapeutic for lung inflammation, especially if inflammation arises from a novel pathogen. In this review, the general mechanisms of each reflex, the evidence for these circuits in the control of lung inflammation, and the key knowledge gaps in our understanding of these neuroimmune circuits will be discussed. These limitations can be overcome not only through a better understanding of neuroanatomy but also through a systematic evaluation of stimulation parameters using immune activation in lung tissues as primary readouts. Our rapidly evolving understanding of the nervous and immune systems highlights the importance of communication between these cells in health and disease. This integrative approach has tremendous potential in the development of targeted therapeutics if specific challenges can be overcome.
INTRODUCTION
Bidirectional communication between the immune and nervous systems serves to initiate and refine host responses to immunological challenges. This communication between neurons and immune cells, or neuroimmune circuits, has been identified in primary and secondary lymphoid organs (1–6) and various organ systems. In the lung, neuroimmune circuits regulate the inflammatory response to bacterial and viral pathogens, as well as modulate the allergic responses (7–11). Although the function of these neuroimmune circuits has been predominantly characterized in the context of systemic inflammation (12–14), common mechanisms appear to regulate a diverse array of immune cells in different tissues (8, 15–20). This commonality is most likely due to the extensive innervation throughout the body by the peripheral nervous system, and the expression of neurotransmitter receptors by immune cells that facilitate the control of inflammation (21, 22). The highly specialized innervation regulates physiology in various organ systems as a function of neuroanatomy, with terminal axons releasing specific neurotransmitters in the target organ. These key features have been shown to regulate immune responses, highlighting the potential for targeted therapeutic intervention with neuronal stimulation by exploiting neuronal circuits that innervate specific tissues (22). Indicative of the therapeutic potential for this approach beyond systemic inflammation, neuronal stimulation reduces inflammation in preclinical models of postoperative ileitis (23), colitis (24), acute kidney injury (25, 26), ventilator-induced lung injury (VILI) (9), and acute respiratory distress syndrome (ARDS) in the lung (27). These promising results are based on the underlying assumption of a common neuronally evoked mechanism to limit inflammation; however, protection afforded by neuronal stimulation can be achieved through several neuronal pathways, differentially modulating immune cell types in the same target organ. As an additional complication, pathogens have also been identified to exploit these neuroimmune pathways to reduce host protective immunity (8), demonstrating the need for pathway-selective targeting. These concerns have not prevented limited clinical trials from demonstrating the efficacy of neurostimulation in the treatment of inflammatory diseases, including Crohn’s disease (28, 29) and rheumatoid arthritis (30). With these successes, and the emergence of SARS-CoV-2 as a novel respiratory virus that can induce significant morbidity and mortality as a consequence of inflammation, unfounded suggestions for the use of neurostimulator devices, such as vagus nerve stimulation (VNS), have been proposed as a possible therapeutic (31). This review seeks to introduce some of these pertinent issues and highlight the continued significant gaps in our understanding of neuroimmune circuits in the control of lung inflammation.
NEUROIMMUNE CIRCUITS
Interest in the impact of neuroimmune cross communication on host physiology has been reinvigorated in recent years. As there is tremendous diversity in the types of neurons in the peripheral nervous system, and in their innervation of organs, it should not be surprising that these neuroimmune circuits and the functional outcomes depend on the neuronal type, tissue innervated, and immune cell targets. It is well established that neurons express receptors for immune cell-derived molecules that can change host physiology and drive pathology. Sensory nociceptors can be directly activated by these proinflammatory mediators, in diverse organ systems ranging from the skin (32) to the lung (33, 34). The close proximity of immune cells to neurons has been well established in the lung to exert control over physiological processes during pathology. Neuronal activation and sensitization can occur in response to histamine, leukotrienes, and proteases released following the activation of mast cells, eosinophils, and neutrophils (35–38). In the lung, the consequences of immune cell-induced neuronal sensitization can include increased airway hypersensitivity during infection or allergic airway inflammation (7, 39). Physical contact between dendritic cells or T cells and neurons has been shown, highlighting the potential for communication between immune cells and the innervation at mucosal tissues such as the lung (40). Here we review the evidence for the regulation of lung inflammation by neuronal signaling.
Expression of Neurotransmitter Receptors by Immune Cells
Fundamental to the control of immune responses by neuronal signaling is the expression of neurotransmitter receptors by immune cells. Ionotropic receptors including nicotinic AChRs in addition to metabotropic G-protein-coupled receptors have recently been described to regulate key immune cells in both innate and adaptive populations. Complicating our understanding of neurotransmitter signaling that alters immune cell function is the structural homology of related receptors and G-protein-coupled receptors (GPCR), which complicates the production of specific antibodies (41). This homology means that studies assessing GPCR expression on immune cells, using antibodies that have not been validated using target gene knockout samples or other biological negatives to demonstrate specificity, should be viewed with some degree of skepticism. In the absence of this information, excellent resources such as RNAseq and single-cell SEQ databases [e.g., the Immunological Genome Project (42)], CellxGene (43), and antibody databases such as the Human Protein Atlas (44) can be used to help assess if specific genes are expressed by a cell type of interest. When using these references, low expression, sequencing depth, and the number of cells analyzed should be carefully considered as these critical factors could result in a “no-call” to be misinterpreted as a gene is not expressed. Here, we discuss select neurotransmitter receptors that are integral to neuroimmune circuits. Readers interested in comprehensive listings of neurotransmitter receptor expression by various immune cells are directed to recent in-depth reviews (45–48). In addition, we have provided a table highlighting critical ligands, receptors, and outcomes of activation on specific populations of immune cells for further clarification (Table 1).
Table 1.
Listing of neurotransmitter ligands and receptors present on immune cell populations
| Class | Subtype | Cell Types | Inflammation |
|---|---|---|---|
| Muscarinic acetylcholine | M1 Gq/11 | Dendritic cells (53) *mouse | ND |
| Monocytes (54) | ND | ||
| Alveolar macrophages (54) | No effect (55) | ||
| M2 Gi/o | Dendritic cells (53) *mouse | ND | |
| Monocytes (54) | No effect (54) | ||
| Alveolar macrophages (54) | No effect (54, 55) | ||
| Neutrophils (56) | ND | ||
| M3 Gq/11 | Dendritic cells (53) | ↓ TNFα (57) | |
| Monocytes (54) | No effect (54) | ||
| Alveolar macrophages (54, 58) | ↑LTB4, ↓NF-κB signaling (55) | ||
| M4 Gi/o | Dendritic cells (53) | ↓ TNFα (57) | |
| Monocytes (54) | ND | ||
| Neutrophils (56) | ND | ||
| Alveolar macrophages (54) | ND | ||
| M5 Gq/11 | Dendritic cells (53) | ↓ TNFα (57) | |
| Monocytes (54) | ND | ||
| Alveolar macrophages (54) | ND | ||
| Neutrophils (56) | ND | ||
| Nicotinic acetylcholine | α2 | Neutrophils (59) | ND |
| Dendritic cells (53) | ND | ||
| Monocytes (60) | ND | ||
| α3 | Neutrophils (59) | ND | |
| α4β2 | Neutrophils (59) | ND | |
| Alveolar macrophages (61) | ↓ IL-6, IL-12, TNFα (61) | ||
| α3β2 | Neutrophils (59) | ↑ IL-8 | |
| α4 | Neutrophils (59) | ↑ IL-8 | |
| α5 | Neutrophils (59) | ND | |
| Dendritic cells (53) | ND | ||
| Monocytes (60) | ND | ||
| α6 | Neutrophils (59) | ND | |
| Dendritic cells (53) | ND | ||
| Monocytes (60) | ND | ||
| α7 | Monocytes (62) | ↓ TNFα, IL-1β, IL-12 (63) | |
| Alveolar macrophages | ↓ TNFα, MIP2, HMGB1 (123) | ||
| Neutrophils (59, 62) | ↓ TNFα, MIP2, HMGB1 (123) | ||
| Dendritic cells (53) | Inhibit antigen processing (64) | ||
| α9 | Neutrophils (59) | ND | |
| Monocytes (60) | ↓TNFα, IL-1β, IL-12 (63) | ||
| α10 | Dendritic cells (53) | ND | |
| Monocytes (60) | ND | ||
| α Adrenergic | α1 | Monocytes (65) | ↑ IL-1β, ↑ p38 MAPK activation (66), ↑ complement (C2) synthesis (67), ↓ TNFα, IL-8, MIP1B (68) |
| Neutrophils (69) | ↑ proliferation (70) | ||
| Dendritic cells (71, 72) | ↓ mobility (71) | ||
| NK cells (73) | ↑ cytotoxicity (74) | ||
| α2 | Neutrophils (69) | NE (70) | |
| Dendritic cells (75) | ↑ mobility (71) | ||
| NK cells (73) | ↑ cytotoxicity (74) | ||
| α3 | Neutrophils (69) | ND | |
| β Adrenergic | β1 | Monocytes (76, 77) | ↑ cAMP, ↑ IL-1β (77) |
| Neutrophils (69) | Inhibition of migration (78) | ||
| Dendritic cells (75, 79) | ND | ||
| β2 | Alveolar macrophages (80) | ↓ TNFα, ↓ JNK phosphorylation (81, 82), ↑ IL-10 (82, 83) | |
| Monocytes (76) | ↓ TNFα, ↓ GM-CSF (84) | ||
| Neutrophils (85, 86) | ↓ IL-8 induced chemotaxis (86), ↓ netosis (87) | ||
| Dendritic cells (72, 75) | ↓ TNFα, ↑ IL-10 (83) | ||
| NK cells (73) | ↑ proliferation (88) | ||
| Eosinophils (89) | ↓ respiratory burst (90), LTC4 (91), No effect on degranulation (89) | ||
| Basophils (92) | Inhibit histamine release (93) | ||
| Mast cells (94) | ↓ histamine (95–97) / leukotriene (98) release | ||
| β3 | Neutrophils (69) | ND |
| Ligand | Receptor | Cell Types | Inflammation |
|---|---|---|---|
| SP | NK1R | Neutrophils (99) | ↑ MIP-1a, MIP-2, CCR1, CXCR2 (100) |
| NK cells (101) | ↓ cytotoxic activity, degranulation, ↓ phospho-ERK (102) | ||
| Monocytes (103) | ↑ NF-κB expression, NLRP3 (104) | ||
| Mast cells (105) | ↑ mast cell accumulation (106) Degranulation (105) | ||
| Dendritic cells (107) | Modulate T-cell proliferation (107), ↑ DC survival (108) | ||
| Cell type not specified | Pro-oncogenic (109) | ||
| NK2R | Airway dendritic cells (110, 111) | ↑ type I IFN expression (110) | |
| Airway eosinophils (112) | ↑ survival (113) | ||
| CGRP | RAMP1/CLR | Alveolar macrophages (114) | ↓ TNFα, FcγR-mediated phagocytosis, ↓ pro-IL-1β (115) |
| Neutrophils (114) | Granule secretion (116) | ||
| Dendritic cells (117) | ↓CCR2 and CCR7 expression (118) | ||
| Monocytes (114) | ↓ MCP-1, TNFα, ↑ IL-6 (149) | ||
| Mast cells (150) | NE on degranulation (105) | ||
| Eosinophil precursors (151) | ↓ recruitment (152) |
ND, not detected.
Cholinergic Anti-Inflammatory Pathway
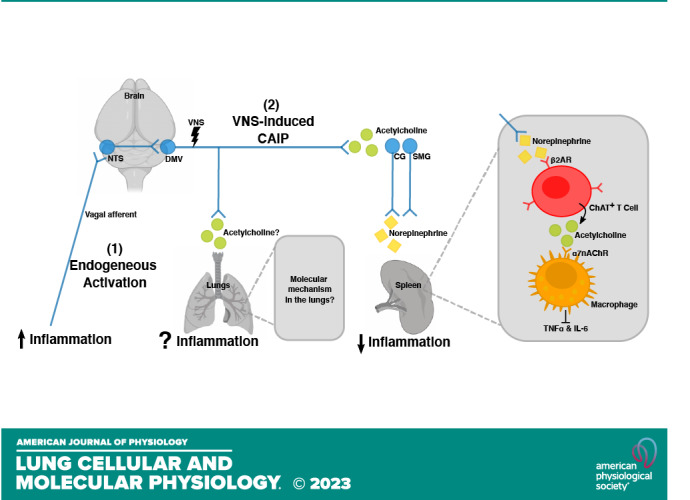
The cholinergic anti-inflammatory pathway (CAIP) is a well-established neuroimmune circuit that regulates inflammation in a variety of organ systems. Similarly, selective efferent VNS, achieved by transection of the vagus and application of electrical stimulation distal to the cut site, significantly inhibits macrophage activation and consequently reduces TNFα production in the spleen and select lymph nodes. Immunomodulatory effects evoked by VNS, however, are indirect, since there is no direct vagal innervation of the spleen or lymph nodes. Instead, vagal efferent fibers activate sympathetic neurons in the superior mesenteric/celiac ganglia complex that project into the spleen and MLN (45). Sympathetic activation results in localized norepinephrine (NE) release and induces a unique T-cell population that expresses the enzyme choline acetyltransferase (ChAT) to release acetylcholine (ACh) (2, 4, 119). This T-cell-derived ACh binds to the nicotinic α 7 receptor (α7R) on macrophages to suppress proinflammatory cytokine production by inhibiting NF-κB (14, 23, 120). Together, these cells constitute the CAIP (Fig. 1), and numerous studies have highlighted the utility of activating this circuitry in models of kidney ischemia-reperfusion injury (27) and intestinal inflammation (23, 24). Although VNS has demonstrated great potential in these preclinical studies and early clinical trials, great care should be taken in ascribing an anti-inflammatory effect of VNS to activation of the CAIP. Recent studies have revealed several neuroimmune circuits, each with unique components, which converge in the spleen and lymph nodes and are capable of regulating immune cells. It should be noted that the composition of the vagus nerve in most animal species is predominately afferent fibers. With respect to the lung, the bronchial vagal afferents are ∼60% afferent and 40% efferent (121), and although the effects of CAIP have been ascribed to activation of vagal efferent fibers, current vagal nerve stimulators activate both efferent and afferent signaling. Highlighting the importance of understanding the circuits activated by VNS, we have shown that blockade of LPS-induced inflammation by efferent VNS required ChAT+ T cells, whereas afferent selective VNS prevented inflammation independently of these specialized T cells (119). Afferent VNS activates a neuroimmune circuit that reduces immune regulation, dependent on β2 adrenergic receptors (β2AR) signaling but independent of the CAIP (119). In addition, afferent VNS has been shown to induce an efferent splanchnic anti-inflammatory mechanism, capable of suppressing LPS-induced proinflammatory cytokine production systemically (122) (Fig. 2). With this in mind, it is possible that prior reports of VNS-induced regulation of inflammation may occur independently of CAIP, through activation of other unknown neuroimmune circuits. We propose that VNS-mediated control of inflammation should not be ascribed to the CAIP unless there is sufficient evidence for components of this neuroimmune circuit such as ChAT+ T cells being required.
Figure 1.
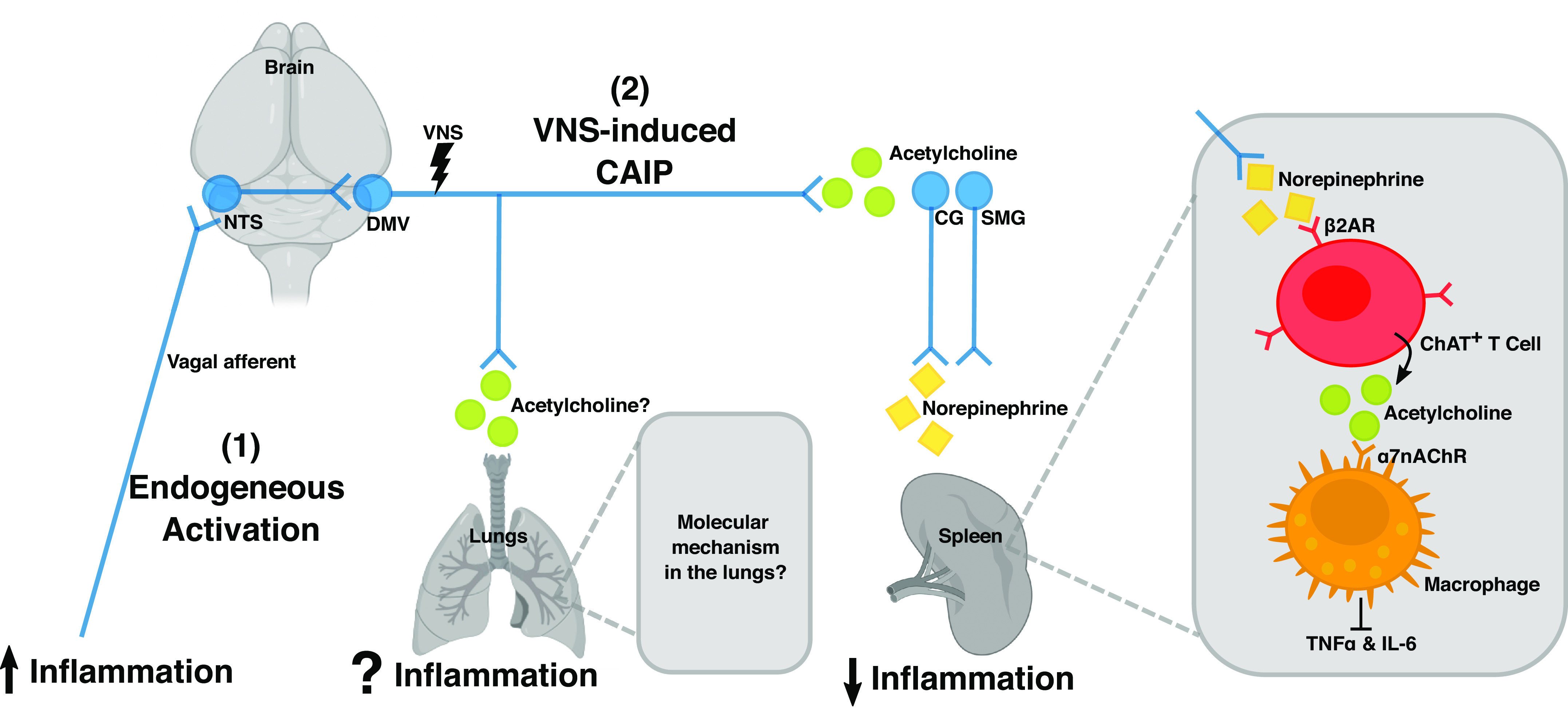
A schematic illustration of the cholinergic anti-inflammatory pathway (CAIP) in the spleen. Vagal efferent fibers activate sympathetic neurons in the superior mesenteric ganglia (SMG) or celiac ganglia (CG) that project into the spleen. This leads to a local release of norepinephrine, which induces choline acetyltransferase (ChAT)-positive T cells to release acetylcholine. Acetylcholine in turn activates macrophages by binding to the nicotinic α 7 receptor (α7nChR), which inhibits the production of proinflammatory cytokine, such as TNFα and IL-6. Together, these cells constitute the CAIP, and selective efferent VNS modulates inflammation in the spleen via this pathway. It remains elusive if the same neuroimmune circuit is present in the lungs. [Image created with BioRender.com and published with permission.]
Figure 2.
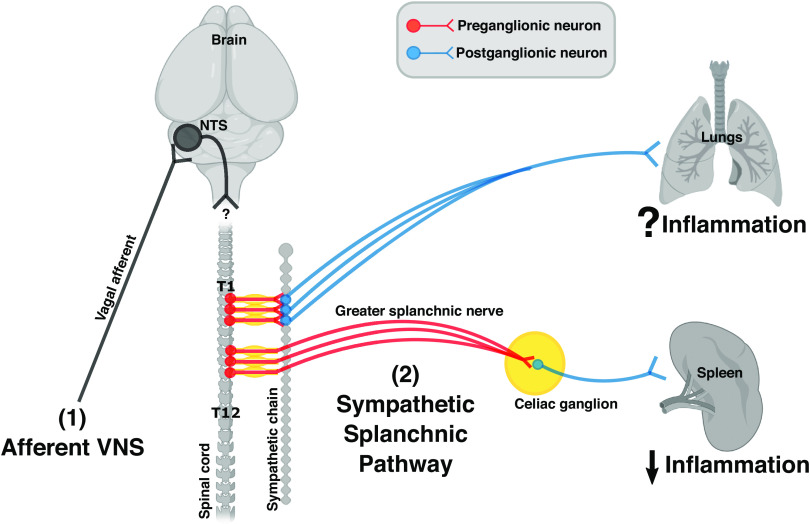
The organization of sympathetic innervations in the spleen and the lungs. Cell bodies of sympathetic preganglionic neurons illustrated here (red) are in the thoracic spinal cord between level T1 and T12. Axons from preganglionic neurons (located in T1 to T5) synapse with postganglionic neurons (blue) at the paravertebral ganglia within the sympathetic chain. Axons from these postganglionic neurons travel to and innerve the lungs. The immunomodulatory effect of this pathway remains unknown. In contrast, axons from preganglionic neurons (located in T6 to T8) enter the greater splanchnic nerve, synapse with postganglionic neurons at the celiac ganglion, and innervate the spleen. Activation of this sympathetic splanchnic pathway is known to suppress inflammation in the spleen. [Image created with BioRender.com and published with permission.]
Evidence for the CAIP in the lung.
Although there is growing evidence for neuroimmune control of lung inflammation, a critical distinction should be made between the CAIP and other neuroimmune circuits. It is exciting to consider that prior literature describing the regulation of inflammation in the lung may have incorrectly ascribed these effects to CAIP, instead of other novel neuroimmune circuits activated by VNS. The vagus nerve is critical in mediating lung inflammation, with vagotomy significantly increasing VILI and inflammation in rats, as indicated by increased IL-6 production and neutrophil recruitment compared with surgical controls (9). Further supporting a lung anti-inflammatory role of the vagus, efferent selective VNS reduced IL-6 production and granulocyte recruitment (9). Although α7R expression and signaling in bronchial epithelial cells were investigated in vitro, these studies did not demonstrate that the anti-inflammatory effects of VNS required α7R to suppress cytokine production in vivo (9). It is unclear from these studies if the effects of VNS required α7R expressed on lung epithelial cells, macrophages, ChAT+ T cells, or the spleen/lymph nodes as components of the CAIP.
Further evidence for a vagal anti-inflammatory pathway in the lung is suggested by the observations that vagotomy increases inflammation during bacterial pneumonia (123). Although these effects were proposed to be dependent on the α7R, this conclusion is based on reduced inflammation after administration of selective α7R agonists, or increased inflammation induced by E. pneumonia in wild-type (WT) compared with α7R−/− mice, without assessing the contribution of the vagus to either effect (123). Increased inflammation has also been observed in α7R−/− mice or mice with right cervical vagotomy during influenza A infection (124). More recently famotidine, an H2 histamine receptor antagonist, reduced inflammation during systemic endotoxemia that was abrogated when the mice underwent a bilateral vagotomy (125). In each of these studies, it remains unclear if the vagal anti-inflammatory effects were due to CAIP, or another anti-inflammatory neuroimmune pathway. It should be noted that experiments using α7R agonists to reduce inflammation during bacterial pneumonia (123) or ventilator-induced injury and inflammation (126) are not direct evidence of the CAIP in the lung, but are instead the direct effects of these compounds, and are in agreement with previously identified anti-inflammatory roles of nicotinic receptors.
Regulation of Lung Inflammation by Catecholamines and Sympathetic Innervation
The release of catecholamines (epinephrine, norepinephrine, and dopamine) occurs following sympathetic neuronal activation, or from chromaffin cells in the adrenal medulla. Although there is abundant sympathetic innervation of the lung, historically this innervation is thought to be associated with the vasculature with few fibers identified beyond the lung hilus in mice and rats (127). Although this would suggest that these fibers are not in close physical proximity to the majority of immune cells, ablation of lung sympathetic innervation by surgical denervation or chemical sympathectomy significantly enhanced LPS-induced lung inflammation (128). It is important to note that, although chemical sympathectomy reduced tyrosine hydroxylase staining in the lung but not in the spleen, thymus, or bone marrow, the adrenal medulla was not assessed (128). These data suggest that chemical sympathectomy was restricted to the lung, but do not exclude potential off-target effects from the adrenal glands. Nevertheless, these exciting results demonstrate that alveolar macrophages (AMs) and innate lymphoid cells (ILC) express β2AR and that NE can block the production of proinflammatory cytokines induced by LPS or IL-33 (128).
As evidence of the complexity in the regulation of immune responses, proinflammatory roles for β2AR have also been described (129). Inhalation of particulate matter (PM) typical in air pollution increases the expression of proinflammatory cytokines, such as IL-6, by AMs and consequently induces thrombosis. This inflammatory state is dependent on PM-inducing catecholamine release and activation of β2AR on macrophages. Observations supporting the critical role of AMs include prevention of PM-induced inflammation on chlodronate liposome intratracheal administration (130), as well as significantly reduced IL-6 in LysM.Cre β2ARf/f mice compared with controls (131). However, as neither approach selectively targeted AMs, it cannot be conclusively determined that this effect is solely restricted to AMs.
Although it is uncertain how β2AR signaling contributes to airway inflammation, noncanonical β arrestin-mediated signaling in AM appears to play a significant role in the pathogenesis of allergic airway inflammation. In β2-arrestin (ARRB2)−/− mice, sensitization and subsequent exposure to ovalbumin (OVA) reduced T-cell chemotaxis, recruitment, and Th2 cytokine production in the lung (e.g., IL-4, IL-5 production) compared with WT mice. This reduction in immune-mediated pathology was not associated with reduced OVA-specific IgE, or with simply increased production of Th1 type cytokines. Moreover, acute inflammation following LPS challenge was identical in ARRB2−/− and WT mice (132). These data suggest that GPCR activation leading to ARRB2 binding can be proinflammatory in specific models of inflammation and unique immune cells and that this aspect of neuronal control of inflammation will require additional careful study.
Control of Lung Inflammation by Sensory Neurons
Similar to many organs, the lung is densely innervated with sensory neurons that induce physiological changes in response to noxious stimuli such as mechanical injury and inflammation (133, 134). The innervation consists of unmyelinated C-fibers arising from the dorsal root ganglia (DRG) in the spinal cord and specialized vagal afferent nociceptive neurons originating from the nodose and intracranial jugular ganglia (34, 135). Anatomically these nociceptors are extensively branched, similar to somatosensory nociceptors, and can be found from the conducting airways through to alveoli (34, 135). In these regions of the lung, vagal nociceptive afferents can form close associations and protrude into clusters of pulmonary neuroendocrine cells (PNECs) in a neuroendocrine bundle (136). This bundle is in contrast to the sensory nociceptors from the DRG that form plexi adjacent to PNECs (136), suggesting that each type responds to divergent stimuli. Activation of these neurons through the nociceptive transient receptor potential cation channel subfamily V member 1 (TRPV1) triggers the release of peptide neurotransmitters such as substance P, calcitonin gene-related peptide, and neurokinin-A. This localized neurotransmitter release is the foundation of neurogenic inflammation, resulting in chemotaxis of immune cells (137), vasodilation (138), increased endothelial permeability (139), and adhesion molecule expression (140). In addition to these classically defined roles, sensory nociceptive neurons have recently emerged as playing a critical role in the coordination of host protective immunity during bacterial infection of the lung or skin, highlighting a previously unappreciated role of neuroimmune communication in maintaining host protection (141, 142).
Despite the well characterized immune cell recruitment and activation during neurogenic inflammation, the mechanistic role of sensory nociceptive neurons in coordinating mucosal immune responses during infection in the lung have only begun to be elucidated. Elegant experiments have demonstrated that Staphylococcus aureus activates TRPV1+ vagal nociceptors to suppress host protective responses by inhibiting neutrophil and γδ T-cell recruitment (8). This effect was attributed to CGRP release by TRPV1+ nociceptors, as a CGRP peptide antagonist improved survival following lethal S. aureus challenge (8). These results are in keeping with the activation of TRPV1+ somatosensory neurons by the bacterial pathogen Streptococcus pyogenes in the skin, which inhibits neutrophil recruitment in a CGRP-dependent manner (142). Although these results suggest TRPV1+ nociceptors could function generally as inhibitory neuroimmune circuits, TRPV1+ neurons are also required for host protection during infection with the enteric bacterial pathogens Citrobacter rodentium and Salmonella enterica serovar Typhimurium (143, 144). Together, these results suggest that the response of a neuroimmune circuit comprised of sensory nociceptors could depend on the type of pathogen.
Sensory nociceptors have also been described to contribute to the development of allergen-induced airway inflammation (35). The transient receptor potential channel TRPA1, which functions as a sensor of both mechanical stress and reactive compounds, such as cinnamaldehyde and hydrogen peroxides, is often coexpressed with TRPV1 by nociceptors (145). Highlighting the divergent roles of these channels in allergic lung inflammation, mice deficient for TRPA1 but not TRPV1 showed a significant reduction in immune cell recruitment into the lung, Th2 type cytokine production, goblet cell hyperplasia, and mucus production in the OVA-sensitization and challenge model (35). The significantly reduced antigen-induced airway inflammation in TRPA1−/− mice was proposed to be due to reduced release of neuropeptides including CGRP and neurokinin-A in the lung and could be recapitulated using WT mice treated with a TRPA1 selective antagonist (35). Thus, although depletion of sensory neurons by targeting TRPV1+ neurons can result in functional changes, one should be careful in ascribing these changes to this particular receptor.
OUTLOOK/POTENTIAL FOR NEURAL STIMULATION IN THE TREATMENT OF LUNG INFLAMMATION
The preclinical advances in understanding of neuroimmune circuitry and, in particular, of VNS-induced suppression of inflammation have led to a push for clinical translation in a variety of inflammatory conditions including COVID-19 (31, 49, 146–148). Despite these successes, it is informative to look at each of the study outcomes, noting differences depending on the site of stimulation and the VNS parameters used. Clinical VNS is typically provided by surgical implantation of an electrode on the cervical vagus, transcutaneous electrical nerve stimulation (TENS) unit, or an electrode on the concha of the ear, which provides stimulation of the auricular branch of the vagus (50). As each paradigm is unique it should not be surprising that different parameters are required. For example, direct contact between the nerve and electrode requires significantly less current to evoke stimulation compared with TENS or auricular devices. These differences in VNS techniques can have significant effects on the ability to selectively induce vagal nerve activation and clinical outcome. Nonetheless, therapeutic effects of VNS are clear in diseases such as rheumatoid arthritis, with significant reductions in inflammatory cytokines, C-reactive protein (CRP), and disease activity scores achieved in patients implanted with a helical electrode to provide cervical VNS (30). Remarkable reductions in clinical disease (primary end point) and serum TNFα have also been observed in a proof-of-concept study using noninvasive auricular VNS (51). With the unique VNS site and parameters selected, it is, however, uncertain if this effect is due to the same mechanism of action. At present, it is unclear how much these factors contribute to VNS nonresponsive patients. A thorough understanding of the parameter space, time from disease onset, and when in the course of disease treatment VNS should be applied are desperately needed in the field.
Although there are several registered VNS clinical trials in the treatment of both acute COVID-19 (NCT04368156 NCT04379037, NCT04514627, NCT05058742) and long COVID-19 (NCT05225220), peer-reviewed results have not been published. At present, results from small open-label studies and preprint publications have suggested mixed benefits of VNS in acute COVID-19. Although limited cases reported improved patient symptoms with transcutaneous VNS (49), a randomized control trial of patients with COVID-19 (47 VNS, 50 control patients) found significantly reduced inflammation by serum CRP, and procalcitonin with VNS treatment compared with standard of care controls (52). Despite these promising findings, no benefit was observed in respiratory or clinical outcomes. These data suggest that VNS may have some therapeutic potential; however, the identification of individual parameters or time for treatment are critical factors. Confirmation of neuronal engagement, such as monitoring heart rate variability (HRV), during stimulation does not appear in many protocols, making it unclear if the lack of benefit was due to a lack of biological effect or inappropriate stimulation parameters. Although no adverse events were reported in these studies, the use of a neural stimulation device without understanding the basic mechanisms of action in the lung is concerning. From preclinical studies with bacterial pathogens where vagal sensory nociceptors in the lung prevented clearance of the pathogen in a host maladaptive process, it is unclear if VNS could engage these circuits as well, preventing desired host responses to viral infection. These uncertainties argue for continued research into the effects of VNS, not only in mice but in other animal species with similar neuroanatomy to humans, with a consideration of the parameters used to evoke neuronal action. Finally, careful control and reporting of these parameters, site of stimulation, and confirmation of neuronal engagement should be undertaken. We would further propose that a closed-loop system whereby HRV is monitored in real-time while adjusting VNS parameters could be used to elicit maximal engagement while minimizing undesired effects.
CONCLUSIONS
Communication between the nervous and immune systems evoked by inflammation or pathogens can significantly affect the host immune response. Although exogenous exploitation of these circuits (typically in the form of VNS) reduces inflammation in a multitude of immunopathologies including VILI-ARDS in the lung, continued research is required to identify new neuroimmune circuits and refine existing ones. These gaps in our knowledge are significant and attempting clinical translation of these approaches when critical parameters remain unknown risks complete failure and abandonment of the technique and its therapeutic potential. These challenges are not insurmountable but instead should instruct and inspire future research in neuroimmunology and neuroimmune circuitry in the treatment of lung inflammation.
GRANTS
This work was supported in part by National Institute of Allergy and Infectious Diseases Grants R01 AI150647 and R21 AI148188 (to C. Reardon) and National Institute of General Medical Sciences Grants T32 GM099608 and GM144303 (to S. Schreiber).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.X.Y.T. prepared figures; M.C., S.S., and C.R. drafted manuscript; M.C., S.S., K.M., and C.R. edited and revised manuscript; C.R. approved final version of manuscript.
ACKNOWLEDGMENTS
Graphical abstract image created with BioRender.com and published with permission.
REFERENCES
- 1. Murray K, Godinez DR, Brust-Mascher I, Miller EN, Gareau MG, Reardon C. Neuroanatomy of the spleen: Mapping the relationship between sympathetic neurons and lymphocytes. PLOS ONE 12: e0182416, 2017. doi: 10.1371/journal.pone.0182416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray K, Barboza M, Rude KM, Brust-Mascher I, Reardon C. Functional circuitry of neuro-immune communication in the mesenteric lymph node and spleen. Brain Behav Immun 82: 214–223, 2019. doi: 10.1016/j.bbi.2019.08.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med 203: 1623–1628, 2006. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334: 98–101, 2011. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bratton BO, Martelli D, McKinley MJ, Trevaks D, Anderson CR, McAllen RM. Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp Physiol 97: 1180–1185, 2012. doi: 10.1113/expphysiol.2011.061531. [DOI] [PubMed] [Google Scholar]
- 6. Martelli D, Farmer DGS, McKinley MJ, Yao ST, McAllen RM. Anti-inflammatory reflex action of splanchnic sympathetic nerves is distributed across abdominal organs. Am J Physiol Regul Integr Comp Physiol 316: R235–R242, 2019. doi: 10.1152/ajpregu.00298.2018. [DOI] [PubMed] [Google Scholar]
- 7. Talbot S, Abdulnour R-EE, Burkett PR, Lee S, Cronin SJF, Pascal MA, Laedermann C, Foster SL, Tran JV, Lai N, Chiu IM, Ghasemlou N, DiBiase M, Roberson D, Von Hehn C, Agac B, Haworth O, Seki H, Penninger JM, Kuchroo VK, Bean BP, Levy BD, Woolf CJ. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron 87: 341–354, 2015. doi: 10.1016/j.neuron.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baral P, Umans BD, Li L, Wallrapp A, Bist M, Kirschbaum T, Wei Y, Zhou Y, Kuchroo VK, Burkett PR, Yipp BG, Liberles SD, Chiu IM. Nociceptor sensory neurons suppress neutrophil and gammadelta T cell responses in bacterial lung infections and lethal pneumonia. Nat Med 24: 417–426, 2018. doi: 10.1038/nm.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. dos Santos CCD, Shan Y, Akram A, Slutsky AS, Haitsma JJ. Neuroimmune regulation of ventilator-induced lung injury. Am J Respir Crit Care Med 183: 471–482, 2011. doi: 10.1164/rccm.201002-0314OC. [DOI] [PubMed] [Google Scholar]
- 10. Bienenstock J, Perdue M, Blennerhassett M, Stead R, Kakuta N, Sestini P, Vancheri C, Marshall J. Inflammatory cells and the epithelium. Mast cell/nerve interactions in the lung in vitro and in vivo. Am Rev Respir Dis 138: S31–S34, 1988. doi: 10.1164/ajrccm/138.6_Pt_2.S31. [DOI] [PubMed] [Google Scholar]
- 11. Horkowitz AP, Schwartz AV, Alvarez CA, Herrera EB, Thoman ML, Chatfield DA, Osborn KG, Feuer R, George UZ, Phillips JA. Acetylcholine regulates pulmonary pathology during viral infection and recovery. ImmunoTargets Ther 9: 333–350, 2020. doi: 10.2147/ITT.S279228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med 265: 663–679, 2009. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci USA 105: 11008–11013, 2008. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421: 384–388, 2003. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 15. Foster SL, Seehus CR, Woolf CJ, Talbot S. Sense and immunity: context-dependent neuro-immune interplay. Front Immunol 8: 1463, 2017. doi: 10.3389/fimmu.2017.01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cyphert JM, Kovarova M, Allen IC, Hartney JM, Murphy DL, Wess J, Koller BH. Cooperation between mast cells and neurons is essential for antigen-mediated bronchoconstriction. J Immunol 182: 7430–7439, 2009. doi: 10.4049/jimmunol.0900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sawatzky DA, Kingham PJ, Court E, Kumaravel B, Fryer AD, Jacoby DB, McLean WG, Costello RW. Eosinophil adhesion to cholinergic nerves via ICAM-1 and VCAM-1 and associated eosinophil degranulation. Am J Physiol Lung Cell Mol Physiol 282: L1279–L1288, 2002. doi: 10.1152/ajplung.00279.2001. [DOI] [PubMed] [Google Scholar]
- 18. Galle-Treger L, Suzuki Y, Patel N, Sankaranarayanan I, Aron JL, Maazi H, Chen L, Akbari O. Nicotinic acetylcholine receptor agonist attenuates ILC2-dependent airway hyperreactivity. Nat Commun 7: 13202, 2016. doi: 10.1038/ncomms13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164: 378–391, 2016. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pirzgalska RM, Seixas E, Seidman JS, Link VM, Sánchez NM, Mahú I, Mendes R, Gres V, Kubasova N, Morris I, Arús BA, Larabee CM, Vasques M, Tortosa F, Sousa AL, Anandan S, Tranfield E, Hahn MK, Iannacone M, Spann NJ, Glass CK, Domingos AI. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med 23: 1309–1318, 2017. doi: 10.1038/nm.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen C-S, Barnoud C, Scheiermann C. Peripheral neurotransmitters in the immune system. Curr Opin Physiol 19: 73–79, 2021. doi: 10.1016/j.cophys.2020.09.009. [DOI] [Google Scholar]
- 22. Hodo TW, de Aquino MTP, Shimamoto A, Shanker A. Critical neurotransmitters in the neuroimmune network. Front Immunol 11: 1869, 2020. doi: 10.3389/fimmu.2020.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud H-R, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol 6: 844–851, 2005. [Erratum in Nat Immunol 6: 954, 2005]. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 24. Koren T, Yifa R, Amer M, Krot M, Boshnak N, Ben-Shaanan TL, Azulay-Debby H, Zalayat I, Avishai E, Hajjo H, Schiller M, Haykin H, Korin B, Farfara D, Hakim F, Kobiler O, Rosenblum K, Rolls A. Insular cortex neurons encode and retrieve specific immune responses. Cell 184: 5902–5915.e17, 2021. doi: 10.1016/j.cell.2021.10.013. [DOI] [PubMed] [Google Scholar]
- 25. Inoue T, Abe C, Sung S-SJ, Moscalu S, Jankowski J, Huang L, Ye H, Rosin DL, Guyenet PG, Okusa MD. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through α7nAChR+ splenocytes. J Clin Invest 126: 1939–1952, 2016. doi: 10.1172/JCI83658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abe C, Inoue T, Inglis MA, Viar KE, Huang L, Ye H, Rosin DL, Stornetta RL, Okusa MD, Guyenet PG. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat Neurosci 20: 700–707, 2017. doi: 10.1038/nn.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li S, Qi D, Li J-N, Deng X-Y, Wang D-X. Vagus nerve stimulation enhances the cholinergic anti-inflammatory pathway to reduce lung injury in acute respiratory distress syndrome via STAT3. Cell Death Discov 7: 63, 2021. doi: 10.1038/s41420-021-00431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sinniger V, Pellissier S, Fauvelle F, Trocmé C, Hoffmann D, Vercueil L, Cracowski J‐L, David O, Bonaz B. A 12-month pilot study outcomes of vagus nerve stimulation in Crohn's disease. Neurogastroenterol Motil 32: e13911, 2020. doi: 10.1111/nmo.13911. [DOI] [PubMed] [Google Scholar]
- 29. Bonaz B, Sinniger V, Hoffmann D, Clarençon D, Mathieu N, Dantzer C, Vercueil L, Picq C, Trocmé C, Faure P, Cracowski J-L, Pellissier S. Chronic vagus nerve stimulation in Crohn's disease: a 6-month follow-up pilot study. Neurogastroenterol Motil 28: 948–953, 2016. doi: 10.1111/nmo.12792. [DOI] [PubMed] [Google Scholar]
- 30. Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ, Tak PP. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci USA 113: 8284–8289, 2016. doi: 10.1073/pnas.1605635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Azabou E, Bao G, Bounab R, Heming N, Annane D. Vagus nerve stimulation: a potential adjunct therapy for COVID-19. Front Med (Lausanne) 8: 625836, 2021. doi: 10.3389/fmed.2021.625836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tamari M, Ver Heul AM, Kim BS. Immunosensation: neuroimmune cross talk in the skin. Annu Rev Immunol 39: 369–393, 2021. doi: 10.1146/annurev-immunol-101719-113805. [DOI] [PubMed] [Google Scholar]
- 33. Lee LY, Yu J. Sensory nerves in lung and airways. Compr Physiol 4: 287–324, 2014. [Erratum in Compr Physiol 5: 1971, 2015]. doi: 10.1002/cphy.c130020. [DOI] [PubMed] [Google Scholar]
- 34. Mazzone SB, Undem BJ. Vagal afferent innervation of the airways in health and disease. Physiol Rev 96: 975–1024, 2016. doi: 10.1152/physrev.00039.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D'Amours M, Witek JS, Fanger CM, Chong JA, Hayward NJ, Homer RJ, Cohn L, Huang X, Moran MM, Jordt S-E. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci USA 106: 9099–9104, 2009. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hsu C-C, Lin YS, Lin R-L, Lee L-Y. Immediate and delayed potentiating effects of tumor necrosis factor-α on TRPV1 sensitivity of rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 313: L293–L304, 2017. doi: 10.1152/ajplung.00235.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu S, Kollarik M, Ouyang A, Myers AC, Undem BJ. Mast cell-mediated long-lasting increases in excitability of vagal C fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 293: G850–G856, 2007. doi: 10.1152/ajpgi.00277.2007. [DOI] [PubMed] [Google Scholar]
- 38. Gu Q, Lim ME, Gleich GJ, Lee L-Y. Mechanisms of eosinophil major basic protein-induced hyperexcitability of vagal pulmonary chemosensitive neurons. Am J Physiol Lung Cell Mol Physiol 296: L453–L461, 2009. doi: 10.1152/ajplung.90467.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tränkner D, Hahne N, Sugino K, Hoon MA, Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci USA 111: 11515–11520, 2014. doi: 10.1073/pnas.1411032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Veres TZ, Shevchenko M, Krasteva G, Spies E, Prenzler F, Rochlitzer S, Tschernig T, Krug N, Kummer W, Braun A. Dendritic cell-nerve clusters are sites of T cell proliferation in allergic airway inflammation. Am J Pathol 174: 808–817, 2009. doi: 10.2353/ajpath.2009.080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jo M, Jung ST. Engineering therapeutic antibodies targeting G-protein–coupled receptors. Exp Mol Med 48: e207, 2016. doi: 10.1038/emm.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heng TS, Painter MW, Immunological Genome Project Consortium. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol 9: 1091–1094, 2008. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 43. Megill C, Martin B, Weaver C, Bell S, Prins L, Badajoz S, McCandless B, Pisco AO, Kinsella M, Griffin F, Kiggins J, Haliburton G, Mani A, Weiden M, Dunitz M, Lombardo M, Huang T, Smith T, Chambers S, Freeman J, Cool J, Carr A. cellxgene: a performant, scalable exploration platform for high dimensional sparse matrices. bioRxiv, 2021. doi: 10.1101/2021.04.05.438318. [DOI]
- 44. Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, Wernerus H, Björling L, Ponten F. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol 28: 1248–1250, 2010. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 45. Reardon C, Murray K, Lomax AE. Neuroimmune communication in health and disease. Physiol Rev 98: 2287–2316, 2018. doi: 10.1152/physrev.00035.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martelli D, Farmer DGS, Yao ST. The splanchnic anti-inflammatory pathway: could it be the efferent arm of the inflammatory reflex? Exp Physiol 101: 1245–1252, 2016. doi: 10.1113/EP085559. [DOI] [PubMed] [Google Scholar]
- 47. McAllen RM, McKinley MJ, Martelli D. Reflex regulation of systemic inflammation by the autonomic nervous system. Auton Neurosci 237: 102926, 2022. doi: 10.1016/j.autneu.2021.102926. [DOI] [PubMed] [Google Scholar]
- 48. Pongratz G, Straub RH. Role of peripheral nerve fibres in acute and chronic inflammation in arthritis. Nat Rev Rheumatol 9: 117–126, 2013. doi: 10.1038/nrrheum.2012.181. [DOI] [PubMed] [Google Scholar]
- 49. Staats P, Giannakopoulos G, Blake J, Liebler E, Levy RM. The use of non-invasive vagus nerve stimulation to treat respiratory symptoms associated with COVID-19: a theoretical hypothesis and early clinical experience. Neuromodulation 23: 784–788, 2020. doi: 10.1111/ner.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kaniusas E, Kampusch S, Tittgemeyer M, Panetsos F, Gines RF, Papa M, Kiss A, Podesser B, Cassara AM, Tanghe E, Samoudi AM, Tarnaud T, Joseph W, Marozas V, Lukosevicius A, Ištuk N, Lechner S, Klonowski W, Varoneckas G, Széles JC, Šarolić A. Current directions in the auricular vagus nerve stimulation II – an engineering perspective. Front Neurosci 13: 772, 2019. doi: 10.3389/fnins.2019.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Genovese MC, Gaylis NB, Sikes D, Kivitz A, Lewis Horowitz D, Peterfy C, Glass EV, Levine YA, Chernoff D. Safety and efficacy of neurostimulation with a miniaturised vagus nerve stimulation device in patients with multidrug-refractory rheumatoid arthritis: a two-stage multicentre, randomised pilot study. Lancet Rheumatol 2: e527–e538, 2020. doi: 10.1016/S2665-9913(20)30172-7. [DOI] [PubMed] [Google Scholar]
- 52. Tornero C, Pastor E, Garzando MDM, Orduña J, Forner MJ, Bocigas I, Cedeño DL, Vallejo R, McClure CK, Czura CJ, Liebler EJ, Staats P. Non-invasive vagus nerve stimulation for COVID-19: Results From a Randomized Controlled Trial (SAVIOR I). Front Neurol 13: 820864, 2022. doi: 10.3389/fneur.2022.820864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci 80: 2314–2319, 2007. doi: 10.1016/j.lfs.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 54. Koarai A, Traves SL, Fenwick PS, Brown SM, Chana KK, Russell REK, Nicholson AG, Barnes PJ, Donnelly LE. Expression of muscarinic receptors by human macrophages. Eur Respir J 39: 698–704, 2012. doi: 10.1183/09031936.00136710. [DOI] [PubMed] [Google Scholar]
- 55. Xu Z-P, Yang K, Xu G-N, Zhu L, Hou L-N, Zhang W-H, Chen H-Z, Cui Y-Y. Role of M3 mAChR in in vivo and in vitro models of LPS-induced inflammatory response. Int Immunopharmacol 14: 320–327, 2012. doi: 10.1016/j.intimp.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 56. Milara J, Cervera A, de Diego A, Sanz C, Juan G, Gavaldà A, Miralpeix M, Morcillo E, Cortijo J. Non-neuronal cholinergic system contributes to corticosteroid resistance in chronic obstructive pulmonary disease patients. Respir Res 17: 145, 2016. doi: 10.1186/s12931-016-0467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Salamone G, Lombardi G, Gori S, Nahmod K, Jancic C, Amaral MM, Vermeulen M, Español A, Sales ME, Geffner J. Cholinergic modulation of dendritic cell function. J Neuroimmunol 236: 47–56, 2011. doi: 10.1016/j.jneuroim.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 58. Sato E, Koyama S, Okubo Y, Kubo K, Sekiguchi M. Acetylcholine stimulates alveolar macrophages to release inflammatory cell chemotactic activity. Am J Physiol Lung Cell Mol Physiol 274: L970–L979, 1998. doi: 10.1152/ajplung.1998.274.6.L970. [DOI] [PubMed] [Google Scholar]
- 59. Safronova VG, Vulfius CA, Shelukhina IV, Mal'tseva VN, Berezhnov AV, Fedotova EI, Miftahova RG, Kryukova EV, Grinevich AA, Tsetlin VI. Nicotinic receptor involvement in regulation of functions of mouse neutrophils from inflammatory site. Immunobiology 221: 761–772, 2016. doi: 10.1016/j.imbio.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 60. Fujii T, Mashimo M, Moriwaki Y, Misawa H, Ono S, Horiguchi K, Kawashima K. Expression and function of the cholinergic system in immune cells. Front Immunol 8: 1085, 2017. doi: 10.3389/fimmu.2017.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Matsunaga K, Klein TW, Friedman H, Yamamoto Y. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J Immunol 167: 6518–6524, 2001. doi: 10.4049/jimmunol.167.11.6518. [DOI] [PubMed] [Google Scholar]
- 62. Zhao C, Yang X, Su EM, Huang Y, Li L, Matthay MA, Su X. Signals of vagal circuits engaging with AKT1 in alpha7 nAChR(+)CD11b(+) cells lessen E. coli and LPS-induced acute inflammatory injury. Cell Discov 3: 17009, 2017. doi: 10.1038/celldisc.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. St-Pierre S, Jiang W, Roy P, Champigny C, LeBlanc É, Morley BJ, Hao J, Simard AR. Nicotinic acetylcholine receptors modulate bone marrow-derived pro-inflammatory monocyte production and survival. PLoS One 11: e0150230, 2016. doi: 10.1371/journal.pone.0150230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fujii YX, Fujigaya H, Moriwaki Y, Misawa H, Kasahara T, Grando SA, Kawashima K. Enhanced serum antigen-specific IgG1 and proinflammatory cytokine production in nicotinic acetylcholine receptor alpha7 subunit gene knockout mice. J Neuroimmunol 189: 69–74, 2007. doi: 10.1016/j.jneuroim.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 65. Heijnen CJ, Rouppe van der Voort C, van de Pol M, Kavelaars A. Cytokines regulate alpha(1)-adrenergic receptor mRNA expression in human monocytic cells and endothelial cells. J Neuroimmunol 125: 66–72, 2002. doi: 10.1016/S0165-5728(02)00034-6. [DOI] [PubMed] [Google Scholar]
- 66. Grisanti LA, Woster AP, Dahlman J, Sauter ER, Combs CK, Porter JE. alpha1-adrenergic receptors positively regulate Toll-like receptor cytokine production from human monocytes and macrophages. J Pharmacol Exp Ther 338: 648–657, 2011. doi: 10.1124/jpet.110.178012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lappin D, Whaley K. Adrenergic receptors on monocytes modulate complement component synthesis. Clin Exp Immunol 47: 606–612, 1982. [PMC free article] [PubMed] [Google Scholar]
- 68. Grisanti LA, Perez DM, Porter JE. Modulation of immune cell function by alpha(1)-adrenergic receptor activation. Curr Top Membr 67: 113–138, 2011. doi: 10.1016/B978-0-12-384921-2.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Konstan MW, Wagener JS, Vandevanter DR, Pasta DJ, Yegin A, Rasouliyan L, Morgan WJ. Risk factors for rate of decline in FEV1 in adults with cystic fibrosis. J Cyst Fibros 11: 405–411, 2012. doi: 10.1016/j.jcf.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Altenburg SP, Martins MA, Silva AR, Cordeiro RS, Castro-Faria-Neto HC. LPS-induced blood neutrophilia is inhibited by alpha 1-adrenoceptor antagonists: a role for catecholamines. J Leukoc Biol 61: 689–694, 1997. doi: 10.1002/jlb.61.6.689. [DOI] [PubMed] [Google Scholar]
- 71. Maestroni GJ. Dendritic cell migration controlled by alpha 1b-adrenergic receptors. J Immunol 165: 6743–6747, 2000. doi: 10.4049/jimmunol.165.12.6743. [DOI] [PubMed] [Google Scholar]
- 72. Maestroni GJ. Sympathetic nervous system influence on the innate immune response. Ann N Y Acad Sci 1069: 195–207, 2006. doi: 10.1196/annals.1351.017. [DOI] [PubMed] [Google Scholar]
- 73. Jetschmann J-U, Benschop RJ, Jacobs R, Kemper A, Oberbeck R, Schmidt RE, Schedlowski M. Expression and in-vivo modulation of alpha- and beta-adrenoceptors on human natural killer (CD16+) cells. J Neuroimmunol 74: 159–164, 1997. doi: 10.1016/S0165-5728(96)00221-4. [DOI] [PubMed] [Google Scholar]
- 74. Xiao J, Huang H-W, Peng Y-P, Bao J-Y, Huang Y, Qiu Y-H. Modulation of natural killer cell function by alpha-adrenoreceptor-coupled signalling. Neuro Endocrinol Lett 31: 635–644, 2010. [PubMed] [Google Scholar]
- 75. Maestroni GJ, Mazzola P. Langerhans cells beta 2-adrenoceptors: role in migration, cytokine production, Th priming and contact hypersensitivity. J Neuroimmunol 144: 91–99, 2003. doi: 10.1016/j.jneuroim.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 76. Ratge D, Wiedemann A, Kohse KP, Wisser H. Alterations of beta-adrenoceptors on human leukocyte subsets induced by dynamic exercise: effect of prednisone. Clin Exp Pharmacol Physiol 15: 43–53, 1988. doi: 10.1111/j.1440-1681.1988.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 77. Grisanti LA, Evanson J, Marchus E, Jorissen H, Woster AP, DeKrey W, Sauter ER, Combs CK, Porter JE. Pro-inflammatory responses in human monocytes are beta1-adrenergic receptor subtype dependent. Mol Immunol 47: 1244–1254, 2010. doi: 10.1016/j.molimm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Garcia-Prieto J, Villena-Gutierrez R, Gomez M, Bernardo E, Pun-García A, García-Lunar I, Crainiciuc G, Fernández-Jiménez R, Sreeramkumar V, Bourio-Martínez R, García-Ruiz JM, Del Valle AS, Sanz-Rosa D, Pizarro G, Fernández-Ortiz A, Hidalgo A, Fuster V, Ibanez B. Neutrophil stunning by metoprolol reduces infarct size. Nat Commun 8: 14780, 2017. doi: 10.1038/ncomms14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yang H, Du R-Z, Qiu J-P, Tang Y-Q, Chen S-C. Bisoprolol reverses epinephrine-mediated inhibition of cell emigration through increases in the expression of beta-arrestin 2 and CCR7 and PI3K phosphorylation, in dendritic cells loaded with cholesterol. Thromb Res 131: 230–237, 2013. doi: 10.1016/j.thromres.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 80. Henricks PA, Van Esch B, Van Oosterhout AJ, Nijkamp FP. Specific and non-specific effects of beta-adrenoceptor agonists on guinea pig alveolar macrophage function. Eur J Pharmacol 152: 321–330, 1988. doi: 10.1016/0014-2999(88)90727-3. [DOI] [PubMed] [Google Scholar]
- 81. Grailer JJ, Haggadone MD, Sarma JV, Zetoune FS, Ward PA. Induction of M2 regulatory macrophages through the beta2-adrenergic receptor with protection during endotoxemia and acute lung injury. J Innate Immun 6: 607–618, 2014. doi: 10.1159/000358524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bosmann M, Grailer JJ, Zhu K, Matthay MA, Sarma JV, Zetoune FS, Ward PA. Anti-inflammatory effects of beta2 adrenergic receptor agonists in experimental acute lung injury. FASEB J 26: 2137–2144, 2012. doi: 10.1096/fj.11-201640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ağaç D, Estrada LD, Maples R, Hooper LV, Farrar JD. The beta2-adrenergic receptor controls inflammation by driving rapid IL-10 secretion. Brain Behav Immun 74: 176–185, 2018. doi: 10.1016/j.bbi.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Donnelly LE, Tudhope SJ, Fenwick PS, Barnes PJ. Effects of formoterol and salmeterol on cytokine release from monocyte-derived macrophages. Eur Respir J 36: 178–186, 2010. doi: 10.1183/09031936.00158008. [DOI] [PubMed] [Google Scholar]
- 85. Scanzano A, Schembri L, Rasini E, Luini A, Dallatorre J, Legnaro M, Bombelli R, Congiu T, Cosentino M, Marino F. Adrenergic modulation of migration, CD11b and CD18 expression, ROS and interleukin-8 production by human polymorphonuclear leukocytes. Inflamm Res 64: 127–135, 2015. doi: 10.1007/s00011-014-0791-8. [DOI] [PubMed] [Google Scholar]
- 86. de Coupade C, Gear RW, Dazin PF, Sroussi HY, Green PG, Levine JD. Beta 2-adrenergic receptor regulation of human neutrophil function is sexually dimorphic. Br J Pharmacol 143: 1033–1041, 2004. doi: 10.1038/sj.bjp.0705972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Marino F, Scanzano A, Pulze L, Pinoli M, Rasini E, Luini A, Bombelli R, Legnaro M, de Eguileor M, Cosentino M. beta2 -Adrenoceptors inhibit neutrophil extracellular traps in human polymorphonuclear leukocytes. J Leukoc Biol 104: 603–614, 2018. doi: 10.1002/JLB.3A1017-398RR. [DOI] [PubMed] [Google Scholar]
- 88. Diaz-Salazar C, Bou-Puerto R, Mujal AM, et al. Cell-intrinsic adrenergic signaling controls the adaptive NK cell response to viral infection. J Exp Med 217: e20190549, 2020. doi: 10.1084/jem.20190549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yukawa T, Ukena D, Kroegel C, Chanez P, Dent G, Chung KF, Barnes PJ. Beta 2-adrenergic receptors on eosinophils. Binding and functional studies. Am Rev Respir Dis 141: 1446–1452, 1990. doi: 10.1164/ajrccm/141.6.1446. [DOI] [PubMed] [Google Scholar]
- 90. Hadjokas NE, Crowley JJ, Bayer CR, Nielson CP. Beta-adrenergic regulation of the eosinophil respiratory burst as detected by lucigenin-dependent luminescence. J Allergy Clin Immunol 95: 735–741, 1995. doi: 10.1016/S0091-6749(95)70179-6. [DOI] [PubMed] [Google Scholar]
- 91. Munoz NM, Vita AJ, Neeley SP, McAllister K, Spaethe SM, White SR, Leff AR. Beta adrenergic modulation of formyl-methionine-leucine-phenylalanine-stimulated secretion of eosinophil peroxidase and leukotriene C4. J Pharmacol Exp Ther 268: 139–143, 1994. [PubMed] [Google Scholar]
- 92. Mannaioni PF, Mastroianni R, Mastrangelo D. Adrenaline inhibits the immunological activation of human basophils at pharmacological and ultra-low doses. Med Sci Monit 16: BR227–32, 2010. [PubMed] [Google Scholar]
- 93. Marone G, Ambrosio G, Bonaduce D, Genovese A, Triggiani M, Condorelli M. Inhibition of IgE-mediated histamine release from human basophils and mast cells by fenoterol. Int Arch Allergy Appl Immunol 74: 356–361, 1984. doi: 10.1159/000233573. [DOI] [PubMed] [Google Scholar]
- 94. Gebhardt T, Gerhard R, Bedoui S, Erpenbeck VJ, Hoffmann MW, Manns MP, Bischoff SC. beta2-Adrenoceptor-mediated suppression of human intestinal mast cell functions is caused by disruption of filamentous actin dynamics. Eur J Immunol 35: 1124–1132, 2005. doi: 10.1002/eji.200425869. [DOI] [PubMed] [Google Scholar]
- 95. Barnes PJ. Effect of beta-agonists on inflammatory cells. J Allergy Clin Immunol 104: S10–S17, 1999. doi: 10.1016/S0091-6749(99)70269-1. [DOI] [PubMed] [Google Scholar]
- 96. Church MK, Hiroi J. Inhibition of IgE-dependent histamine release from human dispersed lung mast cells by anti-allergic drugs and salbutamol. Br J Pharmacol 90: 421–429, 1987. doi: 10.1111/j.1476-5381.1987.tb08972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Drury DE, Chong LK, Ghahramani P, Peachell PT. Influence of receptor reserve on beta-adrenoceptor-mediated responses in human lung mast cells. Br J Pharmacol 124: 711–718, 1998. doi: 10.1038/sj.bjp.0701897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chong LK, Cooper E, Vardey CJ, Peachell PT. Salmeterol inhibition of mediator release from human lung mast cells by beta-adrenoceptor-dependent and independent mechanisms. Br J Pharmacol 123: 1009–1015, 1998. doi: 10.1038/sj.bjp.0701703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wozniak A, McLennan G, Betts WH, Murphy GA, Scicchitano R. Activation of human neutrophils by substance P: effect on FMLP-stimulated oxidative and arachidonic acid metabolism and on antibody-dependent cell-mediated cytotoxicity. Immunology 68: 359–364, 1989. [PMC free article] [PubMed] [Google Scholar]
- 100. Sun J, Ramnath RD, Bhatia M. Neuropeptide substance P upregulates chemokine and chemokine receptor expression in primary mouse neutrophils. Am J Physiol Cell Physiol 293: C696–C704, 2007. doi: 10.1152/ajpcell.00060.2007. [DOI] [PubMed] [Google Scholar]
- 101. Feistritzer C, Clausen J, Sturn DH, Djanani A, Gunsilius E, Wiedermann CJ, Kähler CM. Natural killer cell functions mediated by the neuropeptide substance P. Regul Pept 116: 119–126, 2003. doi: 10.1016/S0167-0115(03)00193-9. [DOI] [PubMed] [Google Scholar]
- 102. Monaco-Shawver L, Schwartz L, Tuluc F, Guo C-J, Lai JP, Gunnam SM, Kilpatrick LE, Banerjee PP, Douglas SD, Orange JS. Substance P inhibits natural killer cell cytotoxicity through the neurokinin-1 receptor. J Leukoc Biol 89: 113–125, 2011. doi: 10.1189/jlb.0410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol 159: 5654–5660, 1997. [PubMed] [Google Scholar]
- 104. Pappa V, Spitsin S, Gaskill PJ, Douglas SD. Neurokinin-1 receptor signaling induces a pro-inflammatory transcriptomic profile in CD16+ monocytes. J Neuroimmunol 353: 577524, 2021. doi: 10.1016/j.jneuroim.2021.577524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 123: 398–410, 2008. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhan M, Zheng W, Jiang Q, Zhao Z, Wang Z, Wang J, Zhang H, He S. Upregulated expression of substance P (SP) and NK1R in eczema and SP-induced mast cell accumulation. Cell Biol Toxicol 33: 389–405, 2017. doi: 10.1007/s10565-016-9379-0. [DOI] [PubMed] [Google Scholar]
- 107. Lambrecht BN, Germonpré PR, Everaert EG, Carro‐Muino I, De Veerman M, de Felipe C, Hunt SP, Thielemans K, Joos GF, Pauwels RA. Endogenously produced substance P contributes to lymphocyte proliferation induced by dendritic cells and direct TCR ligation. Eur J Immunol 29: 3815–3825, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 108. Janelsins BM, Mathers AR, Tkacheva OA, Erdos G, Shufesky WJ, Morelli AE, Larregina AT. Proinflammatory tachykinins that signal through the neurokinin 1 receptor promote survival of dendritic cells and potent cellular immunity. Blood 113: 3017–3026, 2009. doi: 10.1182/blood-2008-06-163121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhang X-W, Li L, Hu W-Q, Hu M-N, Tao Y, Hu H, Miao X-K, Yang W-L, Zhu Q, Mou L-Y. Neurokinin-1 receptor promotes non-small cell lung cancer progression through transactivation of EGFR. Cell Death Dis 13: 41, 2022. doi: 10.1038/s41419-021-04485-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kitamura H, Kobayashi M, Wakita D, Nishimura T. Neuropeptide signaling activates dendritic cell-mediated type 1 immune responses through neurokinin-2 receptor. J Immunol 188: 4200–4208, 2012. doi: 10.4049/jimmunol.1102521. [DOI] [PubMed] [Google Scholar]
- 111. Ohtake J, Kaneumi S, Tanino M, Kishikawa T, Terada S, Sumida K, Masuko K, Ohno Y, Kita T, Iwabuchi S, Shinohara T, Tanino Y, Takemura T, Tanaka S, Kobayashi H, Kitamura H. Neuropeptide signaling through neurokinin-1 and neurokinin-2 receptors augments antigen presentation by human dendritic cells. J Allergy Clin Immunol 136: 1690–1694, 2015. doi: 10.1016/j.jaci.2015.06.050. [DOI] [PubMed] [Google Scholar]
- 112. Kraneveld AD, Nijkamp FP, Van Oosterhout AJ. Role for neurokinin-2 receptor in interleukin-5-induced airway hyperresponsiveness but not eosinophilia in guinea pigs. Am J Respir Crit Care Med 156: 367–374, 1997. doi: 10.1164/ajrccm.156.2.9608101. [DOI] [PubMed] [Google Scholar]
- 113. Raap M, Rüdrich U, Ständer S, Gehring M, Kapp A, Raap U. Substance P activates human eosinophils. Exp Dermatol 24: 557–559, 2015. doi: 10.1111/exd.12717. [DOI] [PubMed] [Google Scholar]
- 114. Li M, Wetzel-Strong SE, Hua X, Tilley SL, Oswald E, Krummel MF, Caron KM. Deficiency of RAMP1 attenuates antigen-induced airway hyperresponsiveness in mice. PLoS One 9: e102356, 2014. doi: 10.1371/journal.pone.0102356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Soultanova A, Mikulski Z, Pfeil U, Grau V, Kummer W. Calcitonin peptide family members are differentially regulated by lps and inhibit functions of rat alveolar NR8383 macrophages. PLoS One 11: e0163483, 2016. doi: 10.1371/journal.pone.0163483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Richter J, Andersson R, Edvinsson L, Gullberg U. Calcitonin gene-related peptide (CGRP) activates human neutrophils–inhibition by chemotactic peptide antagonist BOC-MLP. Immunology 77: 416–421, 1992. [PMC free article] [PubMed] [Google Scholar]
- 117. Carucci JA, Ignatius R, Wei Y, Cypess AM, Schaer DA, Pope M, Steinman RM, Mojsov S. Calcitonin gene-related peptide decreases expression of HLA-DR and CD86 by human dendritic cells and dampens dendritic cell-driven T cell-proliferative responses via the type I calcitonin gene-related peptide receptor. J Immunol 164: 3494–3499, 2000. doi: 10.4049/jimmunol.164.7.3494. [DOI] [PubMed] [Google Scholar]
- 118. Mikami N, Matsushita H, Kato T, Kawasaki R, Sawazaki T, Kishimoto T, Ogitani Y, Watanabe K, Miyagi Y, Sueda K, Fukada S-I, Yamamoto H, Tsujikawa K. Calcitonin gene-related peptide is an important regulator of cutaneous immunity: effect on dendritic cell and T cell functions. J Immunol 186: 6886–6893, 2011. doi: 10.4049/jimmunol.1100028. [DOI] [PubMed] [Google Scholar]
- 119. Murray K, Rude KM, Sladek J, Reardon C. Divergence of neuroimmune circuits activated by afferent and efferent vagal nerve stimulation in the regulation of inflammation. J Physiol 599: 2075–2084, 2021. doi: 10.1113/JP281189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Guarini S, Altavilla D, Cainazzo M-M, Giuliani D, Bigiani A, Marini H, Squadrito G, Minutoli L, Bertolini A, Marini R, Adamo EB, Venuti FS, Squadrito F. Efferent vagal fibre stimulation blunts nuclear factor-κb activation and protects against hypovolemic hemorrhagic shock. Circulation 107: 1189–1194, 2003. doi: 10.1161/01.CIR.0000050627.90734.ED. [DOI] [PubMed] [Google Scholar]
- 121. Jammes Y, Fornaris E, Mei N, Barrat E. Afferent and efferent components of the bronchial vagal branches in cats. J Auton Nerv Syst 5: 165–176, 1982. doi: 10.1016/0165-1838(82)90037-6. [DOI] [PubMed] [Google Scholar]
- 122. Komegae EN, Farmer DGS, Brooks VL, McKinley MJ, McAllen RM, Martelli D. Vagal afferent activation suppresses systemic inflammation via the splanchnic anti-inflammatory pathway. Brain Behav Immun 73: 441–449, 2018. doi: 10.1016/j.bbi.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Su X, Matthay MA, Malik AB. Requisite role of the cholinergic alpha7 nicotinic acetylcholine receptor pathway in suppressing Gram-negative sepsis-induced acute lung inflammatory injury. J Immunol 184: 401–410, 2010. doi: 10.4049/jimmunol.0901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gao Z-W, Li L, Huang Y-Y, Zhao C-Q, Xue S-J, Chen J, Yang Z-Z, Xu J-F, Su X. Vagal-α7nAChR signaling is required for lung anti-inflammatory responses and arginase 1 expression during an influenza infection. Acta Pharmacol Sin 42: 1642–1652, 2021. doi: 10.1038/s41401-020-00579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yang H, George SJ, Thompson DA, Brines M, Pavlav VA, Andersson U, Chavan SS, Tracey KJ. Famotidine exerts anti-inflammatory effects via a vagus nerve-dependent mechanism. FASEB J 36, 2022. doi: 10.1096/fasebj.2022.36.S1.R5540. [DOI] [Google Scholar]
- 126. Kox M, Pompe JC, Peters E, Vaneker M, van der Laak JW, van der Hoeven JG, Scheffer GJ, Hoedemaekers CW, Pickkers P. α7 Nicotinic acetylcholine receptor agonist GTS-21 attenuates ventilator-induced tumour necrosis factor-α production and lung injury. Br J Anaesth 107: 559–566, 2011. doi: 10.1093/bja/aer202. [DOI] [PubMed] [Google Scholar]
- 127. Kummer W. Pulmonary vascular innervation and its role in responses to hypoxia. Proc Am Thorac Soc 8: 471–476, 2011. doi: 10.1513/pats.201101-013MW. [DOI] [PubMed] [Google Scholar]
- 128. Liu T, Yang L, Han X, Ding X, Li J, Yang J. Local sympathetic innervations modulate the lung innate immune responses. Sci Adv 6: eaay1497-eaay1497, 2020. doi: 10.1126/sciadv.aay1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Walker JK, DeFea KA. Role for β-arrestin in mediating paradoxical β2AR and PAR2 signaling in asthma. Curr Opin Pharmacol 16: 142–147, 2014. doi: 10.1016/j.coph.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Mutlu GM, Green D, Bellmeyer A, Baker CM, Burgess Z, Rajamannan N, Christman JW, Foiles N, Kamp DW, Ghio AJ, Chandel NS, Dean DA, Sznajder JI, Budinger GRS. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J Clin Invest 117: 2952–2961, 2007. doi: 10.1172/JCI30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chiarella SE, Soberanes S, Urich D, Morales-Nebreda L, Nigdelioglu R, Green D, Young JB, Gonzalez A, Rosario C, Misharin AV, Ghio AJ, Wunderink RG, Donnelly HK, Radigan KA, Perlman H, Chandel NS, Budinger GRS, Mutlu GM. Adrenergic agonists augment air pollution–induced IL-6 release and thrombosis. J Clin Invest 124: 2935–2946, 2014. β2 doi: 10.1172/JCI75157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Walker JKL, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, Lefkowitz RJ. Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest 112: 566–574, 2003. doi: 10.1172/JCI200317265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Alawi K, Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther 125: 181–195, 2010. doi: 10.1016/j.pharmthera.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 134. Zheng J. Molecular mechanism of TRP channels. Compr Physiol 3: 221–242, 2013. doi: 10.1002/cphy.c120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kollarik M, Ru F, Brozmanova M. Vagal afferent nerves with the properties of nociceptors. Auton Neurosci 153: 12–20, 2010. doi: 10.1016/j.autneu.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Brouns I, Van Genechten J, Hayashi H, Gajda M, Gomi T, Burnstock G, Timmermans J-P, Adriaensen D. Dual sensory innervation of pulmonary meuroepithelial bodies. Am J Respir Cell Mol Biol 28: 275–285, 2003. doi: 10.1165/rcmb.2002-0117OC. [DOI] [PubMed] [Google Scholar]
- 137. Ruff MR, Wahl SM, Pert CB. Substance P receptor-mediated chemotaxis of human monocytes. Peptides 6 Suppl 2: 107–111, 1985. doi: 10.1016/0196-9781(85)90142-1. [DOI] [PubMed] [Google Scholar]
- 138. Newby DE, Sciberras DG, Ferro CJ, Gertz BJ, Sommerville D, Majumdar A, Lowry RC, Webb DJ. Substance P-induced vasodilatation is mediated by the neurokinin type 1 receptor but does not contribute to basal vascular tone in man. Br J Clin Pharmacol 48: 336–344, 1999. doi: 10.1046/j.1365-2125.1999.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bowden JJ, Garland AM, Baluk P, Lefevre P, Grady EF, Vigna SR, Bunnett NW, McDonald DM. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci USA 91: 8964–8968, 1994. doi: 10.1073/pnas.91.19.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Nakagawa N, Sano H, Iwamoto I. Substance P induces the expression of intercellular adhesion molecule-1 on vascular endothelial cells and enhances neutrophil transendothelial migration. Peptides 16: 721–725, 1995. doi: 10.1016/0196-9781(95)00037-k. [DOI] [PubMed] [Google Scholar]
- 141. Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Bubeck Wardenburg J, Hwang SW, Carroll MC, Woolf CJ. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501: 52–57, 2013. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Pinho-Ribeiro FA, Baddal B, Haarsma R, O'Seaghdha M, Yang NJ, Blake KJ, Portley M, Verri WA, Dale JB, Wessels MR, Chiu IM. Blocking neuronal signaling to immune cells treats streptococcal invasive infection. Cell 173: 1083–1097.e22, 2018. doi: 10.1016/j.cell.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lai NY, Musser MA, Pinho-Ribeiro FA, Baral P, Ma P, Potts DE, Chen Z, Paik D, Soualhi S, Shi H, Misra A, Goldstein K, Sivanathan KN, Jacobson A, Wallrapp A, Lagomarsino V, Kuchroo VK, Nowarski R, Starnbach MN, Surana NK, An D, Wu C, Huh JR, Rao M, Chiu IM. Gut-innervating nociceptor neurons protect against enteric infection by modulating the microbiota and Peyer’s patch microfold cells. bioRxiv 580555, 2019. doi: 10.1101/580555. [DOI]
- 144. Ramirez VT, Sladek J, Godinez DR, Rude KM, Chicco P, Murray K, Brust-Mascher I, Gareau MG, Reardon C. Sensory nociceptive neurons contribute to host protection during enteric infection with citrobacter rodentium. J Infect Dis 221: 1978–1988, 2020. doi: 10.1093/infdis/jiaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Talavera K, Startek JB, Alvarez-Collazo J, Boonen B, Alpizar YA, Sanchez A, Naert R, Nilius B. Mammalian transient receptor potential TRPA1 channels: from structure to disease. Physiol Rev 100: 725–803, 2020. doi: 10.1152/physrev.00005.2019. [DOI] [PubMed] [Google Scholar]
- 146. Kaniusas E, Szeles JC, Kampusch S, Alfageme-Lopez N, Yucuma-Conde D, Li X, Mayol J, Neumayer C, Papa M, Panetsos F. Non-invasive auricular vagus nerve stimulation as a potential treatment for Covid19-originated acute respiratory distress syndrome. Front Physiol 11: 890, 2020. doi: 10.3389/fphys.2020.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bonaz B, Sinniger V, Pellissier S. Targeting the cholinergic anti-inflammatory pathway with vagus nerve stimulation in patients with Covid-19? Bioelectron Med 6: 15, 2020. doi: 10.1186/s42234-020-00051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Leitzke M, Stefanovic D, Meyer J-J, Schimpf S, Schönknecht P. Autonomic balance determines the severity of COVID-19 courses. Bioelectron Med 6: 22, 2020. doi: 10.1186/s42234-020-00058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ma W, Dumont Y, Vercauteren F, Quirion R. Lipopolysaccharide induces calcitonin gene-related peptide in the RAW264.7 macrophage cell line. Immunology 130: 399–409, 2010. doi: 10.1111/j.1365-2567.2009.03239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kawashima-Takeda N, Ito Y, Nishizawa N, Kawashima R, Tanaka K, Tsujikawa K, Watanabe M, Majima M. RAMP1 suppresses mucosal injury from dextran sodium sulfate-induced colitis in mice. J Gastroenterol Hepatol 32: 809–818, 2017. doi: 10.1111/jgh.13505. [DOI] [PubMed] [Google Scholar]
- 151. Fairfax KA, Bolden JE, Robinson AJ, Lucas EC, Baldwin TM, Ramsay KA, Cole R, Hilton DJ, de Graaf CA. Transcriptional profiling of eosinophil subsets in interleukin-5 transgenic mice. J Leukoc Biol 104: 195–204, 2018. doi: 10.1002/JLB.6MA1117-451R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Wallrapp A, Burkett PR, Riesenfeld SJ, Kim S-J, Christian E, Abdulnour R-EE, Thakore PI, Schnell A, Lambden C, Herbst RH, Khan P, Tsujikawa K, Xavier RJ, Chiu IM, Levy BD, Regev A, Kuchroo VK. Calcitonin gene-related peptide negatively regulates alarmin-driven type 2 innate lymphoid cell responses. Immunity 51: 709–723.e6, 2019. doi: 10.1016/j.immuni.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]